Register for free to join our community of investors and share your ideas. You will also get access to streaming quotes, interactive charts, trades, portfolio, live options flow and more tools.
There is a big difference that many people seem to oversee between NEED and WANT. They do not need to do nothing because they don’t have nothing to provide in Nupelo or Dubai. It never happened!!! They are in the want business now and that includes shareholders money, etc. I hope I get proven wrong
Their IR guy sucks and has proved to be totally useless to the company and shareholders. What is the point of employing Rich Inza for IR if he won't even answer the shareholders and is the guy putting out the PRs that seem very suspect.
They need to provide valid updates with proof about Nupelo, International sales and march delivery orders per tweets and PRs, Dubai, VA, HMO distributor, licensing in all 50 states. If not, then it's safe to say their PRs and tweets have been fraudulent and it is probably going to take a big lawsuit against the company to see what's really going on here. Start talking to your lawyer if you haven't already.
Now 4 weeks and counting without promised response from IR, regarding state licensing revenue discrepancy answer. Another follow-up email was sent this morning.
Per 3/2/23 PR, state licensing revenue should be $165,000 for 2Q23 and 3Q23, and not $150,000 (10% understatement discrepancy each quarter).
Per 10/24/23 PR, state licensing revenue should be $210,000 for 4Q23 and 1Q24, and not $150,000 (40% understatement discrepancy each quarter).
Either, like the financial reports' Section 4)’s being embarrassing 3-7 years out of date, the financial reports’ Income Statement have been erroneous for 4 straight quarters, regarding state licensing revenue, with it understated, while being signed off as correct;
OR
Official PRs on 03/02/23 and 10/24/23 were both erroneous in the information reported, with reported number of state licensing agreements being overstated, with no issued correction.
Yeah, you got me. I was working and didn't realize markets were closed. My bad. Please strike my rant from the record...until it becomes applicable.
Ort maybe it was a market holiday?
VDRM does suck but your rant is totally misplaced. Happy Juneteenth!
Really? OTIKO celebrate Juneteenth anniversary in VDRM. It means, today ViaDerma celebrate those who has been bent over and submitted With lube by the Nigerian Vampire. Every bags in here is full of joy today because it is the only day market is closed in here that nobody get molested.
Don’t hold your breath, more is coming tomorrow. Dilution as fuckkk
Mkt was closed, freedom day.
Can't tell if you are joking or serious. Stock market closed today.
No volume today?? Maybe trading has been halted due to an impending announcement; possible merger between Pfizer and VDRM; or an announcement by the World Heath Organization recognizing Vitastem as the worlds leading topical antibodic cream; or the US military has placed an order for 10,000,000 units; or the FDA has passed regulations requiring VItastem be administered in every hospital in the US.
The other possiblity is that the company sucks and nobody is interested investing.
You didn't really go out on a limb with that prediction. A PR will definitely be issued. The question is when. It could be tomorrow or 2 years from now.
"Pending News / PR on the way" typically. But, doubt otiko has any serious news to share. No tweets in months and nothing from the IR guy. But who knows. Maybe there will be a surprise coming.
That is my fear as well.
911 shares at .00595. Is that a signal? Or is it a hoax?
Based on Otiko's track record, definitely not.
I think so. Next reporting should increased
Is that cash figure really believable?
This is allegedly a new design for suppositories. Sloppy and DB (who I believe are one in the same) were part of the testing, but theirs kept falling out, so it was redesigned several times. This is gonna be a deal breaker so everyone should load up. Only one to a box because of the large size. PR should be released soon. GLTA
We take a Vitastem suppository every time the Doc issues a PR!!
Been repeating the same unsubstantiated nonsense for years. Beyond embarrassing really. Needs to buy another bottle of Ultra and take a swig. By the way....the Doc is going to be announcing a new version of Ultra in a suppository form. Details to follow.
I'd be quite embarrassed.....
Just stop! You have been wrong about everything!!! Never see .007’s again huh?
.0051 don't make it look appealing 4 new buyers
Not the first time we have been in these levels, buying opportunity in here. Once he submit next fillings for better clarity of situation, this shall go back to 0.02…
Seems today some weak hands walked away. Don’t blame them, Otiko makes it hard to swallow
He is the one debating with me. Please check original message
In Post #73,528, misunderstanding became clear. Attention is not being paid to calendar year and OTCM column heading in the included screenshot in Post #73,528, thereby causing misinterpretation. The screenshot accurately depicts 2Q23 financial report for 04/01/23-06/30/23, was not issued until ~7 months late (03/21/24), and thus is not supportive of incorrect claim (2nd Quarter filing Due June 30th).
No he doesn’t. This is just good business. Milking cow business…. The golden egg nest… That makes us the cow and the nest for OTIKO
Exactly, easy to buy and I think soon this will of been great buying opportunity. But if wrong impossible to unload.
Dude, I even gave you the link of the doc. We are talking about 2 different things.
Check
03/21/2024 Quarterly Report 06/30/2023
Your screenshot is about March period, which is the first quarter. I am talking the next (Which per history. 03/21/2024 Quarterly Report 06/30/2023) it is on June 30 this time, 2nd Q per OTIKO’s pattern. I ain’t talking about SEC mate, I am just saying what OTIKO been doing. Not replying to you on this again, I feel I am interacting to a wall.
Agreed. Invariably, someone here will claim this to be a buying opportunity. Been there done that. But when do we get the selling opportunity?? When does our CEO create shareholder value which increases buying interest in the stock which increases the price so that we can capitalize on these so-called buying opportunities?? When you own large blocks of shares, it is hard to flip for small blips in price.
At least .01 with over 1 million in cash and monthly income, no way we should be at these levels.
The price action is infuriating!!! Does Otiko give a sh** at all??
Correct....not sure what he is trying to debate with you.....
One last attempt:
SEC sets due dates for reporting. CEOs do not always meet them. VDRM has very spotty record, but has been doing better last two issuances.
2023 Annual Report (1/1/23-12/31/23) was issued on time on 4/1/24.
1Q24 Report (1/1/24-3/31/24) was issued two days early on 5/13/24.
(see OTCM screenshot below)
In no way, shape or form is a financial report due on last day of a reporting period, in the upcoming case, June 30th, for 2Q24 (4/1/24-6/30/24), nor will a CEO issue a financial report on the last day of the reporting period, again, in the upcoming case June 30th, as was stated in Post #73,517.

