Thursday, January 26, 2017 7:12:13 PM
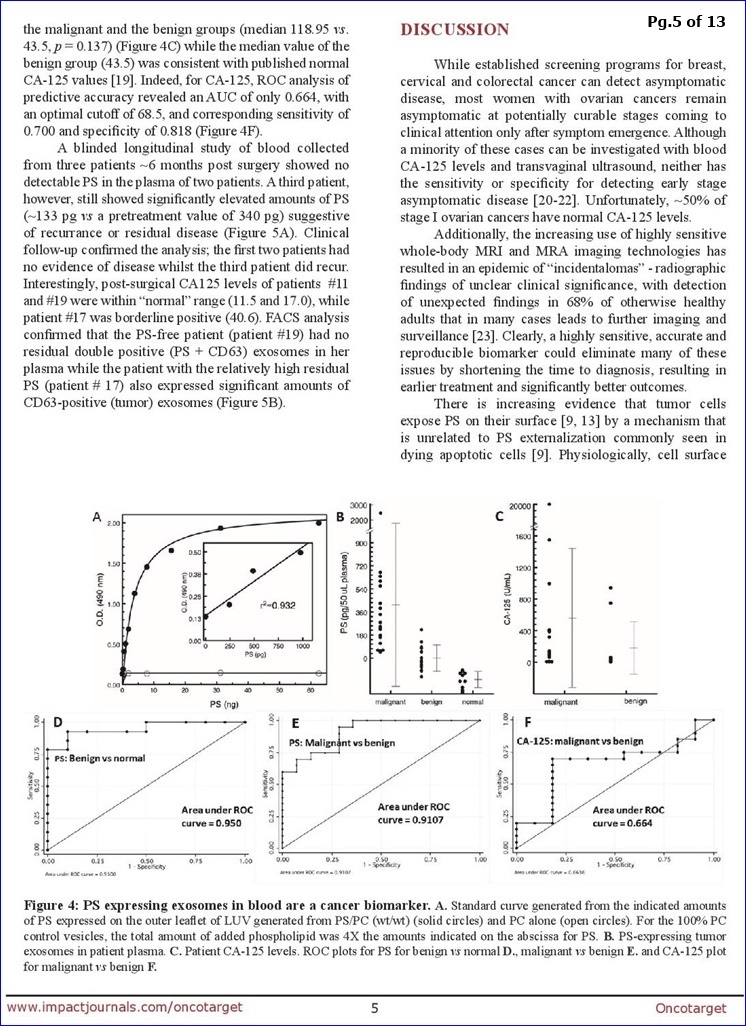
FROM PG.8: “The data summarized in Fig. 4 show that quantification of PS-exosomes in blood distinguishes, with 100% accuracy, healthy tumor-free individuals from patients with ovarian malignancies.”
1-22-17 Oncotarget: “Detection Of Phosphatidylserine-Positive Exosomes as a Diagnostic Marker for Ovarian Malignancies: A Proof of Concept Study”
Lea J 1, Sharma R 2, Yang F 2, Zhu H 1, Ward ES 3,4, Alan J. Schroit (PPHM SAB, UTSW Profile: http://tinyurl.com/jlrkxma ) 1,3
1 Harold Simmons Comprehensive CC, UTSW-MC/Dallas
2 Hamon Center for Therapeutic Oncology Res., UTSW-MC/Dallas
3 Dept of Immunology, UTSW-MC/Dallas
4 Dept of Molecular & Cellular Medicine, Texas A&M Univ. Health Science Ctr
https://www.ncbi.nlm.nih.gov/pubmed/28122335
ABSTRACT
There are no suitable screening modalities for ovarian carcinomas (OC) and repeated imaging and CA-125 levels are often needed to triage equivocal ovarian masses. Definitive diagnosis of malignancy, however, can only be established by histologic confirmation. Thus, the ability to detect OC at early stages is low, and most cases are diagnosed as advanced disease. Since tumor cells expose phosphatidylserine (PS) on their plasma membrane, we predicted that tumors might secrete PS-positive exosomes into the bloodstream that could be a surrogate biomarker for cancer. To address this, we developed a highly stringent ELISA that detects picogram quantities of PS in patient plasma. Blinded plasma from 34 suspect ovarian cancer patients and 10 healthy subjects were analyzed for the presence of PS-expressing vesicles. The nonparametric Wilcoxon rank sum test showed the malignant group had significantly higher PS values than the benign group (median 0.237 vs. -0.027, p=0.0001) and the malignant and benign groups had significantly higher PS values than the healthy group (median 0.237 vs -0.158, p<0.0001 and -0.027 vs -0.158, p=0.0002, respectively). ROC analysis of the predictive accuracy of PS-expressing exosomes/vesicles in predicting malignant against normal, benign against normal & malignant against benign revealed AUCs of 1.0, 0.95 and 0.911, respectively. This study provides proof-of-concept data that supports the high diagnostic power of PS detection in the blood of women with suspect ovarian malignancies.
