Thursday, November 05, 2015 4:22:35 PM
Nov4-8 2015: “(SITC) Society for Immunotherapy of Cancer 30th Annual Meeting”, Natl-Harbor MD
”The premier destination for scientific exchange, education, and networking in the Cancer Immunotherapy Field”
SITC = The Society for Immunotherapy of Cancer http://www.sitcancer.org
SITC 2015 Meeting: http://www.sitcancer.org/2015
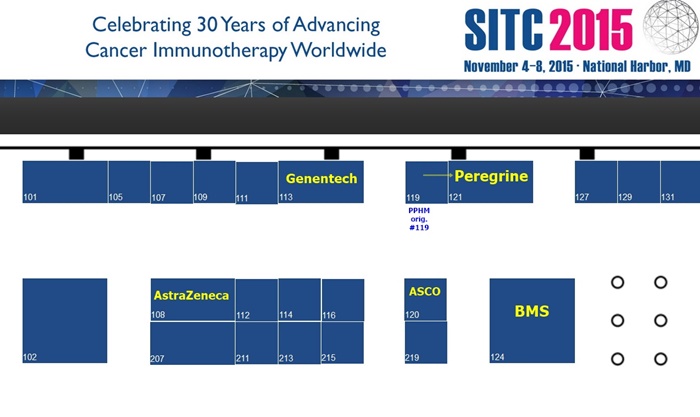
SITC'15 PPHM's Booth #121...
NOTE: Join us for a Scientific Session - FRI Nov6 2015 7:30-9:00pm
“The PS Signaling Pathway: A Promising
Therapeutic Target Exploited by
Tumors for Immune Evasion”
PGM:
* Raymond Birge (PhD, Rutgers) http://birgelab.org
* Douglas Graham (MD, PhD, Emory) http://choa.org/Childrens-Hospital-Services/Cancer-and-Blood-Disorders/Meet-the-Team/Physicians-and-Researchers/Douglas-Graham
* Dmitry Gabrilovich (MD, PhD, Wistar Inst.) http://www.wistar.org/our-science/scientists/dmitry-gabrilovich-md-phd
* Rolf Brekken (PhD, UTSW/Dallas) http://www.utsouthwestern.edu/labs/brekken
* Maria Karasarides (PhD, AstraZeneca - Sr. Director, ImmunoOncology, Global Medicines Dev.) http://www.linkedin.com/pub/maria-karasarides-ph-d/6/769/136
= = = = = = = = = = = = = = = = = =
SITC’15(Nov4-8): Peregrine-Exhibiting; 2 Posters(UTSW/DUKE/PPHM), “Optimizing Combination Immunotherapy” by UTSW's X.Huang/Rolf Brekken/etal, DUKE's Dr.Kim Lyerly/etal, PPHM Scientists…
[Note: Duke's Dr. Kim Lyerly is the senior author of Poster P357]
Nov4-8 2015: “(SITC) Society for Immunotherapy of Cancer 30th Annual Meeting”, Natl-Harbor MD
”The premier destination for scientific exchange, education, and networking in the Cancer Immunotherapy Field”
SITC = The Society for Immunotherapy of Cancer http://www.sitcancer.org
SITC 2015 Meeting: http://www.sitcancer.org/2015
EXHIBITOR: Peregrine Pharm. - booth #121 (directly across from #120/ASCO & BMY/124; AstraZeneca/108 & DNA/113 are close by)
...Floorplan: http://www.eventscribe.com/2015/sitc/exhibitors/index.asp
- - - - - - - -
Track: “Optimizing Combination Immunotherapy”
11-7-15/Sat./12:45-2:00pm
PPHM#1: Poster P358, “Targeting Phosphatidylserine Synergizes with Immune Checkpoint Blockade by Inducing De Novo Tumor Specific Immunity”
Presenting Author: Xianming Huang, PhD (UTSW-MC/Dallas)
Xianming Huang 1, Jian Gong 2, Dan Ye 1, Van Nguyen 2, Michael Gray 2, Steven King 2, Jeff Hutchins 2, Rolf Brekken 1, Bruce Freimark 2
...1 UTSW-MC/Dallas
...2 Peregrine Pharmaceuticals, Tustin CA
Direct Link: http://bit.ly/1LS4VYk
http://www.immunotherapyofcancer.org/content/pdf/2051-1426-3-S2-P358.pdf
BACKGROUND: Extensive studies have demonstrated that the inside-out membrane phospholipid phosphatidylserine (PS) actively drives global immunosuppression in the tumor microenvironment and is a major contributor to tumor resistance to immune checkpoint blockade. We have shown that PS targeting antibodies can re-program the tumor microenvironment from immunosuppressive to immune potentiating by reducing the number of myeloid-derived suppressor cells (MDSCs), repolarizing tumor associated macrophages from M2 to M1 and by promoting the maturation of dendritic cells. We have found that PS targeting antibodies synergize with and significantly enhance the therapeutic efficacy of immune checkpoint blockade in multiple tumor models. In the present study, we examined the effect of combination of a PS targeting antibody and anti-PD-1 or anti-CTLA-4 on tumor-specific CD8 T cell immunity.
