Register for free to join our community of investors and share your ideas. You will also get access to streaming quotes, interactive charts, trades, portfolio, live options flow and more tools.
There are a few other things to consider
1)ZYGELS SIDE EFFECTS vs other drugs
2)ZYGEL Not being used with another drug,(as far as I know)
3)Zygel cost per year
4)Zygels trial results numbers
Im also wondering about other markets @ Australia,& UK.One would certainly assume Zanerba will be working with Australian officials to be green lighted for the same indications FDA approves here.Then on to the UK.
LOL,Stockfun I'm not sure about your house pay off,but looking at potential revenues at this point,yes I believe Zynerba can reach those share price numbers,the possible rev.numbers I posted a few days ago did not include DEES,nor any other possible future indications that Zygel may be found to
work on.plus they were consevate numbers. Then you throw in the collaboration ,or pure buy out picture.
Lots to consider,I enjoy speculating about it,but one step,at a time is what gets us there.... Zanerbas biggest news to this day is coming in June..Final FXS phase 3 results.
GL
And yes... I’m hoping for $75. Im just a little unsure of time it takes to get there. I’m interested to know what others say is the time frame for the $50+ PPS.
So dhbuzz, since GW went from $8 to $125 over 7 years ..... can you see Zynerba going from $6 to $100 over similar time period? Is that reasonable? Just want to know when I’ll pay off my house![]()
I look at Zynerba in a similar light as GWPH, with hopes that we have the same success. Could we go over $100 a share, over time I’d like to think so. I’m sure I’d part with some in that $50-$75 range with hopes of hitting triple digits one day with the rest of my shares.
I'm with you ... maybe half at $50 but I really think it's going higher..
Oh well,guess not lol.
CYA Fri.
So,anyone care to play the game of;How Much Would I Sell My Stock For
I gave my price this week 50.00 a share ,and that would not be easy,and it would not be more than half of my holdings.
Here is a look at GW Pharmas max time chart for SP
https://us.search.yahoo.com/yhs/search?hspart=omr&hsimp=yhs-001&type=853263319¶m1=y6bdVFVIsvuYsgEClQfz8J%2F6YJ8S85dv%2FZeM8NDKnXeoSu7q4%2FqOVTUieSbUfZbUNAIaXZeQMHIpVs6aPeG%2FiiYmQKGg%2BPtjG4uos6pgpYWICt0Onl4EnQVAVPBu3q0CAZqVglQM%2Fop4uZ9dneBr%2FtyHaC0I3BI1wAmq7WhsCoPNWKDd5CHmC7zJwDW%2BYDNZ&p=5+year+gw+pharma+chart
HEY,HEY....Lumpy! Great to see you.Looks like baby Zanerba is about to transform into an adult.lol
GL my friend,and many thanks to you in the very early stages for all your DD,and help here.
It's going to start to pop up on more and more radar screens.
When phase 3 FX syndrome trial results release in June
Zanerba enters a new world.
Looking good indeed! Everything is going well for this company lately. Been a long road, but all the patient shareholders will be richly rewarded in my opinion.
Haven't posted here in a while, I hope DH and all longs are doing well. Our time is coming!
Cantor Fitzgerald maintains Zyne as Overweight.. Looking good !
Also want to ask Will about company plans to seek out approval
in Australia,and UK.
So,if you are sitting on the board for a huge insurance company that covers patients looking at drug costs and say you come up with a company who offers a drug that cost less, has fewer side effects and stands alone/
Wonder which on gets picked to cover?
Zanerbas current market cap is 139.47 million.
Chuckle sounds...
So....conservative number for ASD anually at 2% market capture is 585 million alone.
Throat cleaing sound...
I posted this recently,sorry for the repost,but
this write up points out so many things.
As the writter points out on the rev.possibilties the ASD looks to be
the leader.Plus the writter points out the numbers are consevative.
As we know yesterdays phase 2 ASD trial results met EVERY idicator.Now on to phase 3.
