Register for free to join our community of investors and share your ideas. You will also get access to streaming quotes, interactive charts, trades, portfolio, live options flow and more tools.
post by TimGDixon » Wed Sep 21, 2022 5:48 am
CTE is an interesting topic. We have reported that as of current we, our group that is, consisting of Dr. Veltmeyer as clinician, have successfully diagnosed and treated five retired US Navy SEALS. This was all done under Right To Try and yet we are still working through animal models with FDA to clear IND for phase 1/2. Dr. James Veltmeyer is the only physician in the USA that can diagnose CTE in a living human being. Just think about that for a moment. FDA has given us green light to diagnose and treat ahead of our IND and phased trial. You would think the NFL (we tag them on twitter on every CTE release) would be curious but I can tell you we have never had any contact. I know a bunch of ex-chargers because I live in San Diego and they know but the problem is they are all in litigation with the NFL. Not one of the players in that case have an actual diagnosis of CTE. Imagine what would happen to that case if we were to diagnose a large % with an incurable and terminal disease? Might change the payout... So because we live near Coronado and Camp Pendleton we have decided to instead see what we can do with out local military. We would love to help the NFL, or anyone else in high risk occupation but so far the only people we have been able to help are those 5 hero's.
https://forum.therapeuticsolutionsint.com/viewtopic.php?t=384&start=15
but vhy do other companies show theirs and we do not show ours? how do we even know if full patient capacity for the trial has even been reached. vhat does Tim say about that?
financing to complete Phase 3, it is still dilution and unfortunately dilution while TSOI is trading near its lows (so greater dilution than if this financing was in place 6 months - 12 months ago). The trick now is if Tim can release positive developments more quickly than he needs to draw down the $10mm funding over the next 24 months. Keep in mind the buyer can’t hold more than 4.99% of TSOI at any time, so they will be selling the bulk of their shares in the open market over the next 24 months -
by TimGDixon » Wed Sep 21, 2022 4:51 am
Codycrusher wrote: ?Wed Sep 21, 2022 4:33 am
I’m curious on the status of general ARDS, that is a big move and it’s very important for phase 3 because you always said the endgame here is to go for general ARDS in phase 3 and for commercialization, so I know how important that is for these trials and all.
I’m excited to see you get the approval and the announcement, I assume it should be any day now because you filed last month and made the PR on the 25th and they have to respond within 30 days I believe so that leaves the end of this month as the timeline and I’m very excited for that.
We have not heard back from FDA yet on amendment but that is just part of the process. Oh I know people want to dig up regulations and then fixate on some number of days the reg's speak of but do what I do and you will see that those things are just ink on paper because they can stop and restart the clock as needed so we just wait patiently. Here's what you need to know and everything in between is called patience/ the minute FDA signed off on our form 1571 that converted the academic phase 2 to a commercial phase 3, the trial began and the trial will continue until we have a BLA with FDA for use in the general population with diagnosed ARDS. Thats it in a nutshell. So everyone needs to come to grips with that. There isn't much else that will come out before BLA other than our published results in peer reviewed journal which will set us up for BLA application. Thats it.
We have a clinical trial to conduct in CTE and Breast Cancer and COPD and no one even talks about that - we just filed a new patent to license to Campbell (note to self, draft license and send to Kalina). Were about to launch the first of several new spin-offs to accommodate those IND's and working on many new patents yet to be filed.
I hope you all can find excitement in other things while we do our things behind the scenes...
https://forum.therapeuticsolutionsint.com/viewtopic.php?t=384&start=15
by TimGDixon » Wed Sep 21, 2022 4:23 am
Thanks - just wrapping up the S3 and hopefully file by the end of week - that depends on audit and legal clearance but all underway and then we wait on SEC to approve... there's a new buyer and seller in town and the times, they are a changin'...
https://forum.therapeuticsolutionsint.com/viewtopic.php?t=384&start=15
their forum has the drift
https://forum.therapeuticsolutionsint.com/viewtopic.php?t=385
TSOI I believe Dixon has already back tracked from on his own private forum
Tim already said it isn't gonna show anywhere
-until they publish the results .
you guys need to watch their forum more
Tim already said it isn't gonna show anywhere
-until they publish the results .
you guys need to watch their forum more
Can somewon show me the P3 Jadicell trial on the FDA clinical trials website. I have some wealthy chinese firms looking
I wonder how many insiders DIDN’T dump yesterday? I will mark my calendar as well!
yesterdays VWAP created some more angry shareholders I bet
I wonder how many insiders dumped yesterday ... dates are marked on my caleneder, looking for form 4 ... plus other thing.s
No followthru ??? Cmon Tim Dixon yu know the drill .
I wish Tim gifted his faithfools shares, I'd have a lot more. I just believe in TSOI, I believe that they will help people around the world. I also hold strong even when there's ups and downs.
Just think of the tens of thousands of dollars people made from this TSOI pump n dump over the last 24 hours! Don’t ever sleep in late because you never know when the penny stock press releases are gonna show up! I am gonna go free motorcycle shopping thanks to all of TSOI managements hard work…
It’s a pump and dump! Everyone knows it. A handful of faithfools on a forum trust him.....wonder how many shares they were gifted for their loyalty. Sad.
We are living in money crisis globally atm.
( YET )
$10,000,000 DOLLARS SECURED FUNDING TO COMPLETE PHASE3 CLINICAL TRIAL..
Data must be rock solid and efficacy must be concrete...
This is just the beginning $TSOI.
Ya BDEZ, and TSOI is #1 Therapeutic Solutions International Inc (TSOI) on the IHub BOB right now ![]()
https://investorshub.advfn.com/boards/breakoutboards.aspx
CLINICAL TRIALS & FUNDING
Not long ago our good friend TT posted all the achievements of TSI without fundings. Around 2.5B shares were issued in about 10 years. I know of companies that just issued 2.8B shares in 3 months and are trading at $0.001, unlike TSI, which speaks of the great capabilities of our CEO Tim Dixon, Thomas, James and the BOD.
Let's see what 2.5B shares did for TSOI, this is without fundings...
65 filed patents
20 published patents
3 issued patents
(5) INDs w #’s
JadiCell/CTE Excl license
Treated (5) Nsvy SEALS successfully/CTE via RTT
International Amazon Prime sales
StemVacs Platform
Campbell Neurosciences
Allogen Biologics
CampbellCell
Campbell Score
ApoptoCyte
NanoStilbene
QuadraMune
Projuvenol
NanoCannabidiol
NanoPlus
NanoPSA
DermalStilbene
Now having $10M available, besides the ongoing Phase III clinical trial, do you know how many other potential clinical trials are waiting in line? And for a company to get a shot for a Clinical Trial, the inventors gotta be really really good at it, let's take a look of what could go thru clinical trials and how effective they are...
THERAPEUTIC MONOCYTES FOR PREVENTION OF SUICIDAL IDEATION
Type: Application
Filed: March 4, 2022
Publication date: September 8, 2022
Applicant: Therapeutic Solutions International, Inc.
Inventors: Thomas E. Ichim, Timothy G. Dixon, Famela Ramos, Kalina O'Connor, James Veltmeyer
Suppression of Inflammation Induced Memory Dysfunction by Cord Blood Derived Monocytes
The lipopolysaccharide administration model was used to replicate neuroinflammation associated with suicidal behavior. Female BALB/c mice were treated with control, daily lipopolysaccharide treatment (10 ng/mouse0, and some with cord blood derived monocytes. Cord blood derived monocytes were extracted by ficoll separation of cord blood mononuclear cells and 4-hour incubation on T175 plastic flasks. Non-adherent cells were washed off by rinsing with phosphate buffered saline and adherent cells where subsequently dissociated using trypsin and placed into another flask. Cord blood monocytes where cultured for 24 hours with 1 IU of oxytocin per million cells in complete DMEM media with 10% fetal calf serum. Cells were dissociated with trypsin, washed in phosphate buffered saline and administered intravenously vial tail vein at a concentration of 500,000 per mouse on days 0, 3, and 6.
To assess memory function, water filled basin which was 120 cm in diameter was broken into 4 quadrants. 10 cm diameter platform placed 1 cm below water. Mice were forced to swim to find the hidden platform, starting from all four different quadrants, each day for a total of 7 days. As seen below, mice receiving umbilical cord monocytes resisted the pathological effects of lipopolysaccharide treatment.
Suppression of LPS Induced Plasma Interleukin-6 by Cord Blood Derived Monocytes
The lipopolysaccharide administration model was used to replicate neuroinflammation associated with suicidal behavior. Female BALB/c mice were treated with control, daily lipopolysaccharide treatment (10 ng/mouse0, and some with cord blood derived monocytes. Cord blood derived monocytes were extracted by ficoll separation of cord blood mononuclear cells and 4-hour incubation on T175 plastic flasks. Non-adherent cells were washed off by rinsing with phosphate buffered saline and adherent cells where subsequently dissociated using trypsin and placed into another flask. Cord blood monocytes where cultured for 24 hours with 1 IU of oxytocin per million cells in complete DMEM media with 10% fetal calf serum. Cells were dissociated with trypsin, washed in phosphate buffered saline and administered intravenously vial tail vein at a concentration of 500,000 per mouse on days 0, 3, and 6.
To assess interleukin 6 levels in plasma, mice were sacrificed at the indicated timepoints and cytokine was assayed by ELISA.
EX VIVO GENERATION OF IMMUNOCYTES RECOGNIZING BROTHER OF THE REGULATOR OF IMPRINTED SITES (BORIS) EXPRESSING CANCER STEM CELLS.
Type: Application
Filed: February 22, 2022
Publication date: August 25, 2022
Applicant: Therapeutic Solutions International, Inc.
Inventors: Thomas E. Ichim, Timothy G. Dixon, Feng LIN, Famela Ramos, James Veltmeyer
EXAMPLES
Stimulation of Immunity to CD133 Positive PC-3 Cancer Stem Cells
Cord blood generated allogeneic dendritic cells were obtained by culture of adherent monocytes with GM-CSF (100 IU/ml) and IL-4 (100 IU/ml) for 7 days. Cells expressed CD11c and possessed typical dendritic morphology. Cells where incubated with full length BORIS protein and treated with 30 ng/ml Poly (IC) for 24 hours to induce dendritic cell maturation. Dendritic cells where cultured with allogeneic peripheral blood mononuclear cells for 7 days in the presence of IL-2 (10 IU/ml) and anti-CD3 and anti-CD28 beads (10(5) beads per ml. T cells where purified by Magnetic Activated Cell Sorting for CD3 (containing both CD8 and CD4 cells). PC-3 prostate cancer cells where cultured and separated into either control (no T cells added), unfractionated, CD133 negative and CD133 positive. Cells where co-cultured at a 1:1 ratio and cytotoxicity was determined by MTT assay.
As observed, T cells possessed a preferential ability to kill CD133+ cells, which corresponds to a cancer stem cell phenotype. Results are shown in FIG. 1.
Stimulation of Immunity to Fik Positive PC-3 Cancer Stem Cells
Cord blood generated allogeneic dendritic cells were obtained by culture of adherent monocytes with GM-CSF (100 IU/ml) and IL-4 (100 IU/ml) for 7 days. Cells expressed CD11c and possessed typical dendritic morphology. Cells where incubated with full length BORIS protein and treated with 30 ng/ml Poly (IC) for 24 hours to induce dendritic cell maturation. Dendritic cells where cultured with allogeneic peripheral blood mononuclear cells for 7 days in the presence of IL-2 (10 IU/ml) and anti-CD3 and anti-CD28 beads (10(5) beads per ml. T cells where purified by Magnetic Activated Cell Sorting for CD3 (containing both CD8 and CD4 cells). PC-3 prostate cancer cells where cultured and separated into either control (no T cells added), unfractionated, flk negative and flk positive. Cells where co-cultured at a 1:1 ratio and cytotoxicity was determined by MTT assay.
As observed, T cells possessed a preferential ability to kill PC-3 flk+ cells, which corresponds to a cancer stem cell phenotype. Results are shown in FIG. 2.
Stimulation of Immunity to Rhodamine Positive PC-3 Cancer Stem Cells
Cord blood generated allogeneic dendritic cells were obtained by culture of adherent monocytes with GM-CSF (100 IU/ml) and IL-4 (100 IU/ml) for 7 days. Cells expressed CD11c and possessed typical dendritic morphology. Cells where incubated with full length BORIS protein and treated with 30 ng/ml Poly (IC) for 24 hours to induce dendritic cell maturation. Dendritic cells where cultured with allogeneic peripheral blood mononuclear cells for 7 days in the presence of IL-2 (10 IU/ml) and anti-CD3 and anti-CD28 beads (10(5) beads per ml. T cells where purified by Magnetic Activated Cell Sorting for CD3 (containing both CD8 and CD4 cells). PC-3 prostate cancer cells where cultured and separated into either control (no T cells added), unfractionated, Rhodamin negative and Rhodamin positive. Cells where co-cultured at a 1:1 ratio and cytotoxicity was determined by MTT assay.
As observed, T cells possessed a preferential ability to kill PC-3 Rhodamin+ cells, which corresponds to a cancer stem cell phenotype.
STIMULATION OF NATURAL KILL CELL MEMORY BY ADMINISTRATION OF DENDRITIC CELLS
Type: Application
Filed: February 8, 2022
Publication date: August 11, 2022
Applicant: Therapeutic Solutions International, Inc.
Inventors: Thomas E. ICHIM, Timothy G. DIXON, James VELTMEYER, Famela RAMOS
Example 1 Homotaurine Increases Ability of StemVacs to Stimulate NK Activity
Peripheral blood mononuclear cells where obtained by ficoll centrifugation and plated with StemVacs umbilical cord derived dendritic cells. StemVacs was generated by culture of umbilical blood adherent cells with interleukin 4 and GM-CSF for 7 days with maturation step induced by 24 hour culture TLR agonist. Assessment of natural killer cell activity was performed subsequent to culture of cells with taurine (10 micrograms/ml) or homotaurine (10 micrograms/ml) for the indicated timepoints. Cytotoxicity against NK target cell line was performed using the flow cytometry Promega assay. Results are shown in FIG. 1.
Example 2 Homotaurine Increases Ability of StemVacs to Stimulate T Cell Activity
Peripheral blood mononuclear cells where obtained by ficoll centrifugation and plated with StemVacs umbilical cord derived dendritic cells. StemVacs was generated by culture of umbilical blood adherent cells with interleukin 4 and GM-CSF for 7 days with maturation step induced by 24 hour culture TLR agonist. Assessment of T cell activity was performed subsequent to culture of cells with taurine (10 micrograms/ml) or homotaurine (10 micrograms/ml) for the indicated timepoints. T cell activity was assessed by ELISA quantification of interferon gamma production after stimulation with 5 micrograms per ml of phytohemagglutinin.
IMMUNOTHERAPY FOR OPIOID ADDICTION
Type: Application
Filed: December 20, 2021
Publication date: June 23, 2022
Applicant: Therapeutic Solutions International, Inc.
Inventors: Thomas Ichim, Timothy G. Dixon, Famela Ramos, Wais Kaihani, James Veltmeyer, Kalina O'Connor
Example 1: Reduction of Brain Microglial Activation by PRP and Pterostilbene
BALB/c mice were anesthetized with isofluorane and administered pterstilbene (0.4 mg/mouse) and/or platelet rich plasma (5 microliter per mouse) via intranasal route subsequent to induction of systemic inflammation by intraperitoneal administration of endotoxin. Mice were sacrificed after 24 hours of treatment and quantification of cytokine IL-18 was performed by ELISA from brain homogenate tissue. As seen below, a synergistic reduction of TNF-alpha was observed. Results are shown in FIG. 1.
Example 2: Reduction of Brain Microglial Activation by PRP and Oxytocin
BALB/c mice were anesthetized with isofluorane and administered oxytocin (0.1 IU/mouse) and/or platelet rich plasma (5 microliter per mouse) via intranasal route subsequent to induction of systemic inflammation by intraperitoneal administration of endotoxin. Mice were sacrificed after 24 hours of treatment and quantification of cytokine IL-18 was performed by ELISA from brain homogenate tissue. As seen below, a synergistic reduction of TNF-alpha was observed. Results are shown in FIG. 2.
TREATMENT OF MAJOR DEPRESSIVE DISORDER AND SUICIDAL IDEATIONS THROUGH STIMULATION OF HIPPOCAMPAL NEUROGENESIS UTILIZING PLANT-BASED APPROACHES
Type: Application
Filed: December 8, 2021
Publication date: June 9, 2022
Applicant: THERAPEUTIC SOLUTIONS INTERNATIONAL, INC.
Inventors: Thomas Ichim, Timothy G. Dixon, James Veltmeyer, Kalina O'Connor
Example
BALB/c mice where exposed to random stress 4 times a day by spinning by the tail for 15 seconds. Controls where not stressed. Stressed mice where treated with Prozac or QuadraMune™ daily. Assessment of endogenous neurogenesis was performed by administering BRDU and assessment of incorporation by histology. Augmented neurogenesis was seen in animals which received Prozac, with enhanced neurogenesis in animals taking QuadraMune™
PROTECTION AND REGENERATION OF NEUROLOGICAL FUNCTION BY USING STEM CELLS
Type: Application
Filed: October 27, 2021
Publication date: April 28, 2022
Applicant: Therapeutic Solutions International, Inc.
Inventors: Thomas E. Ichim, Timothy G. Dixon, Amit N. Patel, Famela Ramos, Kalina O'Connor
Examples
JadiCell Conditioned Media Inhibits Endothelial Death.
Injury associated with inflammation. Inflammation causes oxidative stress. Oxidative stress induces death of endothelial cells. Endothelial death exposes basement membrane, induces coagulopathy. Culture of HUVEC cells with upstream inflammatory agent TNF-alpha or downstream H2O2 results in death. Conditioned media (CM) from JadiCells decreases endothelial cell death. Results are shown in FIGS. 1 and 2.
JadiCell™ Reduces Immunogenicity of Endothelial Cells
Endothelial cells can act as antigen presenting cells. Stimulation of T cells by endothelial cells results in inflammation and breaking of blood brain barrier. HLA expression on HUVEC was utilized to quantify one aspects of endothelial antigen presentation. Mixed lymphocyte reaction used as a test of T cell activation. Results are shown in FIGS. 3 and 4.
JadiCell™ Reduces Thrombogenicity of Activated Endothelial Cells
In response to inflammation endothelial cells induce clotting by stimulation of extrinsic coagulation pathway. Tissue factor is activator of extrinsic pathway. HUVEC cells were treated with endotoxin to stimulate activation. Assessment of Tissue Factor performed by flow cytometry. Results are shown in FIG. 5.
CELLULAR, ORGAN, AND WHOLE-BODY REJUVENATION UTILIZING CORD BLOOD PLASMA AND PTEROSTILBENE
Type: Application
Filed: November 4, 2020
Publication date: May 6, 2021
Applicant: Therapeutic Solutions International, Inc.
Inventors: Thomas E. Ichim, Timothy G. Dixon
EXAMPLE
Cord Blood Plasma Suppression of Senescence Marker Beta Galactosidase is Augmented by Pterostilbene
Foreskin fibroblasts where obtained from ATCC and cultured according to manufacture instructions. Accelerated senescence was induced by exposure to indicated concentrations of H2O2 for 48 hours. Cells where cultured in control media (DMEM) or 10% cord blood plasma, or combination of cord blood plasma with 3 uMol Pterostilbene. Senescence was detected by fixing cells with 4% paraformaldehyde and SA-ß-Gal was stained using senescent cells histochemical staining kit (Sigma Aldrich, St. Louis, Mo., USA). Three images per each well were collected, and the SA-ß-Gal-stained cells were counted.
THIS IS JUST TO SHOW A FEW OF THE EXPERIMENTS AND RESULTS SEEN ON THE EXAMPLES, THOUGH TSOI ALREADY HAD SUCCESSFUL TRIALS IN THE PAST, WITH THIS REPUTATION,.I HAVE NO DOUBTS THAT TSOI IS GOING TO SHAKE THE WHOLE BIOPHARMA INDUSTRY.
Nice day eh.....
TSOI
Totally agree Cents, it won't be too long.
with a 34m m/c really holding us down
A2Z...IMO it's the ultra high O/S that is holding the pps down. There is no quick solution to that.
just to have a record as of 8k filing & PR
Authorized Shares 3,500,000,000 09/07/2022
Outstanding Shares 2,565,802,627 09/07/2022
Restricted 1,171,830,128 09/07/2022
Unrestricted 1,393,972,499 09/07/2022
Held at DTC 1,350,524,672 09/07/2022
https://www.otcmarkets.com/filing/html?id=16088583&guid=v6--kKXJEDAIB3h
https://www.otcmarkets.com/stock/TSOI/news/story?e&id=2328196
Yes today was great news on family. Even though we supposedly had funding before but wasn’t going to be announced who it was till the 10 K we now have something possibly to work on to start the phase 3.
Of course tomorrow I do look for this to fall down in price per share as it’s only a penny stock. It will be interesting to see if they always show up on clinical trial.gov. I know we’re waiting on the FDA on the COPD news if it will be excepted into the phase 3 for the Covid I hope it is but again I’m not holding my breath it will leave it here by Thursday.
This stock has been beaten down so much and clobbered shorted and Cetra that it’s just that you don’t know what to believe anymore. I guess all of the selling today was from the ones that bought in the ones trying to unload some profit.
It amazes me that even with this great news of funding that we couldn’t even reach two cents how pathetic. In the past I have seen other bios get funding and one in particular rows from $.02- $.67 with only one product in mine. Here we have a great product that has been proven with many other products behind it coming up in time and put them all together 66 of them and we can’t reach two cents.
In my opinion with all this company has to offer we should be setting at a minimum of $.25 at least if not way more.
nice day for wise shareholders w/ liquidity & daily spread
Volume: 25,186,335 Day Range: 0.0101 - 0.0197
Dubs y Subs were so sweet...lil bank same # of shares w/ an overall lower cost basis
#3 Therapeutic Solutions International Inc (TSOI), on the IHub BOB, Lookin good doog ![]()
https://investorshub.advfn.com/boards/breakoutboards.aspx
Good job TSOI News: Current Report Filing (8-k)
https://ih.advfn.com/stock-market/USOTC/therapeutic-solutions-TSOI/stock-news/89104715/current-report-filing-8-k
Right perfect understanding of what truly defines the float
Any seasoned investor knows that the actual float is what is Held at DTC 1,350,524,672 09/07/2022.
So much avoidance & deflection when the answer is quite simple & EZ to find
Held at DTC 1,350,524,672 9/07/2022
Float 303,593,877 08/16/2019
yea
1,350,524,672 is what matters its what is out in the float
Nice list yet to save time & perhaps inspire the investing public how much revenue to date have all those Patents (Intellectual Property(s)) added to the TSOI Coffers since they 1st started On July 8, 2015, up till On September 19, 2022,
https://www.otcmarkets.com/filing/html?id=16030841&guid=JF--kWqp5N5HJth
https://www.otcmarkets.com/stock/TSOI/disclosure
links provided for factual data
I also believe today's news is a positive advancement while now the investing public does not have to wait on the 10K to find out who the funder is. Wonder what started & why we had that 10k quote from ......CEO Timothy G Dixon
I've found over the decades that any company by just having an honest transparent CEO usually serves both the company & investing public quite well as it relates to instilling investor confidence in both the aforementioned. (company/management)
from their website :
https://therapeuticsolutionsint.com/patents/
Intellectual Property
On September 19, 2022, the Company filed a patent application titled “Treatment of Bipolar Disorder Using Mesenchymal Stem Cells and Modification of Mesenchymal Stem Cells” that discloses the utilization of mesenchymal stem cells, exosomes from mesenchymal stem cells, conditioned media from mesenchymal stem cells, apoptotic bodies from mesenchymal stem cells, and modified mesenchymal stem cells for treatment of bipolar disorder. In one embodiment mesenchymal stem cells isolated from umbilical cord tissue are treated with carbon monoxide at a concentration sufficient to induce activation of heme-oxygenase I and infused into a patient at risk or suffering from bipolar disorder.
On September 12, 2022, the Company filed a patent application titled “Treatment of COPD by Stimulation of Stem Cell Mobilization” which discloses means of inducing pulmonary regeneration and/or protection from oxidative stress by stimulation of endogenous stem cell mobilization together with one or more inhibitors of NF-kappa B and/or one or more inhibitors of oxidative stress. The invention discloses the unexpected finding that G-CSF administration enhances oxidative stress and pulmonary damage, however, coadministration with pterostilbene, results in synergistic suppression of COPD pathology.
On August 29, 2022, the Company filed a patent application titled “Gene Silencing Therapy of Acute Respiratory Disorder” that teaches treatment means, compositions of matter and protocols useful for suppression of acute respiratory disorder (ARDS) through induction of RNA interference in the pulmonary microenvironment alone and/or in conjunction with mucolytic and/or DNA disrupting agents. In one embodiment short interfering RNA (siRNA) is prepared which targets complement receptors C3R and/or C5R together with TNF-receptor, IL-6 receptor and/or TLR4 and TLR9. In some embodiments NanoStilbene is utilized as a delivery vehicle for siRNA delivery.
