Register for free to join our community of investors and share your ideas. You will also get access to streaming quotes, interactive charts, trades, portfolio, live options flow and more tools.
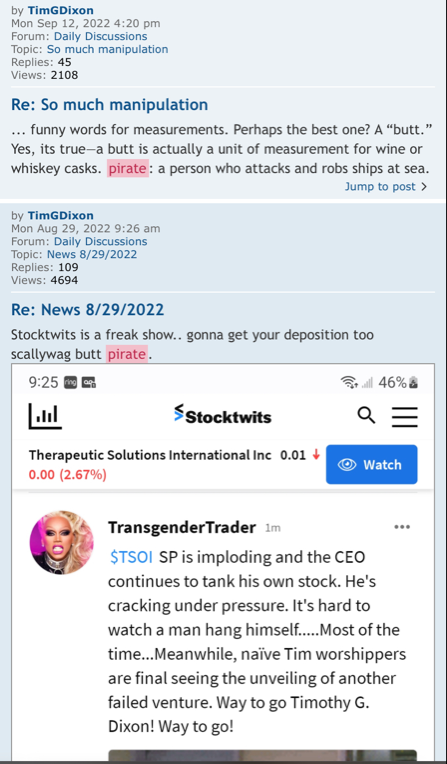
It's really hard to imagine that anyone could believe this is a scam company. The good TSOI will do in the future is mind boggling.
Cellular Manufacturing and Cell Banking
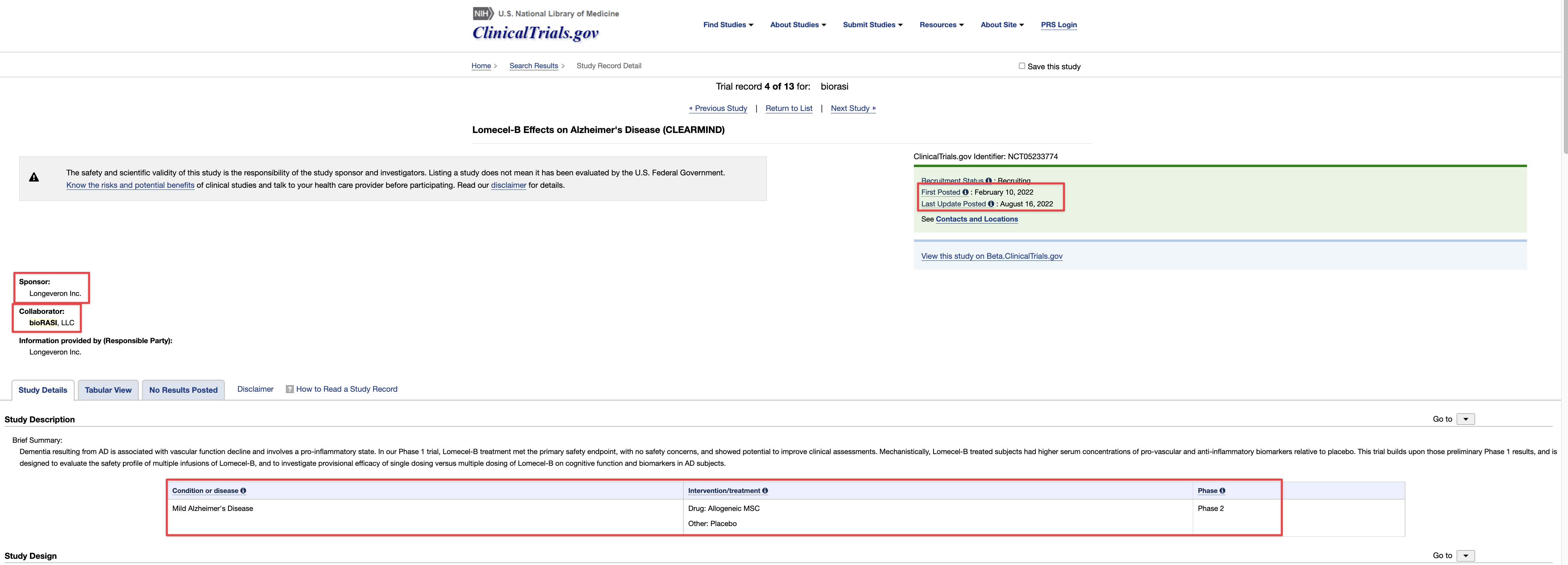
On October 18, 2021, the Company announced the formation of Allogen Biologics Inc, a wholly owned subsidiary of TSOI. Allogen Biologics will house intellectual property and Standard Operating Procedures related to generation of the Company’s existing and anticipated cellular therapeutics. In addition, Allogen will house and maintain all relevant cell banks.
