Register for free to join our community of investors and share your ideas. You will also get access to streaming quotes, interactive charts, trades, portfolio, live options flow and more tools.
Call the office I did They can explain.
There has never been doubts about such. The problem is the shareholders: We do have the hard stick in, for years, without lube man. You know it AND the company doing well
Hence......
Watching for "Red Flags" Revealing Unreliable Microcap Press Releases
Given press release misinformation risks, savvy microcap investors watch for certain "red flags" that may reveal a release contains unreliable claims:
- Announcements lacking specifics or relying on vague claims that would not allow objective independent verification.
- Press releases strangely timed late in the day after markets close or late on Fridays, suggesting an aim to avoid scrutiny.
- Material announcements failing to provide customary supporting evidence like verifiable names of referenced customers or vendors.
- Major claims that seem to directly contradict objective data in the company's actual financial disclosures and filings.
Figure it out yet DD master?
Good luck getting an answer. I highly doubt it.
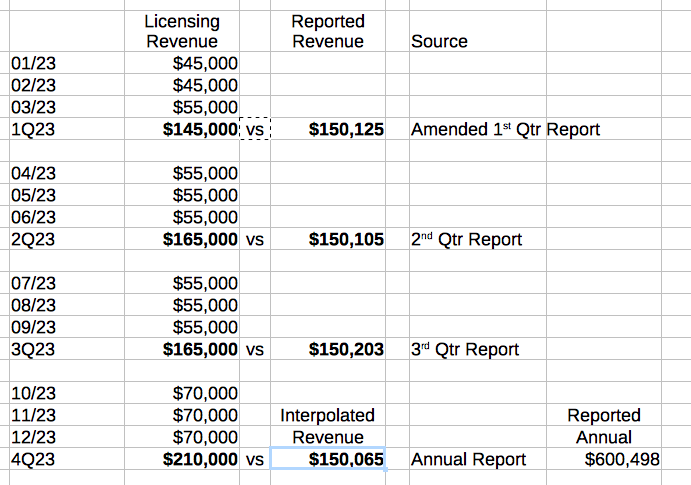
Like too many things with VDRM, revenue numbers lead to only to more questions to put it politely. The reported numbers for 2023 look like licensing revenue from 10 states/month, with a trickle of online sales. However, 06/24/21 PR stated increase in states to nine (9); 03/02/23 PR stated increase in states to eleven (11); and 10/24/23 PR stated licensing agreement up and running in fourteen (14) states. The below table depicts the projected licensing revenues vs reported total revenues. I have made inquiry to IR about the discrepancy.
Hey BoBo… Biogenix charged your credit card😂
Please let us know per the financials how much revenue comes in outside of the licensing....
We will wait.....
Sorry snowflake
Fact company has revenue besides states.
He isn't wrong. Per financials it looks like the only revenue is from licensing.....
Sales of this product benefits Biogenix. After all they collect the money. VDRM is a SCAM.
Good then lets move the decimal point (to the right)!! DOC we are all waiting....let's go!!!!!!!!!
Watching for "Red Flags" Revealing Unreliable Microcap Press Releases
Given press release misinformation risks, savvy microcap investors watch for certain "red flags" that may reveal a release contains unreliable claims:
- Announcements lacking specifics or relying on vague claims that would not allow objective independent verification.
- Press releases strangely timed late in the day after markets close or late on Fridays, suggesting an aim to avoid scrutiny.
- Material announcements failing to provide customary supporting evidence like verifiable names of referenced customers or vendors.
- Major claims that seem to directly contradict objective data in the company's actual financial disclosures and filings.
Ohh noo, I am sorry. I was being sarcastic. Of course that will never happen. It was all BS by the Doc
Doubt either of those will ever materialize. IR needs to give an update.
Sorry ya won’t be getting any of mine but I’m a buyer @ that price 😀
Sorry guys but it looks like I’ll be getting some of those 75s sooner than later
.009...WTF!! We've had enough buying opportunities. Let's get this show on the road. Doc??? Earth to Doc. Come-in. We need a status update right away.
