Register for free to join our community of investors and share your ideas. You will also get access to streaming quotes, interactive charts, trades, portfolio, live options flow and more tools.
Fuji building $30million astaxanthin facility in Washington State
July 17, 2013
AlgaeIndustryMagazine.com

President Nishida meets with Washington’s Governor Inslee about the astaxanthin project on July 11th, 2013.
Fuji Chemical Industry Co. Ltd., AstaReal Technologies Inc., Grant County, and the City of Moses Lake, Washington have announced that AstaReal Technologies Inc. will build a microalgae-based biotechnological manufacturing plant for the production of natural astaxanthin in Moses Lake, WA. During the initial construction phase, $30 million will be invested and 45 permanent local jobs will be created.
Fuji established its manufacturing subsidiary, AstaReal Technologies, Inc., in order to expand its astaxanthin business in the United States, and in the City of Moses Lake in Grant County primarily because of the abundant water resource and competitive costs of renewable hydropower energy. For decades, Grant County International Airport in Moses Lake was home to training operations for Japanese Airlines, the international passenger and cargo carrier. A skilled labor force, an open culture welcoming to Japanese companies, and supportive local and state governments were also contributing factors for the decision to locate in Moses Lake.
AstaReal has been developing its technology for astaxanthin since the 1990s. The natural astaxanthin produced in Moses Lake will be marketed in the United States and worldwide through Fuji’s various subsidiaries. “Astaxanthin is an effective anti-inflammatory agent for living organisms.We want to make people healthier and happier by delivering astaxanthin raw materials for health foods to people across the world.” said Mitsunori Nishida, President and CEO of Fuji Chemical Industry.
“We see the 21st century as the era of anti-aging,” he said. “We believe that preventive medicine will play an even greater role in the future of healthcare as advances in anti-aging, lifestyle and disease research drive improvements both in the treatment and prevention of disease and illness. To that end, we have succeeded in realizing the world’s first industrial production of natural astaxanthin, which is both safe and high in antioxidant potency. We have devoted more efforts than any other research organization in advancing astaxanthin research.”
AstaReal Technologies will produce natural astaxanthin using state of the art photo-bioreactors to cultivate microalgae. The 45 skilled people AstaReal Technologies plans to hire include manufacturing operators, quality control, facilities maintenance and administration. Most employees will be hired locally in mid-2014. The manufacturing plant is anticipated to be completed and start operation in the third quarter of 2014.
President Nishida said, “We are honored to be a community member of Grant County and the City of Moses Lake. We believe that this community will experience significant growth and want to contribute to this future development. This 30 million dollar investment shows our strong commitment to the future of this community.”
Fuji is a global company based near Toyama City in Japan.
http://www.algaeindustrymagazine.com/fuji-building-30million-astaxanthin-facility-in-washington-state/
A ‘theory of everything’ for disease?
$10 million grant is awarded by the Blavatnik Family Foundation to explore inflammation’s role in diverse illnesses
Theoretical physicists have long sought a grand “theory of everything,” which would account for all the physical phenomena in the universe by unifying Einstein’s general relativity with the so-called standard model based on quantum mechanics.
In recent years, some biomedical scientists, including School of Medicine immunobiologists Richard A. Flavell, Ph.D., and Ruslan M. Medzhitov, Ph.D., have proposed that deeply understanding inflammatory processes might provide similar unifying insights into a great range of seemingly dissimilar chronic diseases: heart disease, cancer, type 2 diabetes, Alzheimer’s disease, and more.
Thanks to a $10 million grant from the Blavatnik Family Foundation, a charitable organization started by American industrialist and philanthropist Leonard Blavatnik, Flavell and Medzhitov will now have the opportunity to put their ideas to the test. “The Blavatnik Family Foundation is proud to support breakthrough scientific discoveries that accelerate the impact of biomedical research,” Blavatnik says. “The theory proposed by Drs. Flavell and Medzhitov represents a paradigm shift in the science of chronic diseases and may lead to new prevention strategies, treatments, and even cures for many disorders.”
The healthy human body regulates its own tissues and organs to maintain key physiological variables in a beneficial balance, a steady state that scientists call homeostasis. The body even gets some outside help from microbes, or commensal microorganisms, that reside on the skin and in the digestive tract and play a part in maintaining core body temperature, blood pressure, blood sugar, sleep patterns, and a host of metabolic processes needed for fitness and survival.
When infection or tissue damage occurs, the body’s innate immune system activates inflammatory mechanisms that help to combat these dangers and restore a proper balance, at least in the short term. Flavell, chair and Sterling Professor of Immunobiology, and Medzhitov, the David Wallace Professor of Immunobiology, postulate that these same inflammatory mechanisms can have a cumulative damaging effect on homeostatic controls—an effect they believe is a root cause of many serious health disorders. With the new grant from the Blavatnik Family Foundation, the scientists plan a detailed study to define the molecular links between inflammation, commensal microorganisms, and chronic disease.
Yale President-elect Peter Salovey expressed the University’s gratitude to the Blavatnik Family Foundation for what he called “an extremely generous and far-sighted” contribution. “The research now under way in the Medzhitov and Flavell laboratories has the potential to transform our understanding of human biology and our approaches to the most intractable diseases. This grant will accelerate their work at Yale’s Department of Immunobiology, which is world-renowned for leading major advances in innate and adaptive immunity,” Salovey said.
Medzhitov and Flavell, both of whom are Howard Hughes Medical Institute investigators, have led pioneering studies on the control of inflammation by the innate immune system.
Medzhitov is widely recognized for classic studies he conducted in the late 1990s with the late Charles A. Janeway Jr., M.D., that clarified the functions and importance of the innate immune system, work for which he received a Blavatnik Award for Young Scientists in 2007. In February, Medzhitov and Flavell were jointly awarded the 2013 Vilcek Prize for Biomedical Science.
Robert J. Alpern, M.D., dean of the School of Medicine, believes that a unified theory of inflammation and chronic disease will be a game-changer. “This work offers a whole new way to look at the causes of many chronic illnesses, including cardiovascular disease, type 2 diabetes, autoimmune diseases, asthma and allergies, neurodegenerative diseases such as Alzheimer’s disease, and cancer,” Alpern says. “A few years from now, I am optimistic that we will be in a position to develop new therapeutics that can broadly impact human health and quality of life.”
The founder and chair of Access Industries, Leonard Blavatnik is deeply committed to supporting innovation in biomedical research and higher education. In 2007, the Blavatnik Family Foundation established the Blavatnik Awards for Young Scientists, awarded through the New York Academy of Sciences, to recognize innovative and high-impact accomplishments in the life sciences, physical sciences, mathematics, and engineering. Blavatnik has supported the Broad Institute at Harvard University and MIT, and he has provided seed funding at Harvard for highly promising, early-stage research in the life sciences. In 2010, he contributed more than $115 million to the University of Oxford to establish the Blavatnik School of Government.
http://www.medicineatyale.org/mayjune2013/features/coverstories/157499
Taking statins could HALVE the risk of osteoarthritis by reducing inflammation in the body
Statins are known have an anti-inflammatory effect
They can also protect against cancer, Alzheimer’s and ease the symptoms of MS, diabetes and heart disease
Now, research by Keele University shows that those on high doses of statins have a lower risk of arthritis
By EMILY PAYNE
PUBLISHED: 09:35 GMT, 28 May 2013 | UPDATED: 09:38 GMT, 28 May 2013
Taking cholesterol-lowering statins every day could more than halve the chances of getting arthritis, according to a new study.
Researchers at Keele University carried out the research on the back of growing evidence that arthritis is not just down to wear and tear as the body ages - but also inflammation in the joints.
The study of more than 16,000 adults found that people on the highest doses of statins – 18.5mg or more a day – had 60 per cent lower osteoarthritis rates than people not taking the drugs.
As well as lowering cholesterol, statins have an anti-inflammatory effect, and are taken by eight million people to ward off strokes or heart attacks.
They are also thought to protect against cancer and Alzheimer’s and ease the symptoms of multiple sclerosis and protect diabetes sufferers against heart disease.
Keele university’s Health Services Research Unit, which is funded by Arthritis Research UK Primary Care Centre, found that people on very low doses seemed to be at higher risk of arthritis than patients not taking statins.
The study, Statin Use and Clinical Osteoarthritis in the General Population:A Longitudinal Study, which is published in the Journal of General and Internal Medicine, said the findings suggest the condition may be more closely linked to heart disease than first thought.
'Our work has shown that the risk factors for cardiovascular disease are also associated with osteoarthritis,' said researchers.
'The co-occurrence of osteoarthritis and cardiovascular disease is common.'
A spokeswoman for Arthritis Research UK said: 'We welcome this study as it contributes to the idea that osteoarthritis (OA) in not simply wear and tear as we get older, and that in the future drug treatments can offer hope to people with OA.'
'OA is an active disease that includes inflammation and active damage to the joint.
'The concept that OA is caused by the way that our body processes lipids is not proven, but this study provides some intriguing data to suggest that this warrants further work.
'Arthritis Research UK is committed to finding the causes of OA, new therapies for OA as well as treatments, such as pain relief, that help to manage the symptoms of OA.
'For example, we are funding a trial to investigate whether a drug called hydroxychloroquinone, commonly used to reduce inflammation in rheumatoid arthritis, is also effective for people living with OA.'