On May 10, 2022, Allogen Biologic, Inc, and Therapeutic Solutions International Inc, entered into an Exclusive Patent License Agreement (EPLA) for Patent Application Serial No. 63/254,469, filed by Licensor and titled as: Umbilical Cord Derived Regenerative and Immune Modulatory Stem Cell Populations.
Schizophrenia/Suicide Clinical Programs
On October 29, 2020, the Company announced publication on the NIH clinical trials website of its newly initiated trial aiming to validate a blood-based diagnostic for predicting suicide risk and is listed as NCT04606875.
The Campbell Score™, which is a patent-pending method of quantifying inflammatory-associated biological markers, has previously been shown in pilot investigator-initiated studies to correlate with propensity for suicide. Based on positive feedback from collaborators, the Company decided to initiate a formal clinical trial to validate correlations between the Campbell Score™ and established psychiatric assessment tools of suicidal propensity. Currently the only means of quantifying predisposition to suicide is based on psychological, question-based techniques.
On December 31, 2020, the Company signed license agreements with Campbell Neurosciences Inc., a partially owned company, for access to the 9 patents filed related to the previous Campbell Neurosciences Division. The patents are:
1. 63/128759 Immunotherapy for Opioid Addiction
2. 63/122862 Treatment of Major Depressive Disorder and Suicidal Ideations Through Stimulation of Hippocampal Neurogenesis Utilizing Plant-Based Approaches
3. 63/105964 Protection/Regeneration of Neurological Function by Endothelial Protection/Rejuvenation using Stem Cells for Treatment of Conditions such as Chronic Traumatic Encephalopathy and Schizophrenia
4. 17/030416 Personalized Immunotherapies for Reduction of Brain Inflammation and Suicide Prevention
5. 63/077723 Immunotherapy of Schizophrenia and Schizophrenia Associated Suicidal Ideation/Suicide
6. 63/071381 Upregulation of Therapeutic T Regulatory Cells and Suppression of Suicidal Ideations in Response to Inflammation by Administration of Nutraceutical Compositions Alone or Combined with Minocycline
7. 63/068388 Methods of Determining Risk of Suicide and/or Suicidal Ideation by Immunological Assessment
8. 63/061202 Prevention of Neuroinflammation associated Memory Loss Using Nutraceutical Compositions
9. 63/057315 Neuroprotection and Neuroregeneration by Pterostilbene and Compositions Thereof
Additionally, Campbell Neurosciences Inc. has entered into purchase agreements with Therapeutic Solutions International ensuring a continued supply, at a discounted rate, of nutraceuticals which are being explored for antiinflammation/suicide prevention activity.
Investigational Drug Applications:
(Revised, after reading the S3)
Treatment of Metastatic Breast Cancer by StemVacs-V Cancer Immunotherapeutic IND # (a number has not been assigned to this one yet)
The Primary Objective is safety and feasibility of StemVacs-V administration at 12 months as assessed by lack of adverse medical events. The Secondary Objective is efficacy as judged by tumor response, time to progression, and immunological monitoring.
Safety, Feasibility, and Immunomodulatory Activities of StemVacs in Patients with Advanced Solid Tumors IND # 17448
The Primary Objective is safety and feasibility of StemVacs administration at 12 months as assessed by lack of adverse medical events. The Secondary Objective is efficacy as judged by tumor response, time to progression, and immunological monitoring.