Well, the week still young and anything can happen, right? Who knows, we might even get the surprise that a Dubai deal went thru or Nupelo is back!!! Let’s see LMAOOOO. Can’t make this up
Someone in here said PR this week. I got the feeling the Doc ran off gas. The Doc got nothing on his tab, I think the pink current update status was the last smoke from his basement
Like any other liquid medication, nothing special. Now, talking about the company, it feels almost like Dilution going on, the last weeks. Someone dumping ugly in here. It feels like manipulated dilution
April 16th and no news about overseas shipping in march and contracts yet. No news from IR or any tweets in weeks. Anyone hear from the IR guy?
That's funny I have complained several times and have never received a response. I bought a bottle of the new ultra and a month later it started turning brown.I have called, emailed and texted with no response.
It's not a big deal But I'll post it below
Shop Logo
Let us know what you think!
Hey John,
We'd love to hear what you thought about your order from ViaDerma, which we fulfilled 14 days ago. It'll only take a few minutes.
Thanks,
The ViaDerma team
Unsubscribe from review request emails
Powered by Judge.me
Assuming he bought a bottle, and there was a follow up email asking for feedback/review?
Been loaded up for a while. BUT LOOKING TEMPTING FOR MORE.
Flippers unloading, could be a good thing for a leg up I suppose
Thanks ,,,NOT SWEETNESSES
Currently e trade not showing all stocks current price appreciated
And here we go, down the hill. Nonsense. This stock is beginning to behave like RGBP
That would be nice! I'm tired of eating franks and beans.
I believe with any news and updates this thing goes above 5 cents and it could be this week!
I would agree to that. This CEO isn’t trustworthy
Fits VDRM to a "T" no debating 😂 😢
That nothing NO MENTION OF VDRM NO RED FLAGS
Watching for "Red Flags" Revealing Unreliable Microcap Press Releases
Given press release misinformation risks, savvy microcap investors watch for certain "red flags" that may reveal a release contains unreliable claims:
- Announcements lacking specifics or relying on vague claims that would not allow objective independent verification.
- Press releases strangely timed late in the day after markets close or late on Fridays, suggesting an aim to avoid scrutiny.
- Material announcements failing to provide customary supporting evidence like verifiable names of referenced customers or vendors.
- Major claims that seem to directly contradict objective data in the company's actual financial disclosures and filings.
👍️. Hopefully, some updates/news next week.
GC, thanks for taking the time to put that all together. Good summary! Regarding perpetual late reporting, I guess we will know by May 15th for the 1st quarterly report if he has changed his ways. Nonetheless, it's good to see:
Pink Open Market Logo
Pink Current Information
Verified Profile IconVerified Profile 04/2024
Transfer Agent Verified IconTransfer Agent Verified
|
Followers
|
622
|
Posters
|
|
|
Posts (Today)
|
4
|
Posts (Total)
|
74799
|
|
Created
|
10/27/08
|
Type
|
Free
|
| Moderators | |||

ViaDerma's proprietary transdermal delivery system allows for rapid mass transfer of the pharmaceutical active ingredient across the skin and into the body to provide immediate localized therapy.
The technology allows transfer of chemicals through the stratum corneum (outer layer of skin) with a diffusion constant which is 10,000 times higher than the diffusion constant which characterizes water movement through the stratum corneum.
This enables ViaDerma to pair almost any active ingredient with the technology and provide rapid transport of the medicine right to the site of action.
The first product is a broad spectrum tetracycline-based topical antibiotic is the only antibiotic in the world that kills bacteria both a physical and a chemical mechanism. All known antibiotics (other than ours) primarily use only a chemical mechanism of kill. The physical mechanism of kill is a key feature of what we call Rapid Active Ingredient Delivery System (RAIDS). One result of RAIDS is that tetracycline is carried in higher concentrations, more quickly, to and through the cell walls, where the tetracycline can become more effective than if conventional antibiotics were used. Conventional antibiotics require more time (usually prescribed for 5 to 7 days for best results), whereas our tetracycline-based products usually produce desirable results in 24 hours (or less) because of the RAIDS effect.

A second important result of RAIDS is that the topical antibiotic kills all harmful Gram positive and Gram negative bacteria that have been available for testing. We believe this is the world’s strongest broad-spectrum topical antibiotic available.
