Register for free to join our community of investors and share your ideas. You will also get access to streaming quotes, interactive charts, trades, portfolio, live options flow and more tools.
>>> Steakholder Foods Brings Sustainable Innovation to the U.S. with 3D-Printed Plant-Based Delicacies
PR Newswire
Apr 3, 20247
https://finance.yahoo.com/news/steakholder-foods-brings-sustainable-innovation-130000363.html
Steakholder Foods Introduces SHMeat and SHFish blends with Advanced 3D Food Printing Technologies after receiving approval from a highly regarded consultant for the ingredients of the SHMeat and SHFish blends in the United States market
REHOVOT, Israel, April 3, 2024 /PRNewswire/ -- Steakholder Foods Ltd. (Nasdaq: STKH), is excited to announce its innovative SHMeat and SHFish blends, designed for 3D-printing exquisite plant-based fish and steak alternatives.
A recent feasibility report prepared by highly regarded consultant confirms the legal status of the ingredients, marking a significant step towards sustainable and ethical food choices.
The comprehensive feasibility report confirms that all ingredients used in Steakholder Foods' SHMeat and SHFish blends are approved for use in the United States. Each ingredient has been carefully selected to ensure compliance with food safety regulations, and they have all achieved Generally Recognized as Safe (GRAS) status. This commitment to using only GRAS ingredients underscores the company's dedication to consumer health and safety.
Introducing SHMeat and SHFish blends
The company's flagship products, SHMeat Beef Steak blend and SHFish White Fish blend, are at the forefront of this innovation. These blends are crafted to mimic the taste and texture of traditional meat and fish, offering a guilt-free and environmentally friendly alternative for consumers.
Expanding the Plant-Based Horizon
Steakholder Foods is not stopping there. The development pipeline includes an exciting range of blends, such as:
SHMeat Beef Asado
SHMeat Beef Tenderloin
SHMeat Beef Flank
SHMeat Chicken Fillet
SHFish Salmon
Each blend is being carefully developed to ensure the highest quality and flavor, catering to a variety of culinary preferences.
Revolutionary Production Technologies
Steakholder Foods' production machines utilize two types of 3D technologies to mimic the texture of meat and fish:
Drop Location in Space (DLS): Used for fish and seafood production, creating delicate textures that closely resemble those found in real seafood.
Fused Paste Layering (FPL): Used for meat production, ensuring that the fibrous texture of meat is accurately replicated in the plant-based products.
These machines are designed and built to work in traditional food factories, matching the same scale production of the industry and are designed according to food safety standards set by the European Hygienic Engineering & Design Group (EHEDG).
It should be noted that the company is actively working to ensure that the production process meets the requirements of FSMA and Good Manufacturing Practices (cGMPs) and any other required regulation, as recommended by the consultant.
Seeking Partnerships to Expand the Future of Food
As Steakholder Foods makes its mark in the U.S. market, we are actively seeking partnerships with like-minded companies and producers. Our goal is to collaborate with those who are already established in the plant-based meat and fish alternatives sector, as well as traditional meat and fish producers who are looking to diversify and expand their product portfolios. Together, we can lead the charge in the food industry's evolution, offering consumers innovative, sustainable, and ethical food choices. We believe that through collaboration, we can achieve greater strides in making the future of food not only delicious but also beneficial for our planet.
Arik Kaufman, CEO of Steakholder Foods
"As we introduce our SHMeat and SHFish blends to the U.S. market, we stand at the cusp of a new era in food technology. Our advanced 3D printing technologies are not just a testament to innovation but also a commitment to sustainability and health. These products represent our dedication to providing consumers with food options that are not only delicious but also responsible choices for our planet. We believe that our entry into the U.S. market is a significant step towards a future where the food we eat contributes to a healthier society and a more sustainable world."
About Steakholder Foods
At Steakholder Foods, we are not just creating food; we are shaping the future of sustainable dining. Our innovative SHMeat and SHFish blends, developed through advanced 3D food printing technologies, are pioneering a new era of culinary excellence. With our flagship products like the SHMeat Beef Steak blend and SHFish White Fish blend, we offer a symphony of taste and texture that rivals traditional meat and fish.
Our proprietary Drop Location in Space (DLS) and Fused Paste Layering (FPL) technologies are the keystones of our production, allowing us to craft plant-based alternatives with unparalleled precision and quality. These technologies enable us to produce a variety of textures and flavors, from the delicate fibrousness of a white fish fillet to the robustness of a beef steak, ensuring that every bite is as satisfying as it is responsible.
Designed to integrate into existing food production lines, our machines are built to the scale of industry demands while adhering to the strictest food safety standards, as outlined by the European Hygienic Engineering & Design Group (EHEDG). This commitment to quality and safety is at the heart of everything we do.
Steakholder Foods stands at the vanguard of food technology, committed to providing delicious, sustainable, and ethical food choices. Our products are more than just alternatives; they are a testament to our vision of a world where indulgence and sustainability go hand in hand.
<<<
---
>>> Autodesk (ADSK) is a prominent software company known for its expertise in 3D design, engineering and entertainment software. Yahoo! Finance has 22 analysts forecast a high of $270 and a low of $180 with an average of $240.
InvestorPlace
byMichael Que
October 18, 2023
https://finance.yahoo.com/news/3-metaverse-stocks-transport-future-001013006.html
The global 3D design industry is set to grow significantly, from $3 billion in 2022 to $13.3 billion by 2030 with a strong 20.6% CAGR. Growth factors include a reduction in manufacturing cost and process downtime, government investments in 3D printing projects, and the development of new industrial-grade 3D printing materials.
In Q3 2023, ADSK posted robust financials. Total revenue reached $1.3 billion, up 8% YoY. Technology development, solutions and consultancy hit $714 million. This doubled with a 104% YoY growth. Other services revenue surged to $704 million, marking a tenfold increase. These results highlight ADSK’s strong financial performance and demand response. Q3 financials were strong, with a trailing 12-month revenue of $5.21 billion. This represents an 8.70% year-over-year quarterly revenue growth.
ADSK’s growth strategy centers on end-to-end digital transformation by listening to customers’ needs to integrate real-time, immersive experiences into design and manufacturing processes. Collaborations, such as its partnership with Epic Games, make processes nearly real-time. This enhances efficiency and turnaround times which is unique to ADSK’s software. ADSK’s software can efficiently produce and run 3D drawings performing better than its competition. Autodesk’s commitment to cutting-edge technologies like artificial intelligence (AI) and machine learning (ML) aligns with its vision for end-to-end digital transformation.
Furthermore, ADSK is one of the strong metaverse stocks with healthy financials and robust growth prospects. The 3D design industry’s explosive growth, ADSK’s impressive performance in Q3 2023 and ADSK’s focus on end-to-end digital transformation all showcase its commitment to innovation and efficiency.
<<<
---
Steakholder Foods (STKH) may be starting to gain some traction (link below). The stock popped on this news, but I see the company quickly did a money raise the next day, thanks to a pre-existing shelf registration. So very resourceful, and in spite of the instant dilution the stock held up pretty well.
>>> Steakholder Foods Signs First Ever Multi-Million-Dollar Agreement with GCC Governmental Body to Commercialize its 3D Bio-Printing Technology
Yahoo Finance
July 24, 2023
https://finance.yahoo.com/news/steakholder-foods-signs-first-ever-110000101.html
<<<
>>> Steakholder Foods Ltd. (STKH), a deep-tech food company, engages in the development of cultivated meat technologies to manufacture cultivated meat without animal slaughter in Israel. The company develops a three-dimensional bioprinter to deposit layers of stem cells and differentiated stem cells, scaffolding, and cell nutrients in a three-dimensional form of structured cultured meat. It intends to license its production technology; provides associated products, such as cell lines, printheads, bioreactors, and incubators; and offers technology implementation, training, and engineering support services directly and through contractors to food processing, food retail, and cultivated meat companies. Steakholder Foods Ltd. is headquartered in Rehovot, Israel. <<<
---
>>> Steakholder Foods® Unveils 3D Bio-printing Business Model
Steakholder Foods Ltd.
May 25, 2023
https://www.prnewswire.com/news-releases/steakholder-foods-unveils-3d-bio-printing-business-model-301834537.html
The business model will focus on selling 3D bio-printers and bio-inks designed to develop delicious, nutritious, safe, and consistent cultivated meat from ethically harvested cells.
3D bio-printers: the Company is developing Ready-to-Cook 3D printers, for the production of hybrid cultivated products, incorporating a unique printing process that gives the product its fibrous texture.
Bio-inks: the Company is developing various bio-inks to print different species, and will offer customization options intended to allow clients to purchase bio-inks for any type of species they would like to produce, tailored to their specific needs and preferences.
REHOVOT, Israel, May 25, 2023 /PRNewswire/ -- Steakholder Foods Ltd. (Nasdaq: STKH), an international deep-tech food company at the forefront of the cultivated meat industry, today announced that it has focused its business model to target B2B meat manufacturers and cultivated meat producers, by offering manufacturers the ability to produce a cultivated meat product that aims to closely mimic the taste, texture, and appearance of traditional meat. The Company intends to monetize its 3D printers and bio-inks that are needed to support printer operation.
The Company's competitive advantage lies in its top-of-the-line expertise in 3D bio-printing technology and its ability to create highly–sophisticated, structured end products that aim to closely mimic real meat in terms of taste, texture, and appearance.
In accordance with Steakholder Foods' new business model, the products it plans to offer:
1. 3D bio-printers
The Company's 3D printers are state-of-the-art technology designed to produce cultivated meat products that mimic the texture, taste, and appearance of conventional meat. The Company is developing two types of printer platforms:
Ready to Cook (RTC) printer
The flagship product, the RTC printer, produces a hybrid cultivated meat product made from a mixture of cultivated and plant-based ingredients.
The Company plans to offer lab- and industrial-scale printers using one of two types of technology to produce different end products. DropJet technology, based on drops of gel-based materials to create a 3D structure, is ideal for producing fish and seafood products, while for all other meat products, Fusion technology extrudes paste materials through a narrow nozzle, enabling the creation of fiber texture that best simulates conventional meat fibers.
3D printer for incubated products
The Company's innovation team is developing a 3D printer for an incubated product, such as tissue-engineered steak, considered the holy grail of the industry. This printer is expected to be a future value proposition for the Company when economies of scale support a market for fully cultivated products. This printer is designed to produce a fully matured, cultivated, printed meat product, which will require live cells to grow, differentiate, and mature, forming complex fibrous tissue that resembles the texture and taste of conventional meat.
2. Bio-inks
Bio-inks are an integral part of the Company's 3D printing technology. Steakholder Foods' bio-inks are made of plant-based ingredients and cultivated cells. They are developed to ensure the production of tasty, safe, and consistent products. The Company plans to offer customization options intended to allow clients to create bio-inks for any type of species they would like to produce, tailored to their specific needs and preferences. The bio-inks will be available for purchase alongside the 3D printers.
Arik Kaufman, CEO of Steakholder Foods: "By offering 3D printing production methodologies to B2B clients, Steakholder Foods has the opportunity to become a backbone supplier that enables the production of products that consumers seek and expect. Our 3D bio-printing technology and customized bio-inks reflect our commitment to revolutionizing the food industry."
About Steakholder Foods
Steakholder Foods Ltd., formerly MeaTech 3D Ltd., is an international deep-tech food company at the forefront of the cultured meat revolution. The company-initiated activities in 2019 and is listed on the Nasdaq Capital Market under the ticker "STKH" (formerly MITC), with headquarters in Rehovot, Israel.
The company is developing a slaughter-free solution for producing cellular agriculture meat products, such as beef and seafood, by offering manufacturers the ability to produce a cultivated meat product that aims to closely mimic the taste, texture, and appearance of traditional meat— as an alternative to industrialized farming and fishing. With its membership in the UN Global Compact, Steakholder Foods is committed to act in support of issues embodied in the United Nations Sustainable Development Goals (SDGs) which include strengthening food security, decreasing carbon footprint, and conserving water and land resources.
<<<
---
Steakholder Foods (STKH) -
>>> MeaTech 3D Ltd., a deep-tech food company, engages in the development of cultivated meat technologies to manufacture cultivated meat without animal slaughter. The company develops a three-dimensional bioprinter to deposit layers of differentiated stem cells, scaffolding, and cell nutrients in a three-dimensional form of structured cultured meat. It intends to license its production technology; provide associated products, such as cell lines, printheads, bioreactors, and incubators; and offer services, such as technology implementation, training, and engineering support directly and through contractors to food processing and food retail companies. The company is headquartered in Rehovot, Israel.
<<<
---
>>> Cultured Meat Company MeaTech 3D Becomes Steakholder™ Foods
Yahoo Finance
August 3, 2022
https://finance.yahoo.com/news/cultured-meat-company-meatech-3d-120000024.html
The new name reflects the company's commitment to cultivating a new community of meat lovers who will participate in the company's mission to make high-quality real meat sustainably
REHOVOT, Israel, Aug. 3, 2022 /PRNewswire/ — MeaTech 3D Ltd. (Nasdaq: MITC), an international deep-tech food company at the forefront of the cultured meat industry, has become Steakholder™ Foods Ltd. (Nasdaq: STKH).
Beginning in 2019, the company set out to develop the technology and scientific processes to produce whole cuts of meat sustainably using animal cell cultivation and 3D bioprinting. Steakholder Foods' initial activities have been primarily focused around developing the "holy grail" of meat ? steak. This has enabled the company to assemble a unique mix of the best engineers and cellular biologists in the field who were motivated by the opportunity to tackle the most complex challenge in this burgeoning industry.
Soon after the company's founding, Steakholder Foods became the first Nasdaq-listed cultured meat company. As a public company, Steakholder Foods is creating an opportunity for people to become "steakholders" in a movement aimed at transforming how meat is sourced and supplied. The company believes that cultured meat production can have a significant positive impact on the future of the planet, the welfare of animals, and the security of nutritious meat for billions of people.
As part of the Steakholder Foods community, anyone can participate in the company's mission to develop high-quality real meat that is delicious, nutritious and sustainable.
Today, Steakholder Foods is a leading technology innovator in the cultured meat industry. The company's proprietary 3D bioprinting technology can print whole cuts of meat with pinpoint precision at an industrial scale to create any desirable ratio of muscle tissue and fat marbling and without damaging cell viability.
In December 2021, the company printed the largest ever cultured steak at 3.67 oz and was recently granted its first patent (among numerous provisional patents) for systems and methods that enhance muscle fiber formation to develop high-quality meat.
Arik Kaufman, Steakholder Foods' Chief Executive Officer: "As Steakholder Foods, our hope is to send a strong message to meat lovers around the globe that together we can and should create a world where people everywhere continue enjoying their favorite meat sustainably — for the health and welfare of the planet and all its inhabitants."
About Steakholder Foods
Steakholder Foods Ltd., formerly MeaTech 3D Ltd., is an international deep-tech food company at the forefront of the cultured meat revolution. The company initiated activities in 2019 and is listed on the Nasdaq Capital Market under the ticker "STKH" (formally MITC). Steakholder Foods maintains facilities in Rehovot, Israel and Antwerp, Belgium and is in the process of expanding activities to the US.
The company is developing a slaughter-free solution for producing a variety of beef, chicken, pork, and seafood products — both as raw materials and whole cuts — as an alternative to industrialized farming and fishing. With its membership in the UN Global Compact, Steakholder Foods is committed to act in support of issues embodied in the United Nations Sustainable Development Goals (SDGs) which include strengthening food security, decreasing carbon footprint, and conserving water and land resources.
<<<
---
>>> Is Autodesk Stock a Buy Now?
Motley Fool
By Leo Sun
Jun 1, 2022
https://www.fool.com/investing/2022/06/01/is-autodesk-stock-a-buy-now/?source=eptyholnk0000202&utm_source=yahoo-host&utm_medium=feed&utm_campaign=article
Autodesk's Q1 numbers beat analysts' expectations.
It provided steady guidance for the rest of the year.
The stock isn't cheap, but its evergreen business model deserves a premium valuation in this tough market.
The cloud-based software company continues to grow.
Autodesk's (ADSK 1.27%) stock price jumped 10% on May 27 after the software company posted its fiscal year 2023 first-quarter results (for the period that ended April 30). Its quarterly revenue rose 18% year over year to $1.17 billion, which beat analysts' estimates by $20 million. Its adjusted earnings increased 39% to $1.43 per share, which also surpassed the consensus forecast by $0.09.
Those growth rates are impressive, but should investors chase Autodesk's post-earnings rally?
What does Autodesk do?
Autodesk's software and services operations can be split into four categories: architecture, engineering, and construction (AEC); AutoCAD and AutoCAD LT; manufacturing (MFG); and media and entertainment (M&E).
During the first quarter, it generated 44% of its revenue from its AEC segment, 30% from AutoCAD and AutoCAD LT, 19% from MFG, and 6% from M&E. It provides most of its software as cloud-based services.
Autodesk's cloud-based subscriptions enabled it to generate stable growth throughout the pandemic. Its revenue rose 16% in fiscal 2021, which ended last January, and increased another 16% to $4.39 billion in fiscal 2022. All four of its core businesses generated robust growth over the past year.
A stable outlook for the rest of the year
Autodesk's sales in Europe initially slowed down following Russia's invasion of Ukraine, which resulted in the suspension of its business in Russia on March 3, but CEO Andrew Anagnost said during the recent conference call that its business in the region recovered as the quarter progressed.
Autodesk expects its revenue to rise 15% to 17% year over year in the second quarter, and grow 13% to 15% for the full year. That outlook includes a $40 million reduction (about 3% of its revenue) from the suspension of its services in Russia.
It expects its adjusted earnings per share (EPS) to increase 27% to 32% in the second quarter, and to grow 27% to 31% for the full year.
High gross margin and rising operating margin
Autodesk's conversion of its desktop software into cloud-based services, which occurred over the past decade, boosted its gross margin above 90% on a non-GAAP (adjusted) basis. Its operating margin has also consistently expanded.
For the full year, Autodesk expects its non-GAAP operating margin to increase 400 basis points to 36%, even after factoring in the suspension of its operations in Russia.
During the conference call, CFO Debbie Clifford also reiterated Autodesk's long-term goals of generating double-digit revenue growth, non-GAAP operating margins in the 38% to 40% range, and double-digit free cash flow growth on a compound annual basis.
A wide moat with a reasonable valuation
Autodesk's AutoCAD is the gold standard of computer-aided design software and that reputation gives it a wide moat against its smaller rivals. It then leveraged that reputation to launch additional software products and lock more subscribers into its sticky cloud-based ecosystem.
That evergreen business model makes Autodesk similar to Adobe, which also converted its suite of creative software into cloud-based services over the past decade.
Autodesk is growing at a similar rate as Adobe, and both stocks trade at about 30 times forward earnings. Those multiples aren't cheap, but their resilient businesses support their premium valuations.
Is it the right time to buy Autodesk?
Autodesk lost about a quarter of its value this year as rising interest rates drove investors away from higher-growth tech stocks. However, I believe Autodesk's well-diversified business, sticky subscriptions, rising margins, and clear targets for the future still make it a rock-solid investment.
<<<
---
>>> MeaTech Reveals Promising Results with Muscle Stem-Cell Differentiation
Yahoo Finance
February 8, 2022
https://finance.yahoo.com/news/meatech-reveals-promising-results-muscle-120000941.html
Successful development of novel technology process fusing muscle cells into significant muscle fibers that better resemble those in whole cuts of meat
NESS ZIONA, Israel, Feb. 8, 2022 /PRNewswire/ -- MeaTech 3D Ltd., (NASDAQ-CM: MITC), an international food technology company at the forefront of the cultured meat industry, today revealed significant improvement in its differentiation process from stem cells to muscle fibers. The company has succeeded in accelerating the formation of real living muscle fibers and enhancing their quality to mirror key characteristics of farm-raised meat.
These results show the process in which bovine stem cells were isolated, proliferated in the lab, and differentiated into matured muscle cells with improved muscle fiber density, thickness and length. Based on these improvements, MeaTech has filed a provisional patent application with the USPTO.
Arik Kaufman, CEO of MeaTech: "Today's achievement is another important step towards our goal of developing a true replacement for conventional meat. We are committed to advancing our cellular technology to attain a cultivated-meat-eating experience that replicates that of farm-raised meat. This achievement follows our recent announcement that we successfully 3D printed an almost 4 oz steak comprised of actual living muscle and fat tissue."
Yaron Kaiser, Chairman of the Board, MeaTech: "MeaTech's wonderful results, led by our technology team, place the company at the forefront of the cultured meat revolution.
The professionalism and capabilities of a small, elite, and goal-oriented technological team are enabling MeaTech to achieve significant and groundbreaking achievements efficiently and quickly, giving the company a clear competitive advantage in an industry that is on a secure path to changing our lives and the way we consume meat. "
About MeaTech 3D
MeaTech is an international food technology company at the forefront of the cultured meat revolution. The company is listed on the Nasdaq Capital Market under the ticker "MITC".
