Register for free to join our community of investors and share your ideas. You will also get access to streaming quotes, interactive charts, trades, portfolio, live options flow and more tools.
I WAS BIDDING FOR 1,5 MILLION AT .007.I GOT ZERO SHARES.AND IT EXPIRED.
Expired Buy 1500000 VDRM Limit 0.007 -- 07/25/22 16:06:51 07/25/22
Order No. 9009269034 Viaderma, Inc. Common Sto...
Entered:13:09:04 07/25/22 Reuse
Big news coming this week!
Testing,etc.
DeerBalls I agree 100% & news very soon
It will or will not happen regardless of any great things type posts. Coming Soon.
$.008 No one is forced to buy/hold VDRM. Glad I bought away while it was down.
I have complete confidence in Dr. Otiko. Others may not and that is just fine!
Been holding stock in this company since Feb ‘17
Otiko has never come through on his promises. To say otherwise, you are living in fantasy land
After a call back he could not remember
will never waste my time listening to BS again
I Bad info from suppose to be good source
Yes and that's exactly what I was told
LET;S HOPE WE GET AN UPDATE FROM OTIKO.AND WE SHOULD.GOOD OR BAD.THE 4 WEEK MARK IS OVER OTIKO.
That's right! Update could be anyday next week!!!
I think if doc doesn't have news soon people should book appointment with him and put him on the spot because he has our money!!!!!!I If I lived with in 500 miles of his practice I would be there
He could be referring to you stating we just received 10k units on 6/14. Obvious bullsh*t
How about posting what you are referring to.
Let's just keep posting the release from June 22nd. Come on man enough. I would think most everyone here wants to see the big boom but posting unsubstantiated claims of orders being filled or retailers who will stock the products is just hearsay right now. I for one hope all these claims come true, but folks are way ahead of their skis at this point. IMO
Large orders being filled!
I bought a few more this week thinking Ok maybe something new will happen. Now I feel like I just through money away. I bought their product for my granddaughter over a year ago from Amazon and it really works but damn . . .
TODAY IS EXACTLY 4 WEEKS.OTIKO COME ON MAN.AT THE LEAST GIVE US AN UPDATE IF THERE IS A DELAY ETC.
