Register for free to join our community of investors and share your ideas. You will also get access to streaming quotes, interactive charts, trades, portfolio, live options flow and more tools.
Price rising and volume picking up! GOOD THINGS coming!!
$VDRM $$$$$
His opportunity for improvement in the latest PR, regards likely issue of report regarding successful testing. Tomorrow will be two weeks after the espoused four weeks of testing. It would be normal that a report or other documentation is being prepared, and two weeks or a measure more time is certainly not unusual to complete/approve it. It just would have been preferred if this step had been mentioned and included in the timing expectations.
Nice close today, missed the last 2 hrs of the market but last trade was 1.1 milly to close the day up.. looking fwd to tomorrow.
Agree. I do think in past years he has put out PRs that were based on half-azz info at best. In VDRMs case with the Dr. at the helm, less is more. Get everything straight before a PR, or make sure you have something worthy before PR
All in my opinion of course. But let’s give them until next week at the latest to give us info. Last 2 PR’s were one on a Wednesday and one on a Thursday…..
Patience is a virtue. All good things come to those who wait they say. So far IR and the DR have been trending in legitimacy by not stating false info. So rather they have all their “ducks in a row” and not put out anything half-assed.
We need the test results!!! Why Dr. O is taking forever ?????
Emphasizing “How great would it be to have more interest in the company and regular and positive business developments to discuss?”
3 million on the bid
Looking great 2 minutes to go @89 over 3.7 million
We are going into some serious times
I, like most, do not prefer to be wrong, but I am glad I was wrong about qtr report being the center of the problem versus the Profile, whereas the fix was quicker. $0.0082 (full retrace before hiccup) or higher close will be nice next step, while waiting for the successful testing announcement, with follow-on resumption of inventory for sale at Amazon, contracts and expansion.
Unless the owners, who sold during the hiccup, had already intended to sell during that period, it is a shame that they were duped.
Separately, hopefully one day the word "soon" will be consistently applicable to upcoming positive business announcements.
How great would it be to have more interest in the company and regular and positive business developments to discuss?
Step in the right direction. Seeing some good buys
Positive update coming soon!
WE NEED AN UPDATE ON THE TESTING.OTIKO HAS TO GIVE US AN UPDATE ON HOW THE TESTING WENT.IT IS PAST DUE.ONCE HE GIVES US AND UPDATE.IF POSITIVE WE ROCK BIG TIME.IF NOT THEN WE ARE IN TROUBLE.
Buying more and more in here, buying more on Monday, we are past due in here. Supernova coming
Believe we are getting closer to a major run here. Next quarter should be good cash flow.
BAHAHAHAHA!! This isn't aging well! PINK CURRENT!!!
Let's go $VDRM $$$$$$$
Yep.....News is once the Grace Period is up we hit the Expert Market where there will be no liquidity as it will not have any MM's.
If so you could move mountians LOL
It’s time for me to vent. I’ve been told by the company since fourth quarter of last year that the PO’s are coming. How long does it take to get a contract signed. There are 4 PO’s and an Amazon press release waiting in the wings for 3/4 of a year. Even for a pink sheet company, we should be much further down the road. Let’s get this company moving.
BIG BIDS ARE IN.IF ANYONE WANTS TO BAIL YOU KNOW WHAT TO DO.LOL.
BITCHING DID THE TRICK.AND MADE THEM MOVE FROM THEIR A##ES.LEARN THE GAME.
DeerBalls I agree bitching did nothing.
No, no "noise" made anything happen. VDRM had been on THE OTC MARKETS' PROBLEM, but OTC Markets is slow to react.
"Expert market"....NOPE!
Be well, things are fine.
THIS IS GREAT NEWS.SOME NOISE MAKES THINGS MOVE FASTER.BACK TO ACCUMULOLOGY MODE STARTING TODAY.
With this time and (money wasting distraction for weaker investors) out of the way, I expect an update by end of next week! And if it’s a week or two after that I really don’t care. Quarterly fins due 8/15, and I expect a worst case scenario to still show revenues from licensing. Something the vast majority of Pennie’s can’t boast or even hope for!!
