Tuesday, June 06, 2017 12:10:40 PM
CHRONOLOGICAL ORDER:
#1 10-10-16/ESMO’16: “B2GPI Biomarker(30%pts) StatSig OS 7.7=>13.2mos.”
David R. Spigel: LEAD AUTHOR: CSO/Dir. Lung Cancer Pgm/Sarah Cannon Res.
David E. Gerber: SENIOR AUTHOR: UTSW/Dallas (Sunrise PI)
CO-AUTHORS: R.Natale/CEDARS-SINAI, R.Sanborn/PROVCC, PPHM’s N.Kallinteris, J.Lai, M.Tang, J.Shan, and 7 Intl. Sunrise PI’s: Ger/2, Ukraine, Greece, Spain, Korea, Hungry.
RESULTS:
~200 of 600 pts (~30%) tested positive for B2GPI(200-240). Those 200 received Stat.Sig. Improvement in O/S 7.7=>13.2mos; ie, lived +70% longer.
MORE DETAILS: http://tinyurl.com/hp73njt
#2 12-7-16/WCLC’16(IASLC): “Complement & IL-10 Pathways Id Pts Benefiting from Bavi+Doce”
PRESENTATION CANCELLED – IR said,”Data analysis not completed in time.”
David E. Gerber: LEAD AUTHOR: UTSW/Dallas (Sunrise PI)
Rachael Sanborn: SENIOR AUTHOR: Dir./Thoracic-Oncology, Providence CC/Portland
CO-AUTHORS: L.Horn/VANDY, G.Losonczy/BUDAPEST, R.Natale/CEDARS-SINAI, H.Roder, J.Roder/BIODESIX, PPHM’s N.Kallinteris, M.Tang, J.Shan.
RESULTS:
104 of 193 pts tested high for complement activation, and from that, a 2nd subgrp isolated that tested low IL-10: O/S 5.9=>12.5mos. Remaining 397pts will be presented at WCLC’16.
* ”The Complement System is an enzyme cascade that is a collection of blood & cell surface proteins to help the abilities of antibodies to clear pathogens from an organism.”
* ”IL-10 is an anti-inflammatory TH2 cytokine that has a critical role in limiting the immune response to pathogens to prevent host damage.”
MORE DETAILS: http://tinyurl.com/z8cq8vx
. . . .
12-12-16 CEO/S.King: “We are actively evaluating addl. potential biomarkers and we hope to identify a profile for patients who will receive therapeutic benefit from treatment with bavituximab.” VP/JOE SHAN: ”Numerous addl. biomarkers are currently being evaluated.” http://tinyurl.com/hhn4gga
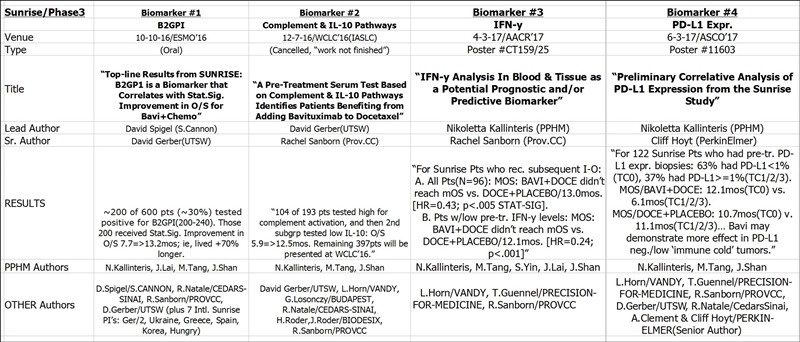
#3 4/3/17 AACR’17 CT159/25 (Session: Phase II/III Clinical Trials in Progress)
“IFN-y Analysis In Blood & Tissue as a Potential Prognostic and/or Predictive Biomarker”
Lead Author: Nikoletta Kallinteris (PPHM); Senior Author: Rachael Sanborn (Dir./Thoracic-Oncology, Providence CC); CO-AUTHORS: L.Horn/VANDY, T.Guennel/PRECISION-FOR-MEDICINE, PPHM’s N.Kallinteris, M.Tang, S.Yin, J.Lai, J.Shan.
NOTE: “IFNy, or type II interferon gamma, is a cytokine that is critical for innate & adaptive immunity against viral, some bacterial & protozoal infections.”
4-4-17/PR: ( http://ir.peregrineinc.com/releasedetail.cfm?ReleaseID=1020046 )
“SUNRISE Data Analysis Demonstrates Stat. Significant Overall Survival (OS) Improvement in Patients Receiving Bavituximab+Docetaxel and Subsequent Immunotherapy Compared to Placebo+Docetaxel and Subsequent Immunotherapy…”
Pts in the study's BAVI+DOCE arm who received subsequent immunotherapy, the mOS was not reached, while mOS was 13.0mos. for patients in the study's DOCE+PLACEBO arm who received subsequent immunotherapy [HR=0.43; p=.005]. These are the first clinical results reported supporting the hypothesis that bavituximab may modulate the tumor microenvironment to enhance the anti-tumor activity of immunotherapy agents... The presentation highlighted an analysis in which the company evaluated the impact of subsequent immunotherapy treatment, as well as patients' pre-treatment interferon gamma (IFN-y) levels on overall survival. Overall, low peripheral IFN-y correlated with more favorable OS in the patients receiving BAVI+DOCE and is a biomarker of interest. Data were also analyzed by low vs. high IFN-y levels. For patients with low pre-treatment IFN-y levels who received subsequent immunotherapy, those in the BAVI+DOCE arm did not reach mOS compared to mOS of 12.1mos. for the DOCE+PLACEBO arm [HR=0.24; p<.001].
