***CAUTION... Many of the stocks listed here are easily subjected to manipulation... http://sharesleuth.com/investigations/2018/03/pretenders-and-ghosts-stealth-promotion-network-exploits-financial-sites-to-tout-stocks
John Maynard Keynes once wrote, “It is safer to be a speculator than an investor in the sense that a speculator is one who runs risks of which he is aware and an investor is one who runs risks of which he is unaware.”
Don't forget to vote...something needs to change in this picture...We need change... for starters we do not need career politicians (aka brown noseing lap dogs that only vote the party line out of fear of not getting re-elected)...we need senators and congressman elected that are willing to represent their State and their people and know how to compromise... Congress needs to stop allllllllllllllllllllllllll these endless wars and take responsibility=vote if troops are needed ... Congress needs to stop letting the lobyist write 1000 page bills that they vote on before they read the bill (infact they sometimes aren't allowed to read the new bill unless they pass it). Pensions and health care need to be take away from Congress and Cabinet Members because they haven't been doing their job in the first place.

Your life and finances have been hijacked. Sold to the highest bidder. Your rights and ability to save and earn have been slowly eroded, picked apart by bankers, lobbyists, politicians and inflation. I've seen the slow decay firsthand. Since the turn of the century, the world has been changing...
International bankers have taken over the world's finances. They print as much paper currency as they want, conspire to manipulate global interest rates, launder money for rogue entities, and generally operate above the law.
Pensions are gone. Social Security will soon be gone. Costs keep rising for food, energy, health care, and other essentials.
But wages barely grow. If you can afford to save at all, investment fees, wild market swings, poor yield, and ever-increasing taxes eat away at what should be your nest egg...
Millions of people are starting to realize that the American Dream is a myth. That it's not those who work hard and obey the rules that get ahead, but rather those who make the rules, and change them as they see fit.
Debt is out of control. We fight trillion-dollar, multi-year wars the majority of people are against.
And now, they're treating citizens like criminals... The Patriot Act has erased your privacy and liberties. There are millions of cameras watching you — on every street corner and traffic light, in every airport, and silently from up in the sky. Your calls are monitored, your Internet traffic observed.
Those truly in charge — the banking elite who control the currency and exercise their will by pulling the strings of puppet politicians — continue to compound their wealth.
They placate a third of the country with handouts: nearly 50 million people are on food stamps, 10 million are on disability, and millions more are taking drugs prescribed for “depression.” The rest of us pay for it all with our hard-earned money and freedoms. We're the Outsiders. Nick Hodge... the OutSider Club
122 Things Everyone Should Know About Investing
The inflation pipeline has three stages. The first stage is the price of raw materials. The second stage is the price companies pay for raw materials (producer price inflation). The third stage is what companies charge consumers for their products (CPI inflation). It all starts with the direction of commodity prices. Strangely, that's the part that economists (and the Fed) pay no attention to. How can economists expect to predict the final stage of CPI inflation, if they ignore the first stage which is the direction of commodity prices? ...John Murphy StockCharts.com
https://wallmine.com/
http://www.stock1010.com/
http://www.valuewalk.com/stock-watchlists/
2018...Incredible Change Is Coming to the Business World
Now, there are opportunities like this unfolding in every sector of our economy right now.
In finance, it’s the emergence of blockchain and mobile payments.
In energy, it’s a shift to natural, sustainable, renewable energy that is local, storable and portable.
In transportation, it’s a shift to electric motors and self-driving cars, trucks, ships and planes.
In food, it’s a shift to local, organic and natural food and meal kits.
For retailers, it’s a shift to online shopping and brands that cater to the millennial generation’s preferences.
In building, it’s the coming use of building blocks and modules and 3-D printing.
Incredible change is coming all at once.
For investors who are open to investing in these changes in the business world, and who are in the right stocks, it’s a time of incredible opportunity. For those who stay invested in the old ways, and the companies whose technologies and products are based on the old ways, they’re going to lose their money as these companies are wiped out. https://banyanhill.com/business-world-revolutionary-change/
The Game in Wall Street and How to Play it Successfully ...by Hoyle...this is a tiny book copyright 1898. The sections on PANICS and Systems/Speculation are very good. Try to find some time to read it...
https://babel.hathitrust.org/cgi/pt?id="hvd.hb1j3s;view=1up;seq=48"
"...we shall adhere to our opinion that 95% of the business (trading) in Wall Street is part of a game.
We will show later that it is NOT A Game of Chance, but a GAME of SKILL."
"There is no business in the world that, win or lose, is so sure to wreck soul, mind and body, as is Wall Street speculation."
"...it seems to be the general opinion that anyone can rush into stock speculation and make a fortune. If this theory were true, if the amateurs could win as a rule ... If the public should take away more money from the Street than it brought, who would pay the expenses of the game? Someone must lose, and if it were the brokers and the pools they would shut up shop after a season or two."
(...I came to this same conclusion (see below) about 10 years ago..about Systems and opinions... kiy)
\
Markets are complicated. Market analysis is complicated. Developing a trading system certainly can be complicated. But trading cannot be complicated. The act of placing trades and evaluating results must be as streamlined and simplified as possible. Here are a few suggestions for things you can do today, without investing an enormous amount of time and energy, to improve your trading or investing. Chances are that you are already doing some of these things, but can you do them better?
1. Make sure you have an edge. I hate to keep coming back to this, but it matters. Furthermore, most things most people do don't actually work. Are you certain your methodology has a statistical edge in the long run? You will make two piles of money from your trades: money you win and money you lose. Are you certain the “money you will win” pile will be bigger? If not, develop and test a methodology until you have confidence in it.
2. Keep good records. Your broker statement is not adequate. You need good records of why you took a trade, what you saw when you took it (or what conditions triggered an entry), how the P&L developed, what decisions you made once you were in a trade, etc. Don’t make this a daunting task; if you create a system that is onerous you will not follow it, so you have to strike a balance.
3. Work on your process. Do you have an established process for finding new trades? What markets to look at, when, how, and what will trigger an entry? How about for evaluating existing trades? When to take partial profits, when to tighten stops, when to exit, when to add, etc.? You can’t be consistent unless you have a defined process you follow each and every time.
4. Think about how you evaluate your results. The conventional wisdom is that we should evaluate our winning trades, but that might not be right. Do you know how to tease out the impact of luck in your results? Do you know how to evaluate the stability of your edge? These things matter far more than looking at charts of winning and losing trades you made.
5. Work on yourself. Though I’m often critical of trading psychology (believing that correct trading has a much clearer impact on psychology than vice versa), there is no doubt that regular exercise, living from a conviction in the abundant bounty of the universe, working on impulse control, being connected to friends and family, and practicing conscious gratitude lead to a happier and healthier you. Consider adding some of these to your daily process, and always work to get better.
5 ways to improve your trading today was originally published on Adam H Grimes http://adamhgrimes.com/blog/
You are only as good as your next trade...Kiy

DAY TRADING IS HARD, an exceptionally difficult undertaking that requires diligence, skill, insight, discipline, flexibility, adaptability . . . . Please note: Kiy is a swingtrader and there is a very big difference between daytrading and swing trading and yes; daytrading is... and all trading is hard.
With Swing Trading you have velocity on your side & compounding... this blows buy and hold returns away... this is FACT. Consider the swings that have taken place. Thousands of points of potential opportunity... but you have to work for it.
Subjective vs. Objective....The truth is... we need to wait for an actual signal instead of jumping the gun and projecting our emotions/opinion into the data. You can then swing trade trying to capture +10-15% from oversold levels and if you catch a good trend you can get more...FAD-fanatics...Either you learn to be very objective (able to look at both sides in your assessment or you remain a member of the FAD-fanatics... A good way to break this addiction of the Fad-fanatic is practice= you argue for the BEAR when you're Bullish...argue for the Bull when you're Bearish...and objectivity starts to show...If you can't point to it on the PRICE Chart for everyone to see; you are being too subjective. Your Subjective feelings and emotions and egothinking have no place in trading...you are the weakest link if you bring these traits into your trading.
........................................................................................................................................................................................
5 simple rules:
-- Don't risk more than you can lose (I'm talking 100%, not some arbitrary stop)
-- Take half off if it doubles (so you can play with the house's money)
-- Don't be afraid to take a loss
-- Don't buy a falling knife
-- Don't fall in love
A key to trading is to wait patiently for a stock to pullback to a key technical or price support level. This will allow you to set a tight stop
while minimizing losses in case a trade goes against you.
***As a reminder, it is estimated that roughly 60%-70% of a stock’s movement over a 12-month period can be attributed to its post-earnings moves. From a trading perspective, this spells opportunity, particularly if one looks to position trade once the earnings report has come out.
Some RULES for this I-Hub Board...........You are totally responsible for your trades...
This board will be used for analysis of charts ranging in different time periods to determine signals for trading of various instruments=individual stocks and options. Please direct your comments towards technical indicators that everyone can see on a price chart: we are talking about signals and technical indicators here, not opinions or predictions or forecasts of when the next grand slam will be... share technical signals and charts with the group and if you have an active position...... Rather than being opinionated and letting your subjective bias determine which side of the market to be on, try to maintain objectivity based on technical analysis and the fundamental story of the stock. Intellect is the capacity for understanding, thinking, and reasoning, as distinct from feeling or wishing. The faculty of reasoning and understanding objectively, especially with regard to abstract or academic matters will serve you best.
One of the key items to remember as investors/traders is that the market doesn't always reflect reality. Oftentimes it reflects belief. And belief can prove to be a delusion. "A belief held without objective examples that everyone can see is a delusion."
“Opinion is that exercise of the human will which 'lets' us make a decision without objective information and objective examples.” And is not a choice that promotes a healthy process.
“Discussion is an exchange of knowledge; an argument an exchange of ignorance.”
If you don't know yourself; the Stock Market is an expensive place to find out...
It is no disgrace to be wrong... disgrace happens when you choose to stay wrong...
YES Symphonic...And You and I
Anticipation vs. Rewards: Is it in the Stock or in the Brain?
If you feel you must GAMBLE and encourage gambling from the 5 minute or lower time frames you will be asked to take your message to the "gamblers board"...If you feel you "must offer an opinion or off topic comment; please do it after trading hours. Charts and signals at signal lines is what we want...and If you feel you do not need to answer questions on this board ...then why are you here.
I-Hub's rules state: It is the burden of each poster to ensure that their posts do not contain content that qualifies them for removal. It doesn't matter if your post contains the cure to the common cold or the best stock tip since (fill in your favorite ticker here), if it contains other content that is a violation of the site's rules of conduct, then it qualifies for removal. ...
Thank You... Kiy...ki...theMatrix You are only as good as your next trade...Kiy
This release may contain "forward-looking statements" that are within the safe harbor provisions of the Private Securities Litigation Reform Act of 1995. Forward-looking statements are identified by certain words or phrases such as "may", "will", "aim", "will likely result", "believe", "expect", "will continue", "anticipate", "estimate", "intend", "plan", "contemplate", "seek to", "future", "objective", "goal", "project", "should", "will pursue" and similar expressions or variations of such expressions. ...
"I am tempted to say that stupid and drunk is another bad combination, but that would be disingenuous. It is after all one of the great combos that humanity has brought forth upon this big blue marble, right up there with high crimes and misdemeanors, drawing and quartering, and the biathlon (frozen snot rules). But stupid, drunk and posting? That's a losing trifecta, always has been and always will be." (...LOL...I won't say who posted this; found it on the "JailHouse Board...I've never been sent there...yet...)
//////////////////////////////////////////////////////////////////////////////////////////////////////////////////////////////////////////////////////////////////////////////////////////////////////////////////////////////////
RISK not... want not...
The key to trading anything is the recognition that you can control risk. Once you embrace that idea, the wild moves in stocks don't seem so random and unpredictable. You can handle them because you will cut your exposure when they don't cooperate. ....Nothing to it...right...?...
... it has been well said, humans think in herds; it will be seen that they go mad in herds, while they only recover their senses slowly, and one by one." Extraordinary Popular Delusions and the Madness of Crowds ...Charles Mackay 1841

...as with anything in a market, anything can be real as long as enough people believe in it....(...comment from SeekingAlpha ...
(...next few links are for the bubble heads that don't understand Market psychology or their own psychology...
If you don't know yourself; the Stock Market is an expensive place to find out...
Argumentation theory...Argument from ignorance...Confirmation bias
Psycho-Therapy
Look What They Done to My Song
( ...and so...its a certainty that you can be fooled...its easy to be fooled...and it is even easier to FOOL yourself...kiy... Is it in the Stock or in the Brain?
Still a man hears what he wants to hear and disregards the rest
Fear, Uncertainty and Doubt (often shortened to FUD) is a disinformation strategy used in sales, marketing, public relations, talk radio, politics, religious organizations, and propaganda. FUD is generally a strategy to influence perception by disseminating negative and dubious or false information and a manifestation of the appeal to fear. While the phrase dates to at least the early 20th century, the present common usage of disinformation related to software, hardware and technology industries generally appeared in the 1970s to describe disinformation in the computer hardware industry, and has since been used more broadly.

... the fact is that quite frequently we behave as if controlled by little green men from outer space. We are the only species on the planet that is always at odds with each other, with practically all other species, and with the planet itself. We are the only species with wars, jails, ghettos and mental institutions, where we act and live worse than animals would anywhere. What have we done to ourselves as a species? What is the force that binds us to selfish deeds?
“Just look at us. Everything is backwards, everything is upside down. Doctors destroy health, lawyers destroy justice, psychiatrists destroy minds, scientists destroy truth, major media destroys information, religions destroy spirituality and governments destroy freedom.” -Michael Ellner It holds true that Humans aren't complicated they just complicate everything...it also holds true that Humans seem to have to go to extremes with everything before concensus asks to draw it in a bit to the middle road = seek some "balance" here people ...to an outside observer humans really do appear to be a bunch of YoYos...(Concensus in government seems to have come to a standstill...gridlock and laws come in packages of thousands of pages...perpetuates the insanity of the people in power ...and they think they're "Normal"...this isn't normal...why aren't there more humanitarians in congress...more mothers in congress...can't just have a bunch of people with law degrees that says they can argue any point about anything to absurdity...just look at who's running for president...just look at who's president 2017...more of the same or outright insanity...that's some great choices YOU PEOPLE have made... isn't it...so don't forget to vote... In philosophy, "the Absurd" refers to the conflict between (1) the human tendency to seek inherent value and meaning in life and (2) the human inability to find any. In this context absurd does not mean "logically impossible", but rather "humanly impossible". ===============================================================================================================================
Bubbles
According to Grantham, "Investment bubbles and high animal spirits do not materialize out of thin air. They need extremely favorable economic fundamentals together with free and easy cheap credit, and they need it for at least two or three years. Importantly, they also need serial pleasant surprises in such critical variables as global GNP growth."
If BUBBLES, Fads, and Mania are a human characteristic (mass hysteria collective obsessive behavior)...Humans be very flawed...Kiy...
\
What would a panic sell of Bitcoin look like...?... A bunch of people trying to get their money back from nowhere. BitCoin...?...Sh!tCoin...?...
"I may be a little bit over exposed, I am using a little bit of spidey sense, instincts etc. and I do not like doing that with the math based monkey brain attatched in my head..." (LOL...:) ...from The 2-1/2 Minute Warning
Some comments from ZeroHedge: Crypto Currency...
Oh, you mean that so called currency based on nothing more than some electricity-wasting calculations? Now that's a real storage of value "=) Seriously... whatever it is you buy, make sure it's something tangible and has actual worth. When this illusion we're living under finally collapses, necessity will demand real things... not empty promises. SPEAKING of RAGING BULLS
Risk management has been completely thrown out the window. Big money is doing this. Retail traders can't drive markets like this. Absolutely stunning. The only way to participate is to chase. Laggards aren't worth buying. That which under performs bull markets will over perform in a bear market to the downside. The decade of easy money is finally exploding the bubble. It is truly a sight to behold. The eventual implosion will end empires. ...Circlem Jan. 2018
Gold/Silver Ratio When empires are about to fail, war looks like a great alternative to fool the masses as to what is really going on. Currency Wars, Trade Wars and Shooting Wars...
On the Madness of Crowds..."Once the crowd gains momentum in the direction of stupid, there is no stopping it." not so famous quote by some guy...Circlem
Bitcoin is going up in price, not in value.

 Goldman’s Sharmin Mossavar-Rahmani and Brett Nelson write. They note cryptocurrencies already dwarf both the dot-com bubble and the notorious Dutch “Tulipmania,” a period where tulip bulbs became a prized commodity between 1634 and 1637 and prices went haywire.
Goldman’s Sharmin Mossavar-Rahmani and Brett Nelson write. They note cryptocurrencies already dwarf both the dot-com bubble and the notorious Dutch “Tulipmania,” a period where tulip bulbs became a prized commodity between 1634 and 1637 and prices went haywire. The bubble logic driving tulipomania has since acquired a name: “the greater fool theory.” Although by any conventional measure it is folly to pay thousands for a tulip bulb (or for that matter an Internet stock, ...a BitCoin), as long as there is an even greater fool out there willing to pay even more, doing so is the most logical thing in the world.
– Michael Pollan, The Botany of Desire: A Plant’s-Eye View of the World (2001)

Albert Einstein http://quoteinvestigator.com/2011/10/31/compound-interest/
Create Half a Million $ With $5 a day
https://medium.com/mcubedblog/create-half-a-million-with-5-a-day-ec73d56f7055
Literally everyone can build wealth the tried and true way.
The S&P500 Index, one of many indicator of stock market performance (by tracking big US businesses), averaged 9.5% annual returns from 1928 to 2015
Now let’s take our $5 a day, which adds up to to $1,825 a year, and put it into an S&P500 or total stock market index or mutual fund. Which just means it should have similar performance, over the long term, as the S&P500’s 9.5% annual returns. We contribute this $5 a day in weekly or monthly chunks (this is important).
You start to accrue both the money you contributed, that $5 a day, and the 9.5% a year returns. Then those contributions and the returns, plus the second year’s contributions, earn another 9.5%. And so on and so fourth. This won’t be the exact case for the year 1 and year 2, because the stock market is SUPER unpredictable in the short term. But in the long term, you can expect decent returns.
After 25 years, we can reach over $180,000 dollars. Your contributions of $5 a day, only make up 1/4 of that total sum. 75% of that is JUST EARNINGS.
After 35 years, we can reach half a million dollars. Can you spare $5 a day for a $500,000 payday? 87% of that is purely earnings!
(There are more examples like these on the S&P board Intro page...)
----------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------
Henry David Thoreau famously stated in Walden that “the mass of men lead lives of quiet desperation.” He thinks misplaced value is the cause: We feel a void in our lives, and we attempt to fill it with things like money, possessions, and accolades.
Henry David Thoreau famously stated in Walden that “the mass of men lead lives of quiet desperation.” He thinks misplaced value is the cause: We feel a void in ourlives, and we attempt to fill it with things like money, possessions, and accolades.
Art isn’t all fairytale photoshoots and landscape shots – it can also act as catalyst of change. And Steve Cutts thinks that many things in the world should be different. Work shouldn’t be a grinding, soul-crushing rat race for the almighty dollar. Consumerism shouldn’t hold a vice-like grip on our lives. And social media, well, we need to throw-off the shackles we so eagerly put on ourselves. Wouldn’t life be better then?
Wish You Were Here ...Comfortably Numb .... Hey You

.... Brain Damage "There's someone in my head, but its not me"...(Recall what was said about ego) ..... Us and Them....
WE Are Borg...you will be assimilated...
Elon Musk and AI experts urge U.N. to ban artificial intelligence in weapons
Earlier this year, Elon Musk unveiled details about his new venture Neuralink, a California company that plans to develop a device that can be implanted into the brain and help people who have certain brain injuries, such as strokes. The device would enable a person’s brain to connect wirelessly with the cloud, as well as with computers and with other brains that have the implant.
The end goal of the device, Musk said, is to fight potentially dangerous applications of artificial intelligence.
“We’re going to have the choice of either being left behind and being effectively useless or like a pet — you know, like a house cat or something — or eventually figuring out some way to be symbiotic and merge with AI,” Musk said in a story on the website Wait But Why.
////////////////////////////////////////////////////////////////////////////////////////////////////////////////////////////////////////////////////////////////////////////
Trading Notes
Swing trades= Always trade in the direction of the daily bias even for short term trades...if you really feel you must trade against the daily bias...you need a hair trigger...get out quick if the position moves against you.
Remember...What you're looking at on an intraday chart is a daily candle or OHLC price bar "uncompressed" from the daily chart. Kind of a "micro" view of the Macro daily chart...and daily chart would be a micro view of the weekly chart's price bar...
Front Running a Daily chart signal (...The 60 minute chart will lead daily chart signals when daily CCI is getting ready to come out of oversold/overbought levels...it just takes patience to get the timing..) This way; rather than only trade the intraday cycle you can use the intraday charts to front run a daily CCI signal. When the Daily CCI is ready to move out of oversold or overbought... your goal eventually is to catch a trade from intraday that carries over onto the daily chart. It just takes some practice and patience becoming comfortable with the CCI cycles= Intraday/Daily/(Weekly) and as these cycles sometimes line up together= synchronized you may consider a larger position. Best trades will be trades that are trading in the same direction as the daily chart's bias...=Commodity Channel Index CCI 20 points the way...
There is a "Method to the Madness of Crowds" and you can find it and define it on the charts...Technical Charting takes away some of the fear/doubt in trading... and then all you need is patience while waiting for the chart to tell you when the bias/momentum/sentiment turns...listen to signals at signal lines(simple enough...yes?...)...
MOMENTUM...looking at the price chart below; the technical indicators above the price bars are momentum indicators...buy low/sell high...oversold/overbought "relative" to what = CCI 20...%B...Stochastics...and ULT are all about "PRICE" MOMENTUM. Technical indicators "indicate"... "point direction" they are best read when the indicator is at one of it's "signal" lines and when price is at certain price levels such as Support/Resistance lines.
Stochastics is an important indicator, learn all you can about this one. Developed by George C. Lane in the late 1950s, the Stochastic Oscillator is a momentum indicator that shows the location of the close relative to the high-low range over a set number of periods. According to an interview with Lane, the Stochastic Oscillator “doesn't follow price, it doesn't follow volume or anything like that. It follows the speed or the momentum of price. As a rule, the momentum changes direction before price.” As such, bullish and bearish divergences in the Stochastic Oscillator can be used to foreshadow reversals. This was the first and most important, signal that Lane identified. Lane also used this oscillator to identify bull and bear set-ups to anticipate a future reversal. Because the Stochastic Oscillator is range bound, it is also useful for identifying overbought and oversold levels. (StockCharts.com)
Regression to the Mean/Mean Reversion is the most powerful law in financial physics
Talking about PRICE here... Mean reversions out of extremes are the most powerful and profitable forces in all the financial markets. Riding one has enormous benefits for your wealth. Financial-market prices and sentiment are like a giant pendulum. The farther they are pulled to one extreme by excessive greed or fear, the farther they necessarily swing to the opposite extreme in the subsequent mean reversion. Like pendulums, these reversions don’t magically stop right in the middle at normal again. Their kinetic momentum carries them through to the opposite ends of their arcs. But overshot extremes don’t last for long, as the universal greed necessary to fuel them quickly burns itself out.
The Market is a giant feedback loop, showing traders (and anyone who views the market) a thermometer reading of the social mood under which traders, and by extension society, are operating. Most traders seem to think of the market as something that has some external value outside of the price attributed to it by traders. I prefer to think of it as a real-time gauge of a society’s view of their own productive capacity…or more simply put–social mood. When Markets are understood, the idea that everyone can make money is not only inaccurate but impossible and laughable. Everyone making money means there is no market, because who would be willing to take the other side of the trade? In addition, most traders feel they can move with the crowd to make a (paper) profit, and then get out before the crowd, turning that trade into a real profit. In theory this is sound, but remember everyone else is setting out to do the same thing. It is this crowd movement which allows traders to make money at times. Without a large portion of traders coming to the same decision markets simply would not move. It takes conviction by many traders to create a trend, then it takes euphoric acceptance that “this is the new norm” to end it and “bend it. ” It then takes mass disillusionment to crash it the other way. (vantagepointtrading.com)

Note how important the 50 day average is as the 10day average is trying to decide to move above or below the 50 day average... It can be this easy... maybe now you are an expert, but don't know it. Test it... You can look back at a chart from 5years ago or look at this chart 5years from now...I will not have to change any parameters on this chart and all the definitions above will remain the same... (please note: these colored price bars are part of the Elder's Impulse System which is based on two indicators, a 13-day exponential moving average and the MACD-Histogram. The moving average identifies the trend, while the MACD-Histogram measures momentum. As a result, the Impulse System combines trend following and momentum to identify tradable impulses. This unique indicator combination is color coded into the price bars for easy reference. I (Kiy) have added some speed to it with the use of the 10day moving average (also the 10day average relates to the Grail averages and it just so happens the colored price bars work very good with the 10day average also.) And (Kiy) does not use MACD for momentum, I like Stochastics....I leave it to you to use the link above to see how Dr. Elder interprets the colored price bars.) (...Any way you look at the colored price bars; they are very helpful...better than Candlesticks ; you can test it and learn.) Next...Note the last graph on the above chart, below the On Balanced Volume indicator... Note how the price bars change color when price closes above or below the 10 day average. Maybe a green price bar is telling you to buy because a potential trend may form; maybe a green price bar is saying don't sell...maybe = its saying both buy-don't sell ... Maybe a red price bar says sell or maybe its saying don't buy... maybe both... Maybe a blue price bar is neutral about Direction/Trend; maybe its saying to listen to the last green or red price bar on the price chart...?... The stickie note "Sectors" ...Daily charts... has a long list of charts based on the technical indicators noted above: https://investorshub.advfn.com/boards/read_msg.aspx?message_id=141247347 "Trader's Sentiment Indicators" *$*$*$*$*Markets are not about beliefs, but about sentiment. And, if you can track sentiment, If you can measure sentiment... then you are in a position to make your investment account grow without the need for excuses. (...this really is all you need to know about the Markets; ... SENTIMENT On the above chart...look at the indicators starting with VOLUME... I use these indicators as SENTIMENT INDICATORS... ...Volume=volume speaks volumes...Accumulation/Distribution... Money Flow Index (MFI) is also both a momentum and sentiment indicator...On Balanced Volume (OBV)...(and on an intraday chart VWAP=Volume Weighted Average Price which I consider both a MOMETUM and SENTIMENT indicator ...from Wikipedia The VWAP can be used similar to moving averages, where prices above the VWAP reflect a bullish sentiment and prices below the VWAP reflect a bearish sentiment. Traders may initiate short positions as a stock price moves below VWAP for a given time period or initiate long position as the price moves above VWAP. Institutional buyers and algorithms will often use VWAP to plan entries and initiate larger positions without disturbing the stock price.) The crowd is always taking action...The sentiment indicators are telling you what the "crowd" is doing/thinking... These Sentiment Indicators "indicate"... "point direction" (it really is ALLLL about UP/Sideways/DOWN...) Sentiment indicators are best read when price is at certain price levels like Support/Resistance Lines or at certain Moving Average Lines like the 10day, 20day and 50day moving average. Markets are not about beliefs/opinions, but about sentiment. And, If you can measure sentiment... then you are in a position to make your investment account grow without the need for excuses. (...this really is all you need to know about the Markets... and it should be carved in stone...kiy) (https://seekingalpha.com/article/1719142-how-should-you-trade-silver-with-all-the-manipulation) (note Kiy; does not advocate Elliot Wave) Investors focus on a wide range of measures to gauge the current state of the stock market. Financial analysts generate elaborate financial models. Technical analysts interpret charts and complex indicators. Quant investors design intricate algorithms.
Each of these approaches is legitimate in its own right. But each ignores the elephant in the room: market sentiment.
Maybe that's because market sentiment is hard to quantify, making it the red-headed stepchild of market analysis.
But in the real world, market sentiment often matters more than a market's fundamentals. https://www.investmentu.com/article/detail/58940/end-of-bull-market-does-not-feel-like-this?src=email#.WuAAJhvRWUs |
...You are now an expert... Enjoy good trades...And always...Give a little back to a good cause. ----------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------
https://wallmine.com/ http://www.stock1010.com/ https://scan.alpaca.ai/symbol/SLV/
http://www.stockta.com/
http://www.buyupside.com/sample_portfolios/lithium.php
**** https://www.biopharmcatalyst.com/calendars/fda-calendar FDA Calender
https://www.tipranks.com/stocks/fcx Price Target
https://finbox.io/fcx fair value
https://swingtradebot.com/equities/FCX
https://www.google.com/finance?q=FCX&ei=6p84WZm8E9aMjAHSlLTYDg
Fair Value (fv) estimates are from https://financhill.com/search/valuation/GG
http://oracledispatch.com/
http://wealthyventurecapitalist.com/
www.insidercow.com What is a Pump-and-Dump Scam
Speculation Stocks ...just KNOW that you're Speculating and not investing... and you just don't buy a stock because you think it will go up in price; buy it because it's worth more than the price at the time, based on fundamentals and growth prospects
https://swingtradebot.com/equities/most-tracked
There isn't a day that goes by on Wall Street when certain stocks trading for under $10 a share don't experience massive spikes higher. Traders savvy enough to follow the low-priced names and trade them with discipline and solid risk management are banking ridiculous coin on a regular basis. Roberto Pedone... Street.com

Color Code of stock symbols/letters....MVIS (T/$3.50H-4.50) example MVIS letter colors "red" indicate the stock price is moving down and all the letters are boxed in with green= oversold oversold MVIS=MVIS and you should be looking/considering a long position...(T/$3.50H-4.50) and someone has put a $4.50 price target on it. (..."T"=target price...the "H" indicates the target price has been hit...
Colors are based on reading the CCI 20 (Commodity Channel Index signal lines) ... if "letters" are colored =Green =UP...Red=down...lighter colors = less confident in direction...if letters/stock symbols are Boxed in=Green =oversold=anticipate an "UP" cycle for the CCI 20 indicator or a mean reversion price bounces = at least to centerline within the Bollinger Bands 20,2= the 20 day moving average... and if the box is red=Red=overbought=anticipate a "DOWN" cycle...
§=Reverse of a Signal line(focus is mainly the CCI centerline...overbought line +100 and oversold line -100)(The CCI signal lines))... ¥= first stock to turn UP/Down after the Group/Sector has been overbought/oversold. Stocks that have a "Target" price are the better choice for a trade...example...MVIS (T/$3.50H-4.50) a target price with an "H" behind it says the target was "hit" since the target price was added...here MVIS has a second target price of $4.50...so if you find MVIS oversold you can consider a long position if you are comfortable with the width of the Bollinger Bands and the target price will give you 10% or more profit... remember this; PRICE= %B and CCI they indicate very much the same and they represent price within the parameters of the Bollinger Bands (box Price in, inside these Bollinger Bands. The %B tends to lead the CCI signals (a little)... ***BAC (T/$24H-26H-30H-35) light blue color is 2017 price targets that where hit=H A few of the more interesting ETFs/Sectors http://www.direxioninvestments.com/products/direxion-daily-sp-500-bull-3x-etf?gclid=CPPB6bX5-s0CFRSUfgodGfQGMA ...Candle Glance Charts= click on the ETF's symbol and you'll get a set of the largest holdings 10-12 stocks in that ETF. FINANCIAL Rising bond yields are good for financials like banks and insurers, but are bad for bond proxies like utilities (and REITs). 03/15... The Fed hiked rates today as expected, with expectations for two more hikes this year. Judging from immediate market reactions, the Fed announcement is being viewed as somewhat dovish. For one thing, bond yields are dropping. With yields falling, bond prices are having a strong day. Junk bonds, which have been correcting lately, are especially strong. That's also positive for stocks. Rate sensitive stock groups like utilities and REITs are leading a strong market response. Falling yields, however, are weighing on bank stocks. The dollar is also dropping. XLF CandleGlance BRK.B,*JPM,WFC, ,C ,SPG,USB,CB,*AIG,*GS,*BAC  XLK CandleXGlance AAPL,MSFT,FB,T,GOOGL,GOOG,VZ,INTC,***CSCO(T/$ 40H-43H-45H-48-52)... ***HDP (T/$21H-24) .. SGH (T/$21H-33H-40H 40H-50H-55H-75 ) ...V ...REW ...2xBear... TECS ...3xBear
XLK CandleXGlance AAPL,MSFT,FB,T,GOOGL,GOOG,VZ,INTC,***CSCO(T/$ 40H-43H-45H-48-52)... ***HDP (T/$21H-24) .. SGH (T/$21H-33H-40H 40H-50H-55H-75 ) ...V ...REW ...2xBear... TECS ...3xBear Short Technolgy



Short Semiconductors
 SMH CandleGlance Semiconductor ...AMD 04/2018 ***AMD(T/$11H-13H-15H-17H),MVDA,CY(T/$15H-18H-20-23),IDTI,IPHI,MPWR,MU,MXIM,TXN,
SMH CandleGlance Semiconductor ...AMD 04/2018 ***AMD(T/$11H-13H-15H-17H),MVDA,CY(T/$15H-18H-20-23),IDTI,IPHI,MPWR,MU,MXIM,TXN,
http://www.buyupside.com/sample_portfolios/semiconductorsmems.php SMH

10-12
*STM (T/$18H-26H)...INTC (T/$40H-47H-55H-62)...AMD 04/2018 ***AMD (T/$11H H-13-15-17) ... ***MRAM (T/$14H-18H-1013-15)Potential Double ... ***RESN (T/$8H-6 -8)...... **ICHR (T/$24H-28H-30H-35-38-40-42)... UCTT... ***IOTS (T/$10-12) ...LSCC (T/$7-9) COHU (T/$24H-28-31) ... CY (T/$14H-15H-18-20) .***AXTI (T/$10H-8-10-12)...AMSC (T/$6H-7.50) ...
*ASYS (T/$
8H-12H-13.50H -8H-10H-13-15-18) equip...
A.I. ***XLNX (T/$ 70H-75H-80)... INTC (T/$54H-57-60-64) ... QUIK (T/$2.5)...
 07/25****
07/25****AMD 04/04/2018 Target $13...(T/$11-13.50)

*SKYY CandleGlance Cloud Computing ...AKAM,*ORCL(T/$55-60),EQIX,NTAP,AMZN,CSCO,OTEX,JNPR,FB ...  ***AMBR (T/$10H-12-14)...
***AMBR (T/$10H-12-14)...June 21, 2018...There’s a lot to be optimistic about in the Technology sector an analyst just weighed in on Amber Road (NYSE: AMBR) . Stifel Nicolaus analyst Tom Roderick maintained a Buy rating on Amber Road (NYSE: AMBR) today and set a price target of $14. The company’s shares closed yesterday at $9.80, close to its 52-week high of $10.29. According to TipRanks.com, Roderick is a 5-star analyst with an average return of 18.7% and a 72.0% success rate. Roderick covers the Technology sector, focusing on stocks such as Nuance Communications, Salesforce.com, and Everbridge Inc.
Amber Road has an analyst consensus of Moderate Buy, with a price target consensus of $14. Amber Road, Inc. provides cloud-based global trade management (GTM) solutions in the United States and internationally. The company's GTM solutions include modules for logistics contract and rate management; supply chain visibility and event management; international trade compliance; and global knowledge trade content database, supply chain collaboration with overseas factories and vendors, and duty management solutions to importers and exporters, nonvessel owning common carriers (resellers), and ocean carriers. It provides its solution to various industries, including chemical/pharmaceutical, high technology/electronics, industrial/manufacturing, logistics, oil and gas, and retail/apparel through a software-as-a-service model.
\
 DTRM (T/$3.50H - 1.50-3.00) ****YEXT (T/$14H-14H-16H-18H-21H) 4 brokers like this stock....Yext, the company only held its initial public offering in April2017. However, Yext could be one of the best up-and-coming AI stocks around. Yext's key product is a knowledge engine platform that lets businesses manage their digital knowledge in the cloud and sync that knowledge to over 100 services, including Bing, Cortana, Google, Google Maps, and Siri. The company calls this "digital knowledge management" and touts its ability to enable businesses to facilitate face-to-face and digital interactions with customers that boost brand awareness and lead to higher sales. Mar. 8th https://seekingalpha.com/article/4155001-yext-still-giving
DTRM (T/$3.50H - 1.50-3.00) ****YEXT (T/$14H-14H-16H-18H-21H) 4 brokers like this stock....Yext, the company only held its initial public offering in April2017. However, Yext could be one of the best up-and-coming AI stocks around. Yext's key product is a knowledge engine platform that lets businesses manage their digital knowledge in the cloud and sync that knowledge to over 100 services, including Bing, Cortana, Google, Google Maps, and Siri. The company calls this "digital knowledge management" and touts its ability to enable businesses to facilitate face-to-face and digital interactions with customers that boost brand awareness and lead to higher sales. Mar. 8th https://seekingalpha.com/article/4155001-yext-still-giving  HACK Cyber Security... IMPV,QLYS,JNPR (T/$24H-28H),SAIC,FTNT,CSCO, CHKP... *** FEYE (T/$16H-18H-20-23) http://www.buyupside.com/sample_portfolios/cybersecurity.php *CYBR(T/$42H-48H-55H),TMICF,PFPT,AVG...
HACK Cyber Security... IMPV,QLYS,JNPR (T/$24H-28H),SAIC,FTNT,CSCO, CHKP... *** FEYE (T/$16H-18H-20-23) http://www.buyupside.com/sample_portfolios/cybersecurity.php *CYBR(T/$42H-48H-55H),TMICF,PFPT,AVG... 

Posted
July 20, 2018 at 8:00PM ...
Jason Simpkins There’s been a lot of talk about hacking this week. America was once again reminded that Russia launched a dangerous cyberattack on our democracy in 2016. But I’m not here to talk politics. I’m here to talk
profits.
Last week, the special counsel appointed to investigate election interference indicted 12 Russian spies, accusing them of hacking into two Democratic Party computer systems.
These Russian spies are part of GRU — Russia’s military intelligence agency. And to achieve their goal, they set up an entire company that employed hundreds of people. It had a budget of $1.25 million per month. It used information (Social Security numbers and birth dates) stolen from U.S. citizens to create fake online personas, like Guccifer 2.0. And it used a virtual private network to disguise its location. This “cyberarmy” infiltrated computer networks at the DCCC and DNC, where it secretly monitored the activity of dozens of employees. It also planted hundreds of files containing malicious computer code to steal passwords and data. That is how sophisticated, how complex, and how well-funded these operations are. And there’s dozens more just like it.
China has a cyberarmy, too. It’s called ‘Unit 61398’. Beijing uses it to spy on political rivals (the United States) and steal trade secrets and technology from companies like Apple and Samsung. In fact, in June, Chinese hackers gained access to U.S. submarine plans after hacking a U.S. Navy contractor’s computer.
But now, here’s the really scary thing: You don’t even have to be a professional to be an effective hacker. Earlier this month, it was revealed that an
amateur hacker stole U.S. military information about combat drones…
and they weren’t even trying. They simply used what
Wired magazine called a “dumb security flaw.” Yet they were able to access top-secret documents stored on Air Force computers and then put that information up for sale on the dark web for a couple hundred dollars. If this person had been targeting this information, or had known what it was they found, they would have charged way more for U.S. military secrets!
When you consider all that, it can’t come as a surprise that
cybersecurity stocks are going gangbusters — doubling the return of the overall market. Indeed, cybersecurity stocks (as tracked by the ETFMG Prime Cyber Security ETF) have returned 30% over the past year —
double the return of the S&P 500. It’s also no coincidence that we’re just a few months shy of the one-year anniversary of the Equifax hack — one of the largest data breaches ever, affecting 145.5 million people.
In fact, corporate entities are even more at risk than governments.
**05/14 REPORTS SPCB (T/$5) Jan. 8th ...crypto services, allows users to load funds into their SuperWallet mobile application through a credit card and bank account or by providing cash at any supporting top-up location or agent. SuperPay's Bitcoin and cryptocurrency capabilities in development will allow users also without a bank account or credit card to load and receive funds and then use those funds to purchase Bitcoin, Ethereum and other cryptocurrencies. They will also be able to sell their cryptocurrency through the suite. https://finance.yahoo.com/news/supercom-develop-bitcoin-other-cryptocurrency-140000884.html
 ***NXTD NXTD (T/$5H-6H -5) SOUNDS LIKE BIG POTENTIAL HERE...BUT WHAT HAPPENED TO PRICE???Nxt-ID, Inc., a security technology company, engages in the development of products and solutions for security, healthcare, finance, and Internet of Things (IoT) markets....
***NXTD NXTD (T/$5H-6H -5) SOUNDS LIKE BIG POTENTIAL HERE...BUT WHAT HAPPENED TO PRICE???Nxt-ID, Inc., a security technology company, engages in the development of products and solutions for security, healthcare, finance, and Internet of Things (IoT) markets....NXT-ID, Inc. (
NXTD) a provider of payment, credential management, and authentication platform services and Fit Pay, Inc., its wholly owned subsidiary,...Jan. 8th, 2018 NXT-ID, Inc. and Fit Pay, Inc. will demo the FitPay Payment Platform
™, the
company's proprietary technology platform that adds contactless payment capabilities to wearable and IoT devices. FitPay recently announced that Garmin Pay™, a contactless payment capability on the Garmin vívoactive 3 smartwatch that is powered by the FitPay Platform, is now live.Management will also highlight with attending media its recently announced
strategy to extend the FitPay Payment Platform to cryptocurrency holders. On December 20
th, Fit Pay, Inc. and Cascade Financial Technology Corp announced an agreement for the joint development of a platform that gives cryptocurrency holders the ability to use the value of their currency to make purchases at millions of retail locations worldwide. The new platform will enable devices with stored value exchanged from cryptocurrency to be used for traditional payment transactions.
May 23/2018 another parent
https://finance.yahoo.com/news/nxt-id-announces-issuance-us-120000094.html  Global Internet Of Things According to researchers, the global market for IoT in 2020 is going to be worth $373 billion in terms of sales. Hardware will account for 52% of sales — devices ranging from personal wearable technology to smart homes to connected cars. The remaining 48% will pour in from the software and analytics required to turn the copious amounts of data generated by hardware into usable information. Leading market research firm IDC forecasts China to spend $128 billion on IoT by 2020. India’s spending will come in around $10 billion to $12 billion by 2020. And forecast sales for the Middle East and Africa are at $11 billion by 2019.
Global Internet Of Things According to researchers, the global market for IoT in 2020 is going to be worth $373 billion in terms of sales. Hardware will account for 52% of sales — devices ranging from personal wearable technology to smart homes to connected cars. The remaining 48% will pour in from the software and analytics required to turn the copious amounts of data generated by hardware into usable information. Leading market research firm IDC forecasts China to spend $128 billion on IoT by 2020. India’s spending will come in around $10 billion to $12 billion by 2020. And forecast sales for the Middle East and Africa are at $11 billion by 2019. 
 SNSR CandleGlance Global Internet Of Things...SWKS,GRMN,ST,BRCD,DXCM,JCI,SLAB,BDC,IDCC,ADI ... **OTIV (T/$ 2.50H-2.00-2.50)... cashless payment and management requirements for the Internet of Payment Things (IoPT), wearables, automated retail and petroleum markets. crypo...
SNSR CandleGlance Global Internet Of Things...SWKS,GRMN,ST,BRCD,DXCM,JCI,SLAB,BDC,IDCC,ADI ... **OTIV (T/$ 2.50H-2.00-2.50)... cashless payment and management requirements for the Internet of Payment Things (IoPT), wearables, automated retail and petroleum markets. crypo...
NDCVF ...SWIR...PTC.. SFE.. 
***
HDP (T/$13H-19H-21-23-25) In addition to its Hortonworks Data Platform (HDP) for processing stored data (data at rest), the company also has a relatively new product called Hortonworks DataFlow (HDF) specifically geared toward the Internet of Things. HDF collects and processes purchase, sensor, and asset data in real time and feeds this information into either a data lake for analysis or directly into streaming applications. In its most recent quarter, Hortonworks installed HDF in eight of its 10 largest new deals, showing that the platform has a lot of promise and that large enterprises are investing in IoT solutions. Hortonworks certainly looks to be in good position to capture its share of the IoT wave.  .*****IOTS (T/$5H-7H-10-12) ultra low power (flash) memory., trademark resistive RAM technology called Conductive Bridging RAM (CBRAM®). CBRAM® is a breakthrough technology platform that enables 100 times less energy consumption than today’s flash memory technologies as well as delivering enhanced performance. Visit: http://www.adestotech.com
.*****IOTS (T/$5H-7H-10-12) ultra low power (flash) memory., trademark resistive RAM technology called Conductive Bridging RAM (CBRAM®). CBRAM® is a breakthrough technology platform that enables 100 times less energy consumption than today’s flash memory technologies as well as delivering enhanced performance. Visit: http://www.adestotech.com 
PRNT 3D Printing Jan 2017 International Data Corporation (IDC) Worldwide Semi-Annual 3D Printing Spending guide is predicting that the rapid expansion of the 3D printing industry is expected to continue without slowing down. According to the IDC analysts, global revenues for the 3D printing market will explode to a massive $35.4 billion by the year 2020. With 2016 revenues expected to reach $15.9 billion which means the industry could nearly double within the next five years. http://www.marketwired.com/press-release/3dx-industries-metal-printing-division-positioned-for-assertive-growth-in-2017-otcqb-dddx-2189898.htm
http://www.buyupside.com/sample_portfolios/3dprinters.php 
 DDD 3D Systems (T/$13H -H10H-13H-15H-18),
DDD 3D Systems (T/$13H -H10H-13H-15H-18),  SSYS (T/$24H-19H-21H-23H) Stratasys ...
SSYS (T/$24H-19H-21H-23H) Stratasys ...  XONE ..ExOne... VJET (T/$6H -6) Voxeljet ...
XONE ..ExOne... VJET (T/$6H -6) Voxeljet ...  .............MTLS Materialise Sftwre Services... **ONVO (liver tissue)(late 2019) HPQ (T/$22H-24H-26-28)sftwre ...HP introduced their new line of Multi Jet Fusion 3-D printers that are available for the public to buy; ironically enough, these printers have been used to produce a number of components to build their “regular” printers.
.............MTLS Materialise Sftwre Services... **ONVO (liver tissue)(late 2019) HPQ (T/$22H-24H-26-28)sftwre ...HP introduced their new line of Multi Jet Fusion 3-D printers that are available for the public to buy; ironically enough, these printers have been used to produce a number of components to build their “regular” printers.  PRLB A unique company in the 3-D printing industry is Proto Labs Inc. (NYSE: PRLB). What makes it unique is that it doesn’t actually make and sell printers. Its business model is making prototypes and test models of products for other companies.
PRLB A unique company in the 3-D printing industry is Proto Labs Inc. (NYSE: PRLB). What makes it unique is that it doesn’t actually make and sell printers. Its business model is making prototypes and test models of products for other companies. Proto Labs has quietly been doing very well over the past year, as its stock has generated a return of about 140% during that time. And even though 3-D printing revenue growth has slowed in the past couple years, Proto Labs has been seeing double-digit sales growth. In fact, its
sales growth for 2018 is expected to be 27%, which would be its highest growth since 2014.
The reason I bring PRLB up is that it could be used as a proxy for demand in the 3-D printing industry. If a lot of companies are making orders for 3-D printing-based models for product prototypes, it suggests that the demand for the technology is still there. The process of creating prototypes and models, what’s known as the product development stage, seems to be the sweet spot for using 3-D printing technology right now, and if the demand is there, we could be seeing a rebound in industry-wide growth starting in the next year or two. This suggestion could be backed up by comments from management of companies around the world that seem very bullish about 3-D printing. In a survey done by Sculpteo, a French 3-D printing company, about 1,000 companies from 62 countries around the world that use 3-D printing as part of their business operations gave their take on the technology. About 90% of the companies said that they consider it an advantage over their competitors that don’t use it. Even better, 72% of the companies said that they expect to spend more on the technology in 2018 than they did in 2017, even after their average budget for 3-D printing technology grew by 55% in 2017.
 IYZ Telecom... The deployment of 5G connectivity It’s different than the now-common 4G connectivity that powers your smartphone in that even as fast as 4G is, only one connection per radio frequency can be made at a time. With fifth-generation signals, the network understands and manages the high-speed connections being made at any given time by a massive number of devices, allowing them to share radio frequencies with minimal degradation of their connection speed. That’s how 5G connections will effectively be up to three times faster than 4G connections are. 5G connectively is in its infancy, however, only being tested in real-world settings rather than sold as an outright feature for wireless phone subscribers. Wireless telecom carriers know, however, the real opportunity in 5G is in providing the backbone for the radio-based connections the real rise of IoT will require. It’s coming though, and soon.
IYZ Telecom... The deployment of 5G connectivity It’s different than the now-common 4G connectivity that powers your smartphone in that even as fast as 4G is, only one connection per radio frequency can be made at a time. With fifth-generation signals, the network understands and manages the high-speed connections being made at any given time by a massive number of devices, allowing them to share radio frequencies with minimal degradation of their connection speed. That’s how 5G connections will effectively be up to three times faster than 4G connections are. 5G connectively is in its infancy, however, only being tested in real-world settings rather than sold as an outright feature for wireless phone subscribers. Wireless telecom carriers know, however, the real opportunity in 5G is in providing the backbone for the radio-based connections the real rise of IoT will require. It’s coming though, and soon.  ***T (T/$36H-38H-34H-36H-40-42-44)AT&T Inc. is setting the stage for a major victory on the 5G front, though you won’t see it until next year 2018; it’s not ready for mass rollout just yet. Ditto for T-Mobile US Inc (NASDAQ:TMUS), which recently caught up with AT&T in its effort to reach 5G speeds in excess of one gigibit per second .Indeed, T-Mobile is on track to unveil the nation’s first narrowband IoT network in 2018, directly addressing and removing one of the key bottlenecks that had been holding the movement back. ***T,VZ,TMUS,CLT,SBAC,LVLT,S,FTR,SHEN ....TDS....(WIN ... RNG... ***QTNA (T/$18-20) ...
***T (T/$36H-38H-34H-36H-40-42-44)AT&T Inc. is setting the stage for a major victory on the 5G front, though you won’t see it until next year 2018; it’s not ready for mass rollout just yet. Ditto for T-Mobile US Inc (NASDAQ:TMUS), which recently caught up with AT&T in its effort to reach 5G speeds in excess of one gigibit per second .Indeed, T-Mobile is on track to unveil the nation’s first narrowband IoT network in 2018, directly addressing and removing one of the key bottlenecks that had been holding the movement back. ***T,VZ,TMUS,CLT,SBAC,LVLT,S,FTR,SHEN ....TDS....(WIN ... RNG... ***QTNA (T/$18-20) ...  ALT ENERGY GEX Global Alt. Energy...TSLA,VWSYF,ETN,GAM,ENS,FSLR,CREE,ITRI,ORA,POWI,CVA,...CLPXF,NDRBF,KTWIF,NIBE B ICLN Global Clean Energy...OEZVF,CHFFF,VWSYF,
ALT ENERGY GEX Global Alt. Energy...TSLA,VWSYF,ETN,GAM,ENS,FSLR,CREE,ITRI,ORA,POWI,CVA,...CLPXF,NDRBF,KTWIF,NIBE B ICLN Global Clean Energy...OEZVF,CHFFF,VWSYF,  PHO Water Resources WAT,ROP,ECL,DHR,HDS,IEX,TTC,AOS,RXN,AWK,VMI...TTEK,WTR, CGW Global Water Index... ...... (CDZI...Water Utility) TAN SOLAR
PHO Water Resources WAT,ROP,ECL,DHR,HDS,IEX,TTC,AOS,RXN,AWK,VMI...TTEK,WTR, CGW Global Water Index... ...... (CDZI...Water Utility) TAN SOLAR  ...FSLR,SCTY,GCPEF,TERP,TSL,SPWR,CAFD,ABY ... RUN (T/11H-12H-15) ... VSLR (T/$6H)... ***SUNW (T/$2.50H-2) ...
...FSLR,SCTY,GCPEF,TERP,TSL,SPWR,CAFD,ABY ... RUN (T/11H-12H-15) ... VSLR (T/$6H)... ***SUNW (T/$2.50H-2) ... 

