Saturday, January 28, 2017 1:32:25 PM
EXOSOME-BASED CANCER DETECTION & MONITORING TECHNOLOGY ("Liquid Biopsy")
...Excellent Exosome (aka microparticles, microvesicles) info: http://www.exosome-rna.com
7-14-16: Peregrine Licenses Exosome-based technology from UTSW (Inventors: Alan Schroit/Philip Thorpe) http://tinyurl.com/zszd4fj
...“relates to assays that are able to detect small amts of PS+ Exosomes in a patient's blood sample as a way to detect cancer at a very early stage of development.”
7-14-16/CC S.King PREPARED: “...In addition to bavituximab program which continues to have tremendous potential value, the company announced earlier today that it has licensed-in a Novel PS Exosome Technology, with the potential to detect & monitor cancer at an early stage through a simple blood test [ 7-14-16: http://tinyurl.com/zszd4fj ]. I recognize that some of you might think this theme is contrary to controlling spending as we move toward profitability, but in fact we believe that given our already existing knowledge base in Targeting PS and our already available infrastructure for developing & validating tests, that so a very modest capital investment we can quickly reach proof-of-concept with a goal of partnering this technology, which could then bring in addl. revenue, with another potential upside of the technology being that it can possibly be useful in the continued dev. of bavituximab. Jeff will talk more about this during his prepared remarks. Given the strategy of R&D targeted toward early partnering, will allow the company to continue its R&D activities with significant upside coming from partnering as we move to our profitability.” http://tinyurl.com/h8eqtg5
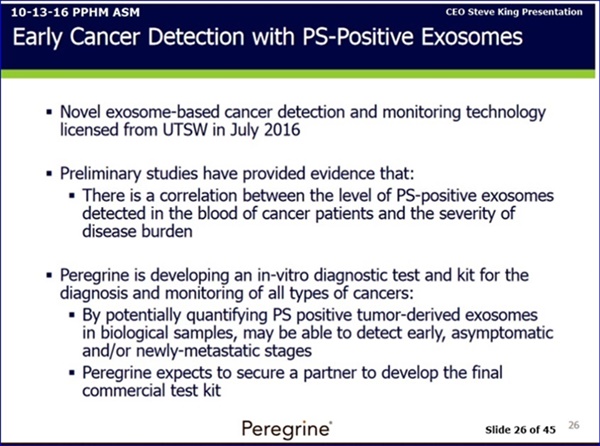
7-14-16/CC J.Hutchins PREPARED: “I'm very happy to be able to discuss a number of exciting developments in our preclinical group. First, as Steve discussed, we have executed the licensing agreement with UT Southwestern Medical Center, for novel exosome technology [ 7-14-16: http://tinyurl.com/zszd4fj ] . While many of you are familiar with exosomes, I’ll provide a brief overview for those who are not. Exosomes are cell-secreted vesicles, or mini-cells if you will, that are present in nearly all bodily fluids, including blood. Likewise, tumor-derived exosomes represent small pieces of tumor cells that are released into the blood as tumors grow. As well, these tumor-derived exosomes have Phosphatidylserine or PS on their surface as a marker and can also contain DNA, RNA and proteins as markers of malignant disease. It is believed that even small tumors begin to release PS-positive exosomes, and thus the ability to detect these exosomes in the blood may be an indicator of presence or progression of a tumor. The licensed technology is designed to detect & monitor PS-positive exosomes in a patient's blood sample, providing clinicians with detection & monitoring information regarding the presence & prevalence of cancer. These exosomes have PS flipped to the outside of the surface and demonstrate immunosuppressive activity, just as we find with tumor cells. Preliminary studies have demonstrated that the levels of PS-positive exosomes present in the blood of cancer patients are higher than levels found in the blood of healthy volunteers. Furthermore, study findings also suggest that there is a correlation between the level of PS-positive exosomes that are detected in the blood of cancer patients and the severity or extent of their disease burden. Given our in-house expertise in PS-targeting, we believe that we are uniquely qualified to advance this technology. As Steve stated, there are significant opportunities to use this technology as both a complimentary tool in bavituximab's ongoing development, which Joe will address later, as well as more broadly as the basis for a novel cancer detection & monitoring test kit that will be the focus of our partnering efforts. It is our goal to develop, optimize, and validate a functional detection & monitoring assay capable of detecting PS-positive exosomes from a simple blood sample, and, given the company's extensive experience in developing assays of this type, we do not anticipate the need for added personnel or any specialized equipment for this project. Once we have successfully validated this assay, we plan to establish proof-of-concept through an efficient preclinical & clinical testing program. We have no intention of conducting further development work beyond the proof-of-concept stage. Rather, we expect to initiate partnering discussions for commercialization of this program in 2017. We're very excited to begin this work on this new program and we'll have more details to offer in the coming months.”
