Register for free to join our community of investors and share your ideas. You will also get access to streaming quotes, interactive charts, trades, portfolio, live options flow and more tools.
Great news, thank you, was hoping for above average results; PV-10 certainly did not disappoint. Realistically, however, I do not see this doing anything for the price other than today's bump. Some second tier biotech has to be getting interested, however, this has to be compelling to them on so many levels as an affordable investment/partnership. Big pharma does not see the financial opportunity in the PVCT approach; more interested in maintaining their reoccurring price gouging regimens.
We are making progress thank goodness, just do not think this data set will be our start. I believe some collaborative news will eventually be forthcoming, just don't think this release is enough. Added on the last dip to $0.08, will do so again if the opportunity presents itself. The ocular and veterinary products might be the first to make noise.
NEWS -- Provectus Biopharmaceuticals Provides Updated Data on Cancer Immunotherapy PV-10 for Metastatic Uveal Melanoma
KNOXVILLE, TN, Nov. 13, 2023 (GLOBE NEWSWIRE) -- Provectus (OTCQB: PVCT) today provided updated data from an ongoing Phase 1 clinical trial of investigational cancer immunotherapy PV-10 (rose bengal sodium) for the treatment of uveal melanoma (UM) metastatic to the liver (mUM) (NCT00986661).
mUM patients enrolled in this study received 1 or more cycles of PV-10 injection into 1 or more hepatic metastases. Where indicated, standard of care immune checkpoint blockade (CB), as either monotherapy pembrolizumab or the combination of ipilimumab and nivolumab (IN), was also administered.
To date, 25 mUM patients have received monotherapy PV-10 or PV-10 in combination with CB:
NEWS -- Provectus Biopharmaceuticals Announces Presentation of Preclinical PV-10 Vaccine Adjuvant Data at Society for Immunotherapy of Cancer (SITC) 2023 Annual Meeting
KNOXVILLE, TN, Nov. 06, 2023 (GLOBE NEWSWIRE) -- Provectus (OTCQB: PVCT) today announced that preclinical data from ongoing research on the potential use of investigational cancer immunotherapy PV-10 (rose bengal sodium) as an adjuvant in vaccines to help them work better was the subject of a poster presentation at the Society for Immunotherapy of Cancer (SITC) 2023 annual meeting, which was held in San Diego, CA from November 1-5. This research has been led by Aru Narendran, MD, PhD and his lab team from the Cumming School of Medicine at the University of Calgary in Alberta, Canada.
A copy of the SITC poster, titled “The iodinated fluorescein derivative PV-10 enhances the antiviral activity of CD8+ T-Cells by inducing STING dimerization: Implications for enhanced vaccine applications,” is available on Provectus’s website at https://www.provectusbio.com/media/docs/2023-SITC-poster.pdf.
Dr. Narendran and his colleagues previously discovered that PV-10 activated stimulator of interferon (IFN) genes (STING), demonstrating its potential as a vaccine adjuvant in PV-10-mediated systemic anti-tumor immune responses. See Thakur et al., American Association for Cancer Research 2020 Virtual Annual Meeting II.
In its SITC work, the Narendran lab showed that PV-10 treatment induced STING activation, upregulated cytokines and chemokines, and increased IFN-? secretion by CD8+ T-cells. Dr. Narendran and his colleagues demonstrated PV-10’s ability to function as an effective adjuvant to enhance T-cell responses and concluded that PV-10’s unique modulation of the STING pathway was a potential mechanism of this activity.
Dominic Rodrigues, Vice Chair of Provectus’s Board of Directors, said “This novel work introduces the potential of PV-10 to be an effective, multi-purpose, vaccine adjuvant for the first time, and conveys the opportunity to potentially use PV-10 in anti-viral, anti-cancer, and possibly other vaccines for greater protection against disease by improving a person’s immune response to vaccination.”
Mr. Rodrigues added, “We remain grateful to Dr. Narendran and his lab team members for their many-sided research to elucidate PV-10’s various potential mechanisms and applications. Provectus’s preclinical and clinical data to date, which may indicate that the response to PV-10 treatment is tantamount to in situ vaccination, may also suggest that PV-10-adjuvanted vaccines could potentially contribute to more effective and durable immune responses, better responses from patient populations with unique characteristics, higher efficacy using less antigen, and making vaccines more accessible, sustainable, and affordable.”
About Provectus
Provectus Biopharmaceuticals, Inc. (Provectus or the Company) is a clinical-stage biotechnology company developing immunotherapy medicines for different diseases that are based on a class of synthetic small molecule immuno-catalysts called halogenated xanthenes (HXs). Provectus’s lead HX molecule is named rose bengal sodium (RBS).
The Company’s proprietary, patented, pharmaceutical-grade RBS is the active pharmaceutical ingredient (API) in the drug product candidates of Provectus’s clinical development programs and preclinical formulations of the Company’s drug discovery programs. Provectus’s pharmaceutical-grade RBS displays different therapeutic effects at different concentrations and can be formulated for delivery by different routes of administration. The International Nonproprietary Names Expert Committee of the World Health Organization selected “rose bengal sodium” for the nonproprietary name of the Company’s API.
RBS may target disease in a bifunctional manner. Direct contact may lead to cell death or repair depending on the disease being treated and the concentration of Provectus’s RBS utilized in the treatment. Multivariate immune signaling, activation, and response may follow that may manifest as stimulatory, inhibitory, or both.
The Company believes that it is the first entity to advance an RBS formulation into clinical trials for the treatment of a disease. Provectus believes that it is the first and only entity to date to make pharmaceutical-grade RBS successfully, reproducibly, and consistently at a purity of nearly 100%.
Provectus’s small molecule HX medical science platform includes clinical development programs in oncology, dermatology, and ophthalmology; proof-of-concept in vivo development programs in oncology, hematology, wound healing, and animal health; and in vitro drug discovery programs in infectious diseases and tissue regeneration and repair.
Information about the Company’s clinical trials can be found at the National Institutes of Health (NIH) registry, https://ClinicalTrials.gov. For additional information about Provectus, please visit the Company’s website at https://www.provectusbio.com.
FORWARD-LOOKING STATEMENTS: The information in this press release may include “forward-looking statements,” within the meaning of U.S. securities legislation, relating to the business of Provectus and its affiliates, which are based on the opinions and estimates of Company management and are subject to a variety of risks and uncertainties and other factors that could cause actual events or results to differ materially from those projected in the forward-looking statements. Forward-looking statements are often, but not always, identified by the use of words such as “seek,” “anticipate,” “budget,” “plan,” “continue,” “estimate,” “expect,” “forecast,” “may,” “will,” “project,” “predict,” “potential,” “targeting,” “intend,” “could,” “might,” “should,” “believe,” and similar words suggesting future outcomes or statements regarding an outlook.
The safety and efficacy of the agents and/or uses under investigation have not been established. There is no guarantee that the agents will receive health authority approval or become commercially available in any country for the uses being investigated or that such agents as products will achieve any particular revenue levels.
Due to the risks, uncertainties, and assumptions inherent in forward-looking statements, readers should not place undue reliance on these forward-looking statements. The forward-looking statements contained in this press release are made as of the date hereof or as of the date specifically specified herein, and Provectus undertakes no obligation to update or revise any forward-looking statements, whether as a result of new information, future events or otherwise, except in accordance with applicable securities laws. The forward-looking statements are expressly qualified by this cautionary statement.
Risks, uncertainties, and assumptions include those discussed in the Company’s filings with the Securities and Exchange Commission (SEC), including those described in Item 1A of:
#####
Contact:
Provectus Biopharmaceuticals, Inc.
Heather Raines, CPA
Chief Financial Officer
Phone: (866) 594-5999
NEWS -- Provectus Biopharmaceuticals Announces Acceptance of PV-10 Poster Presentation at Society for Immunotherapy of Cancer (SITC) 2023 Annual Meeting

KNOXVILLE, TN, Aug. 07, 2023 (GLOBE NEWSWIRE) -- Provectus (OTCQB: PVCT) today announced that preclinical data from ongoing research on investigational cancer immunotherapy PV-10 (rose bengal sodium) as an immune vaccine adjuvant will be presented on a poster presentation at the Society for Immunotherapy of Cancer (SITC) 2023 annual meeting to be held in San Diego, CA from November 1-5. This PV-10 research has been led by Aru Narendran, MD, PhD and his team of researchers at the Cumming School of Medicine at the University of Calgary in Alberta, Canada.
