Friday, May 09, 2014 8:08:41 PM
>>> Organovo's Bioprinting More Than A 'Stupid Exercise'
May. 8, 2014
http://seekingalpha.com/article/2202063-organovos-bioprinting-more-than-a-stupid-exercise
Summary
•Misleading short pieces and a cooling sector have had a negative effect on Organovo's share price.
•Organovo's fully cellular bioprinting method provides crucial benefits over competing approaches. This will distinguish Organovo as a 3D bioprinting leader.
•Data that the company has released has been compelling enough to warrant contracts without third-party validation and product launch.
Organovo (ONVO) is down 50% YTD due to a combination of short sellers calling out the company's valuation and the struggles of the biotech and 3D printing sectors. The latest critic, Simeon Research, is an anonymous equity research firm created in February 2014 entirely to issue negative reports about Organovo. Reading through the 81 pages of Simeon's "research"; I wondered what vendetta Simeon had against Organovo. The blatantly negative tone and misleading information stood out, taking away from the report's objective.
I started covering Organovo back in September 2012, when the company was trading $2.10 and regularly criticized. Is the company real? Does the NovoGen bioprinter even exist? Is the facility in San Diego just a cover up? Looking back, it's laughable what shorts were critical of. According to Simeon, which gave a price target of $1.35, not much seems to have changed with regards to sentiment surrounding Organovo. Till now, the company has proved doubters wrong. I expect this to continue.
Bioprinting: A Stupid Exercise?
In a 2012 seminar, one of Organovo's founders, Dr. Gabor Forgacs, who was also the company's chief scientific officer at the time, uttered the words "it's nothing, it's a stupid exercise" when talking about bioprinting. Simeon took this quote out of context and ran with it, claiming it expressed the public views of Organovo's scientific founder.
What was not mentioned, however, was the fact that Forgacs spoke for over an hour about Organovo's bioprinting process and the future of bioprinting in a very positive manner. Have a look at the YouTube clip. The 24-minute mark is where Forgacs mentions "stupid exercise". I highly recommend you watch the whole clip to get the FULL views of Organovo's scientific founder, instead of nitpicking certain parts. Simeon Research uses ten second of a lecture that's an hour and seventeen minute to come to Forgacs' "public views" of bioprinting. Quite misleading.
The "Magic" of Bioprinting
The natural properties of cells are the magic behind the bioprinting concept. When placed in close proximity, cells aggregate and form their own extracellular matrix. This natural process finalizes the tissue from the printed cell. This is a visual (from Organovo's April 2014 investor presentation) to explain the process.
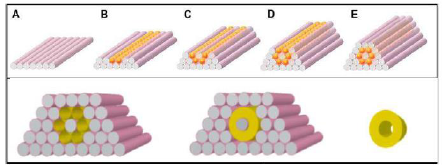
(Source: Organovo April 2014 Presentation)
Steps A-E is Organovo's layered printing process of cell aggregates (yellow) supported by agarose molecule hydrogel (grey cylinders). The bottom half of the picture is where the "magic" happens as the cell neutral agarose is removed and the cellular material fuses naturally to form the living cell.
To get to this "magic" step though, a special delivery device is needed. You can't just clump cells together and hope they aggregate naturally. Forgacs says a "good printer" optimizes the natural process by depositing cell aggregates in the right position to fuse. To trigger the magic of bioprinting, cells must be printed in a specific position; otherwise, the natural process will not occur. This is what Organovo's NovoGen bioprinter does.
Replicating such a process is easier said than done, due to Organovo's fully cellular approach (where no artificial scaffolds are being used) and intellectual property. Organovo's patent from Clemson University provides rights to use inkjet printer technology to dispense cells, and to create matrices of bioprinted cells on agarose hydrogel materials. Though there are competitors out there who also follow a similar bioprinting process, Forgacs refers to Organovo as "champions" in creating cell aggregates.