Phase 3 with FX is done ,and will be next news.My main focuss is,and will remain to be...BUY MORE DOWN HERE.??
The benefits in the behavioral components of FXS have led to the development of Zygel for other syndromes with behavioral manifestations. These include ASD and 22q. ASD affects nearly 1 million patients, and can include symptoms such as anxiety, repetitive patterns of behavior, impairment in social communication, and social impairment. There are limited products approved for ASD as of present time. The BRIGHT study is a 14 week trial evaluating Zygel in ASD patients. The trial is a single arm trial that will evaluate the effect of Zygel on several aspects of behavior and anxiety. Results of this trial are expected in the second quarter. Finally, we have 22q, which affects around 81,000 patients in the United States. This disorder results in neuropsychiatric illnesses including anxiety. Presently, there are no approved drugs for the treatment of 22q, which paves the way for Zygel to be a first to market product. The INSPIRE trial is evaluating the use of Zygel for patients with 22q11.2 deletion syndrome, and will look at the neuropsychiatric effect at 14 weeks, similar to the BRIGHT trial. Results of this trial are expected in the third quarter of 2020. So lets look at the market opportunity here. The current WAC price of Epidiolex is around $32,500 annually. We will use a similar price point for our estimates. Using the market opportunities as estimated by Zynerba, we arrive at these conservative annual revenue for Zygel:
FXS (10% market penetration): $210 million annually
ASD (2% market penetration): $585 million annually
22q (10% market penetration): $240 million annually
These estimates do not include any potential revenue from a DEE indication either. As you can see, these potential revenues far exceed the current valuation (market cap of $100 million). So where is the disconnect? Well when Zynerba released their most recent DEE, the stock sold off sharply on what is perceived to be safety concerns. Most people will point to the infection-related adverse events. First, in clinical trials, all adverse events are reported, whether they are related to trial drug or not (runny nose, ingrown toenail, scrapes, etc.). So the absolute rate of adverse events is misleading. Furthermore, what people need to understand is that these patients are medically fragile. Many of these patients have co-morbid conditions such as cerebral palsy, chronic respiratory infections, tracheostomies (breathing tubes), and feeding tubes. Many of these conditions predispose patients to frequent health care visits (risk factor for infection) and infections (breathing tubes). To be honest, it is not surprising these patients had infections, but it is more likely related to their other conditions and not related to treatment with Zygel. All of this concern overshadowed the fact that Zygel resulted in a 44% median reduction in seizures by month 2. Furthermore, only one patient discontinued therapy with Zygel (due to application site reaction).
I plan to try and speak with Will Roberts today.There are a few questions I have.
I've seen estimated (WAC)wholesale Avg. cost... for Zygel dose for ASD ,and have seen does cost with GW drug,if memory seves me correctly Zygel was considerably less.That tells me we have another advantage ,plus the fact Zygel looks to not have the side effects GW drug has ,and with better results.I also think Zygel is being used as a stand alone drug,and not combined with other drugs when being used.These are the questions Im checking on today with Will.
Hmmm? Not sure I’d agree with that. The stock was $15 @ year ago. No revenues then and much better data out. My avg PPS is low $6 so it is regaining some of its losses but just frustrating. I am curious what others feel is a reasonable target price with an NDA announcement.
Yes, actually bought more this afternoon. Just would like to see it hold some of its gains with a trend up. It simply doesn’t belong down here. I’ve seen $20 as Target but with these most recent results and all the indications do you think this hits higher than that. GW is well over $100 ??
Usually the market will not react appropriately until true revenue is shown.
It is sell on the news. people taking some off the table who bought in lower.Im not concerned with short term,as you said this phase 2 BRIGHT trial news was outstanding.Met every point. Now comes FDA lookover and on to phase 3 for ADS final.The phase 3 FX is finished,and that news,and results will most likly be out in june and most likly be good,and you go to FDA for NDA.
The revenue projection is what gets my attention.This share price is temporary,take advantage when you can, this is a gift down here.
