Register for free to join our community of investors and share your ideas. You will also get access to streaming quotes, interactive charts, trades, portfolio, live options flow and more tools.
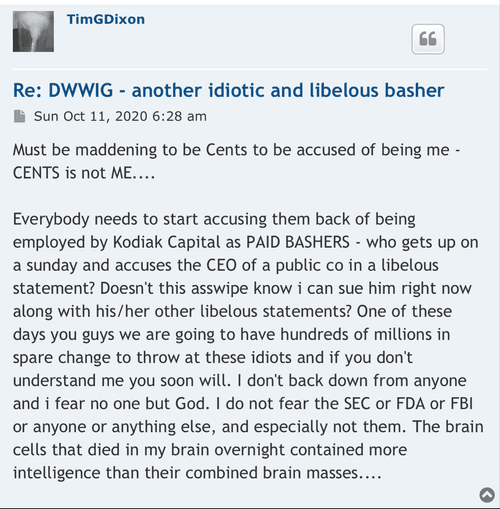
Not true? LMAO. Why don't you get back to not dealing with the likes of me. It doesn't work out well for you when you attempt to.
What do you know about truth? Your post history suggests that it is not much at all.

You didn't answer my question. Surely we can agree that anyone who has been silly enough to follow your financial advice (or any of the other TSOI bulls/longs) has not fared well to this point. Right?
Not true, from what the stock was up to at that time, I was down that much. But, when it was up, I sold enough to get my investment back. I can't lose anything. You should know how in invest. I don't usually say things like this, but, like I said, I have a hard time dealing with people like you.
Oh sure, sure. You won. If you were down 26K on 4.20.21, you have likely lost a lot more since. If that is winning, I'd hate to see what losing looks like

If you don't deal with people like me and no one should pay me any attention, why do you respond to so many of my posts? Surely we can agree that anyone who has been silly enough to follow your financial advice (or any of the other TSOI bulls/longs) has not fared well to this point.
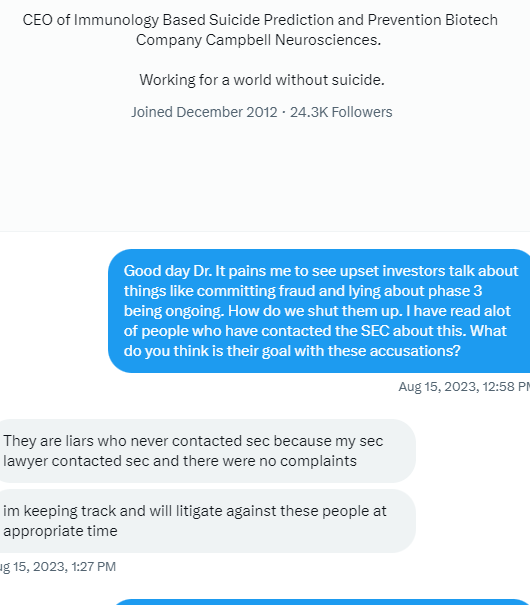
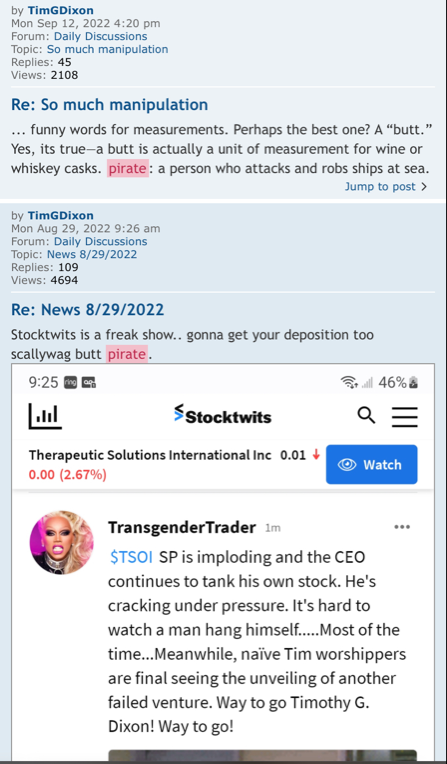
Why do I attack TSOI? By "attack" do you mean sharing information that casts TSOI in a negative light? Well this is a stock board that deals with TSOI and most of the information pertaining to TSOI is negative (have you read the recent SEC filings?!). I have never invested in a stock that is more rotten and deserving of exposure and consequences.
And why do you just attack Tsoi, there’s many more to attack. You should look into that.
Looks like I won, the way you play, anyways. Believe me, you’re someone that no one should pay attention to. Sad.
-If you don't deal with "people like me," why don't you just ignore me. If you keep leaving your silly little clown emojis and discussing people instead of the company and stock then don't get surprised when you get called on it.
-You have no idea whether or not I am an investor. It is none of your business.
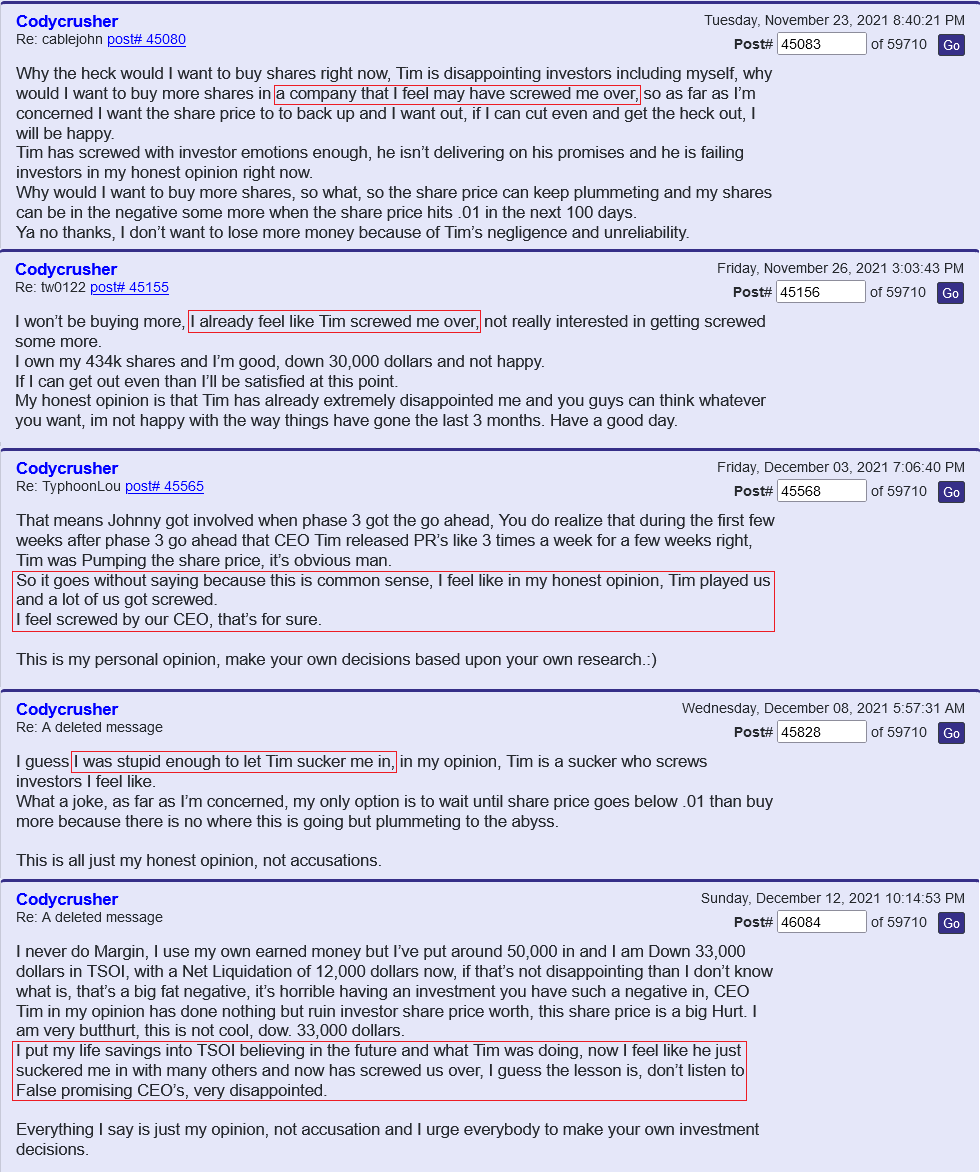
-You have been saying it is only a matter of time before TSOI makes people money for years. Meanwhile TSOI stock has plummeted. Help people? Shareholders have lost 99% of TSOI market cap and TSOI has nothing near commercialization. Who has it helped? When do you accept your forecasting is horrible?
-Glad the products have "worked" for you. Given the decreasing/small sales it does not appear that many people share your confidence in TSOI's products. How have they fared in registered clinical trials....oh never mind.
-What I do in my life is none of your business either. This is a stock board. I am here to discuss TSOI. If you don't like that why don't you move over to the FB page or get Dixon to start the forum up again so you can censor views you don't like and group bully people you disagree with?
-People are free, of course, to look at my post history. I am clearly bearish and don't like lies, incompetence, and group think. As we have already agreed you should just ignore me, but if you want to discuss any of my post history, I would be happy to do so.
-Let's take a brief look at your post history.

Why all the scammy "buy alerts"? How have all those people you advised to buy (all the way back to 3.28.22 at least) fared? Given your history and MO should anyone be taking your advice seriously at this point?
I've said my piece many times, I don't deal with people that are like you. You're not an investor. I'll again say: It's only a matter of time before TSOi makes us a lot of money, and better yet help many people. I've tried some of their products, and know they work. What have you done, except say your piece over, and over and over. What's the point of you even being on this board? All a person has to do is look at your post history.
