Register for free to join our community of investors and share your ideas. You will also get access to streaming quotes, interactive charts, trades, portfolio, live options flow and more tools.
Is that a random hope, or are they in talks or something? TIA and GL!
NEWS -- Provectus Biopharmaceuticals Announces Release of Preprint Manuscript Describing Preclinical Study of PV-10® Immunotherapy and Chemotherapy for Treatment of Pancreatic Cancer
KNOXVILLE, TN, Jan. 20, 2021 (GLOBE NEWSWIRE) -- Provectus (OTCQB: PVCT) today announced that H. Lee Moffitt Cancer Center (Moffitt) has released a manuscript preprint describing how investigational autolytic cancer immunotherapy PV-10, an injectable formulation of Provectus’ proprietary small molecule rose bengal disodium (RBD), in combination with gemcitabine may enhance the chemotherapy’s efficacy against pancreatic tumors. Chemotherapy regimens that include gemcitabine are the standard of care for the treatment of pancreatic cancer.
The manuscript, entitled “Intralesional injection of Rose Bengal augments the efficacy of gemcitabine chemotherapy against pancreatic tumors,” is available at the following link: https://assets.researchsquare.com/files/rs-140621/v1/cb1df721-b58b-4f5d-9ba6-1b8b2880391d.pdf.
Moffitt co-authors/researchers Patrick Innamarato, Jennifer Morse, Amy Mackay, Sarah Asby, Matthew Beatty, Jamie Blauvelt, Scott Kidd, John Mullinax, Amod Sarnaik, and Shari Pilon-Thomas showed that:
NEWS -- Provectus Biopharmaceuticals Announces Oral Presentation of Updated Data from Combination Therapy Trial of PV-10® and Keytruda® for Checkpoint-Refractory Advanced Cutaneous Melanoma at Melanoma Bridge 2020
NEWS -- Provectus Biopharmaceuticals Announces Presentation of PV-10® Pancreatic Cancer Data at 2020 Society for Immunotherapy of Cancer (SITC) Annual Meeting
KNOXVILLE, TN, Nov. 16, 2020 (GLOBE NEWSWIRE) -- Provectus (OTCQB: PVCT) today announced that H. Lee Moffitt Cancer Center (Moffitt) presented non-clinical data from ongoing research on investigational autolytic cancer immunotherapy PV-10, an injectable formulation of Provectus' proprietary small molecule rose bengal disodium (RBD), as a single-agent and in combination with gemcitabine chemotherapy for the treatment of pancreatic cancer at the Society for Immunotherapy of Cancer’s (SITC) 35th Anniversary Annual Meeting & Pre-Conference Programs (SITC 2020), held online from November 9-14, 2020.
RBD selectively accumulates in the lysosomes of cancer cells upon contact, disrupts these lysosomes, and causes the cancer cells to die. Intralesional (IL) (aka intratumoral) administration of PV-10 causes acute destruction of injected tumors, resulting in the release of danger-associated molecular pattern molecules (DAMPs) and tumor antigens. These signaling factors may initiate an immunologic cascade, where the innate immune system response may facilitate systemic anti-tumor immunity by the adaptive immune system. PV-10-mediated DAMP release may activate CD8+ T cells, CD4+ T cells, and NKT cells.
Moffitt’s poster presentation, authored by Innamarato et al. and entitled “Intralesional injection of rose bengal augments the efficacy of gemcitabine chemotherapy against pancreatic tumors,” concluded:
Encouraging, like to see some of this optimism transition into the stock price. Holding for over a year now, hoping this continues to proceed to investment status instead of the lotto category. Phase I results early next year will definitely be a price mover. Thanks for the update.
NEWS -- Provectus Biopharmaceuticals Announces Acceptance of PV-10® Melanoma Abstract for Oral Presentation at Melanoma Bridge 2020
KNOXVILLE, TN, Nov. 12, 2020 (GLOBE NEWSWIRE) -- Provectus (OTCQB: PVCT) today announced that data from the Company's ongoing Phase 1b/2 study of autolytic cancer immunotherapy PV-10, an injectable formulation of Provectus' proprietary small molecule rose bengal disodium (RBD), in combination with KEYTRUDA® (pembrolizumab) for the treatment of advanced cutaneous melanoma in patients refractory to immune checkpoint blockade (CB) will be presented at Melanoma Bridge 2020, to be held online from December 3-5, 2020.
The abstract accepted for oral presentation is entitled “Response for combination of PV-10 autolytic immunotherapy and immune checkpoint blockade in checkpoint-refractory patients.”
