Register for free to join our community of investors and share your ideas. You will also get access to streaming quotes, interactive charts, trades, portfolio, live options flow and more tools.
cuz you weren't looking @ TSOI sales YOY
which was pointed out before.
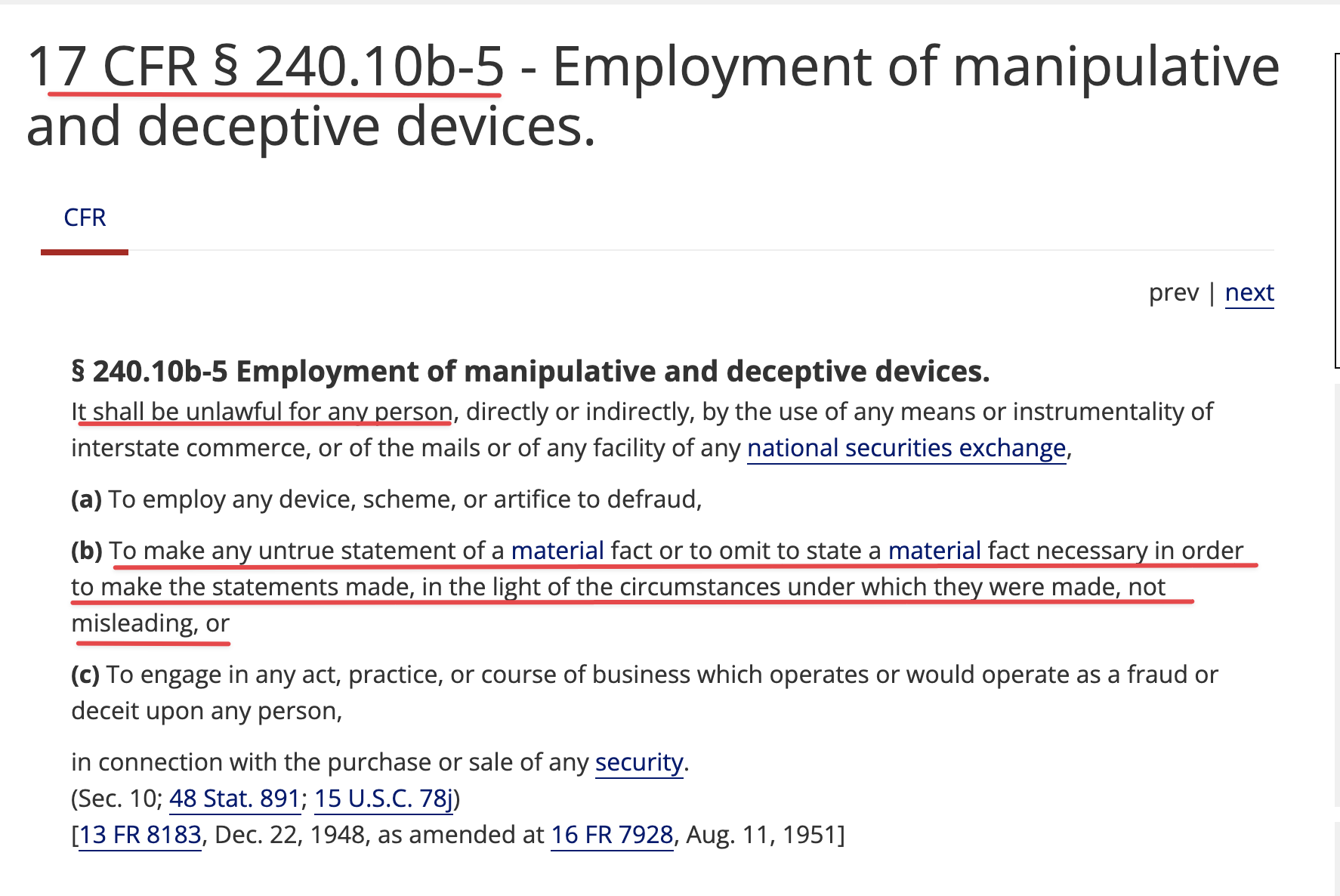
purchase notice means with respect to a purchase pursuant to section 2(a) hereof
an irrevocable written notice from the company to the investor, directing the investor to buy a specified amount of purchase shares .
https://www.sec.gov/Archives/edgar/data/1419051/000149315222026362/ex99-1.htm
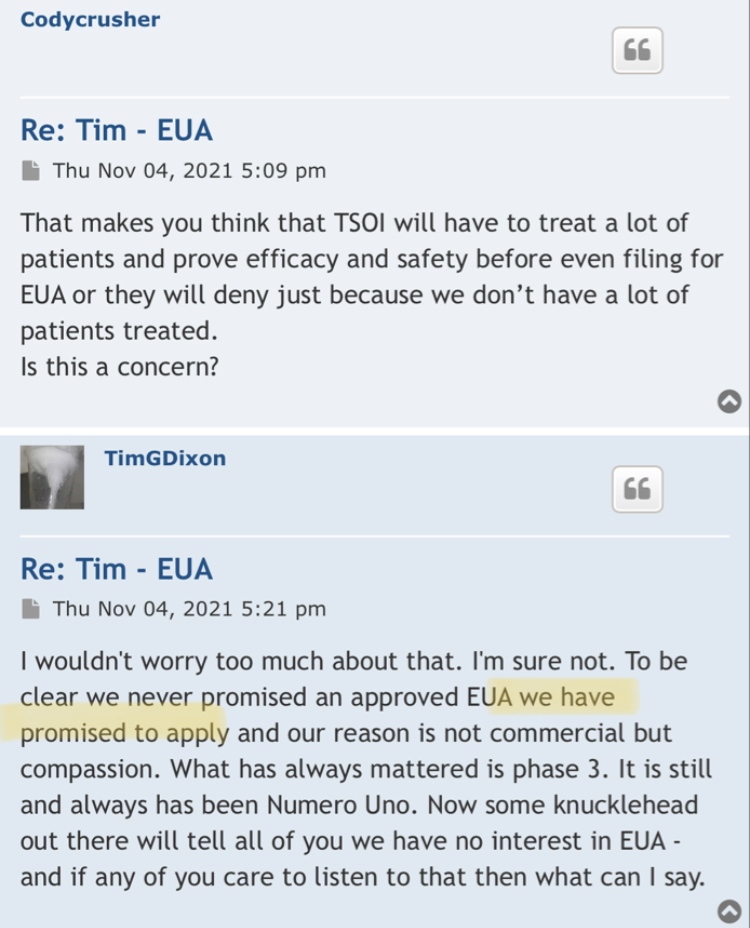
let me educate the investing public 1st this is what I said
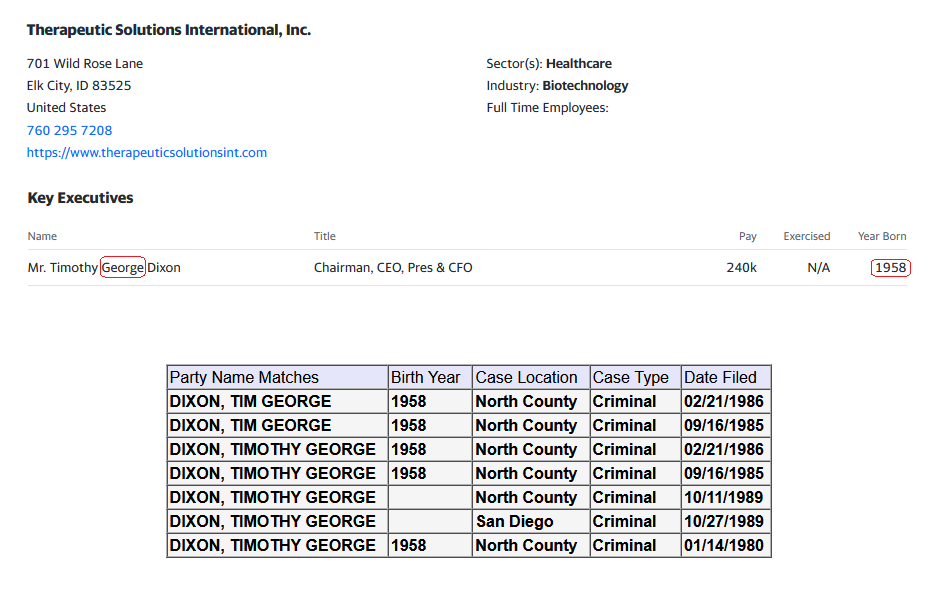
none have generated any positive revenue as per filings w/ Timothy G Dixon's signature.
Accumulated deficit (15,785,926)
Loss from operations (1,075,383)
For the Three
Months Ended
June 30, 2022
(u) "Purchase Price" means with respect to a Purchase made pursuant to Section 2(a) hereof 80% of the lowest traded price of the Common stock during the Valuation Period. With respect to a purchase made pursuant to Section 2(a) following such time as the Company's common stock is listed on a National Exchange, 90% of the lowest WVAP during the Valuation Period, subject to a floor of $[ ], below which the Company cannot deliver a Purchase Notice
https://www.otcmarkets.com/filing/html?id=16088583&guid=FkX-kFGGBz2pJth#ex99-1_htm
Zoom that Purchase Agreement out & ALL will see as I stated earlier Funder's cannot lose, in this case GHS especially.
by noslippas808 » Thu Sep 22, 2022 8:49 am
https://forum.therapeuticsolutionsint.com/viewtopic.php?t=384&start=45
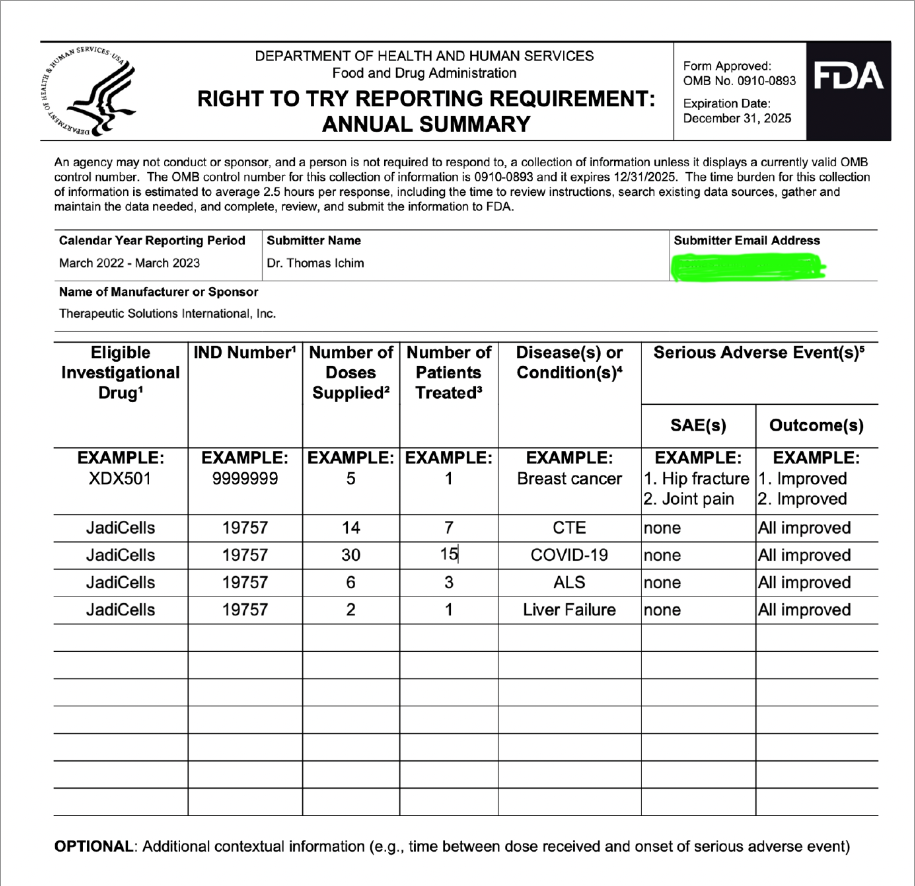
CTE is an interesting topic. We have reported that as of current we, our group that is, consisting of Dr. Veltmeyer as clinician, have successfully diagnosed and treated five retired US Navy SEALS. This was all done under Right To Try and yet we are satill working through animal models with FDA to clear IND for phase 1/2. Dr. James Veltmeyer is the only physician in the USA that can diagnose CTE in a living human being. Just think about that for a moment. FDA has given us green light to diagnose and treat ahead of our IND and phased trial. You would think the NFL (we tag them on twitter on every CTE release) would be curious but I can tell you we have never had any contact. I know a bunch of ex-chargers because I live in San Diego and they know but the problem is they are all in litigation with the NFL. Not one of the players in that case have an actual diagnosis of CTE. Imagine what would happen to that case if we were to diagnose a large % with an incurable and terminal disease? Might change the payout... So because we live near Coronado and Camp Pendleton we hve decided to instead see what we can do with out local military. We would love to help the NFL, or anyone else in high risk occupation but so far the only people we have been able to help are those 5 hero's.
