Thursday, August 27, 2015 11:34:39 AM
…See Images of Dr. Hutchins’ 29-screen Presentation Slideshow below…
8-26-15: Peregrine Pharmaceuticals Presents Data at Annual Immunotherapy and Vaccine Summit (ImVacS) Supporting Ability of Bavituximab to Mediate Anti-Tumor T Cell Responses Across Multiple Tumor Types
• Increasing Activated T Cells in Tumors Demonstrates Potential Complement to anti-PD-1 and anti-PD-L1 Checkpoint Inhibitors
• Clinical and Preclinical Studies Demonstrate Estimated Survival Curves that Plateau
http://ir.peregrineinc.com/releasedetail.cfm?ReleaseID=928906
TUSTIN, Aug. 26, 2015: Peregrine Pharmaceuticals, Inc. (NASDAQ:PPHM, PPHMP), a biopharmaceutical company focused on developing therapeutics to stimulate the body's immune system to fight cancer, today announced the presentation of a range of clinical, translational and pre-clinical study results highlighting the ability of bavituximab, Peregrine's investigational phosphatidylserine (PS)-signaling pathway inhibitor, to promote anti-tumor T cell mediated activity in several tumor types. The data were presented today by Jeff T. Hutchins, Ph.D., VP, Preclinical Research at Peregrine Pharmaceuticals and chairperson of the Combination Immunotherapy Strategies session at the 10th Annual Immunotherapy and Vaccine Summit (ImVacS), being held August 24-28, 2015 in Boston, Massachusetts.
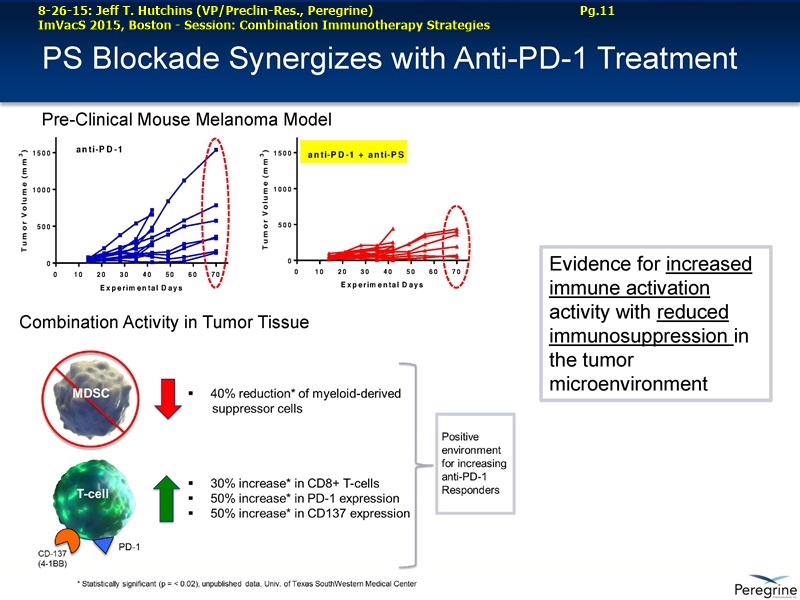
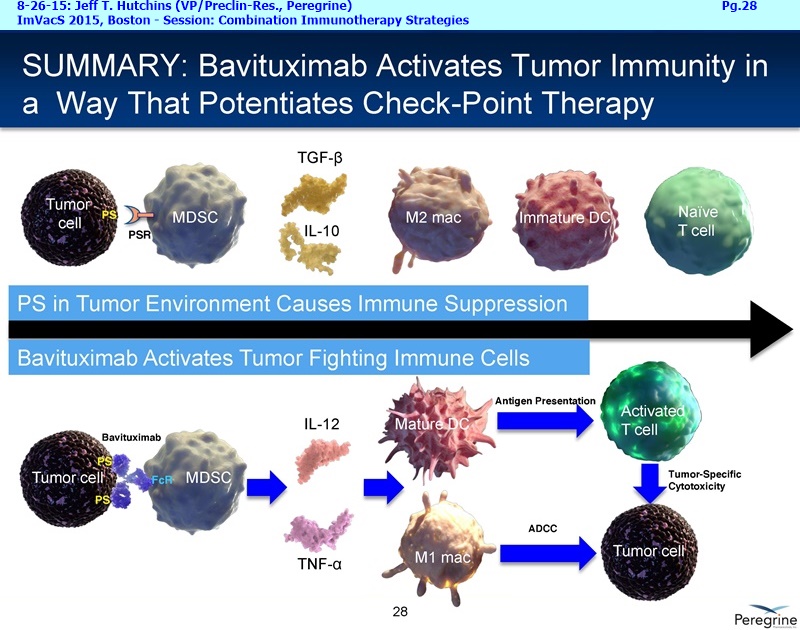
Bavituximab is an investigational immunotherapy designed to assist the body's immune system by targeting and modulating the activity of phosphatidylserine (PS), a highly immune-suppressive signaling molecule expressed broadly on the surface of cells in the tumor microenvironment. Peregrine's PS signaling pathway inhibitor candidates, including bavituximab, reverse the immunosuppressive environment that many tumors establish in order to proliferate and fight cancer by activating macrophages and cytotoxic T cells in tumors. Preclinical data