Intralesional (IL) (aka intratumoral) administration of PV-10 for the treatment of solid tumor cancers can yield immunogenic cell death and induce tumor-specific reactivity in circulating T cells.1,2,3 This IL PV-10-induced functional T cell response may be enhanced and boosted in combination with CB.4 In CB-refractory advanced cutaneous melanoma, IL PV-10 may restore disease-specific T cell function, which may also be prognostic of clinical response.
About the Phase 1b/2 Combination Therapy Trial (NCT02557321)
A first expansion cohort of the Phase 1b portion of the study began enrolling patients with metastatic cutaneous melanoma who were CB-refractory in December 2018. This CB-Refractory Cohort extended an exploratory group of refractory patients enrolled into the study's main cohort that primarily enrolled CB-naïve patients. Patients with at least one injectable lesion and who were candidates for KEYTRUDA were eligible. Eligible subjects received the combination treatment of PV-10 and KEYTRUDA every three weeks for up to five cycles (i.e., over a period of up to 12 weeks), followed by only KEYTRUDA every three weeks for up to 24 months. The protocol allows for additional cycles of PV-10 beyond the fifth cycle for patients with additional injectable disease. The primary endpoint for the Phase 1b trial was safety and tolerability. ORR and progression-free survival were key secondary endpoints (both assessed via RECIST 1.1 after five treatment cycles, and then every 12 weeks thereafter).
About Rose Bengal Disodium
RBD is 4,5,6,7-tetrachloro-2',4',5',7'-tetraiodofluorescein disodium, a halogenated xanthene and Provectus’ proprietary lead molecule. Provectus' current Good Manufacturing Practices (cGMP) RBD is a proprietary pharmaceutical-grade drug substance produced by the Company's quality-by-design (QbD) manufacturing process to exacting regulatory standards that avoids the formation of uncontrolled impurities currently present in commercial-grade rose bengal. Provectus' RBD and cGMP RBD manufacturing process are protected by composition of matter and manufacturing patents as well as trade secrets.
An IL formulation (i.e., by direct injection) of cGMP RBD drug substance, cGMP PV-10, is being developed as an autolytic immunotherapy drug product for solid tumor cancers. By targeting tumor cell lysosomes, RBD treatment may yield immunogenic cell death in solid tumor cancers that results in tumor-specific reactivity in circulating T cells, including a T cell mediated immune response against treatment refractory and immunologically cold tumors.1,2,3 Adaptive immunity can be enhanced by combining CB with RBD.4 IL PV-10 is undergoing clinical study for relapsed and refractory adult solid tumor cancers, such skin and liver cancers.
IL PV-10 is also undergoing preclinical study for relapsed and refractory pediatric solid tumor cancers, such as neuroblastoma, Ewing sarcoma, rhabdomyosarcoma, and osteosarcoma.5,6
A topical formulation of cGMP RBD drug substance, PH-10®, is being developed as a clinical-stage immuno-dermatology drug product for inflammatory dermatoses, such as atopic dermatitis and psoriasis. RBD can modulate multiple interleukin and interferon pathways and key cytokine disease drivers.7
Oral formulations of cGMP RBD are undergoing preclinical study for relapsed and refractory pediatric blood cancers, such as acute lymphocytic leukemia and acute myelomonocytic leukemia.8,9
Oral formulations of cGMP RBD are also undergoing preclinical study as prophylactic and therapeutic treatments for high-risk adult solid tumor cancers, such as head and neck, breast, pancreatic, liver, and colorectal cancers.
Different formulations of cGMP RBD are also undergoing preclinical study as potential treatments for multi-drug resistant (MDR) bacteria, such as Gram-negative bacteria.
Tumor Cell Lysosomes as the Seminal Cancer Drug Target
Lysosomes are the central organelles for intracellular degradation of biological materials, and nearly all types of eukaryotic cells have them. Discovered by Christian de Duve, MD in 1955, lysosomes are linked to several biological processes, including cell death and immune response. In 1959, de Duve described them as ‘suicide bags’ because their rupture causes cell death and tissue autolysis. He was awarded the Nobel Prize in 1974 for discovering and characterizing lysosomes, which are also linked to each of the three primary cell death pathways: apoptosis, autophagy, and necrosis.
Building on the Discovery, Exploration, and Characterization of Lysosomes
Cancer cells, particularly advanced cancer cells, are very dependent on effective lysosomal functioning.10 Cancer progression and metastasis are associated with lysosomal compartment changes11,12, which are closely correlated (among other things) with invasive growth, angiogenesis, and drug resistance13.
