Register for free to join our community of investors and share your ideas. You will also get access to streaming quotes, interactive charts, trades, portfolio, live options flow and more tools.
VXRT .62 - Up to $453 million to Vaxart of San Francisco, California, developing an oral pill vaccine candidate, adenovirus serotype 5 (Ad-5). BARDA will provide an initial $65.7 million for early trial milestones, with remaining funds provided as the effort successfully advances toward trial execution. Vaxart will execute its own Phase 2b clinical trials.
https://www.hhs.gov/about/news/2024/06/13/barda-awards-500-million-project-nextgen-funding-vaccine-clinical-trials.html
Small Biotechs always dilute to raise money. Daytrade it only
I got out before today.
Thanks!
Thanks. Just saw LGVN.
I am tempted to jump in. Have a nice weekend.
VXRT: But nevertheless --- assuming that your iHub Profile PICTURE is in fact YOU --- well, at least you are still unfairly BEAUTIFUL!!!!
I got fooled again. Thought this was
a winner. Could not uinderstand why the price didn't surge higher. Now, I know why.
Invest-in America: and now it’s a complete Buy Reset into Buying Territory.
VXRT: Indeed, Bro!! VERY dirty trick!! (And yes, everybody on iHub is well AWARE of that trick, but I just had to blow-off some 'steam' about it since we ALL have witnessed that 'trick' so many times during this past trading year.)
It’s a dirty trick already pre planned by the company and current large stock holders.
Step 1. Release Good News and Sell current shares into the open Market.
Step 2. Use those funds and Buy back Shares with the offering.
End result is the large stock holders Make Money and the Company Makes Money. No out of pocket expense for large stock holders to fund the company.
VXRT: And, their "Outstanding Shares" is HUGE in the first place --- as they periodically DUMP yet more & more shares upon the Public!!
VXRT: However, their OTHER "news" of late today --- about DUMPING $40-mil. more shares to Market --- will DESTROY any hopes of this Covid-19-Circus-Act ever SOARING in price tomorrow!!!
Buying in AH. Picked some up
at $1.24. Let's see what happens. I will have my finger on the trigger tomorrow AM to buy more.
Offering of 50 million common shares at .80 cents
News released AFTER the close indicates you could be VERY right.
Many Short shares. This is due
for a short squeeze. 19M shares Short, out of 180M OS. Phenomenal NEWS today should push this north. Very oversold!
Vaxart Receives BARDA-Funded Project NextGen Award Valued Up to $453 Million to Conduct a Phase 2b Study Evaluating Its COVID-19 Oral Pill Vaccine Candidate
June 13 2024 - 4:15PM
Alert
Print
Share On Facebook
Vaxart, Inc. (Nasdaq: VXRT) announced today that it received a project award valued at up to $453 million through the Rapid Response Partnership Vehicle (RRPV). The RRPV is a Consortium funded by the Biomedical Advanced Research and Development Authority (BARDA), part of the Administration for Strategic Preparedness and Response (ASPR) in the U.S. Department of Health and Human Services (HHS).
The funds will be used to conduct a Phase 2b comparative study evaluating Vaxart’s oral pill COVID-19 vaccine candidate against a U.S. Food and Drug Administration (FDA)-approved mRNA vaccine comparator. In preparation for the trial, Vaxart created and manufactured under Good Manufacturing Practice (GMP) standards a next-generation oral COVID-19 vaccine tablet candidate that — based on preclinical data — is more potent than Vaxart’s prior COVID-19 vaccine constructs.
Funding under the award will be provided in two parts with approximately $65.7 million available immediately to continue study start-up activities, and the remainder of approximately $387.2 million provided when Vaxart and BARDA have determined that the study may further proceed and paid over the course of the study. Currently, Vaxart anticipates initiating enrollment as early as summer 2024. An interim analysis for vaccine efficacy compared to an approved mRNA comparator may occur as early as the first quarter of 2025.
“We are grateful to BARDA for this funding, which will enable Vaxart to conduct a Phase 2b trial for our COVID-19 oral pill vaccine candidate. This trial will evaluate whether our oral pill vaccine candidate compares favorably against an approved mRNA injectable vaccine,” said Dr. James F. Cummings, Vaxart’s Chief Medical Officer. “We are excited to explore the results of this head-to-head comparison. Previous research showed that our earlier COVID-19 vaccine constructs triggered long-lasting immune responses and induced a cross-reactive immunogenic response against all tested SARS-CoV-2 variants.”
