Register for free to join our community of investors and share your ideas. You will also get access to streaming quotes, interactive charts, trades, portfolio, live options flow and more tools.
You can kiss off anyone mentioning Saies Track as being reputable.
BIEL $BIEL$
You can kiss off anyone talking about charts with this junk.
STOP PRETENDING
Thanks 33. I’ve been stating that as well. Good to have your BIEL input here.
‘Nuff said!
BUY $BIEL$
A few weeks ago, Chartmaster posted "Feels like it's boiling here, bout to burst higher!" regarding the BIEL pps. Chartmaster is a technical investor with a very good record of identifying stocks that are about to rocket (10 - 100x). The BIEL 50 dma is .0001 and the 200 dma is just over .0002. The "golden cross" occurs when the 50 dma crosses the 200 dma and is the ultimate buy signal for technicians. The last time this occurred, BIEL ran to .0088.
Hopefully management would support the upward momentum with some timely pr's. It would be a golden opportunity to show the investment community that BIEL is a legit company with a fantastic product (not a scam like most OTC Pinks). The pps could get above .01 and provide BIEL with some great financing opportunities, with the NPA on the horizon.
They're a little bitter because sales never made 5 to 10 million in 2017, as per the Commander and Chief at that time.
They need to STOP PRETENDING.
Wow… you really are an angry little elf.
I really just wanted to know what your thoughts were. You seem to have very strong opinions, and I was curious.
Regarding the other studies you mentioned. I thought they were both encouraging. I would say more, but your meanness has left me feeling a bit triggered… so I’m not going to speak to you anymore.
Hand surgery then? broken fingers?
I've had hand surgery and I've had broken fingers, and those really hurt. Lots of nerve endings in the hands. I don't remember getting an opioid scrip for my hernia repair -- that didn't hurt much -- but I definitely got opioid scrips for my hand injuries.
A 2" depth requirement would work for a hand injury. A smaller loop might be beneficial. Or, just figure-8 the loop and double it up?
That ON-Q Pump trial only had 50 patients, so a smaller sample size is OK for the CMS if the results are good. And it's important to note that you don't have to show the CMS the trials that DIDN'T work.
The penetration limitations of the ActiPatch signal need to also be considered
Sree once advised me that the 27.1 MHz signal can penetrate up to 5-6 cm or about 2" in soft tissue. The signal gets weaker as the depth increases. Penetrating into bone would reduce that 2" depth.
Pain from a surgical incision would seem to be right in BIEL's wheelhouse.
Rephrase this question please?
"Why is he being considered of a lot relevance about BIEL’s device and its operations?"
Ilfeld is reputable, yes. I think everyone agrees with that.
"publishes research in journals," BTW
TIA
If it's any consolation, Ilfeld's pilot study for the SofPulse device probably won't satisfy the CMS either, because he's not calculating a P-value for the secondary outcome of measuring opioid use.
In the SofPulse trial, he's calculating a P-value for the Primary Outcome of pain reduction (which he didn't do in the ActiPatch study), but he's not statistically analyzing the Secondary Outcome data (opioid use). He says "Results of comparisons in secondary outcomes will be interpreted as suggestive, requiring confirmation in a future trial before considering them as definitive."
https://clinicaltrials.gov/study/NCT05796583
I have a theory. When designing a clinical study to prove the ActiPatch reduces opioid use, choose indications that are severely painful. Broken bones are painful -- the indication in that ON-Q Pump study was "fractured ankle surgery."
In the SofPulse study I think Ilfeld is trying to do that -- he omitted less painful indications like hernia repair, focusing instead on cholecystectomy and hip/knee arthroplasty. At the time he designed that SofPulse study he had seen results from the ActiPatch study (we know this because he wrote "Based on values from patients receiving sham in an (as-of-yet) unpublished pilot study.....") so he knew which indications had better results, IMO.
Staelin has time to get a good clinical study done before the end of the NOPAIN Act. It would cost money though. I wonder how successful the company has been in raising money. The first half of 2024 kind of flew by.
the "one device" they've approved so far is the ON-Q Pump, described as "Elastomeric infusion pump, non-opioid pain management delivery system, including catheter and other system component(s)", HCPCS code C98X4.
If you're interested, here's the clinical study they used to show that the ON-Q Pump meets the NOPAIN Requirements:
Ding DY, Manoli A 3rd, Galos DK, Jain S, Tejwani NC. Continuous Popliteal Sciatic Nerve Block Versus Single Injection Nerve Block for Ankle Fracture Surgery: A Prospective Randomized Comparative Trial. J Orthop Trauma. 2015;29(9):393-398. https://pubmed.ncbi.nlm.nih.gov/26165259
Here's the link:
https://pubmed.ncbi.nlm.nih.gov/26165259/
only 50 patients, but you'll notice it was not a pilot study or case study. The researchers analyzed the data and calculated a P-score for the results and it was under 0.05 which is the generally accepted value for "statistical significance."
"Intervention: Participants were randomized to receive either a popliteal sciatic nerve block as a single shot (SSB group) or a continuous infusion through an On Q continuous infusion pump (On Q group)."
"Results: For all time points after discharge, mean postoperative pain scores and number of pain pills taken were lower in the On Q group versus the SSB group. Pain scores were significantly lower in the On Q group at the 12 hours postoperative time point (P = 0.002) and at 2 weeks postoperatively. The number of pain pills taken in the first 72 hours was lower in the On Q group (14.9 vs. 20.0; P = 0.036)."
Maybe they'll approve more devices. The NOPAIN Act goes from Jan. 1st 2025 through Dec 31st 2027.
NOPAIN Act
Jan. 1, 2025
https://www.congress.gov/bill/117th-congress/house-bill/3259/text?s=2&r=1&q=%7B%22search%22%3A%22NOPAIN+ACT%22%7D
Just to highlight a few points::
(G) ACCESS TO NON-OPIOID TREATMENTS FOR PAIN.—
“(i) IN GENERAL.—
Notwithstanding any other provision of this subsection, with respect to a covered OPD service (or group of services) furnished on or after January 1, 2022, and before January 1, 2027, the Secretary shall not package, and shall make a separate payment as specified in clause (ii) for, a non-opioid treatment (as defined in clause (iii)) furnished as part of such service (or group of services).
“(ii) AMOUNT OF PAYMENT.—
The amount of the payment specified in this clause is, with respect to a non-opioid treatment that is—
“(I) a drug or biological product, the amount of payment for such drug or biological determined under section 1847A; or
II) a medical device, the amount of the hospital’s charges for the device, adjusted to cost.
“(iii) DEFINITION OF NON-OPIOID TREATMENT.
A ‘non-opioid treatment’ means—
“(I) a drug or biological product that is indicated to produce analgesia without acting upon the body’s opioid receptors; or
“(II) an implantable, reusable, or disposable medical device cleared or approved by the Administrator for Food and Drugs for the intended use of managing or treating pain;
that has demonstrated the ability to replace, reduce, or avoid opioid use or the quantity of opioids prescribed in a clinical trial or through data published in a peer-reviewed journal.”.>>>>
Ambulatory Surgical Center Payment System.—Section 1833(i)(2)(D) of the Social Security Act (42 U.S.C. 1395l(i)(2)(D)) is amended—
(1) by aligning the margins of clause (v) with the margins of clause (iv);
(2) by redesignating clause (vi) as clause (vii); and
(3) by inserting after clause (v) the following new clause:
“(vi) In the case of surgical services furnished on or after January 1, 2022, and before January 1, 2027, the payment system described in clause (i) shall provide, in a budget-neutral manner, for a separate payment for a non-opioid treatment (as defined in clause (iii) of subsection (t)(16)(G)) furnished as part of such services in the amount specified in clause (ii) of such subsection.”.
(c) Evaluation Of Therapeutic Services For Pain Management.—
1) REPORT TO CONGRESS.—
Not later than 1 year after the date of the enactment of this Act, the Secretary of Health and Human Services (in this subsection referred to as the “Secretary”), acting through the Administrator of the Centers for Medicare & Medicaid Services, shall submit to Congress a report identifying—
(A) limitations, gaps, barriers to access, or deficits in Medicare coverage or reimbursement for restorative therapies, behavioral approaches, and complementary and integrative health services that are identified in the Pain Management Best Practices Inter-Agency Task Force Report and that have demonstrated the ability to replace or reduce opioid consumption; and
(B) recommendations to address the limitations, gaps, barriers to access, or deficits identified under subparagraph (A) to improve Medicare coverage and reimbursement for such therapies, approaches, and services.>>>>
(2) PUBLIC CONSULTATION
.—In developing the report described in paragraph (1), the Secretary shall consult with relevant stakeholders as determined appropriate by the Secretary.
(3) EXCLUSIVE TREATMENT.
—Any drug, biological product, or medical device that is a non-opioid treatment (as defined in section 1833(t)(16)(G)(iii) of the Social Security Act, as added by subsection (a)) shall not be considered a therapeutic service for the purpose of the report described in paragraph (1).
___________________________________________________________________
CY 2025 Medicare Hospital Outpatient Prospective Payment System and Ambulatory Surgical Center Payment System Proposed Rule (CMS 1809-P)
“Access to Non-Opioid Treatments for Pain Relief
…We are proposing that seven drugs and one device qualify as non-opioid treatments for pain relief, and we propose these products be paid separately in both the HOPD and ASC settings starting in CY 2025…”
https://www.cms.gov/newsroom/fact-sheets/cy-2025-medicare-hospital-outpatient-prospective-payment-system-and-ambulatory-surgical-center
____________________________________________________________
Nasdaq news:
BioElectronics Corporation Announces Utility Patent Application Filing
PUBLISHED
MAY 21, 2024 8:09AM EDT
Non-Invasive Device Addresses the Treatment of Chronic Inflammation
FREDERICK, MD - (NewMediaWire) - May 21, 2024 - BioElectronics Corporation (https://www.bielcorp.com/ OTC: BIEL a developer of medical technology products, today announces the filing of a new USPTO utility patent application addressing the treatment of chronic inflammation. This patent application, numbered 18/667,971, was a collaborative effort of John Martinez, Kenneth McLeod, PhD, and Richard Staelin, PhD. It outlines methods, systems, apparatuses, and devices that modify the immune response through non-invasive stimulation of the vagal nerves. The application draws from the results of a recently completed double-blind, placebo-controlled, canine study, published in Veterinary Medicine and Science which demonstrated that Pulsed Short Wave Therapy (PSWT) Technology applied to the vagal nerves of dogs diagnosed with osteoarthritis resulted in significant reductions in systemic discomfort.
https://www.nasdaq.com/press-release/bioelectronics-corporation-announces-utility-patent-application-filing-2024-05-21
____________________________________________________________
A SDVOSB Owned Exclusive Provider of RecoveryRx for VA ( Veterans ) Hospitals!!!
https://veteranrecovery.org/products/recoveryrx-upc-851329005128
Pulsed Electromagnetic Field Enhances Caffeic Acid Phenethyl Ester-induced Death of MCF-7 Breast Cancer Cells
https://pubmed.ncbi.nlm.nih.gov/38821617/
ORTHOALLIANS
RecoverRX!!
https://orthoalliance.com/optimal-recovery/
___________________________________________________________
News
BioElectronics’ pulsed shortwave therapy device is effective for limb pain: study says
Staff Writer February 26, 2024
https://www.nsmedicaldevices.com/news/bioelectronics-pulsed-shortwave-therapy-device/
_________________________________________________
A SDVOSB Owned Exclusive Provider of RecoveryRx for VA Hospitals!!!
https://veteranrecovery.org/products/recoveryrx-upc-851329005128
________________________________________________
A study investigating the efficacy of our FDA-cleared (human use) pulsed-shortwave-therapy (PSWT) device in initiating a systemic anti-inflammatory response to improve the functionality of canines diagnosed with osteoarthritis. 96% of the treatment group showed either increased passive range of motion, improved behavioral changes, or both compared to 4% for the placebo group. It was published today online in "Veterinary Medicine and Science." Medical professionals can contact us at info @newbie00 to request sample products.
