Register for free to join our community of investors and share your ideas. You will also get access to streaming quotes, interactive charts, trades, portfolio, live options flow and more tools.
Register for free to join our community of investors and share your ideas. You will also get access to streaming quotes, interactive charts, trades, portfolio, live options flow and more tools.
Heck of a chart, heck of a week- SOLID RED, down another 6% today on pretty high vol, making ever lower bids. LOWER highs and LOWER lows, continually. A 9 month new low and 50% loss in about 2.5 months and an 80% plus loss since just March/April of this year. Wow. And no support in sight? Looks like breaking 1 CENT is a very real possibility here.
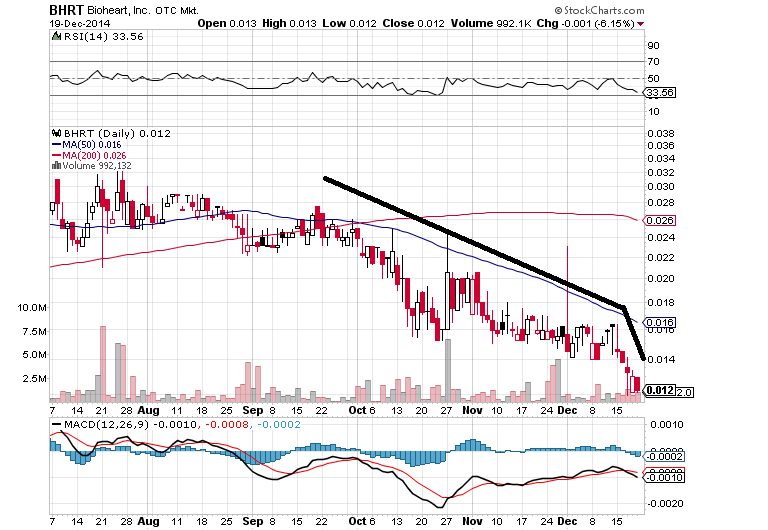
That's a heck of a down-trend to try and reverse and no PR or anything issued so far has seemed to make a difference ("Nigeria" PR yesterday, didn't make a blip on the radar?). The bid is continually dropping but no buying interest has materialized?
Look at the increase in drop from about the time the Magna $200K "note" was announced as being funded. Seems to coincide pretty tight IMO. Plus, it seems like Asher, and Daniel James and Fourth Man and the convertible, toxic debt folks like to "close out" at end of year each time (speculating, but it always seems that way) as the selling and share dumping volume picks way up. A month or so ago, this thing was lucky on a lot of days to be trading maybe 50K shares, sometimes less, like $1200 bucks a day total, for quite a while there. Now it's doing over a million shares on a lot of these days where it's getting buried.
Too coincidental IMO, that this would all the sudden just be Joe Q. Public retail buyers/sellers all of the sudden doing all this down-side volume when all this good news and super duper PR's keep coming out?? Seems strange to me?
Remember too, the lower the share price drops like this- the more shares the toxic, convertible debt folks get if they choose to convert, which means just that much more dilution. Thus, the term "death spiral" financing. Also, if they were to tap another finance line here, or the Magna "credit line" became active, it's gonna be just that much more dilutive the lower the share price keeps dropping. Looking real weak in here.
"Nigeria" PR seems to have gone well seeing how it's solid red yesterday and now making the low bid again today.
This has PR "fatigue" IMO. They were issuing a PR about every week, literally for like a yr or more, then every 2 weeks minimum. Just PR "about something", anything.
Months of continual PR releases now and it hasn't budged this slide/decline in the slightest or made even a hint at reversing this major down-trend this is in for many months now.
I just think these vague "PR's" don't hold much water anymore IMO. Not when stacked against the massive amount of low priced share over-hang caused by endless dilution and use of toxic convertible debt financing, paying common bills by issuing 10's of millions of shares and all the rest of it.
That's my 2 cent take on it. It's going lower from here, more than likely IMO. Magna dilution hasn't even really gotten started yet. I'd be looking for 2 zeros after the decimal pretty soon.
OTC MARKET site has it at $6.70 and about 12K shares traded.
http://www.otcmarkets.com/stock/OCAT/quote
Looks live and updating to me?
"OCAT patients went from degression to "improvement"."
All 18 of um or whatever the tiny number is. So I guess that's pretty much settled "science" for all 6 BILLION plus people on planet earth?
Not quite how the "scientific method" works. Takes a bit more than a single, micro "study", with a very small sample size and little time of follow-on to even know what the mid-term or long term effects are, potential side effects, if it even lasts, etc. There's literally 1000's of seemingly "miracle treatments" that look promising when first tried, but that get derailed and prove to be failures when tested and studied in larger population sets and over much longer periods of time. It's why out of every 10,000 drug candidates that look "promising" in the lab or very early staged, "maybe" 2 or 3 ever see the light of day as commercialized and salable, approved products- and even many of them prove later to be failures and unsafe. Remember the "miracle" called fen-phen? A lot of young people, who lost a lot of weight, but are now walking time bombs with permanently damaged hearts. Fen-phen was heralded as a literal "miracle" and had made it through all FDA trials and testing and gained approval.
Not a "cure" for blindness yet by a million miles.
"curing blindness is just
such a bad thing."
WHO has actually "cured blindness"??
Where and when? What FDA approved or similar Euro approved or phase III completed, proven safe on humans, etc "cure for blindness" exists anywhere on planet earth?
Would be fascinated to read about this supposed "blindness cure"? Where is it documented and proven? Where can one go and get this proven "cure" for blindness and how much does it cost?
Here's another past business venture of Tomas, it's where he came from while being appointed to run Bioheart. He's still mentioned in even recent SEC filings as controlling (almost like he just "assumed" the shares to himself now, confusing IMO) the shares of stock of this "group" and was listed as President of the company/group in even recent filings. It's not even clear to me if he still runs this "Astri" group to this day, while still being CEO of Bioheart- a recent Bioheart breakdown of BHRT share ownweship shows the Astri group shares as more or less being "assumed" or taken over by Tomas, greatly adding to his ownership stake in BHRT, but it's not clear if he bought those shares from Astri group (which would be a separate company of which he was just President and some sort of partner in) or did he just "assume" the shares to himself now- which makes no sense IMO, unless he personally had paid for all those shares with his own money originally.
http://www.bizjournals.com/southflorida/stories/2010/09/20/daily51.html?page=all
http://www.corporationwiki.com/Florida/Coral-Gables/the-astri-group-llc-6306255.aspx
http://www.manta.com/c/mm833gg/astri-group-llc?ftoggle-frontend-prod-on=abTests.revenue.responsive_12162014_b&utm_expid=82789632-30.8Ue3RXoXRoWAwC0cgSs_wg.3&utm_referrer=https%3A%2F%2Fwww.google.com%2F
Last filed 10-K, 3/25/2014 PAGE 67:
". Mike Tomas was appointed as the Company’s President and Chief Executive Officer, and as a director on June 19, 2010. Mr. Tomas has been President for the past nine years of The ASTRI Group, an early stage private equity investment company in Florida with an investment in Bioheart since 2001. In 2003, he joined Bioheart’s Board of Directors as the independent representative of The ASTRI Group. ASTRI provides capital, business development and strategic marketing support to emerging private companies. Mr. Tomas will continue to serve as President of The ASTRI Group"
Same 10-K filing, PAGE 77: (table showing insider share ownership, note 1 at bottom)
"Includes shares are held by The Astri Group over which Mr. Tomas has shared voting and investment power and includes (i) includes 6,578,947 shares of common stock and (ii) 10,900,000 shares of common stock issuable upon exercise of presently exercisable stock options."
"
"Great Job BHRT!!!!."
An 80% plus loss in about 8 months is certainly a "great job" - it takes a lot of work to get that kind of price decline during one of the greatest bull markets in history, yes.
The market was up what, about 300 points today and BHRT still closed solid red, making a new 8 month low bid of .0120 and consistently LOWER HIGHS and LOWER LOWS. Quite impressive IMO.
The common shares have "only" lost 99.98% of their value since their IPO on the NASDAQ at $5 per share in 2008 (an IPO that raised almost no money, another impressive "great job"), then BHRT was delisted almost exactly 1 yr later in 2009 and have been on the OTC and in a steady decline ever since. Now losing 99.98% of their value and being diluted out to 650 MILLION plus common stock shares with the available shares upped to 2 BILLION as massive dilution continues unabated. Those common stock, public traded shares have "only" lost about 98% since the present CEO took over around 2010. Yes, a $million plus per yr compensation for those kind of results, seems low IMHO. More pay and bigger bonuses would make sense to me. A 98% loss to common shareholder value in a huge bull market is an impressive sign of business success and superior Sr. mgt skills, yes. It's what all public companies strive for as far as I'm familiar? But what do I know?
Their major phase II/III trials have stalled out and never progressed in over 4 yrs, almost 5 yrs now. A "great job" and very impressive to say the least. Yes. Amazing.
Yes, those are impressive statistics and signs of superior Sr mgt and a fantastic business plan IMHO. Yes.
http://venturebeat.com/2008/02/20/bioheart-a-new-record-for-ipo-futility/
http://globenewswire.com/news-release/2009/02/26/393349/160467/en/Bioheart-Inc-Receives-Notice-of-Delisting-From-The-NASDAQ-Capital-Market.html
Yes, incredible signs of success and fantastic leadership IMO. Amazing actually. They finished the most recent qtr with a grand total of $46K cash on hand, despite numerous PR about "revenues". Again, very impressive IMO. And they only have immediate debt (accounts payable) of more than $2 million per their last 10-Q filing and $10 million plus total debt- with essentially little to no cash at any given time. All signs of business success and a "great job", yes of course.
Thus, more base pay and bigger bonuses for just 2 people, of the 4 full time employees left at the company (at the big "world wide" 4000 sq-ft headquarters, the $6K per month leased massive main headquarters) yes, pay more to those 2 incredible managers rather than funding any actual trials or research (per latest 10-Q, $3K per month, total, spent on R&D, but recently they spent $5K for one month of a penny stock promotion service called smallcapvoices. Gotta have one's priorities straight- a sign of incredible Sr mgt IMHO of course) so yes, more base pay and ever bigger cash bonuses seems like the best plan in my humble opinion.
