Register for free to join our community of investors and share your ideas. You will also get access to streaming quotes, interactive charts, trades, portfolio, live options flow and more tools.
USRM: MIKEY'S NEW GIG LMAO !!!
Hey Reagan - was trying to get time and reply to you. Hope you also have had a good summer and some time in the sun and with family.
DIG THIS - besides a NEW JUDGEMENT AGAINST THIS BUSTED-OUT RENT-a-DESK NON EXISTENT SCAM SCAM SCAM....ole DIRTY MIKE aka Mike Michael Miguel Tomas has a "new gig" Bwaaaa ha ha ha ha .....
YOU KNOW - cause all "CEO's of public traded companies" have time to jack around and side-gig and work another hustle etc. WTF ????
AND in EVERY COURT PROCEEDING ole MIKEY never ever ever "shows" or answers the subpoenas etc - he passes the buck to the imaginary "VP" of USRM-a-CRIME the clown Julietta Radich or whatever the hell her names is....yeah....a FIVE ZERO...aka .000001 per share shit heap has a VP let alone a "CEO"....
Mike Tomas - 3.0 Capital
LinkedIn · Mike Tomas
6.6K+ followers
Miami-Fort Lauderdale Area · 3.0 Capital
Mike Tomas, 3.0 Capital, University of Miami, About, President & CEO, US Stem Cell, Inc (fka Bioheart Inc 2010 to present), Partner.
08/05/2024 Motion for Garnishment After Judgment Sub Garnishee: United Community Bank/ $ 112,803.88
Party: Plaintiff Daily Wellness LLC
/6
08/05/2024 eWrit Issuance - Garnishment Sub Garnishee: United Community Bank/ $ 112,803.88
/2
07/30/2024 Notice of Appearance Supplementary, Dr. KRISTIN COMELLA, MARY ELLEN COMELLA, and BIOHACKERS HEALTH AND FITNESS, LLC, and
equests that all future correspondence and pleadings be
/2
07/22/2024 Amended Complaint
/23
07/19/2024 Order Granting Leave to Amend Complaint
/2
07/18/2024 Final Judgment in Garnishment AGAINST GARNISHEE, US STEM CELL. INC
/2
FINAL GARNISHMENTJUDGMENTAGAINST US STEM CELL. INC.
THIS MATTER came before the Court for hearing on July 17, 2024 on the Plaintiff's Motion
for Entry of Final Garnishment Judgment against Garnishee, US Stem Cell, Inc. ("Motion") and the
Court, having reviewed the Motion, been advisedthat neither the Garnishee, U.S Stem Cell, Inc. nor the
Judgment Debtor, US Stem Cell Clinic, LLC, object to the Motion, and otherwise being fully advised in
the premises, it is hereby
ORDERED and ADJUDGED that the Motion is hereby GRANTED. Accordingly, a Final
Garnishment Judgment is entered in favor of Plaintiff, DAILY WELLNESS, LLC (whose address is
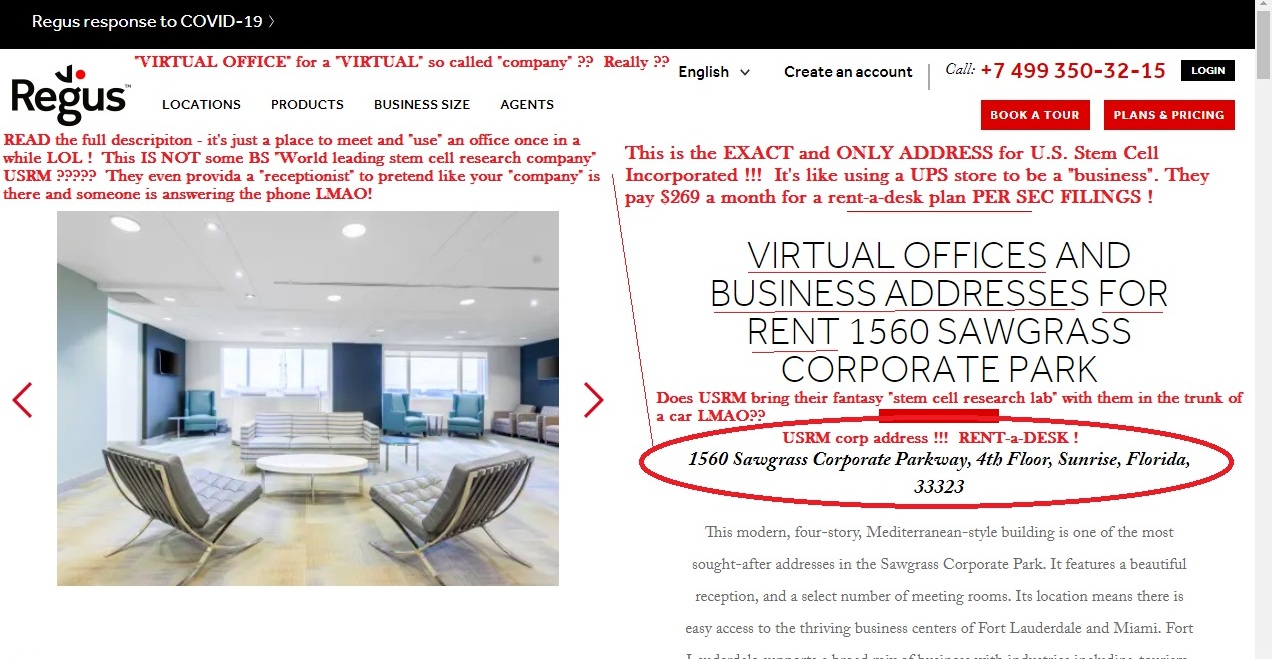
2676 Edgewater Dr., Weston, FL 33332 ) and against Garnishee, US STEM CELL, INC. (whose
address is 1560 Sawgrass Corporate Pkwy., 4h Flr., Sunrise, FL 33323) in the amount of $112,803.88
for which sum let execution issue.
Hi DragonLady
I hope you had a great summer!! I wish you a safe Labor Day upcoming. May you continue to expose that absolute filth scum of society. Ergo filthy Mike, Miguel, Michael Tomas. Who preyed upon dying for financial gains and is no better than that of a two bit hustler pushing meth on 16yo children. May God have a plan for such filth. May Brenda get her day in court and rip the flesh of this scum.
God Bless America
- REAGAN
$USRM: DIRTY MIKE TOMAS 5 ZERO SPECIAL LMAO !!