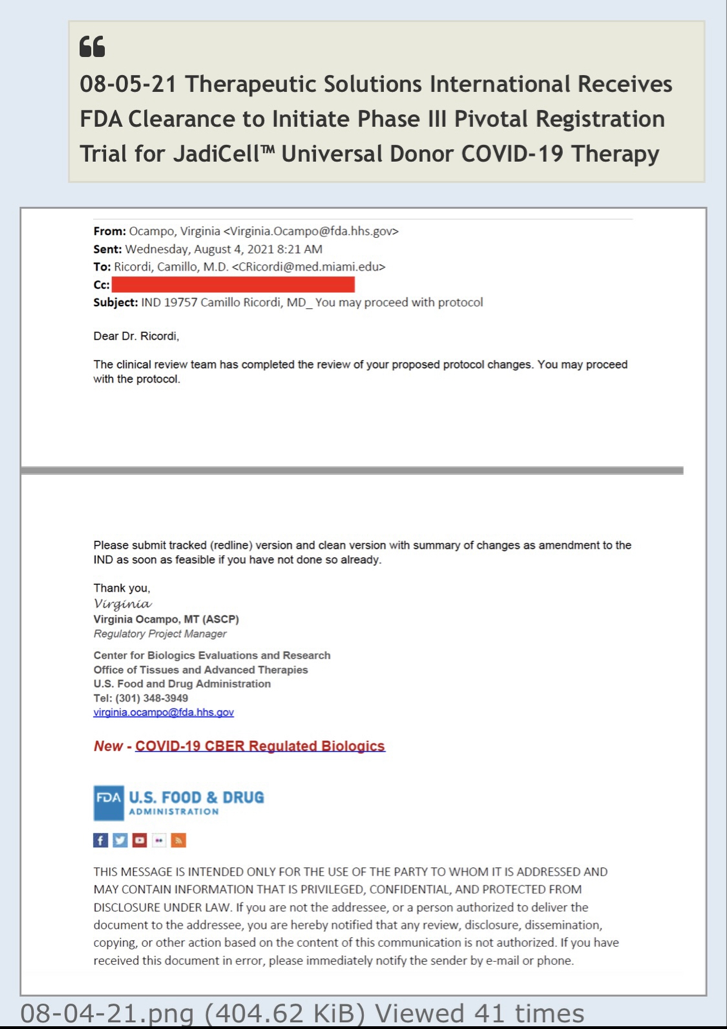
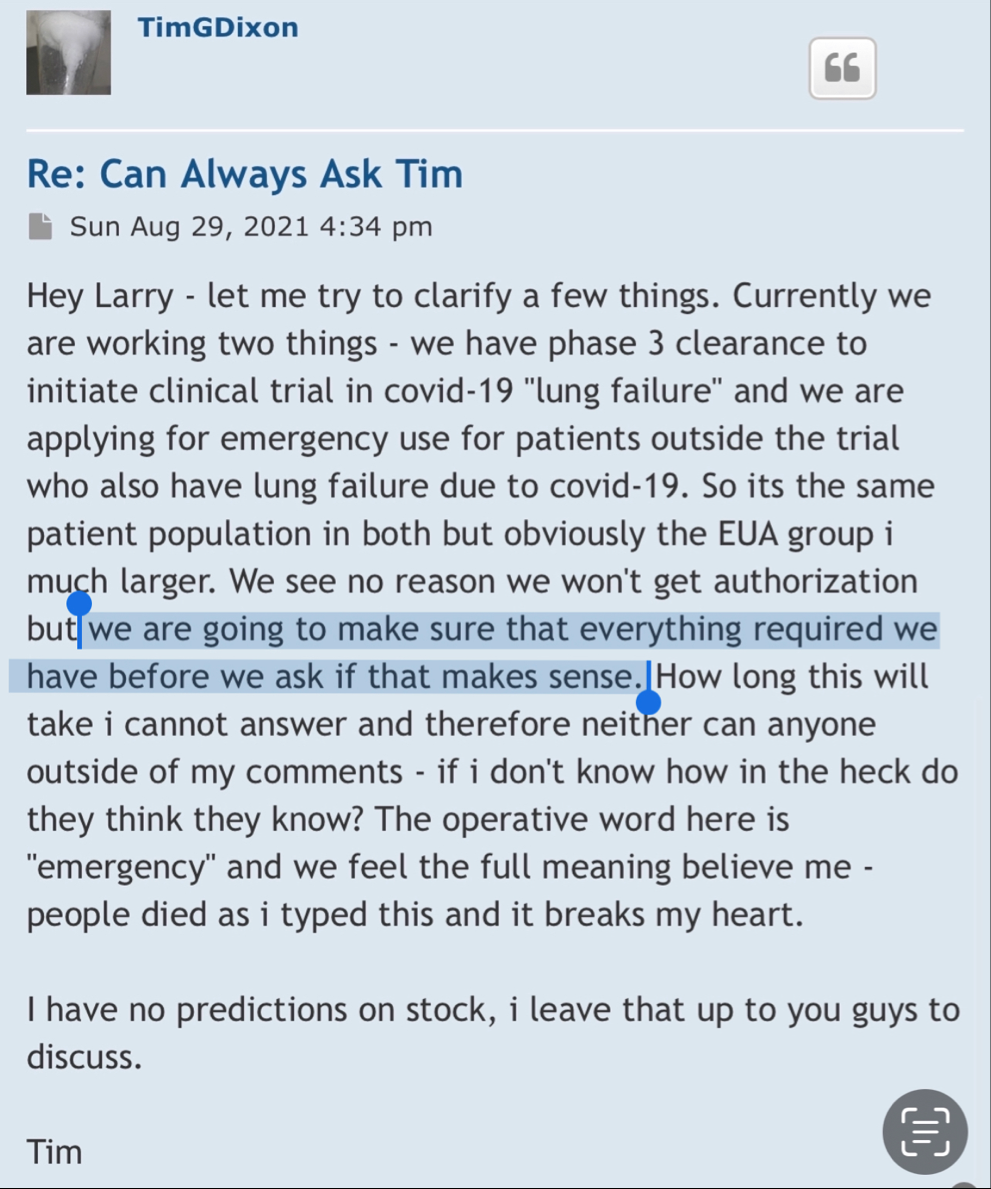
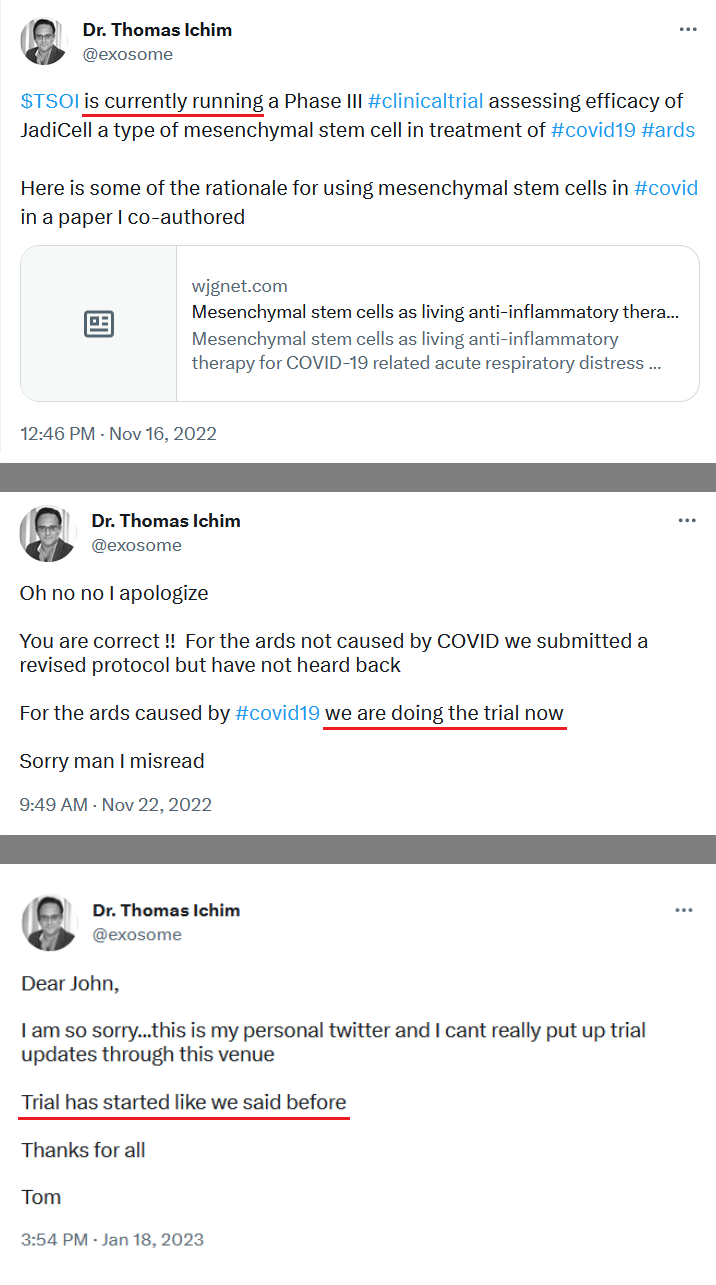
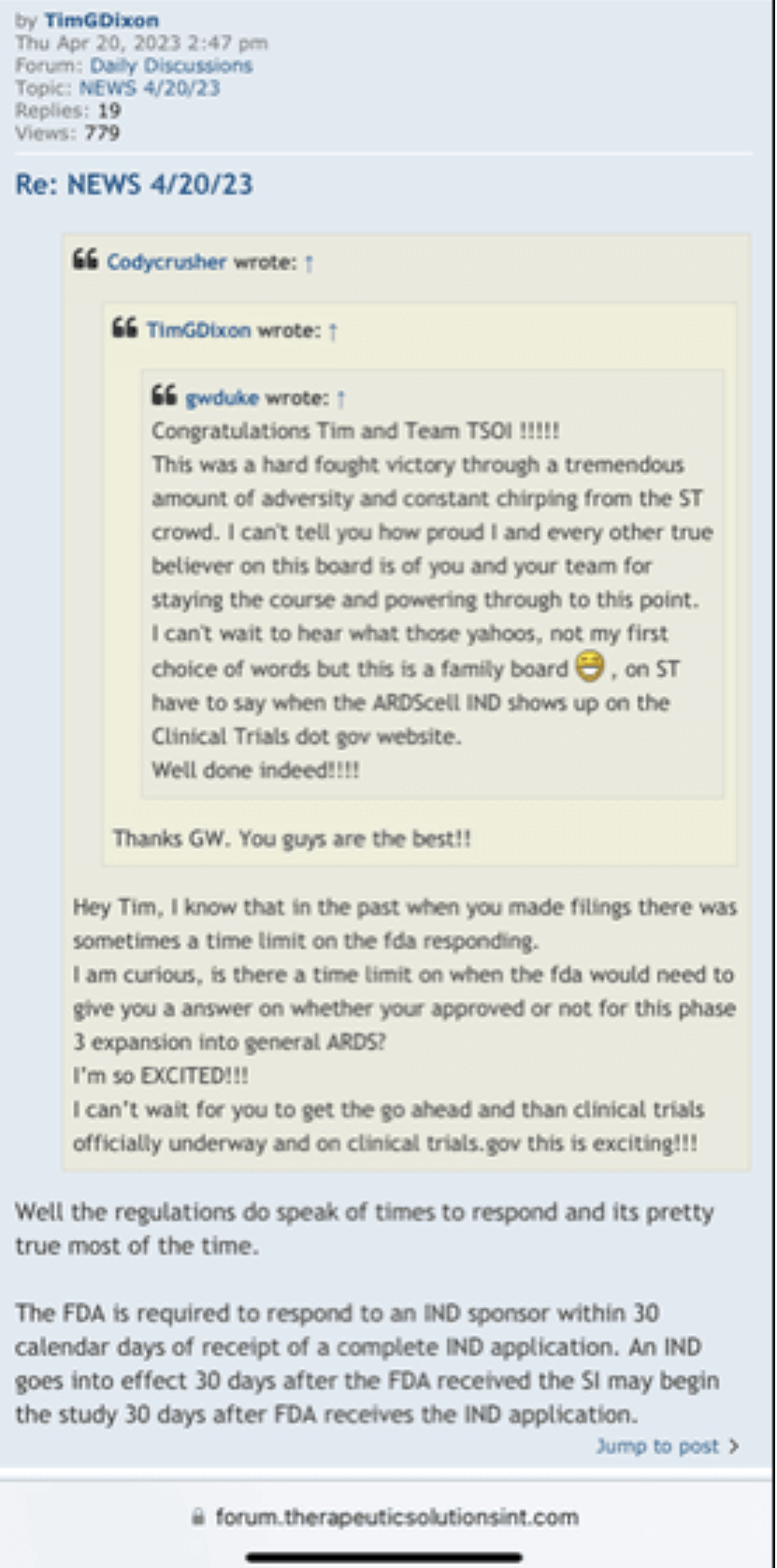
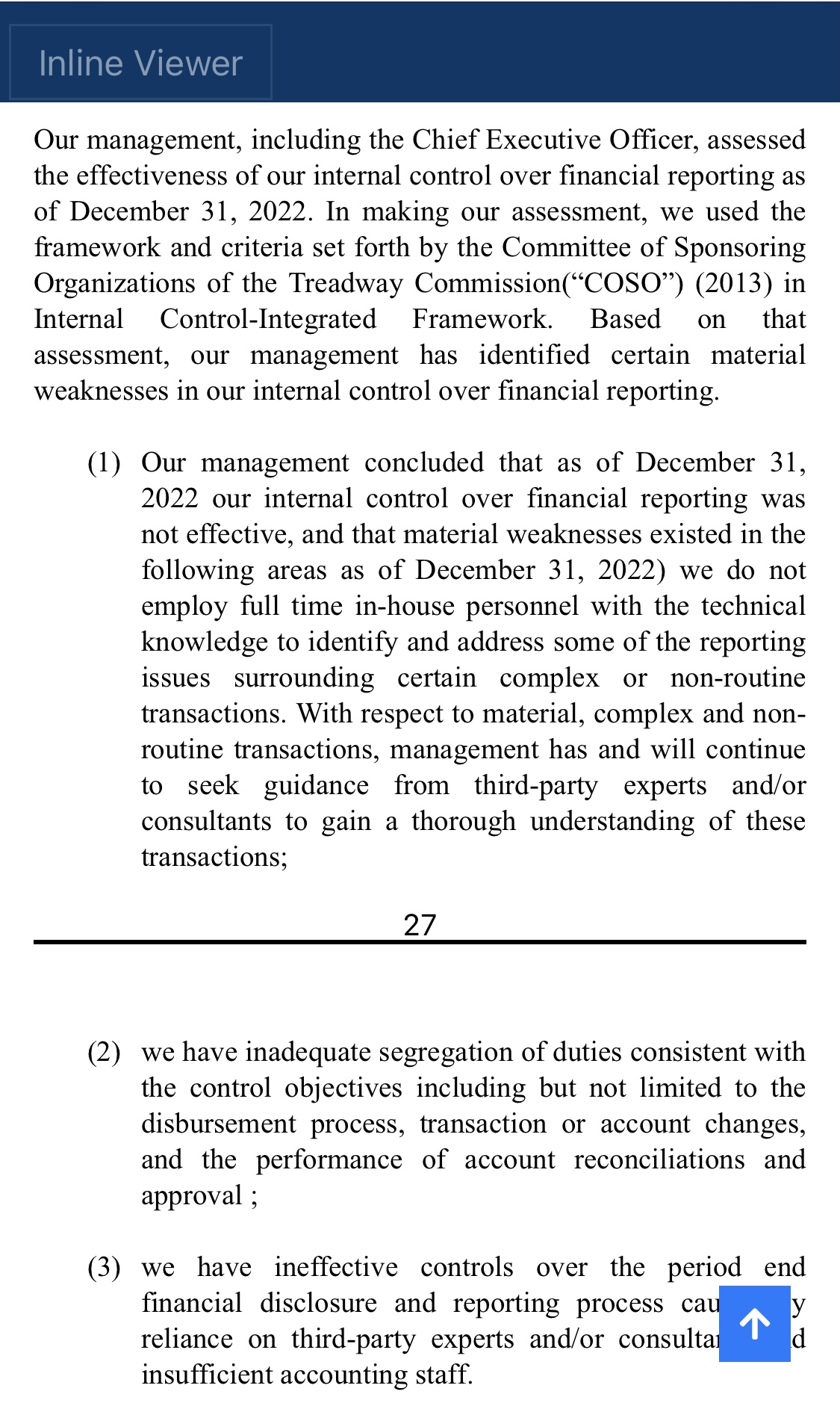
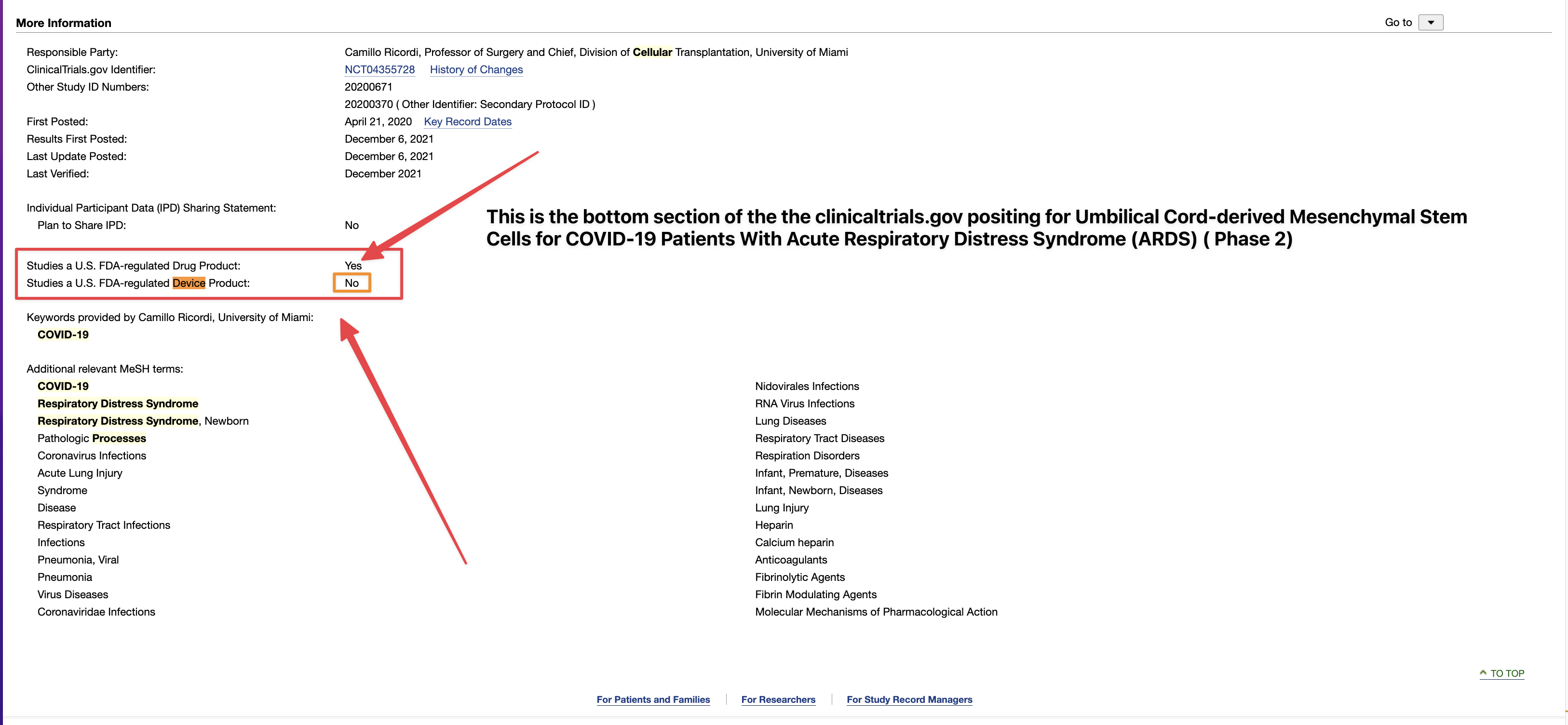
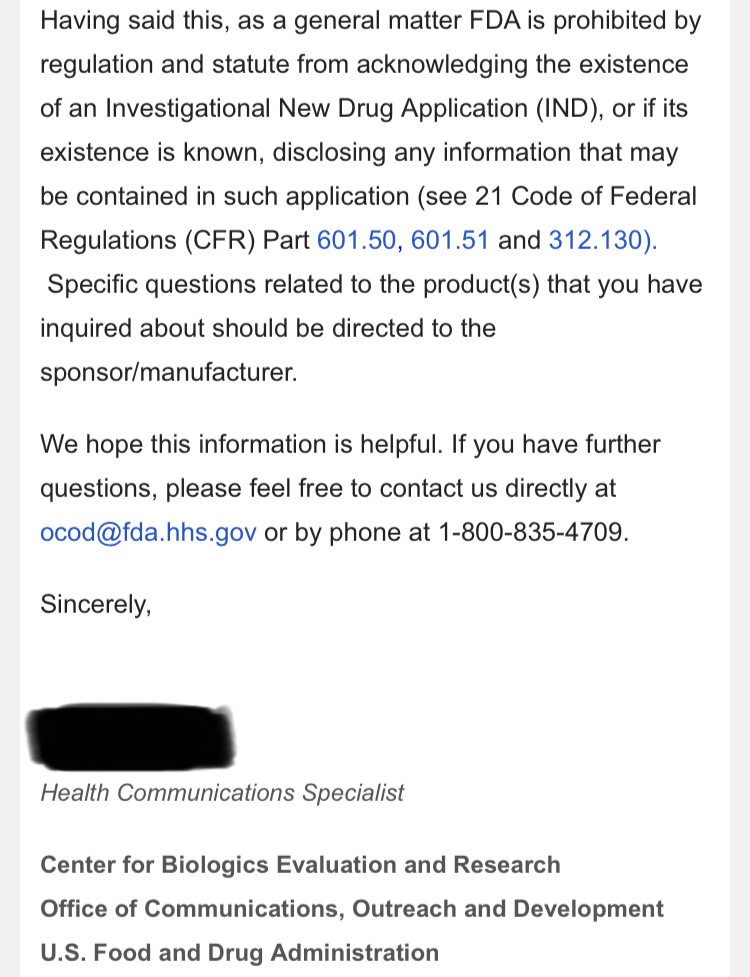
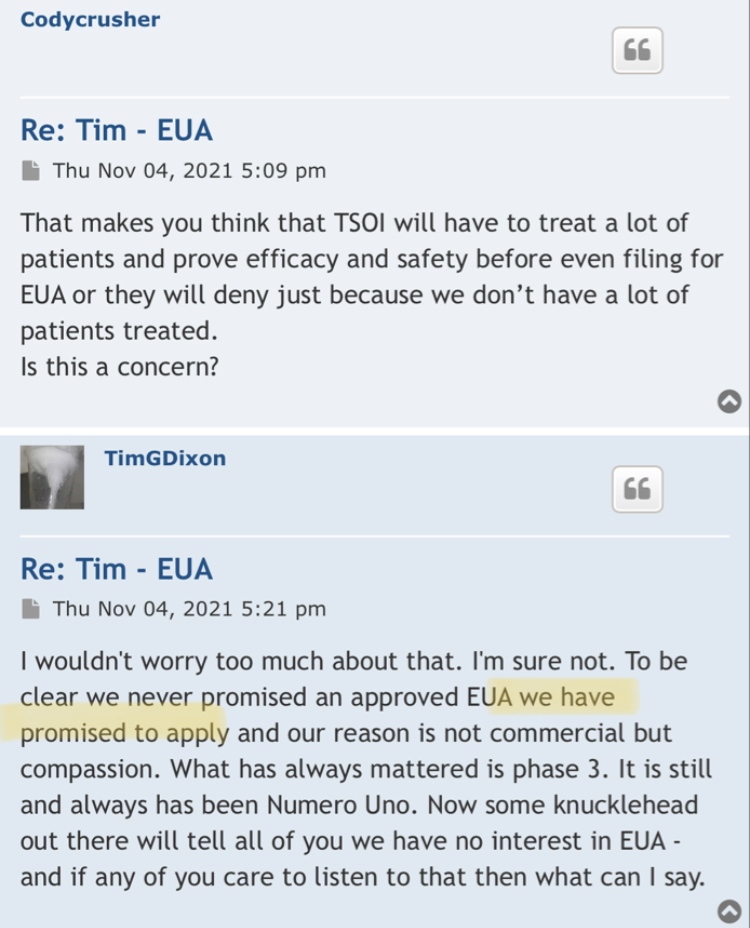
We, of course, do not know why the application was not approved. The fact remains, however, that the FDA does not have a general "aging" requirement. The fact also remains that TSOI rarely provides transparent updates on FDA communications. TSOI, in fact, has a history of providing false and misleading updates along such lines. It is also worth noting that TSOI has never completed a registered clinical trial of its own, has none underway and will be very hard pressed to fund one if it ever gets the opportunity because of its horrible financial straits.