All very impressive IMHO and certainly a "great job" by all business metrics and standard business practices that I'm familiar with??? I mean a $MILLION a yr in compensation for 1 person and $1,575,000 to just 2 people for producing those kind of results? Why not double that? Just issue more dilution shares and use more toxic convertible debt financing as needed- makes great sense. Don't worry about paying bills or debt or actually conducting clinical trials (even though your twitter feed and main corp website for example "claims" your'e the UNDISPUTED PHASE II/III WORLD LEADER in stem something or other)- I mean just funnel all that money to those 2 superior leaders as the results are beyond impressive. Yes.
IMHO of course.
"This is a company wout endless funds but WILL NOT QUIT and as dire as people make it seem are not as close to insolvent as they try to portray BHRT... They went from a company with over a 100 million dollars invested and 0 money coming back to a company that is close to making a million dollars a quarter."?????
What?
1) Their financial situation is past dire, they are teetering on insolvency and except for massive, endless dilution and ability to dump shares (unlike a private business, non public traded company) - they'd of been BK and done a long, long, long time ago. They dilute shares like water- more than doubling the outstanding share count in less than 1 yr. They just finished a qtr q/ $46K total cash in the bank and over $2 MILLION in just immediate debts (accounts payable, normally due in 30 days or less). Thus, it's correctly pointed out that they in desperation often offer millions, if not 10's of millions of shares to others as payments. When a company is teetering on BK, when it's not spending anything on R&D which is the business they "claim" to be in, but they are handing out lavish, large pay raises and cash bonuses to 2 people, out a micro sized company of 4 full time people total- then it's a huge problem in most people's book. It shows the company is more than likely nothing but a vehicle being used to self enrich insiders. They appear to to little or no actual "business" in the fields they claim to be pursing in their own SEC filings. Trials that are dead and parked for 5 yrs now for "lack of funding" - but money flowing like water to select insiders is OTC-ville and extremely problematic IMO and that of many others. Further, executives are paid for results- look at the share price and market cap of this stock, it's an unmitigated failure- right in the middle of one of the greatest bull market periods in history.
2) They are nowhere near "close to making a million dollars a quarter.". They have never, ever, ever "made", as in a profit or a return on investment or as in positive cash flow so much as ONE CENT in their entire existence. They LOSE money and that's all they ever do. They're not even remotely close to a million a qtr in revenue and last qtr made a lousy 10% gross margin on what revenue they did bring in. Meaning costs/expenses (cost of sales) ate up nearly all revenue, making it irrelevant for the most part. READ THE 10-Q FILING.
3) 2 of the people who get the massive salaries and cash bonuses sit on the BOD as 1/3 of the vote. This company has no independent compensation committee or similar. They self-deal to each other in essentially everything they do- voting their own pay raises, creating Northstar LLC who is one in the same with BOD, etc Again, READ THE SEC filings.
As to some supposed history of Tomas or whoever "making millions" for others in the past or whatever- I don't know of any concrete proof of that or how one would know that w/o specific insider information? Neither Tomas nor Comellas has any background or published experience or successes at ever successfully running a bio-tech company, and especially not at ever bringing an FDA approved product to market successfully. Most, if not all of their experience in "C" level, Sr mgt positions at running/managing public traded companies is at Bioheart (100% certain in the case of Comella).
Also, I could care less about a bunch of self-gloating and often trumped up "awards" or whatever- who freaking cares? RUN THE BUSINESS, it's not an awards who or popularity contest- the stock's market cap is barely $7 million dollars and the share price is sub basement at 1.3 pennies. And we're supposed to care about some wash list of awards? I've got a cabinet full of "awards" but I don't go around in public boasting about them- my ego is fine w/o that.
From the companies most recent 10-Q filing: PAGE 12 (their own auditor and Sr mgt think and state their financial condition as DIRE, teetering on insolvency, which is the step prior to bankruptcy)
"NOTE 2 — GOING CONCERN MATTERS
The accompanying unaudited condensed financial statements have been prepared on a going concern basis, which contemplates the realization of assets and the satisfaction of liabilities in the normal course of business. As shown in the accompanying unaudited condensed financial statements, during nine months ended September 30, 2014, the Company incurred an operating loss of $1,247,199 and used $747,184 in cash for operating activities. As of September 30, 2014, the Company had a working capital deficit (current liabilities in excess of current assets) of approximately $10.0 million. These factors among others may indicate that the Company will be unable to continue as a going concern for a reasonable period of time.
The Company’s existence is dependent upon management’s ability to develop profitable operations and to obtain additional funding sources. There can be no assurance that the Company’s financing efforts will result in profitable operations or the resolution of the Company’s liquidity problems. The accompanying statements do not include any adjustments that might result should the Company be unable to continue as a going concern."
See those magic words, "liquidity problems"?? That's a nice way of saying inability to PAY ONE'S BILLS ON-TIME or WHEN and IF THEY COME DUE, which then leads to insolvency which ends in BANKRUPTCY. It's in their own words. They are teetering on being ill-liquid.
Look at the massive O/S share count (and they recently upped the available shares to 2 BILLION on a 1.3 cent stock, a joke and past desperation IMO)
10-Q, PAGE 9:
"Fully diluted shares outstanding were 659,543,477 and 323,296,916 for the three months ended September 30, 2014 and 2013, respectively and 605,015,919 and 336,682,241 for the nine months ended September 30, 2014 and 2013, respectively."
This "company" essentially exists as nothing more than a share dilution machine IMO. A hallmark of many OTC penny stocks. Look at those numbers, more than 300 MILLION shares, more than a doubling of shares O/S in less than a 1 yr period. And for what? What trial(s) progressed or were advanced- there entire "claimed" business and purpose?
"Postponed indefinitely" indicates they couldn't get any pricing power or demand on the offer. Simple as that in my book.
It's a weak stock with a pretty tainted (recent) past. The underwriters, I've speculated from day one, are gonna want a steep discount for that risk. They don't want to get stuck holding a bag of 10 million shares of a micro cap that they can't get unloaded for their profit margin targets and guidelines.
Thus, higher risk = lower offering price. Management, my guess, balked at the offering price. The stock traded in the $5's a few days ago- so any offer is gonna be lower than that. My guess, $5 a share was tops, and probably lower. Netting OCAT only $45 mil, maybe as low as $40 mil for 30% pure, instant dilution. The mgt maybe fears that if the share price gets buried further after that- they'll end up a $2 or $3 dollar stock pretty rapidly.
Their pickle is they need cash and need it bad. They can't even begin to fund a serious phase II on Lincoln money- all Lincoln money does is pay the basic salaries and stuff and keep the doors open- it doesn't get into even making a dent in a serious phase II.
So question now is what do they do? They haven't been able to attract any non dilutive equity stake partners, so they're parked in the squeeze. They're sitting in what's still one of the greatest bull market runs in the history of markets- but can't crack $7 bucks a share. If you can't place an offer now, then IMO you can't place it ever. Money's been flowing like water right now on the street- it's the roaring bull ride times, but that window might be closing. Companies have been issuing record bond debt as interest rates are essentially zero- so they've been using debt instead of equity to expand or just buy back shares (even a cash cow like Apple did it, cause it's prudent use of cheap money) - but OCAT can't attract debt as they have no cash flow.
So, what do they do now? They gotta get off the dime and get into phase II or the share price is sunk anyway. I'd say they gotta bight the low priced share offer bullet and take the hit and move ahead.
They've messed this past week up about as royally as it could be messed IMO. If they mess things up much more, or leave confusion and uncertainty hanging out there much longer- they're gonna make a self induced sell off IMO. One thing people and markets hate- is uncertainty and a feeling of being misled. They better get off the dime and slice some bread one way or another or get on the public horn and explain in detail what the heck they're doing. My 2 cents
"article"?? There's no "article" to read? It's a PR, that's a carbon copy of several PR's issued just like it (just the doctor name changed, the country, and the population count, simple as that. Read the S. Africa PR, the one that did not happen according to the latest 10-Q filing, it's near word for word verbatim of this one)
Again, WHAT does BHRT have as proprietary nohow, or a salable product/device/therapy, whatever that relates to cell preservation that would have anything to do with an invitro practice and OBGYN practice or anything else? In what SEC filing does BHRT claim that "cell preservation" is their line of business or expertise or anything else?
I can buy a cold storage vat, used, on-line for a few grand. They're used in every hospital and even small doctor's offices all over the world. What's proprietary in cold storing biological anything?
Again, what's the specific details on what BHRT is selling this doctor or how they monetize this or what this has to do with anything "stem cell" related? What?
"bhrt for their cell preservation techniques. "??
BHRT is not in the "cell preservation" biz and has no proprietary tech there? Especially as it would relate to an OBGYN practice?
Cryo-cell preservation is as old as time, there's nothing new or unique there that this Nigerian doc wouldn't already posses or know about?
What proprietary methods, technology, equipment or whatever does BHRT sell or posses as related to "cell preservation", especially as needed for invtro or anything to do with OBGYN or fertilization processes/issues, etc?
SEC filing pages or references or other published data and information would be great.
Nigerian doctor is an OBGYN, who specializes in "reproductive and fertility" medicine and shows ZERO "stem cell" anything on his resume or background.
He is Board certified in Obstetrics and Gynecology. His area of specialization is Infertility and Assisted Conception.
WHAT is he going to be "treating" using Bioheart supposed "stem cell therapies" or whatever?
From the Bioheart PR:
" a biotechnology company focused on the discovery, development and, subject to regulatory approval, commercialization of autologous cell therapies for the treatment of degenerative diseases, announced today that it has entered into a joint venture with Dr. Prosper Igboeli of Nigeria. "
How many "Dr. Prosper Igboeli of Nigeria" does one think exists, with that exact name and practicing in Nigeria? Well a simple Google search reveals ONE and he's a OBGYN who practices in reproductive and fertility issues and shows ZERO background, training or interest in "stem cell" anything on his self published bio and other information??? He actually runs his own fertility, aka BABY making and delivery, reproductive hospital. WHAT does this guy have to do with Bioheart and "stem cells"??
http://www.mmfertilityhospital.com/DynamicPage.aspx?pageid=70
http://www.mmfertilityhospital.com/DynamicPage.aspx?pageid=22
http://people.rit.edu/mrppph/collaborators_2/My%20Collaborators/content/d_large.html
Another Biheart "PR" that's "fascinating" to say the least, IMO.
"or Kristin Comella was just awarded the Nobel Prize for her work with stem cell biology"??
What? It's now being inferred that Comella is some Nobel potential candidate? Really?