The potential commercial impact is immense. Drug developers believe it takes much longer for bacteria to develop drug resistance to a physical kill mechanism. This is because it is relatively easy for bacteria to change their response to a chemical threat, but it takes numerous generations for bacteria to grow a new kind of cell wall structure to respond to a physical threat.
Our novel approach to overcome drug resistance of antibiotics is designed to sustain the effectiveness of antibiotics and other topical drugs for many years. This gives new topical drug products a longer useful lifetime and therefore more commercial value. This technology, when licensed to larger pharmaceutical companies, may provide stronger incentive for the discovery and development of new antimicrobial drugs. In recent years, the dearth of new antibiotics has been largely due to the uncertain new-drug commercial lifetime which is diminished when bacteria develop immunity to that drug.
In addition the technology can be licensed to other companies to convert existing oral drugs to transdermal medications extending the profitably of the existing drug.

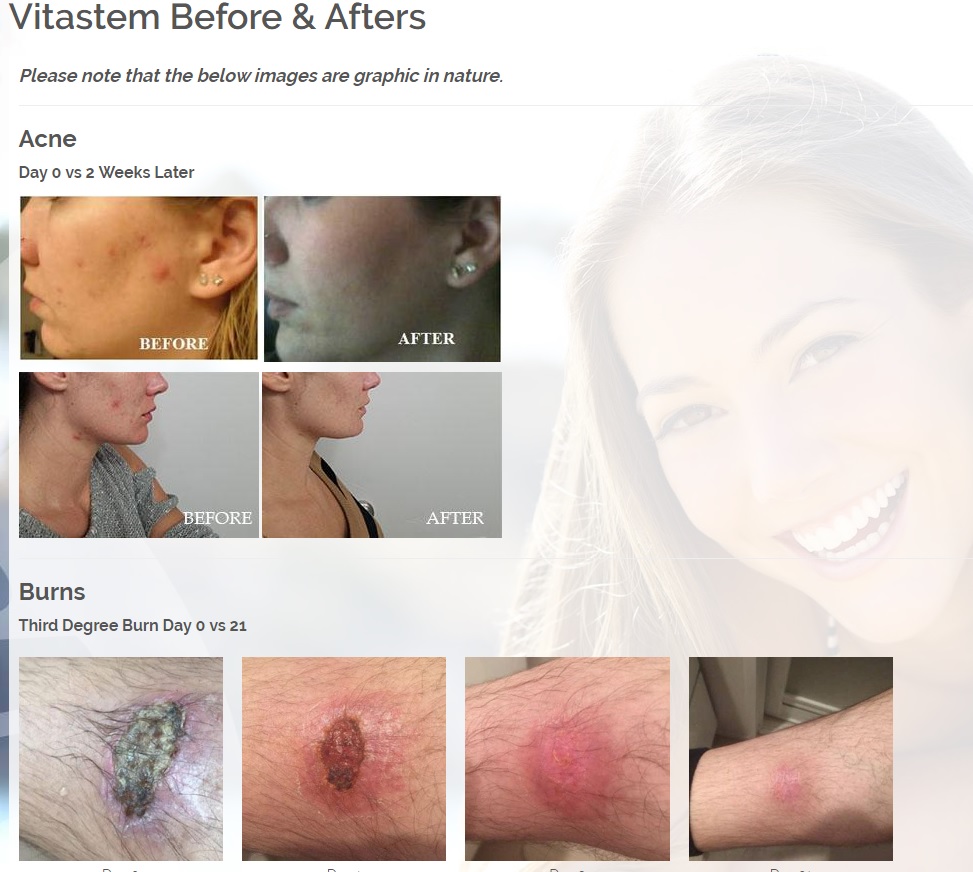
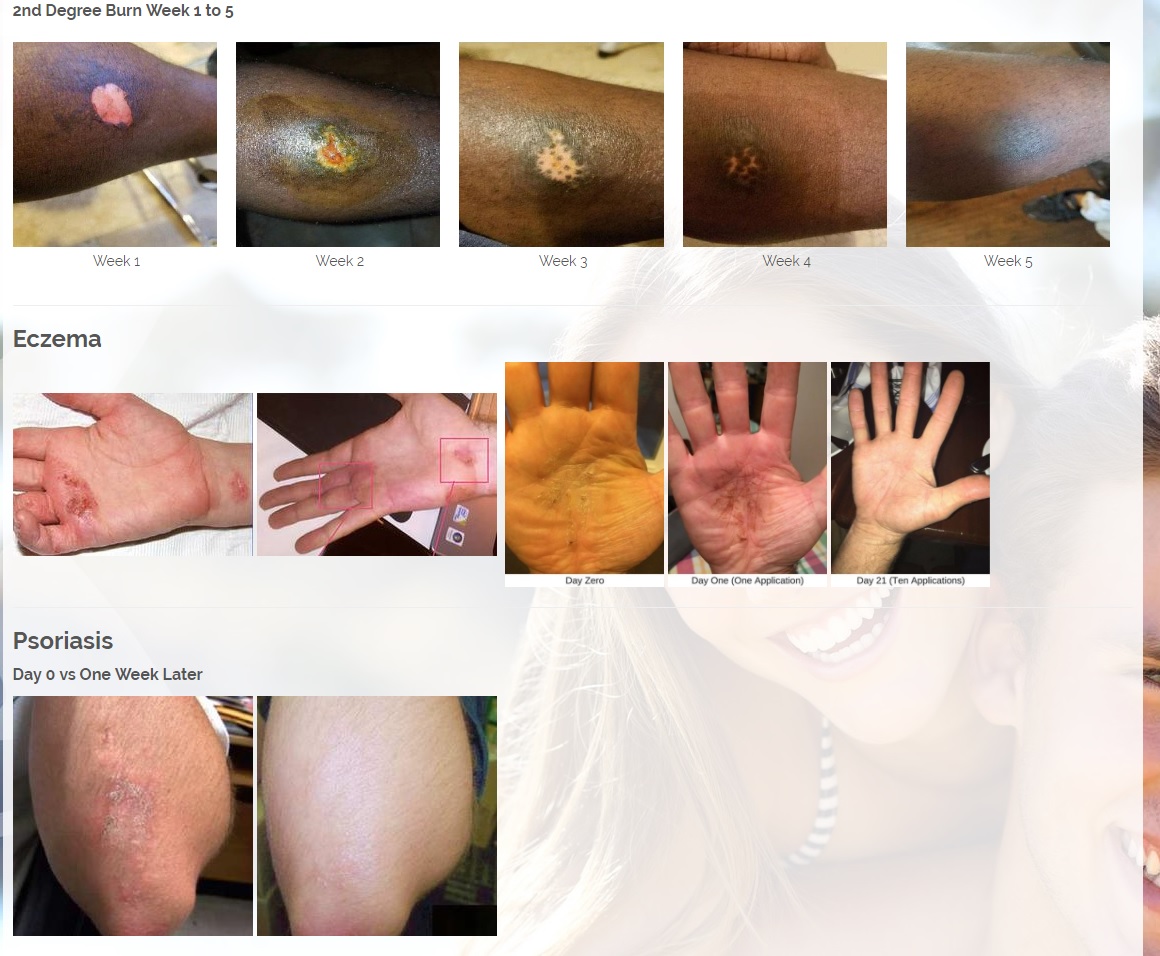
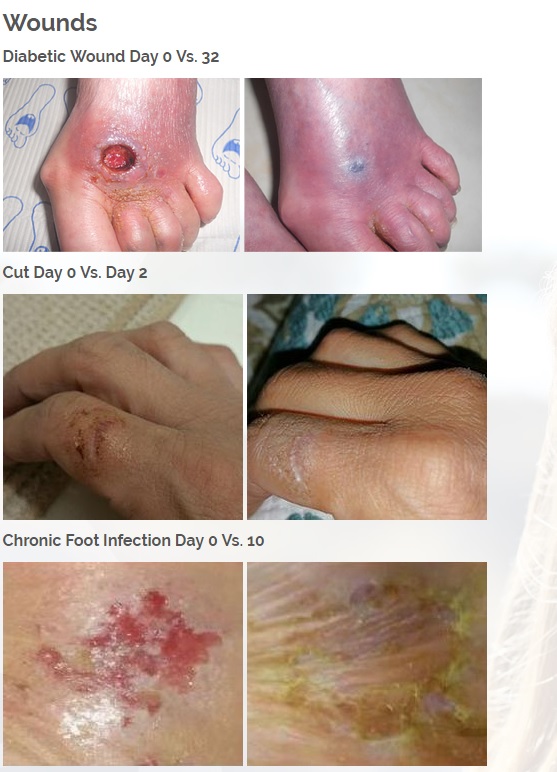

We are developing products in the following fields of use;
Treats all types of bacteria including MRSA, VRE and other flesh eating bacteria. Fights drug resistant bacteria. $6B/year global market.