Press Release | 06/22/2022
The Company expects the additional testing will be completed within four weeks and product distribution is expected to commence soon after.
LOS ANGELES, June 22, 2022 (GLOBE NEWSWIRE) -- ViaDerma, Inc., (“Company”) (OTC Pink: VDRM) announces plans to conduct additional internal quality control testing for their new Vitastem products. Vitastem and Vitastem Ultra are manufactured at a contract facility in the United States and have successfully undergone all standard testing required by regulators. Following extensive consultation with the Company’s manufacturing partners and potential purchasers and distributors of the products, the decision was made to implement further testing as an additional precautionary measure since both products are using new formulations.
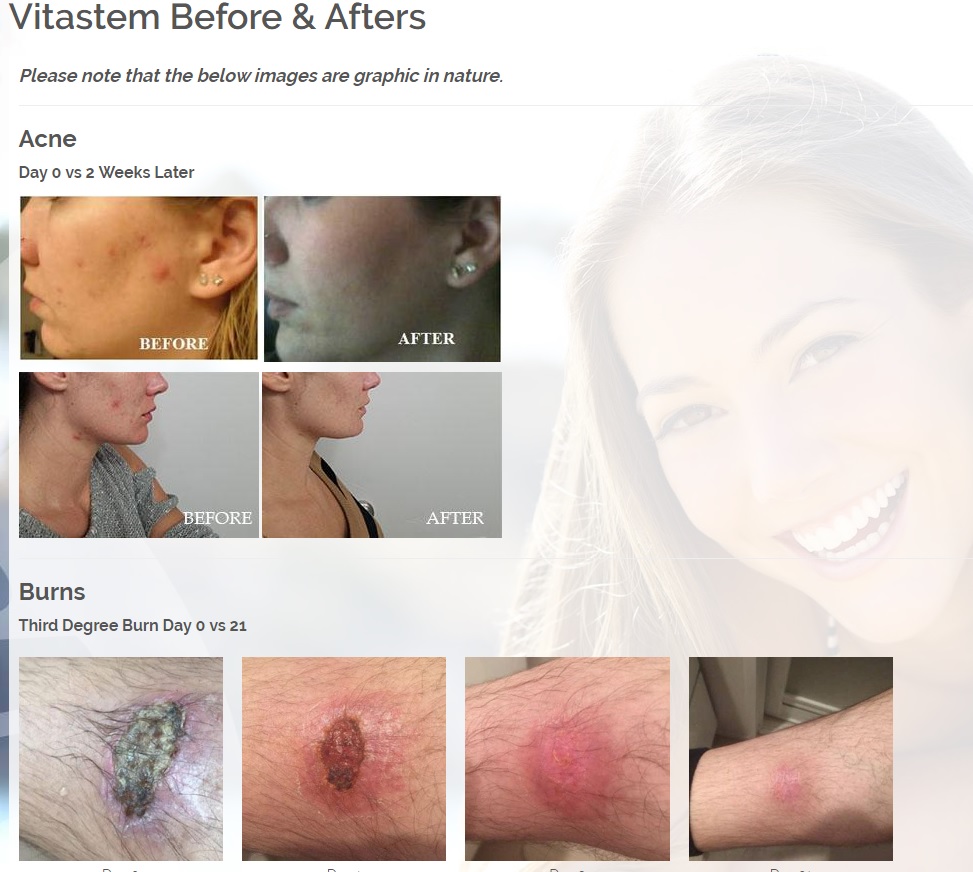
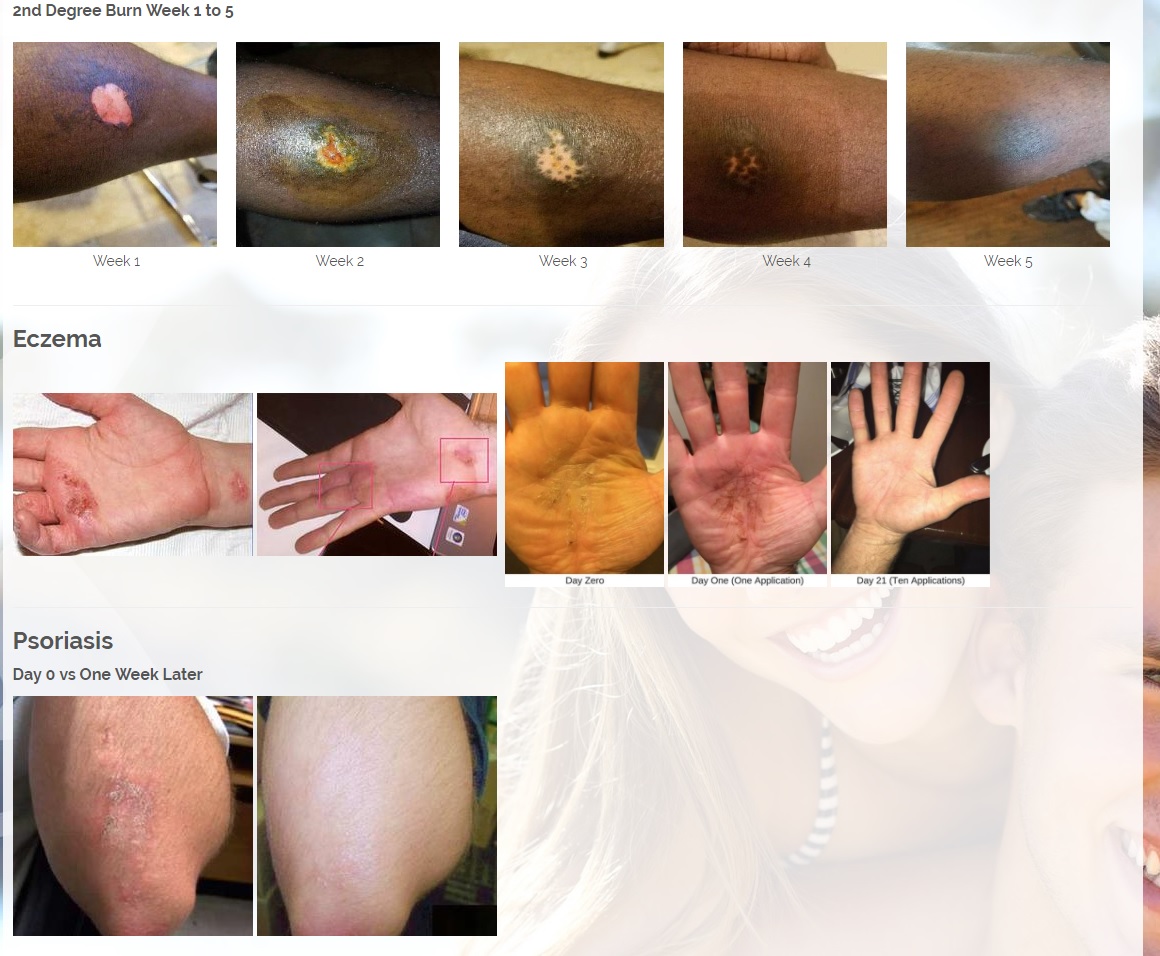
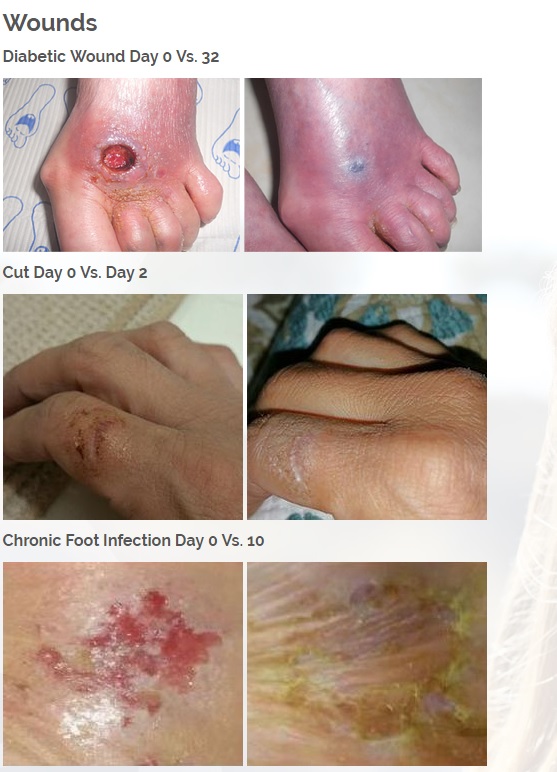
The new and improved Vitastem and Vitastem Ultra are FDA-registered drugs and are among the strongest and most effective topical antibiotics available on the market. Vitastem kills all harmful Gram-positive and Gram-negative bacteria that have been available for testing (bacteria that is associated with acne, cuts, scrapes, burns, and secondary infections associated with psoriasis and eczema). Vitastem Ultra uses bacitracin as the active ingredient and the new formula is clear and colorless compared to the brownish color of the original (which has tetracycline as the active ingredient), so it will not stain the skin, fingernails, toenails, or anywhere else it is applied. Both Vitastem and Vitastem Ultra work similarly and provide the same great results. The new formulation with bacitracin provides an alternative for consumers who are allergic or resistant to one or the other.
President and CEO, Dr. Chris Otiko said, “Since both of these products are a new and improved formulation, we decided now would be the best time to conduct additional testing before we begin large scale distribution.”