The accepted abstract is:
NEWS -- Provectus Biopharmaceuticals Establishes Research Collaboration with University of Tennessee College of Veterinary Medicine to Investigate Pharmaceutical-Grade Small Molecule Immunotherapy Rose Bengal Sodium for Canine Soft Tissue Sarcomas

KNOXVILLE, TN, June 28, 2023 (GLOBE NEWSWIRE) -- Provectus (OTCQB: PVCT) today announced that the Company has initiated a new sponsored research program with the University of Tennessee College of Veterinary Medicine (UTCVM) to assess the safety and preliminary efficacy of intralesional injection of a formulation of Provectus’s pharmaceutical-grade RBS for canine soft tissue sarcomas. UTCVM’s lead principal investigator of this work is clinical pathologist, comparative cancer biologist, and Assistant Professor Nora Springer, DVM, PhD, DACVP.
This preclinical and clinical veterinary study is being undertaken as part of the State of Tennessee’s funding to develop animal health drug products in partnership with state universities that have agriculture and veterinary medicine programs and the Company.
Soft tissue sarcomas are common malignant neoplasms in dogs that are locally invasive and have a high prevalence of recurrence after surgical excision. These tumors are often located on the extremities, where complete surgical excision is challenging because minimal soft tissues hinder the ability to gain adequate tumor margins while maintaining the ability to close the surgical wound. Adjuvant chemotherapeutic protocols do not improve either time to recurrence or overall survival with incompletely excised soft tissue sarcomas. Radical measures, such as limb amputation, are often required to ensure complete excision for limb sarcomas and this option is frequently unpalatable to patient owners. Therefore, intralesional injection resulting in tumor ablation may be an ideal treatment modality for soft tissue sarcomas.
Dr. Springer graduated from Marietta College with a Bachelor of Science in Chemistry, LaGuardia Community College with an Associate in Applied Science in Veterinary Technology, Kansas State University with a Doctor of Veterinary Medicine (DVM), and Cornell University with a Doctor of Philosophy (PhD) in Translational Medicine. She completed the American College of Veterinary Pathologists (ACVP) certifying examination to become a Diplomate Member (DACVP), and has co-authored a number of medical journal publications to date.
Dominic Rodrigues, Vice Chairman of Provectus’s Board said, “We are excited to commence this canine cancer study with the University of Tennessee College of Veterinary Medicine and Dr. Nora Springer to accelerate our animal health drug development program. We believe that the Company’s adult cancer clinical and pediatric preclinical data to date can meaningfully contribute to Dr. Springer’s canine cancer work, and vice versa because spontaneous cancers in dogs share many features with human cancers.”
About Provectus
Provectus Biopharmaceuticals, Inc. (Provectus or the Company) is a clinical-stage biotechnology company developing immunotherapy medicines for different diseases that are based on a class of synthetic small molecule immuno-catalysts called halogenated xanthenes (HXs). Provectus’s lead HX molecule is named rose bengal sodium (RBS).
The Company’s proprietary, patented, pharmaceutical-grade RBS is the active pharmaceutical ingredient (API) in the drug product candidates of Provectus’s clinical development programs and preclinical formulations of the Company’s drug discovery programs. Provectus’s pharmaceutical-grade RBS displays different therapeutic effects at different concentrations and can be formulated for delivery by different routes of administration. The International Nonproprietary Names Expert Committee of the World Health Organization selected “rose bengal sodium” for the nonproprietary name of the Company’s API.
RBS may target disease in a bifunctional manner. Direct contact may lead to cell death or repair depending on the disease being treated and the concentration of Provectus’s RBS utilized in the treatment. Multivariate immune signaling, activation, and response may follow that may manifest as stimulatory, inhibitory, or both.
The Company believes that it is the first entity to advance an RBS formulation into clinical trials for the treatment of a disease. Provectus believes that it is the first and only entity to date to successfully, reproducibly, and consistently make pharmaceutical-grade RBS at a purity of nearly 100%.
Provectus’s small molecule HX medical science platform includes clinical development programs in oncology, dermatology, and ophthalmology; proof-of-concept in vivo drug discovery programs in oncology, hematology, wound healing, and animal health; and preclinical in vitro drug discovery programs in infectious diseases and tissue regeneration and repair.
Information about the Company’s clinical trials can be found at the National Institutes of Health (NIH) registry, https://ClinicalTrials.gov. For additional information about Provectus, please visit the Company’s website at https://www.provectusbio.com.
FORWARD-LOOKING STATEMENTS: The information in this press release may include “forward-looking statements,” within the meaning of U.S. securities legislation, relating to the business of Provectus and its affiliates, which are based on the opinions and estimates of Company management and are subject to a variety of risks and uncertainties and other factors that could cause actual events or results to differ materially from those projected in the forward-looking statements. Forward-looking statements are often, but not always, identified by the use of words such as “seek,” “anticipate,” “budget,” “plan,” “continue,” “estimate,” “expect,” “forecast,” “may,” “will,” “project,” “predict,” “potential,” “targeting,” “intend,” “could,” “might,” “should,” “believe,” and similar words suggesting future outcomes or statements regarding an outlook.
The safety and efficacy of the agents and/or uses under investigation have not been established. There is no guarantee that the agents will receive health authority approval or become commercially available in any country for the uses being investigated or that such agents as products will achieve any particular revenue levels.
Due to the risks, uncertainties, and assumptions inherent in forward-looking statements, readers should not place undue reliance on these forward-looking statements. The forward-looking statements contained in this press release are made as of the date hereof or as of the date specifically specified herein, and Provectus undertakes no obligation to update or revise any forward-looking statements, whether as a result of new information, future events or otherwise, except in accordance with applicable securities laws. The forward-looking statements are expressly qualified by this cautionary statement.
Risks, uncertainties, and assumptions include those discussed in the Company’s filings with the Securities and Exchange Commission (SEC), including those described in Item 1A of:
#####
Contact:
Provectus Biopharmaceuticals, Inc.
Heather Raines, CPA
Chief Financial Officer
Phone: (866) 594-5999
Thank you for your write up Wehalls, I remain overall encouraged even though I am a bit deflated that the TGA route seems to be rapidly dwindling. More likely, it appears to be a probable dead end since we would not likely put limited resources into a foreign drug approval agency if a `fast track' avenue is not an option for us.
I always thought the ocular route with Miami would be our first revenue producer despite the statement that Management is "trying to stay focused and putting everything on the back burner that is not related to their current oncology trials". I forgot about the animal/pet possibility, glad to hear that is moving forward, I honestly thought that had great merit as well.
Don't know what to think about the reverse stock split, I do not recall one company off hand that has used it successfully; I realize there are certainly outliers. Sounds like Ed and Dom are not to concerned about financing, that is certainly positive news. And having potential investors helping out with clinical trials is very positive. I like the idea that any financial decision they make has at least some consideration for the shareholders. PHR has been wonderful to us so far, I think they are a stand up management team, no one else would finance a company like they do by buying the convertible shares over market value. Would just like to see some traction in clinical outcomes; just seems like a consistent one foot forward, another step backwards approach.
Appreciate your write-up, I remain pretty upbeat about the company despite knowing it remains a long shot for commercial success (inherent with the industry). One can dream though, and patients may one day may have medical options that can truly extend and improve quality of life thanks to PVCT. Thank you for your efforts.
Might be over 1 to 100 R/S.
I attended the AM and below are my notes from the 2023 Provectus Annual Meeting.
All five directors were present in person this year.
All six proposals approved.
Dominic went over the slides that updated the progress of the company. Slides provided below but you must go past the first few pages to get to the slides. (See slides PVCT sent to investors)
After the presentation, Ed made a few comments, and one interesting remark is that PVCT is going to explore the possibility of having a conference call every 6 months to try and keep the investors more up to date on what’s happening with the company.