Scientists believe that inflammation plays a larger role in osteoarthritis than first thought.
They are investigating whether drugs used in rheumatoid arthritis, which occurs when the immune system attacks the joints, can also help in osteoarthritis.
Around 10 million Britons suffer from arthritis.
The charity Arthritis Care estimates the number of patients will rise to 17 million in the next 20 years.
http://www.dailymail.co.uk/health/article-2332016/Taking-statins-HALVE-risk-osteoarthritis-reducing-inflammation-body.html?ito=feeds-newsxml
Foul-smelling gas shows health benefits in reducing joint swelling
A gas associated with the smell of rotten eggs has proven to effectively reduce joint swelling, in research which could lead to advances in the treatment of arthritis.
Scientists at the University of Exeter Medical School have discovered that a novel drug molecule, which slowly generates the gas hydrogen sulfide (H2S), effectively reduces swelling and inflammation in arthritic joints.
For years, H2S has been regarded as a highly poisonous by-product which is corrosive, flammable and explosive. But research is now showing an altogether more benign side to the substance.
Professor Matt Whiteman, of the University of Exeter Medical School, said the research, which is published online in the Journal of Cellular and Molecular Medicine, could pave the way for more effective treatments of arthritis and other inflammatory conditions. Prof Whiteman said: “H2S is widely dismissed as a toxic and foul-smelling environmental pollutant, but it has recently been shown to be created in humans and animals by a specific set of enzymes. Why would the body do this if it had no benefit? Our research has shown that the key to unlocking the therapeutic qualities of H2S is through slow release, mimicking the body’s own production.”
The team has previously shown that H2S levels were increased by up to four times in the knee joints of patients with joint diseases such as rheumatoid arthritis, but intriguingly the higher H2S levels strongly correlated with a lower number of inflammatory cells in the joint. The latest study provides further evidence that the real role for H2S may be to combat inflammation, swelling and joint destruction.
Prof Whiteman added: “A patient will usually visit their doctor with a joint already inflamed, swollen and painful. Since the compound worked after arthritis was established, it may be useful in treating arthritis in the future. Many compounds can prevent arthritis in the laboratory, but of course nobody knows when they will get arthritis. Having a class of compounds which reduce inflammation and swelling when arthritis is already active is extremely exciting. These molecules may also be useful in other inflammatory conditions, and even in the inflammatory aspects of diabetes and obesity.”
The study was part of a large collaboration funded by the Wellcome Trust and Arthritis Research UK, involving Professor Philip K Moore and Dr Julie Keeble from King’s College London, as well as researchers at the National University of Singapore and Queen’s University, Belfast. The team used primary human cells as well as a model of arthritis. Rheumatoid arthritis causes some cells to proliferate too quickly in the joint and secrete substances which promote tissue inflammation, swelling and eventually joint destruction. However, the H2S donor molecule prevented this secretion, and inhibited the activity of several enzymes which cause inflation. In the arthritis model, the compound did not prevent arthritis, but was highly effective at reducing joint inflammation and swelling once arthritis was established, suggesting H2S-based compounds may one day be useful in clinic.
The same team has previously found that people who are overweight or have diabetes have lower levels of H2S in their bodies than healthy adults resulting in higher blood pressure, poorer insulin sensitivity and higher levels of sugar in their blood. It has also been reported to promote ulcer healing and reduce lung injury in smokers.
Co-author Dr Mark E Wood, at the University of Exeter, added: “Despite its reputation for being hazardous, H2S could in fact hold the key to solving some of the widespread health problems affecting the country. Our work is a major step in proving that it can be more hero than villain to the human body, providing it is administered in the right way, at the right time. We currently have several more efficient H2S donor molecules being evaluated with collaborators and this is a very exciting time for us.”
Dr Julie Keeble, co-author from King’s College London, commented: “The finding that H2S is able to reduce joint inflammation in experimental models makes it a very exciting prospect for treating arthritis. Many patients with arthritis do not respond effectively to current treatments or suffer side-effects from their medication. We hope that H2S-releasing drugs like the one tested in this study will be effective in treating arthritis without uncomfortable side effects.”
http://scienceblog.com/62645/foul-smelling-gas-shows-health-benefits-in-reducing-joint-swelling/
Discovery Of Immunity Protein That Ramps Up Inflammation - And Agents That Can Block It - Offers Potential To Improve Treatments For Pneumonia
Article Date: 03 Apr 2013 - 0:00 PDT
Scientists at the University of Pittsburgh School of Medicine have discovered a new biological pathway of innate immunity that ramps up inflammation and then identified agents that can block it, leading to increased survival and improved lung function in animal models of pneumonia. They reported their findings in Nature Immunology.
Pneumonia and other infections sometimes provoke an inflammatory response from the body that is more detrimental than the disease-causing bacteria, said senior author Rama Mallampalli, M.D, professor and vice chair for research, Department of Medicine, and director of the Acute Lung Injury Center of Excellence at Pitt.
"In our ongoing studies of pneumonia, we found infecting bacteria activate a previously unknown protein called Fbxo3 to form a complex that degrades another protein called Fbxl2, which is needed to suppress the inflammatory response," said Dr. Mallampalli, who is also chief of the pulmonary division of the VA Pittsburgh Healthcare System. "The result is an exaggerated inflammatory response that can lead to further damage of the lung tissue, multi-organ failure and shock."
The research team, led by Bill B. Chen, Ph.D., associate professor, Division of Pulmonary, Allergy and Critical Care Medicine, conducted experiments in which mice that lacked the ability to make Fbxo3 were infected with a strain of Pseudomonas bacteria, and found that they had better lung mechanics and longer survival than mice that still made the protein.
Research team members Bryan J. McVerry, M.D., and Yingze Zhang, Ph.D., both of the Acute Lung Injury Center of Excellence, found that blood samples from 16 people who had sepsis, a condition of systemic inflammation, revealed higher levels of Fbxo3 and other inflammatory proteins and lower levels of Fbxl2 than samples from seven patients who did not have sepsis or lung infection.
Based on the structure of Fbxo3, the researchers developed a family of small molecules with the aim of inhibiting its activity. Administration of one of them, called BC-1215, led to reduced inflammatory markers and improved lung mechanics in mouse models of pneumonia and sepsis.
"The key is to find ways to help the body temper its inflammatory response so that it's able to kill the infectious agent without causing injury to healthy tissue," Dr. Mallampalli said.
"The F-box protein Fbxo3, and other related proteins, represent ideal targets for treatment of acute lung injury, because it controls the innate immune response, is upstream of important inflammatory signaling pathways, and is more selective than traditional drugs that regulate protein turnover," noted Mark T. Gladwin, M.D., chief of the Division of Pulmonary, Allergy and Critical Care Medicine, Pitt School of Medicine.
The team is beginning to study the effects of BC-125 on other conditions of systemic inflammation, such as colitis and arthritis.
http://www.medicalnewstoday.com/releases/258454.php
Did Evolution Give Us Inflammatory Disease?
Mar. 22, 2013 — In new research published in the April 4, 2013 issue of The American Journal of Human Genetics, researchers from Brigham and Women's Hospital (BWH) demonstrate that some variants in our genes that could put a person at risk for inflammatory diseases such as multiple sclerosis, Crohn's disease or rheumatoid arthritis, have been the target of natural selection over the course of human history.
The research team, led by Philip De Jager, MD, PhD, BWH Department of Neurology, and Barbara Stranger, PhD, University of Chicago looked at genome-wide association studies along with protein-protein interaction networks, as well as other data and found 21 places in the genome that bear a 'signature' for both inflammatory disease susceptibility and natural selection.
Towfique Raj, PhD, BWH Department of Neurology, is the lead author on this study. The findings suggest that in the past these variants rose in frequency in the human population to help protect us against viruses, bacteria and other pathogens. But now in our modern world, the environment and exposure to pathogens has changed, and the genetic variants that were originally meant to protect us, now make an autoimmune reaction more likely. These results are consistent with the hygiene hypothesis in which our cleaner environment is thought to contribute to the increasing prevalence of inflammatory diseases.
Journal Reference:
Towfique Raj, Manik Kuchroo, Joseph M. Replogle, Soumya Raychaudhuri, Barbara E. Stranger, Philip L. De Jager. Common Risk Alleles for Inflammatory Diseases Are Targets of Recent Positive Selection. The American Journal of Human Genetics, 2013; DOI: 10.1016/j.ajhg.2013.03.001
http://dx.doi.org/10.1016/j.ajhg.2013.03.001
http://www.sciencedaily.com/releases/2013/03/130322104255.htm
New biodegradable nanoparticles deliver inflammation-resolving drugs to sites of tissue injury
Published on March 20, 2013 at 1:57 PM
A multicenter team of researchers, including scientists at Columbia University Medical Center (CUMC), Brigham and Women's Hospital (BWH), Mount Sinai School of Medicine, and Massachusetts Institute of Technology, has developed biodegradable nanoparticles that are capable of delivering inflammation-resolving drugs to sites of tissue injury. The nanoparticles, which were successfully tested in mice, have potential for the treatment of a wide array of diseases characterized by excessive inflammation, such as atherosclerosis. The study was published today in the online edition of the Proceedings of the National Academies of Sciences.