“The fact that the FDA accepted our definition of CTE as diagnosed in living patients is in our opinion, a major breakthrough” said Dr. James Veltmeyer, Chief Medical Officer of the Company. “The experiments conducted demonstrate safety of the JadiCells™ in several different systems. We applaud the diligence and high level of scrutiny that the FDA has applied to our clinical trial and look forward to utilizing this novel approach to addressing this serious unmet medical need.”
“We are pleased that the FDA has thoroughly reviewed our proposed clinical trial and agrees with us that chronic traumatic encephalopathy (CTE) is a valid disease indication for potential therapeutic intervention,” stated Dr. James Veltmeyer, Chief Medical Officer of the Company and Top Doctor of San Diego. “Classically, the perception is that CTE can only be diagnosed post-mortem. The fact that the FDA is open to allowing our protocol, with minor changes, strongly supports development of regenerative-based approaches for this terrible condition.”
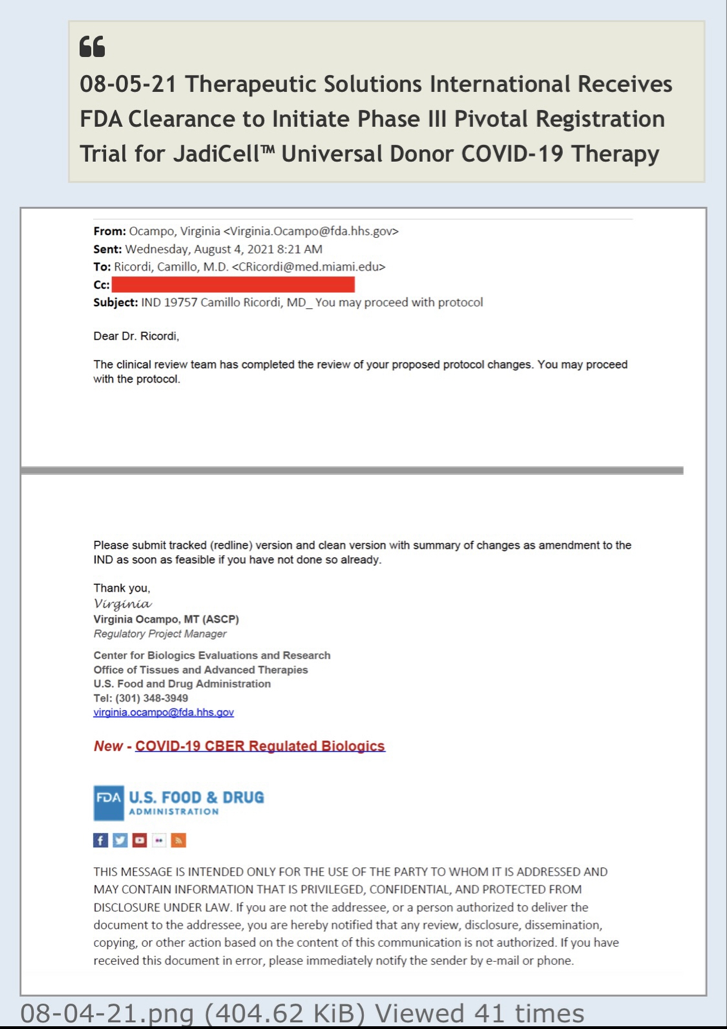
The FDA requested several changes to the IND and clarifications centered around manufacturing, delivery, and monitoring of the cellular product before patient treatment can be initiated.
In regard to recognition of CTE as a valid clinical indication, the FDA stated, “To limit the number of subjects being exposed to unknown but potentially significant risk(s), please modify your protocol to include an appropriate staggering period between consecutive subjects for the first several subjects.” Additionally, the FDA requested that the Company potentially exclude pediatric patients and patients unable to provide proper informed consent.
by TimGDixon » Thu Sep 22, 2022 9:03 am
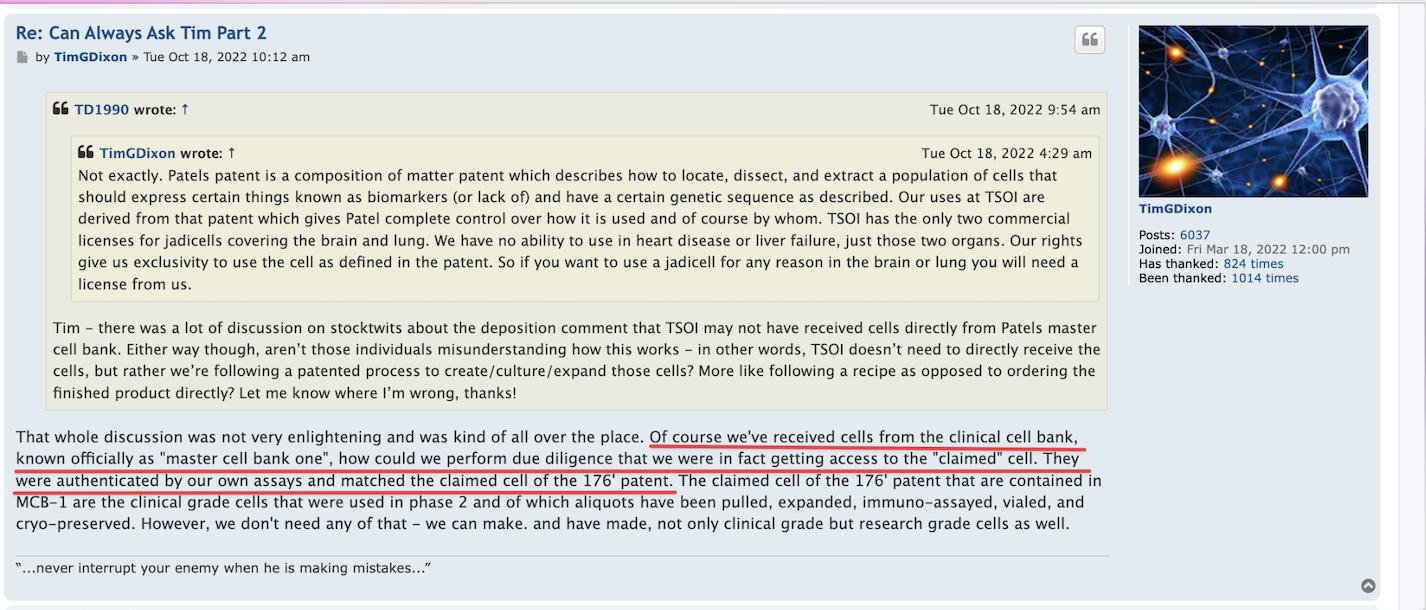
Not exactly sure what you mean. The IND # 27377 has already been filed and issued serial number. FDA asked for additional animal studies (as they always do) and I think we will soon have the data to supplement with. Outside the IND the FDA had asked us to limit (under RTT) the first few patients with ample time between each which we did and then diagnosed and treated 3 more. Once we submit this data then the drug master file kicks in and there won't be any more delays to study mice and we should get green light to proceed. But, with FDA anything can change any time.
“To limit the number of subjects being exposed to unknown but potentially significant risk(s), please modify your protocol to include an appropriate staggering period between consecutive subjects for the first several subjects.”
yes, and this sets up a run
get aboard
the R and D is tied into this company and they do have revenue YOY
get a grip
WoW & yet none have generated any positive revenue as per filings w/ Timothy G Dixon's signature.
https://www.otcmarkets.com/stock/TSOI/disclosure
GHS gets to buy the shares @par value.
so isn't it better to wait for a good run if they were gonna dump .
the purchase agreement is for GHS to buy 10M shares minimum to maximum
per daily volume.
https://www.sec.gov/Archives/edgar/data/1419051/000149315222026362/ex99-1.htm
they buyin in restricted shares @ par value .
what does that tell you? - restrictions aside
the most profit to be made is on a humongous run
the godzilla of all runs i look at , and wonder about.
-and how long i have to wait for it.
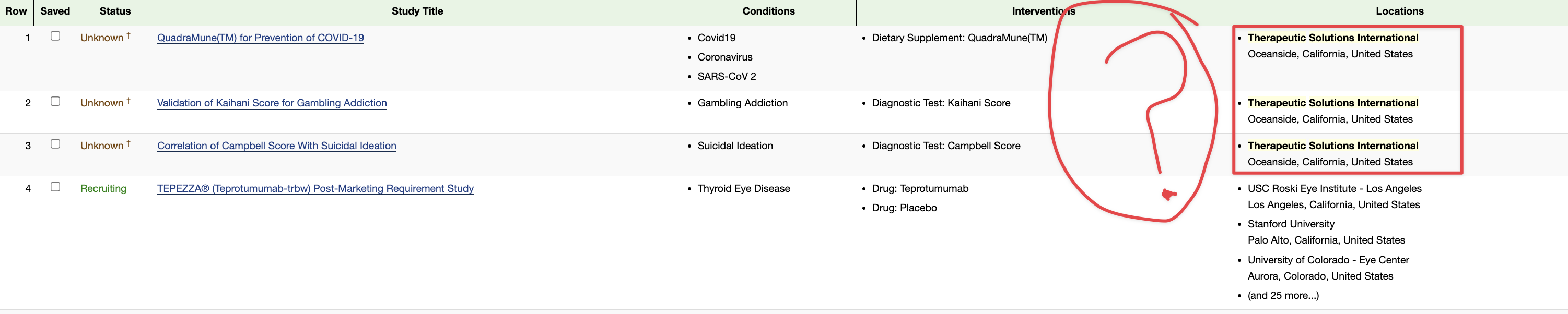
Therapeutic Solutions International has 66 filed and issued patents, multiple IND’s covering Chronic Traumatic Encephalopathy (CTE), Acute Respiratory Distress Syndrome (ARDS), Chronic Obstructive Pulmonary Disease (COPD), and solid tumor Cancer’s as well as numerous preclinical and pilot clinical data around immunotherapy, oncology, and stem cell therapeutics.
https://www.nasdaq.com/press-release/therapeutic-solutions-international-secures-financing-to-complete-current-phase-iii
TSOI 00954 4,990,079 avg @ 13:10:19
Agreed, the when matters most. Either way though, unless there’s serious price appreciation between now and then, they would be dumping a huge amount of shares over time, in addition to the ongoing dilution. I haven’t read enough - what is the time restriction on them selling shares? Are they restricted for 60, 90 days, etc? We need enough time for the cash to be put to use on the trial, to in-turn create value, BEFORE GHS starts dumping
Yes, and most important....the financing allows TSOI to forge ahead with Phase 3 FDA clinical trials. That's the bottom line. Everything else is just noise. This is a long term investment. It's NOT a good swing trade.