The 2022 Reverse Split was discussed and PRH stated that the events they hoped would be in place didn’t happen and they simply decided to not go forward with the R/S.
This is a good place to discuss the TGA event that we all thought would have triggered the R/S. I asked whether an application had been submitted to the TGA and Dominic commented that it was their policy not to comment on these types of actions by management. This is understandable based on previous actions by Dees and their public announcement that they had submitted a package to the FDA which was turned down because of the lack of significant patient data. However, there were comments from other investors in private that they had heard that PVCT had decided to not submit the package because TGA had decided that Intermittent Melanoma was not a rare disease and therefore was not eligible for being fast tracked. Consequently, management decided to not pursue this path of TGA regulatory approval and to go forward with the FDA or maybe with the normal TGA regulatory path (not sure).
There was an interesting discussion about whether or not the delays related to COVID was a blessing or a curse. Management believes that in the long run it was a blessing because it allowed them to open up many different avenues of potential RB use such as dermatology, virology, ophthalmology, and others. They believe without the COVID delays they would have gone down the oncology path and any other venues would not have been explored.
One of my biggest concerns has been funding. Dominic commented that they could go and get the money they needed at a price point that wouldn't necessarily be advantageous to the current stockholders, but that was always an option. They seemed fairly confident that they could find the money they needed to get the trials done without destroying the current stockholders' share position. The bottom line is the money that Dominic and Ed have been putting into the company was not to keep the lights on, but to progress the company's objectives towards regulatory approval by the FDA and maybe the TGA. Also of interest, there are individuals who have the ability to fund specific trials that interest them, and Ed is in discussions with them.
The question was asked regarding Bascom Palmer and negotiations for rights to use their equipment with RB to treat fungal keratitis. Many investors believed that negotiations have broken down. Dominic replied that this was definitely not the case and their relationship with Bascom Palmer was strong and that it was taking longer than anticipated because of the bureaucracy associated with the University of Miami (For Profit University) which is who Bascom Palmer is a part of. He also commented that their equipment is not what is doing the heavy lifting in treating fungal keratitis, but it is PV-10 and there are other equipment options.
A question was asked about operational costs associated with operations and trial costs and Ed commented that the company’s current spend rate is $250K per month.
In regard to the University of Tennessee’s grant for exploring PV-10 with animal sciences, Dr. Lacey stated it has taken a while to find the lead investigator for the study. However, they have now found the individual they need, and she is not only a veterinarian but also a PhD and they have every confidence in her and believe the outcome will be fruitful. In essence, the study is just now getting off the ground, but progress will be made. Unfortunately, the use of PV-10 in animal sciences in Australia will not be of much use in the study other than lessons learned from clinic use and practical applications. The lead investigator will have to start from scratch due to academic rigor although she will have the advantage of the information from the veterinarian’s use of RB in dogs (or other animals) in Australia.
The board of directors stated that they are trying to stay focused and putting everything on the back burner that is not related to their current oncology trials. They are concentrating on pancreatic cancer, metastatic uveal melanoma, stage III melanoma combo, and intransit metastatic melanoma (possibly TGA route, not sure) and will be moving forward with these trials. Also of interest, PVCT is trying to initiate PET scans in their trials because only PET scans are able to determine if the tumor is truly dead. A dead tumor may show up on a regular scan, but the Radiologist can’t tell if the tumor is dead or not and therefore won’t declare a CR without a PET scan. Regarding the date of conclusion of trials, there are a lot of unknowns such as patient recruitment, funding, successful readout of data and application to FDA/TGA, etc. Investor guesses is sometime within the first 6 months of 2024 and that is only a guess.
These are my notes, so if someone else was at the meeting and wants to correct or elaborate on my notes, please feel free to do so and my feelings will not be hurt.
Conclusion: I know we are all very frustrated with the slow pace that this is taking and the low share price and low volume. Management acknowledged our frustration and stated they are also frustrated with the low stock price because they are also stockholders. My impression is that by the next annual meeting, things will be significantly different. I also acknowledge that we have all thought this year after year. I've often said if making money in this stock was easy everybody would be doing it. However, this has been a very difficult investment for all of us and I believe in the long run it could very well be an investment of a lifetime. The Board Members believe PV-10 could be an industry disrupter and could completely change oncology. Not only do we have a great drug, but I believe we have good men on the board of directors. These thoughts are my opinion and based on years of being invested in PVCT, so take it for what it's worth.
NEWS -- Provectus Biopharmaceuticals Announces Stockholder Approval to Undertake Reverse Split of Outstanding Equities and Reduce Number of Authorized Equities by Same Ratio

KNOXVILLE, TN, June 26, 2023 (GLOBE NEWSWIRE) -- Provectus (OTCQB: PVCT) today announced that the Company’s shareholders have approved the proposals of Provectus’s Board of Directors (Board) to seek the authority to undertake a reverse stock split and an authorized share reduction.
At the Company’s Annual Meeting of Stockholders held in Knoxville, Tennessee on June 21, 2023, shareholders also approved the Board’s recommendations of four other proposals: the election of directors, an advisory vote on the approval of the compensation of Provectus’s named executive officers, an advisory vote on the frequency of the aforementioned advisory vote on compensation, and the ratification of the Company’s independent registered public accounting firm.
Ed Pershing, Chairman of Provectus’s Board said, “I want to express my sincere appreciation and the sincere appreciation of our directors, officers, employees, and staff to all of our stockholders for your active participation in the voting process as well as your support of our business direction and management, as evidenced by these favorable voting results. Your continued interest in and support of Provectus is welcomed and greatly appreciated.”
Mr. Pershing added, “The Company’s Board understands its responsibility to evaluate and determine whether and when a reverse stock split of Provectus’s outstanding equities and reduction of authorized shares are in the best interests of the Company and our stockholders.”
A copy of Provectus’s Form 8-K filed on June 22, 2023, which provided details of shareholder voting on the Board’s six proposals and included a brief description of and the vote tabulation for each proposal, may be found here:
https://www.sec.gov/ix?doc=/Archives/edgar/data/315545/000149315223022116/form8-k.htm.
Proposal #1 (election of directors) passed with [an average of] 44% FOR of shares outstanding and 96% FOR of shares voted.
Proposal #2 (advisory vote on compensation) passed with 43% FOR of shares outstanding and 94% FOR of shares voted.
Proposal #3 (one-year frequency of advisory vote on compensation) passed with 43% FOR of shares outstanding and 94% FOR of shares voted.
Proposal #4 (independent accounting firm) passed with 67% FOR of shares outstanding and 99% FOR of shares voted.
Proposal #5 (reverse stock split) passed with 61% FOR of shares outstanding and 90% FOR of shares voted.
Proposal #6 (authorized share reduction) passed with 61% FOR of shares outstanding and 91% FOR of shares voted.
About Provectus
Provectus Biopharmaceuticals, Inc. (Provectus or the Company) is a clinical-stage biotechnology company developing immunotherapy medicines for different diseases that are based on a class of synthetic small molecule immuno-catalysts called halogenated xanthenes (HXs). Provectus’s lead HX molecule is named rose bengal sodium (RBS).
The Company’s proprietary, patented, pharmaceutical-grade RBS is the active pharmaceutical ingredient (API) in the drug product candidates of Provectus’s clinical development programs and preclinical formulations of the Company’s drug discovery programs. Provectus’s pharmaceutical-grade RBS displays different therapeutic effects at different concentrations and can be formulated for delivery by different routes of administration. The International Nonproprietary Names Expert Committee of the World Health Organization selected “rose bengal sodium” for the nonproprietary name of the Company’s API.
RBS may target disease in a bifunctional manner. Direct contact may lead to cell death or repair depending on the disease being treated and the concentration of Provectus’s RBS utilized in the treatment. Multivariate immune signaling, activation, and response may follow that may manifest as stimulatory, inhibitory, or both.
The Company believes that it is the first entity to advance an RBS formulation into clinical trials for the treatment of a disease. Provectus believes that it is the first and only entity to date to successfully, reproducibly, and consistently make pharmaceutical-grade RBS at a purity of nearly 100%.