Can one list all the peer reviewed medical journals she's published in, or any text books she's authored or what chair or even teaching positions she holds at a major university, what her Ph.D. or M.D. degree is in, in what specialty and from where, etc. Can one list her curriculum vitea and entire self authored body of medical research and self authored patents and papers, journal writings, etc? All of them please, the entire lengthy list. What original research and discovery is she well known for in academia circles, list all of it if one can?
You know, to even be in the remote, remote, remote running for a Nobel Prize in medicine or chemistry or whatever imagined field is being referenced?
Would love to see that information, really would be fascinated to see it in all its detail. Where can I find that, does anyone know?
"Setting up business in Nigeria"??
What exact "business" have they "set up"?? Where's the details, the money that was spent to "assume" this 49% ownership of this "vapor" location and some single, doctor's office practice at best or whatever this "entity" is they "assumed"? Kinda like the ole S. Africa "joint venture"? Like that one?
Overlay the S. Africa PR or the Honduras PR or any of um, and they're cut n paste templates except for changing the doctor's name and the population count of the "new" country. Does one really believe that the entire population of Nigeria, all 173 million, every man, woman and child and infant living there, the 173 million that they list in that "PR" (like they do in every carbon copy PR just like it) - that every person in Nigeria is now a "customer" or even remotely a potential candidate/customer for some "Stem cell" whatever via 4 person Bioheart and one "doctor's office" in ole Nigeria? Really?
Or, does one think the so called Nigerian "Ministry of Health" (if they even exist) is actually speaking with or cares one wit about "Bioheart" and stem cell whatever in Nigeria?
What exact "treatments" is Bioheart supposedly selling in Nigeria since their own SEC filings say they have 1) NO approved stem cell anything and 2) State in their own SEC filing they carry no product liability insurance for the very reason they have nothing proven safe or approved or salable yet and 3) State clearly their stem cell candidates have yet to be proven safe for use in humans???
So what exactly are they peddling down in ole Nigeria? What exactly?
From last filed BHRT 10-K PAGE 31:
"Our product candidates may never be commercialized due to unacceptable side effects and increased mortality that may be associated with such product candidates.
Possible side effects of our product candidates may be serious and life-threatening. A number of participants in our clinical trials of MyoCell have experienced serious adverse events potentially attributable to MyoCell, including six patient deaths and 18 patients experiencing irregular heartbeats. A serious adverse event is generally an event that results in significant medical consequences, such as hospitalization, disability or death, and must be reported to the FDA. The occurrence of any unacceptable serious adverse events during or after preclinical and clinical testing of our product candidates could temporarily delay or negate the possibility of regulatory approval of our product candidates and adversely affect our business. Both our trials and independent trials have reported the occurrence of irregular heartbeats in treated patients, a significant risk to patient safety. We and our competitors have also, at times, suspended trials studying the effects of myoblasts, at least temporarily, to assess the risk of irregular heartbeats, and it has been reported that one of our competitors studying the effect of myoblast implantation prematurely discontinued a study because of the high incidence of irregular heartbeats. While we believe irregular heartbeats may be manageable with the use of certain prophylactic measures including an ICD, and antiarrhythmic drug therapy, these risk management techniques may not prove to sufficiently reduce the risk of unacceptable side effects.
Although our early results suggest that patients treated with MyoCell do not face materially different health risks than heart failure patients with similar levels of damage to the heart who have not been treated with MyoCell, we are still in the process of seeking to demonstrate that our product candidates do not pose unacceptable health risks. We have not yet treated a sufficient number of patients to allow us to make a determination that serious unintended consequences will not occur."
But hey, I guess it's "good enough" for those folks down in ole Nigeria? Makes no sense to me? Selling un-proven and potentially very risky and unsafe "treatments" to be used on humans, per their own words in their own duly filed, legal SEC documents? What can possibly go wrong with that business model?
DOWN, SOLID RED making new 8 month lows on a day the market is literally on fire. Looks like the market place is just sucking up that "NIGERIA" joint venture, whatever ole news PR like a sponge?
Nigeria???
Oh no, not again from these guys?? They gotta be kidding? Nigeria?
Kinda like the ole S. Africa big "joint venture" that had (supposedly) a "grand opening" and then many patients "treated" and "facility expansion" take place, all put out in numerous PR, well- all except for when that pesky 10-Q just got filed and told a bit of a different "story".
http://www.marketwired.com/press-release/bioheart-announces-joint-venture-in-south-africa-otcbb-bhrt-1923668.htm
http://www.marketwatch.com/story/bioheart-announces-successful-grand-opening-of-facility-in-south-africa-2014-09-24
THEN, here comes the 10-Q and the "story" kinda changed a bit of the tune, in my humblest of opinions.
Most recent 10-Q, PAGE 23:
"We announced a joint venture in South Africa and the facilities called “South African Stem Cell Institute” were successfully opened in September, 2014 with the intention to retain a 49% ownership of the new entity. As of September 31, 2014, however, there was no formal legal entity established and no formal operating agreement for this joint venture. In additional the Company has not yet incurred any material expenses associated with this venture. Management has concluded that as of September 31, 2014 this announcement is not material to the Company’s financial statements."
What? Huh? How can that be? Suddenly all that PR that stated IT ACTUALLY HAPPENED, then became an "intention" and "no money was spent" and "no formal operating agreement", blah, blah, blah.
NIGERIA? What's the annual avg income in ole Nigeria. Lets check- so we can see who's gonna be rushing around down their to get expensive "stem cell treatments" and what not.
Take about the last 5 of these 3rd world "joint venture PR" releases- and overlay them on each other. Just cut n past the new name of some country and some doctor in, they're verbatim copies, right down to the phrase "laser like focus" and "will work with the (insert country name here) ministry of health" and then insert the entire population of the particular country, in this case 173 million. EVERY PR is a template of the last one, even the "Bioheart will assume 49% ownership". Always wondered how one just "assumes" ownership? They don't buy it and no money trades hands? Oh, then the S. Africa 10-Q facts come out- and they didn't "assume" 49% of anything after all. Funny how that "works"?? No?
World bank says it's about $3K PER YEAR annual income per person in Nigeria. Oh yeah, LOTS of money in their pockets for "stem cell treatment" down there. I'd think they're more worried about eating and surviving maybe? Perhaps?
And, lets check the PR for the ole "we're gonna work with the Nigerian govt", the line that's been in every PR about some 3rd world country "joint venture". Yep, THERE IT IS.
"Bioheart's partnership will establish a critical relationship with the Nigerian government and the joint venture will work closely with the Ministry of Health to make Bioheart protocols part of the standard of care for patients in Nigeria. Bioheart will provide the necessary training and expertise to transfer Bioheart therapies to the new facility.."
The ole Nigerian "MINISTRY OF HEALTH" is gonna be "working" with little ole Bioheart. Yep. Sure thing. I'm gonna write that "Ministry of Health" today and ask um if they've ever even heard of a U.S. based company called Bioheart. Will write it and mail if myself.
Now bout this from Bioheart's own 10-K filing??
Last 10-K, PAGE 39:
"We do not currently have product liability insurance because none of our product candidates has yet been approved for commercialization. While we plan to seek product liability insurance coverage if any of our product candidates are sold commercially, we cannot assure you that we will be able to obtain product liability insurance on commercially acceptable terms, if at all, or that we will be able to maintain such insurance at a reasonable cost or in sufficient amounts to protect against potential losses.
Claims may be made by consumers, healthcare providers, third party strategic collaborators or others selling our products if one of our products or product candidates causes, or appears to have caused, an injury. We may be subject to claims against us even if an alleged injury is due to the actions of others. For example, we rely on the expertise of physicians, nurses and other associated medical personnel to perform the medical procedures and processes related to our product candidates. If these medical personnel are not properly trained or are negligent in using our product candidates, the therapeutic effect of our product candidates may be diminished or the patient may suffer injury, which may subject us to liability. In addition, an injury resulting from the activities of our suppliers may serve as a basis for a claim against us.
We do not intend to promote, or to in any way support or encourage the promotion of, our product candidates for off-label or otherwise unapproved uses. However, if our product candidates are approved by the FDA or similar foreign regulatory authorities, we cannot prevent a physician from using them for any off-label applications. If injury to a patient results from such an inappropriate use, we may become involved in a product liability suit, which will likely be expensive to defend."
Sure seems to me like they're "promoting" the use of un-approved so called "products/therapies" or whatever that have yet to be proven safe in humans? Pretty crystal clear to me, IMO, from reading the ole "NIGERIA" PR. Seems in direct contradiction to their own SEC filed 10-K?? Confusing to me, to say the least.
Nigeria?? Sorry, doesn't do it for me personally. NOT SEEING IT and totally don't "get it". Makes ZERO sense and looks like every other PR put out in the last yr about some 3rd world country supposed "joint venture" or whatever. My 2 cent opinion.
.0121 right out of the gate. WOW !
Another new 8 or 9 month low bid on a day the markets are on fire.
This is total free-fall IMO. The markets are on fire and this thing is still dropping like a hot rock. No bid support or even a bump appears to be in sight anywhere.
Awfully close to 1 cent now. Market cap is getting shaved down daily and their ability to use the dilution financing gets worse with everyday the price drops lower. They gotta be really, really tight for cash at this point, IMO.
Really, really looking weak here. Now it appears the Magna "credit line" deal is delayed and has not become active yet per the recent SEC S-1/A filing, so they must be looking for cash somewhere? That Magna $200K note only lasts a little over a month or so given their cash burn rate and high debts and just immediate bills they showed like over $2 million in accounts payable as of last 10-Q filing.
How long can they survive in this mode I wonder? I mean what costs can they cut when you only have 4 full time and 1 part time employee and work from a leased, very small facility already?? Also, they got these recent law suits and appear to be hiring/retaining big law firm types, that can't be cheap either.
I'd find it hard to believe, IMO, that they're spending anything on so called "trials" or even conducting any actual medical research or "trials" at this point- the total R&D spending last 10-Q was already down to $3K a month total, and they appear in worse financial shape now probably. What exactly is their actual "business" they're in then, if they don't run phase II/III trials and similar as their twitter feed and other PR "claim"?? I really wonder at this point? I mean what do they actually do all day as a "business" and for these large salaries and bonuses collected by just 2 people- when they seem to have little to no cash and no ability or even desire to direct money to actual trials and medical R&D which is their stated business per their own SEC filings?
Makes zero sense to me anymore. None.
OTC, FINRA just updated 12/18/14.
It's interesting, that FINRA IMO, appears to have the spot light on this stock now, and it appears they are now making OCAT file an "update" daily, since the Friday "thing" occurred (mess, disaster, manipulation trading, whatever one wants to call it. It was, and still is a royal mess in my book and opinion).