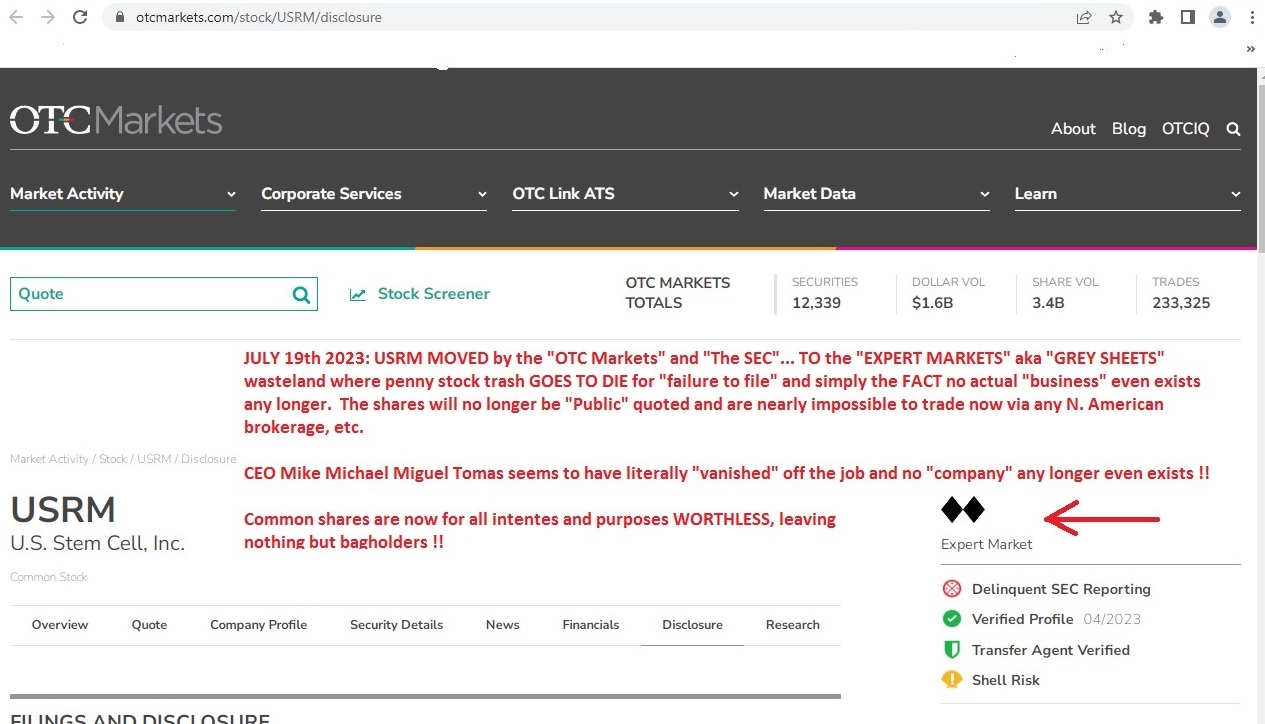
https://www.otcmarkets.com/stock/USRM/profile
Expert Market Icon
Expert Market
Delinquent SEC Reporting
Shell Risk
Broward County Case Number: CACE20016818State Reporting Number: 062020CA016818AXXXCECourt Type: CivilCase Type: Other
Incident Date: N/AFiling Date: 10/09/2020Court Location: Central CourthouseCase Status: Reopened - CVMagistrate Id / Name: N/AJudge ID / Name: Haimes, David A.
06/25/2024 Motion for Entry of Judgment
Against U.S. Stem Cell Inc
USRM
The foreclosure lawsuit does not name Tomas. A call to Tomas at Bioheart was not immediately returned.
Serious statement...Here are your all your qualifications as a basher in the parentheses ( )
BTW... what does " Dragon and have known each other" in your post really mean? Dragon and ???????. Try spell check dufus/doofus...seems you can't put more than 3 words together that make any sense...BBBBWWAAAAAAAAAAAAAAAAAAAAAAAAAAAAAAAAAAA!!!!!!!! What a simpleton ( you may have to look that word up dummy)BBBWAAAAAAAAAAAAAAAAAAAAAAAAAAAAAAAAAAAAAAAAAAAAAAAAAAAAAAAAAAAAAAAAAAAAAAAAAAAAAA!!!!!!!!!
You are ignored now!!!!!!!! Enjoy you dimwit!!!!
Serious question, do you have downs? Your syntax and grammar remind of a friends brother who had downs. Great little buddy of mine. Dragon and have known each other since our 1st accounts in 2000’s. We are bashers 100%.
USRM-a-CON: COLLECTION/GARNISHMENT ACTION LMAO !!!
💀 ☠️ 💀 ☠️ 💀 ☠️ 💀 ☠️ 💀 ☠️ 💀 ☠️ 💀 ☠️ 💀 ☠️ 💀 ☠️ 💀 ☠️ 💀 ☠️ 💀 ☠️
Well - the Dr Rosemary Daly case is COMING FOR MIKE MICHAEL MIGUEL TOMAS aka "CEO" clown of this POS train wreck......the Kristin Comella CON-Mella lawsuit WON BY TRIAL is "on the hunt" for monies owed and is COMING FOR MIGUEL AND THE CREW....and for this POS CRIME SCENE USRM aka US Stem Cell INC aka RENT-a-DESK INC Bwaaaa ha ha ha.....
Funny as shit - is MIGUEL MIKE MICHAEL TOMAS will never ever ever ever "appear" as in be DEPOSED and/or EVEN ANSWER THE LEGAL ISSUES - he sends his lackey Julietta Radiche...... whatever the F her name is...... aka PRETEND "EXECUTIVE VP"....you know....cause all $99 buck RENT-a-DESKS need and have a "VP" for a SCAM that conducts ZERO day-to-day "business" etc. WTF .....Miguel is such a filthy dirty con......always ......
SHIT SHOW STILL ON-GOING....MIGUEL CAN'T BK THIS POS as then Brenda Leonhardt will have FULL GO at the personal assets of MIGUEL and the DIRTY NORTHSTAR BOYS CLUB CRIME CREW.....yepper......where's Miguel on any given day....as he sure as shit ain't showing up to "work" at this turd pile .00000X per share special HE created and HE ran into the ground in spectacular crash n burn fashion......:))
05/23/2024 Reply to Answer
/2
05/22/2024 Notice of Service
/5
05/20/2024 Answer to Writ of Garnishment Julieta Radiche SVP of Operations U.S. Stem Cell, Inc
/3
05/15/2024 Notice of Service
/12
05/01/2024 eWrit Issuance - Garnishment Garnishee: US Stem Cell Inc / Deft: US Stem Cell Clinic LLC / Total $ 379,325.46
Party: Plaintiff Daily Wellness LLC
/1
05/01/2024 eSummons Issuance - On Amended US STEM CELL, INC
What's the point though?
USRM probably no revenues, no income and certainly no updates, no return of phone calls or emails, nothing. The company is done.
REAGAN...dont understand US STEM...