Provectus’s small molecule HX medical science platform includes clinical development programs in oncology, dermatology, and ophthalmology; in vivo proof-of-concept programs in oncology, hematology, wound healing, and animal health; and in vitro programs in infectious diseases and tissue regeneration and repair.
Information about the Company’s clinical trials can be found at the National Institutes of Health (NIH) registry, https://ClinicalTrials.gov. For additional information about Provectus, please visit the Company’s website at https://www.provectusbio.com.
FORWARD-LOOKING STATEMENTS: The information in this press release may include “forward-looking statements,” within the meaning of U.S. securities legislation, relating to the business of Provectus and its affiliates, which are based on the opinions and estimates of Company management and are subject to a variety of risks and uncertainties and other factors that could cause actual events or results to differ materially from those projected in the forward-looking statements. Forward-looking statements are often, but not always, identified by the use of words such as “seek,” “anticipate,” “budget,” “plan,” “continue,” “estimate,” “expect,” “forecast,” “may,” “will,” “project,” “predict,” “potential,” “targeting,” “intend,” “could,” “might,” “should,” “believe,” and similar words suggesting future outcomes or statements regarding an outlook.
The safety and efficacy of the agents and/or uses under investigation have not been established. There is no guarantee that the agents will receive health authority approval or become commercially available in any country for the uses being investigated or that such agents as products will achieve any particular revenue levels.
Due to the risks, uncertainties, and assumptions inherent in forward-looking statements, readers should not place undue reliance on these forward-looking statements. The forward-looking statements contained in this press release are made as of the date hereof or as of the date specifically specified herein, and Provectus undertakes no obligation to update or revise any forward-looking statements, whether as a result of new information, future events or otherwise, except in accordance with applicable securities laws. The forward-looking statements are expressly qualified by this cautionary statement.
Risks, uncertainties, and assumptions include those discussed in the Company’s filings with the Securities and Exchange Commission (SEC), including those described in Item 1A of:
#####
Contact:
Provectus Biopharmaceuticals, Inc.
Heather Raines, CPA
Chief Financial Officer
Phone: (866) 594-5999
I'm planning on going to the Annual Meeting on 6/21/23. Anyone else going? If so, several of us investors usually get together for lunch prior to the meeting if you are interested. Let me know and I'll give you the details.
NEWS -- Provectus Biopharmaceuticals Announces Publication of Activity of Pharmaceutical-Grade Rose Bengal Sodium (RBS) Against Colistin-Resistant Gram-Negative Bacteria

Strong today with decent volume at midday. New 20 day high. Wants to push higher.
In for a few bushels. Thanks for the recommendation
Yet another bounce off of $0.10 support. Stock is reacting well after recent Dec highs. Good call on support Wehalls, I have an order in at $0.10, seems like that is the new line in the sand. Saw on the other board that Pershing bought additional shares ($125k of $0.286 convertible notes). That certainly shows conviction. Still a long wait ahead but exciting nonetheless.
Look at the tweet under the newsletter tweet
$PVCT Releases 2023 Stockholder Letter https://t.co/JkO7ssLsGB #rosebengal #rosebengalsodium
— Provectus Biopharmaceuticals, Inc. (@ProvectusBio) January 9, 2023
NEWS -- Provectus Biopharmaceuticals Releases 2023 Stockholder Letter

KNOXVILLE, TN, Jan. 09, 2023 (GLOBE NEWSWIRE) -- Provectus (OTCQB: PVCT) today announced that it had issued a beginning-of-the-year letter for 2023 to the Company’s stockholders, which may be found below.
2023 Letter to Stockholders
Dear Provectus Stockholders,
Thank you for your continued support of Provectus in 2022.
As fellow, longtime shareholders, our leadership team is committed to maximizing the long-term, fundamental value of the Company through the prospective 2023 efforts described below, and other activities at Provectus, by basic medical, translational, and clinical research collaborators, and with key vendor-partners.
Provectus Biopharmaceuticals, Inc. (Provectus or the Company) is a clinical-late-stage biotechnology company headquartered in Knoxville, Tennessee. The Company is developing immunotherapy medicines for different diseases that are based on a class of synthetic small molecule immuno-catalysts called halogenated xanthenes (HXs). Provectus’ lead HX molecule is named rose bengal sodium (RBS). Provectus’ lead clinical-stage indication and primary focus is oncology (i.e., solid tumor cancers). Drug development work at the Company is ongoing on nine (9) clinical development and drug discovery programs (i.e., nine disease areas) that are validating our small molecule HX medical science as an immunotherapy platform and could potentially generate co-development and/or out-licensing opportunities.
Follow link here for full Press Release -- https://finance.yahoo.com/news/provectus-biopharmaceuticals-releases-2023-stockholder-130000805.html
Our little bio is still in the holiday spirit, closed even in a broadly down market. 2-0-1 for the new year, nice batting average. Volume has declined depreciably though, fortunately with little price movement. Must mean not many motivated sellers. Still need a few more shares, I should have ample opportunities in the months ahead. This year I feel price appreciation will be on our side.
Merry Christmas Everyone!
Isaiah 9:6-7
"For to us a child is born, to us a son is given, and the government will be on his shoulders. And he will be called Wonderful Counselor, Mighty God, Everlasting Father, Prince of Peace. Of the increase of his government and peace there will be no end. He will reign on David's throne and over his kingdom, establishing and upholding it with justice and righteousness from that time on and forever. The zeal of the LORD Almighty will accomplish this."
Boom here we go
I wouldn't worry too much about that tweet. I have no idea if or who PVCT is talking to at this time in regard to the Big Pharmas. There is speculation that there is a partner with the P2/3 combo trial, but I think I would wait for an announcement before I put too much promise on that. I think we will get there; we just have to be patient. There are several potential big events in 2023 to include the TGA announcement, the P2/3 combo trial, and potentially things we don't know about. PVCT doesn't reveal much to their investors which everyone finds frustrating. As I've written before, it's got to mean something that Dominic recently put $3M of his own money into the company. JMO
PVCT's tweets this morning listed some superior PV-10 drug attributes, differentiating themselves from miRNA research and method of actions, that I would have thought would be more likely provided when in direct meetings with a bp to potentially secure their interest and financing.
By going public with it like we did, does that flag that no credible discussions are ongoing? It seems to me that one would not go public with that info unless they are purposely trying to mass market themselves to the bp community at large . . . . meaning promising discussions may not be currently underway with a specific bp. Do you have a read on the matter?? I'm probably getting to wrapped up over nothing, just do not want the momentum to stall. I'm hoping some clinical phase announcement is only a couple of quarters out at most.
Good call. Wild gyrations again in the last 20 minutes of trading. Wehall's 10 cent base nicely held yet again as well. Things looking promising, but it may be months off (hopefully shorter) before we get some meaningful clinical trial discussions going.
As soon as we get some details about the upcoming P2/3
Volume back down to pre-news levels, now we wait. 10 cent base continues to build out, hopefully it holds. Cautiously optimistic for the upcoming year. Overall market looking like it will be fighting headwinds well into first quarter.
Yes, the price range seems to be settling around 10 cents. I have also been told that there is institutional buying at this time so that's good. I'm really hoping for some good results from the P2/3 coming up.
For the second day in a row, the stock closed relatively flat by session end. Today was the exact opposite of yesterday, today's mid-session loss recovered in the last 15 minute segment with the highest volume of the day, as well as the biggest green candle of the session. Yesterday's trading was the exact opposite; giving away mid-afternoon gains with the tallest red candle combined with the highest candle volume, all in the last half hour of trading. Low 8 cents the past two days held up. Don't quite understand what all the last minute price/volume market fervor is all about. Today's volume was the second weakest of the last ten days, beating only yesterday's total volume (per trading view).
Just musing out loud where the new price channel range could possibly settle in for this new period; do we see 7 cents for the low while we await further news? If so, that would still be a decent improvement over our last hibernation zone of 4 cents. I need to be more patient this time in lowering my cost per share. Just feel this company has a realistic chance for success and I want to make sure I have a decent seat; management has demonstrated their opinion with recent purchases. We will undoubtedly learn a considerable bit about this company in 2023, hopefully for the best.
