Register for free to join our community of investors and share your ideas. You will also get access to streaming quotes, interactive charts, trades, portfolio, live options flow and more tools.
I wasn’t all in, but a little more than I could afford to lose...to tune of 20k. Now, I can sleep. I hope it works out for everyone...especially the patients. ![]()
Well if no reply from N.W perhaps you could start a coalition of shareholders, with a large voting block. IMHO
Interesting tweet from Bigger:
$12 million of commitment with no ability to sell until x,y,z happens Think about that ....
— Michael Bigger (@biggercapital) December 8, 2017
Thanks, Re: Neil Woodford.
Looks like over 1 million shares interested in the first 20 minutes.
I shall approach as a direct investor in Neil Woodford Patient Capital Trust.... just pointing out that there is interest from retail investors. The main thing is to try and gather a focal point and to stop this “divide into thousands of small parts and ignore” business. It may come to nothing .. but better to have tried and lost than never to have tried at all. NW may have no interest in it in which case it just sinks and we wait for LP to look on us munificently as good long term employees.
Woodford seems to be more of passive investor. But if he wanted to pursue a proxy battle with Linda, it would be interesting to hear what he has to say. Honestly, if I was Woodford I would pursue better governance, especially to avoid conflicts of interest, and add more independent board members.
hi, Thanks for reply to the Neil Woodford Enquiries”
I am afraid that hearing that LP “holds all the cards’ and “might” look kindly on investors and “may” want to reward us is part of the diversionary fluff that I have described. “We” as retail investors have been thoroughly divided and our comments can be fatuous, vapid or outstandingly prescient AND YET have no worth at all. I am not comfortable with that. This is NOT a family business so someone trying to run it like it is needs to be challenged before it becomes a family piggy bank.
Neil Woodford is the ONLY other significant (25?million shares.. $160 million invested) investor I am aware of that has a history of running businesses properly (he openly admits to mistakes... and that is quite a distinction is it not?). I feel that I need to ask him if he can use my shares at an ASM. I have asked if anyone else likes this idea. I have also said that I would consider Neil Woodford my first port of call if shareholder value disappeared overnight. I will keep you posted.
Thanks for your observation. I hope that glove fits! ![]()
I suspect the "NDA" exemption is much more narrow than people think just by reading the plain language there. I'd bet it has more to do with the particularities of the offering, until it's disclosed by the company, rather than an exemption on them that allows them to selectively disclose information on trials or other data, which I suspect they don't even have yet, but even if they did, I doubt that exemption applies to that particular kind of information. I think that is beyond the scope of what is likely intended and meant there.
It's of course possible, and I won't claim to have deeply researched it, but just say, from experience of a different sort, if someone told me, and asked me to rely on just reading that language alone, I'd go get some experienced securities law counsel, and even they'd probably have to do an evening's worth of intermediate research to give me the answer.
Looking forward to your non or response from N.W.
Incliedampforest - I like your idea. I am fine with NW representing my 928k shares. BTW, I have also emailed NW two months ago and still waiting for his response.
Re Neil Woodford
Thank you for responding. If there is a lot of support for this idea by 10am (East Coast) tomorrow I will make sure it is referenced.
I believe Linda Powers may reward those who have stuck with her through all of this after the dust has settled. She has the ability to do this and just like situations where family businesses might reward long term employees with bonuses based on the length of time with the business
hi, Thanks for reply to the Neil Woodford Enquiries”
I am afraid that hearing that LP “holds all the cards’ and “might” look kindly on investors and “may” want to reward us is part of the diversionary fluff that I have described. “We” as retail investors have been thoroughly divided and our comments can be fatuous, vapid or outstandingly prescient AND YET have no worth at all. I am not comfortable with that. This is NOT a family business so someone trying to run it like it is needs to be challenged before it becomes a family piggy bank.
Neil Woodford is the ONLY other significant (25?million shares.. $160 million invested) investor I am aware of that has a history of running businesses properly (he openly admits to mistakes... and that is quite a distinction is it not?). I feel that I need to ask him if he can use my shares at an ASM. I have asked if anyone else likes this idea. I have also said that I would consider Neil Woodford my first port of call if shareholder value disappeared overnight. I will keep you posted.