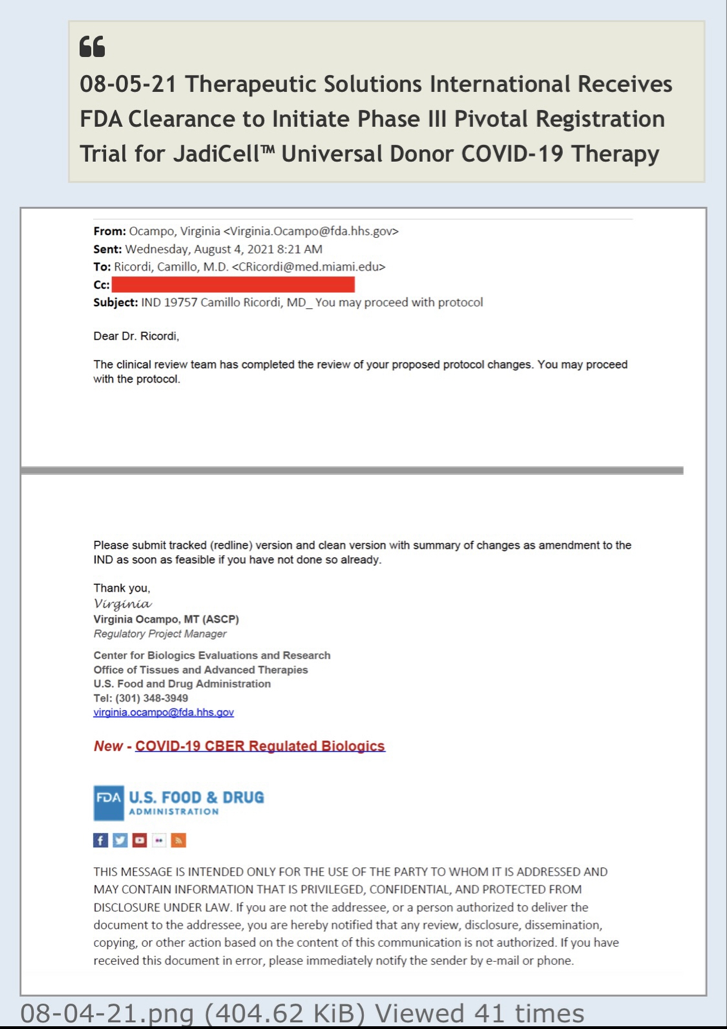
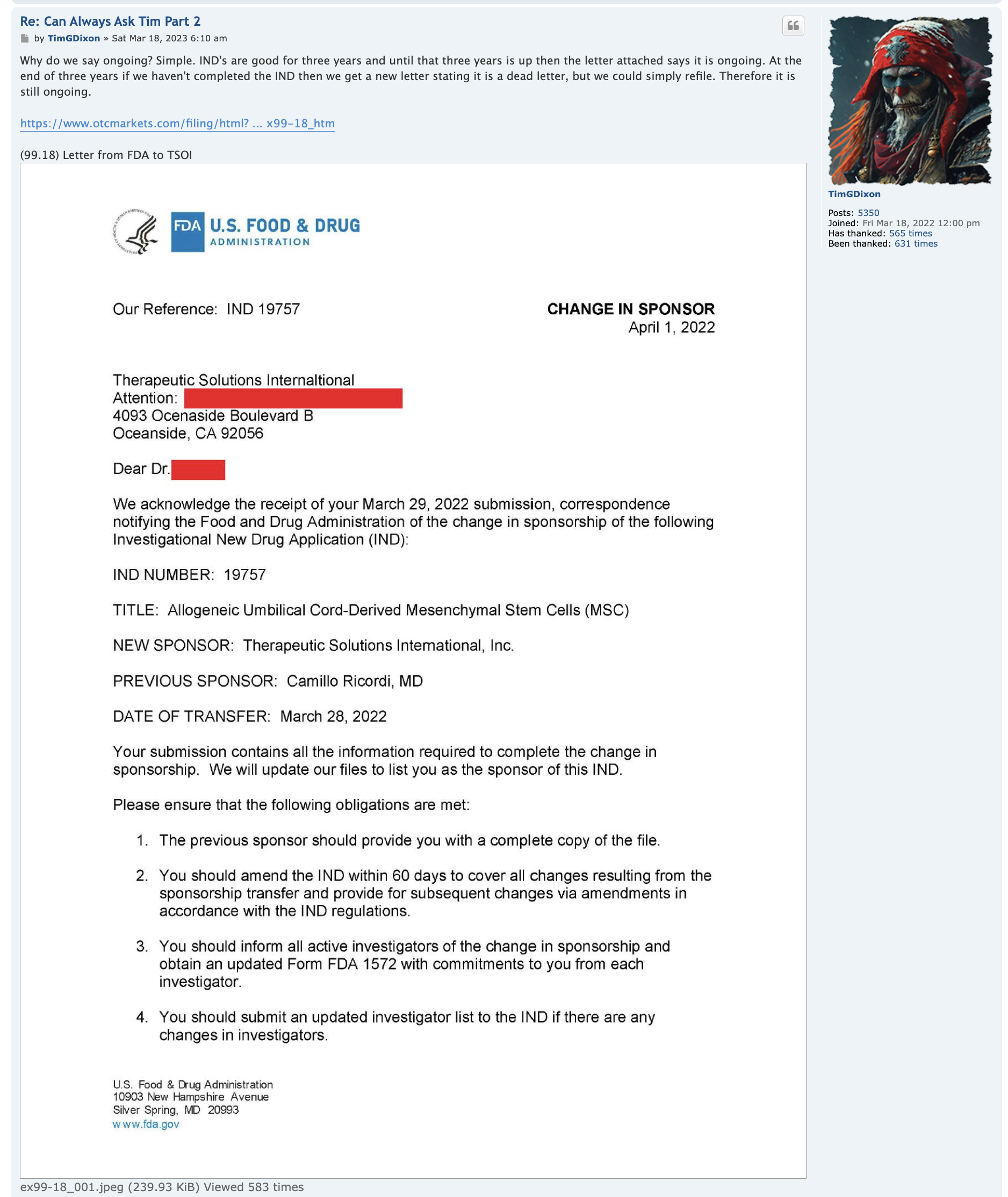
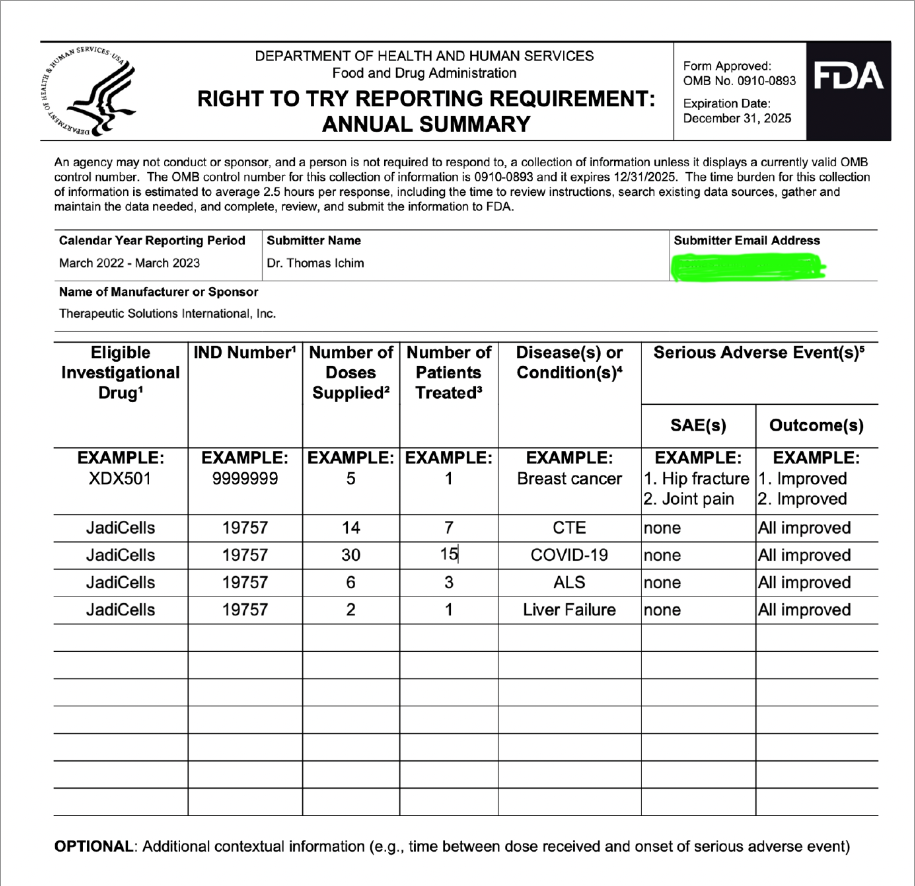
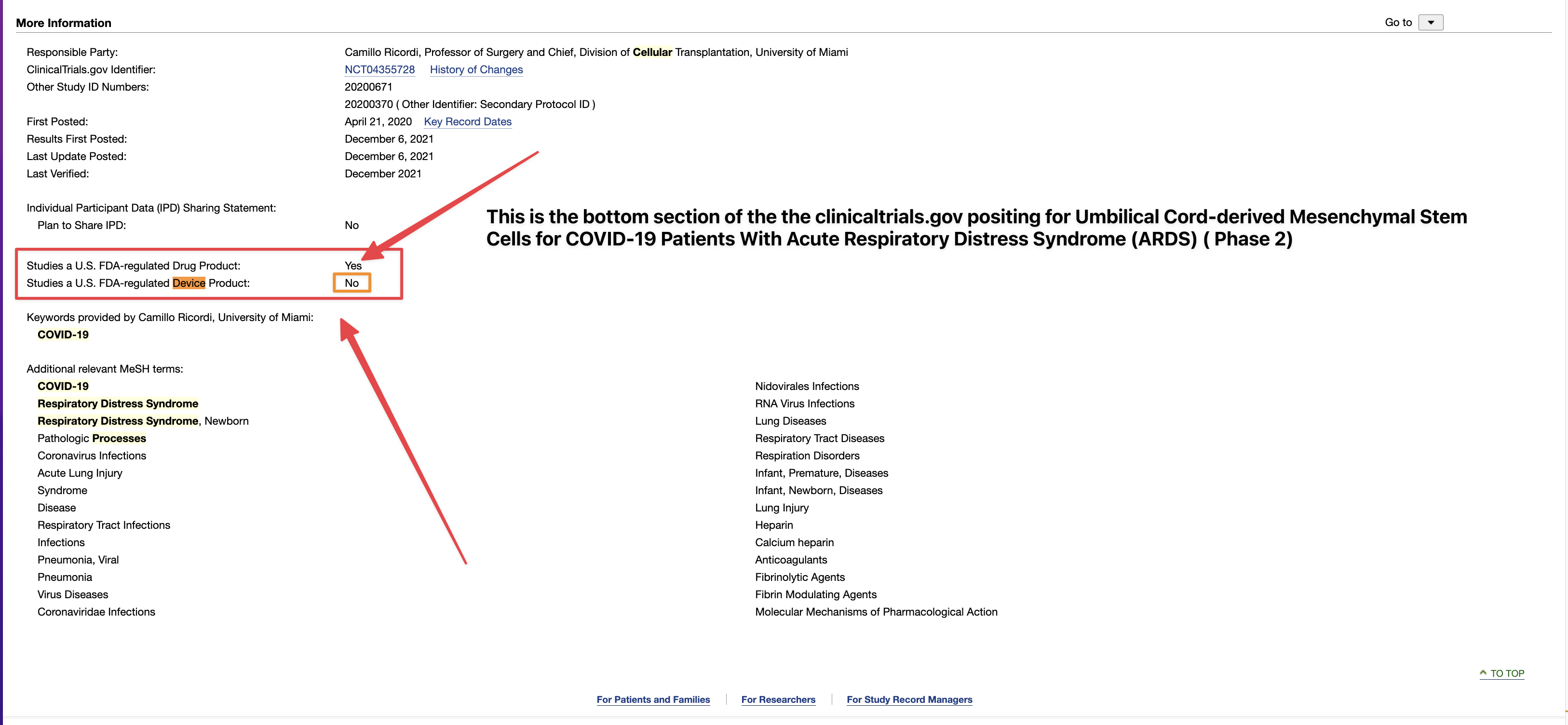
Umbilical Cord-derived Mesenchymal Stem Cells for Patients with COVID-19 (“UC-MSC for COVID-19”) IND # 19757
The primary objective will be to assess effectiveness of UC-MSC treatment on proportion of patients alive and free of respiratory failure at Day 60 after randomization. The secondary objectives will be to assess all-cause mortality at Day 60, survival at day 31, number of subjects experiencing serious adverse events (SAEs) by day 31, SAE-free survival, time to recovery (evaluated until day 60), and time to oxygen requirement equal or below 40% oxygen.
Investigation of Umbilical Cord-derived Mesenchymal Stem Cells for the Treatment of Chronic Traumatic Encephalopathy Patients IND # 27377
To determine safety and efficacy of 100 million intravenously administered JadiCell™ allogeneic umbilical cord mesenchymal stem cells. Efficacy will be determined by behavioral scores, brain imaging, and reduction in inflammatory markers. Toxicity of treatment was evaluated for the duration of the study and will be graded according to the criteria of the World Health Organization.