Tiers of Bioprinting
Printing scaffold with no cells
A lot of 3D bioprinting can actually be classified as 3D printing of gels/polymers into a shape with no cells inside. The cells are added afterwards. An example of this would be EnvisionTec's 3D Bioplotter. In their list of materials printed, living cells are not mentioned. The Bioplotter prints a polymer scaffold into a shape, which they can later add cells into the network to mimic natural harvesting and end up with a tissue. Mimicking proximity of cellular network is complicated, which is why the accomplishments of scaffold-based tissue engineering are more exception than rule. EnvisonTec's approach can be classified closer to 3D printing rather than bioprinting, since no cells are technically printed.
Simeon claims that EnvisionTec has a significant competitive advantage over Organovo, due to greater accuracy. The "accuracy" and "complexity" of the Bioplotter can print up to 5 different materials at once at a range of 1 micron. Compare this to Organovo's 20-micron precision. Again, this is misleading, because Organovo prints living cells and the Bioplotter prints materials like gelatin. Generally, cells are 20-50 microns in size to begin with, so it's not even possible to print 1 micron cells. An apples to apples comparison cannot be made.
Printing biomaterial with cells included
Another tier of bioprinting can be classified as 3D printing a gel, with cells included in the gel. These cells are embedded into the printed gel shape. Though this can be considered more cellular in nature, there are still issues trying to form tissue from this approach. These non-natural materials are foreign to human bodies, and thus, may reduce effectiveness of tissue regeneration by limiting cellular interaction. An example of a company utilizing this approach is privately-held Swiss RegenHU.
Fully (or substantially) cellular bioprinting
This approach does not use any artificial scaffolds, instead utilizes cells and hydrogel as support. This is Organovo's bioprinting method.
Using a recent cancer study, I will show the difference between Organovo's fully cellular bioprinting approach and that of competitors who use a combination of artificial biomaterials and cells.
Three-dimensional printing of Hela cells for cervical tumor model in vitro
A study from Drexel University compared 3D printing of Hela cells for cervical tumor model in vitro with a 2D model. The study used a 3D cell printer developed by the group at Drexler to print Hela cells (cervical tumor cells) and biomaterials (gelatin, alginate, fibrinogen) to mimic the cervical cancer microenvironment.
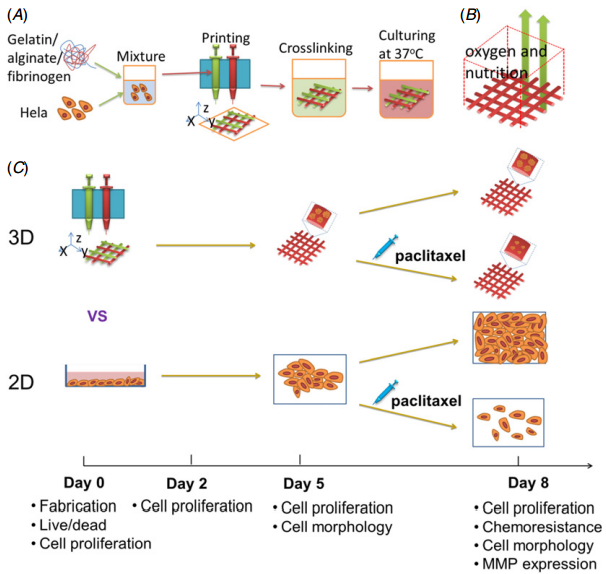
(Source: Y Zhao et al. Three-dimensional printing of Hela cells for cervical tumor model in vitro)
As seen in the images above, Drexel's bioprinter is shaping fibrinogen/gelatin gel rods into a grid shape. Within these gels, there are Hela cells which are being used as the cancer model. This bioprinting approach is using a combination of living cells and gels.
