Register for free to join our community of investors and share your ideas. You will also get access to streaming quotes, interactive charts, trades, portfolio, live options flow and more tools.
CRSP will climb tremendously over the years.
SMMT went from $1.00-30.00+ this year cancer drug superior to Mercks Keytruda
ICCM company being over looked that kills cancers by freezing them 97% non recurrence rate for breast cancer after 5 years and superior data to lung cancer safe cryoblation is done in less than hour.
https://www.prnewswire.com/il/news-releases/icecure-medical-reports-positive-topline-results-from-ice3-cryoablation-breast-cancer-study-achievement-of-96-39-recurrence-free-rate-brings-company-one-step-closer-to-providing-women-a-non-surgical-alternative-to-lumpectomy-302092712.html
https://www.prnewswire.com/il/news-releases/icecure-medical-reports-positive-topline-results-from-ice3-cryoablation-breast-cancer-study-achievement-of-96-39-recurrence-free-rate-brings-company-one-step-closer-to-providing-women-a-non-surgical-alternative-to-lumpectomy-302092712.html
Buy range again thanks a bunch
Buy range again thanks a bunch
Hey Monk, Hopefully you bought some shares on Friday@ Sold at 10% discount!
LC
CRISPR!!!
Crispr added some $120 Vertical debit spreads in 2026
$CRSP
I agree.....solid company$$$$$$$$
It may take some time for CRSP returns to a $200 price tag
The same could be said for hundreds of other stocks that soared during Covid mania
Since then the stock has rallied from low points
With all that money the company has to do incredible things with its biotechnology the stock isn’t going to zero

Strong balance sheet with approximately $2.1 billion in cash, cash equivalents, and marketable securities as of March 31, 2024
https://finance.yahoo.com/news/crispr-therapeutics-provides-business-reports-200000059.html
(OT): A new AI paper from Princeton and Stanford https://www.biorxiv.org/content/10.1101/2024.04.25.591003v2.full
CRISPR-GPT demonstrated a marked improvement in gene-editing experiments, increasing the accuracy of target gene modifications by up to 30% compared to conventional methods. In validation tests, it achieved a specificity rate exceeding 95%, with a significant reduction in off-targets. The system also reduced the time required to design and plan experiments by approximately 40%, streamlining the workflow for researchers.
Business Update and Q1 Financial Results https://uk.finance.yahoo.com/news/crispr-therapeutics-provides-business-reports-200000300.html
Highlights (plus new programs utilising in vivo gene editing) https://finance.yahoo.com/news/crispr-therapeutics-highlights-asgct-oral-110000066.html
https://www.globenewswire.com/news-release/2024/04/22/2867236/0/en/CRISPR-Therapeutics-to-Present-Oral-Presentation-at-the-American-Society-of-Gene-Cell-Therapy-ASGCT-2024-Annual-Meeting.html
EDIT is also pursuing myocilin-related glaucoma https://www.globenewswire.com/news-release/2024/04/22/2867244/0/en/Editas-Medicine-to-Present-Pre-clinical-Data-Demonstrating-Progression-of-in-vivo-Medicines-Pipeline-at-the-American-Society-of-Gene-and-Cell-Therapy-Annual-Meeting.html
Hi MD,
CRSP is now down around 1/3 from its February high. The price is where I usually start to accumulate more shares.
Best wishes,
OAG
They entered into an investment agreement for the sale of ~$280 million of its shares to a select group of institutional investors in a registered direct offering, at $71.5 apiece. The financing is being led by EcoR1 Capital and SR One with participation from existing investors and a healthcare specialist investor https://finance.yahoo.com/news/crispr-therapeutics-announces-280-million-133000932.html
So there has been some discussion since my last post
More in this podcast
Good morning OAG,
Surprising and interesting. Share price sudden "jack-rabbit" moves in quick price reversals. The technical picture for today changed from positive breakout yesterday to "High Pole Warning" with sudden drop in price. Apparently the dominating group of participants seem to dictate this pace a bit unusual and different from other stocks. Yet it offers opportunity for profit once one has a good plan and strategy.
Good you have a plan and strategy for your preferred limits and ranges for your trading. Let's see how it goes. Have fun & GLTY
Good morning JJ8,
It needs to crest $85 or better to get me interested in reducing my share inventory. A drop to $57 will trigger an addition of 12% to share count.
I'd like to see it crest $72 and maintain that price right now.
Best wishes,
OAG
CRSP share price Double Top Breakout today on 19-Dec-2023. Happy Holidays & GLTA
Oscar Wilde once observed that a cynic knows the price of everything and the value of nothing
Windbag1014,
Thanks for your views and informative response. GLTY & GLTA
OldAIMGuy,
Thanks for your clear and detailed response.
Your approach and strategy dealing with what Crispr T AG stock price movement over time shows, and the Crispr company activity makes good sense. More power and good luck to you.
John
Hi JJ8, Re: CRSP price action..........................