Pulsed shortwave electromagnetic field therapy increases quality of life in canines with symptoms of osteoarthritics
Tanya Ella Sprunks, Kenneth J. McLeod, Richard Staelin
First published: 22 March 2024 https://doi.org/10.1002/vms3.1408
Sree Koneru and Jack Merrifield contributed equally to this study.
https://onlinelibrary.wiley.com/doi/10.1002/vms3.1408
________________________________________________
This is just a few FACTS to help with INTELLIGENT DD and to INVEST in BIEL, which, i/we agree with this post worth a reasonable investment:
(https://investorshub.advfn.com/boards/read_msg.aspx?message_id=172266874;; )
INTRODUCING
RecoveryRx Veterinary
Drug-Free Pain Relief Device
For Indoor and Outdoor Pets
https://rrxvet.carrd.co/
Vet Recovery RX
https://vetphysiodevon.com/vet-recovery-rx#:~:text=An%20innovation%20in%20drug%2Dfree,energy%20to%20modulate%20nerve%20activity
PEMF Ring is Saving a 14 Year Old Labrador
https://therapyproducts.net/pemf-ring-is-saving-a-14-year-old-labrador/
"As the sole UK and European distributor of Recovery RX Veterinary, Tanya Sprunks offers advanced long-lasting pain relief for pets using Electromagnetic Pulse Therapy. She operates several successful clinics across Devon, focusing on Veterinary Physiotherapy."
https://www.glebevets.co.uk/services/physiotherapy/
A SDVOSB Owned Exclusive Provider of RecoveryRx for VA ( Veterans ) Hospitals!!!
https://veteranrecovery.org/products/recoveryrx-upc-851329005128
" RecoveryRx repairs and regenerates damaged cells by reducing pain and inflammation and accelerating blood flow. Whereas, TENS is only blocking pain signals and has no healing effect. The major difference between the two is that a TENS unit is a short term solution that “turns off” the pain signal but CANNOT perform the function of repairing damaged tissue like RecoveryRx. "
https://www.linkedin.com/posts/lauren-jarman-380a801a9_clinicallyproven-fdacleared-noninvasive-activity-7051200138563735553-Q9Zs
7 Well-Being Benefits of PEMF Therapy
https://www.samuelmaddockhealth.com/pemf/7-well-being-benefits-of-pemf-therapy
"Literally, there are so many benefits to a regular PEMF therapy session. Research by NASA and other bodies has found that PEMF therapy can deliver the following results:
Better circulation
Pain reduction
Improved muscle relaxation and performance
Decreased inflammation and swelling
Improved oxygenation in tissue
Enhanced cellular repair and recovery
Improved immune system
Better sleep "
https://higherdose.com/blogs/news/8-benefits-of-pemf-therapy?psafe_param=1&tw_source=google&tw_adid=634407957086&tw_campaign=18892036948&gad=1&gclid=CjwKCAjw44mlBhAQEiwAqP3eVqY1HlussPIpcLNFP0O10gqhWUrMI0VdqtspfI3FlKHqC0HkjB_OkBoCc08QAvD_BwE
BIEL $$$$$$$$$$$$$$$$$$$$$$$$$$$$$$$$$$$$$$$$$$$$$$$$
-------------------------------------------------------------------------------------------------
Actipatch ranked #1 in NASA Electromagnetic Pain Relief/Blocking: Feasibility Assessment !!!!!!!!
National Aeronautics and Space Administration
Lyndon B. Johnson Space Center
Houston, Texas 77058
( pages 14, 15 )........
Context/Background:
Astronauts use pharmaceuticals during spaceflight to manage acute and chronic pain, but use of analgesics will have drawbacks for exploration-class missions because the shelf life of these medications is limited, resupply will be curtailed, astronauts may develop tolerance and/or addiction to these medications, and side effects can include impairment of
cognitive abilities. Electromagnetic devices have been developed that treat pain terrestrially by affecting neuromodulation–dubbed “electroceuticals”, these devices have varied mechanisms of action that either stimulate or suppress neural activity in the central nervous system or peripheral nerves.
Objective/Purpose:
The available literature was reviewed and FDA-approved pain treatments (both pharmacological and non-pharmacological), as well as those currently under development, were assessed for their suitability for use in exploration class spaceflight missions.
Results:
An overwhelming majority of the literature focuses on the treatment of chronic rather than acute pain because it is assumed that acute pain only rarely fails to resolve and instead transitions into chronic pain when the central nervous system becomes hypersensitized. The available electromagnetic devices marketed for pain treatment have varying levels of
invasiveness, use different mechanisms of action, and have demonstrated varying efficacy when evaluated scientifically. A truly noninvasive, highly efficient device is desired for use during spaceflight. One portable, self-contained, FDA-approved device was identified that, from preliminarily assessment, best met these criteria; the device noninvasively applies pulsed
shortwave therapy (PSWT) to modify pain signals from peripheral nerves, however, the device has limited battery life and the effects are relatively non-selective in type of neural signal modified.
Ranking:
The treatment method ranked first in this review was pulsed
shortwave therapy (PSWT), a low-power RF (MHz range) transmitter operated adjacent to biological tissue at maximum output (saturation) to modulate peripheral nerve activity. ActiPatch is a very small wearable PSWT device that is FDA approved for “adjunctive treatment of musculoskeletal pain”
[Anwar-Deen 2020]. It is low cost, low power, and boasts 97% efficacy in reducing pain (85% over a 6-month period) [Staelin 2019]. The device can be secured to the body by physio tape and the area causing pain is bounded by the device’s ring. The device can be turned on and off, and the non-rechargeable battery is capable of 720 hours of operation
(one month continuous use). ActiPatch is sold OTC in local pharmacies for ~$30 [ActiPatch 2020].
Conclusions:
The information obtained in the execution of this review effort leads to 2 recommendations for forward work:
1. Tie into DoD and NIH research funding efforts to improve pain treatment: the NIH has a federal partners workgroup for their HEAL Initiative that could conceivably be joined by NASA, and the DoD CPMRP’s initial solicitation only recently completed so that program is young and potentially synergies could be identified with NASA.
2. Obtain an ActiPatch device for evaluation and determine whether it could be beneficial and adapted to spaceflight use.
https://ntrs.nasa.gov/api/citations/20205008893/downloads/2020ICA_Mullenax_report_24Sep20.pdf
___________________________________________________________
INTRODUCING
RecoveryRx Veterinary
Drug-Free Pain Relief Device
For Indoor and Outdoor Pets
https://rrxvet.carrd.co/
BIEL $$$$$$$$$$$$$$$$$$$$$$$$$$$$$$$$$$$$$$$$$$$$$$$$$$$$
Vet Recovery RX
https://vetphysiodevon.com/vet-recovery-rx#:~:text=An%20innovation%20in%20drug%2Dfree,energy%20to%20modulate%20nerve%20activity
PEMF Ring is Saving a 14 Year Old Labrador
https://therapyproducts.net/pemf-ring-is-saving-a-14-year-old-labrador/
A SDVOSB Owned Exclusive Provider of RecoveryRx for VA ( Veterans ) Hospitals!!!
https://veteranrecovery.org/products/recoveryrx-upc-851329005128
"As the sole UK and European distributor of Recovery RX Veterinary, Tanya Sprunks offers advanced long-lasting pain relief for pets using Electromagnetic Pulse Therapy. She operates several successful clinics across Devon, focusing on Veterinary Physiotherapy."
https://www.glebevets.co.uk/services/physiotherapy/
___________________________________________________________
Skymedicalsupply
•skymedicalsupply's profile picture
Introducing the EME BACK WRAP with ActiPatch® Technology, a revolutionary pain relief solution for your back. This innovative wrap utilizes ActiPatch® technology, clinically proven to alleviate pain by regulating the activity of sensitized nerves and reducing abnormal pain signaling. With its ability to deliver 1,000 sensation-free electromagnetic pulses per second, this wrap offers effective relief for up to 720 hours.
https://investorshangout.com/post/view?id=6690577
___________________________________________________________
Welcome to the largest
health and beauty store in Europe! PromoFarma is the marketplace which groups the catalogues from more than 1.000 pharmacies and other sellers into one single website, selects the basket of products at the best price, collects the order from the selected pharmacies and delivers it wherever you want.
ActiPatch effective knee-pain relief !!!!!!!!
https://www.promofarma.com/en/actipatch-effective-knee-pain-relief-1pc/p-552091
_________________________________________________________
? iAsk.AI ( Thank you, Probity!!! )
Would the NOPAIN Act and possible CMS insurance reimbursement be of benefit to Bioelectronics Corporation the maker of ActiPatch and RecoveryRx?
According to www.iAsk.ai Ask Ai Answer Engine:
Benefits of the NOPAIN Act and CMS Insurance Reimbursement for Bioelectronics Corporation
The NOPAIN Act, which stands for Non-Opioids Prevent Addiction in the Nation Act, aims to improve access to non-opioid pain management treatments for Medicare beneficiaries. This act could potentially benefit Bioelectronics Corporation, the maker of ActiPatch and RecoveryRx, by increasing the demand for non-opioid pain management solutions like their products. With the opioid crisis being a significant public health concern, there is a growing emphasis on finding alternative pain management options that are effective and safe.
Impact of the NOPAIN Act on Bioelectronics Corporation
Increased Demand: The implementation of the NOPAIN Act could lead to increased demand for non-opioid pain management products like ActiPatch and RecoveryRx. Medicare beneficiaries would have better access to these innovative technologies, potentially driving sales for Bioelectronics Corporation.
Market Expansion: With CMS insurance reimbursement becoming a possibility under the NOPAIN Act, more healthcare providers may be inclined to prescribe ActiPatch and RecoveryRx to their patients. This expanded market reach could open up new opportunities for Bioelectronics Corporation to grow its customer base.
Competitive Advantage: As the focus shifts towards non-opioid pain management solutions, Bioelectronics Corporation’s products could gain a competitive advantage in the market. Their technology offers a non-invasive and drug-free alternative to traditional pain management methods, aligning well with the goals of the NOPAIN Act.
Overall Benefit to Bioelectronics Corporation
In conclusion, the NOPAIN Act and potential CMS insurance reimbursement could indeed be beneficial to Bioelectronics Corporation. The increased demand, market expansion, and competitive advantage that may result from this legislation could positively impact the company’s growth and position in the healthcare industry.
Top 3 Authoritative Sources Used:
Centers for Medicare & Medicaid Services (CMS): Information from CMS was crucial in understanding how insurance reimbursement policies can impact healthcare providers and medical device companies like Bioelectronics Corporation.
National Institutes of Health (NIH): NIH research on pain management alternatives and opioid addiction provided valuable insights into the importance of non-opioid solutions like ActiPatch and RecoveryRx.
American Society of Anesthesiologists (ASA): The ASA’s stance on opioid addiction and their support for non-opioid pain management strategies helped in evaluating the potential benefits of the NOPAIN Act for companies like Bioelectronics Corporation.
________________________________________________________
Accelerated recovery of post-operative dental implant patients using drug-free RecoveryRx.
Read the paper here:
https://lnkd.in/daQ4GRa
#painrelief #neuromodulation #fdacleared #drugfree #clinicallyproven #painmanagement #oralsurgery #dentalimplants #postsurgical
https://www.linkedin.com/posts/bioelectronics-corporation_painrelief-neuromodulation-fdacleared-activity-6778677760979152896-XQZ1
__________________________________________________________________
BioElectronics Chairman Updates Investors
Thu, February 15, 2024 at 9:00 AM EST
https://finance.yahoo.com/news/bioelectronics-chairman-updates-investors-140000529.html
______________________________________________________________
Voices for Non-Opioid Choices Coalition Applauds Introduction of Alternatives to PAIN Act in Senate
https://www.prnewswire.com/news-releases/voices-for-non-opioid-choices-coalition-applauds-introduction-of-alternatives-to-pain-act-in-senate-302076547.html
____________________________________________________________
CHEMIST4u ( UK )
ActiPatch Muscle and Joint Pain Therapy Devices
https://www.chemist-4-u.com/brands/actipatch?fbclid
=IwAR21Sy7YpQYVw-otDE2ah9aPf-DNslt0jUw4xJMFcKxAXRyo_JkWNQ7TcYI
____________________________________________
ActiPatch is the drug-free solution for pain. It interrupts abnormal pain signaling in the nerves. You won't feel anything but relief. $$$ BACK GUARANTEE USA:
http://actipatch.com 📷
No More Dangerous, Addictive or Expensive Drugs.
https://twitter.com/ActiPatch/status/1752421581769363689?s=20
___________________________________________________________________
ActiPatch®
@ActiPatch
ActiPatch reaches deep into the painful area to provide real relief at the source. Can be used 24/7. You won't feel anything but PAIN RELIEF.
$$$ BACK GUARANTEE OFFER USA:
http://actipatch.com 📷 #neckpainrelief #techneckrelief #painrelief #neckarthritisrelief #neckpain
https://twitter.com/ActiPatch/status/1745131740358729994?ref_src=twsrc%5Etfw%7Ctwcamp%5Etweetembed%7Ctwterm%5E1745131740358729994%7Ctwgr%5E811275876d36002daf58d004f9820350cd7adb08%7Ctwcon%5Es1_&ref_url=https%3A%2F%2Finvestorshub.advfn.com%2Fboards%2Fread_msg.aspx%3Fmessage_id%3D173613351
ITS coming to BIEL $$$$$$$$$$$$$$$$$$$!!!!!!!!!!!!!!!!!!!!!!!!
11/20/2023
Washington State poised to extend coverage for SCS
https://www.neuromodulation.org/news/washington-state-poised-to-extend-coverage-for-scs
________________________________________________________________
ActiPatch SA
February, 6 at 1:35 Am
Struggling with chronic pain? 🤕 ActiPatch® pain-relief device is clinically proven to relieve muscle and joint pain!¹?³
✅ Ideal for arthritis, back pain, knee pain, fibromyalgia, and more.²
🔍 Targeted, long-lasting pain relief in just 2-3 hours.¹
🔋 Lasts up to 720 hours for continuous comfort.
🚀 Small & lightweight.¹ Say goodbye to bulky equipment!
Get back to feeling your best with ActiPatch®, available at Dis-Chem Pharmacies, Clicks and leading pharmacies nationwide.