If his reasons are good to vote one way or another, then I believe everyone will follow.IMHO
Shareholders are entitled to vent and make demands. It happens all the time and it is for the better,of all invested. It is investors that are making NWBO possible. IMHO
inclineddampforest,
Linda Powers holds the cards right now and Mr. Woodford still has some explaining to do. I don't think he is capable of doing much of anything right now except wait and watch. Without the context of knowing the big picture of Linda Power's complete strategy we are all basically at a loss with knowing how to interpret current moves in the context of longer term goals. This leads us to impose our beliefs or fears into our interpretations of what the company moves mean with added weight given to historical patterns as evidence. From my own perspective, I believe Linda Powers may reward those who have stuck with her through all of this after the dust has settled. She has the ability to do this and just like situations where family businesses might reward long term employees with bonuses based on the length of time with the business, comments attributed to management about faithful long term longs lead me to believe that she might just have something similar planned but not advertised for various reasons. I don't count on this happening and no one else should either but just like a family run business owner, Linda could do this if she wanted to. Best wishes.
Wonder if Barcode knows what the latest reads. He has access to Pacer. Are they pulling out of settlement court for December 18, or is the Motion to Dismiss just parallel preparation?
Just an observation about Cognate. From their website.
"Developed Innovative Closed Systems for Manufacturing of Adherent and Non-adherent Cells" -- Cognate Bioservices
The present invention it was determined that dendritic cells could be derived from various sources including peripheral blood monocytes in the presence of only GM-CSF without other cytokines if the monocytes were not activated. By preventing activation, such as by preventing binding of the cells to the surface of the culture vessel, the monocytes do not require the presence of additional cytokines, such as IL-4 or IL-13, to prevent differentiation into a non-dendritic cell lineage. -- Marnix Bosch
Monocytic dendritic cell precursors and immature dendritic cells can also be prepared in a closed, aseptic system. As used herein, the terms “closed, aseptic system” or “closed system” refer to a system in which exposure to non-sterile, ambient, or circulating air or other non-sterile conditions is minimized or eliminated. Closed systems for isolating dendritic cell precursors and immature dendritic cells generally exclude density gradient centrifugation in open top tubes, open air transfer of cells, culture of cells in tissue culture plates or unsealed flasks, and the like. In a typical embodiment, the closed system allows aseptic transfer of the dendritic cell precursors and immature dendritic cells from an initial collection vessel to a sealable tissue culture vessel without exposure to non-sterile air. -- 2016 Benjamin A. Tjoa, Marnix L. Bosch
Enquiries to Neil Woodford. Ihave read with interest your (bored lawyer) post and those associated with it directly or by subject matter.
My own position has been laid out over several months with infrequent, but I think pertinent , observations,
I control 170,000 shares at an average that I wish was lower.
I think this message board has been designed or manipulated to waste vast amounts of retail investor energy ; in particular it allows steam to be vented while no one actually does anything .
It is clear to me that the science here is remarkable.
It is clear that the management of this company have a complete disregard to the reasonable concerns and interests of retail investors. To what extent this is due to the personal psychologies of the Officers I do not know. I find it very very odd. It may be perfectly honest ... but still very very odd. Like having a friend with Asperger’s(this is not intended as a flippant comment).
Tomorrow I am contacting Neil Woodford’s team and enquiring if there is any way in which he can use my shares voting rights at the ASM. I don,t know if this has any mileage in it but it seems , to me, absurd that no one can get a squeak out of the company while all the while deals are being done that dilute our interest to hell. Such dilution might be fair in the interests of the original huge stake holders , but if so , say it out loud. .
It I also clear to me that in the event of something “tricksy” occurring that we need some leverage ready and waiting. This should not be an ambulance chasing lawyer... the stakes are far too high. Woodford is the obvious RV point if we , as retail investors, suddenly find ourselves out in the cold.
I will contact NW regardless. If anyone thinks there is mileage in asking him to represent individual voters please say. If anyone thinks there is mileage in asking him to be an RV point in the event that we wake up one morning to have no share value please indicate.
Just to be clear... no-one is shaking my shares out of me. This is all my honest opinion . I have no idea if NE will respond. The only way I have of contacting him is on the message board at his NWBO holding fund.
Despite the Charles Payne twitter traffic about NWBO, this company is probably indeed more likely to be featured on American Greed than on the Fox Business Channel.
Exactly, we're really not asking that much. I am not even saying that they have to unblind the data before I hand in my proxy vote. All I am freaking asking for is maximum date certain for that unblinding and perhaps some information on the many other topics that they can legally discuss. The maximum date would probably even suffice as long as there was no wiggle room in the way they worded it and it was not say 5 years from now.
The idea that ordinary shareholders are bad people or stupid for finally standing up for themselves and saying,
"If you cannot even be just a little honest and transparent with us, when you are asking us to take an action in your favor and the favor of your inside clique, then maybe the DCVax L IP that Linda Liau (not Linda Powers but Linda Liau) developed should be in the hands of a management team that will actually do something with it, instead of stringing along hapless investors year after year with "stay tuned" (remember 'stay tuned' is not my quote but Linda Powers')."
