Register for free to join our community of investors and share your ideas. You will also get access to streaming quotes, interactive charts, trades, portfolio, live options flow and more tools.
Lol at DUMB idiots learning how to play Russian Roulette with grand daddy!!!
Funny how each time I throw in 100, these dumb fools fret like a ghoulish pig....
The also criminalize at BKYI, AIKI and AFH, as well as several others you'd seen my history. These idiots steal my personal trading data and swear to go bankrupt selling against us. I started loading this yesterday and today, the CRIMINALS stole my info and are at work. That's the reason for the below-the-line sells. Buy, if you care, but I know what's going on here!!!
Apparently the idiots you'd see when you click my name also make markets here. No wonder the stock is deep trouble. Mgmt need to flush out the Market Makers....these guys are the worst that we thought we cleaned out but still lurking everywhere.
Dhbuzz ....just know your input and DD is still welcome. You should be proud of your input on this board. If you got out ahead of the plum-it based on dd you never shared... well I’m not hating the player. Just the game I hate.
It is odd. However, the stock is not dead so not sure why he’d be gone even if he were a paid Zynerba poster. Zynerba is clearly still seeking FDA meeting for FX and there is still other trial results pending. Maybe he’s just heart broken and needs a moment. Time will tell.
Was he merely a shill for Zynerba? Odd her disappears mid June .
Is dhbuzz just gone??
That would be awesome because I just bought more yesterday
Drugs do get approval from FDA that don’t meet statistically significant results on there primary endpoints, but they must have something else to ride on for there approval. Zygel has this. To comment on why they did not go back to FDA in the middle of trial and ask to change endpoints.... your not allowed. But I suspect that the company did recognize the way there earlier trials were going and that’s why they had the ADHOC trial splicing out the High Methylated Group. They knew this and prepared. They likely even already know what the FDA will say and this was the best pathway to be able to prove there drug works to FDA. IMO this drug is going to market. For FX, ASD, & Epilepsy. IMO this will be a block buster billion dollar drug.
Totally agree
There is an old saying
“I’d rather be luck than good”
In this case, imo, company’s mgt., unfortunately not so good
Tons of money spent over many years with no real results
But with these findings for the sub group, maybe the shareholders do get lucky, as well as the methylated fragile x patients most importantly
Totally agree that it's going to take time now. Also agree with your point that it would take great results to move the stock higher. I really expected a gradual move higher throughout the day Tuesday, as investors looked past the bad headline and realized that Zygel being statistically significant for 80% of patients in the trial could be good enough for approval. I was wrong on that thought clearly.
I do wonder something though, and am curious if anyone has insight into this: has a drug ever gotten approval after failing to meet its primary endpoint in the clinical trial, but the secondary info showed it warranted approval for a specific subset of the patient group? It's not like we're talking 10% of all patients....this was 80% of the trial participants and 60% of the world's population with FX. And this is a condition with zero drugs currently approved to treat it. But will the FDA care, or simply say "run another trial with ONLY patients with full methylation", and then we'll talk.
There is something that bothers me here, in the name of full disclosure. After all the testing and first two phases of the clinical trials, it never became clear to management that good results were only happening with the sickest patients (well, not "sickest" but rather with more of the condition)? And if 80% of the trial participants were all in that group, they didn't think to maybe have the primary endpoint be catered to them?? It's possible they thought the worse off the person, the worse Zygel would do. But if that's the case, then why stack your trial with 80% of those people? I would think that after a deep dive into the phase II trial results, it would become evident that those full methylation patients were getting the most benefit. I'd have then gone to the FDA and asked to change the primary endpoint of the phase III trial to be "show stat sig benefit to those with full methylation, and secondary endpoints of showing benefit to those less afflicted by FX". Would the FDA have allowed them to change the criteria? Maybe not, but it had to be worth a shot.
Fragile X: zero approved drugs on the market, and Zygel shows real clinical benefit to 60% of all people who have it. And you can test BEFORE being prescribed the drug to see if you're in that 60% or not. If the trial was set up differently, we're looking at slam dunk approval and ZYNE stock through the roof. Disappointing to think of it that way. I'm holding tight though, hoping they do get approval for the 60% and really hoping for good things with ASD.
