Wednesday, November 07, 2018 1:25:36 PM
Is data presented at CTAD suffiecient? If not, the TGA might come back with requirements for the P2b.
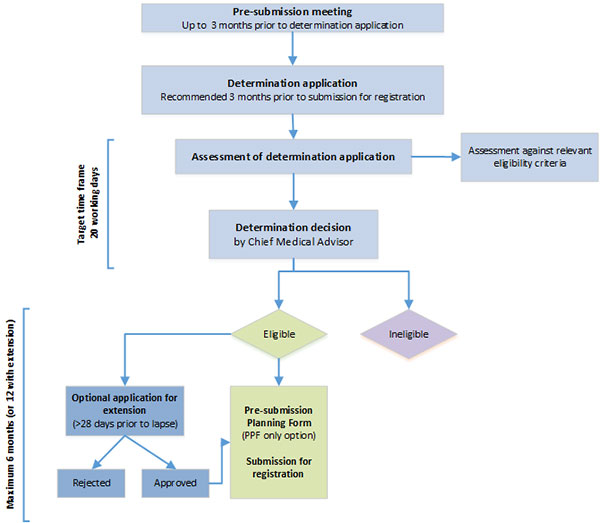
https://www.tga.gov.au/publication/provisional-determination
The P3 plan would have to be in place to apply -
the person who made the application under subsection 22C(1) of the Act has provided sufficient evidence of the plan to submit comprehensive clinical data on the safety and efficacy of the medicine before the end of the 6 years (starting on the day that provisional registration of the medicine would commence if the Secretary were to provisionally register the medicine).
https://www.tga.gov.au/publication/provisional-determination-eligibility-criteria
Recent AVXL News
- Anavex’s Blarcamesine Achieves Pre-specified Efficacy in Phase IIb/III Alzheimer’s Trial: Data Presented at CTAD Conference 2024 • GlobeNewswire Inc. • 10/31/2024 08:00:00 AM
- ZenaTech, Inc. (NASDAQ: ZENA) Software Company Acquisition • InvestorsHub NewsWire • 10/23/2024 12:03:31 PM
- Anavex Life Sciences Announces Encouraging Preliminary Biomarker Results from Ongoing Phase 2 Study of ANAVEX®3-71 for the Treatment of Schizophrenia • GlobeNewswire Inc. • 10/17/2024 11:30:00 AM
- ZenaTech, Inc. (NASDAQ: ZENA) First US Trial of IQ Nano Drone for Inventory Management • InvestorsHub NewsWire • 10/15/2024 12:21:48 PM
- ZenaTech, Inc. (NASDAQ: ZENA) To Commence Trading Today • InvestorsHub NewsWire • 10/01/2024 11:00:00 AM
- Anavex Life Sciences to Present at the H.C. Wainwright 26th Annual Global Investment Conference 2024 • GlobeNewswire Inc. • 09/03/2024 11:30:00 AM
- Form 10-Q - Quarterly report [Sections 13 or 15(d)] • Edgar (US Regulatory) • 08/06/2024 09:21:05 PM
- Anavex Life Sciences Reports Fiscal 2024 Third Quarter Financial Results and Provides Business Update • GlobeNewswire Inc. • 08/06/2024 11:30:00 AM
- Anavex Life Sciences to Announce Fiscal 2024 Third Quarter Financial Results on Tuesday, August 6, 2024 • GlobeNewswire Inc. • 08/01/2024 11:30:00 AM
- Form 8-K - Current report • Edgar (US Regulatory) • 07/30/2024 09:20:53 PM
- Anavex Life Sciences Announces Translational Biomarker Data for ANAVEX®2-73 (blarcamesine) in Fragile X Syndrome (Major Cause of Autism) at the 19th NFXF International Fragile X Conference • GlobeNewswire Inc. • 07/30/2024 11:30:00 AM
- Form S-3 - Registration statement under Securities Act of 1933 • Edgar (US Regulatory) • 07/29/2024 09:21:49 PM
- Results from Anavex Life Sciences Landmark Phase IIb/III Trial of Blarcamesine Presented at Alzheimer's Association Conference • GlobeNewswire Inc. • 07/28/2024 09:00:00 PM
- Anavex Life Sciences to Present at the H.C. Wainwright 5th Annual Neuro Perspectives Virtual Conference • GlobeNewswire Inc. • 06/20/2024 11:30:00 AM
- Form 4 - Statement of changes in beneficial ownership of securities • Edgar (US Regulatory) • 06/17/2024 11:30:10 AM
- Anavex Life Sciences Announces Expansion of Leadership Team • GlobeNewswire Inc. • 05/22/2024 11:30:00 AM
- Form 4 - Statement of changes in beneficial ownership of securities • Edgar (US Regulatory) • 05/17/2024 10:01:00 AM
- Anavex Life Sciences to Present at the H.C. Wainwright 2nd BioConnect Investor Conference at NASDAQ • GlobeNewswire Inc. • 05/14/2024 11:30:00 AM
- Shareholders that lost money on Anavex Life Sciences Corporation(AVXL) Urged to Join Class Action - Contact The Gross Law Firm to Learn More • PR Newswire (US) • 05/10/2024 09:45:00 AM
- Form 10-Q - Quarterly report [Sections 13 or 15(d)] • Edgar (US Regulatory) • 05/09/2024 08:35:55 PM
- Form 8-K - Current report • Edgar (US Regulatory) • 05/09/2024 12:00:30 PM
- Anavex Life Sciences Reports Fiscal 2024 Second Quarter Financial Results and Provides Business Update • GlobeNewswire Inc. • 05/09/2024 11:30:00 AM
- The Gross Law Firm Announces the Filing of a Securities Class Action on Behalf of Anavex Life Sciences Corporation(AVXL) Shareholders • PR Newswire (US) • 05/07/2024 09:45:00 AM
- Form DEFA14A - Additional definitive proxy soliciting materials and Rule 14(a)(12) material • Edgar (US Regulatory) • 05/06/2024 10:03:33 AM
CBD Life Sciences Inc. (CBDL) Launches High-Demand Mushroom Gummy Line for Targeted Wellness Needs, Tapping into a Booming $20 Billion Market • CBDL • Oct 31, 2024 8:00 AM
Nerds On Site Announces Q1 Growth and New Initiatives for the Remainder of 2024 • NOSUF • Oct 31, 2024 7:01 AM
Innovation Beverage Group Receives Largest Shipment of its Top-Selling Bitters to Date in the U.S.-Ready to Meet Growing Demand from Expanding Distribution Network • IBG • Oct 30, 2024 12:22 PM
Element79 Gold Corp to Update Investors on the Emerging Growth Conference on October 31, 2024 • ELMGF • Oct 30, 2024 9:08 AM
CBD Life Sciences Inc. (CBDL) Announces Grand View Research Report Findings on High - Growth CBD Equine Market, Aiming to Drive Unprecedented Shareholder Value • CBDL • Oct 29, 2024 10:19 AM
Integrated Ventures Announces Partnership And Lease Agreement with Driptide Wellness - Leading Health and Wellness Provider. • INTV • Oct 29, 2024 8:45 AM