They have already let DCVax Prostate languish despite being approved for a Phase 3 trial. How many years has that been pending partnership again? And this despite the fact that prostate surgery has debilitating effects on men and furthermore is one of the biggest cancer indications.
Right now, absent some clarity provided by NWBO management, a lot of us believe we're going to lose either way, so we might as well at least try to fight.
The idea that NWBO either has to tell us everything or tell us nothing is also a falsehood.
Transparency is a continuum and not an on or off switch.
They can answer a lot of questions without answering all of them and the main thing is to give clear and unambiguous answers to whatever they do answer instead of this vague language that creates more uncertainty than it resolves.
They could provide a clear road map for the way forward etc. without unblinding any data.
Issues like Sawston are likewise not blinded, as are things like whether the hiring for Cognate positions is relevant to NWBO or a separate matter.
Rebuilding confidence during bad situation demands transparent and accountable leadership and not executives behaving like criminal defendants adhering to the advice of counsel.
Instead NWBO management is so far towards the "disclose nothing more than the minimum we can legally get away with" end of the transparency continuum it is not even funny.
Thanks for your post Bored Lawyer.
I agree with your interpretation. I'd written, some time back as this subject often comes up, that it was my opinion they didn't have to disclose, so long as they've not disclosed to anyone.
Sounds like a reasonable demand any investor should make, before allowing the amount of authorized shares, to be increased.IMHO
Do you know does all info communicated to the investors who signed the NDA have to be divulged in the 8K?
I saw that. I wonder if one or more of the $12m preferred investors put in $2million, and they also had 6.6 million .26¢ warrants. Perhaps as an incentive for contributing $2m in the $12m deal, they were able ALSO to exchange their .26¢ warrants from a previous deal for .22¢ Class D-1 warrants.
That's how it reads to me. However, it isn't an even exchange.
Concurrent with the closing of the Series A Offering, the Company also exchanged previously issued warrants for the purchase of 6,686,342 shares of our Common Stock, exercisable at $0.26 per share, for Class D-1 Warrants for the purchase of 11,792,482 shares of our common stock, exercisable at $0.22 per share, and 120,590 shares of Series A Preferred Stock. Such securities are being exchanged and issued to certain investors in consideration of approximately $2,000,000 in additional investments received by the Company in this offering
The Burden of Proof.
The burden of proof, when you claim someone is a criminal or a fraud, is you have the duty to prove it. As we know already, cases have been brought in court, at the lowest standard of review, on a motion to dismiss, plaintiffs making the exact same arguments you have made, have had their cases dismissed, having likely spent many hundreds of thousands on both sides in attorney and court fees. People making your same arguments have wasted the resources of every investor here, and run down the stock with fake claims.
Even if that were not the case, the burden of proof, to prove that people are honest, would not rest with Kabuni, it would rest with you and your outrageous claims, not on a bulletin board, but in a court of law.
But having run the company and every investor here through that ordeal, those costs, the outrageous claims that turned out to be scurrilous and not provable with even a "scintilla" of evidence, which is the general standard for keeping a court case in court... you and those making the arguments you make, have no grounds to stand on China.
I really don't want to spend any more time on this issue. I understand the requirement for or not for correcting or updating. But, are you confident you have the correct reference, that for secondary offerings by public companies?
There is so much interpretation, which is why I am fine to go with the 9 out of 10 time rule and continue to use my common sense.
So you are saying Bigger is not legitimate???
Looks like you will have to pay more if you want to get back in. That is the problem with selling, it always goes up. IMHO
Hope your right about that and NWBO can do something for shareholders. The ones who are making all of this possible. IMHO
Kabunushi, it is my understanding that the exemption from Reg FD for offerings is because they are satisfactorily covered by the original securities act. And, secondary offerings, or, some secondary offerings are not exempted. I hate these legal searches. We are such a country defined by lawyers for lawyers. There are typically multiple areas to look with multiple interpretations to work through. Bottom line for me is it is quite illogical to permit "material" disclosures to a select audience and in this case it could be information that would conflict with what has already been communicated to current investors in favor of new (or repeat) select investors. I will continue this silly legal search and deal with the legal terms. The SEC website is the first stop. It does take time.
TY senti. You are the best. :)
I did get the chance to listen to the entire presentation. There wasn't really anything new and riveting in the testimony there.
There was something worth noting and I don't think it was noted on this board yet.
One of the senators asked Dr. Gottlieb about a laboratory test that received approval. It had been given breakthrough designation, and it was given simultaneous CMS coverage at the same time (which is new, I guess).
