Register for free to join our community of investors and share your ideas. You will also get access to streaming quotes, interactive charts, trades, portfolio, live options flow and more tools.
Wow mate. Ur brain is fried
Reach IR and ask!!! Nobody is concerned about that mate. We are here waiting about the profits of the sales. He sent 500K units of something at, let’s say $10 each and next month 500K more AND NIGERIAN likes the Koolaid!!! That’s $10M between now and October shipment… AND we know it won’t be sold at $10 a piece, it will be more expensive. Supply and demand results is what we care. Relax on details… let the doc worry about that. Eye on the ball mate, FOCUS ON THE PPS and the money
Reach IR and ask!!! Nobody is concerned about that mate. We are here waiting about the profits of the sales. He sent 500K units of something at, let’s say $10 each and next month 500K more AND NIGERIAN likes the Koolaid!!! That’s $10M between now and October shipment… AND we know it won’t be sold at $10 a piece, it will be more expensive. Supply and demand results is what we care. Relax on details… let the doc worry about that. Eye on the ball mate, FOCUS ON THE PPS and the money
Does anyone know the name of the product in Viaderma’s lineup that contains tetracycline?
If not, how do you expect them to benefit financially from a product that they don’t offer?
Through a licensing fee? Nobody wants to talk about this
Otiko has cried wolf one too many times. Now investors need more than just nice sounding words. File the financial statements, report significant revenues and cash balances to corroborate the nice words; then this thing will take off IMHO. As much as I said I wouldn't, I couldn't resist adding a little last week. My thinking is that here has to be some truth to his announcement (only a brash fraudster would lie about such significant sales). I hope I am right. I (as many here do) have an aweful lot of money at stake. Lets go Otiko...get off your ass.
THIS IS WHY WE TRADE DOWN AT THESE STUPID LEVELS.BECAUSE OTIKO LOST ALL CREDIBILITY.NO ONE BELIEVES ANY OF HIS GARBAGE.THUS NO ONE IS BUYING.ITS STRANGE TO THINK THAT PEOPLE WERE BUYING THIS THING IN THE .01 AND .009 LIKE CRAZY.WHEN IT HAD NOTHING GOING.NOW AT 50% HAIR CUT WITH ALL THESE HUGE CONTRACTS AND NO ONE IS TOUCHING IT.NO TO MENTION PEOPLE WERE BUYING IT ALSO LIKE CRAZY IN THE .02S TO .03S.LOL.
I don't know if it is incompetence or Otiko simply doesn't care or appreciate what it means to operate a publically held company. When someone has no accountability to anyone and he can continue to do so with impunity, then why change the behavior?
Can’t understand why or how the incompetence can continue. It’s unbelievable in my opinion!
Wow, must be Billions in revenues which is holding up the Q2 report from being filed since the Grace period from the NT filing is WAAAAAAAAAAAAAAAY overdue.
SLOPS IS IT YOUR SHARES I KEEP BUYING??HOW MUCH HAVE YOU LEFT WITH.SELL THE REST BRO.ORCA HAS BEEN EATING UP THE FLOAT.ALMOST DAILY.KEEP THEM COMING.
Yes indeed a 10% range of last posted trade
The Bid and ASK gap ridiculous
MM's ,,,gotta laugh ,,for sure,,, 400 share trade, cannot even buy a coffee,,LOL,
Here we go with SPOOFING THE BID again!!!!!!!!! JUST HIT THE ASK IF YOU WANT SHARES!!!!!!
WE GOT TWO MORE MONTHS UNTIL Q3 REPORT.SO MAYBE THEY KEEP HER DOWN HERE,AND WE ADD 20, 30 MILLION SHARES MORE:))))YOU CAN NOT FIX STUPID THESE DAYS.THEY DID THE SAME CRAP WITH AMMX,RJDG,
THEY KEPT AMMX DOWN FOR A LONG TIME TO SHAKE ME OUT.I WAS LMAO.I KEPT ADDING.THEN FROM .08 GOES TO .42 IN A DAY.LOL.AND ON RJDG THEY KEPT HER IN THE .005 FOR A LONG TIME.I WAS BUYING MILLIONS.NEXT THING YOU KNOW IT GOES TO .0145.LMAO.LIKE I SAID YOU CAN NOT FIX STUPID.