RBD selectively accumulates in the lysosomes of cancer cells upon contact, disrupting the lysosomes and causing the cells to die. Provectus1,14, external collaborators5, and other researchers15,16,17 have independently shown that RBD triggers each of the three primary cell death pathways: apoptosis, autophagy, and necrosis.
Cancer Cell Autolytic Death via RBD: RBD-induced autolytic cell death, or death by self-digestion, in Hepa1-6 murine hepatocellular carcinoma (HCC) cells can be viewed in this Provectus video of the process (ethidium homodimer 1 [ED-1] stains DNA, but is excluded from intact nuclei; lysosensor green [LSG] stains intact lysosomes; the video is provided in 30-second frames, with a duration of approximately one hour). Exposure to RBD triggers the disruption of lysosomes, followed by nucleus failure and autolytic cell death. Identical responses have been shown by the Company in HTB-133 human breast carcinoma (which can be viewed in this Provectus video of the process, with a duration of approximately two hours) and H69Ar human multidrug-resistant small cell lung carcinoma. Cancer cell autolytic cell death was reproduced by research collaborators in neuroblastoma cells to show that lysosomes are disrupted upon exposure to RBD.5
Tumor Autolytic Death via RBD: RBD causes acute autolytic destruction of injected tumors (via autolytic cell death), mediating the release of danger-associated molecular pattern molecules (DAMPs) and tumor antigens; release of these signaling factors may initiate an immunologic cascade where local response by the innate immune system may facilitate systemic anti-tumor immunity by the adaptive immune system. The DAMP release-mediated adaptive immune response activates lymphocytes, including CD8+ T cells, CD4+ T cells, and NKT cells, based on clinical and preclinical experience in multiple tumor types. Mediated immune signaling pathways may include an effect on STING, which plays an important role in innate immunity.9
Orphan Drug Designations (ODDs)
ODD status has been granted to RBD by the U.S. Food and Drug Administration for metastatic melanoma in 2006, hepatocellular carcinoma in 2011, neuroblastoma in 2018, and ocular melanoma (including uveal melanoma) in 2019.
Intellectual Property (IP)
Provectus’ IP includes a family of US and international (a number of countries in Asia, Europe, and North America) patents that protect the process by which cGMP RBD and related halogenated xanthenes are produced, avoiding the formation of previously unknown impurities that exist in commercial-grade rose bengal in uncontrolled amounts. The requirement to control these impurities is in accordance with International Council on Harmonisation (ICH) guidelines for the manufacturing of an injectable pharmaceutical. US patent numbers are 8,530,675, 9,273,022, and 9,422,260, with expirations ranging from 2030 to 2031.
The Company's IP also includes a family of US and international (a number of countries in Asia, Europe, and North America) patents that protect the combination of RBD and CB (e.g., anti-CTLA-4, anti-PD-1, and anti-PD-L1 agents) for the treatment of a range of solid tumor cancers. US patent numbers are 9,107,887, 9,808,524, 9,839,688, and 10,471,144, with expirations ranging from 2032 to 2035; US patent application numbers include 20200138942.
About Provectus
Provectus Biopharmaceuticals, Inc. (Provectus or the Company) is a clinical-stage biotechnology company developing immunotherapy medicines for different disease areas based on an entirely- and wholly-owned family of small molecules called halogenated xanthenes. Information about the Company’s clinical trials can be found at the National Institutes of Health (NIH) registry, http://www.clinicaltrials.gov. For additional information about Provectus, please visit the Company's website at http://www.provectusbio.com.
References
1. Wachter et al. Functional Imaging of Photosensitizers using Multiphoton Microscopy. Proceedings of SPIE 4620, 143, 2002.
2. Liu et al. Intralesional rose bengal in melanoma elicits tumor immunity via activation of dendritic cells by the release of high mobility group box 1. Oncotarget 7, 37893, 2016.
3. Qin et al. Colon cancer cell treatment with rose bengal generates a protective immune response via immunogenic cell death. Cell Death and Disease 8, e2584, 2017.
4. Liu et al. T cell mediated immunity after combination therapy with intralesional PV-10 and blockade of the PD-1/PD-L1 pathway in a murine melanoma model. PLoS One 13, e0196033, 2018.
5. Swift et al. Potent in vitro and xenograft antitumor activity of a novel agent, PV-10, against relapsed and refractory neuroblastoma. OncoTargets and Therapy 12, 1293, 2019.
