Register for free to join our community of investors and share your ideas. You will also get access to streaming quotes, interactive charts, trades, portfolio, live options flow and more tools.
Time to STOP lending Your $NWBO Shares
as the trap is set
The $NWBO Critical MASS is in place
Time to STOP lending Your $NWBO Shares
— maverick_1 (@maveric92283613) May 3, 2024
as the trap is set
The $NWBO Critical MASS is in place
Lots MultiTasking done only by Femme PhenomS
that CEO Linda Powers & UCLA’s Dr. Linda Liau
has BOTH mentored a cadre of Esprit de Corp
For The New Era of Personalized Immuno Oncology
Eventually. Narratives never immediately change price. This is just your way of trying to steer the conversation back to nonsense. A change in narrative can be quite subtle after years and years of misinformation.
DEF NOT THE SP showing at fintel, maybe a calculation between absurd spreads.
wouldn't the sp / the trading in germany reflect THAT sp ! ?
the sp in germany is between 41 and 43 euro/cents
Thanks Eagle. Obviously there are a lot of vermin circulating within the Ihub stock boards trying to cause FUD on disruptive technologies! I see it daily on a different technology stock I own a large position in, and many are on ignore or I just follow the informed folks to keep in touch with evolving advances, as the time eats into my day/life. It is unfortunately the world we live in, and Adam Far*st&^b was one of the earlier ones, and pseudo-professional too, that got away with biotech murder.
GLTU and All longs/patients
First see then believe ilovetech.
GLTU
May 2024 Neurology Recall: Topics in Neuro-Oncology
— Ida Bogac (@albtur_mnymkrs) May 3, 2024
Dr. Linda Liau on the study of vaccine-based therapy for GBM @ min 00:32. $nwbo #dcvax https://t.co/yvdsBq0gvx
See this results on pediatric patients from the combination of DCVax-L with CYT107. You don't want DCVax-L available to all cancer patients including children, do you? How could you sleep at night?
Results: Forty-three patients enrolled and 29 received immunotherapy. The regimen was well tolerated. Intent-to-treat analysis demonstrated 5-year OS of 51% with significant differences based upon histologic group (63% vs. 0% for Ewing/rhabdomyosarcoma vs. other sarcomas) and response to standard therapy (74% no residual disease vs. 0% residual disease). Five-year intent-to-treat OS of patients with newly diagnosed metastatic Ewing/rhabdomyosarcoma was 77%, higher than previously reported in this population and higher than observed in a similar group treated with an earlier adjuvant immunotherapy regimen (25% 5-year OS). T-cell responses to autologous tumor lysate were identified in 62% of immunotherapy recipients, and survival was higher in those patients (73% 5-year OS with vs. 37% without immune response, P = 0.017). Immune reconstitution, measured by CD4 count recovery, was significantly enhanced in subjects treated with recombinant human IL7.
We know they have St. Gobain's Glass for production of the disposable cassette, I believe they can produce virtually any quantity that NWBO wants. In terms of the EDEN unit itself, I'm sure that several companies would be capable of either building the entire unit, or building components that the Flaskworks personnel could assemble, test and certify.
I believe the EDEN units will be leased to those authorized to make the vaccine, NWBO will be responsible for all maintenance and upgrades, and I think they'll track each cassette to the machine that makes the vaccine and it's proper disposal. The question is whether Flaskworks will assemble the EDEN's from contracted components, or just certify each unit prior to delivery to the producer, and then maintaining and updating it as applicable. I think that Flaskworks will be a profit center of it's own.
Gary
Manibio -
So not sure what those trades posted on Stocktwits represent
will put NWBO and partners on a logarithmic trajectory.