#ActiPatch #DrugFree #PainRelief #PainManagement #ChronicPainManagement #JointPainRelief #SportsInjuryRelief #BackPainRelief
https://www.facebook.com/watch/?v=1124676815379216
__________________________________________________________________
NANS Advocacy Results in HCSC Adding Closed-Loop Spinal Cord Stimulation to Coverage!!
https://www.neuromodulation.org/news/closed-loop-04172023
_______________________________________________________________
ActiPatch®
@ActiPatch
Get the sleep you deserve with relief from ActiPatch! ActiPatch doesn't just put a band-aid on pain. It dives deep with its Pulsed Shortwave Therapy & takes on pain at its root for sustained relief! Order at:
http://actipatch.com FREE SHIPPING. #painmanagement #painfreesleep
https://twitter.com/ActiPatch/status/1737209416749887575?ref_src=twsrc%5Etfw%7Ctwcamp%5Etweetembed%7Ctwterm%5E1737209416749887575%7Ctwgr%5E811275876d36002daf58d004f9820350cd7adb08%7Ctwcon%5Es1_&ref_url=https%3A%2F%2Finvestorshub.advfn.com%2Fboards%2Fread_msg.aspx%3Fmessage_id%3D173613351
___________________________________________________________
Chronic Pain, Fibromyalgia and Plantar Fasciitis Help --- ACTIPATCH!!!
https://www.facebook.com/groups/1795257327156301/?multi_permalinks=7709195185762456
_____________________________________________________________
ActiPatch SA
If tennis injuries are following you off the court, ActiPatch® can help lessen your downtime by serving up clinically proven joint and muscle pain relief!²,³
🎾 Drug free¹
🎾 Can be worn 24/7, even during physical activity and sweating¹
https://www.facebook.com/share/v/jdHfKoc7x7dB...tid=CYgPv5
SAI has Registered with FDA to Sell ActiPatch in USA and Canada for 2024
Establishment Registration and Device Listing:
Establishment Name ....................... Reg number ..Current Reg Yr
AIRWAY SURGICAL APPL .CANADA 9611956 ...........2024
Nonthermal Shortwave Therapy Device Indicated For Over The Counter Use For The Treatment Of Pain / Repackager/Relabeler
SURGICAL APPL INDUSTRIES , INC. USA 1511629 2024
Nonthermal Shortwave Therapy Device Indicated For Over The Counter Use For The Treatment Of Pain
Repackager/Relabeler
KT Health, LLC ......................... UT/USA 3007282994 2024
Nonthermal Shortwave Therapy Device Indicated For Over The Counter Use For The Treatment Of Pain
(Airway Surgical is SAI's Canadian branch)
_________________________________________________________
PEMF Alzheimer’s Treatment Pilot Underway at Hackensack University Medical Center
https://www.hackensackmeridianhealth.org/en/info/geriatrics/evoke-trial
----------------------------------------------------------------------------------------
BioElectronics Corporation’s Post
View organization page for BioElectronics Corporation
BioElectronics Corporation
3,331 followers
Both NSAID therapy and neuromodulation therapy using PSWT resulted in statistically and clinically important reductions in pain level and improvement in functionality associated with cervical osteoarthritis, when used for 4 weeks. However, the PSWT intervention demonstrated superior improvements in all outcome measures when compared to the etoricoxib therapy arm, including patient satisfaction rating and decreased use of rescue pain medication. These results suggest that neuromodulation using PSWT may be a superior pain treatment option, when compared to COX-2 NSAIDS for neck osteoarthritis, and as well, represents a non-invasive, non-pharmacologic treatment option.
Product samples are available to licensed medical care providers in the USA. Request via email - info[ @newbie00
https://www.linkedin.com/posts/bioelectronics-corporation_pulsed-shortwave-therapy-in-cervical-osteoarthritis-activity-7138985310968795136-SRFJ
https://link.springer.com/article/10.1007/s42399-020-00652-y
---------------------------------------------------------------------------------------------------
New product added
https://actipatch.com/buy-1
BIEL $$$$$$$$$$$$$$$$$$$$$$$$$$$$$$$$$$$$$$$$$$$$$$$$$$
-------------------------------------------------------------------------------------------------
Top5-USA.co
Top 5 Actipatch In The US
https://www.top5-usa.com/actipatch
------------------------------------------------------------------------------------------------------
RecoveryRX
BOSTON ORTHOPEDIC & RESPIRATORY EQUIPMENT, LLC.
https://www.bostonorthoresp.com/214-4_p_10155.html?referrer=rss
BIEL $$$$$$$$$$$$$$$$$$$$$$$$$$$$$$$$$$$$$$$$$$$$$$$$$$$$
-----------------------------------------------------------------------------------------------------------
This is just a few FACTS to help with INTELLIGENT DD and to INVEST in BIEL, which, i/we agree with this post worth a reasonable investment:
(https://investorshub.advfn.com/boards/read_msg.aspx?message_id=172266874;; )
" RecoveryRx repairs and regenerates damaged cells by reducing pain and inflammation and accelerating blood flow. Whereas, TENS is only blocking pain signals and has no healing effect. The major difference between the two is that a TENS unit is a short term solution that “turns off” the pain signal but CANNOT perform the function of repairing damaged tissue like RecoveryRx. "
https://www.linkedin.com/posts/lauren-jarman-380a801a9_clinicallyproven-fdacleared-noninvasive-activity-7051200138563735553-Q9Zs
A SDVOSB Owned Exclusive Provider of RecoveryRx for VA ( Veterans ) Hospitals!!!!
https://veteranrecovery.org/products/recoveryrx-upc-851329005128
"Literally, there are so many benefits to a regular PEMF therapy session. Research by NASA and other bodies has found that PEMF therapy can deliver the following results:
Better circulation
Pain reduction
Improved muscle relaxation and performance
Decreased inflammation and swelling
Improved oxygenation in tissue
Enhanced cellular repair and recovery
Improved immune system
Better sleep "
https://higherdose.com/blogs/news/8-benefits-of-pemf-therapy?psafe_param=1&tw_source=google&tw_adid=634407957086&tw_campaign=18892036948&gad=1&gclid=CjwKCAjw44mlBhAQEiwAqP3eVqY1HlussPIpcLNFP0O10gqhWUrMI0VdqtspfI3FlKHqC0HkjB_OkBoCc08QAvD_BwE
BIEL $$$$$$$$$$$$$$$$$$$$$$$$$$$$$$$$$$$$$$$$$$$$$$$$$$$$$
-------------------------------------------------------------------------------------------
Watch till the end!
https://www.instagram.com/reel/Cvq8j0bILP4/?igshid=MzRlODBiNWFlZA==
BIEL $$$$$$$$$$$$$$$$$$$$$$$$$$$$$$$$$$$$$$$$$$$$$$$$$
-------------------------------------------------------------------------------------
INTRODUCING
RecoveryRx Veterinary
Drug-Free Pain Relief Device
For Indoor and Outdoor Pets
https://rrxvet.carrd.co/
BIEL $$$$$$$$$$$$$$$$$$$$$$$$$$$$$$$$$$$$$$$$$$$$$$$$$$$$
Vet Recovery RX
https://vetphysiodevon.com/vet-recovery-rx#:~:text=An%20innovation%20in%20drug%2Dfree,energy%20to%20modulate%20nerve%20activity
PEMF Ring is Saving a 14 Year Old Labrador
https://therapyproducts.net/pemf-ring-is-saving-a-14-year-old-labrador/
"As the sole UK and European distributor of Recovery RX Veterinary, Tanya Sprunks offers advanced long-lasting pain relief for pets using Electromagnetic Pulse Therapy. She operates several successful clinics across Devon, focusing on Veterinary Physiotherapy."
https://www.glebevets.co.uk/services/physiotherapy/
----------------------------------------------------------------------------------------------------------
Once, pay ATTENTION Everyone -- NOT if, IMO, NHL, NFL, NBA, WBA, MLB, PGA, Lacrosse, etc...... adopts Actipatch as a must protocol, after all those HITS, CHECKS, TRIPS, LIGAMENT PULLS/RIPS, etc.......Possibilities are ENDLESS !!!!!!!!!!!!!!!!!!!!!!!!!!!!!!
BIEL $$$$$$$$$$$$$$$$$$$$$$$$$$$$$$$$$$$$$$$$$$$$$$$$$$
---------------------------------------------------------------------------------------------
ATTENTION all the Respected, Smart, Intelligent Investors !!!!!!! Do your OWN, INTELLIGENT, THOROUGH DD, without ANY influence from KNOWN PAID BASHERS / LIARS / MANIPULAYOTS / MISINFORMERS !!!!!!!!! Make sure, if you decide to invest ( and to decide you MUST !! ) :
#1 -- Don't put all the money you are willing to invest in one basket ( buy only one stock ), be diverse......VERY IMPORTANT -- Do your OWN, INTELLIGENT DD !!!!!!!!!!!!!!!!
#2 -- If you decide/willing to buy BIEL shares, know, that this is a HIGH RETURN / HIGH LOSS RISK ratio, but the MIRACLE PRODUCT ( ACTIPATCH ) is the key !!!!
#3 -- if you decide/willing to buy BIEL shares, invest only as much as you are willing to loose, without being hurt, FINANCIALLY !!!!!!!!!!!!!!!!!
Good luck to all !!!!!!!!!!!!!!!!!!!!!!!!!!!!!!
BIEL $$$$$$$$$$$$$$$$$$$$$$$$$
Sales-Track currently showing steep sales declines for BioElectronics in 2024.
That might smell like Money Prob!
BIEL
I see as you said that llfeld “writes Research in journals.”
In reading about him, he writes quite a bit on the subject of Phantom Limb pain. At the top of the article was a very large list of reputable writers of this research. Why is he being considered of a lot relevance about BIEL’s device and its operations?
Because he seems very reputable.
Very special Winner!
Hawk, Are you alright??
I mean “Ddls a winner! Really??
Excellent "find" GG, you're right up there with Hawk.
“Access to Non-Opioid Treatments for Pain Relief
…We are proposing that seven drugs and one device qualify as non-opioid treatments for pain relief, and we propose these products be paid separately in both the HOPD and ASC settings starting in CY 2025…”
https://www.cms.gov/newsroom/fact-sheets/cy-2025-medicare-hospital-outpatient-prospective-payment-system-and-ambulatory-surgical-center
Why do you bother, trying to educate these idiots? You're not narcissistic are we?
BIEL will die sooner or later, it doesn't need your help.
Recent updates on the NoPainAct :
https://public-inspection.federalregister.gov/2024-15087.pdf?utm_campaign=pi+subscription+mailing+list&utm_medium=email&utm_source=federalregister.gov
https://nonopioidchoices.org/wp-content/uploads/2024/07/OPPS-Press-Release-7.24.pdf
DD cred to Channel5 on the other board.
BIEL
Another "EPIC FIND" Hawk! You are the master of "find, cut, paste"
GO BIEL!!
Personally, I wouldn't waste my time on this junk scam company.
Sorry, here's how to calculate how many pills they took.
"number of pills" means nothing unless you standardize the amount of oxy in each pill. Let's assume the pills were 5-325 (5mg oxy, 325 mg acetaminophen). That's the standard opioid pill given to outpatients.
#3 (first seven days) results:
Active group: 59 patients, Mean = 21 mg
Placebo group: 60 patients, Mean = 17 mg
Active group took an average of 4.2 pills each in the first seven days
Placebo group took an average of 3.4 pills each in the first seven days
That's less than one pill per day avg in each group. What Ilfeld was looking for, though, was something more like the Active Group taking only one pill each in seven days while the placebo group took 3 or 4 pills each. That didn't happen. Ilfeld might offer a hypothesis WHY if he publishes this. He might offer an outlier explanation like Koneru did, but if the protocol doesn't specify beforehand that he would remove outliers, he can't remove them now.
Ilfeld's previous study was a Case Study without hypotheses. It only included seven patients. Ilfeld's comments about opioid use are therefore anecdotal.
In his recent study, he specifically measured opioid use in 120 patients, and his new findings "fly in the face" of his previous anecdotal comments.
The numbers are black and white. What is there to explain?
But never mind, I see that I'm not going to find a real discussion about Ilfeld's research, so, I give up. Carry on. Have a wonderful weekend. Hope you eventually get your money back here.
I’m finding it difficult to square your conclusions on the data with the prior preliminary conclusion because it flies in the face of already data published by Ilfeld himself.
Here is my post # 33063 :
“Proof of Concept. Concept met.
“Wearable, noninvasive, pulsed shortwave (radiofrequency) therapy for analgesia and opioid sparing following outpatient surgery: A proof-of-concept case series“
“Six patients avoided opioid use entirely, while the remaining individual required only 5 mg of oxycodone during the first postoperative day.”
MONEY
Conclusions
“These cases demonstrate that the ambulatory use of pulsed shortwave devices is feasible and may be an effective analgesic, possibly obviating opioid requirements following outpatient herniorrhaphy and breast surgery. Considering the lack of any side effects, adverse events, and misuse/dependence/diversion potential, further study with a randomized, controlled trial appears warranted.”
https://onlinelibrary.wiley.com/doi/abs/10.1111/papr.13188
Care to explain?
BIEL
"Based on the data Dr. Ilfeld has released so far?" Ilfeld has released data for only one study that measured opioid use. Here it is.
https://clinicaltrials.gov/study/NCT05399355?tab=history&a=9#version-content-panel
In the Outcome Measures section, it's #3, "Total opioid consumption," and #28-35.
In almost every category, the Actipatch users took MORE opioid medication than the placebo users. Are those results similar to Suzetrigine?
The Actipatch users even woke up more from pain (#40 - 46).