Back to Pink Current:
https://www.otcmarkets.com/stock/VDRM/news
Agreed all of my emails are in correct form pushing blame no where and have replied to.
Most of mine over the last year plus were followups on announced items. Two did have somewhat sarcastic quips in it. In any event, glad to know Investor Relations is on top of it!
Thanks again for your information.
It always has been working. Only time I would think anyone should never receive a reply would be if asking insider information questions or if the email came off as nasty.
Your report was absolutely correct about the Profile Update. My assumption was dead wrong on report. I just received a second email response from Investor Relations, see below text:
The Yield sign is scheduled to come off tomorrow. As you can see the profile has now been verified which was the reason for the status change.
Separately, I am glad Investor Relations is functioning again!
100%! All good though, bought a bunch of cheapies!
Correct. The bullshit Yield sign issue was because the Company Profile had not been updated in a while. Check now. You will notice that it now shows the Company Profile as being updated with 7/2022 date.
https://www.otcmarkets.com/stock/VDRM/profile
So hopefully by tomorrow or Monday the Yield sign should be gone.
Probably a Market Maker who needs shares thought of this quick hit-type piece to get this designation to show on the OTC so as to scoop up shares from panicked sellers in my opinion. What a crock of shit!
Correct. The bullshit Yield sign issue was because the Company Profile had not been updated in a while. Check now. You will notice that it now shows the Company Profile as being updated with 7/2022 date.
https://www.otcmarkets.com/stock/VDRM/profile
So hopefully by tomorrow or Monday the Yield sign should be gone.
Yield sign coming off soon! Fingers crossed for Friday or Monday next week..
GREAT time to BUY!!
$VDRM $$$
Back to the business for a moment. It would seem that the best possibility, in the current situation, for the 2nd Quarter Report is $135K revenue from the continuing licenses in the nine (9) states, same as1st Quarter Report.
Excerpt from Rule 15c2-11, regarding what is required:
Issuer Information
The nature of the issuer information that is required to be reviewed for material accuracy and reliability, and to be current and publicly available, turns on the nature of the issuer under federal securities laws related to issuer disclosures. For example, the issuer information would be a Securities Act of 1933 (Securities Act) Section 10(a) prospectus within 90 days of effectiveness, a Securities Act Regulation A offering circular that was qualified less than 40 days prior to the quote date, an annual report filed with the SEC under Section 13 or 15(d) of the Securities Exchange Act of 1934 (Exchange Act) and OTHER PERIODIC and CURRENT REPORTS, AN ANNUAL REPORT FILED PURSUANT TO REGULATION Crowdfunding, an annual statement of an insurance company per Section 12(g)(2)(G)(i), or information published as required by a private foreign issuer exempt from registration under SEC Rule 12g3-2(b).
https://www.wilmerhale.com/insights/client-alerts/20201029-sec-amends-rule-15c2-11-to-enhance-publicly-available-information-for-securities-quoted-in-the-over-the-counter-markets
My first response from Investor Relations in over a year was received overnight. It is to an email that I sent 7/25/22. I had inquired:
"Is it expected that the 2nd quarter report will be issued on time (on or before 8/15/22)."
Response received:
"Yes, probably before. We are usually early on our filings."
Note: It was sent to info@viadermalicensing.com.
I have NOT yet received response to emails sent on 7/26/22 and yesterday. I will pass along responses if, and when received.
It's going to the Expert Market.
They don't understand the new Regulation.
Has NOTHING to do with the upcoming Quarterly Report LMFAO.....
Then will immediately display no quotes and become illiquid.
DeerBalls good post I agree 100%
Relax everything is moving at a slow pace
And the hits just keep on coming. Wow! Again, I feel so much better. NOT! All, and I mean ALL evidence to the contrary. Great to talk about what used to be and how great the product was but what good is the memory of a product the public can't buy or find anywhere. Generating revenue? Really where? Except for maybe some minor runs this company seems to be circling the drain. Just excuses after excuses after more excuses. But what remains humorous is the nonsense through the prism of smoking mirrors. As always just my opinion but at least it's honest and from someone with a sizable position here.