NOW THEY ARE KEEPING DOWN VPLM,AND VDRM.I AM LOADING ON BOTH.SOMETIMES DAILY.
WE WILL SEE WHAT HAPPENS ON BOTH.
I GOT PARCIAL FILL TODAY.NOT MUCH.BUT KEEP THEM COMING.I HAD ANOTHER ORDER FOR 5 MILLION SHARES
IN THE .0041S.IT IS FUNNY WHAT THE SCAMBAGS DID.LET ME TELL YOU WHAT THEY DID.
SO I PLACE THE 5 MILLION ORDER AT 12:44 P.M.THE BID WAS AT .0052.AS SOON AS THEY SAW MY 5 MILLION WHAT DID THEY DO???THEY HIT THE BIDS AT .0052 AND .005 AND .0049.AND THEY OFFER AROUND 150K SHARES AT .0049
I WAS IN DISBELIEF.I WAS FURIOUS.I SAID GET READY TO GET FD MFs.SO I HIT THE ASK AT .0049.AND FOR THE REST OF THE DAY.THEY STOPPED THE BS.I GOT PARCIAL.THEY ARE COMPLETE SCAMBAGS.NO QUESTION ABOUT IT.THE MANIPULATION IS UNBELIEVABLE ON PENNY STOCKS LAST TWO YEARS.ORDERS EXPIRED.
IF THEY CAME DOWN TO FILL MY 5 MILLION.NEXT ORDER WOULD BE FOR 10 MILLION AND SO ON.UNTIL I BROKE THEIR BACKS.
Date Time Price Quantity Amount
Sep-18-2024 12:46:28 PM ET $0.0049 37,500.000 $183.75
Sep-18-2024 12:46:57 PM ET $0.0049 105,000.000 $514.50
Net Total: 142,500.000 $698.25
See no matter how bad this stock treats us we can still laugh. 👍
Ok that sounds alot better. Lol
Hmm, I think not titties this time, maybe next year. This looks manipulated again, it is stuck
lol not driving drunk i hope 🙂 post us pictures if you buy those new tits
lol, typo I’m driving
I am going to get her some!
Your going to look funny with tits.🤣
If we hit .02 my wife is getting me titts. Let’s go!
I’m not here to make a little money. I’m hear to make a lot of money
I HAD SAID LAST POST UNTIL THE BS ENDS.TODAY IT SHOWED THAT THE BS WAS NOT WHERE TO BE FOUND THUS I AM POSTING AGAIN.THE FLOAT IS GETTING VERY TIGHT.THE CHEAP SHARES SHOWED TODAY THAT WERE NOT WHERE TO BE FOUND.TIME TO MOVE IN THE .01 AREA.LET;S HOPE WE WILL HIT .03 BY YEAR END.OR .07 BY END OF MARCH 2025 ON THE YEARLY 2024 DUE FILING.WHICH WILL HAVE BOTH 500K ORDERS IN IT.
ORCA
Re: ORCA post# 74408
Thursday, September 12, 2024 10:46:31 AM
Post#
74409
of 74434
LAST POST HERE UNTIL THE BS ENDS.GOOD LUCK.KEEP HITTING THE BIDS.WE WILL SEE HOW LONG THIS BS WILL GO ON.BUT BE PREPARED TO GET FD.NO QUESTION ABOUT IT.REALLY REALLY FD.
Regarding overdue report, see https://investorshub.advfn.com/boards/read_msg.aspx?message_id=175066444
No that I am aware of. Why are you asking for an IR interaction Slops?