On August 12, 2022, the Company filed a patent application titled “Treatment of Chronic Obstructive Pulmonary Disease by Mesenchymal Stem Cell Apoptotic Bodies and Compositions Thereof” that discloses means, treatments and compositions of matter useful for treatment of chronic obstructive pulmonary disease (COPD). In one embodiment the invention provides the administration of mesenchymal stem cell apoptotic bodies alone or in combination with “regenerative adjuvants” to prevent and/or reverse reduction in lung function associated with COPD. In other embodiments the invention teaches the utilization of stem cell apoptotic bodies for induction of pulmonary regeneration directly or indirectly.
On July 29, 2022, the Company filed a patent application titled “Gene Modified iPSC Derived Cellular Compositions for Regeneration and Immune Modulation” that disclosed cells and cellular compositions useful for treatment of degenerative and/or autoimmune diseases derived from gene edited/gene modified pluripotent stem cells. In one embodiment pluripotent stem cell such as inducible pluripotent stem cells are gene modified to express tissue associated transcription factors such as pdx-1 if endodermal tissue is desired and cells are differentiated into regenerative-type cells such as along the mesenchymal lineage. In one embodiment the invention teaches transfection with IL-27 to induce expression of coinhibitory molecules for suppression of autoimmunity. In some embodiments the invention provides generation of iPSC derived MSC which cannot stimulate inflammation due to gene-editing based removal of inflammatory associated transcription factors.
On May 12, 2022, the Company filed a patent application titled “Inhibition and Reversion of Chronic Obstructive Pulmonary Disease (COPD) by Endothelial Cell Regeneration” that teaches means, treatment methods, and compositions of matter useful for prevention and/or reversion of chronic obstructive pulmonary disease (COPD). In one embodiment the invention provides the administration of mesenchymal stem cells and exosome thereof as a means of augmenting endogenous endothelial regeneration and/or endothelial regeneration stimulated by exogenous means. In some embodiments the invention provides administration of allogeneic mesenchymal stem cells together with autologous endothelial progenitor cells and/or mobilization of said autologous endothelial progenitor cells.
On March 7, 2022, the Company filed a patent application titled “Treatment of Trauma Associated Cognitive Dysfunction Using Mesenchymal Stem Cell Apoptotic Bodies and Compositions Thereof” which teaches means, treatments and compositions of matter useful for treatment of chemotherapy/radiotherapy associated cognitive dysfunction. In one embodiment the invention provides the administration of mesenchymal stem cell apoptotic bodies alone or in combination with “regenerative adjuvants” to prevent and/or reverse cognitive dysfunction associated with chemotherapy and/or radiation therapy. In other embodiments the invention teaches the utilization of stem cell apoptotic bodies for induction of neuroregeneration directly or indirectly.
On February 7, 2022, the Company filed a patent application titled “Treatment of COVID-19 Associated Cognitive Dysfunction by Nutraceutical Preparations” that teaches means and methods of treating cognitive dysfunction associated with COVID-19 and/or other associated with inflammatory conditions. In one embodiment treatment of COVID-19 cognitive dysfunction performed by administration of nutraceutical means, wherein said nutraceuticals are administered at a frequency and/or concentration sufficient to induce proliferation of endogenous neural progenitor cells and/or protect cells from inflammatory damage. In one embodiment said nutraceuticals are comprised of green tea extract, and/or nigella sativa, and/or pterostilbene, and/or sulforaphane. In some embodiments nutraceutical compositions are utilized to overcome treatment resistant of currently used antidepressants.
On November 1, 2021, the Company filed a patent application titled “Induction of Concurrent Pulmonary Immune Modulation and Regeneration by Protein Mediated Conjugation of Immune Regulatory Cells with Endogenous Progenitor Cells” that discloses means, methods and compositions of matter useful for treatment of inflammatory pulmonary diseases such as COVID-19 through administration of agents that facilitate interaction between immune modulatory cells and endogenous pulmonary progenitor cells. In one embodiment a bispecific antibody capable of facilitating the interaction between CD25 on T regulatory cells and CD47 on pulmonary epithelial stem cells is described.
On October 11, 2021, the Company filed a patent application titled “Umbilical Cord Derived Regenerative and Immune Modulatory Stem Cell Populations” which provides universal donor cellular populations derived from umbilical cords possessing ability to elicit immune modulation and evoke regeneration when administered into a mammalian host. Generation of cellular products for clinical use are provided including methodologies of expansion, characterization, and means of therapeutic implementation.
On October 4, 2021, the Company filed a patent application titled “Reduction of Neutrophil Extracellular Trap formation by Mesenchymal Stem Cells and their Exosomes” that disclosed methods of reducing lung inflammation in acute respiratory distress syndrome elicited by various factors such as COVID-19 infection by reduction of neutrophil extracellular trap formation through administration of mesenchymal stem cells and/or exosomes thereof. The invention provides means of inhibiting neutrophil release of extracellular traps by mesenchymal stem cells and/or exosomes derived from said mesenchymal stem cells. Additionally, synergies are provided between mesenchymal stem cells and/or exosomes derived from mesenchymal stem cells and agents approaches which reduce neutrophil extracellular trap formation.
On September 22, 2021, the Company filed a patent application titled “Stimulation of Mesenchymal Stem Cell Therapeutic Activities by T Regulatory Cells” teaches novel means of enhancing mesenchymal stem cell regenerative activities including, intra alia, production from pulmonary leakage and suppression of scar tissue formation by co-administration with T regulatory cells. In some embodiments the invention provides an interaction between T regulatory cells and mesenchymal stem cells in which T regulatory cells stimulate upregulation of mesenchymal stem cell activity in a GITR dependent manner.
On September 16, 2021, the Company filed a patent application titled “Ivermectin Compositions for Treatment of COVID-19” that discloses novel mechanisms of action of ivermectin therapy as related to treatment of COVID-19 and means of augmenting therapeutic activities by co-administration with one or more of the following: pterostilbene, thymoquinone, epigallocatechin-3-gallate, and sulforaphane. In one embodiment the invention provides enhanced reduction of inflammation induced pulmonary leakage without augmenting immune suppressive mechanisms.
On August 23, 2021, the Company filed a patent application titled “Umbilical Cord Mesenchymal Stem Cells for Treatment of Chronic Obstructive Pulmonary Disease and Lung Degeneration” that discloses means of treating lung degenerative diseases including chronic obstructive pulmonary disease (CODP) using umbilical cord mesenchymal stem cells such as JadiCells alone, and/or using said cells under conditions that are activated in order to endow enhanced regenerative activity. In one embodiment said activation of said mesenchymal stem cells is performed through stimulation with a toll like receptor agonist at a concentration and duration sufficient to induce a >50% increase in keratinocyte growth factor expression from said stem cells. In another embodiment the invention provides the use of JadiCells as a means of producing exosomes, wherein said exosomes possess therapeutic properties capable of reducing inflammation, fibrosis and degeneration associated with COPD, as well as stimulation of regenerative activity. In some JadiCells are activated by a treatment with Activated Protein C.
On August 18, 2021, the Company filed a patent application titled “Enhancement of Umbilical Cord Mesenchymal Stem Cell Therapeutic Activity by Stimulators of T Regulatory Cells and/or Cells Expressing CD73” that teaches compositions of matter and protocols useful for treatment of COVID-19 and/or other inflammatory pathologies through stimulation of T regulatory cells and/or T cells expressing CD73 using administration of umbilical cord derived mesenchymal stem cells such as JadiCells. In one embodiment dosage of JadiCells needed to treat a patient is determined by the increase of T regulatory cells and/or CD73 expressing cells that are increased in number and/or activity subsequent to a test dose of JadiCells. In another embodiment stimulators of T regulatory cells and/or CD73 expressing T cells are utilized together with JadiCells in order to augment therapeutic activity. In some embodiments administration of JadiCell is performed with low dose interleukin-2 as a treatment for COVID-19 or other inflammatory related pathologies.
On August 11, 2021, the Company filed a patent application titled “Induction of Neurogenesis using Umbilical Cord Derived Mesenchymal Stem Cells and Derivatives Thereof” that disclosed compositions of matter and protocols useful for treatment of neurological dysfunctions through stimulation of adult neurogenesis using administration of umbilical cord derived mesenchymal stem cells such as JadiCells. In one embodiment viral induced neuropathy is reduced by administration of JadiCells to stimulate neurogenesis. In another embodiment the neurogenic activity of selective serotonin reuptake inhibitors is enhanced by administration of JadiCells. In some embodiments administration of JadiCell exosomes, conditioned media, microvesicles and/or apoptotic bodies is utilized to stimulate neurogenesis.
On July 6, 2021, the Company filed a patent application titled “Treatment of Parkinson’s Disease by Immune Modulation and Regenerative Means” in which we describe and disclose means, methods and compositions of matter for treatment Parkinson’s Disease through concurrent immune modulation and regenerative means. In one embodiment Parkinson’s Disease is treated by augmentation of T regulatory cell numbers and/or activity while concurrently providing regenerative cells such as mesenchymal stem cells, and/or dopamine secreting cells. In one embodiment administration of immunoglobulins such as IVIG together with low dose interleukin-2 and/or low dose naltrexone is disclosed as a preparatory means prior to administration of therapeutic cells such as stem cells. Other therapeutic means utilized in an adjuvant manner are also provided for hormonal rebalancing, transcranial magnetic stimulation, and deep brain stimulation.
On May 24, 2021, the Company filed a patent application titled “Immunotherapies for Targeting of Tumor Vasculature” that disclosed novel means, protocols, and compositions of matter for creating targeted immune responses and/or induction of immunological memory towards the tumor vasculature. In one embodiment pluripotent stem cells are transfected with one or more genes capable of eliciting immunity, induced to differentiate into endothelial-like cells which resemble the tumor endothelial cells, and utilized as a vaccine. In some embodiment’s genes are engineered under control of specific promoters to allow for various specificities of activity. In one specific embodiment pluripotent stem cells engineered to endow properties capable of inducing expression of the a- Gal epitope (Gala1,3Gala1,4GlcNAc-R). Addition of adjuvants to enhance antigen presentation of the vaccine composition, as well as means of stimulating systemic enhancement of circulating endothelial specific T cells are also disclosed.
On May 21, 2021, the Company filed a patent application titled “Lithium as a Monotherapy and/or Stem Cell Adjuvant Therapy for Pulmonary Fibrosis” that disclosed compositions of matter, therapeutics, and protocols useful for reduction and/or reversion of pulmonary fibrosis. In one specific embodiment lithium chloride is administered together with a regenerative cell in a patient suffering from, or at risk of pulmonary fibrosis. In one embodiment said lithium chloride is administered as an adjuvant to a regenerative therapy, wherein said regenerative therapy is a gene therapy, a protein therapy, a cell therapy, or a tissue transplant. In one embodiment lithium chloride, or a salt thereof is utilized alone, or with a regenerative means, to evoke preservation and/or elongation of telomere length in pulmonary tissue. In one embodiment the invention teaches administration of umbilical cord mesenchymal stem cells (MSC) and/or products derived from said cells in order to induce an inhibition of natural or pathological reduction of telomere length, to preserve telomere length or to enhance telomere length. In one embodiment the MSC described in the invention as useful are umbilical cord derived MSC.
On May 17, 2021, the Company filed a patent application titled “Treatment of Major Depressive Disorder by Low Dose Interleukin-2” which teaches methods, compositions of matter, and protocols useful for treatment of major depressive disorder through administration of low dose interleukin- 2 at a concentration and/or frequency sufficient to increase expansion of T regulatory cell numbers and/or enhancement of T regulatory cell activity. In some embodiments administration of interleukin-2 is provided as means of enhancing efficacy of standard antidepressant therapies. Furthermore, administration of interleukin-2 receptor agonists is also described in the current invention as a treatment of major depressive disorder.
On April 13, 2021, the Company filed a patent application titled “Amelioration and Treatment of Opioid Addiction” that discloses compositions of matter, protocols and treatment means for reducing and/or preventing opioid addiction. In one embodiment the invention teaches intranasal administration of umbilical cord blood plasma, or extracts thereof, together with pterostilbene or pterostilbene containing nanoparticles, and/or oxytocin, and/or human chorionic gonadotropin.
On March 29, 2021, the Company filed a patent application titled “Compositions Capable of Stimulating Immunity Towards Tumor Blood Vessels” which discloses novel means, protocols, and compositions of matter for eliciting an immune response against blood vessels supplying neoplastic tissue. In one embodiment pluripotent stem cells are transfected with one or more genes capable of eliciting immunity. In some embodiments such genes are engineered under control of specific promoters to allow for various specificities of activity. In one specific embodiment pluripotent stem cells engineered to endow properties capable of inducing expression of the a-Gal epitope (Gala1,3Gala1,4GlcNAc-R).
On March 23, 2021, the Company filed a patent application titled “Chimeric Cells Comprising Dendritic Cells and Endothelial Cells Resembling Tumor Endothelium” which disclosed are means, methods and compositions of matter useful for induction of immunological responses towards tumor endothelial cells. In one embodiment the invention teaches fusion of dendritic cells and cells resembling tumor endothelial cells and administration of such chimeric cells as an immunotherapy for stimulation of tumor endothelial cell destruction. In other embodiments pluripotent stem cells are utilized to generate dendritic cells, wherein said dendritic cells are fused with pluripotent stem cell derived endothelial cells created in a manner to resemble tumor endothelial cells.
On March 16, 2021, the Company filed a patent application titled “Pluripotent Stem Cell Derived Dendritic Cells and Engineered Dendritic Cells for Cancer Immunotherapy” which disclosed are populations of dendritic cells generated from stem cells capable of inducing immunity towards cancer. In one embodiment said dendritic cells are generated from allogeneic inducible pluripotent stem cells, for some uses, said pluripotent stem cells are genetically engineered/edited to induce cancer specific immunity and/or resist immunosuppressive effect of tumor derived microenvironment. In one embodiment pluripotent stem cells are transfected with cancer stem cell antigens such as BORIS and/or NR2F6.
On March 4, 2021, the Company filed a patent application titled “Therapeutic Monocytes for Prevention of Suicidal Ideation” that discloses ompositions of matter, protocols, and therapeutic means for treatment of suicidal ideations and/or suppression of suicidal attempts. In one embodiment the invention provides the use of umbilical cord derived monocytes as a means of treatment. In another embodiment, monocytes are de-differentiated from adult monocytes using reprogramming means to create monocyte capable of producing anti-inflammatory as well as regenerative properties useful in reducing suicidal ideations and/or attempts. Published on September 8, 2022 https://patents.justia.com/patent/20220280574
On February 2, 2021, the Company filed a patent application titled “Ex Vivo Generation of Immunocytes Recognizing Brother Of The Regulator of Imprinted Sites (BORIS) Expressing Cancer Stem Cells” that discusses means, methods and compositions of matter useful for induction of immunity towards cancer stem cells by providing a dendritic cell, wherein said dendritic cells express BORIS and/or peptides derived from BORIS, wherein said dendritic cell is cultured in the presence of one or more immunocytes. In one embodiment said dendritic cells are derived from umbilical cord blood sources and allogeneic to T cells, which are expanded ex vivo and used for the purposes of immunotherapy. Published on August 25, 2022 https://patents.justia.com/patent/20220267730
On February 8, 2021, the Company filed a patent application titled “Stimulation of Natural Kill Cell Memory by Administration of Dendritic Cells” which disclosed means, methods and compositions of matter useful for induction of natural killer cell memory by administration of dendritic cells and/or exosomes thereof. In one embodiment a mammal suffering from cancer is administered allogeneic cord blood derived dendritic cells that are not pulsed exogenously. In one embodiment the dendritic cells are stimulated to possess chemotactic activity towards the tumor by culture of dendritic cell progenitors in hypoxia. Natural killer cell memory is induced, in part, by triggering of upregulation of cytokines associated with homeostatic expansion such as interleukin 7 and interleukin 15. Published on August 11, 2022 https://patents.justia.com/patent/20220249551
On January 26, 2021, the Company filed a patent application titled “Stimulation of Dendritic Cell Activity by Homotaurine and Analogues Thereof” which discloses means, methods, and compositions of matter useful for enhancement of dendritic cell activity. In one embodiment the invention provides the use of GABA agonists such as homotaurine for stimulation of dendritic cell activity. In one embodiment said dendritic cell activity is enhancement of natural killer cell activity and/or of T cell activity. In one embodiment NK cell activity is ability to induce cytotoxicity in neoplastically transformed cells, whereas T cell activity is either cytokine production for CD4 cells or cytotoxicity for CD8 cells. Published on July 28, 2022 https://patents.justia.com/patent/20220235325
On December 21, 2020, the Company filed a patent application titled “Immunotherapy for Opioid Addiction” which teaches means, methods and compositions of matter useful for reduction of brain inflammation and prevention of opioid addiction and/or tolerance. In one embodiment the invention provides utilization of platelet rich plasma (PRP), alone, or admixed with regenerative/anti-inflammatory adjuvants, for reduction of neural inflammation. In one embodiments PRP is admixed with oxytocin and administered intranasally in a patient at risk of opioid addiction. In another embodiment, PRP is admixed with fortified and non-fortified nigella sativa oil, and/or pterostilbene and administered intranasally. Other embodiments include utilization of autologous stromal vascular fraction cells alone and/or admixed with regenerative/anti-inflammatory adjuvants. Published on June 23, 2022, https://patents.justia.com/patent/20220193127
On December 8, 2020, the Company filed a patent application titled “Treatment of Major Depressive Disorder and Suicidal Ideations Through Stimulation of Hippocampal Neurogenesis Utilizing Plant-Based Approaches” that teaches means and methods of treating major depressive disorder and/or other disorders that predispose to suicide by administration of nutraceutical means, wherein said nutraceuticals are administered at a frequency and/or concentration sufficient to induce proliferation of endogenous neural progenitor cells. In one embodiment said nutraceuticals are comprised of green tea extract, and/or nigella sativa, and/or pterostilbene, and/or sulforaphane. In some embodiment’s nutraceutical compositions are utilized to overcome treatment resistant of currently used antidepressants. Published on June 9, 2022 https://patents.justia.com/patent/20220175701
On November 24, 2020, the Company filed a patent application titled “Stimulation of NK Cell Activity by QuadraMune Alone and together with Metformin” that disclosed means, compounds, and compositions of matter useful for stimulation of natural killer cell activity. In some embodiments the invention teaches the administration of a therapeutic combination of ingredients comprising of metformin, pterostilbene, nigella sativa, sulforaphane, and epigallocatechin-3-gallate (EGCG) to a mammal in need of natural killer cell immune modulation. In another embodiment, the invention teaches administration of said therapeutic combination to a mammal infected with said SARS-CoV-2. In some embodiments dosage of said therapeutic combination is based on inflammatory and/or immunological parameters observed in patients with COVID-19. Published on May 26, 2022 https://patents.justia.com/patent/20220160809
On October 27, 2020, the Company filed a patent application titled “Protection/Regeneration of Neurological Function by Endothelial Protection/Rejuvenation” using Stem Cells for Treatment of Conditions such as Chronic Traumatic Encephalopathy and Schizophrenia” which therapeutic compounds, protocols, and compositions of matter useful for treatment of neurological conditions. In one embodiment the invention teaches the treatment of chronic traumatic encephalopathy (CTE) through protecting/regenerating the endothelial by administration of cells such as stem cells. In one embodiment stem cells are administered in order to protect the endothelium from apoptosis and to preserve the blood brain barrier. In another embodiment stem cells are administered together with endothelial progenitor cells in order to regenerate neural endothelium. In other embodiments preservation of brain integrity in conditions of degeneration is accomplished by administration of stem cells and/or endothelial cells. Published on April 28, 2022 https://patents.justia.com/patent/20220125852
On October 18, 2020, the Company filed a patent application titled “Nutraceutical Reduction Prevention and/or Reversion of Multiple Sclerosis” that discloses compositions of matter, protocols, and treatment means for preventing and/or reversing multiple sclerosis in a mammal. In one embodiment administration of compositions containing pterostilbene, and/or nigella sativa, and/or sulforaphane, and/or epigallocatechin-3-gallate (EGCG) are provided. Published on June 23, 2022 https://patents.justia.com/patent/20220193170
On September 24, 2020, the Company filed a patent application titled “Personalized Immunotherapies for Reduction of Brain Inflammation and Suicide Prevention” that discloses means, methods and compositions of matter useful for reduction of brain inflammation and prevention of suicidal ideations and suicidal attempts. In one embodiment the invention provides utilization of autologous platelet rich plasma, alone, or admixed with regenerative/anti-inflammatory adjuvants, for reduction of neural inflammation. In one embodiment autologous PRP is admixed with oxytocin and administered intranasally in a patient at risk of suicidal ideation. In another embodiment, PRP is admixed with fortified and non-fortified nigella sativa oil and administered intranasally. Other embodiments include utilization of autologous stromal vascular fraction cells alone and/or admixed with regenerative/anti-inflammatory adjuvants. Published on March 24, 2022 https://patents.justia.com/patent/20220088086
On September 14, 2020, the Company filed a patent application titled “Immunotherapy of Schizophrenia and Schizophrenia Associated Suicidal Ideation/Suicide” Disclosed are methods, means, and protocols of modifying the immune system so as to induce an immunologically tolerant state insofar as T regulatory cell number and/or activity is augmented in a patient suffering from schizophrenia. In one embodiment T regulatory cells are administered to the patient from exogenous sources, be they allogeneic or autologous. In other embodiments, T regulatory cells are generated endogenously through administration of immature dendritic cells, mesenchymal stem cells, and/or pharmaceutical means.
On August 28, 2020 the Company filed a patent application titled “Upregulation of Therapeutic T Regulatory Cells and Suppression of Suicidal Ideations in Response to Inflammation by Administration of Nutraceutical Compositions Alone or Combined with Minocycline” which discloses compositions of matter, treatments and protocols useful for induction of T regulatory cells in response to inflammation, as well as inhibition of suicidal ideations and/or neuroinflammation. In some embodiments the invention teaches the administration of a therapeutic combination of ingredients comprising of minocycline, pterostilbene, nigella sativa, sulforaphane, and epigallocatechin-3-gallate (EGCG) to a mammal undergoing upregulation of inflammatory mediators. Published on March 3, 2022 https://patents.justia.com/patent/20220062367
