Register for free to join our community of investors and share your ideas. You will also get access to streaming quotes, interactive charts, trades, portfolio, live options flow and more tools.
Xenetic Biosciences Inc NASDAQ: XBIO
GoSymbol lookup
Health Care : Biotechnology | Small Cap BlendCompany profile
Xenetic Biosciences, Inc. is a biopharmaceutical company. The Company is focused on advancing its immune-oncology technologies addressing hard to treat cancers. The Company's DNase platform is designed to improve outcomes of existing treatments, including immunotherapies, by targeting neutrophil extracellular traps (NETs), which are weblike structures composed of extracellular chromatin coated with histones and other proteins. The Company is also developing its personalized Chimeric Antigen Receptor (CAR) T platform technology, XCART, to develop cell-based therapeutics targeting the B-cell receptor on the surface of an individual patient’s malignant tumor cells, for the treatment of B-cell lymphomas. It is also focused on developing its proprietary drug delivery platform, PolyXen, which uses the biological polymer polysialic acid (PSA) to prolong the drug's half-life and potentially improve the stability of therapeutic peptides and proteins.
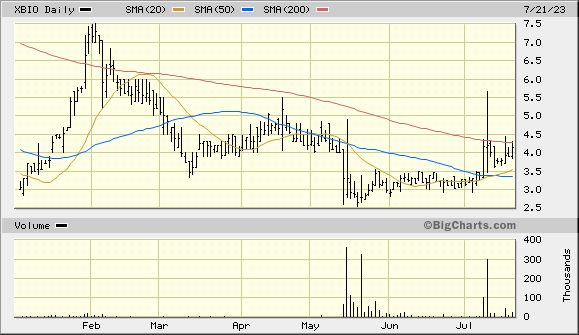
whats going on over here?????? nice moves this year.
XBIO: Great price to invest here. Count me in.
Amazing patents. The stock was recently at $5.00/share. It is egregiously shorted, for no good reason, which means I'm coming in to own it. Heavily.
$XBIO
#XBIO
yesir you are correct good job
looks like nobody cares about this pile of poo
big new out everyone miss it ?
xbio news out !
I may move this money and cut my loses
XBIO is off the SHO list. I may reevaluate
Most sells all day today. Done for the day here will hopefully see some green next week. Enjoy the weekend
We need to get out of these 2s if we want to see any volume anytime soon it seems
This will run like crazy when MMs decide to cover their naked shorts. Until then we churn
XBIO: Reasons to invest into Xenetic Biosciences Inc
- ortex numbers
- extremely low float
- on the threshold list
- great patents
- good price point for growth
- low market cap (good for ownership)
- squeeze potential
$XBIO
#XBIO
XBIO: Great buy here at these levels
$XBIO
#XBIO
Just picked up 1000
Short exempts were not that high today but volume was half of what it was yesterday. However Wednesday and Thursday were 1.3% and 4.3% of the float respectively. The extremely low volume today was ok because nobody is selling and shares are hard to locate. MMs are behind on delivering so soon they will be slapping the ask
If XBIO has another high short exempt day on this low volume it means MMs can't locate shares. It is already on the SHO list with lots of FTDs so MMs can't afford to get too behind on shares.
Remember the float is 8.89 million so the MMs soon will have to slap that ask hard.
APE STRONG
In for 10k shares
I actually forgot I still have shares in the 5s from a while back when I looked in my other account to see if anything cleared to buy yet. Lol
Whats the short interest over here against the float?
Im Holding a boat, will load another boat when i see some movement.
If short exempts are huge again this is going to explode. Shares are very hard to locate
Sold some to get a starter over here. Looks really interesting and actually if you look back in time this has been well over $400 per share. The company is in great shape, can’t wait to see how this plays out.
Sold some more junk yesterday. Thought I would get funds today but don't see any. Going to be a player over here Monday
XBIO had an unusual amount of short exempts today on low volume. 370,000 short exempts. This could make a move soon.
Any thoughts on the S3 filing? This may hurt short term but long term
Outlook is good. The lender obviously has confidence in the company
Just slapped it for 1200 more. Enjoy the ride
Yes just getting started
More room to run on this?