MeaTech initiated activities in 2019 and maintains facilities in Ness Ziona, Israel and Antwerp, Belgium. The company believes cultivated meat technologies hold significant potential to improve meat production, simplify the meat supply chain, and offer consumers a range of new product offerings.
MeaTech aims to provide an alternative to industrialized animal farming, circumventing the serious issues surrounding conventional animal husbandry, such as carbon footprint, water resources and animal welfare. By adopting a modular factory design, MeaTech will be able to offer a sustainable solution for a variety of species, including beef, chicken, and pork, both as raw materials and whole cuts; and also provide the production equipment
<<<
>>> 3D Bioprinting: Eradicating Transplantation Waiting Lists And Testing Drugs On Living Tissues
The Medical Futurist
11 March 2021
https://medicalfuturist.com/3d-bioprinting-overview/
From time to time, news arises about 3D-printed organs. On such occasions, people usually think that a machine can already create readily available, implantable human organs. However, the reality is far from this optimistic image.
Researchers worldwide are working on possible solutions: from a group that printed a miniature kidney, through technological solutions like BioAssemblyBot we wrote about earlier, to entirely new methods that can lead to patient-specific heart tissue printing. The list is long and set in a clinical setting. We checked out where the technology stands today and where it might lead us in healthcare tomorrow.
3D bioprinting can be the response to worldwide organ shortages and the increasing reluctance to test new cosmetic, chemical, and pharmaceutical products on animals. Whether it will become a reality anytime soon is not certain, although research efforts have grown rapidly over the past years. As seen in this video, the technology behind bioprinting is getting better and (much) faster. However, this hydrogen-based technology is still not bioprinting – it is not the content but the printing technology itself. However, a breakthrough might be just around the corner.
Here’s how bioprinting will break into healthcare revolutionising organ donations and animal testing.
What is bioprinting?
Put the term bioprinting next to Earth-invader androids, shiny spaceships in a post-apocalyptic setting, and you’ll get the next Hollywood blockbuster. However, as opposed to malevolent aliens, bioprinting not only exists in sci-fi movies, but it will also transform healthcare in the following decades. Before going into details, though, let’s dissect the technology itself.
Three-dimensional (3D) bioprinting is a state-of-the-art technology that means creating living tissues, such as blood vessels, bones, heart or skin, via the additive manufacturing technology of 3D printing. Traditional 3D printing implies the production of three-dimensional solid objects from a digital file, using a layering process. In its most common version, a source material, such as plastic, is liquefied, and then the machine adds layer after layer on the platform until you have a fully formed object.
Needless to say, printing organs is a “little bit” more complicated. In the early 2000s researchers discovered that living cells could be sprayed through the nozzles of inkjet printers without damaging them. However, it is not enough to have the cells themselves, they need a nurturing environment to stay alive: food, water, and oxygen. Nowadays, these conditions are provided by a microgel – think of gelatin enriched with vitamins, proteins, and other life-sustaining compounds. Moreover, to create conditions fostering the fastest and most efficient cell growth, researchers plant the cells around 3D scaffolds made of biodegradable polymers or collagen so they can grow into fully functional tissue.
How to 3D print an organ
Let’s take the example of the bladder, a simpler organ consisting of only two types of cells. At first, researchers scan the patient’s organ to determine personalised size and shape. Then they create a scaffold to give cells something to grow on in three dimensions and add cells from the patient to this scaffold. That’s painstakingly labour-intensive work and could take as long as eight weeks. Finally, a bioreactor creates the optimal environment for the cells to grow into an organ. When doctors finally place the organ in the patient, the scaffold has already disappeared or disappears soon after surgery.
This description cannot demonstrate how difficult and time-consuming the entire process is. We are a long way, even decades away, from bioprinting fully functioning, complex organs; however, 3D bioprinting is already used to generate model tissues for research and is also used in regenerative medicine.
A solution for organ shortages
The solution to alarming worldwide organ shortages comes from technology. 3D bioprinting is the response of technology to critical tissue shortages hampering medical professionals’ tasks and endangering many lives. In the U.S., a new person is added to the waiting list of organ donors every 9 minutes. The number of patients waiting for an organ donor has multiplied five-fold in the last 26 years. 17 people die every day due to the lack of available organs in the U.S. alone.
Other countries are not better off either. In 2018, over 150 000 patients in Europe were registered on organ waiting lists. In the U.K., 408 patients died while waiting for an organ in 2019. In 2020, the U.K.’s organ donation policy changed to an opt-in system, meaning every adult agrees to become an organ donor when they die unless they state that they do not wish to donate. This has remarkably decreased the waiting lines in the country.
Closing the gaps
A team of researchers at Carnegie Mellon University have created a 3D bioprinting technique called “Freeform Reversible Embedding of Suspended Hydrogels” (FRESH). During a panel discussion on the virtual AMS Summit in March 2021, the project’s team leader, Professor Adam Feinberg, noted that “we could have a bioprinted heart in an animal in 12 years.” Talking further about the possible future timeline for the technology, another expert, Katie Weimer, VP of Regenerative Medicine at 3D Systems, said the issue is merely “an engineering problem.”
In a recent interview for our Patreon site, Erik Gatenholm, CEO of CELLINK, estimated the same. He said, “we will see fully functioning organs within the next decade or so.” Gatenholm added, “scientists have been able to bioprint hearts, lungs, kidneys, skin, corneas and more throughout the last 5 years and are currently working towards developing full functioning organs. As of now, the industry is progressing at a steady pace due to the democratisation of 3D bioprinting technology.”
Bioprinting making waves on the market
At the AMS Summit, industry leaders went into an in-depth discussion on the commercialisation of 3D bioprinting. Several companies that focus on bioprinting tissues or implants rather than organs themselves already have go-to-market products – like Particle3D, Aspect Biosystems, Rokit Healthcare, Viscient Biosciences, Dimension Inx, and Poietis. But of course, major players on the field like Swedish CELLINK or US-based Organovo also have their technologies on the market already.
The pioneers of 3D bioprinting
The most well-known tissue engineering company is the San Diego-based company, Organovo. It has been actively developing a line of human tissues for use in medical research and drug discovery. They create highly customised 3D human tissues as dynamic models of healthy and diseased human biology for drug development and recently have been in the news for creating miniature human kidneys in the lab, together with the Murdoch Children’s Research Institute.
Today the company still lacks the much-talked-about FDA-approval to its technology. It focuses on replacing animals in testing processes, novel drug discovery and custom disease models, as well as single-cell RNA sequencing. Organovo seeks to have multiple IND filings with the FDA by the end of 2025.
CELLINK has been doing great over the past months. The company develops both bioprinters and bioprinting materials for providing ready-to-print or use models for researchers and healthcare providers to enable 3D cell culture, personalised medicine, and enhanced therapeutics. Their approach helped to decrease the price of bioprinting devices enormously, which allowed more and more research or education institutions and organisations to buy one of these machines – and this inevitably led to even more research and more breakthrough in the industry. Through acquisitions and cooperations, CELLINK indeed became a bioconvergence powerhouse.
Biotech veteran, US-based United Therapeutics develops pharmaceuticals to treat vascular diseases and cancer. The company started bioprinting kidneys, together with Israeli CollPlant, to use the latter’s unique bioink technology and human collagen in the process that aims to reduce worldwide organ shortages.
With 3D bioprinting against testing drugs on animals
Bioprinting can also help eliminate the need for testing new drugs on animals. Replacing lengthy and expensive clinical trials, which often have no results, is a good market opportunity whereby pharma companies could save billions of dollars. Testing medication on mice, rabbits or other animals is in many cases not efficient as the particular drug could still have a different effect on people.
On the other hand, 3D printed tissue proves to be an effective means of testing new pharmaceuticals, meaning that drugs can be thoroughly assessed and brought to market more quickly, all without harming animal test subjects. Moreover, as testing of cosmetics on animals has always been even more controversial than testing for medical purposes, with the emergence of 3D printing human skin, testing cosmetics on animals could disappear once and for all.
In situ bioprinting
The solution means 3D printing tissues directly at the point of injury – no matter whether it’s about bones, tissues or skin. In the next decade, doctors may, therefore, be able to scan wounds and spray on layers of cells to heal them very rapidly. The first results are already out: Australian researchers from UNSW Sydney developed a ceramic-based ink that can print bone-like structures without the use of chemicals or radiation. This technology may help surgeons to 3D print bone parts with living cells – practically to print a replacement bone, including living cells, directly inside a patient’s body.
Scientists in China are also working on “in vivo in situ” solutions and developed a tool that can carry out tissue repair inside the body, used on patients with gastric wounds.
The challenges of 3D bioprinting
The Medical Futurist doesn’t like to ruin optimistic and positive visions for the future, but bioprinting faces severe challenges from technological, financial, and regulatory perspectives.
The most burning issue is the question of regulation. Leaving the market unregulated might lead to a thriving black market. As soon as scaffolds are available and methods are open source, people worldwide might be tempted to start printing unregulated and untested biomaterials and sell them to desperate people.
The FDA has a short segment on 3D bioprinting, but it does not explicitly cover living cells. Those applications are due to go through the FDA’s Center for Biologics Evaluation and Research. As the FDA does not regulate bioprinting but the medical devices and solutions coming out of the printers, regulations still lag behind the technology’s speed.
A 2020 October decision to give 510k clearance to A.D.A.M., a Ukrainian company to work on 3D printed bone implants out of bioceramics, however, indicated that the regulatory body is about to speed up in the field. A.D.A.M. is already in the testing phase and expected to be on the market in 2022 – after getting the FDA approval.
3D Bioprinting is an overly complicated technology, and its many technological, biological challenges; ethical and regulatory issues can already be seen from this brief introduction. It won’t be applied in practice overnight, but it’s going to be a reality to deal with within a decade.
<<<
>>> Big Gains for 3D Printing in Manufacturing, ASME and Carbon Survey Finds
Report shows that nearly all respondents now use a 3D printing process for manufacturing
Carbon
Sep 16, 2021
https://www.prnewswire.com/news-releases/big-gains-for-3d-printing-in-manufacturing-asme-and-carbon-survey-finds-301378972.html
REDWOOD CITY, Calif., Sept. 16, 2021 /PRNewswire/ -- Carbon, a leading 3D printing technology company, and the American Society of Mechanical Engineers (ASME) announced today the findings from a survey which found that the 3D printing process has become an increasingly integral technology in the development of plastic parts and products for production use.
The ASME survey of practicing engineers involved in the design or development of plastic parts found that 88% of respondents now use 3D printing/additive manufacturing in some way. And the technology, previously best known for its use in creating prototypes, is increasingly being used for the production of finished parts. 40% of respondents said they now use additive manufacturing to produce products.
The report, "Additive Manufacturing/3D Printing Adoption from Prototype to Production," includes findings that the technology has secured footholds broadly across industries and that its growing adoption is based on three categories of attributes: its speed, flexibility in timing and design and cost-efficiency. In various ways, digitally-driven product development is making major inroads into industries and manufacturing processes long dominated by two older methods that are less flexible and tend to be more capital intensive: milling and injection molding.
"Additive manufacturing is being broadly implemented in many varied industries, and is having an incredible impact on the supply chain and manufacturing across the world," said Phil DeSimone, cofounder and Chief Product & Business Development Officer at Carbon. "We have seen OEMs embrace 3D printing as a strategic advantage to develop better products and bring them to market in less time. Advances in materials, software and hardware are making it possible for these companies to design products, validate market fit and move to production faster and with better results."
Other key findings from the report include:
For the development of polymer products, 3D printing is used more often than either injection molding or milling.
3D printing is still the most often used process for prototyping.
Engineers within the life sciences and industrial machinery sectors reported the highest levels of familiarity with 3D printing.
Use of and familiarity with 3D printing is heavily influenced by age and company size — the younger the engineer and the larger the company, the greater the embrace of 3D.
"Additive manufacturing, especially in the polymer space, has grown by an incredible amount over the past couple of years," said Lauralyn McDaniel, head of industry strategy and engagement with Metrix, an ASME Company. "Seeing the strong use of 3D printing in polymer production is evidence that additive manufacturing is moving into the mainstream, especially in areas like athletic equipment where the unique lattice design capabilities of 3D printing have been used to increase performance and safety, as well as in healthcare and aerospace, which have been leaders in production use of additive manufacturing."
For the full report, which was commissioned by Carbon, and a deeper look into the state of additive manufacturing and 3D printing please visit: https://resources.asme.org/am3dp-polymer
About Carbon
Carbon is a 3D printing technology company helping businesses to develop better products and bring them to market in less time. The Carbon DLS™ process combines versatile printers, advanced software, and best-in-class materials to deliver functional parts with end-use performance and aesthetics, helping engineers and designers to create products that outperform expectations. From prototyping and low-volume production to production-at-scale, global organizations, including adidas, Ford Motor Company, and Becton, Dickinson and Company, use the Carbon process to create a wide range of functional end-use parts and print them reliably wherever and whenever they need them through Carbon's production network partners. Carbon is a venture-backed company headquartered in Redwood City, CA. To learn more, follow Carbon on Twitter, LinkedIn and Facebook.
About ASME
ASME helps the global engineering community develop solutions to real world challenges. Founded in 1880 as the American Society of Mechanical Engineers, ASME is a not-for-profit professional organization that enables collaboration, knowledge sharing and skill development across all engineering disciplines, while promoting the vital role of the engineer in society. ASME codes and standards, publications, conferences, continuing education, and professional development programs provide a foundation for advancing technical knowledge and a safer world. In 2020, ASME formed the International Society of Interdisciplinary Engineers (ISIE) LLC, a new for-profit subsidiary to house business ventures that will bring new and innovative products, services, and technologies to the engineering community, and later established the holding company, Global Knowledge Solutions LLC. In 2021, ASME launched a second for-profit subsidiary, Metrix Connect LLC, an industry events and content platform to accelerate digital transformation in the engineering community and the exclusive agent for the Mechanical Engineering® brand of media products. For more information, visit www.asme.org.
<<<
>>> Carbon, Inc brings together innovations in software, hardware, and molecular science to deliver industry-leading digital manufacturing solutions. With proprietary Carbon Digital Light Synthesis (DLS) technology and family of programmable liquid resins, manufacturers can unlock new business opportunities such as mass customization, on-demand inventory, and previously impossible product designs. The Carbon Platform allows customers to build uniquely differentiated products while reducing waste and speeding time to market.
Carbon was founded by Dr. Joseph DeSimone and Philip DeSimone in 2013.
<<<
>>> Is this the future of vaccines? UNC researchers create 3D printed vaccine patches
9-25-21
Zachery Eanes
The News & Observer
https://www.msn.com/en-us/health/medical/is-this-the-future-of-vaccines-unc-researchers-create-3d-printed-vaccine-patches/ar-AAONfbS?ocid=uxbndlbing
Sep. 25—CHAPEL HILL — Scientists at UNC-Chapel Hill and Stanford University said this week that they've successfully created a 3D-printed vaccine patch that delivers a stronger immunity response than a standard vaccine shot.
The patch, which would be placed on the skin like a Band-Aid, is covered in microneedles that deliver vaccines directly into the skin.
The researchers tested the patches on animals, and, in a study published in the Proceedings of the National Academy of Sciences, reported an antibody response 50 times higher than the traditional jab. The patches were applied with thumb pressure for two minutes and then left on the skin for 24 hours, according to the study.
The findings could have a profound impact on the logistical rollout of vaccines in the future, said Joseph DeSimone, a professor of chemical engineering at Stanford University and professor emeritus at UNC.
The patches are virtually painless and could eliminate one of the main reasons people refuse to get vaccines, which is a fear of needle injections.
But more important, DeSimone said, is that they don't require extremely cold temperatures like some vaccines, making them easier and cheaper to ship all over the world.
"I think it totally is" the future of vaccines, DeSimone said in a telephone interview with The News & Observer. "I think microneedles can be the OS for vaccine design — the operating system. And I think we're putting too much weight on the traditional way of delivery even in the design stage of vaccines."
Whether the patch will ever be used to deliver COVID-19 vaccines remains to be seen. DeSimone told The N&O the patch might be ready for human clinical trials in 18 to 14 months.
DeSimone is a prominent figure in the world of 3D printing.
During his time at UNC — where he worked from 1990 to 2014 — DeSimone pioneered a new type of 3D printing called Continuous Liquid Interface Production, or CLIP.
The breakthrough that CLIP brought helped DeSimone launch the 3D printing company Carbon Inc., which has raised more than $680 million from investors and has customers ranging from Adidas to Ford Motor Co.
The microneedles for the patches were made using a Carbon CLIP 3D printer, UNC said.
DeSimone said that the patches create a stronger immune response than needles because they deliver the vaccine to the skin rather than the muscle.
"The target cells for vaccines are way more common in our skin than in our muscle," he said. "And that's because of the way we've evolved. You know, if you fall and cut yourself, the first line of defense for avoiding infection is in the skin and those immune cells are the targets for vaccine. There's literally 100 to 1,000 times more per unit volume in the skin than in the muscle."
The patches were tested with a model vaccine, but DeSimone believes they could carry any type of vaccine, including the mRNA vaccines that have been used so effectively during the coronavirus pandemic.
The work behind 3D printing of vaccine patches predates the COVID-19 pandemic by a few years. But the struggles of delivering vaccines to the entire world have shown how critical vaccine technology is going forward, DeSimone said.
"Despite how terrible this pandemic has been — and it's been awful, really awful — it could have been a lot worse," he said. "This thing could have been avian flu with a 30% death rate, and we would be scrambling way more than we have been.
"I think a lot of people believe it's just a matter of time (before the next pandemic), and therefore technologies like this need to be readied for the future."
There could be significant cost savings as well, according to DeSimone's own projections. "I've heard numbers of syringe needles and glass vials and everything being north of $3 to $7 (per vaccine). And I think we can make these patches for less than 10 cents," he said.
DeSimone, striking an optimistic tone, said the ease of transportation of 3D patches could revolutionize the way vaccines are administered.
"We think — and our corporate partners that are emerging think — that the whole direction of this is you're going to receive a vaccine via like Amazon or the U.S. Postal Service in the future," he said.
The next step for the patches is a clinical trial in non-human primates. That could come as early as the first part of 2022, and eventually, the trials would focus on specific vaccines rather than model ones.
Commercial partners, though, are already reaching out to DeSimone about working with the technology, and the universities are eager to commercialize it.
"We love to commercialize stuff, and so we're eager to help facilitate building strategic partnerships to make this happen," he said. "We're in those dialogues now."
<<<
Nano Dimension Ltd - >>> 10 Best Cheap Tech Stocks to Buy According to Cathie Wood
Insider Monkey
May 26, 2021
https://finance.yahoo.com/news/10-best-cheap-tech-stocks-145316098.html
Number of Hedge Fund Holders: 11
Nano Dimension Ltd. (NASDAQ: NNDM) is an Israel-based 3D printing company founded in 2012. It is placed ninth on our list of 10 best cheap tech stocks to buy according to Cathie Wood. The company stock has returned more than 150% to investors over the course of the past twelve months. ARK Investment holds close to 13 million shares in the company worth over $111 million. This represents 0.22% of their portfolio. Nano Dimension primarily focuses on research and development related to 3D printed electronics.
In earnings results for the first three months of 2021, posted on May 20, Nano Dimension Ltd. (NASDAQ: NNDM) reported earnings per share of -$0.05 and a revenue of $0.8 million. The revenue was up over 15% compared to the same period last year.
Out of the hedge funds being tracked by Insider Monkey, New York-based investment firm Renaissance Technologies is a leading shareholder in Nano Dimension Ltd. (NASDAQ: NNDM) with 6.6 million shares worth more than $57 million.
<<<
NanoDimension - >>> Have $3,000? Buying These 2 Stocks Would Be the Smartest Move You Ever Made
Both companies are using nanotechnology to revolutionize healthcare and manufacturing. Plus, they have considerable upside.
Motley Fool
by Taylor Carmichael
Jan 31, 2021
https://www.fool.com/investing/2021/01/31/have-3000-buying-these-3-stocks-would-be-the-smart/?source=eptyholnk0000202&utm_source=yahoo-host&utm_medium=feed&utm_campaign=article
You don't need to invest a lot of money to have amazing returns in the stock market. If you invested $3,000 in Amazon (NASDAQ:AMZN) 20 years ago, you could have bought 300 shares. At a Jan. 29 price of $3,203 per share, your $3,000 investment would now be worth $960,900. To pull off an investment like that, you had to be willing to take risks (Amazon was unprofitable in its early years), practice patience, and let the growth story play out.
Are there any stocks in 2021 with Amazon-sized futures? Yes, there are. A few that come to mind are small companies using nanotechnology to disrupt the healthcare and manufacturing industries. Nano-X (NASDAQ:NNOX) has a device that could make X-rays a lot cheaper and more accesible. And Nano Dimension (NASDAQ:NNDM) uses nanotechnology (and 3-D printing) to revolutionize the way companies manufacture electronic circuit boards. Read more to find out why these stocks might be wonderful for patient, risk-tolerant investors.
1. Smaller is better for Nano-X
Nanotechnology led to the breakthrough.