I never bought a share...i have no clue what they do... just exposing Dragon posts bashing all different boards. Nothing to do with US STEM, he is just a basher. However, YOU seem to be "projecting"...Sounds like you lost your shirt here,35K???? you seem to know the scam, as a victim no doubt... you should do your dd before you put money into these things
Thanks for the kind words. Can't say it's "your company" though. I was a simple investor who believed the company (thus stock) was going to grow.
Wrong decision. I own up to it.
USRM-a-CRIME: BACK TO 5 ZERO SPECIAL LMAO !!
Well - THAT scam lil pump the trash didn't last long Bwaaaa ha ha ha !!
Back to rock solid Mike Michael Miguel Tomas good ole .00000X per share gutter garbage...WHERE IT BELONGS......
Can't wait to see this shit heap to to CAVEAT EMPTOR and/or just get the ticker revoked finally.....police tape this crime scene and bring in the mop up crew and turn off that lone light bulb swinging from a lamp cord at their rent-a-desk WORLD HEADQUARTERS....LOL....WTF....like scammer Tomas "shows up to work each day" at this scam machine still......please......
SHUT IT DOWN FOR GOOD- pull the ticker and DO NOT give Miguel even a 1% chance of cooking up some new scam to re-light this fraud ticker....it needs to be buried for good at 6 ft under and read its last rights never to be seen again....end this scammary once and for all.....TICKER REVOKED for the amount of crime and pure gutter fraud this POS inflicted on God knows how many people.....
Worse than holding up liquor stores for meth money - the bunch that ran this filthy shit show....hope there's still some CLUB FED in their futures....!!
You have always been professional and kind in your responses. Your company is still a stock scam and criminals ran it though
Wow. You lost a lot eh? Did Miguel, Mike Michael CONman CEO make you feel special? Did email and call you on the phone once in a while? Tell you something. Really good was coming and to fasten your seatbelt? LoL. You lost at least $35k in this scam. Kicking yourself for not selling when. It went $0.08. The stock has so many victims. Old, you, average, single moms and so many dumb people that kept buying when all the red red flags were waving.
Maybe you can kindly share some pertinent information regarding USRM. Last I recall, they haven't even filed in the past year.
Not only that, they don't respond to phone calls, emails, etc. My guess is this company is finished.
Your name is quite appropriate.
USRM likeable...but Dragon Lady the paid basher FRAUD!!!
DRAGON LADY IS AN ALL CAPS BASHER WHO HAS NOTHING FACTUAL OR TRUE TO POST.
The person that bought 32.7k of this shit stain scam criminal enterprise should either be committed to a mental institution for his entire life or be arrested for crimes against humanity and set for trial infront of a Military tribunal and if convicted they should be executed.
This !!
I think Karma is coming down the tracks in a Casey Jones fashion when it comes to the DOJ on this. Hopeful asf that the criminal justice system lands these charlatans in a minimum security prison on an AFB in Minnesota next to that FPOS we put in jail.
Let me know when you find a stock a scam for you and I to go to work on. I ready to take another one down
Hi DragonLady
What a wonderful surprise to see your news! 100% Brenda has read this board prior and if she is reading today THANK YOU for going after them Brenda. I am sorry that Howard did this to you and I am sorry that this has most likely cost you upwards of $100k plus in legal fees trying to recover. I know you will never give up until you recover every last penny owed. Good for you! I hope the judge issues a summary judgement and you win. I know it will be appealed but 18% is a very nice return on your money.
100% about Dr M. A victim in this scam. He children and grandchildren have had $10’s of millions stolen from them since 2006 from this stock scam. They/Company/People never had any intention of Cody ting science or healing people. It was all just another stock scaaaaaaaaaaaaam.
I think Karma is coming down the tracks in a Casey Jones fashion when it comes to the DOJ on this. Hopeful asf that the criminal justice system lands these charlatans in a minimum security prison on an AFB in Minnesota next to that FPOS we put in jail.
Let me know when you find a stock a scam for you and I to go to work on. I ready to take another one down
All the best to You Dragon…
-Reagan
In a few words, can explain in simple terms what all this means ? Thx
Going against the estate of a deceased person is common practice.
USRM-a-CON: BRENDA LEONHARDT GOES AFTER MURPHY ESTATE !!
Hey Reagan - checking in and touching base, hope all is well with you.
DIG THIS - ole Brenda ...she is NOT backing the hell down or off chasing the sorry asses of Miguel Michael Mike Tomas .....the fantasy "GREATEST CEO" in the dude's own bad wet dream fantasy.....she's going after Miguel and the ole gang aka the Northstar Biotech boys and the entire shit show once known as Bioheart aka US Stem Cell INC this here scam ticker.....
She filed a motion to "NAME THE ESTATE OF WILLIAM MURPHY" in her pending action for failure to ever pay her ONE CENT of what is now WELL OVER $2 MILLION LARGE LARGE LARGE with all the defaulted 18% interest accrued.......
Broward County Clerk Of Court Site:
https://www.browardclerk.org/web2
Search term under business name: US STEM CELL
03/08/2024 Motion for Substitution of Party(S) PLAINTIFF'S MOTION TO SUBSTITUTE DEFENDANT DR. WILLIAM P. MURPHY JR.'S ESTATE
/2
02/28/2024 Notice of Service of Answers to Interrogatories
/2
Hi Whigs, yes, there is proof on the public court portals..
Thanks very much for all of the information. It seems this company has practically no hope anymore IMO. When they were generating revenues they probably could've paid off some of their debts. This company makes no money and basically their patents have practically no value.
IMO their best hope is to expand internationally where laws are different and work on their veterinarian products. Not that I think it will happen.
I guess I'll have to give you credit for stating this for quite sometime.
I "invested" in this stock many moons ago when "stem cell therapy" at the time was an upcoming big thing. I expect this stock to be a tax-write off for me down the road when I start selling my profitable stocks.
Oh well, that's life. It's best not to dwell on past mistakes but rather to learn from them.
".Comella is off running another "Stem clinic SCAM" on her own now and getting her sorry ass sued off also in several lawsuits....she already lost one at trial....and is "Trying" to avoid paying by pulling a shit-show BK scam and fraud.....same old same old with these 2-bit scammer charlatans.....:))"
-Do you have any proof/links for that?
".Comella is off running another "Stem clinic SCAM" on her own now and getting her sorry ass sued off also in several lawsuits....she already lost one at trial....and is "Trying" to avoid paying by pulling a shit-show BK scam and fraud.....same old same old with these 2-bit scammer charlatans.....:))"
-Do you have any proof/links for that?