“Vaccine delivery has relied primarily on injection for more than 150 years. This funding from BARDA will assist us in determining whether we can bring a transformational, next-generation approach to global vaccination,” said Steven Lo, Vaxart’s Chief Executive Officer. “We believe our oral pill vaccine platform can better meet societal needs not just for COVID-19, which is now in the endemic phase, but for other infectious diseases that present significant endemic and pandemic threats.”
Vaxart was the first U.S. company to complete a Phase 2 clinical trial of an oral vaccine for COVID-19. In earlier clinical trials, Vaxart demonstrated its COVID-19 vaccine candidates generated robust cross-reactive mucosal IgA responses, boosted immune responses to existing COVID-19 vaccines, increased neutralizing antibodies against Omicron 4/5, and had a benign tolerability profile.
Funding for this award was received under Project NextGen, a $5 billion initiative by HHS to develop new, innovative vaccines and therapeutics that provide broader and more durable protection against COVID-19 than the first generation COVID-19 vaccines and medicines. This project has been funded with federal funds from HHS; ASPR; BARDA, under Other Transaction (OT) number 75A50123D00005.
About the COVID-19 Phase 2b Trial
The Phase 2b trial is a double-blind, multi-center, randomized, comparator-controlled study to determine the relative efficacy, safety, and immunogenicity of Vaxart’s oral pill COVID-19 vaccine candidate against an approved mRNA COVID-19 injectable vaccine in adults previously immunized against COVID-19 infection. The study design anticipates enrolling approximately 10,000 healthy adults 18 years and older in the United States with 5,000 receiving Vaxart’s COVID-19 vaccine candidate and 5,000 receiving an approved mRNA comparator. At least 25% of the participants should be at least 65 years old.
The study will measure efficacy for symptomatic and asymptomatic disease, systemic and mucosal immune induction, and the incidence of adverse events. The primary endpoint is relative efficacy of Vaxart’s COVID-19 vaccine candidate compared to an approved mRNA comparator for the prevention of symptomatic disease. Primary efficacy analysis will be performed when all participants have either discontinued or completed a study visit 12 months post-vaccination.
An independent Data and Safety Monitoring Board (DSMB) will review safety data of the participants.
Execution of this Phase 2b study will be funded by BARDA through the RRPV.
About Vaxart
Vaxart is a clinical-stage biotechnology company developing a range of oral recombinant vaccines based on its proprietary delivery platform. Vaxart vaccines are designed to be administered using pills that can be stored and shipped without refrigeration and eliminate the risk of needle-stick injury. Vaxart believes that its proprietary pill vaccine delivery platform is suitable to deliver recombinant vaccines, positioning the company to develop oral versions of currently marketed vaccines and to design recombinant vaccines for new indications. Vaxart’s development programs currently include pill vaccines designed to protect against coronavirus, norovirus and influenza, as well as a therapeutic vaccine for human papillomavirus (HPV), Vaxart’s first immune-oncology indication. Vaxart has filed broad domestic and international patent applications covering its proprietary technology and creations for oral vaccination using adenovirus and TLR3 agonists.
Note Regarding Forward-Looking Statements
This press release contains forward-looking statements that involve substantial risks and uncertainties. All statements, other than statements of historical facts, included in this press release regarding Vaxart's strategy, prospects, plans and objectives, receipt of funding from BARDA for the Phase 2b study, results from preclinical and clinical trials and the timing of such trials and results, commercialization agreements and licenses, and beliefs and expectations of management are forward-looking statements. These forward-looking statements may be accompanied by such words
so, what changed to drive this significant drop? Today marks the new definition of volatility. We, who have no vision of what is happening internally, have been caught, again.
VXRT...............................https://stockcharts.com/h-sc/ui?s=VXRT&p=W&b=5&g=0&id=p86431144783
Leadership announcement is a good step. Unfortunately the stock dropped 4%. Hopefully the performance news will bring more enthusiasm and a confidence builder. I wonder what the institutional investors are chatting about since the trajectory appears to be rather cyclical.
no news on the performance or leadership front. hopefully we are not slow walking back to the cents world. Any positive thinking out there?
VXRT...................................https://stockcharts.com/h-sc/ui?s=VXRT&p=W&b=5&g=0&id=p86431144783
VXRT..................................................................https://stockcharts.com/h-sc/ui?s=VXRT&p=W&b=5&g=0&id=p86431144783
everything hinges on March reporting so again, much patience needed.