The problem with high-end X-ray machines like those using magnetic resonance imaging (MRI) or computerized axial tomography (CAT) is that the machines are massive and cost hospitals over $1 million, and in some cases as much as $3 million. These high-end medical X-ray systems have to generate a tremendous amount of heat for the X-ray to happen -- up to 2,000 degrees Celsius (3,600 degrees Fahrenheit). Using micro-electrical-mechanical-systems (MEMS), Nano-X was able to fabricate millions of nanoscale gates on a silicon chip. Each one of these microscopic "nano-spindts" digitally creates and controls the electrons that power an X-ray device.
The result is that we no longer need to generate heat for an X-ray to work. Instead of having to power up to 3,600 degrees Fahrenheit -- and then cool down -- the Nano-X device stays at room temperature. Thus the company was able to build a much smaller, and cheaper, X-ray machine. And yes, the engineers who designed the sleek machine were inspired by Star Trek.
Nano-X says the device -- once it's cleared by the U.S. Food and Drug Administration (FDA) -- will cost about $10,000 to manufacture. But what's exciting the market is not how cheap the device is, or even how popular it might be. What's truly getting investors jazzed is that Nano-X will take a small percentage of the fee every time its X-ray device is used. That's the classic razor-and-blades pricing model that helped make Intuitive Surgical (NASDAQ:ISRG) investors rich. Nano-X can actually give its machine away, because it's making money on the use of the device, not on the original sale.
Will the device work as well as the MRI and CAT scans common in hospitals today? Skeptics abound. The company has no profits (or even revenues) yet. Shorts and other traders have made Nano-X's stock price incredibly volatile. But early investors who took the plunge upon its IPO in August are doing quite well so far.
2. Using Nanotech to transform 3D printing
3D printing has been around for decades. It's now become fairly commonplace in many industries. For instance, SmileDirectClub (NASDAQ:SDC) uses 3D printers to create aligners for your teeth. Nonetheless, this revolution in manufacturing is just beginning. And Nano Dimension might just be the most important company in 3D manufacturing today.
Nano Dimension uses nanoparticles to transform the inks used in 3D printing. Its Dragonfly device allows designers and engineers to print functional circuit boards and other electronic devices.
This is huge, for any number of reasons. Research and design labs can keep their intellectual property secret, because they can manufacture in-house cheaply, quickly, and easily. But 3D printing has an added benefit -- you can print devices with parts that are so tiny, they were impossible to manufacture before. As the company puts it on its website, designers can "pack more functionality in smaller footprints."
Nano Dimension has customers in aerospace and defense industries, but also in healthcare. Medical device companies can now 3D print noninvasive sensors and micro devices.
The most enticing thing about Nano Dimension for investors is its business model. The company has sold only 60 devices so far. But, as with Nano-X and Intuitive Surgical, the real money will be made in service revenues. Nano Dimension will make its money by supplying the miraculous inks that allow these systems to work. The more companies use its device to print circuit boards, the more money Nano Dimension will make.
This recurring revenue model is a wonderful business to invest in. While these small and unprofitable companies are certainly risky -- and highly volatile in the short term -- patient investors with a long-term outlook can easily make a fortune if either company pulls it off. That's the key to investing in early-stage companies -- don't put all your eggs in one basket. And hold on to your shares to see how it all unfolds. Patient investors who understand the risks might just see some amazing returns down the road.
<<<
>>> Nano Dimension Ltd. (NNDM), together with its subsidiaries, provides additive electronics in Israel and internationally. Its flagship product is the proprietary DragonFly lights-out digital manufacturing system, a precision system that produces professional multilayer circuit-boards, radio frequency antennas, sensors, conductive geometries, and molded connected devices for prototyping through custom additive manufacturing. The company also provides nanotechnology based conductive and dielectric inks; and DragonFly and Switch software to manage the design file and printing process. It markets and sells products and services to companies that develop products with electronic components, including companies in the defense, automotive, consumer electronics, semiconductor, aerospace, and medical industries, as well as research institutes. The company was founded in 2012 and is headquartered in Ness Ziona, Israel.
<<<
>>> Cellink AB (CLLKF) designs and develops bioprinting technologies that enable researchers to 3D print organs and tissue for applications primarily in pharmaceutical to cosmetic industries worldwide. It offers bioprinters, imaging systems, dispensing solutions, and bioinks in the field of academic and clinical medicine for labs and scientists worldwide. The company's bioprinting solutions include BIO X, a bioprinter for life-science companies, researchers, and innovators; BIO X6, a bioprinting platform for printing complex structures; INKREDIBLE and INKREDIBLE+, which are bioprinting solutions for skin, cartilage tissue, and others; Holograph X, a solution that leverages high-resolution holographic stereolithography to bioprint small structures; Lumen X, a solution that offers applications in microfluidics, cell-laden hydrogels, macroporous structures, and others; and tool heads and print heads. It also offers biofluidics solutions, which include I-DOT, a solution for liquid-handling tasks; single-cell printer, an automated laboratory instrument; c.sight, a single-cell dispensing technology for cell line development and single-cell genomics; b.sight, a solution that enables prokaryotic cell isolation in the sub-micron range; and f.sight, a solution that dispenses unlabeled and fluorescent cells. The company offers bioinks for various applications, such as drug screening, tissue models, tissue regeneration, organoids, immunotherapy, microfluidics, scaffolds, encapsulation, wound healing, cosmetics, educational models, and others; CELLCYTE X, a live cell microscopy imaging system; and accessories, such as consumables, needles, and nozzles. Its customers also include universities, hospitals, and public and commercial laboratories. Cellink AB (publ) has a collaboration with MedImmune; and strategic partnership with Made In Space, Inc. It also offers products online. The company was founded in 2016 and is based in Gothenburg, Sweden.
<<<
>>> Organovo and Tarveda Therapeutics Announce Definitive Merger Agreement
Business Wire
December 16, 2019
https://finance.yahoo.com/news/organovo-tarveda-therapeutics-announce-definitive-120000691.html
Organovo and Tarveda Therapeutics Announce Definitive Merger Agreement
Combined company to operate as Tarveda Therapeutics upon closing of merger
Transaction to advance Tarveda’s proprietary Pentarin® miniature drug conjugates including its two clinical programs for the treatment of solid tumor malignancies
Companies to host conference call today at 8:30 AM ET
Organovo Holdings, Inc. ("Organovo") (Nasdaq: ONVO) and Tarveda Therapeutics, Inc. ("Tarveda"), a privately-held, clinical stage biopharmaceutical company developing a new class of potent and selective precision oncology medicines, which it refers to as Pentarin miniature drug conjugates, today announced that they have entered into a definitive merger agreement under which Tarveda would merge with a wholly-owned subsidiary of Organovo in an all-stock transaction. Upon completion of the merger, the merged company would operate under the name Tarveda Therapeutics, Inc. and trade on the Nasdaq Stock Market LLC under the ticker symbol "TVDA."
This press release features multimedia. View the full release here: https://www.businesswire.com/news/home/20191216005228/en/
Tarveda is primarily focused on the development of its pipeline of Pentarin miniature drug conjugates designed to selectively accumulate and retain anti-cancer payloads in solid tumor malignancies. Following the closing of the merger, Tarveda intends to continue to focus on advancing its two clinical stage oncology programs, PEN-866 and PEN-221, and on further development of novel conjugates from its proprietary miniature drug conjugate platform. At the closing of the merger, it is estimated that the combined company will have approximately $35 million of cash on hand that is expected to provide sufficient funding into the second half of 2021 to achieve key upcoming clinical data milestones on both clinical programs.
"After completing an extensive and thorough review of strategic alternatives, we are extremely pleased to announce this transaction with Tarveda, which we believe is in the best interest for our stockholders," said Taylor J. Crouch, President and Chief Executive Officer, Organovo. "Tarveda is advancing an innovative pipeline of clinical stage cancer therapies derived from the company’s proprietary miniature drug conjugate platform. Tarveda is supported by a strong syndicate of investors including Novo A/S, Versant Ventures and ND Capital (NanoDimension) and a highly seasoned management team with prior public company experience."
"Our growing portfolio of miniature drug conjugates has the potential to represent much needed new treatment options for patients with solid tumor malignancies," said Drew Fromkin, President and Chief Executive Officer of Tarveda. "We are encouraged by the activity and tolerability demonstrated in Phase 1 human studies of our two clinical programs, PEN-866 and PEN-221. Our Pentarin miniature drug conjugates are designed to incorporate the best properties of small molecule drugs and antibody drug conjugates to form miniature drug conjugates that are effective at rapidly and deeply penetrating solid tumors while minimizing damage to healthy tissue. We are excited about this merger with Organovo and believe that this is the right point in Tarveda’s trajectory to move forward as a publicly traded company given several upcoming clinical data milestones that we expect to be achieved in 2020 and 2021."
Tarveda expects the merger to provide the capital required to advance its two lead programs through the next set of clinical milestones and to generate novel conjugates from its Heat Shock Protein 90 (HSP90) binding miniature drug conjugate platform. PEN-866, the initial clinical program from Tarveda’s HSP90 binding miniature drug conjugate platform, is designed to bind to the activated form of HSP90 in solid tumors to accumulate and retain its potent topoisomerase 1 inhibitor (SN-38) payload. PEN-866 is completing the Phase 1 dose escalation and safety portion of its "all comers" trial in various types of solid tumors and has shown to be well tolerated and demonstrated early clinical activity in heavily treated, advanced patients with a range of solid tumor malignancies. Beginning in early 2020, it is expected that PEN-866 will be evaluated in a Phase 2a study both as a single agent and as a combination therapy across a range of solid tumors that are sensitive to topoisomerase 1 inhibitors. PEN-221 is a miniature drug conjugate in clinical evaluation for the treatment of patients with solid tumors expressing somatostatin receptor 2 (SSTR2) on the cell surface and is linked to the potent tubulin inhibitor payload, DM1. In a Phase 1 study, PEN-221 was well tolerated and demonstrated early clinical activity. PEN-221 is currently being evaluated in a Phase 2a study for the treatment of patients with neuroendocrine tumors and small cell lung cancer.
About the Proposed Merger
Under the terms of the merger, it is anticipated that Tarveda stockholders will own approximately 75% of the combined company and current Organovo stockholders will own approximately 25% of the combined company on a fully-diluted basis. The exchange ratio is based on valuation assumptions for both companies subject to potential adjustments for certain financial metrics prior to the completion of the merger.
The transaction has been approved by the boards of directors of both companies. The merger is anticipated to close in the first quarter of 2020, subject to the approval of Organovo and Tarveda stockholders as well as other customary closing conditions.
Roth Capital Partners served as financial advisor, and Gunderson Dettmer Stough Villeneuve Franklin & Hachigian, LLP served as legal counsel to Organovo. Canaccord Genuity served as financial advisor, and Cooley LLP served as legal counsel, to Tarveda.
Management and Organization
Following the merger, the combined company will be led by the current Tarveda management team, including Drew Fromkin as President, Chief Executive Officer and Chairman; Jeffrey D. Bloss, M.D., Chief Medical Officer; Brian Roberts, Chief Financial Officer; Mark Bilodeau, Ph.D., Chief Scientific Officer; and Sudhakar Kadiyala Ph.D., Executive Vice President, Strategy.
The Board of Directors of the combined company will be comprised of eight directors, including six directors to be named by Tarveda and two directors to be named by Organovo. The corporate headquarters will be located in Watertown, MA.
Conference Call
Organovo and Tarveda will host a conference call at 8:30 a.m. ET on December 16, 2019, to discuss the proposed transaction. The conference call may be accessed by dialing (866) 405-4577 (domestic) or (602) 563-8680 (international) and using the conference ID 3679123. To help ensure the conference call begins in a timely manner, please dial in five minutes prior to the scheduled start time. The conference call will also be simultaneously webcast at http://www.organovo.com.
<<<
>>> The Best 3D Printing ETF
Interested in 3D printing stocks? You might consider investing in the 3D Printing ETF.
6-22-17
Motley Fool
Beth McKenna
https://www.fool.com/investing/2017/06/22/the-best-3d-printing-etf.aspx
3D printing stocks are having a great 2017, after several very tough years. The stocks of the two largest players, 3D Systems (NYSE:DDD) and Stratasys (NASDAQ:SSYS), for example, have gained 66.3% and 66.6%, respectively, this year through June 19, versus the S&P 500's 10.7% return.
Investors who are interested in 3D printing stocks but don't want to bet on just one player or even a couple of companies, have another option: a 3D-printing exchange-traded fund (ETF). We're going to explore the best (and only, to my knowledge) ETF focused on this space, The 3D Printing ETF (NYSEMKT:PRNT), to see if it's worth investing in.
The 3D Printing ETF, issued by Ark Investment Management, is an index-based fund designed to track the Total 3D-Printing Index. This index is composed of stocks of companies based in the United States and other developed markets that are engaged in 3D printing-related businesses, specifically, 3D-printing hardware, computer-aided design software and 3D-printing simulation software, 3D-printing service centers, scanning and measurement equipment, and 3D-printing materials.
The ETF, which is rebalanced quarterly, has 42 holdings. The weighted-average market cap of the portfolio is $30 billion, while the median market cap is $3 billion. The fund's expense ratio is 0.66%, which is fairly reasonable.
The 3D Printing ETF: Top 10 holdings
Holding No.
Company
Ticker
Market Cap
Country
Weight (% of Portfolio)
1
3D Systems
DDD
$2.5 billion
U.S.
7.08%
2
ExOne
NASDAQ: XONE $209.4 million
U.S.
6.59%
3
MGI Digital Graphic Technology*
ALMDG*
$300 million
France
6.39%
4
Stratasys
SSYS
$1.5 billion
Israel/U.S.
5.60%
5
SLM Solutions**
AM3D**
$700.1 million
Germany
5.36%
6
K2M Group Holdings
NASDAQ: KTWO
$991.1 million
U.S.
5.35%
7
Organovo Holdings
NASDAQ: ONVO
$276.1 million U.S. 4.77%
8
HP Inc.
NYSE: HPQ
$29.9 billion U.S. 4.46%
9
Autodesk
NASDAQ: ADSK
$23.2 billion U.S. 3.93%
10
Trimble
NASDAQ: TRMB
$9.5 billion
U.S.
3.91%
Investors should be clear that this ETF is not a pure play on 3D printing. I've read such a claim on several financial outlets, and it just isn't so. A quick glance at the top 10 holdings should make this obvious: No. 8, HP Inc., for example, is a well-known huge player in 2D printing, with 3D printing no doubt comprising a minuscule part of its business, as it entered the market just last year.
Of the top 10 holdings, only three are 3D printing pure plays, in my opinion: 3D Systems, ExOne, and Stratasys.
3D Systems and Stratasys, the industry's two largest players, are quite diversified. Both make 3D printers for commercial and industrial markets and provide on-demand 3D-printing services. Stratasys also produces desktop 3D printers for the education and professional markets. ExOne makes heavy-duty industrial 3D printers that primarily print in sands (to make molds) and metals; it also provides 3D-printing services. SLM Solutions makes metal 3D printers powered by its selective laser melting technology and vacuum casting equipment.
MGI Digital Graphic Technology specializes in digital 2D-printing and finishing equipment. Apparently, it's included in the ETF because one MGI Group subsidiary, Ceradrop, manufactures equipment for the 3D-printed-electronics market.
K2M and Organovo are involved in the medical space. K2M is a medical-device company that uses 3D printing to produce some of its spine products. Organovo uses its proprietary 3D printing tech to "3D bioprint" human tissues for pharmaceutical testing, though its ultimate goal is to bioprint organs for people in need of transplants.
HP, as I mentioned, entered the 3D-printing market last year, with the launch of two enterprise-focused 3D printers. Autodesk makes design software for 3D printing and other uses, and has several 3D-printing initiatives. Trimble, a company traditionally focused on GPS, owns SketchUp, an extremely popular 3D modeling and design platform. It also partners with Belgian 3D-printing company Materialise on initiatives to streamline 3D-printing workflows. (Materialise -- a 3D-printing pure play that makes 3D-printing software and provides 3D-printing services -- is conspicuously missing from the ETF.)
Takeaway
An ideal 3D-printing ETF, in my opinion, would be more heavily weighted toward 3D-printing pure plays. That said, The 3D Printing ETF does a decent job representing the quite expansive 3D-printing realm. It seems a solid option for investors who want broad exposure to 3D printing -- a technology that is widely expected to revolutionize the manufacturing sector. As previously mentioned, the ETF's expense ratio is 0.66%, which is fairly reasonable.
<<<
>>> Autodesk, Inc. operates as a design software and services company worldwide. The company offers AutoCAD, a professional design, drafting, detailing, and visualization software; AutoCAD Civil 3D, a surveying, design, analysis, and documentation solution for civil engineering, including land development, transportation, and environmental projects; AutoCAD LT, a professional drafting and detailing software; BIM 360, a construction management cloud-based software; computer-aided manufacturing (CAM) software for computer numeric control machining, inspection, and modelling for manufacturing; Fusion 360, a 3D CAD, CAM, and computer-aided engineering tool; and Industry Collections software products for professionals in architecture, engineering and construction, product design and manufacturing, and media and entertainment industries. It also provides Inventor tools for 3D mechanical design, simulation, analysis, tooling, visualization, and documentation; Maya and 3ds Max software products that offer 3D modeling, animation, effects, rendering, and compositing solutions; and PlanGrid, a cloud-based field collaboration software, which provides general contractors, subcontractors, owners, and architects access to construction information in real-time. In addition, the company offers Revit software for building information modeling; and Shotgun, a cloud-based software for review and production tracking in the media and entertainment industry. Autodesk, Inc. sells its products and services to customers directly, as well as through distributors and resellers. The company was founded in 1982 and is headquartered in San Rafael, California.
<<<
Autodesk - >>> 2 Tech Stocks That Have Defied the Downturn
Barrons
By Jon Swartz
Nov. 23, 2018
While FAANG shares were declawed this earnings season, a pair of tech companies with rich histories escaped the wreckage.
The third-quarter earnings of Autodesk (ADSK) and Citrix Systems (CTXS) offer parallel narratives of reinvention, helmed by dynamic new CEOs who have led successful turnarounds by pursuing new markets that have led to profitability and significant bumps in share prices.
Look no further than their stocks this year: Autodesk is up 28% and Citrix 21%, while the S&P 500 is down 0.5%.
Autodesk shares spiked 9% in extended trading Tuesday after the company reported earnings of 29 cents a share on revenue of $660.9 million, compared with a loss of 12 cents on revenue of $515.3 million a year ago.
The results and fourth-quarter guidance—42 cents a share on sales of $705 million, at the midpoint—beat Wall Street forecasts. Autodesk also announced the intention of its largest acquisition, $875 million, for PlanGrid, a maker of construction-productivity software.
“It’s a story about the evolution of Autodesk, the promise of the cloud, and how any company of importance needs to have a software-as-a-service element,” Autodesk CEO Andrew Anagnost told Barron’s in a phone interview.
His plan, to pivot Autodesk from Oscar- and Emmy-winning computer-aided-design software maker to a cloud and software-as-a-service subscription model for “people who make things” such as buildings, roads, movies, games, and bridges, has led to a record $2 billion in fiscal 2018 revenue and guidance for $2.5 billion in its current fiscal 2019. Its stock has nearly tripled to $135.04 per share from $47 in September 2015, giving it a current market value of $29.5 billion. (It was up 9.7% on Wednesday alone.)
Anagnost did not make changes blindly: Before becoming Autodesk CEO in June 2017, the former rocket scientist worked in product and marketing for 20 years at the 36-year-old company, based in San Francisco.
Anagnost and Chief Financial Officer Scott Herren wanted to flip Autodesk’s reliance on software licenses, which accounted for 60% of its total revenue as recently as 2013, to a subscription model, while at the same time implementing a cloud-computing platform to make it easier for contractors, architects, and engineers to collaborate on building projects. Today, 96% of Autodesk’s revenue is via product subscriptions.
The story is eerily similar at Herren’s previous employer, Citrix.
Another long-time employee took over the reins as CEO about a year ago, and devised a plan to transform the virtual-private-network company to a hybrid-cloud solution one while also shifting to a subscription model.
CEO David Henshall, a 15-year company veteran who previously was chief financial officer and chief operating officer, told Barron’s that Citrix is “well ahead” of its plan to double revenue by 2020.
One of America’s Richest Marijuana Companies Has Deep Russian Roots
The 29-year-old company’s third-quarter results last month marked its fifth consecutive quarter of beating Wall Street expectations. Citrix recorded earnings of $1.40 per share and revenue of $732.5 million. Subscription-based revenue jumped 37%, year-over-year, to $112 million, and now accounts for 15% of total revenue, versus 10% a year ago.
The results pushed shares of the company up 2% the day after it announced results. In the past 12 months, Citrix has gained more than $2.5 billion in market value, to its current $14.4 billion. Shares closed up 0.7% on Wednesday, to $106.89.
