Register for free to join our community of investors and share your ideas. You will also get access to streaming quotes, interactive charts, trades, portfolio, live options flow and more tools.
Grabar Law Office Investigates Claims on Behalf of Long-Term Shareholders of ImmunityBio, Inc. (IBRX)
See https://grabarlaw.com/the-latest/immunitybio-shareholder-investigation/
09:45 AM EDT, 06/20/2024 (MT Newswires) -- ImmunityBio (IBRX) said Thursday that it has launched Anktiva, its immunotherapy to treat Bacillus Calmette-Guerin-unresponsive non-muscle invasive bladder cancer, with multiple patients across the US receiving the initial commercial doses.
The US Food and Drug Administration approved Anktiva in April to treat patients with BCG-unresponsive non-muscle invasive bladder cancer with or without papillary tumors, according to ImmunityBio.
Any thoughts on how much revenue FDA approval will bring?
Still +54M shares short and time is ticking. 80% inside ownership with a small float.
up and down.. no problem.. ima staying put
we know there is a very large short position here.. i think that since the earnings post yesterday and the reaction to it that there is now very little reason to stay short,,lets see that spike
Pancreatic Cancer next!!
I like Oncolytics on that list
7. ImmunityBio Inc (NASDAQ:IBRX)
Float Shorted: 40.37%
With over 53 million of its shares shorted, ImmunityBio Inc’s (NASDAQ:IBRX) short interest stands at 40.37% as of April 8.
From Jan 19, 2023: ''ImmunityBio also announced that it held two productive Type B meetings with the FDA in December. The first was to present the recent data and obtain guidance toward a registration pathway in metastatic pancreatic cancer.'' https://www.businesswire.com/news/home/20230119005337/en/ImmunityBio-Announces-Presentation-at-ASCO-GI-2023-of-Fully-Enrolled-Trial-in-Third-Line-and-Greater-Pancreatic-Cancer-and-Update-on-FDA-Type-B-Meetings-Regarding-Paths-to-Registration
What happened?
Nothing listed in the pipeline.
Pancreatic Cancer next!! WOW!!
https://www.prnewswire.com/news-releases/biotechs-role-in-addressing-the-pancreatic-cancer-emergency-302089052.html
IBRX monster bio on the weekly break out
IBRX Friday Close $7.35+$2.24 (+43.84%)
This morning Pre-Mkt
Bid x Size
$8.13 x 2
Ask x Size
$8.20 x 212
Volume
862,349
90 Day Avg. Vol.
4,851,152
Bona fide squeeze underway. Millions of shares short are trapped.
Pancreatic will be even better!!In January 2023, ImmunityBio announced that it held two meetings with the FDA in December 2022 to present data and get guidance on a registration pathway for metastatic
pancreatic cancer
. The company's combination immunotherapy
, Nant Cancer Vaccine, has shown potential effectiveness in pancreatic cancer, more than doubling median overall survival (OS) compared to historical OS.
That’s because IHUB is for pump and dumps.
Good ole ihub. We have a REAL company here with a remarkable game changing cancer drug whose stock price will not only explode but retain it's stock price and no one here is even talking about it. As per my experience here several years ago.......everyone here is only interested in shells and scam companies. What a shame. That's fine. I'll be making bank. Join me if you want.......or don't. I'm not selling till it's somewhere above 40
This a simple bar/volume with 60-minute intervals
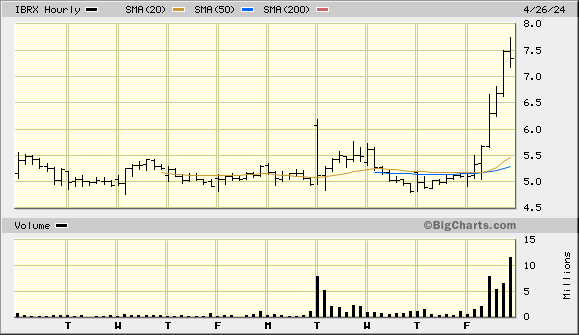
The stock is actually trading counter to a recent bearish trend for most tickers according my bit of DD from the Barchart website that would be considered med tech companies
This a link for the sector that is made up 459 tickers
https://www.barchart.com/stocks/quotes/IBRX/competitors?quoteSectors=-MEBI&viewName=main&orderBy=weightedAlpha&orderDir=desc
I have a free membership so I can use the flipcharts function for the past six months of price discovery
One can observe from the sector graph at the top of the page the best time to buy any old med stock was last November
The best time to sell was at the peak last March which isn’t that long ago
I can observe the same recurring pattern in the more than 200 tickers I flipped through
Anktiva is the next-generation immunotherapy drug, according to ImmunityBio executive chairman Dr. Patrick Soon-Shiong: "This drug is the first drug, as far as I know, that activates the natural killer cell that talks to the T-cell and generates complete remission..." pic.twitter.com/DIVc3A8luy
— Yahoo Finance (@YahooFinance) April 23, 2024
The FDA has accepted the BLA resubmission and will now review the therapy. It has set a Prescription Drug User Fee Act (PDUFA) date of 23 April 2024.