Anyhow... this laboratory test approval is for F1CDx by Foundation Medicine. Why this seems relevant to me is that their test is for genetic mutations to help identify which treatments might be best for patients. And there has been a lot of talk about the importance of such tests in the future treatment of GBM.
FDA Announces Approval, CMS Proposes Coverage of First Breakthrough-designated Test to Detect Extensive Number of Cancer Biomarkers
FDA approves test to detect mutations in 324 genes, two genomic signatures. Second to be approved with proposed coverage under FDA/CMS Parallel Review Program.
The U.S. Food and Drug Administration today approved the FoundationOne CDx (F1CDx), the first breakthrough-designated, next generation sequencing (NGS)-based in vitro diagnostic (IVD) test that can detect genetic mutations in 324 genes and two genomic signatures in any solid tumor type. The Centers for Medicare & Medicaid Services (CMS) at the same time proposed coverage of the F1CDx. The test is the second IVD to be approved and covered after overlapping review by the FDA and CMS under the Parallel Review Program, which facilitates earlier access to innovative medical technologies for Medicare beneficiaries.
“By leveraging two policy efforts aimed at expediting access to promising new technologies, we’ve been able to bring patients faster access to a breakthrough diagnostic that can help doctors tailor cancer treatments to improve medical outcomes and potentially reduce health care costs,” said FDA Commissioner Scott Gottlieb, M.D. “The FDA’s Breakthrough Device Program and Parallel Review with CMS allowed the sponsor to win approval for this novel diagnostic and secure an immediate proposed Medicare coverage determination within six months of the FDA receiving the product application.”
Compared to other companion diagnostics previously approved by the FDA that match one test to one drug, the F1CDx is a more extensive test that provides information on a number of different genetic mutations that may help in the clinical management of patients with cancer. Additionally, based on individual test results, the new diagnostic can identify which patients with any of five tumor types may benefit from 15 different FDA-approved targeted treatment options. Its results provide patients and health care professionals access to all of this information in one test report, avoiding duplicative biopsies.
“The F1CDx can help cancer patients and their health care professionals make more informed care decisions without the often invasive process of extracting tumor samples multiple times to determine eligibility for a single treatment or enrollment in a clinical trial,” said Jeffrey Shuren, M.D., director of the FDA’s Center for Devices and Radiological Health (CDRH). “With the run of one test, patients and health care professionals can now evaluate several appropriate disease management options.”
Today, CMS also issued a proposed national coverage determination of the F1CDx and other similar NGS IVDs for Medicare beneficiaries with advanced cancer (i.e., recurrent, metastatic or advanced stage IV cancer), who have not been previously tested using the same NGS technology and continue to seek further cancer therapy. The proposed national coverage determination provides coverage of NGS IVD tests to assist patients and their treating physicians in making informed cancer treatment decisions that improve health outcomes. Use of a test as a diagnostic also includes the ability to help patients and their treating physicians determine candidacy for cancer clinical trials.
“Through parallel review and collaboration, we speed access to innovative diagnostics, so that doctors are better able to deliver the best quality care to their patients and patients have access to these state-of-the-art tests,” said Seema Verma, Administrator of CMS. “Our proposal establishes clear expectations, while at the same time delivering better outcomes for the people we serve.”
This determination was made under the FDA-CMS Parallel Review Program, where the agencies concurrently review medical devices to help reduce the time between the FDA’s approval of a device and Medicare coverage. This voluntary program is open to certain premarket approval applications for devices with new technologies and to medical devices that fall within the scope of a Part A or Part B Medicare-benefit category and have not been subject to a national coverage determination.
The F1CDx detects gene mutations that may be found in any solid tumor and this information can be used by physicians according to professional guidelines to manage cancer patients. Moreover, it can be used as a companion diagnostic to identify patients with specific mutations who may benefit from certain FDA-approved treatments for non-small cell lung cancer, melanoma, breast cancer, colorectal cancer or ovarian cancer. Importantly, the F1CDx can detect genetic mutations that are indicated for multiple FDA-approved treatments, which extends beyond the previous “one test for one drug” model.
The device works by sequencing DNA from a patient’s tumor sample to determine the presence of gene mutations and alterations. It also detects certain molecular changes (microsatellite instability and tumor mutation burden). Clinical performance of the test was established through a least burdensome means by comparing the F1CDx to previously FDA-approved companion diagnostic tests that are currently used to determine patient eligibility for certain treatments. Results indicated that the test’s ability to detect select mutation types (substitutions and short insertions and deletions) representative of the entire 324 gene panel is accurate approximately 94.6 percent of the time.