6. Swift et al. In vitro and xenograft anti-tumor activity, target modulation and drug synergy studies of PV-10 against refractory pediatric solid tumors. 2018 ASCO Annual Meeting, J Clin Oncol 36, 2018 (suppl; abstr 10557).
7. Krueger et al. Immune Modulation by Topical PH-10 Aqueous Hydrogel (Rose Bengal Disodium) in Psoriasis Lesions. Psoriasis Gene to Clinic, 8th International Congress, Br J Dermatol 177.
8. Swift et al. In Vitro Activity and Target Modulation of PV-10 Against Relapsed and Refractory Pediatric Leukemia. 2018 ASH Annual Meeting, Blood 132, 2018 (suppl; abstr 5207).
9. Thakur et al. Association of heat shock proteins as chaperone for STING: A potential link in a key immune activation mechanism revealed by the novel anti-cancer agent PV-10. 2020 AACR VAM II, (abstr 5393).
10. Piao et al. Targeting the lysosome in cancer. Annals of the New York Academy of Sciences. 2016; 1371(1): 45.
11. Nishimura et al. Malignant Transformation Alters Intracellular Trafficking of Lysosomal Cathespin D in Human Breast Epithelial Cells. Pathology Oncology Research. 1998; 4(4): 283.
12. Gocheva et al. Distinct roles for cysteine cathepsin genes in multistage tumorigenesis. Genes & Development. 2006; 20(5): 543.
13. Fehrenbacher et al. Lysosomes as Targets for Cancer Therapy. Cancer Research. 2005; 65 (8): 2993.
14. Wachter et al. Imaging Photosensitizer Distribution and Pharmacology using Multiphoton Microscopy. Proceedings of SPIE 4622, 112, 2002.
15. Koevary. Selective toxicity of rose Bengal to ovarian cancer cells in vitro. International Journal of Physiology, Pathophysiology and Pharmacology 4(2), 99, 2012.
16. Zamani et al. Rose Bengal suppresses gastric cancer cell proliferation via apoptosis and inhibits nitric oxide formation in macrophages. Journal of Immunotoxicology, 11(4), 367, 2014.
17. Luciana et al. Rose Bengal Acetate photodynamic therapy-induced autophagy. Cancer Biology & Therapy, 10:10, 1048, 2010.
Trademarks
PV-10® and PH-10® are registered trademarks of Provectus, Knoxville, Tennessee, U.S.A.
KEYTRUDA® is a registered trademark of Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc. Kenilworth, New Jersey, U.S.A.
FORWARD-LOOKING STATEMENTS: The information in this press release may include “forward-looking statements,” within the meaning of U.S. securities legislation, relating to the business of Provectus and its affiliates, which are based on the opinions and estimates of Company management and are subject to a variety of risks and uncertainties and other factors that could cause actual events or results to differ materially from those projected in the forward-looking statements. Forward-looking statements are often, but not always, identified by the use of words such as “seek,” “anticipate,” “budget,” “plan,” “continue,” “estimate,” “expect,” “forecast,” “may,” “will,” “project,” “predict,” “potential,” “targeting,” “intend,” “could,” “might,” “should,” “believe,” and similar words suggesting future outcomes or statements regarding an outlook.
The safety and efficacy of the agents and/or uses under investigation have not been established. There is no guarantee that the agents will receive health authority approval or become commercially available in any country for the uses being investigated or that such agents as products will achieve any particular revenue levels.
Due to the risks, uncertainties, and assumptions inherent in forward-looking statements, readers should not place undue reliance on these forward-looking statements. The forward-looking statements contained in this press release are made as of the date hereof or as of the date specifically specified herein, and Provectus undertakes no obligation to update or revise any forward-looking statements, whether as a result of new information, future events or otherwise, except in accordance with applicable securities laws. The forward-looking statements are expressly qualified by this cautionary statement.
Risks, uncertainties, and assumptions include those discussed in the Company’s filings with the SEC, including those described in Item 1A of the Company’s Annual Report on Form 10-K for the year ended December 31, 2019 and Provectus’ Quarterly Report on Form 10-Q for the quarter ended June 30, 2020.
###
Contact:
Provectus Biopharmaceuticals, Inc.
Heather Raines, CPA
Chief Financial Officer
Phone: (866) 594-5999
NEWS -- Provectus Biopharmaceuticals Announces Acceptance of PV-10® Pancreatic Cancer Abstract at 2020 Society for Immunotherapy of Cancer (SITC) Annual Meeting
KNOXVILLE, TN, Oct. 15, 2020 (GLOBE NEWSWIRE) -- Provectus (OTCQB: PVCT) today announced that H. Lee Moffitt Cancer Center will present non-clinical data from ongoing research on investigational autolytic cancer immunotherapy PV-10, an injectable formulation of Provectus' proprietary rose bengal disodium (RBD), as a single-agent and in combination with chemotherapy for the treatment of pancreatic cancer at the Society for Immunotherapy of Cancer’s (SITC) 35th Anniversary Annual Meeting & Pre-Conference Programs (SITC 2020), to be held online from November 9-14, 2020.