The secret to its success? “We want to help customers face the future of a mobile work force,” Henshall told Barron’s, noting a recent McKinsey Group study that predicts a shortage of 90 million to 95 million medium-to-high-skilled workers globally by 2020. “Companies want to embrace the cloud, but it takes a while to manage the past while embracing the future.”
<<<
>>> Autodesk stock slides 18% after mixed quarter, but analysts are unfazed
By Emily Bary
Nov 30, 2017
https://www.marketwatch.com/story/autodesk-stocks-slides-18-after-mixed-quarter-but-analysts-are-unfazed-2017-11-29?siteid=bigcharts&dist=bigcharts
Software maker surprised investors with news of plan to cut more than 1,000 jobs
Autodesk Inc.’s stock tumbled 18% in Wednesday trading, after the company posted mixed results for its fiscal third quarter and announced that it was cutting more than 1,000 jobs as part of a surprise restructuring plan.
The software maker ADSK, -0.12% reported a net loss of $119.8 million for the quarter, on revenue of $515.3 million, which came in above analysts’ estimates for $116.4 million and $513.6 million respectively. But Autodesk only added 146,000 net new subscribers during the period, below expectations for 147,000, and slightly lowered its full-year forecast for that metric.
Analysts, who were a bullish bunch heading into the earnings report, for the most part weren’t troubled by the subscriber miss or restructuring announcement.
“In our opinion, the reason for the weaker-than-expected subscriber and billing metrics was the emergence of larger enterprise customers,” wrote analysts at William Blair, who have an outperform rating on Autodesk shares.
“These customers are skewing the company’s business model toward fewer new subscribers and larger deals extended over multiple years, resulting in increasing unbilled deferred revenue ($148 million in the third quarter versus $63 million in the prior quarter).”
Analysts at Morgan Stanley also urged investors not to read so much into the subscriber miss, since the company is on a path to nabbing more “higher value” customers. The group there said Wall Street may be trying to compare Autodesk’s transition to a subscription model to a similar one made by Adobe Systems Inc., when in fact Autodesk’s is more complicated.
“The Autodesk transition story was never going to be as straightforward as Adobe’s, where subscribers and the average revenue per subscriber told most of the story,” wrote the Morgan Stanley analysts, who have an overweight rating and a $143 price target on the stock.
“The complexity of Autodesk’s model, with multiple types of subscriptions, a much broader set of products, and more complex set of promotions and bundling became readily apparent in Q3 when net subscription adds fell short of consensus expectations, but the Autodesk management team still proclaimed a solid quarter,” they wrote.
Morgan Stanley thinks that the job reductions could help the company yield almost $6 a share in free cash flow for the 2020 fiscal year, the company’s target.
Autodesk has 18 buy ratings, four hold ratings, and two sell ratings from analysts polled by FactSet. Despite Wednesday’s drop, shares have still gained 46% this year, compared with a 17% rise for the S&P 500 SPX, +0.61% in that time.
<<<
>>> Autodesk, Inc. is a design software and services company, offering customers productive business solutions through technology products and services. The Company's segments include Architecture, Engineering and Construction (AEC), Platform Solutions and Emerging Business (PSEB), Manufacturing (MFG), and Media and Entertainment (M&E). The Company serves customers in the architecture, engineering and construction; product design and manufacturing; and digital media and entertainment industries. The Company's product development and manufacturing software provides manufacturers in automotive, transportation, industrial machinery, consumer products and building products with digital engineering solutions. The Company's product offerings include, AutoCAD, AutoCAD LT, Industry Collections, 3ds Max, Maya, Revit, Inventor, AutoCAD Civil three dimensional (3D), CAM Solutions, Fusion 360, BIM 360 and Shotgun. <<<
>>> New 3D printed “ovaries” enabled mice to give birth to live young
Popular Science
Claire Maldarelli
http://www.msn.com/en-us/health/medical/new-3d-printed-%E2%80%9Covaries%E2%80%9D-enabled-mice-to-give-birth-to-live-young/ar-BBBd3nY?OCID=ansmsnnews11
The ultimate goal of 3D printed organs is to be able to churn one out on-demand for any person in need. And we’ve gotten pretty close. Researchers have been growing organs in a lab for the past few decades—and have even successfully transplanted a select few into humans. But it hasn’t yet become routine. This week, in a paper out in the journal Nature Communications, researchers have figured a new technique to grow 3D printed “ovaries”. Mice with these implants were able to give birth to live pups. The researchers say with more work, this technique, or one similar to it, could be used to help women with infertility.
The 3D printed ovaries aren’t exactly what you might be picturing. The technique the researchers used works like this: First they 3D printed a biodegradable scaffold, which holds the cells in and eventually breaks down inside the body. They then placed the mouse’s follicle cells—egg-producing cells that are naturally found in the ovaries—into the scaffold, and then surgically placed that scaffold into the mouse. The new follicle cells allowed the female mouse to ovulate, and eventually give birth to a litter of live young.
That’s all incredibly cool, but this technique is not entirely new. Previous studies have already had success implanting follicle cells into mice to get them to ovulate, and this isn't the first time researchers have reported live pups as a result. But while previous studies have encapsulated follicle cells in a gel-like substance to hold them in place, the new technique relies on 3D printed scaffolding instead. Once inside the scaffolding, the cells are more stable and controlled, so the researchers wanted to see if this would help the follicle cells survive better once inside the animal.
What they found, says Ramille Shah, a materials science researcher at Northwestern University and a co-author of the study, is that the more sophisticated structure of the scaffolding architecture allowed for a more stable base for the cells. And this stability paid off. The follicle cells survived much better when inside the 3D scaffolding than during previous attempts.
This is all good news for the future of in vitro fertilization techniques in humans. But it doesn’t mean we are there yet. Because this was the first study of this kind, researchers will need to perform many more studies like it before it can be safely tried in humans. But it does represent a step forward. And a needed one at that. The researchers say they hope the technique can help survivors of childhood cancers whose treatments have damaged their ovaries, making them infertile. In the future, they'll need to determine how long the 3D printed ovaries remain alive inside the body and whether or not they can keep functioning for a lifetime, which is the ultimate goal, Shah says. For now, though, this study is an important stepping stone for others in the field. If this new architectural scaffolding is indeed the most successful, then future work can focus on how to improve it, and make it even more effective
<<<
>>> Fast Bioprinting of Human Cartilage Implants
March 25, 2015
https://3dprinting.com/news/fast-bioprinting-of-human-cartilage-implants/
At ETH Zürich’s Cartilage Engineering and Regeneration laboratory they have made some notable speed advancements in 3D printing of human cartilage which should lead to implantable replacements for trauma victims. Team of researchers led by Professor Marcy Zenobi-Wong’s and Matti Kesti developed a process that would enable hospitals to make a full size nose implant under 20 minutes. Any cartilage implant could be produced with nose, ear and knee implants being the ones most used in surgeries while significantly reducing the need for transplant donors.
In ETH Zürich news article, Kesti describes how this technology may revolutionise reconstructive surgery in the future:
“A serious car accident results in a passenger’s nose being shattered. It is possible to reconstruct this as a 3D model on the computer. At the same time, a biopsy is performed on the patient and cartilage cells removed from his or her own body, for example from the knee, finger, ear or splinters of the shattered nose. The cells are spawned in the laboratory and mixed with a biopolymer. From this toothpaste-like suspension, a nose cartilage transplant is created using the bioprinter, which is implanted in the patient during surgery. In this process, the biopolymer is used merely as a form of shaping mould; it is subsequently broken down by the body’s own cartilage cells. After a couple of months, it is impossible to distinguish between the transplant and the body’s own nose cartilage. This procedure has significant benefits compared to traditional implants, for instance those made from silicone: the risk of the body rejecting the implant is a lot lower. A particularly crucial factor for young patients is that the cellular implant grows together with the patient, because it is controlled by the patient’s internal growth engine, as is the case for other body parts.
Nose cartilage implant 3d printed on a bioprinter tray from human cells and hydrogel (Picture credits: Cartilage Engineering and Regeneration Group)
Here is a RuptlyTV report:
Their 3D printer is not just a simple three axis system with the single syringe attached but a very complex bioprinting medical device. They use “Bio Factory” made by RegenHu from Villaz St-Pierre, Switzerland, which is equipped with three printheads for normal viscosities, one for high viscosities and a UV lamp. The system is also equipped with a 355 nm UV-Laser for photopolymerization.
You can see more details about BioFactory in this video:
If you want to learn more about 3D printing of cartilage tissues and medical applications you can watch this 20 minute presentation from Dr. Jos Malda who talks about it at the ICRS Focus Meeting 2014 at the FIFA Auditorim in Zürich.
Cartilage. Learn to appreciate it!
With pre-clinical animal trials planned for the near future this medical 3D printing technology will hopefully help many victims of heavy injuries.
Source: https://www.ethz.ch/en/news-and-events/eth-news/news/2015/03/nose-made-by-bioprinters.html
<<<
>>> Scientists hit milestone in 3D printing human cartilage
5-5-17
CBS News
http://www.msn.com/en-us/health/healthtrending/scientists-hit-milestone-in-3d-printing-human-cartilage/ar-BBAMoEK?OCID=ansmsnnews11
The story of 3D printing in medical care is quickly moving from abstract hope to concrete progress, as Swedish researchers announced that they have successfully 3D-printed stem cells that multiplied and differentiated into a material very similar to human cartilage tissue.
The stem cells survived the printing process, which researchers are celebrating as a breakthrough.
The research, a collaboration between scientists from Belgium's Sahlgrenska Academy and Chalmers University of Technology, is published in the latest issue of the journal Scientific Reports.
The researchers designed a new process for producing cartilage. They started with cartilage cells taken from real patients who underwent knee surgery. Those cells were manipulated to revert to stem cells, which were then encapsulated in cellulose material and printed using a 3D bioprinter. When they survived the printing process, the stem cells were treated with growth stimulants, which prompted them to multiply and differentiate — transforming into cartilage.
"In nature, the differentiation of stem cells into cartilage is a simple process, but it's much more complicated to accomplish in a test tube. We're the first to succeed with it, and we did so without any animal testing whatsoever," lead researcher Stina Simonsson said in a statement.
3D bioprinting is an area of explosive interest in medical resesarch. In 2013, Cornell University scientists used a 3D printer to grow human ears from cow cells, a step towards one day growing custom ears for individuals with ear defects. That same year, Princeton University researchers claimed that some of the 3D printed ears they produced could hear at frequencies that are far out of range for the normal human ear.
3D printing is also making waves in facial reconstructive surgery, burn treatment, and more.
The latest research points toward a future in which scientists can generate cartilage based off a patient's own stem cells, and use that cartilage in the patient's body. Researchers say that the cartilage produced in their procedure was so similar to human cartilage that experienced surgeons could not tell the difference.
But researchers warned of one roadblock they still need to overcome: the kind of cellulose they used in the printing process might not be well-suited for the human body. They said they hope to explore another material that can be broken down and absorbed by the body, safely leaving only the endogenous cartilage behind. They also noted that this method requires large amounts of live stem cells.
<<<
>>> Organovo Holdings Inc.'s 36% Ascent in 2016 Can Be Attributed to These 3 Factors
Things have finally begun to gel for Organovo, but investors shouldn't get too excited just yet.
Sean Williams
Jan 6, 2017
http://www.fool.com/investing/2017/01/06/organovo-holdings-incs-36-ascent-in-2016-can-be-at.aspx?source=yahoo-2&utm_campaign=article&utm_medium=feed&utm_source=yahoo-2&yptr=yahoo
What happened
Shares of Organovo Holdings (NASDAQ:ONVO), a life sciences company primarily focused on the development of bioengineered human tissues for the pharmaceutical and research industries, surged 36% in 2016 according to data from S&P Global Market Intelligence. The reason for Organovo's standout year can be traced to three factors.
So what
As outlined by my Foolish colleague Keith Speights, the biggest catalyst for Organovo in 2016 was the commercial launch of its ExVive human kidney tissue assay in September. Organovo's human kidney tissue product joins its ExVive human liver tissue assay as its only marketed products. These assays can be quite handy to drug developers since they can help determine tissue toxicities without having to run costly, time-consuming preclinical and/or phase 1 trials. Organovo's management team believes its kidney assay could bring in $100 million annually in peak sales, if not more.
Secondly, Organovo's top and bottom lines are finally beginning to show improvement with sales of its liver assay maturing and its kidney assay kicking off. For instance, after reporting $1.5 million in fiscal 2016 sales, the company now expects between $4.5 million and $6.2 million in full-year revenue in fiscal 2017. With a net cash utilization of between $31 million and $34 million, Organovo has about two years of cash runway left before it may need to seek additional financing. By then, sales of its core products may have roared even higher.
Finally, Organovo has been a busy bee when it comes to collaborations and agreements. In addition to recognizing revenue from its collaboration with Merck, announced in 2015, Organovo announced collaborations with the University of California, San Francisco to develop 3D bio-printed tissues for skeletal disease research , and in December signed a distributor agreement with Cosmo Bio in Japan for its NovoView Preclinical Services. The point of these collaborations and agreements is to get its name out into the mainstream, and to that end Organovo appears to be doing a good job.
Now what
The big question, though, is whether Organovo can take its Star Trek-like technology and apply it to the business world to make money. Even though we're seeing a nice surge in revenue and a slight decline in net operating losses, Organovo isn't anywhere near being profitable on a recurring basis. In fact, Wall Street's prediction of $31 million in sales by 2019 probably won't be enough to put Organovo into the black. This means Organovo is going to need more cash at some point in the future, which probably means more share offerings that dilute existing investors.
On the other hand, Organovo has technology with the capability to really save drug developers money. What's unanswered at this point is whether it'll catch on. While I'd certainly like it to from the standpoint that it quickens the drug-development process and makes it safer, my brain also tells me that we're very early in the game and Organovo has a lot to prove before it's investment-worthy. Until we see additional drug developers latch onto Organovo's assays, or we see a substantive decline in net operating losses, my suggestion would be to monitor Organovo stock safely from the sidelines.
<<<
>>> Organovo on Track to Commercialize Kidney Tissue; Company Signs First Customer Orders for Kidney Tissue Early Access Program
July 20, 2016
http://finance.yahoo.com/news/organovo-track-commercialize-kidney-tissue-120500277.html
SAN DIEGO, July 20, 2016 (GLOBE NEWSWIRE) -- Organovo Holdings, Inc. (ONVO) (“Organovo”), a three-dimensional biology company focused on delivering scientific and medical breakthroughs using its 3D bioprinting technology, today announced that it is on track to commercialize its second tissue, the kidney proximal tubule, in the third calendar quarter of 2016. As part of its pre-commercialization activities, Organovo introduced an early access program for preferred partners and has signed the first customer orders to study the effects of drug exposure on the kidney proximal tubule. The Company has also actively engaged with additional biopharmaceutical companies, indicating strong interest in this program and its kidney tissue.
“The power and versatility of our technology platform allows us to create multiple tissues that address a number of different customer needs,” said Paul Gallant, general manager of commercial operations, Organovo. “Kidney is a natural expansion of our preclinical product and service portfolio, providing a better solution than traditional in vitro models for drug research. Our initial customers are using the kidney model to study key aspects of kidney pharmacology, including compound transport, metabolism and toxicity.”
Gallant continued, “With a robust sales footprint in place, and our ability to leverage the strong relationships we’ve built with core customers in the liver toxicology business, we’re confident that we’ve positioned ourselves for early success with the kidney tissue. We look forward to bringing this groundbreaking product to market in the coming months.”
About Organovo Holdings, Inc.
Organovo designs and creates functional, three-dimensional human tissues for use in medical research and therapeutic applications. The Company develops 3D human tissue models through internal development and in collaboration with pharmaceutical, academic and other partners. Organovo's 3D human tissues have the potential to accelerate the drug discovery process, enabling treatments to be developed faster and at lower cost. The Company’s initial product is the exVive3DTM Human Liver Tissue for use in toxicology and other preclinical drug testing. Additional products are in development, with the anticipated release of the exVive3D Human Kidney Tissue scheduled for the third quarter of calendar year 2016. The Company also actively conducts early research on specific tissues for therapeutic use in direct surgical applications. In addition to numerous scientific publications, the Company's technology has been featured in The Wall Street Journal, Time Magazine, The Economist, Forbes, and numerous other media outlets. Organovo is changing the shape of life science research and transforming medical care. Learn more at www.organovo.com.
<<<
>>> Organovo and Roche Researchers Publish Data Demonstrating Superiority of 3D Bioprinted Human Liver Tissues in Assessing Drug-Induced Toxicity
http://finance.yahoo.com/news/organovo-roche-researchers-publish-data-120500326.html
SAN DIEGO, July 11, 2016 (GLOBE NEWSWIRE) -- Organovo Holdings, Inc. (NYSE MKT:ONVO) (“Organovo”), a three-dimensional biology company focused on delivering scientific and medical breakthroughs using its 3D bioprinting technology, today announced a publication in the scientific journal, PLOS One, which demonstrates the superiority of Organovo’s 3D bioprinted human liver tissues to effectively model drug-induced liver injury and distinguish between highly-related compounds with different toxicity profiles.
Using Organovo’s 3D bioprinted human liver tissues, researchers from Organovo and Roche Pharmaceutical Research and Early Development (“Roche”) were able to detect significant dose-dependent toxicity of trovafloxacin at clinically relevant doses, compared to levofloxacin, a structurally related, but non-toxic compound. The hepatotoxic potential of trovafloxacin was not originally identified by traditional preclinical tests including animal studies. Trovafloxacin is a third-generation anti-infective drug that was withdrawn from the market one year following its approval due to liver failure and death in a small proportion of patients. Deborah G. Nguyen, Ph.D., Senior Director of Research & Development, was Organovo’s lead author for the publication.
“This publication clearly shows the outstanding performance of Organovo’s 3D bioprinted human liver tissues when compared to traditional preclinical tests in replicating human drug response at the tissue level,” said Dr. Sharon Presnell, chief scientific officer, Organovo.
Drug-induced liver injury is a major cause of late-stage clinical failures and post-market drug withdrawals. Existing preclinical tests include conventional 2D cell culture models, which lack longevity and cannot replicate the complex cell-cell interactions and environment of human tissue. Animal models can miss compounds that are toxic in humans because of species variations and other factors.
“Organovo’s bioprinting technology creates an in vitro system comprising multiple liver cell types in a defined spatial architecture that can be used over time to gather both histopathological and biochemical data for preclinical toxicity testing,” explained Dr. Presnell. “Our durable and reproducible model can also be used to measure cell-type specific responses and investigate toxicity mechanisms to develop alternative solutions.”
Organovo’s 3D bioprinted human liver tissues are composed of patient-derived parenchymal (hepatocyte) and non-parenchymal (endothelial and hepatic stellate) cell populations. In addition, the Company’s 3D bioprinting technology creates tissues that are both spatially patterned and three-dimensional. This solution allowed researchers to perform histologic analyses in this study that revealed distinct intercellular hepatocyte junctions, CD31+ endothelial networks, and desmin-positive, smooth muscle actin-negative quiescent stellates. Unlike 2D cell cultures, Organovo’s tissues maintained metabolically relevant levels of ATP and albumin and drug-induced enzyme activity of cytochrome P450s for more than four weeks in culture.
Dr. Presnell concluded, “This data set clearly supports the use of Organovo’s 3D bioprinted human liver tissues in preclinical testing for drug-induced liver toxicity to potentially reduce the risk of toxic drugs reaching patients and avoid costly late-stage clinical failures.”
The publication entitled, “Bioprinted 3D primary liver tissues allow assessment of organ-level response to clinical drug induced toxicity in vitro,” was published online on July 7 and can be found here: http://journals.plos.org/plosone/article?id=10.1371/journal.pone.0158674
About Organovo Holdings, Inc.
Organovo designs and creates functional, three-dimensional human tissues for use in medical research and therapeutic applications. The Company develops 3D human disease models through internal development and in collaboration with pharmaceutical and academic partners. Organovo's 3D human tissues have the potential to accelerate the drug discovery process, enabling treatments to be developed faster and at lower cost. The Company recently launched its initial product of the planned exVive3D portfolio offering, the exVive3D Human Liver Tissue for use in toxicology and other preclinical drug testing. Additional products are in development, with the anticipated release of the exVive3D Human Kidney Tissue scheduled for the third quarter of calendar year 2016. The Company also actively conducts early research on specific tissues for therapeutic use in direct surgical applications. In addition to numerous scientific publications, the Company's technology has been featured in The Wall Street Journal, Time Magazine, The Economist, Forbes, and numerous other media outlets. Organovo is changing the shape of medical research and practice. Learn more at www.organovo.com.
<<<
n3D Biosciences -- >>> Organovo Isn't Worried About the Competition, But Should It Be?