A key way in which the body protects itself against infection or injury is through acute inflammation. Ideally, this response first promotes the clearance of pathogens or damaged tissue; then, through a process called inflammation resolution, it clears cellular debris and inflammatory mediators and restores the tissue to its normal state. However, in many conditions, including heart disease, arthritis, and neurodegenerative diseases, the inflammatory process never resolves, leading to tissue damage.
"A variety of medications can be used to control inflammation. Such treatments, however, usually have significant side effects and dampen the positive aspects of the inflammatory response," said co-senior author Ira Tabas, MD, PhD, the Richard J. Stock Professor, Department of Medicine, and professor of Pathology & Cell Biology (in Physiology and Cellular Biophysics) at CUMC. The other co-senior author is Omid Farokhzad, MD, Associate Professor of Anesthesiology and Director of Laboratory of Nanomedicine and Biomaterials at Brigham and Women's Hospital (BWH).
To overcome these obstacles, the researchers incorporated two advances. First, based on an idea from co-lead author Gabrielle Fredman, PhD, a postdoctoral fellow at CUMC, they took advantage of a 24-amino-acid peptide, Ac2-26, which is derived from a naturally occurring protein mediator of inflammation resolution called annexin A1. Second, rather than simply inject the "naked" peptide into injured mice, they packaged the peptide into nanoparticles, designed by the BWH group, that are able to target drugs to sites of tissue injury. The nanoparticles were given this ability through the addition of two components: one that gives them stealthlike properties, enabling them to avoid detection and clearance by white blood cells and the liver; and a second that gives them the ability to target collagen IV, a protein found at sites of tissue injury.
Each nanoparticle is less than 100 nanometers in diameter, or 1/100,000th the diameter of a human hair.
The nanoparticles were tested in mice with peritonitis (inflammation of the peritoneum, the thin tissue that lines the inner wall of the abdomen) or hind-limb ischemia-reperfusion injury (tissue damage caused when blood supply returns to tissue after a period of ischemia, or lack of oxygen). In the mice with peritonitis, intravenous administration of the Ac2-26-containing nanoparticles was significantly more effective at limiting recruitment of neutrophils (a type of inflammatory white blood cell) and at increasing the resolution of inflammation than was intravenous administration of naked Ac2-26. In mice with reperfusion injury, the nanoparticles reduced tissue damage in comparison with either of two types of control nanoparticles: those with a peptide in which the 24 amino acids were scrambled to render it biologically inactive and Ac2-26 nanoparticles without the collagen IV-targeting component.
"These targeted polymeric nanoparticles are capable at very small doses of stopping neutrophils, the most abundant form of white blood cells, from infiltrating sites of disease or injury," said co-lead author Nazila Kamaly, PhD, a postdoctoral fellow at BWH. "This action stops the neutrophils from secreting further signaling molecules that can lead to a constant hyper-inflammatory state and further disease complications."
"The beauty of this approach is that, unlike many other anti-inflammatory approaches, it takes advantage of nature's own design for preventing inflammation-induced damage, which does not compromise host defense and promotes tissue repair," said Dr. Tabas.
While the nanoparticles do spread to tissues throughout the body, they tend to concentrate in areas of inflammation. "In theory, this should allow physicians to use smaller-than-usual doses of medications and reduce unwanted side effects," said Dr. Fredman.
The team is currently designing nanoparticles for the treatment of atherosclerosis. Preliminary studies show that the nanoparticles are capable of targeting atherosclerotic plaques.
The authors have filed a patent for targeted polymeric inflammation-resolving nanoparticles to treat a variety of chronic inflammatory diseases, including atherosclerosis, autoimmune disease, type 2 diabetes, and Alzheimer's disease.
The paper is titled, "Development and in vivo efficacy of targeted polymeric inflammation-resolving nanoparticles." The other contributors are Manikandan Subramanian (CUMC), Suresh Gadde (BWH), Aleksandar Pesic (BWH), Louis Cheung (BWH), Zahi Adel Fayad (Mount Sinai School of Medicine), and Robert Langer (Massachusetts Institute of Technology).
This research was supported by a Program of Excellence in Nanotechnology Award from the National Heart, Lung, and Blood Institute (HHSN268201000045), a grant from the National Institutes of Health (CA151884), and a David Koch-Prostate Cancer Foundation Award
in Nanotherapeutics.
In compliance with the Brigham and Women's Hospital and Harvard Medical School institutional guidelines, Dr. Farokhzad discloses his financial interest in BIND Biosciences, Selecta Biosciences, and Blend Therapeutics, three biotechnology companies developing nanoparticle technologies for medical applications. BIND, Selecta and Blend did not support the research described above, and currently these companies have no rights to any technology or intellectual property developed as part of this research. BIND, Selecta, and Blend were founded by Drs. Farokhzad and Langer, who serve as members of the Boards of Directors and Scientific Advisory Boards.
Source: Columbia University Medical Center
http://www.news-medical.net/news/20130320/New-biodegradable-nanoparticles-deliver-inflammation-resolving-drugs-to-sites-of-tissue-injury.aspx
Clearing up inflammation with pro-resolving nanomedicines
Posted: Mar 18th, 2013
(Nanowerk News) Inflammation is the body's natural defense mechanism against invading organisms and tissue injury. In acute inflammation, the pathogen or inflammatory mediators are cleared away and homeostasis is reached, however in chronic inflammatory states, this resolving response is impaired, leading to chronic inflammation and tissue damage. It is now widely believed that an impaired resolution of inflammation is a major contributing factor to the progression of a number of devastating diseases such as atherosclerosis, arthritis, and neurodegenerative diseases, in addition to cancer. Since the level of inflammation in these diseases is very high—targeted therapeutic solutions are required to help keep inflammation contained.
A new study from researchers at Brigham and Women's Hospital (BWH), Columbia University Medical Center, Icahn School of Medicine at Mount Sinai, and Massachusetts Institute of Technology presents the development of tiny nanomedicines in the sub 100 nm range (100,000 times smaller than the diameter of a human hair strand) that are capable of encapsulating and releasing an inflammation-resolving peptide drug. The authors showed that these nanoparticles are potent pro-resolving nanomedicines, capable of selectively homing to sites of tissue injury in mice, and releasing their therapeutic payload in a controlled manner over time. Uniquely, these nanoparticles are designed to target the extracellular microenvironment of inflamed tissues. The particles then slowly release their potent inflammation-resolving payload such that it can diffuse through the inflamed tissue. There the drug binds to receptors on the plasma membrane of activated white blood cells and causes them to become more quiescent. This study will be electronically published in Proceedings of the National Academy of Sciences the week of March 18, 2013.
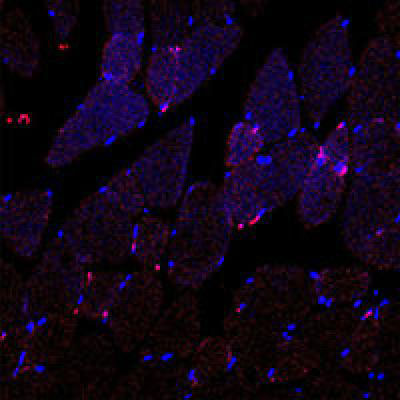
Collagen IV-targeted polymeric nanoparticles (shown in pink) are home to injured tissue, post-injection, in the blood. (Image: Farokhzad Lab)
"The beauty of this approach is that it takes advantage of nature's own design for preventing inflammation-induced damage, which, unlike many other anti-inflammatory strategies, does not compromise host defense and promotes tissue repair," said Ira Tabas, MD, PhD, physician-scientist at Columbia University Medical Center and co-senior author of this study.
"The development of self-assembled targeted nanoparticles which are capable of resolving inflammation has broad application in medicine including the treatment of atherosclerosis," said Omid Farokhzad, MD, physician-scientist at BWH, and a co-senior author of this study.
Polymers consisting of three chains attached end-to-end were developed as building blocks for the engineering of self-assembled targeted nanoparticles; one chain enabled the entrapment and controlled release of the therapeutic payload, in this case a peptide which mimics the pro-resolving properties of the Annexin A1 protein. Another chain conferred stealth properties to the nanoparticles, enabling their long-circulation after systemic administration. Yet a third chain gave homing capability to the nanoparticles to target the collagen IV protein to the vascular wall. As such these nanoparticles are capable of selectively sticking to injured vasculature allowing their therapeutic anti-inflammatory cargo to be released where it is needed to effectively promote inflammation resolution in a deliberate and targeted manner.
"These targeted polymeric nanoparticles are capable of stopping neutrophils, which are the most abundant form of white blood cells, from infiltrating sites of disease or injury at very small doses. This action stops the neutrophils from secreting further signaling molecules which can lead to a constant hyper-inflammatory state and further disease complications," said Nazila Kamaly, PhD, a postdoctoral fellow at BWH and co-lead author of this study.
"Nanoparticles that selectively bind to injured vasculature could have a profound impact in prevalent diseases, such as atherosclerosis, where damaged or comprised vasculature underlie the pathology. This work offers a novel targeted nanomedicine to the burgeoning field of inflammation-resolution, a field previously pioneered by BWH's Dr. Charles Serhan," said Gabrielle Fredman, PhD, a post-doctoral fellow at Columbia University Medical Center and co-lead author of this study.