On August 21, 2020 the Company filed a patent application titled “Methods of Determining Risk of Suicide and/or Suicidal Ideation by Immunological Assessment” which discloses means and methods of identifying risk of suicide and/or suicidal ideation by assessment of immunologically related cytokines and cells. In one embodiment, a score, termed the “Campbell Score” is devised based on assessment of serum cytokines, ability of immune cells to make cytokines when stimulated ex vivo, and ability of immune cells to produce neurotransmitters when stimulated ex-vivo. In one embodiment the concentration of interleukin-6 is utilized as a means of assessing suicidal propensity along, and/or in combination with metabolites of the enzyme indolamine 2,3 deoxygenase.
On August 05, 2020 the Company filed a patent application titled “Prevention of Neuroinflammation associated Memory Loss Using Nutraceutical Compositions” which discloses means, methods, and therapeutic compositions for prevention of memory loss during situations of neuroinflammation. In one embodiment the invention teaches administration of the therapeutic combination of ingredients comprising of pterostilbene, Nigella sativa, sulforaphane, and epigallocatechin-3-gallate (EGCG) to a mammal suffering from inflammation in order to preserver memory function. Published on February 10, 2022 https://patents.justia.com/patent/20220040248
On July 28, 2020, the Company filed a patent application titled “Neuroprotection and Neuroregeneration by Pterostilbene and Compositions Thereof” with new data demonstrating that the blueberry derived compound pterostilbene possesses numerous brain protective and potentially brain regenerative activities. The data disclosed by the Company indicates: a) pterostilbene suppresses inflammatory cytokines TNF-alpha, IL-1 beta and IL-6; b) pterostilbene inhibits death of neurons caused by inflammatory mediators; c) pterostilbene stimulates production of regenerative factors from cells in the brain such as BDNF, NGF, FGF-1, and FGF-2; and d) pterostilbene allows/enhances proliferation of endogenous brain stem cells. Published on February 3, 2022 https://patents.justia.com/patent/20220031793
On July 22, 2020, the Company filed a patent application titled “Additive and/or Synergistic Combinations of Metformin with Nutraceuticals for the Prevention, Inhibition and Treatment of SARS-Cov-2 and Associated COVID-19” showing potent synergy between QuadraMune™ and the antidiabetic drug metformin in treating COVID-19 associated lung damage models. It was discovered that the ability of QuadraMune™ to protect the lungs from inflammation that resembles coronavirus-induced pathology is markedly amplified by concurrent administration of metformin. At a mechanistic level, it was shown that metformin increased the ability of QuadraMune™ to a) increase the number of “healing macrophages” (“M2” macrophages); b) augment production of anti-inflammatory and regenerative proteins; and c) suppress production of pathological inflammatory proteins. Published on January 27, 2022 https://patents.justia.com/patent/20220023237
On July 13, 2020, the Company filed a patent application titled “Prevention of Pathological Coagulation in COVID-19 and other Inflammatory Conditions” s directed to the utilization of pterostilbene, and/or nigella sativa extract, and/or sulforaphane, and/or Epigallocatechin gallate (EGCG) alone or in combination, for the prevention of pathological coagulation. In on embodiment a composition containing all four ingredients is administered to a patient at risk of hypercoagulation in order to prevent aberrant expression of pro-coagulation molecules and/or induce expression of molecules known to suppress coagulation. In one embodiment the invention teaches administration of pterostilbene, thymoquinone, sulforaphane, and EGCG as a means of decreasing expression of tissue factor. Published on May 12, 2022 https://patents.justia.com/patent/20220143123
On June 30, 2020, the Company filed a patent application titled “Augmentation of Natural Killer Cell Activity and Induction of Cytotoxic Immunity Using Leukocyte Lysate Activated Allogeneic Dendritic Cells: StemVacs™” which describes the process of preparing allogeneic dendritic cells utilizing a leukocyte lysate based approach. These data support development of StemVacs for conditions that would benefit from NK activation such as cancer and COVID-19. Published on March 31, 2022 https://patents.justia.com/patent/20220096542
On June 22, 2020, the Company filed a patent application titled “Treatment of SARS-CoV-2 with Dendritic Cells for Innate and/or Adaptive Immunity” that disclosed means, methods, and compositions of matter for prophylaxis and/or treatment of SARS-CoV-2 by administration of dendritic cells in a manner and frequency sufficient to induce activation of innate and/or adaptive immune responses. In one embodiment the invention teaches administration of dendritic cells pulsed with one or more innate immune stimulants in a manner endowing said dendritic cell with ability to induce augmentation of natural killer (NK) cell number and/or activity. In another embodiment the invention teaches the use of dendritic cells stimulated with innate immune activators in a manner to allow for uptake of viral particles and presentation of viral epitopes to T cells in order to stimulate immunological activation and/or memory responses. Published on December 23, 2021 https://patents.justia.com/patent/20210393681
On June 15, 2020, the Company filed a patent application titled “Nutraceuticals for Suppressing Indolamine 2,3 Deoxygenase” from new data showing QuadraMune™ significantly inhibited inflammation associated with memory impairment, as well as reduced levels of kynurenine. Elevation of kynurenine is associated with activation of indolamine 2,3 deoxygenase, an enzyme associated with inflammation and depression. Granted on January 25, 2022 https://patents.justia.com/patent/11229674
On June 11, 2020, the Company filed a patent application titled “Nutraceuticals for Reducing Myeloid Suppressor Cells” which disclosed compositions of matter, treatments and protocols useful for reduction of number and/or activity of myeloid suppressor cells (MSC). In some embodiments the invention teaches the administration of a therapeutic combination of ingredients comprising of pterostilbene, Nigella sativa, sulforaphane, and epigallocatechin-3-gallate (EGCG) to a mammal at possessing an increased number and/or activity of said MSC in which reduction of number and/or activity is desired. In another embodiment, the invention teaches administration of said therapeutic combination to a mammal infected with viral and/or bacterial infections and/or neoplasia. In some embodiments dosage of said therapeutic combination is based on inflammatory and/or immunological parameters observed in patients. Published on December 16, 2021 https://patents.justia.com/patent/20210386815
On May 11, 2020, the Company filed a patent application titled “Treatment of COVID-19 Lung Injury Using Umbilical Cord Plasma Based Compositions” which disclosed means, methods, and compositions of matter useful for the treatment of lung inflammation associated with viral and bacterial infections, as well as with systemic inflammation, through the administration of umbilical cord blood derived plasma-based compositions. In one embodiment the invention teaches administration of umbilical cord blood plasma together with pterostilbene, and/or sulforaphane, and/or thymoquinone, and/or Epigallocatechin gallate (EGCG) and/or n-acetylcysteine in an aerosolized manner to patients suffering from COVID-19 associated pulmonary deficiencies. In another embodiment, umbilical cord blood plasma is administered with immune-stimulatory agents in order to concurrently inhibit propagation of viral load in the lung while suppressing pulmonary deficiencies.
On May 4, 2020, the Company filed a patent application titled “Nutraceuticals for the Prevention, Inhibition and Treatment of SARS-Cov-2 and Associated COVID-19” which teaches compositions of matter, treatments and protocols useful for prevention of SARS-CoV-2 infection, as well as inhibition of viral propagation and acceleration of viral cure. In some embodiments the invention teaches the administration of a therapeutic combination of ingredients comprising of pterostilbene, nigella sativa, sulforaphane, and epigallocatechin-3-gallate (EGCG) to a mammal at risk of infection with SARS-CoV-2. In another embodiment, the invention teaches administration of said therapeutic combination to a mammal infected with said SARS-CoV-2. In some embodiments dosage of said therapeutic combination is based on inflammatory and/or immunological parameters observed in patients with COVID-19. Granted on March 8, 2022 https://patents.justia.com/patent/11266707
On November 4, 2019, the Company filed a patent application titled “Cellular, Organ, and Whole-Body Rejuvenation Utilizing Cord Blood Plasma and Pterostilbene” that disclosed methods, means, and protocols for stimulation of rejuvenation in single cells, organs, and organisms by administration of cord blood derived plasma, cord blood plasma concentrates, and cord blood derived exosomes together with pterostilbene. The invention describes the previously unexpected finding that addition of pterostilbene to cord blood enhances the rejuvenation properties of cord blood. Said rejuvenation properties include telomere preservation, reduction in beta galactosidase, and retention of cellular activities. Published on May 6, 2021 https://patents.justia.com/patent/20210128638
On September 9, 2019, the Company filed a patent application titled “Pterostilbene and Formulations Thereof for Protection of Hematopoiesis from Chemotherapy and Radiation” which disclosed compositions of matter useful for treatment and/or prevention of hematopoietic injury using pterostilbene and formulations thereof. In one embodiment nanoparticle delivered pterostilbene is administered subsequent to chemotherapy induced neutropenia in order to accelerate recovery of the hematopoietic compartment. In another embodiment, pterostilbene is provided concurrently with chemotherapy in order to concurrently assist the neoplasia killing action of the chemotherapy while protecting the bone marrow from suppression. In contrast to conventionally used agents that protect from neutropenia such as G-CSF and GM-CSF, the products disclosed can be chronically administered, thus allowing for concurrent use with chemotherapeutic or radiotherapeutic agents.
On January 21, 2019, the Company filed a patent application titled “Prevention and Reversion of Chronic Traumatic Encephalopathy through Administration of “Educated” Monocytes and Progenitors Thereof” that provides means of preventing and/or reversing chronic traumatic encephalopathy in a patient through the modulation of monocytes as well as monocytic progenitors. In one embodiment the invention teaches administration of monocytes that have been previously “educated” by exposure to mesenchymal stem cells in order to endow onto said monocytes properties associated with stimulation of neuroregenerative properties. In some embodiments monocytes are educated by treatment of monocytic progenitors with conditions capable of endowing anti-inflammatory and regenerative conditions, said conditions include culture with epigenetic modifying agents. In other embodiments, the invention teaches the manipulation of cord blood derived monocytes as a starting population of cells for education by culture with mesenchymal stem cells.
On January 21, 2019, the Company filed a patent application titled “Autologous Neurogenic Cells and Uses Thereof for Professional Athletes at Risk of Chronic Traumatic Encephalopathy” which disclosed are means, compositions of matter and methods of business for treating Chronic Traumatic Encephalopathy (CTE) using autologous primary cells and modified cells of autologous origin which have been banked. In one embodiment of the invention autologous dedifferentiation cells are generated and stored for future administration in patients which have suffered CTE. In other embodiments, dedifferentiated cells are differentiated into neurons or neuronal progenitor cells and subsequently administered locally or systemically or in a combination. In other embodiments autologous cells are maintained in an undifferentiated manner and/or neurologically differentiated state and utilized as a conditioning source in an extracorporeal circulatory system replicating clinical stage extracorporeal liver perfusion (ECLP) with substitution of autologous dedifferentiated, neurologically differentiated or a combination of said cells instead of hepatic cells.
On December 18, 2018, the Company filed a patent application titled “Treatment of Chronic Traumatic Encephalopathy via RNA Administration” which disclosed are protocols, treatment means, and compositions of matter useful for treatment of Chronic Traumatic Encephalopathy through administration of RNA or modified RNA molecules. In one embodiment said RNA is generated to activate various toll like receptors (TLR), of which said activation leads to production of cytokines which paradoxically lead to protection from Chronic Traumatic Encephalopathy, wherein said protection constitutes a) reduction in glial cell activation, b) neuronal apoptosis due to excitotoxicity; and c) stimulation of endogenous regenerative processes including endothelial progenitor cell mobilization, proliferation of neuronal progenitor cells in the dentate gyrus and subventricular zones. In one particular embodiment targeting of RNA molecules is performed to specific brain cells including pyramidal neurons through the use of liposomes, exosomes, apoptotic bodies, nanoparticles and shark or cameloid antibodies is disclosed.
On September 25, 2018, the Company filed a patent application titled “Pterostilbene and Formulations Thereof for Treatment of Pathological Immune Activation” that teaches treatments, protocols, and compositions of matter are described for reduction of pathological immune system activation. In one embodiment, pterostilbene and/or formulations thereof are administered in a patient suffering from cytokine release syndrome at a concentration and frequency sufficient to reduce abnormal cytokine production and thus treat the cause of said cytokine release syndrome. Formulations of pterostilbene are disclosed for rapid release, enhanced biodistribution, and targeting to cytokine releasing effectors are disclosed for use in the practice of the invention.
On September 17, 2018, the Company filed a patent application titled “Pterostilbene and Compositions Thereof for Prevention and Treatment of Chronic Traumatic Encephalopathy” that teaches means, methods, and compositions of matter useful for prevention of chronic traumatic encephalopathy. In one embodiment of the invention, disclosed is utilization of pterostilbene and/or pterostilbene based compounds for prevention and/or treatment of chronic traumatic encephalopathy. In one embodiment, the invention teaches administration of pterostilbene and/or pterostilbene based compounds for reduction of taupathy associated with chronic traumatic encephalopathy.
On August 13, 2018, the Company filed a patent application titled “Enhancement of Ozone Therapy using Pterostilbene” that disclosed methods, means and compositions of matter using pterostilbene for enhancing therapeutic efficacy of ozone therapy in the field of oncology. The invention provides previously unknown synergies between ozone administration together with pterostilbene at inducing direct and indirect cytotoxicity to cancer cells. The invention provides means of delivery, administration, and therapeutic protocols for treatment of cancer patients. In one embodiment combination of ozone therapy together with pterostilbene is utilized to overcome drug resistance.
On October 08, 2017, the Company filed a patent application titled “Synergistic Inhibition of Glioma Using Pterostilbene and Analogues Thereof” that teaches methods, means and compositions of matter for utilizing pterostilbene and analogues thereof for suppression of viability, metastasis and proliferation of glioma cells alone, or together with immunotherapy, chemotherapy, or radiotherapy means. In one embodiment said pterostilbene augments immunogenicity of glioblastoma cells so as to enhance killing by immune cells or complement subsequent to damage of said glioblastoma cells by chemotherapy, radiotherapy, or immunotherapy.
On April 26, 2017, the Company filed a patent application titled “Augmentation of Stem Cell Activity using Pterostilbene and Compositions Containing Pterostilbene“ that disclosed means of augmenting circulating endogenous stem cells through administration of an effective amount of pterostilbene or derivatives thereof. In one embodiment a patient with reduced levels of circulating endothelial progenitor cells is treated with pterostilbene at a concentration and frequency sufficient to restore, and/or enhance levels of circulating endothelial progenitor cells (EPC). In another embodiment endogenous levels of stem cells are restored or enhanced by administration of pterostilbene, said endogenous stem cells comprising cells of the dentate gyrus, subventricular zone, hepatic stem cells, cardiac stem cells, and hematopoietic stem cells.
On March 29, 2017, the Company filed a patent application titled “Stimulation of Immunity to Tumor Stem Cell Specific Proteins by Peptide Immunization” that discloses treatment of cancer is disclosed through administration of proteins or specific peptides found on tumor stem cells in vivo, in a matter eliciting monocyte or dendritic cell migration in order to allow uptake of said administrated proteins or peptides, followed by administration of a maturation signal in vivo. The invention provides for treatment of cancer through induction of anticancer immunity and/or immunity towards tumor initiating stem cells.
On March 29, 2017, the Company filed a patent application titled “Targeting the Tumor Microenvironment through Nutraceutical Based Immunoadjuvants” that disclosed compositions useful for the treatment of cancer which modulate tumor associated immunosuppression, thus acting as immunoadjuvants. In one embodiment a composition containing apigenin, is provided, said composition useful for inhibition of tumor associated immune suppression mediated through the molecule indolamine 2,3 deoxygenase (IDO). In another embodiment, liposomal apigenin is administered as a means of decreasing IDO expression.
On March 29, 2017, the Company filed a patent application titled “Activated Leukocyte Extract for Repair of Innate Immunity in Cancer Patients” that disclosed are compositions, methods of use, and pharmaceutical preparations useful for modulation of immune responses. In one embodiment a composition is extracted polyvalently activated peripheral blood mononuclear cells through dialysis. Said immune modulator is useful for treatment of cancer and alleviation of cancer associated immune depression. In one embodiment, said immunomodulator acts as a costimulatory of T cell activation by modulation of cytokine production. In one embodiment said immune modulator is concentrated for miRNA species capable of activating innate immune cells.
On March 29, 2017, the Company filed a patent application titled “Augmentation of Anti-Tumor Immunity by Mifepristone and Analogues Thereof” which relates to compositions of matter and methods useful for improving a treatment outcome and/or an alteration of immunity in a condition that benefits from immune stimulation. In particular, one embodiment of the invention teaches administration of sufficient doses of mifepristone or a derivative, alone, or in combination with an immunotherapeutic such as, but not limited to, an antibody, a vaccine, a cytokine, or a medicament whose therapeutic activity is associated with immune modulation.
On March 29, 2017, the Company filed a patent application titled “Methods of Re-Activating Dormant Memory Cells with Anticancer Activity” that disclosed methods, protocols and compositions of matter useful for stimulation of anticancer immune responses. In one embodiment of the invention culture of buffy coat cells is performed in an environment resembling non-physiological conditions. Buffy coat derived products are subsequently harvested, concentrated, and added to a culture of monocytes and lymphocytes. Conditioned media from said second culture is subsequently utilized as an injectable solution for stimulation of anticancer immunity.
On March 29, 2017, the Company filed a patent application titled “Modulation of Oral Microbiome for Treatment of Periodontitis” that disclosed methods, means, and compositions of matter useful for inhibition of, reduction in progression and reversion of periodontitis. In one embodiment the invention provides prebiotic and/or probiotic compositions which modulation the oral microbiome in order to ameliorate, prevent or reverse periodontitis. In one embodiment a composition is administered into the oral cavity containing Actinomyces naeslundii, Actinomyces odontolyticus, Streptococcus thermophilius, Lactobaccilus brevis and Lactobacilius plantarum. Administration may be performed using various means including a mouthwash, a patch, a toothpaste, or in a preferred embodiment said prebiotic and/or probiotic compositions are delivered via a mouth tray.
On July 20, 2016, the Company filed a patent application titled ”Prevention of Pregnancy Complications by Probiotic Administration” which disclosed methods, protocols and compositions of matter for the treatment of pregnancy complications through immune modulation of a mammal in need. In one embodiment the invention provides probiotic compositions for immune modulation to decrease risk of pregnancy complications. Pregnancy complications include recurrent spontaneous abortions (RSA), preterm birth, pre-eclampsia including hemolysis elevated liver enzymes low platelets (HELP), premature rupture of the membrane, Antepartum hemorrhage including placental abruption, chorioamnionitis, Intrauterine growth restriction, placenta pravaevia, sequalae of intraamniotic infection. Published on January 26, 2017 https://patents.justia.com/patent/20170020930
On July 20, 2016, the Company filed a patent application titled “Exosome Mediated Innate and Adaptive Immune Stimulation for Treatment of Cancer” that teaches means of stimulating innate and/or adaptive immunity to cancer by administration of exosomes. Stimulation of innate immunity involves modifying exosomes by chemical addition of innate immune stimulators, whereas stimulation of adaptive immunity involves pulsing dendritic cells generating exosomes with antigens, in some cases, pulsing with Brother of the Regulator of Imprinted Sites (BORIS) proteins, peptides, or altered peptide ligands thereof.
On July 8, 2015, the Company filed a patent application titled “Augmentation of Oncology Immunotherapies by Pterostilbene Containing Compositions” that disclosed compositions of matter and methods useful to augmentation of immune responses to tumors. In one embodiment, a pterostilbene containing composition is administered to a cancer patient at a sufficient concentration and frequency to induce de-repression of tumor targeting immune responses. In one specific embodiment of the present invention, pterostilbene enhances antibody dependent cellular toxicity (ADCC) and in turn augments efficacy of FDA approved antigen specific immunotherapeutics such as trastuzumab (Herceptin) and other monoclonal antibody therapies used for treating cancer.
The above list of intellectual properties are original works of Thomas E. Ichim and Timothy G. Dixon who have generously assigned them to the Company.
after all of these TSOI patents filed/ pending
very few know the real deal on
let's say half of what they have churning in the fire here .
the valuation is coming
it's not gonna impress me again until it crosses 20
if the funding is a go, so does the interest , and the queries - imo
Each of these TSOI products should proof out their values.
Penny stocks like TSOI don’t hold gains! Especially after the stock rises 90% during the day! Believe it or not but profit taking happened again! Many professionals planned accordingly…
MM's crushed it to scare shares, with Phase III funding, it will turn back up, imo
Nice news just not jumping up and down quite yet. Cause .......history. Will probably close up 30% or so is my guess. Seems it lost its steam. Ugh
Dollar Volume $ 306,531 so far today & will be climbing higher by days end
Ur fine...much can happen here in short term, that news and vol will lure new $$$ and some big pharma will be watching..
cashing in some subs near the HOD while using the proceeds to sit bid seems to have been a good choice.
GL to us ALL as another question was finally answered, well part of it. All about the details of said agreement
Bought at 0.0190 and now down to 0.0154….smh
It is GREAT news that Tim has now gone public with HOW he will fund Phase 3 of the FDA clinical trials. This has always been an open issue and concern for me. Congratulations to Tim for keeping the shareholders informed !!!
TSOI what should the market cap be for a company that is in Phase III Trial and funded?
![]()
Tripping here may cross 02 huge got funding for phase III clinicals
TSOI Therapeutic Solutions International Inc () 0.017625
0.007525 (74.50%)
Volume: 13,643,191
Day Range: 0.0101 - 0.0192
TSOI MM's shaking it hard, lets see how thin to .02+ now
TSOI Lets see if .02 gets annihilated ![]()
TSOI He He, getting some Whales attention yet?
|
Followers
|
523
|
Posters
|
|
|
Posts (Today)
|
2
|
Posts (Total)
|
65717
|
|
Created
|
10/04/08
|
Type
|
Free
|
| Moderators BigBadWolf johnnytrader33 JMC$ Yooperman Hogwarts | |||