JadiCell Therapy for COPD IND # 28508
To determine safety and efficacy of intravenously administered allogeneic JadiCell umbilical cord blood mesenchymal stem cells in patients with moderate-to-severe COPD. The Primary Endpoint, which is toxicity, will be assessed by number of adverse events (AEs). The Secondary Endpoint, which is efficacy will be evaluated at baseline and days 30, 60, and 90.
Yes and this time it held see if we can test 02 today
the hope and dream traders going to get lured in another time before they pull the rug
$TSOI Really broke out yesterday..has a little more to go before it tests resistance..
https://stockcharts.com/c-sc/sc?s=TSOI&p=D&b=5&g=0&i=0&r=1664358947979
Right now? Hard to say. Hope it continues up.
Let’s hope it continues tomorrow
JMC$ do you like this line from the filing? Let me know your thoughts.
In addition, the Company has filed data with the FDA, as part of IND #17448, which demonstrated that treatment of cancer patients with StemVacs™ resulted in enhanced activity of a type of immunological cell called “natural killer” cells, otherwise known as “NK cells.”
Now that is something I can agree on...... today that is
Bell it would take an arm full of good news to drive this app and keep it up. It’s just like tractor for sale said it’s a penny stock penny stocks go up and then come down you got to take advantage of it while it’s up and when it comes down by back in cheaper so what I’ve been doing
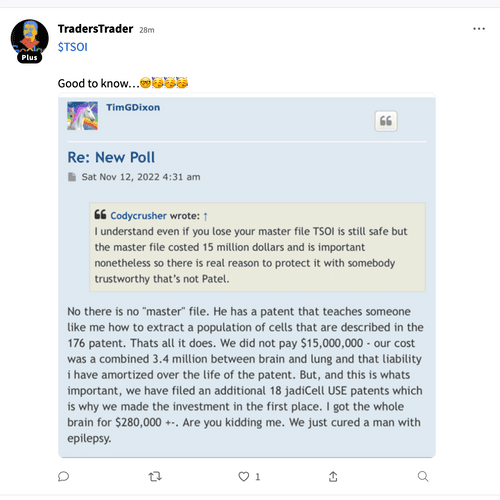
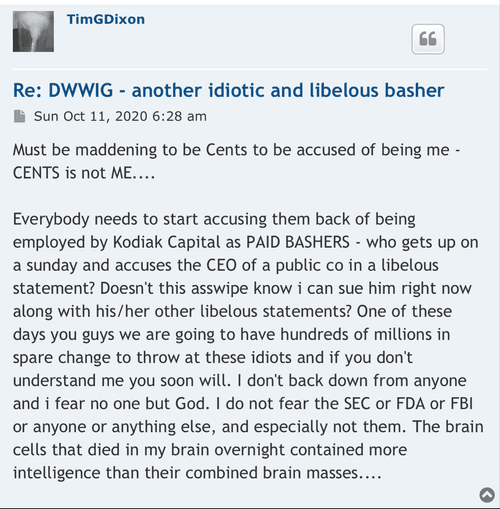
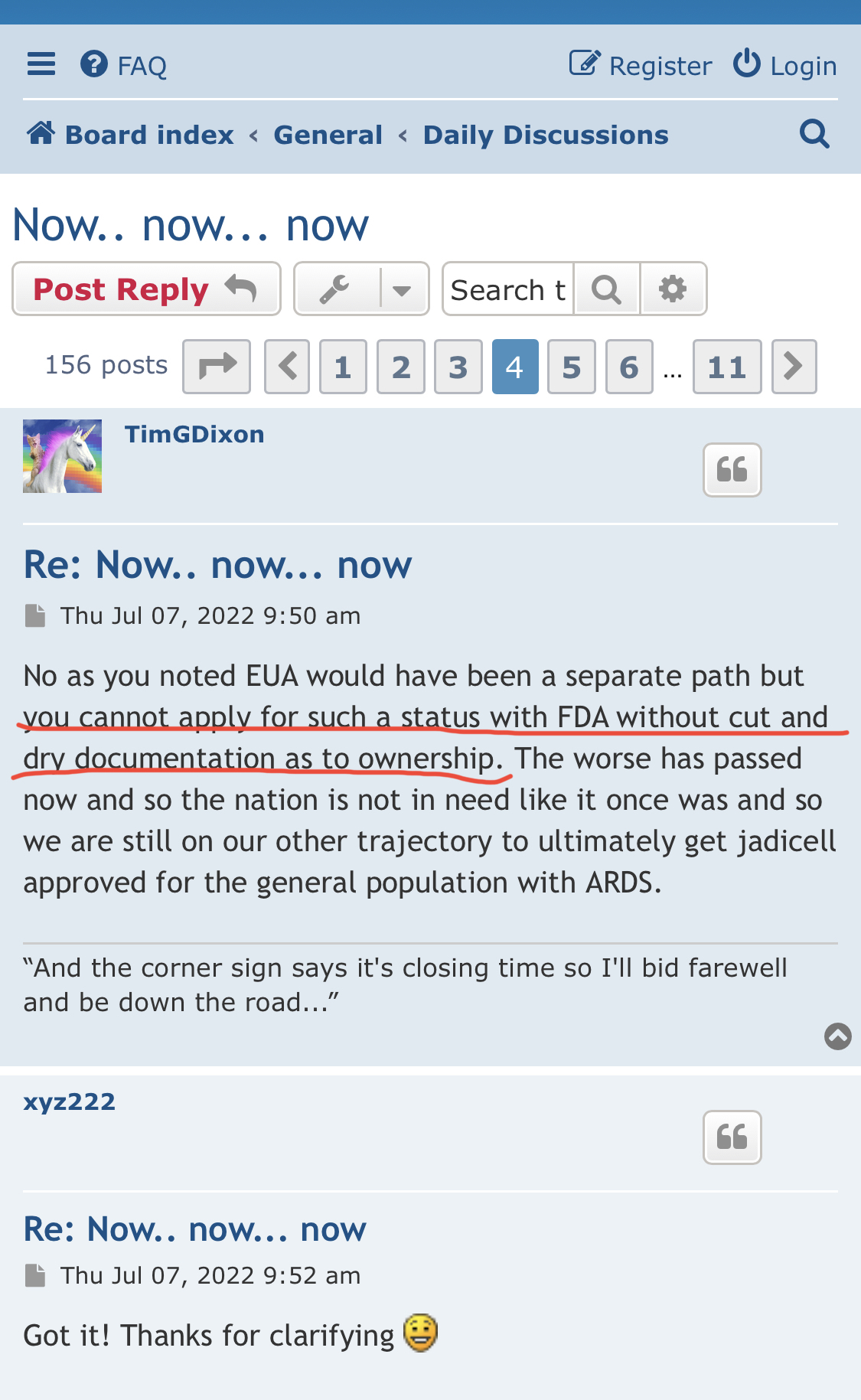
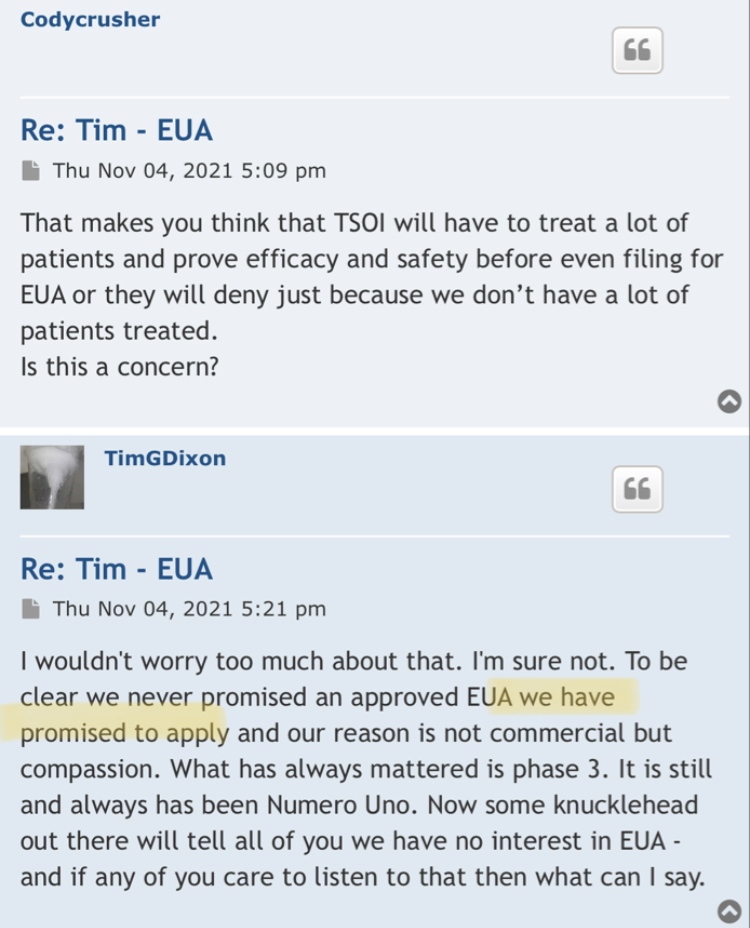
There’s a chance that we get some small bumps along the way but basically it’s going to be another year before phase 3 is completed and successful with word from the FDA. Those intellectual properties he keeps filing do not do any good right now but they will down the line but right now all that we have can’t even hold us at two cents which is pathetic.