Dr. Otiko continued, “The products have tested well so far, and we expect they will continue to do so without issue, but because they are both different formulations than the original, and since we are moving much closer to having to fulfill large purchase orders, we felt we should implement these precautionary measures at this time in an abundance of caution. The last thing we want is to have a problem down the road when a correction would be much harder to address. Taking this step now will minimize the potential for such problems later.”
The Company expects the additional testing will be completed within four weeks and product distribution is expected to commence soon after.
ViaDerma Announces the Implementation of Internal Quality Control Measures for its New Vitastem Products
Press Release | 06/22/2022
LOS ANGELES, June 22, 2022 (GLOBE NEWSWIRE) -- ViaDerma, Inc., (“Company”) (OTC Pink: VDRM) announces plans to conduct additional internal quality control testing for their new Vitastem products. Vitastem and Vitastem Ultra are manufactured at a contract facility in the United States and have successfully undergone all standard testing required by regulators. Following extensive consultation with the Company’s manufacturing partners and potential purchasers and distributors of the products, the decision was made to implement further testing as an additional precautionary measure since both products are using new formulations.
The new and improved Vitastem and Vitastem Ultra are FDA-registered drugs and are among the strongest and most effective topical antibiotics available on the market. Vitastem kills all harmful Gram-positive and Gram-negative bacteria that have been available for testing (bacteria that is associated with acne, cuts, scrapes, burns, and secondary infections associated with psoriasis and eczema). Vitastem Ultra uses bacitracin as the active ingredient and the new formula is clear and colorless compared to the brownish color of the original (which has tetracycline as the active ingredient), so it will not stain the skin, fingernails, toenails, or anywhere else it is applied. Both Vitastem and Vitastem Ultra work similarly and provide the same great results. The new formulation with bacitracin provides an alternative for consumers who are allergic or resistant to one or the other.