Joseph Shan, Peregrine’s VP of Clinical & Regulatory Affairs (4-4-17/PR):
"We are extremely encouraged by the results of these exploratory analyses which provide further clinical rationale for combining bavituximab and checkpoint inhibitors. This will be the key focus for upcoming early phase clinical trials, which includes a study of bavituximab & pembrolizumab in Head & Neck cancer through our ongoing collaboration with the NCCN.”
---------
**NOTE: Per JDM find, we know that 96 Sunrise Pts received “Subsequent Immunotherapy” - see 10-13-16 ASM CEO S.King Slide#14: http://tinyurl.com/n2bajew
-----------
Nikoletta Kallinteris 1, Leora Horn 2, Min Tang 1, Tobias Guennel 3, Shen Yin 1, Jennifer Lai 1, Joseph Shan 1, Rachel E. Sanborn [Providence CC, Dir./Thoracic-Oncology http://cancergrace.org/faculty/rachel-sanborn-md - Dr. Sanborn's Conflicts of interest: DNA, AZN]
1=Peregrine Pharmaceuticals
2=Vanderbilt-Ingram Cancer Center, Nashville, TN
3=Precision for Medicine, Frederick, MD
4=Providence Cancer Center, Portland, OR
ORIG. AACR’17 ABSTRACT:
BACKGROUND: SUNRISE, a global, double-bind, Phase III trial of docetaxel (D) plus bavituximab (B) or D plus placebo (P) in previously treated non-squamous non-small cell lung cancer (NSCLC), demonstrated similar overall survival (OS) in both treatment arms. Immune correlate analyses including pre-treatment IFN-y levels in blood and tumor tissue were used to potentially identify prognostic and/or predictive correlation with clinical outcome.
METHODS: Serum was isolated from all randomized NSCLC patients at screening, periodically during treatment and at disease progression for evaluation of IFN-y levels using the Simoa TM assay (Myriad RBM, Austin, TX). Available archival tissue was also tested for 91- immune gene activation markers, including IFN-y by the Fluidigm-based gene-expression platform (Sirona Dx, Lake Oswego, OR). Kaplan-Meier statistical methods and Cox proportion hazards models were utilized to evaluate and contrast the correlation of peripheral and intratumoral IFN-y levels with OS. Patients were classified paradoxically as IFN-y "low" with a favorable disease prognosis vs. "high" associated with more aggressive disease based on the median.
RESULTS: Pretreatment serum results were available for 582 out of the 597 randomized patients. Each patient was classified to be pre-treatment IFN-y high or low (< cut-off) using cut-off 0.093 pg/ml, which is the median IFN-y value in the D+B group. Median overall survival (mOS) in all patients with IFN-y low is 11.3mos. (95% CI, 10.1-13.5) vs. 10.4mos. (95% CI, 8.4-11.3) in all IFN-y high; p=0.047. mOS of D+B arm is 11.6mos. (95% CI, 10.2-13.9) and 11.1mos. (95% CI, 9.1-14.7) in the D group; p=0.982 for IFN-y low. mOS of D+B arm is 9.0mos. (95% CI, 6.7-11.2) and 10.6mos. (95% CI, 8.9-13.0) in the D group; p=0.252 for IFN-y high. With the limited intratumoral IFN-y gene expression data (n=33), no statistically significant correlation with OS was observed.
CONCLUSIONS: Correlative approaches identified low peripheral low IFN-y at pretreatment as a biomarker of interest correlating with more favorable clinical outcomes and is consistent with the hypothesis that bavituximab may demonstrate more immunomodulatory effects in patients with “immune cold” tumors.
POSTER #159/25 IMAGE:
http://www.peregrineinc.com/images/stories/pdfs/aacr2017kallinteris.pdf
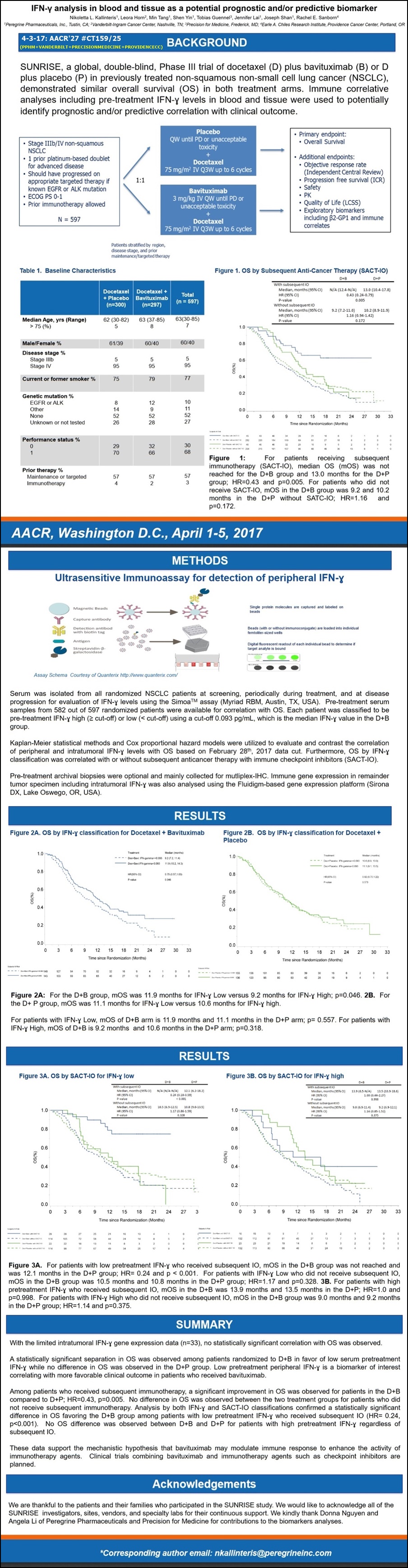
#4 6/3/17 ASCO’17 #11603 (Session: Tumor Biology)
“Preliminary Correlative Analysis of PD-L1 Expression from the Sunrise Study”