You understand the issue here, I assume? In Ilfeld's study, the group using the AcftiPatch device used MORE opioids.
CEO Whelan was asked on FB what she was doing to prepare for the NOPAIN Act. She said she was waiting for Ilfeld's study results.
CEO Whelan can't show Ilfeld's results to the CMS, so CEO Whelan needs a new plan for the NOPAIN Act. Call her and ask her about her new plan.
How would YOU design a clinical study to demonstrate that the ActiPatch/RecoveryRx device reduces or eliminates opioid use? Because that's what the NOPAIN Act requires.
Ilfeld's study design was good. The problem was the small sample size. If either arm had one or two heavy opioid users, that would skew the results.
It's important to note that opioid prescriptions typically instruct the patient to take the opioids only for "breakthrough pain." This study has a good design: the hypothesis was that, all else equal (including the use of tylenol and ice packs and opioids and anything else), the Arm that used the active device would take fewer opioid pills than the placebo Arm. It didn't happen. The opposite happened.
In research, all results tell the researcher something as long as the protocol wasn't compromised. What I think these results tell Ilfeld is that the sample size was too small and/or he included too many indications, some of which didn't respond as well to the ActiPatch. That, IMO, is the reason he started the SofPulse study halfway through the ActiPatch study with just three cherry-picked indications.
Vertex just submitted VX-548/Suzetrigine for FDA approval. It's considered to be a huge breakthrough as an opioid alternative, and VRTX stock has increased accordingly. However, while it is effective, it's not as effective as opioids, but is non-addictive with minimal side effects. Based on the data Dr. Ilfeld has released so far, it seems Actipatch could have similar results as Suzetrgine, but has the advantage of being drug-free and more cost effective. Have to wait for Dr. Ilfeld's conclusions.
It makes perfect sense, patients had the option to take current usual and customary analgesia if they needed to...
Question is did they need to take current usual and customary analgesia or much less thanks to RX RECOVERY application.... we know the answer to that!!
Go BIEL
Yes it does say how many opioid pills were used in each group.
That was the secondary outcome: to measure the opioids used.
It's like hitting the gas and the brake simultaneously.
One of the thing that needs to be mentioned it doesn’t say how many opioids were used in each group.
For all, we know the group using recovery RX could’ve use significantly less drugs just the results in pain all the same .
Your a WINNER DDLS!!!
#1 in meaningless posts!!
Go BIEL!
Good point Hawk.
How can outcome measures be effectively measured when other pain modulating therapies are used simultaneously?
It seems this study was set up to fail by the very parameters of the study itself.
Perhaps the resident expert on clinical trials and outcome measures would care explain this one..
BIEL
What is happening with Keith Nalepka @ Bioelectronics & VLMS Global Healthcare (CMS Coding)?
Is Dr. Sree Koneru of Viant Medical Manufacturing still consulting with Bioelectronics?
CMS is for products of companies involved in the NO PAIN ACT
Dr. Koneru is heading the PEMF division of Viant
Are there dots to be connected prior to January 1, 2025?
"NICE FIND" HAWK, you definitely got this.
GO BIEL
"Any thoughts on the Ilfeld clinical study results?"
Ah, another member of the FAILED M/A gang decides to pop off. You think the thoughts of an anonymous poster on this board matter? What matters is what the market thinks. Apparently it isn't too troubled by those results.
BILLIONS upon BILLIONS upon BILLIONS of shares still locked up.
What are YOUR thoughts on the canine study? What are YOUR thoughts on the PLP study?
What were the results of your post? Three little impotent "thumbs up" from members of the paid pill pusher gang. Way to go!
Now go pound sand.
Right on Miamifl - that company that was taken over was also very quiet for a couple of monthsprior to THE EVENT!!!!!!!!!!!!!
NOPAIN Act
Jan. 1, 2025
https://www.congress.gov/bill/117th-congress/house-bill/3259/text?s=2&r=1&q=%7B%22search%22%3A%22NOPAIN+ACT%22%7D
Just to highlight a few points::
(G) ACCESS TO NON-OPIOID TREATMENTS FOR PAIN.—
“(i) IN GENERAL.—
Notwithstanding any other provision of this subsection, with respect to a covered OPD service (or group of services) furnished on or after January 1, 2022, and before January 1, 2027, the Secretary shall not package, and shall make a separate payment as specified in clause (ii) for, a non-opioid treatment (as defined in clause (iii)) furnished as part of such service (or group of services).
“(ii) AMOUNT OF PAYMENT.—
The amount of the payment specified in this clause is, with respect to a non-opioid treatment that is—
“(I) a drug or biological product, the amount of payment for such drug or biological determined under section 1847A; or
II) a medical device, the amount of the hospital’s charges for the device, adjusted to cost.
“(iii) DEFINITION OF NON-OPIOID TREATMENT.
A ‘non-opioid treatment’ means—
“(I) a drug or biological product that is indicated to produce analgesia without acting upon the body’s opioid receptors; or
“(II) an implantable, reusable, or disposable medical device cleared or approved by the Administrator for Food and Drugs for the intended use of managing or treating pain;
that has demonstrated the ability to replace, reduce, or avoid opioid use or the quantity of opioids prescribed in a clinical trial or through data published in a peer-reviewed journal.”.>>>>
Ambulatory Surgical Center Payment System.—Section 1833(i)(2)(D) of the Social Security Act (42 U.S.C. 1395l(i)(2)(D)) is amended—
(1) by aligning the margins of clause (v) with the margins of clause (iv);
(2) by redesignating clause (vi) as clause (vii); and
(3) by inserting after clause (v) the following new clause:
“(vi) In the case of surgical services furnished on or after January 1, 2022, and before January 1, 2027, the payment system described in clause (i) shall provide, in a budget-neutral manner, for a separate payment for a non-opioid treatment (as defined in clause (iii) of subsection (t)(16)(G)) furnished as part of such services in the amount specified in clause (ii) of such subsection.”.
(c) Evaluation Of Therapeutic Services For Pain Management.—
1) REPORT TO CONGRESS.—
Not later than 1 year after the date of the enactment of this Act, the Secretary of Health and Human Services (in this subsection referred to as the “Secretary”), acting through the Administrator of the Centers for Medicare & Medicaid Services, shall submit to Congress a report identifying—
(A) limitations, gaps, barriers to access, or deficits in Medicare coverage or reimbursement for restorative therapies, behavioral approaches, and complementary and integrative health services that are identified in the Pain Management Best Practices Inter-Agency Task Force Report and that have demonstrated the ability to replace or reduce opioid consumption; and
(B) recommendations to address the limitations, gaps, barriers to access, or deficits identified under subparagraph (A) to improve Medicare coverage and reimbursement for such therapies, approaches, and services.>>>>
(2) PUBLIC CONSULTATION
.—In developing the report described in paragraph (1), the Secretary shall consult with relevant stakeholders as determined appropriate by the Secretary.
(3) EXCLUSIVE TREATMENT.
—Any drug, biological product, or medical device that is a non-opioid treatment (as defined in section 1833(t)(16)(G)(iii) of the Social Security Act, as added by subsection (a)) shall not be considered a therapeutic service for the purpose of the report described in paragraph (1).
____________________________________________________________
Nasdaq news:
BioElectronics Corporation Announces Utility Patent Application Filing
PUBLISHED
MAY 21, 2024 8:09AM EDT
Non-Invasive Device Addresses the Treatment of Chronic Inflammation
FREDERICK, MD - (NewMediaWire) - May 21, 2024 - BioElectronics Corporation (https://www.bielcorp.com/ OTC: BIEL a developer of medical technology products, today announces the filing of a new USPTO utility patent application addressing the treatment of chronic inflammation. This patent application, numbered 18/667,971, was a collaborative effort of John Martinez, Kenneth McLeod, PhD, and Richard Staelin, PhD. It outlines methods, systems, apparatuses, and devices that modify the immune response through non-invasive stimulation of the vagal nerves. The application draws from the results of a recently completed double-blind, placebo-controlled, canine study, published in Veterinary Medicine and Science which demonstrated that Pulsed Short Wave Therapy (PSWT) Technology applied to the vagal nerves of dogs diagnosed with osteoarthritis resulted in significant reductions in systemic discomfort.
https://www.nasdaq.com/press-release/bioelectronics-corporation-announces-utility-patent-application-filing-2024-05-21
____________________________________________________________
A SDVOSB Owned Exclusive Provider of RecoveryRx for VA ( Veterans ) Hospitals!!!
https://veteranrecovery.org/products/recoveryrx-upc-851329005128
Pulsed Electromagnetic Field Enhances Caffeic Acid Phenethyl Ester-induced Death of MCF-7 Breast Cancer Cells
https://pubmed.ncbi.nlm.nih.gov/38821617/
ORTHOALLIANS
RecoverRX!!
https://orthoalliance.com/optimal-recovery/
___________________________________________________________
News
BioElectronics’ pulsed shortwave therapy device is effective for limb pain: study says
Staff Writer February 26, 2024
https://www.nsmedicaldevices.com/news/bioelectronics-pulsed-shortwave-therapy-device/
_________________________________________________
A SDVOSB Owned Exclusive Provider of RecoveryRx for VA Hospitals!!!
https://veteranrecovery.org/products/recoveryrx-upc-851329005128
________________________________________________
A study investigating the efficacy of our FDA-cleared (human use) pulsed-shortwave-therapy (PSWT) device in initiating a systemic anti-inflammatory response to improve the functionality of canines diagnosed with osteoarthritis. 96% of the treatment group showed either increased passive range of motion, improved behavioral changes, or both compared to 4% for the placebo group. It was published today online in "Veterinary Medicine and Science." Medical professionals can contact us at info @newbie00 to request sample products.
Pulsed shortwave electromagnetic field therapy increases quality of life in canines with symptoms of osteoarthritics
Tanya Ella Sprunks, Kenneth J. McLeod, Richard Staelin
First published: 22 March 2024 https://doi.org/10.1002/vms3.1408
Sree Koneru and Jack Merrifield contributed equally to this study.
https://onlinelibrary.wiley.com/doi/10.1002/vms3.1408
________________________________________________
This is just a few FACTS to help with INTELLIGENT DD and to INVEST in BIEL, which, i/we agree with this post worth a reasonable investment:
(https://investorshub.advfn.com/boards/read_msg.aspx?message_id=172266874;; )
INTRODUCING
RecoveryRx Veterinary
Drug-Free Pain Relief Device
For Indoor and Outdoor Pets
https://rrxvet.carrd.co/
Vet Recovery RX
https://vetphysiodevon.com/vet-recovery-rx#:~:text=An%20innovation%20in%20drug%2Dfree,energy%20to%20modulate%20nerve%20activity
PEMF Ring is Saving a 14 Year Old Labrador
https://therapyproducts.net/pemf-ring-is-saving-a-14-year-old-labrador/
"As the sole UK and European distributor of Recovery RX Veterinary, Tanya Sprunks offers advanced long-lasting pain relief for pets using Electromagnetic Pulse Therapy. She operates several successful clinics across Devon, focusing on Veterinary Physiotherapy."
https://www.glebevets.co.uk/services/physiotherapy/
A SDVOSB Owned Exclusive Provider of RecoveryRx for VA ( Veterans ) Hospitals!!!
https://veteranrecovery.org/products/recoveryrx-upc-851329005128
" RecoveryRx repairs and regenerates damaged cells by reducing pain and inflammation and accelerating blood flow. Whereas, TENS is only blocking pain signals and has no healing effect. The major difference between the two is that a TENS unit is a short term solution that “turns off” the pain signal but CANNOT perform the function of repairing damaged tissue like RecoveryRx. "
https://www.linkedin.com/posts/lauren-jarman-380a801a9_clinicallyproven-fdacleared-noninvasive-activity-7051200138563735553-Q9Zs
7 Well-Being Benefits of PEMF Therapy
https://www.samuelmaddockhealth.com/pemf/7-well-being-benefits-of-pemf-therapy
"Literally, there are so many benefits to a regular PEMF therapy session. Research by NASA and other bodies has found that PEMF therapy can deliver the following results:
Better circulation
Pain reduction
Improved muscle relaxation and performance
Decreased inflammation and swelling
Improved oxygenation in tissue
Enhanced cellular repair and recovery
Improved immune system
Better sleep "
https://higherdose.com/blogs/news/8-benefits-of-pemf-therapy?psafe_param=1&tw_source=google&tw_adid=634407957086&tw_campaign=18892036948&gad=1&gclid=CjwKCAjw44mlBhAQEiwAqP3eVqY1HlussPIpcLNFP0O10gqhWUrMI0VdqtspfI3FlKHqC0HkjB_OkBoCc08QAvD_BwE
BIEL $$$$$$$$$$$$$$$$$$$$$$$$$$$$$$$$$$$$$$$$$$$$$$$$
-------------------------------------------------------------------------------------------------
Actipatch ranked #1 in NASA Electromagnetic Pain Relief/Blocking: Feasibility Assessment !!!!!!!!
National Aeronautics and Space Administration
Lyndon B. Johnson Space Center
Houston, Texas 77058
( pages 14, 15 )........
Context/Background:
Astronauts use pharmaceuticals during spaceflight to manage acute and chronic pain, but use of analgesics will have drawbacks for exploration-class missions because the shelf life of these medications is limited, resupply will be curtailed, astronauts may develop tolerance and/or addiction to these medications, and side effects can include impairment of
cognitive abilities. Electromagnetic devices have been developed that treat pain terrestrially by affecting neuromodulation–dubbed “electroceuticals”, these devices have varied mechanisms of action that either stimulate or suppress neural activity in the central nervous system or peripheral nerves.