President and CEO, Dr. Chris Otiko said, “Since both of these products are a new and improved formulation, we decided now would be the best time to conduct additional testing before we begin large scale distribution.”
Dr. Otiko continued, “The products have tested well so far, and we expect they will continue to do so without issue, but because they are both different formulations than the original, and since we are moving much closer to having to fulfill large purchase orders, we felt we should implement these precautionary measures at this time in an abundance of caution. The last thing we want is to have a problem down the road when a correction would be much harder to address. Taking this step now will minimize the potential for such problems later.”
The Company expects the additional testing will be completed within four weeks and product distribution is expected to commence soon after.
About ViaDerma, Inc.
ViaDerma, Inc. (OTC: VDRM) is a publicly traded specialty pharmaceutical company committed to bringing new products to market and licensing its innovative technology to current leaders in the pharmaceutical industry in a wide variety of therapeutic areas. For more information, visit: www.viadermalicensing.com
Any forecast of future performance is a "forward looking statement" under securities laws. Such statements are included to allow potential investors the opportunity to understand management’s beliefs and opinions with respect to the future so that they may use such beliefs and opinions as one factor among many in evaluating an investment.
Contact information:
Investor Relations
Email: info@viadermalicensing.com
Phone: 310-734-6111
Follow us on Twitter: ViaDerma Official @viaderma
Like us on Facebook: ViaDerma-VDRM @viadermapharma
https://www.otcmarkets.com/stock/VDRM/news/ViaDerma-Announces-the-Implementation-of-Internal-Quality-Control-Measures-for-its-New-Vitastem-Products?id=362199
VDRM SECURITY DETAILS
Share Structure
Market Cap Market Cap
7,553,714
07/21/2022
Authorized Shares
1,000,000,000
07/08/2022
Outstanding Shares
983,556,451
07/08/2022
Restricted
69,456,186
07/08/2022
Unrestricted
914,100,265
07/08/2022
Held at DTC
902,867,184
07/08/2022
Float
911,600,265
07/23/2021
Par Value
0.001
https://www.otcmarkets.com/stock/VDRM/security
Based on pr from last year, before things went haywire, and seemingly backed up by 1st qtr revenue of $135K, we are still at nine (9) state licenses ($5K * 9 * 3 or $135K).
Aside from our expected news regarding the testing and distribution, I look forward to hearing more about our licensing expansion into other states. Obviously that generates recurring revenue which should help propel VDRM to the next level. Someone correct me, but believe we have 8states licensed generating $5K per month. Is that right?
Do believe that good things are coming soon.
Agreed 100%. Just stating it would not surprise me to see a PR tomorrow or next week stating that all went well and shipments are flowing out the door!!!
Not his fault if it takes a few days longer
Of course it is PR on June 22nd said WITHIN 4 weeks. Today is the end of week 4 :)
4 weeks from last PR is today. So maybe the Dr will update us tomorrow or possibly next week. Either way, this stock is primed for an explosion in my opinion.
I think the 4 weeks not due yet... Give it time mate, that PR was done on stone, it will happen AND it will be EPIC. I hope it does not get you with your pants down. GL
You will soon see VDRM is now solid & secure
Like the Nigerian Company that never existed....or the Nameless/vague Hospital Chains that never happened?
LMFAO!
Oh yeah. 4 weeks is up. Nothing as expected :)
I would love to see a huge contract $$$
I would love to see a huge contract $$$
NEWS ARE VERY CLOSE.DID YOU GUYS GET TO SEE THE 7 MILLION BID SUPPORT AT .0075S?WOW.WE WILL SEE HOW HIGH IT WILL RUN NO THE NEWS SOON.FORWARD LOOKING EVENT IS DUE ANY DAY NOW.
Over one million on the bid
0.01's this week before news would be nice. Hopefully, 0.02 - 0.05+ after this news.
Hopefully we get over two and hold.
I SEE .02 TO AS HIGH AS .10 THIS YEAR IMO.PER WHAT I READ IN THE LAST PR FROM CEO.HUGE CONTRACTS.COMING IMO.WE SHOULD MOVING UP TO .01 ARE THIS WEEK IMO.