We are talking 2 different things in here. Not sure what you mean with falsely equated!! I do write down every time OTIKO submit such and it does match OTCM. You can open his quarterly fillings, dates also match my data. Facts.
Yes, SEC has their general agenda but that’s far from reality in some real scenarios. ViaDerma is proof of such. His delays and history of submitting such. I am only talking about VDRM. Not general SEC data
End of quarter and filing date are being falsely equated.
The end of the quarter is June 30th.
The filing due date per SEC requirements is, as stated, August 14th, with possible 15 Day Extension with Filing of NT 10-K. This information is from the SEC's EDGAR system, as per following link, that was also included at the end of the original reply:
https://edgarsolutions.com/resources/sec-filing-calendar/
Hopefully, he will not be late filing this quarter.
Not sure where did you get that from… That’s general scenario expected from the SEC, in a perfect word… I do follow the Doc patterns and historical submissions on the filings
https://www.otcmarkets.com/otcapi/company/financial-report/394787/content
For the period ending of June 30 2023
So….. Same period on June 30 2024
He does March, then June, then September and so On, check his history filings please
OTIKO has done in like I explained. It is all in OTCM
2nd Qtr 10-K is not due to August 14, with possible 15 Day Extension with Filing of NT 10-K.

https://edgarsolutions.com/resources/sec-filing-calendar/
2nd Quarter filing Due June 30th, we’ll see what he got by then
I could not agree more. My point is Dr. O is aware of the share holders complaints.
Right or wrong he will do it his way. He is the shot caller not the S/H's.
He will stay within his legal limits as this is not his first rodeo.
Long story short we wait.
Ohh yeah, not doubt about it. He’s a good dancer
Unofficial Board Procedure For A Few:
When you have NOT ONE fact to support your contrary position, childish emojis are the ONLY response alternative, since factual, intelligent debate is not a remotely possible option.
I, on the other hand, can defend my contention with those pesky things called facts.
If, and ONLY if, what he is doing has nothing to do with his actual job
(fiduciary responsibility to shareholders;
growing sales and company;
growing shareholder value;
forthright and timely communications with shareholders through financial reporting and press releases and investor relations,
etc.).
|
Followers
|
622
|
Posters
|
|
|
Posts (Today)
|
5
|
Posts (Total)
|
74785
|
|
Created
|
10/27/08
|
Type
|
Free
|
| Moderators | |||