These new developments have led the researchers to start investigating the potential of these pro-resolving nanomedicines for their effects on shrinking atherosclerotic plaques, and these studies are currently underway.
Source: Brigham and Women's Hospital
http://www.nanowerk.com/news2/newsid=29590.php
Chronic inflammation is making America obese, unhappy and stricken with the deadliest modern diseases
Monday, March 18, 2013 by: Jonathan Benson, staff writer
(NaturalNews) One of the claims constantly being made by mainstream health authorities and the media is that foods high in saturated fat and cholesterol are among the primary causes of modern illnesses such as obesity, diabetes and heart disease. But scientific evidence increasingly points to saturated fat and cholesterol as the actual saviors of health, while everyday triggers of chronic inflammation are the true culprits that often lead to depression, disease and for some early death.
What exactly are these inflammatory triggers? A growing body of research suggests that relatively modern foods like grains, including whole grains, refined sugars and vegetable oils, all of which are commonly recommended as being "healthy" alternatives to foods high in saturated fat and cholesterol, are directly responsible for triggering systemic inflammation. And if left unchecked, the constant barrage of C-reactive proteins, cytokines, and other inflammatory markers that result can lead to more serious illnesses.
According to Dr. Beverly Teter, a lipid biochemist at the University of Maryland (UM), as well as many other renowned experts in the field, having so-called "high cholesterol" is not the cause of chronic illness, but rather the symptom. The real problem, it turns out, is an underlying inflammatory condition that all-too-often leads an ailing body to generate high circulating levels of cholesterol, a nutrient that is actually being sent to help repair the damage caused by this inflammation.
"It's the inflammation in the vessels that starts the lesion," not the cholesterol, says Dr. Teter about this widespread misunderstanding, as quoted by CBN News. "The body then sends the cholesterol like a scab to cover over it to protect the blood system and the vessel wall from further damage."
Everything you've been told about the 'dangers' of saturated fat and cholesterol is a lie
The entire system of thought that pegs saturated fat and cholesterol as causes of chronic disease was birthed from errant observations that many years ago, first identified these two nutrients in conjunction with inflammation. At the time, researchers observed a correlation between high levels of circulating cholesterol and conditions like heart disease and obesity, and basically jumped to the false conclusion that cholesterol was the cause of these diseases.
Though this delinquent theory has since been debunked by the more progressive branches of modern science, the idea that avoiding foods high in saturated fat and cholesterol is healthy still pervades popular thought. And the resulting consequence of this mistaken view has been a continued rise in chronic illnesses of all sorts, including a sharp rise in deteriorating brain conditions like Huntington's disease and Alzheimer's disease.
"Saturated fat and cholesterol in the diet are not the cause of coronary heart disease," adds Dr. George V. Mann, M.D., a professor of medicine and biochemistry at Vanderbilt University in Tennessee, about the issue. "That myth is the greatest scientific deception of this century, perhaps of any century."
Your brain needs cholesterol in order to function, which means it is vital to consume plenty of it in conjunction with a diet high in omega-3 fatty acids and low in omega-6 fatty acids. Since the typical American consumes 15 times more omega-6s compared to omega-3s, it is crucial to intake more grass-fed meat and butter, coconut oil, wild fish, chia and hemp seeds, and other rich sources of omega-3s and healthy fats.
"I come from a family that has, my mother's side, had naturally high cholesterol," adds Dr. Teter. "Her cholesterol was between 380 and 420 when I started watching her medical records, and she died at 97. So I don't think that cholesterol was too bad for her."
http://www.naturalnews.com/039527_chronic_inflammation_obesity_America.html
Negative thinking linked to increased inflammation
Published on 15 March 2013
People with a habit of dwelling on negative events may experience increased levels of inflammation in their bodies, new research suggests.
Inflammation is involved in a number of health complaints, including heart disease, autoimmune conditions and inflammatory joint diseases.
One marker of tissue inflammation is C-reactive protein, which is produced as part of the immune system's initial inflammatory response.
Researchers at Ohio University found that people who were asked to think about a stressful incident experienced increases in their levels of C-reactive protein.
The study involved 34 healthy young women and revealed that levels of C-reactive protein continued to rise for at least an hour after the stressful event among those who dwelt on it, whereas the marker quickly started to return to normal in those who focused on nicer thoughts.
Lead author Dr Pegga Zoccola, who presented the findings at the annual meeting of the American Psychosomatic Society, said: "More and more, chronic inflammation is being associated with various disorders and conditions.
"The immune system plays an important role in various cardiovascular disorders such as heart disease, as well as cancer, dementia and autoimmune diseases."
Researchers at the university are now continuing to study the effects of rumination on other markers of inflammation and in population groups that are particularly prone to dwelling on negative thoughts, such as older people.
A spokesman for Arthritis Research UK said the study had looked at very small numbers of patients and was unpublished. "It's an interesting concept, but is still fairly speculative, and these results need to be replicated in larger numbers of people," he added.
http://www.arthritisresearchuk.org/news/general-news/2013/march/negative-thinking-linked-to-increased-inflammation.aspx
Aspirin May Lower Melanoma Risk
Mar. 11, 2013 — A new study has found that women who take aspirin have a reduced risk of developing melanoma -- and that the longer they take it, the lower the risk. The findings suggest that aspirin's anti-inflammatory effects may help protect against this type of skin cancer. The study is published early online in Cancer, a peer-reviewed journal of the American Cancer Society.
In the Women's Health Initiative, researchers observed US women aged 50 to 79 years for an average of 12 years and noted which individuals developed cancer. At the beginning of the study, the women were asked which medications they took, what they ate, and what activities they performed.
When Jean Tang MD, PhD, of Stanford University School of Medicine in Palo Alto, and her colleagues analyzed available data from 59,806 Caucasian women in the study, they found that women who took more aspirin were less likely to develop melanoma skin cancer during the 12 years of follow up. Overall, women who used aspirin had a 21 percent lower risk of melanoma relative to non-users. Each incremental increase in duration of aspirin use (less than one year of use, one to four years of use, and five or more years of use) was associated with an 11 percent lower risk of melanoma. Thus, women who used aspirin for five or more years had a 30 percent lower melanoma risk than women who did not use aspirin. The researchers controlled for differences in pigmentation, tanning practices, sunscreen use, and other factors that may affect skin cancer risk.
"Aspirin works by reducing inflammation and this may be why using aspirin may lower your risk of developing melanoma," said Dr. Tang. Other pain medications, such as acetaminophen, did not lower women's melanoma risk. Dr. Tang noted that the findings support the design of a clinical trial to directly test whether aspirin can be taken to prevent melanoma.
Journal Reference:
Christina A. Gamba et al. Aspirin is associated with lower melanoma risk among postmenopausal Caucasian women. Cancer, 2013 DOI: 10.1002/cncr.27817
http://dx.doi.org/10.1002/cncr.27817
http://ca.wiley.com/WileyCDA/PressRelease/pressReleaseId-107806,descCd-release_about_journal.html
http://www.sciencedaily.com/releases/2013/03/130311091531.htm
Red wine's link to health gains support
Evidence found for resveratrol’s mechanism of action.
Heidi Ledford
07 March 2013
The discovery that a compound in red wine may provide a healthier and longer life had guaranteed popular appeal, but the suggestion has been attacked from all sides. One such skirmish — a debate about the hypothesized benefits of one particular compound — may now be resolved.
In Science this week1, researchers show that the compound, called resveratrol, acts directly on a protein that has been linked to cell metabolism and inflammatory diseases.
“This will be a major step forward for the field,” says David Sinclair, a molecular biologist at Harvard Medical School in Boston, Massachusetts, and lead author of the study. ”The controversy has no doubt scared people off from studying these molecules.”
Unhealthy glow
A decade ago, Sinclair and his co-workers reported that resveratrol activated SIRT1, a member of a family of enzymes that remove acetyl groups from proteins2 and are thought to be involved in ageing and metabolism. The team's result was based on an assay that used a peptide bearing a tag that would fluoresce when the acetyl group was removed.
Shortly afterwards, Sinclair co-founded Sirtris, a company in Cambridge, Massachusetts, that used the assay to search for SIRT1-activating compounds that might serve as potential therapies for ageing-related diseases. Just four years later, GlaxoSmithKline (GSK), a large pharmaceutical company based in London, snatched up the company for US$720 million. It was a controversial acquisition, in part because GSK’s internal scientists couldn't reproduce the SIRT1 activation with untagged peptides.
They were not the only ones: other groups had reported that resveratrol boosted the activity of SIRT1 only when a bulky, hydrophobic tag was present on the peptide. “People thought it was an artefact,” says Brian Kennedy, chief executive of the Buck Institute for Research on Aging in Novato, California, and one of the researchers who initially reported concerns about the assay.
Direct evidence
But Sinclair and his colleagues now report that some of the naturally occurring targets that are amenable to SIRT1 activation by resveratrol and other such compounds have a common feature: bulky, hydrophobic amino acids at a key position.
The team also identified a SIRT1 mutation that blocked the effects of resveratrol and other SIRT1-activating compounds on mitochondria
The study provides an important clarification, says Matt Kaeberlein, who studies ageing at the University of Washington in Seattle. But resveratrol interacts with numerous proteins, he adds, and it is still unclear to what extent the compound's biological effects are caused by its interaction with SIRT1.