Sure would be nice if Ms Zia Choe would update and post the Q2….. Unbelievable the incompetence that seems to occur. Sort of feel bad because it makes it seem like Ms Choe is incompetent by not having the fins posted on-time since she already knows what she needs and should have asked the Dr for it prior to him going on any trips!!!
We need the great news to bring in NEW SHARE HOLDERS,,,,,I think everyone here is happy with current positions.
Updated Fins and revenue numbers from the Nigeria contracts would be a good start. Then 2025 projections for sales and revenue would be even better.
Without these, this isn't moving up much. Think shareholders and those on the sidelines aren't buying the recent news entirely yet.
Was hopefully thinking , but cannot even get EVEN.
Let's hope for a good day with a big price jump
i agree with you...someone was selling shares last week for whatever reason. you never know why someone does something like that but there is no dilution. there doesn't need to be any dilution from now on
Well would be nice if Dr would have the fins posted and actually audited.
So place your bets, how high or low do you think we are going to hit Tomorrow Monday? It is a key day. Mid of the month!!! Favorite for OTC CEOs to drop PRs and fillings… We’ll see
Seems very strange prices not going ularge volumes trading I hopnot selling sharese
I don’t worry thinking about that until I do check the Fins. Holding long
You don't think the good Dr. Is selling his shares do you?
What's up guess they don't believe the Dr. sold 500k units and 500k more on the way next month.... Not getting any of my shares I'm holding tight....
Level 2 showed such at the moment I pulled such. No sure where did you buy such but at the moment I pulled the volume you didn’t buy that amount per level 2 tool
Of course it is.
It is easy, level two and Time And Sales, real time.
Not sure where you got the buys and sells. I bought 815,000 shares myself.
Guys, I think this is being manipulated
Buy: 272K: Sell: 3M
This is not due to weak hands. This seems somewhat, someone is……. I better wait until shared update or next quarter… I don’t want to say dilution because not too many shares for such. But the gap is interesting between buy and sell in VDRM
|
Followers
|
617
|
Posters
|
|
|
Posts (Today)
|
10
|
Posts (Total)
|
74461
|
|
Created
|
10/27/08
|
Type
|
Free
|
| Moderators | |||

ViaDerma's proprietary transdermal delivery system allows for rapid mass transfer of the pharmaceutical active ingredient across the skin and into the body to provide immediate localized therapy.
The technology allows transfer of chemicals through the stratum corneum (outer layer of skin) with a diffusion constant which is 10,000 times higher than the diffusion constant which characterizes water movement through the stratum corneum.
This enables ViaDerma to pair almost any active ingredient with the technology and provide rapid transport of the medicine right to the site of action.
The first product is a broad spectrum tetracycline-based topical antibiotic is the only antibiotic in the world that kills bacteria both a physical and a chemical mechanism. All known antibiotics (other than ours) primarily use only a chemical mechanism of kill. The physical mechanism of kill is a key feature of what we call Rapid Active Ingredient Delivery System (RAIDS). One result of RAIDS is that tetracycline is carried in higher concentrations, more quickly, to and through the cell walls, where the tetracycline can become more effective than if conventional antibiotics were used. Conventional antibiotics require more time (usually prescribed for 5 to 7 days for best results), whereas our tetracycline-based products usually produce desirable results in 24 hours (or less) because of the RAIDS effect.

A second important result of RAIDS is that the topical antibiotic kills all harmful Gram positive and Gram negative bacteria that have been available for testing. We believe this is the world’s strongest broad-spectrum topical antibiotic available.
The potential commercial impact is immense. Drug developers believe it takes much longer for bacteria to develop drug resistance to a physical kill mechanism. This is because it is relatively easy for bacteria to change their response to a chemical threat, but it takes numerous generations for bacteria to grow a new kind of cell wall structure to respond to a physical threat.
Our novel approach to overcome drug resistance of antibiotics is designed to sustain the effectiveness of antibiotics and other topical drugs for many years. This gives new topical drug products a longer useful lifetime and therefore more commercial value. This technology, when licensed to larger pharmaceutical companies, may provide stronger incentive for the discovery and development of new antimicrobial drugs. In recent years, the dearth of new antibiotics has been largely due to the uncertain new-drug commercial lifetime which is diminished when bacteria develop immunity to that drug.