AIMO,
GLT ALL
Should say gapped up to almost $8
Dhbuzz, what’s your take on the pps? I understand it gapped you to almost $8 then fell but usually it would recover some during the day on such phenomenal news. Do you believe its this just a matter of time and it’s going to hit hit 20+ ??
Strong pre market with great news. Makes perfect sense for a sharp drop. That 52k+ sell right at open hurt
Hard to imagine how much this is going to change peoples lives.
“I am very impressed with the improvements my patients made over the 14-week treatment period while receiving Zygel; the reduction in irritability, communication deficits, and repetitive movements were especially noteworthy since some of these are core autistic behaviors,” said Helen Heussler, FRACP, Associate Professor at Children’s Health Queensland, Medical Director Child Development and principal investigator in the BRIGHT trial. “The magnitude of effect on autistic behaviors in this trial is significant, including hyperactivity and stereotypy, which are among the most difficult behaviors to improve with therapeutic intervention. The results of this study strongly suggest the potential of this drug as an important treatment for ASD and I look forward to participating in future clinical studies with Zygel.”
Now for the big one,phase 3 FX results.
CONGRATULATIONS TO ZANERBA TEAM!
BRIGHT TRIALS 2,RESULTS ARE A+
Amazing news this morning
I'm off to bed before I get into trouble...lol...
See ya'll at the market.
I guess once ZANERBA anounces that zygel cures everything
the share price will dip into the pennies.
A SHOUT OUT to Lumpy,hope all's well in your world.em
HEY,BIZARROW WORLD is on planet ATRA too,lol.
https://ih.advfn.com/stock-market/NASDAQ/atara-biotherapeutics-ATRA/stock-news/82531597/atara-biotherapeutics-presents-data-demonstrating
So,with that time line in mind it looks like Zygell could go to market
in 2020 vs mid 2021 if phase 3 FX trial results are in line.
Zygel is a clear gel formulation of CBD designed for transdermal administration The Food and Drug Administration (FDA) has granted Fast Track designation to Zygel (ZYN002; Zynerba), a cannabidiol gel for the treatment of behavioral symptoms associated with Fragile X syndrome (FXS).
CBD Gel Gets Fast Tracked for Behavioral Symptoms Associated ...
www.empr.com/home/news/drugs-in-the-pipeline/cbd-gel-gets-fast-tracked-for-behavioral-symptoms-associated-with-fragile-x-syndrome/
How long does it typically take for the FDA to review a NDA?
***********************
Remember Zygel has been fast tracked by FDA for FX Syndrome ,that could mean that 12 month period is cut to 6 month.
***********************
A standard review means that the FDA can take as long as 12 months after the NDA is submitted to review the data and grant approval. With a priority review, the FDA calls on a larger number of staff to review the NDA, reducing the approval time to less than six months.
I think that at this point,not that I really think Zynerba has to,and not sure I want them to,but I think the possibility of a very large pharma wanting to become involved here is going up.It would depend on the deal
for me.I would not take 50 a share here right now,at 50 I would give it looooooooots of thought.
Ya know,if one takes todays news ,and combines it withthe upcoming phase 3 fx results,along with phase 2 BRIGHT ASD results,one could surmize that what the media has been saying recently,and that is a huge transfer of money,welth is taking place in the markets at this point. Hmmmmmmmmm,well IMO if you are able to load ZYNE at this point you could be a part of that transfer,and on the good side of it.LOL
Thanks to things just like todays market reaction to GREAT news..
I'm a buyer again today..........
..and now back to BIZARROW WORLD,and the drop in share price with this mornings news..LOL
THANK YOU!!!!!!!!..IT DOES NOT GET MUCH EASIER .
“It’s very exciting to see that the observed early benefits of Zygel appear to be sustained for over two years in patients who enrolled in the open label extension of FAB-C; these data suggest the potential for a sustained and measurable benefit for those patients who experience an early response,” said Zynerba’s Chief Medical Officer, Joseph M. Palumbo, MD, FAPA, MACPsych. “It’s also reassuring to see these responses in the context of a strong tolerability profile. We look forward to the results of our pivotal CONNECT-FX study in children and adolescents with FXS late this quarter.”