Not a big confidence builder IMO, and all this has occurred and all this screwy trading and inability to get accurate quotes and even brokerage firms not knowing where or how it's even trading or how to process orders efficiently for their clients- all while "trying" to price and place a large, dilutive, 10 million share secondary offering? Talk about perfect timing?
From FINRA, a few minutes ago:
http://otce.finra.org/DailyList
12/18/2014 05:25:43 Reinstatement 12/17/2014 21:00:00 OCAT Ocata Therapeutics, Inc. Common Stock undefined Other OTC
Daily List Date 12/18/2014 08:25:43
Event Type Reinstatement
Effective/Execution (Eff/Ex) Date 12/18/2014 00:00:00
Issue Type
Symbol OCAT
Issue Name Ocata Therapeutics, Inc. Common Stock undefined
Class
ADR Ratio
Maturity Date
Market Category Other OTC
Offering Type No Restrictions
OATS Reportable (Rptbl) Flag Yes
Unit of Trade 100
Reg Fee Flag Yes
Daily List Comment Market Center moved to NASDAQ postponed.
12/18/14, that's today's date last time I checked. So, OTC IT IS and "NASDAQ POSTPONED" it is. That's the final word from FINRA.
Oh, it's gets even better and sweeter than that, as it's even gone up since then. They just granted Tomas and Comella large cash bonuses in 2014, as well as, big increases to their base salaries, as the common stock sinks like a boat anchor and it made it's all time low of .0063 in late 2013 and is now at a 9 month low for 2014, heading to near all time lows. (example, as fast as they started this PR of reporting "revenues", they handed out in just bonuses, more than all the revenue for the yr will amount too, especially given the high cost of sales, meaning low net on those "revenues". Thus, nothing about their precarious financial situation changed in the slightest IMO, they're as cash broke as ever and still issuing "notes" for "bonuses owed" or "loans owed" to insiders or whatever)
As you stated, for what do only 2 people get these huge base salary increases and cash bonuses? Look at the market cap and present share price and status of their key trials for instance.
From the last 10-Q, PAGE 23:
"Employment agreements
On July 28, 2014, the Company’s Board of Directors approved the 2014/2015 salary for Mike Tomas, Chief Executive Officer, at $525,000 per year, beginning July 1, 2014 with an incentive bonus ranging from $150,000 to $500,000. In addition, the Board of Directors will grant Mr. Tomas options to be determined on or before June 30, 2015. The Company’s Board of Directors approved a bonus of $500,000 and options to acquire 10,000,000 shares of the Company’s common stock for ten years with four year vesting and a cashless exercise provision at an exercise price equal to the five day average closing price of the Company’s common stock as of August 1, 2014. The cash bonus may be paid in the form of a six month promissory note.
On July 28, 2014, the Company’s Board of Directors approved the 2014/2015 salary for Kristin Comella, Chief Scientific Officer, at $250,000 per year, beginning July 1, 2014 with an incentive bonus ranging from $100,000 to $300,000. In addition, the Board of Directors will grant Ms. Comella options to be determined on or before June 30, 2015. The Company’s Board of Directors approved a bonus of $300,000 and options to acquire 5,000,000 shares of the Company’s common stock for ten years with four year vesting and a cashless exercise provision at an exercise price equal to the five day average closing price of the Company’s common stock as of August 1, 2014. The cash bonus may be paid in the form of a six month promissory note."
So, just those 2 alone (out of a company with qty-4 full time employees per their own 10-Q filing)- those two consume $525K + $250K + $500K + $300K = $1,575,000 dollars a yr for a near CASH BROKE, almost R&D spending ($3K per month), no approved product, 4 full time and one part time "company" that work out of a tiny, 4000 sq-ft leased facility (about a $6K per month facility lease payment per their SEC filing).
Yep, not hard IMO to figure out where most of the money is going and it's not "trials" or "medical research" that I can see per their SEC filings.
Totally agree. They're so cash poor ($46K TOTAL cash end of last qtr against over $2 MILLION in just immediate accounts payable), they pay common bills in shares of stock and have been doing so for years, for everything from "accounts payable" to "services rendered" and to "related party notes" (insider loans), and other vague terms, etc. 10's of millions of shares of common stock, continually, for years.
A big chunk of any cash that does come in to the company via financing or reported "revenue" or whatever, just goes straight to the salaries and large cash bonuses of just 2 people, while they spend next to nothing on R&D ($3K per month spent on R&D per the last 10-Q filing, less than the monthly lease payment on their small facilities), meaning there's no way IMO they're conducting any FDA level phase II or phase III trials, all of which stalled out years ago. Then they claim to be doing all these new additional "studies" and who knows what- which is supposed to lead to what, when they can't even fund and pay their daily bills w/o issuing common stock in the 10's of millions of shares? Makes zero sense IMO.
Also, the lower this share price goes- the harder and harder it gets for them to raise small amounts of cash via the convertible, toxic debt deals they've been surviving on, and the more dilutive each deal gets.
From their last filed 10-K, PAGE F-28:
During the year ended December 31, 2013, the Company issued 50,029,227 shares of common stock for proceeds of $865,000.
During the year ended December 31, 2013, the Company issued 31,052,141 shares of common stock issued under its standby equity distribution agreement with Greystone Capital Partners.
During the year ended December 31, 2013, the Company issued an aggregate of 5,656,340 shares of its common stock for settlement of $82,339 of accounts payable. In connection with the settlement, the Company recorded a loss on settlement of debt of $74,877.
During the year ended December 31, 2013, the Company issued 2,500,000 shares of its common stock in connection with the issuance of a note payable.
During the year ended December 31, 2013, the Company issued 57,967,906 shares of its common stock in connection with the settlement and/or conversion of various notes payable.
During the year ended December 31, 2013, the Company issued 34,890,348 shares of its common stock in connection with the settlement of related party notes payable and advances.
During the year ended December 31, 2013, the Company issued 9,408,718 shares of its common stock in settlement of interest and penalty in connection with convertible debt.
During the year ended December 31, 2013, the Company issued 6,220,263 shares of its common stock services rendered valued at $85,151.
During the year ended December 31, 2012, the Company issued an aggregate of 952,851 shares of its common stock, valued at $34,600, in exchange for services rendered.
During the year ended December 31, 2012, the Company issued an aggregate of 51,751,138 shares of its common stock in exchange for $720,214 of outstanding notes payable and related accrued interest.
During the year ended December 31, 2012, the Company issued an aggregate of 700,000 shares of its common stock in exchange for $14,000 of accrued liabilities.
During the year ended December 31, 2011, the Company issued an aggregate of 1,982,995 shares of its common stock on exercise of options.
During the year ended December 31, 2011, the Company issued an aggregate of 27,120,856 shares of its common stock in exchange for $1,542,109 of outstanding notes payable and related accrued interest.
During the year ended December 31, 2011, the Company issued an aggregate of 1,000,000 shares of its common stock, valued at $115,035, in exchange for services rendered.
During the year ended December 31, 2011, the Company issued an aggregate of 1,272,730 shares of its common stock in settlement of outstanding related party advance in the amount of $140,000."
And most recent 10-Q, PAGE 27:
Subsequent issuances
On October 3, 2014, the Company issued 514,886 shares of its common stock as payment of $70,521 interest on its Northstar (related party) debt.
In October 2014, the Company issued 1,818,182 shares of its common stock in settlement of $20,000 of convertible debt.
In October 2014, the Company issued 1,293,103 shares of its common stock in settlement of $15,000 of convertible debt.
In October 2014, the Company issued 2,260,764 shares of its common stock in settlement of $18,000 of convertible debt and accrued interest of $2,120.
In October 2014, the Company issued 552,846 shares of its common stock in settlement of $5,500 of convertible debt and accrued interest of $1,300.
In October 2014, the Company issued an aggregate 2,773,549 shares of common stock for consulting services.
In October 2014, the Company issued 538,875 shares of common stock in settlement of accounts payable."
There's sections like that in every 10-Q and 10-K they've filed for at least the past several years.
"So for now we are listed on nasdaq but still trade on the pink sheets? "
There is no such thing. There's no mythological hybrid of being a "NSADAQ" stock but trading as "OTC" or whatever??
OCAT is an OTC stock still. Simple as that.
See the FINRA web site. What FINRA says is all that matters and they are clear, that OCAT "nasdaq listing is POSTPONED, trades on OTC". When it comes to the U.S. public traded markets and who has power, you got GOD, then the SEC and at the right hand of the SEC is FINRA and at the left hand is the DTC (Depository Trust Corp). If they say it, then it's true, if they say it's not on the NASDAQ yet, then it hasn't happened. FINRA is who often prosecutes brokerages, companies for stock trading compliance issues, etc- then the SEC steps in if it gets real big time. DTC "clears" and processes literally every trade in all U.S. markets- they can "freeze or chill" a stock and shut it down, literally rendering a company to the ash heap of history. Then the SEC is basically the power of the Federal Govt above those two watchdog agencies they use as their main, market police forces. So, FINRA wields tremendous power. Just get the info straight from FINRA and cut out any middleman noise like E-trade or whatever.
http://otce.finra.org/DailyList
12/17/2014 05:25:29 Reinstatement 12/16/2014 21:00:00 OCAT Ocata Therapeutics, Inc. Common Stock undefined Other OTC
Daily List Date 12/17/2014 08:25:29
Event Type Reinstatement
Effective/Execution (Eff/Ex) Date 12/17/2014 00:00:00
Issue Type
Symbol OCAT
Issue Name Ocata Therapeutics, Inc. Common Stock undefined
Class
ADR Ratio
Maturity Date
Market Category Other OTC
Offering Type No Restrictions
OATS Reportable (Rptbl) Flag Yes
Unit of Trade 100
Reg Fee Flag Yes
Daily List Comment Market Center move to NASDAQ postponed.
It smacks REAL BAD of being manipulated again today. Numerous testimony of retail buyer/sellers getting bad quotes via major on-line brokerages, testimony of getting bad quotes and told they can not buy/sell via phone support at places such as E-trade.
Meanwhile, since Friday, they've run it up near $7.50, down to $5.90 or so and appear to be running another "blind" day for retail folks, but heavily traded by "someone" who then sinks it with some sell orders. Like a classic P&D and use of heavy shorting again.
I just don't see how they're gonna try and sell a secondary, a large, dilutive secondary of 10 million shares, when the price is being jerked all over the map- let alone what FINRA and the SEC must be thinking of this "mess" since Friday.