And for now, those biological effects remain controversial, as laboratories quarrel over whether SIRT1 activation really does boost lifespan, notes Kaeberlein.
“The field is overly polarized right now,” agrees Kennedy. “We need to find the correct middle ground.”
Nature
doi:10.1038/nature.2013.12563
http://www.nature.com/news/red-wine-s-link-to-health-gains-support-1.12563
Science
http://www.sciencemag.org/content/339/6124/1156.summary
Anti-Aging Drug Breakthrough

Drugs that combat aging may be available within five years. Four thousand synthetic activators, which are 100 times as potent as a single glass of red wine, have been developed -- the best three are in human trials. (Credit: © vgstudio / Fotolia)
Mar. 8, 2013 — Drugs that combat aging may be available within five years, following landmark work led by an Australian researcher.
The work, published in the March 8 issue of Science, finally proves that a single anti-aging enzyme in the body can be targeted, with the potential to prevent age-related diseases and extend lifespans.
The paper shows all of the 117 drugs tested work on the single enzyme through a common mechanism. This means that a whole new class of anti-aging drugs is now viable, which could ultimately prevent cancer, Alzheimer's disease and type 2 diabetes.
"Ultimately, these drugs would treat one disease, but unlike drugs of today, they would prevent 20 others," says the lead author of the paper, Professor David Sinclair, from UNSW Medicine, who is based at Harvard University. "In effect, they would slow aging."
The target enzyme, SIRT1, is switched on naturally by calorie restriction and exercise, but it can also be enhanced through activators. The most common naturally-occurring activator is resveratrol, which is found in small quantities in red wine, but synthetic activators with much stronger activity are already being developed.
Although research surrounding resveratrol has been going for a decade, until now the basic science had been contested. Despite this, there have already been promising results in some trials with implications for cancer, cardiovascular disease and cardiac failure, type 2 diabetes, Alzheimer's and Parkinson's diseases, fatty liver disease, cataracts, osteoporosis, muscle wasting, sleep disorders and inflammatory diseases such as psoriasis, arthritis and colitis (inflammatory bowel disease).
"In the history of pharmaceuticals, there has never been a drug that tweaks an enzyme to make it run faster," says Professor Sinclair, a geneticist with the Department of Pharmacology at UNSW.
The technology was sold to pharmaceutical giant GlaxoSmithKline in 2008. Four thousand synthetic activators, which are 100 times as potent as a single glass of red wine, have been developed -- the best three are in human trials.
"Our drugs can mimic the benefits of diet and exercise, but there is no impact on weight," says Professor Sinclair, who suggests the first therapeutic to be marketed will be for diabetes.
There have been limited trials in people with type 2 diabetes and the skin inflammatory disease, psoriasis. There were benefits to the metabolism in the first group and a reduction in skin redness in the second.
The drugs can be administered orally, or topically. So far, there have been no drugs developed targeting aging skin, but one major skin care range has developed a crème with resveratrol in it.
While any drug would be strictly prescribed for certain conditions, Professor Sinclair suggests that one day, they could be taken orally as a preventative. This would be in much the same way as statin drugs are commonly prescribed to prevent, instead of simply treating, cardiovascular disease.
In animal models, overweight mice given synthetic resveratrol were able to run twice as far as slim mice and they lived 15 per cent longer.
"Now we are looking at whether there are benefits for those who are already healthy. Things there are also looking promising," says Professor Sinclair, who also heads the Lowy Cancer Research Centre's Laboratory for aging Research at UNSW.
"We're finding that aging isn't the irreversible affliction that we thought it was," he says. "Some of us could live to 150, but we won't get there without more research."
Professor Sinclair formed a started up company Sirtris to develop the anti-aging technology. This was subsequently sold to GlaxoSmithKline (GSK). Professor Sinclair is now a scientific advisor to GSK. Several other authors on the paper work for GSK or an affiliated company.
Story Source:
The above story is reprinted from materials provided by University of New South Wales.
Note: Materials may be edited for content and length. For further information, please contact the source cited above.
Journal Reference:
B. P. Hubbard, A. P. Gomes, H. Dai, J. Li, A. W. Case, T. Considine, T. V. Riera, J. E. Lee, S. Y. E, D. W. Lamming, B. L. Pentelute, E. R. Schuman, L. A. Stevens, A. J. Y. Ling, S. M. Armour, S. Michan, H. Zhao, Y. Jiang, S. M. Sweitzer, C. A. Blum, J. S. Disch, P. Y. Ng, K. T. Howitz, A. P. Rolo, Y. Hamuro, J. Moss, R. B. Perni, J. L. Ellis, G. P. Vlasuk, D. A. Sinclair. Evidence for a Common Mechanism of SIRT1 Regulation by Allosteric Activators. Science, 2013; 339 (6124): 1216 DOI: 10.1126/science.1231097
http://dx.doi.org/10.1126/science.1231097
http://www.sciencedaily.com/releases/2013/03/130308111312.htm
Weight Loss May Prevent, Treat Osteoarthritis in Obese Patients
Mar. 8, 2013 — Weight loss may prevent and significantly alleviate the symptoms of osteoarthritis, a progressive disease of the joints known as "wear and tear" arthritis, according to a literature review appearing in the March 2013 issue of the Journal of the American Academy of Orthopaedic Surgeons(JAAOS).
According to the article, obesity actually may trigger the biomechanical and inflammatory changes that cause osteoarthritis, and the pain and loss of mobility associated with the condition.
"There's a clear link between obesity and osteoarthritis, and the link is both from biomechanical factors as well as systemic factors. The systemic component appears to be significant," said Ryan C. Koonce, MD, an orthopaedic surgeon at Skagit Regional Clinics in Mount Vernon, Wash., and one of the authors of the literature review. Approximately one half of osteoarthritis cases of the knee could be avoided in the U.S. if obesity was removed as a risk factor, according to the article. Other highlights include:
Greater weight and load bearing across a particular joint leads to increased wear.
White adipose tissue (WAT), a powerful endocrine organ that can trigger inflammation, is found in abundance in obese adults.
Obesity is considered to be an underlying cause of hypertension, insulin resistance and other metabolic syndrome conditions.
Obesity is a strong independent risk factor for pain, especially in soft-tissue structures such as tendons.
Weight loss can diminish pain, and restore function and quality of life in osteoarthritis patients, and possibly avert approximately 111,206 total knee replacements each year.
"It's important that doctors are aware of the different ways that obesity causes arthritis not only for treatment but for prevention of the condition," said Jonathan T. Bravman, MD, assistant professor in the Department of Orthopaedics at the University of Colorado, an orthopaedic surgeon, and a co-author of the study. "We are underutilizing weight loss as a primary treatment option for arthritis and joint pain."
Story Source:
The above story is reprinted from materials provided by American Academy of Orthopaedic Surgeons.
Journal Reference:
R. C. Koonce, J. T. Bravman. Obesity and Osteoarthritis: More Than Just Wear and Tear. Journal of the American Academy of Orthopaedic Surgeons, 2013; 21 (3): 161 DOI: 10.5435/JAAOS-21-03-161
http://dx.doi.org/10.5435/JAAOS-21-03-161
http://www.sciencedaily.com/releases/2013/03/130308143846.htm
Turmeric protects against diabetes, cancer, inflammation and free radical damage

March 8, 2013
By: Anne Seccombe
Turmeric contains a raft of health benefits from protection against diabetes, cancer, osteoarthritis and digestive problems to Alzheimer's disease
Turmeric, or curcuma longa as it is known botanically, is a very common spice in curry dishes around the world, as well as giving mustard it’s distinctive colour and staining powers. It has been a staple in Ayurvedic medicine for over 4000 years and most of the world’s turmeric is grown in India. Botanically, it is a part of the ginger family. Turmeric is native to the tropical areas of South Asia where it can grow up to six feet tall. It grows best between 20-30 degrees Celsius with plenty of rain and produces dull-yellow, trumpet-shaped flowers. The root or rhizome is the part of the plant which is used both medicinally and in cooking. It has a distinct mustard-like smell, and an earthy flavour, slightly hot and bitter. A turmeric plant can be successfully grown from a small section of the fresh rhizome.
(Cont.)
http://www.examiner.com/article/turmeric-protects-against-diabetes-cancer-inflammation-and-free-radical-damage
Obesity Makes Fat Cells Act Like They're Infected
Mar. 5, 2013 — The inflammation of fat tissue is part of a spiraling series of events that leads to the development of type 2 diabetes in some obese people. But researchers have not understood what triggers the inflammation, or why.
In Cell Metabolism this month (cover), scientists from The Methodist Hospital report fat cells themselves are at least partly to blame -- high calorie diets cause the cells to make major histocompatibility complex II, a group of proteins usually expressed to help the immune system fight off viruses and bacteria. In overweight mice and humans the fat cells, or adipocytes, are issuing false distress signals -- they are not under attack by pathogens. But this still sends local immune cells into a tizzy, and that causes inflammation.
"We did not know fat cells could instigate the inflammatory response," said principal investigator and Methodist Diabetes & Metabolism Institute Director Willa Hsueh, M.D. "That's because for a very long time we thought these cells did little else besides store and release energy. But what we have learned is that adipocytes don't just rely on local resident immune cells for protection -- they play a very active role in their own defense. And that's not always a good thing."