METHODS: B16 tumor-bearing mice were treated weekly i.p. at 5 mg/kg with each antibody or combination. Splenocytes were harvested after three treatments. The number of tumor-specific IFNg-producing splenocytes was evaluated by ELISPOT in the presence or absence of irradiated B16 tumor cells. Tumors were harvested and single cell suspensions were obtained and immune profiled by FACS.
RESULTS:
In the absence of B16 tumor cell stimulation, the combination of ch1N11 and anti-PD-1 resulted in the highest number of IFNg Elispots (109+/-25), significantly better than anti-CTLA-4 (29.4±4, p < 0.005), ch1N11 (16.6+/-3.2, p < 0.001), anti-PD-1 (41.6+/-5.5, p < 0.001), and ch1N11+anti-CTLA4 (73.6+/-13.9, p < 0.05); in addition, the combination of ch1N11 and anti-CTLA-4 was significantly better than anti-CTLA-4 alone (29.4+/-4.2, p < 0.01), indicating that there were significantly more functional T cells in the spleens of mice treated with combination therapy. Importantly, stimulation with irradiated B16 tumor cells resulted in robust induction of IFNg production from splenocytes harvested from mice treated with ch1N11 and anti-PD-1. Tumor cell stimulation resulted in a >2-fold increase in IFNg Elispots (198+/- 39 vs 70+/-5, p < 0.01), which was also 13-fold higher than that of control group (15+/-2.1, p < 0.001).
These data demonstrate that combination treatment induced significantly more tumor specific T cells. FACS analysis of tumor infiltrating lymphocytes (TILs) indicated that combination of antibody-mediated blockade of PS and PD1 significantly enhanced effector function of tumor infiltrating CD8+ T cells, as demonstrated by significant increases in TILs producing IFN-g, TNFa, IL-2, granzyme B, and Ki-67.
CONCLUSIONS:
PS targeting antibodies synergize with immune checkpoint blockade to induce strong tumor specific CD8 T cell immunity.
- - - - - - - -
Track: “Optimizing Combination Immunotherapy”
11-7-15/Sat./12:45-2:00pm
PPHM#2: Poster P357, “Targeting of Phosphatidylserine by Monoclonal Antibodies Augments the Activity of Paclitaxel & anti-pd1/pd-L1 Therapy in the Murine Breast Model E0771”
Presenting Author: Michael Gray, PhD (Sr.Scientist, Peregrine Pharmaceuticals)
Michael Gray 1, Jian Gong 1, Van Nguyen 1, Takuya Osada 2, Zachary Hartman 2, Jeff Hutchins 1, Bruce Freimark 1, Kim Lyerly** 2
...1=Peregrine Pharmaceuticals, Tustin CA
...2=Duke University, Durham, NC - **Dr. Kim Lyerly: http://surgery.duke.edu/faculty/details/0117267 (Professor of Surgery, Assistant Professor in Immunology, Associate Professor of Pathology)
http://www.immunotherapyofcancer.org/content/pdf/2051-1426-3-S2-P357.pdf
INTRODUCTION: The phospholipid lipid phosphatidylserine (PS) normally resides in the inner plasma membrane leaflet in most mammalian cells, including tumor and tumor associated vascular cells. Inducers of cellular stress, such as hypoxia and oxygen radicals encountered in tumors, and treatments by cytotoxic therapies promote PS relocation to the outer leaf of the plasma membrane. In tumors this re-localization allows PS recognition by a number of receptors on myeloid and lymphoid cells in the microenvironment, promoting tumor growth and metastatic disease through the development of an immunosuppressive environment. Currently the PS-targeting antibody bavituximab is being used to treat patients with solid tumors in multiple late-stage clinical trials. Bavituximab’s anti-tumor properties are attributed in part through alleviating PS- receptor mediated immunosuppression and assisting in generating an Fc-FcR mediated pro-inflammatory response.
METHODS: Immune competent mice with E0771 induced tumors were administered either paclitaxel, or anti-PD-1/PD-L1 single agent therapy, or in triple combination with PS targeting antibody (ch1N11) to evaluate the efficacy of PS & PD-1/PD-L1 immune checkpoint inhibitors blockade in combination with cytotoxic chemotherapeutics. The levels of PS and PD-L1 expression were also evaluated on E0771 tumor cells following in vitro treatment via FACS analysis.