 .....AVOID?... WNDW (T/$5H-8H-10H) ...SolarWindow Technologies, Inc. has developed transparent electricity generating coatings that can go onto any glass surface, such as windows or sunroofs. Imagine a Tesla being able to top off its batteries while on the road. And since these coatings generate electricity under natural, low and even shaded light conditions, that Tesla could be charging up all day.
.....AVOID?... WNDW (T/$5H-8H-10H) ...SolarWindow Technologies, Inc. has developed transparent electricity generating coatings that can go onto any glass surface, such as windows or sunroofs. Imagine a Tesla being able to top off its batteries while on the road. And since these coatings generate electricity under natural, low and even shaded light conditions, that Tesla could be charging up all day. 90-plus SolarWindow International and U.S. patent and trademark filings include:
- Electricity generating windows that can:
- self-tint to darker or lighter shades, based on user preferences;
power various devices such as smart sensors for delivering data to intelligent energy management systems in buildings;
Electricity-generating coatings for: - powering mission-critical systems on military and commercial aircraft;
providing emergency power to pilots;
application to various three-dimensional objects;
thin and flexible surfaces, using various application methods;
Processes and the processing of SolarWindow™ device architectures;
Enhancements to SolarWindow™ device architecture; and
Improvements to various SolarWindow™ module performance; among others.
The company’s scientists, engineers, and attorneys continually identify, develop and pursue intellectual property with the objective of providing the high commercial value and strategic position when compared to other technologies. High-value patents and trademarks are actively developed while legacy assets are either repurposed to add value, or eventually retired.
The solar cell is no longer the dominant cost of the energy it produces. The majority of the cost increasingly lies in everything else—the inverter, installation labor, permitting fees
Power inverters have become a critical component of solar power systems large and small. They're what converts the direct current (DC) generated by a solar cell into the alternating current (AC) the grid uses, inverters may be the key to unlocking value for investors. They can be the smart hubs that integrate energy production with energy storage and the grid, a critical component of any installation. Motley Fool
SEDG ...ABB
***** ENPH (T/$1H-4H-6H-8-9) Enphase’s new IQ microinverters 
.....
----------------------------------------------------------------------------------------------------------------------- --------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------Speculation Stocks RISK...
"DILUTION RISK, DRUG FAILURE RISK, poor management decision making... risk" ...
“Promoted stocks tend to rise because of the work of the stock promoter. But, later on, usually share price declines when the promo dollars dry up. Thus, buyers beware! The share price could keep creeping up as more emails are sent out and new market participants buy shares. Some will make money. However, in a few days, weeks or months, the stock promotion will stop or the exchange will warn the market about it, and the share price may fall sharply. Just be careful... Pumps & Dumps 101
This is your brain on stocks

5 Brain Flaws that make you a lousy investor

=========================================================================================================================
RoBoSapien

*****Virtual Reality/Augmented Reality/Artificial Intelligence In the real world, Artifical Intelligence9(AI) will enter our lives in three phases and forms:
Portfolio of Artificial Intelligence (AI) Chip Stocks --- Portfolio of Artificial Intelligence (AI) Stocks
https://agorafinancial.com/2017/02/17/the-next-mega-tech-transformation-10-times-bigger-than-microsoft/
PHASE #1 Assisted Intelligence: AI that replaces repetitive and mundane tasks traditionally done by humans. A timely example would be the proliferation of robots used at Amazon to “pick” items from warehouse shelves, which we mentioned in last month’s issue. You’ll recall we’re already double-digit gains in the space (with more to come) in the
Robo Global Robotics & Automation ETF (ROBO) and
Cognex Corp. (CGNX). PHASE #2 Augmented Intelligence: AI that incorporates machine learning to supplement and support human analysis. One area where this type of AI is being implemented is legal discovery. That is, the period of time in which both legal parties gather evidence. Seventy-five percent of the costs associated with discovery are tied to human review of documents. And people are expensive. Using AI to pre-screen — and reduce the amount of relevant documents before humans even start reviewing the files — can dramatically reduce costs. We profited from this very technology when our portfolio position Epiq Systems was acquired for a 40% gain in July 2016.
PHASE #3 Autonomous Intelligence: AI that takes over for humans in a highly specific task. Self-driving cars would be the ultimate example of this in action.
AI appears on our smartphones, with personal assistants, augmented and virtual reality headsets, robots, in data centers, and in semi and fully autonomous vehicles.
AI will be dominated by the big names such as Apple, Amazon, Alphabet Google, Facebook, and Samsung; however some smaller names like Nvidia and AMD can also do well.
AI will be invisible, yet it will be everywhere. https://seekingalpha.com/article/4086169-look-artificial-intelligence-companies-top-5
AI = "machines with brains"


7/25 AMD (T/$8H-11H-13H 15H-13H-15H-18-20-23) Advanced Micro Devicesis a semiconductor manufacturer. The company's new AI chips are the Radeon Instinct series. AMD is also racing to put its chips into AI applications. AMD will release its ROCm deep-learning framework, as well as an open-source GPU-accelerated library called MIOpen. The company plans to launch three products under the new brand in 2017. ***08/04 https://www.thestreet.com/story/14256965/1/amd-falls-in-premarket-as-abu-dhabi-s-sells-part-of-stake.html?puc=yahoo&cm_ven=YAHOO&yptr=yahoo Abu Dhabi is largest share holder after recent sells still owns 13% of outstanding shares...
04/05/18
AMD...ITS WEAK...no volume...price is up more than 10% off its recent low... 300shares I'd consider selling 200...Getting a good entry you have a chance to catch a move of more than one dollar...grab some profit with 200 shares and let 100 shares do the work for now... That's where I say I don't like dead money...I take the money out and go find another stock that I can hit UP for a quick 10%...it may take AMD 3 months to get above $12...I may get in for 2-3 swing trades before that. And with the Market so volatile... I'm a trader...buy and hold is too slow...as a swing trader you're swing trading in order to own 100 shares at no cost...so you use 300 or more shares on a $10 stock and play hit and run for 10% or more... 60 minute...AMD is going to perform along with the overall MARKET and then its going to over re-act to any bad news about itself...so swing trade it...to fill time...and you just have to get some trades in to at least lower you stop... 07/25
AMD 
 Softbank (OTC:SFBTF)(they bought out chip designer ARM, and own 4.95% of Nvidia)
Softbank (OTC:SFBTF)(they bought out chip designer ARM, and own 4.95% of Nvidia) - AAOI (T/$32H-35H-40H)Applied Optoelectronics Inc. (NASDAQ:AAOI) - Design and manufacture high speed data and fiber-optic network products, such as transmitters and receivers. 2017 PE is 14.2, and 2018 PE is 12.6 representing excellent value for a growth stock. Analyst consensus price target is US$75.78, representing 23% upside.
Fabrinet FN (T/$30-35-37-42) Design and manufacture transceivers, transponders, optical amplifiers, lasers, and sensors. Fabrinet provides optical packaging and precision optical, electro-mechanical, and electronic manufacturing services to original equipment manufacturers of optical communication components, modules and sub-systems, industrial lasers, medical devices, and sensors.

- NeoPhotonics (NPTN),
Lumentum LITE.
MU(T/$50H-55H-60H-65-72-76-82-86-90-95-100) memory based processing is the future.

It is no secret that Automotive makers are racing each other to mass produce the first autonomous car. To achieve fully autonomous driving, cars need to have a variety of sensors to perceive near and far fields of visions in every direction. The sensors include radar, LIDAR, ultrasonic, cameras, and night vision devices. The data collected by these sensors are fed into highly complex System on Chips (SoCs) with compute performance that is equivalent to what only supercomputers could deliver a few years ago. Complex, state of the art algorithms that are increasingly based upon artificial intelligence ensure vehicle, driver, passenger, and pedestrian safety based on factors such as traffic, weather, dangerous conditions, etc https://www.micron.com/solutions/automotive
Next level memory is an integral part of AI. I recommend you getting up to speed on the demands we're talking about in memory today. this is not about storage capacity. This is about on demand, real time access to data that has to solve a problem immediately. This is the next level of memory that only 2 or 3 companies can do. It's a workload solution not just a storage solution. Think about a car that needs to calculate what is happening around it immediately. Thats not a storage solution, thats a storage plus speed and cross referenced access in real time. Only 3 or 4 companies in the world have this capability and innovation, and Micron is one of the only american ones. This is why Nvidia and Intel are partnered with Micron.  Virtual Reality "VR"...Augmented Reality. "AR" 2018 and 2023, Markets expect the AR market to grow from $11 billion to $60 billion, and for the VR market to expand from $7.9 billion to $34 billion.
Virtual Reality "VR"...Augmented Reality. "AR" 2018 and 2023, Markets expect the AR market to grow from $11 billion to $60 billion, and for the VR market to expand from $7.9 billion to $34 billion. 02/28/17 ...
potential profits in the VR/AR industry are enormous. Goldman Sachs (GS) estimates that the market will reach $80 billion by 2025, with the potential for that figure to actually soar much higher, to more than $180 billion. Many of the companies developing VR/AR hardware and software are among the biggest names in Silicon Valley including Apple (AAPL), Alphabet (GOOG), Amazon (AMZN), Facebook (FB) and Sony ***SNE T/$85
. Autoliv ALV Augmented reality superimposes digital images in a person's field of vision. It provides the user with information for navigation, to complete tasks and other purposes. By contrast,
virtual reality is an immersive media that places the wearer of special headsets into a completely digital world, mostly for entertainment purposes. In November,
Bloomberg reported that Apple had already ordered "small quantities of near-eye displays from one supplier for testing." It said Apple was in the exploration phase of wearable computing. The device being tested would connect wirelessly to the iPhone. Also in November, KGI analyst Ming-Chi Kuo said Apple is looking to leapfrog the competition in AR by three to five years with its offering. But Kuo said it could take Apple one to two years to come out with an AR product.
....Sony Facebook's Oculus virtual reality device Samsung, Microsoft Hololens...Google Glass
Head worn devices...ZBRA Zebra Technology... INTC Intel...FJTSY Fujitsu...

Sales of virtual reality and augmented reality headsets will surge by 2021, when 100 million a year of the devices may be sold, according to a new report by research firm IDC.
****FNSR FNSR (T/$18H-20H-22-25-29) ... AAPL's pre-paytment of $390million boost in the company's standing in the fast-growing VCSEL and 3D sensing module market, which could hit $14 billion in revenue by 2020 as compared to just $1.5 billion this year. Investors, therefore, should definitely think of capitalizing on Finisar's stock price weakness this year to ride the VCSEL gravy train. https://finance.yahoo.com/news/apple-apos-millions-could-finisar-163200140.html Finisar was rumored to be in line to supply Apple with VCSEL arrays to enable the Face ID feature in the latest iPhone X, but rival Lumentum (NASDAQ: LITE) ran away with this business as Finisar reportedly had trouble meeting Apple's requirements. Not surprisingly, Lumentum is witnessing a big boost in its business thanks to the massive uptake of its 3D sensing chips by Apple. The company's revenue in the December-ended quarter could rise around 36% year over year as it reportedly got $300 million worth of 3D sensing orders from Apple since April.

***CY(T/$18H-18H-20-23) Cypress is the market leader in Wi-Fi/Bluetooth combo chips for Internet of Things (IoT) devices, USB-C controllers, NOR flash memory chips, and auto instrument cluster microcontrollers (MCUs). All these components are being installed in new high-end vehicles. Wi-Fi/Bluetooth combo chips and USB-C controllers tether mobile devices to the dashboard, NOR flash memory is installed in advanced driver-assistance systems (ADAS) platforms, and auto instrument cluster MCUs sync all of a vehicle's data onto its dashboard. Cypress notes that newer cars require more semiconductors than ever, with "basic" models using about $300 worth of chips, and "high-end" models using about $1,000 in chips. That's why the company expects its automotive business to grow at a compound annual growth rate (CAGR) of 8%-12% between 2016 and 2021. The strength of Cypress' auto chip business, which posted 16% sales growth in 2017, complements its strength in the industrial and IoT markets.

***HIMX HIMX (T/$6H-10H-8H-10-12-15) Himax Technologies
10/06/2017 Himax could soon tap the mass-market AR opportunity on the back of its recent partnership with Qualcomm. The two companies are going to develop a 3D camera system to enable vision-based functions for different use cases such as biometric face authentication and scene perception, among others, for use in smartphones, cars, and Internet of Things applications.
Qualcomm and Himax believe that they can start manufacturing the new platform on a mass scale from the first quarter of 2018. This is a big deal for Himax, as a partnership with Qualcomm opens the doors to tapping the fast-growing AR opportunity in smartphones. Motley Fool

9/14 KOPN (T/$3.70H-5) May 31, 2018 Kopin Unveils World's First Voice Controlled Wearable Smart Screen; Weighing 1.5 Ounces. Golden-i™ Infinity smart screen, the first voice- and gesture-controlled wearable device that supports Android and Windows 10-based computing solutions. Kopin Corporation semvismicndtr...wearable technologies and display products...develops, makes and markets virtual and augmented reality gaming, training, and simulation products. Kopin’s Whisper™ Voice Chip, Solos™ smart glasses and Pupil LCD display modules will be among the Kopin products displayed on the CES show floor. Kopin has transitioned to a wearable-centric company - they have positioned themselves to be entirely levered to success in Enterprise and Consumer head-worn AR with components and licensable IP. When you combine high level AR market forecasts with Kopin's device share dominance with design wins inside 5 Industrial AR headsets, I believe it is clear this stock is the one to own if you are looking to lever your portfolio to the massive growth possible in the head-worn wearable segment. As I have noted previously and pointed out in my blog posting "The Wearables Acquisition Intel Needs To Make - And Fast!" I believe a current reasonable market capitalization value for Kopin would be $394M or north of $5.00/share based on the following: https://seekingalpha.com/article/4070837-small-private-company-disrupting-industrial-ar-market "The Wearables Acquisition Intel Needs To Make - And Fast!" I believe a current reasonable market capitalization value for Kopin would be $394M or north of $5.00/share based on the following: IP portfolio I have valued at at least $300M: Kopin holds over 300 granted & pending wearables patents; I believe they possess the strongest head-worn wearables IP portfolio in the industry.

OLED (T/$) Universal Display Corporation (NASDAQ: OLED) jumped as much as 17% early Tuesday before settling to close up 4% following reports that Apple (NASDAQ: AAPL) has chosen to adopt OLED displays across its entire iPhone line. More specifically, according to "industry sources" speaking to South Korea's Electronic Times, Apple will expand its use of OLED displays from just a single model -- as it currently does with only its high-end iPhone X -- to all three of its newer iPhone models to be launched in 2019. OLED market is poised to take off. The company expects the market for OLED displays to have a CAGR of 21% from 2015 to 2020. At the moment, what is driving this growth is mobile, and we are fairly certain that Apple is still ahead of us for an OLED iPhone. But also just ahead is another wave of growth from TVs, as costs come down and volumes increase. We could see this in the amount of OLED activity at the Consumer Electronics Show this year. Then there should be a third wave of growth from lighting applications. I like when we have some visibility of growth that extends long into the future, and that appears to be the case with Universal Display.
****LITE (T/$45H-50H-60H-68H-79)

LPL (T/$
16H-15H) LG Display
thin-film transistor liquid crystal display and organic light-emitting diode (OLED) technology-based display panels.  ****MVIS (T/$3.00H-2.50-3.50-4.50) Microvision makes PicoP scanning technology for three-dimensional sensing and image capture. MicroVision’s IP portfolio has been recognized by the Patent Board as a top 50 IP portfolio among global industrial companies and has been included in the Ocean Tomo 300 Patent Index. The company is based in Redmond, Wash.
****MVIS (T/$3.00H-2.50-3.50-4.50) Microvision makes PicoP scanning technology for three-dimensional sensing and image capture. MicroVision’s IP portfolio has been recognized by the Patent Board as a top 50 IP portfolio among global industrial companies and has been included in the Ocean Tomo 300 Patent Index. The company is based in Redmond, Wash. 
 IMMR (T/$8H-13) Immersion Corporation...the creation of the sense of touch to the user through the use of vibrations or motions. Immersion is one of the leaders in this technology, also known as kinesthetic communication. (very weak guidance for all of 2017) ****OCLR (T/$9H-8H-11) (wait for 3rd quarter...) 02/6 Although the current quarter could also be weak, Oclaro sees better conditions in the business by midyear, and investors are already factoring those high expectations into their forecasts. ...lasers and optical components partner with CEIN...Oclaro provides differentiated solutions for optical networks and high-speed interconnects driving the next wave of streaming video, cloud computing, application virtualization and other bandwidth-intensive and high-speed applications....The Company provides various solutions for optical networks and interconnects driving the next wave of streaming video, cloud computing, application virtualization and other bandwidth-intensive and high-speed applications.
IMMR (T/$8H-13) Immersion Corporation...the creation of the sense of touch to the user through the use of vibrations or motions. Immersion is one of the leaders in this technology, also known as kinesthetic communication. (very weak guidance for all of 2017) ****OCLR (T/$9H-8H-11) (wait for 3rd quarter...) 02/6 Although the current quarter could also be weak, Oclaro sees better conditions in the business by midyear, and investors are already factoring those high expectations into their forecasts. ...lasers and optical components partner with CEIN...Oclaro provides differentiated solutions for optical networks and high-speed interconnects driving the next wave of streaming video, cloud computing, application virtualization and other bandwidth-intensive and high-speed applications....The Company provides various solutions for optical networks and interconnects driving the next wave of streaming video, cloud computing, application virtualization and other bandwidth-intensive and high-speed applications.  ACIA Acacia Communications simplifies the process by taking data from lasers and moving it between devices using standard silicon chips. And those chips can work with virtually any form of optical networking equipment and also plug into existing networks that aren't designed to transmit data with laser beams. (Motley Fool) Its customers include Cisco Systems Inc. (CSCO), Huawei, Alcatel-Lucent (NYSE:ALU) and Amazon.com Inc. (AMZN). Applied Optoelectronics Inc. (AAOI) and Finisar Corp. (FNSR) are two of its main competitors. NPTN (T/$7-13) NeoPhotonics high speed digital optical signals... FN ... *EMAN (T/$4) light emitting diode (OLED) on silicon micro displays; virtual imaging products that utilize OLED micro displays; and related products. It offers super video graphics array (SVGA) + OLED micro displays; digital SVGA OLED-XL; super extended graphics array OLED-XL and OLED-XL/XLS; video graphics array OLED-XL; widescreen ultra-extended graphics array OLED-XL; and WF05 prism optic with mounting brackets or combined with OLED micro displays to form an optic-display module. The company also provides design reference kits, which include a micro display and associated electronics... Earnings
ACIA Acacia Communications simplifies the process by taking data from lasers and moving it between devices using standard silicon chips. And those chips can work with virtually any form of optical networking equipment and also plug into existing networks that aren't designed to transmit data with laser beams. (Motley Fool) Its customers include Cisco Systems Inc. (CSCO), Huawei, Alcatel-Lucent (NYSE:ALU) and Amazon.com Inc. (AMZN). Applied Optoelectronics Inc. (AAOI) and Finisar Corp. (FNSR) are two of its main competitors. NPTN (T/$7-13) NeoPhotonics high speed digital optical signals... FN ... *EMAN (T/$4) light emitting diode (OLED) on silicon micro displays; virtual imaging products that utilize OLED micro displays; and related products. It offers super video graphics array (SVGA) + OLED micro displays; digital SVGA OLED-XL; super extended graphics array OLED-XL and OLED-XL/XLS; video graphics array OLED-XL; widescreen ultra-extended graphics array OLED-XL; and WF05 prism optic with mounting brackets or combined with OLED micro displays to form an optic-display module. The company also provides design reference kits, which include a micro display and associated electronics... Earnings
9/8 *** VUZI (T/$10-11) VUZI smart glasses ...The Company's products include personal display and wearable computing devices that offer users a portable high-quality viewing experience, provide solutions for mobility, wearable displays and virtual and augmented reality. Vuzix holds 59 patents and 42 additional patents pending and numerous IP licenses in the Video Eyewear field. The Company has won Consumer Electronics Show (or CES) awards for innovation for the years 2005 to 2018 and several wireless technology innovation awards among others.
Jan 29 3million shares at $10...  *NEON
*NEON (T/$3.50) touch screen and ability to sense an object's size,deph,velocity, pressure, proximitty to any type surface....
SYNA ...ACIA... AAOI...
INTC (T$38H-42H -47H -50H-60)..Buying as of03/13 =Mobileye...*MBLY Triple Digit Stock develops computer vision/machine learning/data analysis/localization/mapping for advanced driver assistance systems and autonomous driving technologies primarily in Israel...
11/2017 Intel's latest AI-enabled platform, the Saffron Anti-Money Laundering (AML) Advisor, gathers gather data from several sources and then uses AI to help banking and insurance investigators explore emerging patterns in the data, enabling them to track anomalies. In Intel's words,
the Saffron AML Advisor will mimic "the associative memory of the human brain to surface similarities and anomalies hidden in dynamic, heterogeneous data sources, while accessing an infinitely larger data set than its human counterparts." The analysis of a large data set using artificial intelligence will help the Saffron AML Advisor reduce the time and effort put in to detect and fight a variety of financial crimes ranging from money laundering to identity theft and fraud. Intel (NASDAQ:
INTC) has been very active acquiring companies involved in the burgeoning wearable technology segment. You will find a great overview from Matt Margolis in his article entitled,
"Intel Is Quietly Emerging As The Augmented Reality World Leader." Despite this acquisition activity, there are still significant gaps if Intel wants to repeat the "Intel Inside" strategy for wearables that it so successfully employed in the PC era.
In my opinion, these are the missing pieces from Intel's current wearables ecosystem:
Proven high yield micro display and complementary optical technology
Next generation noise cancellation hardware and software
Head-worn reference designs - the new Intel Inside motherboard!
Licensable user interface IP covering voice and gesture input methods
Software middleware or a wearables operating system
Broad wearables patent protection
Interestingly, there is a way for Intel to achieve gap closure in all of the areas above with a single transaction: they could acquire Kopin Corp. (NASDAQ:
KOPN).

General Motors announced it will begin offering unlimited data for owners for just $20 per month. That’s a steal, considering rates for in-car data started at about $500 for 30 gigabytes in 2014. Considering the average person spends 42 hours per year in traffic — and upward of 80 hours in major metropolitan areas — the car represents the next battleground for media and telecom companies. In short, there’s a lot of revenue up for grabs — over $80 billion by 2021. The sooner companies can get consumers to start connecting in the car, the more market share they stand to capture. Bottom line: Price is always a barrier to adoption. But GM just eliminated it. ...5G networks promise to offer 10–100 times faster data speeds and lower latency. Our recommendation of PCTEL stands to benefit as initial rollouts begin, since it sells equipment vital to testing new wireless networks. However, 5G network progression also bodes favorably for our investments in the internet of things, virtual personal assistants, connected cars and associative computing. Faster processing speeds make these technologies more compelling. That includes Memex Inc. (OEE:TSX-V), Control4 Corp. (CTRL), GSI Technology Inc. (GSIT) and Visteon (VC). So keep buying
https://agorafinancial.com/2017/03/18/prepare-yourself-for-the-invisible-technology-revolution/?pdf=1 VSH ...(T/$15H-16.25H-20H...) Vishay Intertechnology ...broadens its optoelectronics portfolio with the introduction of a new high speed silicon PIN photodiode with enhanced sensitivity for visible light. Vishay Semiconductors VEMD5080X01 offers fast switching times and low capacitance for precise signal detection in wearable devices and medical, industrial, and automotive applications. *ISNS (T/$3H)... Image Sensing Systems, Inc. develops and markets software-based computer enabled detection products and solutions for the intelligent transportation systems industry. Its video and radar processing products are used in traffic, security, police, parking applications, such as intersection control, highway, bridge, tunnel traffic management, venue security, entry control, LPR, traffic data collection. NVDA (T/$145H-200H-225-250-270....) Nvidia neural imprinting and all forms of virtual reality... http://traderprep.com/2016/08/04/4-automakers-betting-on-nvidia/ ...Tesla’s co-founder and CEO, Elon Musk, has promoted NVIDIA’s GPUs as a key element for driverless car technology. At the 2015 GPU Technology Conference last year, Musk said that what NVIDIA is doing with its Tegra GPU’s will prove “really important for self-driving in the future.” While Tesla uses NVIDIA’s GPUs, it doesn’t currently use NVIDIA’s Drive PX system, though there’s no reason why it couldn’t in the future. Why all of this matters for NVIDIA investors NVIDIA’s self-driving car focus is becoming increasingly important because of the technology’s quick adoption by both automakers and consumers. IHS Automotive estimates that between now and 2035, there will be 76 million vehicles on the road with some level of self-driving capabilities. Cheaper technology is spurring growth, as is evidenced by Honda’s $20,040 Civic, which can drive itself on the highway. The driverless-car market is expected to grow to $42 billion by 2025, according to Boston Consulting Group, and it’s clear that NVIDIA’s mapping out a plan to grab as much of that market as possible with its current automotive partnerships. PCTI (T/$8.50) PCTEL Inc. (PCTI) and Visteon Corp. (VC).
http://www.buyupside.com/sample_portfolios/mobilegames.php VOICE ... the iPhone kicked off what can affectionately be dubbed the “Touchscreen Era.” But the sun is setting on that period.
We’re now entering the “Smart Voice Era.” And once again, it’s being pioneered by Apple and its introduction of a virtual assistant in 2011. Very soon, the days of pulling your smartphone from your pocket... swiping to unlock it… opening an app… and then tapping what you want will seem burdensome. Instead, just speak your desire into the air — anywhere — and it will be done. Thanks to key advances in artificial intelligence, voice interfaces can now learn in real-time, enabling them to accomplish an ever-increasing number of difficult and nuanced tasks.
companies like Nvidia and GSI Technology Inc. (GSIT) are at the forefront of this development.
******GSIT (T/$
9H) Remember how Nvidia leveraged the benefits of “parallel processing” to net windfall sales and profits for investors in 2016? Well, GSI’s in-place associative computing chips will leverage “massive parallel processing” capabilities.
****Think Nvidia on steroids.***SO WHERE ARE THE STEROIDS ...?...  ***DSPG
***DSPG (T/$
12.50H-15-17) DSP Group Inc. (DSPG) is a leading provider of digital signal processing chips. Its core know how is
enabling better voice communications. HDClear solution. It’s a comprehensive noise-suppression and voice quality-enhancement chipset for mobile devices. HDClear incorporates the company’s proprietary noise-cancellation algorithms to deliver unrivaled voice quality in any situation. And it does so using very low power, which is a key consideration for device makers. Simply put, HDClear enables voice interfaces to hear clearly and process commands efficiently. No matter how noisy the environment. Management estimates there are currently over 2 billion potential devices that could benefit from its HDClear chipset. And they’re not wasting any time capturing that upside potential. Case in point: New product sales increased 48% in 2016. And they’re headed higher still, as DSP secured two design wins in the voice interface market. These will enter mass production in the second half of 2017. With over 30 years of voice processing expertise — and 227 patents and applications — we couldn’t ask for a better-positioned company to benefit from the rapid transition to voice interfaces. A Rapidly Growing and High-Probability Pipeline: DSP’s new engagement pipeline for
HDClear covers voice-enabled products in every product category — IoT, smart assistants, PCs, wearables, smart speakers, home appliances and automotive. As management said, “We are excited with the market dynamics and the greenfield opportunity in the voice activation and control space.” Given DSP’s technology leadership, it’s only a matter of time before these opportunities are converted into design wins. But don’t just take my word for it. As management confirmed, “We actually feel a lot more bullish about our ability to penetrate and get design wins in this market.” Naturally, more design wins will lead to steadily increasing sales — and, in turn, profits. Speaking of which… New Products Already Driving Growth and Margin Expansion: After debuting with gross margins in the high 30% range, last quarter they increased to 56.6%. Higher margins lead to higher profits, which ultimately leads to higher share prices.
https://agorafinancial.com/2017/03/18/prepare-yourself-for-the-invisible-technology-revolution/?pdf=1  *****HEAR
*****HEAR (T/$1.30-12.50h) 4D sound...

***IZEA (T/$7H-???)Artificial Intelligence CurationEngine ...operates online marketplaces that facilitate transactions between marketers and content creators. Its technology solutions enable the management of content workflow, creator search and targeting, bidding, analytics, and payment processing. The company helps brands to engage online influencers for influencer marketing campaigns, or to create content for distribution through their channels

***XGTI (T/$2.5H-3-4) xG Technology Dec. 27, 2017 /PRNewswire/ -- xG Technology, Inc. ("xG" or the "Company") (Nasdaq: XGTI, XGTIW), a leading provider of wireless video solutions to broadcast, law enforcement and defense markets, and private mobile broadband networks for critical communications, announced today that its IMT Vislink business has completed delivery of the first order against the previously announced $12.5 million U.S Army contract award. The contract covers the supply of hand-held intelligence, surveillance and reconnaissance receiver devices. Delivery of the first order was completed significantly in advance of the required delivery date of Feb. 15, 2018.

***CPST (T/$2-3)
Capstone Turbine Corporation develops, manufactures, markets, and services microturbine technology solutions for use in stationary distributed power generation applications worldwide. It offers microturbine units, components, and various accessories for applications, including cogeneration comprising combined heat and power (CHP) and integrated CHP, as well as combined cooling, heat, and power; and renewable energy, natural resources, and critical power supply. The company's microturbines are also used as battery charging generators for hybrid electric vehicle applications.  Other Tech Stocks AMBA (T/$60H-62H)... Ambarella produces systems on a chip for various camera applications such as GoPro action cams and drones. These chips incorporate numerous patented technologies that allow them to work with very low power, in low ambient light conditions, and with highly efficient compression capabilities. GLNNF GLNNF () GlancePay see crypto/bloskchain stocks at top of page... MEET (T/$6H) SOCIAL NETWORK...
Other Tech Stocks AMBA (T/$60H-62H)... Ambarella produces systems on a chip for various camera applications such as GoPro action cams and drones. These chips incorporate numerous patented technologies that allow them to work with very low power, in low ambient light conditions, and with highly efficient compression capabilities. GLNNF GLNNF () GlancePay see crypto/bloskchain stocks at top of page... MEET (T/$6H) SOCIAL NETWORK... ***PRCP (T/$11) Perception Inc... 3D scanning products, laser scanners ...
*****WATT WATT (T/$
22H-32H-38-45)
Energous...wire-free charging of electronic devices at a distance... Dec. 28,2017 Roth Capital
raises its Energous (NASDAQ:
WATT) price target from $22.80 to $45.80 and maintains a Buy rating.
Energous share are up 8.6% premarket to $25.74. Shares are up 156% this week.
Previously: Energous skies 89% after winning FCC certification (Dec. 27)
Update with analyst comments: Roth analyst William Gibson cites the FCC certification as setting the stage for new WattUp tech with more power, functionality, and scale.
Gibson notes that the first products are expected to launch in Q1, and he expects the FCC approval process to speed up over time.
The analyst says Energous needs more money with only $20.2M in cash as of Q3. Funds could arrive through strategic partnerships such as Dialog exercising its warrants.
 MU Micron is the third largest mobile DRAM and fourth largest NAND manufacturer in the world. It's arguably the most straightforward play on the memory-chip market for investors, Samsung Electronics (NASDAQOTH:SSNLF), and Western Digital (NASDAQ:WDC) icron is currently developing next-gen memory technologies such as 3D NAND and 3DXPoint with Intel (NASDAQ:INTC), Radio Frequency Identification (RFID) isn't just a gamble. It's real, and the stakes are high. Identifying worthy players in disruptive technologies. Motley Fool GPIC Gaming Partners (NASDAQ:GPIC) -- The company makes gaming equipment, and that included at the time a thriving business embedding RFID microchips in casino chips to facilitate immediate inventory tallies and prevent counterfeit currency. It continues to offer RFID readers for cage, table, and vault applications as well as RFID technologies for casino readers. RFID isn't just a gamble. It's real, and the stakes are high. Identifying worthy players in disruptive technologies IDSY () The company offers integrated wireless solutions that utilize radio frequency identification, Wi-Fi, satellite or cellular communications, sensor technologies, and software to control, track, monitor, and analyze industrial vehicles, rental vehicles, and transportation assets. It provides industrial and rental fleet asset management products, including on-asset hardware with mounting and user-interface options that provide an autonomous means of asset control and monitoring; wireless asset managers that link the mobile assets being monitored with the customer's computer network or to a remotely hosted server; server software, which manages data communications between the system's database and wireless asset managers or on-asset hardware; and client software. The company also offers transportation asset management products comprising on-asset hardware configurations to address various remote asset types; VeriWise Intelligence Portal, a hosted Website that provides Internet access to client asset information; and a direct data feed through XML or Web services. In addition, it provides hosting, maintenance, and customer support and consulting services, as well as software as a service. IIJI () MANH () ...the company provides training and change management services; and resells computer hardware, radio frequency terminal networks, radio frequency identification chip readers, bar code printers and scanners, and other peripherals. ORCL () PHG () Koninklijke Philips N.V. QRVO () Qorvo, Inc. provides radio frequency (RF) solutions and technologies for mobile device, infrastructure, and defense and aerospace applications worldwide. ROP ...Roper Technologies ... Roper makes a wide array of products including water meters, energy pump testers, and medical imaging equipment. Roper makes RFID equipment used at tollbooths to facilitate the reading of prepaid express passes. This is naturally a booming market. The days of bypassing tolls are long gone as cars are scanned through tollbooths, and the fact that RFID technology requires fewer tollbooth operators is gravy to controlling costs. SCSC The company's Worldwide Barcode, Networking & Security segment focuses on automatic identification and data capture (AIDC), point-of-sale (POS), networking, electronic physical security, 3D printing, and other specialty technologies. Its AIDC and POS products are used to automate the collection, processing, and communication of information for commercial and industrial applications, such as retail sales, distribution, shipping, inventory control, materials handling, warehouse management, and health care applications; electronic physical security products, including identification, access control, video surveillance, intrusion-related, wireless, and networking infrastructure products; and 3D printing solutions to replace and complement traditional methods, as well as reduce the time and cost of designing new products. *** SPCB (T/$4.50H) SuperCom Ltd. ... provides identity, machine-to-machine, cyber security device, payment, and connectivity products and solutions to governments, and private and public organizations worldwide. The company offers MAGNA, a common platform for ID registries, e-passports, biometric visas, automated fingerprint identification systems, digitized driver's licenses, and electronic voter registration and election management. Its PureRF suite is a solution based on radio-frequency identification (RFID) tag technology to identify, locate, track, monitor, count, and protect people and objects. The company's PureRF suite comprises PureRF tags, hands-free long-range RFID asset tags, hands-free long-range RFID vehicle tags, PureRF readers, PureRF activators, and PureRF initializers. In addition, it provides house arrest monitoring systems, PureTag RF bracelets, PureCom RF base stations, GPS offender tracking systems, PureTrack smartphone device, PureBeacon, PureMonitor offender electronic monitoring software, inmate monitoring systems, DoorGuard tracking station, and personnel tags. Further, it offers domestic violence victim protection systems; SuperPay, a mobile payment hybrid suite; PureMoney Suite that provides mobile money applications and services; SuperPOS, a platform to perform mobile payments; SafeMoney, a mobile security threat scanner; and PowaPOS, an integrated design incorporating retail peripherals. Additionally, it provides Safend's Encryption Suite that protects the organization's sensitive data; and designs solutions for carrier wi-fi, enterprise connectivity, smart city, smart hospitality, connected campuses, and connected events. ZBRA Zebra Technologies (NASDAQ:ZBRA) -- Using RFID to track inventory is made easier through Zebra's solutions. It offers mobile and handheld readers as well as fixed readers. It also makes reader antennas to send data across various points of business activity. Zebra is also a leading distributor of RFID printers that allow businesses to encode and print out the RFID-backed labels that can be tracked with its readers. Semi/Cloud/Biometrics ... ADI... (T/$93H )Biometrics, drones..semicondtrs ANY (T/$3-4-5) tech. cloud, storage...July 11,2017 did a 1 for 25 reverse split CNIT..Internet service company that provides cloud-based platform China... ****SFUN (T/$4.50H) Chinese real estate Internet portal CUDA (T/$25H-29) Barracuda ... cloud-enabled solutions that enable customers to address security threats, improve network performance, and protect and store their data. It provides various security solutions, including Barracuda Email Security (01/09 earns/profit beat) DTRM
MU Micron is the third largest mobile DRAM and fourth largest NAND manufacturer in the world. It's arguably the most straightforward play on the memory-chip market for investors, Samsung Electronics (NASDAQOTH:SSNLF), and Western Digital (NASDAQ:WDC) icron is currently developing next-gen memory technologies such as 3D NAND and 3DXPoint with Intel (NASDAQ:INTC), Radio Frequency Identification (RFID) isn't just a gamble. It's real, and the stakes are high. Identifying worthy players in disruptive technologies. Motley Fool GPIC Gaming Partners (NASDAQ:GPIC) -- The company makes gaming equipment, and that included at the time a thriving business embedding RFID microchips in casino chips to facilitate immediate inventory tallies and prevent counterfeit currency. It continues to offer RFID readers for cage, table, and vault applications as well as RFID technologies for casino readers. RFID isn't just a gamble. It's real, and the stakes are high. Identifying worthy players in disruptive technologies IDSY () The company offers integrated wireless solutions that utilize radio frequency identification, Wi-Fi, satellite or cellular communications, sensor technologies, and software to control, track, monitor, and analyze industrial vehicles, rental vehicles, and transportation assets. It provides industrial and rental fleet asset management products, including on-asset hardware with mounting and user-interface options that provide an autonomous means of asset control and monitoring; wireless asset managers that link the mobile assets being monitored with the customer's computer network or to a remotely hosted server; server software, which manages data communications between the system's database and wireless asset managers or on-asset hardware; and client software. The company also offers transportation asset management products comprising on-asset hardware configurations to address various remote asset types; VeriWise Intelligence Portal, a hosted Website that provides Internet access to client asset information; and a direct data feed through XML or Web services. In addition, it provides hosting, maintenance, and customer support and consulting services, as well as software as a service. IIJI () MANH () ...the company provides training and change management services; and resells computer hardware, radio frequency terminal networks, radio frequency identification chip readers, bar code printers and scanners, and other peripherals. ORCL () PHG () Koninklijke Philips N.V. QRVO () Qorvo, Inc. provides radio frequency (RF) solutions and technologies for mobile device, infrastructure, and defense and aerospace applications worldwide. ROP ...Roper Technologies ... Roper makes a wide array of products including water meters, energy pump testers, and medical imaging equipment. Roper makes RFID equipment used at tollbooths to facilitate the reading of prepaid express passes. This is naturally a booming market. The days of bypassing tolls are long gone as cars are scanned through tollbooths, and the fact that RFID technology requires fewer tollbooth operators is gravy to controlling costs. SCSC The company's Worldwide Barcode, Networking & Security segment focuses on automatic identification and data capture (AIDC), point-of-sale (POS), networking, electronic physical security, 3D printing, and other specialty technologies. Its AIDC and POS products are used to automate the collection, processing, and communication of information for commercial and industrial applications, such as retail sales, distribution, shipping, inventory control, materials handling, warehouse management, and health care applications; electronic physical security products, including identification, access control, video surveillance, intrusion-related, wireless, and networking infrastructure products; and 3D printing solutions to replace and complement traditional methods, as well as reduce the time and cost of designing new products. *** SPCB (T/$4.50H) SuperCom Ltd. ... provides identity, machine-to-machine, cyber security device, payment, and connectivity products and solutions to governments, and private and public organizations worldwide. The company offers MAGNA, a common platform for ID registries, e-passports, biometric visas, automated fingerprint identification systems, digitized driver's licenses, and electronic voter registration and election management. Its PureRF suite is a solution based on radio-frequency identification (RFID) tag technology to identify, locate, track, monitor, count, and protect people and objects. The company's PureRF suite comprises PureRF tags, hands-free long-range RFID asset tags, hands-free long-range RFID vehicle tags, PureRF readers, PureRF activators, and PureRF initializers. In addition, it provides house arrest monitoring systems, PureTag RF bracelets, PureCom RF base stations, GPS offender tracking systems, PureTrack smartphone device, PureBeacon, PureMonitor offender electronic monitoring software, inmate monitoring systems, DoorGuard tracking station, and personnel tags. Further, it offers domestic violence victim protection systems; SuperPay, a mobile payment hybrid suite; PureMoney Suite that provides mobile money applications and services; SuperPOS, a platform to perform mobile payments; SafeMoney, a mobile security threat scanner; and PowaPOS, an integrated design incorporating retail peripherals. Additionally, it provides Safend's Encryption Suite that protects the organization's sensitive data; and designs solutions for carrier wi-fi, enterprise connectivity, smart city, smart hospitality, connected campuses, and connected events. ZBRA Zebra Technologies (NASDAQ:ZBRA) -- Using RFID to track inventory is made easier through Zebra's solutions. It offers mobile and handheld readers as well as fixed readers. It also makes reader antennas to send data across various points of business activity. Zebra is also a leading distributor of RFID printers that allow businesses to encode and print out the RFID-backed labels that can be tracked with its readers. Semi/Cloud/Biometrics ... ADI... (T/$93H )Biometrics, drones..semicondtrs ANY (T/$3-4-5) tech. cloud, storage...July 11,2017 did a 1 for 25 reverse split CNIT..Internet service company that provides cloud-based platform China... ****SFUN (T/$4.50H) Chinese real estate Internet portal CUDA (T/$25H-29) Barracuda ... cloud-enabled solutions that enable customers to address security threats, improve network performance, and protect and store their data. It provides various security solutions, including Barracuda Email Security (01/09 earns/profit beat) DTRM (T/$
3H-5)
*EIGI (T/$8.50H)...cloud *FTR (T/$15-25)telecom fiber-to-the-home and cloud backup, sharing, identy protection... GSB () software cloud based ... HPE (T/$15-18-20) Hewlett Packard Enterprise Co This was the company a bunch of investors all but wrote off a couple of years ago, convinced it was no longer relevant in a cloud-centric world, with or without its consumer oriented PC arm (which it split with in 2015). The doubters were wrong.
See, though the enterprise-oriented outfit still has plenty of work to do, it has found its place in the modern era of technology. Hybrid cloud computing, which combines the best of in-house and outsourced data management, is something no other player does quite as well as HPE. Last quarter’s hybrid IT business was up 10% last quarter, and is now the company’s breadwinner. It’s also the reason HPE stock gained 23% during the first quarter of this year. InvestorPlace.com

*HIVE (T/$9-10) cloud-managed mobile platform... *INVE (T/$7-10) Identiv, Inc. operates as a security technology company that secures and manages access to physical places, things, and information worldwide. It operates through four segments: Premises (PACS), Identity, Credentials, and All Other. The PACS segment offers modular Hirsch MX controllers that allow customers to start with a small system and expand over time; Hirsch Velocity software platform for centralized management of access and security operations across an organization; Federal Identity, Credential and Access Management architecture, an access control system; and TouchSecure door readers that provide various features to support security standards. The Identity segment provides smart card readers, which include various contact, contactless, portable, and mobile smart card readers, as well as tokens and terminals to enable logical access, and security and identification applications, such as national ID, payment, e-health, and e-government. It also offers access cards and other devices related to its reader products. The Credentials segment provides NFC and radio frequency identification products, including inlays and inlay-based, and other cards; and labels, tags, and stickers, as well as other radio frequency and integrated circuits components for use in various applications, such as virtual reality, games, loyalty cards, mobile payment systems, transit and event ticketing, and brand authenticity from pharmaceuticals to consumer goods, hospital resource management, cold-chain management, and others. *MODN (T/$16) ...provides revenue management cloud solutions for the life science and technology industries *NXTD (T-$4) biometrics security mobile devices, electronic wallet virtually any magnetic stripe card allow single electronic card to replicate cards for MobileBio VoiceMatch ...http://finance.yahoo.com/news/nxt-id-inc-demonstrates-module-130000868.html PI (T/$40H) Impinj ...radio frequency ID chip-maker, maker of RAIN RFID chips ***PSTG (T/13H-15) Provides flash memory-based enterprise storage hardware... ****STM (T/$14H-$20) STMicroelectronics Semicndctr ****USAT (T/$6) wireless networking, cashless transactions, asset monitoring... BioMetrics biometrics (fingerprint, retinal, voice and facial recognition) stocks --- http://www.buyupside.com/sample_portfolios/biometrics.php AWRE (T/$5.50) Aware software products are used in government and commercial biometrics systems to identify or authenticate people. It offers biometrics software products, including biometric services platform, search and match products, software development kits, and application products for fingerprint, facial, and iris modalities that enable various functions in biometrics systems, such as enrollment, analysis, and processing of biometrics images and data on workstations or mobile devices; integration of peripheral biometric capture devices; centralized workflow, transaction processing, and subsystem integration; matching of biometric samples against biometric databases to authenticate or verify identities; and analysis and processing of text-based identity data. *BKYI (T/$5.50) BIO-KEY...fingerprint biometric identification and identity verification technologies, cryptographic authentication-transaction security technologies, as well as related identity management and credentialing software solutions. The Company is also engaged in developing automated, finger identification technology that supplements or compliments other methods of identification and verification, such as personal inspection identification, passwords, tokens, smart cards, identity cards, public key infrastructure (PKI), credit card, passports, driver's licenses, one-time password (OTP) or other form of possession or knowledge-based credentialing. Its solutions identify individuals and verify, or confirm, their identity before granting access to, among other things, corporate resources, subscribed data and services, Web portals, applications, physical locations or assets. FBI uses this?
IWSY ImageWare Systems...biometrically enabled software-based identity management ... *NXTD (T-$4) biometrics security mobile devices, electronic wallet virtually any magnetic stripe card allow single electronic card to replicate cards for MobileBio VoiceMatch ...http://finance.yahoo.com/news/nxt-id-inc-demonstrates-module-130000868.html SMME () Smart Metric SYNA
Portfolio of Airport Security Stocks Portfolio of Cyber Security Stocks Portfolio of Security Stocks Portfolio of Video Surveillance Stocks
ROBOTICS Myria Research forecast (~2012) the robotics and automation market to grow from US$ 64b to US$ 1.2 trillion in the next ten years. I calculate that to be an 18.75 fold growth. Robots may replace 800 million workers by 2030. https://seekingalpha.com/article/4147101-look-robotics-sector-robot-companies?li_source=LI&li_medium=liftigniter-widget
Love and Sex with Robots “I believe it is going to be perfectly normal that people will be friends with robots, and that people will have sex with robots,” says Cheok. “All media will touch humanity.” ****BOTZ...Global Robotics and Artificial Intelligence.. ISRG 7.9%, MBLY 4.8%,TRMB5.4%,IRBT,BRKS,FARO  ROBO CandleGlance Global Robotics&Automation...KYCCF,CYBQF,YASKF,CGNX,ISRG, OMRNF,FARO,DAIUF, NCTKF,YASKF,RAVN, FANUF,KRNNF,DAIUF,ROCK,ABLZF,NCTKF,AVAV,AMANF,ARAY,HOLI,JNPKF,BRKS .... GOSY Robot...CareBOT... GMED
ROBO CandleGlance Global Robotics&Automation...KYCCF,CYBQF,YASKF,CGNX,ISRG, OMRNF,FARO,DAIUF, NCTKF,YASKF,RAVN, FANUF,KRNNF,DAIUF,ROCK,ABLZF,NCTKF,AVAV,AMANF,ARAY,HOLI,JNPKF,BRKS .... GOSY Robot...CareBOT... GMED 
ABB
CGNX Cognex engages in the provision of vision systems, vision software, vision sensors, and surface inspection systems used in manufacturing automation.
It also offers industrial identification readers. Put simply, Cognex is a major player in robotic machine vision systems.
(OTCPK:FANUY) (OTCPK:FANUF) Fuji Automatic NUmerical Contro (FANUC) is a Japanese global leader in industrial robots. Their Factory Automation (FA) business, which develops servomotors and NC apparatuses for machine tools, is said to have 50% of the global market share. The FANUC Robodrill (machine) is used to help make many smartphone housings including the Apple iPhone. FANUC is somewhat sensitive to orders from the Asian electronics sector for its Robo Machine. FANUC robots are very commonly found in factories all over the world and that division is growing strongly. FANUC is one the best bets in the industrial robotic sector, but it does not come cheap trading on a 2018 PE of 36.72.
GE General Electric have moved into robots in recent years. In early 2016 GE planned to open a new AI, robotics, and product management labs In New York. GE Aviation recently Acquiredhmc leading robotics manufacturer OC Robotics who specializes in "snake-arm robots". GE's Avitas Systems is a GE new business that will use robotics, artificial intelligence and predictive analytics to take the inspection-services industry to a higher level especially in the area of drones.
HMC ASIMO, an acronym for Advanced Step in Innovative MObility, a humanoid robot with human gestures. More recently the company has developed their 3E robot strategy. 3E stands for empower, experience and empathy. They have also developed a robot all terrain vehicle (ATV).
INTC "Intel wants to use sensors and robots to overhaul in-store shopping. The web of connected gadgets will let companies more closely track inventory, collect data on shopper habits and eventually personalize offerings much like their online counterparts already do."
SFBTF) Designed and built by Softbank and Aldebaran Robotics (78.5 percent owned by Softbank), "Pepper" is a cooing, gesturing humanoid on wheels that can decipher emotions. Pepper has integrated Nuance's (NUAN) voice technology to accurately hear and respond in 19 different languages. Pepper stands at just 4 feet (120cm) tall, and has a 14 hour battery life. Aldebaran has also developed other robots. Investing in Softbank stocks will give you some small exposure only as the robot business revenues will be small compared to their total company revenues. Therefore would be best to invest in Softbank if you liked the company's mobile business.
Softbank's billionaire founder Masayoshi Son sees Pepper's future evolving to become a kind, friendly home companion. You can watch Pepper on video here, or read this MasterCard (NYSE:MA) press release, MasterCard Powers First Commerce Application within SoftBank Robotics' Humanoid Robot "Pepper." In 2017 Softbank bought Boston Dynamics from Alphabet. Boston Dynamics is a military-industrial robotics company who has several robots. BigDog is a quadruped robot designed for the U.S. military. Atlas is a 6'2 human-like robot, unlike all of its competitors, which are more child sized.
TER Teradyne made a big move into the robotics market when they bought Universal Robots (UR). Universal Robots is the world’s leading maker of collaborative robots (cobots). Cobots are low-cost, easy-to-deploy, simple-to-program robots that work side by side with production workers to improve quality and increase manufacturing efficiency. They automate tasks including machine tending, packaging, gluing, painting, polishing and assembling parts and are deployed in the automotive, food and agriculture, furniture and equipment, metal and machining, plastics and polymers, and pharma and chemicals industries. According to Teradyne - "Collaborative robotics is a $100 million segment of the industrial robotics market growing at more than 50% per year. Universal Robots is the leader in this segment and has shipped more than 4,000 cobots to date." Note: The amount shipped has now risen to 20,500 according to Universal More More...Robots. https://seekingalpha.com/article/4147101-look-robotics-sector-robot-companies?li_source=LI&li_medium=liftigniter-widget
Qinetiq Group Plc. ADR (OTCPK:QNTQY) Qinetiq is a British multinational defense technology company that offers a wide range of products and services including robots used to remotely locate and disable roadside bombs, and a solar-powered unmanned aerial vehicle. Based on the fact that Qinetiq offers a vast range of products and services, most of which are unrelated to robotics, it really doesn't concern me that ROBO does not hold this stock.
More ... https://seekingalpha.com/article/4046237-battle-robot-etfs
GLOBAL MEDICAL ROBOTICS is expected to reach USD 20.56 billion by 2022 from USD 6.36 billion in 2016 at a CAGR of 21.6% during the forecast period. Neurology segment is expected to register the fastest growth during the forecast period. This growth is attributed to the advent of newer applications in neurosurgery and the growing popularity of robotic neurosurgery. http://crweworld.com/newsroom/prnewswire?rkey=20170915IO82767&filter=10284
A medical robot is the one which is used by surgeons for greater access to areas under operation using more detailed and less invasive methods. They are in most telemanipulators, which use the surgeon's actions on one side and to control the "effector" on the other side. The medical robotic systems market is majorly fuelled by the enhancement of automation technologies, the rising incidence of disabilities in human beings and rising baby boomer population, that impede their day-to-day physical motions. North America has the highest market share of the medical robotics market in 2016. Its large share is attributed to the high adoption rate of medical robots in the region and the government's strong focus on medical robots to improve the quality of healthcare. Asia Pacific medical robotic systems market is expected to grow at the fastest rate during the forecast period due presence of untapped market/opportunities. Key factors that drive the demand for medical robots market include rapidly growing demand for precise and efficient minimally invasive surgeries. Growth in the volume of these surgeries, as a consequence of growing disease prevalence levels is also likely to drive the market growth. However, the high cost of surgery, negative publicity, and wariness among people continue to challenge the growth. Medical nanotechnology is employing nanorobots that when injected into the patient work at a cellular level. Instruments & Accessories Robotic Systems Surgical Robots Laparoscopy Surgical Robotic Systems Orthopedic Surgical Robotic Systems Neurosurgical Robotic Systems Steerable Robotic Catheters Rehabilitation Robots Therapeutic RobotsAssistive Robots Prosthetics Orthotics Exoskeletons Non-invasive Radiosurgery Robots Hospital and Pharmacy Robots Pharmacy Robots\ IV Robots Telemedicine Robots...
Companies: Intuitive Surgical, Inc. Stryker corporation Accuray, Inc Hansen Medical, Inc. Mazor Robotics IRobot Corporation ReWalk Robotics Siemens Healthcare Aurora Biomed MAKO Surgical Corp. Abbot Diagnostics ZOLL Medical Corp Agilent Technologies Baxter International Roche Holding Kirby Lester Biotek Instruments Carefusion Aesynt Inc Hocoma AG...
ISRG Intuitive Surgical is a leading global designer and manufacturer of surgical instruments as well as healthcare robots.
TransEnterix, Inc. (NYSEMKT:TRXC) TransEnterix is a medical device company that is pioneering the use of robotics to improve minimally invasive surgery. I don't want to be crude, but this is one medical device company that may die on the operating table. There is one round of financing (i.e. share dilution). Based on the current state of clinical trials and sales, there will likely be more before the company starts making a profit.
RWLK RWLK (T/$1.50-3.00) Robotics exoskeleton ReWalk Robotics Ltd. develops, manufactures and markets wearable robotic exoskeletons for individuals with spinal cord injury. ReWalk’s mission is to fundamentally change the quality of life for individuals with lower limb disability through the creation and development of market leading robotic technologies. The Company offers ReWalk, which is an exoskeleton that uses its tilt-sensor technology and an on-board computer and motion sensors to drive motorized legs that power movement. ReWalk designs are intended for people with paraplegia, a spinal cord injury resulting in complete or incomplete paralysis of the legs, having the use of their upper bodies and arms. www.rewalk.com ... MARLBOROUGH, Mass. and YOKNEAM ILIT, Israel, June 12, 2017 /PRNewswire/ -- ReWalk Robotics Ltd. (RWLK) ("ReWalk"), leading manufacturer of exoskeleton systems, today announced a Florida court ruling that found Blue Cross Blue Shield of Florida must provide coverage of a ReWalk exoskeleton system for a plan member with a spinal cord injury ("SCI"). This court decision is the latest of several in the United States that have ruled in favor of the individual after original denial of claims by commercial insurers.
Exoskeletons explained https://finance.yahoo.com/news/exoskeletons-explained-113919072.html Advances in suits for industry "will speed the development of low cost, more functional rehabilitation/enable systems," said Dan Kara, research director at technology market intelligence firm ABI Research. "It's a virtuous circle."
ABI projects that
robotic exoskeleton sales will jump from $97 million globally in 2016 to $1.9 billion by 2025. It predicts almost a quarter of the 100,000 suits sold in 2025 will be for people with disabilities. Better and cheaper battery technologies and stronger, lightweight materials will help drive this growth, analysts say.
Dozens of firms are developing medical exoskeletons, from start-ups to large companies like Korea's Hyundai, which plans to launch a demo version of its H-Mex prototype in 2019. Soft exo-suits worn like garments are being tested in the university labs of ETH Zurich and Harvard.
Nasdaq-listed
ReWalk Robotics is a market leader. Like most exoskeletons, ReWalk has a hard frame with motors at the hip and knee joints that enable users to get up and walk, mimicking a natural gait. A battery pack is worn on the back and crutches are used for balance.
The U.S. Food and Drug Administration has approved the suit, with a list price of $77,000, for clinical and home use. Competitors include
Parker Hannifin with its $80,000 Indego, and s
imilarly-priced Ekso by Ekso Bionics, a pioneer of exo-suit rehabilitation for stroke victims.
The REX, made by New Zealand-based company
REX Bionics, is self-balancing and requires no crutches. Its price starts at $99,000. The Phoenix suit I tested uses two motors at the hips and mechanical hinges at the knee. SuitX, the company behind Phoenix, aims to sell the Phoenix at around $30,000 initially but wants to cut the price by about half over time, according to its chief executive, Homayoon Kazerooni, a professor at the University of California.
Kazerooni said that when the cost of an exoskeleton becomes comparable to the price of a powered wheelchair, health insurers could be persuaded to pay for exo-suits. "Technology has to reach people faster than the rate we see these days," he said.
 EK EKSO (T/$4) Ekso Bionics Holdings, Inc. designs, develops, and sells exoskeletons for use in the healthcare, industrial, military, and consumer markets in North America, Europe, the Middle East, and Africa. The company operates through Medical Devices, Industrial Sales, and Engineering Services segments. It primarily offers Ekso GT, a bionic suit that provides the ability to stand and walk over ground with a reciprocal gait using a cane, crutches, or a walker to individuals with spinal cord injuries, hemiplegia due to stroke, and lower limb paralysis or weakness. The company's Ekso device is primarily used in a clinic or rehabilitation setting. It also performs research and development work on human exoskeletons and related technologies. The company has a license agreement with Lockheed Martin Corporation to develop products for military applications, as well as with OttoBock Healthcare Products Gmbh.
EK EKSO (T/$4) Ekso Bionics Holdings, Inc. designs, develops, and sells exoskeletons for use in the healthcare, industrial, military, and consumer markets in North America, Europe, the Middle East, and Africa. The company operates through Medical Devices, Industrial Sales, and Engineering Services segments. It primarily offers Ekso GT, a bionic suit that provides the ability to stand and walk over ground with a reciprocal gait using a cane, crutches, or a walker to individuals with spinal cord injuries, hemiplegia due to stroke, and lower limb paralysis or weakness. The company's Ekso device is primarily used in a clinic or rehabilitation setting. It also performs research and development work on human exoskeletons and related technologies. The company has a license agreement with Lockheed Martin Corporation to develop products for military applications, as well as with OttoBock Healthcare Products Gmbh.  ***TRXC
***TRXC (T/$2.75) ...
TransEnterix ... focuses on the development and commercialization of surgical robotic systems. The company offers Senhance System, a multi-port robotic surgery system, which allows up to four arms to control robotic instruments and a camera in Europe. It also develops SurgiBot System, a single-port, robotically enhanced laparoscopic surgical platform. In addition, the company develops and manufactures flexible and rigid laparoscopic surgical instruments that are used in abdominal surgery, such as scissors, graspers, clip appliers, and suction and irrigation instruments. The system features advanced technology to enable surgeons with haptic feedback and the ability to move the camera through eye movement. The system replicates laparoscopic motion and integrates three-dimensional high definition (3DHD) vision technology. The ALF-X System also offers responsible economics to hospitals by offering robotic technology with reusable instruments.  *** NMTC NeuroOne Medical Technologies Corporation is a developmental stage company committed to providing minimally invasive and hi-definition solutions for EEG recording, brain stimulation and ablation solutions for patients suffering from Epilepsy, Parkinsons Disease, Essential Tremors and other related neurological disorders that may improve patient outcomes and reduce procedural costs.
*** NMTC NeuroOne Medical Technologies Corporation is a developmental stage company committed to providing minimally invasive and hi-definition solutions for EEG recording, brain stimulation and ablation solutions for patients suffering from Epilepsy, Parkinsons Disease, Essential Tremors and other related neurological disorders that may improve patient outcomes and reduce procedural costs. June 15, 2018 / Elon Musk is investing millions in technology to enhance and connect the brain to robots and prosthetics. So far, this kind of technology has been limited by our current hardware. Upstart NeuroOne Medical (NMTC) could change that with their first FDA approved device, anticipated by the end of this year - a thin-film electrode that was pioneered at The Mayo Clinic. This prestigious institute owns 10% of NMTC, validating the tech.
NMTC is still new to the public markets and has yet to get much investors attention. A planned FDA product filing and approval, expected in the second half of 2018, could send NMTC ripping by 2X or 3X. The potential to "hack" the brain has been a dream of scientists and futurists for years. We're still far from pairing the intricate human mind with the calculating qualities of computers, but progress is being made, and it's attracting some of the smartest minds of the century.
Tesla's (TSLA) own Elon Musk has launched a new company called Neuralink Corp to pursue implanting tiny brain electrodes that may one day upload and download information straight into the human mind. Braintree co-founder Bryan Johnson has launched his own brain hacking startup called Kernel, where the prevailing question is when, not if, humans will have brain implants improving our lives. Both founders are realistic about how much work there is to be done, and the reality today is much less sexy. Very few people have multiple electrodes implanted IN their skulls. The few applications today are in Parkinson's and other neurological conditions, where they can help to alleviate symptoms and restore movement to some paralyzed patientThs. The limitations are mostly due to a lack of high-quality, flexible electrodes that can stay in the brain for long periods of time. That could change in 2018. A new upstart biotech company with backing from the prestigious Mayo Clinic (a 10% equity holder) is bringing a unique implantable electrode array to the market, with a goal of FDA approval by the end of this year. NeuroOne Medical (NMTC) isn't on most investors' radars, even though the company could be generating revenue by this time next year in a large market - they could easily capture tens of millions in sales within a few year's time considering the substantial improvements in their thin-film electrodes over old, bulky technology. 2018 could be a breakout year for NMTC, and the stock could easily double with a possible FDA clearance. Factoring in multiple FDA catalysts, commercial traction and a possible uplisting to a senior exchange within the next 12 months, the stock could be valued at multiples of where it currently trades.
Neural Hacking Could Be Closer Than You Think
The first brain interfacing devices are already here, though they're not exactly mainstream. Deep brain stimulation, which uses small electrodes and low current, can reduce the symptoms of Parkinson's disease, Epilepsy, and certain movement disorders. Amputees are able to operate increasingly impressive prosthetic limbs using their thoughts. Second Sight Medical Products, Inc. (EYES) is helping the blind "see" with their Argus II retinal prosthesis already, for example, and a recent DARPA-funded project actually resulted in improved memories for patients with epilepsy; the doctors are calling it a "Prosthetic Memory."