http://abstracts.asco.org/199/AbstView_199_190902.html
AUTHORS:
LEAD: Nikoletta Lea Kallinteris (PPHM)
Rachel E. Sanborn (Co-Dir., Thoracic Oncology Pgm, Robert W. Franz Cancer Res. Center, Earle A. Chiles Res. Inst., Providence CC, Portland; Honoraria=AstraZeneca; Consulting=Amgen/Ariad/DNA/PPHM/SeaGen; Funding=BMS/MedImmune/Merck)
Leora Horn (Oncologist/Vanderbilt-Ingram CC; Honoraria=Biodesix; Funding=AZN)
David E. Gerber (UTSW)
Ronald B. Natale (Med.Dir/Lung-Cancer-Pgm/Cedars-Sinai Medical Center; consult/funding=AstraZeneca)
Min Tang (PPHM)
Sean Downing (Scientist/Molecular Pathology, Foundation Medicine Inc., Cambridge MA) http://www.foundationmedicine.com ; disclosure=PerkinElmer)
Amanda Clement (Scientist/PerkinElmer; disclosure=Abbvie)
Tobias Guennel (Sen.Dir., Translational Informatics & Biometrics, Precision for Medicine, Frederick MD https://www.precisionmedicinegrp.com/pfm )
Joseph Shan (PPHM, VP/Clin+Reg)
SENIOR AUTHOR: Cliff Hoyt (Oncology Fellow/PerkinElmer)
- - - - - - - - - - - - - -
6-5-17/PR Excerpts – see http://tinyurl.com/yd2c9tty
“Peregrine Presents Prelim. Correlative Analysis of PD-L1 Expression from SUNRISE Trial at ASCO 2017”
**Negative PD-L1 Expression was Associated with a Significantly Longer mOS Compared to Positive PD-L1 Expression in Patients Receiving Docetaxel+Bavituximab
**Presented Results Support Hypothesis that Bavituximab May Demonstrate Greater Effect in "Cold" Tumors Expressing Low to No PD-L1
“...today announced the presentation of promising new data from its Phase III SUNRISE trial of bavituximab in patients with previously treated locally adv./metastatic NSCLC. ...patients in the bavituximab arm who had low baseline PD-L1 expression levels had a statistically significant improvement in mOS as compared to patients who had higher baseline levels of PD-L1… patients in the D+B arm with a pre-treatment PD-L1 expr. level of < 1% (TC0) had a mOS of 12.1 mos. compared 6.1 mos. for patients with PD-L1 expr. >=1% (TC1/2/3) (HR=.42 p=0.007). There was no difference in mOS based on PD-L1 expr. levels observed in the D+P ctl. arm (10.7 mos. for TC0 vs. 11.1 mos. for TC1/2/3; HR=.87; p=0.609).
"We believe that these latest observations from the SUNRISE trial further support the hypothesis that bavituximab, through its immune modulating mechanism, may have more effect on tumors without pre-existent immunity. These ‘cold' tumors suppress normal anti-tumor immune response and are categorized by very low to no PD-L1 expression on tumor cells," said Joseph Shan, VP/Clin+Reg Affairs. "These latest findings, along with other recently announced clinical & preclin. data from our PS-targeting program, inform our clinical development strategy going forward and provide additional rationale for combining bavituximab with checkpoint inhibitors."
As part of the SUNRISE clinical study protocol, researchers requested but did not require that patients provide a tumor tissue sample at the time of diagnosis. In total, tissue samples were collected from 129 of the trial's 597 patients and were assessed retrospectively for baseline PD-L1 expression levels on tumor cells. Of the 129 tissue samples collected, 122 were evaluable for PD-L1 expression on tumor cells (54 in D+B arm and 68 in D+P ctl. arm). Of the evaluable samples in the D+B arm, 69% demonstrated PD-L1 expression levels < 1%, as compared to 59% in the D+P arm.

Poster Image:
http://www.peregrineinc.com/images/stories/pdfs/asco2017kallinteris.pdf
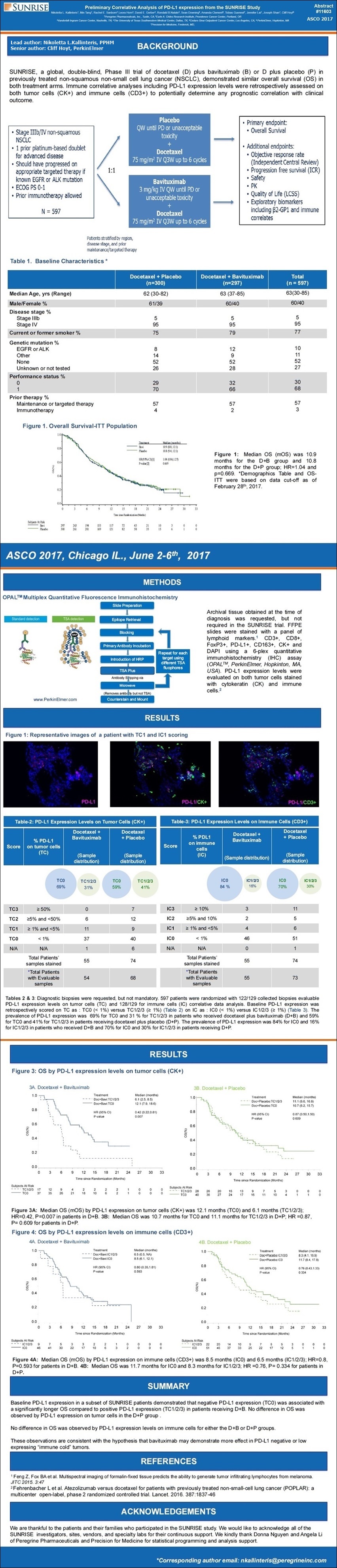
- - - - - - - - - - - - - -
ORIG. ABSTRACT (superceded by 6-5-17 PPHM PR)...