In pinpointing major histocompatibility complex II (MHCII) as a cause of inflammation, the researchers may have also identified a new drug target for the treatment of obesity. Blocking the MHCII response of adipocytes wouldn't cure obesity, Hsueh said, "but it could make it possible for doctors to alleviate some of obesity's worst consequences while the condition itself is treated."
Could the inflammation caused by a high fat diet serve any purpose, or is it a senseless response to an unnaturally caloric diet?
"The expression of MHCII in adipocytes does not seem to be helpful to the body," said co-lead author Christopher Lyon, Ph.D. "It is not at all clear what the advantage would be, given all the negative long-term consequences of fat tissue inflammation in people who are obese, including insulin resistance and, eventually, full diabetes. This just appears to be a runaway immune response to a modern high calorie diet."
Hsueh added, "The bottom line is, you're feeding and feeding these fat cells and they're turning around and biting you back. They're doing the thing they're supposed to do -- storing energy -- but reacting negatively to too much of it."
The scientists studied fat cells from obese, female humans (via biopsy) and overfed male mice. The researchers said that while they expect similar MHCII expression to occur in overweight male humans and female mice, further studies are needed to establish this.
The immunology of adipocyte inflammation is complex. It begins with the import of excess nutrients from the bloodstream, which are converted and stored as fat and stimulate the production of the hormone leptin. Excess leptin, spurred by a high calorie diet, excites CD4 T cells to produce a second signaling molecule, interferon gamma, which causes adipocytes to produce MHCII. This dialogue between adipocytes and T cells appears to initiate the inflammatory response to high fat diet -- Hsueh and her group found that overfed mice lacking MHCII experienced less inflammation.
Interferon gamma from T cells exacerbates the inflamed adipocytes' behavior and causes another type of immune cell, M2 macrophages, to be converted to their pro-inflammatory (M1) version.
"It was known that macrophages and T cells are major players," said lead author Tuo Deng, Ph.D. "But no one knew what the start signals were to ignite inflammation."
RNA was extracted from adipocytes purified from fat tissue biopsies and subjected to microarray analysis, which allowed the researchers to see what genes were increased in overweight subjects. The researchers found high expression of most MHCII complex and MHCII antigen processing genes. Similar gene expression patterns were observed in mice within two weeks of starting a high-fat diet, and this mirrored pro-inflammatory changes in fat tissue CD4 T cells. Hsueh says her group plans to investigate whether the inflammatory response in overfed mice can be blocked when MHCII expression is specifically reduced in adipocytes.
Hsueh says that if she and her group can identify the antigen(s) that MHCII is presenting to T cells in fat tissue, medical researchers would have a new approach to target adipose inflammation in obese patients. The hypothesis is that if a treatment can interfere with the production or MHCII presentation of these antigens, this would reduce the activation of fat tissue immune cells and thus reduce inflammation. Determining the MHCII antigen(s) involved in the inflammatory response of fat tissue to weight gain is one of her group's next goals, she says.
Also contributing to the Cell Metabolism paper were Laurie Minze, Jianxin Lin, Jia Zou, Joey Liu, Yuelan Ren, Zheng Yin, Dale Hamilton, Patrick Reardon, Vadim Sherman, Helen Wang, Kevin J. Phillips, Paul Webb, Stephen Wong, and Rong-fu Wang. The project was supported by grants from the John T. MacDonald Foundation, the National Institutes of Health, and the American Diabetes Association.
Story Source:
The above story is reprinted from materials provided by Methodist Hospital, Houston.
Note: Materials may be edited for content and length. For further information, please contact the source cited above.
Journal Reference:
Tuo Deng, Christopher J. Lyon, Laurie J. Minze, Jianxin Lin, Jia Zou, Joey Z. Liu, Yuelan Ren, Zheng Yin, Dale J. Hamilton, Patrick R. Reardon, Vadim Sherman, Helen Y. Wang, Kevin J. Phillips, Paul Webb, Stephen T.C. Wong, Rong-fu Wang, Willa A. Hsueh. Class II Major Histocompatibility Complex Plays an Essential Role in Obesity-Induced Adipose Inflammation. Cell Metabolism, Volume 17, Issue 3, 5 March 2013, Pages 411-422 DOI: 10.1016/j.cmet.2013.02.009
http://dx.doi.org/10.1016/j.cmet.2013.02.009
http://www.sciencedaily.com/releases/2013/03/130305145145.htm
That is why. Res DOES survive the gut, but bioavailability is low. Won't make it to the parts of the body where you need it.
Injections are another story. Works wonderfully if you can tolerate the discomfort. If it didn't sting so bad, I would still be using it.
I'm curious. How did you take the resveratrol? Oral or subq?
Well, we must compare results, here, of course. Just had my 2nd Omega3 this AM (have been taking the baby aspirin daily for years). Must say, I feel pretty good! Some slight aches and pains linger - hope they improve with time. But so far, so good, can't expect immediate 100% improvement in one day!!
Take a baby aspirin already as part of my daily regime and started the fish oil (1000mg). I'm following your lead
Got a big bottle of Omega3 Fish Oil 1000mg gelcaps today - had one - directions say 3 times daily - I'll try one a day for starters.
Expecting great things!!
Thanks Nano...went to work early this morning after talking with you and BAM when I got home the inflame board was their...
Fantastic new board. Congrats to all who contribute.
Diet change helps reduce inflammation
By Staff Reporter 16 hours 8 minutes ago
Appropriate change of diet can help in reducing inflammation within six weeks, a study has revealed.
According to University of Auckland Nutrition Professor Lynnette Ferguson, the research, based on short-term studies, could affect the cost of human clinical trials.
“Inflammation can be a catalyst for chronic human diseases, including Alzheimer’s, cardiovascular diseases and some cancers, as well as various autoimmune diseases, such as rheumatoid arthritis, Crohn’s Disease and type 2 diabetes,” she said.
Proper food
Professor Ferguson said that the diet should be high in long-chain omega-3 fatty acids, fruit and vegetables, nuts and whole grains, and low in refined grains, saturated fats and sugars.
“Many of these dietary components characterise the ‘Mediterranean diet’, which has been shown to protect against chronic disease,” she said.
Professor Ferguson looked for evidence of inflammation in apparently healthy New Zealanders and whether changing their diet for just six weeks would reduce this evidence.
Her research included biomarkers, including the C-reactive protein (CRP), which is a standard marker for inflammation that could be measured through blood tests.
Healthy volunteers
She encouraged 30 healthy volunteers, selected for their initially poor diets, to cut out refined and processed foods and follow a Mediterranean-type diet over the six weeks of the study, with increased amounts of fish, vegetables, unrefined cereals and ‘good’ fats such as olive oil and avocado.
She gave the participants salmon (for one meal a week), and recipes for healthy eating with gluten-free foods.
The participants, randomly assigned to high and lower-intervention groups, provided blood and urine samples at the beginning and end of the study, completed a four-day diary in the final days, and answered a questionnaires about their diet and lifestyle, as well as attending workshops led by expert dieticians.
Larger study
“This was a small study, intended to be a pilot for a much larger study of patients with Inflammatory Bowel Diseases such as Crohn’s Disease, but the results turned out to be statistically significant. Overall, the average daily fat intake was considerably reduced, while lower percentages of saturated fat were consumed,” Professor Ferguson said.
The self-reporting of volunteers was corroborated by the blood tests, which showed a corresponding reduction in the biomarkers for inflammation. It demonstrated that the high-intervention diet had altered gene expression within six weeks.
“This is a remarkable result,” says Professor Ferguson, “since it shows that average people, many of them young and with no health conditions, can, through an improvement in diet, significantly modify the biomarkers that indicate the risk that they could develop a chronic disease later.”
Differing responses
She said that a larger research project would involve people suffering from Inflammatory Bowel Disease.
According to Professor Ferguson, there are several genotypes characteristic of people suffering from Inflammatory Bowel Disease, each of which responds differently to particular types of diet or dietary items.
“The current research project concentrates on the most common genotype for the disease, though the ultimate aim is to formulate different diets tailored to the needs of the whole range of genotypes. Results are being analysed now and look highly encouraging,” she said.
The results of her findings will be published this month.
http://www.indiannewslink.co.nz/index.php/educationlink/8426.html?print
Ease Chronic Inflammation Easily!
Divya Subramanian
Most of the time, inflammation protects health. It is a part of the body’s natural defense system against injury and disease. In case of an injury the immune cells are invited to the site to battle with the invading bacteria. The immune system’s reaction involves more than 20 proteins to oust the invaders.
However, chronic inflammation harms rather than heal. “It is like being shot by friendly fire during a perpetual war raging inside the body”, says Dr. Philip Schauer, MD, Director of the Bariatric and Metabolic Institute at the Cleveland Clinic.
WARNING SIGN OF CHRONIC INFLAMMATION
Measure your waist. A circumference more than 35 inches for a woman or 40 inches for a man, indicates that you could be at risk for a variety of dangerous diseases linked to chronic inflammation.
“Excessive visceral fat is very different from fat in other parts of the body”, says Dr.Schauer.
Go for Anti-inflammatory foods

GOOD OIL: Ditch the vegetable oil for healthier options like olive, avocado and grape seed oils. They are good for your heart and your brain too. Since Olive oil is rich in oleic acid, an omega-9 fatty acid, it keeps the inflammation down.