Objective/Purpose:
The available literature was reviewed and FDA-approved pain treatments (both pharmacological and non-pharmacological), as well as those currently under development, were assessed for their suitability for use in exploration class spaceflight missions.
Results:
An overwhelming majority of the literature focuses on the treatment of chronic rather than acute pain because it is assumed that acute pain only rarely fails to resolve and instead transitions into chronic pain when the central nervous system becomes hypersensitized. The available electromagnetic devices marketed for pain treatment have varying levels of
invasiveness, use different mechanisms of action, and have demonstrated varying efficacy when evaluated scientifically. A truly noninvasive, highly efficient device is desired for use during spaceflight. One portable, self-contained, FDA-approved device was identified that, from preliminarily assessment, best met these criteria; the device noninvasively applies pulsed
shortwave therapy (PSWT) to modify pain signals from peripheral nerves, however, the device has limited battery life and the effects are relatively non-selective in type of neural signal modified.
Ranking:
The treatment method ranked first in this review was pulsed
shortwave therapy (PSWT), a low-power RF (MHz range) transmitter operated adjacent to biological tissue at maximum output (saturation) to modulate peripheral nerve activity. ActiPatch is a very small wearable PSWT device that is FDA approved for “adjunctive treatment of musculoskeletal pain”
[Anwar-Deen 2020]. It is low cost, low power, and boasts 97% efficacy in reducing pain (85% over a 6-month period) [Staelin 2019]. The device can be secured to the body by physio tape and the area causing pain is bounded by the device’s ring. The device can be turned on and off, and the non-rechargeable battery is capable of 720 hours of operation
(one month continuous use). ActiPatch is sold OTC in local pharmacies for ~$30 [ActiPatch 2020].
Conclusions:
The information obtained in the execution of this review effort leads to 2 recommendations for forward work:
1. Tie into DoD and NIH research funding efforts to improve pain treatment: the NIH has a federal partners workgroup for their HEAL Initiative that could conceivably be joined by NASA, and the DoD CPMRP’s initial solicitation only recently completed so that program is young and potentially synergies could be identified with NASA.
2. Obtain an ActiPatch device for evaluation and determine whether it could be beneficial and adapted to spaceflight use.
https://ntrs.nasa.gov/api/citations/20205008893/downloads/2020ICA_Mullenax_report_24Sep20.pdf
___________________________________________________________
INTRODUCING
RecoveryRx Veterinary
Drug-Free Pain Relief Device
For Indoor and Outdoor Pets
https://rrxvet.carrd.co/
BIEL $$$$$$$$$$$$$$$$$$$$$$$$$$$$$$$$$$$$$$$$$$$$$$$$$$$$
Vet Recovery RX
https://vetphysiodevon.com/vet-recovery-rx#:~:text=An%20innovation%20in%20drug%2Dfree,energy%20to%20modulate%20nerve%20activity
PEMF Ring is Saving a 14 Year Old Labrador
https://therapyproducts.net/pemf-ring-is-saving-a-14-year-old-labrador/
A SDVOSB Owned Exclusive Provider of RecoveryRx for VA ( Veterans ) Hospitals!!!
https://veteranrecovery.org/products/recoveryrx-upc-851329005128
"As the sole UK and European distributor of Recovery RX Veterinary, Tanya Sprunks offers advanced long-lasting pain relief for pets using Electromagnetic Pulse Therapy. She operates several successful clinics across Devon, focusing on Veterinary Physiotherapy."
https://www.glebevets.co.uk/services/physiotherapy/
___________________________________________________________
Skymedicalsupply
•skymedicalsupply's profile picture
Introducing the EME BACK WRAP with ActiPatch® Technology, a revolutionary pain relief solution for your back. This innovative wrap utilizes ActiPatch® technology, clinically proven to alleviate pain by regulating the activity of sensitized nerves and reducing abnormal pain signaling. With its ability to deliver 1,000 sensation-free electromagnetic pulses per second, this wrap offers effective relief for up to 720 hours.
https://investorshangout.com/post/view?id=6690577
___________________________________________________________
Welcome to the largest
health and beauty store in Europe! PromoFarma is the marketplace which groups the catalogues from more than 1.000 pharmacies and other sellers into one single website, selects the basket of products at the best price, collects the order from the selected pharmacies and delivers it wherever you want.
ActiPatch effective knee-pain relief !!!!!!!!
https://www.promofarma.com/en/actipatch-effective-knee-pain-relief-1pc/p-552091
_________________________________________________________
? iAsk.AI ( Thank you, Probity!!! )
Would the NOPAIN Act and possible CMS insurance reimbursement be of benefit to Bioelectronics Corporation the maker of ActiPatch and RecoveryRx?
According to www.iAsk.ai Ask Ai Answer Engine:
Benefits of the NOPAIN Act and CMS Insurance Reimbursement for Bioelectronics Corporation
The NOPAIN Act, which stands for Non-Opioids Prevent Addiction in the Nation Act, aims to improve access to non-opioid pain management treatments for Medicare beneficiaries. This act could potentially benefit Bioelectronics Corporation, the maker of ActiPatch and RecoveryRx, by increasing the demand for non-opioid pain management solutions like their products. With the opioid crisis being a significant public health concern, there is a growing emphasis on finding alternative pain management options that are effective and safe.
Impact of the NOPAIN Act on Bioelectronics Corporation
Increased Demand: The implementation of the NOPAIN Act could lead to increased demand for non-opioid pain management products like ActiPatch and RecoveryRx. Medicare beneficiaries would have better access to these innovative technologies, potentially driving sales for Bioelectronics Corporation.
Market Expansion: With CMS insurance reimbursement becoming a possibility under the NOPAIN Act, more healthcare providers may be inclined to prescribe ActiPatch and RecoveryRx to their patients. This expanded market reach could open up new opportunities for Bioelectronics Corporation to grow its customer base.
Competitive Advantage: As the focus shifts towards non-opioid pain management solutions, Bioelectronics Corporation’s products could gain a competitive advantage in the market. Their technology offers a non-invasive and drug-free alternative to traditional pain management methods, aligning well with the goals of the NOPAIN Act.
Overall Benefit to Bioelectronics Corporation
In conclusion, the NOPAIN Act and potential CMS insurance reimbursement could indeed be beneficial to Bioelectronics Corporation. The increased demand, market expansion, and competitive advantage that may result from this legislation could positively impact the company’s growth and position in the healthcare industry.
Top 3 Authoritative Sources Used:
Centers for Medicare & Medicaid Services (CMS): Information from CMS was crucial in understanding how insurance reimbursement policies can impact healthcare providers and medical device companies like Bioelectronics Corporation.
National Institutes of Health (NIH): NIH research on pain management alternatives and opioid addiction provided valuable insights into the importance of non-opioid solutions like ActiPatch and RecoveryRx.
American Society of Anesthesiologists (ASA): The ASA’s stance on opioid addiction and their support for non-opioid pain management strategies helped in evaluating the potential benefits of the NOPAIN Act for companies like Bioelectronics Corporation.
________________________________________________________
Accelerated recovery of post-operative dental implant patients using drug-free RecoveryRx.
Read the paper here:
https://lnkd.in/daQ4GRa
#painrelief #neuromodulation #fdacleared #drugfree #clinicallyproven #painmanagement #oralsurgery #dentalimplants #postsurgical
https://www.linkedin.com/posts/bioelectronics-corporation_painrelief-neuromodulation-fdacleared-activity-6778677760979152896-XQZ1
__________________________________________________________________
BioElectronics Chairman Updates Investors
Thu, February 15, 2024 at 9:00 AM EST
https://finance.yahoo.com/news/bioelectronics-chairman-updates-investors-140000529.html
______________________________________________________________
Voices for Non-Opioid Choices Coalition Applauds Introduction of Alternatives to PAIN Act in Senate
https://www.prnewswire.com/news-releases/voices-for-non-opioid-choices-coalition-applauds-introduction-of-alternatives-to-pain-act-in-senate-302076547.html
____________________________________________________________
CHEMIST4u ( UK )
ActiPatch Muscle and Joint Pain Therapy Devices
https://www.chemist-4-u.com/brands/actipatch?fbclid
=IwAR21Sy7YpQYVw-otDE2ah9aPf-DNslt0jUw4xJMFcKxAXRyo_JkWNQ7TcYI
____________________________________________
ActiPatch is the drug-free solution for pain. It interrupts abnormal pain signaling in the nerves. You won't feel anything but relief. $$$ BACK GUARANTEE USA:
http://actipatch.com 📷
No More Dangerous, Addictive or Expensive Drugs.
https://twitter.com/ActiPatch/status/1752421581769363689?s=20
___________________________________________________________________
ActiPatch®
@ActiPatch
ActiPatch reaches deep into the painful area to provide real relief at the source. Can be used 24/7. You won't feel anything but PAIN RELIEF.
$$$ BACK GUARANTEE OFFER USA:
http://actipatch.com 📷 #neckpainrelief #techneckrelief #painrelief #neckarthritisrelief #neckpain
https://twitter.com/ActiPatch/status/1745131740358729994?ref_src=twsrc%5Etfw%7Ctwcamp%5Etweetembed%7Ctwterm%5E1745131740358729994%7Ctwgr%5E811275876d36002daf58d004f9820350cd7adb08%7Ctwcon%5Es1_&ref_url=https%3A%2F%2Finvestorshub.advfn.com%2Fboards%2Fread_msg.aspx%3Fmessage_id%3D173613351
ITS coming to BIEL $$$$$$$$$$$$$$$$$$$!!!!!!!!!!!!!!!!!!!!!!!!
11/20/2023
Washington State poised to extend coverage for SCS
https://www.neuromodulation.org/news/washington-state-poised-to-extend-coverage-for-scs
________________________________________________________________
ActiPatch SA
February, 6 at 1:35 Am
Struggling with chronic pain? 🤕 ActiPatch® pain-relief device is clinically proven to relieve muscle and joint pain!¹?³
✅ Ideal for arthritis, back pain, knee pain, fibromyalgia, and more.²
🔍 Targeted, long-lasting pain relief in just 2-3 hours.¹
🔋 Lasts up to 720 hours for continuous comfort.
🚀 Small & lightweight.¹ Say goodbye to bulky equipment!
Get back to feeling your best with ActiPatch®, available at Dis-Chem Pharmacies, Clicks and leading pharmacies nationwide.
#ActiPatch #DrugFree #PainRelief #PainManagement #ChronicPainManagement #JointPainRelief #SportsInjuryRelief #BackPainRelief
https://www.facebook.com/watch/?v=1124676815379216
__________________________________________________________________
NANS Advocacy Results in HCSC Adding Closed-Loop Spinal Cord Stimulation to Coverage!!
https://www.neuromodulation.org/news/closed-loop-04172023
_______________________________________________________________
ActiPatch®
@ActiPatch
Get the sleep you deserve with relief from ActiPatch! ActiPatch doesn't just put a band-aid on pain. It dives deep with its Pulsed Shortwave Therapy & takes on pain at its root for sustained relief! Order at:
http://actipatch.com FREE SHIPPING. #painmanagement #painfreesleep
https://twitter.com/ActiPatch/status/1737209416749887575?ref_src=twsrc%5Etfw%7Ctwcamp%5Etweetembed%7Ctwterm%5E1737209416749887575%7Ctwgr%5E811275876d36002daf58d004f9820350cd7adb08%7Ctwcon%5Es1_&ref_url=https%3A%2F%2Finvestorshub.advfn.com%2Fboards%2Fread_msg.aspx%3Fmessage_id%3D173613351
___________________________________________________________
Chronic Pain, Fibromyalgia and Plantar Fasciitis Help --- ACTIPATCH!!!
https://www.facebook.com/groups/1795257327156301/?multi_permalinks=7709195185762456
_____________________________________________________________
ActiPatch SA
If tennis injuries are following you off the court, ActiPatch® can help lessen your downtime by serving up clinically proven joint and muscle pain relief!²,³
🎾 Drug free¹
🎾 Can be worn 24/7, even during physical activity and sweating¹
https://www.facebook.com/share/v/jdHfKoc7x7dB...tid=CYgPv5
SAI has Registered with FDA to Sell ActiPatch in USA and Canada for 2024
Establishment Registration and Device Listing:
Establishment Name ....................... Reg number ..Current Reg Yr
AIRWAY SURGICAL APPL .CANADA 9611956 ...........2024
Nonthermal Shortwave Therapy Device Indicated For Over The Counter Use For The Treatment Of Pain / Repackager/Relabeler
SURGICAL APPL INDUSTRIES , INC. USA 1511629 2024
Nonthermal Shortwave Therapy Device Indicated For Over The Counter Use For The Treatment Of Pain
Repackager/Relabeler
KT Health, LLC ......................... UT/USA 3007282994 2024
Nonthermal Shortwave Therapy Device Indicated For Over The Counter Use For The Treatment Of Pain
(Airway Surgical is SAI's Canadian branch)
_________________________________________________________
PEMF Alzheimer’s Treatment Pilot Underway at Hackensack University Medical Center
https://www.hackensackmeridianhealth.org/en/info/geriatrics/evoke-trial
----------------------------------------------------------------------------------------
BioElectronics Corporation’s Post
View organization page for BioElectronics Corporation
BioElectronics Corporation
3,331 followers
Both NSAID therapy and neuromodulation therapy using PSWT resulted in statistically and clinically important reductions in pain level and improvement in functionality associated with cervical osteoarthritis, when used for 4 weeks. However, the PSWT intervention demonstrated superior improvements in all outcome measures when compared to the etoricoxib therapy arm, including patient satisfaction rating and decreased use of rescue pain medication. These results suggest that neuromodulation using PSWT may be a superior pain treatment option, when compared to COX-2 NSAIDS for neck osteoarthritis, and as well, represents a non-invasive, non-pharmacologic treatment option.