Think of the brain as a massive network of electrical connections. Figuring out how to interface with this giant computer is a matter of identifying the right connections and inserting electrodes that can either replace damaged connections - as is the case with some current prosthetics - or enhance and expand on some connections, which is what Kernel and Neuralink hope to do.
This is an emerging market, but research firm Grand View Research believe it to be immense. The global microelectronic medical implants market size is anticipated to reach $57 billion by 2025, with a 9.8% CAGR during the forecast period. This includes things like pacemakers, but cochlear and ocular implants as well as neurostimulators are expected to be a major growth driver as this tech evolves in the next ten years.
One Problem Is Connectivity There are a few major hurdles.
First, we still have a LOT to learn about the brain and how it works. The brain has approximately 100 billion connections, like small wires, that pass electrical signals back and forth to control bodily functions. These nerve cells are how sight, touch and sound are measured and then translated to the brain. Mapping the brain to figure out what connections do what, and where, is a monumental task, and it's only just begun. Akin to the mapping of the human genome in the late 20th century, we've only scratched the surface of understanding how to interact with and potentially control these connections in the brain. Many common disorders are the result of damage to these physiological wires. Second, electrode technology hasn't evolved as rapidly as the chips and infrastructure that have made computers smaller and cheaper over the last 30 years. One of the major fundamental roadblocks to implantable replacement or enhancement "wiring" has been a lack of capable electrodes that can be placed within or on the brain and work reliably for the lifetime of a subject. These would have to be strong, but soft enough to conform and match the tissue of the brain to avoid damaging this critical infrastructure. Today's electrodes are bulky and often cause inflammation in the brain over time - they simply don't always hold up.
NMTC's New Mayo Clinic-Backed Tech Could Change Everything
What's going on at NeuroOne is highly interesting in light of the push by visionaries like Musk and Johnson. NeuroOne's proprietary technology, which is anticipated to have an FDA 510k approval by the end of the year, can provide exactly what may be missing today - a platform where thousands of soft wires can both record and generate electrical signals safely in the human brain. NTMC's core technology lies in manufacturing thin-film electrodes; the company just went public in 2017 with The Mayo Clinic as a key backer and equity holder. The prestigious institute owns 10% of the company's outstanding shares, and the company's lead technology actually emerged from a partnership with Mayo's neurology division. A number of Mayo researchers are on the company's advisory board.
NeuroOne's thin-film electrode technology has a more brain-friendly material than today's devices, which can reduce inflammation and improve electrical signal clarity for better diagnosis and electroencephalography readings. This offers high-resolution EEG recording to increase the accuracy of diagnostics, improving resection planning and outcomes - it's like comparing the high-definition television displays of today to older tube technology of the 70's. The tech also utilizes disposable, sterile cables, saving EEG techs time and extensive costs related to cable management, which is part of the process today. And most importantly to many patients, the technology may be implanted through non-invasive methods, without the need to remove part of the brain.
Completely Undiscovered, NMTC Could Fly in 2H18 NMTC is a micro-cap stock and carries certain risks. The company could have trouble financing the business, and they still face competition from larger device companies. You should view NMTC as a high-risk high-reward option on the medical device space.
NMTC is planning their first U.S. Food and Drug Administration (FDA) device submission for the second half of this year, and they could receive a clearance from the agency by the end of the year. This is a major event for any healthcare company, and we've see how rapidly things can change for small-cap medical stocks: GalMed Pharmaceuticals (GLMD) recently rallied over 200% with some new clinical trial data in liver disorders. In fact, robotics-focused Ekso Bionics (EKSO) last year rallied over 3X in the course of one week when their own bionics product was rumored to be impemented by Ford (F) motor company; could NMTC see a similar implementation by the likes of Neuralink or Kernel? NMTC could be set up for their own surprise rally in 2018 as their potential FDA clearance approaches.
About One Equity Stocks One Equity Stocks is a leading provider of research on publicly traded emerging growth companies. Our team is comprised of sophisticated financial professionals that strive to find the companies and management teams that will outperform the market and deliver investment returns to our subscribers. We are not a licensed broker-dealer and do not publish investment advice and remind readers that investing involves considerable risk. One Equity Stocks encourages all readers to carefully review the SEC filings of any issuers we cover and consult with an investment professional before making any investment decisions. One Equity Stocks is a for-profit business and is usually compensated for coverage of issuers we cover as well as other advisory work we perform. In the case of NMTC, we are reimbursed for actual costs we incur and anticipate receiving up to 250,000 shares of restricted stock from NMTC for Business Development, Capital Markets, and Research Services. Please contact us at info@investorclick.net for additional information or to subscribe to our intelligence service.
Wearable technologies/components WEAR IRTC,DXCM,BSX,AAPL,GGNDF,BEAT,KN,TASR,PODD,ABT http://www.buyupside.com/sample_portfolios/wearables.php wearable devices that will ship this year to enterprise customers from public companies including Fujitsu (OTCPK:FJTSY), Vuzix (NASDAQ:VUZI), Google (NASDAQ:GOOG), and Intel (NASDAQ:INTC). Market estimates vary widely, but a recent report by Grand View Research estimates the enterprise wearables market will be valued at more than $20B by 2025. SKYY CandleGlance ETF Cloud Computing ...AKAM,ORCL,EQIX,NTAP,AMZN,CSCO,OTEX,JNPR,FB ... *****VERI (T/$13H-17H-19) ...Veritone, Inc., a cloud-based cognitive software company, develops a proprietary artificial intelligence platform for various markets in the United States and internationally. Its cloud-based open platform integrates and orchestrates an ecosystem of cognitive engines to reveal multivariate insights from various amounts of audio, video, and structured data; and incorporates proprietary technology to manage and integrate a range of AI processes to mimic human cognitive functions comprising perception, reasoning, prediction, and problem solving to transform unstructured data into structured data. The company offers media agency services, including media planning and strategy, media buying and placement, campaign messaging, clearance verification and attribution, and custom analytics to manage, deliver, optimize, verify, and quantify advertising campaigns and content distribution for various clients across multiple channels comprising broadcast radio, satellite audio, streaming audio, broadcast and cable television, digital video, and podcasting. It also provides Software-as-a-Service solutions for media owners and broadcasters to automatically index and organize audio and video content to search, discover, and analyze their media for programming and optimization; political organizations to analyze public and private media, conduct research, and provide access to previously inaccessible data; legal professionals to find the critical details of their cases by storing, organizing, and analyzing evidentiary media; and police and other government authorities to organize and gain insight from the large amount of audio, video, and structured data they accumulate on a daily basis, as well as offers solutions for other markets, including commercial security and retail. Veritone® Inc. (NASDAQ:VERI), a leading provider of artificial intelligence (AI) based solutions, announced today that they have introduced extensive language translation support for virtually any type of media, including audio and video files, emails and text documents, within Relativity®, kCura’s e-discovery software that helps organizations worldwide manage and analyze large volumes of unstructured data.  With the Veritone Media Player, Relativity users can now employ machine translation for over 100 languages, including Chinese, French, German, Italian, Japanese, Russian and Spanish, for evidentiary media. The Veritone Media Player also now incorporates Relativity’s Structured and Conceptual Analytics for expedited and efficient discovery of content and key topics. Initially integrated into the Relativity Ecosystem at the beginning of 2017, Veritone unlocks the power of AI-based cognitive computing, enabling unstructured evidentiary media such as audio and video files to be processed, transformed, and analyzed to generate truly actionable intelligence. The platform can render every second and frame of audio and video content searchable for things like words, phrases, faces, sentiment, and voice identification. Veritone now also provides machine translation, in addition to its existing redaction and transcription capabilities, to produce an index of the processed data within minutes – a process that as recently as 2015 could take thousands of hours. “With Relativity, we deliver an easy and convenient solution that both accelerates and increases the capabilities of the eDiscovery process, generating actionable information from data that was previously inaccessible,” said Mike McDonald, senior vice president of Veritone Legal. “The improvements to the early case assessment and e-discovery processes alone are impressive, but what’s truly valuable is the impact this technology will have on the legal industry and its potential to influence trials, evidentiary use and case outcomes.”
With the Veritone Media Player, Relativity users can now employ machine translation for over 100 languages, including Chinese, French, German, Italian, Japanese, Russian and Spanish, for evidentiary media. The Veritone Media Player also now incorporates Relativity’s Structured and Conceptual Analytics for expedited and efficient discovery of content and key topics. Initially integrated into the Relativity Ecosystem at the beginning of 2017, Veritone unlocks the power of AI-based cognitive computing, enabling unstructured evidentiary media such as audio and video files to be processed, transformed, and analyzed to generate truly actionable intelligence. The platform can render every second and frame of audio and video content searchable for things like words, phrases, faces, sentiment, and voice identification. Veritone now also provides machine translation, in addition to its existing redaction and transcription capabilities, to produce an index of the processed data within minutes – a process that as recently as 2015 could take thousands of hours. “With Relativity, we deliver an easy and convenient solution that both accelerates and increases the capabilities of the eDiscovery process, generating actionable information from data that was previously inaccessible,” said Mike McDonald, senior vice president of Veritone Legal. “The improvements to the early case assessment and e-discovery processes alone are impressive, but what’s truly valuable is the impact this technology will have on the legal industry and its potential to influence trials, evidentiary use and case outcomes.” ****CISN (T/$16H) Cision Ltd. provides public relations (PR) software, media distribution, media intelligence, and related professional services worldwide. It offers Cision Communications Cloud, an earned media cloud-based platform that brands can use to build relationships with influencers and buyers in order to amplify their marketplace influence; and provides media database that offers access to influencers when planning a campaign, as well as to schedule and record various interactions with contacts 
OOMA (T/$10.50H-12H)... Smart office, Smart homes..., Cloud platform Ooma creates new communications experiences for small businesses and consumers. Its smart platform serves as a communicationthe Ooma Telo – the system includes motion, water, door and window sensors in addition to the unique ability to remotely place a local 911 call from the home, thus providing all-encompassing protection s hub, which offers cloud-based telephony, internet security, home security and other connected services. Ooma combines PureVoice HD call quality and innovative features with mobile applications for reliable anytime, anywhere calling. The company has been ranked the No. 1 home phone service for overall satisfaction and value for five consecutive years by the leading consumer research publication. added to the Russell 2000® and Russell 3000® Indexes  YEXT (T/$14H-16H-18H) 4 Brookers like this...
YEXT (T/$14H-16H-18H) 4 Brookers like this...  IPAS...(T/$1.40-2) iPass Inc. provides mobile connectivity that enables Wi-Fi access on various mobile devices in the United States and internationally. It offers mobile connectivity services that provide cloud-based solution allowing customers and their users access to its Wi-Fi network to stay connected to the people and information. The company provides business to business mobile connectivity solutions to large and small enterprises, as well as to strategic partnerships comprising original equipment manufacturers, loyalty programs, software product and service providers, and communication companies; and iPass SmartConnect that takes the guesswork out of Wi-Fi automatically connecting customers to the hotspot for their needs.
IPAS...(T/$1.40-2) iPass Inc. provides mobile connectivity that enables Wi-Fi access on various mobile devices in the United States and internationally. It offers mobile connectivity services that provide cloud-based solution allowing customers and their users access to its Wi-Fi network to stay connected to the people and information. The company provides business to business mobile connectivity solutions to large and small enterprises, as well as to strategic partnerships comprising original equipment manufacturers, loyalty programs, software product and service providers, and communication companies; and iPass SmartConnect that takes the guesswork out of Wi-Fi automatically connecting customers to the hotspot for their needs. 
................................................................................................................................................................................................................................................................................
BIO/Pharma/Medical ... President Donald Trump has stated on multiple occasions that he plans to eliminate restrictions on drug development for the FDA. This policy would positively affect biomedical companies, as their products would reach the open market more easily and frequently. Certain biomedical companies will pose a stronger investment as the FDA revises their drug development standards.
With the promise of regulatory reform on the horizon, investors have been excited about the biomedical space throughout the year.
Terminolgy ..you master the terminology and then you can master the subject...
A
reasonable price-to-sales ratio for biotechs is 4.0 (MF) According to the FDA, around 30% of drugs that make it to late-stage studies win approval. FDA had granted Breakthrough Therapy designation. The status allows for promising drug candidates to undergo expedited development and review processes from regulators, sometimes even based on mid-stage trial results. European Medicines Agency (EMA) and Committee for Medicinal Products for Human Use (CHMP) Prescription Drug User Fee Act (PDUFA) goal date adult growth hormone deficiency (AGHD) Epinephrine Pre-filled Syringe “PFS," EpiPen PAD
peripheral artery disease Pulmonary Hypertension (PH-COPD). “Pulmonary Hypertension with IPF or COPD Biotech Stocks - I ---
Biotech Stocks - II ---
Biotech Stocks - III ---
Biotech Stocks - IV ---
Biotech Stocks - V Portfolio of Big Pharmaceutical Stocks: Cancer Portfolio of Big Pharmaceuticals Stocks: Arthritis Portfolio of Big Pharmaceuticals Stocks: Diabetes Natural killer cells are a critical component of the innate immune system and the first line of defense against cancer and viral infections. HER2.taNK is a natural killer cell based therapeutic that has been engineered to incorporate a novel Chimeric Antigen Receptor (CAR) specific for the human epidermal growth factor receptor 2 (HER2). *****ADMP (T/$7.50-10)...June FDA APPROVAL HAPPENED=http://www.investopedia.com/investing/betting-adamis-pharmaceuticals-and-ocular-therapeutix-ahead-fda-decisions-admp-ocul/?partner=YahooSA&yptr=yahoo Adamis has a vision for its Epinephrine Pre-filled Syringe “PFS," The drug maker hopes to compete with the likes of Mylan's EpiPen that has been raking in billions. Late last year, generic pharmaceuticals giant, Mylan (NASDAQ:MYL) found itself caught in the crosshairs of a nationwide controversy involving steep increases in the pricing of its life-saving drug injector, EpiPen. Since 2009, the price of an EpiPen two-pack has risen from $100 to over $600 today. These results have been great for Mylan's financials, as last year, EpiPen was responsible for approximately $1 billion in revenue and accounted for 40% of Mylan's total operating profits. But it has been a public relations nightmare. On the backs of this controversy, Mylan reacted by announcing that the company would be launching a generic version of the EpiPen late last year. This generic version would be priced at half the cost of the branded EpiPen -- around $300. Even so, at half the sales, this would still be a $500 million indication. And one tiny biotech is chomping at the bit in its efforts to dethrone Mylan's EpiPen empire. With the FDA's decision expected on June 15, Maxim analyst Jason Kolbert seems to not be concerned by the Complete Response Letter the company received from the regulator last year. While the FDA expressed concerns and pushed the firm to work on expansion of the human factors study, Adamis has since handled these challenges successfully. The expansion was just a "low hurdle" to the analyst, as he does not see this to be a meaningful roadblock in any way to Adamis' success. The analyst is also excited about the market opportunity the company is pursuing, noting, "We see a billion dollar market, and in Adamis PFS a superior product [...] If Adamis can acquire just 10% market share and generate sales approaching $100M (which we believe is the low end), it suggests shares are undervalued." Ultimately, Kolbert believes Adamis' valuation is compelling. As such, the analyst reiterates a Buy rating on Adamis shares, with a $10.00 price target, which implies 185% potential upside from current level  ***AGRX (T/$8-10) Women's contrception...weekly patch approvedalternative for those suffering from anaphylactic reactions. ADRO
***AGRX (T/$8-10) Women's contrception...weekly patch approvedalternative for those suffering from anaphylactic reactions. ADRO (T/$18)
Phase Ib clinical trials for the treatment of unresectable malignant pleural mesothelioma; that has completed Phase II clinical trials for the treatment of pancreatic cancer; and that is in Phase I/II clinical trials for the treatment of ovarian cancer. The company is also developing ADU-214 that is in Phase I clinical trials for the treatment of lung cancer; ADU-741, which is in Phase I clinical trials for the treatment of prostate cancer; and a product candidate for the treatment of patients with cancers of the gastrointestinal tract. In addition, it is developing STING Pathway Activator product candidates that are synthetic small molecule immune modulators, which target and activate Stimulator of Interferon Genes receptor under collaboration with Novartis Pharmaceuticals Corporation; and product candidates that address other therapeutic areas, such as autoimmune and infectious diseases. Further, the company's products pipeline comprises BION-1301, a B-select mAb novel therapy for multiple myeloma; and antibody product candidates, including APRIL for the treatment of multiple myeloma, as well as oncology therapies, such as CD27, PD-1, and CTLA-4. Aduro BioTech, Inc. has development and commercialization agreement with Genmab to evaluate five bispecific antibody product candidates targeting immune checkpoints; and collaboration agreement with Janssen Biotech, Inc. and Merck. Dec 30FDA AEZS (T/2H -4 for 2018) AEterna FDA has accepted the drug maker's New Drug Application (NDA) for Macrilen (macimorelin), a novel orally-active ghrelin agonist intended to help in monitoring treatment for adult growth hormone deficiency (AGHD) ***AGRX (T/$10) SHARE DILUTION Aug. 3rd 5.33 million shares at $3.75 ...Women's contrception...weekly patch December 26, 2017 as the Prescription Drug User Fee Act (PDUFA) goal date. ALDR () ...Alder Biopharmaceuticals... A wave of new preventive migraine medications are on the horizon.The migraine market is huge. An estimated 38 million Americans have migraines — and Dr. Eric Pearlman, a medical fellow at Lilly, one of the companies developing these treatments, told Business Insider that about 15 million of those migraine sufferers would be eligible for preventive treatment. Alder Biopharmaceuticals, eptinezumab which would be administered on a quarterly basis, is in phase 3 trials. The company plans to submit to the FDA in the second half of 2018.****08/11 ADMA (T/$13) ADMA Biologics plasma-based biologics for the treatment and prevention of immune deficiencies and infectious diseases. Its lead product candidate is RI-002 derived from human plasma, which has completed Phase III clinical study for the treatment of primary immune deficiency disease. The company also operates source plasma collection facilities in Norcross and Marietta, Georgia.
ALIM (T/$3-5) Alimera Sciences... The Company focuses on diseases affecting the back of the eye or retina. The Company's product is ILUVIEN, which is developed to treat diabetic macular edema (DME). DME is a disease of the retina that affects individuals with diabetes and can lead to severe vision loss and blindness.
AKTX (T/$6) Akari Therapeutics... focuses on therapeutics to treat rare and orphan autoimmune and inflammatory diseases. Its lead drug candidate is Coversin, a second-generation complement inhibitor that is in Phase II clinical trial for the treatment of autoimmune and inflammatory diseases, including paroxysmal nocturnal hemoglobinuria, guillain barre syndrome, and atypical hemolytic uremic syndrome.
AMAG (T/$20h-34) “Severe preeclampsia can be a life-threatening condition and is a contributing factor in a significant number of preterm births in the U.S., yet no FDA-approved treatment options currently exist,” said Julie Krop, M.D., chief medical officer and senior vice president, clinical development and regulatory affairs at AMAG. “The development and potential approval of DIF for the treatment of severe preeclampsia could have a significant impact on the lives of high-risk pregnant women and their babies, and the therapy would nicely complement AMAG’s existing portfolio of maternal health products. ...focus on maternal health, anemia management and cancer supportive care. Its offerings focus on maternal health, ... Makena (hydroxyprogesterone caproate injection); services related to the collection, processing and storage of umbilical cord blood stem cell and cord tissue units operated through Cord Blood Registry (CBR); its product, Feraheme (ferumoxytol), for intravenous (IV) use, and MuGard Mucoadhesive Oral Wound Rinse. It is engaged in the development of Digoxin immune fab, a polyclonal antibody for the treatment of severe preeclampsia in pregnant women.
AMRN (T/$7-10) Ireland drug manufacturer... company's lead product is Vascepa, a prescription-only omega-3 fatty acid capsule, used as an adjunct to diet for reducing triglyceride levels in adult patients with severe hypertriglyceridemia. It is also involved in developing Vascepa for the treatment of patients with high triglyceride levels who are also on statin therapy for elevated low-density lipoprotein cholesterol levels.
***APDN (T/$6) Applied DNA Sciences Inc.
APRI (T/$4-5) commercialization of product candidates in the areas of urology and rheumatology. Its lead product is Vitaros, a topically-applied cream formulation of alprostadil used for the treatment of erectile dysfunction. The company also engages in developing RayVa, which is in Phase 2 development for the treatment of Raynaud's Phenomenon associated with scleroderma; and Fispemifene, a tissue-specific selective estrogen receptor modulator for the treatment of secondary hypogonadism, chronic prostatitis, and lower urinary tract symptoms in men. It operates in Europe, Canada, Latin America, and the Asia Pacific.
ARDM (T/$7-10) ... drugs for the prevention and treatment of severe respiratory diseases. The company's lead development candidates are proprietary formulations of the potent antibiotic ciprofloxacin, including Linhaliq (ARD-3150) and Lipoquin (ARD-3100) that are delivered by inhalation for the management of infections associated with severe respiratory diseases, including non-cystic fibrosis bronchiectasis and cystic fibrosis. It is also developing inhaled ciprofloxacin formulations for treatment of patients with cystic fibrosis and non-tuberculous mycobacteria, as well as the prevention and treatment of high threat and bioterrorism infections, such as inhaled tularemia, pneumonic plague, melioidosis, Q fever, and inhaled anthrax.
**ARNA (T/$19H) ...weight loss drug...pulmonary arterial hypertension...auto immune disease...ulcerative colitis
*****ARLZ (T/5-6) Aralez Pharmaceuticals June 7, 2017, Aralez announced the national commercial launch of Zontivity® in the U.S. The company had previously commenced a phased launch of Zontivity® in late April 2017 with 15 sales representatives targeting high volume physicians who treat post-myocardial infarction and peripheral artery disease (PAD) patients. .. Following a very rough first half of 2017 for Aralez, it appears as though the company has at least stabilized, and may be in the process of heading in the right direction. The early numbers for Zontivity® look promising, and the company’s focus on high-risk PAD patients may end up paying dividends. While YOSPRALA® is unlikely to ever live up to its original billing, its nice to see patients and prescribers responding favorably to the new pricing strategy. We will continue with updates on sales for Zontivity® and YOSPRALA® throughout the year, and are cautiously optimistic this may be the beginning of a turn around for the company. Our valuation remains $6.00, and now may be a good time for those with a high-risk tolerance to consider an investment in Aralez.
https://finance.yahoo.com/news/arlz-looking-capitalize-successful-launch-190000407.html
*****AUPH (T/$10-14) ...treatment lupus nephritis ....new duration-of-remission data from a mid-stage study that's good enough to suggest it could eventually change how doctors treat kidney failure in lupus patients. In the United States, there are over 500,000 people with systemic lupus erythematosus (SLE), and up to 60% of these patients will develop LN, or inflammation of the kidneys that can lead to end-stage kidney disease. Today, patients with LN are treated with steroids and CellCept, but for many patients this approach falls short. Steroids can result in unwanted side effects, and CellCept's efficacy can be hit-and-miss.Because current treatments are inadequate, there's an important need for new treatments, and based on Aurinia Pharmaceuticals' trial data, that need may be addressed by voclosporin. Earlier this year, the company reported that adding voclosporin to CellCept improved patient response rates and reduced patients' need for steroids. Specifically, the two-drug combination delivered a complete response rate of 49% at the 23.7 mg dose that was far better than the 24% complete remission rate observed in the trial's control group. https://www.fool.com/investing/2017/06/17/new-data-suggests-aurinia-pharmaceuticals-is-onto.aspx ... https://www.fool.com/investing/2017/08/17/3-reasons-to-buy-aurinia-pharmaceuticals-stock-rig.aspx?yptr=yahoo
*******AVXL (T/$10-16) Anavex Life Sciences ...Sept. 05, 2017 Anavex Life Sciences Corp. (“Anavex” or the “Company”) (AVXL) today announced that the United States Patent and Trademark Office has issued U.S. Patent Number 9,750,746 entitled “ANAVEX 2-73 and certain anticholinesterase inhibitors composition and method for neuroprotection.“ This patent provides the Company with intellectual property protection covering composition of matter for ANAVEX™ 2-73 directed to a novel and synergistic neuroprotective compound combined with donepezil. The issued patent offers protection through 2033.“This newly issued patent expands the proprietary position of ANAVEX™ 2-73,” said Christopher U. Missling, PhD, President and Chief Executive Officer of Anavex. “This new patent is a continuation of Anavex’s vigorous pursuit of intellectual property protection for our neuroprotective therapeutics and we look forward to continued progress, including advancing ANAVEX™ 2-73 in clinical studies this year.“ ANAVEX™ 2-73 is an orally available, small-molecule activator of the sigma-1 receptor which, data suggest, is pivotal to restoring neural cell homeostasis and promoting neuroplasticity.1 Donepezil is currently the leading compound for the treatment of Alzheimer’s disease in more than 50 countries and is postulated to exert a therapeutic effect by enhancing cholinergic function. ANAVEX™ 2-73 is to advance this year into a Phase 2/3 clinical trial for the treatment of Alzheimer’s disease, as well as other neurodegenerative and neurodevelopmental CNS indications, including the orphan designation Rett syndrome.
1 Advances in Experimental Medicine and Biology Volume 964 2017 Sigma Receptors: Their Role in Disease and as Therapeutic Targets
About Alzheimer’s disease
Alzheimer's disease is a progressive, irreversible neurological disease and the most common cause of dementia. In the U.S., there are over five million individuals living with Alzheimer's disease and an estimated 50 million people live with dementia worldwide. Today, there are no commercially available therapies to address the underlying cause of Alzheimer's. According to the World Alzheimer Report 2016, the global cost of dementia was $818 billion in 2015 and is expected to become a trillion-dollar disease by 2018.
The Company's SIGMACEPTOR Discovery Platform produced small molecule drug candidates with unique modes of action. Sigma receptors may be targets for therapeutics to combat many human diseases, including Alzheimer's disease. When bound by the appropriate ligands, sigma receptors influence the functioning of multiple biochemical signals that are involved in the pathogenesis (origin or development) of disease. ...drug candidates for the treatment of Alzheimer's disease, other central nervous system diseases, pain, and various cancers. The company's lead drug candidates include ANAVEX 2-73, a Phase 2a clinical trial for the treatment of Alzheimer's disease; and preclinical stage to treat Parkinson's disease. Its preclinical drug candidates include ANAVEX 3-71, which uses ligands that activate sigma-1 and M1 muscarinic receptors to treat Alzheimer's disease; ANAVEX 1-41, a sigma-1 agonist that protects nerve cells from degeneration or death; ANAVEX 1037 for the treatment of prostate cancer; and ANAVEX 1066, a mixed sigma-1/sigma-2 ligand for the treatment of neuropathic and visceral pain. To our knowledge, this is the first use of this approach in the design of clinical trials for Alzheimer’s disease, Parkinson’s disease and Rett syndrome, and marks a shift towards advancing implementation of precision medicine for these and other neurodegenerative and neurodevelopmental diseases."
*****AXSM (T/$14-18) (fv $4.79)...fast track designation Tx for agitation in patiennts with alzheimer disease...
BCRX (T/$7-10) develops small molecule drugs that block key enzymes involved in the pathogenesis of diseases. The company markets peramivir, an intravenous neuraminidase inhibitor, which is approved for uncomplicated seasonal and acute influenza in the United States and Canada under the name RAPIVAB, in Japan and Taiwan as RAPIACTA, and in Korea as PERAMIFLU. It also has various ongoing development programs, including BCX7353 and second generation oral inhibitors of plasma kallikrein for hereditary angioedema; and galidesivir, a broad spectrum viral RNA polymerase inhibitor that is indicated to treat filoviruses, as well as forodesine, an oral purine nucleoside phosphorylase inhibitor for use in oncology. It has collaborative relation Shionogi & Co., Ltd. and Green Cross Corporation for the development and commercialization of peramivir in Japan, Taiwan, and South Korea; Seqirus UK Limited for the development and commercialization of RAPIVAB worldwide, except Japan, Taiwan, Korea, and Israel; and the University of Alabama at Birmingham for the development of influenza neuraminidase and complement inhibitors
**BLFS hypothermic storage and cryopreservation cells and tissues, reduce post-preservation necrosis and apoptosis...Bothel,Wa.
BLPH (T/$5)... new clinical data from a Phase 2 clinical trial that investigated INOpulse in idiopathic pulmonary fibrosis patients with pulmonary hypertension (PH-IPF) at the American Thoracic Society (ATS) 113th International Conference, currently taking place in Washington DC. The company also presented preliminary data from a second, ongoing study evaluating INOpulse in COPD patients with Pulmonary Hypertension (PH-COPD). “Pulmonary Hypertension with IPF or COPD has no approved therapies and has been associated with increased hospitalizations and mortality. The therapies used in other pulmonary hypertension populations, which act systemically, have failed to show a benefit for these patients, creating a critical unmet need for effective and safe long-term treatment options,” said Prof. W. De Backer MD, Director in the Department of Pulmonary Medicine, University Hospital and University of Antwerp. “Pulsed iNO has a potentially unique and distinguishing advantage as the data from these trials shows that it can provide selective vasodilation to the well-functioning parts of the lung, allowing for improvement in hemodynamic measures as well as increased exercise capacity without worsening oxygenation.”
AZRX (T/$8-10) developing non-systemic, recombinant protein therapies for treatment of gastrointestinal and infectious diseases. The company's lead candidate is MS1819, a Phase 2 autologous yeast recombinant lipase (Yarrowia lipolytica LIP2) being developed to target the $1.5 billion global market for treating Exocrine Pancreatic Insufficiency (EPI) in patients with cystic fibrosis (CF) and chronic pancreatitis (CP). AzurRx's second program is AZX1101, a pre-clinical beta-lactamase for prevention of hospital infections from resistant bacterial strains. AzurRx has several upcoming clinical events in 2018, including final data from its ongoing Phase 2 MS1819 clinical trial, initial data expected by year-end 2018 from a new Phase 2 CF trial for MS1819, and proof of concept data for AZX1101.
BLRX (T/$3-4)... stem cell etal...
*****APHB (T/$5) AUG. 21, 2017 =
AmpliPhi Biosciences Corporation (NYSE MKT: APHB), a clinical-stage biotechnology company focused on the development of therapies for antibiotic-resistant infections using bacteriophage technology, announces publication of a case study highlighting the successful treatment of a critically ill patient with a multidrug-resistant (MDR)
Acinetobacter baumannii (
A. baumannii) infection. The manuscript, “Development and use of personalized bacteriophage-based therapeutic cocktails to treat a patient with a disseminated resistant
Acinetobacter baumannii infection,” was published in the peer-reviewed journal
Antimicrobial Agents and Chemotherapy and can be found
here. This case study was also featured in a July 2, 2017
Washington Post article, which can be found
here. The case study details a patient suffering from an abdominal
A. baumannii infection whose condition deteriorated over a four-month period, despite multiple courses of antibiotics, and became comatose. AmpliPhi was involved in a joint effort, which included several academic institutions and a U.S. Navy laboratory, to produce a bacteriophage therapy targeted to the bacterial strain infecting the patient.
The therapy was administered under a U.S. Food and Drug Administration (FDA) Emergency IND, and the patient emerged from his coma. The infection was cleared and the patient returned to health. Robert T. “Chip” Schooley, M.D., Professor of Medicine at University of California, San Diego, who treated the patient and is the corresponding author of the paper, remarked, “Phage therapy is a very well-tolerated and potent therapeutic approach that can benefit patients with multidrug-resistant bacterial infections. It has the potential to help patients with multiple types of infections who have limited therapeutic options and, as a consequence, face severe disability or death." “At AmpliPhi, we are now developing our lead therapeutic candidates, AB-SA01 and AB-PA01 targeting multidrug-resistant
S. aureus and
P. aeruginosa infections, under expanded access guidelines by treating individual patients who have failed multiple courses of antibiotics and have few or no satisfactory treatment options,” said Paul C. Grint, M.D., CEO of AmpliPhi Biosciences. “We expect this strategy to validate the clinical utility of our therapies by early 2018 and position us to initiate further efficacy clinical trials later that year.”
BPMX () NO DEBT
BVXV () ... focuses on developing and commercializing immunomodulation therapies for infectious diseases primarily in Israel. The company offers M-001, a synthetic peptide-based protein, which is in Phase 2 clinical development stage targeting seasonal and pandemic strains of the influenza virus. Phase-3 inTandem3 clinical trials The study demonstrated that sotagliflozin 400 mg was superior to the placebo in the patients with A1C <7.0% at Week 24. The study also did not record any episode of severe hypoglycemia or diabetic ketoacidosis (DKA) after randomization. Severe hypoglycemia is a diabetic emergency where blood glucose level becomes so low that patients need help from another person for treatment. Diabetic ketoacidosis is a serious diabetes complication where the body produces excess blood acids (ketones). “Strategy for approving a universal flu vaccine” in the journal Future Virology (Ben-Yedidia et al., 2017). The authors discuss the strategy to get M-001 through the regulatory process by using a stepwise approach that first utilizes an existing regulatory marker before using newly defined markers. Currently, the efficacy of all seasonal influenza vaccines is tested based on induction of hemagglutination inhibition (HAI) antibodies that target the outer variable regions of the viral hemagglutinin protein. Since M-001 is targeted against conserved regions of the influenza virus, it does not elicit HAI antibodies but instead induces a cellular response. Thus far, BiondVax has taken the conservative approach of using M-001 as a primer to be given ahead of the seasonal influenza vaccine. This has allowed the HAI antibody response to continue to be used to verify the efficacy of M-001. When given has a primer, M-001 enhances and broadens the HAI antibody response induced by the seasonal influenza vaccine. CANF (T/$6-7)
Can Fite Biofarma... Company develops new treatments for autoimmune diseases and cancer. The Company's drugs are CF101 for Psoriasis treatment, RA treatment, for the treatment of Keratoconjunctictivitis Sicca, for the treatment of Glaucoma, among others; and CF102 for the treatment of liver diseases. ****CAPR (T/$2) Capricor Therapeutics, ...granted FDA Rare Pediatric Disease Designation for CAP-1002, the company’s treatment for Duchenne muscular dystrophy (DMD) and cardiology indications. In reaction, CAPR stocks soared by 132%. CDTX (T/$10-14-20) ...class of antifungals for the treatment and prevention of serious, invasive fungal infections. The company also develops CD201, a novel bispecific antimicrobial immunotherapy for the treatment of multidrug-resistant gram-negative bacterial infections, including those caused by pathogens harboring the mcr-1 plasmid. In addition, it develops a proprietary immunotherapy technology platform Cloudbreak, which is designed to create compounds that direct immune system to attack and eliminate bacterial, fungal or viral pathogens. CATB (T/$4-9)...Cystic Fibrosis... also involved in developing SMART linker conjugates that are in preclinical stage of development, including CAT-5571 for cystic fibrosis (CF) trafficking and function of CF transmembrane conductance regulator, as well as for the clearance of Pseudomonas aeruginosa; and CAT-4001 for the treatment of severe and rare neurodegenerative diseases, such as Friedreich's ataxia and amyotrophic lateral sclerosis. CBAY (T/$12-15) liver disease...
most significant was the lack of safety concerns associated with the drug ****CBIO (T/$11) focuses on developing medicines to address hematology indications, ... hemophilia ****CERS (T/$7-9) ommercializing the INTERCEPT Blood System to enhance blood safety. Its INTERCEPT Blood System is based on its proprietary technology for controlling biological replication; and targets and inactivates blood-borne pathogens, such as viruses, bacteria, and parasites, as well as harmful white blood cells, while preserving the therapeutic properties of platelet, plasma, and red blood cell transfusion products. The company's INTERCEPT Blood Systems for platelets and plasma are designed to inactivate blood-borne pathogens in platelets and plasma donated for transfusion; and INTERCEPT Blood System for red blood cells to inactivate blood-borne pathogens in red blood cells donated for transfusion. It markets platelet and plasma systems through its direct sales force and distributors in the United States, Europe, the Commonwealth of Independent States, the Middle East, Latin America, and internationally CLBS (T/$7) ...provides development and manufacturing services to the cell therapy industry in the United States... also developing CLBS03, a T regulatory cell clinical Phase II therapy targeting adolescents with type 1 diabetes. *****CHRS (T/$40) Coherus BioSciences, Inc. shares dropped sharply to close the week down 28% to $14.85 following its announcement that the FDA issued a CRL (Complete Response Letter) for its Biologics License Application (BLA) for CHS-1701, a pegfilgrastim (Neulasta) biosimilar candidate. No further trials were requested by the FDA and the company expects to respond to the agency in the coming months. The market for biosimilars -- drugs designed to be interchangeable with branded biologically based medicines -- is expected to become one of the fastest-growing segments of the pharmaceutical industry over the next decade. After all,
there is $100 billion worth of biologically based drugs on the market that have either lost exclusivity or will lose patent protection by 2020, according to a report by Markets and Markets. Unfortunately, there aren't a lot of great ways for American investors to gain direct access to this emerging market. The late-stage biologics company Coherus Biosciences, however, is one glaring exception.
Coherus is presently working on resolving the outstanding issues regarding its regulatory filing for a biosimilar to
Amgen's megablockbuster bone marrow drug Neulasta. The good news is that U.S. Food and Drug Administration reportedly isn't going to require the company run any additional trials in order to refile its application. So, depending on the nature of all the outstanding issues, Coherus could have an FDA-approved biosimilar for a top-selling drug on the market within the next twelve months. Although it's hard to say how much of the Neulasta market Coherus can actually capture with its copycat medicine, the drugmaker's shares should get a nice boost even if it only goes on to grab a couple hundred million dollars in annual sales at peak. Coherus, after all, presently sports a market cap of a mere $794 million following its latest dip in the wake of that regulatory setback. In all,
Coherus is well-positioned to take advantage of the rapidly evolving biosimilar market, making it an outstanding growth play for savvy investors.
Coherus is researching copycat versions of a handful of multi-billion dollar drugs, including Amgen's Neulasta and Enbrel as well as AbbVie's Humira. If Coherus succeeds at bringing one of these drugs to market and can steal even a small amount of market share then it could be a financial bonanza for the business. https://www.fool.com/investing/2017/06/16/3-stocks-that-could-double-your-money.aspx?yptr=yahoo http://stockcharts.com/c-sc/sc?s=CHRS&p=D&yr=1&mn=0&dy=0&i=p48111706416&r=1497823356920
*****CMRX (T/$8) (fv $7.65oral antivirals herpes virus and adeno virus. ****CNAT (T/$9-18) ...Liver disease
****CORT ()
Corcept Therapeutics Incorporated, a pharmaceutical company, discovers, develops, and commercializes drugs for the treatment of severe metabolic, oncologic, and psychiatric disorders in the United States. It offers Korlym (mifepristone) tablets as a once-daily oral medication for the treatment of hyperglycemia secondary to hypercortisolism in adult patients with endogenous Cushing syndrome, who have type 2 diabetes mellitus or glucose intolerance, and have failed surgery or are not candidates for surgery. The company is also developing Korlym in combination with eribulin, which is in Phase I/II clinical trial to treat patients with metastatic triple-negative breast cancer; Korlym in combination with drug Abraxane that is in Phase II clinical trial to treat patients with triple-negative breast cancer; and Korlym combined with the androgen deprivation agent enzalutamide, which is in Phase II clinical trial to treat patients with metastatic castration-resistant prostate cancer. In addition, it develops CORT125134 for the treatment of patients with Cushing's syndrome and solid-tumor cancers; and CLIA-validated assay to measure expression of the gene FKBP5, which is stimulated by cortisol activity at glucocorticoid receptor.
...(Motley Fool ... Roughly 90% of biotech stocks aren't profitable, but Corcept isn't part of that group. The company's only Food and Drug Administration-approved product is Korlym, a treatment for Cushing's syndrome. Specialty drugs typically have very strong pricing power and minimal competition, suggesting that the company will be able to grow sales both organically in terms of volume, and via price increases, in the years to come. Because of that,
it's forecast that sales will nearly triple between 2016 and 2020 to approximately $211 million. If there is one concern with a company like Corcept, it's that the company is reliant on a single drug. But there are two key catalysts that could change that. To begin with, Corcept ended the first quarter with nearly $57.3 million in cash and cash equivalents ($47 million in net cash), and it's probably on track to generate in the neighborhood of $25 million in annual free cash flow over the next couple of years. This would give Corcept somewhere near $150 million in net cash, if not more, to work with by the end of the decade -- funds it could use to fuel internal research, or potentially even purchase new therapies to broaden its portfolio. Investors also shouldn't overlook the outside chance that Korlym's profitability and Corcept's net cash position might act as a dangling carrot, luring in larger acquirer.
The other catalyst is Corcept's pipeline, which offers a number of next-generation products that, like Korlym, block glucocorticoid receptors, but that have potentially fewer side effects. Some of these studies are focused on breast cancer in combination with a chemotherapeutic agent, Alzheimer's disease, and muscular dystrophy. Many are still in the very early stages, but they offer the promise of a more diverse portfolio to come.
**CPRX (T/$5)
Catalyst Pharmaceuticals,Company has completed the Phase III trial of Firdapse. The Company's CPP-109 (vigabatrin) is a gamma-aminobutyric acid (GABA) aminotransferase inhibitor. CPP-109 is indicated for the treatment of Tourette's Disorder. The Company's CPP-115 is a GABA aminotransferase inhibitor. CPP-115 is indicated for the treatment of selected neurological indications, such as complex partial seizures and Tourette's Disorder, and epilepsy. *****CTRV(T/$4) Hepatitis B
CYDY (T/$2)
CytoDyn Inc ...focused on the clinical development and commercialization of humanized monoclonal antibodies to treat Human Immunodeficiency Virus (HIV) infection. The Company's lead product candidate, PRO 140, belongs to a class of HIV therapies known as entry inhibitors that block HIV from entering into and infecting certain cells. The Company's product pipeline also includes Cytolin and CytoFeline. Cytolin is a mouse monoclonal antibody developed to identify a specific type of immune cell called a cytotoxic T cell, or cytotoxic T lymphocyte (CTL). CytoFeline is an anti-lymphocyte function-associated antigen-1 (LFA-1) antibody for the treatment of Feline Immunodeficiency Virus (FIV) infection. PRO 140 blocks HIV from entering a cell by binding to a molecule called C-C chemokine receptor type 5 (CCR5). The Company has finished Phase II clinical trials for PRO 140 with demonstrated antiviral activity in man. CYTK (T/$24)
Cytokinetics, a late-stage biopharmaceutical company, focuses on discovering, developing, and commercializing muscle activators as potential treatments for debilitating diseases in which muscle performance is compromised and/or declining. The company is developing small molecule drug candidates primarily engineered to increase muscle function and contractility. Its lead drug candidate is Tirasemtiv, a fast skeletal troponin activator, which is in Phase III clinical trial in patients with amyotrophic lateral sclerosis. Tirasemtiv has been granted orphan drug designation and fast track status by the U.S. Food and Drug Administration and orphan medicinal product designation by the European Medicines Agency. The company is preparing for the commercialization of Tirasemtiv in North America and Europe, as well as has granted an option to Astellas Pharma Inc. for development and commercialization in other countries. ****CYTR (T/$10)
scleroderma etal... DRRX (T/$3) Durect Corp... DVAX DVAX(T/$26)Dynavax hepatitis B vaccine, skin cancer... ®***Nov 10th will find out by August 10 if the FDA approves the drug for use on hepatitis B patients. EBS...(T/$40) Emergent BioSolutions,Biodefense division... BioThrax to treat anthrax disease; Anthrasil treatment of inhalational anthrax; BAT treat botulinum disease; VIGIV address adverse events from smallpox vaccination; RSDL removal or neutralization of chemical agents, T-2 toxins, and various pesticide-related chemicals from the skin. NuThrax, next generation anthrax vaccine; UV-4B for dengue and influenza infections; GC-072 compound in the EV-035 series of broad spectrum antibiotics for Burkholderia pseudomallei; VAX161C, a recombinant pandemic influenza vaccine; PreviThrax, a recombinant protective antigen anthrax vaccine, as well as other products addressing public health threats and emerging infectious diseases. Its Biosciences division markets IXINITY prevent bleeding episodes in people with hemophilia B; WinRho SDF treat autoimmune platelet disorders and hemolytic of the newborn; HepaGam B post-exposure prophylactic treatment of hepatitis-B; VARIZIG post-exposure prophylactic treatment of varicella zoster virus. This division's investigational stage product candidates include otlertuzumab, protein therapeutic for Chronic Lymphocytic Leukemia; MOR209/ES414 metastatic castration resistant prostate cancer under collaboration with MorphoSys AG; ES210, a protein therapeutic for inflammation-related indications; 5E3, a monoclonal antibody therapeutic for Alzheimer's disease; and other protein therapeutic product candidates primarily for the treatment of immuno-oncology. The company also provides contract manufacturing services to third-party customers. ****EDGE (T/$21) Developing a sustained-release therapy for limiting brain injury after aneurysms. ***ENDV (T/1.50)... Bioelectronic device, treat acute liver failure (ALF) and other inflammatory conditions in vital organs ... stem cells... **** EYEG (T/$10/) Diseases of the eye...
ocuses on developing and commercializing drug compositions and drug delivery systems for treating diseases and disorders of the eye. The company is developing EGP-437 for the treatment of various inflammatory conditions of the eye, including the treatment of ocular inflammation and pain in post-surgical cataract patients; and uveitis, a debilitating form of intraocular inflammation of the anterior portion of the uvea. It is also developing the EyeGate Ocular Bandage Gel, a topically-applied eye drop formulation that has completed its first-in-man clinical trial for the management of corneal epithelial defects, as well as to accelerate re-epithelization of the ocular surface following surgery, injection, and other traumatic and non-traumatic conditions. EVOK.(T/$9)
FBIO (T/$11)
Fortress Biotech The Company's product, CNDO-109, is a lysate (disrupted Closteroviridae (CTV)-1 cells, cell membrane fragments, cell proteins and other cellular components) that activates donor Natural Killer (NK) cells. CTV-1 is a leukemic cell line re-classified as a T-cell acute lymphocytic leukemia (ALL). Company holds the license to develop and commercialize CNDO-109 to activate NK cells for the treatment of cancer-related and other conditions, and a non-exclusive license to certain clinical data solely for use in the Investigational new drug (IND) for CNDO-109. The Company is conducting the Phase I clinical studies of CNDO-109. FOLD AMICUS Therapeutics...biotech rare diseases... GEMP (T/$18)
clinical-stage biopharmaceutical company, focuses on developing and commercializing therapies for the treatment of dyslipidemia cardiovascular disease. Its product candidate is gemcabene (CI-1027), an oral therapy for patients who are unable to achieve normal levels of LDL-C or triglycerides with currently approved therapies. GLMD (T/$18) liver disease
GNCA (T/$12-40) ....THIS IS A $6 STOCK today...
Genocea Biosciences, lead product candidate is GEN-003, a therapeutic vaccine or immunotherapy that is in Phase 2 trial for the treatment of genital herpes infections. The company is also developing GEN-009, which is in pre-clinical stage for the treatment of immuno-oncology-neoantigen; and GEN-007 that is in research stage for the treatment of Epstein-Barr virus. https://finance.yahoo.com/news/cowen-sings-praises-genocea-biosciences-211052793.html The reason? Genocea announced data from GEN-003 Phase 2b trial in HSV2 demonstrating significant reductions in several measures of genital lesions through 12 months. Nadeau commented, "We are optimistic that GEN-003 will succeed in Phase III, and think that it addresses a significant commercial opportunity. The CDC estimates that 16% of the U.S. population aged >14 has been infected with HSV-2 and that 20-25% of infected people are aware of their status. This implies that GEN-003 could address a market oppy of 8MM - 11MM people in the U.S. alone. Assuming a price of $300 per course, GEN-003's total addressable market would be $2B+." (07/24) All the 5 analysts polled in the past 12 months rate Genocea stock a Buy. With a return potential of 323%, the stock's consensus target price stands at $24.67. ***June 26th FDA...
HALO (T/$14-20) ...
focused on creating products that help other drugs work better ...The company's human enzymes are used to facilitate the delivery of injected drugs and fluids, enhancing the efficacy and the convenience of other drugs or can be used to alter tissue structures for clinical benefit. Its products are based on the Enhanze technology, a patented recombinant human hyaluronidase enzyme (rHuPH20) that enables the subcutaneous delivery of injectable biologics, such as monoclonal antibodies and other therapeutic molecules, as well as small molecules and fluids. The company offers Hylenex recombinant, a formulation of rHuPH20 to facilitate subcutaneous fluid administration for achieving hydration; to enhance the dispersion and absorption of other injected drugs; in subcutaneous urography; and to enhance resorption of radiopaque agents. It also develops PEGylated recombinant human hyaluronidase for the treatment of pancreatic ductal adenocarcinoma, non-small cell lung cancer, gastric cancer, and metastatic breast cancer. Recent share dilution...10million shars sold $12.50 below market price $14.16... ** HEB biotech for the treatment of viral and immune based chronic disorders...chronic fatigue syndrome=myalic encephalomyelitis ****KDMN (T/$3.25)
Kadmon Holdings markets and distributes a portfolio of branded generic ribavirin products for chronic hepatitis C virus infection; co-promotes a product for chronic weight management; distributes products for chorea, cytomegalovirus retinitis, and for the prevention of CMV disease. It is also developing product candidates in autoimmune and fibrotic diseases, oncology, and genetic diseases. KMDA (T/$7) AUG. 28, 2017 ...
Kamada FDA has approved Kedrab [rabies immune globulin (Human)] for post-exposure prophylaxis against rabies infection. The company will launch Kedrab in the United States in early 2018. ***HZNP (T/$13H) ...arthritis, pain, and inflammation and inflammatory diseases in the United States and internationally. The company's marketed medicine portfolio consists of ACTIMMUNE for the treatment of chronic granulomatous disease and malignant osteopetrosis; RAVICTI and BUPHENYL/AMMONAPS to treat urea cycle disorders; PROCYSBI for the treatment of nephropathic cystinosis; QUINSAIR for the treatment of chronic pulmonary infections due to pseudomonas aeruginosa in cystic fibrosis patients; and KRYSTEXXA to treat chronic refractory gout. Its products also include RAYOS/LODOTRA for the treatment of rheumatoid arthritis, polymyalgia rheumatic, systemic lupus erythematosus, and multiple other indications; DUEXIS to treat signs and symptoms of osteoarthritis and rheumatoid arthritis; MIGERGOT for the treatment of vascular headache; PENNSAID 2% to treat pain of osteoarthritis of the knees; and VIMOVO for the treatment of signs and symptoms of osteoarthritis, rheumatoid arthritis, and ankylosing spondylitis. The company has collaboration agreements with Fox Chase Cancer Center to study ACTIMMUNE in combination with PD-1/PD-L1 inhibitors for use in the treatment of various forms of cancer; and Alliance for Lupus Research (ALR) to study the effect of RAYOS on the fatigue experienced by systemic lupus erythematosus (SLE) patients. MESO ()
specialized cells, known as mesenchymal lineage adult stem cells, to establish a portfolio of late-stage product candidates. Its allogeneic off-the-shelf cell product candidates target advanced stages of diseases with high, unmet medical needs including cardiovascular diseases, immune-mediated and inflammatory conditions, orthopedic disorders, and oncologic/hematologic conditions. MCRB (T/$19)
MDWD (T/11) Burn treatment...
MDXG (T/$18)
The Company's gross margin for the quarter ended June 30, 2017, was 89%, as compared to the 87% gross margin in the second quarter of 2016. MiMedx is the leading supplier of placental tissue, having supplied over 900,000 allografts to date for application in the Wound Care, Burn, Surgical, Orthopedic, Spine, Sports Medicine, Ophthalmic and Dental sectors of healthcare MiMedx Group, Inc. develops, processes, and markets patent protected and proprietary regenerative biomaterial products and bioimplants processed from human placental tissue, skin, and bone. The company collects the donated human placental tissue and converts into a sterile product through its proprietary PURION process. Its biomaterial platform technologies include AmnioFix and EpiFix that are tissue technologies processed from human amniotic membrane derived from donated placental tissue for homologous applications; OrthoFlo, an amniotic fluid-derived allograft for homologous applications; Physio, a bone grafting material comprising 100% bone tissue with no added carrier; and CollaFix, a technology platform derived from collagen fiber technology designed to mimic the natural composition, structure, and mechanical properties of musculoskeletal tissues to augment their repair. The company also offers EpiCord, an umbilical cord allograft that provides a connective tissue matrix to replace or supplement damaged or inadequate integumental tissue; AmnioCord, an umbilical cord allograft that offers a protective environment for the healing process; and AmnioFill, a cellular tissue matrix allograft that enhances healing. Its products have applications in the areas of wound care, burns, surgery, orthopedics, spine, sports medicine, ophthalmology, and dentistry ***MHTX () nanomedicine... MDGL 10/15 WOULDN'T CHASE IT...September 19, Madrigal reported that its independent Data Safety Monitoring Board (DSMB) completed a review of two phase 2 studies of experimental drug MGL-3196. One of those studies targeted treatment of heterozygous familial hypercholesterolemia (HeFH), with the other focusing on treatment of non-alcoholic steatohepatitis (NASH). The DSMB recommended that both studies continue without any changes. While the DSMB recommendation was certainly encouraging,
Madrigal stock didn't respond immediately. Instead, it was a few days later before shares skyrocketed by nearly 70%. That momentum has carried over into this week. My hunch is that shares are being driven up in anticipation of a potential acquisition of Madrigal. There are quite a few major players especially interested in the NASH indication. For now, though, that's just a hunch. Motley Fool
*** MNKD commercial-stage diabetes drug and device maker.... MNTM Anti-Aging Drug ... **MRNS 1.13 low (T/$3) biopharm epilepsy and neuropsychiatric disorders... . MTNB (T/$6) ...focuses on identifying and developing therapeutics for the treatment of fungal and bacterial infections. The company develops orally delivered therapeutics based on a proprietary, lipid-based drug delivery platform called cochleate delivery technology. Its lead product candidate is MAT 2203, an orally-administered cochleate formulation of the broad spectrum anti-fungal drug amphotericin B that is in Phase II study for the treatment of fungal infections. The company is also involved in developing MAT2501, an orally-administered, encochleated formulation of aminoglycoside antibiotic agent amikacin for acute bacterial infections, including non-tuberculous mycobacterium (NTM) and multi-drug resistant gram negative bacterial infections. In addition, it is exploring strategic partnership options for MAT8800 discovery program, which intends to identify product candidates derived from omega-3 fatty acids for the treatment of non-alcoholic fatty liver disease NAVB (0.60) Navidea Biopharmaceuticals, Inc. is a biopharmaceutical company focused on the development and commercialization of precision immunodiagnostic agents and immunotherapeutics. The Company is developing multiple precision-targeted products based on the Manocept platform to help identify the sites and pathways of undetected disease. The Manocept platform is predicated on the ability to specifically target the CD206 mannose receptor expressed on activated macrophages. NAV5001 is an iodine-123 (I-123) radiolabeled single-photon emission computed tomography (SPECT) imaging agent being developed as an aid in the diagnosis of Parkinson's disease (PD) and other movement disorders, with potential use as a diagnostic aid in dementia. NAV4694 is a fluorine-18 (F-18) radiolabeled positron emission tomography (PET) imaging agent being developed as an aid in the diagnosis of patients with signs or symptoms of Alzheimer's disease (AD) and mild cognitive impairment (MCI). ***NBRV (T/13-22) ...Developing a novel antibiotic for bacterial pneumonia. NBY (T/5-6) ...arkets anti-infective products for the eye care market in the United States. Its commercial products include the Neutrox family of products, Avenova for the eye care market; Aganocide compounds patented synthetic molecules with a range of spectrum of uses against bacteria, viruses, and fungi; Auriclosene Irrigation Solution for urology; CelleRx for the dermatology market; intelli-Case, a device for soft and rigid gas permeable contact lenses; and NeutroPhase for wound care. *****NEOS (T/$8H-15-20) treatment of attention deficit / hyperactivity disorder (ADHD)Given... FDA approval June 19th “With this approval, Neos will be the only company to have both a branded methylphenidate and a branded amphetamine product available in an extended-release orally disintegrating tablet dosage form. We look forward to having Cotempla XR-ODT join our Adzenys XR-ODT®(amphetamine) extended-release orally disintegrating tablets on the market this fall.” Neos Therapeutics is a small-cap pharmaceutical company developing modified-release drugs for the treatment of ADHD and other neurodevelopmental disorders. Currently, the company's only approved branded product is Adzenys XR-ODT, an orally dissolvable tablet (ODT) form of extended-release amphetamine indicated for treatment of attention deficit / hyperactivity disorder (ADHD). Since its launch in May of 2016, sales of Adzenys have been growing well, with a total of 32,296 prescriptions filled between the months of January through March of this year. Monthly prescriptions increased 20% month-over-month during this timeframe, with total Adzenys revenue coming in at $3.1 million. As the company estimates that 54% of kids have difficulty swallowing tablets or capsules, and the total ADHD medication market to be worth $10.4 billion, these sales could have much more room to grow. NEOT Neothetics...LIPO-202 phase 2 TRIALS. ..specialty pharmaceutical company, develops therapeutics for the aesthetic market. Its lead product candidate is LIPO-202, an injectable formulation of salmeterol xinafoate for the reduction of localized fat deposits under the chin, treatment of attention deficit / hyperactivity disorder (ADHD). submental fat, as well as for reduction of central abdominal bulging due to subcutaneous fat in non-obese patients. *NNVC nano-biopharmaceutical viral infections, shingles cream will be its first drug to start human testing... NTEC (T/$10) ...Parkinson treatment...
NVAX (T/$1.50) (insider buying 05/11) recombinant nanoparticles vaccines... NSTG NanoString Tech (biotech)NVCR (T/$15) Mesothelioma OCRX...Ocera Therapeutics (T/$3-4) (fv $1.69) treatment of hyperammonemia and resultant hepatic encephalopathy *****OCLR (T/$35) OCULAR THERAPEUTIC JULY 19th FDA should approve Dextenza that treats eye pain after surgery... OMER... (T/$15H-22H) ...pupil-dilating drug called Omidria, which helps eye surgeons perform cataract removal and lens replacement procedures. While the initial ramp-up was slow, Omidria's sales growth exploded by 212% last year to $41.4 million -- and that could be just the beginning. Since millions of eye surgeries are performed every year, it's possible that Omeros could have a blockbuster on its hands. Omeros is a compound drug called OMS721. This product candidate is being researched as a treatment option for a number of disorders that includes atypical hemolytic-uremic syndrome (aHUS). There is currently only one treatment option for this ultra-rare condition: Alexion Pharmaceuticals' Soliris. Alexion rang up more than $2.8 billion in total Soliris sales last year in part because of its aHUS monopoly. That hints that OMS721 could potentially ring up nine figures in sales on its aHUS opportunity alone. *****ONCS (T/$3-5) ...gene therapies...electroporation delivery device, melanoma... ONTX 1.78 low(T$5.00) (fv $2.39)Onconova targets RAS cellular pathways which have been linked to cancer cells while causing minimal damage to healthy cells.