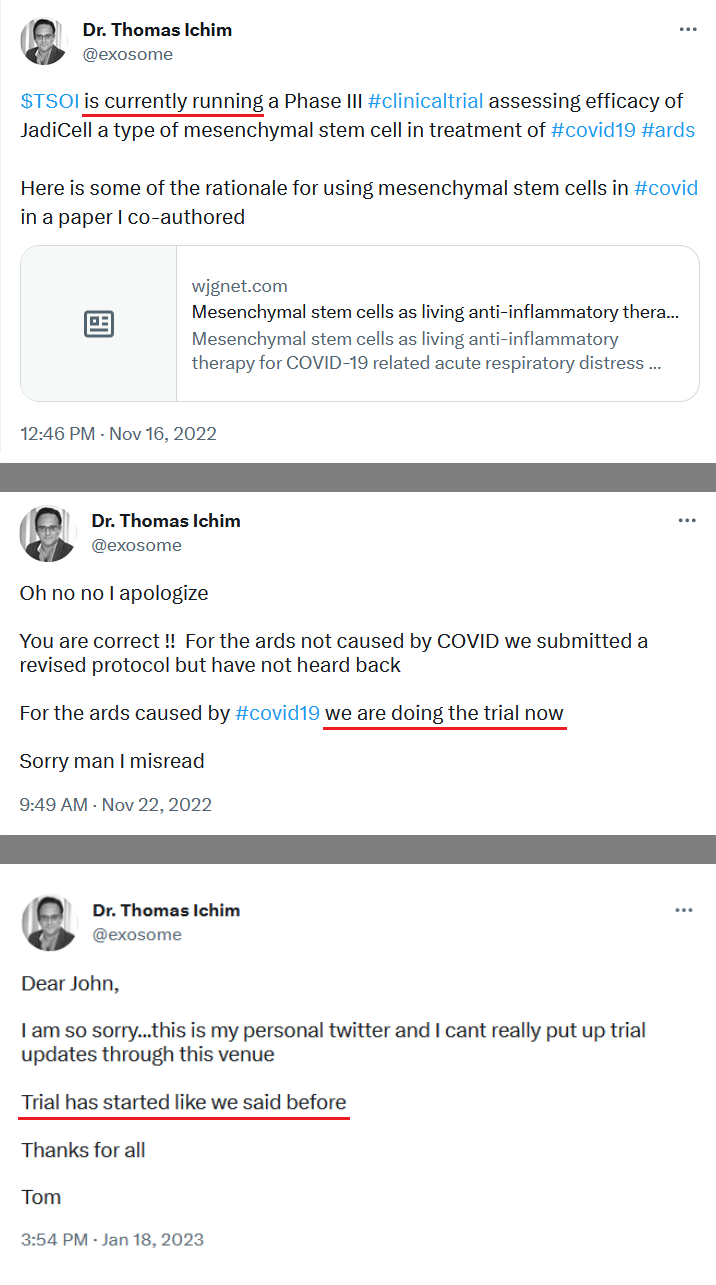
You keep misstating this, so to be clear: TSOI has requested that the phase 3 trial be expanded to all causes of ARDS (not just COVID), this has nothing to do with COPD.
and so what
really matters is when , not the how , or the why.
who cares about that right now?
not I , but there are several here that make it sound like they are coming onto the market yesterday - and that has no bearing on today's market.
Who is doing all this selling now does anyone really know. I’m waiting on word from the FDA on the COPD situation. I think it should be out no later than tomorrow. I’m hoping they except it and I don’t know why they wouldn’t.
I just checked on volume and we traded over 9 1/2 million shares and lucky to be at a penny. Mini have their own speculation of what’s happening to tell you the truth I don’t think none of you know I know I don’t.
This is a great company with great scientific new ways and it’s harder than hell they even hold a penny now. With all its potential why in the world with anyone cell now.
99.999999 % of the time that’s what they do. They aren’t investing for the long term. Never happens. They ain’t waiting to see phase 3 listed. They aren’t liking for honesty from the ceo. They are looking to make a buck. They dump. Period.
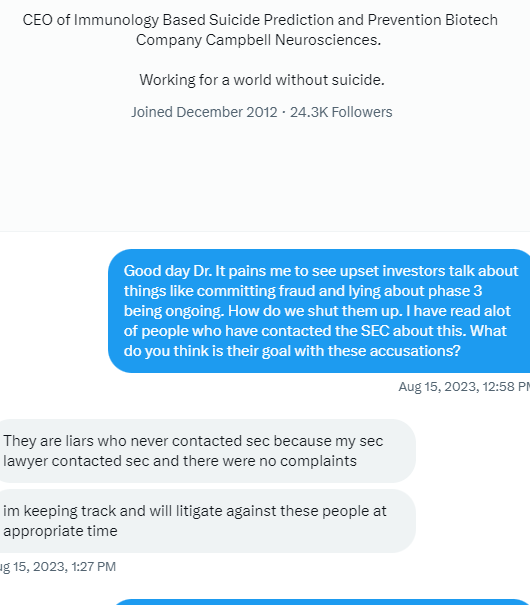
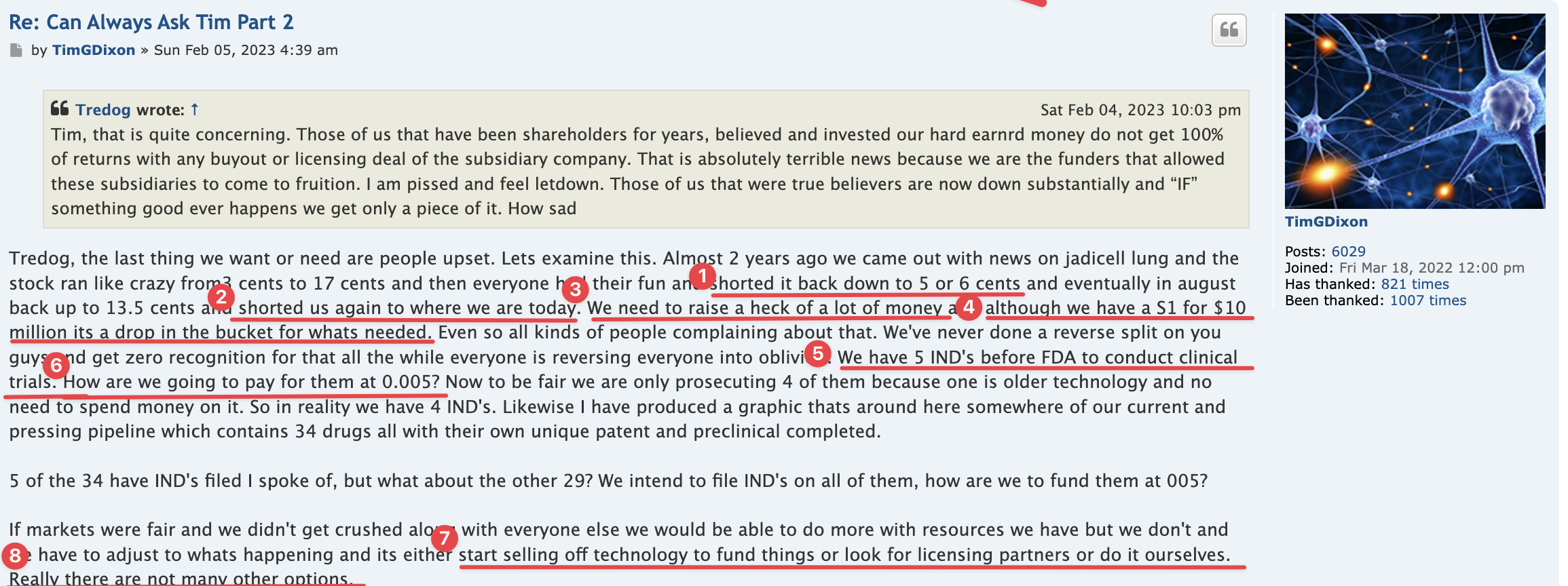
NO speculation needed all about the 4.99% clause & why ![]() it was placed in the Purchase Agreement
it was placed in the Purchase Agreement
other stuff moving right along
by TimGDixon » Thu Sep 22, 2022 6:48 am
Konghusker wrote: ?Thu Sep 22, 2022 6:10 am
Great job Tsoi!! How soon could we potentially start seeing cancer trials starting?
Working to clear breast cancer IND at the moment.
Hey let's see what actually happens first.
speculation on if and when they are gonna dump their shares is just that - speculation.
they are restricted shares to boot. they can't even unload them yet.