RBD selectively accumulates in the lysosomes of cancer cells upon contact, disrupts these lysosomes, and causes the cells to die. Intralesional (IL) (aka intratumoral) administration of PV-10 for the treatment of solid tumors can yield immunogenic cell death and induce tumor-specific reactivity in circulating T cells.1-3
The abstract accepted for poster presentation at SITC 2020 is entitled “Intralesional injection of rose bengal augments the efficacy of gemcitabine chemotherapy against pancreatic tumors” (Abstract ID: 585; Category: Immune-stimulants and immune modulators). Moffitt co-authors include Patrick Innamarato, PhD, Jennifer Morse, MS, Amy Mackay, Sarah Asby, Matthew Beatty, PhD, Jaime Blauvelt, Scott Kidd, John Mullinax, MD, Amod Sarnaik, MD, and Shari Pilon-Thomas, PhD.
This pancreatic cancer work was led by Dr. Pilon-Thomas and members of the Pilon-Thomas Lab, whose previous peer-reviewed published work on RBD includes:
Paulness, totally agree that this is not a scam company.. BUt a billion dollar company, no chance.
I am hoping MRK buys them for $5 a share to combine with Keytruda and we get MRK shares :)
Does Provectus have the capability with its patented PV10 technology to piggyback half-life radiotherapy on its platform to selectively kill cancers in the body? If so this is not just a one-trick pony show. We are looking at a billion-dollar company unfolding right before our eyes.
It was the X CEO that was dishonest. The new people in management are a Godsend for this company and will bring Provectus to a new level, PV 10 is no scam.
Because of the HUGE scam rhat has gone on for many years with ceo and management. May never recover
one day the stock price will match the true potential of this drug to treat cancer.
How this stock is not over $1 when so many other stocks that are not even nearly as close as PVCT is are trading for $1 - $10/share, I will never understand.
NEWS -- Provectus Biopharmaceuticals Completes Enrollment of PV-10® Study for Treatment of Symptomatic Neuroendocrine Tumors Metastatic to the Liver Refractory to Somatostatin Analogs and Peptide Receptor Radionuclide Therapy
Man, I hope we move up sooner than that... Have been in this stock forever, and would like to one day look down on this experience as a positive one.
Yep. I'm thinking so. I think once we are close to the expiration of the warrants in Aug next year that this stock will take off again. Dom and the gang are methodically going after it in a big way.
I am guessing he is kidding.
Either way, I still love the fact that this stock years ago ran to $6 with the hopes of it being a real drug
Now with positive after positive news in its combination trial with Keytruda the stock is bogged down in the .08-.10 area.
crazy how the market works.
Is this something that they are really working on ? Lots of money has been put into PV10.
Get ready for PV20,20% RB could yield a 100% better cure rate of cancers versus the PV10 which is 10% rose bengal, this small molecule is a miracle for cancer burdened population.
BREAKING OUT!!!!!
GET OVER .10 and who knows?
Independent Contributions Account for Clinical Activity of PD-1 Checkpoint Inhibitor Combination Therapies
Emmett Schmidt, PhD, Distinguished Scientist & Executive Director, Merck Research Labs
Clinical combination studies including PD-1 checkpoint inhibitors exploded in the 7 years since the first PD-1 checkpoint inhibitor approvals. Expectations of combination success were high at first. Seven years on, it is important to assess these studies more realistically. The presenter recently published a cross sectional study of PD-1 pathway checkpoint inhibitor clinical trials. Encouraging and discouraging results will be reviewed to guide thinking about future combination strategies.
NEWS -- Provectus Biopharmaceuticals Highlights Stage IV M1c Patient Outcome from Combination Therapy Trial of PV-10® and KEYTRUDA® for Checkpoint-Naïve Advanced Cutaneous Melanoma at ESMO Virtual Congress 2020
This one is tightly held must be insiders telling their friends and relatives about the successes of PV10, PVCT has a great potential to hit .50 cents in 6 months, management said this was an exciting time in the history of the company. great news today and the company that owns keytruda will want PV10.