OUTSTANDING! Statistically significant reductions from baseline in mean ELDQOL-modified subscale scores for seizure severity, behavior, and mood were observed at week 26 (P < 0.01 for all measures).
Good Day/Bad Day Assessment
At month six, the combined proportion of “good day” and “fantastic day” reports increased from 52% at baseline to 70%, and the combined proportion of “terrible day” and “bad day” reports decreased from 12% at baseline to 4%.
An infographic accompanying this announcement is available at https://www.globenewswire.com/NewsRoom/AttachmentNg/5afa2b80-2b14-49f0-bccd-f62c93a1583b
Qualitative Caregiver Feedback
The qualitative caregiver assessment was administered to parents/caregivers for 43 patients. Improvement in summary measures of qualitative assessments was observed in most patients for most measures:
Any improvement: 84% (n = 36)
I’m in for the long haul too. The news looks good to me. Thanks.
Everyone has their own plan.I'm total long here
till FX results hit.\ as the potential with that ,and the rest of the Zenerba pipe line is huge.This company is about to transform
one way or the other,the odds due to past trial results lean toward a
positive outcome short and long term IMO.
The potential with ASD BRIGHT TRIAL)is BIG BIG BIG as far as market value from the numbers projected.As we know though FX is done with trials now and would be the first to enter the market in 2021.BRIGht is just finished phase 2.
Its a very undervalued stock bellow 20 IMO
|
Followers
|
91
|
Posters
|
|
|
Posts (Today)
|
0
|
Posts (Total)
|
2676
|
|
Created
|
08/06/15
|
Type
|
Free
|
| Moderators | |||

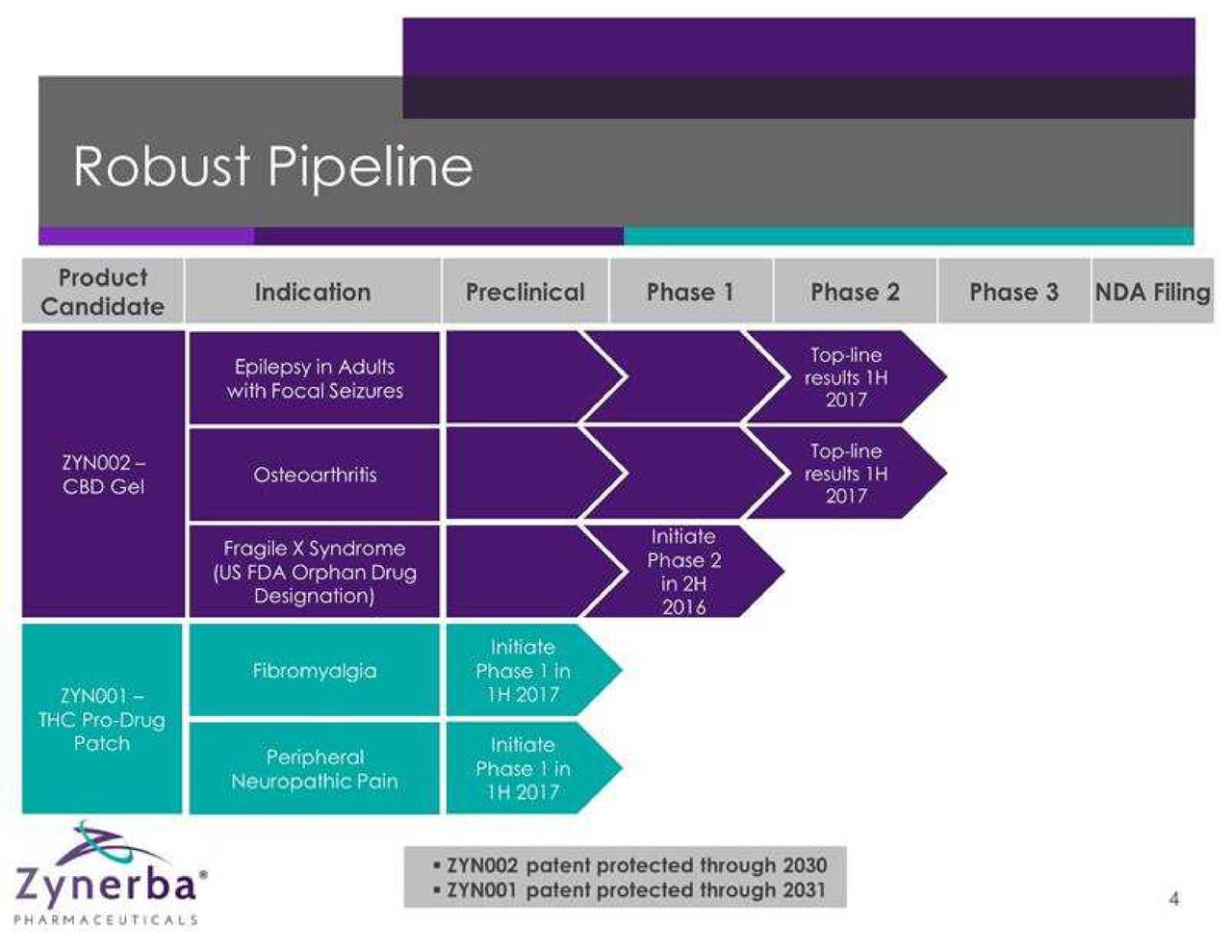
Zynerba Pharmaceuticals, Inc. is a specialty pharmaceutical company focused on developing and commercializing synthetic cannabinoid therapeutics formulated for transdermal delivery. The Company is evaluating approximately two product candidates, ZYN002 and ZYN001, in over five indications. The Company intends to study ZYN002 in patients with