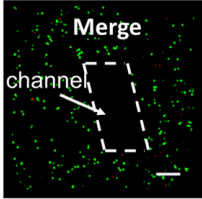
(Source: Y Zhao et al. Three-dimensional printing of Hela cells for cervical tumor model in vitro)
This image shows the 3D space, in which the green lights are cells and the rest of the space between the cells is gel material. By looking at the image, it can be estimated that the density of the model may be as low as 1% cellular, and the rest, gel material. This percentage may be a bit more as spheroids grow, however, even then, the model may be pushing 10% cellular.
The study demonstrated that Hela cells formed round spheroids with smooth surfaces and tight cell-to-cell connections within the 3D hydrogel, whereas Hela cells cultured on the 2D tissue culture plates showed a ?at and elongated morphology. Even with such a low volume of cellular makeup, 3D models revealed that the Hela cells showed a higher proliferation rate, MMP protein expression and chemoresistance than those in 2D culture. In conclusion, the results revealed that the printed 3D models have more simulated tumor characteristics compared with the 2D planar cell culture models.
Organovo's 3D bioprinted human breast cancer for in vitro drug screening
"Various techniques, such as multicellular spheroids, cell-seeding 3D scaffolds, hydrogel embedding, micro?uidic chips and cell patterning have been developed for construction of 3D in vitro tumor models." (Y Zhao et al., Three-dimensional printing of Hela cells for cervical tumor model in vitro) However, it is difficult to simulate a complex 3D physiological tumor microenvironment in most of the listed models, due to the limitations of the fabrication techniques.
Organovo's technique prints a mass of cells that is ALL cellular in nature. This makes Organovo's printed cells very architecturally similar to what is in the body, and as a result, more relevant to the human system. Last month, Organovo updated on the progress of the company's bioprinted breast cancer models at the American Association for Cancer Research (AACR) annual meeting.
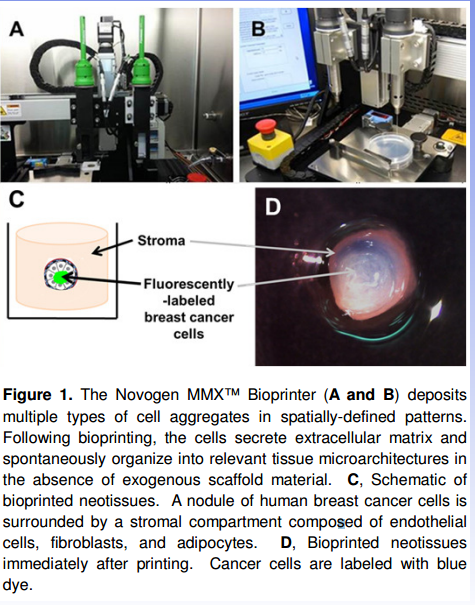
(Source: Organovo 2014 AACR Poster)
Image C above (from Organovo's 2014 AACR poster) shows that Organovo's bioprinted breast cancer tissue has regions within it. The exterior region, called the stroma, of the tumor builds a wall around the core. At the core is where you will find the mass of cancer cells. These are normal features of tissues that provide resistance to drug penetration. This model recreated the tissue as it is in the human body, at 100% cell density. Thus, it provides better understanding on how to approach drug discovery when compared to other bioprinting models that create a cube of gel with a fraction of cell volume. Though the combination of gel/cell structures (such as the Hela spheroids shown above) may have some similarities to actual human systems, they are not recreating the tissue. Organovo is. A spheroid is certainly a step into 3D, but lacks aspects that native tissues have, such as the outside layer of non-cancer cells.
Organovo's breast cancer model measured the early effects of small molecules (defined by molecular rate) on cancer cells, as well as different cell types in the breast microenvironment. The biological response profiles of chemotherapeutic agents cisplatin, paclitaxel, methotrexate, and tamoxifen were measured. Early data on performance of smaller drugs showed that they can penetrate tumor cell and have a differential effect, thus offering a better understanding on how the tested drugs may affect the tissue. The responses of the 3D breast cancer model to chemotherapeutic agents were compared to the response of 2D breast cancer cell lines to determine the relative efficacy of compounds in 2D versus 3D. Updated data is expected in the coming months.