Product samples are available to licensed medical care providers in the USA. Request via email - info[ @newbie00
https://www.linkedin.com/posts/bioelectronics-corporation_pulsed-shortwave-therapy-in-cervical-osteoarthritis-activity-7138985310968795136-SRFJ
https://link.springer.com/article/10.1007/s42399-020-00652-y
---------------------------------------------------------------------------------------------------
New product added
https://actipatch.com/buy-1
BIEL $$$$$$$$$$$$$$$$$$$$$$$$$$$$$$$$$$$$$$$$$$$$$$$$$$
-------------------------------------------------------------------------------------------------
Top5-USA.co
Top 5 Actipatch In The US
https://www.top5-usa.com/actipatch
------------------------------------------------------------------------------------------------------
RecoveryRX
BOSTON ORTHOPEDIC & RESPIRATORY EQUIPMENT, LLC.
https://www.bostonorthoresp.com/214-4_p_10155.html?referrer=rss
BIEL $$$$$$$$$$$$$$$$$$$$$$$$$$$$$$$$$$$$$$$$$$$$$$$$$$$$
-----------------------------------------------------------------------------------------------------------
This is just a few FACTS to help with INTELLIGENT DD and to INVEST in BIEL, which, i/we agree with this post worth a reasonable investment:
(https://investorshub.advfn.com/boards/read_msg.aspx?message_id=172266874;; )
" RecoveryRx repairs and regenerates damaged cells by reducing pain and inflammation and accelerating blood flow. Whereas, TENS is only blocking pain signals and has no healing effect. The major difference between the two is that a TENS unit is a short term solution that “turns off” the pain signal but CANNOT perform the function of repairing damaged tissue like RecoveryRx. "
https://www.linkedin.com/posts/lauren-jarman-380a801a9_clinicallyproven-fdacleared-noninvasive-activity-7051200138563735553-Q9Zs
A SDVOSB Owned Exclusive Provider of RecoveryRx for VA ( Veterans ) Hospitals!!!!
https://veteranrecovery.org/products/recoveryrx-upc-851329005128
"Literally, there are so many benefits to a regular PEMF therapy session. Research by NASA and other bodies has found that PEMF therapy can deliver the following results:
Better circulation
Pain reduction
Improved muscle relaxation and performance
Decreased inflammation and swelling
Improved oxygenation in tissue
Enhanced cellular repair and recovery
Improved immune system
Better sleep "
https://higherdose.com/blogs/news/8-benefits-of-pemf-therapy?psafe_param=1&tw_source=google&tw_adid=634407957086&tw_campaign=18892036948&gad=1&gclid=CjwKCAjw44mlBhAQEiwAqP3eVqY1HlussPIpcLNFP0O10gqhWUrMI0VdqtspfI3FlKHqC0HkjB_OkBoCc08QAvD_BwE
BIEL $$$$$$$$$$$$$$$$$$$$$$$$$$$$$$$$$$$$$$$$$$$$$$$$$$$$$
-------------------------------------------------------------------------------------------
Watch till the end!
https://www.instagram.com/reel/Cvq8j0bILP4/?igshid=MzRlODBiNWFlZA==
BIEL $$$$$$$$$$$$$$$$$$$$$$$$$$$$$$$$$$$$$$$$$$$$$$$$$
-------------------------------------------------------------------------------------
INTRODUCING
RecoveryRx Veterinary
Drug-Free Pain Relief Device
For Indoor and Outdoor Pets
https://rrxvet.carrd.co/
BIEL $$$$$$$$$$$$$$$$$$$$$$$$$$$$$$$$$$$$$$$$$$$$$$$$$$$$
Vet Recovery RX
https://vetphysiodevon.com/vet-recovery-rx#:~:text=An%20innovation%20in%20drug%2Dfree,energy%20to%20modulate%20nerve%20activity
PEMF Ring is Saving a 14 Year Old Labrador
https://therapyproducts.net/pemf-ring-is-saving-a-14-year-old-labrador/
"As the sole UK and European distributor of Recovery RX Veterinary, Tanya Sprunks offers advanced long-lasting pain relief for pets using Electromagnetic Pulse Therapy. She operates several successful clinics across Devon, focusing on Veterinary Physiotherapy."
https://www.glebevets.co.uk/services/physiotherapy/
----------------------------------------------------------------------------------------------------------
Once, pay ATTENTION Everyone -- NOT if, IMO, NHL, NFL, NBA, WBA, MLB, PGA, Lacrosse, etc...... adopts Actipatch as a must protocol, after all those HITS, CHECKS, TRIPS, LIGAMENT PULLS/RIPS, etc.......Possibilities are ENDLESS !!!!!!!!!!!!!!!!!!!!!!!!!!!!!!
BIEL $$$$$$$$$$$$$$$$$$$$$$$$$$$$$$$$$$$$$$$$$$$$$$$$$$
---------------------------------------------------------------------------------------------
ATTENTION all the Respected, Smart, Intelligent Investors !!!!!!! Do your OWN, INTELLIGENT, THOROUGH DD, without ANY influence from KNOWN PAID BASHERS / LIARS / MANIPULAYOTS / MISINFORMERS !!!!!!!!! Make sure, if you decide to invest ( and to decide you MUST !! ) :
#1 -- Don't put all the money you are willing to invest in one basket ( buy only one stock ), be diverse......VERY IMPORTANT -- Do your OWN, INTELLIGENT DD !!!!!!!!!!!!!!!!
#2 -- If you decide/willing to buy BIEL shares, know, that this is a HIGH RETURN / HIGH LOSS RISK ratio, but the MIRACLE PRODUCT ( ACTIPATCH ) is the key !!!!
#3 -- if you decide/willing to buy BIEL shares, invest only as much as you are willing to loose, without being hurt, FINANCIALLY !!!!!!!!!!!!!!!!!
Good luck to all !!!!!!!!!!!!!!!!!!!!!!!!!!!!!!
BIEL $$$$$$$$$$$$$$$$$$$$$$$$$
In addition to the pulsed shortwave device(s), participants will receive standard-of-care supplemental analgesics which can include acetaminophen, ibuprofen, ketorolac, opioids, gabapentin (this is provider- and patient-dependent). Therefore, all patients of this study-regardless of the treatment arm they are randomized to-will continue to receive current usual and customary analgesia: all will receive the same combination of supplemental analgesics they would regardless of study participation.
This study doesn’t make sense!
Just curious, What was the frequency of Press Releases from the company that you’re referring to. They must have been very quiet with an NDA because of the Buyout .
What I find interesting is that 2 of BIEL’s employees went to 2 different companies to also assist in eventually helping Biel. And both of them are in still good terms with Biel. This thought that I’m referencing is similar to a post that “The Pro” mentioned if I read him right.
Patience.... give it a break here notoohot. I have been in the PIG for years and its a depressing piece of shit . Nuff fnnnn said
You and toohot have managed to ignite this worthless company today with 2 trades thus far.
Go KELLY...Go BIEL...Go Commander and Chief
Any thoughts on the Ilfeld clinical study results?
"I thought your suggested business plan was absurd..."
Just like how BIEL thought your group's plan was absurd when you failed MISERABLY to acquire the company.
Gosh! I wonder why you haven't shared that plan with the rest of the board?
Fascinating!
Well, it fits this junk company to a tee.
One important clarification: Ilfeld doesn't "write opinions in journals." He publishes research in journals.
I don't think anyone questions Ilfeld's integrity. He is unbiased in all his research.
|
Followers
|
1057
|
Posters
|
|
|
Posts (Today)
|
0
|
Posts (Total)
|
330774
|
|
Created
|
05/18/05
|
Type
|
Free
|
| Moderators misanthrope Capsule JustGoDeep toohot JNdouble1 gimmee greenbacks | |||
PHOENIX, June 13, 2018 (GLOBE NEWSWIRE) -- Uptick Newswire welcomed Andy Whelan, President of BioElectronics Corporation (OTC Pink:BIEL) ("the Company") back onto the "Stock Day" podcast. Andy spoke with Everett about their progress in the US market, UK market, the new product, the US FDA, and new distributors and potential partnership opportunities.
BioElectronics Corporation is a leader in non-invasive electroceuticals and the maker of an industry leading family of disposable, drug-free, pain therapy devices: ActiPatch(R) Therapy, over-the-counter treatment for back pain and other musculoskeletal complaints; RecoveryRx(R) Devices for chronic and post-operative wound care; Allay(R) Menstrual Pain Therapy. For more information, please visit http://www.bielcorp.com/
The Company is currently pursuing multiple US partnership opportunities that are partially contingent on additional FDA clearance for the treatment of musculoskeletal pain, back pain, knee pain, muscle, hip, carpal tunnel, etc. Whelan said,"Patience is key throughout this process. It's going as well as it can." Additionally, the Company anticipates solidifying deals with two new major distributors in the Asian and Eastern European markets.
The Company's product development and regulatory clearances are going well. Now, with pre-submission clearance, the 510(k) relief of musculoskeletal pain market clearance application will be filed by the end of June.
The US FDA has recently implemented an Innovation Program seeking "Breakthrough Devices" to Prevent and Treat Opioid Use Disorder. Opioids are routinely used for post-operative pain. Our United Kingdom ActiPatch User Registry accounts that 71% of opioid customers report a moderate (50%) to complete (100%) elimination in opioid medication use. The Company plans to submit its post-operative pain relief market clearance application into this program. The FDA's assistance, participation, and approval will provide credence and accelerate product market acceptance.
The Company has begun shipments of its new single box kinesiology tape product to be used for back, knee, hip, and muscles and joints in lieu of the back wrap, knee wrap and muscle and joint adhesives. The kinesiology tape is easier to use, reduces product costs, and retail shelf space requirements.
The Company is transitioning its United Kingdom over-the-counter sales and marketing from the front of the store to behind the counter to expand product availability from a few stores to all 14,000 pharmacies. Most pharmacies obtain daily product deliveries and most people in the UK qualify for free prescriptions.
Whelan is confident in the direction the Company is moving, and cites the unique nature of their products as a factor that helps the Company stand out in the global medical electronics industry. Whelan then said, "This is one busy little Company."
"Stock Day" host Everett Jolly stands by his assessment from his April interview with the company that the stock is very undervalued. BioElectronics Corporation trades on the OTC Pink market under the ticker symbol BIEL. Shares are currently selling at 0.0037, up over 100% since April.
For more details about the Company's recent FDA meetings, potential partnership opportunities, and attempts to combat the ongoing opioid crisis, follow the link below to hear the full interview.
https://upticknewswire.com/featured-interview-ceo-andrew-whelan-of-bioelectronics-corp-otc-pink-biel-3/ ;
About BioElectronics Corporation
BioElectronics develops and manufactures unique nonprescription affordable neuromodulation medical devices. The Company's technology platform is for the treatment of central sensitization, which is now widely accepted as the physiological explanation for many neurological disorders, and specifically chronic pain. Central sensitization is difficult, if not impossible, to address through pharmacotherapy without having a detrimental impact on normal physiologic function. Moreover, pharmacological and masking therapies (creams, heat patches, TENS etc.) have limited effectiveness for chronic pain due to their transient nature. BioElectronics' electromagnetic stimulation therapy has the distinct advantage of being safe, affordable, long lasting, and effective pain relief.