In addition the technology can be licensed to other companies to convert existing oral drugs to transdermal medications extending the profitably of the existing drug.

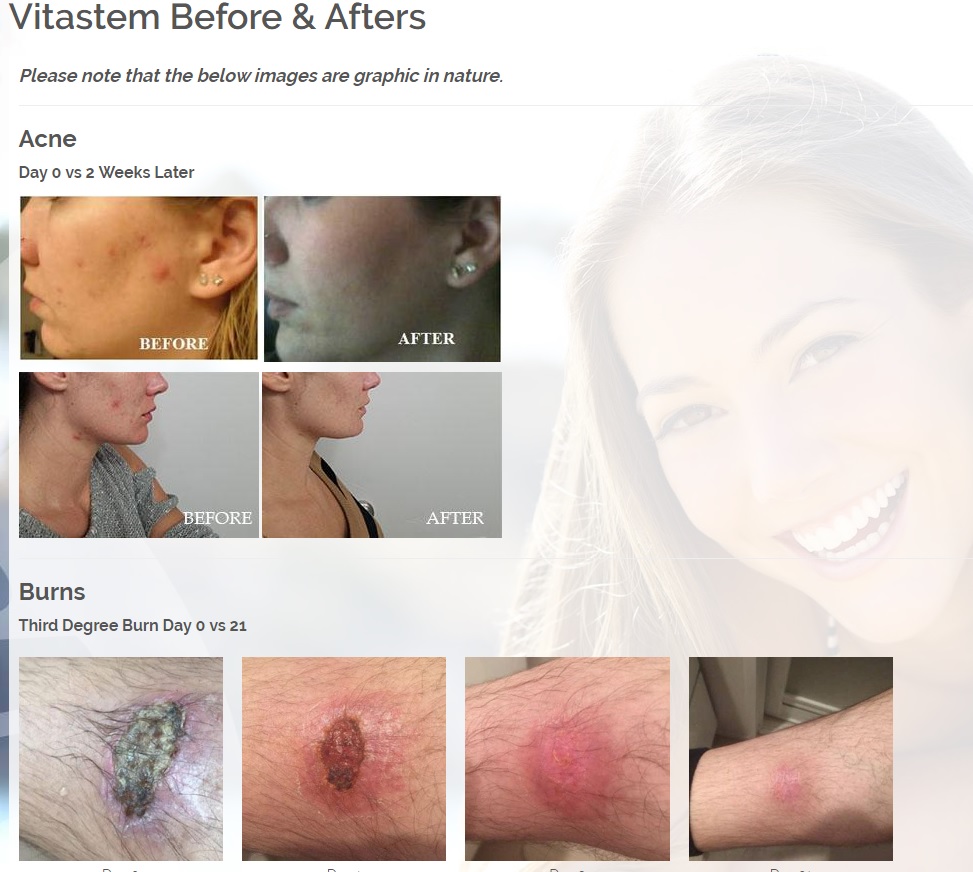
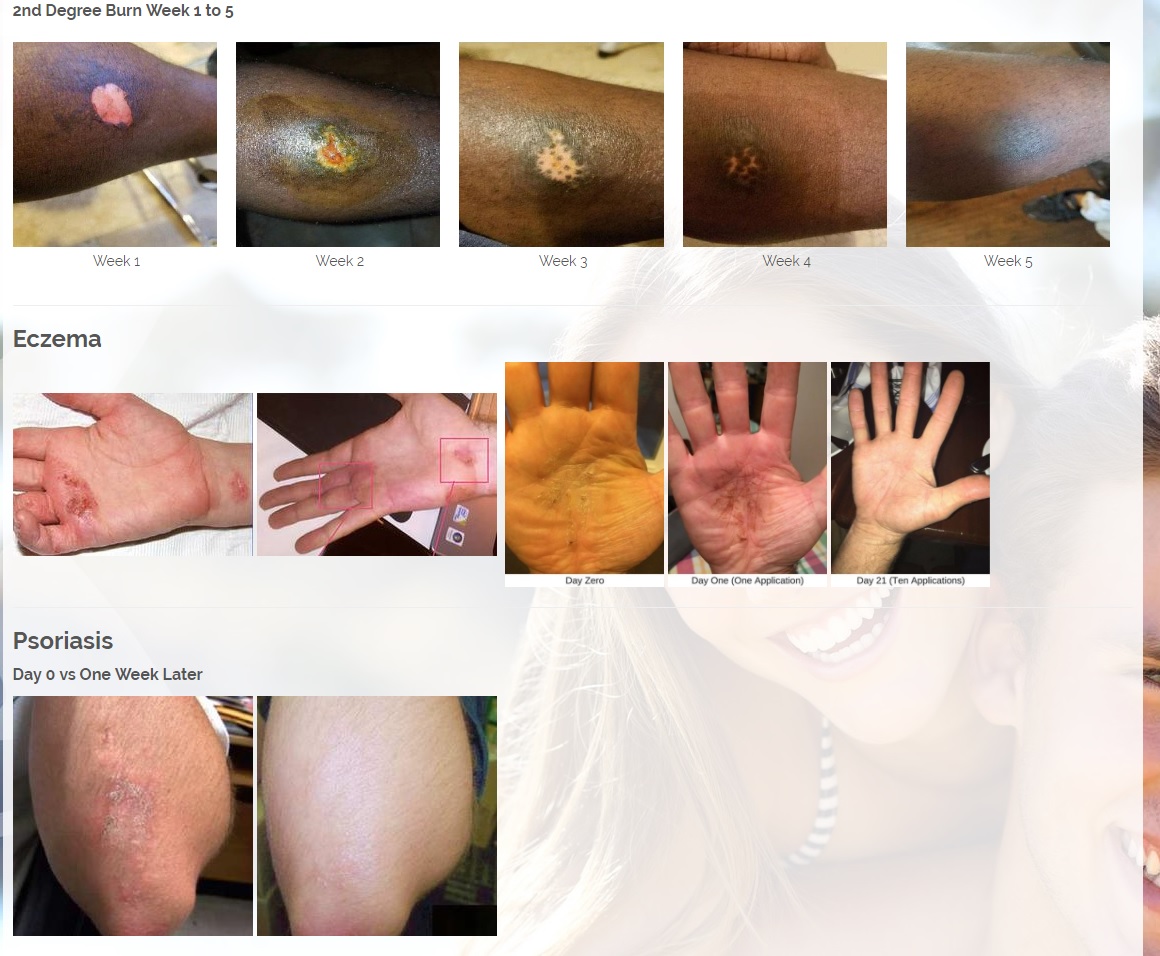