Talk about pulling defeat from the jaws of victory .. wow
NEWS -- Provectus Biopharmaceuticals Announces Decision to Not Undertake Reverse Split of Outstanding and Authorized Equities in 2022; Expects to Seek Same Stockholder Approval Again in 2023

KNOXVILLE, TN, Dec. 12, 2022 (GLOBE NEWSWIRE) -- Provectus (OTCQB: PVCT) today announced that the Company’s Board of Directors (Board) has decided to not undertake the reverse stock split and authorized share reduction, which Provectus stockholders approved at the Company’s 2022 Annual Meeting of Stockholders, by the approved date of December 31, 2022.
Ed Pershing, Chair of Provectus’ Board said, “The Board assessed the prospective factors that it reasonably believed would be necessary to support the potential successful follow-through from undertaking a reverse stock split, and that could also potentially mitigate negative capital markets behavior.”
Mr. Pershing added, “The Board ultimately concluded that it would be in the best interests of the Company’s stockholders to not undertake the reverse stock split and authorized share reduction at this time. The Board expects to seek the same authority from the stockholders to undertake these same measures in 2023 at either a special meeting or the annual meeting of Provectus stockholders.”
About Provectus
Provectus Biopharmaceuticals, Inc. (Provectus or the Company) is a clinical-stage biotechnology company developing immunotherapy medicines for different diseases that are based on a class of synthetic small molecule immuno-modulators called halogenated xanthenes (HXs). Provectus’ lead HX molecule is named rose bengal sodium (RBS).
The Company’s proprietary, patented, pharmaceutical-grade RBS is the active pharmaceutical ingredient (API) in the drug product candidates of Provectus’ clinical development programs and the preclinical formulations of the Company’s drug discovery programs. Importantly, Provectus’ pharmaceutical-grade RBS displays different therapeutic effects at different concentrations and can be formulated for delivery by different routes of administration. The International Nonproprietary Names (INN) Expert Committee of the World Health Organization (WHO) selected “rose bengal sodium” for the nonproprietary name of the Company’s API.
RBS may target disease in a bifunctional manner. First, direct contact may lead to cell death or repair depending on the disease being treated and the concentration of Provectus’ RBS utilized in the treatment. Secondly, multivariate immune signaling, activation, and response may follow that may manifest as stimulatory, inhibitory, or both.
The Company believes that it is the first entity to advance an RBS formulation into clinical trials for the treatment of a disease, such as those trials reported on the clinical trials registry ClinicalTrials.gov. Provectus also believes that it is the first and only entity to date to successfully, reproducibly, and consistently make pharmaceutical-grade RBS at a purity of nearly 100%.
Provectus’ small molecule HX medical science platform includes clinical development programs in oncology, dermatology, and ophthalmology; proof-of-concept in vivo drug discovery programs in oncology, hematology, wound healing, and animal health; and preclinical in vitro drug discovery programs in infectious diseases and tissue regeneration and repair.
Information about the Company’s clinical trials can be found at the National Institutes of Health (NIH) registry, https://www.clinicaltrials.gov. For additional information about Provectus, please visit the Company’s website at https://www.provectusbio.com.
FORWARD-LOOKING STATEMENTS: The information in this press release includes “forward-looking statements,” within the meaning of U.S. securities legislation, relating to the business of Provectus and its affiliates, as well as its 2023 annual meeting, which are based on the opinions and estimates of Company management and are subject to a variety of risks and uncertainties and other factors that could cause actual events or results to differ materially from those projected in the forward-looking statements. Forward-looking statements are often, but not always, identified by the use of words such as “seek,” “anticipate,” “budget,” “plan,” “continue,” “estimate,” “expect,” “forecast,” “may,” “will,” “project,” “predict,” “potential,” “targeting,” “intend,” “could,” “might,” “should,” “believe,” and similar words suggesting future outcomes or statements regarding an outlook.
The safety and efficacy of the agents and/or uses under investigation have not been established. There is no guarantee that the agents will receive health authority approval or become commercially available in any country for the uses being investigated or that such agents as products will achieve any particular revenue levels.
Due to the risks, uncertainties, and assumptions inherent in forward-looking statements, readers should not place undue reliance on these forward-looking statements. The forward-looking statements contained in this press release are made as of the date hereof or as of the date specifically specified herein, and Provectus undertakes no obligation to update or revise any forward-looking statements, whether as a result of new information, future events or otherwise, except in accordance with applicable securities laws. The forward-looking statements are expressly qualified by this cautionary statement.
Risks, uncertainties, and assumptions include those discussed in the Company’s filings with the Securities and Exchange Commission (SEC), including those described in Item 1A of:
Participants in Solicitation and Additional Information and Where to Find It
This press release may constitute soliciting material under SEC Rule 14a-12, and the Company and its directors, executive officers, and advisors may be deemed to be participants in the solicitation of proxies from the holders of the Company’s common stock, Series D Convertible Preferred Stock, and Series D-1 Convertible Preferred Stock. Investors may obtain additional information regarding the interest of those participants by reading the Company’s 2023 proxy statement (when available) and other relevant proxy materials, and the Company’s annual reports on Form 10-K and quarterly reports on Form 10-Q, as filed with the SEC. Stockholders are urged to read carefully and, in its entirety, the Company’s 2023 proxy statement (when available) and other relevant materials, because they will contain important information about the Company and the 2023 annual meeting. Stockholders may obtain free copies of the Company’s proxy statement and its other SEC filings electronically by accessing the SEC’s home page at http://www.sec.gov. Copies can also be obtained, free of charge, upon written request to Provectus Biopharmaceuticals, Inc., Attn: Secretary, 800 S. Gay Street, Suite 1610, Knoxville, TN 37929, (866) 594-5999.
#####
Contact:
Provectus Biopharmaceuticals, Inc.
Heather Raines, CPA
Chief Financial Officer
Phone: (866) 594-5999
Feeling positive something big just over the horizon. Maybe the Australian TGA will expand limited PV-10 use, or possibly a big pharma starts nosing around, or the Univ of Miami ocular licensure takes off, or even the possible reverse split gives us a bump. But 2023 looking promising in my book. Its been a long wait, hopefully this upcoming year will show some serious momentum. The limited study results released this past week sure were encouraging, patients the true beneficiaries. Makes me feel like holding long term may not be so bad an idea after all.
The best part is that you're going to miss out.
Up. Its loading 1.00 coming
This Scam finally went up
200000 AND 172000 SHARE BUY. LITTLE MONSTER HERE. UP UP AND AWAY $$$$$$$$$$$$$$$$$$$$$
NEW HIGH TODAY
NEWS -- Provectus Biopharmaceuticals Announces New Data from Combination Therapy Trial of Small Molecule Cancer Immunotherapy PV-10® and Keytruda® for First-Line Stage III Melanoma at Melanoma Bridge 2022

6 year high above $ 0.13. I think we could get it soon.
Pershing is the Board Chairman and Rodrigues is the Vice Chairman. Rodrigues recently put in $3M of his own money and Pershing also put some money in but I don't remember how much. This week's presentation will tell us a lot about what's going on with the company's combo Keytruda/PV-10 trial.
I have been out of this for years but still watch it. Nice move today for those who have stuck it out. Is Dom still the big guy. And does he still have the connecting the dots blog? TYIA
$PVCT
Pokerpro05
Nice move today. Pershing buying more shares. Something is up, volume is multiples of avg daily. Must be the update to be presented in Italy next week. Might be a nice move in store for us.
NEWS -- Provectus Biopharmaceuticals Announces Acceptance of Small Molecule Cancer Immunotherapy PV-10® Stage III Melanoma Abstract at Melanoma Bridge 2022

KNOXVILLE, TN, Nov. 10, 2022 (GLOBE NEWSWIRE) -- Provectus (OTCQB: PVCT) today announced that data from the Company’s ongoing, multi-cohort, Phase 1b/2 study of the combination therapy of cancer immunotherapy PV-10, an intratumoral formulation of Provectus’ small molecule rose bengal sodium (RBS), and immune checkpoint inhibitor KEYTRUDA® (pembrolizumab) for the treatment of Stage III cutaneous melanoma will be presented at Melanoma Bridge 2022, to be held in Naples, Italy and online from December 1-3, 2022.