Memory serves me right he gave the Dolphin greats a SEVENTY FIVE percent discount on stock issued. Smartly Miguel then ran a pump and dump stock scam
Pump to literally hurt thousands of people that can ill afford to lose a dime let alone millions in combined losses. It went up 400%. They made a ton of money. Ole Howard was neighbors of Danny just like
Dr. M (RIP). I LOVE BRENDA. I LOVE TGAT SHE HAS ENOUGH MONEY IN RESERVES TO GET JUSTICE NOT ONLY FOR HERSELF AND TENS OF THOUSANDS OF PENNY PEOPLE THESE STOCK SCAAAAAAAAM VICTIMIZED. Notice how they have not filed Chapter 7???? They can not. The Federal magistrate will a f*ck ton of questions.
***STOCK-SCAAAAAAAAAAAAM***
Memory serves me right he gave the Dolphin greats a SEVENTY FIVE percent discount on stock issued. Smartly Miguel then ran a pump and dump stock scam
Pump to literally hurt thousands of people that can ill afford to lose a dime let alone millions in combined losses. It went up 400%. They made a ton of money. Ole Howard was neighbors of Danny just like
Dr. M (RIP). I LOVE BRENDA. I LOVE TGAT SHE HAS ENOUGH MONEY IN RESERVES TO GET JUSTICE NOT ONLY FOR HERSELF AND TENS OF THOUSANDS OF PENNY PEOPLE THESE STOCK SCAAAAAAAAM VICTIMIZED. Notice how they have not filed Chapter 7???? They can not. The Federal magistrate will a f*ck ton of questions.
Well..LOL..uh.... I'm not even sure this company exists anymore. I've called, emailed, checked anything related to the company...crickets.
No filings, etc. Might be time to put a fork in this one and call it a "tax write-off". A shame given I was up quite a bit on this at one time. Oh well.
Uhmmm....NOT SURE how to break it to you here pardner....BUT this cough cough uh "company" aka BIG OLE STINKY PINKY SCAM...aka the MIKE Michael MIGUEL TOMAS ole SHIT SHOW SPECIAL.......uh.....
IT AIN'T EXISTED FOR WELL WELL WELL OVER A FREAKING YR NOW and in reality about FIVE PLUS YEARS....other than as a INSIDER DILUTION MILL SHARE PRINTING CON n SCAM MACHINE and a SELF ENRICHMENT FRAUD for a few insiders and nothing much else.....
https://www.otcmarkets.com/stock/USRM/disclosure
USRM-a-CON and their $99 buck a month rent-a-desk SHIT SHOW FRAUD only "exists" now as a Corp filing in FL and a cheap shit website FULL OF LIES in order to protect the CEO ASS CLOWN Tomas and clown-show BOD from being PERSONALLY SUED which is really already happening....
IF Miguel Mike Tomas were to LET THE CORPORATION LAPSE...then the LAWSUIT THEY WILL 100% LOSE and LOSE BIG which is "live" in FL as we speak - will TAKE THE INSIDER'S PERSONAL ASSETS....
Other than that - NO ONE and certainly not Mike Tomas is "showing up to work" and doing a DAMN FREAKING THING related to this shit show SCAM TICKER and STINKY PINKY FRAUD.....it's a DEAD TICKER.....nothing else to say....
Hard to even get a shitty tax loss as it's 100% ILL-LIQUID now that THE SEC and OTC sent this POS CRIME SCENE to the gray market where it belonged about 5 or more years ago LMAO :))
USRM IS 100% A TOTALLY DEAD TICKER - sorry for your losses....this was a TOMAS/COMELLA SCAM from at least 10 years ago......Comella is off running another "Stem clinic SCAM" on her own now and getting her sorry ass sued off also in several lawsuits....she already lost one at trial....and is "Trying" to avoid paying by pulling a shit-show BK scam and fraud.....same old same old with these 2-bit scammer charlatans.....:))
I'm not even sure this company exists anymore. I've called, emailed, checked anything related to the company...crickets.
No filings, etc. Might be time to put a fork in this one and call it a "tax write-off". A shame given I was up quite a bit on this at one time. Oh well.
Well Said Reagan ! Speaking of Brenda Lawsuit -
She is TAKING IT FULL TILT TO MIGUEL and "THE GANG" aka Northstar Bio boys and the rest of the dirty charlatans.....
DIG THIS - Brenda and her legal team...... just issued SUBPOENAS TO ole DAN MARINO and JASON TAYLOR LMAO .....I'm sure you remember that SCAM-o-RAMA and how MIGUEL SURE AS HELL "PAID THEM BACK AND THEN SOME" while he stiffed ole Brenda out of some sort of sick spite or whatever the F rubbed that POS the wrong way....NEVER was Miguel ever intending to pay Brenda back BUT he paid HIMSELF and THE SPIN DOCTOR and everyone else who stroked his sick ego.....
Broward County Clerk Of Court - I'll print the page out tomorrow and post it :
"
Brenda Leonhardt, et al Plaintiff vs. US Stem Cell Inc, et al Defendant
Broward County Case Number: CACE20012095State Reporting Number: 062020CA012095AXXXCECourt Type: CivilCase Type: Contract and Indebtedness
Incident Date: N/AFiling Date: 07/27/2020Court Location: Central CourthouseCase Status: PendingMagistrate Id / Name: N/AJudge ID / Name: Casey, Daniel A.
01/26/2024 Subpoena Returned Served DANIEL CONSTANTINE MARINO, JR.,, 19th day of January, 2024, 10:25 am
/4
01/24/2024 Subpoena Returned Served served on -chris Carman on the 18th day of January 2024 @10:45am
/3
01/22/2024 Case Management Order CASE MGMT CONF: 3/26/2024 @ 8:45 AM
/3
01/18/2024 Subpoena Returned Served JASON PAUL TAYLOR,, 12th day of January, 2024, 4:10 pm
/4
01/11/2024 Notice of Taking Deposition OF Daniel Constantine Marino Jr
/2
01/11/2024 Notice of Taking Deposition OFJason Paul Taylor
/2
01/11/2024 Subpoena Issued By Attorney TO Daniel Constantine Marino Jr
/3
01/11/2024 Subpoena Issued By Attorney TO Jason Paul Taylor
/3
"
Bwaaa ha ha ha YOU GO GIRL BRENDA.....DIG THAT DIRTY LAUNDRY OUT AND LET THE WORLD SEE THIS STINK SHOW ......she ain't quitting buddy....she's a WOMAN SCORNED if you ask me and intends to GET HER HUNK OF FLESH out of Miguel's DIRTY HIDE and BUST HIM BROKE IF IF IF there's ANY WAY ON PLANET EARTH TO DO IT......God bless her if you ask me.....:)))
HNY as well to your DragonLady, when a person trudges in the filth like I have since 2006 on IHUB I grow wary. I lost my original account on the SPNG Spongetech account. Lost a few more after that as well. I provided MUCH information to FED’s on that one Spongetrch account. I have helped bust several scams over the years. I have not felt the urge to Bash the stock scams since that shell Miller stock scam we brought down in 9/2021 ( you did a magnificent job on that one. I could not for the life of me get thru to the OTC, but You did thankfully!! That was a good one. We made bag holders of paid pumps and insiders. The overt death threats and “we’re gonna sue “ threats have always been my favorite parts of bashing stock scaaaaaaaaaaaams. lol. In this stock scam the Pumps on the Miguel scam were mostly decent bag holders. Heart in right place and truly believed in the “science” although no science existed since Phase 1/2 Myocell trial from 2006. Liar Miguel always promised to restart that Trial but it was all just a lie. He was all about the “scam” Putrid scam kits of his poison Goo he was pushing on the terminally ill and get his terminally ill victims to leave the USA for TJ. Nothing more than a Civil War carpet bagger preying upon the old and down trodden that FOOS is. My thinking is he is thinks putting his home in a trust will protect his asset. But it will not. The 3yr look back period does not apply to Brenda since the scam was ongoing and defrauding her since 2010. We will NEVER see a USRM BK filing. Doing so opens up the DOJ arm. I would think that the FDA investigator never pursued this stock scam because of lack of resources and that Cali court took em down and besides the DOJ has more teeth to put Miguel, Spin Doctor and others in a Fed Pen for 12/24 months. Concerned that the statute of limitations is gonna run out on this and ANOTHER bag of shit CEO will get away with a massive crime spree. Hopeful that Brenda gets them. And yes RIP Dr M. Everyone remembers you for your pacemaker and blood transfusion bags not this stock scam. I noticed that your people did a great job of removing Your name from news releases on this scam.