BACKGROUND:
SUNRISE, a global, double-bind, Phase III trial of docetaxel (D) + bavituximab (B) or D plus placebo (P) in previously treated non-squamous NSCLC, demonstrated similar overall survival (OS) in both treatment arms. Biomarkers including pre-treatment PD-L1 expression are being retrospectively assessed in on-going exploratory analyses.
METHODS:
Archival tissue obtained at the time of diagnosis was requested but not required in the SUNRISE trial. FFPE slides were stained with a panel of lymphoid cell markers: CD3+, CD8+, FoxP3+, PD-L1+, CD163+, CK+ and DAPI using a 6-plex quantitative immunohistochemistry (IHC) assay (OPAL, PerkinElmer, Hopkinton, MA). Baseline PD-L1 expression was retrospectively scored on tumor cells (TC) as a percentage of PD-L1 expressing tumor cells: TC3>=50%, TC2>=5% and < 50%, TC1>=1% and < 5%, and TC0 < 1%. Cox regression models for PD-L1 IHC subgroup populations were used for correlation with OS.
RESULTS:
In the subset of patients with available diagnostic biopsies (110 out of 597 randomized patients), the prevalence of PD-L1 expression was 5% for TC3, 18% for TC2/3, 35% for TC1/2/3, 65% for TC0. Median OS (mOS) of the D+B arm is 11.5 months (TC0, < 1%) and 6.0 months (TC1/2/3, >=1%) with HR 0.38 (95% CI, 0.19-0.76); p-value = 0.004. mOS of the D+P arm is 11.1 months (TC0, < 1%) and 10.4 months (TC1/2/3, >=1%) with HR 0.93 (95% CI, 0.47-1.87); p value = 0.844.
CONCLUSIONS:
Baseline PD-L1 expression in a subset of SUNRISE patients demonstrated that PD-L1 expression (TC0) was associated with a significantly prolonged OS compared to positive PD-L1 expression (TC1/2/3) in patients receiving D+B. No difference in OS was observed in the D+P group by PD-L1 expression. These observations are consistent with the hypothesis that bavituximab may demonstrate more effect in PD-L1 negative or low expressing “immune cold” tumors.
Clinical trial info: https://www.clinicaltrials.gov/ct2/show/NCT01999673
.
.
BIOBS2012’S 6-6-17 SUMMARY OF THE 4 KNOWN SUNRISE BIOMARKERS:
http://investorshub.advfn.com/boards/read_msg.aspx?message_id=131942267
SUNRISE Topline Results (at 70% cut-off ; presented at ESMO October, 2016)
BAVI Arm = 10.7
Placebo Arm = 10.8
Biomarker #1 (B2GP1)
B2GP1 Levels between 200 and 240 ug/ml (~30% of randomized patients)
BAVI Arm = 13.2
Placebo Arm = 7.7
---
B2GP1 Levels > or = 240 ug/ml (~50% of randomized patients)
BAVI Arm = 11.9
Placebo Arm = 10.1
---
Biomarker #2 (197 patients)
Subgroup with high Complement Activation (subgroup IL-10 activation)
BAVI Arm = 12.5 (n=50)
Placebo Arm = 5.9 (n=54)
---
Remaining subgroup
BAVI Arm = 5.6
Placebo Arm = 10.4
Data from remaining 397 patients remains unknown at this time.
Biomarker #3 (IFN-y)
Low pre-treatment IFN-gamma levels
BAVI Arm = 11.9
Placebo Arm = 11.1
---
High pre-treatment IFN-gamma levels
BAVI Arm = 9.2
Placebo Arm = 10.6
---
Subsequent I/O therapy (low pre-treatment IFN-gamma levels)
BAVI Arm = NOT REACHED
Placebo Arm = 12.1
---
Subsequent I/O therapy (high pre-treatment IFN-gamma levels)
BAVI Arm = 13.9
Placebo Arm = 13.5
---
NO Subsequent I/O therapy (low pre-treatment IFN-gamma levels)
BAVI Arm = 10.5
Placebo Arm = 10.8
---
NO Subsequent I/O therapy (high pre-treatment IFN-gamma levels)
BAVI Arm = 9.0
Placebo Arm = 9.2
Biomarker #4 (PD-L1 Expr.)