demonstrate that combining the enhanced T cell anti-tumor activity of bavituximab-like antibodies with checkpoint inhibitors, such as anti-PD-1 antibodies, results in significantly improved tumor control in multiple models of cancer.
Dr. Hutchins' presentation highlighted key findings from several recent bavituximab-focused studies including:
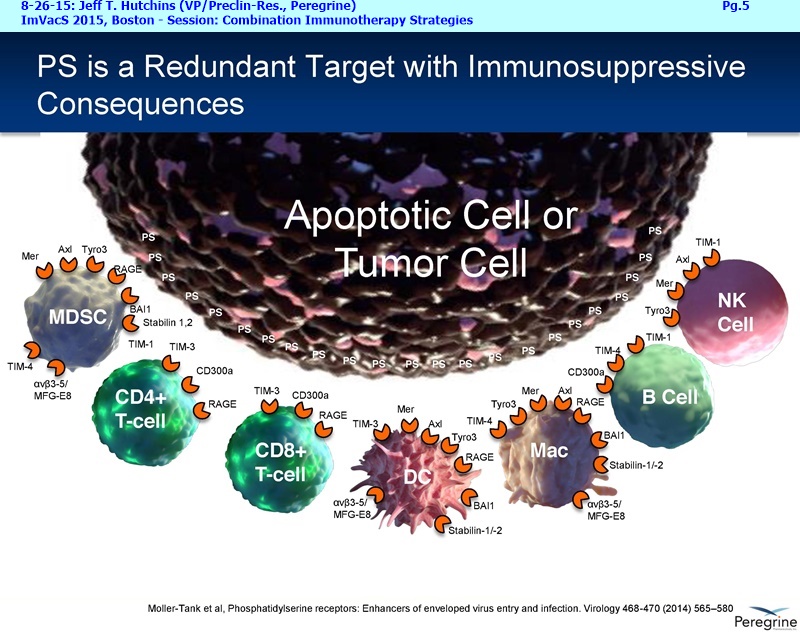
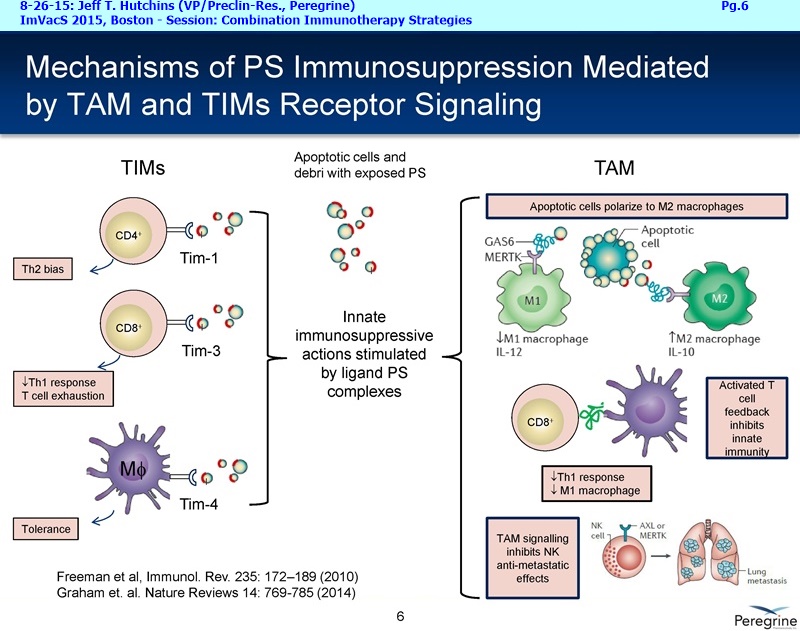
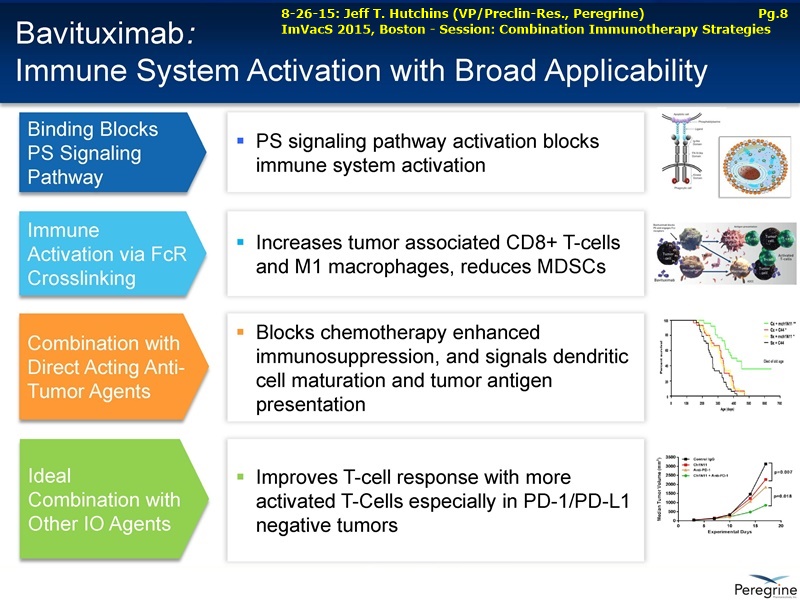
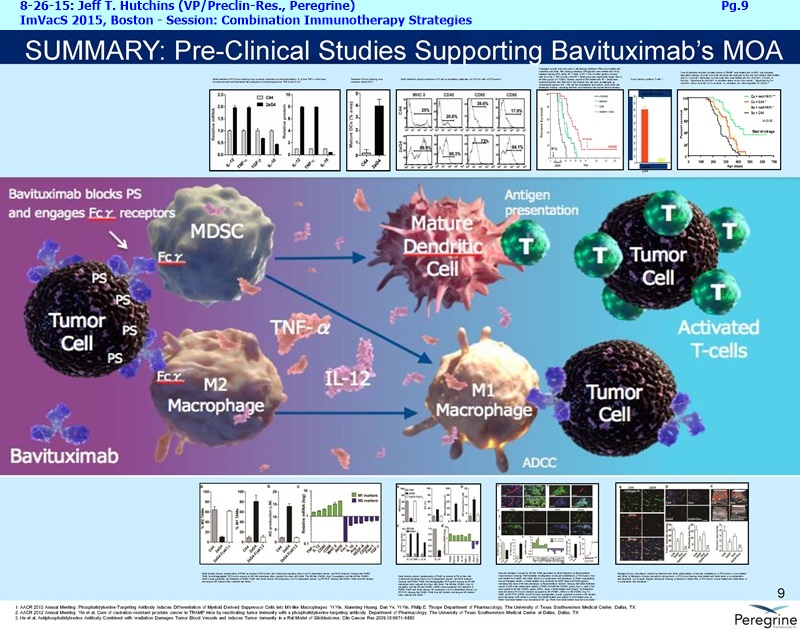
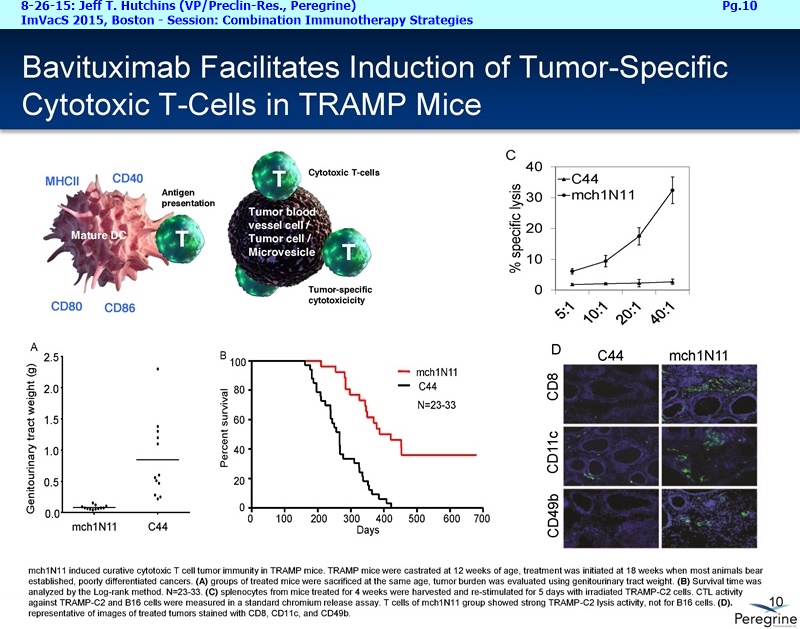
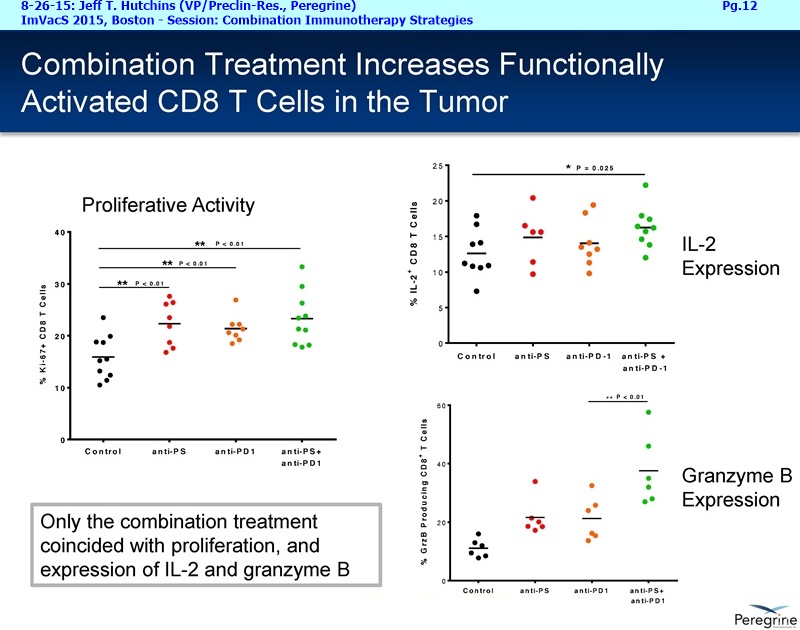
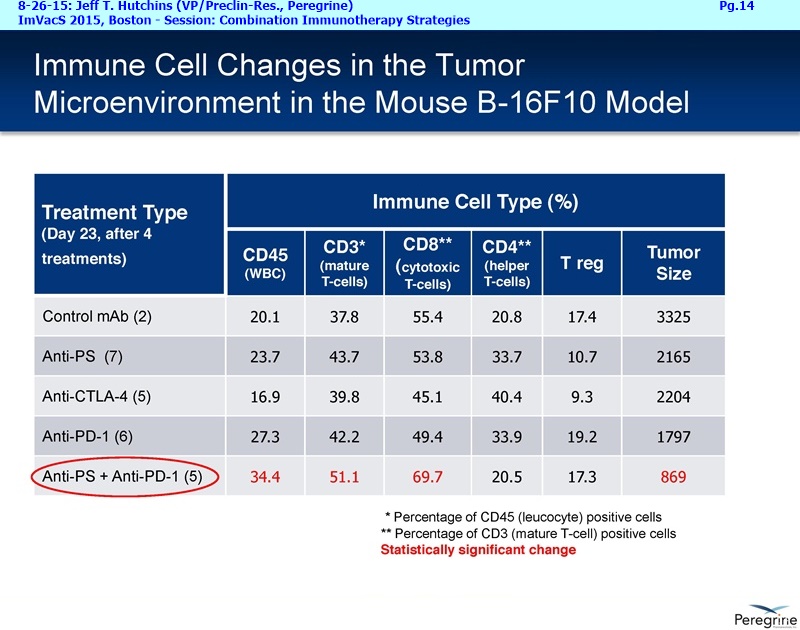
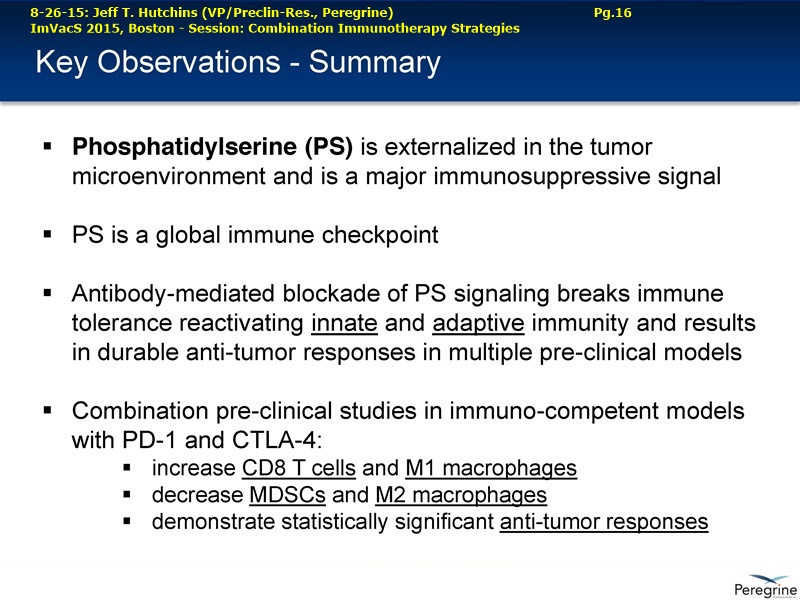
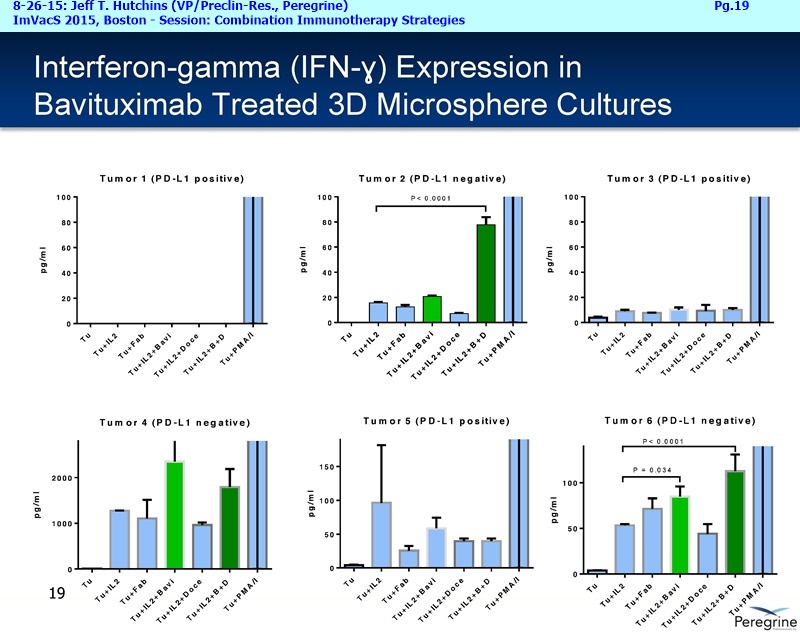
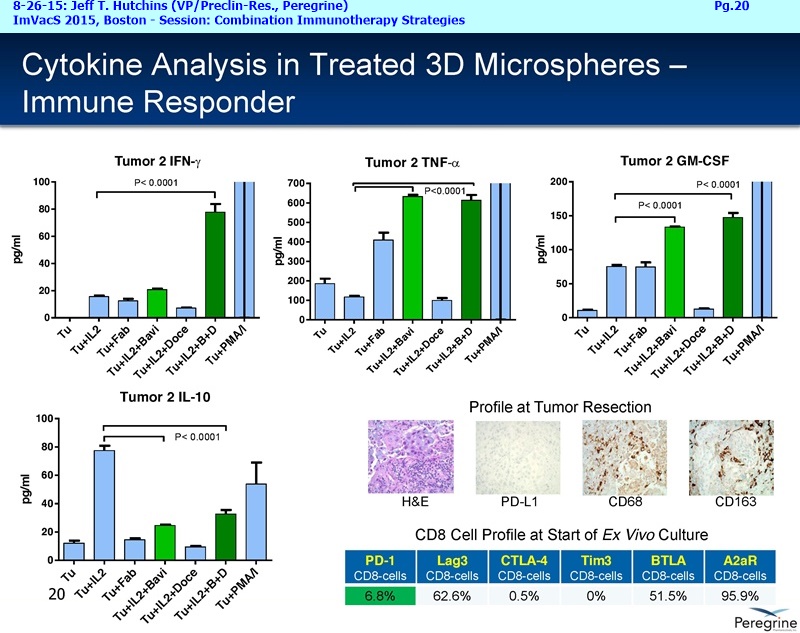
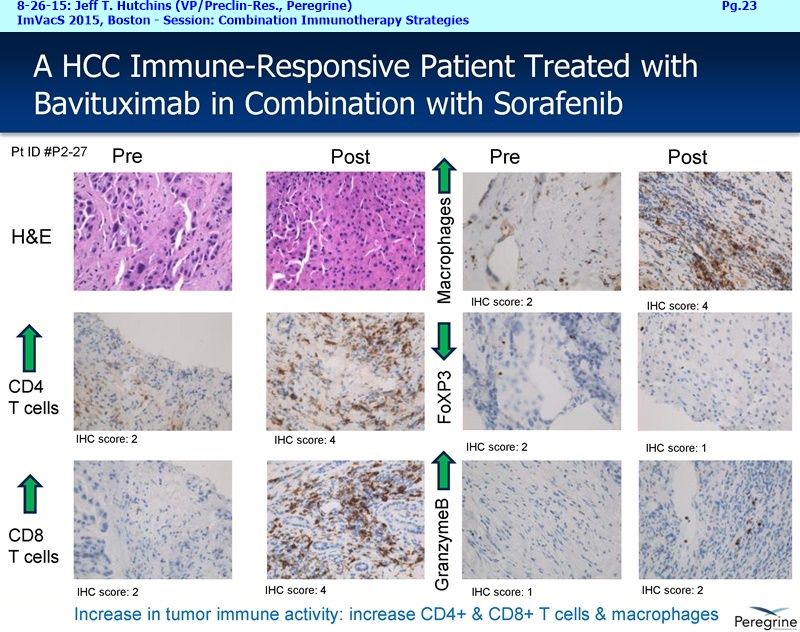
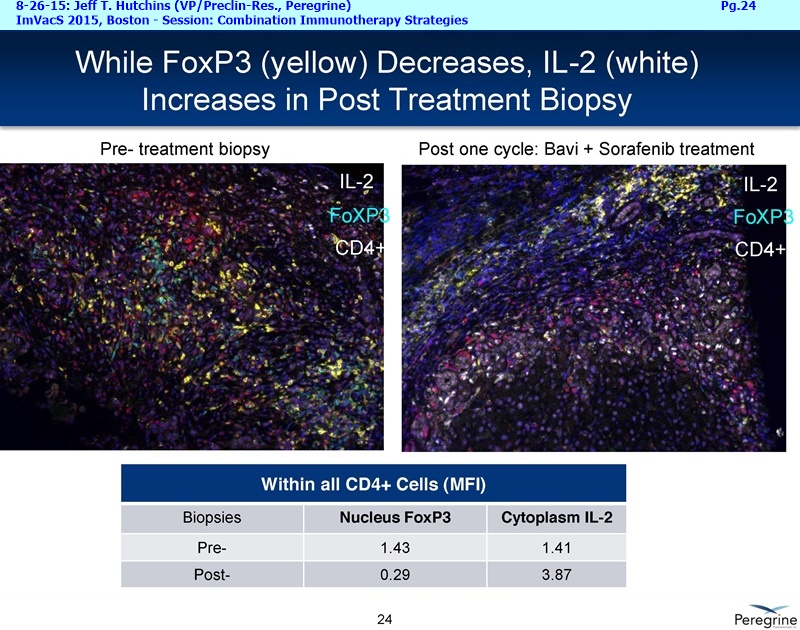
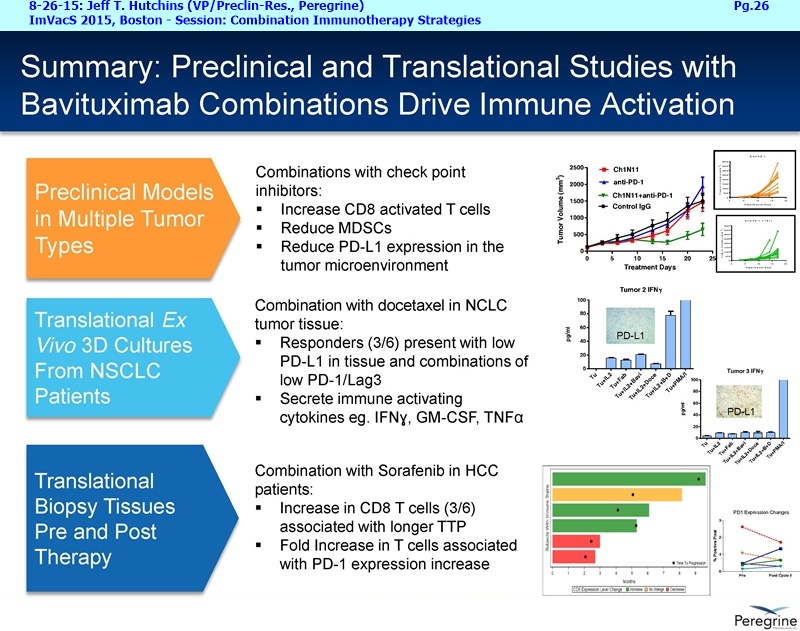