I work in the Pharmaceutical Industry. The general pat answer from CBER does not mean that this was not requested by the application reviewer.
@Yooperman, why don't you try and progress beyond the clown emoji and present a perspective, formulate an idea or counterpoint, marshal some evidence or something along those lines? Give it a try.
Right, you have dealt with the FDA. For what? Regardless, you are mistaken. The response below is directly from the FDA itself. Email them yourself to confirm if you'd like,

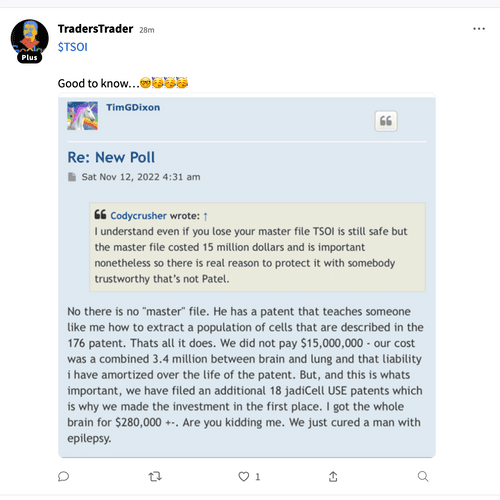
It sounds like TSOI never even had its own MCB (which is crazy given how long they misled investors about ongoing clinical trials)
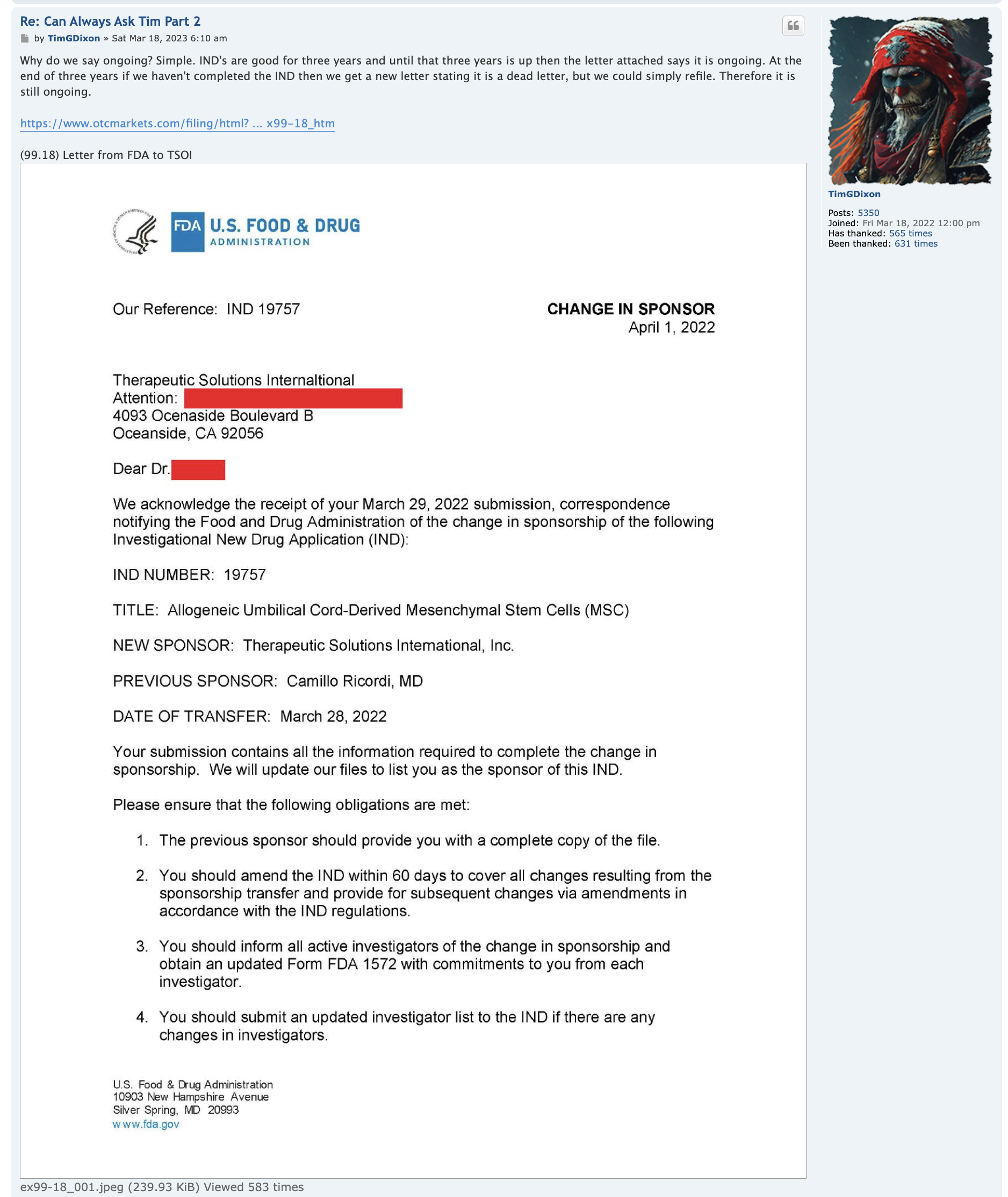
As the following document makes clear, "Master Cell Bank (MCB) You should include in the IND information regarding MCB history, source, derivation and characterization, including testing to adequately establish the safety, identity, purity, and stability of the cells."