Contact: Paul Knopick, 940.262.3584, pknopick@eandecommunications.com
FREDERICK, MD., May 31, 2018 (GLOBE NEWSWIRE) — BioElectronics Corporation (OTC PINK: BIEL), maker of advanced medical devices announces that B. Braun Ltd, of Sheffield, UK has recently completed development of its same day surgical TOTAL Pathway program for joint replacements which includes BioElectronics medical devices. The program is being launched by B. Braun’s UK […]
FREDERICK, MD, May 29, 2018 (GLOBE NEWSWIRE) — BioElectronics Corporation (OTC PINK: BIEL), www.bielcorp.com reports that it has cancelled today’s scheduled US Food and Drug Administration meeting. “The FDA’s response to our questions, suggestions, and instructions are more than adequate for us to proceed with filing of the formal 510(k) Market Clearance Application,” stated Ian […]
FREDERICK, MD , May 11, 2018 (GLOBE NEWSWIRE) — BioElectronics Corporation (OTC PINK: BIEL), www.bielcorp.com is pleased to report on the outcome of its Pre-Submission meeting on May 9th, 2018 with the US Food and Drug Administration (US FDA). The FDA’s Pre-Submission Program is designed to organize and give guidance and feedback on clinical data and what […]
FREDERICK, MD, May 02, 2018 (GLOBE NEWSWIRE) — BioElectronics Corporation (OTC PINK: BIEL), www.bielcorp.com is pleased to announce that two meetings have been scheduled with the US Food and Drug Administration (FDA) to seek expanded over-the-counter (OTC) clearances for their drug-free, wearable medical devices. The first meeting will take place on May 9th, 2018 to […]
PHOENIX, April 26, 2018 (GLOBE NEWSWIRE) — Uptick Newswire was thrilled to have Keith Nalepka, VP of Marketing & Sales at BioElectronics (OTC Pink:BIEL) (“the Company”), return to the “Stock Day” podcast. The company has been working hard lately to expand the United Kingdom market with B. Braun in the surgical market and the product […]
FREDERICK, MD, March 19, 2018 (GLOBE NEWSWIRE) — BioElectronics Corporation (OTC PINK: BIEL), www.bielcorp.com is pleased to announce the commencement of a clinical study investigating the efficacy of its RecoveryRx® device for postoperative pain management and recovery following total knee arthroplasty surgery. Currently there are 700,000 total knee replacement surgeries in the US alone and are projected to grow 673% to 3.5 million procedures per […]
PHOENIX, Jan. 22, 2018 (GLOBE NEWSWIRE) — The Uptick Newswire “Stock Day” podcast keeps investors up to date on the latest penny stock news by bringing transparency in the micro-cap side of the market. Connect with “Stock Day” and to over 600+ CEO interviews on the OTC, Pink Sheets and micro-cap news from around the world […]
FREDERICK, MD, Jan. 09, 2018 (GLOBE NEWSWIRE) — BioElectronics Corporation (OTC Pink: BIEL), the maker of advanced wearable consumer pain management medical device, ActiPatch, announced today that CARE Pharmacies has committed to introducing ActiPatch, Musculoskeletal Pain Therapy medical device to its patients. “With the current opioid crisis here in the United States, as pharmacists, we […]
FREDERICK, Md., Jan. 04, 2018 (GLOBE NEWSWIRE) — BioElectronics Corporation (OTC PINK: BIEL), www.bielcorp.com makes wearable, drug-free chronic pain therapy medical devices. It is pleased to announce that the UK’s government funded public health service, National Health System (NHS), has approved our application to cover and pay for ActiPatch® Musculoskeletal Pain Therapy. The NHS estimates that almost half […]
FREDERICK, MD, Dec. 12, 2017 (GLOBE NEWSWIRE) — BioElectronics Corporation (OTC Pink: BIEL), the maker of advanced wearable consumer pain management medical device, ActiPatch, announced today the launch of ActiPatch for Over-The-Counter, drug-free pain relief as an alternative to Opioids. ActiPatch is approved by the FDA for Over-The-Counter sales for the adjunctive treatment of pain […]
BioElectronics Receives FDA Pre-Submission Approval for its Relief of Musculoskeletal Pain Market Clearance Application
FREDERICK, MD , May 11, 2018 (GLOBE NEWSWIRE) -- BioElectronics Corporation (BIEL) , www.bielcorp.com is pleased to report on the outcome of its Pre-Submission meeting on May 9th, 2018 with the US Food and Drug Administration (US FDA). The FDA’s Pre-Submission Program is designed to organize and give guidance and feedback on clinical data and what regulatory pathway should be followed to get market clearance. The focus of the May 9 pre-submission meeting was in discussing the FDA’s feedback on the clinical outcomes and statistical techniques used in reporting the back-pain study.
Upon reviewing pre-submission information on the ActiPatch® back-pain study (https://clinicaltrials.gov/ct2/show/NCT03240146), the FDA provided positive feedback on the clinical results, and guidance on a 510(k) submission to obtain expanded market clearance for over-the-counter (OTC) treatment of musculoskeletal pain. This would make ActiPatch available as a drug-free, safe, pain relief option for the 126 million Americans (one in two adults) who are suffering with some form of musculoskeletal pain. While ActiPatch is already FDA cleared for treatment of pain from knee osteoarthritis (25 million) and plantar fasciitis (1 million annually), expanded market clearance would allow additional products for the back (42 million), neck (19 million), hip (9 million), shoulder (11 million), carpal tunnel (12 million) and many other musculoskeletal complaints.
The company was represented by their clinical R&D team comprising: Sree Koneru, Ph.D., VP Product Development, Ian Rawe, Director Clinical Research of BioElectronics (BIEL), Kenneth McLeod, Ph.D. Director of Clinical Science and Engineering Research, State University of New York at Binghamton and Richard Staelin, Ph.D. Professor Duke University. Dr. Koneru, who led the discussion, expressed confidence in the FDA’s constructive feedback. “We are pleased that the FDA viewed our data and statistical methods favorably. They have provided guidance on how to combine the back-pain study results, along with our previously cleared 510(k), into a single 510(k) submission for obtaining expedited expanded market clearance.”, he said.
An additional pre-submission meeting is scheduled with the FDA on May 29th, 2018 to seek expanded indications in a separate application for OTC treatment of pain and edema following surgical procedures for its RecoveryRx® medical device.
About BioElectronics Corporation
BioElectronics Corporation is a leader in non-invasive electroceuticals and the maker of an industry leading family of disposable, drug-free, pain therapy devices: ActiPatch® Therapy, over-the-counter treatment for back pain and other musculoskeletal complaints; RecoveryRx® Devices for chronic and post-operative wound care; Allay® Menstrual Pain Therapy. For more information, please visit www.bielcorp.com
BioElectronics VP of Sales Discusses Pre & Post-operative Pain Relief Surgical Program in "Stock Day" Interview
PHOENIX, April 26, 2018 (GLOBE NEWSWIRE) -- Uptick Newswire was thrilled to have Keith Nalepka, VP of Marketing & Sales at BioElectronics (OTC Pink:BIEL) ("the Company"), return to the "Stock Day" podcast. The company has been working hard lately to expand the United Kingdom market with B. Braun in the surgical market and the product opportunity from the recent National Health Service payment approval for its ActiPatch® medical device.
BioElectronics is with B. Braun Medical, Ltd.UK to initiate the "Total Pathways Program" program into B. Braun's 26 geographic markets. The program is for pre-habilitation (treatment that happens prior to surgery) and post-operative recovery. This new program will benefit the majority of the 160,000 UK annual knee, hip, or joint replacements patients. According to Keith, BioElectronics is not just trying establishing a brand, but "to establish the process to a standardize care for pre and post-operative pain."
The company is immensely proud of their team for getting their concept through to the National Health Service (NHS) in the UK. In addition to successes here, the company is hoping to utilize their overseas success by applying data to their US-based efforts.
There are some great potential partnerships currently being discussed at BioElectronics, with the closing of a significant deal just on the horizon.
BioElectronics Corp is approaching their upcoming FDA meetings with confidence. "We have the data to make a very strong case for both broader musculoskeletal and post-operative pain relief indications," said Nalepka. "It's an exciting time for the company, and a really cool little product."
For more details on the Total Pathways Program, news about a partnerships, and information about the company's upcoming meetings with the FDA, follow the link below to the full interview.
https://upticknewswire.com/featured-interview-vp-of-sales-keith-nalepka-of-bioelectronics-corp-otcpink-biel-3/ ;
About BioElectronics
BioElectronics Corporation is a leader in non-invasive electroceuticals and the maker of an industry leading family of disposable, drug-free, pain therapy devices: For more information, please visit www.bielcorp.com.
Contact: Paul Knopick, 940.262.3584, pknopick@eandecommunications.com
Safe Harbor Statement
Certain statements contained herein are "forward-looking statements," (as defined in the Private Securities Litigation Reform Act of 1995). BioElectronics Corp. cautions that statements and assumptions made in this news release constitute forward-looking statements; the company makes no guarantee of future performance. Forward-looking statements are based on estimates and opinions of management at the time statements are made. These statements may address issues that involve significant risks, uncertainties, and other estimates made by management. Actual results could differ from current projections or implied results. BioElectronics Corp. undertakes no obligation to revise these statements following the date of this news release.
About Uptick Newswire
Uptick Newswire is a private company reaching out to the masses keeping investors and shareholders up to date on company news and bringing transparency to the undervalued, undersold, micro-cap stocks of the market and is the sole producer of the Uptick Network "Stock Day" Podcast. The Uptick Network "Stock Day" Podcast is an extension of Uptick Newswire and has recently launched the Video Interview Studio located in Phoenix, Arizona.
Contact Information
Uptick Newswire LLC
Everett Jolly, CEO/Founder
602-441-3474
10000 N. 31st Avenue C307 Phoenix, AZ 85051
info@upticknewswire.com
www.upticknewswire.com
| Release #:812-170554-rl-1141231: |
| BioElectronics Up-Date |
| Dear Fellow Shareholders, This letter is to report on our recent progress in marketing and product development. We have established the following:
We are continuing to solicit new international and domestic sales and marketing partners for distribution and licensing, while strengthening our relationship with existing partners. To aid in this effort we have engaged an accomplished sales and marketing pharmaceutical consultant to assist in introduction and negotiations of sales and marketing partnerships. US Market Clearance ActiPatch®, Musculoskeletal Pain Therapy We are in the process of expanding the US market clearance from relief of osteoarthritis of the knee pain and plantar fasciitis. Our goal is to obtain general clearance for musculoskeletal pain. To achieve this, we have submitted a chronic back pain clinical study to the US FDA as a formal Pre-Sub. The initial meeting with the FDA is tentatively scheduled for May 9th. Prior guidance from the FDA indicated that a third clinical study was required before expanding the current market indications to cover all musculoskeletal pain. We are hopeful that the process will be completed rapidly, however we will not have a good estimate of this market clearance date until the completion of the May 9th meeting. Postoperative Recovery and Pain We have submitted a formal Pre-Sub to the US FDA for guidance in obtaining market clearance for the palliative treatment of postoperative pain and edema. The application is supported by postoperative clinical studies on breast augmentation and caesarean section. Marketing Expansion We believe that expanded market clearance from the FDA will enhance the market value and attractiveness of the ActiPatch product line. Our economic and clinical studies for the National Health Services allowed us to gain a drug tariff listing for reimbursement in England and Wales allowing the following:
The ActiPatch registry studies report that people buy the device because it is effective, drug-free, has no harmful side effects and overall improves quality of life. We are continually working to solve existing minor issues with the use of ActiPatch. To improve the product, we are introducing custom-design kinesiology tape strips to attach the device to the body. Aside from improving user comfort, this allows us to promote a single multi-purpose box with the added benefit of reducing retail shelf space. We believe this will then allow us to sell the 7-day trial devices from pharmacy shelves. B. Braun UK This is an important development for BioElectronics. B Braun has been using RecoveryRx to improve recovery following joint replacement for many years, although in limited surgeries. However, new advances in day surgery for joint replacement have expanded our opportunity. B. Braun has recently completed a pilot program in the UK evaluating day for joint replacement. The United Kingdom's Group day surgery program for knee and hip replacement patients and is now being implemented on a national basis. Currently there are 80,000 procedures per year involving B Braun. The "fast-track" same day hip and knee replacements are being supported by hospital physiotherapy teams visiting the patient at home and communicating using a special wireless technology tablet. Upon hospital discharge, each patient will be given an ActiPatch medical device and a prescription for 6 additional devices to help accelerate recovery and mitigate the postoperative pain. A similar program is in development for spinal surgeries. On an international level, B. Braun's 54,000 employees are in 60 countries and are separated into additional divisions for hospital care, surgical products, outpatient care, and home care services. It is anticipated that B. Braun's German Group will roll out the same day hip and knee replacement program in the next few months.
Smart Insole – Heel Pain Relief We have redesigned the Smart™ Insole plantar fasciitis insole to enhance user comfort. We are awaiting a written License and Supply Agreement for the product. Clinical Research to Expand Market Clearance and Acceptance Allay®, Menstrual Pain Therapy 28% of women are in the menstrual phase of life and 60% have moderate to severe menstrual pain, or 17% of women. Previously our medical devices were classified as high risk class III by the US FDA. While our existing pilot clinical study for menstrual pain reported exceptional results for menstrual pain, to publish a clinical study and obtain US market clearance, we are collaborating with the University Hospital at Birmingham, UK. The researchers here are world renowned thought leaders in women's health and are conducting a double-blind randomized controlled trial to evaluate the efficacy of ActiPatch in reducing menstrual pain (clinicaltrials.gov listing NCT03394547). You can view the existing clinical evidence and our commercials at https://www.myallay.com/ Prevention of Episodic Migraines Headaches Migraines affect 36 million men, women and children in the United States alone. The facts are:
Chronic pain is now widely understood to be due to central sensitization, which leads to exaggerated pain perception. Migraine is no exception; since it is well known that sensitization of the trigeminovascular pain pathway can occur during a migraine attack. There is pilot data that ActiPatch can help mitigate this sensitization, so a study has been completed to determine the efficacy of ActiPatch in preventing chronic, episodic migraines. The data from the study will be analysed in the next few weeks. We believe this data will allow us to work towards developing a product as a migraine therapy and allowing us to obtain market clearance. Postoperative Recovery and Pain Working with our distributor in Lebanon we are conducting a double blind placebo randomized controlled trial on total knee replacement (clinicaltrial.gov NCT03395444). Through our long standing association with B. Braun UK, RecoveryRx has been determined to be a valuable aid in joint replacement surgery. This clinical study will allow us to gain worldwide recognition and allow for expanded marketing and acceptance of the RecoveryRx as a standard of post-operative care following joint replacement. Interstitial Cystitis (Overactive Bladder and Pelvic Pain) This study is a University of Texas, McGovern Medical School sponsored double blind randomized controlled trial. The goal is to determine how well the ActiPatch therapy works in treating patients with interstitial cystitis, bladder pain syndrome and overactive bladder. Interstitial cystitis and bladder pain syndrome are chronic bladder health conditions that greatly affect quality of life. These conditions create intermittent feelings of pain and pressure in the bladder area. The study is expected to recruit 60 women who are urology patients of the University's hospital. At least 35 million Americans have overactive bladder. Lower urinary tract symptoms, urgency, and pelvic pain are common complaints to urologists and primary care physicians. Additional Bioelectronic Product Opportunities As we develop the organizational structure we envision additional opportunities in chronic wounds, neuropathy, hypertensive therapy, etc. Most importantly future growth is not dependent on large capital outlays for research and development. Immediate Sales Growth We anticipate imminent solid sales growth from the following programs:
Thank you for your support. Sincerely,
______________________ |
FREDERICK, MD., May 31, 2018 (GLOBE NEWSWIRE) — BioElectronics Corporation (OTC PINK: BIEL), maker of advanced medical devices announces that B. Braun Ltd, of Sheffield, UK has recently completed development of its same day surgical TOTAL Pathway program for joint replacements which includes BioElectronics medical devices. The program is being launched by B. Braun’s UK Group to improve care and reduce the UK healthcare cost of its 160,000 annual hip and knee replacement procedures.