THERE IS NO EXCUSE.THAT FPOS OTIKO SHOULD UPDATE SHAREHOLDERS OF WHAT THE F IS GOING ON.GOOD OR BAD.1)WHAT HAPPENED WITH THE TESTING???
2)UPDATE US ABOUT THE OTC PROBLEM.LIKE ALL HE HAS TO SAY IS:DEAR SHAREHOLDERS WE ARE AWARE WITH THE PROBLEM,AND WE ARE ON IT,AT THE MOMENT TO FIX IT,AND GET BACK TO CURRENT STATUS.
$.0067 Just a suggestion from someone who was a broker for most of my career and have been around thousands of stocks/situations: Investors contacting the company/IR expecting it to be productive in the sense of, "this is a problem/fix this/are you aware that" is TOTAL FOLLY! It may make some feel they are doing something, but it is as productive as moving papers around on a messy desk.
VDRM will be fine, things will get fixed, in my view. VDRM is tiny, underfunded and has had some bumps, ok. VDRM is aware of the OTC Markets screwup, OTC Markets fixes it when they do. IT WILL GET FIXED!
This all reminds me of a buddy(broker I worked with) who passed a few years back. Nicest, low key guy you'd ever meet, but would stand up for himself when pushed. His wife was Type-A and would pick/pick/pick...my buddy would take it for days, until he had enough and BOOM, the "SHUT-THE-F-UP" would come. The wife would for a few days. My point, don't be that wife as a shareholder, it is counterproductive.
VDRM has a great product and we will be happy in the relative near-term! CHILL!
No or, hopefully, not yet.
Did you get a reply?
$.006635 BULLSHIT! There are revenues, when product is available. It will be soon. Because a pink sheet, VDRM, doesn't have the resources of pfe, and misses being spot-on time-wise means little.
I know folks who have used the product and they rave about it.
Licensing revenues, are they for NOTHING? I don't think so.
Hey, no one is forced to buy/hold... I'M DEFINITELY HOLDING!
|
Followers
|
627
|
Posters
|
|
|
Posts (Today)
|
12
|
Posts (Total)
|
74901
|
|
Created
|
10/27/08
|
Type
|
Free
|
| Moderators | |||

ViaDerma's proprietary transdermal delivery system allows for rapid mass transfer of the pharmaceutical active ingredient across the skin and into the body to provide immediate localized therapy.
The technology allows transfer of chemicals through the stratum corneum (outer layer of skin) with a diffusion constant which is 10,000 times higher than the diffusion constant which characterizes water movement through the stratum corneum.
This enables ViaDerma to pair almost any active ingredient with the technology and provide rapid transport of the medicine right to the site of action.
The first product is a broad spectrum tetracycline-based topical antibiotic is the only antibiotic in the world that kills bacteria both a physical and a chemical mechanism. All known antibiotics (other than ours) primarily use only a chemical mechanism of kill. The physical mechanism of kill is a key feature of what we call Rapid Active Ingredient Delivery System (RAIDS). One result of RAIDS is that tetracycline is carried in higher concentrations, more quickly, to and through the cell walls, where the tetracycline can become more effective than if conventional antibiotics were used. Conventional antibiotics require more time (usually prescribed for 5 to 7 days for best results), whereas our tetracycline-based products usually produce desirable results in 24 hours (or less) because of the RAIDS effect.

A second important result of RAIDS is that the topical antibiotic kills all harmful Gram positive and Gram negative bacteria that have been available for testing. We believe this is the world’s strongest broad-spectrum topical antibiotic available.
The potential commercial impact is immense. Drug developers believe it takes much longer for bacteria to develop drug resistance to a physical kill mechanism. This is because it is relatively easy for bacteria to change their response to a chemical threat, but it takes numerous generations for bacteria to grow a new kind of cell wall structure to respond to a physical threat.