Yep it happens on OTC often , that those trades are balanced after hours , but the price difference is not more than 5-10% at the most that I have seen . But Fidelity site says that one can trade OTC after hours . So not sure what those trades posted on Stocktwits represent
skitahoe,
As you know Corning has had some past experience with Flaskworks so yes, there can be a scale up under the right circumstances which were obviously not in place at the time Corning sold their rights back. The cassettes will also be mass produced at some point. It was the initial layout that took so long and the GMP produced final product. We seem to be about ready to move at a steady forward pace with all of this and that, coupled with approval and reimbursement agreements, will put NWBO and partners on a logarithmic trajectory. Like I have said consistently, big pharma is in no hurry to announce that they have become second fiddles but when they are forced to because of NWBO achievements the fireworks really begin. Best wishes.
Could it have been a trade made during hours but posted after hours?
Same. Certainly appears one can, but....who dun it?
-Work continues- The funds will be used for the Company’s ongoing business operations, including beginning initial construction works for the first grade C lab in the Company’s Sawston, UK facility, ordering certain initial long lead-time equipment for the first grade C lab, and facility preparations for delivery of the initial GMP units of the Flaskworks system.
https://www.otcmarkets.com/filing/html?id=17505671&guid=hdQ-k6p3kt77B3h
We can only hope it is time
I was also under impression that OTC can’t be traded after hours
Thanks, I think I may have after hours authority at Fidelity, but didn't think OTC was covered, clearly it is. Pink's aren't, and that's fine with me.
Let's hope that purchase is based on someone knowing something. The other possibility is someone put in a market order after hours and the MM's took total advantage of them. Market orders have risk anytime, but after hours I believe you're exceptionally vulnerable to MM's playing games as practically no trading is going on, especially in a stock like NWBO.
Gary
Believe it if we see it on the open and it holds, anything short of that is meh, more tricks?
Haaa by, bye or buy?
I didn't know that you could buy after hours on the OTC, but perhaps on a foreign exchange. If this is real, someone knows something.
Gary
You ever heard of 1 share ah paint. That's more dot connecting for you newbies. This doesn't really trade AH. Can anyone on this board by after hours? Nope they cant.
NWBO up 72.16% after hours. Not much interest on this board: https://fintel.io/so/us/nwbo
Doc logic,
I think you knew this. J. Kelly Ganjei has been the board member of INmune Bio Inc for many years. INmune Bio Inc has trials on both cancer and Alzheimer which strike me as two completely different fields. Is Alzheimer an autoimmune disease?
https://www.inmunebio.com/index.php/about-us
StonkMaster - Thx. A poster on Stocktwits responded just before you did. Looks pretty real to me. Very interesting 🤔
He doesn't show up on 13F/G. They don't really have any institutional funds....379k share is what their biggest institutional holder has and that is pathetic. No legit institutions want any part in this flea ridden company. Where are all these so called investors Thermo and bigger??? I don't see any 13s from these guys. Maybe they are just pumpers too. I thought Thermo said he has shares. I don't see any evidence of it. Where are biggers 13 filings?. Nothing surprises me with regard to how low this company stoops at stock promotion.
TTsr - Do you have the link?
Check out @jstiltz22 message on Stocktwits http://stocktwits.com/jstiltz22/message/571657977
HELLO… Anyone else see the FINTEL #s tonight?
REAL TIME PRICE
May 2, 2024 at 3:59:59
0.4659 0.0059 (1.28%)
EXTENDED HOURS
May 2, 2024 at 4:19:13 PM EDT
0.8049 0.339 (72.76%)!!!!
Yeah, this came up when he first started doing business with NWBO. Seems like he paid some fines and is back in business doing more straightforward deals, but I could be wrong.
Doc,
To my way of thinking, the real question regarding production of the vaccine is what the capacity is for whatever company, or companies, is for making the EDEN units and disposable cassettes. I don't know if estimates that some made of the EDEN costing tens of thousands each when mass produced, but if true, well over the cost of making it is returned in the first batch of vaccine that's made in it. I believe that if worldwide demand were a million batches a year, we could find, or build cleanrooms capable of supporting 20,000 EDEN units which at 50 batches a year give us 1 million batch capacity. I suspect that CRL could house all, or most of the EDEN's in existing facilities they have worldwide that already have cryogenic, and other support needed.