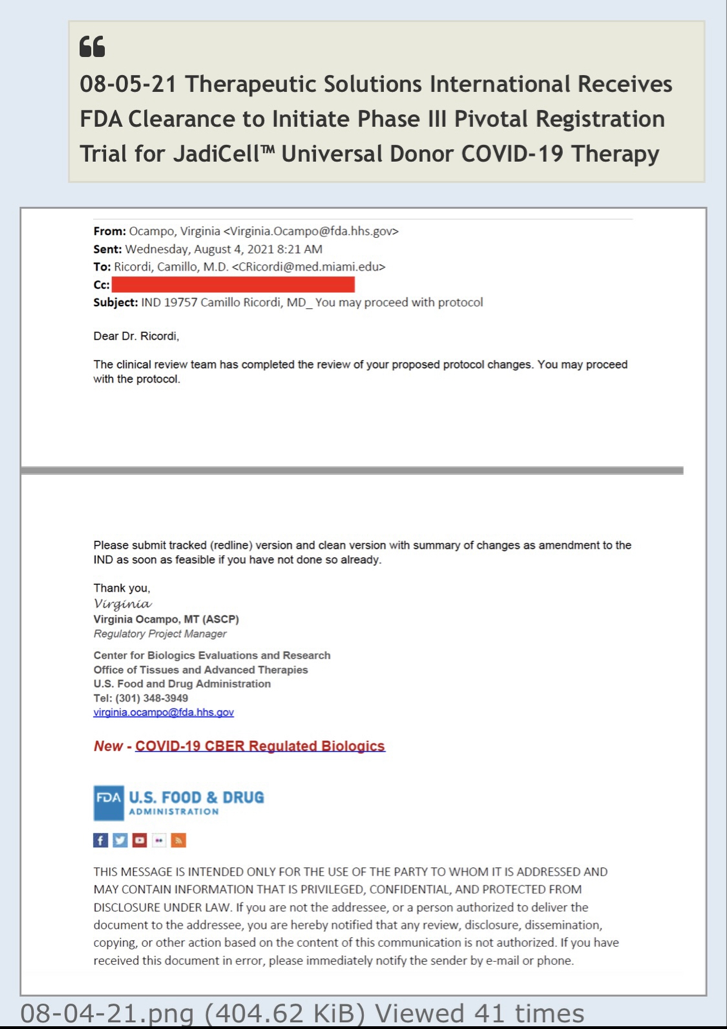



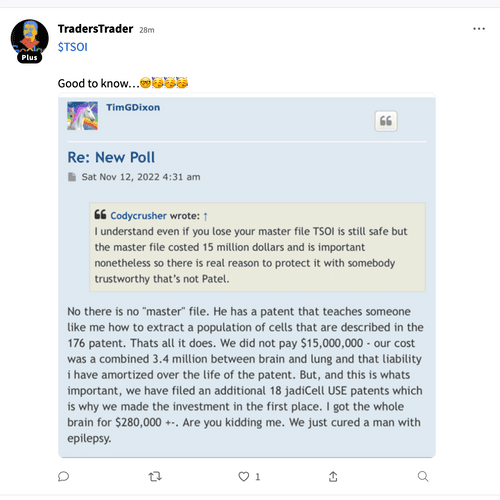
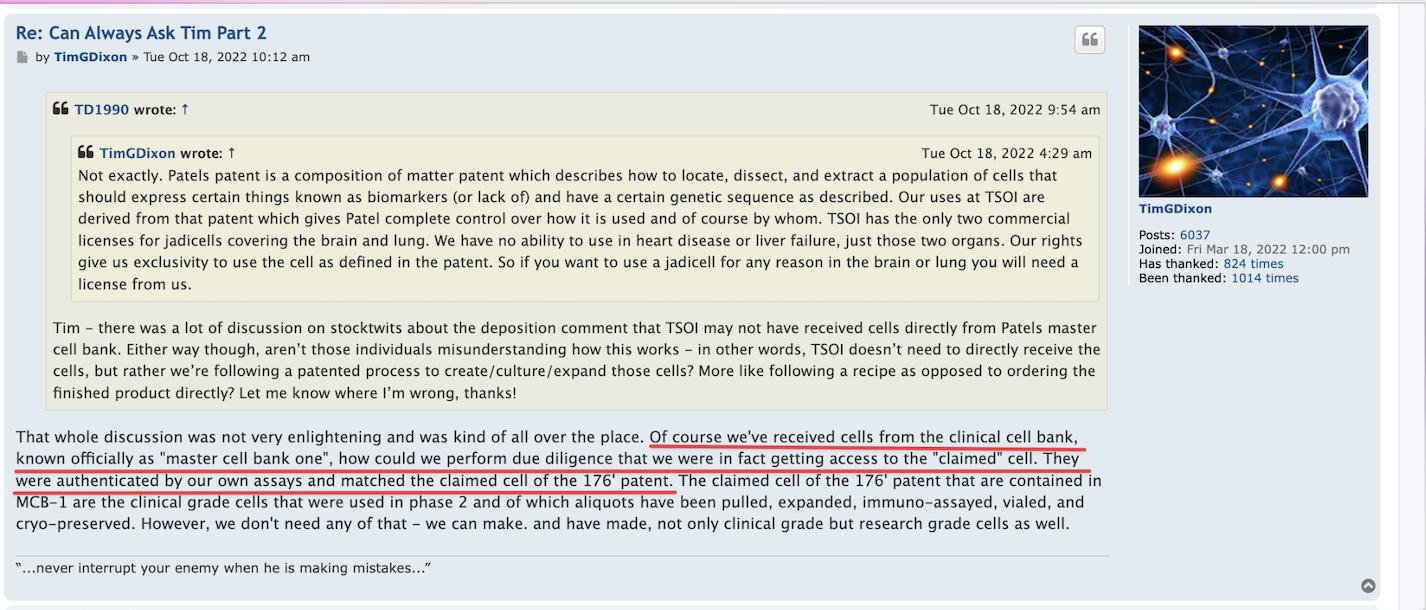
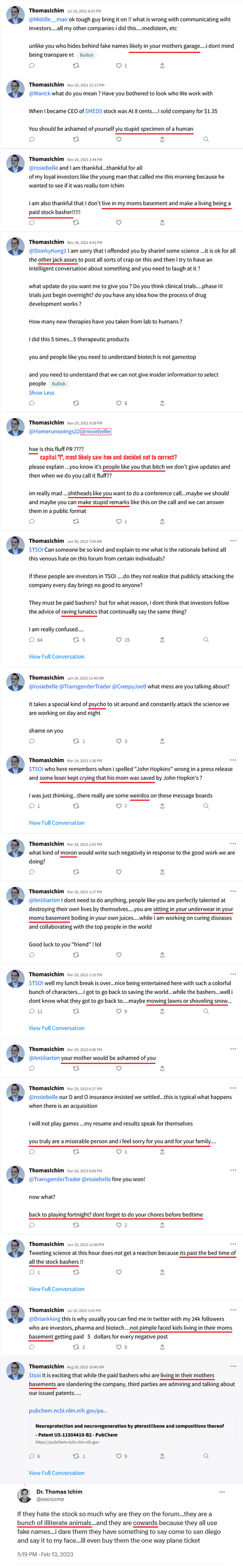
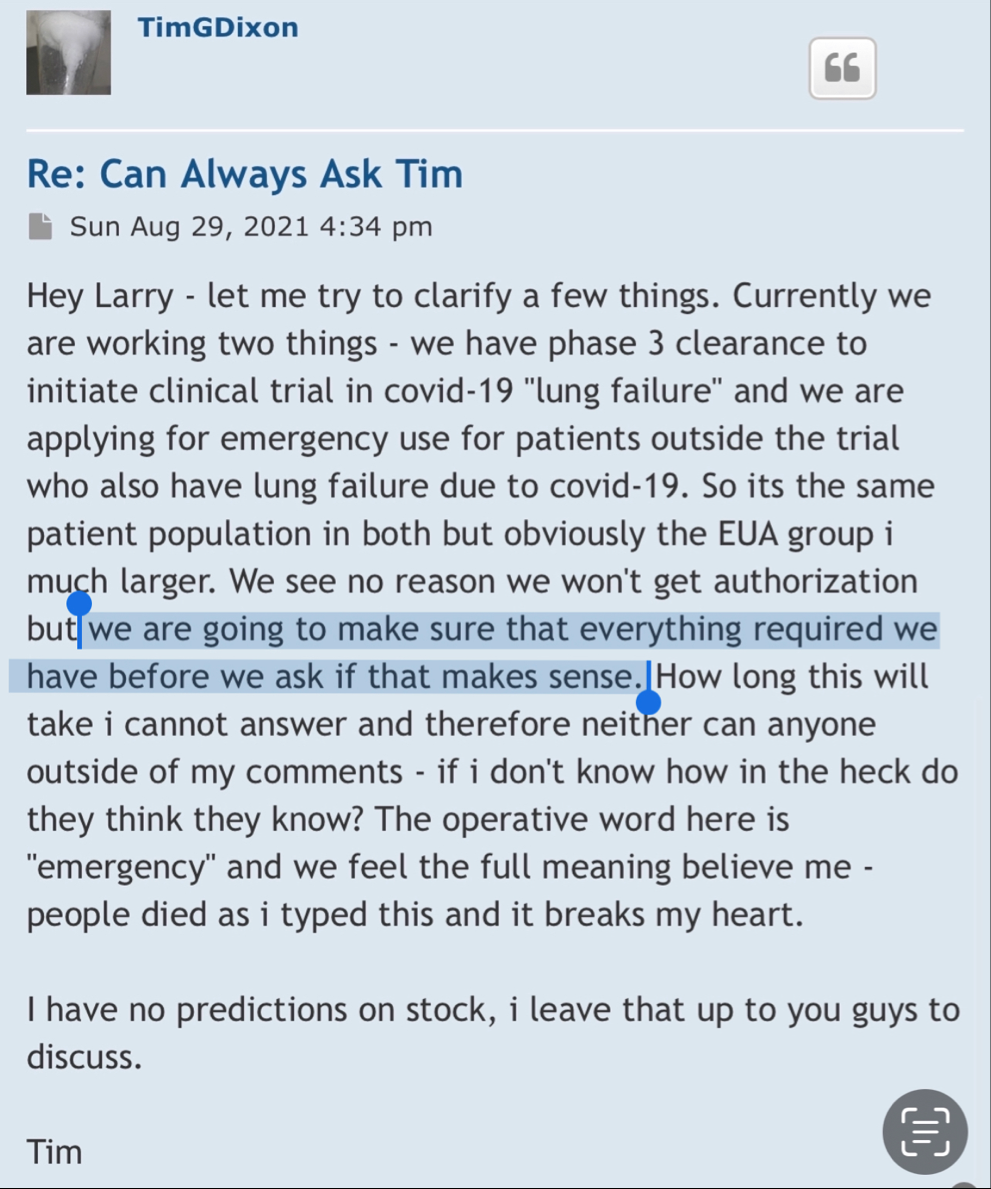
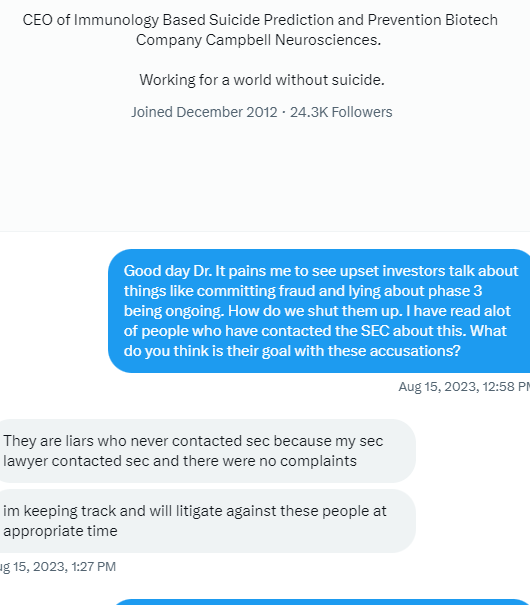