Our novel approach to overcome drug resistance of antibiotics is designed to sustain the effectiveness of antibiotics and other topical drugs for many years. This gives new topical drug products a longer useful lifetime and therefore more commercial value. This technology, when licensed to larger pharmaceutical companies, may provide stronger incentive for the discovery and development of new antimicrobial drugs. In recent years, the dearth of new antibiotics has been largely due to the uncertain new-drug commercial lifetime which is diminished when bacteria develop immunity to that drug.
In addition the technology can be licensed to other companies to convert existing oral drugs to transdermal medications extending the profitably of the existing drug.

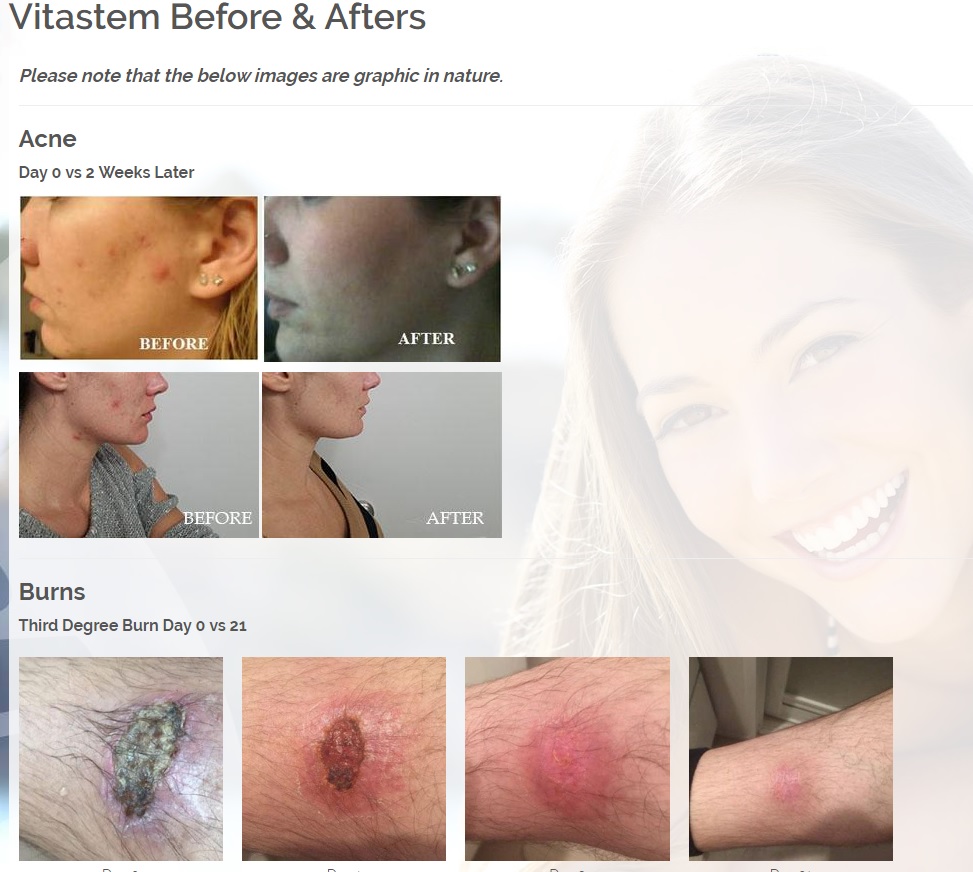
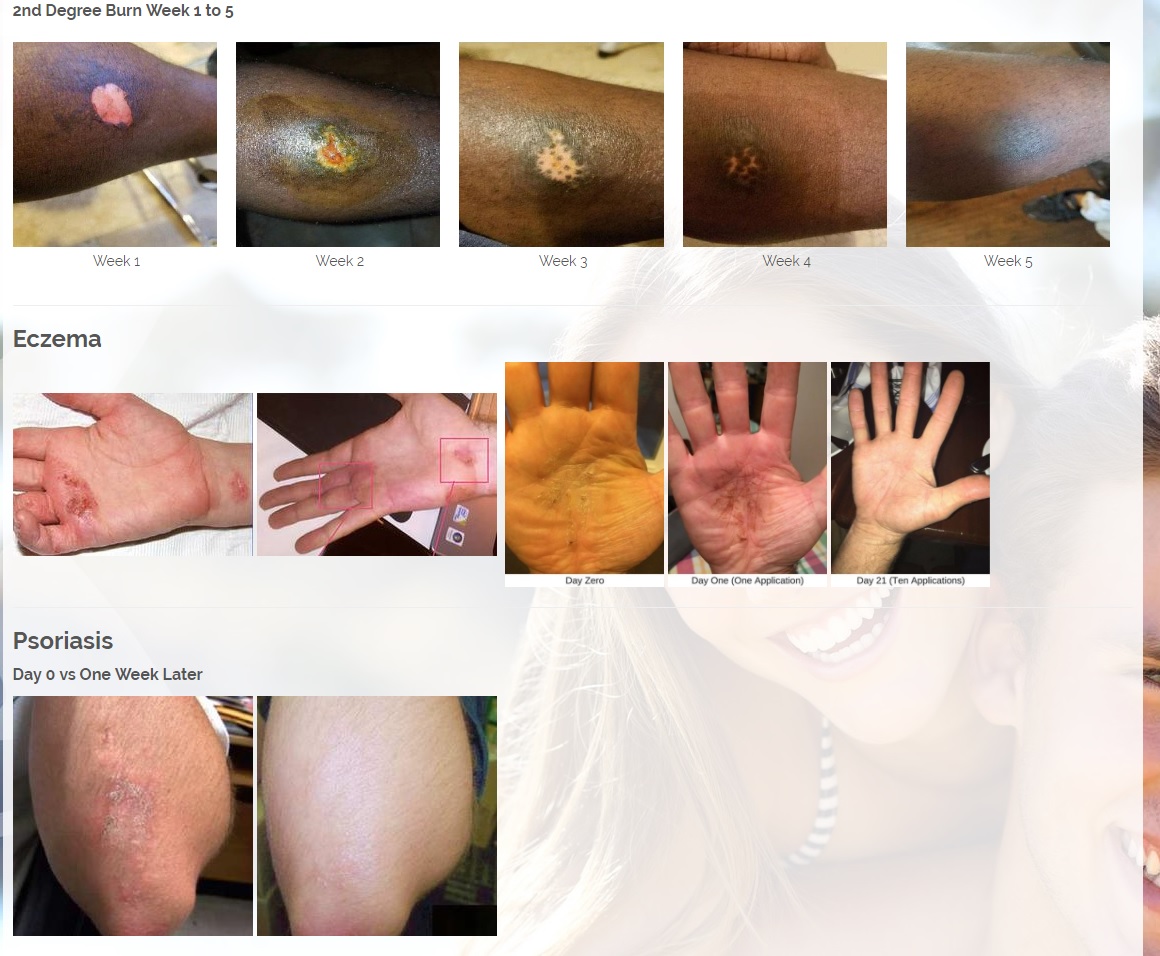
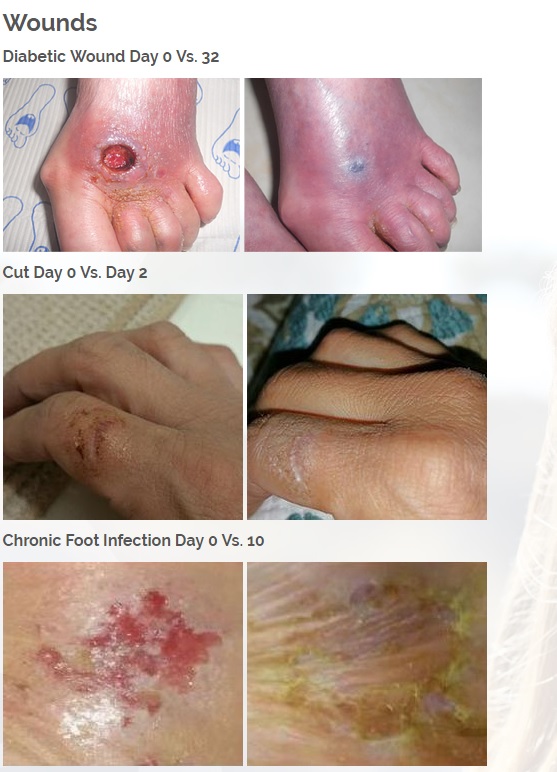

We are developing products in the following fields of use;
Treats all types of bacteria including MRSA, VRE and other flesh eating bacteria. Fights drug resistant bacteria. $6B/year global market.