Yes it does. On the SHO list with zero shares available to borrow. This puppy is gonna run
Got in looks like it’s ready to burst $$$
Buy the dip. This is a low volume MM take down
|
Followers
|
25
|
Posters
|
|
|
Posts (Today)
|
0
|
Posts (Total)
|
1689
|
|
Created
|
02/24/14
|
Type
|
Free
|
| Moderators | |||

Xenetic Biosciences is a biopharmaceutical company focused on the acquisition, research and development of novel oncology therapeutics, including next-generation cellular immunotherapies for difficult to treat cancers, including B-cell Lymphomas, as well as improved biologic drugs. The Company recently announced its plans to acquire the XCART platform, a novel CAR T technology engineered to target patient- and tumor- specific neoantigens. The acquisition is subject to conditions typical for a transaction of this kind, including appropriate stockholder approvals, and is expected to close in the first half of 2019. The Company plans to initially apply the XCART technology to develop cell-based therapeutics for the treatment of B-cell Lymphomas. We believe our personalized T cell therapies have the potential to offer cancer patients substantial benefits over the existing standard of care and currently approved CAR T therapies.
Xenetic's Phase 2 oncology asset, XBIO-101 (sodium cridanimod), is a small-molecule investigational immunomodulator and interferon inducer which, in exploratory clinical studies, has also been shown to increase progesterone receptor (PrR) and estrogen receptor (ER) expression in certain tumor tissues. The Company plans to pursue collaborations with immuno-oncology (I-O) companies in which it would seek to use XBIO-101 in combination with approved or developmental I-O compounds such as checkpoint inhibitors.
Additionally, Xenetic's proprietary drug development platform, PolyXen™, enables next-generation biologic drugs by improving their half-life and other pharmacological properties. The Company has ongoing business development activities to explore partnerships utilizing its PolyXen delivery platform.
| Program | Indication | Preclinical | Phase 1 | Phase 2 | Phase 3 | Next Catalyst |
| XCART Platform | B-Cell Non-Hodgkin Lymphoma | Preclinical Phase in progress | | | | Meet with regulatory authorities to discuss development plan |
| XBIO-101 | Progesterone Resistant Endometrial Cancer | Preclinical Phase complete | Phase 1 Phase complete | Phase 2 Phase in progress | | Data analysis |
| Triple-Negative Breast Cancer | Preclinical Phase complete | Phase 1 Phase in progress | | | Exploratory study of estrogen receptor and I-O biomarkers | |
| Immuno-Oncology | Preclinical Phase in progress | Phase 1 Phase not started | Phase 2 Phase not started | Phase 3 Phase not started | Exploratory Phase 1 study | |
| PolyXen™ | Next Generation Delivery Platform for Biologics | Potential for non-dilutive milestone and royalty payments from partners |
Chief Executive Officer
Mr. Eisenberg was appointed Chief Executive Officer of Xenetic in October 2017. He joined Xenetic's management team in December 2016 as Chief Operating Officer and has served on the Company's Board of Directors since July 2016. He is a seasoned life science executive with over 20 years of broad operational expertise. Over the course of his career, Mr. Eisenberg has led all crucial areas of R&D, operations, manufacturing/quality, business development, strategic partnering, product development, commercialization, and talent management. Prior to joining Xenetic, his most recent position was Chief Executive Officer of Noven Pharmaceuticals, where during his tenure as CEO revenues more than doubled, the company's cash increased by more than 300%, and two new products were launched following the successful filings of New Drug Applications (NDAs) submitted to the U.S. Food and Drug Administration. Mr. Eisenberg also was responsible for leading Noven's Novogyne joint venture with Novartis (NYSE: NVS), an entity that generated over $300 million in revenue in its last full year of operation.