I would think that if the demand is there to build 10,000 EDEN units a month, at the right price manufacturers will materialize who can do it. I'm not saying that many are necessary if production proceeds approvals, but if substantial numbers don't exist at the time of approvals, rapid production may be needed, especially if off label use in other solid cancers is available.
Gary
Nobody here ever talks about Billionaire Robert Hefner anymore. Did he exit?
Do you coach kids soccer? Feels like a pep talk
The Danish Dude,
I have been expecting something to be announced for years with regard to Dr. Mark Lowdell and INmune Bio and other ever since the picture of him, Linda Powers, Kristyn Powers and her dad who was a patient and now a long term survivor after being treated within weeks of expected death from rGBM. He has helped advance manufacturing of L past product release roadblocks too. Lots of lesser known pieces of the puzzle coming together right now. Great job and best wishes.
Hi this is NRNR, am I speaking with Nemesis or is this semen?
IMO today really good day for nwbo and getting closer to great possibilities for gbm patients! 😇
The real dilution hasn't even come yet...
JMHO
Hoooray!!!! I'm not as "Seniored" as I thought!!! When I saw your brief summary of the 8K and the mention of Sawston buildout of C Suites, I remembered talking to the company after hearing Linda Powers talking about the Sawston expansion plan and learned that a single C Suite buildout could double or triple annual vaccine production from 500 vaccines to potentially 1000 to 1500 per year and maybe more!! I also know from friends of mine in London that 1000 vaccines translates into $150 million in revenues to the company. Additionally, I learned that expansions to the plant are scheduled/permitted twice a year in conjunction with "Spit Shine" cleaning before and after completion AND those 2 windows are the last couple of weeks in May and the last 2 weeks in November. So you definitely do not want to miss those windows!!! Hence the "Safety Net" loan that assures shareholders that the company can build the C Suite and upon anticipated approval can begin manufacturing vaccines and start "Raking In The Chips"!!!! Also, with the new loan, 100% of the proceeds can be used because no payments are due for 8 months!! What a "Suite" deal, PUN INTENDED!!!
So in spite of KG and the bad boys trying to kill us, Northwest is screaming down that "Track of Success" like a locomotive fueled with NITRO!!!!! Bravo to the Northwest team and "You know what ... to Griffin and the rest of that "Pond Scum!"
Cheers,
BB
NWBO has nowhere else to go for the money. Seems they were made for each other.
More weakness in share price and more dilution will come when the stock issued for that loan starts hitting the market.
You poor boys have been begging for "news" and the only news you get is more dilution via toxic loans, and bigger salaries and more bonuses to come for management.
I feel sorry for anyone losing money here.
My posts are my opinions
It got some serious history....Those who wish to understand the issues that lie behind Streeterville’s precipitous action, should refer to the current charges brought against Streeterville by the US Securities and Exchange Commission. In those charges, the SEC refers to Defendant John Fife (the owner of Chicago Venture Partners/Streeterville) as a “recidivist violator of federal securities laws. The SEC charges Fife in various ways with manipulating the stock of microcap companies (such as NRx) for profit and is seeking to restrain such action. In 2007, the SEC charged Mr. Fife and his prior entity Clarion Capital with various violations of securities laws and entered into an agreement with him not to violate securities laws in the future. Fife subsequently sued FINRA to overturn FINRA’s decision to bar and censure him. He is currently appealing that case to the US Supreme Court.
https://www.sec.gov/ix?doc=/Archives/edgar/data/1719406/000110465924055041/tm2413153d1_8k.htm
original link won't copy for update on law case
so
Google the following:
Twitter NWBO Hoffman
for Hoffman's synopsis of MTD from defendants
flipper44,
So it seems the reason we have been waiting all this time is the… manufacturing… manufacturing… manufacturing “bump in the road”. Mr. Neil Woodford said what happened with the hold was an expected part of development. However, this became a much more important and lengthy wait because of Covid and cost constraints associated with additional treatment of patients on a label that expanded from GBM to GBM, rGBM perhaps lower grade gliomas and probable significant off label use in trials which creates a much greater initial demand upon approval.