All just my opinion, as always
Agree with your observations here
Old saying
“Rather be lucky than good”
And those are my thoughts regarding Zynerba’s management
That being said, they may really have something here
What’s your thoughts on the path forward. IMHO, because this is still passing the MDA with FDA and still will be used heavily by physicians for FX. Even those without 90% methylation. Plus...because there are 3 more indications ( particularly ASD, this is still a winner. Market over reaction has made this a very good buy ( if anyone has powder to spend now) . Curious about your thoughts.
* * $ZYNE Video Chart 06-30-2020 * *
Link to Video - click here to watch the technical chart video
Picked up 9850 shares. OVERREACTION $ZYNE
added some down here, best to all here
Too many shorts in on this stock for it to do anything but go down unless the results were perfect. Unfortunately, we will have to wait it out now.
Disappointing results and I understand why the stock is taking a hit. With that said, Zygel DID reach stat sig with patients showing "full methylation". That was 80% of the trial participants, and 60% of all Fragile X cases in the world (estimate, according to ZYNE management).
Bottom line (for me)....if Zygel works for 60% of the Fragile X population, the drug WILL get approved and WILL bring in significant money to Zynerba. Obviously not as good as helping everyone with FX, but 60% is a big number. And with no serious adverse side effects, it's a no-brainer for doctors to prescribe to patients. Maybe I'm just looking through rose colored glasses here, but I think sales to potential 60% of the group is good enough to warrant the stock going UP today, not down. Time will tell where this heads....
IMHO, This is not over. Zygel still going to FDA and will likely be approved for FX under more narrow indication. Once on market will be used in all FX patients. Also likely to have other indications approved in future.
Yes , and the smaller group make up 60% of FX population overall and 80 % of the study group. This drug is still going to FDA for this indication. And Will get approved.
Shorts were right on this one.
Well - it failed in wider population - bad luck
The smaller targeted population met primary outcome - will it have any impact in long term unlikely - this is not a good stock now
From company presentation
“ As of now, our timelines for delivery of top line results from all of our ongoing trials remain unchanged
• Includes our expectation of results from our pivotal CONNECT-FX trial in FXS late in late June 2020”
Tomorrow is last day of Jun - will we get good news tomorrow?
Thanks
Your concern is valid in terms of buyout - if FX results are as good as phase 2, this stock can cross $20 easily as it translates into market cap of $500 million and this is pivotal.
This also confirms the MOA in CNS and also ASD - this can fly and GWPH can offer $30 easily and I will not be happy.
Just for ASD, this is $ 1.0 B market cap stock. I am ready to wait for 1-2 years for that.
Welcome to the board Amateur17. Great post, and spot on in my opinion. I completely agree that ASD gives ZYNE the best potential for highest stock price. Fragile X is the nearest term catalyst though, as you are aware. Even though FX is far more rare than ASD, the opportunity to be "first in class" is huge. Good top line results in the FX trial and this stock is off to the races immediately. Honestly, my biggest fear at the moment....bigger even than getting bad results in clinical trials (because I dont think we will)....is a buyout. GWPH is already nuts for not trying to buy ZYNE already, unless they have but didn't make it public knowledge. If they made an offer of $20 a share right now, it would be difficult for management to turn down. I don't think they would offer $20 per share, and yet it's way too low. I do fear a buyout capping the ultimate potential here. If ZYNE can avoid being bought out, there's no doubt this can be a $100 stock if the results are good in all phase III trials. $100 is even cheap.....
Exciting times to be a ZYNE shareholder
As per their Jun 2nd presentation, they were supposed to announce FX pivotal trial results by end of the quarter which is tomorrow.
If the results are as good phase 2 - this could really jump very high
As I read about ZYNE, I started liking it more - only on ASD and FX this can become next AXSM. I like it more for ASD
Autism Specturm Disorder is not rare disease (affects 1 in 59 adults) -
Each year approx 3.85 million children are born - which means approx 50,000 to 60,000 new ASD patients each year. As the ASD patients have same life span, the ASD population keeps increasing.