Chief Scientific Officer
Curtis A. Lockshin, Ph.D. joined the Xenetic Management team as Chief Scientific Officer in January 2017, after having served as the Vice President of Research and Operations of the Company since March 2014. From July 2015 to July 2016, Dr. Lockshin served as Chief Executive Officer and Director of SciVac Therapeutics Inc., and its subsidiary SciVac, Ltd., a commercial-stage biologics and vaccine company in Rehovot, Israel, where he had been serving as CEO and Director since September 2014. Subsequent to SciVac Therapeutics' merger with VBI Vaccines, Inc. in July 2016, Dr. Lockshin served as Chief Technical Officer of VBI Vaccines and its subsidiary SciVac Ltd. In addition, he has served as President and CEO of Guardum Pharmaceuticals, LLC, a private pharmaceutical company, and previously as Vice President of Corporate R&D Initiatives for OPKO Health, Inc., a multinational pharmaceutical and diagnostics company. Dr. Lockshin has served as a member of the Board of Directors at a number of companies including RXi Pharmaceuticals, Corp. (now Phio Pharmaceuticals Corp.), ChromaDex, Inc., and Sorrento Therapeutics, Inc., and as a Director of the Ruth K. Broad Biomedical Research Foundation, a Duke University Support Corporation that supports basic research related to Alzheimer's disease and neurodegeneration via intramural, extramural and international grants.
Chief Financial Officer
Mr. Parslow is a seasoned financial executive with 30 years of experience providing financial and business leadership to the biotech, clean tech, business-to-business e-commerce and high-tech manufacturing industries. Over the course of his career, Mr. Parslow has demonstrated expertise with strategic planning and operations, budgeting, financial planning and analysis, accessing capital markets, M&A, investor relations, risk management, SOX compliance, and SEC/GAAP reporting. Prior to joining Xenetic, Mr. Parslow most recently served as Chief Financial Officer, Treasurer and Secretary of World Energy Solutions, Inc., a publicly-traded business-to-business e-commerce company brokering energy and environmental commodities, from 2006 until the company was acquired by EnerNOC, Inc. in 2015.
Chairman
Adam Logal joins the Xenetic Board of Directors with over 15 years of experience in the biopharmaceuticals industry. Since April 2014, Mr. Logal has served as Senior Vice President, Chief Financial Officer, Chief Accounting Officer and Treasurer of OPKO Health, Inc. and from March 2007 until April 2014 served as OPKO's Vice President of Finance, Chief Accounting Officer and Treasurer. Mr. Logal is a director of VBI Vaccines, Inc. and serves as its Audit Committee Chairman. Prior to joining OPKO, Mr. Logal served in various financial management roles at Nabi Biopharmaceuticals, a commercial stage biopharmaceutical company. Mr. Logal is a strategic finance executive with extensive experience in SEC compliance and reporting, domestic and international finance, strategic planning, cash flow management, budgeting, taxation, treasury and business development.
Chief Executive Officer
Mr. Eisenberg was appointed Chief Executive Officer of Xenetic in October 2017. He joined Xenetic's management team in December 2016 as Chief Operating Officer and has served on the Company's Board of Directors since July 2016. He is a seasoned life science executive with over 20 years of broad operational expertise. Over the course of his career, Mr. Eisenberg has led all crucial areas of R&D, operations, manufacturing/quality, business development, strategic partnering, product development, commercialization, and talent management. Prior to joining Xenetic, his most recent position was Chief Executive Officer of Noven Pharmaceuticals, where during his tenure as CEO revenues more than doubled, the company's cash increased by more than 300%, and two new products were launched following the successful filings of New Drug Applications (NDAs) submitted to the U.S. Food and Drug Administration. Mr. Eisenberg also was responsible for leading Noven's Novogyne joint venture with Novartis (NYSE: NVS), an entity that generated over $300 million in revenue in its last full year of operation.
Non-Executive Director
James Eric Callaway, Ph.D., joins the Xenetic Board of Directors with over 30 years of experience in the execution of product development operations for biotherapeutics. Dr. Callaway has an established track record of achievement against challenging technologies and aggressive project timelines. He currently serves as a Corporate Strategy Consultant at Callaway Innovations. Dr. Callaway is a seasoned CEO within the venture-backed biotech community and over the course of his career he has built and operated two companies, transforming each from research companies to clinical stage operating entities. Prior to these efforts, Dr. Callaway held multiple senior leadership positions at Elan Pharmaceuticals, including simultaneously acting as Head of Development and overseeing the complex partnership with Wyeth Pharmaceuticals in the Alzheimer’s disease immunotherapy program. He has developed antibodies for a wide-range of therapeutic applications over the past two decades, including treatments of multiple sclerosis (Tysabri®: pharmaceutical development), Alzheimer’s disease (bapineuzumab: Program Executive), and blood-brain barrier transport, and has worked with the United Stated Food and Drug Administration on multiple orphan drug development programs.