• The potential of bavituximab to shift the tumor microenvironment from immuno-suppressive in which tumors evade immune detection to a state of immune activation in which the immune system recognizes and fights the tumor. Presented findings demonstrate that bavituximab-like antibodies significantly increase the prevalence of tumor infiltrating CD8+ T-cells and immune-activating cytokines, while decreasing macrophages and myeloid cells that allow the tumor to evade immune detection. This elucidation and confirmation of bavituximab's mechanism of action highlights the potential of bavituximab to enhance the anti-tumor effects of both chemotherapy and immune checkpoint inhibitors.
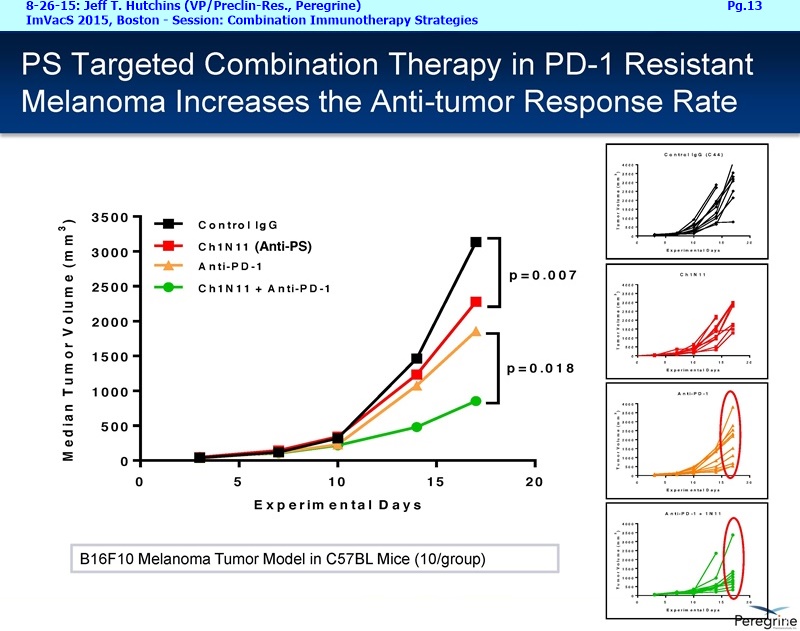
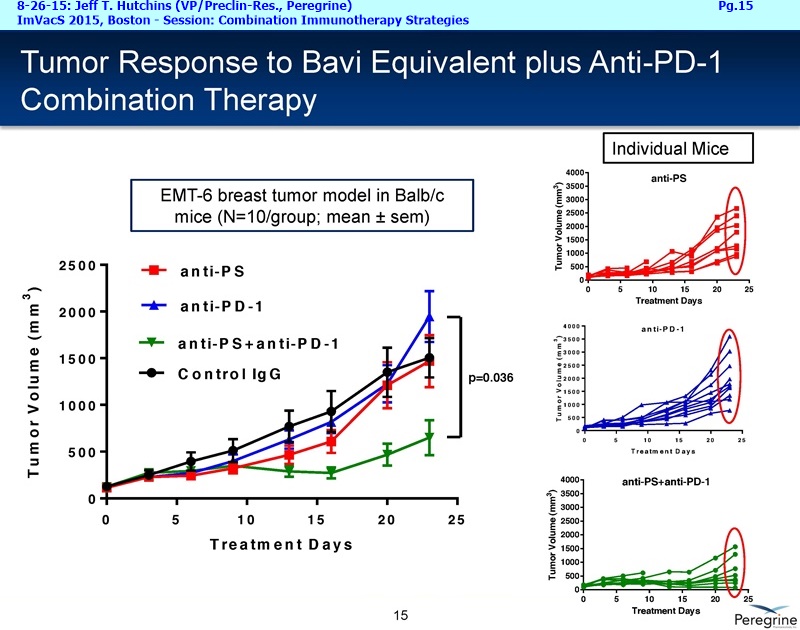
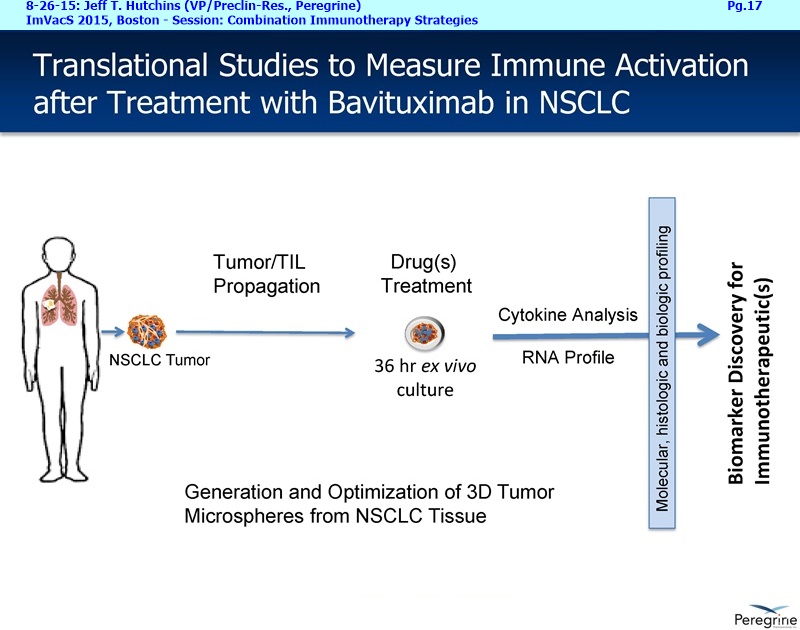
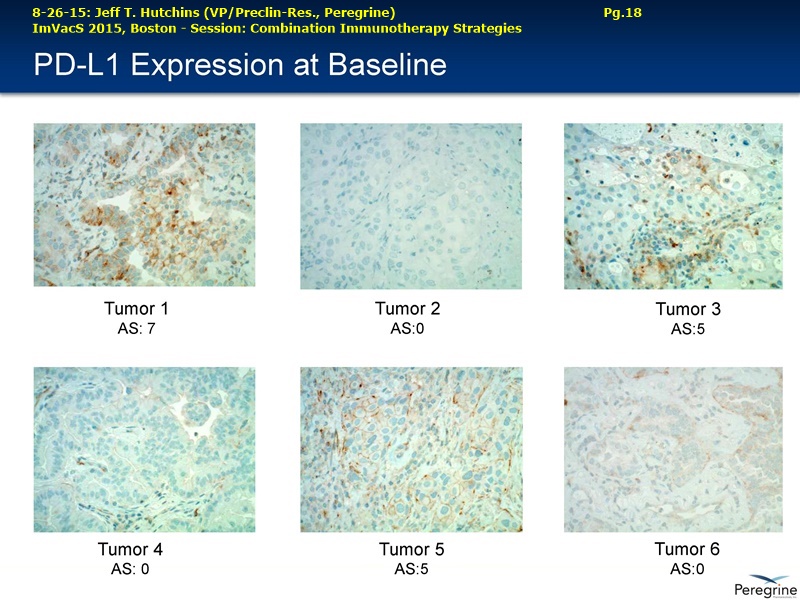
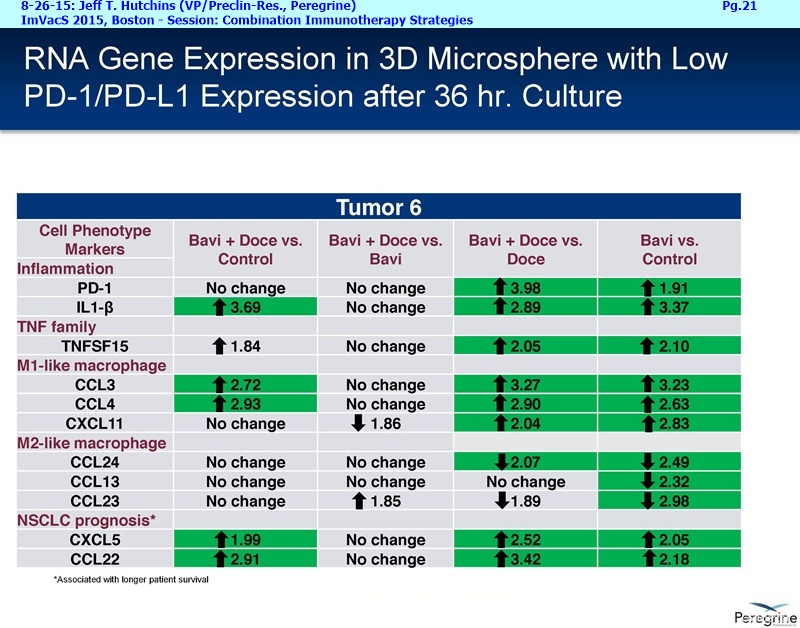
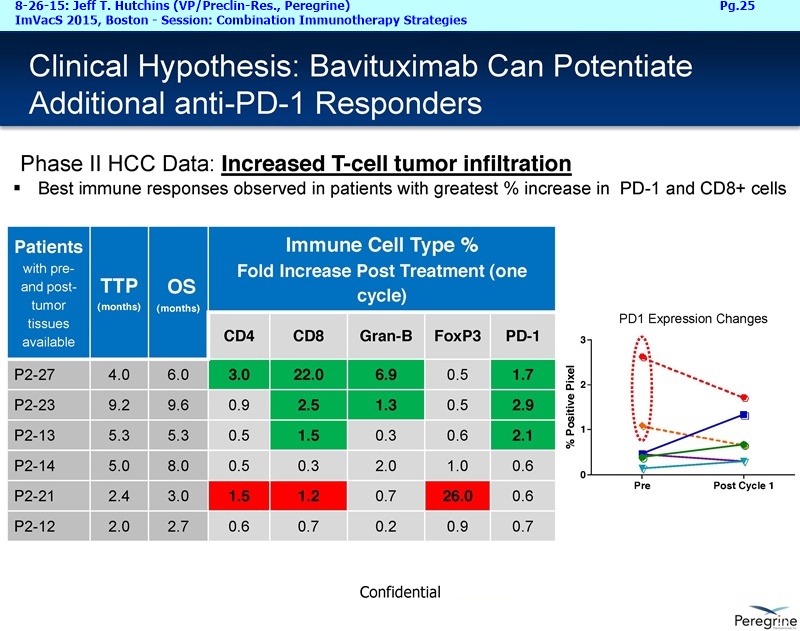
• Bavituximab increases the number of activated CD8+ cells in the tumor, which stimulates PD-1 expression, thereby upregulating the target for checkpoint inhibitors such as anti-PD-1 and anti-PD-L1. Importantly, translational study data across multiple cancers indicated that tumors with low PD-L1 or PD-1 expression on tumor infiltrating T cells showed promising signs of immune activation after treatment with bavituximab. This suggests the potential for bavituximab to activate a tumor specific immune response in patients with PD-L1 negative tumors that generally do not respond as well to PD-1 and PD-L1 inhibitors. By doing so, it is believed that bavituximab may hold potential to increase the number of patients able to respond to PD-1 and PD-L1 targeting immunotherapies. Furthermore, the combination of bavituximab-like antibodies and anti-PD-1 antibodies resulted in enhanced, synergistic anti-tumor activity in animal models of multiple tumor types, as compared to either agent alone. In some cases, complete tumor regressions were achieved, highlighting the anti-tumor potential of bavituximab in combination with checkpoint inhibitors such as anti-PD-1 antibodies.
