Register for free to join our community of investors and share your ideas. You will also get access to streaming quotes, interactive charts, trades, portfolio, live options flow and more tools.
2 year play
ELTP
X 1. Cash Flow Positive Status - 5 years
X 2. Purchase main building housing their cGMP registered facility for research, development, manufacturing and packaging of pharmaceutical products.
X 3. Adderall IR $335 Million Approved and Launched
X 4. Adderall XR $1.56 Billion Approved and Launched
X 5. Double digit quarterly revenues in millions
X 6. In house marketing and distribution: Kirkov
X 7. Prasco/Burel Adderall agreement starting January 1st 2024
X 8. First shipment Adderall XR to PRASCO Dec 2023
X 9. DEA increases manufacturing quotas for Adderall & Vyvance
X 10. Generic Vyvanse - $5.1 BILLION - FDA submission Dec 2023
X 11. FDA Acceptance of Generic OxyContin Sept 2023
__12. $50 million in yearly revenues
__13. Generate revenues over $20 million/quarter
__14. Generic OxyContin Approval -;FIRST TO FILE Aug 17, 2023 $720 Million
__15. Prevail over Purdue in Generic OxyContin infringement suit - 6 month stay
X 16. Lease additional manufacturing space and storage vault for new Needle Mover ANDAs Jan 2024
__17. European distribution - Dexcel partnership approval by Israeli Health
__18. Full ownership of Adderall IR $ 335 Million
__19. Full ownership of Adderall XR $ 1.56 Billion
__20. Generic Concerta- $1.2 BILLION FDA submission
__21. Vigabatrin - VigPoder approved Pyros $233 Million trade mark challenge
__22. $100 million in yearly revenues
__23. Dopamine Agonist (probably Requip XL or Mirapex ER). $12 Million
__24. Patented Unique ADF (w/o naltrexone)-- NDA
__25. Mikah ANDA (s)
__26. Undisclosed ANDAs/NDAs
__27. Antimetabolite ANDA- Methotrexate -$600 Million - unconfirmed
__28. Undisclosed Antimetabolite ANDA- $42 Million
__29. Generic Vyvanse Approval
__30. DollarLand PPS
__31. Uplist to the NASDAQ Exchange
__32. ELTP Elite Pharmaceutical Buyout - less than 2 1/2 years from Feb 2024
__33. Vegas Baby !!!!!!!
What would Mr. Hakim say is the likelihood that Elite will get bought out by a large rival within the next five years?
Two things I'll say to that. One, cut that prediction in half or more than half. Five years is a very long time to do either. We are at a point where our fundamentals are solid, and we are in a growth stage, as I said earlier. We're not flattening out at $30 million and $40 million. We skip the 40s, went to 50s. And we'll talk in June once I have a little more data, what I expect for the future. But definitely, the year after, we're going to beat that. And the year after, we're going to be that.
So we're at a growth stage. So the fundamentals are looking great, a buyout is looking great. And five years is way too long for that. So I would say either of this will happen in a couple of years.
MWG may be an interesting bottom play. Connections to OMH?
https://twitter.com/eyeofthetigerpa/status/1660311512420962306?s=46&t=mg6_rOBFeh2qKc7TszacvQ
MWG may be an interesting bottom play. Connections to OMH?
https://twitter.com/eyeofthetigerpa/status/1660311512420962306?s=46&t=mg6_rOBFeh2qKc7TszacvQ
AGREE = $BETS finally down to their last funder
Im told Apollo Capital last of the last conversion shares should be out anytime now, once he is out should be nice a climb
Apollo was put on a leak out agreement= http://finance.yahoo.com/news/seaniemac-signs-leek-agreement-apollo-140500236.html
and his only other note isnt due for 4 months...(nice Dilution Break to roam freely)
August 25, 2016 (the “Maturity Date”).
http://investorshub.advfn.com/boards/read_msg.aspx?message_id=122168380
Multiple news coming starting this Weds
http://investorshub.advfn.com/boards/read_msg.aspx?message_id=122168380
SS update from TA
Sent: Mon, Apr 25, 2016 1:18 pm
Subject: RE: Seaniemac (BETS)
There are currently 986,258,885 shares outstanding.