...SUMMURY: “And lastly, we've in-licensed a new exosome technology for a minimal cost that leverages our existing in-house expertise and provides us with another opportunity for us to create value to product development. Together, we believe the strategy will provide success, as it will allow us to focus the majority of our resources on achieving our primary corporate goal, future sustainable profitability within 24mos. At same time, we will focus our R&D efforts on small early stage trials and development of the exosome technology in an effort to attract partners. We believe this strategy will allow us to build near-term revenues through Avid, while maintaining the potential for significant addl. value creation associated with our R&D efforts.” http://tinyurl.com/h8eqtg5
7-14-16/CC J.Shan PREPARED: “I'd first like to comment on our new exosome program. One of the most exciting aspects of this technology is the potential synergy that it offers with our bavituximab clinical dev. program. Through our ongoing work with bavituximab, we have gained significant understanding of PS-mediated immuno-suppression in cancer. The availability of the PS specific biomarker, which can be implemented in our planned future bavituximab clinical trials, aligns nicely with our refocused bavituximab dev. strategy aimed at generating the most meaningful data possible from small, early stage clinical trials to support partnering efforts. We are very anxious to bring this new technology to Peregrine and we look forward to the value it brings to our bavituximab program.” http://tinyurl.com/h8eqtg5
7-14-16/CC Q&A/Pantginis – SK: “(On the Exosome pgm), …it fits right in; we’re not having to hire addl. people, we’re not having to bring in addl. equipment. This really fits in with everything we are doing on both studying PS, as well as on the assay development side of the business. We think it’s actually going to be complementary. There are other technologies out there looking at exosomes; they're are all taking a very different approach to what we’re doing and we actually think they could be very complementary to each other. We also see a need, even as interest in exosomes begins to pick up, to actually utilize this in conjunction with other things that are in development.” http://tinyurl.com/h8eqtg5
7-14-16/CC Q&A/T.Yip – SK: “(On the Exosome pgm), the next step will be to validate that through patient samples. The beauty of this is that while you do need IRB approval of course, you’re not running really clinical trials, so this can be done in conjunction with either our ongoing trials or partners' trials, or there are many other sources of just receiving these types of blood samples. This gives us the ability to very quickly go through and test hundreds or thousands of patient samples as part of the validation process. At that point, we can zero in on what are the potential applications of the technology, outside of what we might do with our own PS Targeting programs – what would be the potential utility of this for patients. Our goal is not to become a diagnostics company, but to put this in the hands of a good organization that's already established in the diagnostics area and then have them finish up the commercialization and expansion of the utility of the actual assay itself. Our benefit at that point would become, hopefully, some residual royalties, milestones and what have you, which feeds back into our revenue goals of becoming profitable. So, we thought that this is a very attractive technology that just fits right in with what we’re doing and requires almost no addl. resources whatsoever…
Q2:/TY: ”when should we expect to see more preclinical data on this front?”… SK: “Our goal is, probably towards for the end of this year, to be in a position to have data that we can present. That will come in a lot of different formats, so it will be in conjunction with other ongoing studies that maybe taking place already to be standalone just on the diagnostic itself. So, you'll be hearing a lot about this, and one of the reasons when to get this news out there is because what you think sooner than later we will build a talk about this technology.” http://tinyurl.com/h8eqtg5
9-8-16/PR: “Peregrine in-licensed a novel exosome technology from UTSW that has potential for cancer detection and monitoring applications. This technology aligns directly with the company's expertise, its proprietary PS-targeting platform and the bavituximab development program. As such, there are opportunities to use this technology as both a complementary tool in bavituximab's ongoing development, as well as more broadly as the basis for novel cancer detection & monitoring tests that can be the focus of partnering efforts.” http://tinyurl.com/jydtkoy
