Thursday, December 13, 2018 10:00:46 PM
In March 2018, the TGA announced a provisional approval pathway. This will allow drugs to be available for up to six years based on preliminary data.9 The anticancer drug olaratumab is the first drug to be considered for provisional approval in Australia.
Access to new therapies is a balance between evidence (determining the risk of acceptable adverse effects versus efficacy) and the speed of availability, intersected by the issue of affordability. Making a drug available early with temporary authorisation is not a new concept, particularly for patients with life-threatening or seriously disabling conditions for which there is a clear unmet therapeutic need. Temporary access is akin to a learner driver receiving their provisional licence – a full licence is only granted after more experience. Rapidly approved drugs should receive provisional registration for a period of three years and the drug company should be required to provide annual data on the postmarketing experience.
In Australia at present, sponsor companies are required to report all negative outcomes that they become aware of, but there is no imperative for them to actively and meticulously seek out adverse events, or confirm efficacy after approval. As pharmacovigilance relies on spontaneous voluntary reporting of adverse effects by clinicians, it is highly likely that safety concerns are under-reported.
Improving the scientific rigor of postmarketing information to track effectiveness and safety outcomes, either through independently monitored registry studies as a condition of initial registration or data linkage (e.g. with linking of Pharmaceutical Benefits Scheme and Medicare Benefits Scheme datasets), will be of paramount importance during any provisional registration period. If efficacy outcomes in the real-world environment are not confirmed or a significant safety problem emerges, then the drug’s registration should be suspended, at least for previously untreated patients, until the sponsor satisfactorily addresses the problems.
https://www.nps.org.au/australian-prescriber/articles/fast-tracking-of-new-drugs-getting-the-balance-right
See also:
https://www.tga.gov.au/provisional-approval-pathway-prescription-medicines
The document with the table below has been posted before:
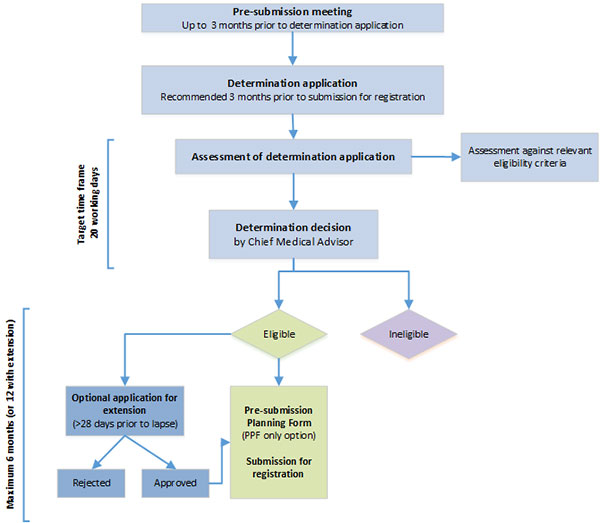
https://www.tga.gov.au/publication/provisional-determination
This drug was approved out of P2 in Austraila and by the FDA approval came on November second:
Pfizer Australia Pty Ltd
Designation/determination: Provisional approval Effective Date: 27/09/2018 Lapse Date: 27/03/2019
Medicine Name: lorlatinib
Therapeutic area: Oncology
https://www.tga.gov.au/ws-designation-notices-index
Recent AVXL News
- Anavex Life Sciences to Present at the H.C. Wainwright 26th Annual Global Investment Conference 2024 • GlobeNewswire Inc. • 09/03/2024 11:30:00 AM
- Form 10-Q - Quarterly report [Sections 13 or 15(d)] • Edgar (US Regulatory) • 08/06/2024 09:21:05 PM
- Anavex Life Sciences Reports Fiscal 2024 Third Quarter Financial Results and Provides Business Update • GlobeNewswire Inc. • 08/06/2024 11:30:00 AM
- Anavex Life Sciences to Announce Fiscal 2024 Third Quarter Financial Results on Tuesday, August 6, 2024 • GlobeNewswire Inc. • 08/01/2024 11:30:00 AM
- Form 8-K - Current report • Edgar (US Regulatory) • 07/30/2024 09:20:53 PM
- Anavex Life Sciences Announces Translational Biomarker Data for ANAVEX®2-73 (blarcamesine) in Fragile X Syndrome (Major Cause of Autism) at the 19th NFXF International Fragile X Conference • GlobeNewswire Inc. • 07/30/2024 11:30:00 AM