I've watched a lot of stocks for a lot of years and never, ever have seen something like this. This "up listing" notice on the FINRA site late into the night (the bait) then the early AM "reinstatement" back to OTC right before market opens, then blank quote screens all over the net on nearly every major portal and people seeing their accounts go blank, then flash NASDAQ, then back to OTC then errors in price quotes, then inability to enter orders or get fills, etc.
This just can't be good for this company right now and I don't see how FINRA and other regulators can't be looking at this with a microscope now. It's smells real, real bad in my book. It wreaks of some bad attempt to manipulate this secondary process- and the silence by the company is deafening.
If it traded as low as $5.90 just yesterday, then what sophisticated underwriter is not going to use that as their point from which to discount the shares even further? They aren't stupid? If they really wanted the stock, then they'd just buy it at retail and have paid $5.90 a share. NO WAY in my book do they pay anywhere near that recent price point. They are wholesalers effectively- paying discount is their entire business. It's like today someone is trying to load the boat again by sucking in some more retail buyers, all the while knowing they're gonna unload on um at any moment or use their cheap, discounted shares later to cover a short. I wonder how much of today was just pure, pro level short selling to amateur buyers? Like to see those numbers.
Where can anyone even get an accurate quote on price and volume right now? Today the OTC Market site isn't even updating and it's been the one reliable source since Friday. FINRA this AM says OTC "other" and yet it's not quoting. I-HUB blank, OTC site blank, numerous other places I checked blank or charts don't look to be updating properly? Who knows even if the prices seen anywhere are even real again today- Friday the bad REDUX PART II. Not good IMO. B A D, BAD.
"tax write off season again"???
But why would anyone be selling and needing and taking a tax write off if this is the greatest medical miracle and breakthrough of probably the past 100 yrs?
I can't make sense of that? Why would it sell off? Why aren't insiders buying using their own money too? One would think they'd buy it all up? Instead they vote themselves huge base pay increases and also very large cash bonuses for just 2 people in the company- but they never buy their own shares, even when they are so cheap, like barely a penny? Why?
Wouldn't they just buy the company and take it private? Doesn't the Leonhardt gut always give "presentations" and all and have money or money people around? How come they never finance this with non-dilutive money for an equity stake in it?? It's market cap is like $7 million and about $10 million in debt- where's some buy-out group to pick this up for $17 million, which is pocket change on Wall Street?
Why only continual dilution and use of toxic, convertible debt, desperation financing to eek along, barely making it and putting no money towards trials for a yr or more? $3K per month R&D on last 10-Q?? $46K cash total, last 10-Q, which doesn't even pay a month or two of the base salary and bonuses for just the 2 people? That's it? How come?
I don't understand the need for "tax loss selling" on a winner stock and winner company? Makes no sense to me, IMO?
Also, the LOWER IT GOES, the more MAGNA will dilute. "IF" they can get this Magna credit line approved- then it's just that much more dilution now as the price drops lower and lower.
Say they wanted to make a "draw" for $500K now with the share price at .0120. Magna then gets to pay no more than .93 X .0120 = .011 per share.
$500K/.011 = 45 MILLION shares of dilution it will take just to get $500K dollars, and that doesn't even pay the 2014 cash bonuses awarded for just the 2 people ($300K + $500K = $800K in bonuses presently owed the CSO and CEO per last 10-Q filing).
45 MILLION shares of pure dilution it would take just to get $500K, plus the 9 million shares already issued to Magna for up-front "fees" plus the 31 MILLION shares for the $200K Magna "note". So that alone is almost 100 MILLION shares more of pure dilution that can happen in a blink, added to the already massively diluted, ever increasing share count.
Not looking too good here IMHO.
"It's in a downtrend could go under .01"
Uh, there it went, just when I hit click- just SANK LIKE A STONE to .0119. A new 8 month low, DOWN, RED 13% plus or more. Holy cow this is collapsing bad in here. Wow !
Yep, I totally agree. It's awfully close to 1 CENT right now and nothing they've done, "PR" or "10-Q" or "blogs" or whatever has made the slightest dent in stopping this falling bid and steady decline in price.
They also must be close desperately low on cash at this point- and now it appears this Magna "credit line" is at least slow or delayed in becoming active, per the S-1/A SEC form just filed.
They got another lawsuit going and appear to be hiring lawyers, so that's just more expenses there IMO, that can't be cheap when looking at the law firm profiles and all. Lots of money these guys seem to spend on lawyers IMO, yet almost nothing on R&D or trials? The last 10-Q had um spending about $3K a month, total, on R&D which is stunning when they claim they're the "phase II/III LEADER" or whatever it says on their twitter page and other places. $3K a month? What does that fund for a medical research company? Small labs spend more than that just on disposables and stuff like gloves and masks and throw away stuff and glassware, etc. Wonder what one of these law firms is costing to defend um, on retainer? I'd guess way more than that R&D budget of $3K per month?
https://www.clerk-17th-flcourts.org/Clerkwebsite/BCCOC2/OdysseyPA/CaseSummary.aspx?CaseID=7155410&hidSearchType=party_case&DisplayCitation=no&CaseNumber=CACE13024037&SearchType=
https://www.clerk-17th-flcourts.org/Clerkwebsite/BCCOC2/OdysseyPA/CaseSummary.aspx?CaseID=7862332&hidSearchType=party_case&DisplayCitation=no&CaseNumber=CACE14021256&SearchType=
It's got "PR fatigue" IMO. It's just not cutting it anymore. Too much low priced share overhang and just raw dilution down pressure on the shares.
It hasn't updated yet, but a spike on the I-HUB chart this AM looks like it might have just taken a dip to .0120? Everyday the bid is ratcheting down it looks like. Lower lows and lower highs.
Extremely weak in here now. No sign of even a slight reversal yet.
"Must be a hell of a bidding war here for those 10M"???
When it traded into the $5's yesterday?
There's no "bidding war" IMO. More like lack of pricing power from a high risk sale and lower demand.
They would NEVER hold back on a secondary and not "strike" if the iron was red hot. They'd get those shares out and sold as fast as humanly possible.
There's no rumored bidding war or any indication such as thing is taking place? The price doesn't show any such thing. The hedge funds and people "in the know" would be all over this as buyers if that was true.
No way IMO. This is a sign of low demand and low pricing power on the secondary the way it looks to me, not the opposite.
The OTC sit as of right now isn't even quoting- like it might be halted, or just a big mess again this AM, since the FINRA "reinstatement". They can't even get daily trading right or smoothed out. This has been a huge mess IMO.
http://www.otcmarkets.com/stock/OCAT/quote
It's a no quote right now? Figure that one out? Another messed up trading day it looks like?
"For the first time E*TRADE showing "
E-Trade can show whatever they want. It NEVER trumps what FINRA says. FINRA rules the market and can shut E-trade down with a simple memo. FINRA and the SEC are essentially one in the same.
FROM FINRA, live this AM:
http://otce.finra.org/DailyList
Daily List Comment Market Center move to NASDAQ postponed.
Market Category Other OTC
12/17/2014 05:25:29 Reinstatement 12/16/2014 21:00:00 OCAT Ocata Therapeutics, Inc. Common Stock undefined Other OTC
Until FINRA says it, then it's moot and has not happened. Nothing trumps FINRA in the markets-they're the ones that regulate all the brokerage houses such as E-trade.
Going to the FINRA site is getting it straight from the source. E-Trade is like getting it 2nd hand news or via the rumor mill.
"go to icell"???
I can't think of a worse place to get accurate information about stocks or companies. More wrong info and pure speculation there than probably any other website source IMO. Nothing but amateurs who are in love with this stock and never post an objective statement that I've ever seen. Most statements appear to be by total financial illiterates who never read SEC filings or similar, or if they do, they get the meaning 100% incorrect more often than not. They only state to buy more, no matter what happens.
The icell track record of "predictions" coming true or being accurate is less than throwing darts at a board of random choices from what I've seen. Last place I'd go for any stock information.
You got far more objective and a better cross section of info and opinions right here on I-HUB than one will ever find on icell, IMO.
My 2 cents.
FINRA, live now, OTC "reinstatement".
http://otce.finra.org/DailyList
They appear to be unable to get the secondary priced and sold IMO. Low demand and low pricing more than likely. If this sold in the $5's yesterday, then how low would the secondary have to be priced? Lower than yesterday's lowest price IMO, because no "sophisticated" buyer would ever pay a premium over what they could have just bought it for on the free market.
Since Friday, this has been a pretty big mess IMO. Can't be helping to sell 10 million shares the way this is going.
From FINRA, the only source that counts IMO (forget what any other brokerage or quote screen says, FINRA is god to the markets)
12/17/2014 05:25:29 Reinstatement 12/16/2014 21:00:00 OCAT Ocata Therapeutics, Inc. Common Stock undefined Other OTC
Market Category Other OTC
Daily List Comment Market Center move to NASDAQ postponed.
"If your reading comprehension was astute"
MY reading comprehension is QUITE astute. Thanks for the tip though.
One needs to read that statement on page S-17/S-18 in full context.
The ONLY thing it's referring to being "locked up" is in regards to the sale OF SIMILAR SECURITIES. It has nothing to do with some supposed "lock up" of shares the underwriters are purchasing as part of the secondary offering.
Here's the ENTIRE PASSAGE and not a "selective" cut n paste where the word "lock up" was found via a search function or similar. It's pretty crystal clear. It has ZIP to do with any restriction of the shares that the underwriters are purchasing in the offering and any "restriction" to sell them at-will, free of any delay or "lock up" period.
PAGES S-17/S-18: THE FULL CONTEXT, titled, "SALE OF SIMILAR SECURITIES"
"No Sales of Similar Securities
We and each of our executive officers and directors have agreed, subject to specified exceptions, not to directly or indirectly:
•
sell, offer, contract or grant any option to sell (including any short sale), pledge, transfer, establish an open "put equivalent position" within the meaning of Rule 16a-l(h) under the Securities Exchange Act of 1934, as amended, or
•
otherwise dispose of any shares of common stock, options or warrants to acquire shares of common stock, or securities exchangeable or exercisable for or convertible into shares of common stock currently or hereafter owned either of record or beneficially, or
•
publicly announce an intention to do any of the foregoing for a period of 90 days after the date of this prospectus supplement without the prior written consent of Jefferies LLC, Cowen and Company, LLC and Piper Jaffray & Co.
