Register for free to join our community of investors and share your ideas. You will also get access to streaming quotes, interactive charts, trades, portfolio, live options flow and more tools.
TRVI....................................https://stockcharts.com/h-sc/ui?s=TRVI&p=W&b=5&g=0&id=p86431144783
I want 2.75s
Come on keep moving
Held after gap fill...Glad I loaded Fri
https://www.tradingview.com/x/TC0syZyI/
Yup no reversal after gap fill then it's toast falling more like a 1,000lb hemorrhoid...Covered my short thanks pumpers on stock wits
Gap fill time making lower lows...I'll wait
Trevi Therapeutics Reports Positive Results from the Ph2b/3 PRISM Trial of Haduvio™ in the Treatment of Prurigo Nodularis
Haduvio (oral nalbuphine ER) demonstrated statistically significant results on the primary efficacy endpoint as measured by a 4-point reduction in the Worst Itch – Numerical Rating Scale (WI-NRS) (p=0.0157)
The trial also met key secondary endpoints, with a safety profile consistent with prior studies
Topline data will be presented by management and study investigator, Jennifer L Parish, MD, on a conference call and webcast on June 29, 2022, at 8:30 am EDT
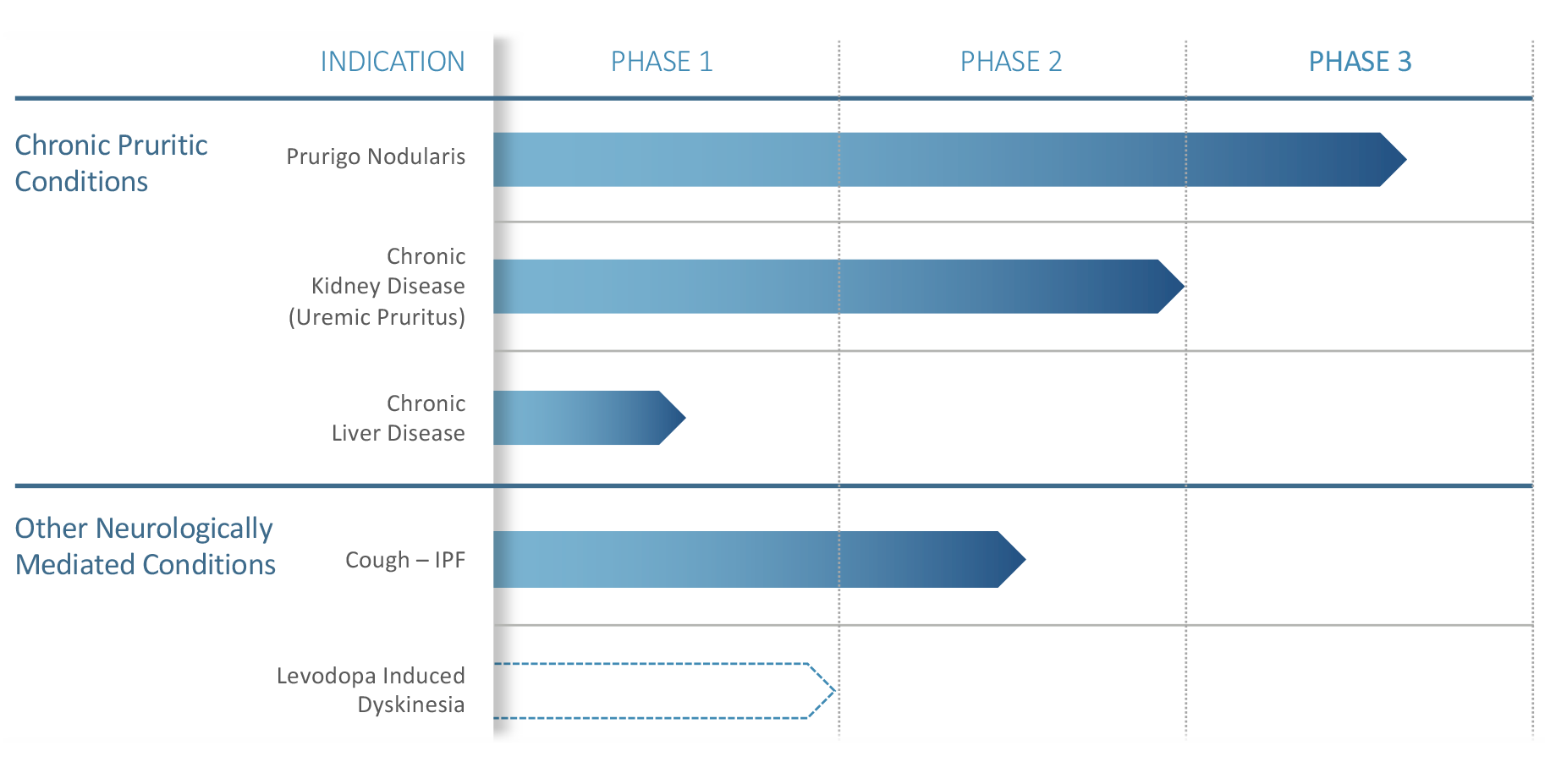
NEW HAVEN, Conn., June 29, 2022 /PRNewswire/ -- Trevi Therapeutics, Inc. (Nasdaq: TRVI), a clinical-stage biopharmaceutical company developing an investigational therapy Haduvio™ (oral nalbuphine ER) for prurigo nodularis and chronic cough in idiopathic pulmonary fibrosis (IPF), today announced positive results from its Phase 2b/3 Pruritus Relief through Itch-Scratch Modulation (PRISM) trial of Haduvio in treating prurigo nodularis.
Trevi Therapeutics Ph2b/3 PRISM trial of Haduvio achieves statistical significance for the treatment of Prurigo Nodularis
"We are very pleased to announce the statistically significant results for Haduvio from the PRISM trial, the first and only oral therapy to demonstrate in a clinical trial a positive result in treating prurigo nodularis," said Jennifer Good, President and CEO of Trevi Therapeutics. "This positive trial in prurigo nodularis, along with the highly statistically significant data from the interim analysis of our Phase 2 trial in IPF chronic cough that we presented earlier this year, further supports our belief that Haduvio could potentially benefit patients across a broad range of refractory chronic pruritic and cough conditions."