TEA TIME: Researchers at Case Western Reserve University, Cleveland recently reported that the Anti-oxidant polyphenols in Green tea stages an anti-inflammatory fight inside the body. Add some lemon juice to perk up the tea’s flavor.

GARLIC: Garlic is best known as a wonderful seasoning to add aroma and taste to your dishes. But over the years it has been used as a medicine to prevent a wide range of diseases. It works great for swollen joints.

GINGER: This simple herb contains almost 500 different compounds, many of which are anti-inflammatory. So incorporate 100mg of this fighter every day.

YUMMY CHOCOLATES: Cocoa based products are among the richest functional foods based upon flavanols. Eating healthy doesn’t mean missing out on the sweet stuff.

Eat more FISH: Red meat leave you with cholesterol and salt. Switch to fish - they are high in Omega-3 fatty acids which effectively fight inflammation.

NUTS and FRUITS: Walnuts, almonds, sunflower seeds and hazelnuts are all great choices. Apples, blueberries, cherries, raspberries, strawberries and pineapple are all wonderful anti-oxidants.
Planning an appropriate diet will certainly help suppress chronic inflammation than relying on medication alone!
http://health.sulekha.com/ease-inflammation-easily_599019_blog
As various companies offering products to alleviate inflammation get noted here on this board I shall slip them into a list I shall form in the board box.
Is inflammation the root of all health evil?
The body's natural response to injury can also cause harm.
By
Jennifer Nelson Mon, Feb 25 2013 at 5:39 PM
Related Topics:
Alternative Medicine, Health & Well Being, Viruses & Diseases

Inflammation is not a disease, but it is a big health problem that appears to be at the root of all sorts of conditions: asthma, allergies, autoimmune disorders and many issues to do with aging.
But how does inflammation relate to conditions like heart disease, stroke and cancer — is it a cause or an effect?
Chicken or the egg?
“Acutely we know inflammation is the body’s natural response to an injury or an outside irritation,” says Jaspreet Mundeir, ND, a naturopathic physician based in Walnut Creek, Calif. If we want our body to mount an immune response, and we do, inflammation is what brings needed resources to the area where it fights infection or gets rid of an injury to help in the healing process, she said.
If you fall and hit your knee, inflammation occurs. You can also have inflammation in response to a virus or bacteria with congestion and nasal swelling. That’s an inflammatory response to a foreign body. These are examples of acute inflammation.
“You can also have chronic inflammation when there is an overreaction by the immune system,” says Christina Shannon, ND, clinical director of naturopathic medicine at cancer treatment centers of America at Midwestern Regional Medical Center. Many autoimmune disorders like lupus and rheumatoid arthritis result in the immune system responding repeatedly to inflammatory attacks.
Ulcerative colitis, for example, is a chronic inflammatory process in the colon. Hepatitis is chronic liver inflammation. Arthritis is inflammation in joints, while reflux disease (persistent heartburn) is a chronic inflammation that occurs as a result of acid. We also see inflammation in many cancers and even age-related changes like wrinkles.
“It’s when inflammation is prolonged and it becomes chronic that it has a detrimental effect, and the reason is because it causes more damage in the body,” Shannon says.
The cycle of inflammation
Inflammation’s role in disease is becoming more apparent. “When you continually have an immune response and it’s causing damage to nearby tissues, it becomes a self-perpetuating situation where those inflammatory chemicals create more inflammation, which creates more damage, which creates more inflammation,” explains Shannon. It’s not clearly understood why.
But is inflammation the original problem or the result of the problem? We don’t quite have the answers yet. Research is beginning to investigate whether catching inflammation earlier could stop the course of disease.
“We know chronic inflammation damages arteries, and that can lead to high cholesterol, heart attack and stroke. We know when it depresses the immune system, your body cannot focus on something else going on, like a cancerous tumor,” Mundeir says. So it’s logical that halting inflammation in its tracks will help fight disease.
Preventing inflammation
We can eat a whole grain diet with a focus on fruits and vegetables, since that helps bring down inflammation. We can also include omega 3 fatty acids, like coldwater fish, seeds and nuts, and increase dietary fiber. Anti-inflammatory herbs like ginger, curcumin, basil, boswellia and bromelain are all tops for halting inflammation.
In addition, limit high trans-fats and omega-6 fats like red meats, dairy products and processed foods with a longer shelf life, like cookies and crackers. Ditto for white carbohydrates like rice and flour. For many people wheat causes inflammation, and eliminating it from their diet helps.
There is also a movement called earthing, which studies show help reduce inflammation by directly touching the Earth for 15 minutes daily. “The Earth has this soothing effect — basically it’s a huge anti-inflammatory — like a Motrin,” says Laura Koniver, MD, a South Carolina physician and author of "From the Ground Up."
The theory is that negatively charged electrons on the Earth’s crust flow into our body through any skin contact with the Earth and neutralize free radicals that may be responsible for inflammation. To try: stand barefoot in your yard; dewy grass is the perfect conductor, as is wet sand. Lean up against a tree. Slip off shoes on a park bench and place feet directly on the ground. Try to get 15 minutes of Earth contact daily, and longer is better.
“The Earth’s crust has a limitless supply of free electrons and that’s what’s missing from our body when we build up chronic inflammation,” explains Koniver. Heck, being in contact with Mother Nature can never hurt.
http://www.mnn.com/health/fitness-well-being/stories/is-inflammation-the-root-of-all-health-evil
Good ones noretreat. I take them along with anatabloc (got a bunch of free samples since I was a health care provider).
Might want to look at the book "THe Wheat Belly" or similar videos on youtube.
Since I'm 68 and in good health it might be hard to give up bread and pizza at this stage of the game!
Ah...to be young again! Then we wouldn't have to worry about any of this! Of course, we'd be flexible, but penniless!
I think with Resveratrol the key is what DOESN'T happen. Tough to prove anything without a longitudinal study aver about 40 years.
A real start to this for sufferers might be a test standard for inflammation and paid for by reputable insurance companies.
http://www.mayoclinic.com/health/sed-rate/MY00343
I continue to take low dose aspirin and Fish Oil as a prophylactic measure against cancer as my low sed-rate has led to a diagnosis of Fibromyalgia a non-inflammatory condition they say. While fibromyalgia medication seems to provide some relief what I would like is my life back. My insistence they look for possible co-morbid conditions and a better quality of life has my doctors somewhat taken aback. Not saying that nurtrasuticals and whatever else comes along aren't worth pursuing, but at least take yourselves seriously enough to follow an established path to its conclusion before the next new thing. Good luck to us all.
Diet and fat
I thought this would be appropriate here - could be an important factor in inflammation:
Something for NNVC to work on!
Discovery Of Protein That Could Be Key To Fighting And Preventing Obesity
Article Date: 08 Jan 2013 - 1:00 PST
University of Florida researchers and colleagues have identified a protein that, when absent, helps the body burn fat and prevents insulin resistance and obesity.
The findings from the National Institutes of Health-funded study were published online ahead of print in the journal Nature Medicine.
http://www.nature.com/nm/journal/vaop/ncurrent/full/nm.3056.html
The discovery could aid development of drugs that not only prevent obesity, but also spur weight loss in people who are already overweight, said Stephen Hsu, M.D., Ph.D., one of the study's corresponding authors and a principal investigator with the UF Sid Martin Biotechnology Development Institute.
One-third of adults and about 17 percent of children in the United States are obese, according to the Centers for Disease Control and Prevention.
Although unrelated studies have shown that lifestyle changes such as choosing healthy food over junk food and increasing exercise can help reduce obesity, people are often unable to maintain these changes over time, Hsu said.
"The problem is when these studies end and the people go off the protocols, they almost always return to old habits and end up eating the same processed foods they did before and gain back the weight they lost during the study," he said. Developing drugs that target the protein, called TRIP-Br2, and mimic its absence may allow for the prevention of obesity without relying solely on lifestyle modifications, Hsu said.
First identified by Hsu, TRIP-Br2 helps regulate how fat is stored in and released from cells. To understand its role, the researchers compared mice that lacked the gene responsible for production of the protein, with normal mice that had the gene.
They quickly discovered that mice missing the TRIP-Br2 gene did not gain weight no matter what they ate - even when placed on a high-fat diet - and were otherwise normal and healthy. On the other hand, the mice that still made TRIP-Br2 gained weight and developed associated problems such as insulin resistance, type 2 diabetes and high cholesterol when placed on a high-fat diet. The normal and fat-resistant mice ate the same amount of food, ruling out differences in food intake as a reason why the mice lacking TRIP-Br2 were leaner.
"We had to explain why the animals eating so much fat were remaining lean and not getting high cholesterol. Where was this fat going?" Hsu said. "It turns out this protein is a master regulator. It coordinates expression of a lot of genes and controls the release of the fuel form of fat and how it is metabolized."
When functioning normally, TRIP-Br2 restricts the amount of fat that cells burn as energy. But when TRIP-Br2 is absent, a fat-burning fury seems to occur in fat cells. Although other proteins have been linked to the storage and release of fat in cells, TRIP-Br2 is unique in that it regulates how cells burn fat in a few different ways, Hsu said. When TRIP-Br2 is absent, fat cells dramatically increase the release of free fatty acids and also burn fat to produce the molecular fuel called ATP that powers mitochondria -the cell's energy source. In addition, cells free from the influence of TRIP-Br2 start using free fatty acids to generate thermal energy, which protects the body from exposure to cold.