Onychomycosis, a fungal infection of the toenails, is a major health problem. It is estimated that there are in excess of 40 million sufferers with this condition in the USA. It is a problem throughout the world. A recent European study showed that the prevalence of Onychomycosis may be as high as 26.9%. Fungal resistance can occur when the oral antifungal agents are used on a long-term basis. Topically applied antifungal drugs may work somewhat better adjunctive to surgical removal or chemical dissolution of the nail plate. Yet, this often ineffective and traumatic procedure leaves the subject without a nail for months at risk for re-infection. Given the limitations of current treatment options in this $3B market, there is a great need for a simple, nontoxic and effective alternative treatment. Estimated Global prevalence rate: 140 million.
Treats symptoms of Influenza which is a$4B/ year global market: Common
Diabetic foot wounds.

Per 3/8/18 PR: ViaDerma’s technology is currently being used in Elixr Cannabis products; Topical Balm, Topical Serum and Topical Spray. Sales have begun in Canada.
Per 12/7/17 PR: The Company has signed an MOU and will start production of its Patent Pending CBD or Cannabinoids product line. The Company would combine its two Patent pending technologies, the MMJ Patent #62466209, a patent for delivering medical marijuana / cannabis to the body by applying the medication onto the skin in an ointment base topical solution and the second Provisional patent # 62433964 for enhanced antibiotic and drug delivery for "Aqueous Topical Applications" for human and veterinary uses. The Company would combine its proprietary solutions with CBD's and other natural products that treat psoriasis, fibromyalgia for pain and other ailments.
Per 1/23/18 PR: The Company has also a licensing and distribution agreement in place with its Canadian partners to produce a CBD Topical Serum, which has a 92% absorption rate powered by ViaDerma's Patent Pending Dual Carrier Technology. The CBD and Terpene profile (enriched with specific essential oils and vitamins C, and D) is crafted to alleviate Chronic Dry Skin, Psoriasis, Eczema, Rosacea, and many other skin conditions. The serum has anti-inflammatory, anti-bacterial, and chronic pain relief properties that are absorbed by the skin and provide overall healing benefits. Sales are expected to begin in the first quarter of 2018 and are projected to generate approximately $2 million annual revenues.
The Patented Pending CBD or Cannabinoids product line. The Company would combine its two Patent pending technologies, the MMJ Patent, a patent for delivering medical marijuana / cannabis to the body by applying the medication onto the skin in an ointment base topical solution and the second Provisional patent for enhanced antibiotic and drug delivery for "Aqueous Topical Applications" for human and veterinarian uses. The Company would combine its proprietary solutions with CBD's and other natural products that treat psoriasis, fibromyalgia for pain and other ailments. The Company plans to continue to expand its (IP) "Intellectual Property Portfolio" in 2018.

CBD is a compound found in the Cannabis family of plants such as hemp. CBD is combined into our topical body care products like Elixr's Topical Balm, Serum, and Spray.
CBD is an antioxidant. Antioxidants protect our skin from damaging exposure to smoke, UV rays, and environmental pollutants. Cannabinoid receptors are located all throughout the skin, Topical CBD products interact with those receptors resulting homeostasis and healing. Studies show that CBD can help treat an array of skin conditions such as eczema, psoriasis, rosacea, and acne.
| "What is Intellectual Property?" - A Publication by the World Intellectual Property Organization -
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Volume | |
| Day Range: | |
| Bid Price | |
| Ask Price | |
| Last Trade Time: |