Thank you,
Kimberly Whiteside
I s l a n d S t o c k T r a n s f e r
$BETS interesting at these levels!
$KNOS was supper thing all the way up to .001!
Great post(s) chain on the history of MVTG partner GE and how the future of GE may be MVTG!!!
http://investorshub.advfn.com/boards/manage_msg.asp?message_id=120164963
Nice article on MJ sector I saw from another poster:
http://countercurrentnews.com/2016/01/obama-tells-supreme-court-to-legalize/#
I believe I have found the best of Ihub post of the decade, but you made need the secret decoder ring from Peachman to translate it for you LOL
http://investorshub.advfn.com/boards/read_msg.aspx?message_id=119726504
QTMM NASDAQ Bound 2016/New presentation on the company website.
http://qmcdots.com/qmcdecemberpresentation.pdf
Trader53 $NESV.... http://investorshub.advfn.com/boards/read_msg.aspx?message_id=119052713
$GHTP Highlights:
Share Structure:
500M AS 144M OS
Upcoming Catalysts:
Meeting with FDA on Monday November 30th
Revenues:
$GTHP guaranteed $14mm in sales for their LuViva patented technology over next 4 years
Guided Therapeutics’ Fourth Quarter Orders of LuViva® Advanced Cervical Scan Disposables Triple 2014 Total Sales
Chart:
$GTHP - CCI nearing cross, williams oversold and RSI sub 30s. Severely oversold and great spot for entry.
Extended DD:
http://www.otcmarkets.com/stock/GTHP/news
http://www.otcmarkets.com/stock/GTHP/financials
$GTHP guaranteed $14mm in sales for their LuViva patented technology over next 4 years:
In july 2015 Turkish Distributor signed a four year deal to sell $10 million of LuViva Devices to the Turkish Ministry of Heath, and has expanded this order to $14 million.

Meeting with FDA on Monday November 30th
http://seekingalpha.com/news/2902666-guided-therapeutics-to-meet-with-fda-on-november-30-to-clarify-requirements-for-pma-amendment-for-luviva-system
The FDA agrees to meet with struggling nano cap Guided Therapeutics (OTCQB:GTHP +0.7%) on November 30 instead of November 6 to determine the specific additional patient data the agency requires for the company's Premarket Approval (PMA) application for its LuViva Advanced Cervical Scan system. The date change was to accommodate the schedules of two key clinical investigators
The company has been unable to gain clearance in the U.S. for LuViva. It received its first Complete Response Letter (CRL) about two years ago and a second CRL in May. A CRL is the official response from the agency stating the PMA is not approvable in its present form.
LuViva, designed to rapidly detect cervical pre-cancer without the need for a tissue sample, is currently cleared for sale in Europe, Canada and Mexico.
FDA Meeting Press Release
FDA Grants Guided Therapeutics’ Request for New Meeting Date to Plan Path Forward for LuViva® Advanced Cervical Scan PMA Application
Nov 05, 2015
OTC Disclosure & News Service
-
Guided Therapeutics, Inc. (OTCQB: GTHP), the maker of a rapid and painless testing platform based on its patented biophotonic technology, announced today the U.S. Food and Drug Administration (FDA) has set a new meeting date of November 30, 2015, to review the company’s plan to submit an approvable application for the LuViva® Advanced Cervical Scan.
The company requested the new date to accommodate the schedules of two physicians who plan to attend the meeting on the company’s behalf. Dr. Leo B. Twiggs, professor emeritus at the Miller School of Medicine, University of Miami and Dr. Daron Ferris, professor at the Medical College of Georgia are principal investigators of the LuViva pivotal clinical trial. Both are past presidents of the American Society of Colposcopy and Cervical Pathology.