What happens to NWBO valuation when expansion to 12,000 patient batches per year to meet this demand, just at Sawston, is announced with FDA approval expected in 2025?; ). I’ll tell you one thing, the forward P/E ratio starts climbing real fast. Maybe Walgreens execs have a number in mind at three years out even if most big analysts have been encouraged to keep their fingers off the keyboards with regard to NWBO until big pharma gets their chance to play; ). Best wishes.
In roughly the next week NWBO should issue their quarterly report. Unless we have action from the UK, it's doubtful if they'll say anything beyond the filing there. I believe that if they wished, they could gain an additional week if they thought there would be more to report in the additional week.
As of today, the 150th day is just under 3 weeks away, the company certainly knows if they got an RFI or not, which would give them a clue when they should hear from the UK, but I doubt they'll say anything unless they have something official from the UK. As for anything new on the EDEN, perhaps if something worthy of discussing has occurred, we might hear of it in the quarterly.
To me it would be big news if they broke their tradition of only releasing the formal report, but no webcast, if in advance they PR'd a quarterly release coming on a specific day, and a webcast very shortly thereafter.
Gary
They are getting paid in stock and selling it... As far as I know
Loans to pay the interest on older loans. Eventually some convertible death spiral is the usual. How do we know the lender isn't shorting?
You're an idiot.
Block.
|
Followers
|
1619
|
Posters
|
|
|
Posts (Today)
|
14
|
Posts (Total)
|
688731
|
|
Created
|
02/02/05
|
Type
|
Free
|
| Moderators XenaLives sentiment_stocks CaptainObvious Poor Man - Doc logic JerryCampbell | |||

“Now this is not the end. It is not even the beginning of the end. But it is, perhaps, the end of the beginning.”
~ Winston Churchill
Stylized Dendritic Cell featured on NWBO board since 2015

- Dr. Linda Liau, PhD, MBA, Professor and Chair, Department of Neurosurgery, David Geffen School of Medicine at UCLA
Clinical Trials
DCVax®-L to Treat Newly Diagnosed GBM Brain Cancer (NCT00045968) - Phase III (Double Blind)
UK (MHRA): DCVax-L to Treat Newly Diagnosed GBM Brain Cancer (EudraCT#) 2011-001977-13
DE (Germany - PEI): DCVax-L to Treat Newly Diagnosed GBM Brain Cancer (EudraCT#) 2011-001977-13
Expanded Access Protocol for GBM Patients with Already Manufactured DCVax®-L Who Have Screen-Failed Protocol 020221 (NCT02146066) (Expanded Access)
Safety and Efficacy Study of DCVax-Direct in Solid Tumors (NCT01882946) - Phase I/Phase II (Open Label)
UK Clinical Trials - Study of a Drug (DCVax®-L) to Treat Newly Diagnosed GBM Brain Cancer
EU Clinical Trials for DCVax-L - Phase III
Dendritic Cell Vaccine for Patients with Brain Tumors (NCT01204684) - Phase II - at UCLA - Randomized (Open Label) testing DCVaccine with Resiquimod and DC Vaccination with Adjuvant polyICLC
Pembrolizumab and a Vaccine (ATL-DC) for the treatment of Surgically Accessible Recurrent Glioblastoma - Phase 1 (NCT04201873)
Dendritic Cell-Autologous Lung Tumor Vaccine (DCVax-L) and Nivolumab in Treating Patients with Recurrent Glioblastoma - Phase 2 (NCT03014804)