S-17
Table of Contents
These restrictions terminate after the close of trading of the common stock on and including the 90th day after the date of this prospectus supplement. However, subject to certain exceptions, in the event that either:
•
during the last 17 days of the 90-day restricted period, we issue an earnings release or material news or a material event relating to us occurs, or
•
prior to the expiration of the 90-day restricted period, we announce that we will release earnings results during the 16-day period beginning on the last day of the 90-day restricted period,
then in either case the expiration of the 90-day restricted period will be extended until the expiration of the 18-day period beginning on the date of the issuance of an earnings release or the occurrence of the material news or event, as applicable, unless Jefferies LLC, Cowen and Company, LLC and Piper Jaffray & Co. waive, in writing, such an extension.
Jefferies LLC, Cowen and Company, LLC and Piper Jaffray & Co. may, in their sole discretion and at any time or from time to time before the termination of the 90-day period release all or any portion of the securities subject to lock-up agreements. There are no existing agreements between the underwriters and any of our stockholders who will execute a lock-up agreement, providing consent to the sale of shares prior to the expiration of the lock-up period."
It's referring to the fact that OCAT INSIDERS are under 90 day "lock" so that THEY can't sell their options or dump other shares, raise cash dumping other shares, etc. It's to protect the underwriters price and sales by not having more dilution shares dumping into the market while THEY, the UNDERWRITERS are busy selling their 10 MILLION shares.
READ THINGS IN CONTEXT, THAT is what is known as "astute reading comprehension". It's about restricting OCAT insiders from selling, NOT the underwriters. It's crystal clear IMO.
In other words- the underwriters are like the "house" in VEGAS and they DO NOT LIKE COMPETITION, so they added that clause to give them 90 days, competition free selling- where no one else can dump shares into THEIR market essentially. The underwrites write everything in these deals to TILT IT ALL IN THEIR FAVOR. That's how it works when YOU GOT THE CASH and someone else DESPERATELY NEEDS IT. You're in the driver's seat.
"Do you think the uplisting will help them unload those shares at higher prices"
I have no idea. I'm a total amateur and as in the dark as anyone. My personal experience is that regardless of exchange (NASDAQ, even NYSE) - is that dilution tends to put downward pressure on the shares (often called share overhang). Unless that dilution is what they call immediately "accretive" (additive) to earnings. Meaning for example the dilution is used to buy a competitor and the synergy of the two new merged companies means they immediately cut costs, increase sales and make more earnings. But pure cash burn dilution like this, are usually a down force on share price in my experience. Meaning first, all those 10 million shares need to kind of get "sucked" up and absorbed initially into the market- sort of an initial shock as that many more free trading shares just hit all at once.
Also, if "institutions" were to buy- I'm not share who they'd be? This is still a very speculative micro-cap and will probably be barely above $5 a share on an uplist if this week's trading action/price is any indication. That kind of price per share is skirting the edge of what "institutions" will buy normally IMO.
Further, the bigger worry IMO is that the underwriters are going to engage in selling to hedge funds and even be making a market in the stock- which to me, spells all kinds of possible shanigans. Shorting being the prime candidate I'd be worried about, with dilution weakness to back them up in their shorting efforts. I've seen a lot of companies raise desperate cash via this very method over the years, one's I owned, and their shares often took a serious pounding for an extended period of time, some recovering, some never getting out from under the thumb of the "big boys".
So I have no idea. Look at the company Wooten, the new OCAT CEO just left and came from as a prime example IMO. It's NASDAQ listed and has about $45 MILLION, cold hard cash in the bank and zero debt, but is trading just above about $2 bucks a share right now and a high of maybe $5 a share. I doubt it's got much "institutional" ownership IMO. I can run a simple filter and find probably 100 NASDAW listed companies in under 5 minutes all who trade for $2 to $3 bucks a share- w/o a doubt in my mind.
In other words, an "up-list" by no means IMO guarantees any price appreciation, or price "stabilization" or freedom from shorting, etc. I'd argue just the opposite- that shorting once on the NASDAQ just gets that much easier to do. Call up any discount broker in the country and ask um for short inventory on an OTC "penny" stock. Almost impossible to get and if they do- it's gonna be super restrictive margin account with reams of paperwork, etc. Call up any discount broker on a NASDAQ stock around $5 or more a share- and even Joe Q. Public can probably get short inventory. When it gets below $5 a share, the brokerages will tighten up on the requirements/margin restrictions typically. But the hedge funds? IMO, they salivate for stocks like this to come along- one with several yrs to a product, one that's gonna be burning cash by the dump truck full to fund these trials and projects, no earnings or cash flow for a distant couple of years and only dilution, dilution, dilution to live on, per their own wording, for at least several yrs. I can see the hedge boys just piling on to this w/o mercy.
Wall Street is a brutal place. Shorting is 100% legal per our Congress. This kind of stock is in a weak spot right now and the sharks on Wall St live to sniff out blood and weakness IMO. I'd say it's speculative in here now which way this will go. I sure don't think they helped themselves either with this Friday "thing" and Monday AM thing, whatever it was.
Can it "pop" and spike along the way- for sure, not a doubt in my mind. But does it face mega headwinds to accomplish what they're trying to do- and a long road, I think for certain w/o a doubt. This is not even close to some slam-dunk, done deal, moving onto easy street from here. No way IMO.
Where it prices and what the price does after the underwriters get their Wall St mitts into it- I have no idea. My experience, maybe a little pop, then they might bury it for a while. That's been the experience of some I've owned prior when I was a lot younger and a lot more naive. But it's all a crap shoot- literally. Wall St and Vegas, very similar placed, unless one is talking about owning Coca Cola or Exxon- Modile or something and buying um for the dividend for life. My 2 cents.
"I believe all of those shares will have restrictions barring them from being sold at least for a year. "
Reading the Prospectus, I don't find any verbiage that any shares are, or will be, "restricted" in any way, shape or form. It is pure dilution of the most basic kind and the 30% dilution shares (10 million) can and will hit the market as "free trading" as soon as the underwriters can "flip trade" um IMO.
In this type of deal, the underwriter (in this case qty-3 underwriters got together, which implies to me they want to spread the risk)- the 3 underwriters purchase all 10 million shares and have a pretty common "over-allotment option" to pick up, as much as 1.5 million more if they feel they can make money on them.
What is the fundamental business model of how an underwriter makes money? (the term underwriter is from old English/British insurance biz language, it's related to understanding, quantifying and then assuming RISK). Risk being the key word. The underwriters hand OCAT say $45 Million dollars (say $50 million gross for 10 million shares at $5 per share - $5 mil in fees and expenses) and then the underwriters now own the 10 million shares and the associated RISK that they can sell those shares FOR A PROFIT, their entire business model.
Underwriters have basically 3 ways they make money:
1) The initial share discount they get on the shares they buy (the "spread")
2) Fees and expenses
3) Over allotment sales
The underwriter is the classic "middle man" who puts up money for RISK and "bets" on "taking a cut of the action" on the "spread" for being in the middle.
Thus, right at the front end, the underwriter pays a "discount" to market for the 10 million shares for their "risk" they're taking. If OCAT closes at $6 today for example, then the underwriter pays say a 10% discount or .9 X $6 = $5.40 a share.
But underwriters are not "investors" or long term holders of stock. They're essentially lenders/banks and want to "turn" their money as fast, and as often as possible and earn effectively a "return", commonly called "interest" or a "rate of return". The more times they can "flip" the same money for a profit in a given yr, the greater their returns.
So prior to buying the 10 million shares, the underwriters "run book" (make sales calls and build a "book" list of potential buyers of the shares) and go out and pound the phones, speak to hedge funds, talk to brokers,etc and try and gauge the demand to re-sell, quickly, all 10 million shares of OCAT, aka on the "flip trade".
Now the underwriter has 10 million shares they paid $5.40 for or whatever and OCAT got the cold, hard cash they wanted, paid to them by the underwriters (minus fees, expenses, etc of course, ie. the "net" amount). Now the underwriter(s) want to unload those shares ASAP, "make the spread" and pocket those profits. Thus the "book" they pre made, they start re-selling those discounted shares, now at say $5.80 a share. BUT WAIT, someone says, a lousy .40 cents per share profit? But DO THE MATH, .40 cents on 10 million shares is $4 MILLION dollars for a month's "work" (if one can call it that), plus large sales commissions, fees and expenses. They might make $10 million for putting up $50 million and do it in a matter of a few months or less. Do that 2X in a yr and you just made $20 MILLION on $50 million dollars, or an annual rate of return of 40% on your money. Other than mafia or payday loans or theft- what else in this world pays 40% annual returns on your money?
See why underwriting is a big business for the big boys of Wall (FRAUD) Street? It's like printing money it can be so lucrative. They're just sales people and actuaries who asses risk and charge handsomely for it.
The higher the risk a stock, then the more the underwriter discount and fees, etc. I'd say OCAT, being cash poor, in biz for yrs but never producing a product, no cash flow from operations, no product approvals, a bit of a tainted past with SEC violations, accounting problems, etc is in the "high risk" category would be my guesstimate. You underwrite Facebook, a massively successful money printing machine, soaked in the who's who of prime venture capital money before even going public- and underwriters beat the door down to get a piece of the action and demand that monster will bring. A tiny OTC company "trying" to eek onto the lowest tier NASDAQ and the underwriters are gonna want more upfront fees and bigger discounts- as they DO NOT want to get stuck with any shares of OCAT, not being able to sell and "flip trade" them quickly.
Further, read the prospectus carefully- it makes it 100% CRYSTAL CLEAR that the underwriters themselves, as well as hedge funds or others they may sell shares too, can and WILL "short" the stock "at will" and "as needed" up to and including "naked shorting" so as to protect their down side, increase their profits, blah, blah, blah as they see fit. It also makes it clear that the underwriters intend to "make a market" in OCAT stock, aka, become MM's themselves in the stock; think trading, shorting, hedging their own holdings as they sell them, etc
THAT is the "underwriting" process in a nut shell. There's no "share lock up" such as a traditional IPO where certain insiders may not be able to sell into the initial IPO- as the underwriters are the share buyers here and the insiders really have no role. This is a secondary "shelf" offering of an already public traded company.
Here's a few highlights from the prospectus that emphasize the points above:
Recently filed OCAT secondary prosepectus:
PAGE S-6:
"Common stock to be outstanding following this offering
44,612,718 shares (or 46,112,718 shares if the underwriters exercise their option to purchase additional shares in full)."