“We appreciate the agency working with us to accommodate the busy schedules of Drs. Ferris and Twiggs,” said Gene Cartwright, CEO of Guided Therapeutics. “Having them both at the meeting will make it more productive and hopefully lead to the most positive outcome.”
The LuViva is designed to detect cervical pre-cancer without taking a tissue sample and providing a result immediately, unlike any other women’s health cancer detection technology on the market. The LuViva is approved in Europe, Canada and Mexico and is currently available to women in 20 countries.
The FDA, in a not approvable letter, recommended that the company include additional patient data as part of its next PMA amendment. A key objective of the scheduled meeting will be to determine the parameters of the additional patient data FDA is seeking.
World-wide, the market for cervical cancer screening and diagnostics, as currently practiced using cytology (Pap test) for primary screening, is estimated at $6 billion and is projected to grow to almost $9 billion by 2020. Worldwide there are about 2.6 billion women aged 15 years and older who are at risk of developing cervical cancer.
Guided Therapeutics’ Fourth Quarter Orders of LuViva® Advanced Cervical Scan Disposables Triple 2014 Total Sales
http://www.otcmarkets.com/stock/GTHP/news/Guided-Therapeutics-rsquo--Fourth-Quarter-Orders-of-LuViva-reg--Advanced-Cervical-Scan-Disposables-Triple-2014-Total-Sales?id=116857&b=y
Guided Therapeutics, Inc. (OTCQB: GTHP), the maker of a rapid and painless testing platform based on its patented biophotonic technology, announced today that it expects to ship 25 LuViva® Advance Cervical Scan units and approximately 40,000 single-patient-use disposables in the fourth quarter of 2015.
The shipments are destined for Turkey, Saudi Arabia and Indonesia with manufacturing underway at the Guided Therapeutics facility in Norcross, Georgia.
For the year, the company expects to have shipped 45 LuViva units and approximately 45,000 disposables generating revenue of $1.1 million. This compares to 41 units and approximately 15,000 disposables in 2014 for revenue of $758,000.
“The tremendous burst of sales in the fourth quarter is reflective of closing the Turkish Ministry of Health order and shows the opportunity for growth based on our strategy of working with governments,” said Gene Cartwright, CEO of Guided Therapeutics. “Our primary targets are countries that need a point-of-care solution for cervical cancer screening of large underserved populations.”
Worldwide, the market for cervical cancer screening and diagnostics, as currently practiced using cytology (Pap test) for primary screening, is estimated at $6 billion and is projected to grow to almost $9 billion by 2020. There are about 2.6 billion women aged 15 years and older who are at risk of developing cervical cancer worldwide.