The F1CDx had not been previously submitted for the FDA’s review because it is a laboratory-developed test, for which the agency has generally not enforced premarket review and other applicable requirements. However, at the test developer’s request, the FDA worked closely with them to help enter it into the agency’s newly established Breakthrough Device Program. Because of the test’s potential to consolidate multiple companion diagnostic claims for patients and health care providers in a single test, the F1CDx was granted Breakthrough Devicedesignation. Under the Breakthrough Device Program, the FDA provides intensive interaction and guidance to the company on efficient device development, which expedites evidence generation and the agency’s review of devices that provide for more effective treatment or diagnosis for life-threatening or irreversibly debilitating diseases for which no approved or cleared treatment exists or that offer significant advantages over the existing standard of care.
The FDA also reviewed the F1CDx application using a coordinated, cross-agency approach; the clinical review was conducted by FDA’s CDRH with support from FDA's Oncology Center of Excellence, while all other aspects of review and the final product approval determination was conducted by the FDA’s CDRH.
The FDA granted approval for the F1CDx test to Foundation Medicine, Inc.
www.practiceupdate.com/c/61478/1/12/?elsca1=emc_enews_daily-digest&elsca2=email&elsca3=practiceupdate_onc&elsca4=oncology&elsca5=newsletter&rid=MjU5OTIzOTQ4MTYxS0&lid=10332481
Clinical utility and treatment outcome of comprehensive genomic profiling in high grade glioma patients.
Blumenthal DT1,2, Dvir A3, Lossos A4, Tzuk-Shina T5, Lior T6, Limon D7, Yust-Katz S8,7, Lokiec A5, Ram Z8,9, Ross JS10,11, Ali SM10, Yair R3, Soussan-Gutman L3, Bokstein F12,8.
Author information
Abstract
Genomic research of high grade glioma (HGG) has revealed complex biology with potential for therapeutic impact. However, the utilization of this information and impact upon patient outcome has yet to be assessed. We performed capture-based next generation sequencing (NGS) genomic analysis assay of 236/315 cancer-associated genes, with average depth of over 1000 fold, to guide treatment in HGG patients. We reviewed clinical utility and response rates in correlation to NGS results. Forty-three patients were profiled: 34 glioblastomas, 8 anaplastic astrocytomas, and one patient with anaplastic oligodendroglioma. Twenty-five patients were profiled with the 315 gene panel. The median number of identified genomic alterations (GAs) per patient was 4.5 (range 1-23). In 41 patients (95?%) at least one therapeutically-actionable GA was detected, most commonly in EGFR [17 (40?%)]. Genotype-directed treatments were prescribed in 13 patients, representing a 30?% treatment decision impact. Treatment with targeted agents included everolimus as a single agent and in combination with erlotinib; erlotinib; afatinib; palbociclib; trametinib and BGJ398. Treatments targeted various genomic findings including EGFR alterations, mTOR activation, cell cycle targets and FGFR1 mutations. None of the patients showed response to respective biologic treatments. In this group of patients with HGG, NGS revealed a high frequency of GAs that lead to targeted treatment in 30?% of the patients. The lack of response suggests that further study of mechanisms of resistance in HGG is warranted before routine use of biologically-targeted agents based on NGS results.
Well I’m out of time but as we continue our oversight, and I’m sure you’re going to do this, we need to think of this as a seamless process. We need to go from idea to the doctor’s office to the patient. And we need to get through the F… through the research and into the FDA, through CMS, to make these things work. And I hope you’ll pay a great deal of attention to that.
The trial is at the endpoint and we're going to get news before the new shares can even be sold.
the difference between us is i don't talk my book. now i did somewhat a month or two ago when i encouraged cherrytree to buy when i was buying. i did feel a twinge of conscience when i sold that position and i regretted i didn't tell him but he is up 30% now on the shares he bought.
NWBO SOMEDAY ON TV ? AMERICAN GREED !
YOUR NOT MISSING ANYTHING IMHO
THIS COULD END UP ON REALITY TV
I think what we need to do is create a document with the names and the amount of shares each person has in this company and send those multi million share account document to the company and the demand that we be updated on the trial.
how do you know the most recent investors will "pillage and dump' as you imply?
your implication is equally baseless.
Maybe it's the same vultures bs but they simply have to wait a few days until the shares are available under a revised shelf. How do you know they aren't going to pillage and dump this down to new all time lows as soon as they can? It is a totally baseless hypothesis that this isn't more of the same toxic dilution, except with a slight delay.
And he mentioned 4-6 weeks and that was two weeks ago???