VXRT.................................https://stockcharts.com/h-sc/ui?s=VXRT&p=W&b=5&g=0&id=p86431144783
VXRT..................................................https://stockcharts.com/h-sc/ui?s=VXRT&p=W&b=5&g=0&id=p86431144783
Well that was disappointing ....down 6% on good news
This could pop today.....$9.27 Million Barda Grant
VXRT
Vaxart Inc
0.7199
-0.0008 (-0.11%)
Volume: 1,935,095
Day Range: 0.6911 - 0.735
Last Trade Time: 7:53:18 PM EDT
VXRT
Vaxart Inc
0.92
-0.0463 (-4.79%)
Volume: 17,930,488
Day Range: 0.8799 - 0.96
Last Trade Time: 7:49:22 PM EDT
DIS LOOKS REALLY BULLISH MY FRIEND $VXRT
DIS LOOKS REALLY BULLISH MY FRIEND $VXRT
VXRT
Vaxart Inc
1.22
0.01 (0.83%)
Volume: 1,474,831
Day Range: 1.17 - 1.248
Last Trade Time: 7:59:46 PM EDT
VXRT
Vaxart Inc
0.8001
0.0327 (4.26%)
Volume: 1,143,206
Day Range: 0.76 - 0.82
Last Trade Time: 7:43:05 PM EDT
disappointing VXRT
Vaxart Inc
0.65
-0.033 (-4.83%)
Volume: 1,139,162
Day Range: 0.6424 - 0.7149
Last Trade Time: 7:54:00 PM EDT
VXRT
Vaxart Inc
0.835
-0.0412 (-4.70%)
Volume: 1,433,342
Day Range: 0.81 - 0.8999
Last Trade Time: 7:59:39 PM EST
VXRT
Vaxart Inc
1.0801
-0.0199 (-1.81%)
Volume: 1,933,691
Day Range: 1.07 - 1.13
Last Trade Time: 7:54:57 PM EST
Too quiet here? The Bill and Melinda Gates Foundation recently provided VXRT a Grant to study if breast feeding babies are transfered Covid protection from/through their mother's milk. The amount was around $2.5 Million. That December 2022 Grant is based on the mother initially having protection. A rather tangental, but non the less, public exposure to what may be happening during this period leading up to the end of 1st quarter. Grants require applications! VXRT was given what they requested! Therefore - - - -
(Just my DD. I am not a professional investor. Don't rely on my opinion)
VXRT
Vaxart Inc
1.10
-0.01 (-0.90%)
Volume: 25,343,116
Day Range: 1.05 - 1.12
Last Trade Time: 7:58:54 PM EST
|
Followers
|
187
|
Posters
|
|
|
Posts (Today)
|
0
|
Posts (Total)
|
6214
|
|
Created
|
01/12/05
|
Type
|
Free
|
| Moderators | |||

The Vaxart tablet vaccine is composed of three basic elements:
The vector: the Ad5 delivery vehicle that carries the DNA “payload” to the gut
The antigen: the pathogen protein designed to trigger the targeted immune response
The adjuvant: a “booster” molecule that stimulates and adds to the immune response
By using the same vector, but with different antigens, Vaxart has designed a modular, scalable and standardized approach to vaccine development.
With this system, we can support the rapid development of numerous vaccines against established targets, as well as against new and emerging pathogens.
We can achieve important synergies – we can use the same manufacturing processes for all of our vaccines.
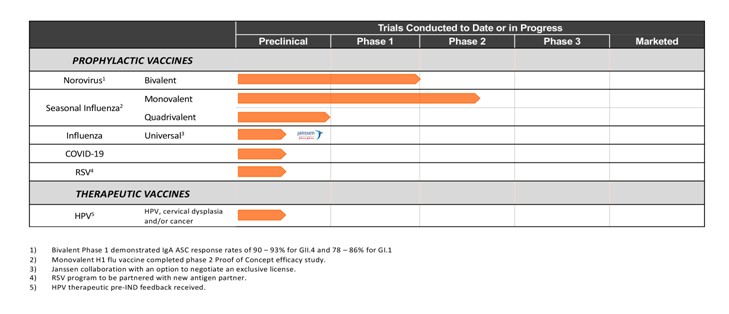
Norovirus
Three Phase 1 studies of Vaxart’s norovirus tablet vaccine indicated it is well tolerated and generated systemic and local immune responses that are both robust and persistent. The most recent Bivalent Phase 1b study demonstrated subjects obtained robust responses to both the mucosal and the systemic immune system.
Influenza
Influenza is a worldwide infectious disease, with symptoms that range from mild to life-threatening and sometimes resulting in death. Serious illness occurs not only in susceptible populations, such as older adults, but also in the general population when unique strains of influenza arise.