The abstract accepted for video oral communication and poster presentation is entitled “Response for combination of PV-10 autolytic immunotherapy and immune checkpoint blockade in stage III cutaneous melanoma.”
About Provectus
Provectus Biopharmaceuticals, Inc. (Provectus or the Company) is a clinical-stage biotechnology company developing immunotherapy medicines for different diseases that are based on a class of synthetic small molecule immuno-modulators called halogenated xanthenes (HXs). Provectus’ lead HX molecule is named rose bengal sodium (RBS).
The Company’s proprietary, patented, pharmaceutical-grade RBS is the active pharmaceutical ingredient (API) in the drug product candidates of Provectus’ clinical development programs and the preclinical formulations of the Company’s drug discovery programs. Importantly, Provectus’ pharmaceutical-grade RBS displays different therapeutic effects at different concentrations and can be formulated for delivery by different routes of administration. The International Nonproprietary Names (INN) Expert Committee of the World Health Organization (WHO) selected “rose bengal sodium” for the nonproprietary name of the Company’s API.
RBS may target disease in a bifunctional manner. First, direct contact may lead to cell death or repair depending on the disease being treated and the concentration of Provectus’ RBS utilized in the treatment. Secondly, multivariate immune signaling, activation, and response may follow that may manifest as stimulatory, inhibitory, or both.
The Company believes that it is the first entity to advance an RBS formulation into clinical trials for the treatment of a disease, such as those trials reported on the clinical trials registry ClinicalTrials.gov. Provectus also believes that it is the first and only entity to date to successfully, reproducibly, and consistently make pharmaceutical-grade RBS at a purity of nearly 100%.
Provectus’ small molecule HX medical science platform includes clinical development programs in oncology, dermatology, and ophthalmology; proof-of-concept in vivo drug discovery programs in oncology, hematology, wound healing, and animal health; and preclinical in vitro drug discovery programs in infectious diseases and tissue regeneration and repair.
Information about the Company’s clinical trials can be found at the National Institutes of Health (NIH) registry, www.clinicaltrials.gov. For additional information about Provectus, please visit the Company’s website at https://www.provectusbio.com.
Trademarks
PV-10® is a registered trademark of Provectus, Knoxville, Tennessee, U.S.A.
Keytruda® is a registered trademark of Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc. Kenilworth, New Jersey, U.S.A.
FORWARD-LOOKING STATEMENTS: The information in this press release may include “forward-looking statements,” within the meaning of U.S. securities legislation, relating to the business of Provectus and its affiliates, which are based on the opinions and estimates of Company management and are subject to a variety of risks and uncertainties and other factors that could cause actual events or results to differ materially from those projected in the forward-looking statements. Forward-looking statements are often, but not always, identified by the use of words such as “seek,” “anticipate,” “budget,” “plan,” “continue,” “estimate,” “expect,” “forecast,” “may,” “will,” “project,” “predict,” “potential,” “targeting,” “intend,” “could,” “might,” “should,” “believe,” and similar words suggesting future outcomes or statements regarding an outlook.
The safety and efficacy of the agents and/or uses under investigation have not been established. There is no guarantee that the agents will receive health authority approval or become commercially available in any country for the uses being investigated or that such agents as products will achieve any particular revenue levels.
Due to the risks, uncertainties, and assumptions inherent in forward-looking statements, readers should not place undue reliance on these forward-looking statements. The forward-looking statements contained in this press release are made as of the date hereof or as of the date specifically specified herein, and Provectus undertakes no obligation to update or revise any forward-looking statements, whether as a result of new information, future events or otherwise, except in accordance with applicable securities laws. The forward-looking statements are expressly qualified by this cautionary statement.
Risks, uncertainties, and assumptions include those discussed in the Company’s filings with the Securities and Exchange Commission (SEC), including those described in Item 1A of:
#####
Contact:
Provectus Biopharmaceuticals, Inc.
Heather Raines, CPA
Chief Financial Officer
Phone: (866) 594-5999
NEWS -- Provectus Biopharmaceuticals Announces World Health Organization Selection of “Rose Bengal Sodium” as International Nonproprietary Name for Active Pharmaceutical Ingredient

KNOXVILLE, TN, Nov. 02, 2022 (GLOBE NEWSWIRE) -- Provectus (OTCQB: PVCT) today announced that the International Nonproprietary Names (INN) Expert Committee of the World Health Organization (WHO) has selected “rose bengal sodium” (RBS) for the nonproprietary name of the Company’s active pharmaceutical ingredient (API). Pharmaceutical-grade RBS is the API in Provectus’ current clinical-stage drug product candidates and preclinical formulations.
The RBS name was selected by the WHO Expert Advisory Panel on the International Pharmacopoeia and Pharmaceutical Preparations, reached the status of recommended INN after a period of public consultation, and was included in INN Recommended List 88 published with the No. 3 issue of the WHO Drug Information, Volume 36 in October 2022.
The aim of the INN system since inception has been to provide health professionals with a unique and universally available designated name to identify each pharmaceutical substance or API, according to the WHO. The existence of an international nomenclature in the form of INN is important for the accurate identification, prescribing, and dispensing of medicines to patients, and for communication and exchange of information among health professionals and scientists worldwide.
Provectus is the first and only entity to have made pharmaceutical-grade RBS at a purity of nearly 100%. This success resulted from:
Paulness, How long have you been invested in PVCT?
NEWS -- Provectus Biopharmaceuticals Expands Research Collaboration with The Rockefeller University to Investigate Clinical-Stage Immuno-Dermatology Agent PH-10 for Skin Inflammation

KNOXVILLE, TN, Oct. 25, 2022 (GLOBE NEWSWIRE) -- Provectus (OTCQB: PVCT) today announced that the Company has expanded its sponsored research program with James G. Krueger, MD, PhD, Co-director, Center for Clinical and Translational Science, D. Martin Carter Professor in Clinical Investigation, Senior Attending Physician, and head of the Laboratory of Investigative Dermatology at The Rockefeller University to investigate the potential for PH-10, a topical formulation of Provectus’ pharmaceutical-grade small molecule rose bengal sodium (RBS) drug substance, to directly alter the growth and differentiation of human keratinocytes, and to block cytokine-mediated signaling that creates different inflammatory skin diseases and may also be important in skin neoplasms.
PH-10 is an immuno-dermatology, multi-indication viable, clinical-stage pharmaceutical asset that the Company has used to treat more than 200 patients in multiple early- and mid-stage clinical trials for psoriasis and atopic dermatitis.
Dr. Krueger and the Laboratory of Investigative Dermatology plan to examine the effects of a wider range of PH-10 concentrations on human keratinocytes in vitro at the level of gene transcription. They also hope to determine PH-10’s interaction with and uptake by blood leukocytes, because these cells mediate inflammatory skin diseases and control or protect against some types of skin cancers.
The Kruger team previously elucidated several PH-10 mechanisms of action from work that it did as part of a Provectus clinical study of psoriasis, showing that:
NEWS -- Provectus Biopharmaceuticals Establishes Research Collaboration with University of Texas Medical Branch at Galveston to Investigate Pharmaceutical-Grade Small Molecule Immunotherapy Rose Bengal for Wound Healing

KNOXVILLE, TN, Sept. 29, 2022 (GLOBE NEWSWIRE) -- Provectus (OTCQB: PVCT) today announced that the Company has initiated a new sponsored research program with Amina El Ayadi, PhD, Assistant Professor, Surgical Sciences Division and Jayson Jay, PhD, Postdoctoral Research Fellow and Jeane B. Kempner Scholar of the Burn, Trauma, and Critical Care Research Laboratory in the Department of Surgery at the University of Texas Medical Branch at Galveston (UTMB) to characterize the effects of Provectus’ proprietary pharmaceutical-grade rose bengal sodium (RBS) on full-thickness cutaneous wounds and during the subsequent phases of wound healing. RBS is the lead member of a class of small molecules called halogenated xanthenes that is entirely owned by Provectus.