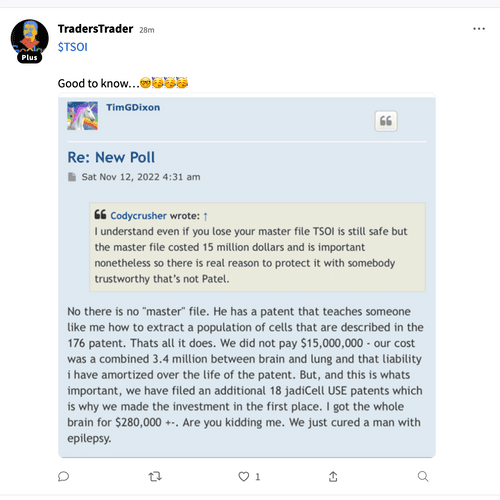
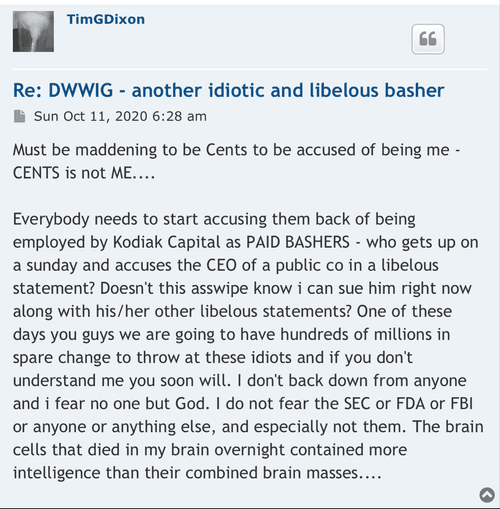
The 4.99% clause tells all intelligent investors/traders out there exactly what will be taking place here. So I could give a rats azz as to any of the investing publics opinions. Again, as I stated I am quite familiar w/ funding deals starting back in the 504D deal days up to present. Any funder (as an example) who would be selling @ 01 would have bought those shares @ a discount to the 01 selling point. Funders do NOT lose & that is the bottom line. FACT
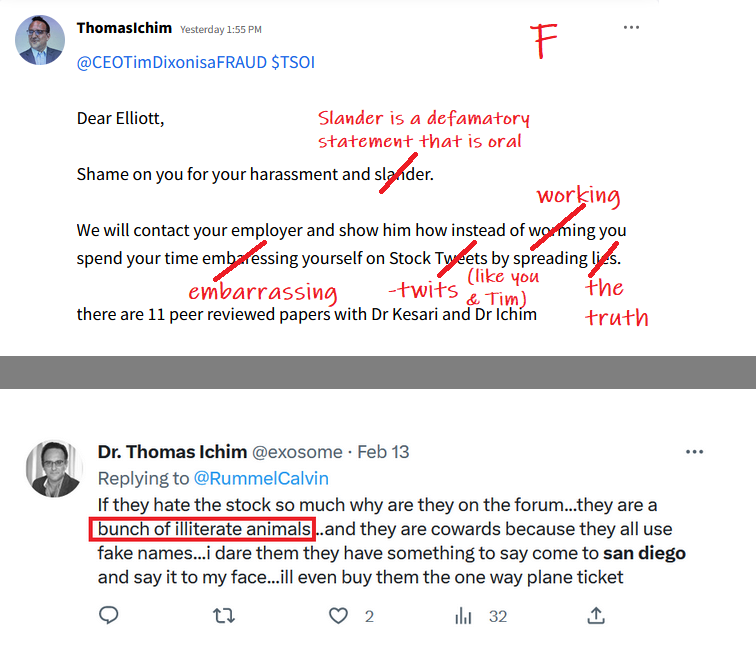
Glad I learned a long time ago from Dixon's original forum (which he deleted) ![]()
What happens if you own 5% of a company?
When a person or group acquires 5% or more of a company's voting shares, they must report it to the Securities and Exchange Commission. Among the questions Schedule 13D asks is the purpose of the transaction, such as a takeover or merger.
I flatly disagree! Anyone who thinks they are going to make a solid ROI by dumping at 1 cent isn't looking at it from a business perspective. If they start dumping at 1 cent, they will quickly be dumping at sub-penny value. That's a losing (poor) business proposition. They will wait for an upward price movement before cashing in on their investment. Otherwise they will destroy their own investment by crushing the PPS.
BS I'm quite familiar w/ financing deals. so NO they do not & will not hold long, the only possibility would be on their last tranche of discounted shares they may decide then to not prior . They make their $ back + only by selling those discounted shares into the market FACT. Especially w/ the 4.99% clause installed in the Purchase Agreement.
https://investorshub.advfn.com/boards/read_msg.aspx?message_id=170012569
I'm sure GHS has multiple entities though, I have seen this done dozens of times.
On the other hand... GHS may plan on holding the shares longer term (as in investment) if they believe in TSOI.
why GHS has the no more than 4.99% clause
in the Purchase Agreement
What happens if you own 5% of a company?
When a person or group acquires 5% or more of a company's voting shares, they must report it to the Securities and Exchange Commission. Among the questions Schedule 13D asks is the purpose of the transaction, such as a takeover or merger.
MM's adjusting the pps, yesterday they could not keep up
also people that bought at .01 are selling
What does BLA approval mean?
For vaccines and therapeutics (a treatment, therapy, or drug), companies file what is called a “biologics license application”—or a BLA. But before filing an application for a vaccine BLA, development and testing must follow a standard set of steps.Mar 7, 2022
How long does it take for a BLA to be approved by FDA?
As per the Prescription Drug User Fee Act (PDUFA), the FDA agreed to review the majority of BLAs within 10 months of 60 day filing and for the priority submissions it has been cut down to 6 months of 60 day filing date.Jun 3, 2019
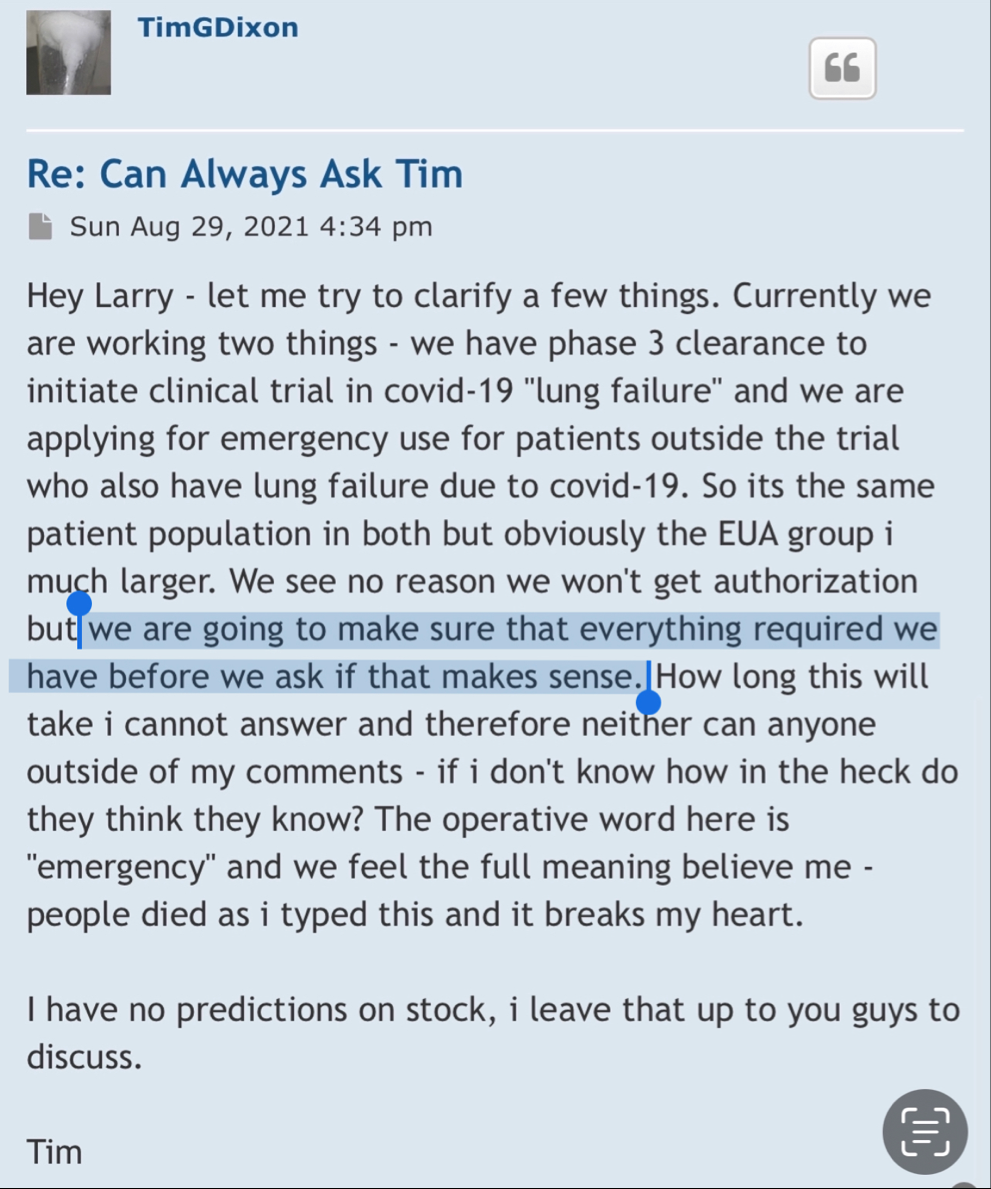
Post by TimGDixon » Wed Sep 21, 2022 7:59 am
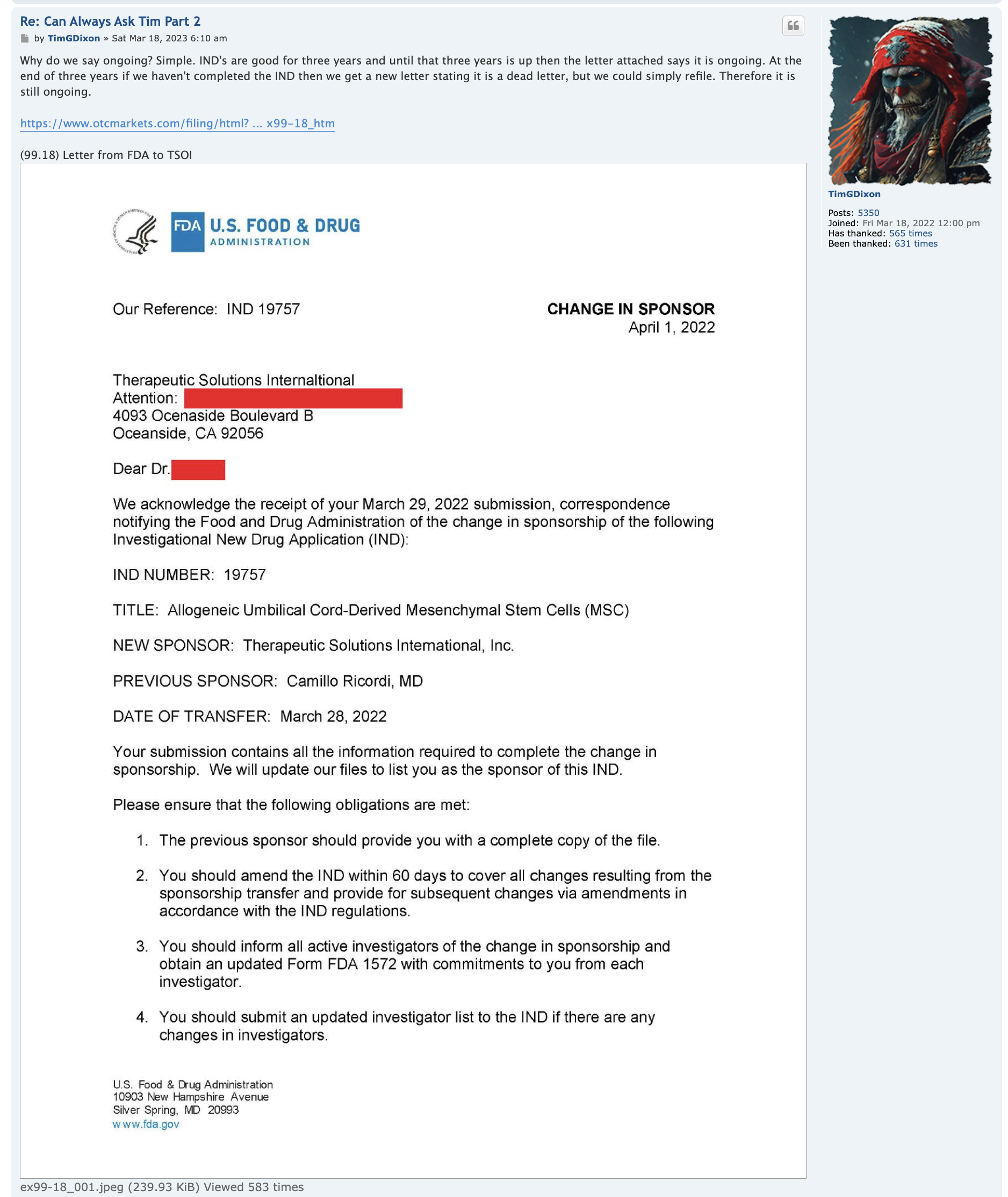
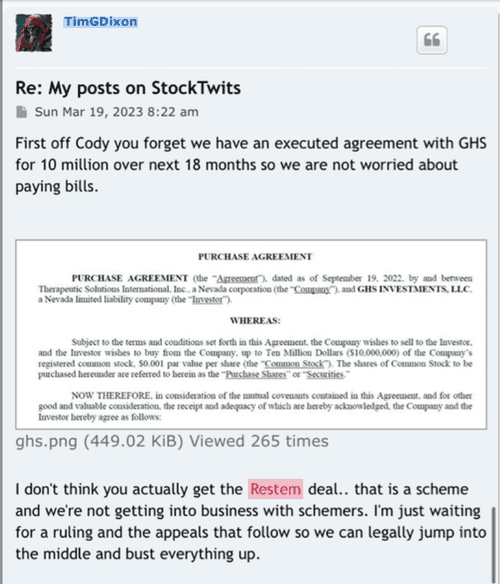
This is a shelf registration that uses Form S-3. Under this shelf registration we may offer and sell, either individually or in combination, in one or more offerings, any of the securities described in the prospectus, for total gross proceeds of up to $10,000,000.