***OPK (T/$6H-11-16-20) CEO Frost is buying up all the shares... clinical laboratory that offers laboratory testing services in the detection, diagnosis, evaluation, monitoring, and treatment of diseases. The Bio-Reference Laboratories also provides core genetic testing and leverage products, such as the 4Kscore prostate cancer test and the Claros 1 in-office immunoassay platform. The company's pharmaceutical segment offers Rayaldee, a treatment for secondary hyperparathyroidism in adults with stage 3-4 chronic kidney disease patients with vitamin D deficiency; and VARUBI for chemotherapy-induced nausea and vomiting. This segment is also developing TT401, which is in Phase II clinical trials for type 2 diabetes and obesity; TT701, an androgen receptor modulator that is in Phase II clinical trials for androgen deficiency indications; and hGH-CTP, a growth hormone injection that is in Phase III clinical trials. In addition, it engages in developing and commercializing hGH-CTP, a recombinant human growth hormone product that is in Phase III; developing Factor VIIa drug for hemophilia that is in Phase IIa, an NK-1 compound, and drugs for treating asthma and chronic obstructive pulmonary disease; developing and producing specialty active pharmaceutical ingredients; and discovery of drugs for the treatment of cancer, heart disease, metabolic disorders, and a range of genetic anomalies. Further, this segment engages in the development, manufacture, and sale of pharmaceutical, nutraceutical, and veterinary products; and markets and distributes pharmaceutical and natural products ***ONVO ONVO human tissues that could be employed in drug discovery and development, tissue patches for repair or replacement damaged tissues/organs... tissue assay toxicities would help companies not have to run Phase 1 trials... OPGN OpGen... VAL-083, an oncology drug for the treatment of refractory glioblastoma multiforme. The company is set to meet with the FDA before the close of the quarter to discuss a Phase 3 study design and has already picked up orphan designation for the drug. On May 5, DelMar reported the closing of a private placement that saw the company pick up gross proceeds of $5.6 million. No company, biotech or otherwise, raises equity-based capital specifically to drive sales efforts if it doesn’t believe there is a thriving market for the product. That OpGen has picked now to raise in order to spend on marketing, and that markets have been responsive to its issue, is indicative of recovery.
****OPK ...hemophilia, human growth hormone ****ORMP(T/$25) Oramed Pharmaceuticals ... field of oral delivery solutions for drugs and vaccines presently delivered via injection. Oramed's flagship product, an orally ingestible insulin capsule in phase II clinical trials, is focused on the treatment of diabetes. The Company is developing orally ingestible protein oral delivery (POD) technology for the delivery of drugs presently administered by way of injection. Oramed's delivery platform protects protein sand enhances their absorption, allowing them to reach the blood stream through the portal vein. OvaScience (OVAS), NovaBay Pharmaceuticals (NBY), Novogen Limited (NVGN), Prana Biotechnology Limited (PRAN), Caladrius Biosciences (CLBS), ImmunoCellular Therapeutics Ltd (IMUC), Pharmacyclics (PCYC) . ****PGNX (T/$14) neuroendocrine tumor affects the hormone-producing cells of the body’s neuroendocrine system that comprises of cells which are a combination of hormone-producing endocrine cells and nerve cells. These cells control the flow of air, blood and food through the different body parts. There are no approved therapies available in the U.S. market for the two diseases. PIRS (T/$9-10) ...proprietary technology, called Anticalins. They are a mimetic antibody that can be used in many of the same ways antibodies can be used but that are far smaller than antibodies and – as a result – can be applied to a far wider range of indications. https://www.insiderfinancial.com/pieris-pharmaceuticals-inc-nasdaqpirs-has-a-near-term-catalyst-that-could-see-it-run-higher/123540/ The company’s strategy is simple – create a treatment based on this technology then find a big name to pay for the rights to it. Execution of this strategy has, so far, been flawless. Pieris has deals in place with Roche Holding Ltd. (VTX:ROG), Sanofi SA (ADR) (NYSE:SNY), Servier and, as per our last coverage, AstraZeneca plc (ADR) *PRAN biotech development of therapeutic drugs in Australia... *****PSDV (T/$9) pSivida Corp. develops drug delivery products primarily for the treatment of chronic eye diseases. The Company operates through the biotechnology sector segment. The Company has developed three products for treatment of back-of-the-eye diseases, which include Medidur for posterior segment uveitis, its lead product candidate that is in pivotal Phase III clinical trials; ILUVIEN for diabetic macular edema (DME), its lead licensed product that is sold in the United States and European Union (EU) countries, and Retisert. Medidur is designed to treat chronic non-infectious uveitis affecting the posterior segment of the eye (posterior segment uveitis). ILUVIEN is an injectable micro-insert that provides treatment of DME from a single injection. Retisert is an implant that provides treatment of posterior segment uveitis. Its product development program is focused on utilizing its two technology platforms, Durasert and Tethadur, to deliver drugs and biologics to treat chronic diseases. AZN ***May 3, 2017 $57 million deal). The combination of these partnerships has the potential to bring more than $4 billion milestone dollars to Pieris and right now the company trades for a market capitalization of less than $200 million. Well, we’re holding out for some fresh partnership announcements, but that’s not all we’ve got our eye on. There’s a near-term data release that could drive this one into the $7-10 range on its release (conservatively) if it hits press as positive. The drug is called PRS-080 and it’s targeting anemia. Back in February, Pieris struck a deal with Japanese drugmaker ASKA Pharmaceutical Co that saw the latter pick up the rights to the drug in Japan and other Asian regions. Pieris retained the rights in the US and Europe, and the drug advanced into a phase IIa multiple dose study almost immediately after the deal hit press. Data from this trial is expected to be released during the third quarter of this year (latest communication says second half 2017, but trial length/expected completion can be used to narrow this a little). PSTI
AZN ***May 3, 2017 $57 million deal). The combination of these partnerships has the potential to bring more than $4 billion milestone dollars to Pieris and right now the company trades for a market capitalization of less than $200 million. Well, we’re holding out for some fresh partnership announcements, but that’s not all we’ve got our eye on. There’s a near-term data release that could drive this one into the $7-10 range on its release (conservatively) if it hits press as positive. The drug is called PRS-080 and it’s targeting anemia. Back in February, Pieris struck a deal with Japanese drugmaker ASKA Pharmaceutical Co that saw the latter pick up the rights to the drug in Japan and other Asian regions. Pieris retained the rights in the US and Europe, and the drug advanced into a phase IIa multiple dose study almost immediately after the deal hit press. Data from this trial is expected to be released during the third quarter of this year (latest communication says second half 2017, but trial length/expected completion can be used to narrow this a little). PSTI (T/$2) ...
manufacture of cell therapeutics products and related technologies for the treatment of various ischemic, inflammatory, and hematologic conditions. The company develops placenta expanded (PLX) cell therapy products, such as PLX-PAD cells, which has been completed Phase-II clinical trial for the treatment of peripheral and cardiovascular diseases, as well as for the treatment of orthopedic diseases. It also develops PLX-R18 cells for hematopoietic cell transplantation in animals, as well as conducts various in-vivo studies for the evaluation of PLX-R18 for the treatment of ARS ******PULM (this may be a sleeper) ...Pulmatrix has developed a technology that allows for the encapsulation of an active compound and the delivery of this encapsulated active compound through an inhaler. The technology is called iSperse, and it is designed to overcome some of the drawbacks associated with inhaled active compounds in standard of care treatments. Specifically, and primarily, these drawbacks are rooted in the active compound clumping up at the back of the throat post-inhalation and causing two distinct problems: one, not enough active drug reaching the lungs (and in turn, a lack of efficacy) and two, toxicity-associated side effects caused by the collection of the active compound at the back of the throat. The iSperse technology, for want of a better phrase, lubricates the active compound so it won’t stick together and doesn’t clump, and by proxy, doesn’t get caught in the throat. This allows for a more compact dose in various indications, as less of the active compound needs to be inhaled to have the same amount of it (when compared to standard of care) reaching the lungs. The primary application of this technology right now is in COPD (Cronic Obstructive Pulmonary Disease).
http://ir.pulmatrix.com/Zacks-Initiates-on-Pulmatrix-NASDAQ-PULM QBIO story stock glaucoma drug... RIGL (T/$5)
development of drugs in the therapeutic areas of immunology, oncology, and immuno-oncology. The company's clinical programs include fostamatinib, an oral spleen tyrosine kinase (SYK) inhibitor, which has completed Phase III clinical program for immune thrombocytopenia purpura; and Phase II clinical study for autoimmune hemolytic anemia and IgA nephropathy. It is also developing two oncology product candidates, which are in Phase I and Phase II. Rigel Pharmaceuticals, Inc. ROKA (T/$10)(NEW LOW )Roka Bioscience molecular assay technologies for the detection of foodborne pathogens ****SBOT (T/$4)... Biotech...keyhole limpet hemocyanin (KLH) protein... POTENTIAL GAME CHANGING TREATMENTS OF SERIOUS DISEASES...When you're developing a vaccine for any indication, you can have a dendritic cell vaccine, you can have a DNA-based vaccine, you can have a KLH-based vaccine or a peptide vaccine, and each one has its own advantages, disadvantages and attributes that you may or may not want as part of the immune response you are targeting. KLH seems to be geared toward generating antibody responses versus T-cell responses. *** SGYP...(T/$9-11-13-15)Synergy Pharmaceuticals ...therapies to treat gastrointestinal diseases and disorders. Its lead product is plecanatide, a novel uroguanylin analog that is traded under the TRULANCE name for the treatment of chronic idiopathic constipation and irritable bowel syndrome SNGX (T/$4) ... objectives of the contract are to advance the development of Soligenix's thermostabilization technology, ThermoVax®, in combination with the Company's ricin toxin vaccine, RiVax®, as a medical countermeasure to prevent the effects of ricin exposure. SPHS (T/$) ...
focuses on the development of products for the treatment of urological diseases. The company's primary product candidate is PRX302, which is in Phase III clinical trial for treatment of lower urinary tract symptoms of benign prostatic hyperplasia (BPH), as well as for the treatment of localized low to intermediate risk prostate cancer. It has a strategic relationship with Kissei Pharmaceutical Co., Ltd. for the development and commercialization of PRX302 and other products for the treatment of the symptoms of BPH, prostate cancer, prostatitis, or other diseases of the prostate; and license agreement with UVIC Industry Partnerships Inc. and The Johns Hopkins University with respect to the use of PRX302 for the development of therapeutics for the symptoms of BPH, prostate cancer, and other non-cancer diseases and conditions of the prostate.
SPPI Sept 29,2017 announced promising results from pre-clinical testing of experimental drug poziotinib in treating non-small-cell lung cancer (NSCLC). Keep in mind, though, that poziotinib is also in a couple of phase 2 studies targeting NSCLC and breast cancer. The pre-clinical results showed that in vitro responses for the drug in NSCLC could be much more effective than AstraZeneca's Tagrisso and Boehringer Ingelheim's Gilotrif. If these results are also demonstrated in Spectrum's phase 2 study for NSCLC, the company could have a huge winner on its hands.Encouraging pre-clinical results sometimes boost small biotech stocks with no approved products, but Spectrum has six products already on the market. No one would have been surprised if late-stage results served as a nice catalyst for Spectrum. However, it's practically unheard of for a stock like Spectrum to soar on pre-clinical data.Motley Fool SRNE...Sorrento Therapeutics STML (T/$11)
TTNP Pharma... treatments for chronic diseases, such as Parkinson's disease. The company offers Probuphine, a product candidate for long term maintenance treatment of opioid dependence, which maintains a stable, around the clock blood level of the medicine buprenorphine in patients for six months following a single treatment. TXMD (T/$10-20) ...hormone therapy drug candidates include TX-001HR, a combination of estradiol and progesterone drug candidate under clinical trials for the treatment of moderate to severe vasomotor symptoms due to menopause; TX-002HR, a natural progesterone formulation for the treatment of secondary amenorrhea without the potentially allergenic component of peanut oil; and TX-004HR, an applicator-free vaginal estradiol softgel drug candidate for the treatment of vulvar and vaginal atrophy in post-menopausal women with vaginal linings that do not receive enough estrogen. ***TTPH (T/$14)
Tetraphase Pharmaceuticals, Inc., a clinical-stage biopharmaceutical company, develops various antibiotics for the treatment of serious and life-threatening multidrug-resistant infections. Its lead product candidate is eravacycline, an intravenous and oral antibiotic for use as a first-line empiric monotherapy to treat resistant and multidrug-resistant infections, including multidrug-resistant Gram-negative infections. The company has completed a Phase III clinical trial of eravacycline with intravenous administration for the treatment of complicated intra-abdominal infections; and initiated a Phase III clinical trial of eravacycline for the treatment of complicated urinary tract infections with intravenous-to- oral transition therapy. It is also developing TP-271, a preclinical compound that is in Phase I clinical trial for respiratory diseases caused by bacterial biothreat pathogens; and TP-6076, a second-generation Gram-negative program, which is in Phase I clinical trial for multidrug-resistant Gram-negative infections. In addition, the company is involved in the discovery and development of additional antibiotics for the treatment of unmet medical needs, including multidrug-resistant Gram-negative bacteria. VCEL (T/$6) ...
markets three autologous cell therapy products, including Carticel and MACI, which are used for the treatment of cartilage defects in the knee; and Epicel, a permanent skin replacement that is used for the treatment of patients with deep-dermal or full-thickness burns comprising greater than or equal to 30 percent of total body surface area in the United States. The company also develops ixmyelocel-T, which is in Phase IIb clinical trial, a patient-specific multicellular therapy for the treatment of advanced heart failure due to ischemic dilated cardiomyopathy. VRX (T/$9-16-18-23)
VSTM (T/$6)
VTVT (T/$13-15) treatment
Alzheimers and type 2 diabetes ... VTV Therapeutics Inc. (
VTVT) announced today that the U.S. Patent and Trademark Office has issued a patent with claims protecting methods of treatment using azeliragon, the Company’s oral antagonist of the Receptor for Advanced Glycation Endproducts (RAGE) for treatment of mild Alzheimer’s disease. The patent number is 9,717,710 (‘710 Patent).The ‘710 Patent, expiring in October 2034, includes claims covering methods of treating patients with mild Alzheimer’s disease by administering about 5 mg per day of azeliragon. vTv expects that the ‘710 Patent will be Orange Book listable. The ‘710 Patent adds to the portfolio of US patents covering azeliragon, including a composition of matter patent that is expected to expire in 2029. vTv continues to prosecute additional patent applications to further enhance its existing patent portfolio covering azeliragon.
“VTV is currently the only company developing a treatment for Alzheimer’s disease by targeting the RAGE receptor, which has demonstrated potential in addressing three key pathologies in Alzheimer's disease: amyloid-β, tau and chronic inflammation,” said Steve Holcombe, president and CEO of vTv Therapeutics. “This new patent is an important milestone as vTv prepares for the results of our upcoming Phase 3 data readout.” Azeliragon is currently being studied in a randomized, double-blind, placebo-controlled Phase 3 trial investigating the efficacy of azeliragon as a potential treatment to slow the decline in cognition and functional activities for patients with mild Alzheimer’s disease. Data from Part A of the trial is expected to read out in late March 2018, data from Part B data is expected to read out in late December 2018.
Precision medicine is going to
transform medicine by matching our unique genetic codes to the information that we are collecting about our health. So now when you are diagnosed with a disease, you’ll get a drug that’s a perfect fit based on your genetic code and the particular symptoms you are showing. The big difference for you between the old way and the new way is this: The new way gives you a higher chance of getting the right diagnosis and drug based on your unique information.
Gene Therapy ... http://www.bizjournals.com/boston/news/2017/05/30/study-raises-concerns-over-gene-editing-hitting.html?ana=yahoo&yptr=yahoo CRISPR stands for Clustered Regularly Interspaced Short Palindromic Repeats. According to the Broad Institute, these are the hallmark of a bacterial defense system which forms the basis for the popular CRISPR-Cas9 genome editing technology. In the field of genome engineering, CRISPR is often used loosely to refer to the entire CRISPR-Cas9 system, which can be programmed to target specific stretches of genetic code and to edit DNA at precise locations. The gene editing technology CRISPR/CAS9 is being used to develop a host of new treatments, mostly for genetic diseases. But a team of researchers from the University of Rochester's Center for RNA Biology are investigating whether gene editing can be used for another purpose: to slow the growth of cancer cells. ARTX (T/$12)
Arcturus Therapeutics Ltd. operates as an RNA medicines company. Its RNA therapeutics platforms could be applied in various types of RNA medicines, including small interfering RNA, messenger RNA, replicon RNA, antisense RNA, microRNA, and gene editing therapeutics. The company owns LUNAR lipid-mediated delivery and Unlocked Nucleomonomer Agent (UNA) technology, including UNA Oligomers, which are covered by its patent portfolio, including 120 patents and patent applications issued in the United States, Europe, Japan, China, and other countries. Its proprietary UNA technology is used to target individual genes in the human genome, as well as viral genes, and other species for therapeutic purposes. ****BTX (T/$6) ...
addressing degenerative diseases based on pluripotent stem cells and HyStem cell/drug delivery platform technologies. Its product candidates include Renevia, a facial aesthetics product that is in pivotal clinical trial for the treatment of HIV related facial lipoatrophy; OpRegen, which is in Phase I/IIa clinical trial for the treatment of the dry form of age-related macular degeneration; HyStem-BDNF, a preclinical development program for the delivery of recombinant human brain-derived neurotrophic factor (BDNF) directly into the stroke cavity of patients for aiding in tissue repair and functional recovery; and ReGlyde that is in preclinical development as a device for viscosupplementation and a combination product for drug delivery in osteoarthritis. The company also develops AST-OPC1, a therapy derived from pluripotent stem cells that is in a Phase I/IIa clinical trial for spinal cord injuries; AST-VAC1, a patient-specific cancer immunotherapy that is in Phase II clinical trial for acute myeloid leukemia; and AST-VAC2, a non-patient specific cancer immunotherapy, which is in Phase I/IIa clinical trial to treat non-small cell lung cancer. In addition, it offers liquid biopsy tests for diagnosis of cancer; bone grafting products to treat orthopedic disorders; and mobile health software products. Further, it markets GeneCards, a human gene database; LifeMap Discovery, a database of embryonic development, stem cell research, and regenerative medicine; MalaCards, a human disease database; VarElect, an application for prioritizing gene variants; and GeneAnalytics, a novel gene set analysis tool. CRSP (T/$23.50) CRISPER Therapeutics AG (Buy rating and price target $23.50 by Chardan Capital) ... focuses on the development of transformative gene-based medicines for serious diseases using its proprietary CRISPR/Cas9 gene-editing platform. The CRISPR/Cas9 technology allows for changes to genomic DNA. The company has strategic collaborations with Bayer AG and Vertex Pharmaceuticals to develop CRISPR-based therapeutics in other diseases. It operates in Switzerland, the United States, and the United Kingdom. CRISPR Therapeutics AG is headquartered in Basel, Switzerland. Both sides claim victory — angling for millions in potential royalties and licenses — in gene-editing patent dispute 02/16/2017  *EDIT (T/$33H-65???) (on hold until MID 2018) Editas Medicine... Buy rating and $65 price target by Chardan. Biotechnology...the ability to play God...gene therapy.... Editas Medicine is a company that should be on a lot of radars, as they’re working on a revolutionary method of genome editing which could conceivably provide cures for the approximately 6,000 diseases which are caused by genetic mutations in our DNA. Of those, only about 5% currently have approved therapeutic treatments (and those don’t address the root cause, but seek to combat the symptoms). Editas’ genome editing platform CRISPR could instead be used to correct the genetic problem at its source: right in our DNA. A protein-RNA complex can be tailored to either repair or replace damaged genetic sequences, and is then guided to the target spot in the genetic link by a guide RNA. The result is a corrected sequence that could completely cure the issues that were being caused by the defect.There are a couple issues surrounding the company however. Firstly, it could be years before any human trials of the platform are launched. Secondly, Editas Medicine is currently caught in the middle of a heated patent dispute over the CRISPR platform itself, which teams of scientists from Berkeley and the Broad Institute each claim to have the patent rights to. Interestingly, members of both of those teams helped found Editas and still serve as founding scientific advisors for the company.Setting aside the patent dispute, there’s some exciting work being done at Editas that could revolutionize medicine, making this one of the most interesting companies in the world.
*EDIT (T/$33H-65???) (on hold until MID 2018) Editas Medicine... Buy rating and $65 price target by Chardan. Biotechnology...the ability to play God...gene therapy.... Editas Medicine is a company that should be on a lot of radars, as they’re working on a revolutionary method of genome editing which could conceivably provide cures for the approximately 6,000 diseases which are caused by genetic mutations in our DNA. Of those, only about 5% currently have approved therapeutic treatments (and those don’t address the root cause, but seek to combat the symptoms). Editas’ genome editing platform CRISPR could instead be used to correct the genetic problem at its source: right in our DNA. A protein-RNA complex can be tailored to either repair or replace damaged genetic sequences, and is then guided to the target spot in the genetic link by a guide RNA. The result is a corrected sequence that could completely cure the issues that were being caused by the defect.There are a couple issues surrounding the company however. Firstly, it could be years before any human trials of the platform are launched. Secondly, Editas Medicine is currently caught in the middle of a heated patent dispute over the CRISPR platform itself, which teams of scientists from Berkeley and the Broad Institute each claim to have the patent rights to. Interestingly, members of both of those teams helped found Editas and still serve as founding scientific advisors for the company.Setting aside the patent dispute, there’s some exciting work being done at Editas that could revolutionize medicine, making this one of the most interesting companies in the world.  *NTLA (T/$19H) Intellia Therapeutics ...lead target is liver disease ...Intellia Therapeutics Inc is a gene editing company, focuses on the development of therapeutics utilizing a biological tool known as the CRISPR/Cas9 system. The company develops in vivo programs focused on liver diseases, including transthyretin amyloidosis, alpha-1 antitrypsin deficiency, hepatitis B virus, and inborn errors of metabolism; and ex vivo applications of the technology in chimeric antigen receptor T cell and hematopoietic stem cell product ...headquartered in Cambridge, Massachusetts.
*NTLA (T/$19H) Intellia Therapeutics ...lead target is liver disease ...Intellia Therapeutics Inc is a gene editing company, focuses on the development of therapeutics utilizing a biological tool known as the CRISPR/Cas9 system. The company develops in vivo programs focused on liver diseases, including transthyretin amyloidosis, alpha-1 antitrypsin deficiency, hepatitis B virus, and inborn errors of metabolism; and ex vivo applications of the technology in chimeric antigen receptor T cell and hematopoietic stem cell product ...headquartered in Cambridge, Massachusetts.  ***CLLS (T/$49) (fv$19-50) Cellectis...focused on developing immunotherapies based on gene edited CAR T-cells (UCART), today announced that U.S. patent 8,921,332, which claims the use of chimeric restriction endonucleases for directing chromosomal gene editing in cells by homologous recombination (HR), initially issued on Dec. 30, 2014, has been upheld by the United States Patent and Trademark Office (USPTO) after a reexamination initiated in October 2015. U.S. patent 8,921,332 claims a general method for modifying chromosomal DNA sequences at a genomic site of interest within a cell by using a chimeric restriction endonuclease such as zing finger nucleases, TAL-effector nucleases, Mega-TALs and CRISPR/Cas9. This technique, commonly referred to as gene targeting or targeted insertion, is now frequently used to modify the genome within plants, animals and cell lines. OPGN
***CLLS (T/$49) (fv$19-50) Cellectis...focused on developing immunotherapies based on gene edited CAR T-cells (UCART), today announced that U.S. patent 8,921,332, which claims the use of chimeric restriction endonucleases for directing chromosomal gene editing in cells by homologous recombination (HR), initially issued on Dec. 30, 2014, has been upheld by the United States Patent and Trademark Office (USPTO) after a reexamination initiated in October 2015. U.S. patent 8,921,332 claims a general method for modifying chromosomal DNA sequences at a genomic site of interest within a cell by using a chimeric restriction endonuclease such as zing finger nucleases, TAL-effector nucleases, Mega-TALs and CRISPR/Cas9. This technique, commonly referred to as gene targeting or targeted insertion, is now frequently used to modify the genome within plants, animals and cell lines. OPGN ()
OpGen, Inc. 06/05/2017 announced the results from additional studies supporting the capabilities of its Acuitas Rapid Test and Acuitas Whole Genome Sequence Analysis to accurately predict antibiotic resistance and for infection control *****QURE (T/$-13H) uniQure... The company's principle programs include AMT-060, a gene therapy that has completed Phase I/II clinical trial for the treatment of hemophilia B; S100A1, a preclinical product candidate for the treatment of congestive heart failure; and AMT-130 for the treatment of huntington's disease. It also provides Glybera, a gene therapy product that has approved for the treatment of patients with lipoprotein lipase deficiency.  *****ABEO (T/$14-17-22) Abeona Therapeutics ...developing novel gene therapies for life-threatening rare genetic diseases. The Company's lead programs include ABO-102 (AAV-SGSH), an adeno-associated virus (AAV) based gene therapy for Sanfilippo syndrome type A (MPS IIIA) and EB-101 (gene-corrected skin grafts) for recessive dystrophic epidermolysis bullosa (RDEB). It is also developing ABO-101 (AAV-NAGLU) for Sanfilippo syndrome type B (MPS IIIB), ABO-201 (AAV-CLN3) gene therapy for juvenile Batten disease (JNCL), ABO-202 (AAV-CLN1) for treatment of infantile Batten disease (INCL), EB-201 for epidermolysis bullosa, ABO-301 (AAV-FANCC) for Fanconi anemia disorder and ABO-302 using a novel CRISPR/Cas9-based gene editing approach to gene therapy for rare blood diseases. The Company also has a plasma-based protein therapy pipeline, including alpha-1 protease inhibitor (SDF Alpha) for inherited COPD, using its proprietary Salt Diafiltration ethanol-free process. Some companies that are related to Abeona Therapeutics include Advaxis (ADXS), NewLink Genetics Corp (NLNK), Compugen (CGEN), Oramed Pharmaceuticals ABUS
*****ABEO (T/$14-17-22) Abeona Therapeutics ...developing novel gene therapies for life-threatening rare genetic diseases. The Company's lead programs include ABO-102 (AAV-SGSH), an adeno-associated virus (AAV) based gene therapy for Sanfilippo syndrome type A (MPS IIIA) and EB-101 (gene-corrected skin grafts) for recessive dystrophic epidermolysis bullosa (RDEB). It is also developing ABO-101 (AAV-NAGLU) for Sanfilippo syndrome type B (MPS IIIB), ABO-201 (AAV-CLN3) gene therapy for juvenile Batten disease (JNCL), ABO-202 (AAV-CLN1) for treatment of infantile Batten disease (INCL), EB-201 for epidermolysis bullosa, ABO-301 (AAV-FANCC) for Fanconi anemia disorder and ABO-302 using a novel CRISPR/Cas9-based gene editing approach to gene therapy for rare blood diseases. The Company also has a plasma-based protein therapy pipeline, including alpha-1 protease inhibitor (SDF Alpha) for inherited COPD, using its proprietary Salt Diafiltration ethanol-free process. Some companies that are related to Abeona Therapeutics include Advaxis (ADXS), NewLink Genetics Corp (NLNK), Compugen (CGEN), Oramed Pharmaceuticals ABUS (T/$6-10 )
developing and commercializing a cure for patients suffering from chronic hepatitis B infection , a disease of the liver caused by the hepatitis B virus (HBV). It currently has negative earnings. It is developing a pipeline focused on advancing Ribo Nucleic Acid interference therapeutics (RNAi) using its Lipid Nanoparticle technology. HSGX (T/$4)
Histogenics Corporation, a regenerative medicine company, focuses on developing and commercializing products in the musculoskeletal segment of the marketplace in the United States. The company offers NeoCart, a tissue implant, which is in Phase III clinical trial to treat tissue injury in the field of orthopedics, specifically cartilage damage in the knee. APTO (/$4)
develops personalized therapies addressing unmet medical needs in oncology in Canada. Its lead clinical program is APTO-253, a small molecule that induces the transcription of the Kruppel-like factor 4 Gene in in vitro studies and in in vivo pharmacodynamic studies of human xenograft tumors in mice. The company's APTO-253 clinical program has completed Phase-I clinical trials for the treatment of various solid tumors; and Phase-Ib clinical trials for relapsed/refractory hematologic malignancies. It also develops APTO-500 program that focuses on discovering and developing small molecule inhibitors of maternal embryonic leucine zipper kinase; and is in a pre-clinical stage for oncology. It has a license agreement with Genentech Inc. to develop and sub-license a specified polypeptide; and collaboration agreement with Eli Lilly and Company to investigate a proprietary series of the company's compounds for veterinary medicine. *****AST (T/$6-12)Stem Cell regenerative medicine neuro...https://finance.yahoo.com/news/asterias-biotherapeutics-completes-enrollment-dosing-110000202.html The Company has two technology platforms. The first is an immunotherapy platform to teach cancer patients' immune systems to attack their tumors. The second is pluripotent stem cell platform. Pluripotent cells are a type of stem cell capable of becoming all of the cell types in the human body. From its immunotherapy platform, the Company is developing over two programs. AST-VAC1 (telomerase loaded, autologous dendritic cells), which allows patient's own cells to recognize and fight cancer cells in acute myelogenous leukemia (AML). Together with Cancer Research United Kingdom (CRUK), it is developing AST-VAC2 (telomerase loaded, -allogeneic dendritic cells), -derived from pluripotent stem cells. From its pluripotent stem cell platform, it is developing AST-OPC1, oligodendrocyte progenitor cells. ATHX (T/$9)
focused on treating neurological conditions, cardiovascular diseases, inflammatory and immune disorders, and pulmonary and other conditions. The company's lead platform product includes MultiStem cell therapy, an allogeneic stem cell product, which has completed Phase 2 study for treating patients suffering from moderate and severe ischemic stroke; that is in Phase 2 clinical study for treating patients with acute myocardial infarction; and, which is in Phase 1/2 clinical study for treating patients with acute respiratory distress syndrome, as well as completed Phase 1 clinical study for patients suffering from leukemia or various other blood-borne cancers. It also develops MultiStem cell therapy to promote tissue repair and healing for animal patients; and 5HT2c agonists for the treatment of obesity and other conditions. AXGN (T/$22)
AxoGen, Inc. provides surgical solutions for peripheral nerve injuries. The company's surgical nerve repair solutions include Avance Nerve Graft, an off-the-shelf processed human nerve allograft for bridging severed nerves without the comorbidities associated with a second surgical site; AxoGuard Nerve Connector, a porcine submucosa extracellular matrix (ECM) coaptation aid for tensionless repair of severed nerves; and AxoGuard Nerve Protector, a porcine submucosa ECM product that is used to wrap and protect injured peripheral nerves, as well as reinforces the nerve reconstruction while preventing soft tissue attachments. Its solutions also comprise Avive Soft Tissue Membrane, a minimally processed human umbilical cord membrane that is used as a resorbable soft tissue covering to separate tissues and modulate inflammation in the surgical bed. In addition, the company offers AcroVal neurosensory and motor testing system, which consists of AcroGrip for use in hand grip strength measurement; AcroPinch for measuring pinch strength; and Pressure-Specified Sensory Device, a somatosensory evaluation and measurement device. Further, it provides AxoTouch two point discriminator, a tool that is used for measuring the innervation density of surface area of the skin. BLRX... **BCLI (T/$8) Brainstorm Cell Therapeutics ...adult stem cell therapies for neurodegenerative disorders ALS...engaged in developing adult stem cell therapies for debilitating neurodegenerative disorders, such as Amyotrophic Lateral Sclerosis (ALS, also known as Lou Gehrig's disease), Multiple Sclerosis (MS) and Parkinson's disease (PD), among others. Its subsidiary, Brainstorm Cell Therapeutics Ltd. (the Israeli Subsidiary), holds rights to commercialize the technology, NurOwn. NurOwn is in clinical development for the treatment of ALS. The Company has completed over two clinical trials of NurOwn in patients with ALS at Hadassah Medical Center (Hadassah). The first study, a Phase I/II safety and efficacy study of NurOwn in ALS patients administered either intramuscularly or intrathecally. *****BPTH (T/$5) NO DEBT
Bio-Path Holdings, Inc. (Bio-Path) is a clinical stage biotechnology company that focuses on developing nucleic acid cancer therapeutics using its proprietary nanoparticle RNAi antisense technology called DNAbilize™. This technology safely distributes nucleic acid based drugs systemically throughout the body via intravenous infusion. Bio-Path's lead product candidate, prexigebersen (BP1001) is in Phase 2 clinical studies for the treatment of acute and chronic myeloid leukemia (AML and CML), and preclinical studies for solid tumors. The Company's second DNAbilize™ drug candidate, Liposomal Bcl2 (BP1002), for the treatment of lymphoma, leukemia, colon, prostate and breast cancers, has completed preclinical studies for non-Hodgkin's lymphoma. Bio-Path is planning a broad Phase 1 clinical trial in lymphoma in 2017. Bio-Path is headquartered in Bellaire, Texas, and currently employs 12 full-time. Despite ongoing advancements in the development of nucleic acid drugs, pharmaceutical developers are still facing the challenge of delivering therapeutics to targeted cells without causing severe side effects in the patient. In clinical studies, Bio-Path's therapeutic platform has delivered a strong, effective therapeutic payload, with no evidence of toxicity. This novel target-based platform has the potential to transform the therapeutic landscape of cancer treatment and also address other diseases that have well-defined targets.
- Bio-Path's pipeline continues to expand with new cancer indications. Importantly, once its DNAbilize™ platform is proven successful for cancer, the core technology can easily be expanded to address new therapeutic areas, including autoimmune diseases.
In contrast to other lipid delivery technologies that have dose-limiting toxicities, DNAbilize™, Bio-Path's next generation oligonucleotide-based technology, enables the delivery of high doses of therapeutics to target cells, while demonstrating no evidence of toxicity. This lack of toxicity enables the development of therapies to address patients, particularly within the growing elderly population, who are unable to withstand aggressive therapeutic regimens, and therefore, have limited available treatment options.
Bio-Path has completed Phase 1 clinical trials for its lead candidate prexigebersen for AML, CML and other blood cancers, and is in the midst of Phase 2 clinical trial for AML and a Phase 1b/2 clinical trial for CML. Importantly, the Company expects to complete an interim analysis in 2H 2017 of the first 19 patients enrolled in the Phase 2 efficacy trial for AML, with the possibility of switching to a registration trial for accelerated approval.
The clinical targets for BP1002 are lymphoma, and potentially breast cancer, colon cancer, and prostate cancer. This novel, non-toxic, specific Bcl2 inhibitor could be a significant advance in cancer therapeutics, with the potential to treat 40% to 60% of solid tumors, according to Bio-Path estimates.
Bio-Path is collaborating with respected academic and clinical institutions, including M.D. Anderson, Thomas Jefferson University and UT Southwestern, to expand indications inside and outside of cancer, which we view as a validation of Bio-Path's DNAbilize™ technology. Going forward, we believe the versatility of the DNAbilize™ platform could represent significant drug development and licensing opportunities for Bio-Path.
Recent results include a net loss of $397K for the quarter ended 3/31/17 vs. a net loss of $1.87M for the comparable quarter of prior year. While operating expenses were similar Y-O-Y, the Company recognized a $1.6M gain for the change in fair value of warrants for the most recent quarter. Management states that cash on hand of approximately $7M is sufficient to fund operations for the next 12 months.
With promising clinical data and several programs in the pipeline addressing sizable markets with unmet needs, our valuation analysis for the BP1001 for AML and CML programs alone results in an estimated range of $3.60-$4.45/share, with a mid-point of approximately $3.99. Stonegate Capital Partners
BLUE...
BSTG () NO DEBT Biostage, Inc., a biotechnology company, engages in developing bioengineered organ implants based on its Cellframe technology. The company's Cellframe technology combines a proprietary biocompatible scaffold with a patient's own stem cells to create Cellspan organ implants. It is developing bioengineered organ implants, which addresses the damage of the esophagus, bronchus, and trachea due to cancer, infection, trauma, or congenital abnormalities. BLRX (T/$3 )
BioLineRx Ltd. Aug 21, 2017 /PRNewswire/ -- (
BLRX) (
BLRX), a clinical-stage biopharmaceutical company focused on oncology and immunology, announced today the filing of regulatory submissions required to commence a randomized, controlled
Phase 3 registrational trial of BL-8040 for the mobilization of hematopoietic stem cells for autologous transplantation in patients with multiple myeloma. The trial, named GENESIS, is expected to commence by the end of 2017, following receipt of regulatory approvals. The Phase 3 GENESIS trial is aimed at evaluating the safety, tolerability and efficacy of the combination treatment of BL-8040 and granulocyte colony-stimulating factor (G-CSF), as compared to the control arm of placebo and G-CSF. The trial will be conducted in two parts: The first part, designed to validate the optimal dosing of BL-8040, is a lead-in, open-label, multi-center study that will include 10-30 patients, in order to assess the efficacy and safety of treatment with BL-8040 and G-CSF. This part will be followed by a randomized, placebo-controlled, multi-center study in approximately 180 patients. The primary endpoint will be the proportion of subjects mobilizing ≥6.0 x 10
6 CD34+ cells/kg with up to 2 apheresis sessions in preparation for autologous transplantation after a single administration of BL-8040 and G-CSF, as compared to placebo and G-CS
CUR (T/$3) 09/07/2017 ...Neuralstem...awarded two additional patents by the United States Patent and Trademark Office (USPTO). These patents broadly protect methods for using neural stem cells to treat neurodegenerative disorders, a key component of the Company’s platform. The first new patent, U.S. Patent No. 9,744,194, covers methods of treating neurodegenerative disorders through transplantation of neural stem cells. The second new patent, U.S. Patent No. 9,750,769, covers neural stem cells engineered to express IGF-1, a neurotrophic molecule with broad therapeutic potential in the treatment of neurodegenerative disorders. ...development of nervous system therapies based on its proprietary human neuronal stem cells and small molecule compounds. The company's stem cell based technology enables the isolation and expansion of human neural stem cells from ... management has great confidence in the strength of its intellectual property, and Neuralstem now owns or licenses over 20 U.S. and 120 foreign issued and pending patents, and it continues to pursue more in the field of regenerative medicine related to its stem cell technologies and neurogenic small molecule program.
It is developing products include NSI-189, a chemical entity, which is in Phase II clinical trial for the treatment of major depressive disorder, as well as is in preclinical programs for the MCAO stroke, type 1 and 2 diabetes related neuropathy, irradiation-induced cognition, long-term potentiation enhancement, and angelman syndrome. The company is also developing NSI-566, which has completed Phase II clinical trial for treating amyotrophic lateral sclerosis disease, as well as is in Phase I clinical trials for the treatment of chronic spinal cord injury and motor deficits due to ischemic stroke. DMTX (T/$3.5)
Developing novel, liver-directed gene therapies for rare genetic disorders. FATE (T/$7)
develops programmed cellular immunotherapies for cancer and immune disorders worldwide. Its immuno-oncology product candidates include FATE-NK100, a natural killer (NK) cell cancer immunotherapy that consists of adaptive memory NK cells; engineered hnCD16 induced pluripotent stem cells (iPSC)-derived natural killer cell therapy candidates for hematologic/solid tumors; and engineered chimeric antigen receptor iPSC-derived T cell therapy product candidates for hematologic/solid tumors. The company's immuno-regulation product candidates comprise ProTmune, an investigational programmed cellular immunotherapy for use as a next-generation allogeneic hematopoietic cell transplantation cell graft; and ToleraCyte for the treatment of autoimmune and inflammatory diseases; engineered iPSC-derived CD34+ cell therapy for immune disorderS. GNMX (T/$1.50-9) the company develops transduced autologous restorative gene therapy (TARGT), a platform to provide protein and peptide therapies to treat a range of chronic diseases. Its lead product candidate is MDGN-201, which is an endogenous erythropoietin secretion TARGT that is in the Phase I/II clinical trials for the treatment of end stage renal diseases and other indications, including ß-thalassemia. HALO (T/$7H) Halozyme Therapeutics, develops, and commercializes human enzymes and other drug candidates in the United States, Switzerland, and internationally. The company's human enzymes are used to facilitate the delivery of injected drugs and fluids, enhancing the efficacy and the convenience of other drugs or can be used to alter tissue structures for clinical benefit. Its products are based on the Enhanze technology, a patented recombinant human hyaluronidase enzyme (rHuPH20) that enables the subcutaneous delivery of injectable biologics, such as monoclonal antibodies and other therapeutic molecules, as well as small molecules and fluids. The company offers Hylenex recombinant, a formulation of rHuPH20 to facilitate subcutaneous fluid administration for achieving to enhance the dispersion and absorption of other injected drugs; in subcutaneous urography; and to enhance resorption of radiopaque agents. It also develops PEGylated recombinant human hyaluronidase for the treatment of pancreatic ductal adenocarcinoma, non-small cell lung cancer, gastric cancer, and metastatic breast cancer. MCAO ...stroke, type 1 and 2 diabetes related neuropathy, irradiation-induced cognition, long-term potentiation enhancement, and angelman syndrome. The company is also developing NSI-566, which has completed Phase II clinical trial for treating amyotrophic lateral sclerosis disease, as well as is in Phase I clinical trials for the treatment of chronic spinal cord injury and motor deficits due to ischemic stroke. HSGX (T/$4) Histogenics Corporation, a regenerative medicine compa ny, focuses on developing and commercializing products in the musculoskeletal segment of the marketplace in the United States. The company offers NeoCart, a tissue implant, which is in Phase III clinical trial to treat tissue injury in the field of orthopedics, specifically cartilage damage in the knee. HTGM (T/$6) instruments molecular profiling applications ...mRNA biomarkers...The HTG EdgeSeq ALKPlus Assay EU is an in vitro diagnostic intended to measure and analyze mRNA ALK gene rearrangements in formalin-fixed, paraffin-embedded lung tumor specimens from patients previously diagnosed with non-small cell lung cancer (NSCLC). This assay may be used to aid the identification of patients eligible for ALK-targeted therapeutics, such as crizotinib, and is automated on the HTG EdgeSeq system using a next-generation sequencer for detection. *****INO (T/$12-13)...Inovio Pharmaceuticals is taking immunotherapy to the next level in the fight against cancer and infectious diseases. We are the only immunotherapy company that has reported generating T cells in vivo in high quantity that are fully functional and whose killing capacity correlates with relevant clinical outcomes with a favorable safety profile. With an expanding portfolio of immune therapies, the company is advancing a growing preclinical and clinical stage product pipeline. Partners and collaborators include MedImmune, Regeneron, Genentech, The Wistar Institute, University of Pennsylvania, DARPA, GeneOne Life Science, Plumbline Life Sciences, ApolloBio Corporation, Drexel University, NIH, HIV Vaccines Trial Network, National Cancer Institute, U.S. Military HIV Research Program and Laval University. Inovio Pharmaceuticals (INO +0.4%
) and Roche's (OTCQX:RHHBY) Genentech will collaborate on a clinical trial assessing the combination of INO-5401, a T cell-activating immunotherapy, and Tecentriq (atezolizumab) in patients with advanced urothelial carcinoma (bladder cancer). INO ...develops active DNA immunotherapies and vaccines in combination with proprietary electroporation delivery devices to prevent and treat cancers and infectious diseases. Its SynCon immunotherapy design has the ability to break the immune system's tolerance of cancerous cells; and SynCon product design is also intended to facilitate cross-strain protection against known, as well as new unmatched strains of pathogens, such as influenza. It has completed, current or planned clinical programs of its proprietary SynCon immunotherapies for HPV-caused pre-cancers and cancers, influenza, prostate cancer, breast/lung/pancreatic cancer, hepatitis C virus, hepatitis B virus, HIV, Ebola, Middle East Respiratory Syndrome, and Zika virus. The company's partners and collaborators include MedImmune, LLC, the Wistar Institute, University of Pennsylvania, Defense Advanced Research Projects Agency, GeneOne Life Science, Plumbline Life Sciences, ApolloBio Corporation, Drexel University, the National Institutes of Health, HIV Vaccines Trial Network, National Cancer Institute, Genentech, and U.S. Military HIV Research Program. HYGS (T/$5-9)
Histogenics Corporation, a regenerative medicine company, focuses on developing and commercializing products in the musculoskeletal segment of the marketplace in the United States. The company offers NeoCart, a tissue implant, which is in Phase III clinical trial to treat tissue injury in the field of orthopedics, specifically cartilage damage in the knee. KPTI ...
****MESO () ...Mesoblast Limited develops cell-based medicines. The company has leveraged its proprietary technology platform based on specialized cells, known as mesenchymal lineage adult stem cells, to establish a portfolio of late-stage product candidates. Its allogeneic off-the-shelf cell product candidates target advanced stages of diseases with high, unmet medical needs including cardiovascular diseases, immune-mediated and inflammatory conditions, orthopedic disorders, and oncologic/hematologic conditions. The company's lead product candidates include MPC-150-IM, a Phase 3 product candidate under investigation for the treatment of chronic congestive heart failure; MPC-06-ID, a Phase 3 product candidate under investigation for the treatment of chronic low back pain due to disc degeneration; MSC-100-IV, a Phase 3 intravenously-delivered product candidate, which is developed for the treatment of acute graft versus host disease following allogeneic bone marrow transplantation; and MPC-300-IV, an intravenously-delivered immunomodulatory product candidate for the treatment of chronic inflammatory conditions, including biologic-refractory rheumatoid arthritis, and diabetic nephropathy, which is under Phase 2 trial. It has a collaboration agreement with JCR Pharmaceuticals Co., Ltd to offer TEMCELL HS. Inj., a product based on its proprietary mesenchymal lineage adult stem cell technology for the treatment of graft versus host disease. OMED (T/$6) develops cancer stem cell (CSC) and immuno-oncology therapeutics. The company's product candidates and preclinical programs include demcizumab (anti-DLL4, OMP-21M18), a humanized monoclonal antibody, in Phase II trial for pancreatic cancer, a randomized Phase II trial in non-small cell lung cancer, and a Phase Ib trial for solid tumor; tarextumab (anti-Notch2/3, OMP-59R5) that targets the Notch2 and Notch3 receptors, which is in Phase II clinical trial for small cell lung cancer; vantictumab (anti-Fzd, OMP-18R5) in two Phase Ib clinical trials for breast and pancreatic cancer; ipafricept (Fzd8-Fc, OMP-54F28), a fusion protein based on Frizzled8 receptor, which is in two Phase Ib clinical trials for ovarian and pancreatic cancer; and navicixizumab (anti-DLL4/VEGF Bispecific, OMP-305B83), a monoclonal antibody that targets DLL4 and vascular endothelial growth factor, which is in Phase Ia single-agent clinical trial for solid tumors, and two Phase Ib clinical trial for ovarian and metastatic colorectal cancer. It is also developing anti-RSPO3 (OMP-131R10), a monoclonal antibody, in Phase Ia/b clinical trial for solid tumor and metastatic colorectal cancer; brontictuzumab (anti-Notch1, OMP-52M51) targeting the Notch1 receptor, in Phase Ib clinical trial for colorectal cancer; anti-tigit (OMP-313M32), a T-cell immunoglobulin and ITIM domain protein, in Phase I clinical trial for tumor; and GITRL-Fc trimer (OMP-336B11), a preclinical product candidate targeting glucocorticoid-induced tumor necrosis factor receptor and its ligand. ONCS (T/5-6) OncoSec Medical ...gene therapies, therapeutics and medical approaches to stimulate an anti-tumor immune response for the treatment of cancer. The Company's lead product candidate, ImmunoPulse IL-12, consists of a plasmid construct encoding the proinflammatory cytokine, IL-12, which is delivered into the tumor through in vivo electroporation. As of July 31, 2016, the Company was pursuing two Phase II trials: ImmunoPulse IL-12 monotherapy in patients with metastatic melanoma and ImmunoPulse IL-12 plus pembrolizumab in patients with advanced, metastatic melanoma. In addition, it is pursuing ImmunoPulse IL-12 monotherapy in patients with triple negative breast cancer. Its ImmunoPulse product candidates are based on its deoxyribonucleic acid (DNA)-based immunotherapy technology, which is designed to stimulate the human immune system, resulting in systemic anti-tumor immune responses. OPGN () in vitro diagnostic tests designed to identify antimicrobial resistant pathogens, as well as XpressFISH diagnostic test products for the identification of various infectious pathogens. It also provides Acuitas MDRO Gene Test, Acuitas CR Elite Test, and Acuitas Resistome Test that are CLIA lab-based tests, which provide a profile of MDRO resistant genes for surveillance and response to outbreaks. In addition, the company offers Acuitas Lighthouse bioinformatics systems, which are cloud-based HIPAA compliant bioinformatics offerings that combine clinical lab test results with patient and hospital information, and provide analytics to enable manage MDROs in the hospital and patient care environment. PSTI (T/$2)
operates as a bio-therapeutics company in Israel. It focuses on the research, development, clinical trials, and manufacture of cell therapeutics products and related technologies for the treatment of various ischemic, inflammatory, and hematologic conditions. The company develops placenta expanded (PLX) cell therapy products, such as PLX-PAD cells, which has been completed Phase-II clinical trial for the treatment of peripheral and cardiovascular diseases, as well as for the treatment of orthopedic diseases. It also develops PLX-R18 cells for hematopoietic cell transplantation in animals, as well as conducts various in-vivo studies for the evaluation of PLX-R18 for the treatment of ARS RGLS (T/2.50-$6)
focuses on the discovery and development of drugs that target microRNAs to treat a range of diseases in the United States. The company uses its microRNA product platform to develop anti-miRs, which are chemically modified and single-stranded oligonucleotides. Its clinical development products include RG-101, a GalNAc-conjugated anti-miR targeting miR-122 to treat patients with hepatitis C virus infection; RG-012, an anti-miR targeting microRNA-21 for the treatment of Alport syndrome; RG-125, a GalNAc-conjugated anti-miR targeting microRNA-103/107 for the treatment of non-alcoholic fatty liver disease; RGLS5040, an anti-miR targeting microRNA-27 for the treatment of cholestatic disease; and RGLS4326, an anti-miR targeting microRNA-17 for the treatment of autosomal dominant polycystic kidney disease. **ROSG (T/$3.50H)(1:12 REVERSE SPLIT 03/17) Rossetta Genomics... MicroRNAs are a group of genes that are produced using instructions encoded in DNA. SNY ...the "immortality gene" treats cholestrol in adults hypercholestrolemia. ****SGMO (T/$4-8-17)
Sangamo Therapeutics...
in May the company announced a new collaboration with Pfizer in hemophilia A. The pair will work together to develop a gene therapy called SB-525, which has received orphan drug designations from the U.S. Food and Drug Administration and the European Medicines Agency. The company's proprietary zinc finger DNA-binding protein (ZFP) technology enables specific genome editing and gene regulation. The ZFPs could be engineered to make ZFP nucleases (ZFNs), proteins that could be used to specifically modify DNA sequences by adding or knocking out specific genes; and ZFP transcription factors (ZFP TFs), proteins that can be used to turn genes on or off. Its therapeutic products include SB-728-T, a ZFN-mediated autologous T-cell product for human immunodeficiency virus and acquired immunodeficiency syndrome (HIV/AIDS), which is in Phase II and Phase I clinical trials; and SB-728-HSPC that is in Phase I/II clinical trials for HIV/AIDS. The company also engages in Phase I/II studies of in vivo genome editing applications of ZFP Therapeutics for hemophilia B, Hemophilia A, and Mucopolysaccharidosis I (MPS) and MPS II, which are lysosomal storage disorder (LSD); proprietary preclinical programs in other LSDs; and research stage programs in certain central nervous system disorders and cancer immunotherapies. SB-525 is Sangamo’s clinical stage cDNA gene therapy candidate for hemophilia A. = fast-track designation from the FDA will facilitate the development and expedite the review of drugs and biologics to treat serious conditions and fill an unmet medical need.
SB-525 already enjoys orphan drug status in the U.S. The FDA has cleared an Investigational New Drug application for this program, and a phase I/II clinical trial evaluating SB-525 in adults with hemophilia A is expected to open and begin screening subjects for enrolment by the end of second-quarter 2017. Data from this study are expected in late 2017 or early 2018. SLNO (GOOGLE) issuance of a new patent (9,757,384) from the U.S. Patent and Trademark Office related to the use of pharmaceutical formulations of diazoxide and its salts, such as diazoxide choline, to reduce one or more aggressive behaviors in a subject with Prader-Willi syndrome (PWS) or Smith-Magenis syndrome (SMS). PWS is a rare and complex genetic neurobehavioral/metabolic disorder affecting appetite, growth, metabolism, cognitive function, and behavior. SMS is a rare disorder with a distinct genetic basis, but a natural history similar to that of PWS. “This patent will further strengthen the intellectual property portfolio for DCCR, our lead product candidate, for the treatment of PWS,” said Anish Bhatnagar, M.D., Chief Executive Officer of Soleno Therapeutics. “Importantly, this most recent patent will provide protection of associated claims into 2035. We expect to leverage additional intellectual property opportunities for DCCR over the coming months.”Soleno expects to initiate a Phase III clinical trial of Diazoxide Choline Controlled Release Tablet (DCCR) in PWS by the end of 2017. The Company is currently finalizing the design of a randomized, double-blind placebo-controlled study that will treat approximately 100 patients. This study is anticipated to take approximately 9-12 months to complete. DCCR has Orphan Designation for the treatment of PWS in the U.S.
About PWS
PWS is a rare and complex genetic neurobehavioral/metabolic disorder affecting appetite, growth, metabolism, cognitive function and behavior. The committee on genetics of the American Academy of Pediatrics states PWS affects both genders equally and occurs in people from all geographic regions: its estimated incidence is one in 15,000 to 25,000 births. This disorder is typically characterized by hyperphagia, a chronic feeling of insatiable hunger, behavioral problems, cognitive disabilities, low muscle tone, short stature (when not treated with growth hormone), the accumulation of excess body fat, developmental delays, and incomplete sexual development. Hyperphagia, in the absence of effective limitations to access to food, can lead to morbid obesity. In a global survey conducted by the Foundation for Prader-Willi Research, 96.5% of respondents (parent and caregivers) rated hyperphagia, which is the unrelenting hunger that severely diminishes the quality of life for patients and their families, as the most important or a very important symptom to be relieved by a new medicine. There are currently no approved therapies to treat the hyperphagia/appetite, metabolic, cognitive function, or behavioral aspects of the disorder.
About Diazoxide Choline Controlled-Release Tablet
Diazoxide choline controlled-release tablet is a novel, proprietary controlled-release, crystalline salt formulation of diazoxide, which is administered once-daily. The parent molecule, diazoxide, as an oral suspension, has been used for decades in thousands of patients in a few rare diseases in neonates, children and/or adults, but not in PWS. Essentialis conceived of and is pursuing an extensive patent portfolio relating to various aspects of the therapeutic use of diazoxide and DCCR in patients with PWS. The DCCR development program is supported by positive data from two completed Phase II clinical studies and five completed Phase I clinical studies in various metabolic indications, as well as a pilot study in PWS patients. In the PWS pilot study, DCCR showed promise in addressing the hallmark symptoms of PWS, most notably hyperphagia
SMMT... SRPT... Nanopore Technologies (private company)
MDXG (T/$20)...