Starting from the Texas City Disaster of 1947, the deadliest industrial accident in U.S. history and one of history’s largest non-nuclear explosions, UTMB clinicians and researchers in the Department of Surgery have developed treatments that improve the survival chances of patients with massive burns, reduce scar formation, and accelerate patient recovery. Many novel treatments discovered by UTMB researchers have been adopted by specialist burn centers around the world. The Department of Surgery’s Burn, Trauma, and Critical Care Research Laboratory is equipped with an array of cutting-edge equipment and technologies that support its research activities, including a dedicated cell culture suite, confocal microscope, flow cytometer, Comprehensive Lab Animal Monitoring System (CLAMS), and bioprinter for 3D cell culture.
Drs. El Ayadi and Jay plan to examine the safety of topically-applied, multi-dosed RBS over the wound healing periods of inflammation and cellular proliferation, determine the efficacy of RBS in a pre-clinical model of wound healing, and elucidate a spatiotemporal immune activation signature over wound healing time in a large animal model of burn and full-thickness cutaneous trauma.
About Provectus
Provectus Biopharmaceuticals, Inc. (Provectus or the Company) is a clinical-stage biotechnology company developing immunotherapy medicines for different disease areas based on a class of small molecules called halogenated xanthenes (HXs). The Company’s lead molecule is RBS. A second HX molecule has been synthesized.
Provectus’ drug discovery and development programs include investigational drugs and drug targets in oncology (clinical-stage), dermatology (clinical-stage), hematology, infectious diseases, ophthalmology (clinical-stage), animal health, tissue regeneration and repair, and wound healing, and use multiple routes of administration, such as intralesional (IL), topical (.top), oral (P.O.), inhaled (.inh), intranasal (IN), and intravenous (IV).
Information about the Company’s clinical trials can be found at the National Institutes of Health (NIH) registry, www.clinicaltrials.gov. For additional information about Provectus, please visit the Company's website at https://www.provectusbio.com.
FORWARD-LOOKING STATEMENTS: The information in this press release may include “forward-looking statements,” within the meaning of U.S. securities legislation, relating to the business of Provectus and its affiliates, which are based on the opinions and estimates of Company management and are subject to a variety of risks and uncertainties and other factors that could cause actual events or results to differ materially from those projected in the forward-looking statements. Forward-looking statements are often, but not always, identified by the use of words such as “seek,” “anticipate,” “budget,” “plan,” “continue,” “estimate,” “expect,” “forecast,” “may,” “will,” “project,” “predict,” “potential,” “targeting,” “intend,” “could,” “might,” “should,” “believe,” and similar words suggesting future outcomes or statements regarding an outlook.
The safety and efficacy of the agents and/or uses under investigation have not been established. There is no guarantee that the agents will receive health authority approval or become commercially available in any country for the uses being investigated or that such agents as products will achieve any particular revenue levels.
Due to the risks, uncertainties, and assumptions inherent in forward-looking statements, readers should not place undue reliance on these forward-looking statements. The forward-looking statements contained in this press release are made as of the date hereof or as of the date specifically specified herein, and Provectus undertakes no obligation to update or revise any forward-looking statements, whether as a result of new information, future events or otherwise, except in accordance with applicable securities laws. The forward-looking statements are expressly qualified by this cautionary statement.
Risks, uncertainties, and assumptions include those discussed in the Company’s filings with the Securities and Exchange Commission (SEC), including those described in Item 1A of:
#####
Contact:
Provectus Biopharmaceuticals, Inc.
Heather Raines, CPA
Chief Financial Officer
Phone: (866) 594-5999
NEWS -- Provectus Biopharmaceuticals Announces Exclusive License Option Agreement with Ophthalmic Biophysics Center of Bascom Palmer Eye Institute at University of Miami for Treatment of Eye Infections
KNOXVILLE, TN, Sept. 21, 2022 (GLOBE NEWSWIRE) -- Provectus (OTCQB: PVCT) today announced that the Company has entered into an option agreement with the University of Miami (UM) for an exclusive worldwide license of intellectual property developed by the Ophthalmic Biophysics Center (OBC) of Bascom Palmer Eye Institute (BPEI), which is part of the UM Health System, for the use of OBC’s photodynamic antimicrobial therapy (PDAT) medical device in combination with Provectus’ proprietary pharmaceutical-grade rose bengal for the treatment of bacterial, fungal, and viral infections of the eye. The Company also initiated a sponsored research program with OBC to investigate Provectus’ rose bengal for the treatment of infectious keratitis.
Established in 1970 by BPEI founding director Edward W.D. Norton, MD and Jean-Marie Parel, IngETS-G, PhD, FARVO, OBC performs translational eye care research in all areas of ophthalmology, from the retina and vitreous to the cornea, glaucoma, cataracts, neuro-ophthalmology, and ocular oncology. OBC has developed more than 350 surgical instruments and clinical devices to date.
Dominic Rodrigues, Vice Chair of the Company’s Board of Directors said, “Provectus is pleased to advance its collaboration with OBC director Dr. Parel, Dr. Guillermo Amescua, MD, a board-certified ophthalmologist and director of Bascom Palmer’s Ocular Surface Center, and the entire OBC team to now include this important option-to-license step. OBC’s innovative and comprehensive work on rose bengal PDAT has, among other things, shown in vitro activity against multiple etiologies of microbial keratitis1,2,3,4,5, including drug-resistant strains3,6, established in vivo safety7, demonstrated in vitro superiority over riboflavin PDAT8,9, and achieved clinical proof-of-concept for the treatment of infectious keratitis10,11.”
Mr. Rodrigues added, “OBC’s clinical work in ophthalmology, Provectus’ clinical trials in oncology and dermatology, and the Company’s wide-ranging preclinical work in hematology, infectious diseases, animal health, tissue regeneration and repair, and other disease areas support a key component of Provectus’ business strategy, which is to demonstrate the broad spectrum therapeutic platform potential of the Company’s proprietary pharmaceutical-grade halogenated xanthene small molecule rose bengal.”
About Bascom Palmer Eye Institute
BPEI serves as the Department of Ophthalmology for the UM Miller School of Medicine in Miami, Florida. Its mission is to enhance the quality of life by improving sight, preventing blindness, and advancing ophthalmic knowledge through compassionate patient care and innovative research. For 2022-2023, U.S. News & World Report (U.S. News) ranked BPEI as the nation’s best in ophthalmology, marking the 21st time and 19th consecutive year that BPEI has received the No. 1 ranking since U.S. News began surveying American physicians for its annual “Best Hospitals” rankings 33 years ago.
About Provectus
Provectus Biopharmaceuticals, Inc. (Provectus or the Company) is a clinical-stage biotechnology company developing immunotherapy medicines for different disease areas based on a class of small molecules called halogenated xanthenes (HXs). The Company’s lead molecule is rose bengal sodium. A second HX molecule has been synthesized.
Provectus’ drug discovery and development programs include investigational drugs and drug targets in oncology (clinical-stage), dermatology (clinical-stage), hematology, infectious diseases, ophthalmology (clinical-stage), animal health, and tissue regeneration and repair, and use multiple routes of administration, such as intralesional (IL), topical (.top), oral (P.O.), inhaled (.inh), intranasal (IN), and intravenous (IV).
Information about the Company’s clinical trials can be found at the National Institutes of Health (NIH) registry, https://www.clinicaltrials.gov. For additional information about Provectus, please visit the Company’s website at https://www.provectusbio.com.
References
1. Arboleda A, Miller D, Cabot F, et al. Assessment of rose bengal versus riboflavin photodynamic therapy for inhibition of fungal keratitis isolates. Am J Ophthalmol. 2014;158:64–70.
2. Durkee H, Arboleda A, Aguilar MC, et al. Rose bengal photodynamic antimicrobial therapy to inhibit Pseudomonas aeruginosa keratitis isolates. Lasers Med Sci. 2019;35:861–866.
3. Halili F, Arboleda A, Durkee H, et al. Rose bengaland riboflavin-mediated photodynamic therapy to inhibit methicillin-resistant Staphylococcus aureus keratitis isolates. Am J Ophthalmol. 2016;166:194–202.
4. Schrier A, Greebel G, Attia H, Trokel S, Smith EF. In vitro antimicrobial efficacy of riboflavin and ultraviolet light on Staphylococcus aureus, methicillin-resistant Staphylococcus aureus, and Pseudomonas aeruginosa. J Refract Surg. 2009;25:S799–S802.