9-8-16/CC J.Hutchins PREPARED: “1st, an an update on our PS Exosome Program. As we announced in July, we executed a licensing agreement with UT Southwestern Medical Center for a novel exosome technology designed to detect PS-positive exosomes in a patient blood sample. It is our belief that this technology can provide clinicians with detection & monitoring information regarding the presence and the prevalence of cancer. Preliminary indep. studies have demonstrated that the levels of PS-positive exosomes present in the blood of cancer patients are higher than levels found in the blood of healthy volunteers. Furthermore, study findings also suggest that there is a correlation between the level of PS positive exosomes detected in the blood of cancer patients and the severity and extent of their disease burden. Given our in-house expertise in PS targeting, we believe that we are uniquely qualified to advance this technology. We believe there are significant opportunities to use this technology as both a complementary tool in bavituximab's ongoing development as well as more broadly as the basis for a novel cancer detection & monitoring test kit that will be the focus of partnering efforts. It is our goal to develop, optimize and validate a functional screening assay capable of detecting PS positive exosomes in a blood sample and to initiate partnering discussions for commercialization of the pgm in 2017. We are on track to achieve this goal and we look forward to providing further updates.” http://tinyurl.com/jydtkoy
10-13-16/ASM REPORT BY ATTENDEE COPPER888: “...I think there is a shift on how the company execs and BOD view the business. As mentioned by multiple posters, SK said that they are still looking to hit a "homerun" with Bavi. But I think that they are now doing that in a framework of risk avoidance, and profitability as their primary goals. With every initiative mentioned, SK would talk about partnering in the next sentence. Exosome testing with a partner; potential of exploring the utility of Beta Bodies - would "advance aggressively with a partner"; If Sunrise data warrants a small study to confirm, they would "partner the next step", etc. I think that for good or bad...the new company directive is the march toward profitability. He also said that the company is worth multiples of its current market cap and that they want to delay the RS as much as they can. "I am focused on getting the Share price over a dollar" He mentioned that there may be many events between now and April that may get us there...” http://tinyurl.com/jx7ouay
12-12-16/CC: No mention of Exosomes!
1-22-17: OncoTarget article on PS-Exosomes: PPHM SAB’r Dr. Alan Schroit (UTSW): Proof-of-Concept data http://tinyurl.com/hv8dcpd
...Data (blinded plasma from 34 O.C. pts & 10 healthy subjects) supports the “high diagnostic power” of PS+ Exosomes in Ovarian Malignancies. “The data (Fig.4) show that quantification of PS-exosomes in blood distinguishes, with 100% accuracy, healthy tumor-free individuals from patients with ovarian malignancies.”
EXCERPTS FROM 1-22-17 ONCOTARGET ARTICLE:
**From DISCUSSION (pg. 8): “The data summarized in Fig. 4 show that quantification of PS-exosomes in blood distinguishes, with 100% accuracy, healthy tumor-free individuals from patients with ovarian malignancies… In summary, this study provides proof-of-concept data that supports the high diagnostic power of PS-expressing tumor exosome detection in blood from women with suspect ovarian malignancies. Ultimately, these studies could lead to earlier stage diagnosis, substantial cost savings, reduced patient exposure to radiation and invasive procedures, and improved clinical outcomes. The assay might also find utility in patients with radiographic abnormalities, even before clinical detection. Indeed, an accurate biomarker predicting the likelihood of malignancy would be extremely beneficial to such a population since they often face long periods of anxiety and uncertainty inherent to a “wait & watch” approach. Finally, if PS-exosome diagnostics are confirmed in a large study to be an accurate and reproducible biomarker of ovarian malignancies, the assay could be applied to the early detection of other visceral malignancies."
**From METHODS (pg.9): “Expression of an engineered tetravalent antibody for PS-detection Monoclonal 1N11 is a human IgG1^ that binds PS through the PS-specific plasma protein B2GP1. A tetravalent variant of 1N11 (1N11-T), with 4 binding sites per molecule was designed to generate a high avidity PS binding agent (Fig.1).”
……...[NOTE: 1N11 is Fully-Human Bavituximab (aka PGN635=AT004), B2GPI-dep. Binding]
**From FINANCIAL SUPPORT (pg.11): “Supported by Cancer Prevention & Res. Inst. of TX (CPRIT) Grant #RP110441, and a Simmons CC Support Grant 5P30 CA142543.”
**EDITORIAL NOTE (pg.11): “This paper has been accepted based in part on peer-review conducted by another journal and the authors’ response & revisions as well as expedited peer-review in Oncotarget."