Liver/Cell Assay Product Market
Organovo identifies the cell assay market as a $500M opportunity by 2018. The company believes that 3D cell assay products will satisfy the unmet customer demand in this market, and is targeting it with its liver product launch in Q4 2014. Organovo has released data to support its upcoming liver product; however, has faced criticism for comparing result to conventional 2D models.
Contrary to the belief of shorts, 2D models are still the conventional method being used in testing for toxicity. As CEO Keith Murphy states in his latest presentation, up to 95% of the market is still using 2D cell cultures. Reason for this lack of uptake is because the "3D models" already in market have not provided compelling enough data to move away from the current methodology. As Organovo prepares to launch its liver assay product, the company will have to show its product superiority to what customers are using (2D models), not a competing product that has not been able to penetrate the market.
Do not get me wrong, I do not think Organovo will suddenly become a market leader in the cell assay product market. The company will face an uphill battle and will need the functional validation of a third-party (key opinion leaders) to show superiority. Functional validation, in the form of journal publications, is expected to be released sometime in 2015, once the product has already been launched. Third-party publications will validate the liver tox data, and in turn, help Organovo's products gain greater sales traction in a new market. Murphy has stated that the pricing of Organovo's product will be in line with that of competitors, so as not to put the company at a price premium.
3D Liver Contracts
On the 3D liver tissue front, Organovo released that it has initiated contracts for research services. Contract services to date totaled in the "low hundreds of thousands of dollars ($100k to $400k range)". Organovo's 3D human liver tissue has shown to have the density of cells and binding capabilities that are the same as the cells in the human system. The data already published by the company has been compelling enough to net contracts before any third-party validation or official product launch. Even with existing market players supposedly having a "first-mover advantage" over Organovo, pre-released contracts indicate interest exists for Organovo's products and services. This early sign bodes well moving forward, as the company will have additional data validating its approach and providing greater chances of converting future contracts.
Risks
Organovo ended calendar year 2013 with ~$50M, and indicated that the quarterly burn rate was roughly $5M. This would place the company's current cash balance at about $45M and signify that, at a current burn rate, Organovo would have more than 2 years of cash on hand. The quarterly burn rate will likely increase with Organovo's expanded R&D efforts and product launch at the end of 2014. However, as shown above, revenue is also expected to rise, thus restoring some of the increased expenses. Trading at a current market cap of sub-$500 million market cap and a 50% drop YTD, dilution does not seem to be a pressing issue.
In my opinion, much of the remaining downside risk will come from volatile trading during downtime, when there is no news or updates released on Organovo's operations. No news is bad news, or at least this is the mindset of those following Organovo. The upcoming catalysts must be communicated with investors, which is something I believe the company PR team has done a good job of thus far.
Conclusion
All in all, the advancement of Organovo's bioprinting technology and released data will determine the value of the company. Investors will face volatility, as there will undoubtedly be skeptics out there who deem the company with foolish price targets of $1.35. Organovo has made great strides to validate its approach in the last couple of years, and I believe this will continue moving forward. Being a catalyst-driven stock, I am betting that ONVO's future catalysts will surprise the market and prove that bioprinting is not a "stupid exercise".
<<<
May. 8, 2014
http://seekingalpha.com/article/2202063-organovos-bioprinting-more-than-a-stupid-exercise
Summary
•Misleading short pieces and a cooling sector have had a negative effect on Organovo's share price.
•Organovo's fully cellular bioprinting method provides crucial benefits over competing approaches. This will distinguish Organovo as a 3D bioprinting leader.
•Data that the company has released has been compelling enough to warrant contracts without third-party validation and product launch.
Organovo (ONVO) is down 50% YTD due to a combination of short sellers calling out the company's valuation and the struggles of the biotech and 3D printing sectors. The latest critic, Simeon Research, is an anonymous equity research firm created in February 2014 entirely to issue negative reports about Organovo. Reading through the 81 pages of Simeon's "research"; I wondered what vendetta Simeon had against Organovo. The blatantly negative tone and misleading information stood out, taking away from the report's objective.