4TH WEEK IS THIS WEEK.READ ALL BOLD MARKS:))THE NEWS WAS JUNE 22ND
MONDAY IS JULY 18TH:))LARGE SCALE DISTRIBUTIONS AFTER THE NEWS ON TESTING.DAMN.WALMARTS???CVs???WALGREENS???PUBLIX??ALDI???THE LIST WILL NOT END.ONCE IT STARTS BAMMMMMMMMMMMMM
The Company expects the additional testing will be completed within four weeks and product distribution is expected to commence soon after.
ViaDerma Announces the Implementation of Internal Quality Control Measures for its New Vitastem Products
Press Release | 06/22/2022
LOS ANGELES, June 22, 2022 (GLOBE NEWSWIRE) -- ViaDerma, Inc., (“Company”) (OTC Pink: VDRM) announces plans to conduct additional internal quality control testing for their new Vitastem products. Vitastem and Vitastem Ultra are manufactured at a contract facility in the United States and have successfully undergone all standard testing required by regulators. Following extensive consultation with the Company’s manufacturing partners and potential purchasers and distributors of the products, the decision was made to implement further testing as an additional precautionary measure since both products are using new formulations.
The new and improved Vitastem and Vitastem Ultra are FDA-registered drugs and are among the strongest and most effective topical antibiotics available on the market. Vitastem kills all harmful Gram-positive and Gram-negative bacteria that have been available for testing (bacteria that is associated with acne, cuts, scrapes, burns, and secondary infections associated with psoriasis and eczema). Vitastem Ultra uses bacitracin as the active ingredient and the new formula is clear and colorless compared to the brownish color of the original (which has tetracycline as the active ingredient), so it will not stain the skin, fingernails, toenails, or anywhere else it is applied. Both Vitastem and Vitastem Ultra work similarly and provide the same great results. The new formulation with bacitracin provides an alternative for consumers who are allergic or resistant to one or the other.
President and CEO, Dr. Chris Otiko said, “Since both of these products are a new and improved formulation, we decided now would be the best time to conduct additional testing before we begin large scale distribution.”
Dr. Otiko continued, “The products have tested well so far, and we expect they will continue to do so without issue, but because they are both different formulations than the original, and since we are moving much closer to having to fulfill large purchase orders, we felt we should implement these precautionary measures at this time in an abundance of caution. The last thing we want is to have a problem down the road when a correction would be much harder to address. Taking this step now will minimize the potential for such problems later.”
The Company expects the additional testing will be completed within four weeks and product distribution is expected to commence soon after.
Wrong!!! You can’t and shouldn’t judge anyone by looking at their past. It has been proven, by experts and millionaires that’s not how you start (The Past) that matters, is how you end (The Future) that counts!!! I ain’t setting myself for a disappointment, I am a business man and investor, if you have experienced such feelings on this before and can’t bare to see such to happen, then you shouldn’t be here. This is not for you mate!!! I have done this for a long time and I know how perfectly the OTC works… Get some popcorn and a Coca!!! Relax mate, chill and enjoy the ride
Would be nice,GL.
|
Followers
|
627
|
Posters
|
|
|
Posts (Today)
|
12
|
Posts (Total)
|
74901
|
|
Created
|
10/27/08
|
Type
|
Free
|
| Moderators | |||

ViaDerma's proprietary transdermal delivery system allows for rapid mass transfer of the pharmaceutical active ingredient across the skin and into the body to provide immediate localized therapy.
The technology allows transfer of chemicals through the stratum corneum (outer layer of skin) with a diffusion constant which is 10,000 times higher than the diffusion constant which characterizes water movement through the stratum corneum.
This enables ViaDerma to pair almost any active ingredient with the technology and provide rapid transport of the medicine right to the site of action.
The first product is a broad spectrum tetracycline-based topical antibiotic is the only antibiotic in the world that kills bacteria both a physical and a chemical mechanism. All known antibiotics (other than ours) primarily use only a chemical mechanism of kill. The physical mechanism of kill is a key feature of what we call Rapid Active Ingredient Delivery System (RAIDS). One result of RAIDS is that tetracycline is carried in higher concentrations, more quickly, to and through the cell walls, where the tetracycline can become more effective than if conventional antibiotics were used. Conventional antibiotics require more time (usually prescribed for 5 to 7 days for best results), whereas our tetracycline-based products usually produce desirable results in 24 hours (or less) because of the RAIDS effect.

A second important result of RAIDS is that the topical antibiotic kills all harmful Gram positive and Gram negative bacteria that have been available for testing. We believe this is the world’s strongest broad-spectrum topical antibiotic available.