Smaller rivals like n3D might not be a big threat to Organovo now, but things can change quickly in a dynamic market.
by Keith Speights
Jun 27, 2016
http://www.fool.com/investing/2016/06/27/organovo-isnt-worried-about-the-competition-but-sh.aspx?source=yahoo-2&utm_campaign=article&utm_medium=feed&utm_source=yahoo-2
What's the first company you think of when you hear the phrase "3D bioprinting"? For many investors, the answer is Organovo Holdings (NYSEMKT:ONVO). One good reason Organovo's name would be at the tip of your tongue is that it's the only publicly traded company focusing on 3D bioprinting of human tissues. That doesn't mean Organovo's the only player in the up-and-coming technology, though.
No worries?
At Organovo's last earnings conference call, CEO Keith Murphy was asked point-blank about his company's competition. An analyst asked Murphy, "If I was president of Merck or one of the big pharmaceutical companies -- why would I choose your company? Are there competitors that have price advantage, or is Organovo's technology such that -- it's very superior to other technology but it tends to do the same thing?"
Murphy replied that Organovo's 3D-bioprinted human liver tissue primarily competes against animal tissues and 2D cell cultures. But what about other 3D bioprinting companies? Murphy said: "There is some use of other technologies that have been around since around 2007, 2008, that people do turn to sometimes, but those are low-single-digit percentages of the market and not major a driver for us in terms of competing with them." (Quotes from the earnings call transcript, by the way, come from S&P Global Market Intelligence.)
As for the analyst's hypothetical question about being Merck's president, the truth is that Merck has already chosen Organovo. The big drugmaker signed a deal last year with Organovo to focus on toxicology and custom tissue disease modeling. Organovo also counts four other of the top 25 pharmaceutical companies as customers.
Organovo doesn't appeared to be too worried about 3D bioprinting competition. I wonder, though, if perhaps it should be looking over its shoulders at least a bit.
Another perspective
I recently spoke with Glauco Souza, the CEO of privately held n3D Biosciences. Like Organovo, n3D focuses on creating 3D cell cultures. Also like Organovo, n3D generated six-figure revenue last quarter, according to Souza. He added that his company's cash burn rate is "much lower" than Organovo's.
Organovo's partnerships with major players like Merck and cosmetics maker L'Oreal have generated buzz for its 3D bioprinting technology. Partnering is also important for n3D Biosciences. Souza pointed to his company's recently formed alliance with Greiner Bio-One, a global medical-technology company.
Souza said that n3D is talking with the same prospects that Organovo targets but didn't name any specific companies. He echoed some of Murphy's statements about the challenges of winning new business with a relatively new technology. In particular, Souza noted that it's tough to gain accounts that have used animal tissues.
In his earnings call remarks, Murphy mentioned that a key step is to "educate the market." Souza had a similar take, telling me that some scientists with prospective customers can be skeptical at first. He said published works about the advantages of 3D tissues help bolster n3D's case. That's right in line with Murphy's statement that publications are "a big driver" that helps with sales efforts.
While both Organovo and n3D Biosciences face the hurdle of educating customers to build market awareness, that should become a lesser challenge over time. As more customers embrace 3D tissues, the question will turn more toward what the analyst asked Murphy: "Why would I choose your company?"
For Souza, probably the most important answer to that question is throughput, or the rate of processing. He said the biggest competitive advantage for n3D's technology is high throughput. Organovo uses a layer-by-layer method in its 3D bioprinting process. N3D instead magnetizes cells and shapes the cells using the magnetic forces, which Souza maintains is a much quicker approach than the one Organovo uses.
Souza acknowledges that n3D and Organovo can compete head-to-head in some cases. However, he also said he sees opportunities for customers to benefit from both companies' approaches -- Organovo's lower throughput and n3D's high throughput.
Looking ahead -- and behind
If you listen to Murphy for a while, you'll hear him talk about the tremendous potential for 3D bioprinting. Souza believes the future is bright for the technology also. While the primary focus now is on toxicity testing and drug research, he pointed out the opportunity for significant advances in precision medicine and regenerative medicine using 3D cells. If Murphy and Souza are right, the 3D bioprinting market should be large enough to support several players.
In the meantime, it's obvious that Souza and n3D are watching Organovo closely. Souza admitted that he's "intrigued with Organovo." It's uncertain, though, to what extent Murphy and Organovo are keeping an eye on n3D and other smaller rivals. As Murphy said, those competitors have only a small percentage of the market right now.
But while Organovo probably doesn't have much to worry about today, underestimating the competition has proved to be a mistake for other companies in other industries. Looking ahead to the future is great. However, looking behind to make sure no one is sneaking up on you isn't a bad idea, either
<<<
>>> Organovo Isn't Worried About the Competition, But Should It Be?
Smaller rivals like n3D might not be a big threat to Organovo now, but things can change quickly in a dynamic market.
by Keith Speights
Jun 27, 2016
http://www.fool.com/investing/2016/06/27/organovo-isnt-worried-about-the-competition-but-sh.aspx?source=yahoo-2&utm_campaign=article&utm_medium=feed&utm_source=yahoo-2
What's the first company you think of when you hear the phrase "3D bioprinting"? For many investors, the answer is Organovo Holdings (NYSEMKT:ONVO). One good reason Organovo's name would be at the tip of your tongue is that it's the only publicly traded company focusing on 3D bioprinting of human tissues. That doesn't mean Organovo's the only player in the up-and-coming technology, though.
No worries?
At Organovo's last earnings conference call, CEO Keith Murphy was asked point-blank about his company's competition. An analyst asked Murphy, "If I was president of Merck or one of the big pharmaceutical companies -- why would I choose your company? Are there competitors that have price advantage, or is Organovo's technology such that -- it's very superior to other technology but it tends to do the same thing?"
Murphy replied that Organovo's 3D-bioprinted human liver tissue primarily competes against animal tissues and 2D cell cultures. But what about other 3D bioprinting companies? Murphy said: "There is some use of other technologies that have been around since around 2007, 2008, that people do turn to sometimes, but those are low-single-digit percentages of the market and not major a driver for us in terms of competing with them." (Quotes from the earnings call transcript, by the way, come from S&P Global Market Intelligence.)
As for the analyst's hypothetical question about being Merck's president, the truth is that Merck has already chosen Organovo. The big drugmaker signed a deal last year with Organovo to focus on toxicology and custom tissue disease modeling. Organovo also counts four other of the top 25 pharmaceutical companies as customers.
Organovo doesn't appeared to be too worried about 3D bioprinting competition. I wonder, though, if perhaps it should be looking over its shoulders at least a bit.
Another perspective
I recently spoke with Glauco Souza, the CEO of privately held n3D Biosciences. Like Organovo, n3D focuses on creating 3D cell cultures. Also like Organovo, n3D generated six-figure revenue last quarter, according to Souza. He added that his company's cash burn rate is "much lower" than Organovo's.
Organovo's partnerships with major players like Merck and cosmetics maker L'Oreal have generated buzz for its 3D bioprinting technology. Partnering is also important for n3D Biosciences. Souza pointed to his company's recently formed alliance with Greiner Bio-One, a global medical-technology company.
Souza said that n3D is talking with the same prospects that Organovo targets but didn't name any specific companies. He echoed some of Murphy's statements about the challenges of winning new business with a relatively new technology. In particular, Souza noted that it's tough to gain accounts that have used animal tissues.
In his earnings call remarks, Murphy mentioned that a key step is to "educate the market." Souza had a similar take, telling me that some scientists with prospective customers can be skeptical at first. He said published works about the advantages of 3D tissues help bolster n3D's case. That's right in line with Murphy's statement that publications are "a big driver" that helps with sales efforts.
While both Organovo and n3D Biosciences face the hurdle of educating customers to build market awareness, that should become a lesser challenge over time. As more customers embrace 3D tissues, the question will turn more toward what the analyst asked Murphy: "Why would I choose your company?"
For Souza, probably the most important answer to that question is throughput, or the rate of processing. He said the biggest competitive advantage for n3D's technology is high throughput. Organovo uses a layer-by-layer method in its 3D bioprinting process. N3D instead magnetizes cells and shapes the cells using the magnetic forces, which Souza maintains is a much quicker approach than the one Organovo uses.
Souza acknowledges that n3D and Organovo can compete head-to-head in some cases. However, he also said he sees opportunities for customers to benefit from both companies' approaches -- Organovo's lower throughput and n3D's high throughput.
Looking ahead -- and behind
If you listen to Murphy for a while, you'll hear him talk about the tremendous potential for 3D bioprinting. Souza believes the future is bright for the technology also. While the primary focus now is on toxicity testing and drug research, he pointed out the opportunity for significant advances in precision medicine and regenerative medicine using 3D cells. If Murphy and Souza are right, the 3D bioprinting market should be large enough to support several players.
In the meantime, it's obvious that Souza and n3D are watching Organovo closely. Souza admitted that he's "intrigued with Organovo." It's uncertain, though, to what extent Murphy and Organovo are keeping an eye on n3D and other smaller rivals. As Murphy said, those competitors have only a small percentage of the market right now.
But while Organovo probably doesn't have much to worry about today, underestimating the competition has proved to be a mistake for other companies in other industries. Looking ahead to the future is great. However, looking behind to make sure no one is sneaking up on you isn't a bad idea, either
<<<
Organovo -- >>> These Companies Prove that Reverse Splits Can Be a Very Good Thing for Stakeholders
June 14, 2016
Contributor: Sam Wentworth
http://news.investornetwork.com/2016/06/14/these-companies-prove-that-reverse-splits-can-be-a-very-good-thing-for-stakeholders/?1=1&1465936593
The Wealthy Biotech Trader (or “WBT”), an investment newsletter focused on showing everyday investors new opportunities in rapidly growing, little-known, biotech, pharma and medical device stocks making news and subsequent market moves, would like to create awareness among investors on why reverse splits aren’t always a bad thing.
Companies included: Advanced Medical Isotope (ADMD); Staffing 360 Solutions (STAF); Organovo Holdings (ONVO); Innovio Pharmaceuticals (INO); Annavex Life Sciences (AVXL)
Few things can get investors’ attention faster than when a company announces a reverse split. This is due to the fact that reverse splits are often associated with penny stocks which most investors assume lack a path to profitability and only want to prop up their share price in order to float more shares. The other scenario could be that a company is desperately trying to maintain its listing in the major exchanges such as NYSE or NASDAQ since these require the stock prices to be maintained at certain levels.
However, many cases of reverse splits should not be viewed as a negative event since they give an opportunity for small over the counter (OTC) stocks to join the ranks of peers at the major exchanges, increasing exposure and liquidity. This essentially means that such stocks will be able to garner much needed attention from retail brokers and institutional investors as well as increasing their access to capital. In the recent past, there have been a couple of reverse splits that have gone on to be quite successful as we will highlight in this article and we believe that we have found another potential winner in Advanced Medical Isotopes (OTC: ADMD).
AMI, a late stage development company engaged primarily in the development of brachytherapy devices and medical isotopes for diagnostic and therapeutic applications with a focus on yttrium-90 brachytherapy treatments has revealed plans to effect a reverse stock split before the end of October. Management further explains that this course of action has been taken as the company plans to list on one of the major exchanges following the highly encouraging results from its product pipeline.
The market for brachytherapy products has been steadily expanding with recent research predicting its growth from $680 million in 2013 to $2.4 billion by 2030. Being one of the few companies developing brachytherapy products, Advanced Medical Isotopes couldn’t be in a better position to make the most of this opportunity.
CEO James Katzaroff has pointed out that the simplest way to describe the company’s lead product, Y-90 RadioGel, is that it’s injected into inoperable tumours and then hardens, effectively stopping the spread of cancer, with the radioactivity effectively killing the cancer cells, while focusing the therapeutic dose to the affected area. He believes that with a successful up-listing, Advanced Medical Isotope will be able to advance its clinical programs, as the company seeks to raise $5-$10 million in the next two years.
Another important aspect that investors should be aware of is the company’s strong commitment to reducing debt and increasing shareholders’ value. AMI reduced total liabilities from $20.2 million in 2014 to about $9.9 million in 2015. For investors with a high risk tolerance, the opportunity to own this stock now before it up-lists may mean the outstanding possibility for above market returns.
Previous reverse stock split winners
Innovio Pharmaceuticals (NASDAQ: INO) announced a 1-4 reverse split almost two years ago and has been on a winning streak ever since up-listing to the NASDAQ later in the same year. The company is taking immunotherapy to the next level in the fight against cancer and infectious diseases being the only immunotherapy company that has reported generating T cells in vivo in high quantity that are fully functional and whose killing capacity correlates with relevant clinical outcomes with a favourable safety profile.
President and CEO, Dr Joseph Kim pointed out that the move to list on the exchange would be instrumental for investors in terms of increasing the stocks liquidity and visibility at a time when the company had made some major breakthroughs in its product pipeline. Since the split, the company has gained 17 percent, grown its product portfolio and grow to a $775 million valuation.
Staffing 360 Solutions (NASDAQ: STAF) is a public company in the staffing sector engaged in the execution of a global buy-and-build strategy through the acquisition of domestic and international staffing organizations with operations in the US and UK. On September last year, the company effected a 1-10 reverse stock split which upon completion would see the stock uplist and trade on the NASDAQ exchange. Since then, the company has had an impressive performance with a number of analysts initiating positive coverage on the stock.
The company has managed to sustain its momentum as it recently reached the halfway mark of its goal of achieving $300 million in annualized revenue by the end of FY16Q3. Revenue rose by 42 percent for the most recent quarter compared to the year ago period also managed to generate $2.2 million in operating cash flow during the first three quarters of the current fiscal year. It is in light of this solid growth that analysts at See ThruEquity gave the stock a price target of $5.65 which translates to upside potential of more than 150 percent from the current share price.
Organovo Holdings (NYSE MKT: ONVO) has surged 12.9 percent on an YTD basis and is another great example of what an OTC stock can achieve after successfully up-listing to a higher exchange. The company is a commercial stage company focused on developing and commercializing functional human tissues that can be employed in drug discovery and development, biological research, and as therapeutic implants for the treatment of damaged or degenerating tissues and organs.
The company’s flagship commercial product is the exVive 3D Human Liver Assays and although management has decided not to reveal information on its sales until later this year, there is no doubt that the potential market is massive. Also, Organovo won’t be banking on this product alone as it plans to develop a kidney which will command higher prices compared to the liver. With the increased visibility of a national listing, the company will be able to raise capital to fund subsequent trials at more favourable terms.
Annavex Life Sciences (NASDAQ: AVXL) is among the more recent companies that have had to do a reverse stock split. The company effected a 1-4 reverse split back in October last year and also uplisted to NASDAQ. Although the clinical-stage biopharmaceutical company developing drug candidates to treat Alzheimer’s disease, other central nervous system (CNS) diseases, pain, and various types of cancer has not been able to get back to its post IPO highs of almost $13 per share, the stock still has plenty of room for growth.
Just last week, the company disclosed that the FDA had granted Orphan Drug Designation to its ANAVEX 2-73 for the treatment of Rett syndrome a clear signal of more upside potential in the stock. Rett syndrome is a devastating disease occurring in early childhood and almost exclusively in girls and since there is currently no cure, ANAVEX stands to become the standard of care in the near term.
This report/release/profile is a commercial advertisement and is for general information purposes only.
<<<
>>> Organovo CEO Keith Murphy: 'People Are Missing The Forest For The Trees'
Benzinga
By Jim Probasco
September 1, 2015
http://finance.yahoo.com/news/organovo-ceo-keith-murphy-people-184235122.html
“I think people are missing the forest for the trees,” Organovo Holdings Inc (NYSE: ONVO) CEO Keith Murphy told Benzinga.
Murphy’s remarks were in reference to Cantor Fitzgerald’s downgrade of Organovo from Buy to Hold following the company’s reported $8.5 million Q1 loss in August.
The conversation with Murphy revealed a CEO anxious to share what he sees as a call for patience on the part of those watching the company, along with enthusiasm for a future he sees as bright.
Related Link: EXCLUSIVE: Organovo CEO Keith Murphy Talks Pipeline, Market Misconceptions
BENZINGA: In regard to Cantor Fitzgerald's downgrade, do Wall Street rankings and opinions have an impact on you and on Organovo?
Keith Murphy: Clearly, we want to maximize our investors' opportunity over time, so these things are important to us.
What I would say is that I think people are missing the forest for the trees here a little bit. We reported about $300K in revenue for the first quarter, and we're seeing some reaction to that, I would say.
The bigger picture is, last quarter we had $2 million in booked contracts. Analyst and shareholder reaction was positive. The fact that these (contracts) take a couple of quarters to be recognized as revenue shouldn't surprise anyone.
I think what people have lost sight of is that since we launched in November, we've been giving guidance that it's about a four- to six-month timeline to get to a contract and then it's a four- to five-month timeline to book the revenue from that deal.
If you add those two time frames together, just use a 10-month number, the 10 months from last November will be September.
BZ: Is the problem that, in this type of industry (biotech), it's a lack of understanding or of forgetting that there is this timeline?
KM: Great question. I think it's very common and not unexpected that investors focus on the revenue line, and can be unforgiving. I think you see with many companies people go through a downturn with reaction to initial product launch.
When we decided to pursue this path, we said to ourselves, "Well, you're gonna get beaten up, because once you start to show revenues, you can't please people for a period of time."
BZ: How do you see the collaborations with Merck & Co., Inc. (NYSE: MRK), L’Oréal and the Yale School of Medicine helping overall?
KM: The Merck deal is one with multiple aspects to what we're doing with them.
One is they've become a customer for the liver tox business. They are actively engaged in contracts with us on using our xVive3D human liver tissue for detecting toxicity of their compounds.
The reason we mentioned that as part of our Merck contract was the fact that it's part of a larger contract. The balance is focused on the development of specific tissues for use in drug discovery.
This is a nice aspect of our platform and something we think will be growing over time. We can work with pharma companies to develop disease models that can be better than animal models for predicting efficacy of a drug.
BZ: What about L’Oréal?
KM: Let me back up a step. With Merck, our goal is building tissues together. Organovo's goal is to deliver tissue that is so promising for Merck that we're going to get into a next larger stage contract with them where they would actively screen compounds in it. We'd like to get royalty and milestone from compounds found with these tissues.
L’Oréal represents just such a next-stage contract. We actually did a first contract with L’Oréal in mid-2013 and delivered on that in mid-2014. What it represents is that kind of next stage where we get backend on what we deliver.
BZ: So, what is the next stage with L’Oréal?
KM: 3D skin prototypes. We need to turn that into a final 3D bio-printed skin models as a first stage of this next contract. The contract anticipates that there will be royalty and milestone for Organovo for things that are discovered and moved forward commercially from those.
L’Oréal is in the beauty industry, so they're looking at finding cosmetic agents that are active and determining the activity of those. The contract anticipates activity back to Organovo for this.
In addition, we can do multiple other things in terms of revenue streams. As L’Oréal does the screening, the contract anticipates (Organovo) to be the commercial supplier of the model.
Further, we can choose together to sell that more broadly, meaning work with other folks in the industry and offer them the tissues if we choose to do that.
Finally, once you build a 3D skin like this, we're talking about the use with cosmetics companies. Organovo has the right to go out and use it in the pharmaceutical industry as well.
BZ: What are you doing with Yale?
KM: That's an early stage research project. Yale is key to the longer-term opportunities here. We can't, today, make large tissues like full organs. To get to the stage where we can do that, we need to cross certain barriers, such as the ability to make a vascular structure inside of a tissue.
With federal research grant dollars, they (Yale) can do big projects with the Organovo bio-printing platform and have opportunities to solve some of these key issues. Then we can work together to leverage them into commercial tissues that would be helpful in the clinic.
BZ: What has you most excited? Is it whole, intact 3D printed organs? Is that the ultimate end game?
KM: It's AN ultimate end game, I would say. One of the things that's excited me most about Organovo is the fact that we can have a big impact very quickly with smaller tissues in an in vitro setting.
For example, with diseases like Alzheimer's, one of the big barriers has been unpredicted toxicology of (medications) that have gotten to Phase II and then had a surprise.
Having better tools to do drug discovery and development really does help patients in a shorter term than these larger tissues will help patients.
Of course, it's safe to say that everyone at Organovo is heavily motivated by that longer-term goal, as well. We certainly want to take bio-printing technology as far as it can go and move it toward those goals longer term.
Related Link: Bayer's Latest Stage 2 Trial Could Be A Boon For Shares
BZ: Looking forward, what other areas are underway?
KM: One big area for us is cancer. We've been building a breast cancer tumor model that we believe has a lot utility in discovering new cancer drugs.
That is also part of a collaboration. We are working with the Oregon Health and Sciences University to do that work.
A breast cancer tumor has a core of cancerous cells surrounded by fat and skin type cells. We can build that structure. It's almost structured like the earth, if you think about it, with a core of molten lava and the crust on the outside.
We can produce that structure and we are looking to leverage that for drug discovery purposes.
<<<
Organovo -- >>> 3 Top Jefferies New Growth Stocks to Buy With Big Upside Potential
By Lee Jackson
July 6, 2015
http://247wallst.com/investing/2015/07/06/3-top-jefferies-new-growth-stocks-to-buy-with-big-upside-potential/2/
Organovo
This stock is initiated at a Buy rating and slipped recently as a result of a secondary stock offering. Organovo Holdings Inc. (NYSEMKT: ONVO) designs and creates functional, three-dimensional human tissues for medical research and therapeutic applications. The company develops 3D human disease models through internal development and in collaboration with pharmaceutical and academic partners. Organovo believes these 3D human tissues have the potential to accelerate the drug discovery process, enabling treatments to be developed faster and at lower cost.