I started covering Organovo back in September 2012, when the company was trading $2.10 and regularly criticized. Is the company real? Does the NovoGen bioprinter even exist? Is the facility in San Diego just a cover up? Looking back, it's laughable what shorts were critical of. According to Simeon, which gave a price target of $1.35, not much seems to have changed with regards to sentiment surrounding Organovo. Till now, the company has proved doubters wrong. I expect this to continue.
Bioprinting: A Stupid Exercise?
In a 2012 seminar, one of Organovo's founders, Dr. Gabor Forgacs, who was also the company's chief scientific officer at the time, uttered the words "it's nothing, it's a stupid exercise" when talking about bioprinting. Simeon took this quote out of context and ran with it, claiming it expressed the public views of Organovo's scientific founder.
What was not mentioned, however, was the fact that Forgacs spoke for over an hour about Organovo's bioprinting process and the future of bioprinting in a very positive manner. Have a look at the YouTube clip. The 24-minute mark is where Forgacs mentions "stupid exercise". I highly recommend you watch the whole clip to get the FULL views of Organovo's scientific founder, instead of nitpicking certain parts. Simeon Research uses ten second of a lecture that's an hour and seventeen minute to come to Forgacs' "public views" of bioprinting. Quite misleading.
The "Magic" of Bioprinting
The natural properties of cells are the magic behind the bioprinting concept. When placed in close proximity, cells aggregate and form their own extracellular matrix. This natural process finalizes the tissue from the printed cell. This is a visual (from Organovo's April 2014 investor presentation) to explain the process.

(Source: Organovo April 2014 Presentation)
Steps A-E is Organovo's layered printing process of cell aggregates (yellow) supported by agarose molecule hydrogel (grey cylinders). The bottom half of the picture is where the "magic" happens as the cell neutral agarose is removed and the cellular material fuses naturally to form the living cell.
To get to this "magic" step though, a special delivery device is needed. You can't just clump cells together and hope they aggregate naturally. Forgacs says a "good printer" optimizes the natural process by depositing cell aggregates in the right position to fuse. To trigger the magic of bioprinting, cells must be printed in a specific position; otherwise, the natural process will not occur. This is what Organovo's NovoGen bioprinter does.
Replicating such a process is easier said than done, due to Organovo's fully cellular approach (where no artificial scaffolds are being used) and intellectual property. Organovo's patent from Clemson University provides rights to use inkjet printer technology to dispense cells, and to create matrices of bioprinted cells on agarose hydrogel materials. Though there are competitors out there who also follow a similar bioprinting process, Forgacs refers to Organovo as "champions" in creating cell aggregates.
Tiers of Bioprinting
Printing scaffold with no cells
A lot of 3D bioprinting can actually be classified as 3D printing of gels/polymers into a shape with no cells inside. The cells are added afterwards. An example of this would be EnvisionTec's 3D Bioplotter. In their list of materials printed, living cells are not mentioned. The Bioplotter prints a polymer scaffold into a shape, which they can later add cells into the network to mimic natural harvesting and end up with a tissue. Mimicking proximity of cellular network is complicated, which is why the accomplishments of scaffold-based tissue engineering are more exception than rule. EnvisonTec's approach can be classified closer to 3D printing rather than bioprinting, since no cells are technically printed.
Simeon claims that EnvisionTec has a significant competitive advantage over Organovo, due to greater accuracy. The "accuracy" and "complexity" of the Bioplotter can print up to 5 different materials at once at a range of 1 micron. Compare this to Organovo's 20-micron precision. Again, this is misleading, because Organovo prints living cells and the Bioplotter prints materials like gelatin. Generally, cells are 20-50 microns in size to begin with, so it's not even possible to print 1 micron cells. An apples to apples comparison cannot be made.