ViaDerma's proprietary transdermal delivery system allows for rapid mass transfer of the pharmaceutical active ingredient across the skin and into the body to provide immediate localized therapy.
The technology allows transfer of chemicals through the stratum corneum (outer layer of skin) with a diffusion constant which is 10,000 times higher than the diffusion constant which characterizes water movement through the stratum corneum.
This enables ViaDerma to pair almost any active ingredient with the technology and provide rapid transport of the medicine right to the site of action.
The first product is a broad spectrum tetracycline-based topical antibiotic is the only antibiotic in the world that kills bacteria both a physical and a chemical mechanism. All known antibiotics (other than ours) primarily use only a chemical mechanism of kill. The physical mechanism of kill is a key feature of what we call Rapid Active Ingredient Delivery System (RAIDS). One result of RAIDS is that tetracycline is carried in higher concentrations, more quickly, to and through the cell walls, where the tetracycline can become more effective than if conventional antibiotics were used. Conventional antibiotics require more time (usually prescribed for 5 to 7 days for best results), whereas our tetracycline-based products usually produce desirable results in 24 hours (or less) because of the RAIDS effect.

A second important result of RAIDS is that the topical antibiotic kills all harmful Gram positive and Gram negative bacteria that have been available for testing. We believe this is the world’s strongest broad-spectrum topical antibiotic available.
The potential commercial impact is immense. Drug developers believe it takes much longer for bacteria to develop drug resistance to a physical kill mechanism. This is because it is relatively easy for bacteria to change their response to a chemical threat, but it takes numerous generations for bacteria to grow a new kind of cell wall structure to respond to a physical threat.
Our novel approach to overcome drug resistance of antibiotics is designed to sustain the effectiveness of antibiotics and other topical drugs for many years. This gives new topical drug products a longer useful lifetime and therefore more commercial value. This technology, when licensed to larger pharmaceutical companies, may provide stronger incentive for the discovery and development of new antimicrobial drugs. In recent years, the dearth of new antibiotics has been largely due to the uncertain new-drug commercial lifetime which is diminished when bacteria develop immunity to that drug.
In addition the technology can be licensed to other companies to convert existing oral drugs to transdermal medications extending the profitably of the existing drug.