Prurigo nodularis is a chronic disease characterized by severe pruritus and the presence of nodules, lesions, and excoriations. Chronic pruritus is a key contributing cause of prurigo nodularis and manifests in an itch-scratch cycle, which is difficult to disrupt. There are no approved therapies for prurigo nodularis where a large unmet need exists due to its impact on patients' quality of life, function, and emotional well-being.
"Prurigo nodularis is one of the most challenging dermatologic diseases for clinicians to manage," said Jennifer L Parish, MD, Dermatologist at Parish Dermatology and study investigator for the PRISM trial. "Patients suffer not only from the itching and the resulting excoriations, but many also suffer from anxiety often caused by the lack of effective treatments. It is exciting to see therapeutic options in development for this potentially devastating condition."
In the Phase 2b/3 PRISM trial, results comparing subjects randomized to Haduvio monotherapy (n=168) or placebo (n=176) showed:
25% of Haduvio subjects evaluated at week 14 met the primary endpoint of a 4-point reduction in WI-NRS from baseline compared to 14% of placebo subjects (p=0.0157).
Haduvio subjects experienced significantly greater improvements in ItchyQoL vs. placebo (p=0.0002) at week 14, which was statistically significant across each of the three domains (symptoms, functional limitations, and emotions). ItchyQoL is used to measure how pruritus impacts a subject's quality-of-life.
55% of Haduvio subjects saw at least a 1-category improvement in the 5-point scale in their Prurigo Activity Scale (PAS) (pruriginous lesions with excoriations), vs. 38% on placebo (p=0.006) as evaluated at week 14.
"In this study, Haduvio demonstrated an early onset of effect to reduce itch, which we believe improved quality of life for the patient as well as the underlying skin healing," said Dr. Bill Forbes, Chief Development Officer at Trevi Therapeutics. "We believe the ability of Haduvio to work both centrally and peripherally helps to break the ingrained itch-scratch cycle, which leads to the difficulty in treating prurigo nodularis. We look forward to analyzing these data further, along with the open label extension data, to help inform continued development in this area of high unmet need."