Very very interesting
I remember Michael Bigger tweeting " Now let's wait for the data" in response to someone but can't find the tweet.
Please tell me why you all keep on trusting LP ?? Please tell me ![]()
A deal with BP would have been better not only for us but also for patients .. I simply can not understand you keep believing in LP as she obviously has herself and Cognate as first priority and not Nwbo ..
If we have the goods as you all say we have, why should a BP not be interested in 50% just to pay costs ..
This deal that has been concluded with Cognate and investors, would LP never have agreed to if she did not own Cognate .. please explain me? What am I missing? ![]()
Thanks GGB. Hope you get a response from NWBO on our behalf. It is interesting that warrants were concurrently exercised bringing some needed funds. Someone must be optimistic to be exercising when the PPS is not deep in the money.
"Concurrent with the closing of the Series A Offering, the Company also exchanged previously issued warrants for the purchase of 6,686,342 shares of our Common Stock, exercisable at $0.26 per share, for Class D-1 Warrants for the purchase of 11,792,482 shares of our common stock, exercisable at $0.22 per share, and 120,590 shares of Series A Preferred Stock. Such securities are being exchanged and issued to certain investors in consideration of approximately $2,000,000 in additional investments received by the Company in this offering "
The entire premise of your long rant is that these new investors are in it for the long haul. What proof do you have of that? Maybe it's the same vultures bs but they simply have to wait a few days until the shares are available under a revised shelf. How do you know they aren't going to pillage and dump this down to new all time lows as soon as they can? It is a totally baseless hypothesis that this isn't more of the same toxic dilution, except with a slight delay.
In reality the float argument is bogus too. You don't think Cognate will sell? Are you familiar with their previous sales?
Now that is very very interesting eh??
Agreed. Investor NDAs covered (a) the existence and terms of the transaction prior to those being released in an 8k (this would include voting arrangements, etc), and (b) a separate NDA covering certain fundamental information about the trial / company.
All investors would have to sign "a". This NDA would prohibit trading until the 8k is released. NDA "b", while optional, would give long term investors comfort that the technology is compelling. The can't sell until the fundamental data is publicly available but why would they? If there is a significant therapeutic benefit, the stock is not going to stop at $0.50
![]() ...you too.
...you too.
Sorry, long post! executive summary, yes but don't overlook the fact that having investors buying the offerings vs vultures is super HUGE good for longs. And Big Kudos to Flipper for pointing out that the float is much smaller than the total shares/warrants outstanding.
News Brief: The bigger guys playing in the capital markets have a HUGE advantage, esp when funding weak companies who have no highly competitive offers to raise capital. We've seen in every single offering for the last 2 years that the bloodsuckers, er funders, get 100% warrant coverage, this is a huge discount. These guys can turn around and sell all the shares and just keep the warrants; or use the warrants as safety against a run up and after selling all the shares, continue selling into shorting against the warrants. It's a huge hazard to invest in financially weak companies but the vulture funders can avoid most of the risk while getting outside rewards.
That having been said, I think it's a big misunderstanding to get pissed off about disclosure! Longs really, really, really want offerings to go into the hands of investors vs the vultures!!! Having the shares go to people who actually believe in the company and will hold the shares is an incredible benefit to us. The 'normal' funding through vulture hedge funds who specialize in quick flips put enormous downward pressure on the stock. I think the action we are seeing with buying before the offering leading to a jump and we don't immediately get slammed right down is totally because investors, not vultures have bought all of the very largely dilutional preferred shares. We can't be certain, but it does look pretty darn good from where I sit, that the stock hasn't gotten slammed at least not yet.
Big Kudos to Flipper for calling a lot of attention to this: while the total share count has gone way up, the number of shares in the float, subject to being traded every day is way less than the total. If the latest preferred offerings all stay off the market, that helps to avoid another collapse and also makes for rocket fuel if the company finally announces data that demonstrates clearly that dcvaxl works!
|
Followers
|
1637
|
Posters
|
|
|
Posts (Today)
|
320
|
Posts (Total)
|
722350
|
|
Created
|
02/02/05
|
Type
|
Free
|
| Moderators flipper44 sentiment_stocks CaptainObvious Poor Man - Doc logic JerryCampbell | |||

“Now this is not the end. It is not even the beginning of the end. But it is, perhaps, the end of the beginning.”