Printing biomaterial with cells included
Another tier of bioprinting can be classified as 3D printing a gel, with cells included in the gel. These cells are embedded into the printed gel shape. Though this can be considered more cellular in nature, there are still issues trying to form tissue from this approach. These non-natural materials are foreign to human bodies, and thus, may reduce effectiveness of tissue regeneration by limiting cellular interaction. An example of a company utilizing this approach is privately-held Swiss RegenHU.
Fully (or substantially) cellular bioprinting
This approach does not use any artificial scaffolds, instead utilizes cells and hydrogel as support. This is Organovo's bioprinting method.
Using a recent cancer study, I will show the difference between Organovo's fully cellular bioprinting approach and that of competitors who use a combination of artificial biomaterials and cells.
Three-dimensional printing of Hela cells for cervical tumor model in vitro
A study from Drexel University compared 3D printing of Hela cells for cervical tumor model in vitro with a 2D model. The study used a 3D cell printer developed by the group at Drexler to print Hela cells (cervical tumor cells) and biomaterials (gelatin, alginate, fibrinogen) to mimic the cervical cancer microenvironment.

(Source: Y Zhao et al. Three-dimensional printing of Hela cells for cervical tumor model in vitro)
As seen in the images above, Drexel's bioprinter is shaping fibrinogen/gelatin gel rods into a grid shape. Within these gels, there are Hela cells which are being used as the cancer model. This bioprinting approach is using a combination of living cells and gels.

(Source: Y Zhao et al. Three-dimensional printing of Hela cells for cervical tumor model in vitro)
This image shows the 3D space, in which the green lights are cells and the rest of the space between the cells is gel material. By looking at the image, it can be estimated that the density of the model may be as low as 1% cellular, and the rest, gel material. This percentage may be a bit more as spheroids grow, however, even then, the model may be pushing 10% cellular.
The study demonstrated that Hela cells formed round spheroids with smooth surfaces and tight cell-to-cell connections within the 3D hydrogel, whereas Hela cells cultured on the 2D tissue culture plates showed a ?at and elongated morphology. Even with such a low volume of cellular makeup, 3D models revealed that the Hela cells showed a higher proliferation rate, MMP protein expression and chemoresistance than those in 2D culture. In conclusion, the results revealed that the printed 3D models have more simulated tumor characteristics compared with the 2D planar cell culture models.
Organovo's 3D bioprinted human breast cancer for in vitro drug screening
"Various techniques, such as multicellular spheroids, cell-seeding 3D scaffolds, hydrogel embedding, micro?uidic chips and cell patterning have been developed for construction of 3D in vitro tumor models." (Y Zhao et al., Three-dimensional printing of Hela cells for cervical tumor model in vitro) However, it is difficult to simulate a complex 3D physiological tumor microenvironment in most of the listed models, due to the limitations of the fabrication techniques.
Organovo's technique prints a mass of cells that is ALL cellular in nature. This makes Organovo's printed cells very architecturally similar to what is in the body, and as a result, more relevant to the human system. Last month, Organovo updated on the progress of the company's bioprinted breast cancer models at the American Association for Cancer Research (AACR) annual meeting.

(Source: Organovo 2014 AACR Poster)
Image C above (from Organovo's 2014 AACR poster) shows that Organovo's bioprinted breast cancer tissue has regions within it. The exterior region, called the stroma, of the tumor builds a wall around the core. At the core is where you will find the mass of cancer cells. These are normal features of tissues that provide resistance to drug penetration. This model recreated the tissue as it is in the human body, at 100% cell density. Thus, it provides better understanding on how to approach drug discovery when compared to other bioprinting models that create a cube of gel with a fraction of cell volume. Though the combination of gel/cell structures (such as the Hela spheroids shown above) may have some similarities to actual human systems, they are not recreating the tissue. Organovo is. A spheroid is certainly a step into 3D, but lacks aspects that native tissues have, such as the outside layer of non-cancer cells.