Respiratory Syncytial Virus (RSV)
Respiratory syncytial virus (RSV) is increasingly recognized as an important cause of respiratory infection. While RSV infections can occur at any age, the elderly and those immunocompromised and living with underlying cardiopulmonary disease are particularly vulnerable.
Human Papillomavirus (HPV)
HPV is a group of more than 100 related DNA viruses that have the ability to infect the skin or mucous membranes. HPV is the most common sexually transmitted virus in the U.S. and infection is very prevalent following the onset of sexual activity. Vaxart is targeting HPV infection that causes cervical dysplasia and cancer due to HPV 16 and 18.
Vaxart’s COVID-19 Vaccine Selected
for the U.S. Government’s Operation Warp Speed
June 26, 2020 at 8:00 AM EDT
OWS to Test First Oral COVID-19 Vaccine in Non-Human Primates
SOUTH SAN FRANCISCO, Calif., June 26, 2020 (GLOBE NEWSWIRE) -- Vaxart, Inc., a clinical-stage biotechnology company developing oral vaccines that are administered by tablet rather than by injection, today announced that its oral COVID-19 vaccine has been selected to participate in a non-human primate (NHP) challenge study, organized and funded by Operation Warp Speed, a new national program aiming to provide substantial quantities of safe, effective vaccine for Americans by January 2021.
The study is designed to demonstrate the efficacy of Vaxart’s oral COVID-19 vaccine candidate.
“We are very pleased to be one of the few companies selected by Operation Warp Speed, and that ours is the only oral vaccine being evaluated. SARS-CoV-2, the coronavirus that causes COVID-19, is primarily transmitted by viral particles that enter through the mucosa - nose, mouth or eyes - strongly suggesting that mucosal immunity could serve as the first line of defense,” said Andrei Floroiu, Chief Executive Officer of Vaxart Inc. “In addition, our vaccine is a room temperature-stable tablet, an enormous logistical advantage in large vaccination campaigns.”
SOUTH SAN FRANCISCO, Calif., Aug. 10, 2020 (GLOBE NEWSWIRE) -- Vaxart, Inc., a clinical-stage biotechnology company developing oral recombinant vaccines that are administered by tablet rather than by injection, today announced that its COVID-19 Investigational New Drug (IND) application has been filed with the US Food and Drug Administration (FDA).
“We are very excited to reach this important milestone in advancing our oral COVID-19 vaccine candidate towards the clinic,” said Andrei Floroiu, chief executive officer of Vaxart. “We expect our upcoming Phase I study to generate data that will further differentiate our oral vaccine from injectable vaccines by substantiating the importance of activating both systemic and mucosal immunity in protecting against COVID-19. We believe that this mechanistic benefit combined with the significant advantages of oral administration to the patient while eliminating the need for cold chain distribution, could make our COVID-19 vaccine an ideal candidate for successful mass vaccination campaigns globally.”
“Filing the IND is the first major step of many we are taking to advance our oral vaccine in the prevention of COVID-19,” said Sean Tucker, Ph.D., chief scientific officer of Vaxart. “We are excited to be moving this project toward clinical trials, and potentially demonstrating similarly potent mucosal and systemic immune responses like we have seen with our other vaccine candidates using the same oral tablet vaccine platform.”

Emergent BioSolutions Signs Development and Manufacturing Agreement
with Vaxart for their Experimental Oral Vaccine Candidate for Coronavirus Disease
GAITHERSBURG, Md., March 18, 2020 (GLOBE NEWSWIRE) -- Emergent BioSolutions Inc. (NYSE:EBS) announced today that it has entered into an agreement with Vaxart, Inc. (Nasdaq: VXRT), a clinical-stage biotechnology company, whereby Emergent has agreed to utilize its molecule-to-market contract development and manufacturing (CDMO) services to develop and manufacture Vaxart’s experimental oral vaccine candidate for coronavirus disease (COVID-19). Development services will begin immediately, and upon Vaxart’s election, Emergent agrees to produce clinical material expected to enable Vaxart to initiate a Phase 1 clinical study anticipated early in the second half of 2020. Vaxart’s oral recombinant vaccine candidate is based on its proprietary VAAST™ platform.
VXRT Investor Relations
F.D.A. great read KindredBio Partners with Vaxart for COVID-19 Oral Vaccine
October 9, 2020
https://www.fdanews.com/articles/199454-kindredbio-partners-with-vaxart-for-covid-19-oral-vaccine

$VXRT THEN 1. Oral formulations vs Liquid inoculations
[remember $KIN has partnered wit $VXRT]
https://www.fool.com/investing/2020/08/13/3-reasons-doctors-will-prefer-vaxarts-coronavirus/
1. Oral formulations make for easier storage and transport
When most people think of vaccines, they think of a syringe filled with liquid which is administered by a healthcare worker into the muscles or veins of the patient.