~ Winston Churchill
Stylized Dendritic Cell featured on NWBO board since 2015

- Dr. Linda Liau, PhD, MBA, Professor and Chair, Department of Neurosurgery, David Geffen School of Medicine at UCLA
Clinical Trials
DCVax®-L to Treat Newly Diagnosed GBM Brain Cancer (NCT00045968) - Phase III (Double Blind)
UK (MHRA): DCVax-L to Treat Newly Diagnosed GBM Brain Cancer (EudraCT#) 2011-001977-13
DE (Germany - PEI): DCVax-L to Treat Newly Diagnosed GBM Brain Cancer (EudraCT#) 2011-001977-13
Expanded Access Protocol for GBM Patients with Already Manufactured DCVax®-L Who Have Screen-Failed Protocol 020221 (NCT02146066) (Expanded Access)
Safety and Efficacy Study of DCVax-Direct in Solid Tumors (NCT01882946) - Phase I/Phase II (Open Label)
UK Clinical Trials - Study of a Drug (DCVax®-L) to Treat Newly Diagnosed GBM Brain Cancer
EU Clinical Trials for DCVax-L - Phase III
Dendritic Cell Vaccine for Patients with Brain Tumors (NCT01204684) - Phase II - at UCLA - Randomized (Open Label) testing DCVaccine with Resiquimod and DC Vaccination with Adjuvant polyICLC
Pembrolizumab and a Vaccine (ATL-DC) for the treatment of Surgically Accessible Recurrent Glioblastoma - Phase 1 (NCT04201873)
Dendritic Cell-Autologous Lung Tumor Vaccine (DCVax-L) and Nivolumab in Treating Patients with Recurrent Glioblastoma - Phase 2 (NCT03014804)
Dendritic Cell Therapy for Brain Metastases From Breast or Lung Cancer (NCT0368765) - Phase 1 - Collaborator: Mayo Clinic
Announcement of DCVax-L and Anti-PD-1 Monoclonal Antibody (Pembrolizumab) for Patients with Liver Metastases of Primary Colorectal Carcinoma Phase 2 Trial - November 17, 2016 - University Medical Center (UMC) of the Johannes Gutenberg University of Mainz
Cognate Bioservices - Owned by Charles River Labs
Website
Company Contact Info
Investor Relations:
Les Goldman (Company) (202) 841-7909 lgoldman@nwbio.com
Sign up for Northwest email list here (hit the subscribe to email list button in the lower right)
Company Headquarters
4800 Montgomery Lane, Suite 800, Bethesda, MD 20814 (240) 497-9024
NW Bio is developing cancer vaccines designed to treat a broad range of solid tumor cancers more effectively than current treatments, and without the side effects of chemotherapy drugs. NW Bio’s proprietary manufacturing technology enables them to produce its personalized vaccine in an efficient, cost-effective manner. NW Bio has a broad platform technology for DCVax dendritic cell-based vaccines.
Their lead product, DCVax-L, is currently in a 331-patient Phase III trial for patients with newly diagnosed Glioblastoma multiforme (GBM), the most aggressive and lethal brain cancer. This trial is currently underway at 69 locations thoughout the United States, Germany and the United Kingdom. NW Bio has also conducted a Phase I/II trial with DCVax-L for late stage ovarian cancer together with the University of Pennsylvania.
Their second product, DCVax-Direct, is currently in a 60-patient Phase I/II trial for direct injection into all types of inoperable solid tumor cancers, with trials currently being conducted at both MD Anderson Cancer Center in Texas, as well as Orlando Health in Florida.
They previously received clearance from the FDA for a 612-patient Phase III trial with its third product, DCVax-Prostate, for late stage prostate cancer.