Just curious, having read this article from June 2022, what makes us think that they will get approval for Antikva.
My concern just stems from the bottom of the article stating.
"Other drugs are in late-stage trials in combination with BCG for the same patient population, including AstraZeneca’s Imfinzi (durvalumab) and Sesen Bio’s Vicinium (oportuzumab monatox). The FDA previously rejected Sesen Bio’s BLA for the treatment of BCG-unresponsive patients, citing multiple issues, including a lack of randomised data comparing Vicinium with an investigator’s choice of intravesical chemotherapy."
https://www.pharmaceutical-technology.com/analyst-comment/immunitybio-first-marketed-drug/?cf-view
There is no comparator in the Antikva trial with any intravesical chemotherapy either, or against anything for that matter. As it's open-label single arm trial, with only Antikva being used. Will this be enough for FDA approval?
I know the prior CRL didn't mention anything about this, but that doesn't mean it isn't a potential issue?
Looking to be convinced wrong on the above :)
Research tool: https://tradingtimeline.com/ibrx.html
This stock is ready to breakout, did awesome today!!
#IBRX 🔥 this stock can go crazy with 30% short interest! $IBRX
#IBRX 🔥 30% short interest and gap fill possibilities for big move! $IBRX
yup, i see that but….
….
i didnt like how each time it runs, they yank it right back down, so i moved on but im happy it ran for you some. just be careful, they dont seem to last long.
News > Up 29%today > The FDA accepted ImmunityBio's resubmission of its Biologics License Application for N-803 as complete.
then the usual dump, lol.
hopped in on this dip. i like the run it had last time. ![]()
IBRX
ImmunityBio Inc
2.72
0.09 (3.42%)
Volume: 1,135,051
Day Range: 2.60 - 2.75
Last Trade Time: 7:59:29 PM EDT
IBRX
ImmunityBio Inc
2.69
-0.12 (-4.27%)
Volume: 6,333,343
Day Range: 2.63 - 2.85
Last Trade Time: 7:51:23 PM EDT
IBRX
ImmunityBio Inc
2.57
0.19 (7.98%)
Volume: 2,354,869
Day Range: 2.35 - 2.57
Last Trade Time: 7:56:03 PM EDT
IBRX an offering done right. bonzzzaaaa
#IBRX ?? gap fill after 3.3? Trade opportunity? $IBRX
-55%
$IBRX received FDA CRL
— Pharmdca (@Pharmdca) May 11, 2023
Recall position was a catalyst play and not an investment which I clearly outlined in my thread.
Multiple times I tweeted that I closed my full position as stock appreciated a lot from entry point (Below $2) to almost 6+
Risk management is important for…
|
Followers
|
57
|
Posters
|
|
|
Posts (Today)
|
0
|
Posts (Total)
|
1627
|
|
Created
|
07/26/15
|
Type
|
Free
|
| Moderators | |||
| Shs Outstand | 98.47M |
| Shs Float | 28.16M |
| Debt/Eq | 0.00 |
| LT Debt/Eq | 0.00 |
| Employees | 153 |


| Volume | |
| Day Range: | |
| Bid Price | |
| Ask Price | |
| Last Trade Time: |