FULL ARTICLE (13pgs):
HTML: http://www.impactjournals.com/oncotarget/index.php?journal=oncotarget&page=article&op=view&path%5B%5D=14795&path%5B%5D=47251
PDF: http://www.impactjournals.com/oncotarget/index.php?journal=oncotarget&page=article&op=download&path%5B%5D=14795&path%5B%5D=47248
EXCERPTS FROM 1-22-17 ONCOTARGET ARTICLE:
**From DISCUSSION (pg. 8): “The data summarized in Fig. 4 show that quantification of PS-exosomes in blood distinguishes, with 100% accuracy, healthy tumor-free individuals from patients with ovarian malignancies… In summary, this study provides proof-of-concept data that supports the high diagnostic power of PS-expressing tumor exosome detection in blood from women with suspect ovarian malignancies. Ultimately, these studies could lead to earlier stage diagnosis, substantial cost savings, reduced patient exposure to radiation and invasive procedures, and improved clinical outcomes. The assay might also find utility in patients with radiographic abnormalities, even before clinical detection. Indeed, an accurate biomarker predicting the likelihood of malignancy would be extremely beneficial to such a population since they often face long periods of anxiety and uncertainty inherent to a “wait & watch” approach. Finally, if PS-exosome diagnostics are confirmed in a large study to be an accurate and reproducible biomarker of ovarian malignancies, the assay could be applied to the early detection of other visceral malignancies."
**From METHODS (pg.9): “Expression of an engineered tetravalent antibody for PS-detection Monoclonal 1N11 is a human IgG1^ that binds PS through the PS-specific plasma protein B2GP1. A tetravalent variant of 1N11 (1N11-T), with 4 binding sites per molecule was designed to generate a high avidity PS binding agent (Fig.1).”
……...[NOTE: 1N11 is Fully-Human Bavituximab (aka PGN635=AT004), B2GPI-dep. Binding]
**From FINANCIAL SUPPORT (pg.11): “Supported by Cancer Prevention & Res. Inst. of TX (CPRIT) Grant #RP110441, and a Simmons CC Support Grant 5P30 CA142543.”
**EDITORIAL NOTE (pg.11): “This paper has been accepted based in part on peer-review conducted by another journal and the authors’ response & revisions as well as expedited peer-review in Oncotarget."
= = = = = = =
EXOSOME-BASED CANCER DETECTION & MONITORING TECHNOLOGY ("Liquid Biopsy") - Excellent Exosome info: http://www.exosome-rna.com
7-14-16: Peregrine Licenses Exosome-based technology from UTSW (Inventors: Alan Schroit/Philip Thorpe) http://tinyurl.com/zszd4fj
...“relates to assays that are able to detect small amts of PS+ Exosomes in a patient's blood sample as a way to detect cancer at a very early stage of development.”
10-13-16 ASM REPORT BY ATTENDEE COPPER888: http://tinyurl.com/jx7ouay
“...I think there is a shift on how the company execs and BOD view the business. As mentioned by multiple posters, SK said that they are still looking to hit a "homerun" with Bavi. But I think that they are now doing that in a framework of risk avoidance, and profitability as their primary goals. With every initiative mentioned, SK would talk about partnering in the next sentence. Exosome testing with a partner; potential of exploring the utility of Beta Bodies - would "advance aggressively with a partner"; If Sunrise data warrants a small study to confirm, they would "partner the next step", etc. I think that for good or bad...the new company directive is the march toward profitability. He also said that the company is worth multiples of its current market cap and that they want to delay the RS as much as they can. "I am focused on getting the Share price over a dollar" He mentioned that there may be many events between now and April that may get us there...”