refractory epilepsy, osteoarthritis and Fragile X syndrome. The Company's ZYN002 is synthetic cannabidiol (CBD) formulated as a permeation-enhanced gel for transdermal delivery. ZYN002 is being developed as a clear that is designed to provide controlled drug delivery with once- or twice-daily dosing. ZYN001 is a pro-drug of tetrahydrocannabinol (THC) that enables transdermal delivery through a patch. The Company intends to test the ZYN001 patch for application to the arm, back and thigh. The Company intends to study ZYN001 in patients with fibromyalgia and peripheral neuropathic pain.
| Corporate Profile |
Zynerba (NASDAQ: ZYNE) is pioneering the development of patent-protected, next-generation synthetic cannabinoid therapeutics formulated for transdermal delivery. Its two lead product candidates in development include ZYN002 and ZYN001, which are being evaluated in five indications. ZYN002 is the first and only synthetic cannabidiol (CBD) formulated as a permeation-enhanced gel for transdermal delivery. In June 2016, the company initiated the STAR 1 Phase 2 clinical trial in refractory epilepsy patients and in August 2016, initiated the STOP Phase 2 clinical trial in patients with osteoarthritis of the knee. A Phase 2 clinical trial in patients with Fragile X syndrome will be initiated in the second half of 2016.
ZYN001, a prodrug of THC that enables transdermal delivery through the skin and circulatory system via a patch, is in preclinical development. A Phase 1 clinical trial is planned in the first half of 2017.
In August 2015, Zynerba completed an initial public offering, raising net proceeds of $42.1 million. As of June 30, 2016, cash and cash equivalents totaled $32.1 million, which is projected to fund five Phase 2 clinical trials through 2017.
| EPS (TTM) | 9/30/2016 | -2.47 |
|---|---|
| P/E Ratio | 9/30/2016 | -- |
| Market Cap | Micro Cap | 146M |
| Shares Outstanding | 9.95M |
| Float | 7.2M |
| Volume | |
| Day Range: | |
| Bid Price | |
| Ask Price | |
| Last Trade Time: |