The safety results of the trial were generally consistent with the known safety profile of Haduvio from previous trials. During the double-blind titration period (weeks 1-2) Treatment-Emergent Adverse Events (TEAE) were more common in the Haduvio-treated subjects (66.1%) vs. placebo-treated subjects (31.3%). During the 12-week fixed-dose period, the occurrence of TEAEs were generally similar between Haduvio and placebo groups (48% Haduvio, 45% placebo). Discontinuations during the 14 weeks of the trial were 36.9% in Haduvio-treated subjects vs. 19.3% in placebo-treated subjects. During the 14-week double-blind portion of the PRISM trial, 8 subjects on Haduvio and 6 subjects on placebo experienced at least one treatment emergent Serious Adverse Event (SAE). None of the SAEs were considered by the investigator to be treatment-related. Adverse events most commonly observed with Haduvio were nausea, dizziness, headache, and constipation.
Conference Call Details
To participate in the live conference call by phone, please dial (888) 317 6003 (domestic) or (412) 317 6061 (international) and provide access code 8601030. A live audio webcast and presentation will be accessible from the 'Investors & News' section on the Company's website at www.trevitherapeutics.com. An archived replay of the webcast and the presentation will also be available for 60 days on the Company's website following the event.
About PRISM
The Phase 2b/3 Pruritus Relief through Itch Scratch-Modulation (PRISM) trial is a randomized, double-blind, placebo-controlled study to evaluate the efficacy and safety of Haduvio in prurigo nodularis. In the trial, subjects are randomized equally across two treatment groups (oral Haduvio 162 mg or placebo, twice daily including an initial 2-week blinded titration period). The primary endpoint of the trial is the proportion of subjects achieving a greater than or equal to 4-point improvement in the weekly mean Worst Itch Numerical Rating Scale (WI-NRS) score at Week 14 compared to baseline. Subjects that complete week 14 are eligible to roll into an additional 38-week open label extension trial. More information about the PRISM trial is available at www.clinicaltrials.gov: NCT03497975
About Trevi Therapeutics, Inc.
Trevi Therapeutics, Inc. is a clinical-stage biopharmaceutical company focused on the development and commercialization of the investigational therapy Haduvio for the treatment of prurigo nodularis and chronic cough in adults with idiopathic pulmonary fibrosis. These conditions share a common pathophysiology that is mediated through opioid receptors in the central and peripheral nervous systems.
Founded in 2011, Trevi Therapeutics is headquartered in New Haven, CT.
About Haduvio
Haduvio, an investigational therapy, is an oral extended-release (ER) formulation of nalbuphine. Nalbuphine is a mixed ?-opioid receptor agonist and µ-opioid receptor antagonist that has been approved and marketed as an injectable for pain indications for more than 20 years in the United States and Europe. The ?- and µ-opioid receptors are kno
private placement pops are different then offerings, offerings always come at top...all out....3.13 resistance.
I'm a bit confused...the news was about dilution, right? And yet the price shot up. Can you explain?
EOD near HOD ... news today .... $10 PT .... we'll see what happens tomorrow ....
Seriously overbought, all out at $1.73
$TRVI 5 times it's avg 10 day volume, so far today.
You still here with me?
Brand new Reddit forum discussion TRVI.
https://www.reddit.com/r/TRVI_Stock/
TRVI
Updated information from the most recent corporate presentation.
I love this stock, been accumulating for about a year.
Prurigo Nodularis – PRISM Trial Update (main indication)
• Approximately 155 subjects enrolled as of 8/13/2020
• COVID-19 screening restrictions lifted in May/June for sites and had
strong enrollment since
• Positive sample size re-estimation read-out this summer. Trial moving
forward with an increase in the sample size to maintain the power
(n=360)
• We have opened more than 60 sites in the US and Europe
• Guidance: Complete Enrollment - Q3 2021
Top-line Data - Q4 2021
• More than 95% of subjects have continued into the one-year open label
extension study
This is a small float, under valued ticker, with Cash position to fund operations into the first half of 2022.
Float 12.0M Shares
Outstanding 18.2M
Institutions Holding Shares 16
% Held by Institutions 79.72%
Up 7%+ on no volume so once we get good news here this extreme low float gem will go through the roof
One of the cheapest biotech you can get at this time .STRONG BUY
|
Followers
|
6
|
Posters
|
|
|
Posts (Today)
|
0
|
Posts (Total)
|
31
|
|
Created
|
05/28/20
|
Type
|
Free
|
| Moderators | |||

| Volume | |
| Day Range: | |
| Bid Price | |
| Ask Price | |
| Last Trade Time: |