The potential commercial impact is immense. Drug developers believe it takes much longer for bacteria to develop drug resistance to a physical kill mechanism. This is because it is relatively easy for bacteria to change their response to a chemical threat, but it takes numerous generations for bacteria to grow a new kind of cell wall structure to respond to a physical threat.
Our novel approach to overcome drug resistance of antibiotics is designed to sustain the effectiveness of antibiotics and other topical drugs for many years. This gives new topical drug products a longer useful lifetime and therefore more commercial value. This technology, when licensed to larger pharmaceutical companies, may provide stronger incentive for the discovery and development of new antimicrobial drugs. In recent years, the dearth of new antibiotics has been largely due to the uncertain new-drug commercial lifetime which is diminished when bacteria develop immunity to that drug.
In addition the technology can be licensed to other companies to convert existing oral drugs to transdermal medications extending the profitably of the existing drug.


We are developing products in the following fields of use;
Treats all types of bacteria including MRSA, VRE and other flesh eating bacteria. Fights drug resistant bacteria. $6B/year global market.

Onychomycosis, a fungal infection of the toenails, is a major health problem. It is estimated that there are in excess of 40 million sufferers with this condition in the USA. It is a problem throughout the world. A recent European study showed that the prevalence of Onychomycosis may be as high as 26.9%. Fungal resistance can occur when the oral antifungal agents are used on a long-term basis. Topically applied antifungal drugs may work somewhat better adjunctive to surgical removal or chemical dissolution of the nail plate. Yet, this often ineffective and traumatic procedure leaves the subject without a nail for months at risk for re-infection. Given the limitations of current treatment options in this $3B market, there is a great need for a simple, nontoxic and effective alternative treatment. Estimated Global prevalence rate: 140 million.
Treats symptoms of Influenza which is a$4B/ year global market: Common
Diabetic foot wounds.

Per 3/8/18 PR: ViaDerma’s technology is currently being used in Elixr Cannabis products; Topical Balm, Topical Serum and Topical Spray. Sales have begun in Canada.
Per 12/7/17 PR: The Company has signed an MOU and will start production of its Patent Pending CBD or Cannabinoids product line. The Company would combine its two Patent pending technologies, the MMJ Patent #62466209, a patent for delivering medical marijuana / cannabis to the body by applying the medication onto the skin in an ointment base topical solution and the second Provisional patent # 62433964 for enhanced antibiotic and drug delivery for "Aqueous Topical Applications" for human and veterinary uses. The Company would combine its proprietary solutions with CBD's and other natural products that treat psoriasis, fibromyalgia for pain and other ailments.
Per 1/23/18 PR: The Company has also a licensing and distribution agreement in place with its Canadian partners to produce a CBD Topical Serum, which has a 92% absorption rate powered by ViaDerma's Patent Pending Dual Carrier Technology. The CBD and Terpene profile (enriched with specific essential oils and vitamins C, and D) is crafted to alleviate Chronic Dry Skin, Psoriasis, Eczema, Rosacea, and many other skin conditions. The serum has anti-inflammatory, anti-bacterial, and chronic pain relief properties that are absorbed by the skin and provide overall healing benefits. Sales are expected to begin in the first quarter of 2018 and are projected to generate approximately $2 million annual revenues.
The Patented Pending CBD or Cannabinoids product line. The Company would combine its two Patent pending technologies, the MMJ Patent, a patent for delivering medical marijuana / cannabis to the body by applying the medication onto the skin in an ointment base topical solution and the second Provisional patent for enhanced antibiotic and drug delivery for "Aqueous Topical Applications" for human and veterinarian uses. The Company would combine its proprietary solutions with CBD's and other natural products that treat psoriasis, fibromyalgia for pain and other ailments. The Company plans to continue to expand its (IP) "Intellectual Property Portfolio" in 2018.

CBD is a compound found in the Cannabis family of plants such as hemp. CBD is combined into our topical body care products like Elixr's Topical Balm, Serum, and Spray.
CBD is an antioxidant. Antioxidants protect our skin from damaging exposure to smoke, UV rays, and environmental pollutants. Cannabinoid receptors are located all throughout the skin, Topical CBD products interact with those receptors resulting homeostasis and healing. Studies show that CBD can help treat an array of skin conditions such as eczema, psoriasis, rosacea, and acne.
| "What is Intellectual Property?" - A Publication by the World Intellectual Property Organization -
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Volume | |
| Day Range: | |
| Bid Price | |
| Ask Price | |
| Last Trade Time: |
