Replies to post #298792 on Avid Bioservices Inc (CDMO)
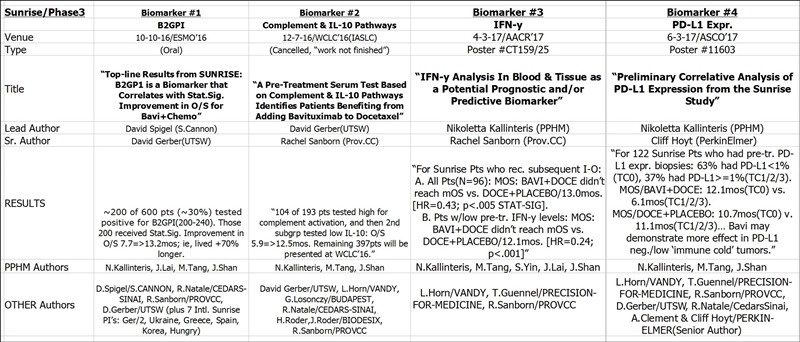
Sunrise Biomarkers (#1/B2GPI #2/COMPL,IL10 #3/IFN-y #4/PD-L1)
...
...
...
#4 6/3/17 ASCO’17 #11603 (Session: Tumor Biology)
“Preliminary Correlative Analysis of PD-L1 Expression from the Sunrise Study”
http://abstracts.asco.org/199/AbstView_199_190902.html
AUTHORS:
LEAD: Nikoletta Lea Kallinteris (PPHM)
Rachel E. Sanborn (Co-Dir., Thoracic Oncology Pgm, Robert W. Franz Cancer Res. Center, Earle A. Chiles Res. Inst., Providence CC, Portland; Honoraria=AstraZeneca; Consulting=Amgen/Ariad/DNA/PPHM/SeaGen; Funding=BMS/MedImmune/Merck)
Leora Horn (Oncologist/Vanderbilt-Ingram CC; Honoraria=Biodesix; Funding=AZN)
David E. Gerber (UTSW)
Ronald B. Natale (Med.Dir/Lung-Cancer-Pgm/Cedars-Sinai Medical Center; consult/funding=AstraZeneca)
Min Tang (PPHM)
Sean Downing (Scientist/Molecular Pathology, Foundation Medicine Inc., Cambridge MA) http://www.foundationmedicine.com ; disclosure=PerkinElmer)
Amanda Clement (Scientist/PerkinElmer; disclosure=Abbvie)
Tobias Guennel (Sen.Dir., Translational Informatics & Biometrics, Precision for Medicine, Frederick MD https://www.precisionmedicinegrp.com/pfm )
Joseph Shan (PPHM, VP/Clin+Reg)
SENIOR AUTHOR: Cliff Hoyt (Oncology Fellow/PerkinElmer)
03/20/20 8:47 AM
| Volume | |
| Day Range: | |
| Bid Price | |
| Ask Price | |
| Last Trade Time: |