Recent CDMO News
- Avid Bioservices Earns Committed Badge from EcoVadis for Sustainability Performance • GlobeNewswire Inc. • 05/23/2024 12:05:46 PM
- Avid Bioservices to Participate at Upcoming Investor Conferences • GlobeNewswire Inc. • 05/07/2024 08:05:11 PM
- Avid Bioservices Reports Financial Results for Third Quarter Ended January 31, 2024 • GlobeNewswire Inc. • 04/24/2024 09:25:33 PM
- Avid Bioservices Announces Receipt of Deficiency Notice from Nasdaq Regarding Late Form 10-Q • GlobeNewswire Inc. • 03/20/2024 11:00:10 AM
- Form 8-K - Current report • Edgar (US Regulatory) • 03/07/2024 11:30:11 AM
- Avid Bioservices Announces Pricing of Private Placement of Convertible Notes • GlobeNewswire Inc. • 03/07/2024 04:58:48 AM
- Avid Bioservices Announces Proposed Private Placement of Convertible Notes • GlobeNewswire Inc. • 03/06/2024 09:32:07 PM
- Avid Bioservices Announces Certain Preliminary Financial Results for Third Quarter Ended January 31, 2024 • GlobeNewswire Inc. • 03/06/2024 09:31:28 PM
- Form 8-K - Current report • Edgar (US Regulatory) • 03/06/2024 09:30:18 PM
- Form SC 13G/A - Statement of acquisition of beneficial ownership by individuals: [Amend] • Edgar (US Regulatory) • 01/26/2024 09:57:52 PM
- Form 4 - Statement of changes in beneficial ownership of securities • Edgar (US Regulatory) • 01/13/2024 12:34:35 AM
- Form 4 - Statement of changes in beneficial ownership of securities • Edgar (US Regulatory) • 01/12/2024 12:39:18 AM
- Form 4 - Statement of changes in beneficial ownership of securities • Edgar (US Regulatory) • 01/12/2024 12:38:30 AM
- Form 4 - Statement of changes in beneficial ownership of securities • Edgar (US Regulatory) • 01/12/2024 12:37:38 AM
- Form 4 - Statement of changes in beneficial ownership of securities • Edgar (US Regulatory) • 01/12/2024 12:36:27 AM
- Form 4 - Statement of changes in beneficial ownership of securities • Edgar (US Regulatory) • 01/12/2024 12:35:47 AM
- Form 4 - Statement of changes in beneficial ownership of securities • Edgar (US Regulatory) • 01/11/2024 12:56:02 AM
- Form SC 13G/A - Statement of acquisition of beneficial ownership by individuals: [Amend] • Edgar (US Regulatory) • 01/08/2024 09:32:36 PM
- Form 4 - Statement of changes in beneficial ownership of securities • Edgar (US Regulatory) • 01/04/2024 12:56:18 AM
- Form 4 - Statement of changes in beneficial ownership of securities • Edgar (US Regulatory) • 01/04/2024 12:55:07 AM
- Form 4 - Statement of changes in beneficial ownership of securities • Edgar (US Regulatory) • 01/04/2024 12:53:58 AM
- Form 4 - Statement of changes in beneficial ownership of securities • Edgar (US Regulatory) • 01/04/2024 12:51:57 AM
- Form SC 13G - Statement of acquisition of beneficial ownership by individuals • Edgar (US Regulatory) • 12/19/2023 09:05:52 PM
- Form 4 - Statement of changes in beneficial ownership of securities • Edgar (US Regulatory) • 12/19/2023 12:34:08 AM
North Bay Resources Announces Composite Assays of 0.53 and 0.44 Troy Ounces per Ton Gold in Trenches B + C at Fran Gold, British Columbia • NBRI • Jun 18, 2024 9:18 AM
VAYK Assembling New Management Team for $64 Billion Domestic Market • VAYK • Jun 18, 2024 9:00 AM
Fifty 1 Labs, Inc Announces Acquisition of Drago Knives, LLC • CAFI • Jun 18, 2024 8:45 AM
Hydromer Announces Attainment of ISO 13485 Certification • HYDI • Jun 17, 2024 9:22 AM
ECGI Holdings Announces LOI to Acquire Pacific Saddlery to Capitalize on $12.72 Billion Market Potential • ECGI • Jun 13, 2024 9:50 AM
Fifty 1 Labs, Inc. Announces Major Strategic Advancements and Shareholder Updates • CAFI • Jun 13, 2024 8:45 AM