RESULTS: Preliminary results in multiple studies demonstrated differential sensitivity to either paclitaxel, or anti- PD-1/PD-L1 single agent therapy while inclusion of ch1N11 triple combination significantly enhanced anti-tumor efficacy over either single agent therapy. Also, FACs analysis demonstrated induction of PS & PD-L1 levels on E0771 cells following in vitro treatment with paclitaxel, suggesting that combining paclitaxel with PS and PD-1 targeting antibodies will have greater anti-tumor activity in triple combination treatments. No in vivo adverse effects were observed in animals given repeated doses of single or triple combinations.
CONCLUSION: These results support combination blockade of PS & PD-1/PD-L1 with paclitaxel to treat breast cancer.
= = = = = = = = = = = = = = = = = = = = = = = = = =
10-15-15: Peregrine & AstraZeneca Expand Collab. w/Ph2/2ndLine-NSCLC Trial, Bavi+durvalumab(MEDI4736), squamous or non-squamous… http://tinyurl.com/q79bkam
10-15-15: AstraZeneca and Peregrine Pharmaceuticals Expand Ongoing Immuno-Oncology Collaboration to Include Phase II Lung Cancer Combination Clinical Trial
Global, Randomized Phase II Trial to Evaluate Immunotherapy Combination of Peregrine's PS-Targeting Bavituximab and AstraZeneca's PD-L1 Inhibitor Durvalumab (MEDI4736) in Previously Treated NSCLC
http://ir.peregrineinc.com/releasedetail.cfm?ReleaseID=936766
TUSTIN, Oct. 15, 2015: Peregrine Pharmaceuticals, Inc. (NASDAQ:PPHM/PPHMP), a biopharmaceutical company focused on developing therapeutics to stimulate the body's immune system to fight cancer, today announced that it has expanded its ongoing cancer immunotherapy clinical collaboration with AstraZeneca to include a second, later-stage trial. The companies will now also evaluate the immunotherapy combination of Peregrine's phosphatidylserine (PS)-targeted immune-activator, bavituximab, and AstraZeneca's anti-PD-L1 immune checkpoint inhibitor, durvalumab (MEDI4736), in a global Phase II study in patients with previously treated squamous or non-squamous non-small cell lung cancer (NSCLC). The randomized Phase II trial will be conducted by Peregrine. . .
ABOUT BAVITUXIMAB: A TARGETED INVESTIGATIONAL IMMUNOTHERAPY
Bavituximab is an investigational chimeric monoclonal antibody that targets phosphatidylserine (PS). Signals from PS inhibit the ability of immune cells to recognize and fight tumors. Bavituximab, the lead compound in Peregrine's immuno-oncology development program, blocks PS to remove this immunosuppressive signal and sends an alternate immune activating signal. Targeting PS with bavituximab has been shown to shift the functions of immune cells in tumors, resulting in robust anti-tumor immune responses.
ABOUT DURVALUMAB (MEDI4736)
Durvalumab is an investigational human monoclonal antibody directed against programmed cell death ligand 1 (PD-L1). Signals from PD-L1 help tumors avoid detection by the immune system. Durvalumab blocks these signals, countering the tumor's immune-evading tactics. Durvalumab is being developed, alongside other immunotherapies, to empower the patient's immune system and attack the cancer.
= = = = = = = = = = = = = = = = = = = = = =
8-24-15: AstraZeneca & Peregrine Collaborate on Bavi+Durvalumab Ph1/1B Trial for “multiple solid tumors”…
Durvalumab=MEDI4736, an anti-PD-L1 immune checkpoint inhibitor.
AZN’s Head/I-O(Robert Iannone): “…Our partnership with Peregrine provides the opportunity to explore an exciting, novel combination that could deliver important clinical benefit to patients across a range of cancers."
8-24-15: AstraZeneca and Peregrine Pharmaceuticals to Collaborate on Immuno-Oncology Combination Clinical Trial
• Collaboration to Focus on Cancer Immunotherapy Combination of Peregrine's PS-Targeting Bavituximab and AstraZeneca's PD-L1 Inhibitor MEDI4736
http://ir.peregrineinc.com/releasedetail.cfm?ReleaseID=928488
TUSTIN, Aug. 24, 2015: AstraZeneca (NYSE:AZN) and Peregrine Pharmaceuticals, Inc. (NASDAQ:PPHM/PPHMP), a biopharmaceutical company focused on developing therapeutics to stimulate the body's immune system to fight cancer, today announced that they have entered into a cancer immunotherapy clinical trial collaboration. The collaboration will evaluate Peregrine's investigational phosphatidylserine (PS)-signaling pathway inhibitor, bavituximab, in combination with AstraZeneca's investigational anti-PD-L1 immune checkpoint inhibitor, durvalumab (MEDI4736). The planned Phase I/Ib trial will evaluate the safety and efficacy of bavituximab in combination with durvalumab in multiple solid tumors.