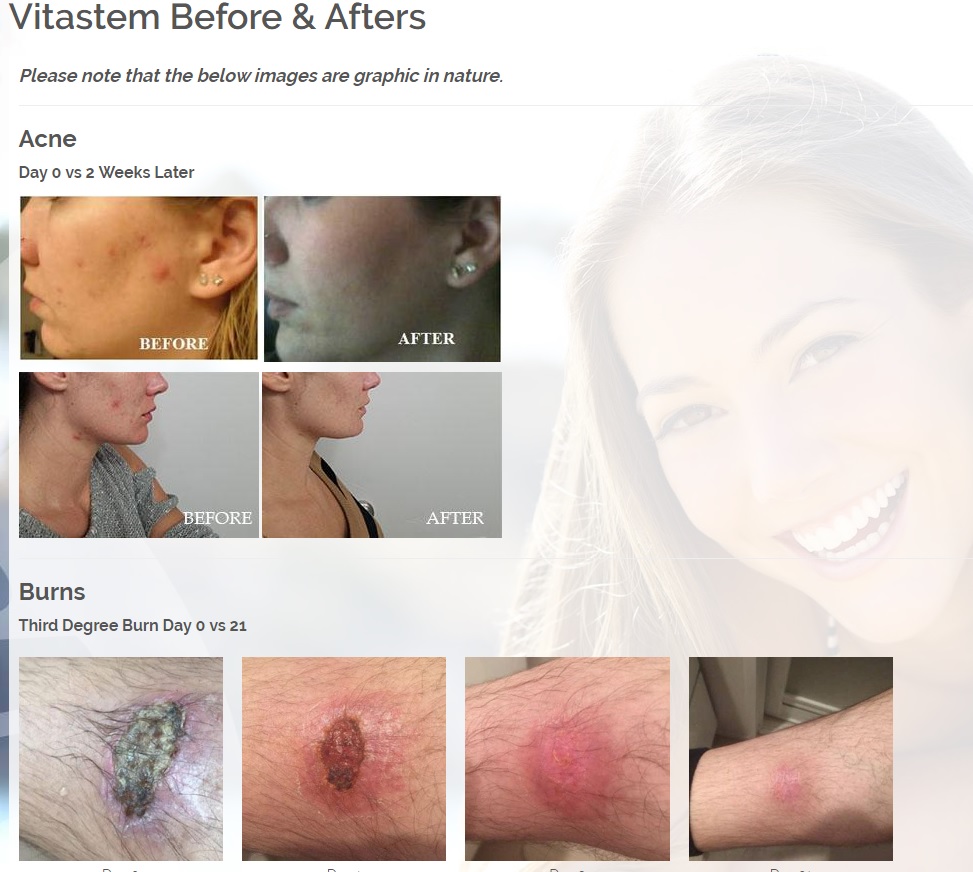
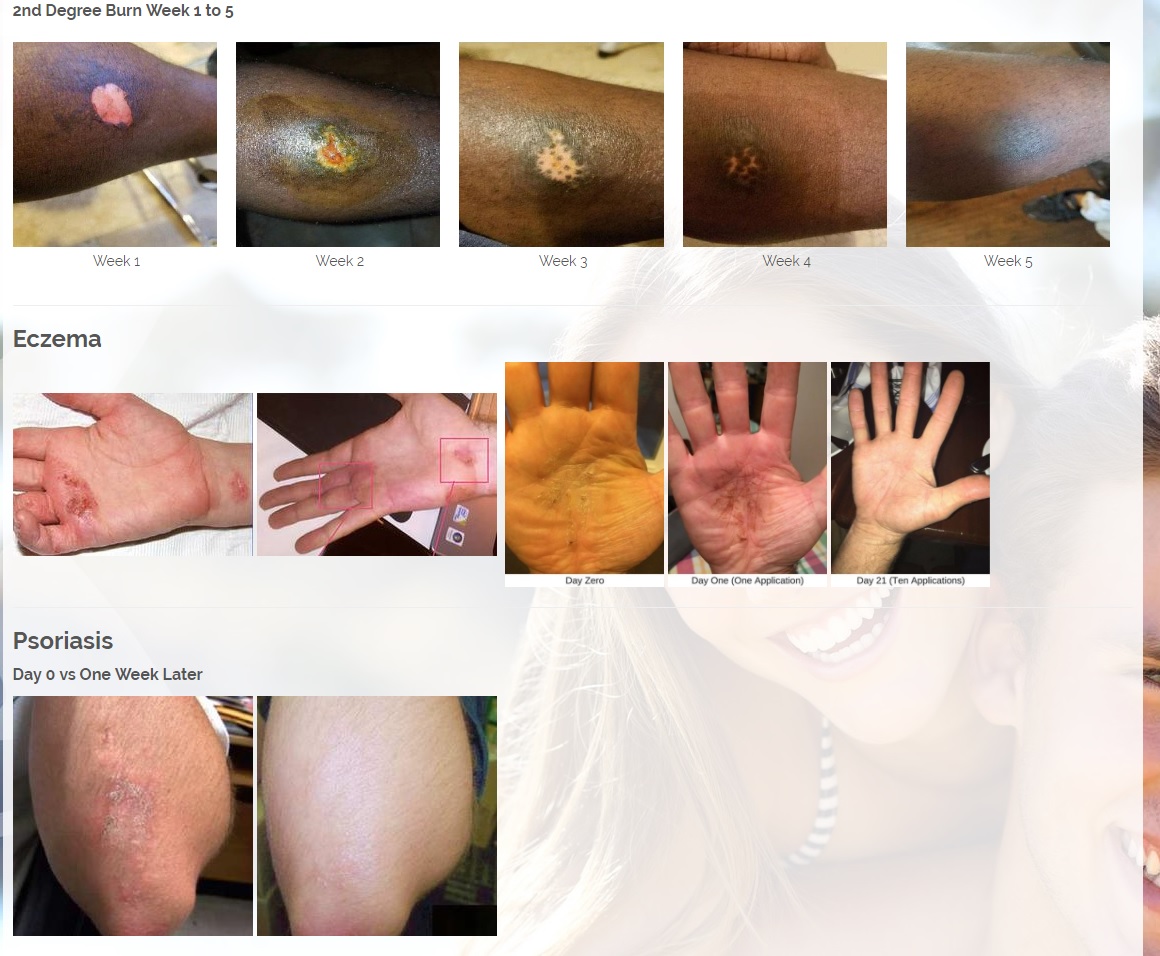
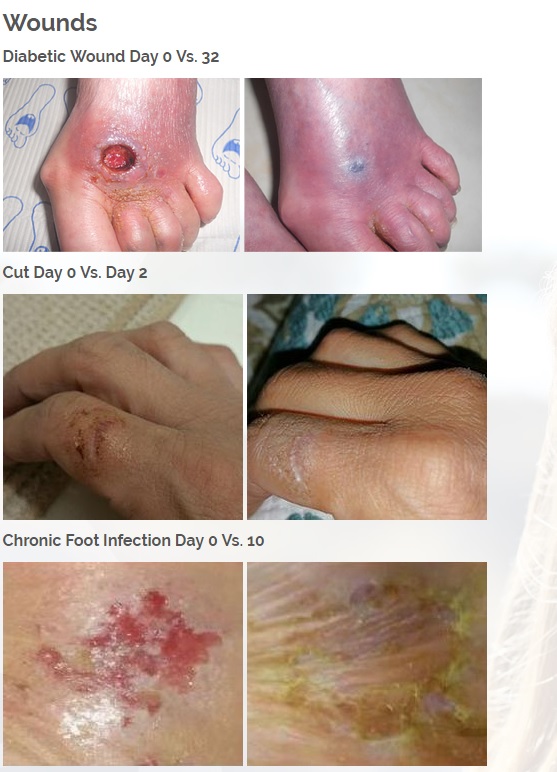