PROSPECTUS
___________________
Therapeutic Solutions International, Inc.
___________________
$10,000,000
COMMON STOCK
PREFERRED STOCK
WARRANTS
UNITS
? common stock;
? preferred stock;
? warrants to purchase our securities; or
? units comprised of, or other combinations of, the foregoing securities.
ELK CITY, Idaho--(BUSINESS WIRE)--Therapeutic Solutions International (TSOI), a clinical stage regenerative medicine and immunotherapy company, announced today it has entered into an agreement with GHS Investments, LLC ("GHS") to purchase up to $10,000,000 of Registered Common Stock.
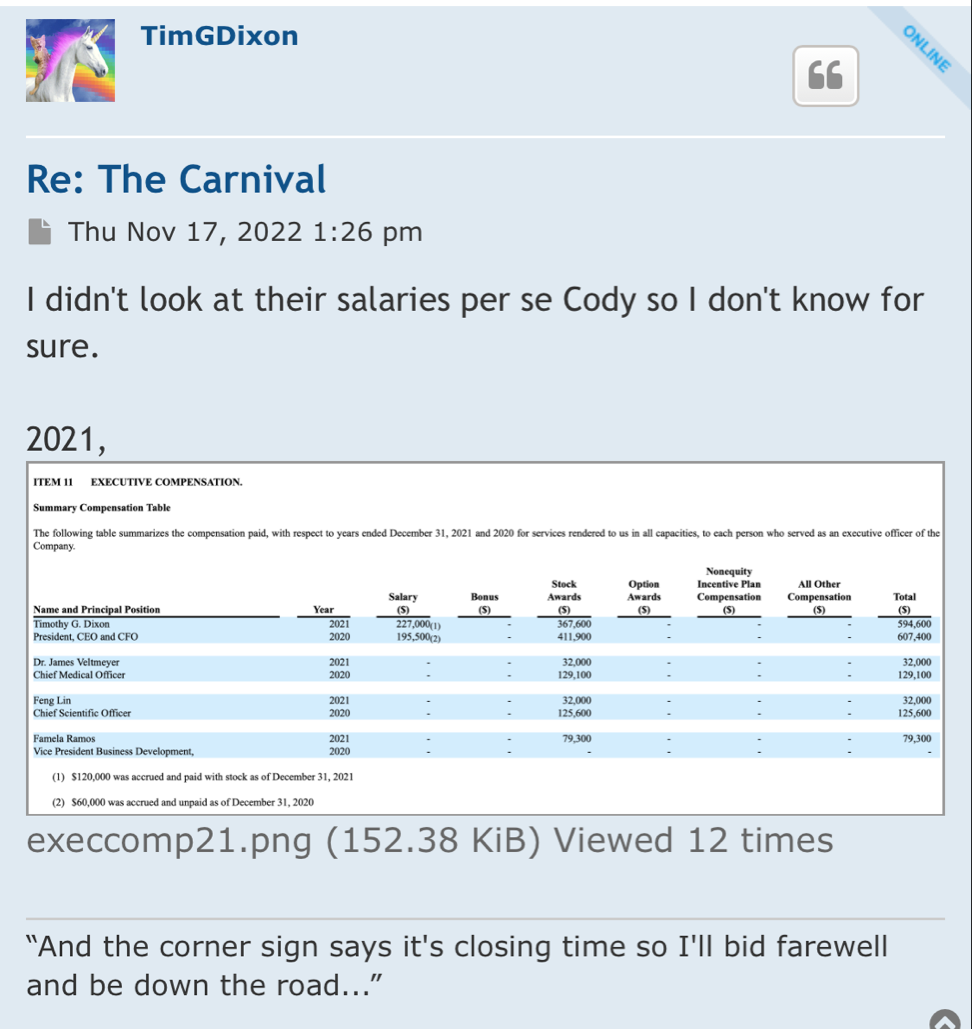
“And the corner sign says it's closing time so I'll bid farewell and be down the road...”
https://forum.therapeuticsolutionsint.com/viewtopic.php?p=10251&hilit=BLA#p10251
What is difference between BLA and NDA?
To formally request approval to market a new drug in the United States, Sponsors must submit either a New Drug Application (NDA) or a Biologics License Application (BLA) to the FDA. As their names suggest, BLAs relate to biological products while NDAs generally pertain to traditional small molecule drugs.
How long does it take for a BLA to be approved by FDA?
As per the Prescription Drug User Fee Act (PDUFA), the FDA agreed to review the majority of BLAs within 10 months of 60 day filing and for the priority submissions it has been cut down to 6 months of 60 day filing date.
well,
everyone can check their own box @ this point in time. -lol
what counts is the funding is done, and let the 20 mule team run through the venue desert , it would not matter.
the TSOI mission continues
https://charts.stocktwits.com/production/original_486125622.png
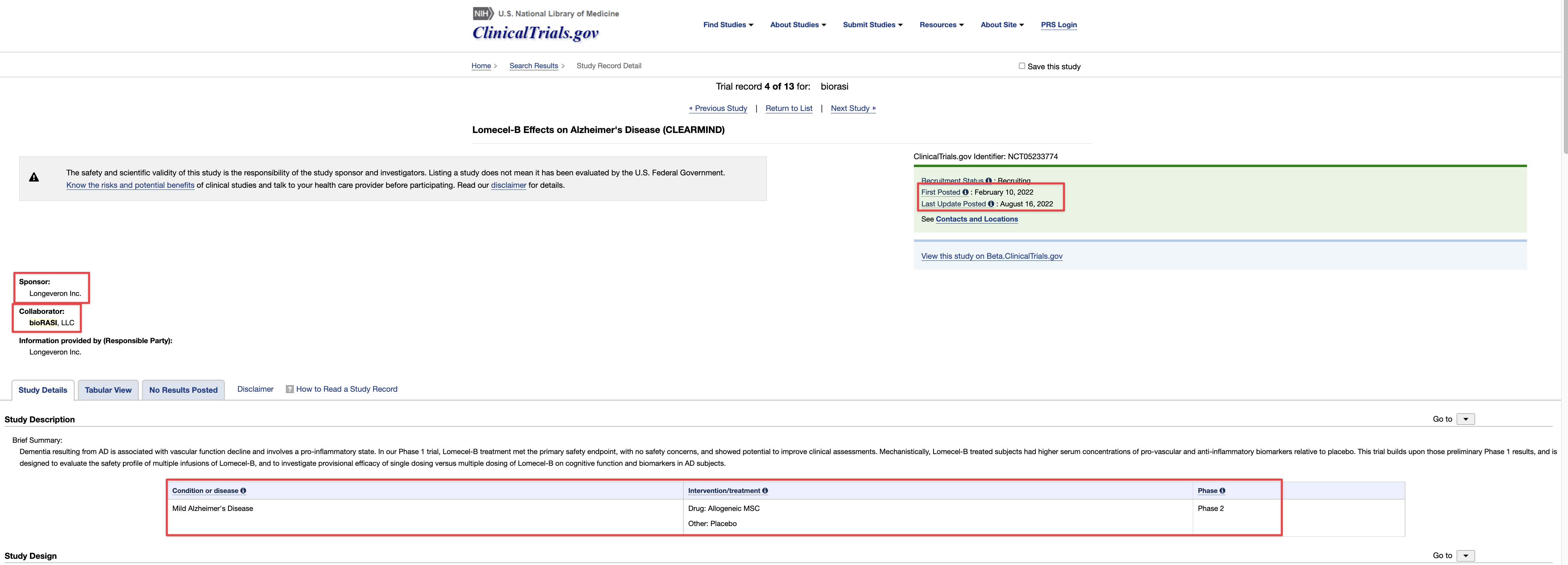
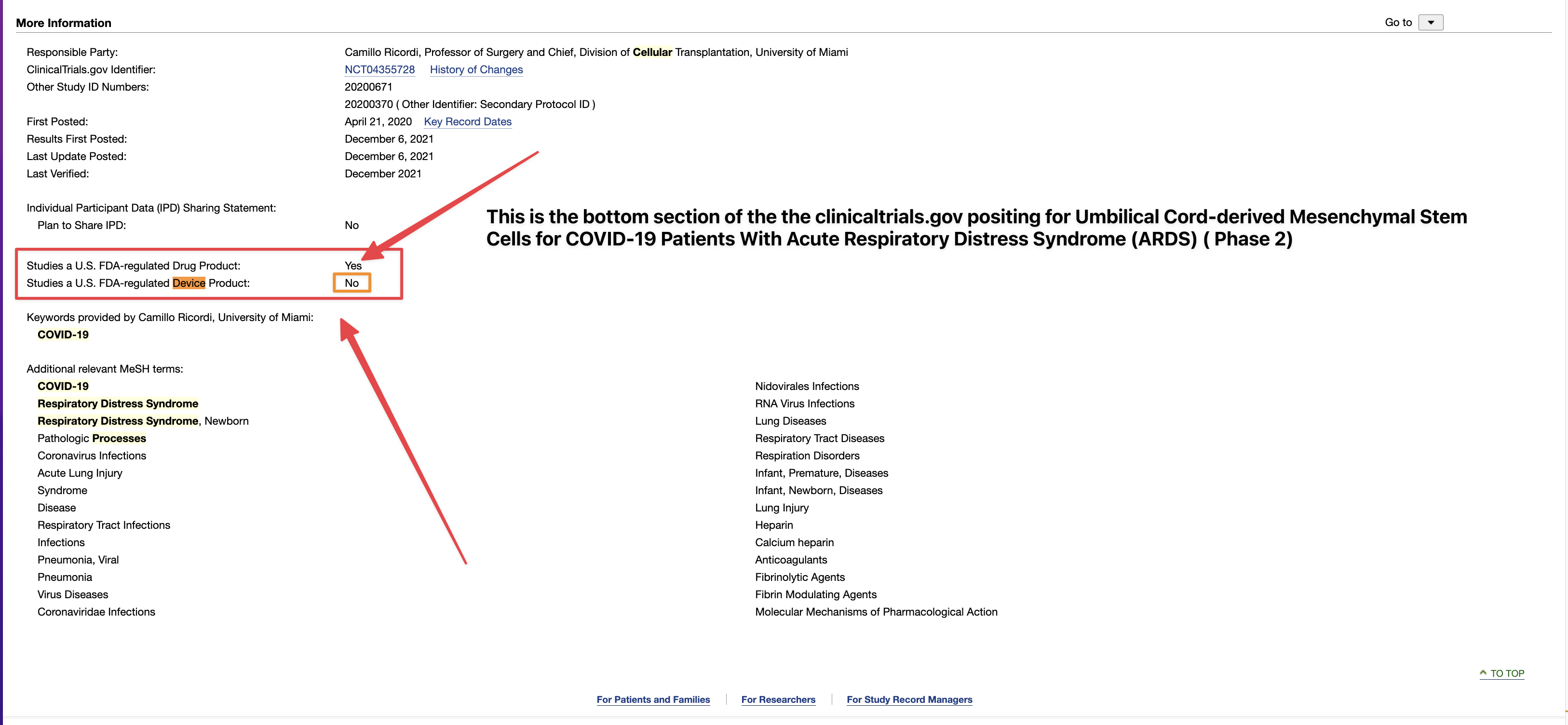
Seems that the trial must be posted to CTDG, it is not a device per phases 1/2. And CTDG itself automatically posts it, according to them there is no option to stop that, or not “check the box” as Tim puts it. Something isn’t adding up
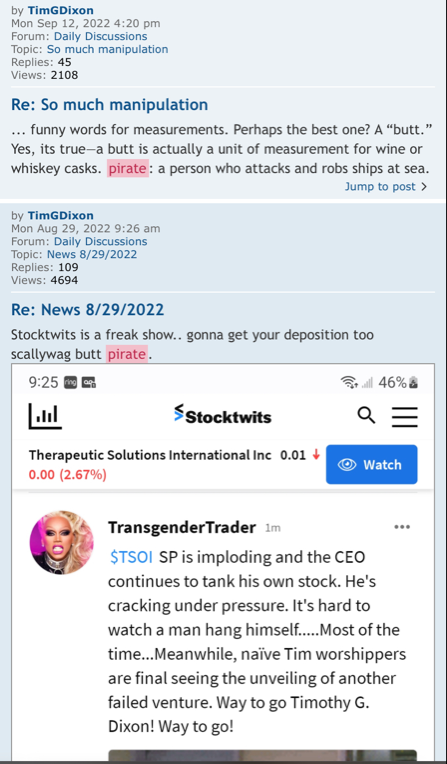
Wow I don’t know why people are running for the hills today? I bought at .0137 yesterday thinking it was a great deal…
TSOI 119 trades Buys 1,611,776 Sells 6,795,971 ? 400,720
https://ih.advfn.com/stock-market/USOTC/therapeutic-solutions-pk-TSOI/trades
VWAP $ 0.011685
TSOI seems to be the overall sentiment of the investing public
.patient enrollment is not fulfilled
by TimGDixon » Wed Sep 21, 2022 6:08 am
It all takes plenty of time which is where great patience comes in. Seven years ago we submitted a clinical protocol to the mexican govt to treat 10 patients with solid tumor cancers using an experimental drug Tom and i developed that you now know as StemVacs - however we are at the 3rd and 4th generation of that discovery that has actually taken us backwards in cell lineage to a cell called an iPSC (induced pluripotent stem cell). Now StemVacs is no longer a single autologous living drug but rather a new platform in which we build all of our living drugs including JadiCell. Patience my young friend. That should be your drug of choice today.
https://forum.therapeuticsolutionsint.com/viewtopic.php?t=384&start=30
Thanks Cents! All your hard work is appreciated by some of us and is one of the reasons we read them in peace and stick around in silence. Have a good one!
this was a wake up call for better appreciation
of the PPS. nobody here can dispute that .
someone is gonna sell theirs along the way , and others will wanna pick em up.
I don't hear 5 NAVY SEALS dissin this stock
i'm with you on that, I don't think the trials have actually started ...patient enrollment is not fulfilled ... i wish there was more clarity but because the lack of clarity, we make assumptions
Bill this is the way I understand it. Enrollment hasn’t been started yet because an addendum was put in on the phase 3 Covid to be able to add COPD to it. Supposedly that was done August 25 and the FDA hast to respond by 30 days which would make it by this Friday. Therefore nothing has been done for actual phase 3 in till the FDA comes out whether they’re going to accept that or not. Now that’s the way I understand it if I’m wrong someone else can come in and say what’s right but I believe that’s the way it is.