MiMedx Group, Inc. develops, patent protected and proprietary regenerative biomaterial products and bioimplants processed from human placental tissue, skin, and bone. The company collects the donated human placental tissue and converts into a sterile product through its proprietary PURION process. Its biomaterial platform technologies include AmnioFix and EpiFix that are tissue technologies processed from human amniotic membrane derived from donated placental tissue for homologous applications; OrthoFlo, an amniotic fluid-derived allograft for homologous applications; Physio, a bone grafting material comprising 100% bone tissue with no added carrier; and CollaFix, a technology platform derived from collagen fiber technology designed to mimic the natural composition, structure, and mechanical properties of musculoskeletal tissues to augment their repair. The company also offers EpiCord, an umbilical cord allograft that provides a connective tissue matrix to replace or supplement damaged or inadequate integumental tissue; AmnioCord, an umbilical cord allograft that offers a protective environment for the healing process; and AmnioFill, a cellular tissue matrix allograft that enhances healing. Its products have applications in the areas of wound care, burns, surgery, orthopedics, spine, sports medicine, ophthalmology, and dentistry. PACB genetic sequencing machine *****SRNE () Sorrento Therapeutics...
primary therapeutic focus is oncology, including the treatment of chronic cancer pain. It is also developing therapeutic products for other indications, including immunology and infectious diseases. Its products in the pipeline include Chimeric Antigen Receptor-T Cell (CAR-T) programs, resiniferatoxin (RTX), and biosimilar/biobetter antibodies clinical development programs. Its pipeline also includes preclinical fully human therapeutic monoclonal antibodies (mAbs), including biosimilars/biobetters, fully human anti-PD-L1 and anti-PD-1 checkpoint inhibitors derived from its G-MAB library platform, antibody drug conjugates (ADCs), bispecific antibodies (BsAbs), as well as CAR-T and Chimeric Antigen Receptor Natural Killer (NK) cells (CAR. NK) for adoptive cellular immunotherapy. VCEL (T/$6)
VCEL (T/$6)
VTGN XON Intrexon Corp is a leader in the bio-tech field. The company’s biologically-based products and processes are some of the most interesting and important revelations in the medical industry. Designing, building, and regulating gene programs, the company’s technological advancements are ahead of most.
One of Intrexon’s most important technologies is its patented UltraVector. The platform is a software-based operating system that accurately maps and assembles genetic programs. The platform allows for deeper understanding of how everything works and allows for experiments not possible otherwise.
As tech continues to fuel medical breakthroughs, Intrexon Corp is definitely worth keeping an eye on.
The company, through a suite of proprietary and complementary technologies, designs, builds, and regulates gene programs, which are DNA sequences that consist of key genetic components. Its technologies include UltraVector gene design and fabrication platform, and its associated library of modular DNA components; Cell Systems Informatics; RheoSwitch inducible gene switch; AttSite Recombinases; Protein Engineering; Laser-Enabled Analysis and Processing; and ActoBiotics platform. It also provides reproductive technologies and other genetic processes to cattle breeders and producers; genetic preservation and cloning technologies; genetically engineered swine for medical and genetic research; biological insect control solutions; technologies for non-browning apple without the use of any flavor-altering chemical or antioxidant additives; commercial aquaculture products; and artwork, children's toys, and novelty goods that are derived from living organisms or enabled by synthetic biology. The company serves health, food, energy, environment, and consumer sectors.
 ZGNX
ZGNX Zogenix stock
more than doubled on Friday after announcing very good results from a late-stage study of experimental drug ZX008 in treating Dravet syndrome, a rare form of epilepsy. Patients taking ZX008 experienced significant improvement in seizure convulsions than did those on placebo in Zogenix's study. In addition, the drug led to meaningful reductions in seizure frequency and longest seizure-free interval.
These results could bode well for Zogenix's second late-stage study for ZX008 in treating Dravet syndrome. Results from that study are expected in the first half of 2018. If all goes well, Zogenix hopes to submit the drug for Food and Drug Administration (FDA) approval in the second half of next year. Motley Fool Genetic-Agriculture *****AMRS (T/$15)
Amyris, Inc. provides various alternatives to a range of petroleum-sourced products worldwide. The company uses its industrial bioscience technology to design microbes primarily yeast, as well as to convert plant-sourced sugars into renewable ingredients. It produces and sells Biofene that converts to squalane, which is used as an emollient in cosmetics and other personal care products; and natural oils and aroma chemicals for the flavors and fragrances market. The company also provides renewable solvents, polymers, and lubricants for industrial markets; Biofene ingredients for nutraceuticals and vitamins market; and renewable fuels for transportation fuels markets. It has a collaboration partnership with Total S.A. to produce and commercialize Biofene-based diesel and jet fuels. CLXT (T/$17) ...
develops healthier specialty food ingredients and agricultural food crops using gene editing technology for plants. It engages in the development of high oleic soybeans, powdery mildew resistant wheat, cold storable potatoes, high fiber wheat, reduced browning potatoes, and herbicide tolerant wheat. YTEN () Yield10 Bioscience, Inc. is focused on developing new technologies to achieve step-change improvements in crop yield to enhance global food security. Yield10 has an extensive track record of innovation based around optimizing the flow of carbon in living systems. Yield10 is leveraging its technology platforms and unique knowledge base to design precise alterations to gene activity and the flow of carbon in plants to produce higher yields with lower inputs of land, water or fertilizer. Yield10 is advancing several yield traits it has developed in crops such as Camelina, canola, soybean and corn. Researchers used the CRISPR genome editing tool to inactivate an enzyme expected to increase seed oil content in Camelina, a trait Yield10 has designated as C3008. There are three copies of this gene in the Camelina genome and complete editing of all copies was achieved. This trait may have further applications when used in combination with other traits that the Company is developing that are expected to increase seed oil content, including C3007. “We believe that genome editing technology represents a fundamentally new way to rapidly deploy desirable, novel traits into commercial agricultural crops by specifically making small changes or removing small pieces of DNA to reduce the activity of target genes without leaving behind any foreign DNA in the plant,”
CANCER 04/15/2018 Robert Beckman of Orgenesis Inc. (ORGS:OTCQB) described CAR-T and similar products as platform products at the embryonic stage, and said the category has the potential to cause major change. Beckman pointed out that his company is modifying liver cells to be used to treat diabetes, and that cellular therapy isn't limited to CAR-T; that there's a lot going on in the space.
Durant added that he thinks the driver of the deal is the promise of where cell and gene therapy could be 20 years' time, and that investors need to see a company make a big bet: "Fifteen years from now, a lot of cancers will be things that you die with, rather than things you die from."
I concluded the discussion by highlighting that the two deals discussed are really not so different, and all of the various elements are folding into one game-changing solution that has the potential to change the way we think about everything related to being healthy.Disruption is convergence, and we need to take a holistic view of healthcare, which includes what we traditionally think of as two different ends of the spectrum—the breakthrough science of cellular therapy and predictive genetics, and "alternative health," which includes preventive care, diet and lifestyle. We need to come together as an industry, start to examine the silos we've created in our heads, and determine how we can take them down, so that the industry will evolve and all of us—patients and consumers—can benefit.
*****ADXS (T/19-24) Advaxis ...Its LM therapies alter live strains of Listeria monocytogenes bacteria to generate cancer-fighting T cells targeting cancer antigens. Clinical trials have shown these immunotherapies to be well tolerated with anti-tumor activity, which could translate to a successful product or partnership in the future if successful in later trials. The downside is that the firm continues to burn cash as it develops its clinical pipeline... Phase II study for the treatment of human papilloma virus associated cancers, including cervical, head and neck, and anal cancer. The company is also developing ADXS-PSA, an Lm-LLO immunotherapy product candidate, which is in Phase I/II clinical trials designed to target the prostate specific antigen associated with prostate cancer; and ADXS-HER2, an Lm-LLO immunotherapy product candidate that is in Phase Ib clinical trials used for the treatment of human epidermal growth factor receptor 2 expressing cancers, including human and canine osteosarcoma. AEZS (T/2H -4 FOR 2018) AEterna FDA has accepted the drug maker's New Drug Application (NDA) for Macrilen (macimorelin), a novel orally-active ghrelin agonist intended to help in monitoring treatment for adult growth hormone deficiency (AGHD) ***AGRX (T/$10) SHARE DILUTION Aug. 3rd 5.33 million shares at $3.75 ...Women's contrception...weekly patch December 26, 2017 as the Prescription Drug User Fee Act (PDUFA) goal date. ****AFMD (T/$7) ...i
ts lead candidate is AFM13, a natural killer cell (NK-cell) TandAb designed for the treatment of CD30-positive (CD30+) B- and T-cell malignancies, including Hodgkin lymphoma. The company's product candidates include AFM24, a NK-cell that treats epidermal growth factor receptor expressing solid tumors, such as lung, head, neck, and colon cancers; AFM26, which binds to B-cell maturation antigen (BCMA) for the treatment of multiple myeloma; and Trispecific Abs for the treatment of multiple myeloma. Its product candidates also comprise AFM11, a T-cell TandAb, which is in Phase I clinical trial for the treatment of various CD19+ B-cell malignancies, including non-Hodgkin Lymphoma and acute lymphocytic leukemia; and AMV564, a CD33/CD3-specific T-cell TandAb for the treatment of acute myeloid leukemia and other hematologic malignancies. AGEN (T/$7) Immuno-oncology
ALRN (T/$19-20) Lymphoma...
****APTO (?) Aptose Bioscience CG’806, a first-in-class FLT3/BTK inhibitor, exerts superior potency against AML cells harboring ITD, TKD and gatekeeper mutated FLT3 or wild-type FLT3, demonstrated the superior potency of CG’806, relative to competitive agents, against hematologic malignancy cell lines driven by various WT or mutant forms of FLT3. In addition, once daily oral dosing of CG’806 in a murine model achieved sustained micromolar plasma concentration over a 24hr period, and was accompanied by complete elimination of AML FLT3-ITD tumors in the absence of toxicity. "Given the potency of CG’806 against all of the mutant forms of FLT3 AML and the ability to eradicate AML tumors in murine xenograft models, CG’806 has demonstrated the potential to be superior to other FLT3 inhibitors and is beginning to differentiate itself as a targeted treatment for AML," commented William G. Rice, Ph.D., Chairman and Chief Executive Officer of Aptose. “We believe CG’806 has the potential to become the best-in-class FLT3 inhibitor, and our internal efforts now are focused on delivering CG’806 to AML patients as soon as practicable.” ARRY (T/$10H-13) Array BioPharma Many drugs in the pipeline cancer...hepatitis C virus...myeloma and many gene cancer inhibitors... *****AST (T/$6-12)Stem Cell regenerative medicine neuro...https://finance.yahoo.com/news/asterias-biotherapeutics-completes-enrollment-dosing-110000202.html The Company has two technology platforms. The first is an immunotherapy platform to teach cancer patients' immune systems to attack their tumors. The second is pluripotent stem cell platform. Pluripotent cells are a type of stem cell capable of becoming all of the cell types in the human body. From its immunotherapy platform, the Company is developing over two programs. AST-VAC1 (telomerase loaded, autologous dendritic cells), which allows patient's own cells to recognize and fight cancer cells in acute myelogenous leukemia (AML). Together with Cancer Research United Kingdom (CRUK), it is developing AST-VAC2 (telomerase loaded, -allogeneic dendritic cells), -derived from pluripotent stem cells. From its pluripotent stem cell platform, it is developing AST-OPC1, oligodendrocyte progenitor cells. ATOS biopharmaceutical breast cancer Seattle... ***ATNM $3-5-8) RECENTLY hyped stock...develops targeted payload immunotherapeutics for the treatment of advanced cancers. The company's proprietary platform utilizes monoclonal antibodies to deliver radioisotopes directly to cells of interest in order to kill those cells safely and effectively. Its lead product candidate is Iomab-B that is in Phase III clinical studies in refractory or relapsed acute myeloid leukemia (AML) patients over the age of 55 for hematopoietic stem cell transplant, commonly referred to as bone marrow transplant. The company is also developing Actimab-A, which is in Phase II clinical trials for the treatment of patients newly diagnosed with AML over the age of 60; and Actimab-M that is in Phase I clinical trials for patients with relapsed or refractory multiple myeloma. In addition, it utilizes its alpha-particle immunotherapy technology platform to generate new drug candidates based on antibodies linked to the element Actinium-225 that are directed at various cancers that are blood-borne or form solid tumors. Acute myeloid leukemia (AML) is a type of cancer of blood-forming cells in the bone marrow in which bone marrow gets filled with excess immature white blood cells (blasts). The cancer spreads rapidly, and the cancer cells impair the production of normal white blood cells, red blood cells, and platelets. AML patients mostly undergo bone marrow transplant, and they need to qualify for the transplant by undergoing an induction therapy using anthracyclines which is a type of antibiotic. AVEO AVEO (T/$3H-$3.5H -4.50) kidney cancer ...European Medicines Agency (EMA) said experts at its Committee for Medicinal Products for Human Use (CHMP) had backed the oral, once-daily drug for marketing approval as a first-line treatment for advanced renal cell carcinoma, or kidney cancer. AVEO has made significant progress with its lead pipeline candidate, tivozanib. It is currently under review in the EU for the first-line treatment of advanced RCC. In Jun 2017, the Committee for Medicinal Products for Human Use (CHMP) recommended the approval of the candidate. A final response on the approval status of the candidate is expected in the third quarter of 2017. https://finance.yahoo.com/news/aveo-pharmaceuticals-aveo-q2-loss-142502771.html The company also announced achievement of enrollment target in a phase III study, TIVO-3, evaluating tivozanib, in June, ahead of its prior deadline of Aug 2017. Top-line data is expected in the first quarter of 2018. The study will compare tivozanib with Bayer’s BAYRY Nexavar in patients with refractory advanced renal cell carcinoma (RCC). The data from this study along with previously completed TIVO-1 study will support the regulatory application for approval of tivozanib in the U.S. as a first- and third-line treatment for RCC. ***BIOC cancer diagnostics company, develops and commercializes proprietary circulating tumor cell (CTC) and circulating tumor DNA assays utilizing a standard blood sample.... ****BPMX (T/$3) ...suggests that molecular iodine can specifically inhibit cellullar proliferation and drive cell death in certain breast cancer cell lines without apparent toxicity to normal breast cells. *****BPTH (T/$2-5)
Bio-Path Holdings, Inc. is a clinical and preclinical stage oncology focused antisense drug development company. The Company utilizes a technology that achieves systemic delivery for target specific protein inhibition for any gene product that is over-expressed in disease. Its drug delivery and antisense technology, DNAbilize, is a platform that uses P-ethoxy, a deoxyribonucleic acid backbone modification. Its lead drug candidate, Liposomal Grb2 (BP1001), targets the protein Growth factor receptor-bound protein 2 (Grb2). Its other liposome delivered antisense drug candidate, Liposomal Bcl2 (BP1002), targets the protein B-cell lymphoma 2 (Bcl2). BP1001 is in Phase II clinical trials for acute myeloid leukemia, and for blast phase and accelerated phase chronic myelogenous leukemia. BP1002 is intended to target the lymphoma and certain solid tumor markets. BP1001 is also in preclinical studies for solid tumors, including triple negative breast cancer and inflammatory breast cancer. ***CALA (T/$9H)...small molecule drugs directed against tumor metabolism and tumor immunology targets... *****CANF (T/$7)
Can-Fite BioPharma ...
Namodenoson is a small orally bioavailable drug that binds with high affinity and selectivity to the A3 adenosine receptor (A3AR). Namodenoson is being evaluated in Phase II trials for two indications, as a second line treatment for hepatocellular carcinoma, and as a treatment for non-alcoholic fatty liver disease (NAFLD) and non-alcoholic steatohepatitis (NASH). A3AR is highly expressed in diseased cells whereas low expression is found in normal cells. This differential effect accounts for the excellent safety profile of the drug. Can-Fite has received Orphan Drug Designation for Namodenoson in Europe and the U.S., as well as Fast Track Status in the U.S. as a second line treatment for hepatocellular carcinoma.= excellent safety profile of the drug=Data also showed that Namodenoson has a liver protective effect that is very unique compared to Nexavar® and other drugs under development for HCC which have shown to induce hepato-toxicity. According to Datamonitor, the market for hepatocellular carcinoma drugs is projected to reach $1.4 billion in 2019. Nexavar® annual sales, as reported by Bayer, were €870 million in 2016.
The Company's lead drug candidate, Piclidenoson, is scheduled to enter a Phase III trial for rheumatoid arthritis in 2017 and a Phase III trial for psoriasis in early 2018. The rheumatoid arthritis Phase III protocol has recently been agreed with the European Medicines Agency. Can-Fite's liver cancer drug Namodenoson is in Phase II trials for patients with liver cancer and is slated to enter Phase II for the treatment of non-alcoholic steatohepatitis (NASH). Namodenoson has been granted Orphan Drug Designation in the U.S. and Europe and Fast Track Designation as a second line treatment for hepatocellular carcinoma by the U.S. Food and Drug Administration. Namodenoson has also shown proof of concept to potentially treat other cancers including colon, prostate, and melanoma. CF602, the Company's third drug candidate, has shown efficacy in the treatment of erectile dysfunction in preclinical studies and is being prepared for an IND submission to the FDA and a Phase I trial. These drugs have an excellent safety profile with experience in over 1,000 patients in clinical studies to date CLSN (T/$7-9) ... focuses on the development and commercialization of directed chemotherapy, DNA-mediated immunotherapy, and RNA based therapy products for the treatment of cancer. The company's lead product includes ThermoDox, a liposomal encapsulation of doxorubicin that is in Phase III clinical trials for primary liver cancer; and under Phase II clinical trials for recurrent chest wall breast cancer. It is also developing GEN-1, a DNA-based immunotherapeutic product for the localized treatment of ovarian and brain cancers.
CGEN (T/$9-14) ... early and preclinical stage immuno-oncology programs based on novel drug targets, such as immune checkpoint and myeloid protein target candidates to harness the immune system to provide treatment solutions in the areas of unmet medical needs in various cancer types ****CGIX (T/$6) Cancer Genetics... Lung cancer is by far the leading cause of cancer death among both men and women; about 1 out of 4 cancer deaths are from lung cancer. It is the second most common cancer in both men and women (not counting skin cancer). About 14% of all new cancers are lung cancers, with NSCLC accounting for 85% of all lung cancers.[1] It is estimated that in 2017, approximately 222,500 Americans will be diagnosed with lung cancer, and almost 156,000 will die from this disease.[2]
Approved by the FDA on June 22 of 2017, the Oncomine Dx Target Test simultaneously evaluates 23 genes clinically associated with NSCLC. Following FDA approval, results from analysis of three of these genes can now be used to identify patients who may be eligible for treatment with one of the following: AstraZeneca's EGFR inhibitor Iressa (gefitinib), Pfizer's ALK and ROS1 inhibitor Xalkori (crizotinib), and the combination therapy of Novartis' MEK inhibitor Mekinist (trametinib) and RAF inhibitor Tafinlar (dabrafenib). With this test, physicians can now match patients to these therapies in days instead of several weeks, which it often takes when screening samples one biomarker at a time.
Recognized as Thermo Fisher's NGS CDx Center of Excellence, CGI is among the first 3 laboratories that will offer the Oncomine Dx Target Test as a service to oncologists. All tests will be run on Thermo Fisher's Ion PGM Dx System, which received FDA 510(k) clearance in parallel for use on formalin-fixed, paraffin-embedded (FFPE) tissue samples. The Oncomine Dx Target Test is a kit and requires as little as 10 nanograms of DNA from FFPE tissues samples - a critical advantage of the test, given the challenge of NSCLC patient samples often being of limited quantity.
CHEK (T/$5-6) medical diagnostics company, engages in the development of an ingestible imaging capsule that utilizes low-dose X-rays for the screening for precancerous polyps and cancer.... CTMX (T/$25) Preclinical biotech developing tumor-activated cancer immunotherapies. CYTR () The company's product candidate is the aldoxorubicin, which is in Phase III clinical trial as a therapy for patients with soft tissue sarcomas (STS) whose tumors have progressed following treatment with chemotherapy; in Phase IIb clinical trial in small cell lung cancer; in Phase II clinical trial in HIV-related Kaposi's sarcoma; in Phase II clinical trial in patients with late-stage glioblastoma (brain cancer); in Phase Ib trial in combination with ifosfamide in patients with STS; and in Phase Ib trial in combination with gemcitabine in subjects with metastatic solid tumors. It also has completed Phase IIb and Phase Ib/II clinical trials with aldoxorubicin as a first-line therapy for STS; a Phase Ib clinical trial of aldoxorubicin in combination with doxorubicin in patients with advanced solid tumors; and a Phase Ib pharmacokinetics clinical trial in patients with metastatic solid tumors. The company is also developing anti-cancer drug conjugates, which utilizes its Linker Activated Drug Release technology DCTH () ...Delcath Systems, an interventional oncology Company focused on the treatment of primary and metastatic liver cancers. I’d also like to highlight that the clinical potential of Melphalan/HDS in the treatment of ocular melanoma liver metastases was repeatedly supported by compelling clinical data presented and published throughout the past year. Most recently, a single-center retrospective review was published in the American Journal of Clinical Oncology in which the authors found that investigational PHP with Melphalan/HDS offered promising results with a doubling of overall survival (OS) and significantly longer progression-free survival (PFS) and hepatic PFS than other targeted therapies. In addition, an oral presentation at the Regional Cancer Therapies 12th International Symposium reported data from a retrospective, multicenter study demonstrating that 45.7% of patients with ocular melanoma that metastasized to the liver who underwent PHP using investigational Melphalan/HDS experienced a complete or a partial response. That same study further showed that among those who responded to treatment, OS was projected to be more than three years. The projected 657-day median OS and 1,207-day median OS in patients with a partial or a complete response is very impressive, and we believe speaks to the potential of our system to provide a meaningful and durable response in a disease that has an average survival of only six to eight months. *****DMPI DMPI DMPI (T/$13) DelMar Pharmaceuticals ... ( John McCain brain cancer is glioblastoma) The Company is engaged in conducting clinical trials in the United States with its product candidate, VAL-083, as a treatment for glioblastoma multiforme (GBM), a form of brain cancer. VAL-083 is being evaluated in a Phase II clinical trial for the treatment of refractory GBM. In addition to its clinical development activities in the United States, the Company has obtained certain commercial rights to VAL-083 in China where it is approved as a chemotherapy for the treatment of chronic myelogenous leukemia (CML) and lung cancer. Its drug discovery research focuses on identifying validated clinical and commercial-stage compounds, and establishing a scientific rationale for development in orphan drug indications. VAL-083 is an alkylating agent, which crosses the blood-brain-barrier. VAL-083 was shown to rapidly introduce irreversible DNA interstrand crosslinks leading to persistent DNA double-strand breaks and cancer cell death. Treatment with VAL-083 induced S/G2 phase cell cycle arrest in all cancer cells tested, including ovarian, prostate, lung and glioma cells. These results suggest the potential for synergy with treatments that depend on a cancer cell to be in the S-phase for activity. Such agents include topoisomerase inhibitors, commonly used in the treatment of brain cancer and other solid tumors, and PARP inhibitors, commonly used in the treatment of ovarian cancer. In combination studies, VAL-083 combined with either topoisomerase inhibitors or PARP inhibitors demonstrated synergy or super-additivity against a range of cancer cells. Topoisomerase inhibitors tested in combination with VAL-083 included etoposide and camptothecin. PARP inhibitors tested in combination with VAL-083 included olaparib, talazoparib and veliparib. "We have previously demonstrated that VAL-083 maintains activity against cancer cells resistant to the most commonly used chemotherapies," stated Dr. Dennis Brown, DelMar's Chief Scientific Officer. "The data presented here expand the opportunity to leverage VAL-083's unique mechanism in combination with agents such as PARP inhibitors and topoisomerase inhibitors that are widely used the treatment of multiple cancers." "Resistance to treatment can occur when cancer cells, or even a small group of cancer cells within a tumor, contain molecular alterations rendering them insensitive to a particular drug," continued Dr. Brown. "In other cases, cancer cells may adapt to the drug while it is being administered, acquiring molecular changes that allow them to escape its effects. We are enthusiastic about our results to date with VAL-083 as a single agent, but these data suggest the potential to further improve patient outcomes by combining VAL-083 with other anti-cancer agents that work by a different molecular mechanism. PARP inhibitors have recently gained significant interest in the treatment of ovarian cancer and are now being explored in multiple cancers. Topoisomerase inhibitors have been established as important components in the treatment of lung, ovarian, prostate and central nervous system tumors, but their utility can be limited by side effects. A synergistic combination with VAL-083 offers the potential to improve outcomes while minimizing toxic side-effects.
****DMTX ($12)
EXAS ...(T/27H) Cologuard, a non-invasive stool-based DNA screening test for the early detection of colorectal cancer and pre-cancer ****EPZM (T/$20-25) ...Epizyme ... Recent activity appears promising and potentially supportive of accelerated approval, considering there is no established standard of care in the setting. There are 25,000 new cases of follicular lymphoma diagnosed in the U.S. annually, and up to 20% of those patients have the EZH2 mutation. Currently, patients are treated with chemotherapy plus Rituxan. However, most patients end up relapsing, and treatment options when the disease returns are limited. Therefore, the findings in these indications are particularly intriguing. https://www.fool.com/investing/general/2017/06/15/why-epizyme-incs-shares-are-skyrocketing-today.aspx?yptr=yahoo ****FBIO (T-9-11) Fortress Biotech, Inc., a biopharmaceutical company, develops novel immunotherapy agents for the treatment of autoimmune diseases and cancer. Its product includes CNDO-109, a lysate that activates donor natural killer cells for the treatment of cancer-related and other conditions. GALE () NO DEBT
******GERN (T/$5) ... lead candidate, imetelstat, appears to have an important advantage over the leading treatment (Jakafi) for a rare form of blood cancer known as myelofibrosis, a disorder marked by overactive blood cell production in bone marrow. In a trial that enrolled 33 myelofibrosis patients, Geron's imetelstat generated a clinical response defined in part by an observed improvement on bone marrow damage among about 21% of patients. Furthermore, 12% experienced a complete remission defined by complete reversal of damage. GLYC (T/$11-21)...leukemia drug ...rocket because of FDA breakthrough designation... According to GlycoMimetics, data announced earlier this month showed high response rates, tolerability, and the first-ever clinical evidence supporting the mechanism of action for GM-1271. It works by disrupting a molecule called E-selectin, which allows circulating cancer cells to attach to healthy cells. The process plays a key role in metastasis and AKL, a cancer of the blood and bone marrow, in which cancer cells interrupt the production of healthy blood cells. Company had multiple programs in preclinical and clinical development focused primarily on the treatment of neurological disorders, with two lead programs in Alzheimer's disease. The Company has two transdermal platforms: Corplex for small molecules and MicroCor, a biodegradable microstructure technology for small molecules and biologics, including vaccines, peptides and proteins. Its late-stage pipeline includes a contraceptive patch, which has completed Phase III trials, and additional transdermal products that are being developed with other partners. Its products include Clonidine TDS, Fentanyl TDS and Crest Whitestrips. GPHT ...see med device...
****GTHX (T/$30) ... engages in the discovery, development, and commercialization of small molecules for the treatment of breast and prostate cancer, and other medical conditions. It focuses on the development of selective androgen receptor modulators (SARMs) for the treatment of breast cancer, stress urinary incontinence (SUI), and Duchenne muscular dystrophy (DMD); and selective androgen receptor degraders (SARDs) to treat progressive castration-resistant prostate cancer (CRPC). The company's lead SARM candidate is the enobosarm GTx-024, a Phase II open-label proof-of-concept clinical trial for patients with advanced androgen receptor (AR) positive triple-negative breast cancer; and estrogen receptor positive and AR positive advanced breast cancer, as well as postmenopausal women with SUI. HALO (T/$7) Halozyme Therapeutics, develops, and commercializes human enzymes and other drug candidates in the United States, Switzerland, and internationally. The company's human enzymes are used to facilitate the delivery of injected drugs and fluids, enhancing the efficacy and the convenience of other drugs or can be used to alter tissue structures for clinical benefit. Its products are based on the Enhanze technology, a patented recombinant human hyaluronidase enzyme (rHuPH20) that enables the subcutaneous delivery of injectable biologics, such as monoclonal antibodies and other therapeutic molecules, as well as small molecules and fluids. The company offers Hylenex recombinant, a formulation of rHuPH20 to facilitate subcutaneous fluid administration for achieving hydration; to enhance the dispersion and absorption of other injected drugs; in subcutaneous urography; and to enhance resorption of radiopaque agents. It also develops PEGylated recombinant human hyaluronidase for the treatment of pancreatic ductal adenocarcinoma, non-small cell lung cancer, gastric cancer, and metastatic breast cancer. ***IDRA 1.57 low (T/$4-8) cancer rare disease.. *****IMDZ (T/$16-18) ... a prime-boost vaccine approach against NY-ESO-1-expressing tumors, designed to generate an integrated, anti-NY-ESO-1 immune response in vivo via a targeted, specific interaction with dendritic cells, a mechanism of action Immune Design believes differs from traditional cancer vaccines. IMGN (T/$9-11) ImmunoGen, Inc. is a clinical-stage biotechnology company that develops targeted cancer therapeutics using its antibody-drug conjugate (ADC) technology. The Company is engaged in the discovery of monoclonal antibody-based anticancer therapeutics. An ADC with the Company's technology comprises an antibody that binds to a target found on tumor cells conjugated to one of its anti-cancer agents as a payload to kill the tumor cell once the ADC has bound to its target. The company's product candidates include mirvetuximab soravtansine, an ADC targeting folate-receptor alpha for the treatment of platinum-resistant ovarian cancer; IMGN529, an ADC that is in Phase 1b/2 clinical trials for B-cell malignancies; coltuximab ravtansine, which is in Phase 2 trials for B-cell malignancies; IMGN779 that is in Phase 1 clinical trials for the treatment of acute myeloid leukemia (AML); and IMGN632, a preclinical CD123-targeting ADC for the treatment of hematological malignancies, including AML ***IMMU (T/$15-16)
monoclonal antibody-based products for the targeted treatment of cancer, autoimmune disorders, and other diseases. 12/07 potential treatment for metastatic triple-negative breast cancer (mTNBC). Data from the 110 patient study showed that sacituzumab govitecan elicited an objective response rate of 31%, with a median duration of response of 9.1 months. That's much better than the 10% to 15% response rate that current standard-of-care therapies provide. *****IMNP ()
Immune Pharmaceuticals findings showing that treatment of mice with Ceplene® efficiently reduces the formation of lung melanoma metastases. Using genetically modified mice, the authors also show that the beneficial effect of treatment with Ceplene® in reducing metastasis was likely explained by its inhibitory effect on NOX2, which is an enzyme expressed by myeloid blood cells. In the article, the investigators additionally report that combination immunotherapy with Ceplene® and interleukin-15 (IL-15) reduced melanoma metastasis by over 70%, an incremental effect over Ceplene alone or IL-15 alone. **INFI (T/$2H) treatment of blood cancers... ***IOVA (T/$11-16) ...
focuses on developing and commercializing cancer immunotherapy products to harness the power of a patient's immune system to eradicate cancer cells. The company's lead product candidate is LN-144, an adoptive cell therapy that is in Phase II clinical trial using tumor-infiltrating lymphocytes (TIL), which are T cells derived from patients' tumors for the treatment of patients with refractory metastatic melanoma. It is also developing LN-145 to treat cervical and head and neck cancers. The company has a patent license agreement with the National Institutes of Health for technologies relating to autologous TIL adoptive cell therapy products for the treatment of metastatic melanoma, lung, breast, bladder, and HPV-positive cancers; cooperative research and development agreement with the National Cancer Institute to develop adoptive cell immunotherapies that are designed to destroy metastatic melanoma cells using a patient's TIL, as well as for the treatment of cervical, head and neck, lung, bladder, and breast cancer; and manufacturing services agreement with Lonza Walkersville, Inc. and WuXi Apptech, Inc. to manufacture, package, ship, and handle quality assurance and quality control of clinical trials for TIL products. In addition, it has collaboration and license agreements with Medimmune, Inc. to conduct clinical and preclinical research in immuno-oncology; H. Lee Moffitt Cancer Center and Research Institute to research and develop adoptive TIL cell therapy; PolyBioCept, AB to develop, manufacture, market, and genetically engineer TIL; and the University of Texas MD Anderson Cancer Center for multi-arm clinical trials for TIL therapy. ****ITUS (T/$).... Sept. 2017 issued U.S. Patent early cancer detection technology. "ITUS's unique liquid biopsy approach utilizes flow cytometry to measure the presence and characteristics of certain circulating immune cells. We then use Artificial Intelligence to analyze the data in a manner that enables us to identify tumor bearing patients. The test utilizes a simple blood draw from the patient. To date, our technology has demonstrated its ability to identify 15 cancer types including breast, prostate, colon, lung, pancreatic, and others. Because we are measuring subtle changes in circulating immune cells, it is not surprising that our technology appears to work on multiple cancer types, and we expect the technology will eventually be able to identify all cancer types. ITUS is performing additional tests including the evaluation of benign conditions which may exist in patients but are not malignant tumors." KPTI ....09/20/17... PHASE 2 SUCCESS...
“Liposarcomas are difficult to treat solid tumors that arise from the body’s fat tissue cells or their precursors,” said Sharon Shacham, PhD, MBA, President and Chief Scientific Officer of Karyopharm. “Dedifferentiated liposarcoma is an aggressive form of the disease that is resistant to both standard chemotherapy and radiation and has a particularly high rate of recurrence following surgery. Most patients who progress following surgery will ultimately succumb to their disease, highlighting the significant unmet need that exists for novel therapies. The FDA has confirmed their acceptance of the proposed Phase 3 SEAL study design, including the PFS primary endpoint, and agreed that positive results from this study could support regulatory approval in this patient population.” ***IOVA (T/11-16)
focuses on developing and commercializing cancer immunotherapy products to harness the power of a patient's immune system to eradicate cancer cells. The company's lead product candidate is LN-144, an adoptive cell therapy that is in Phase II clinical trial using tumor-infiltrating lymphocytes (TIL), which are T cells derived from patients' tumors for the treatment of patients with refractory metastatic melanoma. It is also developing LN-145 to treat cervical and head and neck cancers. The company has a patent license agreement with the National Institutes of Health for technologies relating to autologous TIL adoptive cell therapy products for the treatment of metastatic melanoma, lung, breast, bladder, and HPV-positive cancers; cooperative research and development agreement with the National Cancer Institute to develop adoptive cell immunotherapies that are designed to destroy metastatic melanoma cells using a patient's TIL, as well as for the treatment of cervical, head and neck, lung, bladder, and breast cancer KMPH (T/$8) ADHD
IOVA (T/$13)
KPTI (T/$15) Karyopharm's blood cancer hopeful. Selinexor is a first-in-class oral treatment slated for a large, late-stage combination study with multiple myeloma patients also receiving Velcade and standard chemo. If successful, results from the pivotal trial could eventually support the company's first New Drug Application, KURA (T/$13)
Developing in-licensed protein inhibitors for solid tumors and blood cancers. United States Patent and Trademark Office has issued a patent protecting the company's lead product candidate, tipifarnib, which is currently being studied in multiple Phase 2 clinical trials. Tipifarnib is an inhibitor of farnesylation, a key cell signaling process implicated in cancer initiation and development, and is under investigation in multiple ongoing clinical trials. Tipifarnib has demonstrated encouraging preclinical and clinical activity, including durable partial responses, in an ongoing Phase 2 clinical trial in patients with HRAS mutant SCCHN. Additional information about this clinical trial can be found at clinicaltrials.gov. MGNX (T/$26)
MRTX (T/$18)
MRUS in Phase 1/2 across three different indications — breast, colorectal and ovarian cancer — and based on data MTRX (T/$18)
oncology products. The company's clinical stage product candidates include glesatinib, an orally-bioavailable, potent, small molecule kinase inhibitor that is in Phase II clinical trials for the treatment of non-small cell lung cancer (NSCLC) patients with genetic alterations of MET; and in Phase Ib clinical trials in patients with genetic alterations of MET and Axl in NSCLC and other solid tumors. Its clinical stage product candidates also comprise sitravatinib, an orally-bioavailable, potent, small molecule spectrum-selective kinase inhibitor, which is in Phase II clinical trials for the treatment of solid tumors, such as NSCLC and metastatic Renal Cell Carcinoma, as well as in Phase Ib clinical trials to treat NSCLC patients with RET, CHR4q12, CBL, and AXL genetic alterations; and mocetinostat, an orally administered spectrum-selective Class 1 histone deacetylase inhibitor, which is in Phase Ib/II clinical trials in combination with durvalumab for the treatment of patients with NSCLC. The company has a collaboration agreement with Foundation Medicine, Inc. and Guardant Health, Inc. to explore development of their platforms as companion diagnostics for glesatinib. 
MTEM (T/$10) ..
Molecular Templates,...
development, and commercialization of immunotoxins called engineered toxin bodies for the treatment of cancer and other serious diseases. It develops MT-3724, a lead drug candidate that is in a Phase 1 clinical trial in heavily pre-treated non-Hodgkin's lymphoma patients; MT-4019, a preclinical drug candidate targeting CD38; and evofosfamide, an investigational hypoxia-activated prodrug of a bis-alkylating agent that is preferentially activated under severe hypoxic tumor conditions, a feature of many solid tumors. NAVB... Lymphoseek, a receptor-targeted small-molecule radiopharmaceutical used in the evaluation of lymphatic basins that may have cancer involvement in patients; and Manocept platform to target the CD206 mannose receptor expressed on activated macrophages. *OBMP () Bio cancer vaccines... **OMED 3.20 low (T/$6) (fv $6.68)OncoMed Pharm...anti-cancer stem cell (CSC) and immuno-oncology therapeutics... Lung...Ovarian...Colon... OPK (T/$11) Prostate cancer test...Human groth hormone...
PGNX (T/$12-15)
Progenics Pharmaceuticals ...pipeline includes therapeutic agents designed to target cancer (AZEDRA and 1095); prostate specific membrane antigen (PSMA)-targeted imaging agents for prostate cancer (1404 and PyL), and imaging analysis tools. It also includes commercial product, RELISTOR (methylnaltrexone bromide) for opioid-induced constipation. AZEDRA is a radiotherapeutic product candidate in development as a treatment for malignant and/or recurrent pheochromocytoma and paraganglioma, rare tumors found in the adrenal glands and outside of the adrenal glands, respectively. RELISTOR is a treatment for opioid induced constipation. PyL is a clinical-stage, fluorinated PSMA-targeted Positron Emission Topography (PET) imaging agent for prostate cancer. PSMA TTC is a thorium-227 labeled PSMA-targeted antibody therapeutic. PBMD (T/$5-7)
Prima BioMed Ltd core technologies are based on the Lymphocyte-activation gene 3 (LAG-3) immune control mechanism, which is involved in regulation of the T cell immune response. Its product, IMP321, is in clinical development for the treatment of a range of cancer indications, such as Metastatic Breast Cancer. IMP321 is in Phase IIb trials for Metastatic Breast Cancer and is being studied in Phase I for the treatment of Metastatic Melanoma. The Company is also focused on development of other products, which include IMP701, which is a blocking anti-LAG-3 antibody for cancer and is in Phase I of its clinical trails, and CVac, which is a personalized immunocellular therapeutic investigated for the treatment of epithelial cancer. SRNE (T/$7-16-20)
Sorrento Therapeutics... focus is oncology, including the treatment of chronic cancer pain. It is also developing therapeutic products for other indications, including immunology and infectious diseases. Its products in the pipeline include Chimeric Antigen Receptor-T Cell (CAR-T) programs, resiniferatoxin (RTX), and biosimilar/biobetter antibodies clinical development programs. Its pipeline also includes preclinical fully human therapeutic monoclonal antibodies (mAbs), including biosimilars/biobetters, fully human anti-PD-L1 and anti-PD-1 checkpoint inhibitors derived from its G-MAB library platform, antibody drug conjugates (ADCs), bispecific antibodies (BsAbs), as well as CAR-T and Chimeric Antigen Receptor Natural Killer (NK) cells (CAR. NK) for adoptive cellular immunotherapy. primary therapeutic focus is oncology, including the treatment of chronic cancer pain. It is also developing therapeutic products for other indications, including immunology and infectious diseases. Its products in the pipeline include Chimeric Antigen Receptor-T Cell (CAR-T) programs, resiniferatoxin (RTX), and biosimilar/biobetter antibodies clinical development programs. Its pipeline also includes preclinical fully human therapeutic monoclonal antibodies (mAbs), including biosimilars/biobetters, fully human anti-PD-L1 and anti-PD-1 checkpoint inhibitors derived from its G-MAB library platform, antibody drug conjugates (ADCs), bispecific antibodies (BsAbs), as well as CAR-T and Chimeric Antigen Receptor Natural Killer (NK) cells (CAR. NK) for adoptive cellular immunotherapy. TROV (T/$4) Myeliod Leukemia ***SNDX (T$/20-27) ...pipeline of combination therapies in multiple cancer indications. Our lead candidate, entinostat, which was granted Breakthrough Therapy designation by the FDA following positive results from our Phase 2b clinical trial, ENCORE 301, being evaluated in a Phase 3 clinical trial for advanced hormone receptor positive, human epidermal growth factor receptor 2 negative breast cancer. Syndax is developing entinostat, which has direct effects on both cancer cells and immune regulatory cells, and SNDX-6352, an anti-CSF-1R monoclonal antibody, to enhance the body's immune response on tumors that have shown sensitivity to immunotherapy. Entinostat is being evaluated as a combination therapeutic in Phase 1b/2 clinical trials with Merck & Co., Inc. for non-small cell lung cancer and melanoma; with Genentech, Inc. for TNBC; and with Pfizer Inc. and Merck KGaA, Darmstadt, Germany, for ovarian cancer. SNDX-6352 is being evaluated in a single ascending dose Phase 1 clinical trial and is expected to be developed to treat a variety of cancers. ...( Motley Fool...Despite the breakout success of
checkpoint inhibitor antibodies in fighting a host of cancers, these highly touted treatments do have a serious drawback -- response rates vary widely across tumor types. In a best case, advanced Hodgkin lymphoma patients receiving an anti-PD-1 checkpoint inhibitor have exhibited response rates as high as 60%. More typical response rates for this new class of treatment, though, have hovered around 20% in head and neck, lung, and bladder cancers.
That's where a company like Syndax Pharmaceuticals and its oral, small-molecule product candidate entinostat could make a big splash. The treatment is part of the third wave of immuno-oncology drugs (behind checkpoint inhibitors and cell-based therapies). Entinostat's job, if you will, is to block the action of immune suppressor cells -- known as myeloid-derived suppressor cells -- as well as regulatory T cells, when used in combination with a checkpoint inhibitor. If it succeeds, it will act synergistically with checkpoint inhibitor antibodies to produce a more robust immune response. At present, Syndax is evaluating entinostat in several clinical trials for breast cancer, non-small-cell lung cancer, advanced melanoma, and ovarian cancer. While it's arguably unreasonable to assume that the drug will prove useful in the majority of these indications (based on the poor track record of experimental cancer therapies in general), it shouldn't need to hit on more than one of these conditions to become a major value driver for a company with a market cap of only $304 million. And if a positive readout happens to come through in the high-value breast cancer market, this developmental biotech should prove to be a true hidden gem.
URGN (T/$28)
Urogen Pharma is engaged in developing therapies designed to care for urological pathologies. Its lead product candidates include MitoGel and VesiGel. MiroGel is a sustained release formulation of the chemotherapy agent Mitomycin C for the treatment of low-grade upper tract urothelial carcinoma, an urothelial cancer in the upper tract. ...
VRML (T/$1.75) Varmillion bio-analytical solutions that help physicians to diagnose, treat, and enhance outcomes for women in the United States. It is developing novel diagnostic tests for gynecologic disease with focus on ovarian cancer. The company's products include OVA1, a blood test for the pre-surgical identification of women who are at high risk of having a malignant ovarian tumor ZYME (T/19)
Zymeworks lead product candidate, ZW25, is a bispecific antibody, which is being evaluated in an adaptive Phase I clinical trial. Its ZW33 is a bi-specific antibody that delivers a cytotoxic payload to cancers cells by binding to different epitopes (bi-paratopic targeting) of the overexpressed HER2 protein. It focuses on developing a pipeline of preclinical product candidates and discovery-stage programs in immuno-oncology and other therapeutic areas. It analyzes protein characteristics to develop the scope of protein engineering to tackle biological systems. Pain Relief The potential market is huge. Over 200 million prescriptions are filled each year for drugs to manage pain outside of the hospital setting. Another 100 million prescriptions are filled annually for managing chronic pain. Over 20 million Americans take prescription drugs for treating uremic pruritus. 07/31 FDA/ 08/03 earnings
ACRX ACRX (T/$7)Zalviso Phase 3 trial ..."Since its launch in the first half of 2016, ZALVISO's patient-focused design has garnered positive feedback from patients, who appreciate taking control over their pain, as well as from physicians who are pleased with the product's usability and the desired level of analgesia delivered. BDSI (T/$4-5)
BioDelivery Sciences International... Company develops pharmaceutical products aimed principally in the areas of pain management and addiction. The Company's products utilize the BioErodible MucoAdhesive (BEMA) drug delivery technology, a small, erodible polymer film for application to the buccal mucosa (the lining inside the cheek). The Company's United Sates Food and Drug Administration (FDA) approved product, ONSOLIS (fentanyl buccal soluble film), as well as its approved products BUNAVAIL (buprenorphine and naloxone buccal film) buccal film and BELBUCA (buprenorphine) buccal film, utilize BEMA technology. ***CARA (T/$21-32) ... Share dilution April... 5 million shares at $18...The company's most advanced product is CR845, an injectable kappa opioid receptor agonist intended for the treatment of acute pain. What makes CR845 special is that unlike traditional pain relief medications (opioids), CR845 targets the "kappa" receptor rather than the "mu" receptor. This means that the drug provides pain relief without many of the side effects associated with traditional opioid therapies such as nausea, vomiting, and respiratory depression. Moreover, because CR845 only acts in the periphery (it cannot enter the brain and spinal cord), the drug is unable to create any of the "euphoric" side effects of traditional opioid pain medications. In layman's terms, this means that CR845 has the potential to provide a non-addictive form of pain relief with fewer of the side effects associated with traditional therapy....its drug development pipeline isn't entirely devoted to cannabinoid or endocannabinoid-mimetic drugs. In fact, Cara Therapeutics, which is entirely clinical-stage at this point, is almost exclusively focused on developing CR845, a kappa opioid receptor agonist (KORA) that has nothing to do with cannabis. Cara's ties to cannabis relate to its preclinical research involving CR701, a cannabinoid receptor agonist that's designed to treat chronic pain. Considering that there were no documented overdose-related deaths from marijuana in 2016, compared to over 20,000 prescription opioid overdose deaths in 2015, there's a clear push to consider CB agonists as a possible solution to chronic pain. In rodent models, CR701 produced "hyperalgesia (sensitization of nerve ending to panful stimuli) and allodynia (painful perception of innocuous stimuli) comparable to human conditions." COLL (T/$15)
develops and commercializes abuse-deterrent products that incorporate its DETERx platform technology for the treatment of chronic pain and other diseases. It offers Xtampza, an oral formulation of oxycodone, for the management of pain. The company also develops Onsolis, a transmucosal immediate-release fentanyl film indicated for the management of breakthrough pain in cancer patients 18 years of age and older. The company is also developing COL-195, a hydrocodone for the treatment of chronic pain; COL-172, an oxymorphone for the treatment of chronic pain; COL-196, a morphine formulation to treat pain; and COL-171, a methylphenidate formulation for the treatment of attention deficit hyperactivity disorder. CORI (T/$10-16) As of September 30, 2016, the Company had multiple programs in preclinical and clinical development focused primarily on the treatment of neurological disorders, with two lead programs in Alzheimer's disease. The Company has two transdermal platforms: Corplex for small molecules and MicroCor, a biodegradable microstructure technology for small molecules and biologics, including vaccines, peptides and proteins. Its late-stage pipeline includes a contraceptive patch, which has completed Phase III trials, and additional transdermal products that are being developed with other partners. Corium has developed six marketed products in the prescription drug and consumer markets: Clonidine Transdermal Delivery System for hypertension, Fentanyl TDS for chronic pain and four Crest Advanced Seal Whitestrips products. The company has two proprietary transdermal technology platforms with applications in multiple drug categories and indications: Corplex(TM) and MicroCor(R) (7/11/2017) IMSYS (T/$14) sublingual fentanyl spray
KMPH (T/$7-13)
lead product candidates are an extended release d-threo-methylphenidate product for the treatment of ADHD; an IR formulation of KP201, a prodrug of hydrocodone and acetaminophen for the treatment of acute pain. ...also involved in developing a prodrug of hydromorphone for the management of pain; and KP303, a prodrug of quetiapine for the treatment of central nervous system disorders. ****TRVN (T/$8-9-10-14) intends to commercialize therapeutics for G protein coupled receptors. The company's central nervous system product pipeline includes Oliceridine, a small molecule G protein biased ligand, which is in Phase III clinical trials for patients experiencing moderate to severe acute pain where IV administration is preferred; and TRV734, a small molecule G protein biased ligand at the mu-receptor that is in Phase I clinical trials for the treatment of moderate to severe acute and chronic pain. Its product pipeline also comprises TRV250, a small molecule G protein biased ligand of the delta-opioid receptor, which is in preclinical development stage for the treatment refractory migraine. In addition, the company develops cardiovascular programs, such a TRV027, a peptide beta-arrestin biased ligand that targets the angiotensin II type 1 receptor and is in Phase IIb clinical trials for the treatment of acute heart failure in combination with standard diuretic therapy. ... ****REPH... (T/$12-21 )Recro Pharma, pharmaceutical company, engages in developing non-opioid products for the treatment of acute pain. *****PTIE ...(7?) Pain Therapeutics a safer oxycontin = FDA has provided the company with a clear path for Remoxy ER approval if the company completes two studies, one clinical and the other non-clinical. The company expects to successfully complete both clinical studies in 2017 and has the resources to do so. The company ended 2016 with $18.7M in cash and burns $3M/quarter.
EGLT... NKTR... INSY (T/$16-22)...see marijuana stocks near bottom of this page...
the potential applications for dronabinol, including sleep apnea and opioid dependency, could catapult this drug to annual sales in the billions. **IPCI (T/) pharmaceutical company,...patent on a way to control overdose of prescribed narcotic medications. KALV (T/$10) Phase III clinical trial designed to provide rapid sustained relief from pain associated with osteoarthritis... RDHL ...STML...blood cancers... AST spinal cord injuries and cancer ... *****FLXN (T/$38)
Flexion Therapeutics ...likely approval of the company's first commercial compound by the FDA on the October 6th PDUFA date. It should be approved for the treatment of osterarthritis of the knee and is in trials for two other indications. A recent study showed that Zilretta at a cost of $500 per treatment "had a greater impact on quality-of-life measures than conventional care, diclofenac (NSAID) and HA treatment". This is already a large market and growing as the developed world ages and as society grows more obese. Zika virus http://www.buyupside.com/sample_portfolios/ebola.php *BCRX (T/$6H)... XON(T$40)... INO (T/$13) biopharmaceutical, zika virus, Ebola...bladder cancer SNY...drug manufactures ***Medical Robotics The robotic-surgery space is forecast to explode at a compound annual growth rate of more than 22% over the next five years, making it one of the fastest-growing industries in the healthcare sector. Nov. 1,2016 Fool.com The National Spinal Cord Injury Statistical Center (NSCISC) estimates that there were 282,000 people in the U.S. with spinal cord injuries (abbreviated as SCI) as of 2016, with about 17,000 new cases per annum. Approximately 42,000 of these patients are veterans and the VA has the largest network of SCI facilities in the country. About 80% of SCIs occur among men, according to the NSCISC. From 2010 to 2015, motor vehicle crashes were the #1 cause of SCI cases. Sports injuries account for about 9% of SCI cases. https://seekingalpha.com/article/4110548-medical-robotics-update-m-strategic-alliances-drive-returns?ifp=1&utm_content=buffer46525&utm_medium=social&utm_source=twitter.com&utm_campaign=buffer
 CYBQY ...Exoskeleton Japan's Cyberdyne EKSO(T/$4H) ...Exoskeleton Ekso Bionics Holdings, Inc. designs, develops, and sells exoskeletons for use in the healthcare, industrial, military, and consumer markets in North America, Europe, the Middle East, and Africa. The company operates through Medical Devices, Industrial Sales, and Engineering Services segments. It primarily offers Ekso GT, a bionic suit that provides the ability to stand and walk over ground with a reciprocal gait using a cane, crutches, or a walker to individuals with spinal cord injuries, hemiplegia due to stroke, and lower limb paralysis or weakness. The company's Ekso device is primarily used in a clinic or rehabilitation setting. It also performs research and development work on human exoskeletons and related technologies.
CYBQY ...Exoskeleton Japan's Cyberdyne EKSO(T/$4H) ...Exoskeleton Ekso Bionics Holdings, Inc. designs, develops, and sells exoskeletons for use in the healthcare, industrial, military, and consumer markets in North America, Europe, the Middle East, and Africa. The company operates through Medical Devices, Industrial Sales, and Engineering Services segments. It primarily offers Ekso GT, a bionic suit that provides the ability to stand and walk over ground with a reciprocal gait using a cane, crutches, or a walker to individuals with spinal cord injuries, hemiplegia due to stroke, and lower limb paralysis or weakness. The company's Ekso device is primarily used in a clinic or rehabilitation setting. It also performs research and development work on human exoskeletons and related technologies.  Globus Medical ***(GMED), a leading musculoskeletal implant manufacturer, announced on January 8 that it has acquired Excelsius Surgical, a company that's developing a next generation surgical robotic positioning platform for spine, brain and therapeutic markets.
Globus Medical ***(GMED), a leading musculoskeletal implant manufacturer, announced on January 8 that it has acquired Excelsius Surgical, a company that's developing a next generation surgical robotic positioning platform for spine, brain and therapeutic markets. 
ISRG
 MYO (T/$9-12) Myomo, Inc., ...myoelectric braces (orthotics) designed to offer extended mobility for people with neurological disorders and upper limb paralysis... Myoelectric-controlled orthotics are externally powered braces, which can be controlled via electrical signals generated naturally by the patient's own muscles...Having raised gross proceeds of $8mn, which the company views as growth capital, Myomo has been focused on completing the next generation version of MyoPro® and establishing its distribution and presence for its go-to-market strategy. Management announced the launch of its next-generation version, MyoPro 2. MyoPro 2 is distinguished by its lighter design, improved grasping and sensor functionality, and interchangeable, extended-life rechargeable batteries for continuous daily use. With CE Mark approval, the company will target Europe, starting with Germany through its sales partnership with Ottobock, a well-established market leader in technical orthopedics and prosthetics (O&P). In the US, Myomo is filling out its sales and marketing teams with critical new hires, and added seven new MyoPro® Centers of Excellence - bringing the total to 31. The company also announced that it booked its first orders from a distribution agreement with Ottobock for Veterans Administration Medical Centers in the United States. a commercial stage medical robotics Company, provides expanded mobility solutions for patients suffering from neurological disorders and upper limb paralysis in the United States. The company develops and markets MyoPro, a myoelectric elbow/wrist/hand orthosis that supports an impaired hands and arms of individuals due to a brachial plexus injury, stroke, spinal cord injury, traumatic brain injury, amyotrophic lateral sclerosis, multiple sclerosis, and other upper limb neuromuscular deficits.
MYO (T/$9-12) Myomo, Inc., ...myoelectric braces (orthotics) designed to offer extended mobility for people with neurological disorders and upper limb paralysis... Myoelectric-controlled orthotics are externally powered braces, which can be controlled via electrical signals generated naturally by the patient's own muscles...Having raised gross proceeds of $8mn, which the company views as growth capital, Myomo has been focused on completing the next generation version of MyoPro® and establishing its distribution and presence for its go-to-market strategy. Management announced the launch of its next-generation version, MyoPro 2. MyoPro 2 is distinguished by its lighter design, improved grasping and sensor functionality, and interchangeable, extended-life rechargeable batteries for continuous daily use. With CE Mark approval, the company will target Europe, starting with Germany through its sales partnership with Ottobock, a well-established market leader in technical orthopedics and prosthetics (O&P). In the US, Myomo is filling out its sales and marketing teams with critical new hires, and added seven new MyoPro® Centers of Excellence - bringing the total to 31. The company also announced that it booked its first orders from a distribution agreement with Ottobock for Veterans Administration Medical Centers in the United States. a commercial stage medical robotics Company, provides expanded mobility solutions for patients suffering from neurological disorders and upper limb paralysis in the United States. The company develops and markets MyoPro, a myoelectric elbow/wrist/hand orthosis that supports an impaired hands and arms of individuals due to a brachial plexus injury, stroke, spinal cord injury, traumatic brain injury, amyotrophic lateral sclerosis, multiple sclerosis, and other upper limb neuromuscular deficits. 
MZOR