(excerpt from fda.gov/media/73624/download)
That might help explain why their NDAs have not gone anywhere and the trials were never "ongoing."
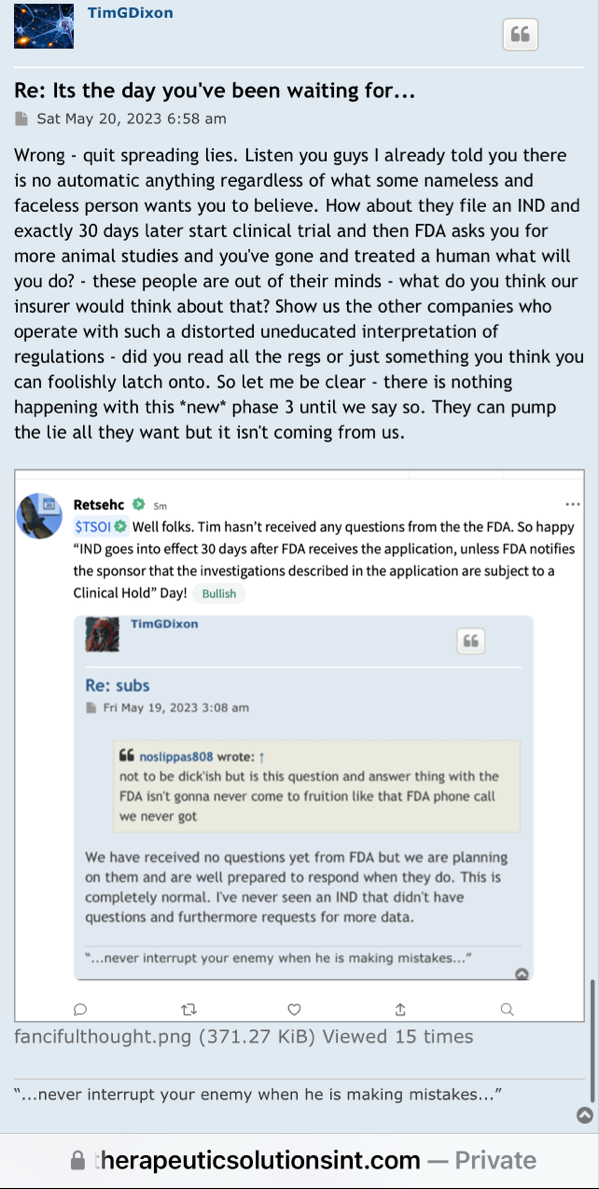
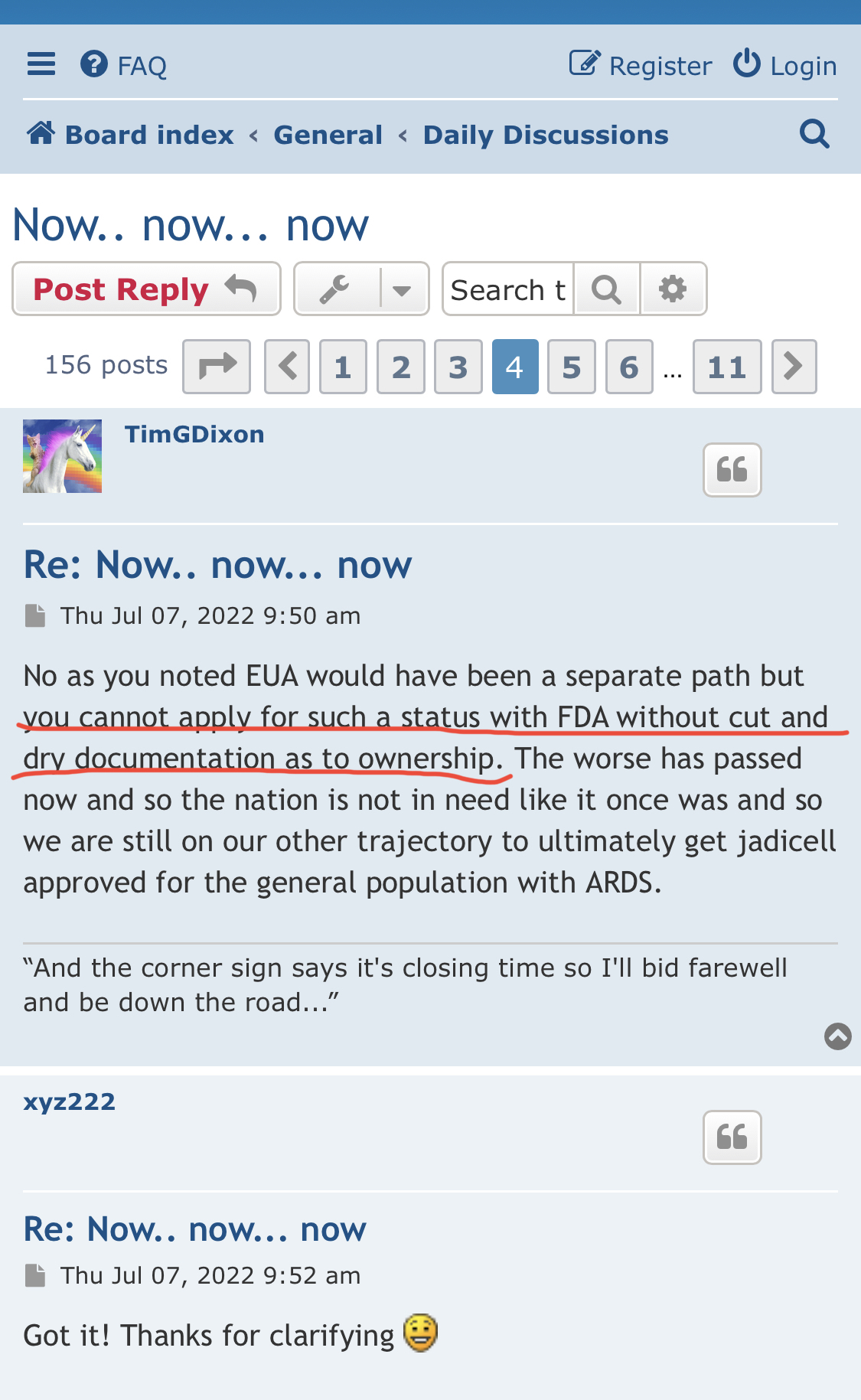
I've dealt with the FDA. To state the FDA does not have any aging requirements is a mistaken notion. FDA Reviewers all the time have specific information and requirements they want to see that are not necessarily outlined in black and white in regulations or FDA guidances. You don't know what the reviewers asked for, especially in a developing field of science.
Plus, stochastics buy signal today!
PPS completely above it's 50 day MA today! First time since Jan!
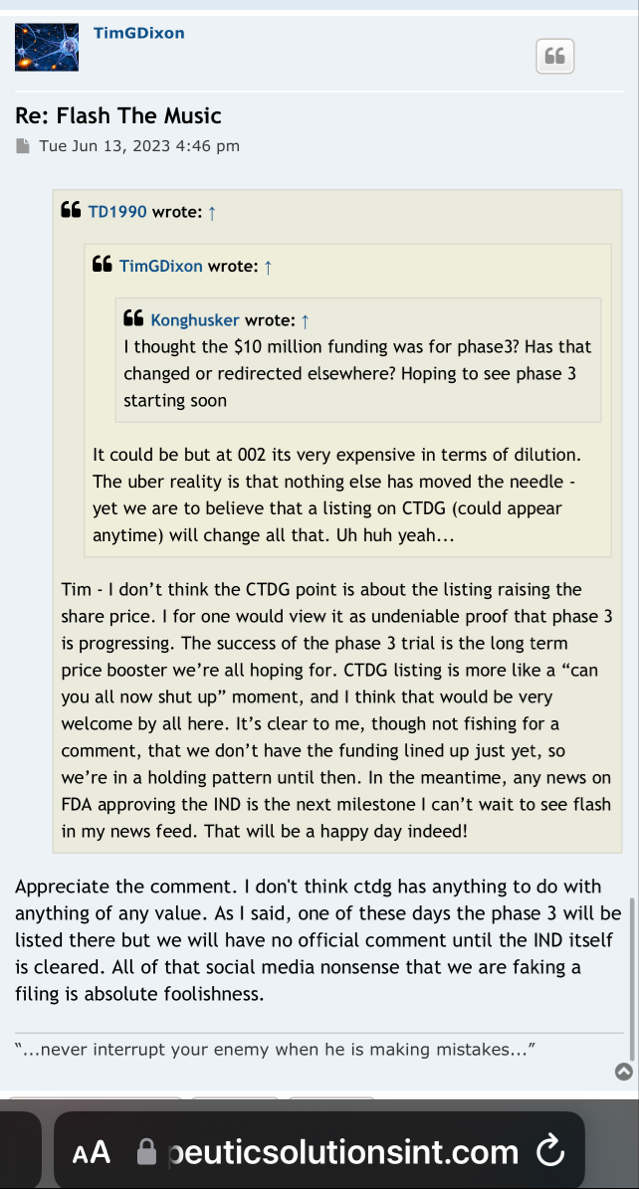
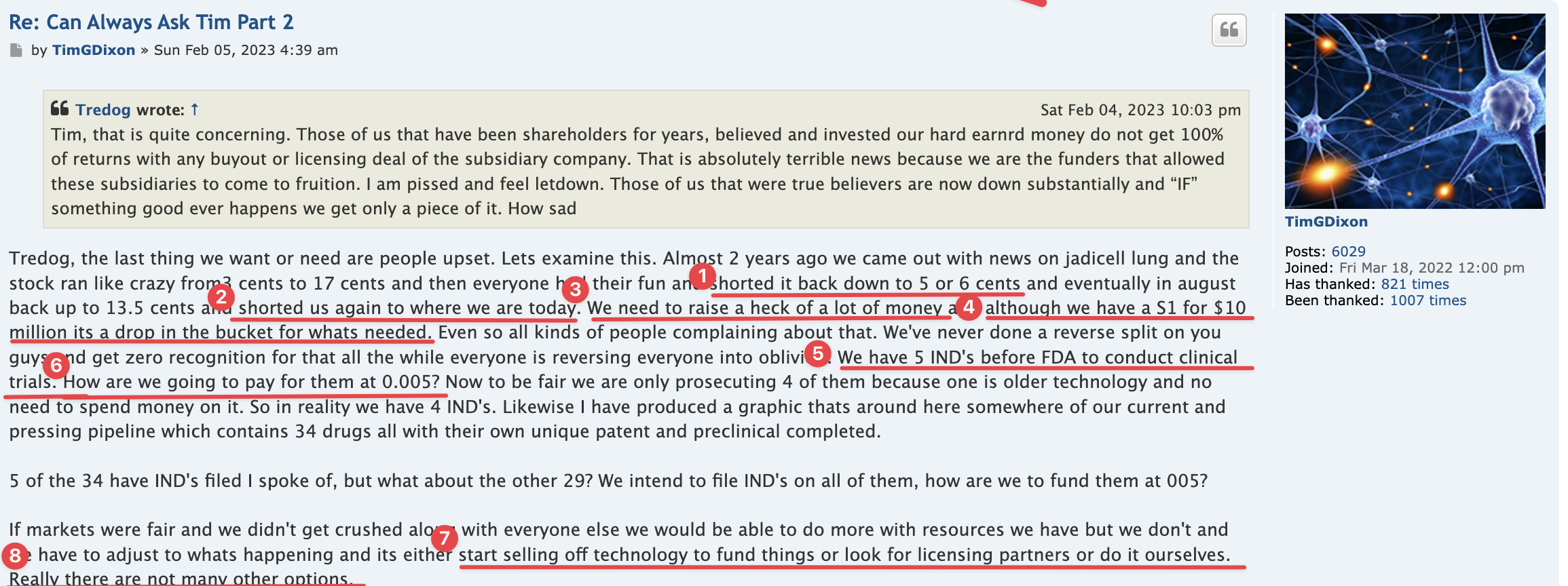
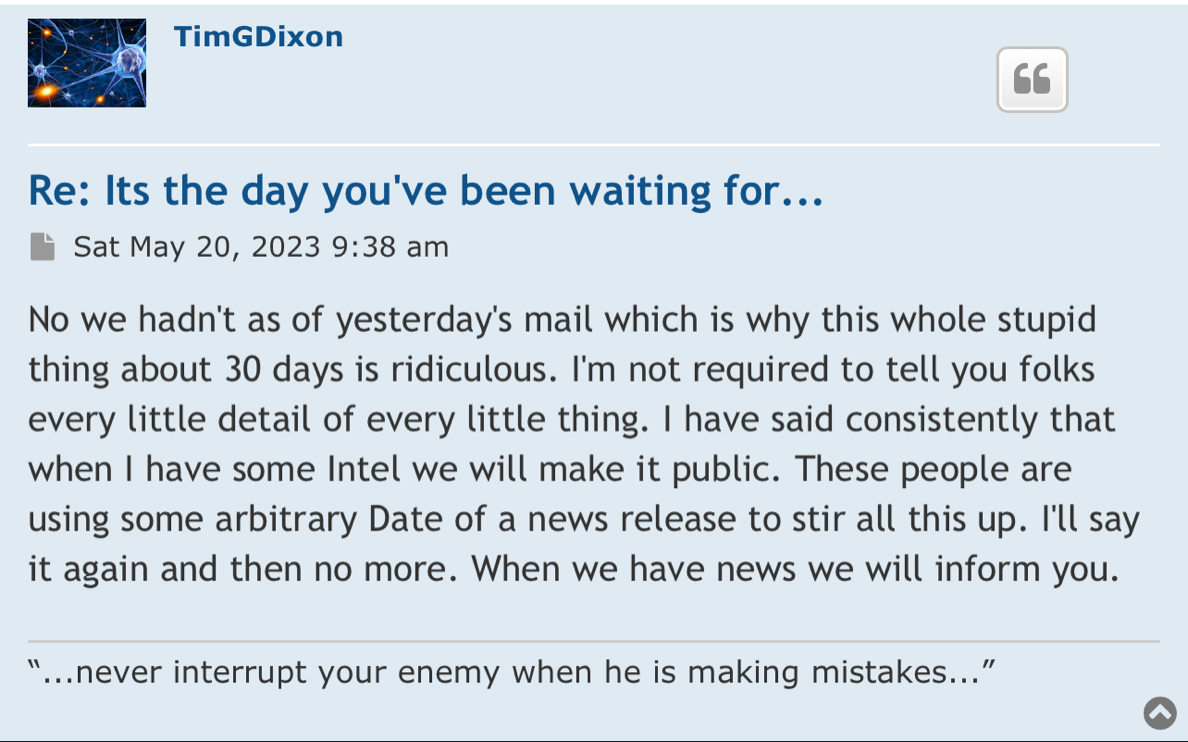