But I also believe there’s a possibility for a small bump very small about like the funding that we thought we already had some time back but I guess we didn’t but anyway we got new finding with the name and etc. right now but it couldn’t make it to two cents. I think if we are approved to add COPD I look for a small bump and then right back down again it’s going to be that way until we start getting some kind of revenues in and we’re phase 3 basically starting we’re still a year to year and a half away from that happening. Now this all is my opinion so take it for what it’s worth.
another
Re: TSOI News 09-20-2022
Post by TimGDixon » Wed Sep 21, 2022 4:23 am
ehrlicheyid wrote: ?Tue Sep 20, 2022 7:47 pm
Congrats Tim and team!
Thanks - just wrapping up the S3 and hopefully file by the end of week - that depends on audit and legal clearance but all underway and then we wait on SEC to approve... there's a new buyer and seller in town and the times, they are a changin'...
TimGDixon wrote: ?Sat Sep 03, 2022 5:08 am
Thank you Steve for the well written and encouraging post. People complain about everything I do. It doesn't alter my plan(s). They tell lies all day and night but it won't stop me. Why do I need 3 new companies? So I don't have to dilute all of you into the oblivion. CTE, breast cancer, COPD. How much dilution will it take to fund them in TSOI? Too much so we go outside and do our thing and when we can bring it home we will. Maybe we simply run the first phases. Exit the investors, and dissolve them. Maybe we continue to next phase and go public. Maybe those companies get bought. No shortage of opportunities. You just have to be bold in execution. If we need to raise 10m each to fund those trials at these prices .01 we would need an additional 3 billion authorized. I could authorize that Tuesday morning with my signature. Thomas and I have the vote. Doesn't mean I need to exercise it and crush all of you.
but vhy do other companies show theirs and we do not show ours? how do we even know if full patient capacity for the trial has even been reached. vhat does Tim say about that?
Commercialization is the BLA (biologic license agreement) which then allows us to ship our "drug" across state lines and engage in interstate commerce. That's the end game here for all of our "living drugs".
post by TimGDixon » Wed Sep 21, 2022 5:48 am
CTE is an interesting topic. We have reported that as of current we, our group that is, consisting of Dr. Veltmeyer as clinician, have successfully diagnosed and treated five retired US Navy SEALS. This was all done under Right To Try and yet we are still working through animal models with FDA to clear IND for phase 1/2. Dr. James Veltmeyer is the only physician in the USA that can diagnose CTE in a living human being. Just think about that for a moment. FDA has given us green light to diagnose and treat ahead of our IND and phased trial. You would think the NFL (we tag them on twitter on every CTE release) would be curious but I can tell you we have never had any contact. I know a bunch of ex-chargers because I live in San Diego and they know but the problem is they are all in litigation with the NFL. Not one of the players in that case have an actual diagnosis of CTE. Imagine what would happen to that case if we were to diagnose a large % with an incurable and terminal disease? Might change the payout... So because we live near Coronado and Camp Pendleton we have decided to instead see what we can do with out local military. We would love to help the NFL, or anyone else in high risk occupation but so far the only people we have been able to help are those 5 hero's.
https://forum.therapeuticsolutionsint.com/viewtopic.php?t=384&start=15
but vhy do other companies show theirs and we do not show ours? how do we even know if full patient capacity for the trial has even been reached. vhat does Tim say about that?