PAGE S-12
"After giving effect to the sale by us of 10,000,000 shares of common stock in this offering at an assumed public offering price of $ per share, and after deducting the underwriting discounts and commissions and estimated offering expenses that we will pay,"
PAGE S-16:
"The underwriting agreement provides that the obligations of the several underwriters are subject to certain conditions precedent such as the receipt by the underwriters of officers' certificates and legal opinions and approval of certain legal matters by their counsel. The underwriting agreement provides that the underwriters will purchase all of the shares of common stock if any of them are purchased. If an underwriter defaults, the underwriting agreement provides that the purchase commitments of the nondefaulting underwriters may be increased or the underwriting agreement may be terminated. We have agreed to indemnify the underwriters and certain of their controlling persons against certain liabilities, including liabilities under the Securities Act, and to contribute to payments that the underwriters may be required to make in respect of those liabilities.
The underwriters have advised us that, following the completion of this offering, they currently intend to make a market in the common stock as permitted by applicable laws and regulations. However, the underwriters are not obligated to do so, and the underwriters may discontinue any market-making activities at any time without notice in their sole discretion. Accordingly, no assurance can be given as to the liquidity of the trading market for the common stock, that you will be able to sell any of the common stock held by you at a particular time or that the prices that you receive when you sell will be favorable.
The underwriters are offering the shares of common stock subject to their acceptance of the shares of common stock from us and subject to prior sale. The underwriters reserve the right to withdraw, cancel or modify offers to the public and to reject orders in whole or in part. In addition, the underwriters have advised us that they do not intend to confirm sales to any account over which they exercise discretionary authority."
PAGE S-18:
"Stabilization
The underwriters have advised us that, pursuant to Regulation M under the Securities Exchange Act of 1934, as amended, certain persons participating in the offering may engage in short sale transactions, stabilizing transactions, syndicate covering transactions or the imposition of penalty bids in connection with this offering. These activities may have the effect of stabilizing or maintaining the market price of the common stock at a level above that which might otherwise prevail in the open market. Establishing short sales positions may involve either "covered" short sales or "naked" short sales.
"Covered" short sales are sales made in an amount not greater than the underwriters' option to purchase additional shares of our common stock in this offering. The underwriters may close out any covered short position by either exercising their option to purchase additional shares of our common stock or purchasing shares of our common stock in the open market. In determining the source of shares to close out the covered short position, the underwriters will consider, among other things, the price of shares of common stock available for purchase in the open market as compared to the price at which they may purchase shares through the option to purchase additional shares.
"Naked" short sales are sales in excess of the option to purchase additional shares of our common stock. The underwriters must close out any naked short position by purchasing shares in the open market. A naked short position is more likely to be created if the underwriters are concerned that there may be downward pressure on the price of the shares of our common stock in the open market after pricing that could adversely affect investors who purchase in this offering.
A stabilizing bid is a bid for the purchase of shares of common stock on behalf of the underwriters for the purpose of fixing or maintaining the price of the common stock. A syndicate covering transaction is the bid for or the purchase of shares of common stock on behalf of the underwriters to reduce a short position incurred by the underwriters in connection with the offering. Similar to other purchase transactions, the underwriters' purchases to cover the syndicate short sales may have the effect of raising or maintaining the market price of our common stock or preventing or retarding a decline in the market price of our common stock. As a result, the price of our common stock may be higher than the price that might otherwise exist in the open market. A penalty bid is an arrangement permitting the underwriters to reclaim the selling concession otherwise accruing to a syndicate member in connection with the offering if the common stock originally sold by such syndicate member is purchased in a syndicate covering transaction and therefore has not been effectively placed by such syndicate member.
Neither we nor any of the underwriters make any representation or prediction as to the direction or magnitude of any effect that the transactions described above may have on the price of our common stock. The underwriters are not obligated to engage in these activities and, if commenced, any of the activities may be discontinued at any time.
The underwriters may also engage in passive market making transactions in our common stock on The NASDAQ Capital Market in accordance with Rule 103 of Regulation M during a period before the commencement of offers or sales of shares of our common stock in this offering and extending through the completion of distribution. A passive market maker must display its bid at a price not in excess of the highest independent bid of that security. However, if all independent bids are lowered below the passive market maker's bid, that bid must then be lowered when specified purchase limits are exceeded."
Just some of the tid-bits in the prospectus. NOT A WORD I find about any "lock up period" or anything similar. IMO, the underwriters will be SELLERS/flippers of OCAT stock the instant they get their discounted shares. That's what they do. To who and at what price they will be willing to sell those shares, who knows? But it's 100% pure, free trading, 10 million shares, 30% dilution to common and will be instantaneous upon the instant the secondary is placed IMO. The Prospectus makes it clear IMO, it lists the share count in black n white at 44 MILLION shares "upon close" of secondary. Doesn't get much clearer than that.
.0138, it just made the new 8 to 9 month LOW BID looks like. Wow.
From .08 in March or so, to .06 and now all the way to .0138. About a 80% loss to common shares since March/April of this year. And that's with a very large amount of share dilution also occurring in that same, 8 or so month time frame.
Appears to be no floor whatsoever under this bid anymore. I can easily see 1 cent before long at the pace it's been in decline and a steady, unbroken downtrend w/ lower highs and lower lows being made.
Market cap is now at about $7.7 million, with just accounts payable debt on last 10-Q of $2 million plus, $46K cash left and $10 million or more in total debt.
Looking pretty dicey in here IMO. And now this Manga "credit line" funding deal appears at least delayed at best, based on the amended S-1/A filing just posted to the SEC.
If the Magna "credit line" were to fund, and they needed to tap it now (which the SEC filing stated they intend to fully tap it for immediate cash needs) -
Then the price per share right now to Magna, at best would be .140 X .93 = .0130 per share. Meaning a lot more dilution to get the money they need, the lower the share price goes.
Wow.
Just traded $5.91, Wow !!
"That is still 50 million dollars in OCAT coffers."
They don't net $50 million if priced at $5 a share, on 10 million shares sold. Underwriter fees and expenses are stiff. The company always nets a fair amount less than the offering gross proceeds.
$45 million, maybe? Who knows?
Just broke down hard below $6, now at $5.91 on OTC.
http://www.otcmarkets.com/stock/OCAT/quote
Might need to lower even that $5 a share target for secondary pricing perhaps? This is looking real weak in here, IMO. Not good.
Dilution, and lots of it (30% in this case, just on round one of this shelf offering), unless it adds positive to a company's earnings, typically puts strong down pressure on a stock's share price IMO. That's historically what I've always learned and observed.
OTC bid $6.01 X $6.05 ask
http://www.otcmarkets.com/stock/OCAT/quote
Looks like some tough sledding in here IMO. "If" they price the secondary now (and they desperately need the cash if they're even to begin all that they've recently presented, especially a down payment on a start of a FDA quality phase II trial)-
If they price the secondary now, a minimum, traditional share discount to an underwriter is about 7% (just Google stocks underwriter discount or the underwriting of a stock offering, numerous sources state 7% is traditional) and probably more for a high risk micro-cap stock trying to move off the OTC, and one with no sales, no cash flow, no ROI, no prospects of cash flows, no earnings for yrs out in the future, lots of on-going dilution, etc
Even at 7% discount that would be $6 per share X .93 = $5.58 that the underwriters "might" be willing to pay to take on the risk of needing to move and sell 10 ma million shares, aka 30% pure dilution to common.
I'd guesstimate they're gonna want a steeper discount than that 7% traditional IMO. Meaning it prices less than $5.50 IMHO.
"according to finra, OCAT postponed uplist another day.. "
Here's the link direct to FINRA if any want to see it/read it for themselves.
http://otce.finra.org/DailyList
OCAT will clearly be seen listed on a row of the "daily table" of events dated today. Go to the right and click the little "notebook" or "page" symbol and it will open a pop-up that lists all the details just given by cty.
Interesting IMO that they've now had to appear twice on this FINRA "daily list" and post as "Reinstatement" to the OTC?? Cause they made the same FINRA table of announcements yesterday with the same "Reinstatement" message. How many times must they "Reinstatement" to the OTC I wonder and why? Are they on some daily review or something now until this Friday thing/mess is sorted out, such that FINRA gives um a daily provision as "Reinstatement" and trading or something? Very interesting IMO. It would seem that once "Reinstatement" was made yesterday at FINRA, then one is on the OTC until further notice and the "NASDAQ postponed" the same until further notice? Why a daily announcement on FINRA?
" boilerplate message"?? I thought they're called SEC FILING FACTS.
Versus slide show "presentations" and vast claims of "we hope" and "we possibly expect" and "we think it might" and "if all goes well, then" and "we may possibly" and "it could result in" and see all our disclosures and safe harbor and "other" disclaimers when reading any of these PR and "presentations".
I prefer to go with the meat of the SEC docs, the ones that bind them legally and hold their feet to the fire. They don't put anything "boiler plate" in a SEC filing. Every last word is in those filings for a reasons- because they know if they fudge it there or stretch it there or over-hype it a bit there or tell the story a little too "rich" there- then they can be held 100% accountable for it. It's the facts versus the sales pitch.
SEC docs for me. All else, just presenting the happy side of the "story". Words like "maybe" and "hope to soon" and "if this, then maybe"- they got 10 plus years of all that. And it just went below $6 bucks a share this AM, the same as 6 CENTS pre R/S split.
That's the reality of it IMO. Where's all the "big money" beating a path to their door if this is all the greatest medical miracle since sliced bread? How many years- yet dilution is all they live on? Why? Why is that? The greatest bull market of the past 100 yrs, swimming in easy money- yet no one has stepped up to pitch these guys a bundle of non-dilutive cash? Why would that be? There are dump trucks full of money in these past 3 plus yrs. Some of the most successful IPOS in history just took place: Facebook, Linkedin, GoPro a lousy camera company just raised a bundle, Alibaba, Twitter, Sprouts market, Michaels stores, numerous bio-tech and med product companies, Dropbox, Spotify, and on and on. SWIMMING in money on the Street and at venture firms. But none has flowed to OCAT, who's supposedly sitting on this "medical miracle"?? Why?
It makes no sense IMO. Money, the "big money" finds it's way if one has the goods- they'd beat a path to your door normally. Yet no "big money" has come anywhere near OCAT. Dilution is what they live on and have lived on and are still planning to live on. Tells a lot of the "story" IMO.
"The ceo leaves his secured excellent paying job to come here and he has no clue about whay you shared with us."
The CEO left a company who's stock was going in the tank on his watch. He was also a prolific seller and dumper of shares at that company. He's receiving very similar compensation here- so what did he "give up"? Who knows? For all anyone knows- he was on his way out at the last company. That's how it usually works at that level.