PD-L1 <1%
BAVI Arm = 12.1
Placebo Arm = 10.7
---
PD-L1 >1%
BAVI Arm = 6.1
Placebo Arm = 11.1
NOTES:
= = = = = = = =BIOMARKER #3 (IFN-y) AND PPHM/NCCN JOHNS-HOPKINS TRIAL TIE-IN (???):
Dr. Ranee Mehra’s (Johns-Hopkins/SidneyKimmelCC) work with Biomarker IFN-y seems to dovetail into PPHM’s newly revealed Sunrise Biomarker #3 that was presented 4-3-17 at AACR’17: #CT159/25, “IFN-y Analysis In Blood & Tissue as a Potential Prognostic and/or Predictive Biomarker”
-------
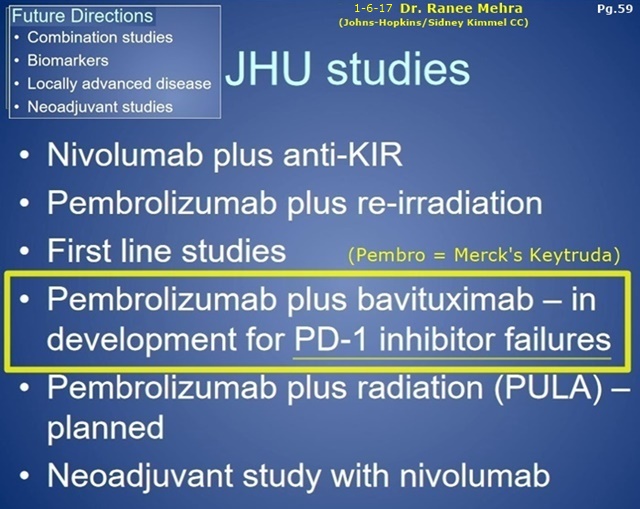
1-6-17 Dr. Ranee Mehra (Johns-Hopkins/SidneyKimmelCC), P.I. for the upcoming NCCN Ph2 Bavi+Keytruda Head&Neck trial. Excerpts from her 1-6-17 talk at GBMC/Greater Balt. MC “H+N Grand Rounds”… I do believe she views this trial is an important part of Johns-Hopkins future anti-cancer direction. Also, look at her Slide #36: “Interferon-y Signature”. Is that an exact tie-in to the newly-revealed AACR’17(4-3-17) Sunrise Biomarker #3 Abstract, “IFN-y Analysis In Blood & Tissue as a Potential Prognostic and/or Predictive Biomarker” (embargoed)? I still totally believe that PPHM has been working with the 3 NCCN Bavi Awardees (Moffitt, MassGEN, JohnsHopkins) to weave in the “chosen” Sunrise Biomarker that is associated with generating “improved outcomes for bavituximab-containing treatments” in future trials.
PDF Link(1-6-17): http://www.gbmc.org/workfiles/HeadNeck/Grand%20Rounds/IO_Therapy_SCCHN02017.pdf
-------
Note: DR. RANEE MEHRA was co-author of ASCO’16, “Biomarkers & Response to Pembro(Keytruda) in Recurrent/Metastatic Head & Neck Cancer” - Conclusion: “The IFN-y signature score was significantly associated with ORR, PFS, and OS (all, P< .001)… PD-L2 & IFN-y signature may be associated with clinical response to Pembro[Keytruda] and may offer addl. strategies to improve prediction of response.” http://meetinglibrary.asco.org/content/165708-176


PPHM’s NCCN#3: Ph2/Progressive Squamous Head+Neck (Bavi+Merck’s Keytruda), JOHNS-HOPKINS(Sidney Kimmel CC) - PI: Ranee Mehra, MD
”Phase II Study of Pembrolizumab[Keytruda] & Bavituximab for Progressive Recurrent/Metastatic Squamous Cell Carcinoma of the Head & Neck" http://tinyurl.com/gutgwb5
Ranee Mehra, MD: Dir., Head & Neck Oncology Therapeutics, Johns Hopkins Medicine https://www.linkedin.com/in/ranee-mehra-34a0467
RANEE MEHRA Disclosures(ASCO’16): GSK, Bayer, BMS, Genentech, Novartis, Mirati Ther.

NCCN Bavituximab Trials Announced 9-6-16 - To Begin "Early 2017" http://tinyurl.com/gutgwb5
...#1: Ph1/HepC-Related Hepatocellular(Liver) (Bavi+RAD+Sorafenib), MOFFITT CANCER CENTER - PI: Jessica Frakes, MD - https://clinicaltrials.gov/ct2/show/NCT02989870
...#2: P1-2/Newly Diag. Glioblastoma (Bavi+RAD+Merck’s Temodar), MASS-GEN. CANCER CENTER - PI: Elizabeth Gerstner, MD
...#3: Ph2/Progressive Squamous Head+Neck (Bavi+Merck’s Keytruda), JOHNS-HOPKINS(Sidney Kimmel CC) - PI: Ranee Mehra, MD
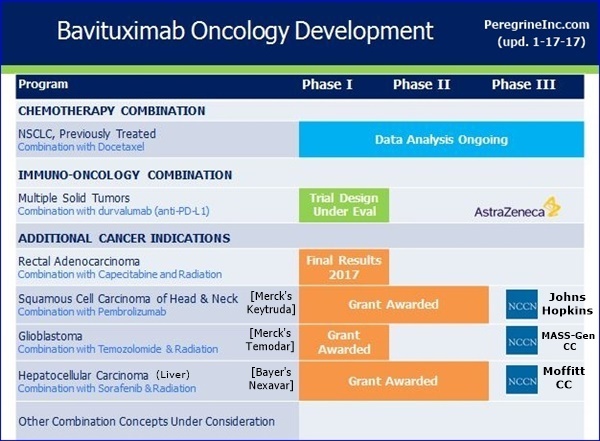
9-9-16/CC/JoeShan: “The 3rd award is for a Phase II study of pembrolizumab [Merck’s Keytruda, anti-PD-1] & bavituximab in Head & Neck Cancer. We are particularly excited about this project, as it will be the 1st clinical trial of bavituximab with a checkpoint inhibitor. In multiple previous preclin. studies, we have observed bavituximab's potential to work synergistically with PD1 inhibitors such as pembrolizumab (Merck’s Keytruda).” http://tinyurl.com/ktrfswj
- - - - - - - - - -
Steve King 9-9-16/CC: “Our collaboration with the NCCN has been an important part of our strategy for advancing the bavituximab clinical program in a cost effective way. We earlier provided NCCN with a $2mm grant to support bavituximab related clinical research with no further financial obligations, and these grant awards represent the outcome of a competitive selection process for the best proposals. These studies will evaluate novel bavituximab combinations in Glioblastoma, Head & Neck Cancer, and Hepatocellular Carcinoma, including an immunotherapy combination [Bavi + Merck’s Keytruda], which is a major focus for advancing the program.” http://tinyurl.com/ktrfswj
- - - - - - - - - -