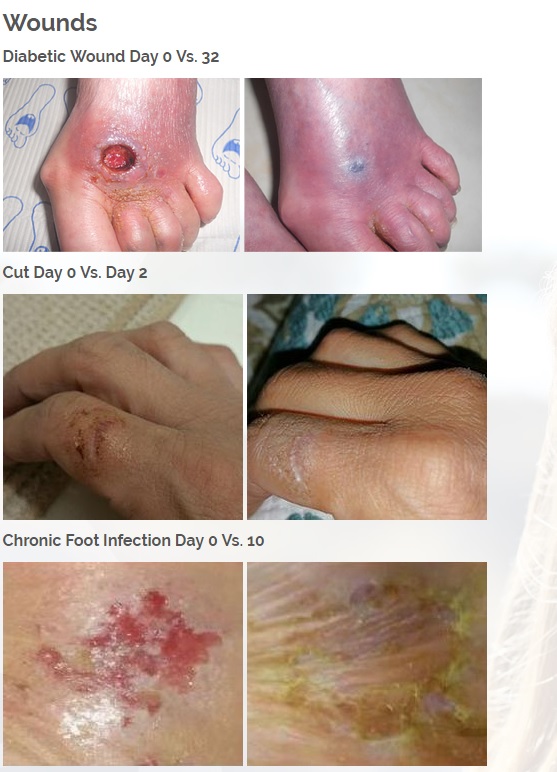

We are developing products in the following fields of use;
Treats all types of bacteria including MRSA, VRE and other flesh eating bacteria. Fights drug resistant bacteria. $6B/year global market.