Non-Executive Director
Firdaus Dastoor was appointed non-executive Director of Xenetic in July 2007. Mr. Dastoor is a Fellow Member of The Institute of Company Secretaries of India. He began his career as the Company Secretary of the Poonawalla Group. He then took on assignments involved in business development strategies and operations. He is on the board of several companies in the fields of engineering products, life sciences and biotech, international trade, financial services and quality standards certifications. Currently, Mr. Dastoor is a Group Director of the Poonawalla Group of Companies and in charge of Finance and Corporate Affairs.
Non-Executive Director
Dmitry Genkin currently serves on the Company’s Scientific Advisory Board and previously served on the Company’s Board of Directors from 2004-2016. He has the Russian equivalent of an MD in Internal Therapy and studied drug delivery under Professor Gregory Gregoriadis at The School of Pharmacy, University of London in 1992, as well as the Department of Clinical Pharmacology at Karolinska Hospital, Stockholm from 1992 until 1993. Since 1993, Dr. Genkin has headed a number of Russia's largest pharmaceutical companies including Pharmavit, which had 27% of the Russian pharmaceutical market. In 1998, he was awarded the silver medal by the Russian Natural Science Academy. Dr. Genkin is currently Chairman of PJSC Pharmsynthez, a public company listed on the Moscow Stock Exchange and Xenetic’s majority stockholder.
Non-Executive Director
Roman Knyazev was appointed to the Board of Directors in May 2014. Mr. Knyazev is an experienced financial professional and senior investment manager at Rusano. Earlier, he worked at PriceWaterhouseCoopers Moscow and at Deloitte. Mr. Knyazev is a member of the SynBio Board of Directors and a Deputy Chairman at Pharmsynthez, PETAR and Nanolek.
Nobel Prize Laureate in Chemistry - Studies of the Molecular Basis of Eukaryotic Transcription
Non-Executive Director
Dr. Kornberg was appointed to the Board of Directors of the Company in February 2016. Dr. Kornberg is a member of the U.S. National Academy of Sciences and the Winzer Professor of Medicine in the Department of Structural Biology at Stanford University. He earned his bachelor's degree in chemistry from Harvard University in 1967 and his Ph.D. in chemical physics from Stanford in 1972. He became a postdoctoral fellow at the Laboratory of Molecular Biology in Cambridge, England and then an assistant professor of biological chemistry at Harvard Medical School in 1976, before moving to his present position as professor of structural biology at Stanford Medical School in 1978. In 2006, Dr. Kornberg was awarded the Nobel Prize in Chemistry in recognition for his studies of the molecular basis of Eukaryotic Transcription, the process by which DNA is copied to RNA. Dr. Kornberg is also the recipient of several awards, including the 2001 Welch Prize, the highest award granted in the field of chemistry in the United States, and the 2002 Leopald Mayer Prize, the highest award granted in the field of biomedical sciences from the French Academy of Sciences.
Director
Dr. Alexey Vinogradov currently serves as Business Development Director and Operations Director at Cantreva LLC, a Russian company with extensive specialized experience of delivering services in the field of renewable energy (solar, wind, hydro power), performing works on a “turnkey” basis, since September 2017. Dr. Vinogradov previously served as General Manager at Togas Middle East LLC in Dubai, UAE from May 2015 to May 2017. Prior to that, Dr. Vinogradov served as branch manager at Togas Group LLC in Russia from March 2012 to November 2016.
Dr. Frigault is a medical oncologist in the Hematologic Malignancy Program at the Massachusetts General Hospital Cancer Center, as well as Assistant Director of the Cellular Therapy Service. In addition, he serves as an Instructor at Harvard Medical School. Dr. Frigault recently completed his oncology fellowship at the combined Massachusetts General Hospital/Dana Farber Cancer Institute training program where he worked with Dr. Marcela Maus, head of the Cellular Immunotherapy Program at Massachusetts General Hospital.