If you look at clinicaltrials site - there are 63 active trials in all phases - however there are only handful of new drugs in trial - Roche, Jansen and ZYNE. All other are repurposed drugs.
The Zygel results in phase 2 are really great compared to any other trials. So if they can repeat phase 2 results in phase 3 - it will be a slam dunk.
The co has only 24 million o/s shares - which translates to $50 for $1 Billion market cap. They have $60 million in cash and think they can go till H2-2021 but will need to raise money for new trials which is not a big deal for biotechs.
My family friend has austistic kid and on somewhat severe side. They have tried many options and few diet based trials. They will be ready to pay for any drug that reduces the intensity of the symptoms even by 20-30%.
With FX NDA in Q4-2020 and another results expected next quarter, the SP can move higher even before NDA submission.
$ZYNE Possible breakout for #Zynerba
Possible entry above $6.70
Upside target $7.86 - $8.50
Very high short interest at 30%
Company Profile
Zynerba Pharmaceuticals , Inc. engages in the provision of pharmaceutically-produced transdermal cannabinoid therapies. It focuses on research and development of rare and near-rare neuropsychiatric conditions. It offers Zygel product which formulated as a permeation-enhanced gel for transdermal delivery. The company was founded by Audra L. Stinchcomb on January 31, 2007 and is headquartered in Devon, PA.
PLEASE GIVE US A LIKE IF YOU FIND OUR CONTENT HELPFUL, THANK YOU.

$ZYNE | #Zynerba Trade Setup
Very high short interest at 30%
Stock is currently well below the average analyst price target of $18 and a BUY rating.
Company profile
Zynerba Pharmaceuticals , Inc. engages in the provision of pharmaceutically-produced transdermal cannabinoid therapies. It focuses on research and development of rare and near-rare neuropsychiatric conditions. It offers Zygel product which formulated as a permeation-enhanced gel for transdermal delivery. The company was founded by Audra L. Stinchcomb on January 31, 2007 and is headquartered in Devon, PA.

ZYNE - looks to be a good CNS play
I like the ASD treatment - that is overlooked area and that impacts the parents for lifetime.
The phase 2 results look very promising. However this is a long term play.
I do not know if they have any warrants - else the o/s are just 25 million - for a biotech that will start phase 3 for such unmet disease, it is very low. A good phase 3 can take market cap to $800 million to $1 B - but that will be in 2021.
However I like the overall company.
ZYNE having a real nice day today. Solid volume behind the move as well. I think we're on the way back to $10 in the next 30 days, even without any news. Just based on the speculation, the stock should keep trending higher. Obviously good news sends this much, much higher than $10.
Hope dhbuzz and all ZYNE longs are doing well. I think our patience will be rewarded
Looks like the people in the last trials were on other meds.Wonder if Zygel
could become a stand alone drug?
More sound advice from the "oracle"
https://www.cnbc.com/2019/06/20/how-warren-buffett-decides-what-to-invest-in.html
Sorry,I cant give the absolute answer to that,but as you point out the fundamentals are pretty darn good at Zanerba.Maybe this guy has a better look at this. lol I continue to buy.
https://video.search.yahoo.com/yhs/search?fr=yhs-omr-001&hsimp=yhs-001&hspart=omr&p=what+makes+a+stock+go+down+even+with+good+news#id=2&vid=67e5622926027e89995905ca581af4e0&action=click
How is it possible that the nasdaq is up almost 200pts, co has put out good news after good news, great float, great finances, and up coming catalyst close in site and we still trade down. It’s a gift but I’ve got no more to spend ![]() Ought to be a crime to manipulate stocks this way. What or who is controlling this PPS??