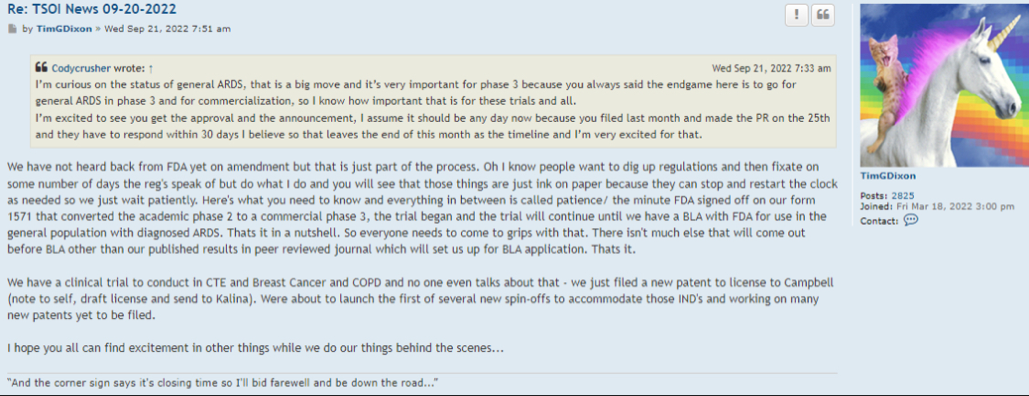
Right, so no updates on an application that was submitted more than a year ago (applications are approved within 30 days if they are a go) for a "new" P3 trial that doesn't have a P2 to stand on.
Hence, the 10Q clearly didn't mention a lost about the p3 ARDS study (certainly nothing of current interest/value).
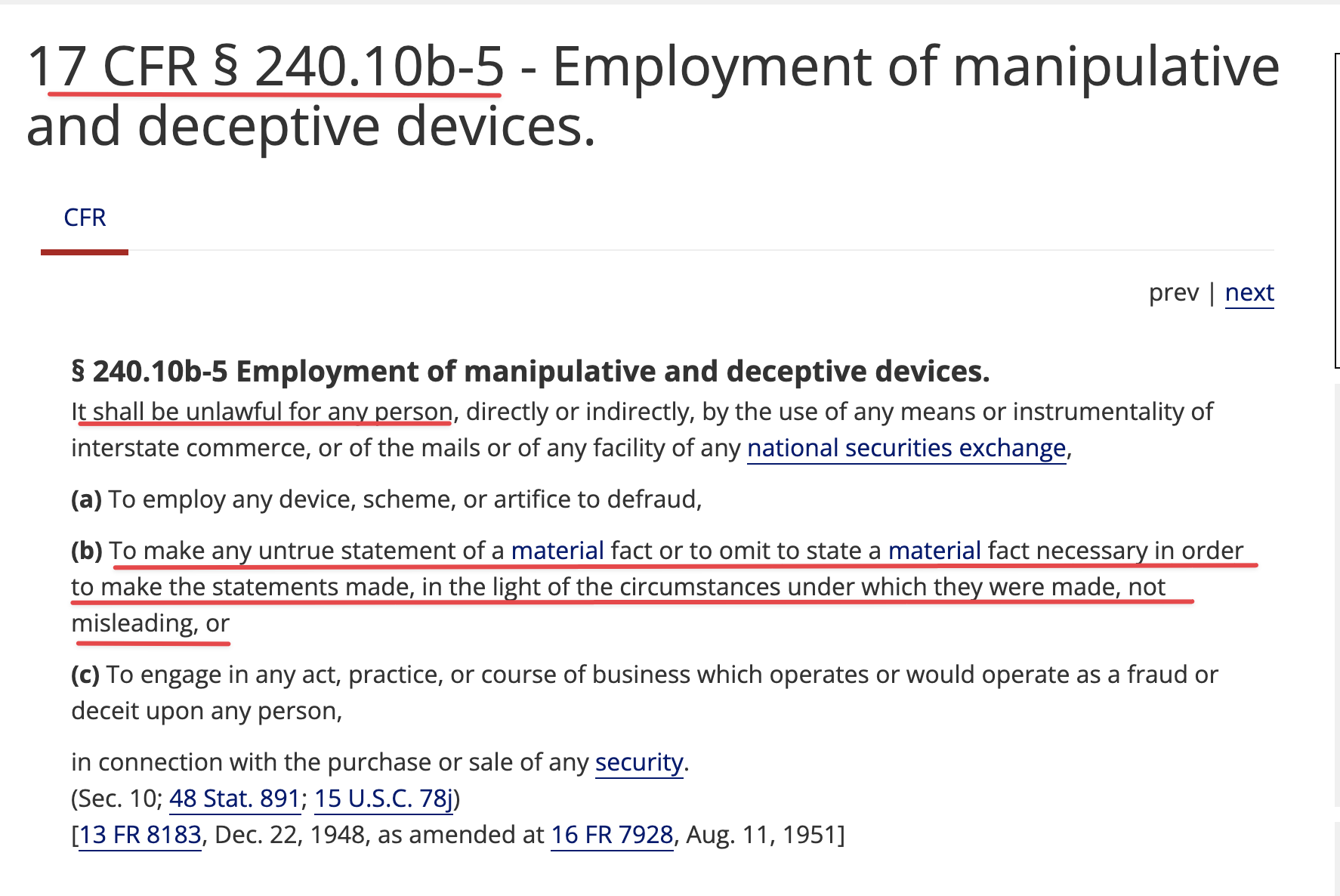
1) The FDA does not have any aging requirements.
2) That communication was issued by the same people who lied about EUA filing, Costco revenue, ongoing clinical trials and more.
3) Why would the FDA approve a P3 when TSOI has never completed a P2?
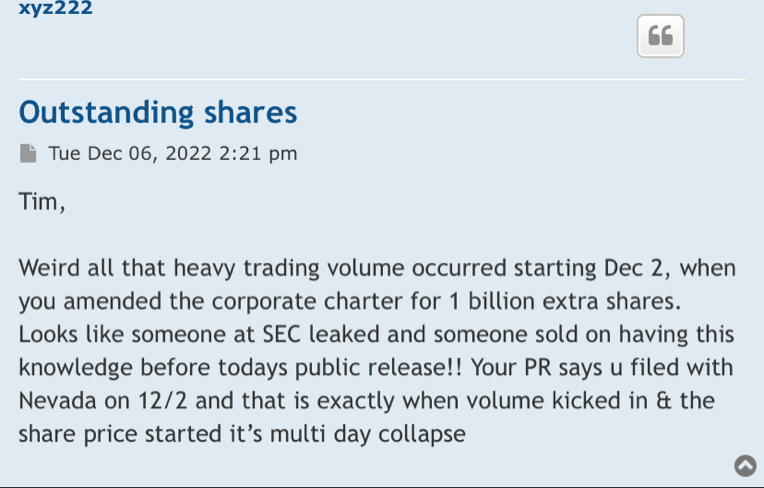
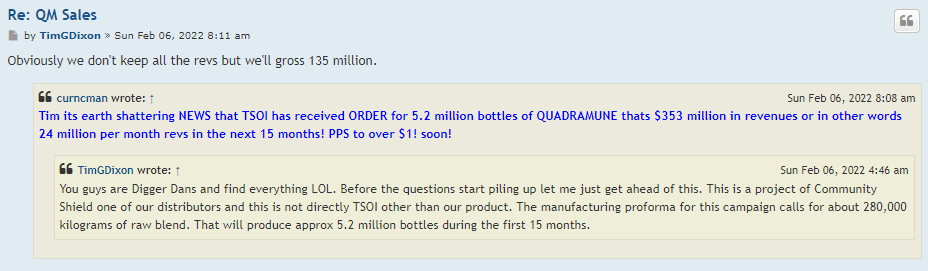
Well, I guess we know where 47,500,000 of volume came from yesterday.