Steve King 9-9-16/CC/Q&A: “I’m very excited about the combinations that were chosen because the Radiation combination is one that in preclinical studies, as was mentioned during the prepared remarks, has always shown a lot of promise. It’s great to be able to now see that put into a clinical setting in a couple of different clinical trials. And the I-O combinations, as Jeff mentioned during his prepared remarks, is a major focus of ours. So to see a Pembro [Merck’s Keytruda] combination picked as well, we are just really excited that these were the 3 winners out of the NCCN selection process.” http://tinyurl.com/ktrfswj
= = = = = = = = = =DETAILS ON SUNRISE BIOMARKERS #1(B2GPI) & #2(COMPLEMENT/IL-10):
#1 10-10-16/ESMO’16: “B2GPI Biomarker(30%pts) StatSig OS 7.7=>13.2mos.” http://tinyurl.com/hp73njt
10-10-16/PR:
“Peregrine Reports Top-Line and Initial Biomarker Data from Phase III SUNRISE Trial of Bavituximab in Oral Presentation at Eur. Society for Medical Oncology (ESMO) 2016 Congress”
-- Company Has Identified Beta-2 Glycoprotein-1 (B2GP1) as a Biomarker that Correlates with Statistically Significant Improvement in Overall Survival for Patients Receiving the Bavituximab Combination Compared to Chemotherapy Alone
-- Ongoing SUNRISE Trial Biomarker Analysis Expected to Identify Addl. Biomarkers Associated with Patients Benefiting from Bavituximab Treatment that Will Help Guide Program's Future Clinical Development. . .
** "With every clinical trial we conduct, we are constantly reminded of the difficulty involved in treating patients with NSCLC. This continues to prove to be a very challenging cancer to combat and the need for effective treatments remains high," David R. Spigel, MD, CSO and PgmDir. of Lung Cancer Res. at the Sarah Cannon Res. Inst. and one of the lead investigators in the SUNRISE trial. "The findings with regard to B2GP1 that have been collected as part of the ongoing SUNRISE trial data analysis are interesting and support further investigation."
** Peregrine intends to further evaluate the role of B2GP1 levels in response to bavituximab therapy in future clinical trials. The company has filed a new patent application directed to the use of this initial biomarker discovery. Addl. patient sample testing & analysis is ongoing and may result in other biomarkers of importance.
--------------
** Data presented at ESMO’16 demonstrated that patients with pre-treatment B2GP1 levels between 200 and 240 (representing approx. 30% of randomized patients) achieved a statistically significant, 5.5-mo. improvement (13.2 mos. vs. 7.7 mos.) in MOS as compared to patients in the ctl. group with the same range of B2GP1 levels [p = 0.049; HR=.67].
-------------
** "We would once again like to thank all of the patients, clinical investigators and scientists who participated in the SUNRISE trial and have made it possible for us to continue to collect and analyze a range of key data from the study. While we were disappointed with the trial being discontinued earlier in the year, we are excited by the fact that we are beginning to learn important information from the trial through the ongoing biomarker analysis program that will be critical in helping guide the future clinical development of bavituximab," said Joseph Shan, VP/Clin&Reg.Affairs at Peregrine. "It is encouraging that the initial biomarker analysis has identified an important biomarker early in the process and we are optimistic that additional biomarkers associated with improved outcomes for bavituximab-containing treatments will be identified as the analysis continues. We expect to be able to share the emerging data over the coming months at scientific & medical conferences as the more results become available.”
http://investorshub.advfn.com/boards/read_msg.aspx?message_id=125687447
= = = = = = = = = = = = = = = =
#2 12-7-16/WCLC’16(IASLC): “Complement & IL-10 Pathways Id Pts Benefitting from Bavi+Doce” <=CANCELLED/”Anal.Not.Done(IR)”
...Lead author (presenter) is UTSW’s Dr. David Gerber (previously presented Ph2/NSCLC data and Prelim. SUNRISE data at AACR’14). The Senior author is Dr. Rachel E. Sanborn, Co-Dir., Thoracic Oncology Pgm, Robert W. Franz Cancer Res. Center, Earle A. Chiles Res. Inst., Providence CC, Portland, OR. Interestingly, one co-author is Heinrich Roder, CTO of Biodesix, Boulder CO...
ABSTRACT CONCLUSION:
“...Proteomic & correlative approaches identified complement activation and low IL-10 levels as important pathways for predicting improved outcomes of patient treatment with Doce+Bavi, in line with preclinical work on Bavi’s MOA...” [Pts=193, within the 1st subgroup of N=104, a 2nd subgroup isolated: MOS 5.9mos => 12.5mos.]