Happy New Yr 2024 Reagan !!
I am just seeing this. I have taken a nice long break from this Stacy filthy scam garbage penny stock shit f^ck pig scams.
Dr M was a great man. Guy invented the Fing Pace Maker and invented the blood transfusion bag…. He is personally responsible for saving millions of lives. The Founder of this stock scam ole Howie another Fake PhD scumbag loser stole his wife’s millions of inheritance and put it into this SCAAAAM in 2006. Then scammed his neighbor Dr M of around $9.5m. Garbage IPO raised $2.5m I think. The Minnesota lake boys were the biggest winners in this stock. Pay millions in stock scam shares that idiots bought for 15yrs and a massive reverse split. Rest in peace Dr M
I am just seeing this. I have taken a nice long break from these filthy scam garbage penny stock shit f^ck pig scams. These people do what they want with zero ramifications. I feel like a clown sometimes for living a clean and honorable life. I could have easily been a Miguel. Stolen millions and did 12 months and a day on a USAF base in Minnesota. What the actual f*ck is wrong with this world? Criminals do what they want.
Dr M was a great man. Guy invented the Fing Pace Maker and invented the blood transfusion bag…. He is personally responsible for saving millions of lives. The Founder of this stock scam ole Howie another Fake PhD scumbag loser stole his wife’s (Deb) millions of inheritance and put it into this SCAAAAM in 2006. Then scammed his neighbor Dr M of around $9.5m. Garbage IPO raised $2.5m I think. The Minnesota lake boys were the biggest winners in this stock. Miguel tried ripping Mareno and Jason Taylor off but they got paid back and actually made money on the loan. Pay millions in stock scam shares that idiots bought for 15yrs and a massive reverse split. Rest in peace Dr M
Happy New Year Dragonlady,
Thank you for staying on top of this stock SCAAAAM. Your post gives me hope that Miguel and his pump Kristy the WORLD FAMOUS PhD in RegMed may actually get into trouble on this. Miguel is a long time Miami criminal and the FBI wants Miguel more than his pump concubine aerobic spin Doctor that participated in this Miami griftathon stock scam.
Jeezus H…. Called the Company??? Pffffffffttttttttt……
How is this still breathing?
USRM-a-CON: BK FILING IS ALL THAT'S LEFT NOW !! I called and left a message I was looking for treatment.
LMAO.....
Dude- this SCAM has no "treatments" or ANYTHING ELSE LEFT.....CEO clown Mike Michael Miguel Tomas DOES NOT EVEN "SHOW UP" at the $99 buck a month scam "WORLD HEADQUARTERS" aka RENT-a-DESK......
LAWSUITS and "on the run in hiding" - THAT is all left going on at USRM-a-FRAUD......WTF...!!!
THIS POS does not own or operate a single "clinic" or ANY OTHER FUNCTIONING ACTUAL "business" of ANY kind and hasn't done so for well well well over a YEAR NOW......
GRAY aka EXPERT MARKET OTC STINKY-PINKY DEAD TRASH mired in too many lawsuits to even count....no damn cash left and DEFAULTING ON ALL LOANS OWED.....thee end :))
https://www.otcmarkets.com/stock/USRM/disclosure
DEMOTED TO "SHELL RISK STATUS" besides EXPERT MARKET TRASH LABEL and frozen NO TRADE at every major brokerage in N. America....!
USRM-a-CON: Old Man Murphy Is Deceased. RIP !
Hey Reagan - don't know if you saw this yet ?
https://www.washingtonpost.com/obituaries/2023/12/06/william-murphy-blood-bags-dies/
Old man Murphy made it to 100 years young - he was a decent dude IMO that somehow got mixed-up with penny cons Miguel and Kristy and this shit show USRM. What a damn shame !!!!
Murphy, HE actually did some good for society unlike the 2-bit penny con charlatans of Mike Michael Miguel and Kristy Con-mella aka The Spin Doctor WTF !!
Only STAIN on that nice obituary write-up about Doctor M. (as in REAL doctor aka NOT fake PhD Con-mella and the gang !!) done in a major newspaper- is scammer Miguel had to go and get his scuzzy name schmattered all over it. WTF AGAIN - couldn't dirty Miguel just leave the old man alone even in death, just to have a fitting tribute, without the shit stain of Miguel's name being attached to Murphy's historic and legit legacy ?
Geez - these cons have no damn shame, no honor, it's ALWAYS about them the 2-bit scammers !!
MY "guess" - with Murphy gone now, I'd say the Vegas odds just got real, real high that Miguel BK's this hunk of shit scam as he no longer has "Money Bags Murphy" as is "go-to" funder in a pinch.
Also, it's highly likely IMO that Murphy was their last "credit line" who acted as "guarantor" to the big Seaside Bank FL, whatever the hell it's called; that old, old $980K loan that Miguel NEVER PAID BACK ONE CENT OF PRINCIPAL ONE, while fleecing this scam dry as a crypt of every last dime and cent of cash !!!!
IF Murphy was the "guarantor" - then IMO that bank can "call the loan" IF his estate or whatever pulls the guarantee or collateral or whatever the hell "arrangements" that had, now past tense most likely.