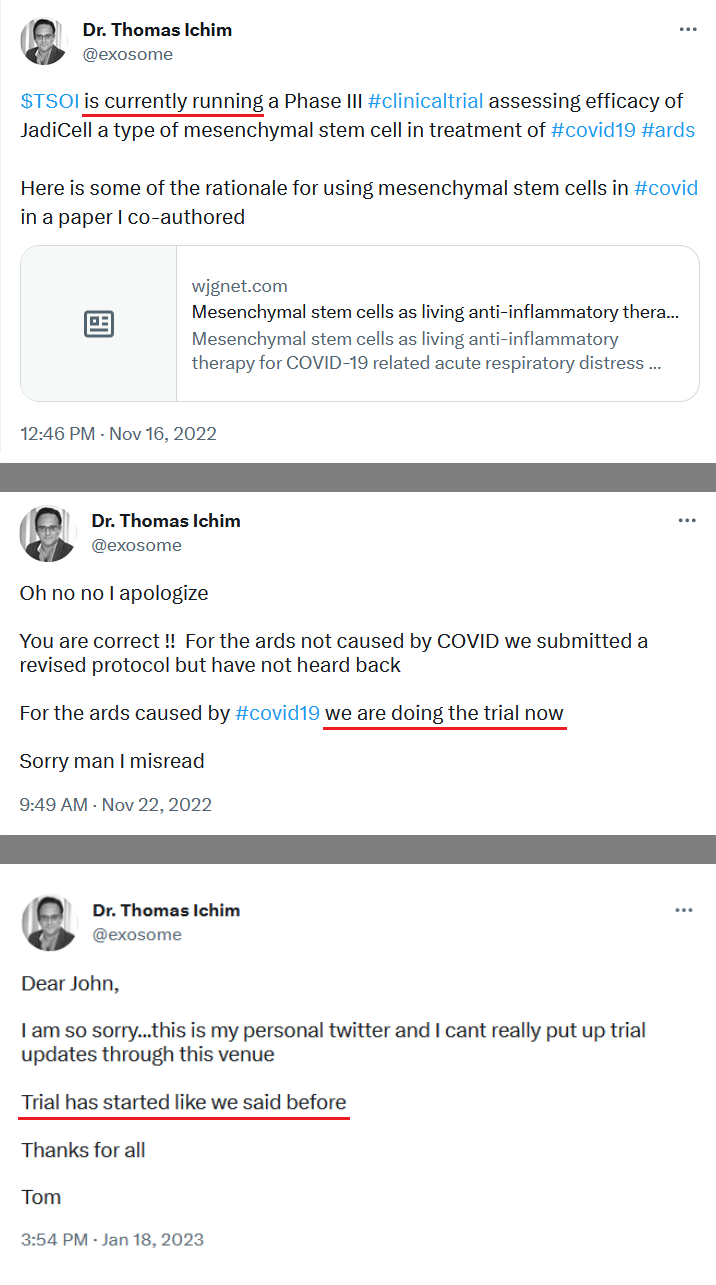

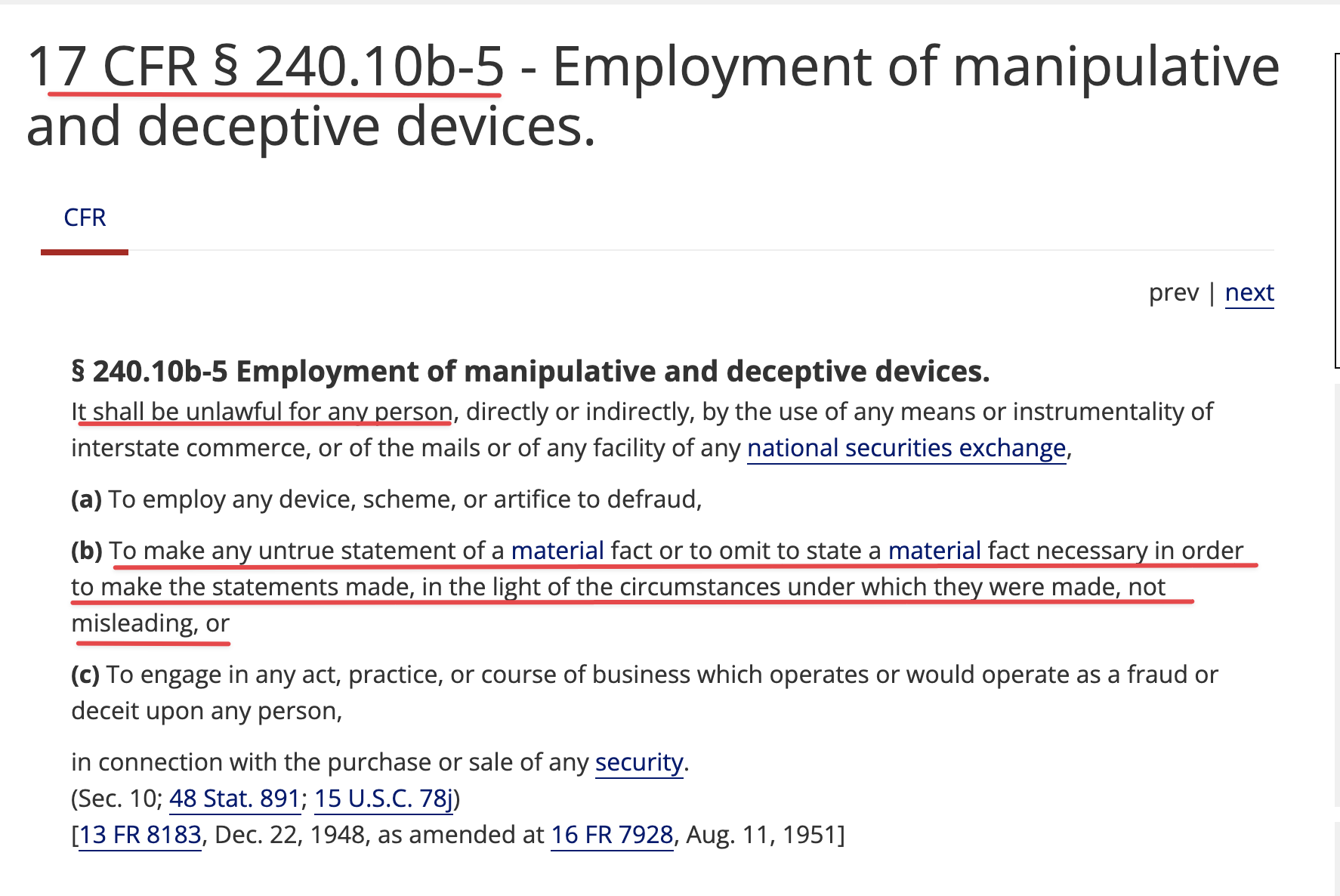


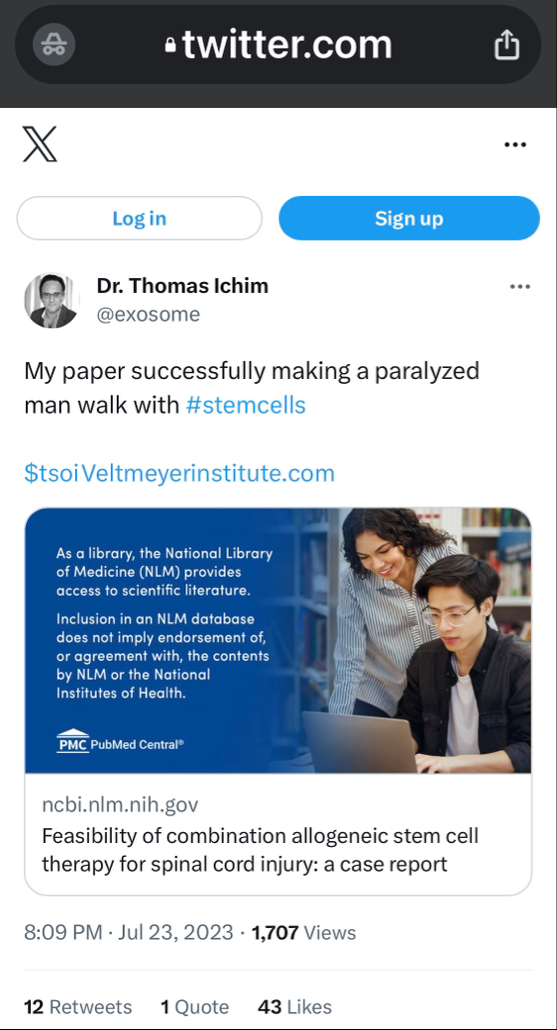


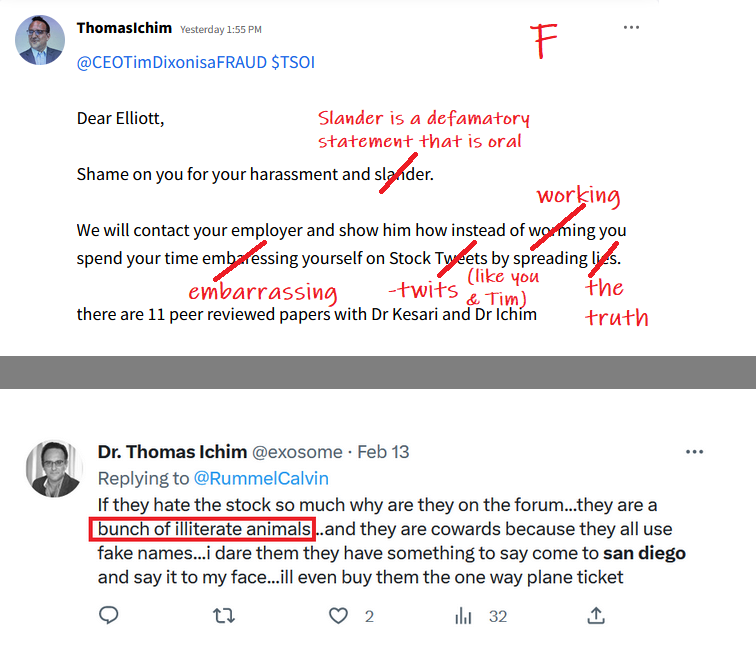
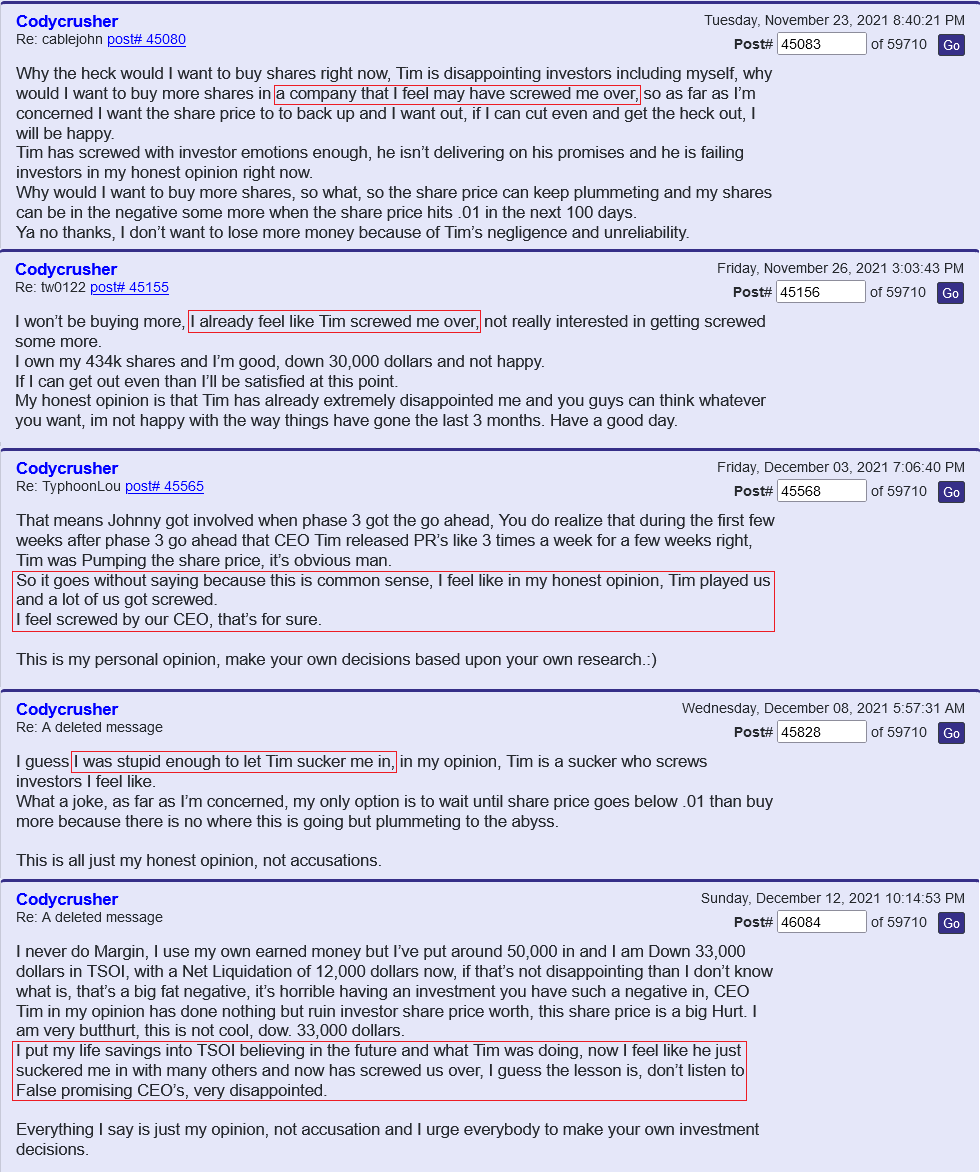





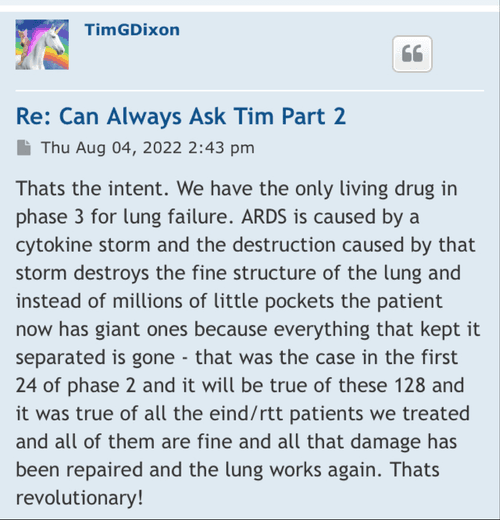


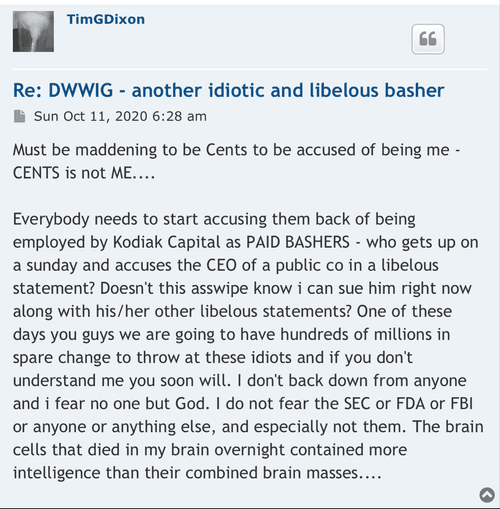

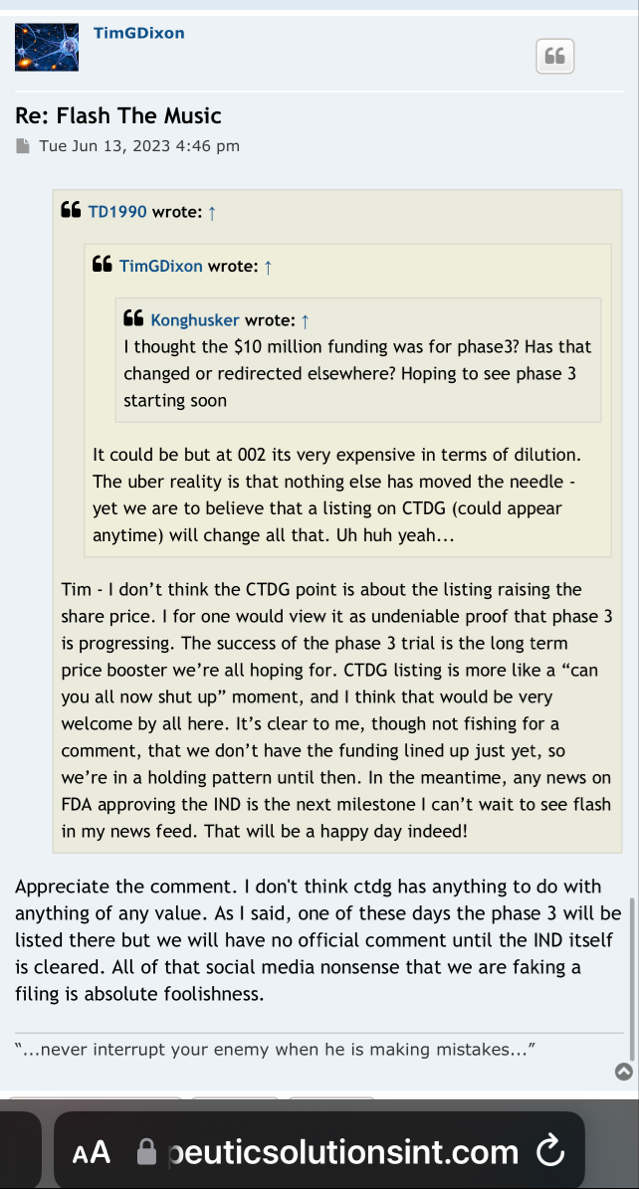
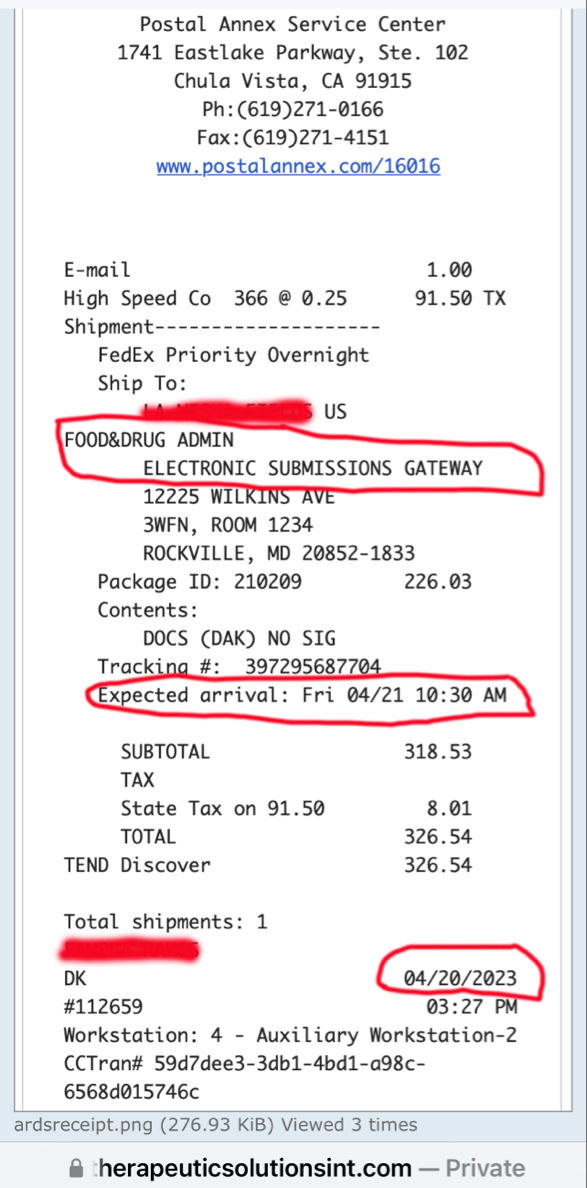

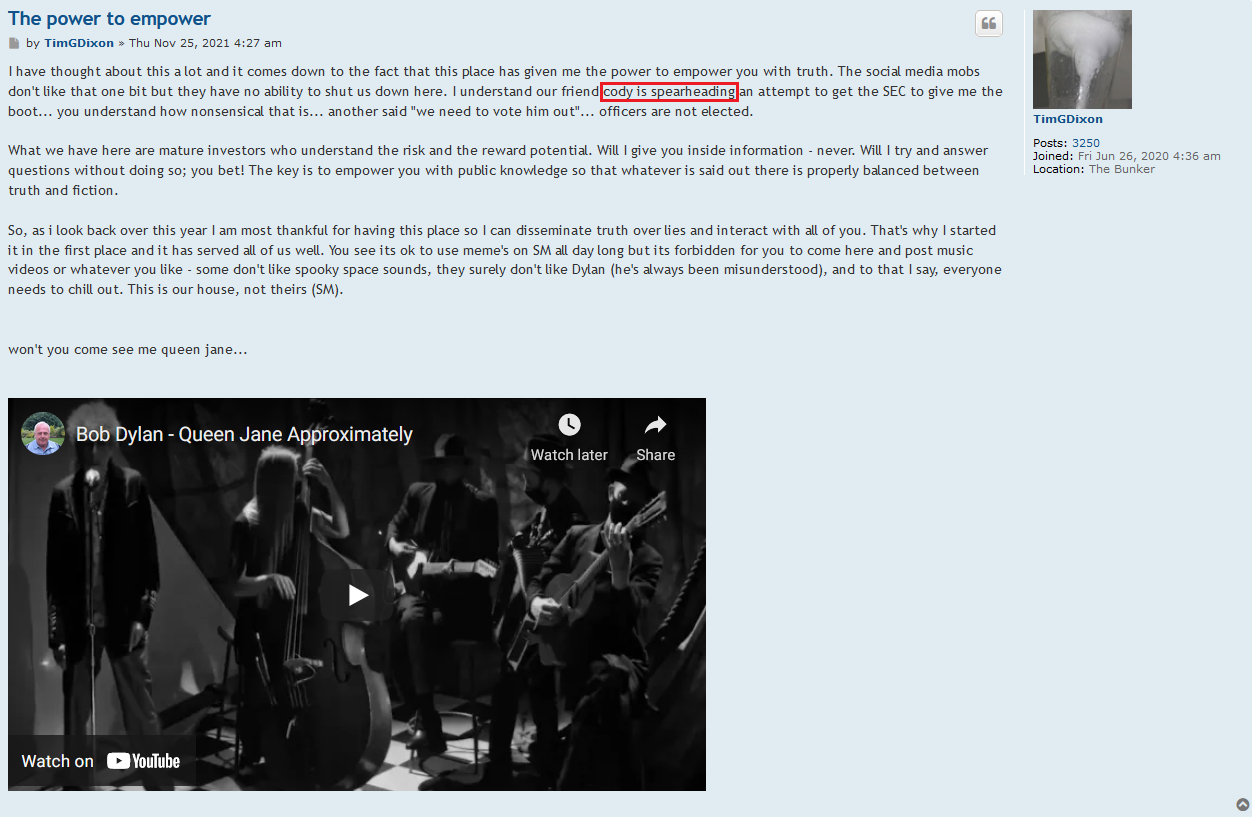
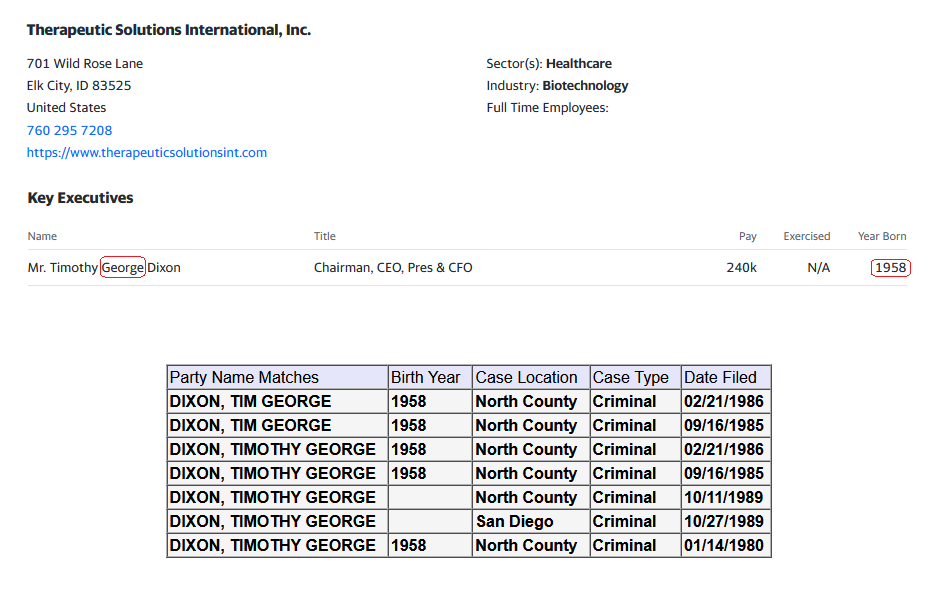