Dendritic Cell Therapy for Brain Metastases From Breast or Lung Cancer (NCT0368765) - Phase 1 - Collaborator: Mayo Clinic
Announcement of DCVax-L and Anti-PD-1 Monoclonal Antibody (Pembrolizumab) for Patients with Liver Metastases of Primary Colorectal Carcinoma Phase 2 Trial - November 17, 2016 - University Medical Center (UMC) of the Johannes Gutenberg University of Mainz
Cognate Bioservices - Owned by Charles River Labs
Website
Company Contact Info
Investor Relations:
Les Goldman (Company) (202) 841-7909 lgoldman@nwbio.com
Sign up for Northwest email list here (hit the subscribe to email list button in the lower right)
Company Headquarters
4800 Montgomery Lane, Suite 800, Bethesda, MD 20814 (240) 497-9024
NW Bio is developing cancer vaccines designed to treat a broad range of solid tumor cancers more effectively than current treatments, and without the side effects of chemotherapy drugs. NW Bio’s proprietary manufacturing technology enables them to produce its personalized vaccine in an efficient, cost-effective manner. NW Bio has a broad platform technology for DCVax dendritic cell-based vaccines.
Their lead product, DCVax-L, is currently in a 331-patient Phase III trial for patients with newly diagnosed Glioblastoma multiforme (GBM), the most aggressive and lethal brain cancer. This trial is currently underway at 69 locations thoughout the United States, Germany and the United Kingdom. NW Bio has also conducted a Phase I/II trial with DCVax-L for late stage ovarian cancer together with the University of Pennsylvania.
Their second product, DCVax-Direct, is currently in a 60-patient Phase I/II trial for direct injection into all types of inoperable solid tumor cancers, with trials currently being conducted at both MD Anderson Cancer Center in Texas, as well as Orlando Health in Florida.
They previously received clearance from the FDA for a 612-patient Phase III trial with its third product, DCVax-Prostate, for late stage prostate cancer.
DCVAX Survival Stories & Testimonials
Alice - Metastic Merkel Cell patient from Florida - ASCO 2018
Brad Silver - GBM patient from Huntington Beach, California - ASCO 2018
Sarah Rigby - GBM patient from Hong Kong - ASCO 2018
Kristyn Power - daughter of GBM patient from Canada - ASCO 2018
Kat Charles - GBM patients from UK - ASCO 2018 - as related by her husband Jason (Kat's Cure)
Prospective patients may contact NW Bio at patients@nwbio.com
UCLA Jamil Newirth DCVax-Patient Video - 2015
Allan Butler Video - National Geographic Vice President - DCVax-Direct patient from Phase 1 Trial with Pancreatic Cancer
NWBO - Patients Sunday Dennis and Jami Newirth - Enrolled at UCLA - Vimeo, Uploaded approx. May 2015
NWBO - Vaccine Helps Keep Brain Cancer Patient Alive (Jennifer Sugioka) - NBC Channel 4, Southern California, February 24, 2015
NWBO - National Geographic's Allan Butler Stage IV Pancreatic Patient using DCVax-Direct at MD Anderson
NWBO GBM Brain Cancer Survival Story of Mark Pace

Presentations
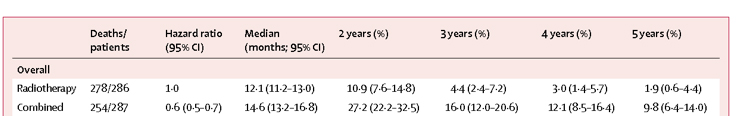
UCLA Agreements
Prostrate
DCVax-Phase II
DCVax-Booster
Upcoming Events
Videos
Linda M. Liau, MD, PhD, MBA - April 24, 2019 at University of Washington, Neurosciences Institute
| Volume | |
| Day Range: | |
| Bid Price | |
| Ask Price | |
| Last Trade Time: |