- Form S-3 - Registration statement under Securities Act of 1933 • Edgar (US Regulatory) • 07/29/2024 09:21:49 PM
- Results from Anavex Life Sciences Landmark Phase IIb/III Trial of Blarcamesine Presented at Alzheimer's Association Conference • GlobeNewswire Inc. • 07/28/2024 09:00:00 PM
- Anavex Life Sciences to Present at the H.C. Wainwright 5th Annual Neuro Perspectives Virtual Conference • GlobeNewswire Inc. • 06/20/2024 11:30:00 AM
- Form 4 - Statement of changes in beneficial ownership of securities • Edgar (US Regulatory) • 06/17/2024 11:30:10 AM
- Anavex Life Sciences Announces Expansion of Leadership Team • GlobeNewswire Inc. • 05/22/2024 11:30:00 AM
- Form 4 - Statement of changes in beneficial ownership of securities • Edgar (US Regulatory) • 05/17/2024 10:01:00 AM
- Anavex Life Sciences to Present at the H.C. Wainwright 2nd BioConnect Investor Conference at NASDAQ • GlobeNewswire Inc. • 05/14/2024 11:30:00 AM
- Shareholders that lost money on Anavex Life Sciences Corporation(AVXL) Urged to Join Class Action - Contact The Gross Law Firm to Learn More • PR Newswire (US) • 05/10/2024 09:45:00 AM
- Form 10-Q - Quarterly report [Sections 13 or 15(d)] • Edgar (US Regulatory) • 05/09/2024 08:35:55 PM
- Form 8-K - Current report • Edgar (US Regulatory) • 05/09/2024 12:00:30 PM
- Anavex Life Sciences Reports Fiscal 2024 Second Quarter Financial Results and Provides Business Update • GlobeNewswire Inc. • 05/09/2024 11:30:00 AM
- The Gross Law Firm Announces the Filing of a Securities Class Action on Behalf of Anavex Life Sciences Corporation(AVXL) Shareholders • PR Newswire (US) • 05/07/2024 09:45:00 AM
- Form DEFA14A - Additional definitive proxy soliciting materials and Rule 14(a)(12) material • Edgar (US Regulatory) • 05/06/2024 10:03:33 AM
- Anavex Life Sciences Corporation Sued for Securities Law Violations - Contact The Gross Law Firm Before May 13, 2024 to Discuss Your Rights - AVXL • PR Newswire (US) • 05/03/2024 09:45:00 AM
- Anavex Life Sciences to Announce Fiscal 2024 Second Quarter Financial Results on Thursday, May 9th, 2024 • GlobeNewswire Inc. • 05/02/2024 11:30:00 AM
- May 13, 2024 Deadline: Contact The Gross Law Firm to Join Class Action Suit Against AVXL • PR Newswire (US) • 04/26/2024 09:45:00 AM
- Contact The Gross Law Firm by May 13, 2024 Deadline to Join Class Action Against Anavex Life Sciences Corporation(AVXL) • PR Newswire (US) • 04/19/2024 09:45:00 AM
- The Gross Law Firm Reminds Shareholders of a Lead Plaintiff Deadline of May 13, 2024 in Anavex Life Sciences Lawsuit - AVXL • PR Newswire (US) • 04/16/2024 09:45:00 AM
BARRON'S COVE to Premier at the Hamptons International Film Festival • APHP • Sep 30, 2024 2:56 PM
Lingerie Fighting Championships Signs Broadcast Deal With Maybacks Global Entertainment • BOTY • Sep 26, 2024 9:00 AM
Maybacks Global Entertainment and Lingerie Fighting Championships Enter Into Broadcast And Revenue Sharing Agreement • AHRO • Sep 26, 2024 8:30 AM
North Bay Resources Commences Operations at Bishop Gold Mill, Inyo County, California; Engages Sabean Group Management Consulting • NBRI • Sep 25, 2024 9:15 AM
CEO David B. Dorwart Anticipates a Bright Future at Good Gaming Inc. Through His Most Recent Shareholder Update • GMER • Sep 25, 2024 8:30 AM
Cannabix Technologies and Omega Laboratories Inc. Advance Marijuana Breathalyzer Technology - Dr. Bruce Goldberger to Present at Society of Forensic Toxicologists Conference • BLOZF • Sep 24, 2024 8:50 AM