"TRIP-Br2 is important for the accumulation of fat," said Rohit N. Kulkarni, M.D., Ph.D., also a senior author of the paper and an associate professor of medicine at Harvard Medical School and the Joslin Diabetes Center. "When an animal lacks TRIP-Br2, it can't accumulate fat."
Because the studies were done mostly in mice, additional studies are still needed to see if the findings translate to humans.
"We are very optimistic about the translational promise of our findings because we showed that only human subjects who had the kind of fat (visceral) that becomes insulin-resistant also had high protein levels of TRIP-Br2," Hsu said.
"Imagine you are able to develop drugs that pharmacologically mimic the complete absence of TRIP-Br2," Hsu said. "If a patient started off fat, he or she would burn the weight off. If people are at risk of obesity and its associated conditions, such as type 2 diabetes, it would help keep them lean regardless of how much fat they ate. That is the ideal anti-obesity drug, one that prevents obesity and helps people burn off excess weight."
http://www.medicalnewstoday.com/releases/254650.php
http://investorshub.advfn.com/boards/read_msg.aspx?message_id=83173979
See also:
NR - nicotinamide riboside
http://investorshub.advfn.com/boards/read_msg.aspx?message_id=76708450
In the Patrick Cox article that introduced a lot of people to Anatabloc he says he was disappointed by Resveratrol. I think we have not heard the last of this. Life Extension Foundation now sells a formulation called CR Mimetic that contains resveratrol but also other things. Intent is to cause genetic expression to move toward the state it assumes on a true calorie restriction diet.
Asthma & Your Immune System
Your Immune System May Be Worsening Your Asthma
From Pat Bass, former About.com Guide
Updated May 07, 2011
The same system that helps protect you from infections, your immune system, can also be responsible for your worsening asthma. You may notice that at the same time you have that runny nose, watery eyes and sinus congestion, your peak flows are lower, you are wheezing more, and you may experience more shortness of breath. So how are my immune system and asthma linked?
The immune system normally protects you against foreign bacteria and viruses. In asthma and other allergic diseases, the immune system may be the cause for your worsening symptoms.
http://asthma.about.com/od/asthmabasics/a/Asthma_Immune.htm
Hey Nano. Good to see you. The only side effects I've heard of are stomach upset. This may be caused by sucralose used as a sweetener in the mint-flavored version of the product. However, there is a flavor-free version now as well that does not include sucralose.
I've had zero problems with it; only benefits of relief from my seborrheic dermatitis (scaly patches of skin on my nose, behind my ears and scalp; lower back pain; and I have a since of well-being, which I have just learned is because it is an MAOI inhibitor. See the following from Patrick Cox. I love the product and am long the stock.
Read more: A "Nutraceutical" Game-Changer http://dailyreckoning.com/a-nutraceutical-game-changer/#ixzz2M7r0GluS - See more at: http://dailyreckoning.com/a-nutraceutical-game-changer/#sthash.4vtxcadl.dpuf
What Is Inflammation?
When you think of arthritis, you think of inflammation. Inflammation is a process in which the body's white blood cells and chemicals help protect us from infection and foreign substances such as bacteria and viruses.
In some diseases, however, the body's defense system (immune system) triggers an inflammatory response when there are no foreign substances to fight off. In these diseases, called autoimmune diseases, the body's normally protective immune system causes damage to its own tissues. The body responds as if normal tissues are infected or somehow abnormal.
Understanding Rheumatoid Arthritis (RA)
What Diseases Are Associated With Inflammation?
Some, but not all types of arthritis, are the result of misdirected inflammation. Arthritis is a general term that describes inflammation in joints. Some types of arthritis associated with inflammation include:
Rheumatoid arthritis
Psoriatic arthritis
Gouty arthritis
Systemic lupus erythematosus
The most common form of arthritis called osteoarthritis (also known as degenerative arthritis) is a bit of a misnomer. It is not believed that inflammation plays a major role in osteoarthritis. Other painful conditions of the joints and musculoskeletal system that are not associated with inflammation include fibromyalgia, muscular low back pain, and muscular neck pain.
http://www.webmd.com/osteoarthritis/guide/arthritis-inflammation
Any bad/any side effects to Anatabloc?
Resveratrol - I was sold - tried it - cost a fortune - stopped using it.
I'm STILL getting older!
Try Anatabloc by Star Scientific (STSI) for inflammatory conditions. It is available from the company's website and through GNC. Both anecdotal and scientific studies are showing it helps multiple conditions. The active ingredient, anatabine citrate, was discovered by the company from the tobacco plant. It was first used as an anti-smoking aid; however, users began reporting relief from numerous inflammation producing conditions and the company switched directions from being in the tobacco to the pharmaceutical space. A tobacco curing patent infringement case brought by the company against RJR was settled for a $5 million to Star, far less than investors had hoped, and the share price dropped. However, product sales of Anatabloc have been going parabolic and the company will most like attain profitability by Q3 2013. There is a heavy short interest against this company and efforts continue to protect those interests. However, I believe the shorts' days are numbered as additional peer reviewed studies are published and sales continue to ramp up.
Here are four peer reviewed studies on the effect of anatabine on inflammation (posted by leifsmith):
1) “Anatabine lowers Alzheimer's Aß production in vitro and in vivo” published in the European Journal of Pharmacology
11/30/11
http://1.usa.gov/YzoXA8
2) “Anatabine Ameliorates Experimental Autoimmune Thyroiditis” published in Endocrinology – Online 7/17/12, Print 9/1/12
http://1.usa.gov/12aMCNj
3) “Anti-inflammatory activity of anatabine via inhibition of STAT3 phosphorylation” published in the European Journal of Pharmacology – 1/5/13
http://1.usa.gov/YzoZrG
4) “Amelioration of Experimental Autoimmune Encephalomyelitis by Anatabine” published in PLOS ONE – 1/30/13
http://bit.ly/12aMAFk
All are OTC. Let's start with 3. All the summaries are lifted from Wikipedia.
Grape seed extract:
Human case reports and results from basic research provide preliminary evidence that grape seed extract may affect heart diseases such as hypertension, high levels of blood cholesterol,[1] platelet aggregation or inflammation.[2] None of these possible effects has been confirmed in vivo.
Resveratrol:
The effects of resveratrol are currently a topic of numerous animal and human studies. Its effects on the lifespan of many model organisms remain controversial, with uncertain effects in fruit flies, nematode worms,[5] and short-lived fish. In mouse and rat experiments, anticancer, anti-inflammatory, blood sugar-lowering and other beneficial cardiovascular effects of resveratrol have been reported. In humans, however, while reported effects are generally positive, resveratrol may have lesser benefits.[6] In one positive human trial, extremely high doses (3–5 g) of resveratrol, in a proprietary formulation designed to enhance its bioavailability, significantly lowered blood sugar.[7] This 28-day Phase 1b study was conducted privately in India by the pharmaceutical company, Sirtris, and was announced at an investor conference in 2008.[8] Although it has been alluded to in review articles, the study has never been published in a peer-reviewed scientific publication. Despite the mainstream press alleging resveratrol's anti-aging effects,[9] there are no accepted data to form a scientific basis for the application of these claims to mammals (see life extension section below). At the present time, research on resveratrol is in its infancy and the long-term effects of supplementation in humans are not known
Alpha Lipoic Acid:
Lipoic acid has been the subject of numerous research studies and clinical trials:
Prevent organ dysfunction[37]
Reduce endothelial dysfunction and improve albuminuria[38][39]
Treat or prevent cardiovascular disease[40]
Accelerate chronic wound healing[41]
Reduce levels of ADMA in diabetic end-stage renal disease patients on hemodialysis[42]
Management of burning mouth syndrome[43][44][45]
Reduce iron overload[46]
Treat metabolic syndrome[47][48][49]
Improve or prevent age-related cognitive dysfunction[50][51]
Prevent or slow the progression of Alzheimer’s Disease[52][53][54]
Prevent erectile dysfunction (animal models)[55][56]
Prevent migraines[57]
Treat multiple sclerosis[58][59][60]
Treat chronic diseases associated with oxidative stress[61]
Reduce inflammation[62]
Inhibit advanced glycation end products (AGE)[63]
Treat peripheral artery disease
Sign me up. Followed over from NNVC/CTIX
What kinds?
Are prescriptions needed?
I take all kinds of anti-inflammatories, and some of them seem to actually work. Cool board idea.
Another treatment I follow to alleviate my arthritis pain is using ginger and turmeric daily - it helps but some pain remains - perhaps aspirin + omega3 will work better.
This posting was the inspiration for this board:
Re --sore joints--
I have THE answer, gleaned recently in my continuous hunt for info:
Aspirin + Omega 3!
An inflammation killer.
InflammationCide!!
Put it in a micelle and deliver pure relief to inflamed areas with an inflammation seeking ligand attached, eh.
NNVC is branching out....or should be!! wink
Ref:
http://www.eurekalert.org/pub_releases/2013-02/cp-aao021313.php
http://investorshub.advfn.com/boards/read_msg.aspx?message_id=85040323
| Volume | |
| Day Range: | |
| Bid Price | |
| Ask Price | |
| Last Trade Time: |