Recent CDMO News
- Avid Bioservices Earns Committed Badge from EcoVadis for Sustainability Performance • GlobeNewswire Inc. • 05/23/2024 12:05:46 PM
- Avid Bioservices to Participate at Upcoming Investor Conferences • GlobeNewswire Inc. • 05/07/2024 08:05:11 PM
- Avid Bioservices Reports Financial Results for Third Quarter Ended January 31, 2024 • GlobeNewswire Inc. • 04/24/2024 09:25:33 PM
- Avid Bioservices Announces Receipt of Deficiency Notice from Nasdaq Regarding Late Form 10-Q • GlobeNewswire Inc. • 03/20/2024 11:00:10 AM
- Form 8-K - Current report • Edgar (US Regulatory) • 03/07/2024 11:30:11 AM
- Avid Bioservices Announces Pricing of Private Placement of Convertible Notes • GlobeNewswire Inc. • 03/07/2024 04:58:48 AM
- Avid Bioservices Announces Proposed Private Placement of Convertible Notes • GlobeNewswire Inc. • 03/06/2024 09:32:07 PM
- Avid Bioservices Announces Certain Preliminary Financial Results for Third Quarter Ended January 31, 2024 • GlobeNewswire Inc. • 03/06/2024 09:31:28 PM
- Form 8-K - Current report • Edgar (US Regulatory) • 03/06/2024 09:30:18 PM
- Form SC 13G/A - Statement of acquisition of beneficial ownership by individuals: [Amend] • Edgar (US Regulatory) • 01/26/2024 09:57:52 PM
- Form 4 - Statement of changes in beneficial ownership of securities • Edgar (US Regulatory) • 01/13/2024 12:34:35 AM
- Form 4 - Statement of changes in beneficial ownership of securities • Edgar (US Regulatory) • 01/12/2024 12:39:18 AM
- Form 4 - Statement of changes in beneficial ownership of securities • Edgar (US Regulatory) • 01/12/2024 12:38:30 AM
- Form 4 - Statement of changes in beneficial ownership of securities • Edgar (US Regulatory) • 01/12/2024 12:37:38 AM
- Form 4 - Statement of changes in beneficial ownership of securities • Edgar (US Regulatory) • 01/12/2024 12:36:27 AM
- Form 4 - Statement of changes in beneficial ownership of securities • Edgar (US Regulatory) • 01/12/2024 12:35:47 AM
- Form 4 - Statement of changes in beneficial ownership of securities • Edgar (US Regulatory) • 01/11/2024 12:56:02 AM
- Form SC 13G/A - Statement of acquisition of beneficial ownership by individuals: [Amend] • Edgar (US Regulatory) • 01/08/2024 09:32:36 PM
- Form 4 - Statement of changes in beneficial ownership of securities • Edgar (US Regulatory) • 01/04/2024 12:56:18 AM
- Form 4 - Statement of changes in beneficial ownership of securities • Edgar (US Regulatory) • 01/04/2024 12:55:07 AM
- Form 4 - Statement of changes in beneficial ownership of securities • Edgar (US Regulatory) • 01/04/2024 12:53:58 AM
- Form 4 - Statement of changes in beneficial ownership of securities • Edgar (US Regulatory) • 01/04/2024 12:51:57 AM
- Form SC 13G - Statement of acquisition of beneficial ownership by individuals • Edgar (US Regulatory) • 12/19/2023 09:05:52 PM
- Form 4 - Statement of changes in beneficial ownership of securities • Edgar (US Regulatory) • 12/19/2023 12:34:08 AM
FEATURED DaBaby and Stunna 4 Vegas's "NO DRIBBLE" Joins Music Licensing, Inc.'s Portfolio • Jun 7, 2024 10:15 AM
Mushrooms Inc. (OTC: MSRM) Announces Significant Share Buy Back by the Board Director and New Strategic Initiatives. • MSRM • Jun 5, 2024 1:32 PM
Hydromer Announces Launch of HydroThrombX Medical Device Coating Technology • HYDI • Jun 5, 2024 10:24 AM
Dr. Michael Dent Finances $1 Million to Drive HealthLynked's Healthcare Transformation • HLYK • Jun 5, 2024 8:00 AM
Avant Technologies Enters Binding LOI to Purchase Dozens of High-Performance, Immersible, AI-Powered Servers • AVAI • Jun 5, 2024 8:00 AM
IQST - iQSTEL Announces $290 Million 2024 Annual Revenue Forecast • IQST • Jun 4, 2024 1:43 PM