USRM-a-CON BK filing - I put it at 90% or better odds now IMO.
Wonder if Miguel is going to file it prior to end of the fiscal yr 2023 here, in less than a few weeks away ?
There's LITERALLY NOTHING LEFT OF USRM-a-CON so the BK filing can probably be done on the back of a napkin from one of Miguel's posh "company paid" dinners or lunches LMAO !!!
Hopefully - the passing of Murphy means the END OF THIS SHIT SHOW ONCE AND FOR ALL !!
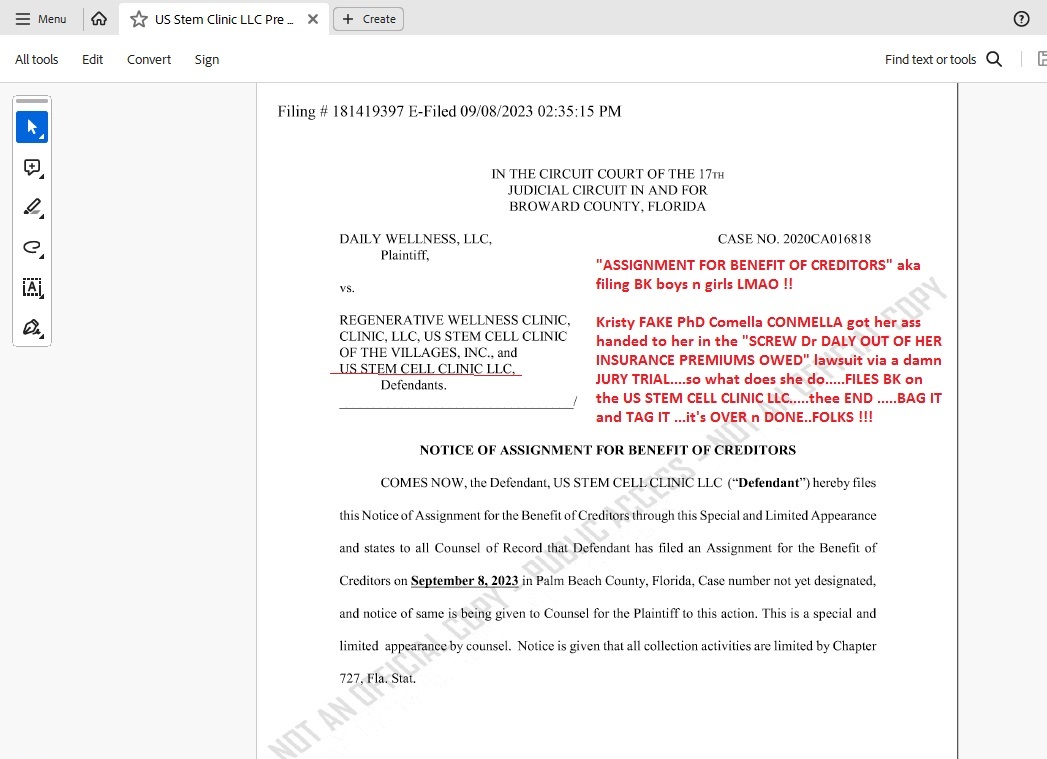
USRM-a-CON: Kristin Comella GETS SPANKED BK COURT FRAUD !!
Hello Reagan - hope you had a great Thanksgiving and a up-coming great Christmas.....:))
Dig this Reagan - ole Kristy aka SPIN DOCTOR AEROBICS QUEEN and FAKE PhD from BLUE MARBLE PANAMA UN-UNIVERSITY.....
Lil Kristin Comella and thus by fiat Michael Mike Mikey Miguel Tomas the "CEO" of nothing and who RAN THIS SHIT SHOW TO LITERAL .000001 AFTER a 1-for-1000 MASSIVE REVERSE SPLIT....
THOSE TWO CONS just got their ASS HANDED TO THEM FOR ATTEMPTING BK FRAUD in Palm Beach County Court.....
Judge TOSSED THE CASE as the attorney for "stiffed" MD Mary Daly showed that Comella "attempted" to BK the US Stem Cell Clinic which had morphed to Regenerwave Clinic and then she moved it to Boca Raton and filed a new micro LLC named INFINITE IMPACT and "tried" to claim THAT SCAM was a "new" company after she LOST A JURY TRIAL in Broward County and owes over $250K to Dr Rosemary Daly.
THEY CAUGHT HER STONE COLD - this could actually be a CRIMINAL CHARGE OF BK FRAUD WTF !!!!
She "tried" to transfer all monies, bank accounts, all the good equipment and anything of value from the US STEM CLINIC and BK IT as she AT THE EXACT SAME MOMENT IN TIME filed a micro LLC INFINITE IMPACT and and and AS A SCAMMER DOES used the EXACT SAME DAMN ADDRESS AS THE CLINIC SHE "tried" to BK and they all CAUGHT IT STONE COLD....the attorney for DALY....the Judge....and SANTA CLAUSE ALL ALL ALL saw the SCAM plain as day.....COMELLA IS IN DEEP SHIT once again LMFAO !!!!!
NOW - the case in Broward County FL is going to SPANK HER SILLY and has asked for a COURT ORDER TO DRAG INFINITE IMPACT TO COURT and TAKE EVERYTHING THEY HAVE TO PAY BACK THE MONEY COMELLA CON-MELLA THE PERP OWES.....
https://www.infiniteimpacthealth.com/
THAT is KRISTY KRISTIN COMELLA CON-MELLAS and MOMMA MARY COMELLA aka THE DUMBSHIT'S LATEST SCAM....NOW getting a subpoena to APPEAR IN BROWARD COUNTY for "trying" to bamboozle a BK aka BENEFIT OF CREDITOR SCAM and stiff paying a COURT ORDERED JUDGEMENT filed against what was US STEM CELL CLINIC .....WTF....DUMBEST CONS TO EVER EXIST are KRISTEN COMELLA and MIKEY MIKE MICHAEL MIGUEL TOMAS...holy hell they are DIRTY AND STUPID AS F....what a F-ing way to go through life Bwaaaa ha ha !!!!!
SUBPOENA TO INFINITE IMPACT TO APPEAR AND RETAIN COUNCIL:
C Plaintiff vs. Regenerative Wellness LLC, et al Defendant
Broward County Case Number: CACE20016818State
Reporting Number: 062020CA016818AXXXCECourt Type: CivilCase Type: Other
Incident Date: N/AFiling Date: 10/09/2020Court Location: Central CourthouseCase
Status: Reopened - CVMagistrate Id / Name: N/AJ
udge ID / Name: Haimes, David A.
REGENERATIVE WELLNESS = US STEM CELL CLINIC = Kristin Comella and momma Mary Ellen Comella aka THE CON FAM SCAM LMAO !!!