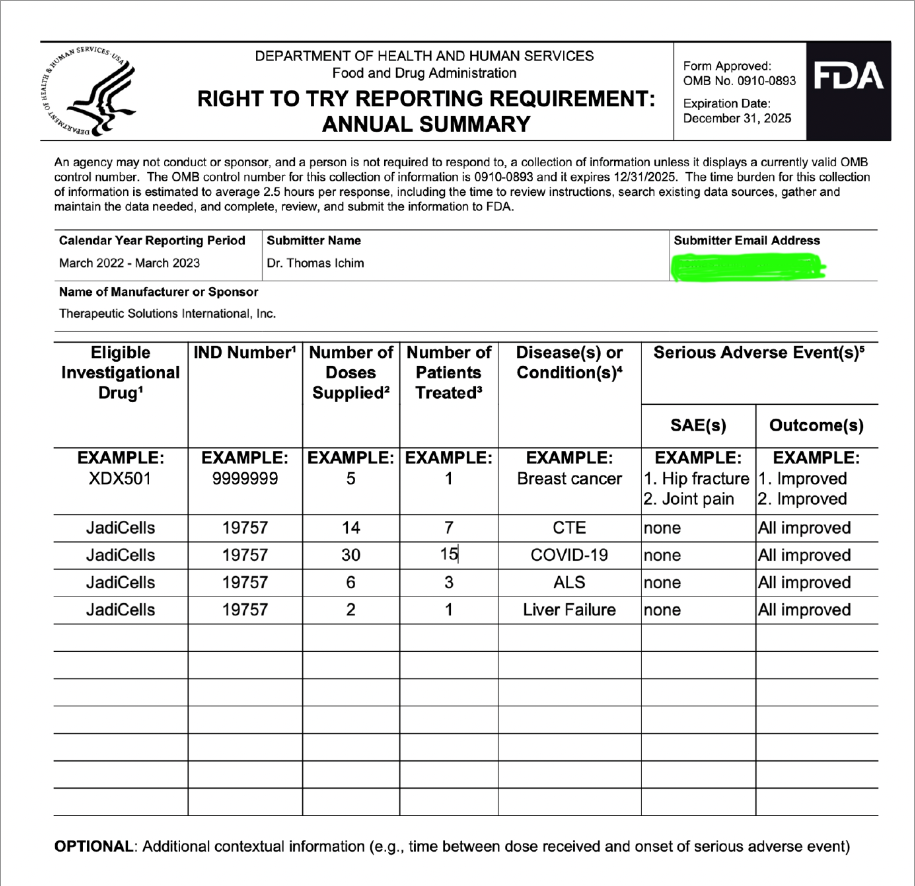
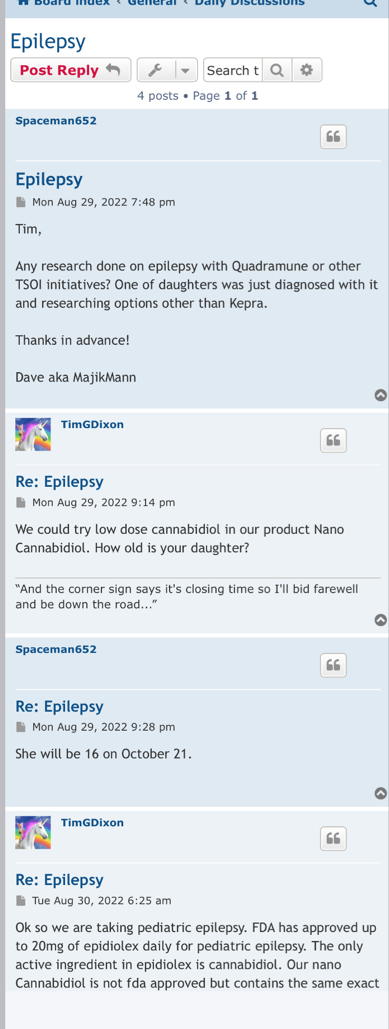
"Now that we have established our own Master Cell Bank with corresponding biomarker assays of CD73, CD90, CD105 >90%, and CD14, CD34, CD45 <10%, these aliquots, which are in process of 3rd party validation will remain in cryopreservation to age the cells for purposes of required immuno-assays to clear phase 3 in the future when cells reach acceptable age to satisfy FDA. MCB was established in August 2023 and our estimate is next August 2024 we will have adequately aged the cells to obtain clearance for phase 3 IND."
$TSOI Pink Current Information
https://www.otcmarkets.com/stock/TSOI/overview
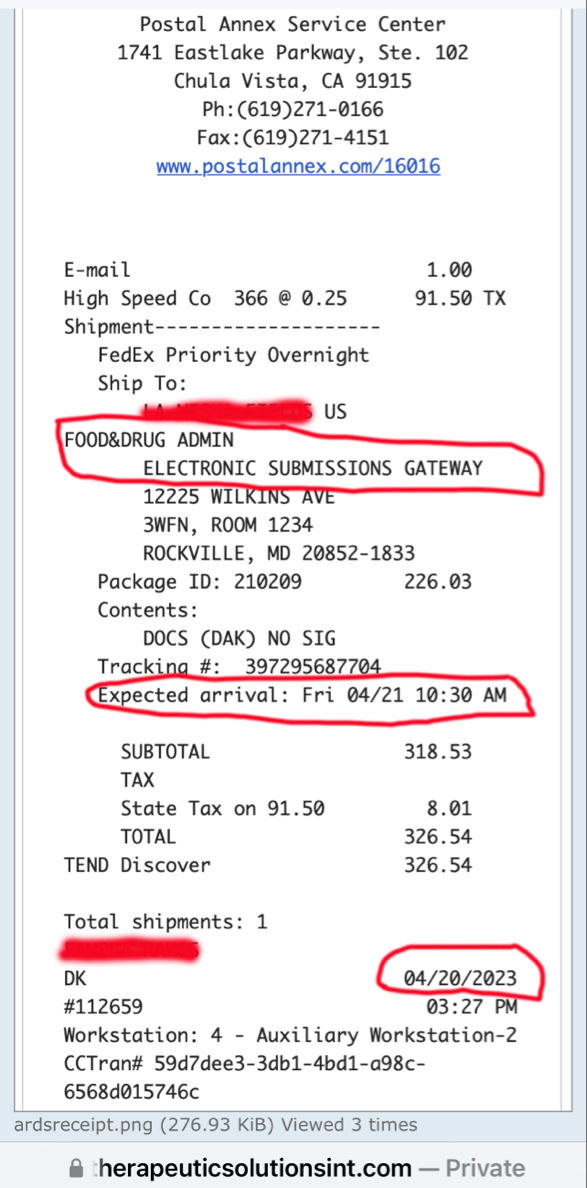
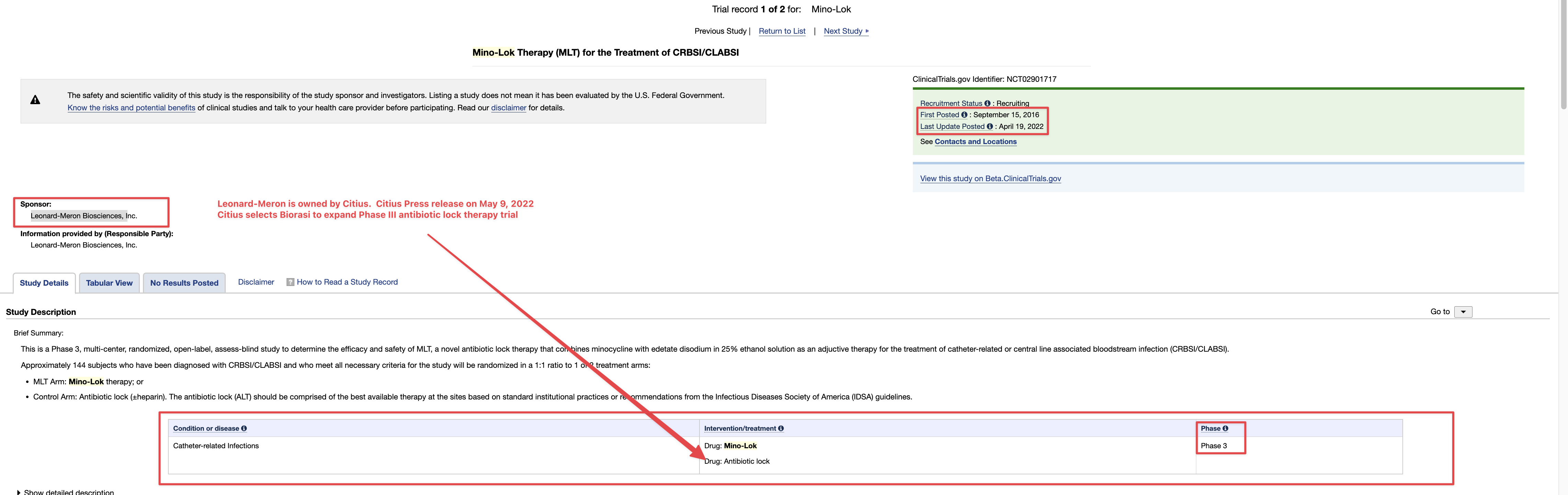
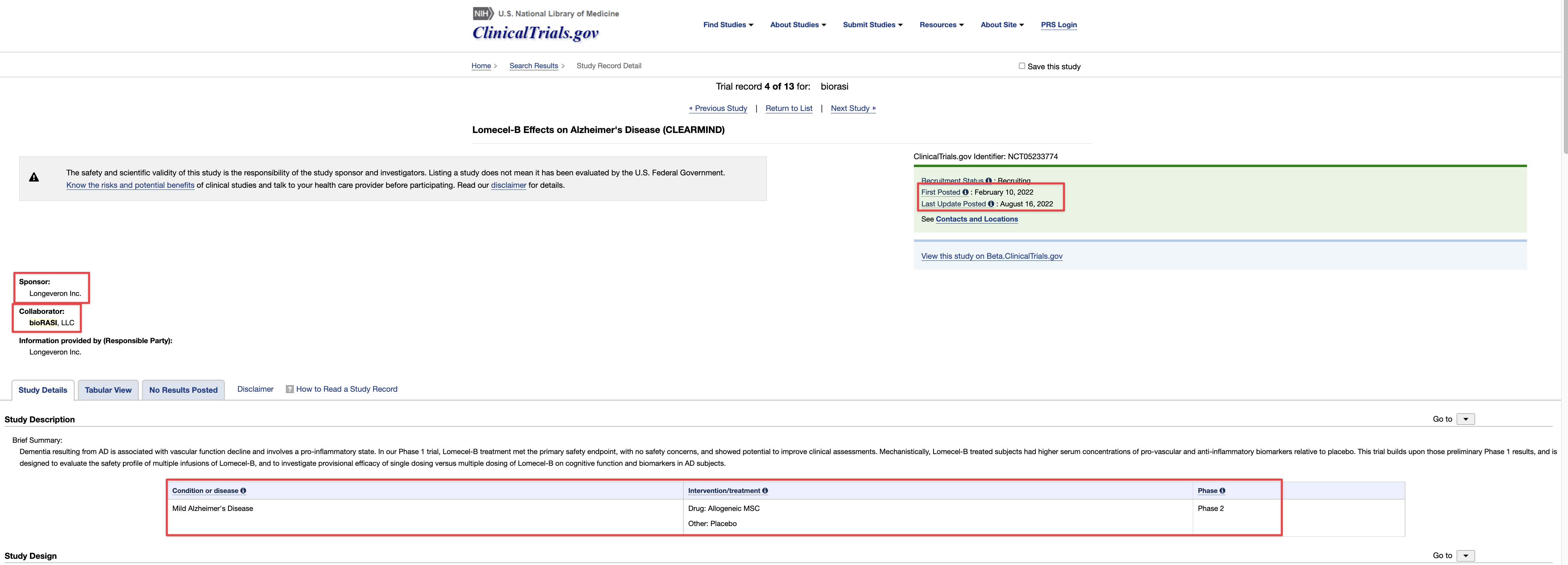
On April 20, 2023, the Company filed with the USFDA an Investigational Drug Application (IND) to initiate a Phase III Clinical Trial for Acute Respiratory Distress Syndrome.
10q didnt really mention a lot about the p3 ARDS study.
No excuse to ever to delinquent in reporting.
I expect that will show a change next week.
O/S No Change as per 10Q
As of May 30, 2024, 4,591,517,624 shares of the registrant’s common stock, par value of $0.001 per shares, were outstanding.
Dividend coming? Nice!