The Jefferies analysts feel that the company’s 3D printed human tissues offer clear advantages over current traditional methods for drug discovery and toxicology testing. They report that Organovo’s first commercial products for the liver uptake has been positive and they have been getting solid interest from top major pharmaceutical companies in recent years. The analysts also think that the liver and kidney could each be $100 million plus products on very minimal penetration.
The Jefferies price target for this very interesting but very aggressive stock is $5, and the consensus is posted at $5.33. Shares ended last week at $3.74.
<<<
>>> Autodesk, Inc. develops and distributes design software. Its Platform Solutions and Emerging Business segment offers AutoCAD software, a computer-aided design application for professional design, drafting, detailing, and visualization; and AutoCAD LT, a professional drafting and detailing software. The company?s Architecture, Engineering and Construction segment offers Autodesk Building Design Suites to manage various phases of design and construction; Autodesk Revit products that provide model-based design and documentation systems; Autodesk Infrastructure Design Suites; AutoCAD Civil 3D products that offer a surveying, design, analysis, and documentation solution; and AutoCAD Map 3D software, which offers direct access to data needed for infrastructure planning, design, and management. Its Manufacturing segment provides Autodesk Product Design Suites for digital prototyping; Autodesk Inventor that allows manufacturers to go beyond 3D design to digital prototyping; AutoCAD Mechanical software to accelerate the mechanical design process; and Autodesk Moldflow family of injection molding simulation software. The company?s Media and Entertainment segment provides Autodesk Maya and Autodesk 3ds Max software products that offer 3D modeling, animation, effects, rendering, and compositing solutions; and Autodesk Flame, Autodesk Smoke, and Autodesk Lustre software applications that offer editing, finishing, and visual effects design and color grading solutions. Autodesk, Inc. also sells consumer products for digital art, personal design and creativity, and home design in various digital storefronts and over the Internet. The company licenses or sells its products to customers in the architecture, engineering, and construction; manufacturing; and digital media, consumer, and entertainment industries directly, as well as through a network of resellers and distributors. Autodesk, Inc. was founded in 1982 and is headquartered in San Rafael, California. <<<
>>> InSphero is a leading supplier of organotypic, biological in vitro 3D microtissues for highly predictive drug testing. The company currently counts all of the top ten global pharmaceutical and cosmetics companies as customers and is helping them to implement its patented microtissue technology in their development work-flow.
InSphero 3D Insight™ Microtissues enable more biologically relevant in vitro applications in efficacy (e.g. in oncology) and toxicology, including long-term and inflammation-mediated toxicity testing. A broad portfolio of tumour and liver microtissues is available, with additional toxicology models in the pipeline. InSphero's 3D microtissues are more predictive, last longer and are more affordable than conventional cell-based models.
A spin-off company of the Swiss Federal Institute of Technology (ETH) Zurich and the University Zurich, InSphero was founded in 2009 by Dr. Jan Lichtenberg, Dr. Jens M. Kelm and Dr. Wolfgang Moritz. It is headquartered in Schlieren, close to Zurich, Switzerland with subsidiaries in USA and Germany. InSphero has been recognised for its scientific and commercial achievements with a number of national and international awards and is also certified to the ISO 9001:2008 standard for its Quality Management System.
InSphero's team combines over 50 years of experience in microtissue engineering, laboratory automation and microfluidics to bring you more biological insights when testing the in vitro effects of your new drugs.
InSphero founders - Dr. Jan Lichtenberg, Dr. Wolfgang Moritz and Dr. Jens M. Kelm
<<<
>>> Organovo to Present at the Morgan Stanley Global Healthcare Conference
http://finance.yahoo.com/news/organovo-present-morgan-stanley-global-120500256.html
SAN DIEGO, Sept. 5, 2014 /PRNewswire/ -- Organovo Holdings, Inc. (NYSE MKT: ONVO) ("Organovo"), a three-dimensional biology company focused on delivering breakthrough 3D bioprinting technology, has been invited to present at the Morgan Stanley Global Healthcare Conference, which will be held in New York September 8-10, 2014.
Organovo's Chairman and Chief Executive Officer, Keith Murphy, is scheduled to present at 9:20am on September 8th. The conference is being held at The Grand Hyatt Hotel, New York, NY.
About Organovo Holdings, Inc.
Organovo designs and creates functional, three-dimensional human tissues for medical research and therapeutic applications. The Company is collaborating with pharmaceutical and academic partners to develop human biological disease models in three dimensions. These 3D human tissues have the potential to accelerate the drug discovery process, enabling treatments to be developed faster and at lower cost. In addition to numerous scientific publications, the Company's technology has been featured in The Wall Street Journal, Time Magazine, The Economist, and numerous others. Organovo is changing the shape of medical research and practice. Learn more at www.organovo.com.
<<<
>>> Organovo Highlights Liver Toxicology Achievement, Reports Q1 Fiscal 2015 Results
http://finance.yahoo.com/news/organovo-highlights-liver-toxicology-achievement-120500885.html
SAN DIEGO, Aug. 12, 2014 /PRNewswire/ -- Organovo Holdings, Inc. (NYSE MKT: ONVO) ("Organovo"), a three-dimensional biology company focused on delivering pioneering 3D bioprinting technology, has reported its first quarter, fiscal 2015, results and provided highlights of its recent activities.
During the quarter, Organovo demonstrated, in collaboration with a major pharmaceutical company, that its 3D Human Liver System could detect the toxicity of a drug that had previously been deemed safe in standard preclinical animal studies and in vitro toxicity tests but caused liver damage upon clinical use. The toxic profile of the compound was only discovered after use in a large number of patients and after significant expense.
Keith Murphy, chairman and chief executive officer of Organovo, commented on the results: "Organovo met a key challenge in this recent quarter. While we knew our liver tissue showed metabolic activity and basic toxicology results comparable to native tissue, we had to ask the question: could it be predictive of drug problems where other methods have failed? These results demonstrate clearly for the first time that our tissue has been able to detect drug-induced liver injury that other methods in the past failed to predict. With this data in hand, we are continuing to push progress in the commercialization of our 3D Liver Tissue. We remain on track for commercial release of our 3D Human Liver Tissue later this calendar year."
Recent Corporate Highlights:
•Key opinion leader reported Organovo's 3D Human Liver Tissue was able to establish the toxicity of toxic drug known to induce liver injury in humans that did not show toxicity in animal and other pre-clinical testing, an achievement historically unmet by animal models or other liver cell model systems
•Initiated contracting for toxicity testing using its 3D Human Liver Tissue, for selected clients, in advance of its scheduled commercial release
•Entered into additional agreements to utilize 3D bio-printed tissue in drug discovery settings
•Reported that its 3D bio-printed tissues enabled researchers to make compartment-specific assessments (i.e., epithelium, stroma, vasculature) of drug response — something that is not currently possible outside of in vivo models to date
•Announced an agreement with the University of Queensland directed toward development of source cell lines for 3D kidney tissue bioprinting activities
•Appointed Gregory T. Lucier, former Chairman and CEO of Life Technologies, as a corporate advisor
•Announced Annual Stockholder meeting will be held on August 20, 2014
On August 6th, the Company announced the appointment of Greg Lucier as a corporate advisor. In the role, Mr. Lucier will advise management relative to the commercial release of the Company's 3D Human Liver Tissue, scheduled to be launched by the end of November, 2014.
On July 11th, the Company announced its annual meeting of stockholders will be held on August 20th, at 9:00 a.m. at its corporate headquarters in San Diego. The notice of meeting and related materials were mailed to stockholders of record on July 11th.
On June 26th, the Company reported that a key opinion leader reported positive results from a collaborative study conducted with Organovo scientists using Organovo's 3D Human Liver Tissues.
On April 11th, the Company reported a sponsored research agreement with the University of Queensland. The agreement calls for the development of source cell lines for 3D kidney tissue bioprinting activities.
On April 10th, the Company announced results of its initial study of 3D breast tissues generated with its NovoGen MMX Bioprinter™ in breast cancer models, which demonstrated that utilizing 3D tissues may enable researchers to make compartment-specific assessments (i.e., epithelium, stroma, and vasculature) of drug response — something that is not currently possible outside of in vivo models. The results were presented at the annual meeting of the American Association for Cancer Research (AACR) held on April 5-9, 2014 in San Diego, California.
Financial Summary
A summary of the Company's financial results for the first fiscal quarter follows, but is not intended to replace the full financial disclosure enclosed in the Quarterly Report on Form 10-Q the Company filed with the Securities and Exchange Commission on August 8, 2014. Please reference that document for additional information. Because Organovo's fiscal year end is March 31, the period from April to June 2014 is considered its first quarter for fiscal year 2015 (Q1 FY2015).
As of June 30, 2014, the Company had cash and cash equivalents of approximately $44.9 million and an accumulated deficit of $98.6 million. The Company also had negative cash flow from operations of $3.4 million during the three months ended June 30, 2014. At March 31, 2014, the Company had cash and cash equivalents of approximately $48.2 million and an accumulated deficit of $92.2 million.
At June 30, 2014, the Company had total current assets of approximately $45.7 million and current liabilities of approximately $3.1 million, resulting in working capital of $42.6 million. At March 31, 2014, the Company had total current assets of approximately $49.2 million and current liabilities of approximately $1.9 million, resulting in working capital of $47.3 million.
Net cash used by operating activities for the three months ended June 30, 2014 was approximately $3.4 million as compared to $2.7 million used in operating activities for the three months ended June 30, 2013. This $0.7 million increase in cash usage can be attributed to a $2.7 million increase in operating expenses, partially offset by an overall increase of $0.7 million of non-cash expenses included in operations, including share-based compensation, depreciation and amortization, and a decrease in working capital.
Net cash used in investing activities was approximately $0.2 million and $0.1 million for the three months ended June 30, 2014 and 2013, respectively. This increase can be attributed to increased capital spending as the Company expands its research capabilities. Net cash from financing activities increased from less than $0.1 million used during the three months ended June 30, 2013 to $0.3 million provided during the three months ended June 30, 2014.
For the three months ended June 30, 2014, total revenues of $0.1 million were consistent with the $0.1 million in revenues for the same period in 2013. For both periods, the majority of revenues were derived from collaborative research agreements.
Operating expenses increased approximately $2.7 million, or 71%, from approximately $3.8 million for the three months ended June 30, 2013 to $6.5 million for the three months ended June 30, 2014. Of this increase, $1.4 million is relates to increased selling, general and administrative expense while the other $1.3 million relates to increased investment in research and development. These increases are attributed to the Company's continued implementation of its business plan, including hiring additional staff to support research and development initiatives, incremental investments associated with strategic growth and commercialization project initiatives, expenses related to operating as a publicly traded corporation, expansion of its facility, and increased stock compensation expense relative to employees and certain consulting services.
For the three months ended June 30, 2014, research and development expenses increased by approximately $1.3 million, or 87%, over the same period in 2013, as the Company increased its research staff to support its obligations under certain collaborative research agreements and to expand its product development efforts in preparation for research-derived revenues. Full-time research and development staffing increased from 24 full-time employees as of June 30, 2013 to 38 full-time employees as of June 30, 2014. In addition to increases in payroll and benefits expense of approximately $0.4 million and an increase in share-based compensation of $0.2 million resulting from increased staffing levels and increasing stock prices from June 30, 2013 to June 30, 2014, the Company increased its spending on lab equipment, supplies and contracted services in proportion to its increased research activities. In addition, the Company has continued to invest additional resources to advance its 3D bio-printing technology during the period.
For the three months ended June 30, 2014, general and administrative expenses were approximately $3.7 million, an increase of $1.4 million, or 61%, over expenses in the same period of 2013 of approximately $2.3 million. Share-based compensation increased $0.6 million due to additional grants and increasing stock prices from June 30, 2013 to June 30, 2014. Staffing expense increased $0.3 million due to an increase in administrative headcount from 11 full-time employees to 14 full-time employees to provide strategic infrastructure in developing collaborative relationships and preparation for commercialization of research-derived product introductions. In addition, the Company incurred additional expenses for investor outreach initiatives and legal costs in the three months ended June 30, 2014 as compared to the three months ended June 30, 2013.
Other expense was less than $0.1 million for the three months ended June 30, 2014 and 2013, and consisted primarily of expense related to the revaluation of warrant derivative liabilities.
About Organovo Holdings, Inc.
Organovo designs and creates functional, three-dimensional human tissues for medical research and therapeutic applications. The Company is collaborating with pharmaceutical and academic partners to develop human biological disease models in three dimensions. These 3D human tissues have the potential to accelerate the drug discovery process, enabling treatments to be developed faster and at lower cost. The Company plans to market the first product of a planned portfolio offering, 3D Human Liver Tissue for use in Toxicology and other preclinical drug testing prior to the end of 2014, and remains on track to bring this breakthrough technology to customers. In addition to numerous scientific publications, the Company's technology has been featured in The Wall Street Journal, Time Magazine, The Economist, and numerous others. Organovo is changing the shape of medical research and practice. Learn more at www.organovo.com
<<<
SC, Saving the stem cells / umbilical cord sounds like a good idea. I haven't been following the stem cell sector directly, but some posters over on Dew's board probably do (Biomaven, etc).
There is a hospital research center in Mexico that reportedly has had amazing results using a patient's own stem cells in treating cancer, autoimmune problems, etc (takes approx 1 month). Lindsey Williams and his wife were both successfully treated there, and he found out about it from his elite friends. He said the 'elites' have been treated at this center for many years, and the treatment is not allowed in the US.
Lindsey doesn't describe the details, but it involves removing one's own stem cells, treating them in some way, and then reinfusing them back into the patient. In the weeks prior to the infusion the patient is also treated nutritionally. Lindsey has a video presentation on the topic over on You Tube and has described it in various audio interviews with the head physician of the Mexican facility. Lindsey's US physicians told him his condition wasn't treatable, so the treatment in Mexico saved his life.
Organovo and J+J/Janssen Research -
>>> 24-Jul-2014
Regulation FD Disclosure
Item 7.01 Regulation FD Disclosure
On July 24, 2014, Organovo Holdings, Inc. (the "Company") announced that it has entered into an agreement with Janssen Research and Development (JRD), a pharmaceutical company of Johnson & Johnson, to evaluate the use of 3D bio-printed tissue in a drug discovery setting, outside of the Company's work in 3D liver tissue for toxicity testing. Terms have not been disclosed.
<<<
http://biz.yahoo.com/e/140724/onvo8-k.html
hello gfp, a friend of mine had her umbellical cord frozen and stored when she gave birth to her child.
how far away do you think, as in her lifetime or not, do you think they will duplicate her, or the babies, stem cells and be able to directly inject them into the brain stem?
I would imagine there would be a long line of folks who would be willing to try this. do you know of it being trialed/attempted now?
The great thing would be if stem cells could be compatible just by blood type, and not direct genetics. if that is the case, then where do I sign up?
just imagine what an einstien, or devinci, could have done with some fresh, usable, new, growing, young, duplicating, cells, in their lifetime.
as usual, sorry for the spelling and hope you are doing well.
take care.
Organovo -- >>> Long On Organovo Artificial Tissues
Jul. 23, 2014
http://seekingalpha.com/article/2332515-long-on-organovo-artificial-tissues
Summary
•3D printing of organs may increase availability of transplants and accelerate the drug discovery process.
•Artificial tissue could save large sums of money by identifying liver toxicity prior to human trials.
•ONVO printed liver tissues can differentiate between similar molecules.
3D printing revolutionized manufacturing and enabled the do-it-yourself regime. And, another type of 3D printing may revolutionize the pharmaceutical industry. This type of printing is 3D bio printing.
3D bio printing uses an ink jet printer to print cells or cells and a support structure into bits of functional tissue. There is quite a bit of speculation around the use of 3D bio printing for manufacturing organs for transplantation purposes. However, the more immediate use of this technology would be for drug discovery.
Currently, drug discovery is an arduous journey. It consists of systematically screening various drug candidates at different concentrations to see if the molecule successfully modifies the drug target. Then, if a promising drug candidate is identified, this molecule is tested in more sophisticated models. The price tag for this research and development process was about $1.8 billion USD in 2010.
Once you are in for about a couple billion, one might be granted a patent on the potential drug candidate and need to find some more money for human trials, which are also expensive.
Needless to say, there is room for improvement.
3D printed tissue may be useful for drug discovery, it provides a more realistic platform for screening drugs candidates. Basically, a potential drug may affect a 3D tissue model differently when compared to current 2D models, and those invested in 3D bio printing believe this printed tissue will more accurately reflect a human response to the drug candidate.
Organovo (NONVO) is a major 3D bio printer player with a "focus on developing a range of tissue and disease models for medical research and therapeutic applications." The current tissue of interest is liver tissue as 10% of all drugs fail in Phase III trials due to liver toxicity. Once the initial R&D is complete and the end of lengthy and expensive human trials is near, there is a 10% chance the drug may adversely affect the liver - some initial testing in an effective liver model could save a lot of time and money.
An effective 3D liver tissue model could allow pharmaceutical companies to screen for liver toxicity during the drug development process or prior to human trials. This would allow a pharmaceutical company to weed out liver toxicity causing drug candidates prior to starting human trials. ONVO's first product, a liver tissue model, can differentiate between similar molecules with different toxicity levels. Meaning, once a target molecule is identified with existing drug screening techniques, the ONVO liver tissue product will allow them to test variations of the molecule to identify the least toxic version of the drug candidate. Thus, reducing the odds of failure due to liver toxicity during Phase III human trials - saving lots of time and money. This is an attractive value proposition for anyone in drug development, a patented molecule pre-screened for liver toxicity is very valuable indeed - Phase III trials represent 40% of pharmaceutical company R&D expenditure.
From a technology perspective, these printed tissues could be quite valuable. However, its income statement could be better. ONVO generated $370,000 in revenue with net negative income of $25,848,000 in 2014. ONVO has plenty of cash with $48,170,000 in the bank and negligible current liabilities. Additionally, ONVO is on track to launch the first viable product in the near future - a 3D liver assay or drug-screening platform can differentiate between similar molecules with different toxicity levels. ONVO has about 2 years' worth of cash reserves as of March 2014 and is schedule to launch its first product in December 2014. R&D expenses may decrease after launch and revenue will increase, however, it will be a while before ONVO turns a profit.
ONVO's first product is promising, could save Big Pharma substantial sums of money on the drug development and human trial processes, and initial testing indicates the liver toxicity assay does what it is supposed to do. ONVO has enough cash to get to product launch plus about a year of additional runway. Going long on ONVO means you believe ONVO's first product will become an essential drug development tool, and the large cash position means there is a healthy margin of error in these early stages.
<<<
>>> Should 3D Systems Corp. Acquire Modern Meadow?
By Steve Symington
July 10, 2014
http://www.fool.com/investing/general/2014/07/10/should-3d-systems-corp-acquire-modern-meadow.aspx
Modern Meadow scientist Karoly Jakab observing a tray of lab-grown meat. Source: Modern Meadow.
One of the biggest draws of additive manufacturing -- or, as we've come to know it, 3-D printing -- is that materials aren't wasted to produce any given product. By contrast, traditional subtractive manufacturing techniques are more like carving a sculpture: Start with a big block of the material out of which the product is to be made, then remove the excess until you achieve the desired form.
And as additive manufacturing leaders like 3D Systems (NYSE: DDD ) , Stratasys (NASDAQ: SSYS ) , and ExOne (NASDAQ: XONE ) continue to narrow the gap with speed and capabilities between their respective technologies and traditional manufacturing, the market for 3-D printing should only continue to grow by leaps and bounds. So far, however, the 3-D printing industry's efforts have largely focused on using materials like plastic, glass, and metal.
Another massive industry ripe for the picking
Speaking of waste, did you know that more than one-third of all available land in the world is used for livestock production? Or that it takes over 50 gallons of water to make a single quarter-pound burger? Or consider the fact that 20% of all leather is wasted in manufacturing and 70% of leather's wet weight is comprised of toxic solid wastes.
But one Brooklyn-based company is aiming to solve these problems: Modern Meadow.
Co-founded by Andras Forgacs, his father, Gabor, and scientists Francoise Marga and Karoly Jacab, Modern Meadow is looking to 3-D bioprinting to reduce the global impact of livestock production -- all without harming animals in the process. Previously, the father-son combo also founded tissue printing specialist Organovo (NYSEMKT: ONVO ) , which went public in 2012 and is working to create functional 3-D human tissue for medical research and therapeutic applications.
As it stands, Modern Meadow's efforts still remain in the early stages and are far from being ready for mass production. But the company has already demonstrated the ability to create lab-grown leather and beef -- the latter of which the elder Forgacs actually ate while giving a live TEDMED talk in 2011.