Just In: $PVCT Provectus Biopharmaceuticals Announces Presentation of Preliminary Data from Combination Therapy Trial of PV-10® and KEYTRUDA® for Checkpoint-Refractory Advanced Cutaneous Melanoma at ESMO Virtual Congress 2020
36% ORR and 64% DCR (RECIST 1.1) PV-10-induced, tumor-specific restoration of T cell function Data compare favorably to approved and investigational therapies for patient population KNOXVILLE, TN, Sept. 17, 2020 (GLOBE NEWSWIRE) -- Provectus (OTCQB: PVCT) today announced that prelimin...
In case you are interested PVCT - Provectus Biopharmaceuticals Announces Presentation of Preliminary Data from Combination Therapy Trial of PV-10® and KEYTRUDA® for Checkpoint-Refractory Advanced Cutaneous Melanoma at ESMO Virtual Congress 2020
Breaking News: $PVCT Provectus Biopharmaceuticals Announces Presentation of 2-Year Landmark Data from Combination Therapy Trial of PV-10® and KEYTRUDA® for Checkpoint-Naïve Advanced Cutaneous Melanoma at ESMO Virtual Congress 2020
62% 2-year OS; median OS not reached Stable, non-overlapping safety profile Clinical synergy between PV-10 and immune checkpoint blockade Data compare favorably to approved therapies for patient population KNOXVILLE, TN, Sept. 17, 2020 (GLOBE NEWSWIRE) -- Provectus (OTCQB: PVCT) toda...
In case you are interested PVCT - Provectus Biopharmaceuticals Announces Presentation of 2-Year Landmark Data from Combination Therapy Trial of PV-10® and KEYTRUDA® for Checkpoint-Naïve Advanced Cutaneous Melanoma at ESMO Virtual Congress 2020
this is poised to make a move.. Whether MRK buys this or not one day, the drugs that are showing positive signs combining with super cancer drug, Keytruda, are all going up in share price.
PVCT is going to have a major move up if this positive news continues.
NEWS -- Provectus Biopharmaceuticals Announces Presentation of 2-Year Landmark Data from Combination Therapy Trial of PV-10® and KEYTRUDA® for Checkpoint-Naïve Advanced Cutaneous Melanoma at ESMO Virtual Congress 2020
NEWS -- Provectus Biopharmaceuticals Announces Presentation of Preliminary Data from Combination Therapy Trial of PV-10® and KEYTRUDA® for Checkpoint-Refractory Advanced Cutaneous Melanoma at ESMO Virtual Congress 2020
NEWS -- Provectus Biopharmaceuticals Establishes Research Collaboration with University of Tennessee Health Science Center to Investigate Small Molecule Rose Bengal Disodium for Antibiotic Resistance
KNOXVILLE, TN, Sept. 09, 2020 (GLOBE NEWSWIRE) -- Provectus (OTCQB: PVCT) today announced that the Company has initiated a new sponsored research program with Michio Kurosu, PhD, Professor, Department of Pharmaceutical Sciences at the College of Pharmacy of the University of Tennessee Health Science Center (UTHSC) in Memphis, Tennessee to investigate rose bengal disodium (RBD) targeting of multi-drug resistant (MDR) bacteria. RBD is Provectus’ proprietary lead molecule, and a member of a class of small molecules called halogenated xanthenes that is entirely and wholly owned by the Company.
Dr. Kurosu’s team will undertake in vitro studies on:
This from another board absolutely fascinating.
Next data release end of September? Melanoma data? Includes stage III?
By November...more data on systemic RB? More Poetic data?
Scheduled ends of all current P1’s by end of 1st qtr 2021....per mgt...must mean that all P1 recruiting has finished by now because you report when you have PFS and OS data. Logic dictates recruiting finished by now at whatever N. Whatever they got they are going forward on PFS and OS data if they plan to report the end of P1 trials.
Per my past posts. Mgt needs to cozy up with mgt a friendly life science fund before all this. I believe this has already started by buying up what I assume was a portion of maxim warrant conversions. I did not expect maxim to convert it all....that would be dumb at this point if they think this science moves ahead.
Mgt published a systemic RB application to deal with leukemia and other indications. They would never have done so without patent application in place. Other papers indicate a systemic application might be interfering with IL-17....which, in turn, blocks IL-6....research derived from Dr. Kruger’s PH-10 studies...therefore new tech to the industry. RB via PV-10/ PH-10 becomes its own checkpoint inhibitor to interleukins.