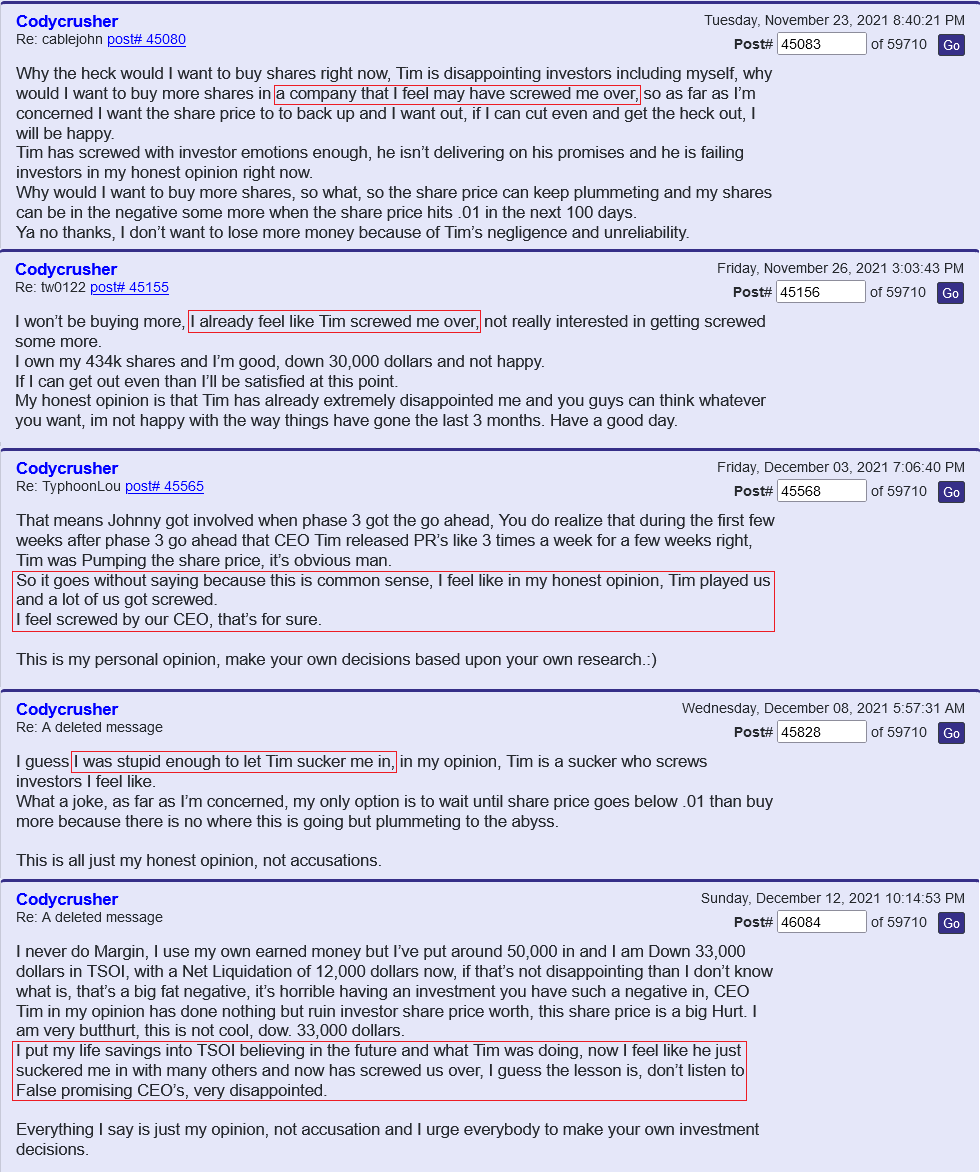
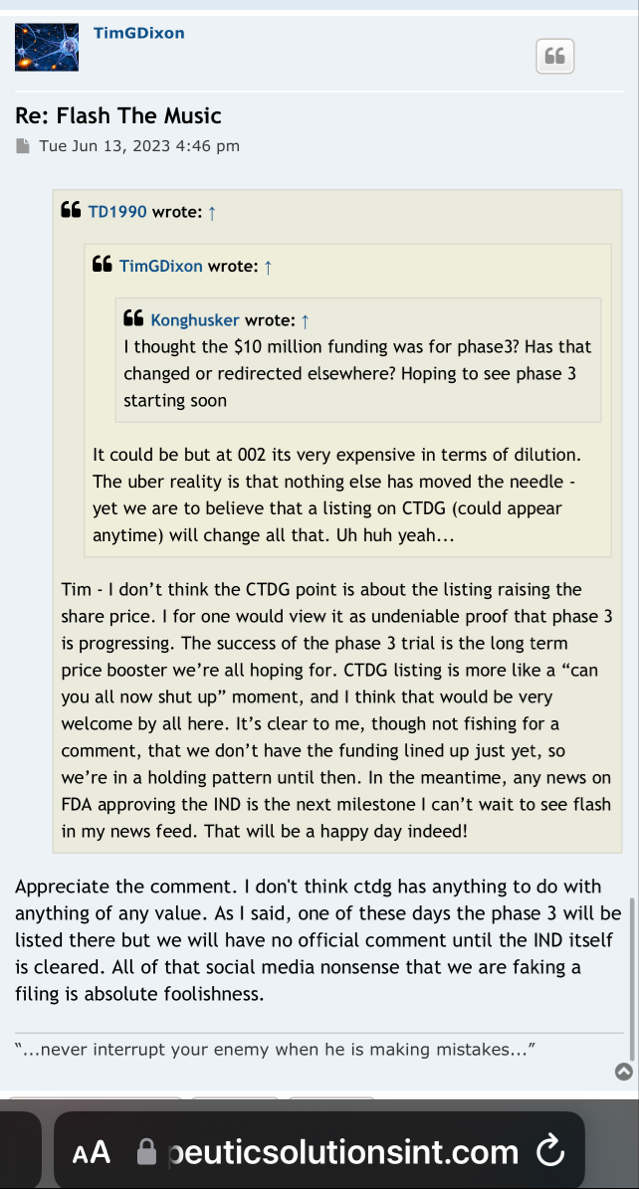
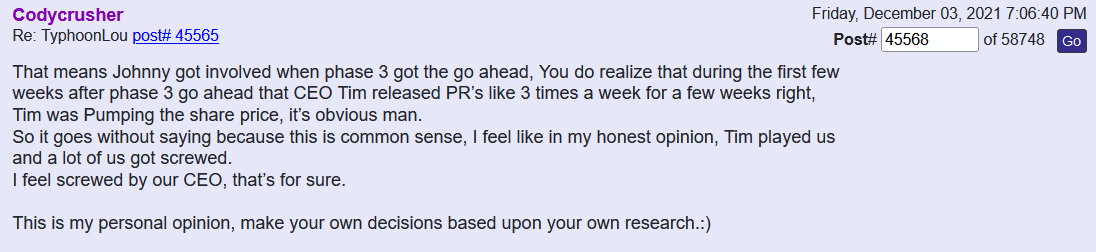
financing to complete Phase 3, it is still dilution and unfortunately dilution while TSOI is trading near its lows (so greater dilution than if this financing was in place 6 months - 12 months ago). The trick now is if Tim can release positive developments more quickly than he needs to draw down the $10mm funding over the next 24 months. Keep in mind the buyer can’t hold more than 4.99% of TSOI at any time, so they will be selling the bulk of their shares in the open market over the next 24 months -
by TimGDixon » Wed Sep 21, 2022 4:51 am
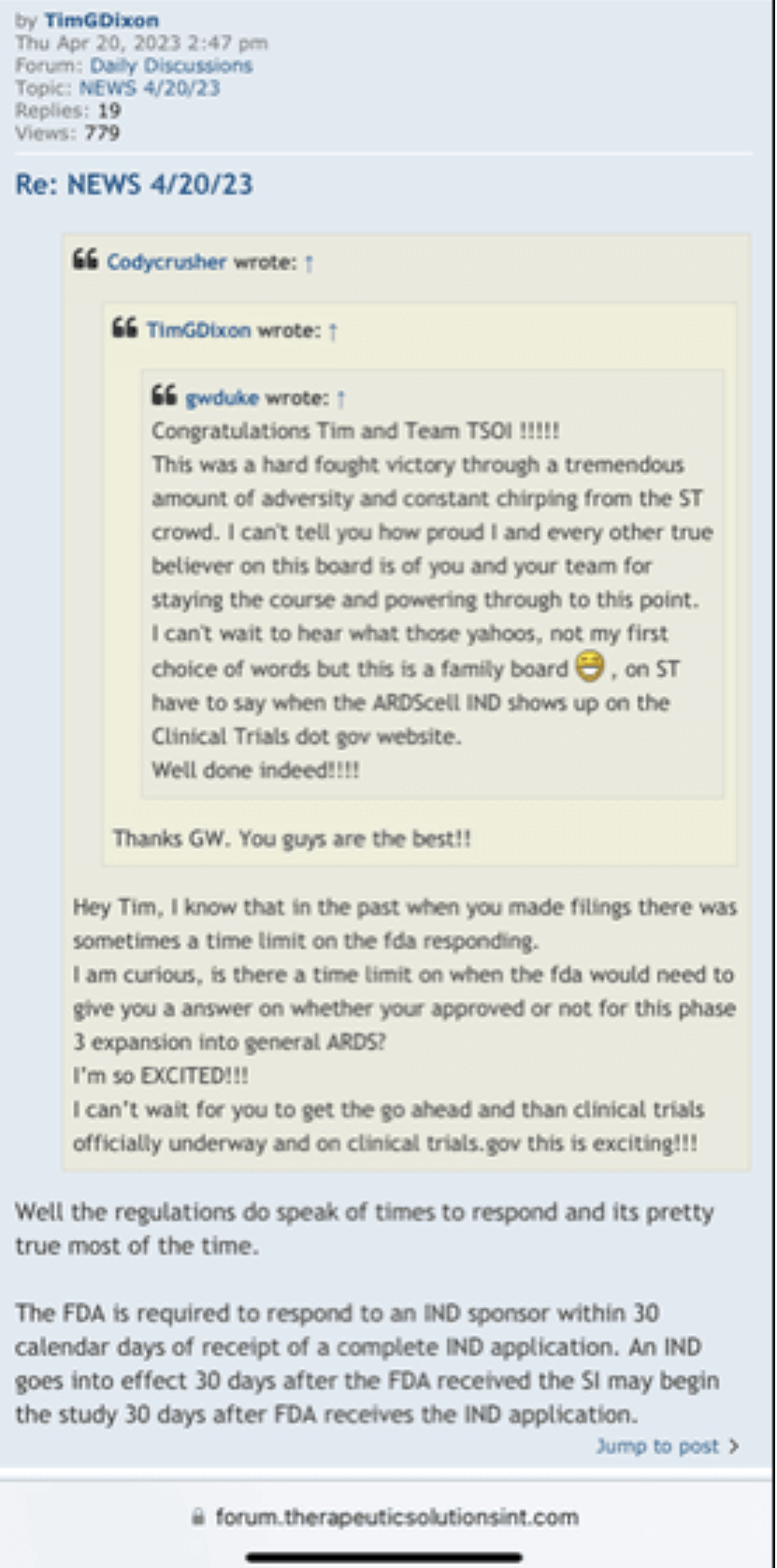
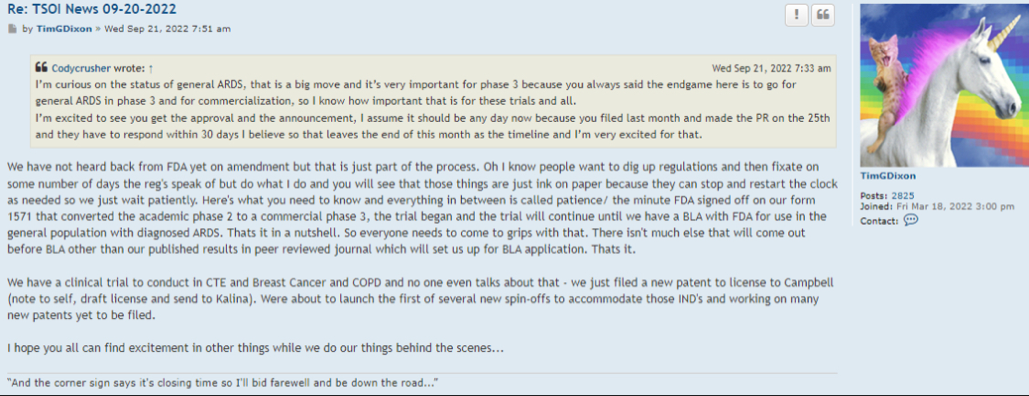
Codycrusher wrote: ?Wed Sep 21, 2022 4:33 am
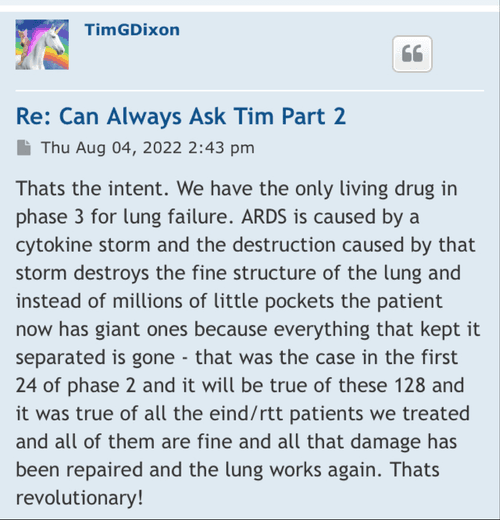
I’m curious on the status of general ARDS, that is a big move and it’s very important for phase 3 because you always said the endgame here is to go for general ARDS in phase 3 and for commercialization, so I know how important that is for these trials and all.