The “fast-track” same day hip and knee replacements are being supported by hospital physiotherapy teams visiting the patient at home and communicating using a specialized wireless tablet. Each patient will be given an ActiPatch medical device and a prescription for 6 additional ActiPatch devices. A similar program is being implemented for spinal surgeries.
Phil Cleary, Senior Product Group Manager, stated that “our TOTAL Pathway program enhances our commitment to patient safety, post-operative care, as well as significantly reducing healthcare costs and hospital stays.” Treating post-surgical pain requires a multifaceted approach. In trials, ActiPatch was well received and produced positive outcomes. We look forward to making this program a success, in the UK and other countries where B. Braun operates.
About BioElectronics Corporation
BioElectronics Corporation is a leader in non-invasive electroceuticals and the maker of an industry leading family of disposable, drug-free, pain therapy devices: For more information, please visit www.bielcorp.com.
About B. Braun Medical Ltd
B. Braun Medical Ltd, UK, https://www.bbraun.co.uk/ is a member of the B. Braun Group, https://www.bbraun.com/en.html one of the world´s leading healthcare companies. On an international level, B. Braun’s 54,000 employees are in 60 countries and are separated into additional divisions for hospital care, surgical products, outpatient care, and home care services.

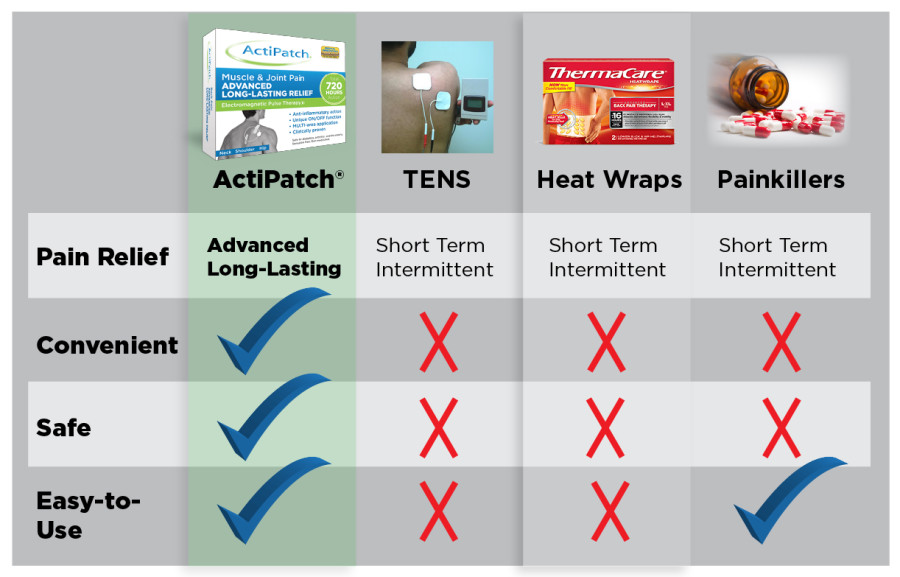
Keep in mind partners are happening and licensed ones at that.
The back study will not go in as a new submission, it is included in the knee and ankle submission so this will happen quicker than most would believe. Well actually the back study will be added to the prior submission, I don't want to confuse you
Watch how huge the Co-Branding gets. How many names besides Pain Gear?
Increasing shareholder value=7 components of shareholder value.
• Revenue
• Operating Margin
• Cash Tax Rate
• Incremental Capital Expenditure
• Investment in Working Capital
• Cost of Capital
• Competitive Advantage Period
Within the next 120 days BIEL will have in place-
Full body clearance minus the Migraine, anticipated clearance at a later date based upon the fact that this is a preventative study and the patients must have signs of a migraine coming, this study is a little more complicated.
Licensed partners (Major)
Equity partners
At least 2 huge retail chains
Let’s add on a few more distributors. Why not? Lol
NHS-first week of April, keep in mind not the only week, this is lifetime!
There will be other EU States that will duplicate the NHS and this will be coupled with licenses being sold to partners. What price, how much in royalties will soon be determined.
The August financials will and should be the strength of evidence that a certain person has established a supply chain that will be second to none.
I should mention that sales makeup only one component of the revenue stream, this is where my opinion may differ from the good posters on the BIEL board.
Revenue is the total amount taken in by a business in a set period of time.
This can include royalties, license fees and equity from partners and sales.
BIEL cannot have millions in sales without including the other revenue streams mentioned above.
My revenue forecast including sales may be different than others.
Great rewards will follow in due time. Waiting on the FDA protocol. Which means pre-submission. Guidance, rules and timing will be part of the protocol.(Looks like May-June clearance.)
Migraine clinical results will still be the huge one for the FDA protocol.(August-September clearance on positive results.)
NHS is the first of many EU States getting on board. Pretty good health systems across the pond. Sweden next!
The back pain study will obtain US FDA clearance for relief of musculoskeletal pain. Migraine headaches are not musculoskeletal. Migraines and musculoskeletal pain are both neurological disorders. Two FDA clearances!!!!!!!!!!!!!
SEC-much ado about nothing!
Partners-much ado about something!
12/7/2017 - Try and Tell US Launch, gets a 7 Day ActiPatch to US residents for shipping and handling costs of $4.95
12/7/17 - BIEL closes a deal with ANDA for National Distribution, ANDA has 60,000 commercial customers and is owned by TEVA Pharma, TEVA has extensive business dealings with the NHS
1/4/2018 - ActiPatch gains admission to the UK NHS Drug Tariff as a covered medical device, The NHS is the largest single healthcare delivery organization in the world
1/9/2018 - CARE Pharmacies will be handling the ActiPatch, CARE is an organization of 90 pharmacies in 17 states, CARE will be educating patients on the benefits of drug-free pain relief through Pain Consultations
1/16/2018 - Allay Menstrual Clinical Study is fully enrolled, taking place at the prestigious Birmingham Women's Hospital,an NHS Foundation Hospital
1/22/2018 - BIEL signs deal with Performance Health, 120 outside sales reps, 40 inside reps, KN interview
1/22/2018 - BIEL signs deal with MundiPharma in Australia and 8 other countries, Mundi is associated with Purdue Pharma, KN interview
1/22/2018 - Back Pain Study is complete, BIEL has dialogue ongoing with FDA for submission of Study to expand ActiPatch indications, KN interview
1/22/2018 - Migraine Study will get the full attention of staff now that the Back Study has been completed
1/22/2018 - BIEL in talks with Walmart
1/22/2018 - Meeting scheduled with CVS
This is the last 2 months of activity for BIEL. Any other OTC company would be dancing in the Street if they had accomplished this much in the last year.
The NHS Approval has opened new doors for BIEL and will continue to do so for many years.

FREDERICK, Md., Jan. 04, 2018 (GLOBE NEWSWIRE) -- BioElectronics Corporation (OTC PINK: BIEL), www.bielcorp.com makes wearable, drug-free chronic pain therapy medical devices. It is pleased to announce that the UK’s government-funded public health service, National Health System (NHS), has approved our application to cover and pay for ActiPatch® Musculoskeletal Pain Therapy. The NHS estimates that almost half of the 50 million UK adults may be living with chronic-pain¹, which is why there are more than 100,000 physician visits every day for musculoskeletal pain alone².
The ActiPatch is a drug-free, wearable medical device that regulates peripheral nerve activity to provide pain relief. The NHS based its decision considering strong clinical evidence and a health economics study, which found that ActiPatch significantly decreased pain and improved quality of life, while reducing overall healthcare costs by 42% (58.5% reduction in physician appointment costs, 35% reduction in prescription medication costs).
“This is a major win for pain sufferers in the UK, since they will now be able to obtain a prescription for ActiPatch, the cost of which will be covered by the government,” stated Ian Rawe, Ph.D., Director of Clinical Research at BioElectronics. “We commend this move by the NHS, as this will open up the doors for reimbursement in the US and other managed care markets. This will likely have a tremendous impact on our sales and marketability of the product in the UK and elsewhere,” said Keith Nalepka, VP of Sales & Marketing at BioElectronics.
With the addition of ActiPatch to list of approved treatments, chronic pain sufferers now have access to paid safe, drug-free chronic pain relief. The ActiPatch will be listed for payment coverage in April.
About BioElectronics Corporation
BioElectronics Corporation is a leader in non-invasive electroceuticals and the maker of an industry leading family of disposable, drug-free, pain therapy devices: For more information, please visit www.bielcorp.com.
References
1 NHS, "NHS Choices," January 2018. [Online].http://www.actipatch.com/actipatch/wp-content/uploads/2014/11/ActiPatch-3-month-Cost-Study-Final.pdf
Available: https://www.nhs.uk/news/medical-practice/almost-half-of-all-uk-adults-may-be-living-with-chronic-pai....
2 Arthritis Research UK, "Muscloskeletal Matters," Keele University, 2009
ActiPatch Cost Assessment Study Used By UK's NHS
http://www.actipatch.com/actipatch/wp-content/uploads/2014/11/ActiPatch-3-month-Cost-Study-Final.pdf
This improvement translated into reduced utilization of healthcare services, leading to a 36.8% reduction in health care costs to the NHS over a 3-month period, even after accounting for the cost of the ActiPatch device
Conclusion
Utilizing ActiPatch® as a chronic pain therapy treatment improves patient quality of life, while reducing the economic burden to the NHS.
"Almost half the adult population is living with chronic pain," the Daily Mail reports. A major new review suggests that around 28 million adults in the UK are affected by some type of chronic pain (pain that lasts for more than three months). Read More Link
https://www.nhs.uk/news/medical-practice/almost-half-of-all-uk-adults-may-be-living-with-chronic-pain/
February 06, 2017 09:05 ET
FREDERICK, MD--(Marketwired - Feb 6, 2017) - BioElectronics Corporation (OTC PINK: BIEL), the maker of wearable pain therapy devices, announced today that it has received over-the-counter use market clearance from the US FDA for ActiPatch® for the adjunctive treatment of musculoskeletal pain related to (1) plantar fasciitis of the heel; and (2) osteoarthritis of the knee.
BioElectronics is an electroceutical company that develops wearable, neuromodulation devices to safely mitigate neurological diseases and improve quality of life. Our innovative pulsed shortwave therapy technology (PSWT) that uses low power pulsed electromagnetic fields regulate electrical activity of the nervous system. The neuromodulation basis of PSWT presents significant opportunities for BioElectronics to develop optimized technology for diabetic neuropathy, postoperative surgery, chronic wounds, and other applications.
Our current OTC product line includes ActiPatch® Musculoskeletal Pain Therapy, Allay® Menstrual Pain Therapy, Smart Insole™ Heel Pain Therapy, and RecoveryRx® Post-operative and Chronic Wounds Therapy. The US FDA clearance is for our flagship product the ActiPatch® Musculoskeletal Pain Therapy, developed to relieve chronic pain. ActiPatch is a drug-free, wearable nonprescription medical device that provides 720-hours (90, 8-hour treatments) of on/off therapy for $30.00 retail. Most users obtain relief with only 8 hours per day of use, so the device will generally last several months, depending on use. ActiPatch Provides:
About BioElectronics Corporation
BioElectronics Corporation is a leader in non-invasive electroceuticals and the maker of an industry leading family of disposable, drug-free, pain therapy devices: For more information, please visit www.bielcorp.com.
How to Order an ActiPatch®?
Visit tryactipatch.com to place your order for the ActiPatch® today.
www.tryactipatch.com
BioElectronics Corporation
301-874-4980
info@bielcorp.com
4539 Metropolitan Court
Frederick, MD 21704
ActiPatch Healthcare Utilization Study Link
http://www.harmonyhealth.se/wp-content/uploads/2017/10/ActiPatchHealthcareCostStudy.pdf
| Volume | |
| Day Range: | |
| Bid Price | |
| Ask Price | |
| Last Trade Time: |