However, according to Modern Meadow business director Sara Sclarsic, "[T]he first range of products to hit the market will be cultured leather and related biomaterials, not cultured meat."
And it appears Modern Meadow wants that to happen sooner rather than later: Only a few weeks ago, Modern Meadow announced it had raised $10 million in additional Series A financing to "accelerate R&D product development as well as to open an expanded research headquarters at the Brooklyn Army Terminal in New York City."
All in favor...
But here's my question: Why hasn't another company acquired Modern Meadow already? More specifically, why hasn't 3D Systems done so yet?
After all, 3D Systems' VP and Chief Strategy Officer Ping Fu already serves as an advisor to Modern Meadow. In fact, without explicitly naming Modern Meadow, Fu even mentioned her involvement with the company and highlighted the promise of its technology in an interview with our very own Brendan Bynes early last year.
3-D printed sugar from 3D Systems ChefJet printers. Source: 3D Sytems Corp.
In addition, 3D Systems already acquired fellow food printing specialist The Sugar Lab last year, so currently stands the largest publicly traded 3-D printing company with any meaningful presence in the 3-D printed food market. Meanwhile, Stratasys has remained busy given both its massive merger of equals with Objet in late 2012, as well as its $400 million acquisition of MakerBot around this time last year. And high-end industrial printing specialist ExOne, for its part, is still facing lumpy quarterly results given the massive costs of its high-end industrial printers, as well as margin pressures from the ongoing implementation of its key ExCast initiative.
I'll admit, however, 3D Systems current line of ChefJet 3-D sugar printers primarily appeals to professional kitchens seeking the ability to create geometrically pleasing edible prints with minimal effort. At the same time, Liz von Hasseln -- who founded The Sugar Lab and is now 3D Systems' creative director of food products -- has already stated they want the technology to evolve "into a variety of flavors and foods, powered by real food printers for both professionals and consumers alike."
And what better way for 3D Systems to do so than to propel its food products business head-first into the multibillion-dollar market opportunity presented by 3-D bioprinting?
All opposed...
That's not to say there wouldn't be drawbacks for 3D Systems in acquiring Modern Meadow.
While we don't have any meaningful information on the precise financials of its business, for example, it's safe to say Modern Meadow's young technology isn't generating any meaningful revenue or earnings at this point. To the contrary, the entire premise of tackling such an acquisition this early in the game lies with Modern Meadow's extraordinary promise for generating results down the road.
But that certainly wouldn't appease investors who are already skeptical of 3D Systems' habit of pursuing growth through acquisitions at the expense of profitability over the past few years. If acquiring Modern Meadow at this stage were to significantly hinder 3D Systems' focus on the rest of its more developed businesses, the market may cry foul.
Foolish takeaway
Alternatively, Modern Meadow could follow Organovo's go-public path. But despite its lack of near-term profitability, keep in mind Organovo currently boasts a $640 million market cap. If Modern Meadow commands anywhere near the same premium, that'd be a big pill to swallow even for the $5.6 billion behemoth that is 3D Systems. Over the long term, though, and if Modern Meadow's world-changing aspirations ultimately bear fruit -- or meat, rather -- it could be the best money 3D Systems ever spent.
<<<
>>> Is Organovo a Great 3D Bioprinting Stock?
By Jason Moser and Brendan Mathews
June 20, 2014
http://www.fool.com/investing/general/2014/06/20/is-organovo-a-great-3d-bioprinting-stock.aspx
In this edition of The Motley Fool's "Ask a Fool" series, Motley Fool analysts Jason Moser and Brendan Mathews take a question from a reader who asks:
I bought Organovo (NYSEMKT: ONVO) at the end of April after they announced demand for their product had increased. Shares have gone up considerably since then. Can you discuss if this is sustainable or just a bubble?
Organovo is a very interesting company in that it provides 3-D bioprinting technology that allows customers to print human tissue for use in drug testing. It’s a fascinating concept, and one with potentially major implications, as it could make the drug approval process potentially much better because it would give companies a better idea whether to pursue an idea, or move on to the next one without wasting additional funds.
Investors need to note that this is a development stage company, meaning it relies heavily on grants, partnerships, and public markets for funding, and there are no guarantees it will succeed. They made mention of the fact that they have interest from a number of different parties, including big pharma and biotech players.
However, Organovo isn’t the only game in town, so it remains to be seen if what they provide is enough of a differentiator. Earnings results out today have sent shares lower, due, in part, to lower sales and higher costs. Jason notes that this isn’t a company that he would be interested in. For those who are, he recommends that it should only be a small allocation in the context of a well-diversified portfolio
<<<
>>> Organovo Reports Fiscal 2014 Financial Results
http://finance.yahoo.com/news/organovo-reports-fiscal-2014-financial-120500761.html
SAN DIEGO, June 12, 2014 /PRNewswire/ -- Organovo Holdings, Inc. (NYSE MKT: ONVO) ("Organovo"), a three-dimensional biology company focused on delivering breakthrough 3D bioprinting technology, has reported its financial results for the fiscal year ended March 31, 2014. The Company also reported on its corporate highlights during fiscal 2014.
FY 2014 Corporate Highlights:
•Expanded independent Board membership with the addition of Tamar D. Howson and Richard A. Heyman, Ph.D.;
•Common stock was approved to list on the NYSE MKT and began trading on the New York Stock Exchange MKT on July 11 , 2013;
•Raised $46.6 Million via a fully underwritten public offering of Common Stock, in which Lazard Capital Markets LLC and Oppenheimer & Co. Inc. acted as joint book-runners for the offering;
•Presented data demonstrating retention of key liver functions in bioprinted tissues for up to 40 days, and demonstrated that Organovo's 3D liver tissues exhibit dose-dependent responses to known liver toxicants, and that the toxic effects can be assessed using both standard screening assays and histopathological assessment of the treated tissue;
•Performed its first 3D Liver tissue delivery, marking the delivery of Organovo's 3D Liver tissue to a laboratory outside of the company to a key opinion leader (KOL) for experimentation;
•Initiated contracting for toxicity testing using its 3D Human Liver Tissue for selected clients prior to full release;
•Announced collaboration with the National Center for Advancing Translational Sciences (NCATS) and the National Eye Institute (NEI) to help scientists develop more reliable tools for bringing safer, more effective treatments to patients on a faster timeline;
•Doubled office and laboratory space to 30,000 sq. feet to accommodate the capacity requirements of recent partnerships and the near-term commercial product launch;
•Celebrated a profile of the company and the 3D bioprinting space as the cover story in the Economist's Technology Quarterly
"Organovo was able to continue our achievement of strong results in fiscal 2014," stated Keith Murphy, chief executive officer of Organovo. "We demonstrated the viability and utility of our 3D liver tissues and breast tumor disease model, expanded our partnerships, uplisted our common stock to the NYSE MKT, raised significant financing, and saw tremendous scientific results from our bioprinting efforts in a variety of tissue types. We will continue to focus in fiscal 2015 on executing our business plan and on striving to deliver long-term shareholder value."
Financial Results:
Comparison of the years ended March 31, 2014 and December 31, 2012
Revenues
Revenues of $0.4 million for the year ended March 31, 2014 decreased approximately $0.8 million, or 67%, over revenues of $1.2 million for the year ended December 31, 2012. This decrease reflects the completion or declining activity under two collaborative research agreements since 2012, partially offset by increasing revenue contributions from three new collaborative research agreements.
Operating Expenses
Operating expenses increased approximately $10.5 million, or 100%, from $10.5 million for the year ended December 31, 2012 to $21.0 million for the year ended March 31, 2014. Of this increase, $5.9 million is related to increased selling, general and administrative expense, while the other $4.6 million relates to increased investment in research and development expense. Those increases are attributed to the Company's continued implementation of its business plan, including hiring additional staff to support its research and development initiatives, incremental investment associated with commercialization project initiatives, expenses related to operating as a publicly traded corporation, expansion to a larger facility, and increased stock compensation expense relative to employees and certain consulting services.
Research and Development Expenses
Research and development expense increased $4.6 million, or 135%, from approximately $3.4 million for the year ended December 31, 2012 to approximately $8.0 million for the year ended March 31, 2014 as the Company significantly increased its research staff to support its obligations under certain collaborative research agreements and grants, and to expand product development efforts in preparation for commercial revenues. Full-time research and development staffing increased from nineteen full-time employees as of December 31, 2012 to thirty-two full-time employees as of March 31, 2014. In addition to the incremental payroll, benefits and stock-based compensation resulting from increased staffing levels, the Company increased its facility space to accommodate its growing research staff, and increased its spending on lab equipment and supplies in proportion to its increased research activities.
Selling, General and Administrative Expenses
Selling, general and administrative expenses increased approximately $5.9 million, or 83%, from $7.1 million for the year ended December 31, 2012 to $13.0 million for the year ended March 31, 2014. Increased staffing expenses of approximately $1.5 million was due to the headcount increase from ten full-time employees as of December 31, 2012 to thirteen full-time employees as of March 31, 2014, to provide strategic infrastructure in developing collaborative relationships and preparing for commercialization of products and services, and to address the additional compliance requirements of operating as a publicly traded corporation. In addition, the year ended March 31, 2014 includes $1.2 million more in payroll taxes related to the vesting of restricted stock units the Company previously granted to certain of its executives. Stock-based compensation costs also increased approximately $2.9 million due to additional grants to employees and consultants as well as an overall increase in the Company's stock price. The remainder of the increase is primarily due to non-recurring external fees and expenses incurred during the year ended March 31, 2014 related to the Company's up-listing to the NYSE MKT and its completion of a secondary public offering during the year.
Other Income (Expense)
The $29.0 million decrease in other expenses as compared to the year ended December 31, 2012 was primarily due to the inclusion of one-time non-cash transaction costs associated with the Merger and 2012 Private Placements in other expense during 2012, including approximately $19.0 million of expense for the excess of the fair value of warrant liabilities over proceeds received, $2.1 million of financing costs in excess of proceeds received and $1.0 million in interest expense from the accretion of debt discount and amortization of deferred financing costs related to the 2011 Private Placement, the Merger and the 2012 Private Placement. In addition, $1.9 million of expense was recorded in 2012 for the loss on inducement to exercise warrants under a tender offer completed during the year. Finally, non-cash expense related to the change in fair value of warrant liabilities decreased by approximately $4.8 million, due to fewer warrants outstanding as of March 31, 2014.
Various factors are considered in the pricing models we use to value the warrants, including the Company's current stock price, the remaining life of the warrants, the volatility of the Company's stock price, and the risk free interest rate. Future changes in these factors may have a significant impact on the computed fair value of the warrant liability. As such, we expect future changes in the fair value of the warrants to continue to vary significantly from quarter to quarter.
Financial Condition, Liquidity and Capital Resources
At March 31, 2014, we had total current assets of $49.2 million and current liabilities of $1.9 million, resulting in working capital of $47.3 million. At March 31, 2013, we had total current assets of $16.1 million and current liabilities of $8.4 million, resulting in working capital of $7.7 million. At December 31, 2012, we had total current assets of $15.9 million and current liabilities of $22.0 million, resulting in a working capital deficit of $6.1 million.
Net cash used in investing activities was approximately $0.3 million, $0.2 million, less than $0.1million, $0.4 million, and $0.1 million for the year ended March 31, 2014, the three months ended March 31, 2013 and March 31, 2012, and the years ended December 31, 2012 and December 31, 2011, respectively. The majority of net cash used in investing activities from inception to date has been for the purchases of equipment for the research lab.
Net cash provided by financing activities was approximately $48.4 million, $3.7 million, $13.6 million, $24.6 million and $2.1 million for the year ended March 31, 2014, the three months ended March 31, 2013 and March 31, 2012, and the years ended December 31, 2012 and December 31, 2011, respectively.
During the year ended March 31, 2014, we raised net proceeds of approximately $43.4 million through the sale of 10,350,000 shares of our common stock in a public offering. In addition, we raised net proceeds of approximately $3.5 million from an at-the-market follow-on offering, $1.0 million from the exercise of warrants, and $0.4 million from stock option exercises during the year ended March 31, 2014.
About Organovo Holdings, Inc.
Organovo designs and creates functional, three-dimensional human tissues for medical research and therapeutic applications. The Company is collaborating with pharmaceutical and academic partners to develop human biological disease models in three dimensions. These 3D human tissues have the potential to accelerate the drug discovery process, enabling treatments to be developed faster and at lower cost. In addition to numerous scientific publications, the Company's technology has been featured in The Wall Street Journal, Time Magazine, The Economist, and numerous others. Organovo is changing the shape of medical research and practice. Learn more at www.organovo.com
<<<
>>> Autodesk, Inc. operates as a design software and services company worldwide. Its Platform Solutions and Emerging Business segment offers AutoCAD software, a computer-aided design application for professional design, drafting, detailing, and visualization; and AutoCAD LT, a professional drafting and detailing software. The company?s Architecture, Engineering and Construction segment offers Autodesk Building Design Suites that give customers the ability to manage various phases of design and construction; Autodesk Revit products, which provide model-based design and documentation systems; Autodesk Infrastructure Design Suites; AutoCAD Civil 3D products that offer a surveying, design, analysis, and documentation solution; and AutoCAD Map 3D software, which provides direct access to data needed for infrastructure planning, design, and management. Its Manufacturing segment provides Autodesk Product Design Suites, a solution for digital prototyping; Autodesk Inventor that allows manufacturers to go beyond 3D design to digital prototyping; AutoCAD Mechanical software to accelerate the mechanical design process; and Autodesk Moldflow family of injection molding simulation software provides tools. The company?s Media and Entertainment segment offers animation products that provide tools for digital sculpting, modeling, animation, effects, rendering, and compositing; and creative finishing products, which offer editing, finishing, and visual effects design and color grading solutions. Autodesk, Inc. also sells consumer products for digital art, personal design and creativity, and home design in various digital storefronts and over the Internet. The company licenses or sells its products to customers in the architecture, engineering, and construction; manufacturing; and digital media, consumer, and entertainment industries directly, as well as through a network of resellers and distributors. Autodesk, Inc. was founded in 1982 and is headquartered in San Rafael, California. <<<
Organovo -- >>> Why Autodesk's CTO Believes Synthetic Biology is More Promising Than 3-D Printing
By Maxx Chatsko
May 27, 2014
http://www.fool.com/investing/general/2014/05/27/why-autodesks-cto-believes-synthetic-biology-is-mo.aspx
What has the brighter future, 3-D printing or synthetic biology?
While 3-D printing is often hyped by the media -- sometimes for good reason -- I don't think it's the most far-reaching, game-changing technology out there. There is significant potential for additive manufacturing to disrupt the world's current manufacturing processes, especially considering that polymers, metals, plasters, and other materials can be used in 3-D printers, but can it really replace traditional manufacturing anytime soon? I have my doubts, and Autodesk (NASDAQ: ADSK ) Chief Technology Officer Jeff Kowalski seems to agree. That could be great news for companies such as Organovo (NYSEMKT: ONVO ) , but it doesn't necessarily have to be bad news for 3D Systems (NYSE: DDD ) .
In an interview earlier this year with the India Times, Kowalski was asked what innovations excited him the most. The software company CTO's answer:
I have a lot of choices, but if I had to pick one that I think is really provocative -- and it's in the really early stages -- I have to say that it is design for synthetic biology. And I think so because it touches everything. It has the potential for changing everything from agriculture to medicine. It is literally reprogramming cells to do fundamentally different things in the way they were naturally created.
That probably shocks some people who would have gone all-in betting 3-D printing would be his answer, but Kowalski was fond of synthetic biology well before that interview. Investors can thank the great visionary Andrew Hessel, now a distinguished researcher at Autodesk, for infecting Kowalski just over two years ago and for keeping the momentum rolling since with Autodesk's Bio/Nano/Programmable Matter Group. What makes designing with biology so powerful, and its potential so limitless? Why might investors want to focus more on synthetic biology than 3-D printing for long-term growth at Autodesk? Allow one very biased author to explain.
3-D printing vs. synthetic biology
Autodesk software for 3-D printing applications certainly represents a major growth area for the company. While not all goods can be produced with additive manufacturing, the opportunity is massive: 3D Systems didn't grow revenue 221% from 2010 to 2013 by mistake or chance. Manufacturing represents roughly $1.6 trillion, or 12%, of American gross domestic product. By comparison, the bioeconomy (biotech crops, biopharmaceuticals, and industrial biotech) represented an estimated $350 billion, or 2.5%, of American GDP in 2012. So why is Autodesk more excited about designing with biology than 3-D printing?
In terms of markets that can be disrupted, synthetic biology has a far greater reach than additive manufacturing. Whether it's agriculture or novel biopesticides, specialty or commodity chemicals, fuels or animal feed, spider silk or algal oils, flavors (traditionally produced from petrochemicals) or fragrances (sourced from unsustainable agricultural practices), or numerous others areas, synthetic biology could have an answer.
You also have to consider not just the markets that can be disrupted, but the markets that can only be created and enabled through more efficient biology. Imagine doubling the efficiency of photosynthesis in agricultural crops, curing the most devastating diseases by creating novel pharmaceuticals from new life forms that could never exist on Earth, or having the ability to grow fully functional human organs for transplantation, as Organovo seeks to do. That's why the broader opportunities presented by synthetic biology have greater potential than anything 3D Systems or 3-D printing have to offer.
However, a drastically improved understanding of biology will be necessary before we can characterize it with lines of software and begin designing with it. That's where Autodesk comes in. Take, for instance, the company's Project Cyborg, which is being developed to become the world's leading platform for designing with biology. There's a long road ahead, but one day engineers will design microbes in much the same way airplanes are designed today. Before the first aircraft prototype gets built, we can be pretty confident that it will fly. We're not that confident with biology just yet, but we have to start somewhere. That somewhere might just be Autodesk's building on the San Francisco Bay.
Foolish final words
If they weren't before, the wheels should be turning for Autodesk investors now. If nothing else, investors should at least be pleased to know they were overlooking the most important long-term growth catalyst at the company. It's not that 3-D printing doesn't offer a great opportunity -- it surely does -- it's that designing with biology has so many potential game-changing applications in its future. Could you imagine how different the world would be if we could grow organs for transplantation? Or if we could double the efficiency of photosynthesis? Or if we could engineer ourselves to be immune from every pathogen on Earth (chiral humans)? It may sound like science fiction, but those are all very plausible with synthetic biology -- and Autodesk could seed the disruption.
<<<
>>> Organovo's Bioprinting More Than A 'Stupid Exercise'
May. 8, 2014
http://seekingalpha.com/article/2202063-organovos-bioprinting-more-than-a-stupid-exercise
Summary
•Misleading short pieces and a cooling sector have had a negative effect on Organovo's share price.
•Organovo's fully cellular bioprinting method provides crucial benefits over competing approaches. This will distinguish Organovo as a 3D bioprinting leader.
•Data that the company has released has been compelling enough to warrant contracts without third-party validation and product launch.
Organovo (ONVO) is down 50% YTD due to a combination of short sellers calling out the company's valuation and the struggles of the biotech and 3D printing sectors. The latest critic, Simeon Research, is an anonymous equity research firm created in February 2014 entirely to issue negative reports about Organovo. Reading through the 81 pages of Simeon's "research"; I wondered what vendetta Simeon had against Organovo. The blatantly negative tone and misleading information stood out, taking away from the report's objective.
I started covering Organovo back in September 2012, when the company was trading $2.10 and regularly criticized. Is the company real? Does the NovoGen bioprinter even exist? Is the facility in San Diego just a cover up? Looking back, it's laughable what shorts were critical of. According to Simeon, which gave a price target of $1.35, not much seems to have changed with regards to sentiment surrounding Organovo. Till now, the company has proved doubters wrong. I expect this to continue.
Bioprinting: A Stupid Exercise?
In a 2012 seminar, one of Organovo's founders, Dr. Gabor Forgacs, who was also the company's chief scientific officer at the time, uttered the words "it's nothing, it's a stupid exercise" when talking about bioprinting. Simeon took this quote out of context and ran with it, claiming it expressed the public views of Organovo's scientific founder.
What was not mentioned, however, was the fact that Forgacs spoke for over an hour about Organovo's bioprinting process and the future of bioprinting in a very positive manner. Have a look at the YouTube clip. The 24-minute mark is where Forgacs mentions "stupid exercise". I highly recommend you watch the whole clip to get the FULL views of Organovo's scientific founder, instead of nitpicking certain parts. Simeon Research uses ten second of a lecture that's an hour and seventeen minute to come to Forgacs' "public views" of bioprinting. Quite misleading.
The "Magic" of Bioprinting
The natural properties of cells are the magic behind the bioprinting concept. When placed in close proximity, cells aggregate and form their own extracellular matrix. This natural process finalizes the tissue from the printed cell. This is a visual (from Organovo's April 2014 investor presentation) to explain the process.
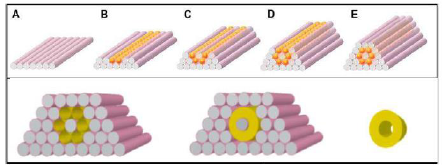
(Source: Organovo April 2014 Presentation)
Steps A-E is Organovo's layered printing process of cell aggregates (yellow) supported by agarose molecule hydrogel (grey cylinders). The bottom half of the picture is where the "magic" happens as the cell neutral agarose is removed and the cellular material fuses naturally to form the living cell.