Dec4-7 2016: “WCLC’16 - IASLC’s 17th World Conf. on Lung Cancer”, Vienna, Austria
http://wclc2016.iaslc.org Pgm: http://wclc2016.iaslc.org/wp-content/uploads/2016/10/WCLC-2016-Poster-Program.pdf
Poster Session with Presenters Present (ID 472) - Track: Advanced NSCLC
12/7/16 2:30-3:45pm David E. Gerber [UTSW], J. Roder, N.L. Kallinteris, L. Horn, G. Losonczy, R. Natale, M. Tang, Heinrich Roder [CTO, Biodesix http://www.biodesix.com/project/heinrichroder ], Joe S. Shan [VP/Clin+Reg], Rachel E. Sanborn [Providence Portland Medical Ctr]
”A Pre-Treatment Serum Test Based on Complement & IL-10 Pathways Identifies Patients Benefiting from the Addition of Bavituximab to Docetaxel”
ABSTRACT Book PDF: http://wclc2016.iaslc.org/wp-content/uploads/2016/12/WCLC2016-Abstract-Book_vF-WEB_revDec12.pdf
ABSTRACT: http://library.iaslc.org/virtual-library-search?product_id=6
BACKGROUND:
SUNRISE, a global, double-bind, Phase III trial of docetaxel (D) plus bavituximab (B) or D plus placebo (P) in previously treated non-squamous non-small cell lung cancer, demonstrated similar overall survival (OS) in both treatment arms. Mass spectrometry and correlative analysis were used to create a test able to identify a subgroup of patients benefitting from the addition of B to D.
METHODS:
Pre-treatment serum samples were available for 197 of the first 200 subjects enrolled in the trial. Mass spectra could be generated for 193 samples using the Deep MALDI method (Duncan et al, ASMS 2013), processed and features (peaks) identified. Mass spectral (MS) features associated with various biological functions were identified using a gene set enrichment analysis approach. Analysis of scores based on these MS feature, subsets indicated that in patients with High Complement Activation outcome depended on IL-10 activation in D+B but not in D+P. A test using the MS features associated with these functions was created to reliably identify a patient subgroup associated with clinical benefit using modern machine learning methods.
RESULTS:
Complement activation, as assessed by a classifier trained using related MS features, was a prognostic factor in both treatment arms, with high activation associated with poorer clinical outcome (OS HR = 0.54, log-rank p = 0.013 for D+B; OS HR = 0.60, log-rank p = 0.040 for D+P). Within the subgroup with high complement activation [N=50 (D+B); N=54 (D+P)], a second classifier using features related to IL-10 activation was able to isolate a subgroup of patients showing numerical benefit from the addition of B [Bavituximab] [median OS 5.9mos.(D+Placebo), 12.5mos.(D+Bavituximab)]. The remaining subgroup showed no benefit from addition of B [MOS 10.4mos.(D+P), 5.6mos.(D+B)]. Blinded validation of the test in the remainder 397 patients randomized in SUNRISE is will be presented.
CONCLUSION:
Proteomic and correlative approaches identified complement activation and low IL-10 levels as important pathways for predicting improved outcomes of patient treatment with D+B, in line with preclinical work on B’s mechanism of action. The test resulting from this work will undergo blinded indep. validation.
- - - - - - - - -
12-20-16/S.Diaz(per Cheynew post #282068): “There was no poster in Vienna. The team was working on a very tight timeframe and, despite their best efforts, couldn’t complete the data analysis in time. Given the crunch, we knew there was a possibility that we wouldn’t meet the timing, so we never issued a PR announcing that we would present. Hence no PR announcing that we did not present.” S.DIAZ/FOLLOWUP: ”Unfortunately, I don’t have a timeline for completion of the analysis or where/when it might be presented. We’ll certainly announce this as soon as we know for sure. Only Joe Shan attended the conference for Peregrine.”
= = = = = = = = = =NOTE:
Ann Oncol (11-8-16/suppl8): ESMO Symposium on Immuno-Oncology, Nov4-6 2016, Lausanne, Switzerland
#30P: “Proteomic Signature Analysis & Application in Clinical Development of the Novel Phosphatidylserine-Targeting Immunotherapy, Bavituximab”
http://annonc.oxfordjournals.org/content/27/suppl_8/mdw525.30
David E. Gerber [UTSW] 1, N.L. Kallinteris 2, L. Horn 3, G. Losonczy 4, R. Natale 5, Heinrich Roder 6 [CTO, Biodesix], M. Tang 7, J. Lai 2, J. Shan 8, Rachel E. Sanborn [9=Providence Portland Medical Ctr]
1 Oncology, UTSW-MC/Dallas
2 Clinical, Peregrine Pharmaceuticals Inc.
3 Oncology, Vanderbilt Ingram CC, Nashville, TN
4 Oncology, Semmelweis Univ., Budapest, Hungary
5 Oncology, Cedars-Sinai M/C, Los Angeles
6 Biodesix, Boulder, CO [CTO, Dr. Heinrich Roder: http://www.biodesix.com/project/heinrichroder ] “Founded in 2005, Biodesix discovers & commercializes cancer tests (diagnostics) that help patients & their doctors make more informed decisions about treatment based on a patient’s unique molecular profile.”