Onychomycosis, a fungal infection of the toenails, is a major health problem. It is estimated that there are in excess of 40 million sufferers with this condition in the USA. It is a problem throughout the world. A recent European study showed that the prevalence of Onychomycosis may be as high as 26.9%. Fungal resistance can occur when the oral antifungal agents are used on a long-term basis. Topically applied antifungal drugs may work somewhat better adjunctive to surgical removal or chemical dissolution of the nail plate. Yet, this often ineffective and traumatic procedure leaves the subject without a nail for months at risk for re-infection. Given the limitations of current treatment options in this $3B market, there is a great need for a simple, nontoxic and effective alternative treatment. Estimated Global prevalence rate: 140 million.
Treats symptoms of Influenza which is a$4B/ year global market: Common
Diabetic foot wounds.

Per 3/8/18 PR: ViaDerma’s technology is currently being used in Elixr Cannabis products; Topical Balm, Topical Serum and Topical Spray. Sales have begun in Canada.
Per 12/7/17 PR: The Company has signed an MOU and will start production of its Patent Pending CBD or Cannabinoids product line. The Company would combine its two Patent pending technologies, the MMJ Patent #62466209, a patent for delivering medical marijuana / cannabis to the body by applying the medication onto the skin in an ointment base topical solution and the second Provisional patent # 62433964 for enhanced antibiotic and drug delivery for "Aqueous Topical Applications" for human and veterinary uses. The Company would combine its proprietary solutions with CBD's and other natural products that treat psoriasis, fibromyalgia for pain and other ailments.
Per 1/23/18 PR: The Company has also a licensing and distribution agreement in place with its Canadian partners to produce a CBD Topical Serum, which has a 92% absorption rate powered by ViaDerma's Patent Pending Dual Carrier Technology. The CBD and Terpene profile (enriched with specific essential oils and vitamins C, and D) is crafted to alleviate Chronic Dry Skin, Psoriasis, Eczema, Rosacea, and many other skin conditions. The serum has anti-inflammatory, anti-bacterial, and chronic pain relief properties that are absorbed by the skin and provide overall healing benefits. Sales are expected to begin in the first quarter of 2018 and are projected to generate approximately $2 million annual revenues.
The Patented Pending CBD or Cannabinoids product line. The Company would combine its two Patent pending technologies, the MMJ Patent, a patent for delivering medical marijuana / cannabis to the body by applying the medication onto the skin in an ointment base topical solution and the second Provisional patent for enhanced antibiotic and drug delivery for "Aqueous Topical Applications" for human and veterinarian uses. The Company would combine its proprietary solutions with CBD's and other natural products that treat psoriasis, fibromyalgia for pain and other ailments. The Company plans to continue to expand its (IP) "Intellectual Property Portfolio" in 2018.

CBD is a compound found in the Cannabis family of plants such as hemp. CBD is combined into our topical body care products like Elixr's Topical Balm, Serum, and Spray.
CBD is an antioxidant. Antioxidants protect our skin from damaging exposure to smoke, UV rays, and environmental pollutants. Cannabinoid receptors are located all throughout the skin, Topical CBD products interact with those receptors resulting homeostasis and healing. Studies show that CBD can help treat an array of skin conditions such as eczema, psoriasis, rosacea, and acne.
| "What is Intellectual Property?" - A Publication by the World Intellectual Property Organization -
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Volume | |
| Day Range: | |
| Bid Price | |
| Ask Price | |
| Last Trade Time: |