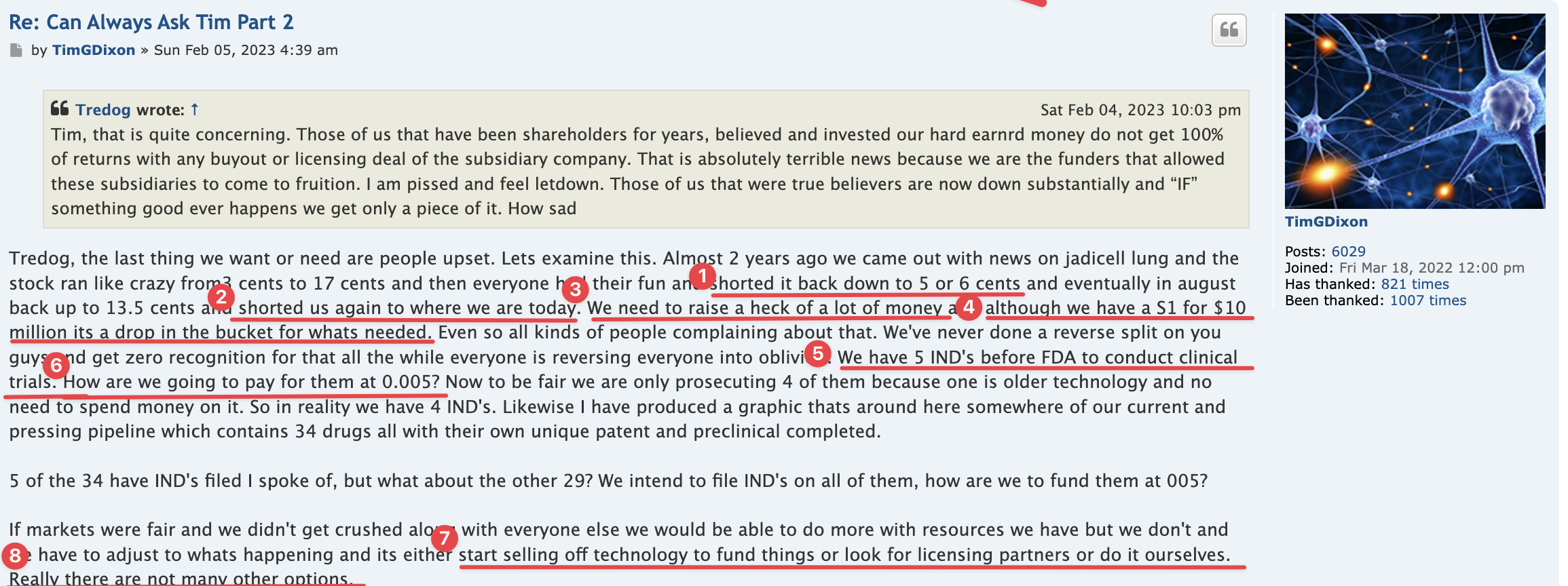



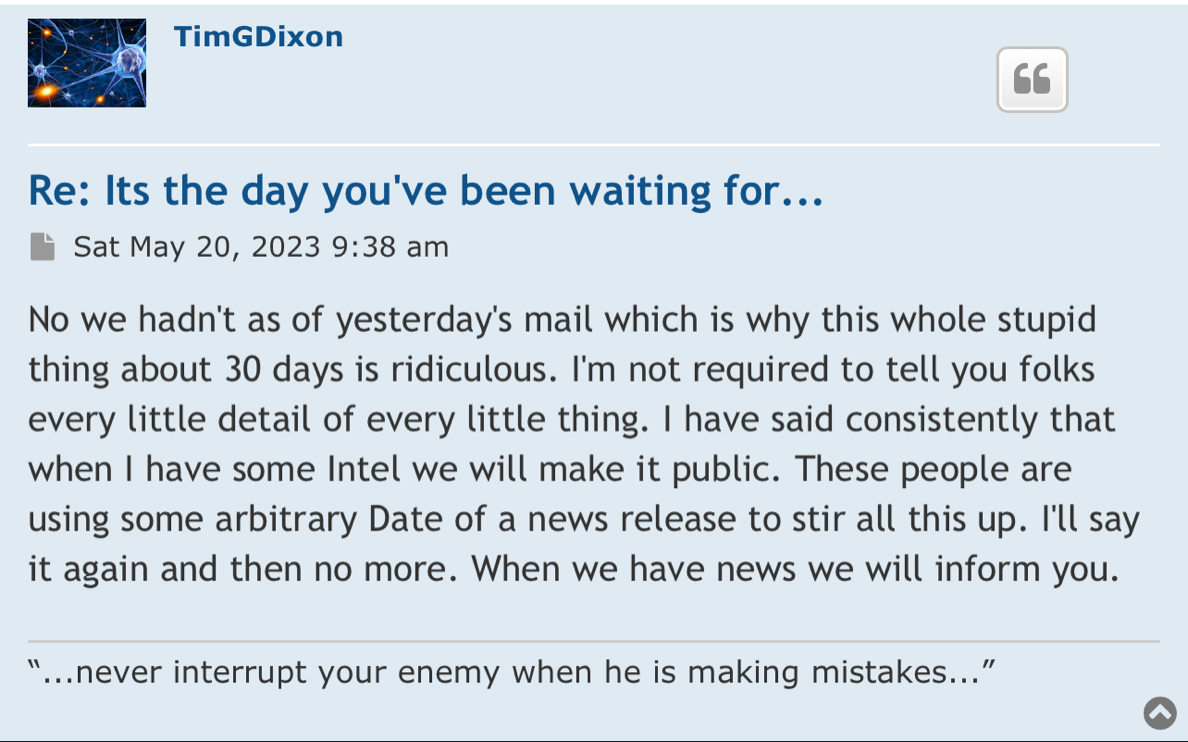
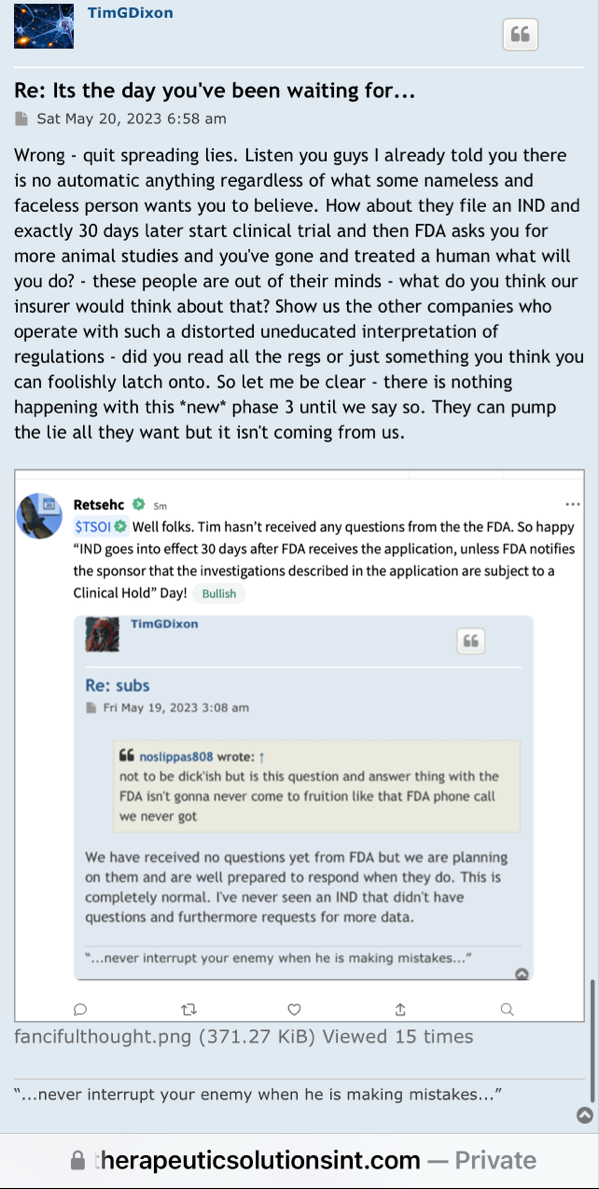
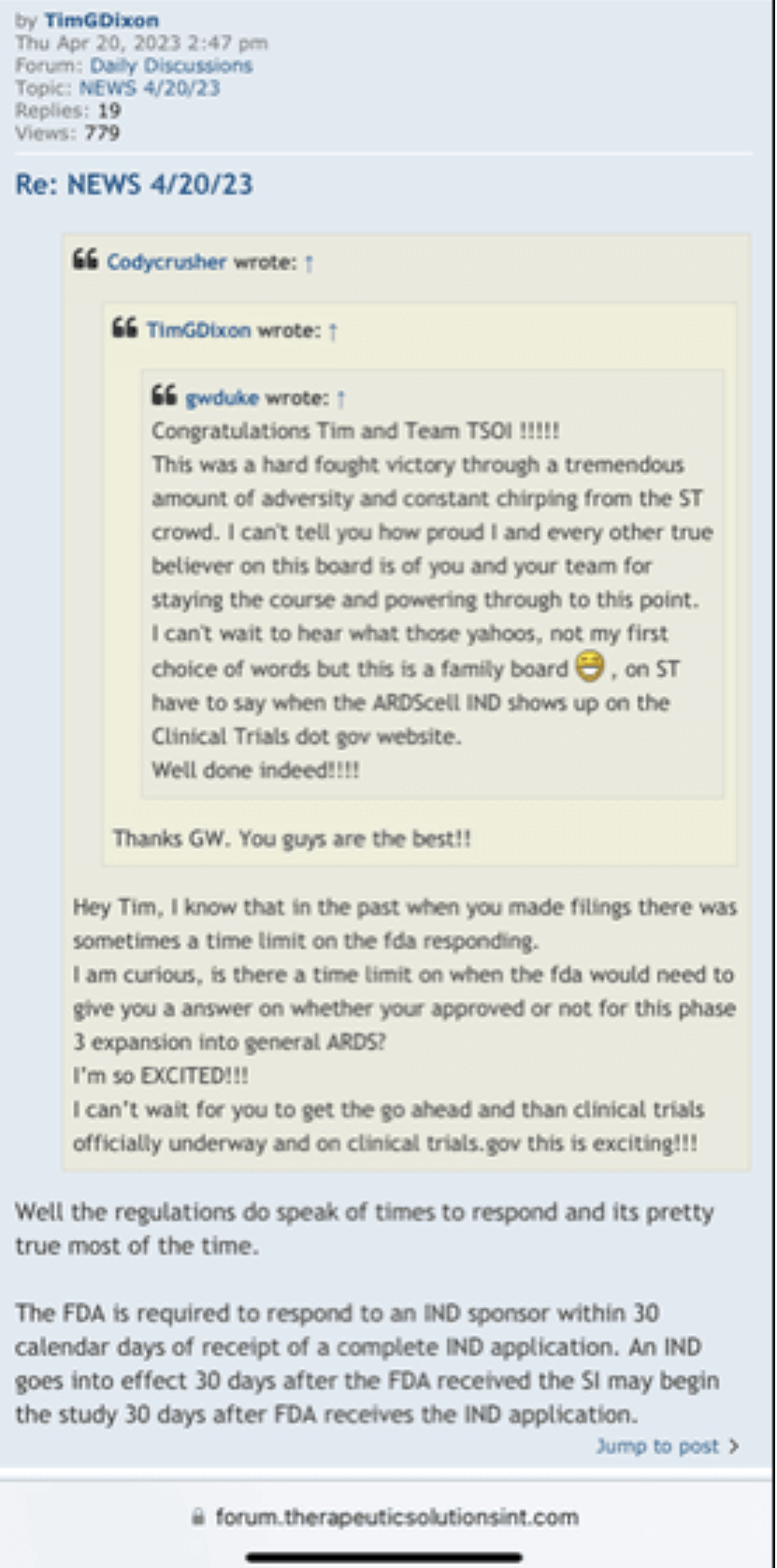


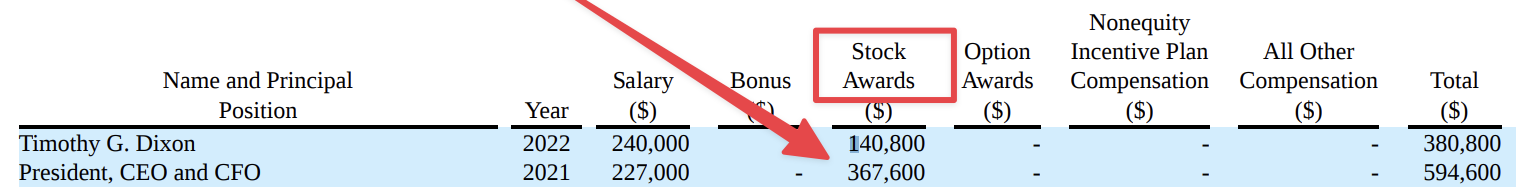



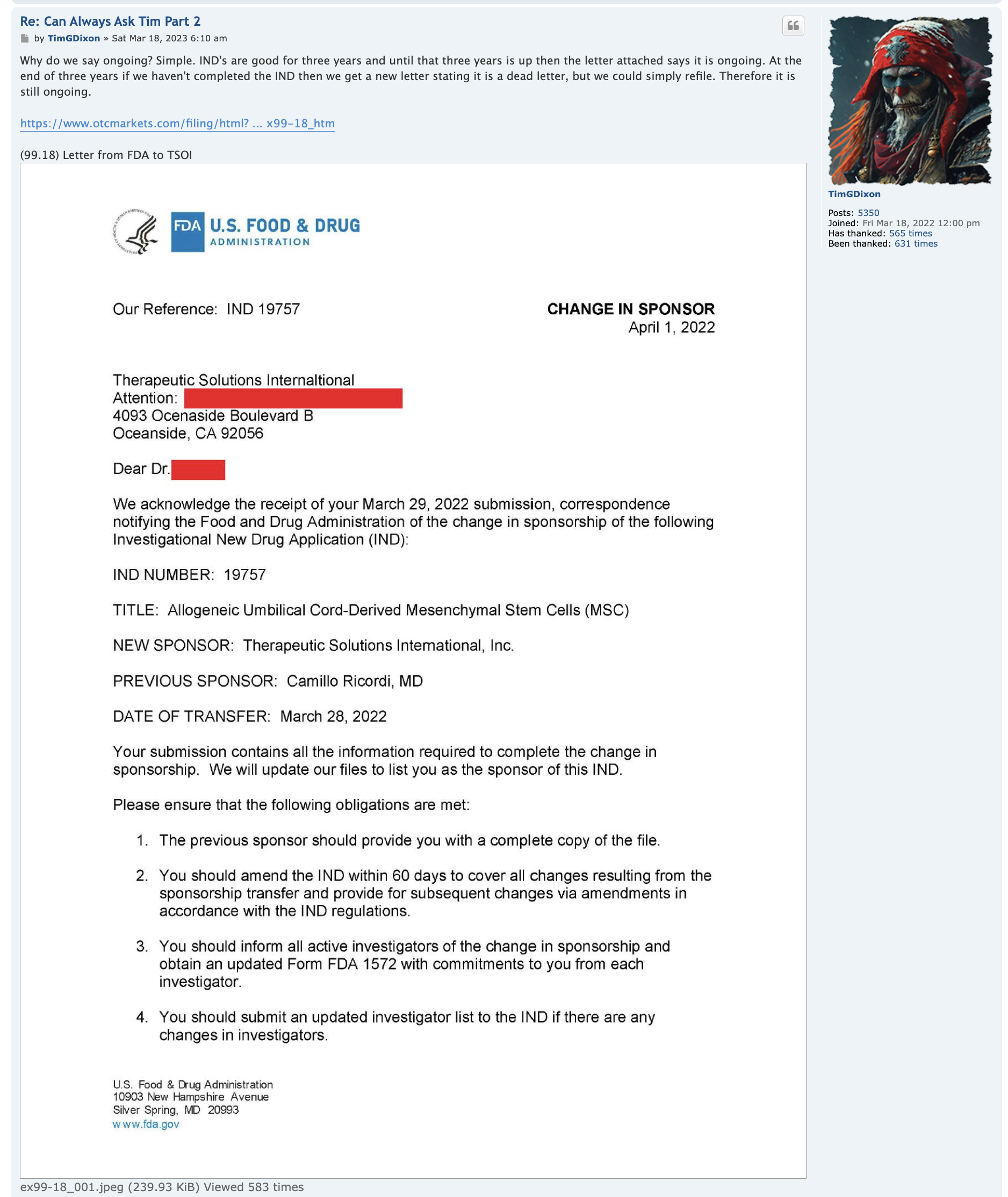
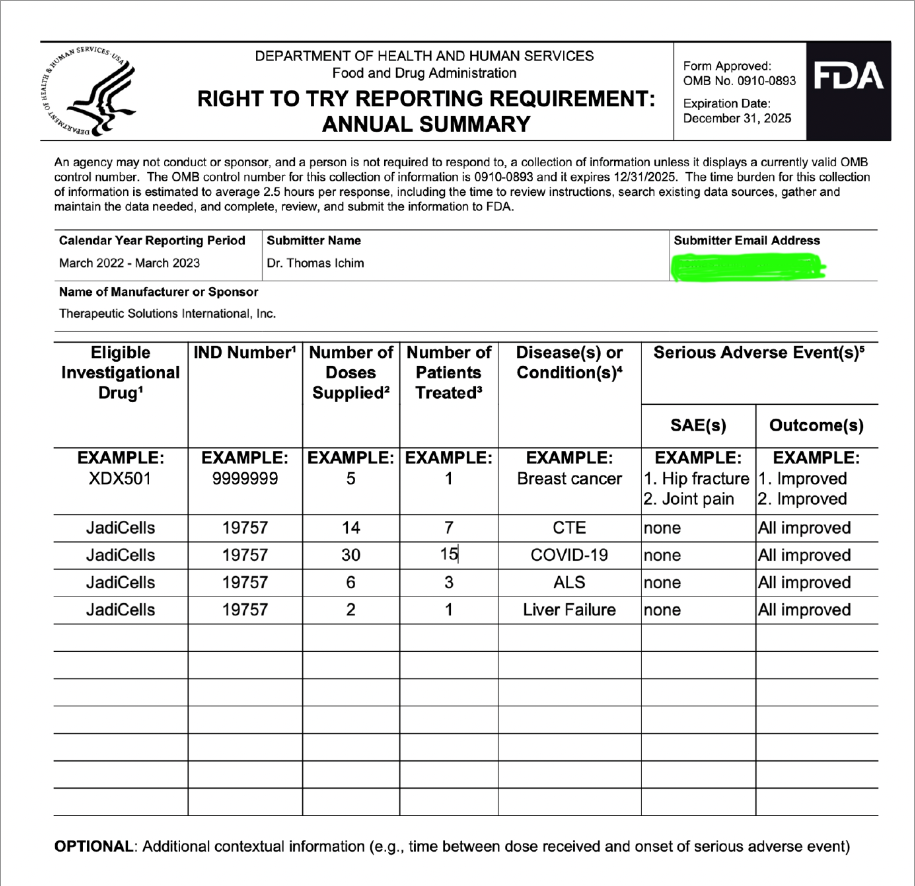