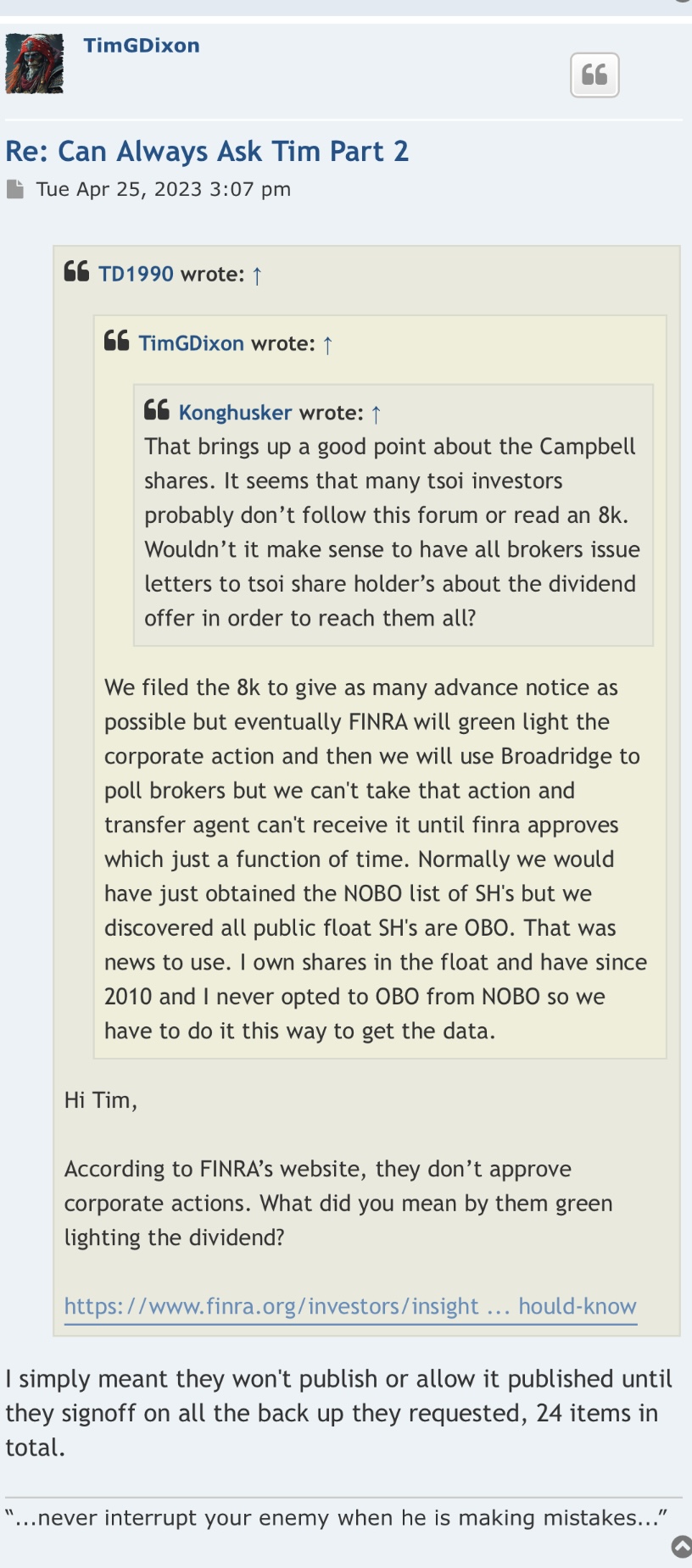
Interested Shareholders Take Note: Any and all correspondence will be mailed to shareholders of record by the Transfer Agent (“Distribution Agent”) if restricted shares, and/or the broker/dealer who holds shares where individual shareholders of record have deposited shares on record with the DTC, which will contain the details of the distribution and how to elect to accept the Dividend Offer for issuance in due course. A Shareholder’s Rights Agreement may be enclosed or will be provided at the time of issuance of the loyalty dividend in CNSI common stock and any associated transactional documents.
The same three MM's on the bid and ask. Trying to slow it down
TSOI Therapeutic Solutions International Inc (PK) 0.0011 0.0005 (83.33%) Volume: 33,545,833 Day Range: 0.00065 - 0.0011
https://ih.advfn.com/stock-market/USOTC/therapeutic-solutions-pk-TSOI/trades
Bio's take time, especially in pinky land.
Yes. Definitely pretty "STALE" 🤣🤣🤣
That is so “yesterday”. lol 😂
Cool. Any word on Costco? "Digger Dan" Thanks. -Pier.
Is there a court date set?
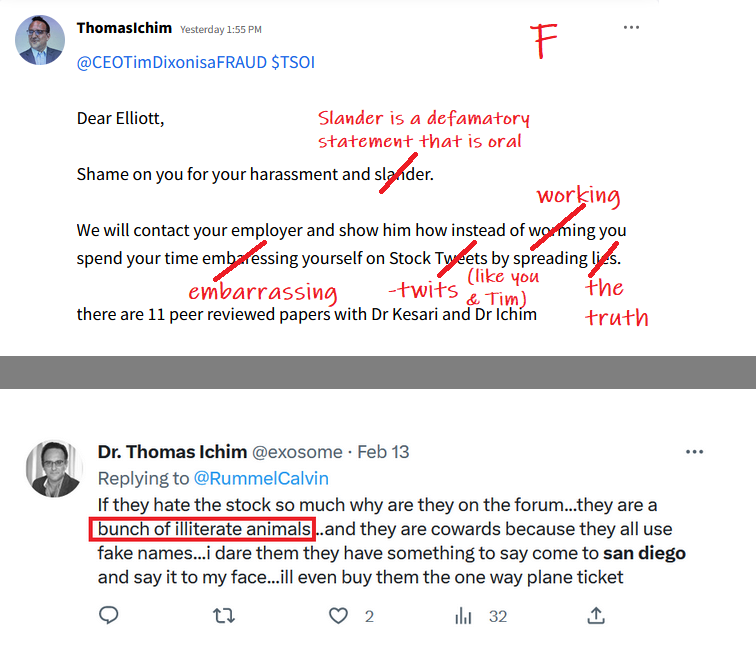
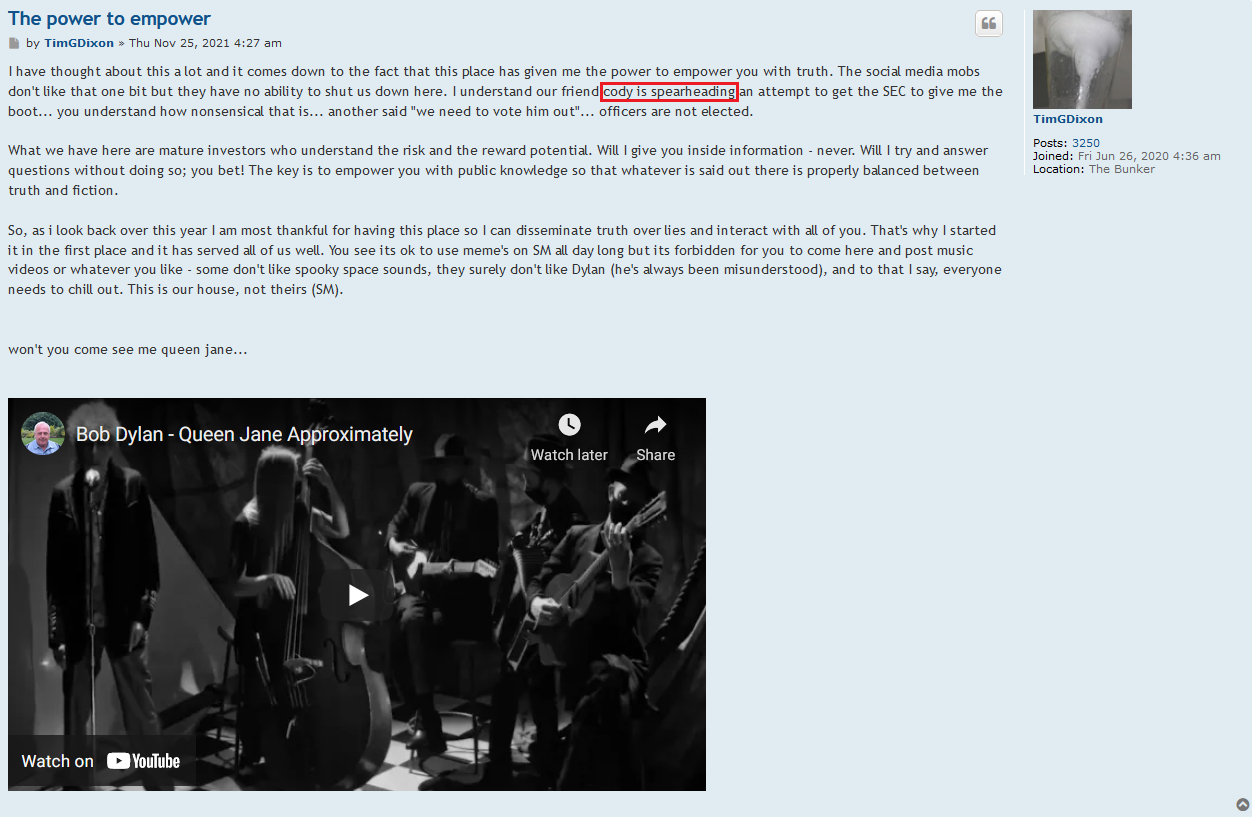
Seems like Thomas Ichim was somehow engaged with these guys before the deal. How did they not know?
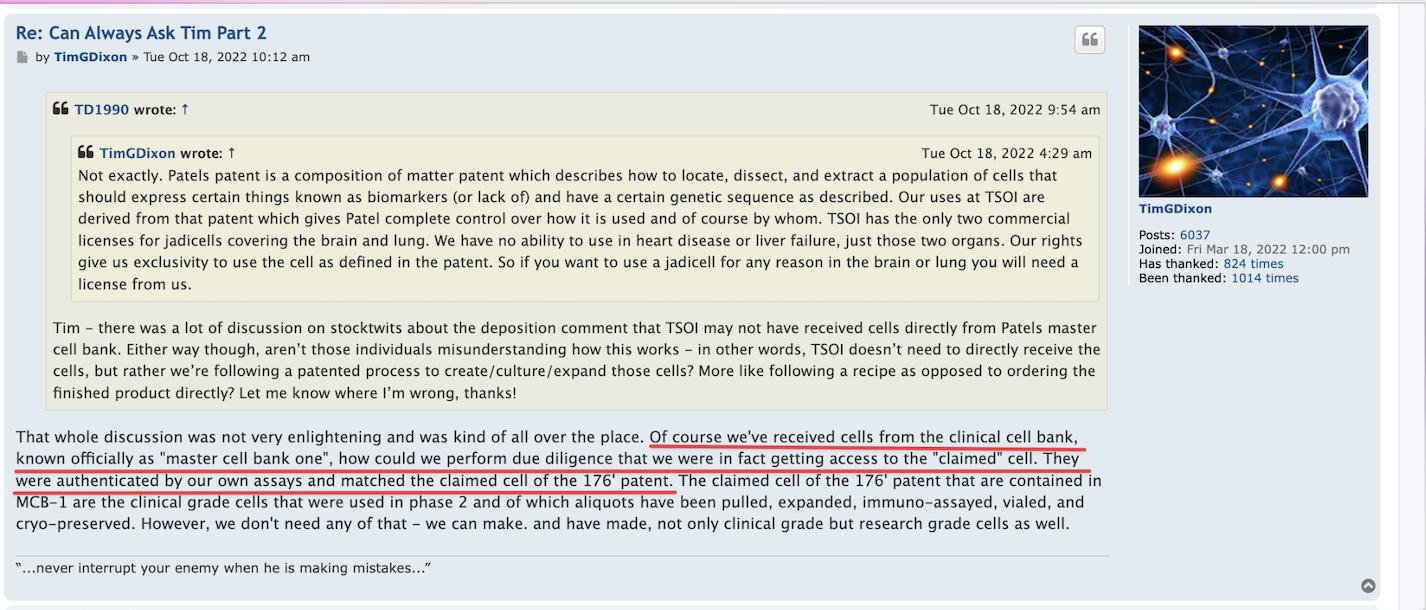
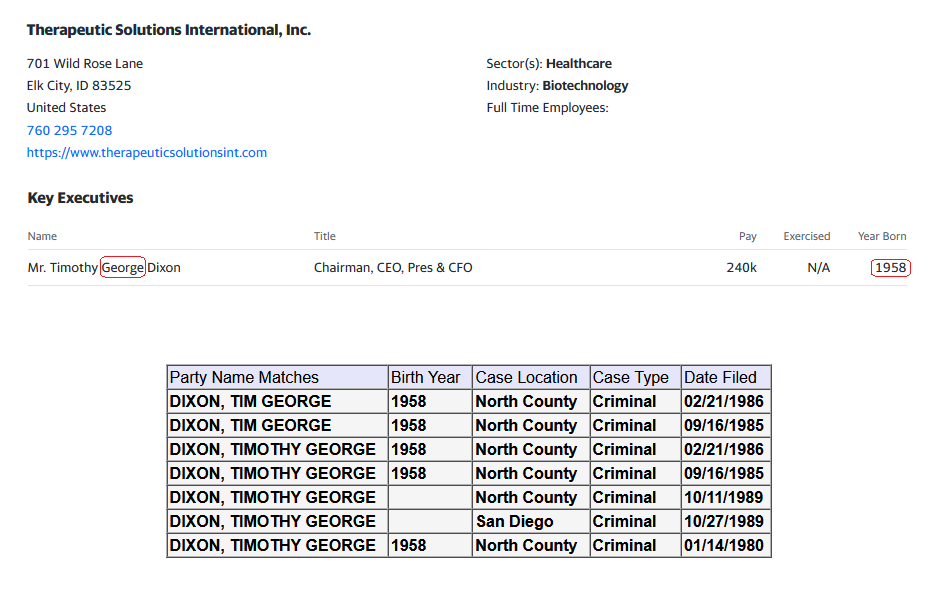
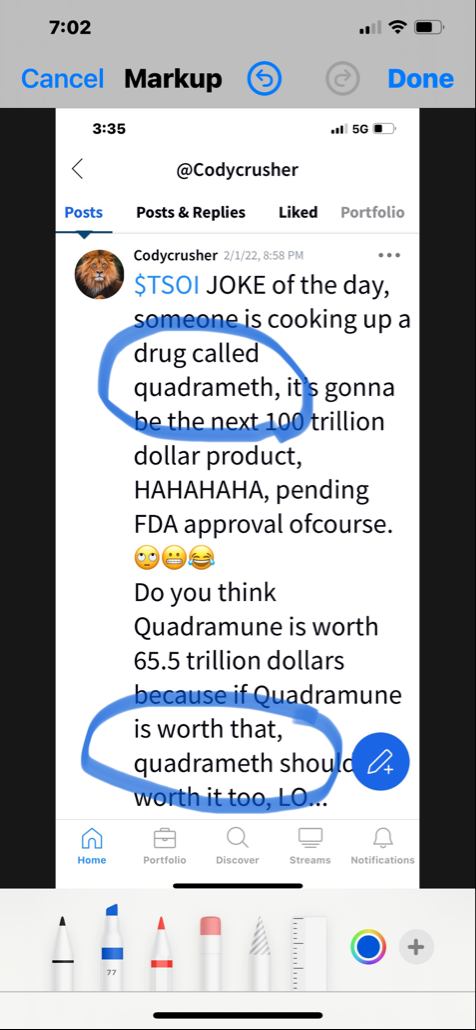
$TSOI in the lawsuit and amicus brief filed by CEO Tim Dixon, clearly states that JADICELLS LLC, RESTEM LLC, and the University of Miami (collectively referred to as "the TRIO") seriously messed up the data in the clinical FDA phase 2a/2b trial and somehow managed to get FDA approval. In February 2021, JADICELLS LLC sold its IP and the clinical 2a/2b JADICELLS trial to $TSOI. However, $TSOI later discovered that the TRIO had used some other inferior cells to pass the phase 2a/2b approval, committing a massive fraud on the FDA. It is in this context that $TSOI brought the lawsuit and amicus brief to light, seeking punishment for the TRIO. Talk about a cellular-level screw-up!