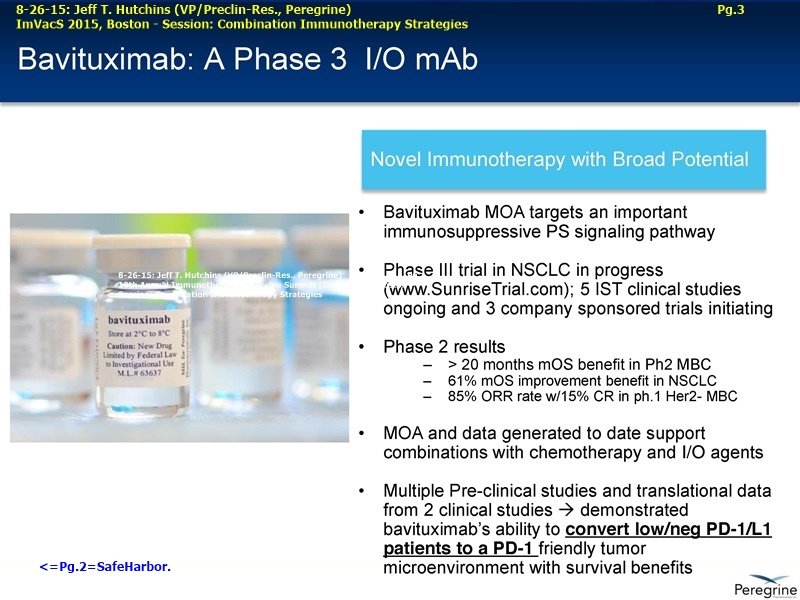
• Results from several clinical and preclinical studies in a range of tumor types show that bavituximab and bavituximab-like antibodies, in combination with conventional therapy, have consistently demonstrated estimated survival curves that plateau.
"We continue to generate a broad collection of pre-clinical, translational and clinical data highlighting bavituximab's novel mechanism of action and synergistic activity for a range of combination treatments. These study results, particularly as they relate to the potential synergies between bavituximab and checkpoint inhibitors, create great excitement for us as we begin work with our new collaborators at Memorial Sloan Kettering Cancer Center and AstraZeneca, while continuing our long-standing relationship with the University of Texas Southwestern Medical Center where this technology was originally developed," said Dr. Hutchins. "By aligning with these world leaders in cancer immunotherapy to study novel immuno-oncology combination therapies, we are best positioning ourselves to maximize the potential role that bavituximab can play in this new era of innovative cancer treatments."
ABOUT BAVITUXIMAB: A TARGETED INVESTIGATIONAL IMMUNOTHERAPY
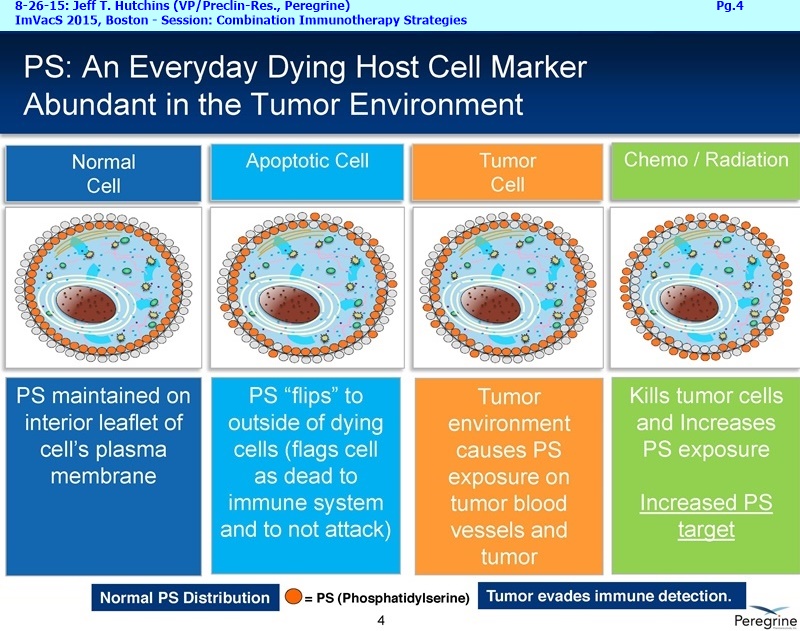
Bavituximab is an investigational chimeric monoclonal antibody that targets phosphatidylserine (PS). Signals from PS inhibit the ability of immune cells to recognize and fight tumors. Bavituximab, the lead compound in Peregrine's immuno-oncology development program, blocks PS to alter this immunosuppressive signal and sends an immune activating signal. Targeting PS with bavituximab has been shown to shift the functions of immune cells in tumors, resulting in anti-tumor immune responses.
ABOUT PEREGRINE PHARMACEUTICALS, INC.
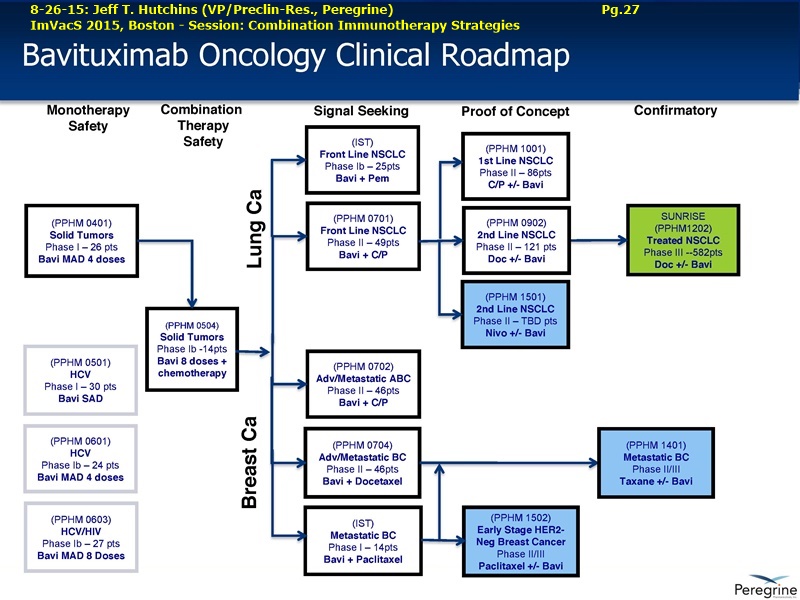
Peregrine Pharmaceuticals, Inc. is a biopharmaceutical company with a pipeline of novel drug candidates in clinical trials focused on the treatment of cancer. The company's lead immunotherapy candidate, bavituximab, is in Phase III development for the treatment of second-line non-small lung cancer (the "SUNRISE trial") along with several investigator-sponsored trials evaluating other treatment combinations and additional oncology indications. Peregrine also has in-house cGMP manufacturing capabilities through its wholly-owned subsidiary Avid Bioservices, Inc. ( http://www.avidbio.com ), which provides development and biomanufacturing services for both Peregrine and third-party customers. For more information, please visit http://www.peregrineinc.com .
Safe Harbor *snip*
Contacts:
• Jay Carlson, Peregrine Pharmaceuticals, Inc., (800) 987-8256, info@peregrineinc.com
• Stephanie Diaz (Investors), Vida Strategic Partners, 415-675-7401, sdiaz@vidasp.com
• Tim Brons (Media), Vida Strategic Partners, 415-675-7402, tbrons@vidasp.com
= = = = = = = = = =8-26-15 Dr. Hutchins Presentation PDF posted to http://www.peregrineinc.com/images/stories/pdfs/imvacs_hutchins-2015.pdf
...Here are images of his 8-26-15 29-screen talk:



= = = = = = = = = = = = = = = = = = =
8-24-15: AstraZeneca & Peregrine Collaborate on Bavi+Durvalumab Ph1/1B Trial for “multiple solid tumors” http://tinyurl.com/owlxpsf
Durvalumab=MEDI4736, an anti-PD-L1 immune checkpoint inhibitor.
…AZN’s Head/I-O(Robert Iannone): “Our partnership with Peregrine provides the opportunity to explore an exciting, novel combination that could deliver important clinical benefit to patients across a range of cancers."