Ought to be a crime to manipulate stocks this way. What or who is controlling this PPS??
not only that the spred is pretty big between the ah price too..geeeeeeeeesh......td and ihub say 5.60 now
Hmmm ihub says up in after hours right now,td ameri says up right now in ah,yahoo says down right now.........lol
A look into Roche Pharma.IMO,this is a name to listen out for going forward in Zanerbas future.Again it is ONLY my opionion.
https://www.roche.com/research_and_development/what_we_are_working_on/rare_diseases/roche-in-rare-disease.htm
Realize there are some MONSTER sized pharmas out ther looking for the fx answer..
night night
I do give this thought about GW Pharmas FDA approved Epidiolex,and what Ive read about the side effects envolved vs. Zanerbas zygel,epidiolex is an oral means of delivery,liguid form.Now my tiny brain ventures into a world of transdermal delivery for epidiolex perhaps like zygel is with very little side effects from what ive read,and who holds that patent for the transdermal means..
Hmmmmmmmmm,just a thought.
Also realize what GW'S DRUG is being used for is not what zanerba is focused on,but all in the same family it looks to me like.
How I do enjoy reading between the lines,and doing a bit of speculation,and so glad our CEO refers to GW Pharma as our friends. ![]()
Also interesting,but not surprising
the fact he mentions possible relations with others working on FX,and the hope it will increase with positive phase 3 fx results.He also mentiond GW pharma,and refered to them as "our friends"...interesting.
When the people involved in looking for sunken ships set there eye on a certain ship they know to have been carrying a huge bounty they have a list of known things on board,if they can find one of those things,its called a 'providence" then they know they have found the mother load and then it gets real lucrative. ; )
FXS phase 3 results are Zanerbas providence...
Our CEO sounds very confident.I like the term "LAUNCH TEAM"
put together ,and adding more for FX,and the future of Zanerba.
Its kind of coincidental we just saw another perfect FX launch a couple of days ago.Falcon X, now FXS launch is on the pad.
Excellent presentation yesterday.
http://wsw.com/webcast/jeff126/zyne/index.aspx
|
Followers
|
91
|
Posters
|
|
|
Posts (Today)
|
0
|
Posts (Total)
|
2676
|
|
Created
|
08/06/15
|
Type
|
Free
|
| Moderators | |||