In contrast, Vaxart's technology platform produces oral tablets containing the equivalent active components.
This may seem like a minor distinction,
but it's actually a critical factor that works strongly to Vaxart's advantage when it comes to currying favor with doctors and other providers.
Liquid inoculations typically require refrigeration during manufacturing, shipping, and storage at the point of use.
If they aren't consistently kept at the correct temperature, their efficacy may drop, or they may spoil completely.
This means that traditional liquid vaccines imply a substantial logistical burden on healthcare systems,
as well as their suppliers, as a result of the additional refrigeration hardware and energy expenditures required.
Vaxart's tablets are stable at room temperature, however,
so healthcare systems won't need to spend nearly as much to transport or store its coronavirus prophylactic,
and the risk of spoilage is much low
2. Tablets don't require any consumables to administer
When patients get an inoculation in tablet form, there's no need
for the clinic that distributed it to purchase additional disposable syringes, sterilizing swabs, bandages, or even gloves.
As a bonus, after the patient has taken the vaccine, clinics won't
need to pay for additional biohazard trash removal services to dispose of the used materials.
Thanks to the sum of these savings, healthcare systems won't need to spend as much to administer Vaxart's candidate as they might with a traditional liquid inoculation,
enabling them to vaccinate more patients than they could otherwise.
If these savings seem like they might be trivial, consider that an effective coronavirus prophylactic program would need to be deployed
to about 3 billion people globally,
which could easily create shortages and price spikes for the basic healthcare materials or services required for traditional liquid vaccinations.
3. Less risky self-administration
You've probably taken pills without a doctor or nurse standing at
your side to supervise,
but it's unlikely that you've ever prepared and self-injected a vaccination on your own.
With an oral tablet, Vaxart hopes that patients will have the freedom
to vaccinate themselves in the safety of their homes.
This would be particularly advantageous because it would nullify the risk of patients getting infected in crowded clinics while waiting for their turn to be inoculated.
Vaxart's inoculations win via superior logistics, but oral vaccines aren't for everyone
Vaxart's shelf-stable, syringe-free, and easy-to-deploy prophylactic tablets would thus be popular among clinicians around the world thanks to their lower costs,
and savvy investors should recognize that these advantages could be decisive for future earnings.
However, there is one reason why doctors in underdeveloped regions might not favor Vaxart's candidate.
While the scientific consensus is still forming,
it may be the case that malnutrition makes oral vaccination less effective compared with intramuscular vaccination, especially in children.
Therefore, clinics in areas with low food security might opt for a traditional formulation instead of Vaxart's solution.
Even with this potential disadvantage, I think that the vaccine's logistical strengths -- should it be approved
-- will give the company an enduring advantage over the competition.
Time will tell whether Vaxart's coronavirus candidate can live up
to the necessary medical standards. In the meantime, look for the company's phase 1 clinical trial to start later in the summer.
Alex Carchidi has no position in any of the stocks mentioned. The Motley Fool has no position in any of the stocks mentioned.
The Motley Fool has a disclosure policy.
https://www.otcmarkets.com/stock/VXRT/disclosure
https://www.otcmarkets.com/stock/VXRT/security
https://www.otcmarkets.com/stock/VXRT/profile
https://www.otcmarkets.com/stock/VXRT/quote
https://www.otcmarkets.com/stock/VXRT/overview
PER MGMT DAVE -
| IH Geek [Dave] DISCLAIMER |
01-31-2021
DISCLAIMER: ONLY FOR MICK
https://investorshub.advfn.com/boards/profilea.aspx?user=1012
*The Board Monitor and herewithin , are not licensed brokers and assume NO responsibility for actions,
investments,decisions, or messages posted on this forum.
CONTENT ON THIS FORUM SHOULD NOT BE CONSIDERED ADVISORY NOR SOLICITATION
AUTHORS MAY HAVE BUYS OR SELLS WITH THE COMPANIES MENTIONED IN TRADING POSTERS SHOULD DUE DILIGENT BUYING OR SELLING.
ALL POSTING SHOULD BE CONSIDERED FOR INFORMATION ONLY. WE DO NOT RECOMMEND ANYONE BUY OR SELL ANY SECURITIES POSTED HEREWITHIN.
ANY trade entered into risks the possibility of losing the funds invested.
• There are no guarantees when buying or selling any security.Any
| Volume | |
| Day Range: | |
| Bid Price | |
| Ask Price | |
| Last Trade Time: |