5. Naranjo A, Pelaez D, Arrieta E, et al. Cellular and molecular assessment of rose bengal photodynamic antimicrobial therapy on keratocytes, corneal endothelium and limbal stem cell niche. Exp Eye Res. 2019;188:107808.
6. Amescua G, Arboleda A, Nikpoor N, et al. Rose rengal photodynamic antimicrobial therapy: a novel treatment for resistant Fusarium keratitis. Cornea. 2017;36:1141–1144.
7. Martinez JD, Arrieta E, Naranjo A, Monsalve P, Mintz KJ, Peterson J, Arboleda A, Durkee H, Aguilar MC, Pelaez D, Dubovy SR, Miller D, Leblanc R, Amescua G, Parel JM. Rose Bengal Photodynamic Antimicrobial Therapy: A Pilot Safety Study. Cornea. 2021 Aug 1;40(8):1036-1043.
8. Arboleda A, Miller D, Cabot F, Taneja M, Aguilar MC, Alawa K, Amescua G, Yoo SH, Parel JM. Assessment of rose bengal versus riboflavin photodynamic therapy for inhibition of fungal keratitis isolates. Am J Ophthalmol. 2014 Jul;158(1):64-70.e2.
9. Adre E, Durkee H, Arboleda A, Alawa K, Maestre J, Mintz KJ, Leblanc RM, Amescua G, Parel JM, Miller D. Rose Bengal and Riboflavin Mediated Photodynamic Antimicrobial Therapy Against Selected South Florida Nocardia Keratitis Isolates. Transl Vis Sci Technol. 2022 Jan 3;11(1):29.
10. Naranjo A, Arboleda A, Martinez JD, Durkee H, Aguilar MC, Relhan N, Nikpoor N, Galor A, Dubovy SR, Leblanc R, Flynn HW Jr, Miller D, Parel JM, Amescua G. Rose Bengal Photodynamic Antimicrobial Therapy for Patients With Progressive Infectious Keratitis: A Pilot Clinical Study. Am J Ophthalmol. 2019 Dec;208:387-396.
11. Sepulveda-Beltran PA, Levine H, Altamirano DS, Martinez JD, Durkee H, Mintz K, Leblanc R, Tóthová JD, Miller D, Parel JM, Amescua G. Rose Bengal Photodynamic Antimicrobial Therapy: A Review of the Intermediate-Term Clinical and Surgical Outcomes. Am J Ophthalmol. 2022 Aug 8;243:125-134.
FORWARD-LOOKING STATEMENTS: The information in this press release may include “forward-looking statements,” within the meaning of U.S. securities legislation, relating to the business of Provectus and its affiliates, which are based on the opinions and estimates of Company management and are subject to a variety of risks and uncertainties and other factors that could cause actual events or results to differ materially from those projected in the forward-looking statements. Forward-looking statements are often, but not always, identified by the use of words such as “seek,” “anticipate,” “budget,” “plan,” “continue,” “estimate,” “expect,” “forecast,” “may,” “will,” “project,” “predict,” “potential,” “targeting,” “intend,” “could,” “might,” “should,” “believe,” and similar words suggesting future outcomes or statements regarding an outlook.
The safety and efficacy of the agents and/or uses under investigation have not been established. There is no guarantee that the agents will receive health authority approval or become commercially available in any country for the uses being investigated or that such agents as products will achieve any particular revenue levels.
Due to the risks, uncertainties, and assumptions inherent in forward-looking statements, readers should not place undue reliance on these forward-looking statements. The forward-looking statements contained in this press release are made as of the date hereof or as of the date specifically specified herein, and Provectus undertakes no obligation to update or revise any forward-looking statements, whether as a result of new information, future events or otherwise, except in accordance with applicable securities laws. The forward-looking statements are expressly qualified by this cautionary statement.
Risks, uncertainties, and assumptions include those discussed in the Company’s filings with the SEC, including those described in Item 1A of:
the Company’s Annual Report on Form 10-K for the year ended December 31, 2021, and
Provectus’ Quarterly Report on Form 10-Q for the quarter ended June 30, 2022.
###
Contact:
Provectus Biopharmaceuticals, Inc.
Heather Raines, CPA
Chief Financial Officer
Phone: (866) 594-5999
|
Followers
|
185
|
Posters
|
|
|
Posts (Today)
|
0
|
Posts (Total)
|
9955
|
|
Created
|
08/09/02
|
Type
|
Free
|
| Moderators | |||
.png)

Provectus Biopharmaceuticals is investigating new therapies for the treatment of skin cancer and liver cancer. Provectus investigational oncology drug, PV-10, is an ablative immunotherapy under investigation in solid tumor cancers. The Company has received orphan drug designations from the FDA for its melanoma and hepatocellular carcinoma indications. PH-10, its topical investigational drug for dermatology, is undergoing clinical testing for psoriasis and atopic dermatitis. Provectus has completed Phase 2 trials of PV-10 as a therapy for metastatic melanoma, and of PH-10 as a topical treatment for psoriasis. In addition, Provectus has begun a Phase 3 trial as a therapy for metastatic melanoma. Information about these and the Company's other clinical trials can be found at the NIH registry, www.clinicaltrials.gov. For additional information about Provectus, please visit the Company's website at www.provectusbio.com.
The red synthetic dye Rose Bengal is shown in a bottle at Provectus Pharmaceuticals, Inc.
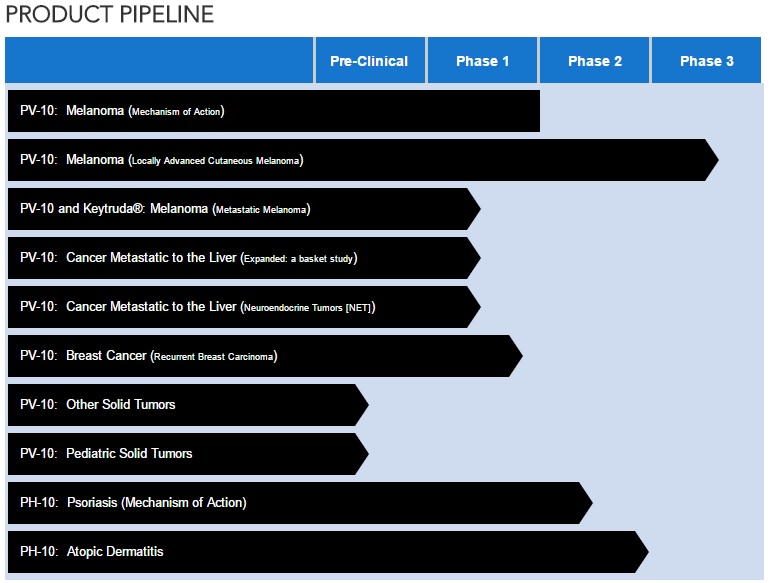
CURRENT PVCT PIPELINE (as of May 23, 2017)
--------------------------------------------------------------
Charts and Technical:
PVCT 6 months chart:
http://stockcharts.com/h-sc/ui?s=PVCT&p=D&yr=0&mn=6&dy=0&id=p03754661229
Technical analysis:
http://www.stockta.com/cgi-bin/analysis.pl?symb=PVCT&num1=7&cobrand=&mode=stock
PVCT News and Analysis:
PVCT News Blog with the latest news and analysis:
http://provectuspharmaceuticalsinc.blogspot.ca/p/news.html
CONNECTING THE DOTS - CURRENT NEWS PAGE
http://provectuspharmaceuticalsinc.blogspot.ca/p/current-news_22.html
CONNECTING THE DOTS - BLOG PAGE
http://provectuspharmaceuticalsinc.blogspot.ca/
PVCT News at OTC
http://www.otcmarkets.com/stock/PVCT/news
CLINICAL TRIALS Updates and Info:
| Short Interest | 167,323 (69.16%) Apr 13, 2017 |
| Significant Failures to Deliver | No |
| Volume | |
| Day Range: | |
| Bid Price | |
| Ask Price | |
| Last Trade Time: |