PDF EXCERPTS 1-22-17/OncoTarget/Schroit/PPHM-Exosomes (5 pgs of the 13):




Recent CDMO News
- Avid Bioservices Earns Committed Badge from EcoVadis for Sustainability Performance • GlobeNewswire Inc. • 05/23/2024 12:05:46 PM
- Avid Bioservices to Participate at Upcoming Investor Conferences • GlobeNewswire Inc. • 05/07/2024 08:05:11 PM
- Avid Bioservices Reports Financial Results for Third Quarter Ended January 31, 2024 • GlobeNewswire Inc. • 04/24/2024 09:25:33 PM
- Avid Bioservices Announces Receipt of Deficiency Notice from Nasdaq Regarding Late Form 10-Q • GlobeNewswire Inc. • 03/20/2024 11:00:10 AM
- Form 8-K - Current report • Edgar (US Regulatory) • 03/07/2024 11:30:11 AM
- Avid Bioservices Announces Pricing of Private Placement of Convertible Notes • GlobeNewswire Inc. • 03/07/2024 04:58:48 AM
- Avid Bioservices Announces Proposed Private Placement of Convertible Notes • GlobeNewswire Inc. • 03/06/2024 09:32:07 PM
- Avid Bioservices Announces Certain Preliminary Financial Results for Third Quarter Ended January 31, 2024 • GlobeNewswire Inc. • 03/06/2024 09:31:28 PM
- Form 8-K - Current report • Edgar (US Regulatory) • 03/06/2024 09:30:18 PM
- Form SC 13G/A - Statement of acquisition of beneficial ownership by individuals: [Amend] • Edgar (US Regulatory) • 01/26/2024 09:57:52 PM
- Form 4 - Statement of changes in beneficial ownership of securities • Edgar (US Regulatory) • 01/13/2024 12:34:35 AM
- Form 4 - Statement of changes in beneficial ownership of securities • Edgar (US Regulatory) • 01/12/2024 12:39:18 AM
- Form 4 - Statement of changes in beneficial ownership of securities • Edgar (US Regulatory) • 01/12/2024 12:38:30 AM
- Form 4 - Statement of changes in beneficial ownership of securities • Edgar (US Regulatory) • 01/12/2024 12:37:38 AM
- Form 4 - Statement of changes in beneficial ownership of securities • Edgar (US Regulatory) • 01/12/2024 12:36:27 AM
- Form 4 - Statement of changes in beneficial ownership of securities • Edgar (US Regulatory) • 01/12/2024 12:35:47 AM
- Form 4 - Statement of changes in beneficial ownership of securities • Edgar (US Regulatory) • 01/11/2024 12:56:02 AM
- Form SC 13G/A - Statement of acquisition of beneficial ownership by individuals: [Amend] • Edgar (US Regulatory) • 01/08/2024 09:32:36 PM
- Form 4 - Statement of changes in beneficial ownership of securities • Edgar (US Regulatory) • 01/04/2024 12:56:18 AM
- Form 4 - Statement of changes in beneficial ownership of securities • Edgar (US Regulatory) • 01/04/2024 12:55:07 AM
- Form 4 - Statement of changes in beneficial ownership of securities • Edgar (US Regulatory) • 01/04/2024 12:53:58 AM
- Form 4 - Statement of changes in beneficial ownership of securities • Edgar (US Regulatory) • 01/04/2024 12:51:57 AM
- Form SC 13G - Statement of acquisition of beneficial ownership by individuals • Edgar (US Regulatory) • 12/19/2023 09:05:52 PM
- Form 4 - Statement of changes in beneficial ownership of securities • Edgar (US Regulatory) • 12/19/2023 12:34:08 AM
Last Shot Hydration Drink Announced as Official Sponsor of Red River Athletic Conference • EQLB • Jun 20, 2024 2:38 PM
ATWEC Announces Major Acquisition and Lays Out Strategic Growth Plans • ATWT • Jun 20, 2024 7:09 AM
North Bay Resources Announces Composite Assays of 0.53 and 0.44 Troy Ounces per Ton Gold in Trenches B + C at Fran Gold, British Columbia • NBRI • Jun 18, 2024 9:18 AM
VAYK Assembling New Management Team for $64 Billion Domestic Market • VAYK • Jun 18, 2024 9:00 AM
Fifty 1 Labs, Inc Announces Acquisition of Drago Knives, LLC • CAFI • Jun 18, 2024 8:45 AM
Hydromer Announces Attainment of ISO 13485 Certification • HYDI • Jun 17, 2024 9:22 AM