AND.....the BK "case" that NEVER WAS AND JUST GOT TOSSED ON ITS ASS FOR "Attempted BK FRAUD"....PALM BEACH COUNTY.....
https://appsgp.mypalmbeachclerk.com/eCaseView/search.aspx
CASE NUMBER: 50-2023-CA-013744-XXXA-MB
CASE STYLE: US STEM CELL CLINIC LLC V ARESTY PA, JOEL M
Submit Submit 2 09/08/2023 CIVIL COVER SHEET
Submit Submit 3 09/08/2023 PETITION
FOR THE ASSIGNMENT OF CREDITORS F/B PET
1 09/14/2023 DIVISION ASSIGNMENT
AH: Circuit Civil Central - AH (Civil)
Submit Submit 4 09/14/2023 PAID $401.00 ON RECEIPT 5042360
$401.00 5042360 Fully Paid
Submit Submit 6 09/14/2023 NOTICE OF APPEARANCE CIVIL
F/B ATTY ARESTY AS ASSIGNEE
Submit Submit 5 09/15/2023 DCM DESIGNATION TO THE STREAMLINE TRACK WITH NON-JURY TRIAL ORDER
JUDGE R. SCOTT DTD 9/15/23
Submit Submit 7 09/15/2023 NOTICE NOTICE OF ASSIGNMENT FOR THE BENEFIT OF CREDITORS TO ALL CREDITORS AND OTHER INTERESTED PARTIES
NOTICE OF ASSIGNMENT FOR THE BENEFIT OF CREDITORS TO ALL CREDITORS AND OTHER INTERESTED PARTIES
Submit Submit 8 09/18/2023 MOTION MOTION TO SET AND FIX AMOUNT OF ASSIGNEE'S BOND
MOTION TO SET AND FIX AMOUNT OF ASSIGNEE'S BOND
Submit Submit 9 09/18/2023 NOTICE OF APPEARANCE CIVIL
F/B ATTY DALY OBO CREDITOR
Submit Submit 10 09/21/2023 NOTICE OF TAKING DEPOSITION
10/6/23 2PM CORPORATE REPRESENTATIVE OF U.S. STEM CELL CLINIC LLC F/B JOEL ARESTY ASSIGNEE
Submit Submit 11 09/22/2023 NOTICE OF APPEARANCE CIVIL
F/B ATTY NOVOSELETSKY OBO PLT
Submit Submit 12 09/25/2023 NOTICE OF TAKING DEPOSITION
ASSIGNMENT FOR BENEFIT OF CREDITORS US STEM CELL CLINIC CORPORATE REPRESENTATIVE 10/06/2023 02:00:00 PM
Submit Submit 13 09/25/2023 NOTICE OF FILING
NOTICE OF FILING BOND
Submit Submit 14 09/27/2023 NOTICE OF APPEARANCE CIVIL
F/B ATTY ARTEAGA-GOMEZ OBO PLT
Submit Submit 16 09/28/2023 NOTICE OF EXAMINATION FILED BY JOEL ARESTY
OF EXAMINATION FILED BY JOEL ARESTY
Submit Submit 15 10/02/2023 MOTION TO DISMISS
PROCEEDING F/B CREDITOR DAILY WELLNESS LLC
Submit Submit 18 10/03/2023 PROOF
OF CLAIM FILED BY JEANNINE MALLARD
Submit Submit 17 10/06/2023 NOTICE OF HEARING
NOTICE OF HEARING 10/11/2023 03:45:00 PM
Submit Submit 19 10/06/2023 ORDER SETTING HEARING
ORDER SETTING HEARING ON MOTION TO DISMISS OCTOBER 11, 2023 3:45 PM ZOOM REID P. SCOTT 10/06/2023
Submit Submit 20 10/06/2023 PROOF OF PUBLICATION
ASSIGNMENT FOR BENEFIT OF CREDITORS
Submit Submit 21 10/06/2023 DECLARATION
F/B ATTY KATHLEEN A DALY
Submit Submit 22 11/17/2023 ORDER OF DISMISSAL BOOK 34683 PAGE 29-30
R SCOTT DTD. 11/17/23 GRANTING MOTION TO DISMISS
23 11/17/2023 DISPOSED AFTER OTHER
DAO - DISPOSED AFTER OTHER
THEE END....this SCAM SCAM SCAM is so so so so OVER OVER OVER OVER OVER....PERP WALK FOR KRISTIN COMELLA and MIGUEL TOMAS are ALL that is left LMAO......!!!
I called and left a message I was looking for treatment.
LMAO !!! I called the number on their website a few weeks ago and left them a message on voicemail (no one picked up the phone). Told them I'm an investor in their company and if there is anything on the horizon regarding the company. Got "crickets".
I'll call again sometime soon.
OK Sport....CALL AWAY WTF.... ??????????
This FIVE ZEROS AFTER THE DECIMAL POINT TOILET PAPER SHARE PRIOR DILUTION MILL INSIDER SELF-ENRICHMENT SCAM...SCAM.....SCAM....... HAS BEEN DOA .... DEAD AS A BENT DOOR NAIL NON-ENTITY FOR A DAMN YR PLUS NOW ...WTF again .....LMAO ????????
Are you dense or what ???
You actually believe that ONE MAN CON SHOW MIKE MICHAEL MIGUEL TOMAS is "showing up to work" at the DAMN SCAM $99 buck a month RENT-a-DESK where EVEN THE PROCESS SERVERS FOR ALL THE LAWSUITS THIS SHIT SHOW IS MIRED IN.....THEY can not even find the faux "CEO" clown-con to serve him papers on the LATEST LAWSUIT....LMAO ??????????
REALITY - there is no damn "company" or SCAM any longer named US STEM CELL "operating as a business" ANYWHERE ON PLANET EARTH - and the clinic filed BK and is in receivership now as I type....IT IS OVER .....THIS CRIME SCENE IS BAKED AND DONE....NO FILINGS OF ANY KIND again this Qtr and GRAY MARKET WORTHLESS SHIT is all that's left.... a tainted dirty lawsuit riddled SHELL.....thee end :)))
I called the number on their website a few weeks ago and left them a message on voicemail (no one picked up the phone). Told them I'm an investor in their company and if there is anything on the horizon regarding the company. Got "crickets".
I'll call again sometime soon.
What's up? It was zero yesterday. 100% loss and now it's breathing. 😀
As always Dragonlady, THANK YOU for your important work you do here and other STOCK SCAAAAAAAAAAMS. I am kinda disappointed Brenda appears to be letting them off the hook? Let’s hope she does not.