Dr. Frigault is responsible for attending on the inpatient bone marrow transplant and leukemia services, managing outpatient cellular therapy patients and overseeing the cellular therapy service in charge of the hospital’s standard of care and clinical research efforts. His current research is focused on the translational aspects of cellular therapies with the goal of developing the next generation of cellular therapies utilizing multi-cistronic lentiviral vectors, CRISPR gene editing and clinical correlatives.
Dr. Frigault’s prior research experience includes preclinical development and correlative studies relevant to T cell immunotherapy in the lab of Dr. Carl June while in graduate school at the University of Pennsylvania. During his post-graduate training at Johns Hopkins, he focused on cellular therapies utilizing marrow infiltrating lymphocytes and chimeric switch receptors in the lab of Dr. Ivan Borrelo.
Dr. Frigault received his Bachelor of Arts degree in Biology and Biological Sciences from the College of the Holy Cross, and his master’s and MD degrees from the University of Pennsylvania.
Since 1997, Dr. Alexander Gabibov has been Head of the Laboratory of Biocatalysis at the Shemyakin & Ovchinnikov Institute of Bioorganic Chemistry at the RAS, and in 2000 he became Professor of Cell Biology at the Moscow State University. In 2003, Dr. Gabibov was named an Associate of the Russian Academy of Sciences, and in 2008 was appointed President of the Russian Biochemical and Molecular Biology Society.
In 2009, Dr. Gabibov took on the role of Foreign Correspondent at the National Academy of Pharmacy in France. In 2012, he was nominated as Head of Department of Industrial Pharmacology at the Lomonosov Moscow State University.
Throughout his career, Dr. Gabibov has been a prolific contributor to the global scientific community as both a frequent contributor to learned journals as well - and especially in the last six years - as a much sought-after speaker on the international conference circuit.
Dr. Gabibov graduated from Moscow State University in 1977, where he studied Chemical Enzymology. He currently holds several senior positions in the Biochemistry sphere in both Russia and France.
Franco Cavalli, born in 1942, was the Scientific Director of the Oncology Institute of Southern Switzerland (IOSI) in Bellinzona (Switzerland) until 2017. He created this institute, which encompasses medical oncology, radio-oncology, nuclear medicine, palliative care, hematology and an important research division. He is currently still President of the Foundation, which manages the Institute of Oncology Research (IOR), located in Bellinzona (Switzerland). He is Professor (Titular professor) of medical oncology at the Medical Faculty in Bern (Switzerland). He has an international reputation for the treatment of and research into malignant lymphoma and new drugs. Every second year he organizes in Lugano the International Conference on Malignant Lymphoma, which is the most important congress on this topic worldwide. He has been very active also in the field of the clinical evaluation of new cancer drugs. The quality of his work has been recognized by the award of 24 national and international prices, including the Petzcoller Award for special dedication to oncology and the ESMO Lifetime Achievement Award. He has published more than 600 articles in peer-reviewed journals and has contributed to many books on cancer, including the Textbook of Medical Oncology, which he edited together with S. Kaye (London), H.H. Hansen (Copenhagen) and D. Armitage (Omaha, Nebraska).
He was Founding Editor and Editor-in-Chief of Annals of Oncology, Europe’s premier medical oncology journal, from 1990 to 2000, and he is on the Editorial Board of several other journals. In 1996, he founded the International Extranodal Lymphoma Study Group (IELSG, www.ielsg.org), which encompasses now more than 200 institutions in 4 continents. IELSG is the leading cooperative group in the field of the biological and clinical studies in the field of extranodal lymphomas.
Franco Cavalli has been President of the Swiss Cancer League and is Chairman of the Scientific Committee of the European School of Oncology (ESO) and of the World Oncology Forum (WOF). He was President of the International Union Against Cancer (UICC) between 2006 and 2008. He has been member of WHO committee of selection of essential medicines for cancers since 2015.
He was member of the Swiss Parliament between 1995 and 2007.