PH () Exoskeleton Parker Hannifin (NYSE:PH) operates the Indego exoskeleton device.

RWLK ()
Exoskeleton ReWalk Robotics ...
commercializes exoskeletons for wheelchair-bound individuals with mobility impairments or other medical conditions. The company offers ReWalk Personal for everyday use to paraplegic individuals at home and in their communities; and ReWalk Rehabilitation for exercise and therapy used in hospitals and rehabilitation centers in the United States and Europe. 
Rex Bionics (RXB.L) Exoskeleton
STXS


SYK...
December 2013, Michigan-based Stryker acquired MAKO Surgical Corporation and its RIO (Robotic Arm Interactive Orthopedic) system. MAKO is "an emerging medical device company that markets [an] advanced robotic arm solution, joint specific applications for the knee and hip, and orthopedic implants for orthopedic procedures." MAKO promoted its system as a minimally invasive approach to knee and hip replacement surgery and indicated that it offered substantial advantages to patients and doctors. 
TITXS

TRXC (T/$3-3.5-4)
Intuitive Surgical (NASDAQ: ISRG) won FDA approval for its da Vinci system. Fast forward to today, and there are now 4,271 da Vinci systems in active use worldwide. Management expects that those systems will be used to perform more than 860,000 procedures in 2017 alone. TransEnterix, whose original answer to the da Vinci was to develop a product called the SurgiBot. This robot had a much smaller footprint than a da Vinci, making it easier (at least in theory) to share the device between multiple operating rooms. The SurgiBot also promised to allow surgeons to do their work right next to the patient instead of from behind a console. When combined with an advanced camera, haptic feedback, and a price tag that was 66% less than the competition, TransEnterix was feeling good about SurgiBot's chance to disrupt the market. However, all of that thinking went out the window when the FDA rejected the SurgiBot. Management was left scrambling and decided to halt development of the SurgiBot altogether and throw their weight behind an acquired product call Senhance (or ALF-X at the time) instead.
TransEnterix successfully won FDA approval for the Senhance in the fall of 2017, and it started the commercialization process soon after. Like the SurgiBot, Senhance offers a number of advantages over the da Vinci. This includes haptic feedback, eye-sensing camera control, an open product architecture, and most importantly, fully reusable .instruments.
***Medical Equip *****AEMD (T/$3.00) medical device company (last reported earnings 02/01...Aethlon Medical, Inc. (AEMD), a therapeutic technology company focused on unmet needs in global health and biodefense, announced today that it has received an Expedited Access Pathway (EAP) designation from the United States Food and Drug Administration (FDA) to support the advancement of the Aethlon Hemopurifier® to treat life-threatening viruses. The FDA EAP program was established to facilitate more rapid patient access to breakthrough technologies with the potential to address life threatening disease conditions for which no approved or cleared treatment alternatives exist. The Company operates through two segments: Aethlon, which represents its therapeutic business activities, and ESI, which represents its diagnostic business activities. The Company's lead product is the Aethlon Hemopurifier, which is a device that selectively targets the elimination of circulating viruses and tumor-secreted exosomes that promote cancer progression. The Aethlon Hemopurifier sheds glycoproteins to treat infectious viral pathogens. In oncology indications, the Hemopurifier targets the removal of circulating exosomes, which are released to promote cancer progression and to seed the spread of metastasis. Through its subsidiary, Exosome Sciences, Inc. (Exosome), the Company is also developing exosome-based product candidates to diagnose and monitor neurological disorders and cancer. https://finance.yahoo.com/news/aethlon-medical-receives-expedited-access-113000571.html ALQA (T/$1.50) regenerative medical products that assist the body in the repair or replacement of soft tissue. It markets MIST Ultrasound Healing Therapy, a painless, noncontact, low-frequency ultrasound to promote healing; Biovance Amniotic Membrane Allograft and Interfyl Human Connective Tissue Matrix that are human biologic regenerative technologies; and TheraBond 3D Antimicrobial Barrier Systems that promotes an optimal wound healing by creating an antimicrobial barrier that helps protect against infection. The company also provides contract manufacturing services, including the development, manufacture, and market of high water content, electron beam cross-linked, aqueous polymer hydrogels, or gels used for wound care, medical diagnostics, transdermal drug delivery, and cosmetics. Alliqua BioMedical, Inc. has a license, marketing, development, and supply agreement with Anthrogenesis Corporation. ANGO (T/$21) APEN (T/$11-12) ...medical devices for the treatment of obesity. The company offers endo-bariatric products, such as Orbera intragastric balloon system, a non-surgical alternative for the treatment of overweight and obese adults; and OverStitch, an endoscopic suturing system that enables endoscopic procedures by allowing physicians to place full-thickness sutures and secure the approximation of tissue through an Olympus dual-channel flexible endoscope. It also provides surgical products, including Lap-Band system, a system designed to provide minimally invasive long-term treatment of obesity; and accessories used in laparoscopic bariatric surgeries. ARAY (T/$5-7) ...Accuray Incorporated designs, develops, and sells radiosurgery and radiation therapy systems for the treatment of tumors in the body. The company offers the CyberKnife System, a robotic stereotactic radiosurgery and stereotactic body radiation therapy system used for the treatment of various types of cancer and tumors in the body. The CyberKnife System automatically tracks, detects, and corrects for tumor and patient movement in real-time during the procedure, as well as enables the delivery of precise, high dose radiation while patients breathe normally. It also offers the TomoTherapy System, which consists of an integrated and versatile radiation therapy system used for the treatment of a range of cancer types. ATEC (T/$4) products for the surgical treatment of spine disorders. Its product portfolio and pipeline addresses the cervical, thoracolumbar, and intervertebral regions of the spine, as well as covers various spinal disorders and surgical procedures. The company offers cervical and cervico-thoracic products, including Trestle Luxe Anterior Cervical Plate System, Solanas Posterior Cervico/Thoracic Fixation System and Avalon Occipital Plate, and Pegasus Anchored Cervical Interbody system. It also provides thoracolumbar fixation products, such as Arsenal Degenerative System, Arsenal CBx Cortical Bone Fixation System, Zodiac Degenerative Spinal Fixation System, Zodiac Deformity Spinal Fixation System, and OsseoScrew Spinal Fixation System. In addition, the company offers spinal spacers comprising Battalion Universal Spacer System, Novel PEEK and Titanium Spinal Spacers, and Alphatec Solus Locking ALIF Spinal Spacer; and MIS Products consisting of Illico Minimally Invasive Surgery System and BridgePoint Spinous Process Fixation System. Further, it offers biologics, such as Neocore Osteoconductive Matrix, a synthetic scaffold for the regeneration of bone. ****ATRS (T/$4-5) Antares Pharma ompany develops, manufactures and commercializes therapeutic products using its drug delivery systems. Its subcutaneous injection technology platforms include VIBEX disposable pressure-assisted auto injector system suitable for branded and generic injectable drugs in unit dose containers, reusable needle-free spring-action injector devices, and disposable multi-use pen injectors for use with cartridges. The Company makes a reusable, needle-free spring action injection device, ZOMA-Jet or Twin-Jector, which is marketed through its partners for use with human growth hormone (hGH). The Company operates through drug delivery segment, which includes self-administered parenteral pharmaceutical products and technologies. ***BLFS
***BLFS (T/$5-7) H
ypothermic storage and cryopreservation solutions for cells and tissues in the United States. Its products are serum-free and protein-free solutions, which are formulated to reduce preservation-induced, delayed-onset cell damage, and death. The company offers HypoThermosol FRS, a hypothermic storage and shipping media product to mitigate temperature-induced molecular cell stress responses that occur during chilling and re-warming of biologics, intermediate products, and final cell products intended for research and clinical applications; and CryoStor cryopreservation freeze media products, which are designed to mitigate temperature-induced molecular cell stress responses during freezing and thawing. It also provides BloodStor freeze media products, such as BloodStor 55-5 and BloodStor 100 for cryopreservation of stem and other cells isolated from umbilical cord and peripheral blood, and bone marrow; and cell thawing media, which offers Dextran and saline for washing cryopreserved cells and tissues to dilute or remove cryoprotectants. In addition, the company provides custom product formulation and packaging services; contract aseptic manufacturing formulation, fill, and finish services of liquid media products; and precision thermal packaging products and cloud-hosted Web applications. It markets its products to the regenerative medicine, bio-banking, drug discovery markets, comprising hospital-based stem cell transplant centers, pharmaceutical companies, cord blood and adult stem cell banks, hair transplant centers, and suppliers of cells to the drug discovery, toxicology testing, and diagnostic markets. BTCY mobile ECG monitoring device and an ECG viewer software package CFMS (T/$6) joint replacement
*****CHEK (T/$6)
Check-Cap Ltd ... development of an ingestible imaging capsule that utilizes low-dose X-rays for screening of the colon to detect polyps, masses, and colorectal cancers. Its C-Scan system consists of C-Scan Cap, an X-ray scanning capsule, which is designed to measure, collect, and transmit structural information; C-Scan Track, a biocompatible unit that is designed to track the capsule and record imaging and positioning data; and C-Scan View, a personal computer-based software package, which is designed to retrieve and process clinical data from the C-Scan Track, and to reconstruct and produce 3D visualization of the colon's inner surface. Utilizing innovative ultra-low dose X-ray and wireless communication technologies, the capsule generates information on the contours of the inside of the colon as it passes naturally. This information is used to create a 3D map of the colon, which allows physicians to look for polyps and other abnormalities. Designed to improve the patient experience and increase the willingness of individuals to participate in recommended colorectal cancer screening... Colorectal cancer is the second leading cause of cancer death in the U.S., with an estimated 135,000 diagnoses and 50,000 deaths in 2017[1]. Despite compelling evidence that screening can detect colorectal cancer and precancerous polyps, nearly one-third of the recommended adult population has never been screened. CHFS ()
early-stage medical device company, develops cardiac/coronary disease products ultrafiltration treatment of patients with fluid overload who have failed diuretic therapy,... ultrafiltration (UF) in reducing hospital readmissions in acute decompensated heart failure (ADHF) patients. The positive results of this meta-analysis, which included CHF Solutions’ Aquadex FlewFlow® system, are consistent with previous publications that demonstrate the importance of considering Aquapheresis as an alternative therapy for diuretic-resistant, ADHF patients suffering from fluid overload. *****CTSO (T/$7-10-13) CytoSorb, which is a blood purification technology with focus in preventing or treating multiple organ failure. The Company's purification technologies are based on biocompatible, porous polymer beads that remove toxic substances from blood and other bodily fluids by pore capture and surface adsorption. The Company's CytoSorb is an extracorporeal cytokine filter and is designed to reduce the cytokine storm that causes inflammation, organ failure and death in common critical illnesses, such as sepsis, burn injury, trauma, lung injury and pancreatitis. In addition, CytoSorb is used in other inflammatory conditions, such as cardiac surgery and autoimmune disease flares and cancer cachexia. It also has other products under development based upon its blood purification technology, ...engages in the research, development, and commercialization of medical devices with its platform blood purification technology incorporating a proprietary adsorbent, porous polymer technology. Its principal product is CytoSorb device, an extracorporeal cytokine filter designed for the adjunctive therapy in the treatment of sepsis; adjunctive therapy in other critical care applications; prevention and treatment of post-operative complications of cardiopulmonary bypass surgery; and prevention and treatment of organ dysfunction in brain-dead organ donors to increase the number and quality of viable organs harvested from donors. 
CVR Medical (TSX:CVM.V; OTC:CRRVF), and its potentially life-saving device is the Carotid Stenotic Scan (CSS)--a technology designed to detect stenosis within arteries, or Ischemia, which is the leading indicator of strokes.Of the 15 million people who suffer stroke every year, some 6 million are killed, while 5 million are rendered permanently disabled, according to the World Heart Foundation. But there has been no cost-effective way to screen for Ischemia. So, when a biotech company offers a potential solution to help prevent the second-leading cause of death in the world--and then releases positive preliminary clinical trial results, investors listen. Now it’s hoping to charge out of the gate and take the market by storm once it manages to gain FDA market clearance.
In the meantime, the catalysts are really lining up:
On 7 September, CVR released updated results from its preliminary clinical trial that showed forward progress for the medical device, which we’ve been watching closely for some time. You can view the results from Thomas Jefferson University here.
And a few weeks prior, they announced another landmark achievement when they signed a letter of intent to manufacture the CSS. The deal gives CVR state-of-the-art manufacturing capabilities with Canon Virginia, Inc (CVI). This would give CVR immediate scalability, and also adds to its credibility: It’s not the first manufacturing deal the company has sealed.Our researchers are keeping a close eye on CVR Medical Corp. because we think this is the turning point. Here’s why:
• It’s had two fresh catalysts in three weeks in an industry that is all about validation
• It’s developing a device that meets critical demand in terms of helping to prevent one of the most debilitating and deadly diseases of our time
• It’s developing a medical device that actually makes sense to the market
• It’s been doing it quietly, without blockbuster hype, and instead painstakingly going for real validation, making it the real deal.
Here are 5 reasons to keep a close eye on CVR Medical (TSX:CVM.V; OTC:CRRVF) in the coming weeks and months:
#1 Catalysts are Lining Up, and News Flow is Gaining Momentum
First, one way to look at the CSS is to think about what 3D seismic imagery did for super quick discoveries in the oil and gas industry. This is exactly what CVR’s sensory system could do for the medical industry in terms of detecting critical stroke symptoms.
This is how it works:
CVR’s Carotid Stenotic Scan (CSS) is a tool to detect blockage within the Carotid Arteries, potentially offering patients and caregivers a device for early detection in a quick and repeatable manner.
CVRS (T/$3) Corindus Vascular Robotics, designs, manufactures, and sells robotic-assisted precision vascular intervention systems for use in interventional vascular procedures. The company offers CorPath system, a medical device with robotic-assisted precision for radial, coronary, and peripheral procedures. Its CorPath system facilitates stent positioning for PCI procedures by allowing a physician to measure, manipulate, and advance devices with robotic precision; and CorPath GRX system enables the precise, robotic-assisted control of coronary guide catheters, guidewires, and balloon/stent devices from the safety of a radiation-shielded interventional cockpit. ELGX (T/5) Endologix, Inc. develops, manufactures, markets, and sells medical devices for the treatment of abdominal aortic aneurysms in the United States and internationally. It offers minimally-invasive endovascular repair (EVAR) products, including AFX (Anatomical Fixation) endovascular AAA system, which is a minimally invasive delivery system; VELA Proximal Endograft, which is designed for the treatment of proximal aortic neck anatomies with AFX; and the ovation abdominal stent graft system. The company also provides endovascular sealing (EVAS) product that is based on the Nellix EVAS system to seal the aneurysm, and provides blood flow to the legs through two blood lumens. In addition, it offers proximal aortic extensions and limb extensions, which attach to the main body of its EVAR device, allowing physicians to customize it to fit the patient's anatomy; and accessories to facilitate the optimal delivery of its EVAR products, including compatible guidewires, inflation devices, and snares. http://oilprice.com/Energy/Energy-General/Could-This-Be-The-Biggest-Biotech-Breakthrough-Of-The-Year.html
*EDAP High Intensity Focused Ultrasound (HIFU)(kidney stones, and Urology Devices and Services. AMDA(T/$1.50) ISRG Intuitive Surgical ... ****ERBA diagnostic test kits automated systems that are used to aid in the detection of disease markers primarily in the areas of autoimmune, infectious diseases, clinical chemistry, hematology, and diabetes testing... EYES (T/$5) bionic eye...WE Are Borg...you will be assimilated... GNMK (T/$13-17) ....Company
provides XT-8 instrument, and related diagnostic and research tests, as well as certain custom manufactured reagents that enable reference laboratories and hospitals to support a range of molecular tests with a workstation and disposable testEYES (T/$5) bionic eye...WE Are Borg...you will be assimilated...cartridges. It offers diagnostic tests for use with its XT-8 system that includes respiratory viral panel, cystic fibrosis genotyping test, warfarin sensitivity test, and thrombophilia risk test, as well as HCV genotyping test and associated custom manufactured reagents, and 2C19 genotyping test. The company also provides ePlex instrument and RP panel, which integrates automated nucleic acid extraction and amplification with its eSensor detection technology to enable operators using ePlex system to place patient sample directly into its test cartridge and obtain results without any additional steps. HTGM (T/$6) 09/20 2 PATENTS...https://finance.yahoo.com/news/htg-announces-issuance-two-method-120000387.html...
instruments molecular profiling applications ...mRNA biomarkers... ICAD (T/$11) *****IVTY (T/$10-13)
PhotonBlade represents a new technology platform for Invuity, delivering directed, thermally cool illumination at the precise point of surgical treatment. PhotonBlade’s novel energy design consists of a proprietary insulated blade for precise cutting and coagulation of tissue, with minimal effect on adjacent tissue. This sophisticated illumination technology does not require a proprietary generator, thereby leveraging widely available operating room equipment and avoiding an institutional capital investment. LMAT () LaMairtre Vascular -- a medical device maker that is profitable, growing quickly, debt-free, and has been a multibagger since its IPO in 2007.
As its name suggests, LaMaitre is a provider of
medical products that are primarily used by vascular surgeons. The company's strategy is to buy or build niche products that can be used to treat peripheral vascular disease (PVD) -- a blood circulation disorder that affects more than 200 million people worldwide. The disease is caused by the buildup of fatty deposits inside blood vessels that can restrict blood flow, which can lead to a range negative outcomes including organ damage, loss of limbs, and even death.
One way to treat PVD is through elective surgery, which is where LaMaitre comes into the picture. The company sells 15 different lines of products used during vascular surgery -- think shunts, grafts, patches, and catheters. What most of its products have in common is that they target niche opportunities. In fact, 97% of the company's revenue comes from segments that rack up less than $125 million in total annual sales. Markets that small typically don't interest the big boys much, which helps to limit LaMaitre's competition, allowing it to capture market share more easily, and to push through regular price increases.
Looking ahead, investors should feel confident that LaMaitre can continue to grow its top line as it adds more sales reps, expands geographically, and makes occasional tuck-in acquisitions. When those factors are combined with its steady margin improvement, planned price increases, and stock buybacks, the company looks to stand a good chance at continuing to deliver on its stated targets of 10% revenue growth and 20% operating income growth annually. If it does, I could easily see its stock continuing to outperform from here. That makes LaMaitre a hidden gem that all growth-focused healthcare investors should get to know. Motley Fool
*****MBOT MicroBot Medical... U.S. Patent and Trademark Office (USPTO) has issued a Notice of Allowance for a key technology patent. The Patent App. No. 14/348,610, covers a device for the prevention of shunt stenosis. Micro-robotic technologies... Self Cleaning Shunt (SCS) for the treatment of hydrocephalus and normal pressure hydrocephalus (NPH); and a self-propelling, semi-disposable endoscope that is being developed initially for use in colonoscopy procedures. **MZOR (T/30H)...Mazor Robotics http://www.fool.com/investing/general/2016/05/28/does-mazor-robotics-deal-with-medtronic-make-it-2.aspx NVCR ...wearable electric field device for treating glioblastoma brain cancer...proprietary therapy called TTFields for the treatment of solid tumor cancers. NovoCure believes it will establish TTFields as an important new treatment modality for a variety of solid cancer tumors by increasing survival without increasing side effects when used in combination with other modalities.
NUVA () Minimally invasive spinal surgery MDT... JNJ...PHG...SYK...ZBH PAVM (T/$10)
The company's product pipeline includes NextFlo, a disposable infusion pump; PortIO, a long-term implantable vascular access device; Caldus, a disposable tissue ablation device; CarpX, a percutaneous device to treat carpal tunnel syndrome; and NextCath, a self-anchoring short-term catheter. The company was formerly known as PAXmed Inc. and changed its name to PAVmed Inc. ******TRXC (T/$2) commercialization of surgical robotic systems company has moved it focus from surgibot to senhance ... http://www.bizjournals.com/triangle/news/2017/06/16/eyeing-fda-approval-transenterix-makes-first.html?ana=yahoo&yptr=yahoo TITXF () robotic surgical system for application in minimally invasive surgery... STXS robotic systems and instruments for the treatment of abnormal heart rhythms...“The Niobe system is perhaps the most revolutionary invention in the field of cardiac ablation, enabling remote anatomic mapping of electrical currents and eradication of difficult-to-reach arrhythmias with unparalleled safety and pinpoint precision,” said Tung. “Our acquisition of Stereotaxis’ latest generation remote magnetic navigation platform will enhance a highly respected electrophysiology (EP) program that is regarded as a global leader in ablation therapy, research and education, especially in the treatment of ventricular tachycardia (VT), and will greatly improve patients’ lives.” NVIV () ...
InVivo Therapeutics... focus on developing and commercializing biopolymer scaffolding devices for the treatment of spinal cord injuries (SCI). It is developing Neuro-Spinal Scaffold implant, an investigational bioresorbable polymer scaffold for acute SCI; and Therapeutic Trails injection program for the treatment of chronic SCI. NVTR (T/$10H) CyBorg technology...neuroscience... ***MLSS (T/$4)
STA Single Tooth Anesthesia Instrument, a computer-controlled local anesthesia delivery instrument that incorporates the pressure feedback elements of its patented CompuFlo technology, which allows dentists to administer injections into the periodontal ligament space; and CompuDent, a computer-controlled local anesthetic delivery instrument that provides painless injections for various routine dental treatments, including root canals, crowns, fillings, and cleanings. Its products also comprise CompuMed, a computer-controlled injection instrument for use in various applications, such as colorectal surgery, podiatry, dermatology, cosmetic surgery, hair restoration surgery, nasal and sinus surgery, orthopedics, and others. In addition, the company provides The Wand, a hand piece that allows bi-directional rotation during injection, which prevents needle deflection that occurs with a traditional syringe. RWLK RWLKRobotics exoskeleton ReWalk Robotics Ltd. develops, manufactures and markets wearable robotic exoskeletons for individuals with spinal cord injury. ReWalk’s mission is to fundamentally change the quality of life for individuals with lower limb disability through the creation and development of market leading robotic technologies. The Company offers ReWalk, which is an exoskeleton that uses its tilt-sensor technology and an on-board computer and motion sensors to drive motorized legs that power movement. ReWalk designs are intended for people with paraplegia, a spinal cord injury resulting in complete or incomplete paralysis of the legs, having the use of their upper bodies and arms. www.rewalk.com EKSO (T/$5)...COMPETITION...Exoskeleton .I-Hub Board
https://investorshub.advfn.com/ReWalk-Robotics-Ltd-RWLK-28903/ 
SIEN (T/$14-15)
Sientra, Inc., a medical aesthetics company, develops and sells medical aesthetics products to plastic surgeons and patients in the United States. The company offers a portfolio of silicone gel breast implants for use in breast augmentation and breast reconstruction procedures; and breast tissue expanders. It also provides body contouring and other implants, including gluteal, pectoral, calf, facial, and nasal implants, as well as nasal stents; silicone elastomer oval carving blocks to treat deformity caused by trauma, congenital and other deformities, or cancer therapy; scar management specialty products under the Medgel brand to treat various types of scars; breast sizers to identify the style and size of implants; and non-breast tissue expanders for expanding tissue and skin surface area for burn care and other reconstructive use. SPNE (T/$15)
SeaSpine Holdings...f
ocuses on the design, development, and commercialization of surgical solutions for the treatment of spinal disorders in the United States and Internationally. The company provides orthobiologics and spinal fusion hardware solutions for the neurosurgeons and orthopedic spine surgeons to perform fusion procedures in the lumbar, thoracic, and cervical spine. Its orthobiologics products include demineralized bone matrices, collagen ceramic matrices, demineralized cancellous allograft bone products, and synthetic bone void fillers to enhance bone fusion rates in a range of orthopedic surgeries, including spine, hip, and extremities procedures. The company offers its orthobiologics products in various forms, such as putties, pastes, and strips. Its spinal fusion hardware products comprise products for spinal fusion in minimally invasive surgery, complex spine, deformity, and degenerative procedures throughout the lumbar, thoracic, and cervical regions of the spine. TROV (T/$4) molecular diagnostic technology for use in disease detection and monitoring across a range of medical disciplines. Its primary internal focus is to leverage its cell-free molecular diagnostic platform to facilitate improvements in the field of oncology, while its external focus includes entering into license agreements or collaborations to develop its technology in areas, such as infectious disease, transplant medicine and prenatal genetics. It has ongoing clinical collaborations to demonstrate the ability to determine and monitor mutational status and response to therapy in lung, colon, pancreatic and skin cancer. It uses its molecular diagnostic technology for the detection of cell-free deoxyribonucleic acid (DNA) originating from diseased cell death that can be isolated and detected from urine, blood and tissue samples to develop disease management. VIVE (T/$11)
Viveve, Inc., designs, develops, manufactures, and markets medical devices for the non-invasive treatment of vaginal laxity. It provides Viveve system, a non-invasive treatment for vaginal laxity that occurs during natural childbirth. XTNT (T/$1.50)
biomaterial products include OsteoSponge provides a natural scaffold for cellular in-growth and exposes bone-forming proteins to the healing environment; OsteoSponge SC to fill bony defects in the subchondral region of joints; OsteoSelect DBM Putty for osteoinductive bone growth; OsteoSelect PLUS DBM putty for use as a bone void filler and bone graft substitute in the pelvis, extremities, and posterolateral spine; and OsteoWrap that wraps around non-union fractures to assist with fusion, as well as could be used in conjunction with a hardware plate system. The company provides BacFast HD facet stabilization dowels; OsteoSTX for posterolateral spine surgery applications, including scoliosis procedures; hMatrix dermal scaffold, an acellular matrix made from donated human dermal tissue that is used to replace a patient's damaged tissue; and 3Demin, a family of allografts that maximizes osteoconductivity and the osteoinductive potential of human bone. Other Medical AEZS (T/$4) 07/19 FDA approval for Adult Growth Hormone
ALQA (T/$1.5)
AMDA (T/$2-5 )biomaterials, Valeo silicon nitride spinal fusion devices.(additional shares/dilution Jan 2017 $0.51... ARDX (T/$12) lead product candidate is tenapanor, which is in Phase III clinical trial for the treatment of patients with irritable bowel syndrome with constipation, as well as in Phase III clinical trial for the treatment of hyperphosphatemia in end-stage renal disease patients on dialysis. The company is also developing RDX7675, which is in Phase III clinical trial for the treatment of people with hyperkalemia; and RDX8940 in Phase I clinical trial for the treatment of patients with NASH and other gastrointestinal indications. Its drug candidates in the stages of research and development include RDX013 for the treatment of patients with hyperkalemia; RDX011 for the discovery and development of second-generation NHE3 inhibitors; and RDX023 for the discovery and development of gut-biased FXR agonists for the treatment of GI and inflammatory diseases. ARNA (T/$5) ulcerative colitis 08/09AMC ATHX ($7-9)... focuses on the research and development activities in the field of regenerative medicine. Its clinical development programs are focused on treating neurological conditions, cardiovascular diseases, inflammatory and immune disorders, and pulmonary and other conditions. The company's lead platform product includes MultiStem cell therapy, an allogeneic stem cell product, which has completed Phase 2 study for treating patients suffering from moderate and severe ischemic stroke; that is in Phase 2 clinical study for treating patients with acute myocardial infarction; and, which is in Phase 1/2 clinical study for treating patients with acute respiratory distress syndrome, as well as completed Phase 1 clinical study for patients suffering from leukemia or various other blood-borne cancers. ATRA (T/$30)
BIOS (T/$3.50)
BLRX (T/$3)
BPMX (T/$3)
address dermatology and women's health markets. The company offers an OTC molecular iodine dietary supplement that addresses cyclic breast discomfort and for the alleviation of symptoms of fibrocystic breast condition (FBC). Its clinical-stage product candidates include BPX03, a molecular iodine tablet, which is in pre-Phase III clinical trial for the treatment of periodic breast pain associated with FBC and cyclic mastalgia; and BPX01, a topical antibiotic gel that is in Phase IIb clinical trial for the treatment of acne vulgaris. The company is also developing BPX02, an injectable product for aesthetic dermatology applications. CLDX biotech... CSLT (T/$5.50) ...operates a health benefits platform in the United States. ***CYTR() CytRx Corp...Soft tissue sarcoma is a cancer occurring in muscle, fat, blood vessels, tendons, fibrous tissues and connective tissue. It can arise anywhere in the body at any age. STS remains a high unmet medical need because of the difficulty in treating the more than 50 types of this aggressive cancer. According to the American Cancer Society, in 2016 more than 12,300 new cases were diagnosed in the U.S. and approximately 5,000 Americans died from this disease. In addition, approximately 40,000 new cases and 13,000 deaths in the U.S. and Europe are part of a growing underserved market. Recently, the company announced the U.S. Food and Drug Administration reached an agreement with CytRx on preparations for a New Drug Application submission for aldoxorubicin in soft tissue sarcomas. 2017 American Society of Clinical Oncology (ASCO) Annual Meeting...the data presented at ASCO this year demonstrate that, unlike any other drugs in this class, aldoxorubicin can be dosed continuously with minimal to no cardiotoxicity," commented Sant Chawla, M.D., F.R.A.C.P., Director of the Sarcoma Oncology Center in Santa Monica, California, and Principal Investigator for the Phase 3 trial. https://finviz.com/chart.ashx?t=CYTR&ty=c&ta=1&p=d&s=l EXEL Exelixis biotech... EYEG (T/$6) EyeGate is developing products using CMHA-S, a modified form of the natural polymer hyaluronic acid (HA), which possesses unique physical and chemical properties such as hydration and healing properties. The ability of CMHA-S to adhere longer to the ocular surface, resist degradation and protect the ocular surface makes it well-suited for treating various ocular surface injuries. EGP-437, EyeGate’s other product in clinical trials, incorporates a reformulated topically active corticosteroid, Dexamethasone Phosphate that is delivered into the ocular tissues through EyeGate’s proprietary innovative drug delivery system, the EyeGate II Delivery System.
GLMD (T/$14) Galmed Pharmaceuticals Ltd., a clinical-stage biopharmaceutical company, focuses on the development of therapeutics for the treatment of liver diseases. The company is developing Aramchol, an oral therapy, which is in ARREST study, a Phase IIb clinical study for the treatment of patients with overweight or obesity, and who are pre-diabetic or type-II-diabetic with non-alcoholic steato-hepatitis. It is also evaluating Aramchol through ARRIVE Study, a proof-of-concept Phase IIa clinical trial, with HIV-associated non-alcoholic fatty liver disease and lipodystrophy. GTHP ...a 0.03cent stock get a lot of volume ....
Guided Therapeutics, Inc., a medical technology company, focuses on developing medical devices. The company focuses on marketing LuViva, a non-invasive cervical cancer detection device that identifies cervical cancers and precancers painlessly, non-invasively, and at the point-of-care by scanning the cervix with light, then analyzing the light reflected and fluorescent light. It is also developing a non-invasive esophageal cancer detection product using its biophotonic technology platform ***HLTH (T/$2.50)...outpatient surgery facilities... MPW (T/$15) Medical Properties RIET
NK NantKwest Inc. (NK), a pioneering, next generation, clinical-stage immunotherapy company focused on harnessing the unique power of our immune system using natural killer (NK) cells to treat cancer, today announced that the first patient has been dosed in a first-in-human, Phase I clinical study in glioblastoma of HER2.taNK, a novel, natural killer cell-based immuno-oncology therapy using CAR technology in patients. NEOS Neos Therapeutics ADHA drugADHA drug approved June 19th 2017...... ... PSDV (T/$5) chronic eye diseases
KURA (T/$16) ...protein inhibitors for solid tumors and blood cancers...
SMED
SNOA (T/$10) ...
markets prescription and over-the-counter products based on its Microcyn platform technology for the dermatology, surgical, advanced tissue, skincare, and animal healthcare markets in the United States, Latin America, Europe, and rest of the world. The Microcyn platform technology is based on electrically charged oxychlorine small molecules, which are designed to target a range of pathogens that cause disease, including viruses, fungi, spores, and bacteria, such as antibiotic-resistant strains. The company's Microcyn has received fourteen 510(k) clearances for use as a medical device in tissue care management consisting of acute and chronic wounds, and in dermatology applications. TXMD (T/$10-15)
BIG short interest 08/25...$5.93) ...
manufactures and distributes prescription and over-the-counter product lines, including prenatal vitamins, iron supplements, and natural menopause relief products under the vitaMedMD brand, as well as generic formulations of its prescription prenatal vitamins products under the BocaGreenMD Prena1 name. Its pipeline of hormone therapy drug candidates include TX-001HR, a combination of estradiol and progesterone drug candidate under clinical trials for the treatment of moderate to severe vasomotor symptoms due to menopause; TX-002HR, a natural progesterone formulation for the treatment of secondary amenorrhea without the potentially allergenic component of peanut oil; and TX-004HR, an applicator-free vaginal estradiol softgel drug candidate for the treatment of vulvar and vaginal atrophy in post-menopausal women with vaginal linings that do not receive enough estrogen. XTNT (T/$1.50)
OsteoSponge that provides a natural scaffold for cellular in-growth and exposes bone-forming proteins to the healing environment; OsteoSponge SC to fill bony defects in the subchondral region of joints; OsteoSelect DBM Putty for osteoinductive bone growth; OsteoSelect PLUS DBM putty for use as a bone void filler and bone graft substitute in the pelvis, extremities, and posterolateral spine; and OsteoWrap that wraps around non-union fractures to assist with fusion, as well as could be used in conjunction with a hardware plate system. The company also provides BacFast HD facet stabilization dowels; OsteoSTX for posterolateral spine surgery applications, including scoliosis procedures; hMatrix dermal scaffold, an acellular matrix made from donated human dermal tissue that is used to replace a patient's damaged tissue RIGL ...
OMER (T/$15H-22H)...FDA Breakout designation for IgA nephrophy
IMGN (T/$6)
Alt-Energy ***ARTX artx (T/$5)defense and security products sells rechargeable and primary batteries, and smart chargers to the military... CPST ($1-2)
Capstone Turbine markets and services microturbine technology solutions for use in stationary distributed power generation applications, including cogeneration (combined heat and power), integrated combined heat and power (ICHP), and combined cooling, heat and power (CCHP), renewable energy, natural resources and critical power supply. The Company's microturbines are used as battery charging generators for hybrid electric vehicle applications. Capstone offers micro turbines for commercial, industrial and utility users with product offerings ranging from 30 kilowatts (kW) to 1 megawatt in electric power output. EFOI (T/$6)
engaged in developing and selling of light-emitting diode (LED) lighting products for military maritime market, and general commercial and industrial markets. It produces, sources and/or markets a range of lighting technologies to serve its primary end markets. It offers military maritime products, such as Military Intellitube, military globe lights and military berth light to serve the United States navy and allied foreign navies. It offers commercial products, such as direct-wire tubular LED (TLED) replacements for linear fluorescent lamps, LED dock lights, low-bay and high-bay lighting for high-intensity discharge applications and LED retrofit kits to serve general commercial and industrial markets. TGEN (T/$6)
Tecogen Inc. designs, manufactures, sells, and services systems that produce electricity, hot water, and air conditioning for commercial installations and buildings, and industrial processes. The company offers cogeneration units that supply electricity and hot water; chillers that provide air-conditioning and hot water; and high-efficiency water heaters. Its products include InVerde, a 100-kilowatt combined heat and power system that primarily provides electricity and hot water; TECOGEN cogeneration system, which produces approximately 500,000 Btu/hr of hot water; TECOCHILL natural gas engine-driven chillers; and Ilios high-efficiency water heaters. The company also provides long-term maintenance contracts, parts sales, and installation services. Tecogen Inc. offers its products and services for hospitals and nursing homes, colleges and universities, health clubs and spas, hotels and motels, office and retail buildings, food and beverage processors, multi-unit residential buildings, laundries, ice rinks, swimming pools, factories, municipal buildings, and military installations. AMRS (T/$15)...
Amyris, Inc The company uses its industrial bioscience technology to design microbes primarily yeast, as well as to convert plant-sourced sugars into renewable ingredients. It produces and sells Biofene that converts to squalane, which is used as an emollient in cosmetics and other personal care products; and natural oils and aroma chemicals for the flavors and fragrances market. The company also provides renewable solvents, polymers, and lubricants for industrial markets; Biofene ingredients for nutraceuticals and vitamins market; and renewable fuels for transportation fuels markets. It has a collaboration partnership with Total S.A. to produce and commercialize Biofene-based diesel and jet fuels. ***ERII (T/$16-21)energy recovery devices, turbochargers for water desalination/makes equipment for oilfield that makes fracking more efficient .. . *AMSC (T/$10-11) now in doubt) wind turbine systems... RVLT (T/$?) sells light emitting diode (LED) lighting solutions... MNGA (T/$) converts liquid waste into hydrogen based fuels... *CWST (T/$13H)water treatment... *HCCI (T/16H) oil clean up parts cleaning... ***MXWL (ultra Capacitors) .. energy storage and power delivery products, ultra-capacitor cells... CDTI CDTI (T/$4)
diesel emissions control systems... ***OPTT (5.3 million share dilution Apr 27)...ocean wave electricity... VTNR recycle industrial waste... FF biofuel SPCL (Google finance)...Solaris Power Cells, Inc. is a developmental stage diversified "Green" Energy Storage manufacturer offering residential and commercial users turnkey, renewable energy storage solutions (ESS). Solaris manufactures the Solaris PESA™ "Passive Electron Storage Array"™, for limited use electric vehicles such as golf cars and Mega Energy Storage Systems. (ESS) The Solaris PESA™ is a 100% lead-free, solid-state storage (ESS) solution that makes renewable energy greener and better by allowing applications to utilize more of the energy generated. The Solaris Power Cell™ is capable of providing energy storage to applications normally reliant and equipped with highly toxic lead acid, nickel metal hydride or lithium-ion batteries. CTRL..(T/$22)...smart home...
ECR (T/$3)... GEVO (T/$12)
Solar....http://finance.yahoo.com/news/why-solar-energy-explosive-potential-202908604.html CAFD (T/$15) November 30, 2015, it owned interests in six utility-scale solar energy projects, two commercial and industrial solar energy projects, and a portfolio of approximately 5,900 residential solar installations with a total capacity of 432 megawatts. IPWR (T/$4)
Ideal Power Inc. develops power conversion solutions with a focus on commercial and industrial grid storage, combined solar and storage, and microgrid applications. The company offers 30kW battery converters for the commercial and industrial grid-tied distributed energy storage market; 30kW grid-resilient alternating current (AC) - direct current (DC) power conversion system (PCS) with two-ports, as well as 30kW grid-resilient AC-DC-DC multi-port model; and 125kW grid-resilient AC-DC PCS. It also focuses on licensing its proprietary power conversion Power Packet Switching Architecture to original equipment manufacturers. RGSE ....02/06 ...$11million dilution and price goes UP... RUN...solar financer and installer... SEDG (T/$25)
SolarEdge Technologies designs, develops, and sells direct current (DC) optimized inverter systems for solar photovoltaic (PV) installations in Israel, Europe, the United States, and internationally. The company's DC optimized inverter systems include power optimizers, inverters, and cloud-based software. SPWR ****SUNW () earnings growth next year...
Sunworks, Company is focused on the design, installation and management of solar power systems for commercial, agricultural, residential and municipal customers. It is a solar systems provider in the western United States, delivering over 2.5 kilowatts to multi-megawatts commercial systems. It provides energy solutions and services to its customers, including project management and installation support, solar energy performance assessments, solutions consultations, materials and equipment procurement support, as well as rebates and incentives management. It is a solar installation and consulting company that offers financing and leasing options. It offers Property Assessed Clean Energy (PACE) financing program for financing home solar energy systems. It provides various system offering solutions, such as roof mounted solar systems, dual-axis trackers, ground mounted systems and tilted single axis trackers WNDW Solar Window Tech.  CandleGlance
CandleGlance  PBW...Clean Energy etf...DQ,TSLA,SQM,OLED,GLBL,BGC,ORA,PWR,HXL... ***AMSC (T/$7) Wind and electrical grid... NRG...energy utility
PBW...Clean Energy etf...DQ,TSLA,SQM,OLED,GLBL,BGC,ORA,PWR,HXL... ***AMSC (T/$7) Wind and electrical grid... NRG...energy utility URANIUM
Is thorium really that safe?
http://www.fairewinds.org/demystify/thorium-reactors?rq=thorium
Thorium ...storage of spent rods is still an issue same as with uranium
Uranium Bull Market
Uranium