Onychomycosis, a fungal infection of the toenails, is a major health problem. It is estimated that there are in excess of 40 million sufferers with this condition in the USA. It is a problem throughout the world. A recent European study showed that the prevalence of Onychomycosis may be as high as 26.9%. Fungal resistance can occur when the oral antifungal agents are used on a long-term basis. Topically applied antifungal drugs may work somewhat better adjunctive to surgical removal or chemical dissolution of the nail plate. Yet, this often ineffective and traumatic procedure leaves the subject without a nail for months at risk for re-infection. Given the limitations of current treatment options in this $3B market, there is a great need for a simple, nontoxic and effective alternative treatment. Estimated Global prevalence rate: 140 million.
Treats symptoms of Influenza which is a$4B/ year global market: Common
Diabetic foot wounds.

Per 3/8/18 PR: ViaDerma’s technology is currently being used in Elixr Cannabis products; Topical Balm, Topical Serum and Topical Spray. Sales have begun in Canada.
Per 12/7/17 PR: The Company has signed an MOU and will start production of its Patent Pending CBD or Cannabinoids product line. The Company would combine its two Patent pending technologies, the MMJ Patent #62466209, a patent for delivering medical marijuana / cannabis to the body by applying the medication onto the skin in an ointment base topical solution and the second Provisional patent # 62433964 for enhanced antibiotic and drug delivery for "Aqueous Topical Applications" for human and veterinary uses. The Company would combine its proprietary solutions with CBD's and other natural products that treat psoriasis, fibromyalgia for pain and other ailments.
Per 1/23/18 PR: The Company has also a licensing and distribution agreement in place with its Canadian partners to produce a CBD Topical Serum, which has a 92% absorption rate powered by ViaDerma's Patent Pending Dual Carrier Technology. The CBD and Terpene profile (enriched with specific essential oils and vitamins C, and D) is crafted to alleviate Chronic Dry Skin, Psoriasis, Eczema, Rosacea, and many other skin conditions. The serum has anti-inflammatory, anti-bacterial, and chronic pain relief properties that are absorbed by the skin and provide overall healing benefits. Sales are expected to begin in the first quarter of 2018 and are projected to generate approximately $2 million annual revenues.
The Patented Pending CBD or Cannabinoids product line. The Company would combine its two Patent pending technologies, the MMJ Patent, a patent for delivering medical marijuana / cannabis to the body by applying the medication onto the skin in an ointment base topical solution and the second Provisional patent for enhanced antibiotic and drug delivery for "Aqueous Topical Applications" for human and veterinarian uses. The Company would combine its proprietary solutions with CBD's and other natural products that treat psoriasis, fibromyalgia for pain and other ailments. The Company plans to continue to expand its (IP) "Intellectual Property Portfolio" in 2018.

CBD is a compound found in the Cannabis family of plants such as hemp. CBD is combined into our topical body care products like Elixr's Topical Balm, Serum, and Spray.
CBD is an antioxidant. Antioxidants protect our skin from damaging exposure to smoke, UV rays, and environmental pollutants. Cannabinoid receptors are located all throughout the skin, Topical CBD products interact with those receptors resulting homeostasis and healing. Studies show that CBD can help treat an array of skin conditions such as eczema, psoriasis, rosacea, and acne.
| "What is Intellectual Property?" - A Publication by the World Intellectual Property Organization -
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Volume | |
| Day Range: | |
| Bid Price | |
| Ask Price | |
| Last Trade Time: |