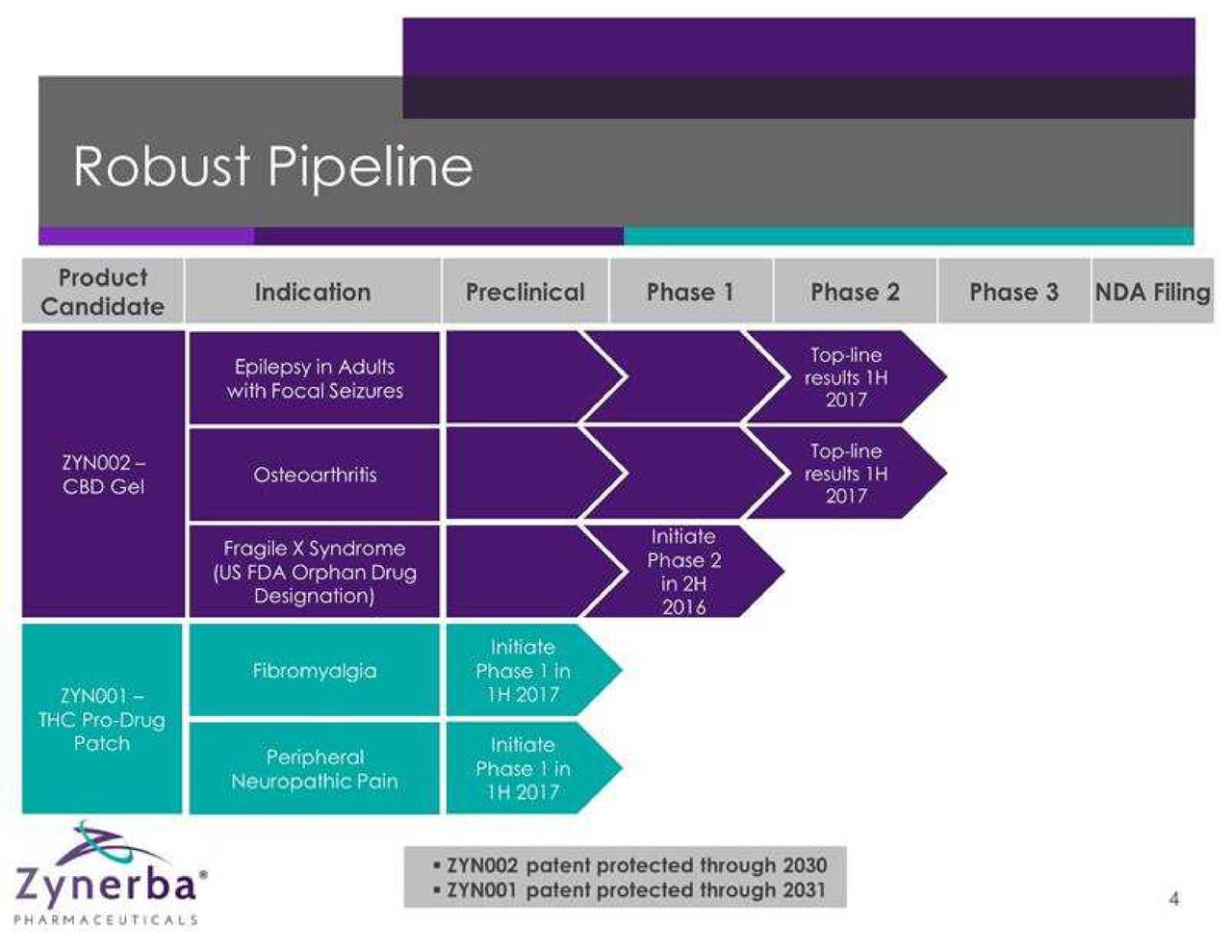
Zynerba Pharmaceuticals, Inc. is a specialty pharmaceutical company focused on developing and commercializing synthetic cannabinoid therapeutics formulated for transdermal delivery. The Company is evaluating approximately two product candidates, ZYN002 and ZYN001, in over five indications. The Company intends to study ZYN002 in patients with refractory epilepsy, osteoarthritis and Fragile X syndrome. The Company's ZYN002 is synthetic cannabidiol (CBD) formulated as a permeation-enhanced gel for transdermal delivery. ZYN002 is being developed as a clear that is designed to provide controlled drug delivery with once- or twice-daily dosing. ZYN001 is a pro-drug of tetrahydrocannabinol (THC) that enables transdermal delivery through a patch. The Company intends to test the ZYN001 patch for application to the arm, back and thigh. The Company intends to study ZYN001 in patients with fibromyalgia and peripheral neuropathic pain.
| Corporate Profile |
Zynerba (NASDAQ: ZYNE) is pioneering the development of patent-protected, next-generation synthetic cannabinoid therapeutics formulated for transdermal delivery. Its two lead product candidates in development include ZYN002 and ZYN001, which are being evaluated in five indications. ZYN002 is the first and only synthetic cannabidiol (CBD) formulated as a permeation-enhanced gel for transdermal delivery. In June 2016, the company initiated the STAR 1 Phase 2 clinical trial in refractory epilepsy patients and in August 2016, initiated the STOP Phase 2 clinical trial in patients with osteoarthritis of the knee. A Phase 2 clinical trial in patients with Fragile X syndrome will be initiated in the second half of 2016.
ZYN001, a prodrug of THC that enables transdermal delivery through the skin and circulatory system via a patch, is in preclinical development. A Phase 1 clinical trial is planned in the first half of 2017.
In August 2015, Zynerba completed an initial public offering, raising net proceeds of $42.1 million. As of June 30, 2016, cash and cash equivalents totaled $32.1 million, which is projected to fund five Phase 2 clinical trials through 2017.
| EPS (TTM) | 9/30/2016 | -2.47 |
|---|---|
| P/E Ratio | 9/30/2016 | -- |
| Market Cap | Micro Cap | 146M |
| Shares Outstanding | 9.95M |
| Float | 7.2M |
| Volume | |
| Day Range: | |
| Bid Price | |
| Ask Price | |
| Last Trade Time: |