Dr. Davide Rossi obtained the specialization in Internal Medicine and his PhD in Clinical and Experimental Medicine at the University of Eastern Piedmont in Novara. Dr. Rossi served as Professor of Hematology and member of the Faculty of the School of Medicine of the University of Eastern Piedmont until 2015. In 2015, Dr. Rossi moved to Switzerland where he is Deputy Head of the Division of Hematology at the Oncology Institute of Southern Switzerland (IOSI), the head of the Experimental Hematology research program at the Institute of Oncology Research (IOR), and the co-chair of the Clinical Lymphoid Tumors Investigation Program (CLIP) of the Oncology Institute of Southern Switzerland (IOSI).
Dr. Rossi’s translational research focuses on lymphomas and chronic lymphocytic leukemia and resulted in more than 250 publications on international peer-reviewed journals (total impact factor: 1678; sum of citations: 14571; H index: 65). Dr. Rossi is also the principal investigator of national and international clinical trials in the field of chronic lymphocytic leukemia.
Positioning and well-recognized standing of Dr. Rossi’s research within the national and international scenario is granted by his contribution to the development of chronic lymphocytic leukemia guidelines and his participation as author of the 2016 WHO Classification of Hematologic Malignancies. Dr. Rossi is regularly invited to give lectures at international meetings, to review grants for national and international funding agencies, and to review manuscripts for high impact medical journals including The Lancet and The New England Journal of Medicine.
Dr. Rossi is currently serving as Associate Editor of Haematologica (the official Journal of the European Hematology Association), as Editorial Board Member of Blood (the official Journal of the American Society of Hematology), as Member of the Scientific Program Committee Advisory Board of the Congress of the European Hematology Association, and as Member of the Organizing Committee of the International Conference on Malignant Lymphoma.
Dr. Rossi’s research activity is funded by a number of successful competitive grants, including grants from the European Research Council, the Swiss National Fund, Oncosuisse, and other national and international funding agencies.
Dr. Guenther Koehne is an internationally recognized cancer specialist and current Deputy Director and Chief of Blood & Marrow Transplant and Hematologic Oncology at the Miami Cancer Institute. He has over 30 years of extensive experience in the treatment of leukemia, myelodysplastic syndrome, multiple myeloma and other lymphoproliferative diseases, including with autologous and allogeneic stem cell transplantations. Over the course of his career, he has established a noteworthy reputation for his work in adoptive immunotherapeutic approaches with antigen-specific, donor-derived T lymphocytes in the treatment of viral complications following allogeneic transplants and has developed new approaches to the treatment of patients with high-risk multiple myeloma, minimal residual disease of leukemia and relapsed disease post-allogeneic bone marrow transplantation.
Prior to joining the Miami Cancer Institute, Dr. Koehne served as both the Assistant Professor of Medicine and Associate Professor of Medicine at Weill Cornell Medicine. Prior to that, Dr. Koehne spent over 20 years at the Memorial Sloan Kettering Cancer Center (MSKCC) and held a multitude of roles, including Medical Director of the Cytotherapy Laboratory (Bone Marrow Transplantation Laboratory) and Hematologic Oncologist. While serving as the Medical Director at MSKCC, he established new T cell depletion techniques and other methods to manipulate donor stem cell products that optimize patient outcomes.
Dr. Koehne has served as Principle Investigator or Principal Co-Investigator for a number of clinical trials involving bone marrow transplantation, particularly in the area of multiple myeloma, to study the effectiveness of T cell-depleted transplants from related and unrelated donors in patients with high-risk and relapsed multiple myeloma. He is regarded in the medical community as a pioneer in developing specific donor-derived immune cells (T lymphocytes) to treat both the viral complications of transplantation and disease relapse following transplantation. His developed treatment approach is a type of adoptive immunotherapy, and is being administered in several active clinical trials.
A physician-scientist, Dr. Koehne received his M.D. and Ph.D. from Medical University of Hamburg, Germany. He completed his residency in Internal Medicine at Medical University of Hamburg and Rush University Medical Center in Chicago. He completed his medical oncology/hematology fellowship at Memorial Sloan Kettering Cancer Center, and an additional research fellowship in the Immunology Program at the Sloan Kettering Institute for Cancer Research, Allogeneic Bone Marrow Transplantation Service, where he also served as a research associate.
| Volume | |
| Day Range: | |
| Bid Price | |
| Ask Price | |
| Last Trade Time: |