7 Biostatistics, Peregrine Pharmaceuticals Inc.
8 Clinical & Regulatory Affairs, Peregrine Pharmaceuticals, Inc.
9 Thoracic Oncology, Providence Cancer Care, Providence, OR
Aim/Background:
Understanding the multi-dimensional characteristics of cancer is essential to patient selection and treatment planning. Topline results from SUNRISE, a global double-blind Phase III trial of docetaxel + bavituximab (D+B) vs. docetaxel + placebo (D) in previously treated non-squamous NSCLC demonstrated mOS of 10.7mos. in the D+B group and 10.8mos. for the D group, which was unexpectedly different from the assumed 9.1mos. for D+B vs. 7.0mos. used for study powering. VeriStrat, a….[must subscribe]
- - - - - - - - - -
5-31-14 ASCO’14: David Gerber/Joe Shan Poster on Ph3/SUNRISE Trial (#TPS8129) http://tinyurl.com/nv4jloo
Recent CDMO News
- Avid Bioservices to Participate in Craig-Hallum Bioprocessing Conference • GlobeNewswire Inc. • 09/12/2024 08:05:27 PM
- Form 10-Q - Quarterly report [Sections 13 or 15(d)] • Edgar (US Regulatory) • 09/09/2024 08:43:56 PM
- Form 8-K - Current report • Edgar (US Regulatory) • 09/09/2024 08:19:30 PM
- Avid Bioservices Reports Financial Results for First Quarter Ended July 31, 2024 • GlobeNewswire Inc. • 09/09/2024 08:05:31 PM
- U.S. Futures Rise Amid Inflation Report Anticipation; Oil Prices Climb on Hurricane Threat and Supply Concerns • IH Market News • 09/09/2024 10:09:14 AM
- Avid Bioservices to Report Financial Results for First Quarter of Fiscal Year 2025 After Market Close on September 9, 2024 • GlobeNewswire Inc. • 09/03/2024 08:05:20 PM
- Form 8-K - Current report • Edgar (US Regulatory) • 08/29/2024 08:30:10 PM
- Form ARS - Annual Report to Security Holders • Edgar (US Regulatory) • 08/28/2024 08:34:04 PM
- Form DEFA14A - Additional definitive proxy soliciting materials and Rule 14(a)(12) material • Edgar (US Regulatory) • 08/28/2024 08:32:18 PM
- Form DEF 14A - Other definitive proxy statements • Edgar (US Regulatory) • 08/28/2024 08:30:28 PM
- Form 4 - Statement of changes in beneficial ownership of securities • Edgar (US Regulatory) • 07/16/2024 11:50:20 PM
- Form 4 - Statement of changes in beneficial ownership of securities • Edgar (US Regulatory) • 07/16/2024 11:48:19 PM
- Form 4 - Statement of changes in beneficial ownership of securities • Edgar (US Regulatory) • 07/15/2024 08:40:04 PM
- Form 4 - Statement of changes in beneficial ownership of securities • Edgar (US Regulatory) • 07/12/2024 08:30:04 PM
- Form 4 - Statement of changes in beneficial ownership of securities • Edgar (US Regulatory) • 07/11/2024 09:02:39 PM
- Form 4 - Statement of changes in beneficial ownership of securities • Edgar (US Regulatory) • 07/11/2024 09:02:27 PM
- Form 4 - Statement of changes in beneficial ownership of securities • Edgar (US Regulatory) • 07/11/2024 09:01:22 PM
- Form 4 - Statement of changes in beneficial ownership of securities • Edgar (US Regulatory) • 07/11/2024 09:01:05 PM
- Form 4 - Statement of changes in beneficial ownership of securities • Edgar (US Regulatory) • 07/11/2024 09:00:54 PM
- Form 4 - Statement of changes in beneficial ownership of securities • Edgar (US Regulatory) • 07/11/2024 09:00:45 PM
- Form 144 - Report of proposed sale of securities • Edgar (US Regulatory) • 07/11/2024 12:23:26 AM
- Avid Bioservices Reports Financial Results for Fourth Quarter and Fiscal Year Ended April 30, 2024 • GlobeNewswire Inc. • 07/02/2024 08:05:04 PM
- Avid Bioservices to Report Financial Results for Quarter and Fiscal Year Ended April 30, 2024, After Market Close on July 2, 2024 • GlobeNewswire Inc. • 07/01/2024 11:00:21 AM
- Avid Bioservices Earns Committed Badge from EcoVadis for Sustainability Performance • GlobeNewswire Inc. • 05/23/2024 12:05:46 PM
- Avid Bioservices to Participate at Upcoming Investor Conferences • GlobeNewswire Inc. • 05/07/2024 08:05:11 PM
VHAI - Vocodia Partners with Leading Political Super PACs to Revolutionize Fundraising Efforts • VHAI • Sep 19, 2024 11:48 AM
Dear Cashmere Group Holding Co. AKA Swifty Global Signs Binding Letter of Intent to be Acquired by Signing Day Sports • DRCR • Sep 19, 2024 10:26 AM
HealthLynked Launches Virtual Urgent Care Through Partnership with Lyric Health. • HLYK • Sep 19, 2024 8:00 AM
Element79 Gold Corp. Appoints Kevin Arias as Advisor to the Board of Directors, Strengthening Strategic Leadership • ELMGF • Sep 18, 2024 10:29 AM
Mawson Finland Limited Further Expands the Known Mineralized Zones at Rajapalot: Palokas step-out drills 7 metres @ 9.1 g/t gold & 706 ppm cobalt • MFL • Sep 17, 2024 9:02 AM
PickleJar Announces Integration With OptCulture to Deliver Holistic Fan Experiences at Venue Point of Sale • PKLE • Sep 17, 2024 8:00 AM