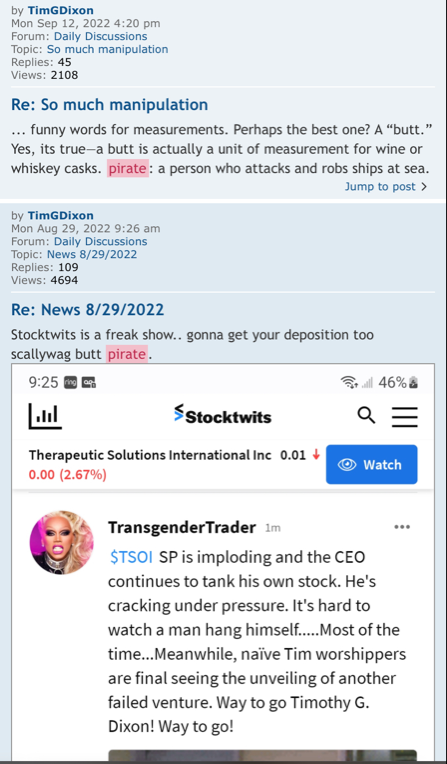
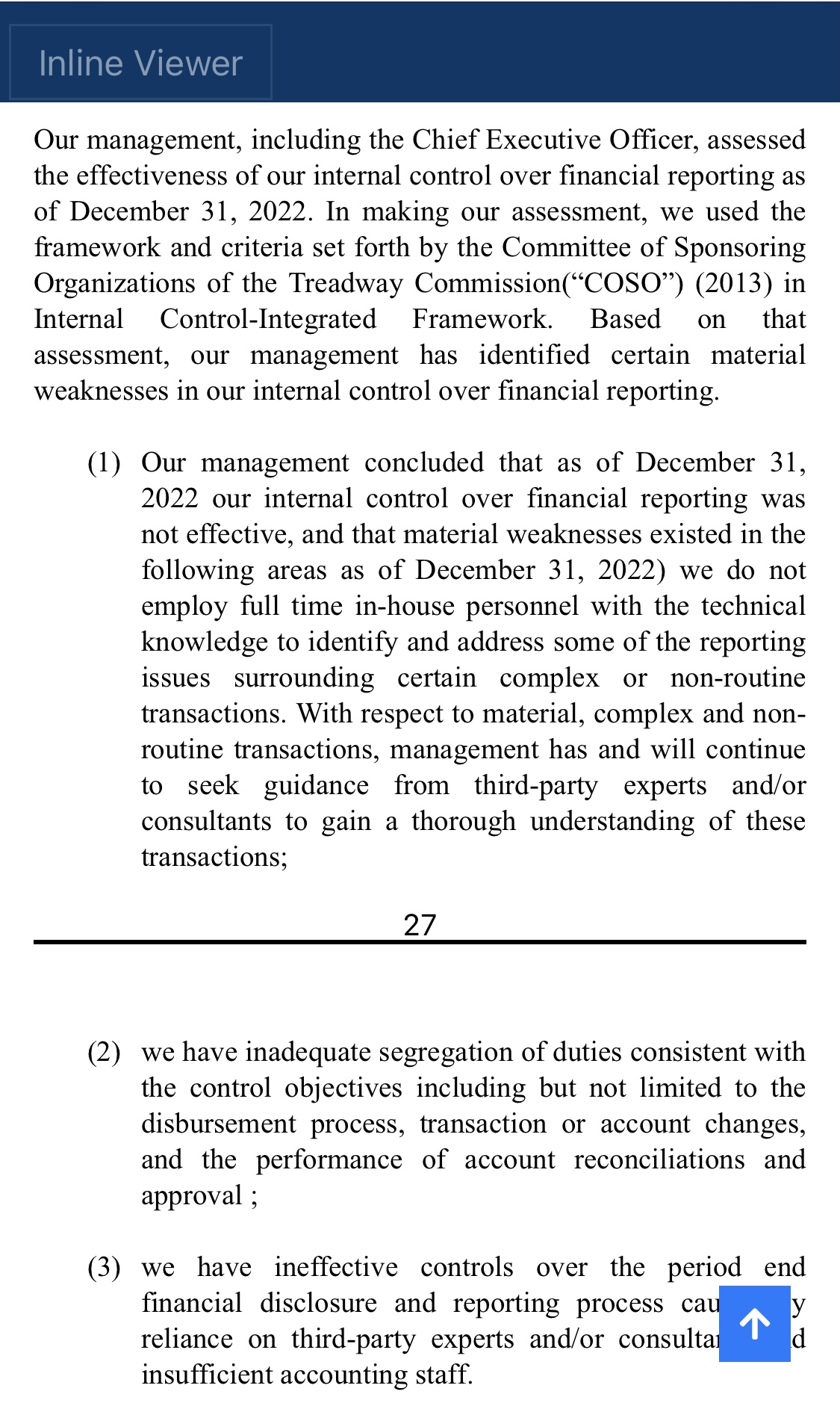
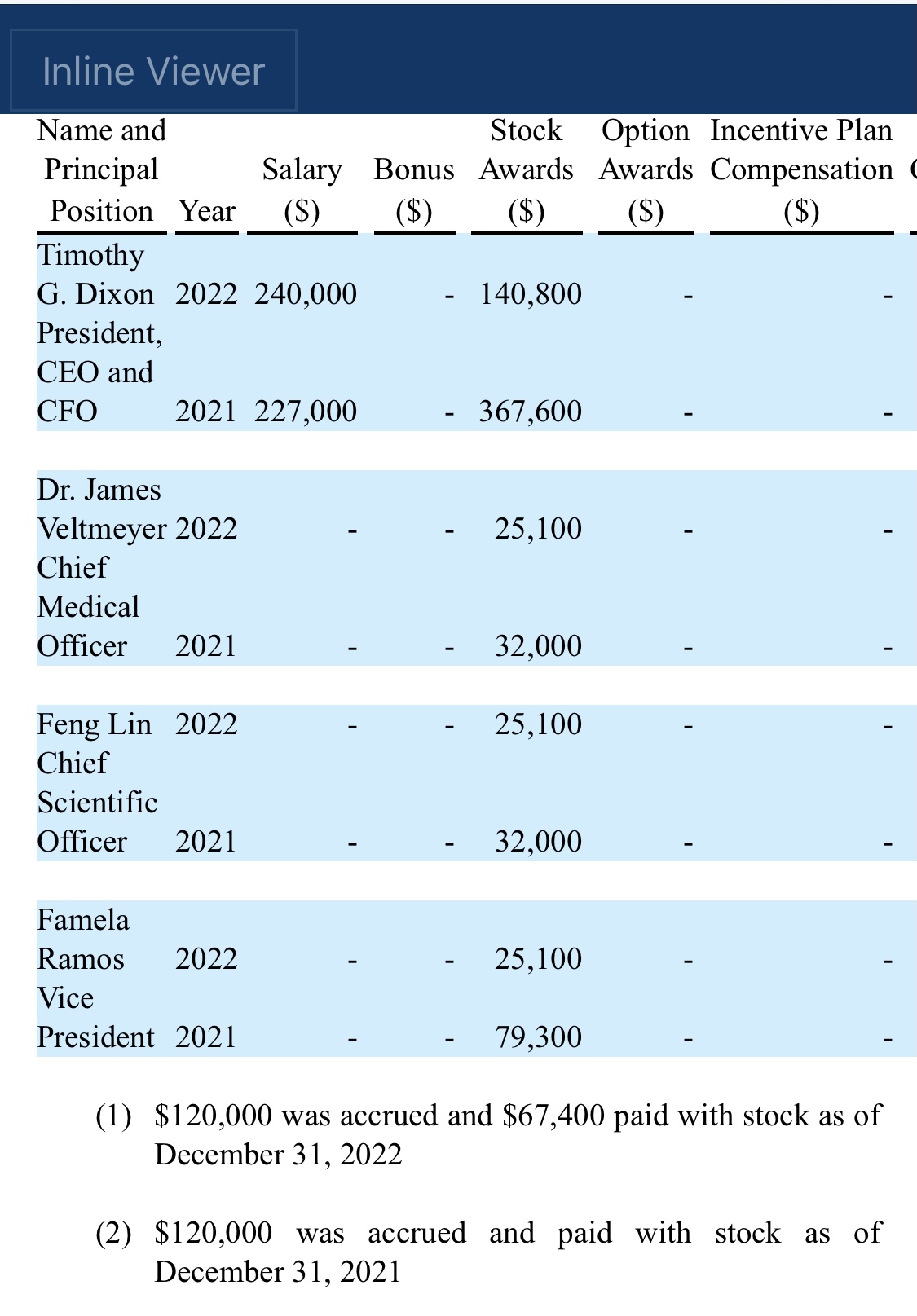


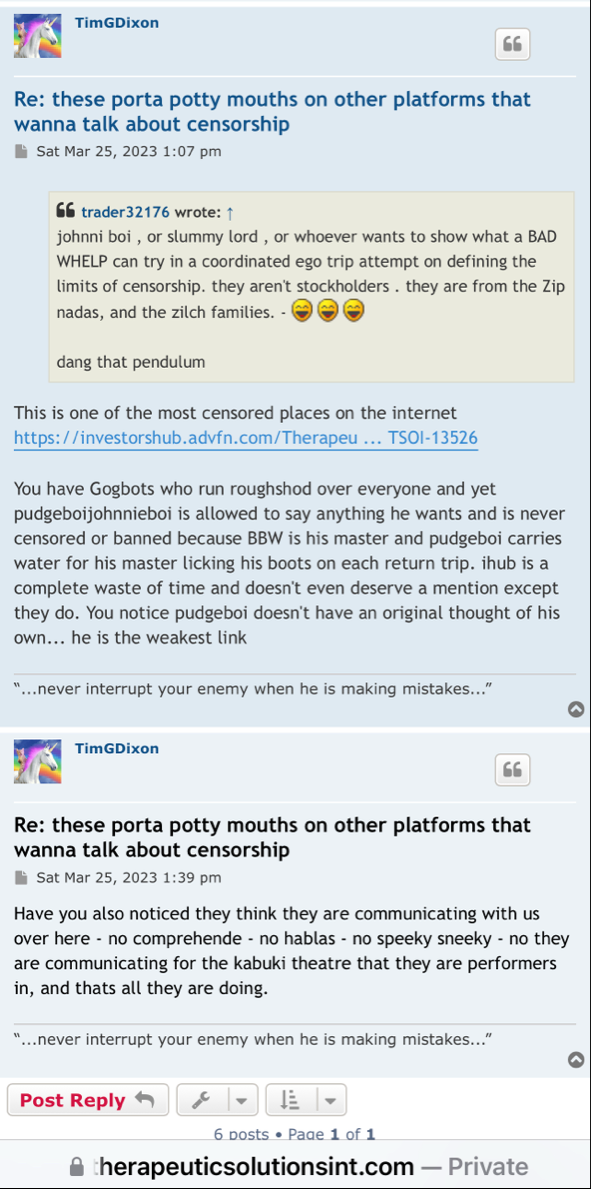

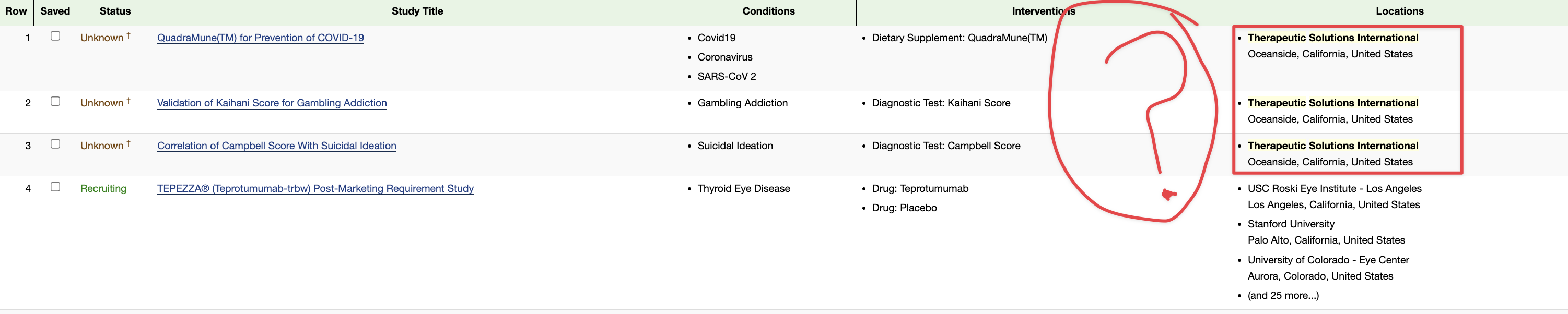
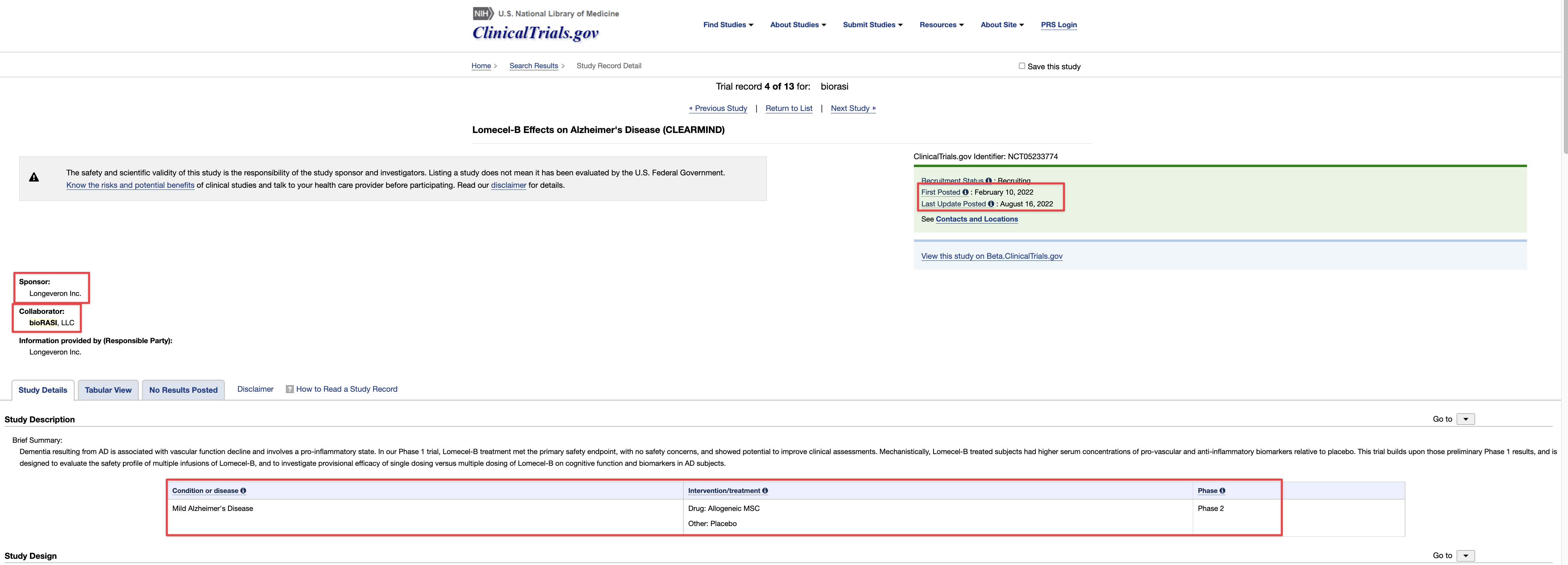
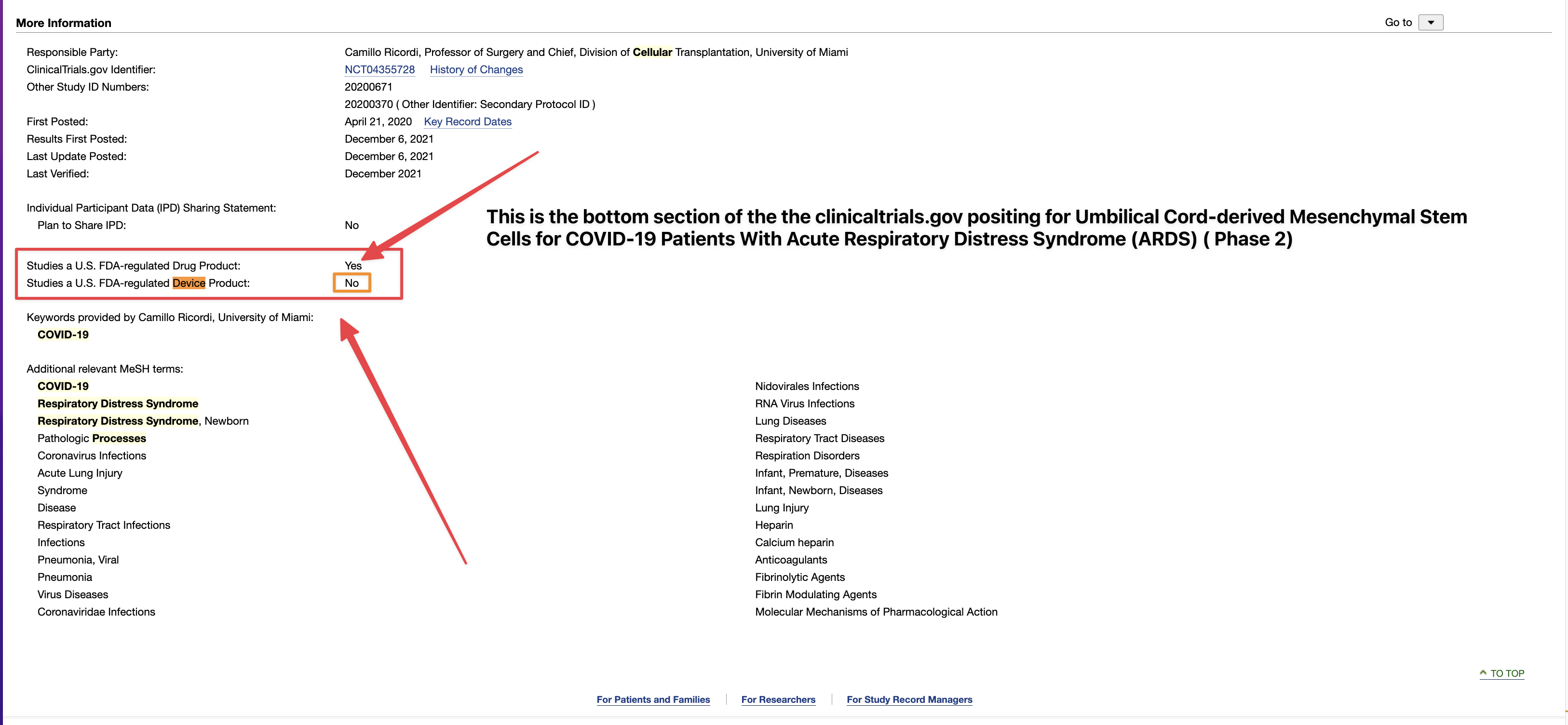
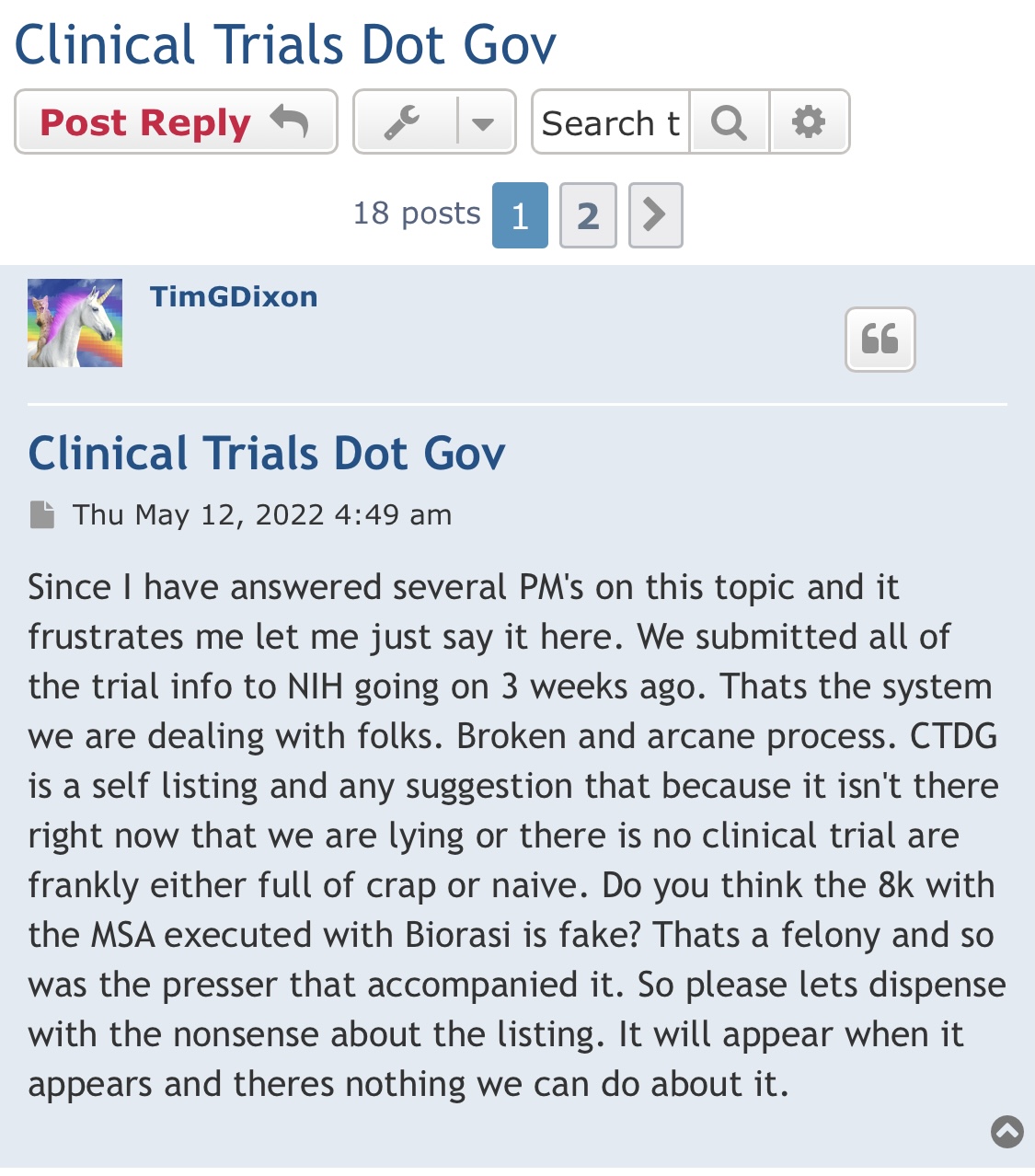
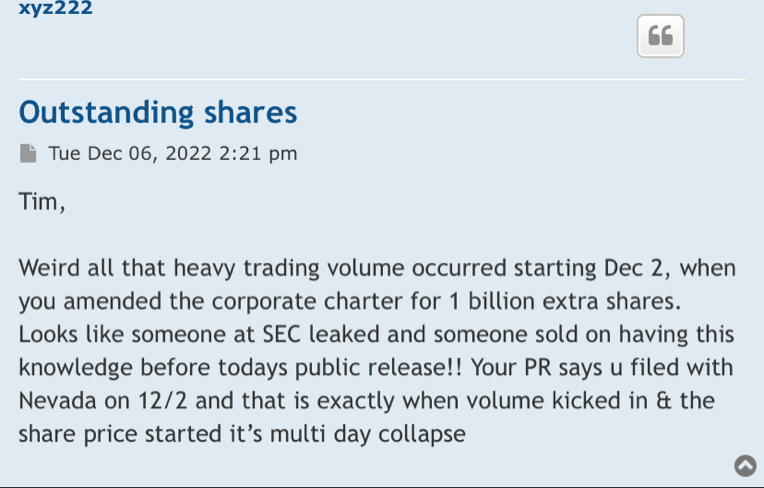
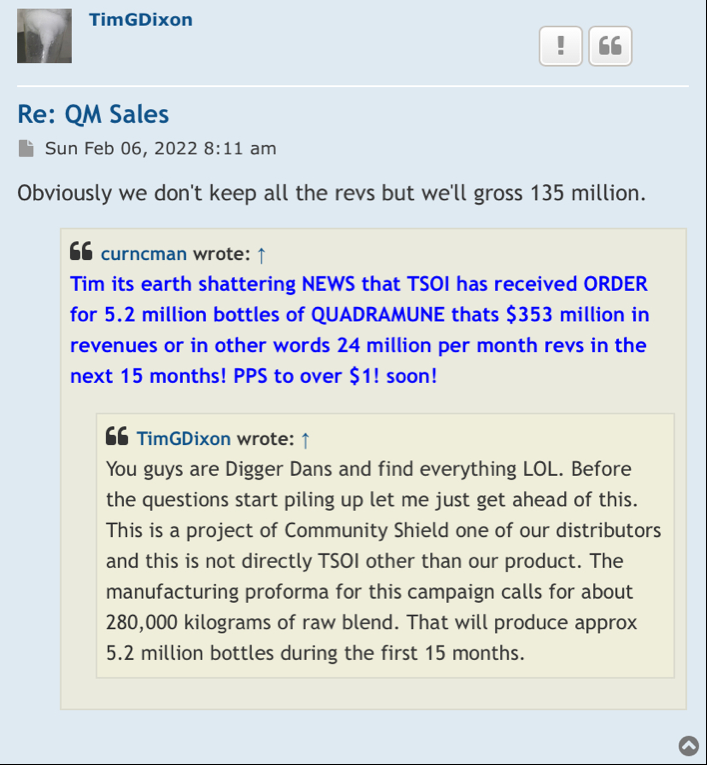