NLR...CandleGlance Uranium... URA Global X Uranium ETF ...........
LTBR Lightbridge Corp... *CCJ(T/$15) .***URG(T/$1) ...UEC ...DNN ... URRE...FCUUF Fission Uranium...
Uranium Energy Corp.(UEC) is one of the lowest-cost producers of uranium in the United States. UEC's specialty is in-situ recovery (ISR), the most cost-efficient and environmentally friendly method of extracting uranium. >>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>
SEMI... AIXG (equip Germany) ... ACLS... Semiconductor equip. and materials... TSEM analog intensive mixed-signal semiconductor devices... CVV (equip) ...materials or coatings for aerospace engine components, medical implants, semiconductors, solar cells, smart glass, carbon nanotubes, nanowires, LEDs, MEMS, and other applications. LEDS... SemiLEDs Corporation develops, manufactures, and sells light emitting diode (LED) chips and LED components. Its products are used primarily for general lighting applications, including street lights, as well as commercial, industrial, and residential lighting; and specialty industrial applications, such as ultraviolet (UV) applications, curing of polymers, LED light therapy in medical/cosmetic applications, counterfeit detection....Taiwan. ***MU semiconductor systems ..... CY CypressSemi................................................................................................................................................................................................................................................................
SINO ...air delivery and frieght services USDP ...Rail...oil...rail
services and oil/gas rail cars... big dividen...
If oil continues to fall, it is likely that the return on investment for new pipelines will fall, leading to greater reliance on crude-by-rail. There remains a shortage of pipeline transportation for Canadian tar sands into the U.S. The oil sands are a better opportunity than U.S. shale plays as the asset life is significantly longer (30+ years). The exposure to Canadian oil sands makes it less sensitive to the vagaries of oil prices (see below added discussion about oil sands). The value of rail over pipelines is relatively unknown by most investors. For one, the movement of oil by rail is cheaper than pipelines with lower fixed costs, and it's less capital-intensive. It is also readily scalable. Faster delivery than pipelines by two-thirds (<10 days v. 30 days) with flexibility to choose destination market once the train load is completed.
https://seekingalpha.com/article/4081496-12-percent-yielder-niche-energy-space-9-straight-quarterly-distribution-hikes Shipping  SEA CandleGlance Shipping TNK,EURN,TGP,NMM,SSW,SFL,TK,TNP, ... SEA vs. VALE
SEA CandleGlance Shipping TNK,EURN,TGP,NMM,SSW,SFL,TK,TNP, ... SEA vs. VALE 
http://marketrealist.com/2016/11/analyzing-crude-tanker-industry-week-46/ ***ANW (T/$6-8) shipping, LNG/LPG carriers a marine fuel logistics company markets supplies refined marine fuel and lubricants to vessels in port, at sea, rivers, worldwide .. ******ASC (T/$10) LNG/LPG carriers ...Ardmore Shipping Corporation, together with its subsidiaries, engages in the seaborne transportation of petroleum products and chemicals through product and chemical tankers worldwide. As of December 31, 2016, the company operated 27 vessels. It serves oil majors, oil companies, oil and chemical traders, and chemical companies. ***DCIX (T/$8) CONTAINER... **DHT (T/$5H, 6)(0.46bk) oil tankers...DLNG (T/$11) LIQUID NAT. GAS ***DRYS(T$2.53...1.64bk) dry bulk ... **DSX (T/$3H, 5) shipping... **EGLE...(T/$5) dry bulk... ***ESEA () dry bulk/containers... FRO (T/$6) ***GLBS (0.49bk)dry bulk,... **GMLP...(T/$23)
Golar LNG Partners LP owns and operates floating storage regasification units (FSRUs) and liquefied natural gas (LNG) carriers under long-term charters in Brazil, the United Arab Emirates, Indonesia, and Kuwait. **GNK (T/$11H) dry bulk, company has 6.51share in cash...NNA **NMM (T/$2)... dry bulk... ***SALT dry bulk, has $3 a share in cash... SHIP() dry bulk...TOPS (0.34bk) oil tankers...**GASS crude oil and liquefied petroleum gas (LPG) ****SFL (T/$16) 11% dividend... fleet of 16 oil tankers, 22 dry bulk carriers, 20 container vessels, 2 car carriers, 2 jack-up drilling rigs, 2 ultra-deepwater drilling units, 5 offshore supply vessels, 2 chemical tankers, and 2 newbuilding oil pro duct tankers. STNG (T/$8)
Scorpio Tankers Inc., engages in the seaborne transportation of refined petroleum products worldwide. As of March 15, 2017, it owned 78 tankers comprising 22 LR2 tankers, 14 Handymax tankers, and 42 MR tankers with an average age of approximately 2.3 years; and 19 time chartered-in tankers, including 9 Handymax, 8 MR, 1 LR1, and 1 LR2 tankers. ***TK () crude oil and LNG carriers..company has $10.81 cash a share, Dividend% 3.20% ***** TNP (T/$6.50) ...Tsakos Energy Navigation Limited provides international seaborne crude oil and petroleum product transportation services worldwide. The company offers marine transportation services to national, major, and other independent oil companies and refiners under long, medium, and short-term charters. As of April 5, 2017, it operated a fleet of 62 double-hull vessels, including 57 conventional tankers, 2 liquefied natural gas carriers, and 3 suezmax DP2 shuttle tankers. Tsakos Energy Navigation Limited has a strategic partnership with Statoil for the crude oil tanker newbuildings. Other Stocks AMRS ...bioscience technology to design microbes primarily yeast, as well as to convert plant-sourced sugars into renewable hydrocarbons. ATTO (T/$14) **AUO liquid crystal displays and other flat panel displays, solar and water re-cycling BKD ($T/16H) senior living ***BTE ...oil Eagle Ford... BRCD Brocade Commucations/Ruckus .. CCIH provider of Internet content and application delivery services and cloud in China... CELP (T/$8) oil gas equip. and services... CHNR iron, zinc, and other nonferrous metals in the Peoples Republic of China
CPSH ...produces and sells advanced material solutions to transportation, automotive, energy, computing/Internet, telecommunication, aerospace, defense, and oil and gas markets. Its products are used in applications that involve energy use or energy generation. The company primarily offers metal matrix composites that are a combination of metal and ceramic, such as baseplates for various applications, including motor controllers used in electric trains, subway cars, wind turbines, and hybrid and electric vehicles; baseplates and housings for use in radar, satellite, and avionics applications, as well as in modules built with wide band gap semiconductors; and lids and heatspreaders used with integrated circuits for use in Internet switches and routers. It also assembles housings and packages for hybrid circuits. CPS Technologies Corporation primarily sells its products to microelectronics systems companies in the United States *CRESY agriculture Argentina.. ESES oil/gas service FEYE (T/$17-20)
FireEye, Inc. provides cybersecurity solutions that allow organizations to prepare for, prevent, respond to, and remediate cyber-attacks. FPI (T/$10-12) ...Farmland Partners REIT...5% Div.
GLUU (T/$3.50) Games GARWF ()Golden Arrow Resources gold, silver, lead, zinc, and copper GRMX GSVC...venture capital pre-IPO IIN...(T/$11) body worn devices...micro-miniature electronics and hearing aids INFN (T/$12-13) INGN oxygen concentrators IPAS Telecom Services... .. JVA. Coffee... ***KTOS KTOS..(T/$7H-14-17) ... provides unmanned aerial, ground, seaborne, command, control, and communications systems.  ****LKAI () mining/explores for gold, silver, lead, and zinc metals... *****LQMT () amorphous metal ( metallic glass/glassy metal) solid metallic material, usually an alloy, disordered atomic-scale structure. MTW farming machinery seasonal construction machinery seasonal ? NE (T/$7.50) ... ... NIOBF () niobium/scandium/titanium project located in Nebraska... NSSC
****LKAI () mining/explores for gold, silver, lead, and zinc metals... *****LQMT () amorphous metal ( metallic glass/glassy metal) solid metallic material, usually an alloy, disordered atomic-scale structure. MTW farming machinery seasonal construction machinery seasonal ? NE (T/$7.50) ... ... NIOBF () niobium/scandium/titanium project located in Nebraska... NSSC (T/$12) security softwar and devices...
*****OTIV (T/$3)
On Track Innovations Ltd. is a developer of cashless payment solutions. The Company's segments include Retail and Mass Transit, Petroleum, Parking and Other. The Company offers solutions for banking, mobile network operators, vending, mass transit, petroleum and parking. The Company provides its customers with training and installation support, customer service and technical support. Its PayEnable technology can be implemented into a range of products. Its Retail and Mass Transit products include TRIO mPOS, Pico BT, WAVE, WAVE PKI, oti SATURN 6700 UNO, oti SATURN 6500 TRIO and oti SCI 6000. It also offers otiMetry and oti CONNECT 3000. Its EasyFuel Plus solution is a wireless, cashless, cardless and paperless refueling tracking and payment solution. Its EasyPark set of parking solutions provides parking fee collection, parking payment enforcement and parking management solution. Its MediSmart solution is an information management and claims submission system for the medical sector. 
PRTS (T/$5) online after market auto parts
RADA () military avionics systems, including flight data recorders for fighter aircraft; digital video/audio/data recorders with data transfer functions; high-rate data recorders for aircraft and airborne pods; video recorders and airborne data servers; a range of head-up-displays color video cameras for fighter aircraft; and various ground debriefing solutions. It also provides avionics solutions comprising integrated avionics upgrade suites for fighters and mission aircraft; mission and display computers; weapon management systems; data interface and processing computers; mission data recorders and debriefing solutions; HUD video cameras; and avionics for unmanned aircraft vehicles (UAVs).
.ROX...sells beverage alcohol products in the United States and internationally Energy Source, Value Lighting, Tri-State LED, E-Lighting, All-Around Lighting and TNT Energy ******RVLT (T/$16) Revolution Lighting Technologies, Light Emitting Diodes

EFOI
 SCON (T/$3) Superconductor technologies SFE venture capital...
SCON (T/$3) Superconductor technologies SFE venture capital...
SNAP (T$8-23)
SSKN (T/$12?) SVU (T/$5-6) wholesale and retail grocer... **UAMY... produces and sells antimony, silver, gold, and zeolite products in the United States. The company's Antimony division offers antimony oxide that is primarily used in conjunction with a halogen to form a synergistic flame retardant system for plastics, rubber, fiberglass, textile good...
VRAY (T/$11)
http://oracledispatch.com/ http://wealthyventurecapitalist.com/ Larger Cap. NUVA (T/$72H) NuVasive Medical Appliance/equip... UBNT (T/$60-66) Ubiquiti wireless equipment... ALGN (T/$110H) AlignMed. Appliance/equip dental...BOFI (T/$33) bank of internet Bofl Holdings savings and loan... ADRO (T/18) Biotech
JUNO (T/29) Biotech... INFN (T/$13.50) Commctn Equip...  UA (T/$30) Under Armour apparel clothing... ***SQ (T/$20h)Mobile payment services WDAY (T/$85H) Workday financial software cloud... PI (T/$40H) IMPINJ Commmunication equip...
UA (T/$30) Under Armour apparel clothing... ***SQ (T/$20h)Mobile payment services WDAY (T/$85H) Workday financial software cloud... PI (T/$40H) IMPINJ Commmunication equip... ---------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------
**Graphene... According to a report by MarketsandMarkets, the global graphite market is projected to reach USD 29.05 Billion by 2022, at a CAGR of 5.2% from 2017 to 2022. Several industries are key end-users of the graphite-based products, including steel companies, automotive and aerospace. The use of graphite in batteries has been increasing in the last 30 years and has become a major component in large batteries. As a result, the demand from manufacturers of electric and hybrid vehicles have increased significantly. Rechargeable lithium-ion batteries utilize roughly twice the amount of graphite than lithium carbonate and as battery technology continues to evolve, the use of graphite in an increasing number of applications are also expected to provide growth opportunities for the graphite market in the future. Berkwood Resources Ltd. (TSX: BKR.V), Panasonic Corporation (OTC: PCRFY), Mason Graphite Inc. (OTC: MGPHF), Graphite One Resources, Inc. (OTC: GPHOF), Northern Graphite Corporation (OTC: NGPHF) The rechargeable batteries for the electric vehicle market, which Tesla is a significant part of, contributes significantly to the graphite market. "Our cells should be called Nickel-Graphite, because primarily the cathode is nickel and the anode side is graphite with silicon oxide [there's] a little bit of lithium in there, but it's like the salt on the salad," explained CEO Elon Musk, according to Benchmark Mineral Intelligence. "The main determinants on the cost of the cell are the price of the nickel in the form that we need it and the cost of the synthetic graphite with silicon oxide coating."
Our rapidly evolving energy future - packed with electric vehicles, massive energy storage solutions and nanotechnology - requires more tech-grade graphite than it does lithium and cobalt. If shortages for lithium and cobalt are looming large, shortages of graphite are even scarier. The United States is the biggest consumer, and it doesn’t even produce any tech-grade graphite. With demand for tech-grade graphite expected to increase 200 percent in less than three years, and 300 percent by 2025, North America will have to go from graphite zero to hero—fast. Lithium-ion batteries have a lithium cathode and a graphite anode. Tesla would probably agree that securing supplies of graphite for its gigafactory is even more critical than securing supplies of lithium and cobalt. Graphene sheets, which comprise the layers of graphite, are 10 times lighter and 200 times stronger than steel. It’s the strongest material in the world, and it’s the backbone of nanotechnology. Isolated only in 2004, graphene is the world’s first 2D material. Take it from Elon Musk himself, who has said: “Our cells should be called Nickel-Graphite, because primarily the cathode is nickel and the anode side is graphite with silicon oxide… [there’s] a little bit of lithium in there, but it’s like the salt on the salad.” Panasonic Corporation (OTC: PCRFY) is a worldwide leader in the development of diverse electronics technologies and solutions for customers in the consumer electronics, housing, automotive, enterprise solutions and device industries. Recently, Panasonic established a global battery cell production system for eco-friendly vehicles in China. In addition, Saint Jean Carbon Inc. announced that it has received their first order from Panasonic Corporation to supply graphite anode material to their manufacturing facility. The order consists of two different material specifications. Panasonic is one of the largest battery manufacturers in the world and makes batteries for companies like; Tesla, Toyota, Volkswagen and many other large corporations.

Mason Graphite Inc. (OTCQX: MGPHF) is a Canadian mining and processing company focused on the development of its 100% owned Lac Guéret natural graphite deposit located in northeastern Québec.

Graphite One Resources, Inc. (OTCQB: GPHOF) announced earlier in May that it has received a site assessment report for its advanced-materials graphite refinery facility prepared by the Alaska Industrial Development and Export Authority (AIDEA). The AIDEA Report is the first product of the Memorandum of Understanding entered into by AIDEA and Graphite One announced on February 16, 2017. Graphite is a critical material for electric vehicle batteries and energy storage systems. The U.S. is presently 100% import-dependent for its graphite supply. "Tapping AIDEA's expertise in helping us assess potential refinery sites is the first step towards making Alaska a key player in the clean-tech energy sector," said Anthony Huston, Graphite One's CEO. "The AIDEA Report confirms the considerable interest Alaska localities have in serving as a base for our advanced-material spherical graphite refinery. With AIDEA's involvement, we intend to move forward to the next phase in our refinery site evaluation."

NGPHF NGPHF Northern Graphite Corporation (OTCQX: NGPHF) has a 100% interest in the Bissett Creek graphite deposit located in southern Canada and relatively close to all required infrastructure. On August 15th, Northern Graphite announced the results of additional metallurgical test work carried out on ore from the Bissett Creek deposit by BGRIMM, a Chinese state owned metallurgical research and development company. BGRIMM confirmed the high recoveries and high purities used in the Feasibility Study and successfully increased the percentage of high value, +50 mesh XL flake in the final concentrate from 48 to 61 per cent. Gregory Bowes, CEO commented that: "We believe Bissett Creek has the best flake size distribution of any new graphite project which will result in concentrate prices that are more than 60 per cent higher than for the "average" project, and we will not have the fines problem that characterizes almost every graphite deposit."

.....***FCSMF... *ZENYF ....
http://seekingalpha.com/article/1729842-investor-enthusiasm-for-graphene-strong-as-graphene?source=yahoo
http://www.forbes.com/sites/tomkonrad/2013/09/18/graphene-stock-investing-what-the-pros-think/ Carbon Fiber http://www.buyupside.com/sample_portfolios/carbonfiber.php CVV (equip) ...materials or coatings for aerospace engine components, medical implants, semiconductors, solar cells, smart glass, carbon nanotubes, nanowires, LEDs, MEMS, and other applications. Graphene Supercapacitor Prototype http://www.technologyreview.com/view/521651/graphene-supercapacitors-ready-for-electric-vehicle-energy-storage-say-korean-engineers/
Graphene update ...... more Graphene
How to Invest in Graphene  FUEL CELL Ceramic/Solid Oxide Fuel Cells http://brucewilds.blogspot.com/2016/04/fuel-cell-car-trump-teslas-vision.html https://1.bp.blogspot.com/-1t_VN2qUibw/Vv0ClMDJiJI/AAAAAAAABj4/Exy_cV4rIYEv4AA4xj3YUARNfQX5EgjLw/s320/fuel%2Bcell%2Bc.jpeg
FUEL CELL Ceramic/Solid Oxide Fuel Cells http://brucewilds.blogspot.com/2016/04/fuel-cell-car-trump-teslas-vision.html https://1.bp.blogspot.com/-1t_VN2qUibw/Vv0ClMDJiJI/AAAAAAAABj4/Exy_cV4rIYEv4AA4xj3YUARNfQX5EgjLw/s320/fuel%2Bcell%2Bc.jpeg  *****BLDP (T/$3.5-6)...09/13 2017 Ballard Power Systems Inc. surged the most in more than a year after announcing it has developed a lightweight fuel cell that uses less platinum. https://finance.yahoo.com/news/ballard-surges-fuel-cell-uses-155901753.html Sept. 13,2017 Ballard Power Systems has sales and partnerships with companies who make buses, trains, trucks, forklifts, drone makers, and even major automakers. It has a diverse pipeline of products as a result, despite the fact that over half of its revenue last year came from just three customers. (Motley Fool) ***PLUG (T/$2h-3)... Plug Power gets all of the media's attention for its huge supply agreements with Walmart and Amazon. Plug Power's business is becoming increasingly reliant on Walmart, which accounted for 34.1% of its business in 2016. As the Amazon deal ramps, the two retailers will likely account for well over half of Plug Power's revenue. That's not a diverse customer base, and since forklifts are the main product being served, it's not a diverse set of products Plug Power is supplying. ***Jeff Bezos' Amazon recently acquired 23% of Plug Power (NASDAQ: PLUG), a New York-based firm focused on developing and commercializing hydrogen fuel cell systems for electric vehicles. ***FCEL (T/$2.50)... Specializing in stationary fuel cell power plants for electricity generation. ****HYGS .HYGS Hydrogenics Hydrogenics Corporation, hydrogen generation products based on water electrolysis technology; and fuel cell products based on proton exchange membrane technology. It operates in two segments, OnSite Generation and Power Systems. The OnSite Generation segment develops products for industrial gas, hydrogen fueling, and renewable energy storage markets. It offers HySTAT Hydrogen Stations that supply on-site hydrogen for various hydrogen applications, including vehicle fueling, distributed power, and various industrial processes; and provides spare parts and services. This segment sells its products to original equipment manufacturers (OEMs), merchant gas companies, end users, and oil and gas companies, as well as to electric power utilities. The Power Systems segment develops products for energy storage, stationary, and motive power applications. This segment offers HyPM fuel cell products comprising HyPM fuel cell power modules that produce direct current (DC) power in standard outputs of 2.5, 5, 8, 12, 16, 30, 90, 120, and 200 kW; and HyPX Fuel Cell Power Pack, which includes a standard HyPM power module integrated with hydrogen storage tanks and ultracapacitors to provide higher power in short bursts. Its HyPM fuel cell products also consist of integrated fuel cell systems that are used for portable and stationary applications, including portable and auxiliary power units for military applications, and DC or DC backup power system for cellular tower sites, as well as provides engineering development services for new or custom products. CEFLF ...Ceramic Fuel Cell... CPWHF... NPWZ... HFCO... SSMFF... ITMPF... EVOMF
*****BLDP (T/$3.5-6)...09/13 2017 Ballard Power Systems Inc. surged the most in more than a year after announcing it has developed a lightweight fuel cell that uses less platinum. https://finance.yahoo.com/news/ballard-surges-fuel-cell-uses-155901753.html Sept. 13,2017 Ballard Power Systems has sales and partnerships with companies who make buses, trains, trucks, forklifts, drone makers, and even major automakers. It has a diverse pipeline of products as a result, despite the fact that over half of its revenue last year came from just three customers. (Motley Fool) ***PLUG (T/$2h-3)... Plug Power gets all of the media's attention for its huge supply agreements with Walmart and Amazon. Plug Power's business is becoming increasingly reliant on Walmart, which accounted for 34.1% of its business in 2016. As the Amazon deal ramps, the two retailers will likely account for well over half of Plug Power's revenue. That's not a diverse customer base, and since forklifts are the main product being served, it's not a diverse set of products Plug Power is supplying. ***Jeff Bezos' Amazon recently acquired 23% of Plug Power (NASDAQ: PLUG), a New York-based firm focused on developing and commercializing hydrogen fuel cell systems for electric vehicles. ***FCEL (T/$2.50)... Specializing in stationary fuel cell power plants for electricity generation. ****HYGS .HYGS Hydrogenics Hydrogenics Corporation, hydrogen generation products based on water electrolysis technology; and fuel cell products based on proton exchange membrane technology. It operates in two segments, OnSite Generation and Power Systems. The OnSite Generation segment develops products for industrial gas, hydrogen fueling, and renewable energy storage markets. It offers HySTAT Hydrogen Stations that supply on-site hydrogen for various hydrogen applications, including vehicle fueling, distributed power, and various industrial processes; and provides spare parts and services. This segment sells its products to original equipment manufacturers (OEMs), merchant gas companies, end users, and oil and gas companies, as well as to electric power utilities. The Power Systems segment develops products for energy storage, stationary, and motive power applications. This segment offers HyPM fuel cell products comprising HyPM fuel cell power modules that produce direct current (DC) power in standard outputs of 2.5, 5, 8, 12, 16, 30, 90, 120, and 200 kW; and HyPX Fuel Cell Power Pack, which includes a standard HyPM power module integrated with hydrogen storage tanks and ultracapacitors to provide higher power in short bursts. Its HyPM fuel cell products also consist of integrated fuel cell systems that are used for portable and stationary applications, including portable and auxiliary power units for military applications, and DC or DC backup power system for cellular tower sites, as well as provides engineering development services for new or custom products. CEFLF ...Ceramic Fuel Cell... CPWHF... NPWZ... HFCO... SSMFF... ITMPF... EVOMF Energy Storage companies - A new and developing area, so not easy to pick the winners here. Certainly the major battery manufacturers and lithium miners discussed above can benefit. Numerous others to choose from include...
****ENPH Enphase Energy (T/$4) The company's semiconductor-based microinverter system converts direct current electricity to alternating current (AC) electricity at the individual solar module level. The Enphase AC Battery is a key component of the Enphase Home Energy Solution, the industry's first and only truly integrated offering that combines solar generation, energy control, and storage, and provides homeowners the unique ability to manage their solar and storage together in an integrated fashion. Advanced Microgrid Solutions (private ), Fronius (private), Imergy (private), Redflow (ASX:RFX), AES Corp. (NYSE:AES), NEC (OTC:NIPNF), Sharp (OTCPK:SHCAY), Younicos (private, interest held by First Solar), Southern California Edison (SCE), General Electric (NYSE:GE), EnerSys (NYSE:ENS), Johnson Controls (NYSE:JCI), Sony (NYSE:SNE), Sonnen (private) and Origin Energy (NASDAQ:ORG). Electrovaya (EFL.TO) could well be the little company that could. Developer and manufacturer of portable Lithium-ion battery power solutions for the automotive, power grid, medical and mobile device sectors. The company is based in Mississauga, Ontario, Canada AES (T/$12) AES Corp... Utility-scale energy storage is expected to surpass 1.6 gigawatts by 2020, edging out the old grid system and making it that much easier to integrate more renewable capacity — once the tech catches up! San Diego Gas & Electric chose a battery design by AES Corp. (NYSE: AES) to begin replacing one of its largest natural gas peaking plants with as much as 300 megawatts of battery storage. The company will also be upgrading the existing plant, but it notes that the end goal is to replace it with renewables later on. Southern California Edison has also contracted Tesla, in a move that should surprise no one by now, to continue building onto its existing 80-megawatt energy storage project in Mira Loma. The system is already online as of this year and can cover peak energy demand for as many as 15,000 California homes. Right now, it’s the biggest lithium battery station operating in the U.S. As it stands, electric cars are still set to be the biggest source of growth in lithium batteries in coming years, but that view could shift if this trend gains traction...
ESU.V ...ZNNMF Capacitor Car
Electrovaya Inc. (EFL.TO) (EFLVF) designs, develops and manufactures proprietary Lithium Ion Super Polymer® batteries, battery systems, and battery-related products for energy storage, clean electric transportation and other specialized applications. Headquartered in Ontario, Canada, Electrovaya is a technology focused company with extensive IP, supplying leading global customers.
MXWL (T/$6) Maxwell Technologies, Inc. develops, manufactures, and markets energy storage and power delivery products worldwide. The company offers ultra-capacitor cells, and multi-cell packs and modules, which provide energy storage and power delivery solutions for applications in various industries, including bus, rail, and truck in transportation; grid energy storage; and renewable wind energy solutions. It also provides lithium-ion capacitors, which are energy storage devices designed to address various applications in the rail, grid, and industrial markets where energy density and weight are differentiating factors. In addition, the company offers CONDIS high-voltage capacitors, such as grading and coupling capacitors, and electric voltage transformers that are used to ensure the safety and reliability of electric utility infrastructure and other applications involving transport, distribution, and measurement of high-voltage electrical energy.
MXWL (T/$6-7) he company offers ultra-capacitor cells, and multi-cell packs and modules, which provide energy storage and power delivery solutions for applications in various industries, including bus, rail, and truck in transportation; grid energy storage; and renewable wind energy solutions. It also provides lithium-ion capacitors, which are energy storage devices designed to address various applications in the rail, grid, and industrial markets where energy density and weight are differentiating factors. In addition, the company offers CONDIS high-voltage capacitors, such as grading and coupling capacitors, and electric voltage transformers that are used to ensure the safety and reliability of electric utility infrastructure and other applications involving transport, distribution, and measurement of high-voltage electrical energy.
POLA The products offered by Polar power have clear competitive advantage over its competitors by offering products with no need for maintenance , higher efficiency charging , longer life cycle , light weight & using a proprietary super capacitor instead of a normal battery . As mentioned in the 10-k form : “ we believe that the number one reliability issue with a generator set is the failure to start. To improve the reliability of our generators, we remove the engine’s starting battery and replace it with a super capacitor. The super capacitor has a 15- to 20-year service life, greater cold cranking amps and withstands greater temperature extremes than conventional starting batteries. “ “ To reduce maintenance and help ensure that there is always adequate oil, we increase the engine’s oil capacity to provide for a 3,000 hour (natural gas / propane) or 1,500 hour (diesel) maintenance interval. Standard oil intervals for typical generators range from 200 to 500 hours. “ Its external shape made from aluminum & stainless steel makes it hard and corrosion resistant . Those qualities are essential in telecommunication , especially for the off-grid sites or sites with potential natural disaster like in Puerto Rico.
Other Metals/Specialty Metals Industry
€***GSM
Rare Earth http://www.buyupside.com/sample_portfolios/rareearth.php
¥REMX...RareEarths

ZINC zinc-based batteries appeal is that they could pack TWICE the power as their lithium-based cousins. ZZZOF ... GLNCY... HL... HBM... MUX... AG... VEDL (India) Colbalt ...cobalt goes in lithium batteries...  Manufacturing 34.5 million electric vehicles, or EVs, to replace the conventional vehicles sold in those jurisdictions every year would require about 276,000 TPY of cobalt. Manufacturing 100 million EVs per year to completely replace ICE technology would require about 800,000 TPY of refined cobalt and exhaust planetary reserves in less than seven years. Since optimistic cobalt growth forecasts only show 41,200 TPY available for EV batteries by 2025, the politicians, demagogues and advocates will be bitterly disappointed.... https://seekingalpha.com/article/4110450-cobalt-cliff-will-crush-teslas-business-may-restore-sanity-ev-industry?li_source=LI&li_medium=liftigniter-widget
Manufacturing 34.5 million electric vehicles, or EVs, to replace the conventional vehicles sold in those jurisdictions every year would require about 276,000 TPY of cobalt. Manufacturing 100 million EVs per year to completely replace ICE technology would require about 800,000 TPY of refined cobalt and exhaust planetary reserves in less than seven years. Since optimistic cobalt growth forecasts only show 41,200 TPY available for EV batteries by 2025, the politicians, demagogues and advocates will be bitterly disappointed.... https://seekingalpha.com/article/4110450-cobalt-cliff-will-crush-teslas-business-may-restore-sanity-ev-industry?li_source=LI&li_medium=liftigniter-widget In June 2017, Morgan Stanley issued a 25-page commodity report on cobalt that surveyed planned capacity additions and forecast primary refined cobalt supply growth from 94.4 tonnes in 2016 to 148,300 tonnes in 2025. On the demand side, Morgan Stanley forecast that in 2025, 59,600 tonnes of cobalt would be used for non-battery applications, and 47,500 tonnes would be used for consumer products batteries, which would leave 41,200 tonnes for use in EV batteries.
Sept. 29, 2017
| Market Price | Cobalt prices have soared from $10.50 per pound in March 2016 to a recent high of $28.35 per pound. |
| Major Mine Purchase | In May 2016 China Molybdenum bought a 56% stake in the DRC’s Tenke Fungurume Mine from Freeport McMoRan for $2.65 billion and gained control over 10% of global cobalt production in a single transaction. In July 2017, China Moly facilitated BHR Partners’ purchase of a 24% stake in the Tenke Mine from Lundin Mining for $1.14 billion. |
| China Dominates Cobalt Refining | Between the Tenke purchase and contracts to finance the Eurasian Resource Group’s Roan Tailings Project, Chinese interests will control at least 60% of the world’s refined cobalt production for the foreseeable future. |
| Updated Chinese Subsidy Regime | In January 2017, China updated its subsidy regime for new energy vehicles to favor higher energy densities and longer travel ranges while permitting the use of nickel-cobalt battery chemistries. As a result, a significant portion of the China market that was previously dominated by cobalt-free batteries is likely to change chemistries. |
| Bernstein’s 2017 Black Book | In March 2017 Bernstein released the latest version of its EV Black Book which devotes 60 of its 271 pages to cathode powder formulations, raw material requirements and the principal players in those markets. In their slow adoption scenario, Bernstein expects the battery market for passenger EVs to exceed 360 GWh per year by 2025, which implies an annual cobalt demand of roughly 55,000 tonnes. Interestingly, Bernstein also forecast a mining industry capital spending requirement of $350 to $750 billion to support a full transition to EVs and noted “the lead time required for conversion of exploration success into an operating mine has lengthened considerably and now stands at ~30 years.” Ultimately, Bernstein’s battery materials discussion concludes, “Either the world must do without EVs or it must pay more for the commodities it consumes, the choice really is as simple as that.” |
| UBS Bolt Teardown | In May 2017, UBS published the results of their teardown analysis of a GM Bolt EV and estimated that global cobalt production would need to increase by 1928% to support an annual EV build of 100 million units. My estimate of a mere 900% global cobalt production growth requirement pales in comparison. |
| Morgan Stanley Cobalt Report | In June 2017, Morgan Stanley issued a 25-page commodity report on cobalt that surveyed planned capacity additions and forecast primary refined cobalt supply growth from 94.4 tonnes in 2016 to 148,300 tonnes in 2025. On the demand side, Morgan Stanley forecast that in 2025, 59,600 tonnes of cobalt would be used for non-battery applications, and 47,500 tonnes would be used for consumer products batteries, which would leave 41,200 tonnes for use in EV batteries. |
| VW's Glencore contract | In July 2017, we learned that Contemporary Amperex Technology and its customer Volkswagen signed a four year 5,000 TPY cobalt offtake agreement with Glencore last fall. |
| VW's Contract Solicitation | In September 2017, we learned that VW is soliciting 10-year requirements-based cobalt offtake commitments for 16,000 to 24,000 TPY and wants contracts in place this year. |
In its “Global EV Outlook 2017” the International Energy Agency summarized the electric car ambitions of the world’s automakers as follows:
| BMW | 100,000 electric car sales in 2017; and
15-25% of the BMW group’s sales by 2025. |
| Chevrolet | 30,000 annual electric car sales by 2017 |
| Chinese OEMs | 4.52 million annual electric car sales by 2020 |
| Daimler | 100,000 annual electric car sales by 2020 |
| Ford | 13 new EV models by 2020 |
| Honda | Two-thirds of the 2030 sales to be electrified vehicles
(including hybrids, PHEVs, BEVs and FCEVs) |
| Renault-Nissan | 1.5 million cumulative sales of electric cars by 2020 |
| Tesla | 500,000 annual electric car sales by 2018; and
1 million annual electric car sales by 2020 |
| Volkswagen | 2-3 million annual electric car sales by 2025 |
| Volvo | 1 million cumulative electric car sales by 2025 |
With an average cobalt content of 8 kg per car, the 41,200 tonnes available for EV batteries in 2025 will only support the manufacture of 5.15 million EVs, a little over half of the aspirational totals set forth above. At this point I have to believe the EV revolution has entered a melee phase where there won’t be enough cobalt to satisfy everybody’s needs and anyone who wants to play the game will have to stand toe-to-toe and compete for cobalt supplies with China Inc. and six of the world’s ten largest automakers. I don’t think Tesla is up to the business challenge establishing robust and reliable supply chains in the face of relentless competition from the big boys. I know it lacks the financial strength to stand toe-to-toe with the big boys in a bidding war.
***Fuel cell alternative... http://brucewilds.blogspot.com/2016/04/fuel-cell-car-trump-teslas-vision.html ECSIF eCobalt Solutions... Cobalt metal https://seekingalpha.com/article/4066726-ecobalt-solutions-know-buying 
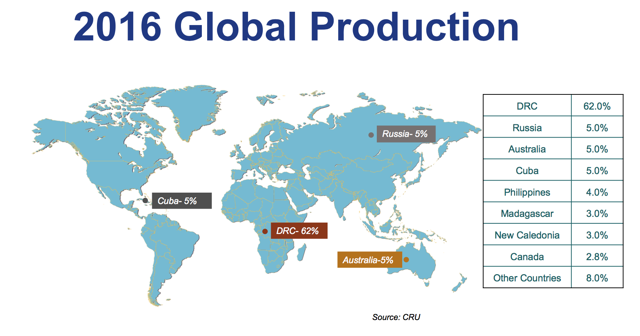 ECSIF eCobalt Solutions... Cobalt metal ...
ECSIF eCobalt Solutions... Cobalt metal ...  http://stockcharts.com/c-sc/sc?s=ARRRF&p=D&yr=0&mn=6&dy=0&i=p81972305457&a=559755852&r=1511908838529
http://stockcharts.com/c-sc/sc?s=ARRRF&p=D&yr=0&mn=6&dy=0&i=p81972305457&a=559755852&r=1511908838529  http://www.infomine.com/investment/metal-prices/cobalt/ https://seekingalpha.com/article/4066102-ecobalt-solutions-getting-u-s-equity-exposure-cobalt-metal-revolution Cobalt metal is in high demand due to growth in the electric vehicle ("EV") market, which uses lithium-ion batteries to power these vehicles (cobalt being a main component). In conjunction, its growing supply deficit is leading the price of the commodity higher. Macquarie Research projected a supply deficit of 885 tonnes in 2018, 3,205 in 2019, and 5,340 in 2020. That comes to a supply deficit increase of 6x in just two years. For large-cap tech companies and automakers looking to produce electric vehicles, the need for cobalt has become increasingly clear given its role in the lithium-ion battery mix. The metal constitutes a little over one-third of a modern lithium-ion battery's composition (and greater than the amount of lithium used itself). FTSSF ...Fist Colbalt... WCTXF How the competition is doing - Larger mining companies also have cobalt operations ongoing, including Freeport-McMoRan (NYSE:FCX) (T/$12-16), Katanga Mining (OTCPK:KATFF), Glencore (GLCNF), Fortune Minerals (OTCQX:FTMDF), Barra Resources (OTC:BRCSF), Tiger Resources (OTC:TRSDF), Lundin Mining (LUNMF), and Cruz Cobalt Corp. (OTCPK:BKTPF).
http://www.infomine.com/investment/metal-prices/cobalt/ https://seekingalpha.com/article/4066102-ecobalt-solutions-getting-u-s-equity-exposure-cobalt-metal-revolution Cobalt metal is in high demand due to growth in the electric vehicle ("EV") market, which uses lithium-ion batteries to power these vehicles (cobalt being a main component). In conjunction, its growing supply deficit is leading the price of the commodity higher. Macquarie Research projected a supply deficit of 885 tonnes in 2018, 3,205 in 2019, and 5,340 in 2020. That comes to a supply deficit increase of 6x in just two years. For large-cap tech companies and automakers looking to produce electric vehicles, the need for cobalt has become increasingly clear given its role in the lithium-ion battery mix. The metal constitutes a little over one-third of a modern lithium-ion battery's composition (and greater than the amount of lithium used itself). FTSSF ...Fist Colbalt... WCTXF How the competition is doing - Larger mining companies also have cobalt operations ongoing, including Freeport-McMoRan (NYSE:FCX) (T/$12-16), Katanga Mining (OTCPK:KATFF), Glencore (GLCNF), Fortune Minerals (OTCQX:FTMDF), Barra Resources (OTC:BRCSF), Tiger Resources (OTC:TRSDF), Lundin Mining (LUNMF), and Cruz Cobalt Corp. (OTCPK:BKTPF). SEPTEMBER 14, 2017 BY
ROSEMARY BECKER WCTXF
http://usanewsgroup.com/2017/12/18/bmw-throws-fuel-on-cobalt-price-fire-well-need-10x-as-much/ CRUZ COBALT CORP COM NPV (OTCMKTS:BKTPF) reported the plunge of -0.15% and closed at $0.194, with the total traded volume of 42,245.00 shares. During last trade its minimum price was $0.19 and it gained its highest price of $0.20 and has a total of 63.06 million outstanding shares.
CRUZ COBALT CORP COM NPV (OTCMKTS:BKTPF) on September 12, 2017 announced that crews have been mobilized on the 100 percent owned Chicken Hawk Cobalt Prospect located in Deer Lodge County, Montana. This prospect consists of 64 contiguous lode claims covering approximately 1,300 acres.
The Chicken Hawk Cobalt Prospect is located on the western edge of the Boulder Batholith and east of the Cordilleran Fold and Thrust Belt in south-western Montana. Covering a boundary between a Cretaceous granodiorite and the Lowland Creek Volcanics from the Eocene; the claims are in the vicinity of a total of four volcanic rock suites. Cobalt, the primary targeted commodity of the Chicken Hawk, is occurring in the pyritized andesite and as cobaltian arsenopyrite; the sulfides are pnuematolytic in origin. The 64 claims surround 4 patented claims, no less than 15 unclaimed prospects, and 3 unclaimed adits.

LICO ENERGY METALS COM NPV (OTCMKTS:WCTXF) reported no change, after closing price for the day was $0.0820. Its total trading volume for the day was 9,500.00 shares, versus its average volume of 133,758.00 shares. Its earnings per share are -$0.07.
LICO ENERGY METALS COM NPV (OTCMKTS:WCTXF) on September 5, 2017 announced that it has entered into a property Purchase Agreement effective August 31st, 2017 with Glencore Canada Corporation (subsidiary of Glencore plc) (“Glencore”) of Baar Switzerland, (GLEN.L) to acquire a 100% interest in mining rights patent #585 (the “Glencore property”) situated in Bucke Township, 6 km east-northeast of Cobalt, Ontario. The Purchase Agreement includes a back-in provision, production royalty and an off-take agreement in favor of Glencore.
Glencore is one of the world’s largest producers of cobalt as a result of by-products created from its copper assets in the DRC and nickel assets in Australia, Canada and Norway.

FIRST COBALT CORP COM NPV (OTCMKTS:FTSSF) showing dropped of -2.48% and closed at $0.515, after gaining total volume of 67,233.00 shares. Its earnings per share (EPS) is -$0.05 and has total market capitalization of $30.23 million and a total of 57.26 million outstanding shares.
FIRST COBALT CORP COM NPV (OTCMKTS:FTSSF) on August 28, 2017 announced the addition of Jason Rickard, MSc., P.Geo. to the new position of Exploration Manager for the Ontario Cobalt Camp. Jason was previously a Senior Geologist for Goldcorp Inc., managing a 100,000+ metre drill program at their Borden Project in Ontario and leading the regional exploration program. Prior to Goldcorp, he was a Senior Geologist for Vale Exploration Canada, where he was heavily involved in exploration program management as well as permitting and community relations.
This is a key addition to the exploration team as the merger transactions with Cobalt One and CobalTech will give First Cobalt a dominant land position in the Cobalt Camp and provide several additional targets for follow up.

MGX Minerals Inc. (“MGX” or the “Company”) (CSE: XMG / FKT: 1MG / OTCQB: MGXMF) and engineering partner PurLucid Treatment Solutions Inc. (“PurLucid”) are pleased to report that, prior to deployment, demonstration of the NFLi-5 commercial-scale rapid lithium extraction system, capable of processing 750 barrels per day (120 cubic meters), is scheduled to take place immediately with oil and gas companies, industrial customers, lithium brine owners and international media scheduled to view the system. Demonstration of the lithium and petrolithium extraction and water treatment system will take place in the first half of June at the PurLucid manufacturing facility in Calgary, Alberta.
Petrolithium Technology PurLucid water treatment technologies, which purify wastewater brine, have been integrated with a newly developed lithium recovery process. Combined, this Cleantech process reduces the capital cost of recovery compared to traditional solar evaporation as it does not require the investment in large, multi-phase, lake sized, lined evaporation ponds, greatly reducing the physical footprint and enhancing the quality of extraction and recovery across a complex range of brines previously considered un-processable due to complexity or geographical location outside of solar evaporation appropriate zones. This includes oil and gas wastewater, natural brine, and other brine sources such as lithium-rich mine and industrial plant wastewater. MGX holds the global rights to the jointly developed lithium extraction technology while PurLucid retains the rights to the pre-treatment water purification and core technology.
PurLucid’s exclusively licensed and patented nanoflotation technology was designed specifically for oilfield environments. The technology separates impurities from oil and gas wastewater and produces clean water as a final product. This allows for the recycling or controlled release of oilfield wastewater and reduces or eliminates downhole and associated transportation costs. Water handling costs are one of the largest operating costs in the oilfield and oilsands operations today. Learn more at www.purlucid.com. MGX currently holds a 51% interest in PurLucid and maintains the right to acquire 100% through successive future investments.  LITHIUM Seven reasons why the Internal Combustion Engine is a dead man walking The lithium industry is still developing, and new discoveries are still being made. Currently, Chile’s Sociedad Quimica y Minera (SQM) produces the majority of the world’s supply, but Bolivia and Afghanistan are believed to have significant reserves as well. Afghanistan is the wild card in that equation; a recent discovery made by Pentagon officials and U.S. geologists indicates that the country is home to nearly $1 trillion in untapped mineral deposits. One government official predicted that Afghanistan would eventually become the “Saudi Arabia of lithium,” but the country’s mining infrastructure is in shambles and it will likely be years before companies develop the ability to tap into these massive resources [also see Afghanistan: The Next Frontier For Mining ETFs?]. Unfortunately for investors, Afghanistan and Bolivia aren’t exactly at the top of the list of most investor-friendly countries, forcing investors to consider high levels of political risk in order to invest in some of the most lucrative markets. Before today, most investors would buy a few select lithium mining companies and hope for the best in terms of political repercussions. Now thanks to the new ETF, investors can put their money in the broad sector and not worry as much about one or two securities sabotaging their total returns. http://etfdb.com/2010/global-x-lithium-etf-lit-hits-the-market/ Nov. 20. 2017, 4-traders (from Baystreet.ca Media Corp) reported, "Li-Ion battery market surge will put further demand on lithium suppliers. Remarkable growth in the Lithium-Ion battery market will see the sector surpass $53 billion by 2024, according to new research. The 2017 report put out by Global Markets Insights highlighted the Lithium Ion Battery market's growth from USD $23 billion in 2015, at a rate of over 9% CAGR to blow past the $53 billion mark by 2024." ****07/25/17 Word out of Japan today is that Toyota is working on launching a new solid-state battery for electric vehicles that will put it solidly in the EV game by 2022. Which leads to a simple question: What is a solid-state battery, and why does it matter? Back in February, John Goodenough observed, "Cost, safety, energy density, rates of charge and discharge and cycle life are critical for battery-driven cars to be more widely adopted." And risking a bad pun on his surname, he seemed to be implying that all of those characteristics weren't currently good enough in autos using lithium-ion batteries. This comment is relevant because Goodenough, professor at the Cockrell School of Engineering at the University of Texas at Austin - it so happens, he turns 95 today - is the co-inventor of the lithium-ion battery, the type of battery that is pretty much the mainstay of current electric vehicles. And he and a research fellow at U of T were announcing they'd developed a solid-state battery, one that has improved energy density (which means a car so equipped can drive further) and can be recharged more quickly and more often (a.k.a., "long cycle life") than a lithium-ion battery. http://www.buyupside.com/sample_portfolios/lithium.php lithium – the silvery-white metal dubbed “The New Gasoline” by Goldman Sachs – is projected to continue rising by as much as 16% per year over the next ten years! http://wallstreetnation.com/featured/soaring-global-demand-lithium-presents-triple-digit-profit-scenario.html China's electric vehicle market is expected to grow at a faster pace than those in the rest of the world, as is its demand for batteries for other applications, such as storing electricity. ***Over the next two years 2016-2018, 25 new makes and models of electric cars are expected to be released. BYD Co. Ltd BYDDF and Tesla TSLA currently lead global sales.Governments all over the world are pushing hard for an increase in the number of electric vehicles – and that has fueled the continued rise in lithium prices. Car industry in China is very fragmented. The traditional "Big Four" domestic car manufacturers are SAIC Motor, Dongfeng, FAW and Chang’an. Other Chinese car manufacturers are Beijing Automotive Group, Brilliance Automotive, BYD, Chery, Geely, Jianghuai (JAC), Great Wall, and Guangzhou Automobile Group. In addition, several multinational manufacturers have partnerships with domestic manufacturers. Here's the rough split: 40% Chinese makers, 23% European makers, 20% Japanese makers (including Nissan), 12% US makers, and 9% Korean makers. India EV, Mahindra is expanding its EV production to 5000 a year and may be looking to a Suzuki/Toshiba/Denso JV for battery. BYD's JV with Goldstone is doing well and looking to produce 400 ebuses a year. BYD should look into becoming a battery supplier there. India announced its move towards full EV by 2030.
LITHIUM Seven reasons why the Internal Combustion Engine is a dead man walking The lithium industry is still developing, and new discoveries are still being made. Currently, Chile’s Sociedad Quimica y Minera (SQM) produces the majority of the world’s supply, but Bolivia and Afghanistan are believed to have significant reserves as well. Afghanistan is the wild card in that equation; a recent discovery made by Pentagon officials and U.S. geologists indicates that the country is home to nearly $1 trillion in untapped mineral deposits. One government official predicted that Afghanistan would eventually become the “Saudi Arabia of lithium,” but the country’s mining infrastructure is in shambles and it will likely be years before companies develop the ability to tap into these massive resources [also see Afghanistan: The Next Frontier For Mining ETFs?]. Unfortunately for investors, Afghanistan and Bolivia aren’t exactly at the top of the list of most investor-friendly countries, forcing investors to consider high levels of political risk in order to invest in some of the most lucrative markets. Before today, most investors would buy a few select lithium mining companies and hope for the best in terms of political repercussions. Now thanks to the new ETF, investors can put their money in the broad sector and not worry as much about one or two securities sabotaging their total returns. http://etfdb.com/2010/global-x-lithium-etf-lit-hits-the-market/ Nov. 20. 2017, 4-traders (from Baystreet.ca Media Corp) reported, "Li-Ion battery market surge will put further demand on lithium suppliers. Remarkable growth in the Lithium-Ion battery market will see the sector surpass $53 billion by 2024, according to new research. The 2017 report put out by Global Markets Insights highlighted the Lithium Ion Battery market's growth from USD $23 billion in 2015, at a rate of over 9% CAGR to blow past the $53 billion mark by 2024." ****07/25/17 Word out of Japan today is that Toyota is working on launching a new solid-state battery for electric vehicles that will put it solidly in the EV game by 2022. Which leads to a simple question: What is a solid-state battery, and why does it matter? Back in February, John Goodenough observed, "Cost, safety, energy density, rates of charge and discharge and cycle life are critical for battery-driven cars to be more widely adopted." And risking a bad pun on his surname, he seemed to be implying that all of those characteristics weren't currently good enough in autos using lithium-ion batteries. This comment is relevant because Goodenough, professor at the Cockrell School of Engineering at the University of Texas at Austin - it so happens, he turns 95 today - is the co-inventor of the lithium-ion battery, the type of battery that is pretty much the mainstay of current electric vehicles. And he and a research fellow at U of T were announcing they'd developed a solid-state battery, one that has improved energy density (which means a car so equipped can drive further) and can be recharged more quickly and more often (a.k.a., "long cycle life") than a lithium-ion battery. http://www.buyupside.com/sample_portfolios/lithium.php lithium – the silvery-white metal dubbed “The New Gasoline” by Goldman Sachs – is projected to continue rising by as much as 16% per year over the next ten years! http://wallstreetnation.com/featured/soaring-global-demand-lithium-presents-triple-digit-profit-scenario.html China's electric vehicle market is expected to grow at a faster pace than those in the rest of the world, as is its demand for batteries for other applications, such as storing electricity. ***Over the next two years 2016-2018, 25 new makes and models of electric cars are expected to be released. BYD Co. Ltd BYDDF and Tesla TSLA currently lead global sales.Governments all over the world are pushing hard for an increase in the number of electric vehicles – and that has fueled the continued rise in lithium prices. Car industry in China is very fragmented. The traditional "Big Four" domestic car manufacturers are SAIC Motor, Dongfeng, FAW and Chang’an. Other Chinese car manufacturers are Beijing Automotive Group, Brilliance Automotive, BYD, Chery, Geely, Jianghuai (JAC), Great Wall, and Guangzhou Automobile Group. In addition, several multinational manufacturers have partnerships with domestic manufacturers. Here's the rough split: 40% Chinese makers, 23% European makers, 20% Japanese makers (including Nissan), 12% US makers, and 9% Korean makers. India EV, Mahindra is expanding its EV production to 5000 a year and may be looking to a Suzuki/Toshiba/Denso JV for battery. BYD's JV with Goldstone is doing well and looking to produce 400 ebuses a year. BYD should look into becoming a battery supplier there. India announced its move towards full EV by 2030. Tesla has already opened their gigafactory in Nevada – and the company is planning on opening as many as 11 more gigafactories in the months ahead…And each one of those “gigafactories” will require a significant amount of lithium in order to sustain production. But there’s another side to this story that virtually ensures higher lithium prices over the next several years:
Lithium supply is right now considerably behind global demand. LIT is more than an ETF proxy on Tesla. In fact, the ETF really is not that. While Tesla is LIT's fourth-largest holding at a weight of just under 6 percent, that is nothing compared to the more than 24 percent the ETF allocates to FMC Corp (NYSE: FMC). That stock is up 34 percent this year.
(Did you ever notice that with time your iPhone keeps less of a charge than it did back when it was shiny and new? That's because it has a limited cycle life. Which is one thing when you're talking about a phone. And something else entirely when it involves a whole car.)
What's more, there is reduced mass for a solid-state battery. And there isn't the same safety concern that exists with li-ion batteries vis-à- vis conflagration (which is why at airplane boarding gates they say they'll check your carryon as long as you remove all lithium-ion batteries).
Lithium-ion batteries may be far more advanced than the lead-acid batteries that are under the hood of essentially every car that wasn't built in Fremont, Calif., but as is the case with those heavy black rectangles, li-ion batteries contain a liquid. In the lithium-ion battery, the liquid, the electrolyte, moves the lithium ions from the negative to the positive side (anode to cathode) of the battery. In a solid-state design, there is no liquid sloshing around, which also means that there's no liquid that would freeze at low operating temperatures.
What Toyota is using for its solid-state battery is still unknown, as is the case for the solid-state batteries that Hyundai is reportedly working on for its EVs.
In the case of Goodenough's solid-state battery, sodium is used to form glass that serves as the electrolyte. Consider the implications of the development of solid-state batteries for powering cars and trucks versus using lithium-ion battery packs.
For one thing, it will certainly mean that those Gigafactories that are sprouting up are going to have to undergo a significant change in what they're producing.
And for another, it will more quickly bring EVs to price parity with internal combustion engine-powered vehicles. A recent UBS study estimated that parity would occur in 2023, but that finding was based on li-ion batteries; Toyota, Hyundai, and Prof. Goodenough could provide the proverbial game-changer.
New Battery better than Lithium...John Goodenough, the 94-year-old father of the lithium-ion battery LIT CandleGlance ...FMC, SQM, TSLA, ALB, BYDDF, PCRFF, GYUAF, OROCF, GALXF, SGPEF, JCI ...OROCF TYHOF LIXXF **NTTHF LACDF AVLIF LIT CandleGlance.  SENTIMENT...GALXF a small lithium stock...
SENTIMENT...GALXF a small lithium stock...  LIT Lithium research Beware of looming electricity and lithium shortages. When Bloomberg did its analysis it predicted, “Electricity consumption from EVs will grow to 1,800 terawatt-hours in 2040, or 5 percent of global power demand, from 6 terawatt-hours in 2016”. This is a staggering 3,000 percent increase. Where will it come from? Better not be coal or possibly even natural gas. At a conference held April 3 in Calgary sponsored by ARC Energy Research Institute (AERI) a representative of Bruce Power, the Ontario nuclear electricity generator, said to economically reduce carbon emissions recharging EVs only made sense at night, not during peak load hours. If everybody drove their EVs to work and tried to plug in at the office it would overload the system. Meanwhile, there is speculation whether the world has enough lithium to build all the batteries skyrocketing EV growth would ensure. One analyst has predicted lithium shortages as soon as 2023 and have already delayed Tesla’s output. The solution, which is not all bad for the oil industry, is dual fuel whereby the battery is smaller, the lithium required per vehicle is lower, and mobility is augmented by a smaller ICE using good old-fashioned gasoline. http://oilprice.com/Alternative-Energy/Renewable-Energy/Electric-Vehicles-No-Threat-To-Oil-Prices-Anytime-Soon.html Related automaker stocks: GELYF, OTCPK:GELYY, OTCPK:BYDDY, OTCPK:BYDDF, KNDI, OTCPK:DNFGY, OTCPK:DNFGF, OTCPK:DDAIF, OTCPK:VLKAY, OTCPK:BMWYY, GM, OTCPK:GNZUF, OTCPK:GNZUY, TSLA, F, OTCPK:NSANY, TM, TTM, HMC, OTCPK:MZDAY, OTCPK:PEUGF, OTCPK:PUGOY, OTC:PUGOF, OTC:RNSDF, OTCPK:RNLSY, MBLY, NVDA, ALV, DLPH.
LIT Lithium research Beware of looming electricity and lithium shortages. When Bloomberg did its analysis it predicted, “Electricity consumption from EVs will grow to 1,800 terawatt-hours in 2040, or 5 percent of global power demand, from 6 terawatt-hours in 2016”. This is a staggering 3,000 percent increase. Where will it come from? Better not be coal or possibly even natural gas. At a conference held April 3 in Calgary sponsored by ARC Energy Research Institute (AERI) a representative of Bruce Power, the Ontario nuclear electricity generator, said to economically reduce carbon emissions recharging EVs only made sense at night, not during peak load hours. If everybody drove their EVs to work and tried to plug in at the office it would overload the system. Meanwhile, there is speculation whether the world has enough lithium to build all the batteries skyrocketing EV growth would ensure. One analyst has predicted lithium shortages as soon as 2023 and have already delayed Tesla’s output. The solution, which is not all bad for the oil industry, is dual fuel whereby the battery is smaller, the lithium required per vehicle is lower, and mobility is augmented by a smaller ICE using good old-fashioned gasoline. http://oilprice.com/Alternative-Energy/Renewable-Energy/Electric-Vehicles-No-Threat-To-Oil-Prices-Anytime-Soon.html Related automaker stocks: GELYF, OTCPK:GELYY, OTCPK:BYDDY, OTCPK:BYDDF, KNDI, OTCPK:DNFGY, OTCPK:DNFGF, OTCPK:DDAIF, OTCPK:VLKAY, OTCPK:BMWYY, GM, OTCPK:GNZUF, OTCPK:GNZUY, TSLA, F, OTCPK:NSANY, TM, TTM, HMC, OTCPK:MZDAY, OTCPK:PEUGF, OTCPK:PUGOY, OTC:PUGOF, OTC:RNSDF, OTCPK:RNLSY, MBLY, NVDA, ALV, DLPH. - Governments all over the world see electric vehicles — and the lithium that fuels them — as the key to a carbon-free future that will slow the pace of climate change.
For every 1% uptake in electric vehicle use, the increase in battery demand will require 70,000 tons of lithium production per year.
That means the equivalent of five new mines will need to be brought online for every 1% increase in electric vehicle usage.
But a 1% increase in electric vehicle usage is just a fraction of expected demand – with Germany, Norway and other nations announcing plans to ensure that all new cars sold there are emissions-free by 2030.
In fact, by 2030, analysts predict that more than 50% of all new vehicles sold will be electric cars.
All of this adds up to mean that more supply needs to be brought online – and quickly – to meet this growing demand!
This extreme imbalance – with soaring demand yet limited supply – has created an opportunity for lithium mining companies to grow at a staggering pace…if they are able to bring a new supply of lithium to market.
For early investors in “junior” lithium exploration companies that can help feed the world’s growing demand…a potential once-in-a-generation profit windfall could await.
MLNLF Millennial Lithium Corp. (TSX: ML ; OTC: MLNLF) MLNLF Millennial Lithium – presents an attractive investment opportunity due to its large pipeline of lithium projects located in a lithium-rich region known as “The Lithium Triangle.” The company’s projects cover 35,000 hectares strategically located near existing major producers in Argentina. And just recently – on June 27 – the company reported positive analytical results from drilling on its Pastos Grandes Project in Salta, Argentina. The results announced confirmed a high grade of lithium beneath the surface…much higher than had been encountered in previous drilling. This news – combined with future drilling results – could mean that “little-known” Millennial Lithium Corp. (TSX: ML ; OTC: MLNLF) may soon see a sharp rise in share price as the market becomes aware of the company’s outstanding lithium potential. Lithium Grades 500% Higher Than in Nevada.
Chart/News... https://www.google.com/finance?q=MLNLF&ei=tQplWanXJIST2AaG24CgDQ
South America’s “Lithium Triangle” is a vast area straddling Argentina, Chile and Bolivia…and it’s where more than half of the world’s identified lithium resources are located. In fact, Argentina has been called “The Saudi Arabia of Lithium” by Forbes because of its vast amount of lithium reserves. But it’s more than just the amount of lithium in the ground that makes property in the Lithium Triangle so attractive – it’s also the grade. Grades in the lithium triangle average 500% higher than in Nevada. This is why the region is home to several world-class lithium mines, which are the most profitable in the industry. And this is precisely where Millennial Lithium Corp. (TSX: ML ; OTC: MLNLF)has a pipeline of highly prospective lithium projects covering 35,000 hectares. Right now, most of the world’s lithium is being produced in Australia, Chile, Argentina, and China. Australia has the largest production by far, coming in at 14,300 tons in 2016, according to the U.S. Geological Survey. Most of Australia’s production comes from hard-rock mines. Chile comes in second place with 10,500 tons, mainly from brine operations in the Salar de Atacama, the country’s largest salt flat. Chile has the world’s largest lithium reserves held in these briny deserts: 7.5 million tons of our metal oil just waiting to be extracted! Despite these high numbers, it’s the last two countries that will be getting all the attention soon. Argentina, with 5,700 tons of production and 2 million tons in reserve. Argentina, meanwhile, has recently opened its doors to a new slew of lithium miners. China, with production at 2,000 tons and reserves around 3.2 million tons, are both looking to ramp up production to meet demand. China already imports much of its lithium supplies from Australian mines. A sudden boom in demand for electric vehicles actually caused a shortage in 2016 and prompted officials to look at increasing domestic production.
... it’s lithium’s difficult nature that makes it so valuable. It’s not rare; it’s just hard to get in the quantities we’re going to need. Which makes Lowry’s point all the more salient: the best thing for the lithium revolution now is to start getting some actual results out of all the energy industry’s talk.
Lirthium demand is being driven by sales of electric vehicles which according to Moringstar, are expected to rise from 0.3million in 2015 to 11million by 2025.
07/28 “Battery demand is rising at the rate of 1-2 new lithium mines per year, growing to 2-3 mines per year by 2020, and current demand will not only absorb all new mine developments in the pipeline, but will require fresh high quality deposits to be investigated for development,” says American Lithium’s Kobler. American Lithium raised capital in early May 2016 and bought prospective brine land in Nevada USA on a previously operating Boron mine in late May 2016.
Juniors such as American Lithium (TSXV:LI), Pure Energy Minerals (TSXV:PE), Nevada Sunrise Gold (TSXV:NEV), Alix Resources (TSXV:AIX), Nevada Energy Metals (TSXV:BFF), Lithium X (TSXV:LIX), Ashburton Ventures (TSXV:ABR), Cypress Development (TSXV:CYP), Sienna Resources (TSXV:SIE), Noka Resources (TSXV:NX), Matica Enterprises (CSE:MMJ) all have claims in the Clayton valley. Other companies that have projects in the state are Dajin Resources (TSXV:DJI), Eureka Resources (TSXV:EUK), and Ultra Lithium (TSXV:ULI).
Since the start of the year, the lithium industry has gained access to over $330 million in funding, whether from private placements, government grants, stock options, or other investment vehicles. The summer drilling season is upon us, and any lithium junior that doesn’t have a drilling program in place by this time is simply not worth looking at. Buying land in highly prospective areas is easy; completing a successful drill program with results takes more expertise.
Neometals (OTC:RRSSF), International Lithium Corp (OTCPK:ILHMF)
*****SQM...SQM is forcefully exerting its dominant position as the lowest cost, highestmargin incumbent lithium producer with aggressive growth plans in Argentina(09/05/16).FMC....**ALB (T/$94H-118H-130-136).. The three largest lithium producers are the Chile-based Sociedad Quimica y Minera (SQM), American FMC Lithium (FMC), which controls the ominously-named Hombre Muerte mine in Argentina, and Albermarle (ALB). 3 companies produce 90% of the world’s lithium with SQM controlling one third of all the world’s lithium reserves.
AABVF... *****ALTAF(T/$0.30) Altura Mining Australia()... ***AVLIF Advantage Lithium.... googleBCRMF Bacanora...
CRE.V Critical Elements lithium and rare earths Quebec.. googleCYDVF...
DJIFF Dajin Resources a minnow now in the lithium space, but their extensive holdings in the lithium triangle are truly astounding. They have some nice Nevada properties as well. *******GALXF... HREEF...google ILHMF... ***ILC.V.. BRGRF
*****LACDF (T/$1.30)Lithium ;Kings Valley Project located in northwestern Nevada... ***LIEG... .********LIXXF ()...
google LRTTF (hype stock 0.50) 07/21 secondary offering ... googleMLNLF ... LTUM Lithium Corp Nevada ****NLC.V... NMKEF .....
***NEV.V Nevada Sunrise http://www.nevadasunrise.ca/projects/nevadalithium/ *******OROCF Orocobre...*****ORRP () Lithium mine/Nevada...
***PEMIF Pure Energy Materials... PKX (Korean steel and now add lithium... googlePNXLF Argentina Lithium Co. ... googleRCKTF... googleSRCAF... SNET rare earths and lithium... googleSNNAF... SSMLF Nevada Energy Metals ...... google RRSSF Neometals... googleBCRMF() Bacanora Minerals
MLNLF Millennial Lithium Neometals (OTC:RRSSF), International Lithium Corp [TSXV:ILC] (OTCPK:ILHMF)
Altura Mining (OTCPK:ALTAF ALTAF $0.30 VERY NEAR PRODUCTION
........................Pilbara Minerals (OTCPK:PILBF PILBF $0.7 (VERY NEAR PRODUCTION)
In the swarm of new entrants on the lithium playing field, International Battery Metals (CSE:IBAT; OTC: RHHNF) stands out—front and center—because it’s sitting on a proprietary advanced technology that could push lithium into the production stage rapidly. It has signed an LOI with North American Lithium (NAL) to acquire all its lithium extraction process intellectual property and be restructured with NAL becoming an integral part of the company.
EVobsession.com/electric car sales KNDI...not seeing much of KANDI on these lists...
https://evobsession.com/electric-car-sales/ https://evobsession.com/tag/zotye-e200/ Batteries https://evobsession.com/ev-lithium-ion-battery-list-topped-by-lg-chem-johnson-controls-aesc/ Lithium/EV/Battery §BYDDF Electric vehicle China ...CBAK China Battery CCGI electric v ehicle (EV) charging stations... ENS EnerSys EGTYF Eguana... ***HPJ (T/$12.78) lithium,and nickel metal hydride rechargeable batteries... HRG... GELYF Geely Holding Co... *OGES lithium ion large format prismatic cells; small format prismatic cells; and battery modules. **PCRFY Panasonic Corporation... TANH electric vehicles and power batteries ... *ULBI lithium battery... UPGI ... OTHER/Related... RDRUY Neometals...LGEAF Korean Battery... FT.TO Fortune Minerals... LUN.TO Lundin Mining...EFLVF Electrovaya  Mar. 28, 2018 Kandi Technologies Group (NASDAQ:KNDI) and Zhejiang Geely Holding Group (OTCPK:GELYF, {GELYY]]) unveiled the all-electric Geely Global Hawk EX3 SUV at a ceremony in Hangzhou earlier this week. https://seekingalpha.com/pr/17114071-kandi-technologies-unveils-electric-suv-geely-global-hawk-ex3-hangzhou
Mar. 28, 2018 Kandi Technologies Group (NASDAQ:KNDI) and Zhejiang Geely Holding Group (OTCPK:GELYF, {GELYY]]) unveiled the all-electric Geely Global Hawk EX3 SUV at a ceremony in Hangzhou earlier this week. https://seekingalpha.com/pr/17114071-kandi-technologies-unveils-electric-suv-geely-global-hawk-ex3-hangzhou 