For us shareholders here...I’m seeing new tech to the industry coming out. Solid science by independent organizations. Much of this new research is expanding new approaches to new applications. To me...this is nothing more than bolt on tech when talking to big pharma...nothing more. To advance this new tech requires a co-development deal. Our prior tech requires this also. There are over 15 big pharmas playing in this field. I assume mgt knows this as well.
Cancer...solid and blood..,,why?
Psoriasis
Possible new checkpoint inhibitor of interleukins
Can PVCT tech rise to such a broad range of applications like aspirin?....how the RB molecule works...I say yes.
Lots of new tech about RB is coming out. One study says it fixes 100% fungal nail disease. That was proven...,proven! No question.
In truth, RB is such a unique molecule with specific targeting abilities. And that targeting can be applied to many targets. Results of those studies has various degrees of efficacy. From uveal to a bad toenail. While efficacy in different indications vary....targeting disfunctional cells has a common theme. And that is bad cells are acidic...cancer...psoriasis...ect. We own a drug that targets acidity first and foremost.
But wait...you also get interleukin binding....and that opens up all the new tech. That tech address cytokine storms associated with Covid. But wait...it possible blocks ligand links in HIV of CD4+T and CD8+T...,that’s being studied now.
So what made RB in saline so important as PV-10/PH-10?
RB being ionic initially. Hydrophilic. But in saline hydrophobic. Robbing the NA+ out of the saline. Then doing a dance on the cell wall on acidic cells. Flipping hydrophilic heads with hydrophilic tails and then the reverse in the cell walls...which puts RB into the lysosomes...in treating cancer.
But what about IL’s and cytokines? Charge difference over atoms. This can possibly lock up IL’s, when you lock up IL’s...by defacto... you become a checkpoint inhibitor. Thus RB is a IL checkpoint inhibitor.
If Provectus shows positive results with COVID 19 they are working on, $1.00 a share is very possible (COVID 19 connection This from the 10q filings (Virology. The Company and research collaborators are undertaking preclinical work on a potential, systemically-administered, cGMP RBD therapy for SARS-CoV-2.)
It looks like the phase 2 trial was done 8 years ago.
Wish they would talk about the combination trial with Keytruda more.
THis could be a massive gamechanger! Keytruda is currently the most promising cancer drug out there and if PVCT's combination with Keytruda forms a more comprehensive, effective drug combination to combat various types of cancer, this company stock could take off.
This stock went to $6 a share on the hopes of it working years ago.
they are so much closer now.
Great Barrons article on Merck and how Keytruda will lead the way.
Would be great for both companies potentially
Wow that's great! Sounds very interesting!
NEWS -- Provectus Biopharmaceuticals Expands Sponsored Research Collaboration with University of Calgary (Canada) to Investigate Oral Administration of Rose Bengal Disodium for Cancer Treatment
Provectus is a clinical-stage biotechnology company developing immunotherapy
medicines based on an entire, wholly-owned family of chemical small molecules
called halogenated xanthenes. The Company's proprietary lead molecule is cGMP
RBD. IL PV-10, the first small molecule autolytic immunotherapy and a
formulation of cGMP RBD, can induce immunogenic cell death ("ICD"), and is
undergoing clinical study for adult solid tumor cancers, such as melanoma and GI
tumors (e.g., HCC, mCRC, mNET, mUM), and preclinical study for pediatric solid
tumor cancers (e.g., neuroblastoma, Ewing sarcoma, rhabdomyosarcoma,
osteosarcoma). Topically-administered PH-10, an immune-modulatory agent and
formulation of cGMP RBD, is undergoing clinical study for inflammatory
dermatoses (e.g., psoriasis, atopic dermatitis). New systemically-administered
formulations of cGMP RBD are being investigated for hematology (e.g., acute
myeloid leukemia, acute monocytic leukemia) and virology (e.g., SARS-CoV-2).
I was right about the COVID 19 connection This from today's 10q filings (Virology. The Company and research collaborators are undertaking preclinical work on a potential, systemically-administered, cGMP RBD therapy for SARS-CoV-2.)
keeping .09-.10/sh and 1 million shares traded, are you kidding me !?! This stock is definitely worth keeping an eye on after its long slumber.
Oops 2 year high
5 year high my friend !
52 wk high! BOOM, here it comes, approaching 2 mil volume, a lot for this stock.
Dom is a genius ! So glad he stepped in to save this company.
Interesting, not sure how long you have been around this stock, but I have multiple years in, went through the name change and hackjob by a couple seeking alpha articles that trashed the company as a rose bengal imitation product, awful stuff. Then, the guy who runs this blog, a D. Rodrigues took over the company I believe for more shares, ever read any of this guy's stuff?
http://provectuspharmaceuticalsinc.blogspot.com/
If we find out the big companies that Provectus is working with this could be the reason also if PV10 can do the same with the corvid 19 virus that it does with cancer this could be the reason it is going up.