My guess is it's a "Sell on the News" action. This isn't the first time CRSP has shown high amplitude price change as this histogram shows:
https://schrts.co/NSfSbXYF
I think it will be important to watch the transition of this company from basically a 'research' business to one of product development and distribution. Somewhere along the way they'll have to show a profit to reward those who've invested in them. I'm fond of watching the Accumulation/Distribution activity to see what is happening with strong and weak hands holding the stock. In the mean time I plan on trimming and backfilling my position as the price moves around. I last added 12% to my position at $47.56 in August and had the opportunity to part with 10% of the holding at $69.57 a few weeks ago. The core position remains.
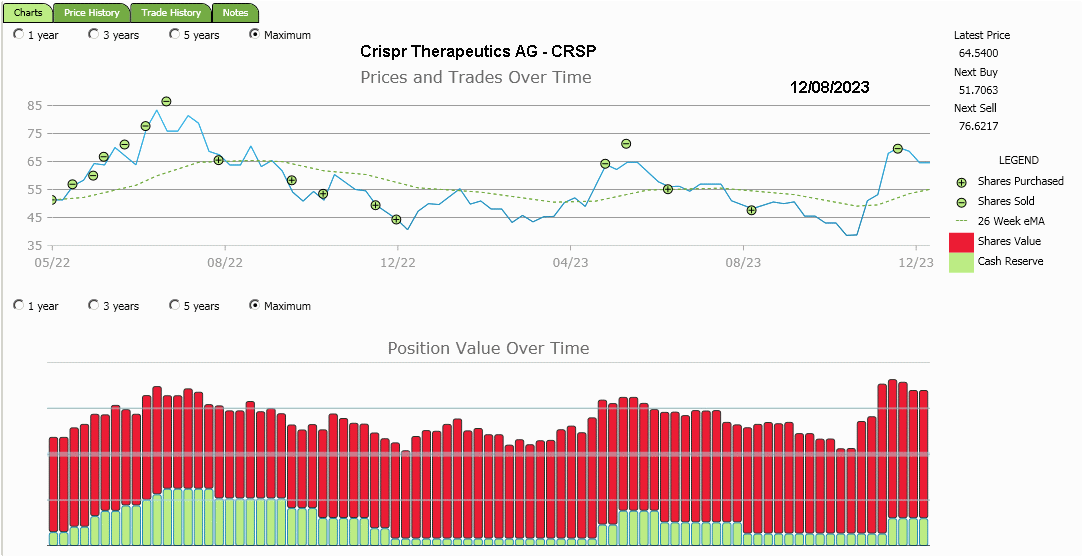
With each cycle of the price the portfolio builds out a bit.
Hope this helps,
OAG
Vertex set the price of Casgevy at $2.2 million compared to $3.1 million for Bluebird’s Lyfgenia. Bluebird’s gene therapy for thalassemia, approved last year, has a list price of $2.8 million.
The therapies are also not easy to receive. Patients must spend weeks, even months, in the hospital before and after the therapy is administered. And some of the preparatory steps can cause serious side effects, including severe infections, nausea, painful mouth sores, and infertility.
Probably just because the share price had run up so much before the approval, so it was kind of baked-in already and some took profits. Also, maybe because of high price tag of the treatment. Still a bright future and should trend upwards.
The dump of Crisp shares last Friday Dec 8th after the "good" news is confusing.
The institutional ownership is 63.48%. Why this response by share owners. I assume it probably was mainly by the institutions. But why at this time? Profit taking? Haven't done a check on insiders transactions yet.
More than 17 million shares traded in one session. The average daily volume of trading happens to be about 2-3 millions of shares daily. Why the exodus a this time of "good" news.
My profits were cut in half in one session. I don't intend to sell at such times.
Actually, I buy usually. That's what I did recently after the sudden drop in Walmart share price.
Any ideas and thoughts? What's going on behind the scene? TIA
GLTA
Casgevy, developed by partners Vertex Pharmaceuticals (VRTX) and CRISPR Therapeutics (CRSP), and bluebird bio's (BLUE.) Lyfgenia were approved for people aged 12 years and older.
https://www.reuters.com/business/healthcare-pharmaceuticals/us-approves-two-gene-therapies-sickle-cell-disease-2023-12-08/
CRSP share price sharp reversal by Double Bottom Breakdown today on 8-Dec-2023. This type of rapid reversals based on company news developments. C'est La Vie in stock trading. GLTA
U.S. approves first gene-editing treatment, Casgevy, for sickle cell disease https://www.cnbc.com/2023/12/08/casgevy-first-crispr-gene-editing-treatment-approved-in-us.html?__source=iosappshare%7Ccom.apple.UIKit.activity.CopyToPasteboard
What are the bleep are you talking about without pasting a chart to back up your assertion
CRSP share price Spread Triple Top Breakout today on 06-Dec-2023. GLTA
Smart move. Don't waste resources, learn from the clinical data and improve the pipeline https://finance.yahoo.com/news/crispr-therapeutics-announces-updates-immuno-211500451.html
Is the 12/8 FDA approval in the price already?
|
Followers
|
60
|
Posters
|
|
|
Posts (Today)
|
0
|
Posts (Total)
|
274
|
|
Created
|
01/20/17
|
Type
|
Free
|
| Moderators | |||
| Volume | |
| Day Range: | |
| Bid Price | |
| Ask Price | |
| Last Trade Time: |