Chart:
$GTHP - CCI nearing cross, williams oversold and RSI sub 30s. Meeting FDA Monday - 500M AS - 14m guaranteed in revs
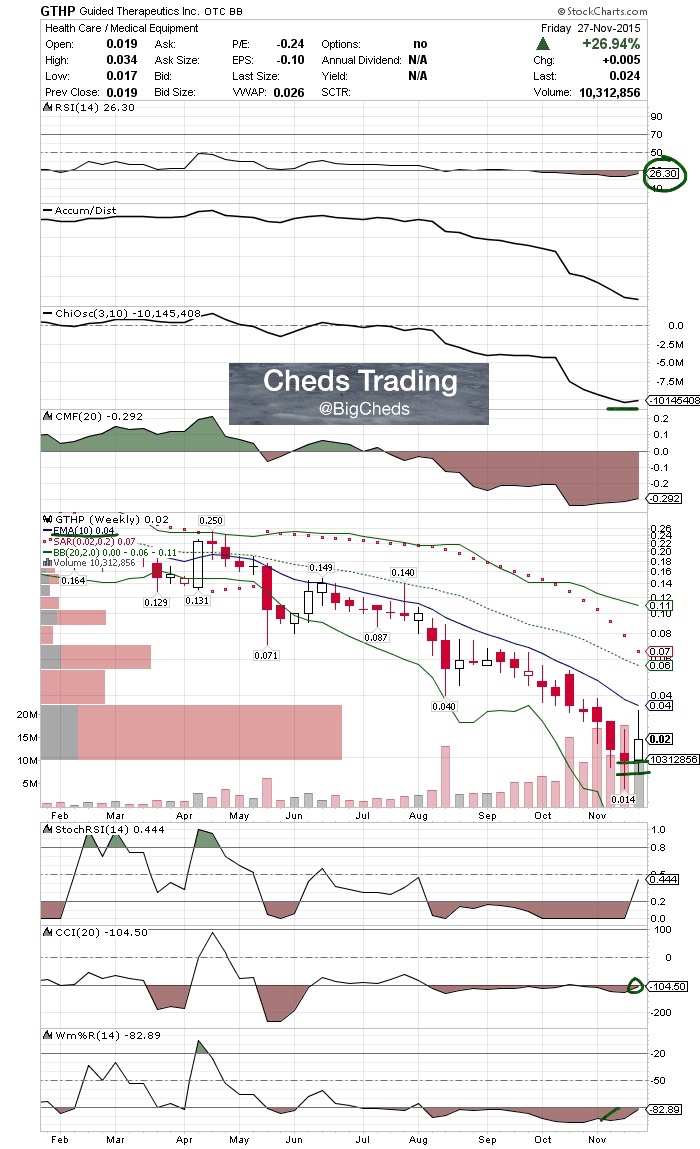
Share structure:
500M AS 144M OS
tankworth $CTLE...http://investorshub.advfn.com/boards/read_msg.aspx?message_id=118711850
RAMO changed to Advantis Corp. (ADVT) goes effective tomorrow.
http://otce.finra.org/DLSymbolNameChanges
$ADVT
See Ramoil / Advantis First Ever CEO Interview:
Awesome $MVTG post today about foot-n-mouth disease LOL
http://investorshub.advfn.com/boards/read_msg.aspx?message_id=118692194
read the news basically says shareholders are screwed
dice Roller $GEAR ... http://investorshub.advfn.com/boards/read_msg.aspx?message_id=118478399
DD_Dempsey $GEAR... http://investorshub.advfn.com/boards/read_msg.aspx?message_id=118467248
RAMO + 30.91% New Website is UP!!! http://www.advantiscorp.com/
ONY 1 TRADING DAY LEFT TILL BIG NEWS HITS
See Ramoil / Advantis First Ever CEO Interview:
$RAMO ONLY 4 TRADING DAYS LEFT UNTIL MONDAY'S ANNOUNCEMENTS
WATCH first ever, Ramoil / Advantis CEO Interview
Watch RAMO "Meet the CEO" interview here:
RAMO + 16.13% "Meet the CEO" interview can be found here:
and Here:
https://www.facebook.com/Advantis-Corp-1675252786083928/?fref=ts
Ramoil Management, Ltd. CEO Interview Details Future, Reveals New Company Name
9:25 am ET November 9, 2015 (Market Wire) Print
RAMOIL MANAGEMENT, LTD. (OTC PINK: RAMO) CEO, Jeremy Foti, detailed recent business developments, expanded on future company prospects, and revealed the company's new name in an interview last Friday. The new company name will be Advantis.
Foti was interviewed at the Ramoil office with the new Advantis logo in the background. When asked if there was any breaking news to announce, Foti was excited to say, "Our name change is finally going to take place. We have changed the name of the corporation to Advantis Corporation... immediately, our symbol (and name) will change on November 16th. We are very excited... with the efforts put forth to rebrand our old identity into Advantis Corporation."
Foti was asked what the next 18-36 months had in store. "The most important change we can bring to the Ramoil platform is turning to profitability," Foti explained, "I will definitely see us reaching profitability, continue to build on exactly what (we are working on) today, and we are heavily moving into the medical field... we have a lot of things coming in that particular industry." Foti went on to say, "In reference to the medical world, it's ground breaking technology that not many people have or have seen, so we are really excited about bringing that into our current business model."Foti mentioned entertainment as the other company focus, stating, "I would say those are the two primary things that we are focusing on."