Organovo's breast cancer model measured the early effects of small molecules (defined by molecular rate) on cancer cells, as well as different cell types in the breast microenvironment. The biological response profiles of chemotherapeutic agents cisplatin, paclitaxel, methotrexate, and tamoxifen were measured. Early data on performance of smaller drugs showed that they can penetrate tumor cell and have a differential effect, thus offering a better understanding on how the tested drugs may affect the tissue. The responses of the 3D breast cancer model to chemotherapeutic agents were compared to the response of 2D breast cancer cell lines to determine the relative efficacy of compounds in 2D versus 3D. Updated data is expected in the coming months.
Liver/Cell Assay Product Market
Organovo identifies the cell assay market as a $500M opportunity by 2018. The company believes that 3D cell assay products will satisfy the unmet customer demand in this market, and is targeting it with its liver product launch in Q4 2014. Organovo has released data to support its upcoming liver product; however, has faced criticism for comparing result to conventional 2D models.
Contrary to the belief of shorts, 2D models are still the conventional method being used in testing for toxicity. As CEO Keith Murphy states in his latest presentation, up to 95% of the market is still using 2D cell cultures. Reason for this lack of uptake is because the "3D models" already in market have not provided compelling enough data to move away from the current methodology. As Organovo prepares to launch its liver assay product, the company will have to show its product superiority to what customers are using (2D models), not a competing product that has not been able to penetrate the market.
Do not get me wrong, I do not think Organovo will suddenly become a market leader in the cell assay product market. The company will face an uphill battle and will need the functional validation of a third-party (key opinion leaders) to show superiority. Functional validation, in the form of journal publications, is expected to be released sometime in 2015, once the product has already been launched. Third-party publications will validate the liver tox data, and in turn, help Organovo's products gain greater sales traction in a new market. Murphy has stated that the pricing of Organovo's product will be in line with that of competitors, so as not to put the company at a price premium.
3D Liver Contracts
On the 3D liver tissue front, Organovo released that it has initiated contracts for research services. Contract services to date totaled in the "low hundreds of thousands of dollars ($100k to $400k range)". Organovo's 3D human liver tissue has shown to have the density of cells and binding capabilities that are the same as the cells in the human system. The data already published by the company has been compelling enough to net contracts before any third-party validation or official product launch. Even with existing market players supposedly having a "first-mover advantage" over Organovo, pre-released contracts indicate interest exists for Organovo's products and services. This early sign bodes well moving forward, as the company will have additional data validating its approach and providing greater chances of converting future contracts.
Risks
Organovo ended calendar year 2013 with ~$50M, and indicated that the quarterly burn rate was roughly $5M. This would place the company's current cash balance at about $45M and signify that, at a current burn rate, Organovo would have more than 2 years of cash on hand. The quarterly burn rate will likely increase with Organovo's expanded R&D efforts and product launch at the end of 2014. However, as shown above, revenue is also expected to rise, thus restoring some of the increased expenses. Trading at a current market cap of sub-$500 million market cap and a 50% drop YTD, dilution does not seem to be a pressing issue.
In my opinion, much of the remaining downside risk will come from volatile trading during downtime, when there is no news or updates released on Organovo's operations. No news is bad news, or at least this is the mindset of those following Organovo. The upcoming catalysts must be communicated with investors, which is something I believe the company PR team has done a good job of thus far.
Conclusion
All in all, the advancement of Organovo's bioprinting technology and released data will determine the value of the company. Investors will face volatility, as there will undoubtedly be skeptics out there who deem the company with foolish price targets of $1.35. Organovo has made great strides to validate its approach in the last couple of years, and I believe this will continue moving forward. Being a catalyst-driven stock, I am betting that ONVO's future catalysts will surprise the market and prove that bioprinting is not a "stupid exercise".
<<<
Join the InvestorsHub Community
Register for free to join our community of investors and share your ideas. You will also get access to streaming quotes, interactive charts, trades, portfolio, live options flow and more tools.