Step 1 - make sense with your posts
You should go silent
Such a shame. As time goes on their true colors come out. Its so obvious when the company goes completely silent.
I think its everything but LEGIT.
Exactly! I would say this company is a joke, but it is too pathetic and crooked to be classified as such.
Me to my canine friend...me too!
Was hoping to see $TSOI closing @ Dubs today & lose a zero...we'll see
Your attention is not supposed to be on the constant dilution, nor the delinquency of the filings, but on the lawsuit of the posters who have called out the company.
GOT IT? 🤣
|
Followers
|
524
|
Posters
|
|
|
Posts (Today)
|
5
|
Posts (Total)
|
65778
|
|
Created
|
10/04/08
|
Type
|
Free
|
| Moderators BigBadWolf johnnytrader33 JMC$ Yooperman Hogwarts | |||









































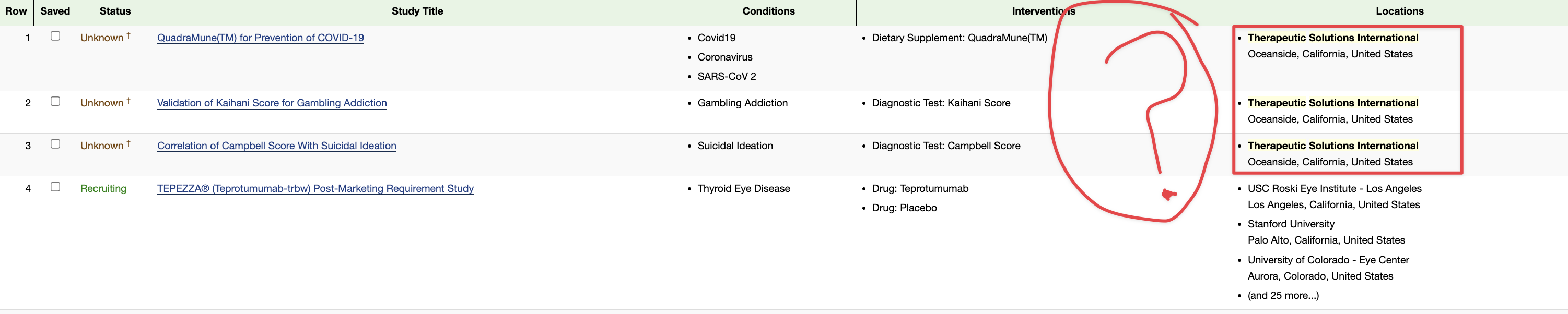







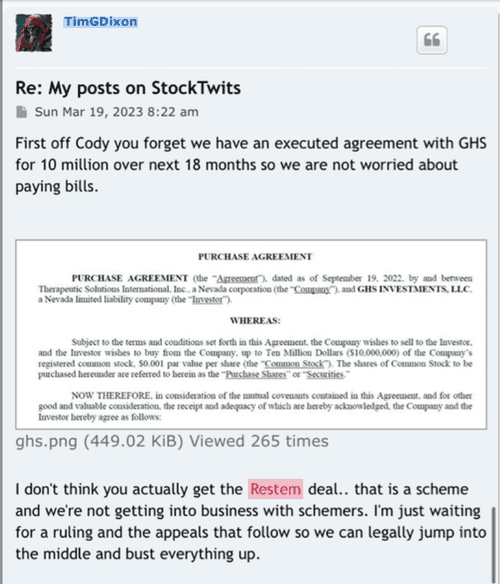



Preclinical Data Suggests QuadraMune™ Prevents Stress-Induced Suppression of Neurogenesis More Effectively than Prozac
OCEANSIDE, Calif., Dec. 9, 2020 /PRNewswire/ -- Therapeutics Solution International, Inc., (OTC Markets: TSOI), announced today new data suggesting the possibility that QuadraMune™ may mediate neuroprotective activity through preserving the ability of regenerative brain cells to proliferate subsequent to psychological stress.
The experiments, which involved exposing mice to established stressors, demonstrated that specific areas of the brain associated with production of new brain cells are damaged by stress. In agreement with previously published research, administration of fluoxetine (Prozac™) protected the brain from stress-induced damage. Surprisingly, QuadraMune™ administration appeared superior to Prozac™ at stimulating proliferation of new brain cells.
"QuadraMune™ which is currently in a clinical trial for prevention of COVD-191, has also been demonstrated to possess anti-inflammatory activity in other clinical trials, suppressing cytokines such as IL-62, which are known to be involved in depression3 and suicide4" said Kalina O'Connor, Director of Campbell Neurosciences and co-inventor on the patent. "Given major depressive disorder causes a significant risk for suicide, we are highly interested in exploring the use of QuadraMune™ for preventing suicide."
"Although much enthusiasm has been generated over the planned distribution of the COVID vaccine, at present little is being done to address mental health issues that are being exacerbated by the current pandemic" said Dr. James Veltmeyer, co-inventor of the patent, and Chief Medical Officer of the Company. "If current results are reproducible, the possibility that a nutraceutical would concurrently boost immunity while preserving mental health is highly enticing."
"It has not escaped us that COVID-19 is associated with increased inflammatory cytokines in the blood of patients, cytokines that also predispose to depression" said Famela Ramos, Vice President of Business Development for the Company. "It may be that the recent increase in suicides and suicide attempts is related biologically to activities of the coronavirus. It will be interesting to examine whether QuadraMune™ may modify putative negative mental effects of the virus."
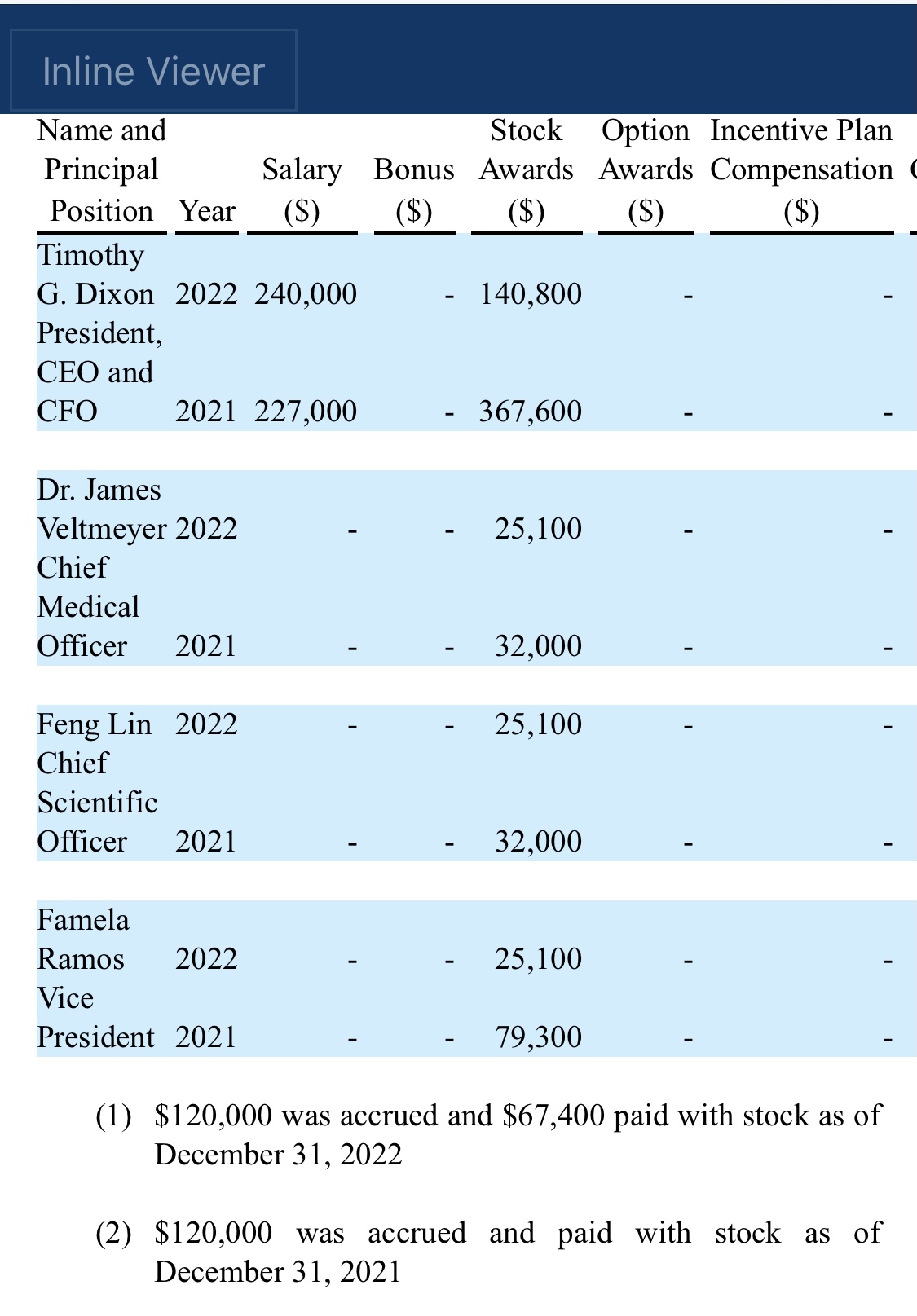
"An estimated 17.3 million adults in the United States had at least one major depressive episode. This number represented 7.1% of all U.S. adults" stated Timothy Dixon, President and CEO of the Company. "We believe the Mission of our Company is not just providing a return on investment to our shareholders, but also increasing the quality of life for Americans. We are extremely pleased to report this unexpected finding with significant potential implications to advancing non-toxic means of helping patients with this terrible condition."
1 QuadraMune(TM) for Prevention of COVID-19 - Full Text View - ClinicalTrials.gov
2 Therapeutic Solutions International Announces Positive Preclinical and Clinical Evaluation of Nutritional Supplement QuadraMune™, Designed to Protect Against COVID-19 | BioSpace
3 Ting et al. Role of Interleukin-6 in Depressive Disorder. Int J Mol Sci. 2020 Mar 22;21(6):2194.
4 O'Donovan et al. Suicidal ideation is associated with elevated inflammation in patients with major depressive disorder. Depress Anxiety. 2013 Apr;30(4):307-14.
| Volume | |
| Day Range: | |
| Bid Price | |
| Ask Price | |
| Last Trade Time: |