Agreed. Dr. Murphy was a great human being that thru his inventions has provided high quality life and saved the lives of millions thru his inventions. His neighbor Howard Leonhardt got a hold of him one day out side his home in Miami and bamboozled him most likely? Howard sucker Dan Marino and Jason Taylor from Dolphins as well. Neighbors? I remember reading Dr M put in north of $12m of his own money. That does not include what he put in after. They actually had a product that worked and showed promise. Took thigh cells and made them ambulatory and those cells would be injected into people with heart attacks to heal tissue. Go read the trials. They went public and nobody wanted the shares. Howard was employing family members in key positions and coupled with the $350m plus needed to get this to trials to market cost. it was a scam fest the entire time. They brought in Mike, Michael Miguel and he ran it like his personal ATM for over a decade. Paying him and fake PhD over a million a year in salary and bonus. The several women suffered permanent injury from the gook they injected into eyes. They had legal settlements and nothing was ever paid. Dragon Lady is much more adept in linguistics talent spelling this out than I am. FYI I never invested in this. I have been bashing scams since I got scammed on
IHUB in 2003. Lost my account in 2015 and kinda lost interest after. Sometimes things peak my interest though. Good luck
DAMN STRAIGHT-UP FACTS REAGAN !!!! The level of SCAAAAAAM however with this one is a whole level. They almost killed and blinded old people and destroyed the legacy of Dr Murphy the inventor of the pacemaker and blood transfusion bag. They gave away all the assets of the company to an employee then dumped stock for almost 2 years with no press releases. This is a higher level of SCAAAAAM
READ THAT FOLKS - because THAT is exactly WTF took place here on this CRIME SCENE in a "Reader's Digest" version....reality....it's even FAR FAR FAR worse than that when one peels back the STINK, ROT and FRAUD on this POS crime scene bad onion.....
PUT IT THIS WAY - the DOJ aka UNITED STATES DEPARTMENT OF JUSTICE and and and THEE FDA went after these ass clowns and BURIED THEM WITH A PERMANENT INJUNCTION and and and were even considering (And STILL may file) CRIMINAL CHARGES......
The DOJ takes to prosecution VERY few cases per year - they choose VERY carefully who to go after and the BULLDOZE THEM TO DUST when they do choose to drag them to court....and USRM-a-CON LOST and LOST BIGLY....all one needs to know about this filthy crime scene....!!!
https://www.justice.gov/opa/pr/department-justice-files-complaints-against-florida-and-california-companies-stop-use
https://www.justice.gov/opa/pr/florida-company-barred-using-experimental-stem-cell-drugs-patients
https://www.fda.gov/news-events/press-announcements/federal-court-issues-decision-holding-us-stem-cell-clinics-and-owner-adulterated-and-misbranded-stem


That does sound pretty shitty. And one of the lowest of low.
The level of SCAAAAAAM however with this one is a whole level. They almost killed and blinded old people and destroyed the legacy of Dr Murphy the inventor of the pacemaker and blood transfusion bag. They gave away all the assets of the company to an employee then dumped stock for almost 2 years with no press releases. This is a higher level of SCAAAAAM
|
Followers
|
746
|
Posters
|
|
|
Posts (Today)
|
0
|
Posts (Total)
|
106841
|
|
Created
|
02/27/08
|
Type
|
Free
|
| Moderators | |||

NEWS ALERT June 2nd 2021:
******** U.S. Stem Cell Inc. LOSES THEIR 11th CIRCUIT FEDERAL APPEAL********
"
III. Conclusion
For these reasons, neither the same surgical procedure exception nor the 361 HCT/Ps exception applies to the Clinic’s surgical practice. The Clinic did not challenge the district court’s judgment upon any other ground. Accordingly, we AFFIRM the judgment of the district court."
https://media.ca11.uscourts.gov/opinions/pub/files/201913276.pdf
https://www.sun-sentinel.com/business/fl-bz-us-stem-cell-loses-appeal-of-shutdown-20210603-bft7txqtkvb5hc5lzgemzh7niu-story.html
https://news.yahoo.com/sunrise-stem-cell-clinic-loses-193500517.html
U.S. Stem Cell Clinic LLC (1290 Weston Rd Suite 230A, Weston, FL 33326) is now under PERMANANT INJUNCTION as issued by the FDA w/ legal backing of the DOJ (U.S. Department of Justice)
https://usstemcellclinic.com/
________________________________________________________________________________________________________________________________________________________________________
STOCK INFORMATION:
https://www.otcmarkets.com/stock/USRM/overview
Symbol: $USRM
(Formerly named Bioheart and former trading symbol $BHRT)
661,507,317
05/23/2023
DILUTION is continual and never ending
OTC Markets Site: "EXPERT MARKET" aka formerly the Grey Market where dead tickers are parked
As of June 14th, 2021 USRM is missing and grossly delinquent on "Timely filing" of the following Qty-6 SEC LEGALLY REQUIRED FILINGS -
1) 2019 SEC 10-K Annual Report (audit required) due March 31st, 2020
2) 2020 SEC 10-Q for Q-1 due May 15th, 2020
3) 2020 SEC 10-Q for Q-2 due July 15th, 2020
4) 2020 SEC 10-Q for Q-3 due Oct 15th, 2020
5) 2020 SEC 10-K Annual Report (Audit required) due March 31st 2021
6) 2021 SEC 10-Q for Q-1 due May 15th, 2021
Main Corporate Website: http://us-stemcell.com/
All SEC filings for $USRM can be found here on the SEC EDGAR database:
https://www.sec.gov/cgi-bin/browse-edgar?action=getcompany&CIK=0001388319&owner=exclude&count=40&hidefilings=0
___________________________________________________________________________________________________________________________________________________________________________
| Name | Age | Title |
| Mark P. Borman | 71 | Director, Chairman of the Board |
| Mike (Michael) Tomas | 60 | Director, President and Chief Executive Officer, Chief Financial Officer |
| Gregory Knutson | 71 | Director |
| Sheldon T. Anderson | 75 | Director |
| William P. Murphy, Jr. M.D. | 100 | Deceased as of Dec 3rd, 2023. Former Chairman Of The Board (Supposedly retired and supposedly replaced by Mark P Borman on March 4, 2022 - OTC profile and their own company website shows NONE of this to be true or accurate ! Murphy is still shown as "Chairman" on all key websites owned and operated by U.S. Stem Cell Inc and on their OTC Profile which CEO Miguel Tomas controls/updates.) |
| Kristen Comella | 49 | Former Director and former so called, "chief science officer" . Now owns and operates Biohacker's Gym Weston, FL and U.S. Stem Cell Clinic LLC Weston, FL. U.S. Stem Cell Clinic LLC appears to have moved to Boca Raton FL and now operates under the name "Infinite Impact Clinic" at 9874 Yamato Road 122, Boca Raton, FL 33434 |
| Volume | |
| Day Range: | |
| Bid Price | |
| Ask Price | |
| Last Trade Time: |