Further, his FIRST STEP upon getting to OCAT was to arrange an insider self-vote to further enrich the Sr mgt members with a perks n goodies booster plan program. Nothing "new" going on IMO.
It makes no difference who the CEO is until he performs and shows some tangible results- none of which has happened yet. The share price, it's parked in the same price range where Gary left it- despite all the "big news" and the "article" being published and all the rest. It's at about 6 CENTS, pre R/S split. It entered the 5's this AM. What has that done for shareholders? It's priced where it was what, 8 or more yrs ago if I'm not mistaken? They're about to dilute the common by another instant 30%, the equivalent of 4.4 BILLION pre R/S shares.
I don't see anything to show "performance" yet IMO. It's like watching a replay of the past 5 or more yrs to this point.
"Just a quick reminder from the horses mouth - not hear say!!! "
Interesting stuff. Here's some other info that's also non "hear say", it's taken straight from the most recently filed OCAT 10-Q qrtly SEC filing:
Just some tid-bits from the 10-Q:
Page 39:
"We have a history of operating losses and we may not achieve future revenues or operating profits.
We have generated modest revenue to date from our operations. Historically we have had net operating losses each year since our inception. As of December 31, 2013, we have an accumulated deficit of $313,844,357 and a stockholders’ deficit of $22,533,610. As of September 30, 2014, we have an accumulated deficit of $342,309,490 and stockholders’ equity of $723,010. We incurred net losses of $31,022,248, $34,584,115, and $55,192,803 for the years ended December 31, 2013, 2012, and 2011, respectively. We have limited current potential sources of income from licensing fees and we do not generate significant revenue from any other source. Additionally, even if we are able to commercialize our technologies or any products or services related to our technologies if approved, it is not certain that they will result in revenue or profitability."
PAGE 40:
"We may not be able to obtain regulatory approvals for any of our products (see the subsection entitled “Regulatory Risks” below), or commence or continue clinical trials for any of our products, or commercialize any products. Any of our therapeutic and product candidates may prove to have undesirable and unintended side effects or other characteristics that could cause adverse effects on patient safety, efficacy or cost-effectiveness that could prevent or limit their therapeutic use, commercialization or acceptance in the medical community. Clinical trial results that we view as positive or proof of safety and/or efficacy may not be viewed in the same manner by regulators or potential collaborators. Any product using any of our technologies may fail to provide the intended therapeutic benefits, or even achieve therapeutic benefits equal to or better than the standard of treatment at the time of testing or production, or may not be safe for use in humans. In addition, we will need to determine whether any of our potential products can be manufactured in commercial quantities or at an acceptable cost, with or without third-party support. Our efforts may not result in a product that can be or will be marketed successfully. Physicians may not prescribe our products, patients may not use our products, or third-party payors may not cover or provide adequate reimbursement for our products. For these reasons we may not be able to generate product revenues."
"We have never generated any revenue from product sales and may never be profitable.
We have limited clinical testing, regulatory, manufacturing, marketing, distribution and sales experience capabilities, which may limit our ability to generate revenues. Due to the early stage of our therapeutic products, including regenerative medical therapies and stem cell therapy-based programs, we have not yet invested significantly in regulatory, manufacturing, marketing, distribution or product sales resources. We cannot assure you that we will be able to invest or develop any of these resources successfully or as expediently as necessary, either alone or with strategic partners, to generate revenue. Our ability to become profitable depends upon our ability to generate revenue. We do not anticipate generating revenues from product sales for the foreseeable future, if ever. "
Page 41:
"We have a limited operating history on which investors may evaluate our operations and prospects for profitable operations.
If we continue to suffer losses as we have in the past, investors may not receive any return on their investment and may perhaps lose their entire investment. Our prospects must be considered speculative in light of the risks, expenses and difficulties frequently encountered by companies in their early stages of development, particularly in light of the uncertainties relating to the new, competitive and rapidly evolving markets in which we anticipate we will operate. A substantial risk is involved in investing in us because, as an early stage company, we have fewer resources than an established company, our management may be more likely to make mistakes at such an early stage, and we may be more vulnerable operationally and financially to any mistakes that may be made, as well as to external factors beyond our control. We also have no experience bringing therapeutics candidates through the regulatory approval process to commercialization, and we operate with little budgetary margin for error. To attempt to address these risks, we must, among other things, further develop our technologies, products and services, successfully implement our research, development, marketing and commercialization strategies, respond to competitive developments and attract, retain and motivate qualified personnel. Any failure to achieve any of the forgoing would result in an inability to achieve profitability."
PAGE 44/45:
"Risks Related to Intellectual Property
Certain aspects of our business are dependent upon maintaining licenses with respect to key technology; if we are unable to obtain or protect intellectual property rights related to our product candidates, we may not be able to compete effectively in our markets.
Several of the patents we utilize are licensed to us by third parties. These licenses are subject to termination under certain circumstances (including, for example, our failure to make minimum royalty payments or to timely achieve spending, development and commercialization benchmarks). The loss of any of such licenses, or the conversion of such licenses to non-exclusive licenses, could harm our operations and/or enhance the prospects of our competitors. Certain of these licenses also contain restrictions, such as limitations on our ability to grant sublicenses that could materially interfere with our ability to generate revenue through the licensing or sale to third parties of important and valuable technologies that we have, for strategic reasons, elected not to pursue directly. The possibility exists that in the future we will require further licenses to complete and/or commercialize our proposed products. We cannot assure you that we will be able to acquire any such licenses on a commercially viable basis.
Certain parts of our technology are not protectable by patent.
Certain parts of our know-how and technology are not patentable. To protect our proprietary position in such know-how and technology, we require all employees, consultants, advisors and collaborators to enter into confidentiality and invention ownership agreements with us. We cannot assure you, however, that these agreements will provide meaningful protection for our trade secrets, know-how or other proprietary information in the event of any unauthorized use or disclosure. Further, in the absence of patent protection, competitors who independently develop substantially equivalent technology may harm our business."
And from the most recent filed secondary offering "Prospectus" PAGE S-2:
"We have no therapeutic products currently available for sale and do not expect to have any therapeutic products commercially available for sale for a period of years, if at all. These factors indicate that our ability to continue research and development activities is dependent upon the ability of management to obtain additional financing as required."
It's a long, long, long way from a tiny, phase I with 18 patients or whatever it is, to even a remote shot at completing phase II and then phase III trial(s) potentially multiple needed, then a shot at even submitting to the FDA, then a remote shot at FDA approval. The number of candidate drug products/therapies that enter phase I and ever make it to market as a FDA approved, commercial, let alone successful product is a VERY, VERY small percentage.
To "claim" this "works" and is conclusively "proven science" now and is "close to market" and all these other things- I think is a 10,000 mile stretch IMO. The number of hurdles to be overcome, the number of places along the way a complex drug/therapy like this can get tripped up along the way is a daunting, daunting gauntlet to be completed- and they're just barely out of the starting gate with a tiny phase I. Thus, to claim it's "settled" and 100% guaranteed "proven" and what not, IMO, is not facing the reality of how far this has to go. They are years, YEARS AWAY per their own commentary and probably $200 MILLION dollars or more away from a "shot" an "attempt" at an approval- it's a long, long, long road ahead to plow. Meantime? Dilution and lots of it- as that's the only source of funds it looks like they got going at this point. 30% dilution for just "round 1" and they'll be going back for more, as that first round won't even fund a high quality phase II trial ramping up, for much more than 1 yr, given their present burn rate, plus the rapid rise in expenses they've already stated will occur once they fully ramp into a phase II trial enrollment and undertaking.
It's far from a done deal or even "settled" anything at this point IMHO. Not even close. This is just the beginning steps the way I see it and per their own words in their own duly filed SEC documents such as those above. My 2 cents.
3rd or 4th tab....
It's a general "blog" little mini mag-article publication that GE puts out, OK? It mentions literally, 100's of names, across a multitude of industries, and little one or two liner blurbs, quotes and who know what else.
It's a million mile stretch IMO to "try" and infer that GE somehow has some formal business relationship or interest in OCAT because of a single slide, among many other slides in a tiny, tiny "article" (if one can even call it that?), discussing many other companies/people, and one of them mentions a few lines about what OCAT "dreams/hopes" to accomplish??
Unless a formal agreement has been made public (SEC filing, formal PR disclosed, etc) it doesn't mean a thing to me. Nothing. I just glanced at blurbs about all kinds of industries and company names on that little "GE blog magazine" and not one of them has a formal investment or biz relationship with GE that I could spot.
I don't know what this little 3 or 4 line "blog" is supposedly supposed to infer about some imagined business relationship between OCAT and GE? Again, if no money changes hands, no contracts, no formal agreements, etc- then it's a non event in my book.
With what just occurred with the OCAT "trading disaster" on Friday (IMO, or whatever one wants to call it) w/ spillage of it still occurring today, including a FINRA AM post about "re-instatement to OTC" and whatnot, I'd say OCAT has way bigger issues on their hands to be concerned about than some micro blog and 3 lines or whatever in some little GE industry mini-magazine/blog. My 2 cents.
"Oh goodness. Here we go..."
Yep, gotta agree, today's collapse in price was dramatic to say the least.
Any delays in funding are pretty critical IMO, given the very precarious financial condition this company is in, with $46K cash left as of last 10-Q filing and very large accounts payable, just as short term debts that are due (over $2 MILLION on 10-Q filing).
So that S-1 being delayed, and thus apparently the Magna "credit line" being delayed, appeared to be taken pretty rough by the market today.
Just lower highs and lower lows continually. The bid now regularly dropping to the .0140 range, even breaking into the .013's now and on pretty good volume.
Nothing has stemmed this multi-month, strong down-trend the stock has been in now for months. It's well below both the 200 and 50 DMA. And no "PR" or "news" or the 10-Q , nothing has appeared to even make a dent at even slightly reversing the trend so far.
Today was a pretty rough looking trading day. Down 10% plus on good volume. Wow.
"OCAT is leading in the science, even GE Healthcare confirms this."???
I don't know, and am not aware of any concrete evidence (actually no evidence at all) that "GE Healthcare" is in any business or any formal relation whatsoever with OCAT? Where has this ever been made public? Where has GE ever "confirmed" anything about the "science" of OCAT?
Is there any SEC filing, or jointly published PR or 8-K or anything similar with a page number that specifically describes the business relationship between OCAT and GE and how specifically GE has "confirmed the science" of OCAT? That would be a big help.