History of KNDI's ups and downs...
https://www.hvst.com/posts/kandi-technologies-chinas-ev-first-mover-completes-upgrades-reveals-new-evs-strong-h2-17-sales-laying-groundwork-for-likely-triple-to-old-high-in-2018-orgTv9QG "
According to Chairman Hu on the last investor Conference Call, KNDI was told by the Government that it was “next in line” to receive a license once the program is reopened in early 2018. " Jan 17, 2018
https://www.hvst.com/posts/kandi-technologies-chinas-ev-first-mover-completes-upgrades-reveals-new-evs-strong-h2-17-sales-laying-groundwork-for-likely-triple-to-old-high-in-2018-orgTv9QG *KNDI ...China electric vehicle...at one time this stock traded above $20...now 2017 there are a ton of lawsuits after Kandi Technologies According to the complaint, throughout the Class Period, the Company issued materially false and misleading statements and/or failed to disclose that: (1) certain areas in Kandi's previously issued financial statements for the years ended December 31, 2015 and 2014, and the first three quarters for the year ended December 31, 2016 required adjustment; (2) in turn, Kandi lacked effective controls over financial reporting; and (3) as a result, defendants' statements about Kandi's business, operations, and prospects, were materially false and misleading and/or lacked a reasonable basis at all relevant times.
On November 14, 2016, Kandi announced the resignation of Cheng Wang as its CFO. On this news, shares of Kandi fell to a close at $3.50 per share on November 14, 2016, damaging investors. On March 14, 2017, Kandi revealed that its previously issued financial statements for the years ended December 31, 2015 and 2014, and the first three quarters for the year ended December 31, 2016 will need to be restated. On this news shares of Kandi fell approximately 7% to close at $4.05 per share on March 14, 2017, further damaging investors.

60minute
 Now what Feb. 14, 2017
Now what Feb. 14, 2017 With the subsidy-payment drama now behind the company, its focus will be on developing new vehicles that won't rely solely on subsidy payments to be economically viable. Kandi is currently developing those EV products for after 2020, when the subsidy payments end. The company also has its hands full launching two new EV models this year and preparing its car-making joint venture company for its initial public offering.
This will be a pivotal year for Kandi and its investors. But don't expect its stock price to regain its lost value until management proves it can take steps to reduce its reliance on subsidy payments while reigniting its revenue.
Daniel Miller has no position in any stocks mentioned. The Motley Fool
On 22 August 2016. Kandi Technologies Group, Inc. (NASDAQ:KNDI) announced that Kandi Electric Vehicles Group Co., Ltd. (the “JV Company,” a 50/50 joint venture between Kandi and Geely Automobile Holdings Ltd.) has unveiled its new pure electric vehicle (“EV”) model named the “Global Hawk K21.” This new all-electric sedan is equipped with automatic braking technology, a voice control system, a infotainment system, remote software update capabilities, and a fully-integrated electrical control system. Packaged with the ternary battery, the vehicle has a top speed of 100+ km/h and a driving range exceeding 150km on a single full charge. The Global Hawk K21 joins the ranks of mid-tier luxury pure electric vehicles offered by the JV Company.
 An Electric Car Vending Machine Four-Passenger Pure Electric Sedan Vehicle
An Electric Car Vending Machine Four-Passenger Pure Electric Sedan Vehicle
 WKHS (T/$5) Workhorse Group Inc., a technology company, engages in the design, development, manufacture, and sale of electric medium duty trucks and unmanned aerial delivery systems that are integrated with electric vehicles. The company's products are used for commercial step vans in package delivery, product delivery, laundry and uniform service, food service, utility, and special use industries; medium duty buses, including para-transit, shuttle, and school buses; special use vehicles, such as emergency vehicles, wreckers, construction equipment, communications equipment, and military base application vehicles; and recreational vehicles. It also produces chassis with electric, propane, compressed natural gas, and hybrid configurations, as well as gasoline drive systems. The company was formerly known as AMP Holding Inc. and changed its name to Workhorse Group Inc. in April 2015. Workhorse W-15 light duty platform design is an extension of the E-Gen electric technology used in Workhorse medium-duty delivery trucks, and is believed to be the first plug-in range-extended electric pickup built from the ground up by an original equipment manufacturer (OEM) in America. With an expected 80-mile all-electric range using Panasonic 18650 Li-ion batteries, the battery pack will cover the vast majority of miles driven in a day by fleet operators. If needed, the gasoline generator will initiate after battery power has been depleted, allowing additional range to complete the day's tasks. The W-15 will also feature the ability for utility fleets to power equipment directly from the vehicle's batteries, allowing a crew to complete their duties that require electricity without an external power source. With the goal of being the safest pickup truck in America for fleet operators, the W-15's safety features include an extra large crumple zone and lower center of gravity while still providing ground clearance. The W-15 also offers crash mitigation technologies, including automatic braking and lane centering. Once production commences, the W-15 is anticipated to have a $52,500 MSRP. After 78 years, the helicopter has been reinvented - SureFly personal helicopter
WKHS (T/$5) Workhorse Group Inc., a technology company, engages in the design, development, manufacture, and sale of electric medium duty trucks and unmanned aerial delivery systems that are integrated with electric vehicles. The company's products are used for commercial step vans in package delivery, product delivery, laundry and uniform service, food service, utility, and special use industries; medium duty buses, including para-transit, shuttle, and school buses; special use vehicles, such as emergency vehicles, wreckers, construction equipment, communications equipment, and military base application vehicles; and recreational vehicles. It also produces chassis with electric, propane, compressed natural gas, and hybrid configurations, as well as gasoline drive systems. The company was formerly known as AMP Holding Inc. and changed its name to Workhorse Group Inc. in April 2015. Workhorse W-15 light duty platform design is an extension of the E-Gen electric technology used in Workhorse medium-duty delivery trucks, and is believed to be the first plug-in range-extended electric pickup built from the ground up by an original equipment manufacturer (OEM) in America. With an expected 80-mile all-electric range using Panasonic 18650 Li-ion batteries, the battery pack will cover the vast majority of miles driven in a day by fleet operators. If needed, the gasoline generator will initiate after battery power has been depleted, allowing additional range to complete the day's tasks. The W-15 will also feature the ability for utility fleets to power equipment directly from the vehicle's batteries, allowing a crew to complete their duties that require electricity without an external power source. With the goal of being the safest pickup truck in America for fleet operators, the W-15's safety features include an extra large crumple zone and lower center of gravity while still providing ground clearance. The W-15 also offers crash mitigation technologies, including automatic braking and lane centering. Once production commences, the W-15 is anticipated to have a $52,500 MSRP. After 78 years, the helicopter has been reinvented - SureFly personal helicopter \\\\\\\\\\\\\\\\\\\\\\\\\\\\\\\\\\\\\\\\\\\\\\\\\\\\\\\\\\\\\\\\\\\\\\\\\\\\\\\\\\\\\\\\\\\\\\\\\\\\\\\\\\\\\\\\\\\\\\\\\\\\\\\\\\\\\\\\\\\\\\\\\\\\\\\\\\\\\\\\\\\\\\\\\\\\\\\\\\\\\\\\\\\\\\\\\\\\\\\\\\\\\\\\\\\\\\\\\\\\\\\\\\\\\\\\\\\\\\\\\\\\\\\\\\\\\\\\\\\\\\\\\\\\\\\\\\\\\
JUNE 14, 2018 The writing is on the wall for non-smokable cannabis. A recent study from Deloitte reveals that smokable marijuana is expected to generate around $5 billion in sales in 2019. That's pretty impressive, and may be a low-ball estimate. Edible products like brownies, candy, and beverages are slated to bring in around $12 to $22 billion in sales. Marijuana Stocks http://marijuanastocks.com/ http://cannabisstocksnews.com/ http://www.fool.com/investing/2016/05/27/is-big-government-about-to-make-big-green-on-marij.aspx Marijuana Stocks EMHTF...the hype is this stock is the AAPL of Cannabis price up 10% (12/04/17...
http://wallst-news.com/cannabis-producer-emerge-apple-inc-industry/?utm_source=push&utm_content=v1&utm_campaign=120417_pm The HYPE will suck you in...so be careful... http://www.buyupside.com/sample_portfolios/cannabisallwithprices.php Database of Marijuana (Cannabis) Stocks ---
Database of Marijuana (Cannabis) Stocks Beginning in 2014, marijuana stocks were in vogue. Investors were scrambling the markets to find almost any stock that promoted themselves as an emerging player in the cannabis industry. The drawback in that investment strategy, however, was that
many investors were too preoccupied with finding a company with a catchy name, rather than a company that actually had some substance. While many investors were drawn in by
the myth that millions would be made by investing in the companies that purportedly planned to cultivate and sell medical and leisure based marijuana, they should have been focused on investing in companies that are intent on utilizing the plant's cannabinoid properties, which are consistently demonstrating extraordinary benefit in treating a host of medical conditions. With that said, if investors had only remained diligent on seeking realistic and lucrative potential through cannabis pharmaceuticals, Vitality Biopharma (
OTCQB:VBIO) would not have been overlooked. In fact,
VBIO is one of only three cannabinoid related companies that I see as actually being able to produce any meaningful revenue within the next few years. Investors need to separate themselves from the nonsense being promoted by companies that are looking to cultivate and sell the product. Despite what they say, once Big Tobacco decides that it's time to enter the market, these small time entrepreneurial bud houses will be pushed out of business faster than a bee to a beetle. For those investors betting a small fortune on any of these penny stock names, consider saving your money while you still can. The real money will be made in cannabinoid pharmaceuticals (CP) https://seekingalpha.com/article/4031914-vitality-biopharma-seriously-undervalued-cannabis-stockhttp://www.fool.com/investing/2016/05/27/is-big-government-about-to-make-big-green-on-marij.aspx 




Marijuana Stock Scams Inside The Pot Stock Bubble There's no two ways about it: As an investor, you'd struggle to find an industry with a more appetizing long-term growth rate than legal marijuana. According to cannabis research firm ArcView, North American legal sales increased by 34%, to $6.9 billion in 2016, albeit black-market sales still totaled $46.4 billion. As more U.S. states push to legalize, and with Canada potentially on the precipice of legalization by 2018, the expectation is more that this $46 billion-plus in illicit sales will shift to the legal and regulated markets in the years to come.
By 2026, investment firm Cowen & Co. anticipates total legal sales could reach $50 billion. If that's the case, we're talking about more than 23% annual growth for a decade. It's these figures that are attracting businesses and investors to marijuana stocks in droves.

ACT...ETF...
invests at least 80% of its net assets (plus any borrowings for investment purposes) in securities of companies that derive at least 50% of their net revenue from tobacco and alcoholic beverages and companies that derive at least 50% of their net revenue from the marijuana and hemp industry or have at least 50% of their company assets dedicated to lawful research and development of cannabis or cannabinoid-related products. It will invest primarily in U.S. exchange listed equity securities, including common and preferred stock and ADRs.  Canopy Growth Corp. (OTCMKTS:TWMJF) impatient. It’s supercharged its future prospects through ambitious acquisitions — such as its 2016 move to purchase Mettrum Health for $430 million — giving it access to roughly half of Canada’s medical marijuana customers and increased its production to include six licensed facilities and two more unique brands of product. It’s a double-edged sword to be such an aggressive acquirer in this budding market, because a few bad deals at big price tags could really set Canopy back. However, if the strategy pays off, it will soon become one of the dominant marijuana companies in North America and operate at a scale few publicly traded companies can match. Constellation Brands — which sells Corona beer, Svedka vodka, and other brands. It ponied up around $191 million for a 9.9% stake in Canopy Growth Corp. They are going to jointly develop and market a series of cannabis-infused beverages. It won't be long until we see more bud-beers and weed-wines. It's only a matter of time. In fact, recent estimates show that legal marijuana sales will outpace soda sales by 2030. It's already slated to be a bigger market than beer in Canada. CRON has become an attractive play in the legalization of recreational cannabis. Taking a valuation call on the stock and the sector, shares now ruling at $6.77, it can move to a level of $13.50 in next few months.
Canopy Growth Corp. (OTCMKTS:TWMJF) impatient. It’s supercharged its future prospects through ambitious acquisitions — such as its 2016 move to purchase Mettrum Health for $430 million — giving it access to roughly half of Canada’s medical marijuana customers and increased its production to include six licensed facilities and two more unique brands of product. It’s a double-edged sword to be such an aggressive acquirer in this budding market, because a few bad deals at big price tags could really set Canopy back. However, if the strategy pays off, it will soon become one of the dominant marijuana companies in North America and operate at a scale few publicly traded companies can match. Constellation Brands — which sells Corona beer, Svedka vodka, and other brands. It ponied up around $191 million for a 9.9% stake in Canopy Growth Corp. They are going to jointly develop and market a series of cannabis-infused beverages. It won't be long until we see more bud-beers and weed-wines. It's only a matter of time. In fact, recent estimates show that legal marijuana sales will outpace soda sales by 2030. It's already slated to be a bigger market than beer in Canada. CRON has become an attractive play in the legalization of recreational cannabis. Taking a valuation call on the stock and the sector, shares now ruling at $6.77, it can move to a level of $13.50 in next few months. Seeks licensed, or actively seeking a license, to produce medical marijuana pursuant to Canada's Marihuana for Medical Purposes Regulations. The firm typically invests in companies based in Canada. The firm is primarily an equity investor, may also advance debt as appropriate.
Daily...saying this
could almost double from 6.80...
\

 Medical Marijuana ***ACBFF (T/$ 3.25H) AURORA CANNABIS is one of the leading players in the raging Canadian cannabis space. We have been strong fans of ACBFF over the past year because of its topline growth and fortress balance sheet –
Medical Marijuana ***ACBFF (T/$ 3.25H) AURORA CANNABIS is one of the leading players in the raging Canadian cannabis space. We have been strong fans of ACBFF over the past year because of its topline growth and fortress balance sheet – current operations are focused largely on harvesting plants for patients with prescriptions, marijuana legalization in the United States (and potentially in Canada) could open up a big growth path for Aurora in the next few years. Yes, the company currently is operating at a loss. But it went from zero revenue in 2015 to over $1 million in sales last year, and is growing fast. If you’re an aggressive investor, this is exactly the kind of company you want to be invested in to get in on the ground floor of the marijuana megatrend. Like Canopy, Aurora could hit $200 million in sales by the end of next year. That, coupled with a U.S. listing, would catapult this stock to instant success.
 APH.TO
APH.TO Aphria Inc is engaged in the production and selling of medicinal marijuana, and while the stock has trended downward since April, the constant profits here suggest there is a lot of upside. The recent pro-marijuana legislation from the Canadian government is sure to boost companies with the reputation of Aphria Inc.Aphria’s $137 million expansion project is well underway to ramp up output to 70,000 kg. If Aphria successfully ramps up production, we believe its share price could go much higher.
GBLX ()
Growblox Sciences is a biotechnology research and development company focused on creating safe, standardized pharmaceutical-grade cannabis-based therapies that target a variety of medical conditions. The company is pioneering technology, industry-leading processes, and a big data-driven clinical research and development algorithm to bring relief to patients in communities across the country. On may 23 GBLX announced the filing of its latest patent application by its wholly-owned subsidiary, Growblox Life Sciences, LLC for the treatment of chronic pain and heart therapies based on myrcene-containing complex mixtures (“MCCM”). The current US opioid epidemic, along with growing public and government concern regarding opioid abuse, makes new pain treatments a promising field of research and development. Our novel pain formulations are also substantially free of delta-9 tetrahydrocannabinol (“THC”), which minimizes their potential for abuse. Pain management represents an estimated health burden of between $560 to $650 billion dollars for chronic pain and between $17 to $20 billion dollars for immunological/neuropathic pain in the US alone. In addition, our novel heart disease formulations address the $316 billion dollar heart therapy market in the US.
http://www.microcapdaily.com/long-and-short-on-growblox-sciences-inc-otcmktsgblx/118916/ *****GWPH GW Pharmaceuticals ... developing, and commercializing cannabinoid prescription medicines using botanical extracts derived from the Cannabis plant. CAMBRIDGE U.K. *****CARA (T/$22-27) chronic pain ...
Cara's ties to cannabis relates to its preclinical research involving CR701, a cannabinoid receptor agonist that's designed to treat chronic pain. Considering that there were no documented overdose-related deaths from marijuana in 2016, compared to over 20,000 prescription opioid overdose deaths in 2015, there's a clear push to consider CB agonists as a possible solution to chronic pain. In rodent models, CR701 produced "hyperalgesia (sensitization of nerve ending to panful stimuli) and allodynia (painful perception of innocuous stimuli) comparable to human conditions."  ****CRBP (T/17-24) Corbus Pharmaceuticals
****CRBP (T/17-24) Corbus Pharmaceuticals is considerably more of a wildcard than GW Pharmaceuticals, but if one specific clinical trial goes its way, it could easily leap to $1 billion in annual sales. Corbus' pipeline consists of a solitary drug known as anabasum, which is a synthetic oral endocannabinoid-mimetic drug that binds to the CB2 receptors expressed on immune cells and fibroblasts. Its drug is being tested in four indications, but one stands out head and shoulders above the rest:
cystic fibrosis (CF). There are few drugs to treat CF, and most that are FDA approved target a very specific mutation, making their impact limited within the CF community. Anabasum is believed to have a global anti-inflammatory effect, meaning it could be taken by most, or all, CF patients.The downside? Even though anabasum hit its primary endpoint of a 75% reduction in the pulmonary exacerbation event rate in phase 2 trials, it didn't provide any improvement in lung function as measured by the forced expiratory volume within the first second (FEV1) test. FEV1 improvement is often seen with FDA approved CF drugs, leaving anabasum's impact on CF somewhat uncertain. (Motley Fool)
 ERBB (Google) American Green is heavily banking on the Arizona marijuana market. ERBB signed a Purchase Agreement for a Class A building in Tempe, Arizona that will be a prime location for a Medical Marijuana Dispensary it will lease to one of its license holder clients. IGC cannabinoid based biopharmaceutical for end of life supportive care,India... *****INSY ...(T-$16-22) Insys ...MEDICAL MARIJUANA, synthesized marijuana cannabidiol...*** 06/16/2017 Insys has been the subject of negative publicity as state and federal investigations continue against six ex-employees regarding the sales and billing practices of the company in the past. The investigations surround the company's drug, Subsys, a fentanyl-based sublingual spray used for the treatment of cancer patients. Insys issued a response to recent media reports by reiterating its commitment towards the highest ethical standards and compliance with all its activities and business practices and complying with governing laws and regulations and cooperating with relevant governmental authorities in ongoing investigations. Management also highlighted that Insys intends to play a meaningful role in providing solutions to address the opioid epidemic by developing innovative products. These potential product solutions include cannabidiol (a novel, non-opioid) for the treatment of opioid dependence, for the treatment of pain and naloxone nasal spray for the treatment of opioid overdose.Recent negative headlines continue to impinge the business risk profile & brand image of the company. Elizabeth Gurrieri, a former manager of reimbursement services for Arizona-based Insys, will plead guilty to one count of wire fraud conspiracy, prosecutors said in a letter filed in Boston federal court. The filing is part of the criminal case against six ex-Insys executives and managers including former Chief Executive Michael Babich, who, prosecutors say, participated in a scheme to bribe doctors to prescribe the drug, Subsys. Gurrieri would become the second former Insys employee nationally to plead guilty in connection with Subsys, an under-the-tongue spray containing fentanyl, a highly addictive and regulated synthetic opioid. On the brighter side, FDA approved the final labeling for Syndros (dronabinol oral solution, a pharmaceutical version of THC), which may classify the drug as a Schedule II controlled substance. Insys expects to launch the product in August 2017. Syndros is indicated for the treatment of anorexia associated with weight loss in patients with AIDS and in the treatment of nausea and vomiting associated with cancer chemotherapy in patients who have failed to respond adequately to conventional antiemetic treatments. Syndros will be a direct competitor to Marinol, a product offered by Abbvie in pill form. Since Syndros is a liquid, it should be easier to swallow and can be administered in prescribed dosages. Dronabinol was approved by the FDA decades ago as a treatment option for cancer patients. Today, the potential applications for dronabinol, including sleep apnea and opioid dependency, could catapult this drug to annual sales in the billions.
ERBB (Google) American Green is heavily banking on the Arizona marijuana market. ERBB signed a Purchase Agreement for a Class A building in Tempe, Arizona that will be a prime location for a Medical Marijuana Dispensary it will lease to one of its license holder clients. IGC cannabinoid based biopharmaceutical for end of life supportive care,India... *****INSY ...(T-$16-22) Insys ...MEDICAL MARIJUANA, synthesized marijuana cannabidiol...*** 06/16/2017 Insys has been the subject of negative publicity as state and federal investigations continue against six ex-employees regarding the sales and billing practices of the company in the past. The investigations surround the company's drug, Subsys, a fentanyl-based sublingual spray used for the treatment of cancer patients. Insys issued a response to recent media reports by reiterating its commitment towards the highest ethical standards and compliance with all its activities and business practices and complying with governing laws and regulations and cooperating with relevant governmental authorities in ongoing investigations. Management also highlighted that Insys intends to play a meaningful role in providing solutions to address the opioid epidemic by developing innovative products. These potential product solutions include cannabidiol (a novel, non-opioid) for the treatment of opioid dependence, for the treatment of pain and naloxone nasal spray for the treatment of opioid overdose.Recent negative headlines continue to impinge the business risk profile & brand image of the company. Elizabeth Gurrieri, a former manager of reimbursement services for Arizona-based Insys, will plead guilty to one count of wire fraud conspiracy, prosecutors said in a letter filed in Boston federal court. The filing is part of the criminal case against six ex-Insys executives and managers including former Chief Executive Michael Babich, who, prosecutors say, participated in a scheme to bribe doctors to prescribe the drug, Subsys. Gurrieri would become the second former Insys employee nationally to plead guilty in connection with Subsys, an under-the-tongue spray containing fentanyl, a highly addictive and regulated synthetic opioid. On the brighter side, FDA approved the final labeling for Syndros (dronabinol oral solution, a pharmaceutical version of THC), which may classify the drug as a Schedule II controlled substance. Insys expects to launch the product in August 2017. Syndros is indicated for the treatment of anorexia associated with weight loss in patients with AIDS and in the treatment of nausea and vomiting associated with cancer chemotherapy in patients who have failed to respond adequately to conventional antiemetic treatments. Syndros will be a direct competitor to Marinol, a product offered by Abbvie in pill form. Since Syndros is a liquid, it should be easier to swallow and can be administered in prescribed dosages. Dronabinol was approved by the FDA decades ago as a treatment option for cancer patients. Today, the potential applications for dronabinol, including sleep apnea and opioid dependency, could catapult this drug to annual sales in the billions.  LHSIF... one of just 13 licensed producers and sellers of medical marijuana in the state of Florida.
LHSIF... one of just 13 licensed producers and sellers of medical marijuana in the state of Florida. 
HMLSF...sm cap stocks....
| Marijuana Stock | Ticker Symbol |
| Abcann Global | (ABCN) |
| Beleave Inc. | (BE) |
| Harvest One Cannabis Inc. | (HVST) |
| Maple Leaf Green World | (MGW) |
| Maricann Group | (MARI) |
 LBUY
LBUY LEAF.TO As a licensed producer of cannabis-based pharmaceutical products, MedReleaf Corp has a head start on the coming boom in Canada. Early July has seen a bounce in the stock price, and investors may look to ride it upward from here. Med Releaf could become Canada’s second biggest medical marijuana company after Canopy Growth Cooperation as many analysts expect the ‘legal weed’ industry to grow 25 percent on an annual basis over the next 10 years. MedReleaf has seen its share price fall since June, but has steadied out in recent months and could be poised for gains as the expected legalization of recreational marijuana materializes.
MJNA MORE HYPE THAN FACTS don't trade ...medical marijuana and industrial hemp...Medical Marijuana Inc (OTCMKTS:MJNA), according to company documents, bills itself as an investment holding company that operates in the medical marijuana and industrial hemp markets. Its products range from patented and proprietary based cannabinoid products to seed and stalk or isolated high-value extracts manufactured and formulated for the pharmaceutical, nutraceutical, and cosmeceutical industries. PRMCF Cronos ...google .. 7% of HMMJ.TO ETF
OGRMF google
TRPX () ... Therapix Biosciences Ltd is a biopharmaceutical Israeli-based company engaged in identifying and investing in biopharma technologies and developing approved drugs based on cannabinoid molecules. Phase 3 trial Tourette's syndrome, a somewhat bizarre disorder that has become something of a counterculture icon of a disease. Sufferers often have uncontrollable tics, which might include twitching, shouting profanity or other sounds, eye blinking, coughing and throat clearing. It's actually a surprisingly common neurological condition. Studies suggest that the prevalence of Tourette’s in the overall population at any time is 0.1% for impairing cases and 0.6% for all cases.  ***VBIO ... https://seekingalpha.com/article/4031914-vitality-biopharma-seriously-undervalued-cannabis-stock
***VBIO ... https://seekingalpha.com/article/4031914-vitality-biopharma-seriously-undervalued-cannabis-stock VBIO is intensely focused on treating Narcotic Bowel Syndrome (NBS) and Inflammatory Bowel Disease (IBD). In the case of NBS, it's been reported that up to 81% of opiate users have functional bowel disorders, with more than 58% reporting chronic abdominal pain during an independently conducted study. From this set of patients, over 6% will ultimately develop NBS. The symptoms associated with NBS can be devastating, and it has been reported that the downward spiral related to quality of life issues has been associated with 61% of all drug overdose deaths.
In prior, non-related studies, cannabinoids have proven the ability to effectively treat Crohn's disease patients, with over 40% of the patients having had the disease put into remission. These prior results, though unrelated to Vitality Biopharma, do have the potential to open additional regulatory pathways for expedited approval. VBIO product pipeline includes Cannabosides – VITA-100 for gastrointestinal disorders, including inflammatory bowel disease and irritable bowel syndrome; Cannabosides – VITA-210 for muscle spasticity in multiple sclerosis and rare white matter disorders; and Cannabosides for epilepsy, schizophrenia, substance abuse, and Huntington’s disease. ??? A prodrug is a medication or compound that, after administration, is metabolized (i.e., converted within the body) into a pharmacologically active drug. Prodrugs are often designed to improve bioavailability when a drug itself is poorly absorbed from the gastrointestinal tract, or to overcome other issues that make molecules unappealing candidates for future development efforts. A prodrug may for instance be used to selectively target a specific tissue or organ, such as the brain or gut, which enables the drug to have a more targeted effect. Prodrugs have been around for a while, with Aspirin, acetylsalicylic acid, first made by Felix Hoffmann at Bayer in 1897, a classic example of a synthetic prodrug of salicylic acid. As of 2015, there were approximately 15 prodrugs that had been classified as blockbusters, defined as having annual sales in excess of $1 billion.
On March 10 VBIO announced it has obtained positive results demonstrating antimicrobial activity of cannabinoids and filed for patent protection on the use of cannabinoid compounds for the treatment of microbes including Clostridium difficile and other “superbug” pathogens. Utilizing a list of the top drug-resistant pathogens from the United States Centers for Disease Control and Prevention (CDC), Vitality researchers screened for antimicrobial activity in their portfolio of compounds. Vitality Biopharma discovered new antimicrobial activities for cannabinoids, and as a result has filed for patent protection on the use of cannabinoids and cannabinoid prodrugs for the treatment of multiple pathogenic bacterial infections.
Vitality Biopharma Inc(OTCMKTS:VBIO) says it is dedicated to unlocking the power of cannabinoids for the treatment of serious neurological and inflammatory disorders. VBIO product pipeline includes Cannabosides – VITA-100 for gastrointestinal disorders, including inflammatory bowel disease and irritable bowel syndrome; Cannabosides – VITA-210 for muscle spasticity in multiple sclerosis and rare white matter disorders; and Cannabosides for epilepsy, schizophrenia, substance abuse, and Huntington’s disease.
Vitality Biopharma has developed a new class of cannabinoid prodrug, known as cannabosides, which upon ingestion can enable the selective delivery of THC and cannabidiol (CBD) to the gastrointestinal tract. Site-specific delivery could enable oral drug formulations of cannabinoids to provide therapeutic benefits while reducing or avoiding the systemic delivery of THC into the bloodstream. Currently, high concentrations of psychoactive THC in the brain limit the dose of cannabinoids that can be used elsewhere in the body for treatment of pain and inflammation.
Independent clinical trial results suggest that cannabinoids will help induce remission in Crohn’s disease patients, and that the vast majority of inflammatory bowel disease patients experience symptomatic relief, including more than 75% of patients who report improvement in visceral pain and abdominal cramping. Approximately 1.4 million Americans are affected by inflammatory bowel diseases, including Crohn’s disease and ulcerative colitis. Most patients are diagnosed before age 30 and require life-long treatment.
In addition to providing targeted delivery, cannabosides could enable a better tasting formulation for improved patient compliance, better oral bioavailability that provides safer and more reliable dosing, and a delayed release mechanism that enables patients using these medications to have long-lasting, overnight relief. http://www.microcapdaily.com/the-gloves-come-off-on-vitality-biopharma-incotcmktsvbio/119372/
*VRTHF...medical marijuana...
ZGNX ()
ZYNE (T/$9H-11H-14-17) medicinal marijuana

Other Marijuana ***AMMJ two divisions within the regulated cannabis industry: consulting and professional services, and the sale of products and equipment utilized in the cultivation, processing, transportation or retail sale of cannabis. The Company offers consulting services for companies associated with the cannabis industry in various stages of development. Its service offerings include Cannabis Business Planning, Cannabis Business License Applications, Cultivation Build-out Oversight Services, Cannabis Regulatory Compliance, Compliance Audit Services, Cannabis Business Growth Strategies and Cannabis Business Monitoring. Its Equipment and Supplies product offerings include The Satchel, SoHum Living Soil, High Density Racking System and The Cultivation Cube. ****APHQF Aphria (NASDAQOTH:APHQF). Aphria has the distinction of being the most prominently profitable marijuana stock, with the company reporting five consecutive quarterly profits. *** CANN General Cannabis... GRNH GreenGro greenhouse/growing equiptment.. ***HEMP marijuana industries... Perhaps no other name has personified the pot stock boom and bust more so than Hemp Inc (OTCMKTS:HEMP) CEO Bruce Perlowin. He relished his role as the “King of Pot” and touted his background as a former drug smuggler who served nine years in prison. Well, it seems he never outgrew his old ways as the SEC charged Hemp Inc, Bruce Perlowin, and others with a “long-running and profitable scheme resulted in the sale of hundreds of millions of unregistered and purportedly unrestricted Hemp shares to public investors. The execution of this scheme involved, among other things, purported gifts and consulting agreements that do not appear to have been bona fide and fraudulent statements made to Commission-registered broker-dealers.” June 22, 2016 The sad part is that Hemp was actually making some progress. Last month and after months of waiting, Hemp Inc finally produced its first environmentally friendly product-to-market from the company’s new decortication plant in Spring Hope, North Carolina. This was a pretty big deal for HEMP and its investors as the company has basically bet the ranch on this plant and the state of North Carolina. We told investors back in February that the company was making a comeback and things were finally starting to come together. https://www.insiderfinancial.com/get-out-of-hemp-inc-otcmktshemp-while-you-can/115823/
LBUY () Leaf Buyer ...hyped as the Priceline of pot...(theStreet.com)
VNNYF... VPRB... UBQU... MCIG... INSY... CARA ...APHQF... *DIGP cannabis testing; and provides cannabis news with a news/talk radio, ... *VRTHF...medical marijuana...CBDS...Cannabis Sativa... HMPQ Hemp America... ERBB, CBIS,PHOT...VBIO... **HTCO ...POTN TBPMF... POTN...March 09, 2018 (GLOBE NEWSWIRE) -- PotNetwork Holding, Inc. (OTC Pink:POTN) (“Company”) is pleased to announce today that PotNetwork the Company and Diamond CBD have been the feature of a recent comprehensive research report projecting a price per share
target of $1.25 by Harbinger Research, LLC, a notable equity research boutique based in Atlanta, Georgia.
March 07, 2018 (GLOBE NEWSWIRE) -- via OTC PR WIRE--PotNetwork Holding, Inc. (OTC Pink:POTN) (“Company”)announced today that its wholly owned subsidiary, Diamond CBD Inc.com, achieved over $500,000 in website- generated orders, surpassing all previous online sales records.
“Even though February is the shortest month of the year, we reached online sales higher than ever before.
Daily...5,000 shares...don't like below $0.35 stop...so risk around $250 to make $2000...
 http://stockcharts.com/c-sc/sc?s=GWPH&p=D&yr=0&mn=6&dy=0&i=p40394851540&r=1398788554470
http://stockcharts.com/c-sc/sc?s=GWPH&p=D&yr=0&mn=6&dy=0&i=p40394851540&r=1398788554470 ****INSY ...
T-$22 MEDICAL MARIJUANA, synthesized marijuana cannabidiol... *VRTHF...medical marijuana...CBDS...Cannabis Sativa... HMPQ Hemp America...
ERBB, CBIS,PHOT...VBIO... **HTCO... TRLFF...***TWMJF http://stockcharts.com/c-sc/sc?s=GWPH&p=D&yr=0&mn=6&dy=0&i=p40394851540&r=1398788554470 http://marijuanaindex.org/ WHO CAN BE A MEDICAL PATIENT IN WASHINGTON? WHO CAN PURCHASE RECREATIONALLY?
Under Washington law, recreational marijuana can be purchased, sold, or transferred by and to anyone 21 years and older. Washington medical patients first must be Washington state residents. Patients are not restricted by age, though in order for someone under 18 to become a medical patient, the parent must consent and register to be the child's caregiver. The physician is required to consult with other physicians before certifying the medical use of marijuana for patients under 18. Furthermore, the physician must see the patient at least twice a year - as opposed to the annual requirement for adults - to evaluate the patient symptoms and effectiveness of treatment.
For all other Washington medical patients, you must be at least 18 years of age. The state of Washington currently recognizes the following ailments and debilitating illnesses in their medical program: - Cancer
Human Immunodeficiency Virus (HIV)
Multiple Sclerosis
Epilepsy or other seizure/ spasticity related disorders
Pain not adequately addressed by current medicine options
Glaucoma, either acute or chronic, that is unresponsive to current treatments
Crohn's disease
Hepatitis C
Anorexia, which can result in nausea, vomiting, wasting, appetite loss, cramping, seizures, muscle spasms, or spasticity
Chronic kidney failure
Posttraumatic stress disorder (PTSD)
Traumatic brain injuries
The Cannabis Patient Protection Act also allows petitions for additional conditions and diseases to be added to the list of approved illnesses. Anxiety, depression, and a variety of other mental health issues, however, have been determined by Washington state not to be adequately addressed by medical marijuana. HOW DO I BECOME A MEDICAL MEMBER IN WASHINGTON?
First, you must be a Washington state resident. The next step is seeking a recommendation from a doctor in good standing with the state. Washington currently recognizes the following health professionals as being eligible to recommend patients: - Medical doctor (MD)
Physician assistant (PA)
Osteopathic physician (DO)
Osteopathic physician assistant (DOA)
Naturopathic physician
Advanced registered nurse practitioner (ARNP)
<<<<<<<<<<<<<<<<<<<<<<<<<<<<<<<<<<<<<<<<<<<<<<<<<<<<<<<<<<<<<<<<<<<<<<<<<<<<<<<<<<<<<<<<<<<<<<<<<<<<<<<<<<<<<<<<<<<<<<
Cannabis Wheaton (TSX.V:CBW) ; (OTC: CBWTF) – the first of its kind to offer access to funding and expertise that could prove critical to licensed producers as they look to expand as quickly as possible. Cannabis Wheaton (TSX.V:CBW) ; (OTC: CBWTF) doesn’t actually grow, harvest or produce marijuana on its own.
By applying a “streaming” business model with a proven history of success in the mining industry, Cannabis Wheaton (TSX.V:CBW) ; (OTC: CBWTF) offers investors a unique chance at high upside potential in this rapidly-growing industry.
 FUAPF (Global Cannabis Applications Corp) *OTCQB*
FUAPF (Global Cannabis Applications Corp) *OTCQB*
$0.18221 as of 04/08/2018 https://www.otcmarkets.com/stock/FUAPF/profile Description Global Cannabis Applications Corp. is a global leader in designing, developing, marketing, and acquiring innovative mobile applications. Used in over 25 countries, Global Cannabis' apps facilitate the evolution of conversation by like-minded people in a digital environment. Managed by digital industry experts, Global Cannabis is focused on global expansion and providing the best user experience in each target market. Its leading mobile platforms are Citizen Green, a platform dedicated to the digital world of all things cannabis; Foro, a peer-to-peer mobile ecommerce student marketplace; Opinit, an app that enables users to socially share their favourite online sentiment-driven content; and Truth, a one-to-one anonymous messaging app.
====================================================================================================== These are ten of Bob Farrell’s most famous observations:
1. “Markets tend to return to the mean over time.” For those of you who’ve taken statistics, you know exactly what this refers to: stocks often move too far in one direction as euphoria or pessimism clouds people’s thinking. Investors lose perspective and start believing the little devil on their shoulders.
2. “Excesses in one direction will lead to an opposite excess in the other direction.” I think of it like bungee jumpers whose cords stretch out and then compress multiple times before they come to rest or achieve equilibrium.
3. “There are no new eras – excesses are never permanent.” As the latest hot sector climbs higher and higher, you inevitably hear variations of the chorus shouting “it’s different this time”. Of course, human nature does not change so it never really turns out to be any different.
4. “Exponential rapidly rising or falling markets usually go further than you think, but they do not correct by going sideways.” The smart money locks in profits which leads to significant selling and inevitably to a correction.
5.“The public buys the most at the top and the least at the bottom.” It’s been this way since humans invented commerce.
6.“Fear and greed are stronger than long-term resolve.” Research in behavioral finance has shown that stock market gains make us exuberant; they enhance well-being and promote optimism which makes investors like to buy. Losses, on the other hand, bring sadness, disgust, fear and regret. Fear increases the sense of risk which thereby makes investors shun stocks.
7.“Markets are strongest when they are broad and weakest when they narrow to a handful of equities.” Think of it as strength in numbers. Broad breadth (i.e. market participation) and big volume is important. When wide ranging momentum channels into a small number of stocks, the top is near.
8.“Bear markets have three stages – sharp down move, reflexive rebound and a drawn-out fundamental downtrend.”
9.“When all the experts and forecasts agree, something else is going to happen.” Farrell suggests that patient buyers who raise cash in frothy markets and reinvest when sentiment is darkest can profit nicely.
10.“Bull markets are more fun than bear markets.” It has been my observation that historically the markets have rewarded optimists to a far larger degree than pessimists. I prefer to play in the bulls’ camp.
Trade well; trade with discipline!-- Gatis Roze
Linda Raschke's 12 Rules for Technical Trading. 1. Buy the first pullback after a new high. Sell the first rally after a new low.
2. Afternoon strength or weakness should have follow through the next day.
3. The best trading reversals occur in the morning, not the afternoon.
4. The larger the market gaps, the greater the odds of continuation and a trend.
5. The way the market trades around the previous day’s high or low is a good indicator of the market’s technical strength or weakness.
6. The previous day’s high and low are two very important “pivot” points, for this was the definitive point where buyers or sellers came in the day before. Look for the market to either test and reverse off these points, or push through and show signs of continuation.
7. The last hour often tells the truth about how strong a trend truly is. “Smart” money shows their hand in the last hour, continuing to mark positions in their favor. As long as a market is having consecutive strong closes, look for up-trend to continue. The up trend is most likely to end when there is a morning rally first, followed by a weak close.
8. High volume on the close implies continuation the next morning in the direction of the last half-hour. In a strongly trending market, look for resumption of the trend in the last hour.
9. The first hour’s range establishes the framework for the rest of the trading day.
10. A greater percentage of the day’s range occurs in the first hour then was the case in the past, and thus it has become increasingly important to trade aggressively if there are early signs of a strong trend for the day.
11. There are four basic principles of price behavior which have held up over time. Confidence that a type of price action is a true principle is what allows a trader to develop a systematic approach. The following four principles can be modeled and quantified and hold true for all time frames, all markets. The majority of patterns or systems that have a demonstrable edge are based on one of these four enduring principles of price behavior. Charles Dow was one of the first to touch on them in his writings.
Principle One: A Trend Has a Higher Probability of Continuation than Reversal
Principle Two: Momentum Precedes Price
Principle Three: Trends End in a Climax
Principle Four: The Market Alternates between Range Expansion and Range Contraction!
12. In the world of money, which is a world shaped by human behavior, nobody has the foggiest notion of what will happen in the future. Mark that word – Nobody! Thus the successful trader does not base moves on what supposedly will happen but reacts instead to what does happen. To better understand and appreciate the trading systems seen on this board Break a bad habit "The truth is that trading, both successful and unsuccessful, is more about psychology than tactics." - Jack Schwager Multicollinearity... Bollinger Bands... CCI... NOISE...RANDOM...CHAOS...ORDER...STRUCTURE ...if you don't know where you are at...how will you know where you're going? Structure= creates Order out of CHAOS Keep Keeping It Simple...Buy low...Sell high...relative to what...? (one day will edit) Patience...Moving averages...CONFLUENCE..
3day moving average study...Grail QUESTion
...it may cure that gambler in you...with understanding ...gambling/fear gets replaced with confidence and patience... http://investorshub.advfn.com/boards/read_msg.aspx?message_id=78642915 SLV...3 cross 5 moving average Adapting Moving Averages to Market Action Quest of the TREND Definition for TREND...and...Momentum Volume speaks VOLUMES... Momentum volume and momentum Stochastics Accumulation/Distribution and how to use the Candle Glance Charts
Price gaps occur when the range of a price bar does not include the range of the previous bar. It acts as a reliable level of support and resistance for subsequent price action and should always be monitored. Does Momentum Investing Work? Momentum Investing With ETFs !PMOBUYALL...Percentage of PMO Crossover Buy Signals Sentiment Extreme Gold Silver www.vectorgrader.com $VIX... "Black Swan" Hot IPO Pullback
Rules (via 'Hit & Run Trading II'): Our proven model does not conclusively show that (any stock) will beat estimates this quarter. That is because a stock needs to have a positive Earnings ESP and a Zacks Rank #1 (Strong Buy), 2 (Buy) or 3 (Hold) for this to happen.



