PV-10 selectively accumulates in the lysosomes of cancer cells upon contact, disrupting the lysosomes and causing the cells to die. Provectus1,13, external collaborators6, and other researchers14,15,16 have independently shown that PV-10 (RB) triggers each of the three primary cell death pathways: apoptosis, autophagy, and necrosis.
so, what is the catalyst, anything new??
Wow, who else noticed their account went up a bit today due to this sleeping giant hopefully waking up?
|
Followers
|
185
|
Posters
|
|
|
Posts (Today)
|
0
|
Posts (Total)
|
9955
|
|
Created
|
08/09/02
|
Type
|
Free
|
| Moderators | |||
.png)

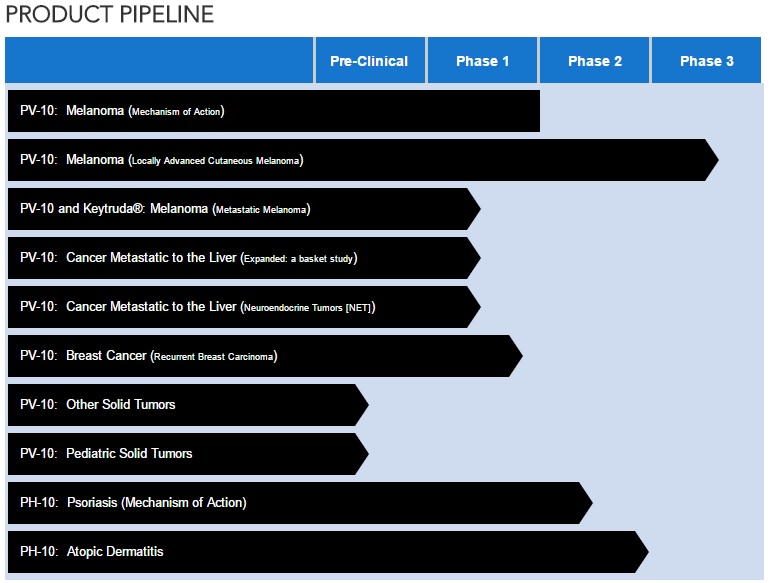
Provectus Biopharmaceuticals is investigating new therapies for the treatment of skin cancer and liver cancer. Provectus investigational oncology drug, PV-10, is an ablative immunotherapy under investigation in solid tumor cancers. The Company has received orphan drug designations from the FDA for its melanoma and hepatocellular carcinoma indications. PH-10, its topical investigational drug for dermatology, is undergoing clinical testing for psoriasis and atopic dermatitis. Provectus has completed Phase 2 trials of PV-10 as a therapy for metastatic melanoma, and of PH-10 as a topical treatment for psoriasis. In addition, Provectus has begun a Phase 3 trial as a therapy for metastatic melanoma. Information about these and the Company's other clinical trials can be found at the NIH registry, www.clinicaltrials.gov. For additional information about Provectus, please visit the Company's website at www.provectusbio.com.
The red synthetic dye Rose Bengal is shown in a bottle at Provectus Pharmaceuticals, Inc.
CURRENT PVCT PIPELINE (as of May 23, 2017)
--------------------------------------------------------------
Charts and Technical:
PVCT 6 months chart:
http://stockcharts.com/h-sc/ui?s=PVCT&p=D&yr=0&mn=6&dy=0&id=p03754661229
Technical analysis:
http://www.stockta.com/cgi-bin/analysis.pl?symb=PVCT&num1=7&cobrand=&mode=stock
PVCT News and Analysis:
PVCT News Blog with the latest news and analysis:
http://provectuspharmaceuticalsinc.blogspot.ca/p/news.html
CONNECTING THE DOTS - CURRENT NEWS PAGE
http://provectuspharmaceuticalsinc.blogspot.ca/p/current-news_22.html
CONNECTING THE DOTS - BLOG PAGE
http://provectuspharmaceuticalsinc.blogspot.ca/
PVCT News at OTC
http://www.otcmarkets.com/stock/PVCT/news
CLINICAL TRIALS Updates and Info:
| Short Interest | 167,323 (69.16%) Apr 13, 2017 |
| Significant Failures to Deliver | No |
| Volume | |
| Day Range: | |
| Bid Price | |
| Ask Price | |
| Last Trade Time: |