Durvalumab (MEDI4736, anti-PD-L1) has a Net Present Value of $6.5B…

7-14-15 Qtly. Conf. Call (King/Shan/Hutchins/Lytle) Transcript http://tinyurl.com/nw2v5u6
...CEO S.King: “We recently entered into collaboration with investigators at Memorial Sloan Kettering Cancer Ctr to continue expanding on this important work, as well as to explore other potential applications of bavituximab and other agents that target PS-signaling pathway.”
5-29-15: Peregrine & Sloan Kettering Enter Collab. to “Investigate Novel PS-Targeting Immunotherapy Combos” http://tinyurl.com/qxu4w2x
= = = = = = = = = = = = = = =
SCIENCE: "Evolution has favored pathogenesis that resembles apoptosis."
Dr. Judah Folkman: ”This [Thorpe’s VTA research] is very promising and very elegant work... The whole goal is really 2-part, reducing the harsh side effects of cancer treatment, and reducing the chance that some cancer cells will evade treatment. That would be a big step in the next decade, and anti-vascular therapy will play a major role." (’97 & ’02 http://tinyurl.com/k5qe96g & http://tinyurl.com/n6vh9hp )
Peregrine's Bavituximab Clinical Trials website: http://PeregrineTrials.com
BAVITUXIMAB MOA & CLINICAL DATA: http://www.peregrineinc.com/pipeline/bavituximab-oncology.html
...Bavi Publications: http://www.peregrineinc.com/publications/publications.html
...Bavi Posters & Presentations: http://www.peregrineinc.com/publications/posters-and-presentations.html
Bavi MOA: Video (3:34) added ~3-2014 http://vimeo.com/87116642 "Bavituximab: A Novel Immunotherapy Candidate Targeting an Upstream Immune Checkpoint to Fight Cancer"
Bavi MOA: Video (1:33) on Bavi’s Immunotherapeutic MOA added to Youtube on 3-27-14 https://www.youtube.com/watch?v=Esewl35JD8s

5-31-15: ASCO’15 Roundtable (webcast), “Raising the Immuno-Oncology Bar” - 7 panel members, incl. 3 Sloan Kettering researchers http://tinyurl.com/qxu4w2x
BAVI MOA 3-25-15: PPHM/VP Dr. Jeff Hutchins’ presentation at "Immune Checkpoint Inhibitors Conf.", Boston - PDF(34 Slides): http://tinyurl.com/ooxkhq7
BAVI MOA 2-9-15: PPHM/VP Dr. Bruce Freimark’s presentation at KEYSTONE "Tumor Immunology Meeting", Banff/CN – PR & Slides: http://tinyurl.com/q6cx4w6
BAVI MOA 12-12-14 San Antomio Breast Cancer Symposium, Dr. Bruce Freimark, Bavi+anti-PD-1 vs. Breast Cancer http://tinyurl.com/p5ng6vs
BAVI MOA 11-2014 SITC’14: 3 posters on preclin. Bavi+aCTLA4/aPD1 combo data (Hutchins Freimark Brekken Huang etal) http://tinyurl.com/pchzr6h
BAVI MOA 8-28-14: Italy’s Dr. Federico Cappuzzo, Bavi/NSCLC profile article (DovePress OpenAccess, 7pgs) - http://tinyurl.com/n3oa7bc
BAVI MOA 8-11-14: PPHM/VP Dr. Jeff Hutchins’ presentation at ImVacS "Immunotherapies & Vaccine Summit", Boston - PR: http://tinyurl.com/lpjy3u7 ; PDF(31 Slides): http://tinyurl.com/oueldme
...MLV’s Dr. George Zavoico’s 8-27-14 report on Dr. Jeff Hutchins’ 8-11-14 ImVacS/Boston Bavi MOA presentation: http://tinyurl.com/l3tw63c
BAVI MOA 5-28-14: Dr. Rolf Brekken’s 47min CRI “Cancer Immunotherapy” webinar about Bavituximab as an Upstream/Global Immune Checkpoint Inhibitor – REPLAY: http://tinyurl.com/lxgftyx
. . .CRI=Cancer Research Institute (NYC – Supported by BMS): http://www.cancerresearch.org - Facebook: http://tinyurl.com/pbmhb2z , https://twitter.com/CancerResearch
. . .CRI launches “Answer to Cancer” (cancer immunotherapy) website http://www.theanswertocancer.org
. . .8-12-14: CRI adds Youtube links to the 5-28-14 CRI Immunotherapy webinar, incl. Dr. Brekken's talk "about Bavi and how it works against lung, liver, and other kinds of cancers" http://tinyurl.com/ps5h6h8
BAVI MOA 3-25-14: Dr. Rolf Brekken’s 40min talk at NYAS Lung Cancer Symposium http://tinyurl.com/lq9stnk (45 Slides)
. . .Dr.Brekken’s talk: “Antibody-mediated Inhibition of PS - A Novel Strategy for Immune Checkpoint Blockade” (the 5 speakers: Jessica Donington, Roy Herbst, Balazs Halmos, Suresh Ramalingam, Rolf Brekken)
12-10-13: With recent scientific insights highlighting bavi’s immunostimulatory moa, these additions to PPHM’s SAB: Dimitry Gabrilovich, Scott Antonia, David Carbone**, Hakan Mellstedt http://tinyurl.com/mw776mk
......**A/o 9-2014, Dr. David Carbone (PPHM SAB/KOL) is President-Elect of IASLC https://www.iaslc.org/about-us/board
BAVI MOA: 12-2013 Bavi’s Immunotherapeutic MOA overviewed by UTSW’s Brekken/Huang in Pan European Networks Jrnl. http://tinyurl.com/lnb46pq
BAVI MOA 11-9-13: Annual SITC (WashDC) – 2 posters about Bavi’s Immunostimulatory MOA http://tinyurl.com/mjaweu5
...“We are actively working towards initiating a clinical trial in the coming months to further investigate the potential synergistic effects of bavituximab and an approved [anti-CTLA-4] immunotherapy in patients with Melanoma."
10-28-13 IASLC/Sydney: “Immune Checkpoints in the Tumor Environment: Novel Targets & the Clinical Promise of Combined Immunotherapies” http://tinyurl.com/mjaweu5
…Symposium speakers: Scott J. Antonia/MD-PhD(H.Lee Moffitt CC), Dmitry I. Gabrilovich/MD-PhD(Wistar Inst), Rolf A. Brekken/PhD(UTSW), David E. Gerber/MD(UTSW)
BAVI MOA: 8-19-13 Data Supporting Bavituximab’s Immunotherapy MOA Published in “Cancer Immunology Research” (AACR) - http://tinyurl.com/mhjftka (PDF)
…“PS-Targeting Antibody Induces M1 Macrophage Polarization & Promotes Myeloid-Derived Suppressor Cell Differentiation” (Thorpe etal)
BAVI MOA: 8-13-13 PPHM/VP Dr. Jeff Hutchins’ Presentation on the Downstream Immunostimulatory Effects/Moa of PS-targeting antibodies (like Bavi) at CHI’s “Immunotherapies Congress”/Boston http://tinyurl.com/m6h2tvt
BAVI MOA: 10-12-12 NMB article on how Bavi "Induces Innate & Specific Anti-tumor Responses" http://tinyurl.com/cw9odb8
BAVI MOA: 5-1-12 Dr. Phil Thorpe's 46min talk at NYAS PS-Targeting Symposium http://tinyurl.com/9792gl5
. . .Symposium title: "Phosphatidylserine (PS) Asymmetry - Therapeutic Apps. in Cancer & Infectious Disease Symposium"
. . .Replays of 5 speakers: Alan Schroit, Chris Reutlingsperger, David Ucker, Ari Helenius, Philip Thorpe
= = = = = = = = = = = = = = = = =
8-26-15: Jeff T. Hutchins (VP/Preclin-Res., Peregrine) - ImVacS 2015, Boston (Session: Combination Immunotherapy Strategies)
Aug 24-28 2015: “CHI’s 10th Annual Immunotherapy & Vaccine Summit (ImVacS ’15)”, Boston
CHI = Cambridge Healthtech Institute, http://www.healthtech.com
“…This year's event has been expanded to 5 days with coverage on adjuvants, vaccine & immunotherapy technologies, immunomodulatory therapeutic antibodies for cancer, combination cancer immunotherapy, and T cell target discovery. ImVacS 2015 promises to be a must-attend event for commercial and academic entities to continue advancing immunotherapies and vaccines through technology and innovation.”