We are developing products in the following fields of use;
Treats all types of bacteria including MRSA, VRE and other flesh eating bacteria. Fights drug resistant bacteria. $6B/year global market.

Onychomycosis, a fungal infection of the toenails, is a major health problem. It is estimated that there are in excess of 40 million sufferers with this condition in the USA. It is a problem throughout the world. A recent European study showed that the prevalence of Onychomycosis may be as high as 26.9%. Fungal resistance can occur when the oral antifungal agents are used on a long-term basis. Topically applied antifungal drugs may work somewhat better adjunctive to surgical removal or chemical dissolution of the nail plate. Yet, this often ineffective and traumatic procedure leaves the subject without a nail for months at risk for re-infection. Given the limitations of current treatment options in this $3B market, there is a great need for a simple, nontoxic and effective alternative treatment. Estimated Global prevalence rate: 140 million.
Treats symptoms of Influenza which is a$4B/ year global market: Common
Diabetic foot wounds.

Per 3/8/18 PR: ViaDerma’s technology is currently being used in Elixr Cannabis products; Topical Balm, Topical Serum and Topical Spray. Sales have begun in Canada.
Per 12/7/17 PR: The Company has signed an MOU and will start production of its Patent Pending CBD or Cannabinoids product line. The Company would combine its two Patent pending technologies, the MMJ Patent #62466209, a patent for delivering medical marijuana / cannabis to the body by applying the medication onto the skin in an ointment base topical solution and the second Provisional patent # 62433964 for enhanced antibiotic and drug delivery for "Aqueous Topical Applications" for human and veterinary uses. The Company would combine its proprietary solutions with CBD's and other natural products that treat psoriasis, fibromyalgia for pain and other ailments.
Per 1/23/18 PR: The Company has also a licensing and distribution agreement in place with its Canadian partners to produce a CBD Topical Serum, which has a 92% absorption rate powered by ViaDerma's Patent Pending Dual Carrier Technology. The CBD and Terpene profile (enriched with specific essential oils and vitamins C, and D) is crafted to alleviate Chronic Dry Skin, Psoriasis, Eczema, Rosacea, and many other skin conditions. The serum has anti-inflammatory, anti-bacterial, and chronic pain relief properties that are absorbed by the skin and provide overall healing benefits. Sales are expected to begin in the first quarter of 2018 and are projected to generate approximately $2 million annual revenues.
The Patented Pending CBD or Cannabinoids product line. The Company would combine its two Patent pending technologies, the MMJ Patent, a patent for delivering medical marijuana / cannabis to the body by applying the medication onto the skin in an ointment base topical solution and the second Provisional patent for enhanced antibiotic and drug delivery for "Aqueous Topical Applications" for human and veterinarian uses. The Company would combine its proprietary solutions with CBD's and other natural products that treat psoriasis, fibromyalgia for pain and other ailments. The Company plans to continue to expand its (IP) "Intellectual Property Portfolio" in 2018.

CBD is a compound found in the Cannabis family of plants such as hemp. CBD is combined into our topical body care products like Elixr's Topical Balm, Serum, and Spray.
CBD is an antioxidant. Antioxidants protect our skin from damaging exposure to smoke, UV rays, and environmental pollutants. Cannabinoid receptors are located all throughout the skin, Topical CBD products interact with those receptors resulting homeostasis and healing. Studies show that CBD can help treat an array of skin conditions such as eczema, psoriasis, rosacea, and acne.
| "What is Intellectual Property?" - A Publication by the World Intellectual Property Organization -
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Volume | |
| Day Range: | |
| Bid Price | |
| Ask Price | |
| Last Trade Time: |