DCVAX Survival Stories & Testimonials
Alice - Metastic Merkel Cell patient from Florida - ASCO 2018
Brad Silver - GBM patient from Huntington Beach, California - ASCO 2018
Sarah Rigby - GBM patient from Hong Kong - ASCO 2018
Kristyn Power - daughter of GBM patient from Canada - ASCO 2018
Kat Charles - GBM patients from UK - ASCO 2018 - as related by her husband Jason (Kat's Cure)
Prospective patients may contact NW Bio at patients@nwbio.com
UCLA Jamil Newirth DCVax-Patient Video - 2015
Allan Butler Video - National Geographic Vice President - DCVax-Direct patient from Phase 1 Trial with Pancreatic Cancer
NWBO - Patients Sunday Dennis and Jami Newirth - Enrolled at UCLA - Vimeo, Uploaded approx. May 2015
NWBO - Vaccine Helps Keep Brain Cancer Patient Alive (Jennifer Sugioka) - NBC Channel 4, Southern California, February 24, 2015
NWBO - National Geographic's Allan Butler Stage IV Pancreatic Patient using DCVax-Direct at MD Anderson
NWBO GBM Brain Cancer Survival Story of Mark Pace

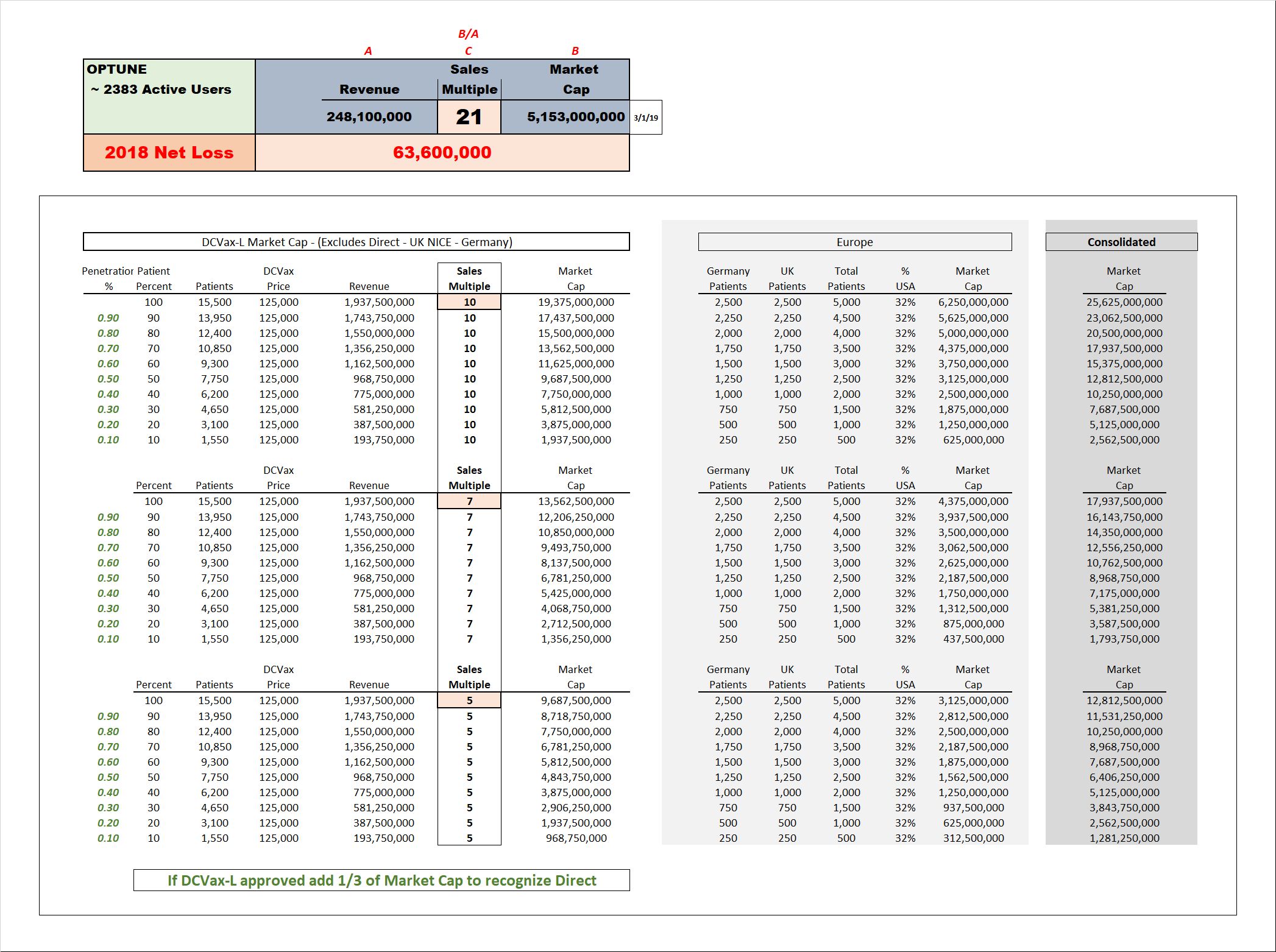
Presentations
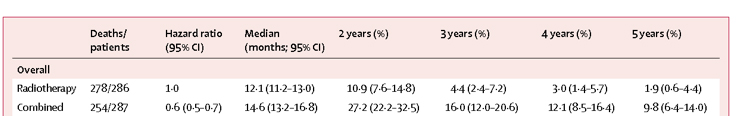
UCLA Agreements
Prostrate
DCVax-Phase II
DCVax-Booster
Upcoming Events
Videos
Linda M. Liau, MD, PhD, MBA - April 24, 2019 at University of Washington, Neurosciences Institute
| Volume | |
| Day Range: | |
| Bid Price | |
| Ask Price | |
| Last Trade Time: |