Event: http://www.imvacs.com/
PGM: Rational Combination Cancer Immunotherapy (1 of 6)
SESSION: COMBINATION IMMUNOTHERAPY STRATEGIES
8-26-15 9-9:30 Jeff T. Hutchins, PhD, VP/Preclin-Res., Peregrine Pharmaceuticals
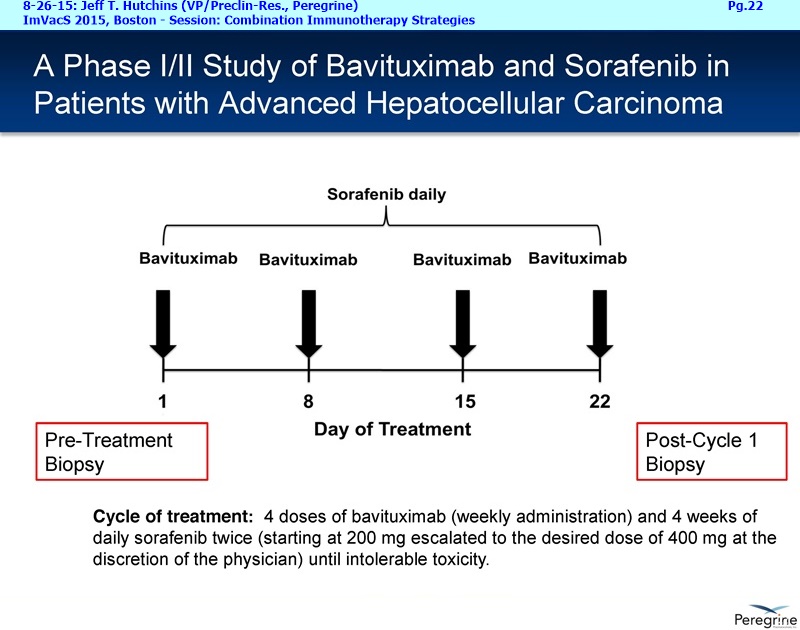
“Expansion and Activation of T Cells via the Targeting of the Immunosuppressive Ligand Phosphatidylserine (PS): Combination Strategy with Conventional, Targeted, and Checkpoint Inhibitor Therapy”
SUMMARY:
The underlying cause for the failure of current therapies is the persistent and multifocal immune suppression in the tumor microenvironment that drives the absence of pre-existing antitumor T cells. Bavituximab blocks PS-mediated immunosuppression (decreasing MDSCs) by reprograming immune cells in the tumor microenvironment to enhance anticancer activity. Pre-clinical, translational, and clinical results using bavituximab with conventional and immunotherapy combinations promotes a robust, anti-tumor T cell mediated response to enhance cancer therapy.
http://ir.peregrineinc.com/events.cfm
Recent CDMO News
- Avid Bioservices Reports Financial Results for Third Quarter Ended January 31, 2024 • GlobeNewswire Inc. • 04/24/2024 09:25:33 PM
- Avid Bioservices Announces Receipt of Deficiency Notice from Nasdaq Regarding Late Form 10-Q • GlobeNewswire Inc. • 03/20/2024 11:00:10 AM
- Form 8-K - Current report • Edgar (US Regulatory) • 03/07/2024 11:30:11 AM
- Avid Bioservices Announces Pricing of Private Placement of Convertible Notes • GlobeNewswire Inc. • 03/07/2024 04:58:48 AM
- Avid Bioservices Announces Proposed Private Placement of Convertible Notes • GlobeNewswire Inc. • 03/06/2024 09:32:07 PM
- Avid Bioservices Announces Certain Preliminary Financial Results for Third Quarter Ended January 31, 2024 • GlobeNewswire Inc. • 03/06/2024 09:31:28 PM
- Form 8-K - Current report • Edgar (US Regulatory) • 03/06/2024 09:30:18 PM
- Form SC 13G/A - Statement of acquisition of beneficial ownership by individuals: [Amend] • Edgar (US Regulatory) • 01/26/2024 09:57:52 PM
- Form 4 - Statement of changes in beneficial ownership of securities • Edgar (US Regulatory) • 01/13/2024 12:34:35 AM
- Form 4 - Statement of changes in beneficial ownership of securities • Edgar (US Regulatory) • 01/12/2024 12:39:18 AM
- Form 4 - Statement of changes in beneficial ownership of securities • Edgar (US Regulatory) • 01/12/2024 12:38:30 AM
- Form 4 - Statement of changes in beneficial ownership of securities • Edgar (US Regulatory) • 01/12/2024 12:37:38 AM
- Form 4 - Statement of changes in beneficial ownership of securities • Edgar (US Regulatory) • 01/12/2024 12:36:27 AM
- Form 4 - Statement of changes in beneficial ownership of securities • Edgar (US Regulatory) • 01/12/2024 12:35:47 AM
- Form 4 - Statement of changes in beneficial ownership of securities • Edgar (US Regulatory) • 01/11/2024 12:56:02 AM
- Form SC 13G/A - Statement of acquisition of beneficial ownership by individuals: [Amend] • Edgar (US Regulatory) • 01/08/2024 09:32:36 PM
- Form 4 - Statement of changes in beneficial ownership of securities • Edgar (US Regulatory) • 01/04/2024 12:56:18 AM
- Form 4 - Statement of changes in beneficial ownership of securities • Edgar (US Regulatory) • 01/04/2024 12:55:07 AM
- Form 4 - Statement of changes in beneficial ownership of securities • Edgar (US Regulatory) • 01/04/2024 12:53:58 AM
- Form 4 - Statement of changes in beneficial ownership of securities • Edgar (US Regulatory) • 01/04/2024 12:51:57 AM
- Form SC 13G - Statement of acquisition of beneficial ownership by individuals • Edgar (US Regulatory) • 12/19/2023 09:05:52 PM
- Form 4 - Statement of changes in beneficial ownership of securities • Edgar (US Regulatory) • 12/19/2023 12:34:08 AM
- Form 4 - Statement of changes in beneficial ownership of securities • Edgar (US Regulatory) • 12/19/2023 12:33:03 AM
- Form 4 - Statement of changes in beneficial ownership of securities • Edgar (US Regulatory) • 12/19/2023 12:32:11 AM
NanoViricides Reports that the Phase I NV-387 Clinical Trial is Completed Successfully and Data Lock is Expected Soon • NNVC • May 2, 2024 10:07 AM
ILUS Files Form 10-K and Provides Shareholder Update • ILUS • May 2, 2024 8:52 AM
Avant Technologies Names New CEO Following Acquisition of Healthcare Technology and Data Integration Firm • AVAI • May 2, 2024 8:00 AM
Bantec Engaged in a Letter of Intent to Acquire a Small New Jersey Based Manufacturing Company • BANT • May 1, 2024 10:00 AM
Cannabix Technologies to Deliver Breath Logix Alcohol Screening Device to Australia • BLO • Apr 30, 2024 8:53 AM
Hydromer, Inc. Reports Preliminary Unaudited Financial Results for First Quarter 2024 • HYDI • Apr 29, 2024 9:10 AM