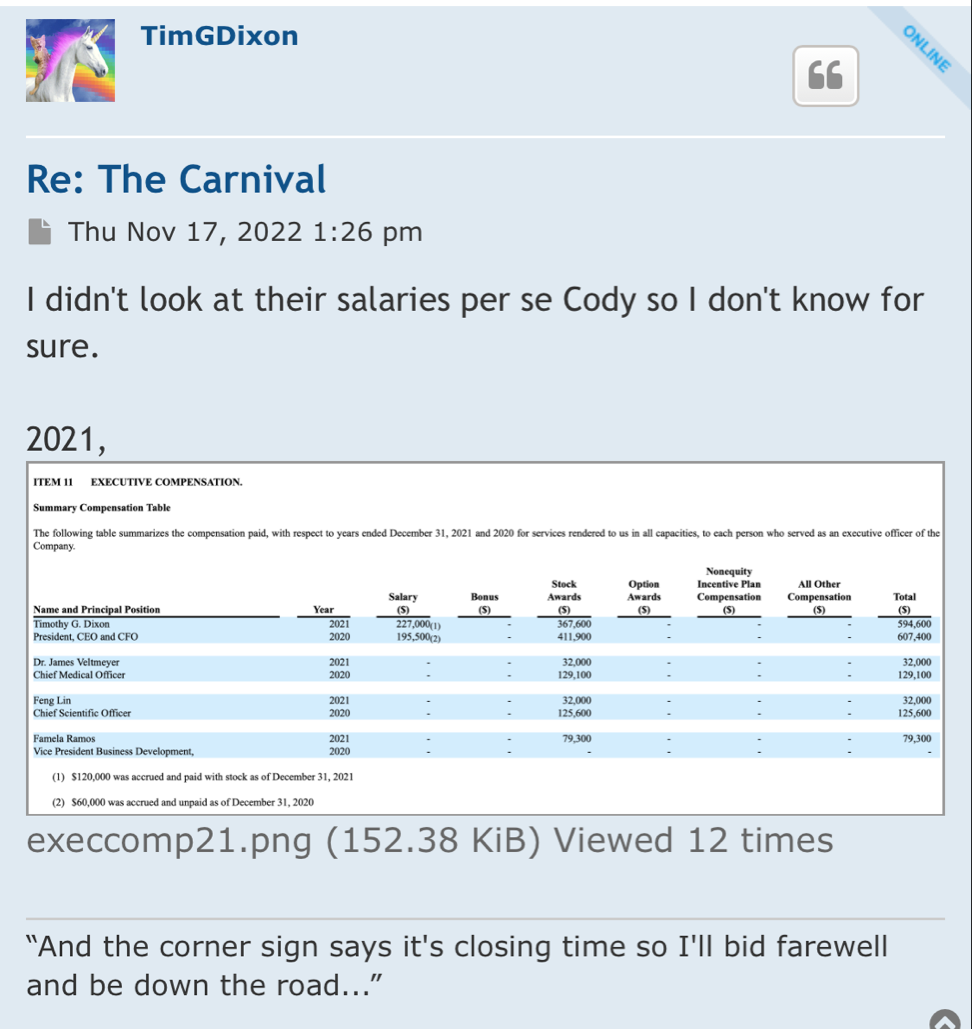
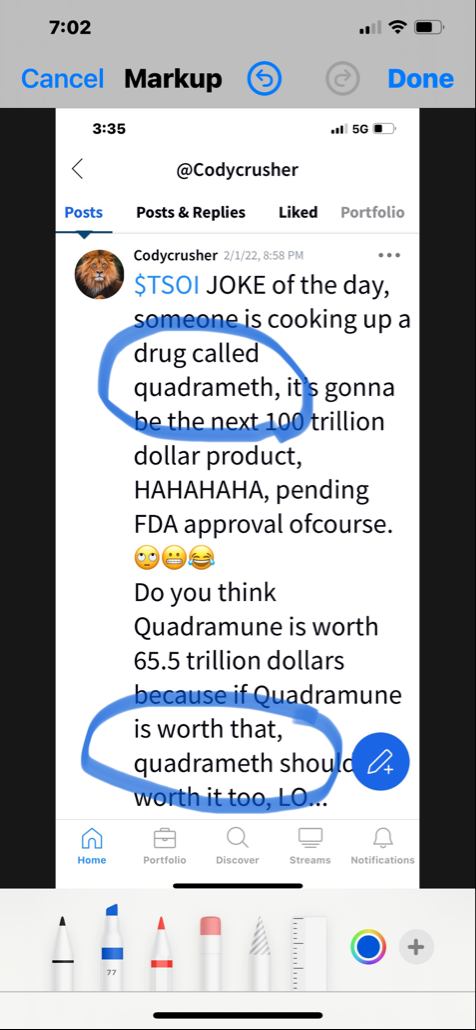


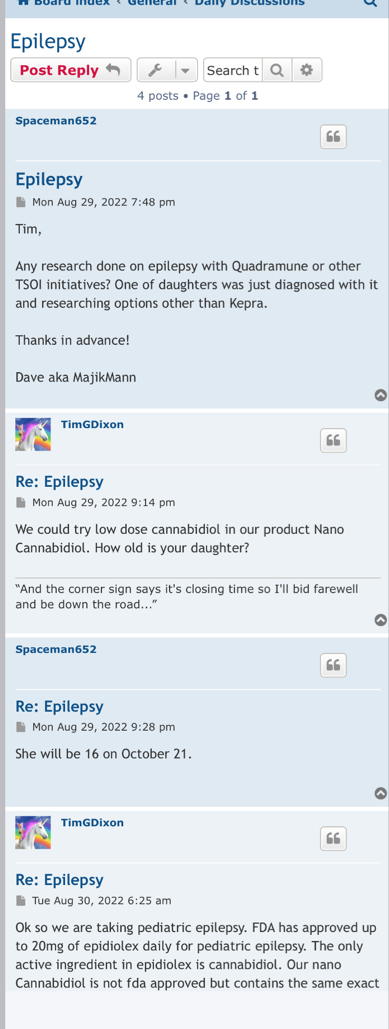
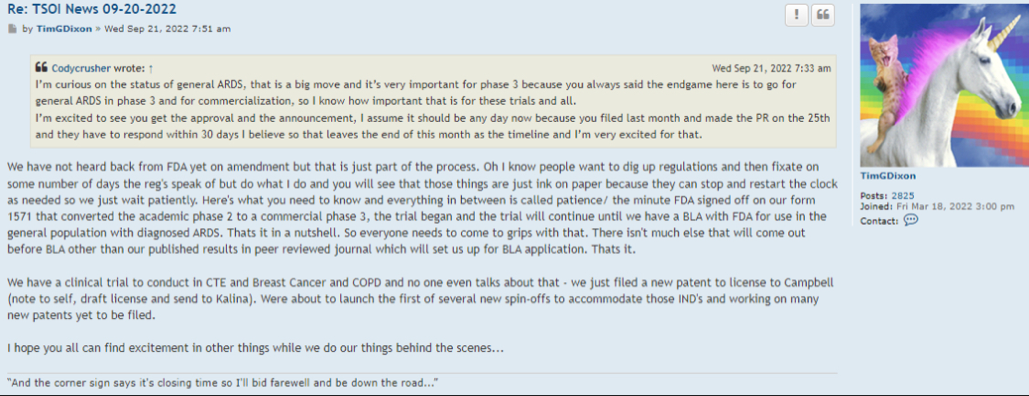



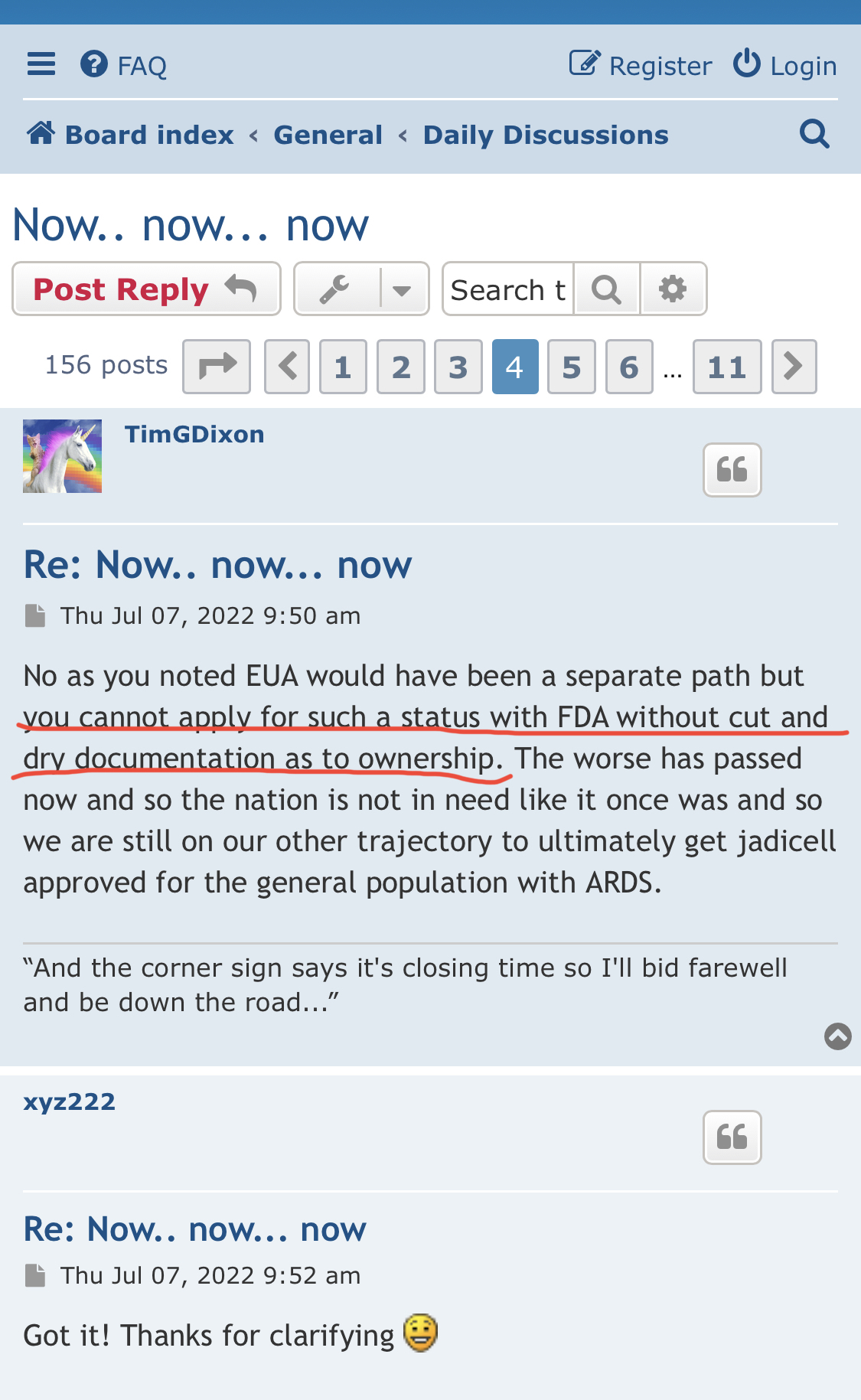
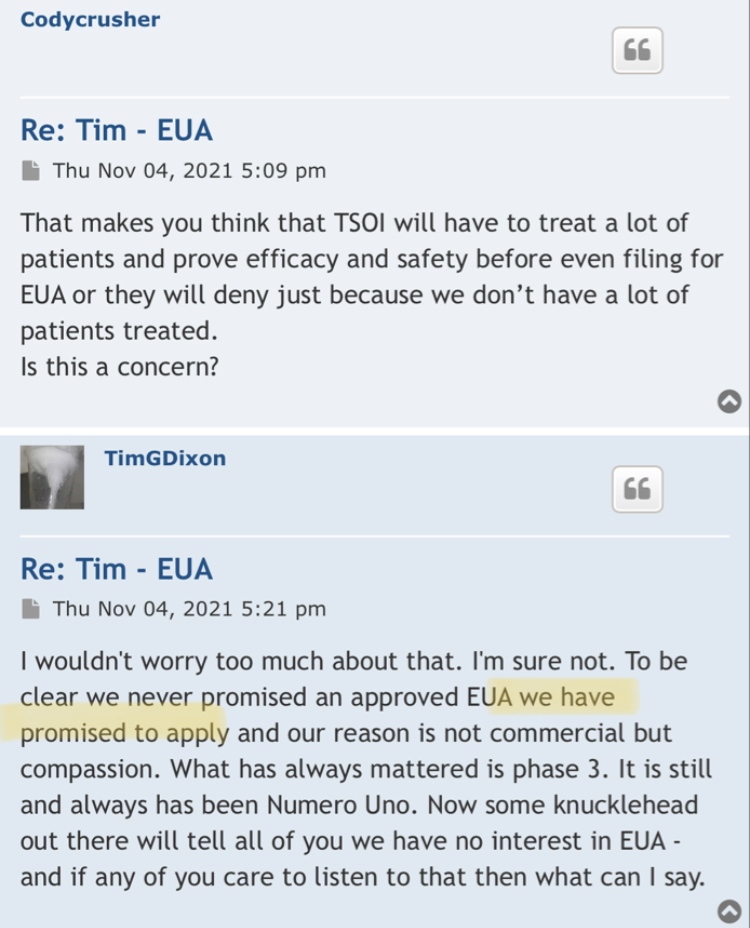

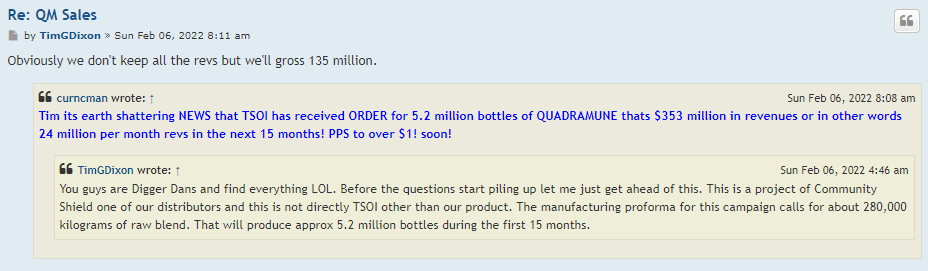
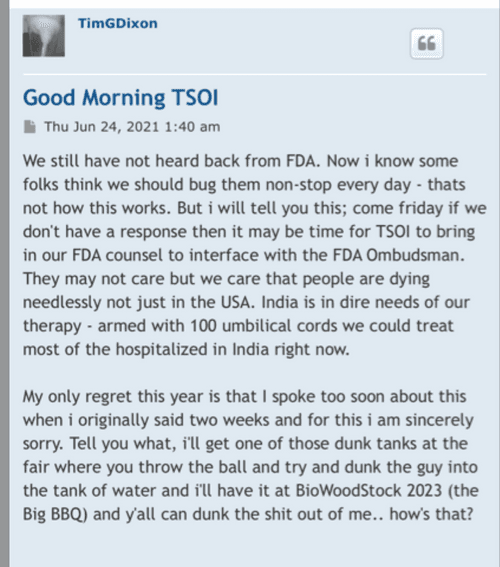
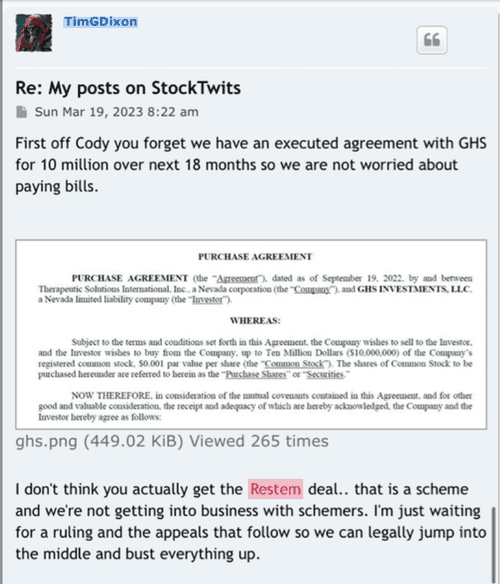



Preclinical Data Suggests QuadraMune™ Prevents Stress-Induced Suppression of Neurogenesis More Effectively than Prozac
OCEANSIDE, Calif., Dec. 9, 2020 /PRNewswire/ -- Therapeutics Solution International, Inc., (OTC Markets: TSOI), announced today new data suggesting the possibility that QuadraMune™ may mediate neuroprotective activity through preserving the ability of regenerative brain cells to proliferate subsequent to psychological stress.
The experiments, which involved exposing mice to established stressors, demonstrated that specific areas of the brain associated with production of new brain cells are damaged by stress. In agreement with previously published research, administration of fluoxetine (Prozac™) protected the brain from stress-induced damage. Surprisingly, QuadraMune™ administration appeared superior to Prozac™ at stimulating proliferation of new brain cells.
"QuadraMune™ which is currently in a clinical trial for prevention of COVD-191, has also been demonstrated to possess anti-inflammatory activity in other clinical trials, suppressing cytokines such as IL-62, which are known to be involved in depression3 and suicide4" said Kalina O'Connor, Director of Campbell Neurosciences and co-inventor on the patent. "Given major depressive disorder causes a significant risk for suicide, we are highly interested in exploring the use of QuadraMune™ for preventing suicide."
"Although much enthusiasm has been generated over the planned distribution of the COVID vaccine, at present little is being done to address mental health issues that are being exacerbated by the current pandemic" said Dr. James Veltmeyer, co-inventor of the patent, and Chief Medical Officer of the Company. "If current results are reproducible, the possibility that a nutraceutical would concurrently boost immunity while preserving mental health is highly enticing."
"It has not escaped us that COVID-19 is associated with increased inflammatory cytokines in the blood of patients, cytokines that also predispose to depression" said Famela Ramos, Vice President of Business Development for the Company. "It may be that the recent increase in suicides and suicide attempts is related biologically to activities of the coronavirus. It will be interesting to examine whether QuadraMune™ may modify putative negative mental effects of the virus."
"An estimated 17.3 million adults in the United States had at least one major depressive episode. This number represented 7.1% of all U.S. adults" stated Timothy Dixon, President and CEO of the Company. "We believe the Mission of our Company is not just providing a return on investment to our shareholders, but also increasing the quality of life for Americans. We are extremely pleased to report this unexpected finding with significant potential implications to advancing non-toxic means of helping patients with this terrible condition."
1 QuadraMune(TM) for Prevention of COVID-19 - Full Text View - ClinicalTrials.gov
2 Therapeutic Solutions International Announces Positive Preclinical and Clinical Evaluation of Nutritional Supplement QuadraMune™, Designed to Protect Against COVID-19 | BioSpace
3 Ting et al. Role of Interleukin-6 in Depressive Disorder. Int J Mol Sci. 2020 Mar 22;21(6):2194.
4 O'Donovan et al. Suicidal ideation is associated with elevated inflammation in patients with major depressive disorder. Depress Anxiety. 2013 Apr;30(4):307-14.
| Volume | |
| Day Range: | |
| Bid Price | |
| Ask Price | |
| Last Trade Time: |