To get to this "magic" step though, a special delivery device is needed. You can't just clump cells together and hope they aggregate naturally. Forgacs says a "good printer" optimizes the natural process by depositing cell aggregates in the right position to fuse. To trigger the magic of bioprinting, cells must be printed in a specific position; otherwise, the natural process will not occur. This is what Organovo's NovoGen bioprinter does.
Replicating such a process is easier said than done, due to Organovo's fully cellular approach (where no artificial scaffolds are being used) and intellectual property. Organovo's patent from Clemson University provides rights to use inkjet printer technology to dispense cells, and to create matrices of bioprinted cells on agarose hydrogel materials. Though there are competitors out there who also follow a similar bioprinting process, Forgacs refers to Organovo as "champions" in creating cell aggregates.
Tiers of Bioprinting
Printing scaffold with no cells
A lot of 3D bioprinting can actually be classified as 3D printing of gels/polymers into a shape with no cells inside. The cells are added afterwards. An example of this would be EnvisionTec's 3D Bioplotter. In their list of materials printed, living cells are not mentioned. The Bioplotter prints a polymer scaffold into a shape, which they can later add cells into the network to mimic natural harvesting and end up with a tissue. Mimicking proximity of cellular network is complicated, which is why the accomplishments of scaffold-based tissue engineering are more exception than rule. EnvisonTec's approach can be classified closer to 3D printing rather than bioprinting, since no cells are technically printed.
Simeon claims that EnvisionTec has a significant competitive advantage over Organovo, due to greater accuracy. The "accuracy" and "complexity" of the Bioplotter can print up to 5 different materials at once at a range of 1 micron. Compare this to Organovo's 20-micron precision. Again, this is misleading, because Organovo prints living cells and the Bioplotter prints materials like gelatin. Generally, cells are 20-50 microns in size to begin with, so it's not even possible to print 1 micron cells. An apples to apples comparison cannot be made.
Printing biomaterial with cells included
Another tier of bioprinting can be classified as 3D printing a gel, with cells included in the gel. These cells are embedded into the printed gel shape. Though this can be considered more cellular in nature, there are still issues trying to form tissue from this approach. These non-natural materials are foreign to human bodies, and thus, may reduce effectiveness of tissue regeneration by limiting cellular interaction. An example of a company utilizing this approach is privately-held Swiss RegenHU.
Fully (or substantially) cellular bioprinting
This approach does not use any artificial scaffolds, instead utilizes cells and hydrogel as support. This is Organovo's bioprinting method.
Using a recent cancer study, I will show the difference between Organovo's fully cellular bioprinting approach and that of competitors who use a combination of artificial biomaterials and cells.
Three-dimensional printing of Hela cells for cervical tumor model in vitro
A study from Drexel University compared 3D printing of Hela cells for cervical tumor model in vitro with a 2D model. The study used a 3D cell printer developed by the group at Drexler to print Hela cells (cervical tumor cells) and biomaterials (gelatin, alginate, fibrinogen) to mimic the cervical cancer microenvironment.
(Source: Y Zhao et al. Three-dimensional printing of Hela cells for cervical tumor model in vitro)
As seen in the images above, Drexel's bioprinter is shaping fibrinogen/gelatin gel rods into a grid shape. Within these gels, there are Hela cells which are being used as the cancer model. This bioprinting approach is using a combination of living cells and gels.
(Source: Y Zhao et al. Three-dimensional printing of Hela cells for cervical tumor model in vitro)
This image shows the 3D space, in which the green lights are cells and the rest of the space between the cells is gel material. By looking at the image, it can be estimated that the density of the model may be as low as 1% cellular, and the rest, gel material. This percentage may be a bit more as spheroids grow, however, even then, the model may be pushing 10% cellular.
The study demonstrated that Hela cells formed round spheroids with smooth surfaces and tight cell-to-cell connections within the 3D hydrogel, whereas Hela cells cultured on the 2D tissue culture plates showed a ?at and elongated morphology. Even with such a low volume of cellular makeup, 3D models revealed that the Hela cells showed a higher proliferation rate, MMP protein expression and chemoresistance than those in 2D culture. In conclusion, the results revealed that the printed 3D models have more simulated tumor characteristics compared with the 2D planar cell culture models.
Organovo's 3D bioprinted human breast cancer for in vitro drug screening
"Various techniques, such as multicellular spheroids, cell-seeding 3D scaffolds, hydrogel embedding, micro?uidic chips and cell patterning have been developed for construction of 3D in vitro tumor models." (Y Zhao et al., Three-dimensional printing of Hela cells for cervical tumor model in vitro) However, it is difficult to simulate a complex 3D physiological tumor microenvironment in most of the listed models, due to the limitations of the fabrication techniques.
Organovo's technique prints a mass of cells that is ALL cellular in nature. This makes Organovo's printed cells very architecturally similar to what is in the body, and as a result, more relevant to the human system. Last month, Organovo updated on the progress of the company's bioprinted breast cancer models at the American Association for Cancer Research (AACR) annual meeting.
(Source: Organovo 2014 AACR Poster)
Image C above (from Organovo's 2014 AACR poster) shows that Organovo's bioprinted breast cancer tissue has regions within it. The exterior region, called the stroma, of the tumor builds a wall around the core. At the core is where you will find the mass of cancer cells. These are normal features of tissues that provide resistance to drug penetration. This model recreated the tissue as it is in the human body, at 100% cell density. Thus, it provides better understanding on how to approach drug discovery when compared to other bioprinting models that create a cube of gel with a fraction of cell volume. Though the combination of gel/cell structures (such as the Hela spheroids shown above) may have some similarities to actual human systems, they are not recreating the tissue. Organovo is. A spheroid is certainly a step into 3D, but lacks aspects that native tissues have, such as the outside layer of non-cancer cells.
Organovo's breast cancer model measured the early effects of small molecules (defined by molecular rate) on cancer cells, as well as different cell types in the breast microenvironment. The biological response profiles of chemotherapeutic agents cisplatin, paclitaxel, methotrexate, and tamoxifen were measured. Early data on performance of smaller drugs showed that they can penetrate tumor cell and have a differential effect, thus offering a better understanding on how the tested drugs may affect the tissue. The responses of the 3D breast cancer model to chemotherapeutic agents were compared to the response of 2D breast cancer cell lines to determine the relative efficacy of compounds in 2D versus 3D. Updated data is expected in the coming months.
Liver/Cell Assay Product Market
Organovo identifies the cell assay market as a $500M opportunity by 2018. The company believes that 3D cell assay products will satisfy the unmet customer demand in this market, and is targeting it with its liver product launch in Q4 2014. Organovo has released data to support its upcoming liver product; however, has faced criticism for comparing result to conventional 2D models.
Contrary to the belief of shorts, 2D models are still the conventional method being used in testing for toxicity. As CEO Keith Murphy states in his latest presentation, up to 95% of the market is still using 2D cell cultures. Reason for this lack of uptake is because the "3D models" already in market have not provided compelling enough data to move away from the current methodology. As Organovo prepares to launch its liver assay product, the company will have to show its product superiority to what customers are using (2D models), not a competing product that has not been able to penetrate the market.
Do not get me wrong, I do not think Organovo will suddenly become a market leader in the cell assay product market. The company will face an uphill battle and will need the functional validation of a third-party (key opinion leaders) to show superiority. Functional validation, in the form of journal publications, is expected to be released sometime in 2015, once the product has already been launched. Third-party publications will validate the liver tox data, and in turn, help Organovo's products gain greater sales traction in a new market. Murphy has stated that the pricing of Organovo's product will be in line with that of competitors, so as not to put the company at a price premium.
3D Liver Contracts
On the 3D liver tissue front, Organovo released that it has initiated contracts for research services. Contract services to date totaled in the "low hundreds of thousands of dollars ($100k to $400k range)". Organovo's 3D human liver tissue has shown to have the density of cells and binding capabilities that are the same as the cells in the human system. The data already published by the company has been compelling enough to net contracts before any third-party validation or official product launch. Even with existing market players supposedly having a "first-mover advantage" over Organovo, pre-released contracts indicate interest exists for Organovo's products and services. This early sign bodes well moving forward, as the company will have additional data validating its approach and providing greater chances of converting future contracts.
Risks
Organovo ended calendar year 2013 with ~$50M, and indicated that the quarterly burn rate was roughly $5M. This would place the company's current cash balance at about $45M and signify that, at a current burn rate, Organovo would have more than 2 years of cash on hand. The quarterly burn rate will likely increase with Organovo's expanded R&D efforts and product launch at the end of 2014. However, as shown above, revenue is also expected to rise, thus restoring some of the increased expenses. Trading at a current market cap of sub-$500 million market cap and a 50% drop YTD, dilution does not seem to be a pressing issue.
In my opinion, much of the remaining downside risk will come from volatile trading during downtime, when there is no news or updates released on Organovo's operations. No news is bad news, or at least this is the mindset of those following Organovo. The upcoming catalysts must be communicated with investors, which is something I believe the company PR team has done a good job of thus far.
Conclusion
All in all, the advancement of Organovo's bioprinting technology and released data will determine the value of the company. Investors will face volatility, as there will undoubtedly be skeptics out there who deem the company with foolish price targets of $1.35. Organovo has made great strides to validate its approach in the last couple of years, and I believe this will continue moving forward. Being a catalyst-driven stock, I am betting that ONVO's future catalysts will surprise the market and prove that bioprinting is not a "stupid exercise".
<<<
>>> Modern Meadow Makes Leather and Meat Without Killing Animals
By Ted Greenwald
June 06, 2013
http://www.businessweek.com/articles/2013-06-06/modern-meadow-makes-leather-and-meat-without-killing-animals
Modern Meadow Makes Leather and Meat Without Killing Animals
Illustration by Steven M. Johnson
Gabor Forgacs holds up a portfolio labeled Global Leathers in shiny gold print. “I want you to feel this,” he says, opening the samples folder to a black square of material. It feels soft and supple, like a kid glove. “Now feel this,” he says, presenting a grayish rectangle the size of a business card. It feels every bit as buttery as the other sample. “Smell it,” he says. Though it has the pungent aroma of freshly tanned animal hide, the swatch is made of biopsied bovine cells multiplied in a flask.
While other companies in the emerging field of tissue engineering focus on supplying the medical market, Forgacs’s business, Modern Meadow, plans to sell lab-grown leather—and eventually meat—to consumers. Forgacs, 65, a biophysicist who heads the University of Missouri’s biological physics lab and Clarkson University’s innovation center, launched the company in 2011 with two Missouri colleagues and his son, Andras, 36, a former venture capitalist and McKinsey consultant. “Leather is a gateway product for us,” Andras Forgacs says amid racks of tissue cultures in their lab at a state-sponsored tech incubator in Columbia, Mo.
The father-son team previously co-founded Organovo, which provides engineered human tissues to drug companies testing new compounds. Gabor Forgacs’s attempts to manufacture blood vessels and muscles convinced him he could enter the $740 billion global meat, fish, and poultry industry. In late 2011 he cooked, salted, and ate a sliver of cultured pork onstage at a TEDMED conference, a taste of what Modern Meadow could develop. His son joined as chief executive officer in January 2012, and the company has since won grants from the federal Small Business Innovation Research program ($242,000) and Peter Thiel’s Breakout Labs ($350,000), plus $1.4 million in investments from angel and seed funds.
Story: The ‘Test Tube’ Burger Is Here, but It Won’t Be an Easy Sell
The challenges of growing meat with the right flavor, texture, and aroma in a lab are daunting. Palatability aside, Modern Meadow’s legal advisers are concerned regulation could stall sales of cultured meat for 5 to 10 years. The company is pushing forward with leather; the CEO intends to show off prototype samples on June 13 at the TEDGlobal conference in Scotland. Pound for pound, leather can be more lucrative than meat, with rare hides fetching hundreds of dollars per square foot. Global leather-product sales total $63 billion a year, according to Bain.
A University of Oxford study of cultured meat estimated a 90 percent savings on resources, including feed, water, land, waste disposal, and greenhouse gas emissions, over the massive environmental costs of animal husbandry. Cultured leather will be equally environmentally friendly and can be grown in uniform shapes, eliminating waste from irregular natural hides as well as the damage wrought by insects, barbed wire, and sunburn. Modern Meadow’s leather will still need to undergo the notoriously toxic process of tanning, but it dispenses with the most chemical-intensive early steps, such as removing hair and flesh.
It will undoubtedly be easier to sell potential customers on lab-grown clothing than food. Designers who don’t use leather in their products, including Stella McCartney and Vivienne Westwood, would have a cruelty-free alternative. Karen Groner, a professor at New York City’s Fashion Institute of Technology, says Modern Meadow leather would have a clear appeal to the fashion business. “We all love animals, and no one wants to see them needlessly slaughtered,” she says.
Blog: A New Kind of Food Truck Makes Millions on Raw Meat
For shoppers concerned about animal cruelty, though, there’s one ingredient that Andras Forgacs calls the “skeleton in the closet” of cultured leather and meat. To multiply, the cells require fetal bovine serum, a fluid derived from calf fetuses. Plant-derived alternatives are costly and produced by genetically modified organisms. While the use of fetal bovine serum can be phased out, genetically modified substitutes may be essential.
For now, Modern Meadow is facing a more fundamental problem: scaling production to a commercial level. On a warm May morning, the Forgacs, senior scientists and co-founders Francoise Marga and Karoly Jakab, plus research assistant Ryan Kaesser gather in a conference room to game out the transition to manufacturing. The process, which takes roughly 30 days, is only just past the proof-of-concept stage and is still largely manual. Jakab excuses himself and ducks into the lab to feed burgeoning films of skin cells, gingerly pouring the red-orange culture medium into dishes. “Efficiency will be the critical hurdle,” says Andras Forgacs. “As we scale up, we need to make sure we can produce a high-quality product at a price the market will accept.”
In late summer, Modern Meadow expects to move from samples the size of credit cards to sheets at least 10 inches square, and the company aims to collaborate with designers next year on accessories and apparel. While many hurdles remain before cultured animal products hit store shelves, some people are eager to try the company’s leather on for size. Ingrid Newkirk, president of People for the Ethical Treatment of Animals, says she’s been following tissue engineering research for two decades and that it’s past time to replace the animal hide used in jackets, shoes, handbags, belts, couches, and car seats. “The impact of cultured leather will be phenomenal and wonderful,” she says.
<<<
>>> 7 Cool Uses of 3D Printing in Medicine
Tanya Lewis
LiveScience
February 04, 2013
http://www.livescience.com/26853-3d-printing-medicine.html
Printing organs?
Printers that spit out three-dimensional human cells and even organs, including the heart and liver, may seem like science fiction. But real scientists are taking real cracks at such a reality. Here are seven cool uses of such printing that could revolutionize medicine.
Printing human embryonic stem cells
Credit: M. Nakamura, Bioprinting Project, Kanagawa Academy of Science and Technology
Stem cells, those magical cells that can develop into many different kinds of tissue in the body, can now be printed, at least in the lab. In a study published Feb. 5, 2013, in the journal Science, researchers from the University of Edinburgh describe a valve-based cell printer that spits out living human embryonic stem cells. The cells could be used to create tissue for testing drugs or growing replacement organs, the scientists report.
Printing blood vessels & heart tissue
Printing some tissue types is already a reality. Gabor Forgacs from the University of Missouri in Columbia and colleagues printed blood vessels and sheets of cardiac tissue that "beat" like a real heart. The work was published in March 2008 in the journal Tissue Engineering. Forgacs and others started a company called Organovo to bring these products to market.
A group at the German Fraunhofer Institute has also created blood vessels, by printing artificial biological molecules with a 3D inkjet printer and zapping them into shape with a laser.
Printing skin
The last 25 years have seen great advances in creating tissue-engineered skin, which could be used to replace skin damaged from burns, skin diseases and other causes. Recently, scientists have added 3D printed skin to their repertoire. Lothar Koch of the Laser Center Hannover in Germany and colleagues laser-printed skin cells, as reported September 2010 in the journal Tissue Engineering Part C: Methods.
Patching a broken heart
Researchers are working on developing a "heart patch" made of 3D-printed cells that could repair damaged hearts. Ralf Gaebel of the University of Rostock, Germany, and colleagues made such a patch using a computerized laser-based printing technique. They implanted patches made of human cells in the hearts of rats that had suffered heart attacks; the rats' hearts that were patched showed improvement in function, the scientists reported in December 2011 in the journal Biomaterials.
Printing cartilage & bone
The skeletal system has also become a popular focus of 3D cell-printing efforts. In 2011, the same group from Germany that made the skin used laser printing to create grafts from stem cells that could develop into bone and cartilage. The work was published in January 2011 in the journal Tissue Engineering Part C: Methods.
Studying cancer with printed cells
Printing cells could lead to better ways of studying diseases in the lab and then developing therapies. For example, one group of researchers used an automated system to print ovarian cancer cells onto a gel in a lab dish where the cells could be grown and studied. The printing approach could enable scientists to study the tumor cells in a more systematic environment and use them to test out drugs. The study, led by biomedical engineer Utkan Demirci, of Harvard University Medical School and Brigham and Women's Hospital, was detailed in February 2011 in Biotechnology Journal.
Printing organs
Can we grow organs instead of transplanting them? That was the question surgeon Anthony Atala asked during a TED talk in 2010 that went viral. Ten Years ago, Atala, who directs the Wake Forest Institute for Regenerative Medicine, took stem cells from a patient with a failing bladder, grew a new bladder and transplanted it into the patient. Atala's more recent efforts have focused on printing organs, and he has since demonstrated an early experiment to print a transplantable kidney.
<<<
>>> Tiny organs printed in 3-D aim for 'body on a chip' for better drug tests
By Jeremy Hsu
9-16-13
http://www.nbcnews.com/tech/innovation/tiny-organs-printed-3-d-aim-body-chip-better-drug-f8C11170018
3D
Hyun-Wook Kang oversees the 3-D printer that will be used to print miniature organs for the "body on a chip" system..
Miniature human organs made by 3-D printing could create a "body on a chip" that enables better drug testing. That futuristic idea has become a new bioprinting project backed by $24 million from the U.S. Department of Defense.
The 2-inch "body on a chip" would represent a realistic testing ground for understanding how the human body might react to dangerous diseases, chemical warfare agents and new drugs intended to defend against biological or chemical attacks. Such technology could speed up drug development by replacing less-ideal animal testing or the simpler testing done on human cells in petri dishes — and perhaps save millions or even billions of dollars from being wasted on dead-end drug candidates that fail in human clinical trials.
"The question is whether can you have a better system to test these drugs, so that you can bypass cell testing and animal testing by going straight to miniature organs," said Tony Atala, director of the Wake Forest Institute for Regenerative Medicine in Winston-Salem, N.C.
Atala's group has pioneered 3-D printing methods that aim to build human organs with layer upon layer of cells. Their bioprinting methods lay down the cell layers along with artificial scaffolding to keep an organ's structure intact as it takes shape — a technique that has allowed the group to make tiny, less complex versions of full-size human organs. [See Photos of the 3-D-Printed 'Body on a Chip' System]
3D1
Developed by scientists at Brigham and Women's Hospital, this system of pumps and fluid channels houses miniature tissues samples that can be exposed to toxins as well as potential treatments. The ultimate goal is to evaluate heart, lung, liver and blood vessel tissue.
"We're printing miniature solid organs: miniature livers, hearts, lungs and vascular structures (blood vessels)," Atala told LiveScience.
The tiny organs intended for the "body on a chip" project don't represent fully functional hearts, livers and kidneys. Instead, they represent small chunks of human tissue from such organs connected together by a system of fluid channels that circulate blood substitute to keep the cells alive — all placed on a 2-inch (5 centimeters) chip with sensors to monitor everything.
Having an artificial circulatory system means researchers can introduce biological or chemical agents into the "blood" to see how it affects the different organs. The system's sensors would measure the temperature, oxygen levels, pH (how acidic or basic a fluid is) and other factors affecting the "body on a chip."
The Wake Forest Institute for Regenerative Medicine is leading the $24 million effort funded by the Space and Naval Warfare Systems Center, Pacific (SSC Pacific), on behalf of Defense Threat Reduction Agency (DTRA).
But the group of experts building the "body on a chip" also hails from Brigham and Women's Hospital in Boston, the University of Michigan, the U.S. Army Edgewood Chemical Biological Center, Morgan State University in Baltimore and the Johns Hopkins Bloomberg School of Public Health. Together, they hope to create a drug development tool for the 21st century that helps modern medicine rapidly respond to fast-moving pandemics or bioterrorism attacks.
"We will know not just how a drug affects one organ, but how a drug affects major body systems together in a chip," Atala said.
<<<
| Volume | |
| Day Range: | |
| Bid Price | |
| Ask Price | |
| Last Trade Time: |