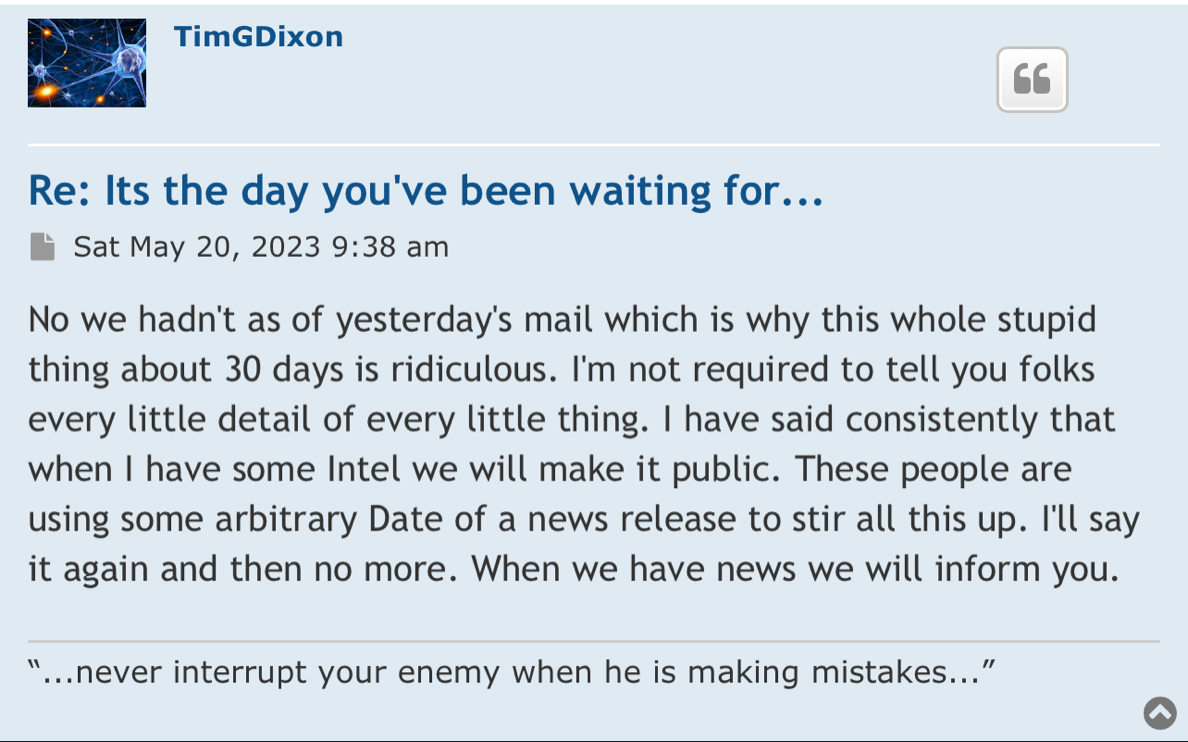
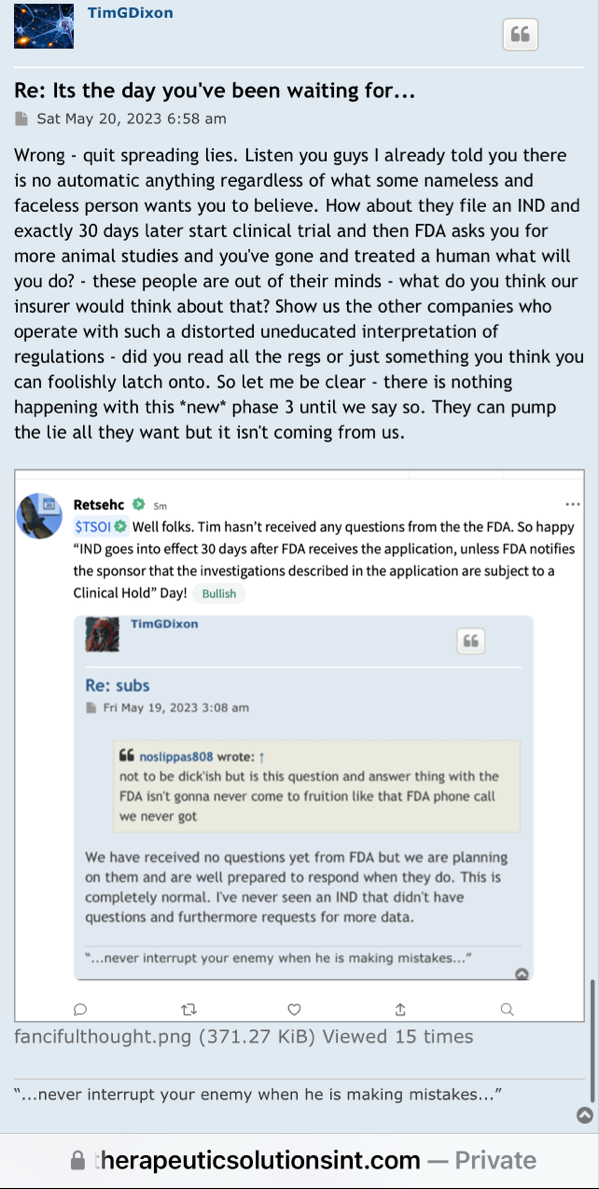
I’m excited to see you get the approval and the announcement, I assume it should be any day now because you filed last month and made the PR on the 25th and they have to respond within 30 days I believe so that leaves the end of this month as the timeline and I’m very excited for that.
We have not heard back from FDA yet on amendment but that is just part of the process. Oh I know people want to dig up regulations and then fixate on some number of days the reg's speak of but do what I do and you will see that those things are just ink on paper because they can stop and restart the clock as needed so we just wait patiently. Here's what you need to know and everything in between is called patience/ the minute FDA signed off on our form 1571 that converted the academic phase 2 to a commercial phase 3, the trial began and the trial will continue until we have a BLA with FDA for use in the general population with diagnosed ARDS. Thats it in a nutshell. So everyone needs to come to grips with that. There isn't much else that will come out before BLA other than our published results in peer reviewed journal which will set us up for BLA application. Thats it.
We have a clinical trial to conduct in CTE and Breast Cancer and COPD and no one even talks about that - we just filed a new patent to license to Campbell (note to self, draft license and send to Kalina). Were about to launch the first of several new spin-offs to accommodate those IND's and working on many new patents yet to be filed.
I hope you all can find excitement in other things while we do our things behind the scenes...
https://forum.therapeuticsolutionsint.com/viewtopic.php?t=384&start=15
by TimGDixon » Wed Sep 21, 2022 4:23 am
Thanks - just wrapping up the S3 and hopefully file by the end of week - that depends on audit and legal clearance but all underway and then we wait on SEC to approve... there's a new buyer and seller in town and the times, they are a changin'...
https://forum.therapeuticsolutionsint.com/viewtopic.php?t=384&start=15
their forum has the drift
https://forum.therapeuticsolutionsint.com/viewtopic.php?t=385
|
Followers
|
524
|
Posters
|
|
|
Posts (Today)
|
0
|
Posts (Total)
|
65738
|
|
Created
|
10/04/08
|
Type
|
Free
|
| Moderators BigBadWolf johnnytrader33 JMC$ Yooperman Hogwarts | |||










































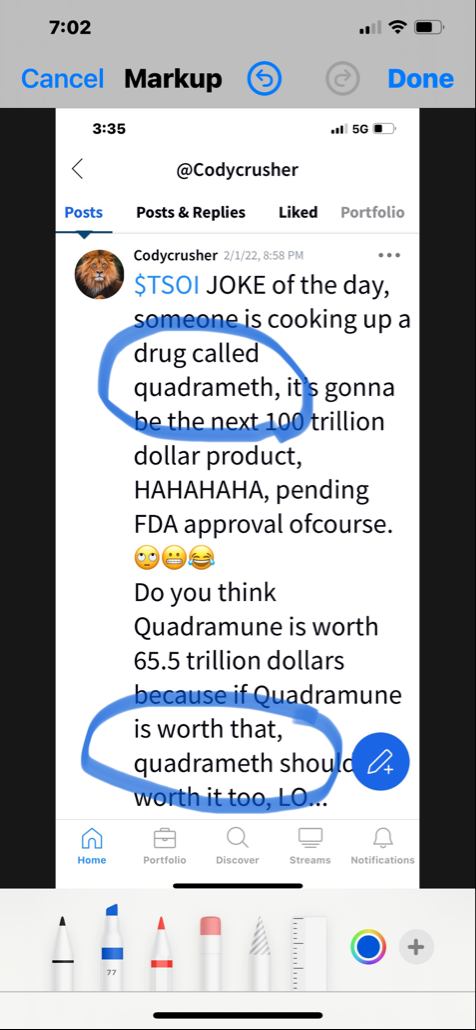









Preclinical Data Suggests QuadraMune™ Prevents Stress-Induced Suppression of Neurogenesis More Effectively than Prozac
OCEANSIDE, Calif., Dec. 9, 2020 /PRNewswire/ -- Therapeutics Solution International, Inc., (OTC Markets: TSOI), announced today new data suggesting the possibility that QuadraMune™ may mediate neuroprotective activity through preserving the ability of regenerative brain cells to proliferate subsequent to psychological stress.
The experiments, which involved exposing mice to established stressors, demonstrated that specific areas of the brain associated with production of new brain cells are damaged by stress. In agreement with previously published research, administration of fluoxetine (Prozac™) protected the brain from stress-induced damage. Surprisingly, QuadraMune™ administration appeared superior to Prozac™ at stimulating proliferation of new brain cells.
"QuadraMune™ which is currently in a clinical trial for prevention of COVD-191, has also been demonstrated to possess anti-inflammatory activity in other clinical trials, suppressing cytokines such as IL-62, which are known to be involved in depression3 and suicide4" said Kalina O'Connor, Director of Campbell Neurosciences and co-inventor on the patent. "Given major depressive disorder causes a significant risk for suicide, we are highly interested in exploring the use of QuadraMune™ for preventing suicide."
"Although much enthusiasm has been generated over the planned distribution of the COVID vaccine, at present little is being done to address mental health issues that are being exacerbated by the current pandemic" said Dr. James Veltmeyer, co-inventor of the patent, and Chief Medical Officer of the Company. "If current results are reproducible, the possibility that a nutraceutical would concurrently boost immunity while preserving mental health is highly enticing."
"It has not escaped us that COVID-19 is associated with increased inflammatory cytokines in the blood of patients, cytokines that also predispose to depression" said Famela Ramos, Vice President of Business Development for the Company. "It may be that the recent increase in suicides and suicide attempts is related biologically to activities of the coronavirus. It will be interesting to examine whether QuadraMune™ may modify putative negative mental effects of the virus."
"An estimated 17.3 million adults in the United States had at least one major depressive episode. This number represented 7.1% of all U.S. adults" stated Timothy Dixon, President and CEO of the Company. "We believe the Mission of our Company is not just providing a return on investment to our shareholders, but also increasing the quality of life for Americans. We are extremely pleased to report this unexpected finding with significant potential implications to advancing non-toxic means of helping patients with this terrible condition."
1 QuadraMune(TM) for Prevention of COVID-19 - Full Text View - ClinicalTrials.gov
2 Therapeutic Solutions International Announces Positive Preclinical and Clinical Evaluation of Nutritional Supplement QuadraMune™, Designed to Protect Against COVID-19 | BioSpace
3 Ting et al. Role of Interleukin-6 in Depressive Disorder. Int J Mol Sci. 2020 Mar 22;21(6):2194.
4 O'Donovan et al. Suicidal ideation is associated with elevated inflammation in patients with major depressive disorder. Depress Anxiety. 2013 Apr;30(4):307-14.
| Volume | |
| Day Range: | |
| Bid Price | |
| Ask Price | |
| Last Trade Time: |