Foti impressed upon developments that will further expand and diversify the company. "We are... moving into the entertainment world... that's something I've dabbled in in the past for quite some time, so we are looking to create a complete new division which is in the entertainment sector," Foti announced. "I think a lot of people will be pleased with the direction we're going with that and who it is that we will bring in that will be working with us. We have a few different people in mind and a few different companies and corporations that we are currently working with that will bring that to fruition."
The interviewer asked Foti to expand on Advantis' core business model. Foti went on to say, "We're looking to completely change our past business model and move into the medical world. We have several medical devices and machinery that we're looking to bring aboard in order to launch our medical division. We've had these products in the making for quite some time. The machinery isn't cheap, it's very, very expensive, so we are doing our due diligence to make sure we bring aboard the right machinery, the right people and the right sophistication that we need to be able to develop it and turn it into a true winner." Foti summarized his efforts, stating with confidence, "We're really going to roll up our sleeves and turn it into a very profitable division."
Foti was sure to acknowledge his right hand man, Vice President, Geoffrey Broderick. "Geoffrey's been a huge help in developing and getting Ramoil off of the ground. His resume basically speaks for itself; he's an attorney, he's an accomplished businessman, he's walked that life. He's really brought a lot of value to what it is we're doing and what we're trying to do, and he continues to impress me daily with everything he brings to the table. I'm very excited to have him by my side."
When asked if he would like to share anything with the shareholders, he said, "I would like to thank each and every one of them for being a shareholder and believing in where the company is going. At the end of the day it is my job as CEO to make it game changing for everybody, and that's what I'm setting out to do. If I can put in the extra mile and make it that much more beneficial for our current shareholders and future shareholders, that's my overall goal."
Outside of running the company, Foti is a devoted husband and father of four. He enjoys spending time with friends and family, and relishes the time he has to coach his sons' baseball teams.
The full "Meet the CEO" interview can be found here on You Tube or on their Facebook website.
$NWAV thanks! I am sure you will like what you find ![]()
Will take a look at it. Thanks!
$NWAV 231 million float confirmed and locked thru 2015
Good info thxs!
$Pistol Pete$ GDSI... http://investorshub.advfn.com/boards/read_msg.aspx?message_id=118346232&txt2find=DD
PUGE 23 million float in the OTC? That's the lowest I've see
PUGE tiny float big board like OTC play. You only find one of these every couple years that could take you from pennies to dollars ahead. Do your DD. God bless cj
thxs for the info! we will give it a check!
Chartman2DRSQ $SNXG...http://investorshub.advfn.com/boards/read_msg.aspx?message_id=118285411
stockdarockk $BIEL....http://investorshub.advfn.com/boards/read_msg.aspx?message_id=118294242&txt2find=DD
BSSP great chart off 52 week lows. This one is ready to run!
DDNOTESMAKER $EEGI.... http://investorshub.advfn.com/boards/read_msg.aspx?message_id=117909592
$PISTOLPETE$ $BSSP... http://investorshub.advfn.com/boards/read_msg.aspx?message_id=118287972
$Nephilim$ $NWAV... http://investorshub.advfn.com/boards/read_msg.aspx?message_id=118266345
|
Followers
|
50
|
Posters
|
|
|
Posts (Today)
|
0
|
Posts (Total)
|
1175
|
|
Created
|
06/07/15
|
Type
|
Free
|
| Moderators OTCRIDDLER Indica norcalpsl Penny Soldier | |||
|
Posts Today
|
0
|
|
Posts (Total)
|
1175
|
|
Posters
|
|
|
Moderators
|
| Volume | |
| Day Range: | |
| Bid Price | |
| Ask Price | |
| Last Trade Time: |