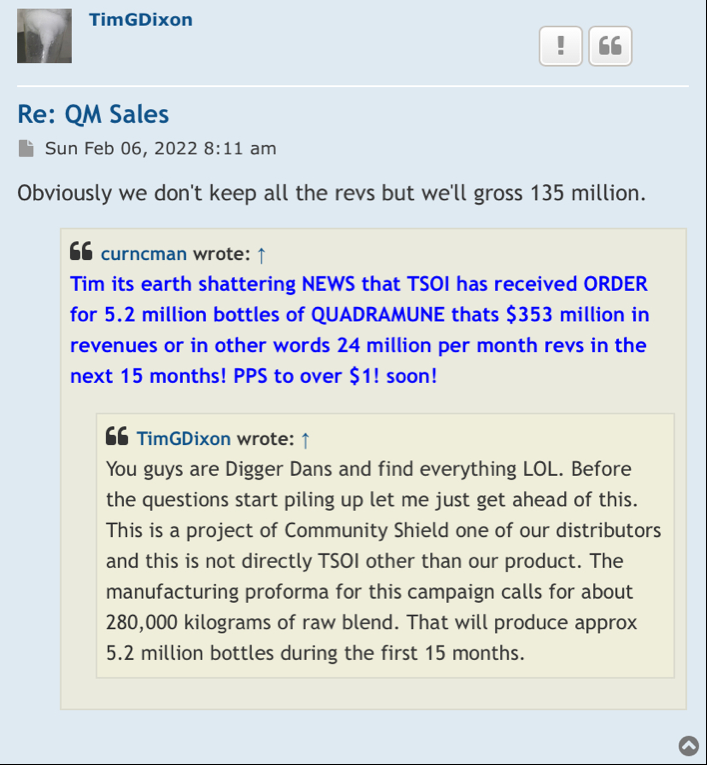
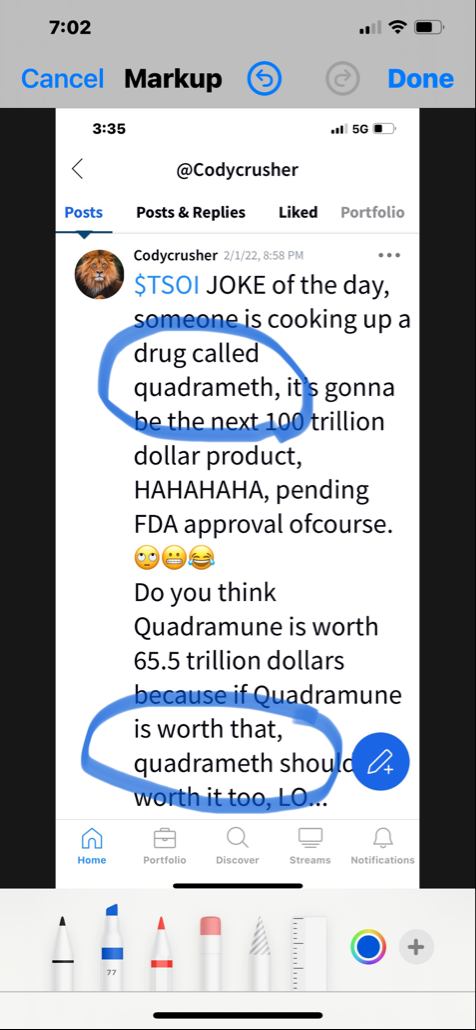
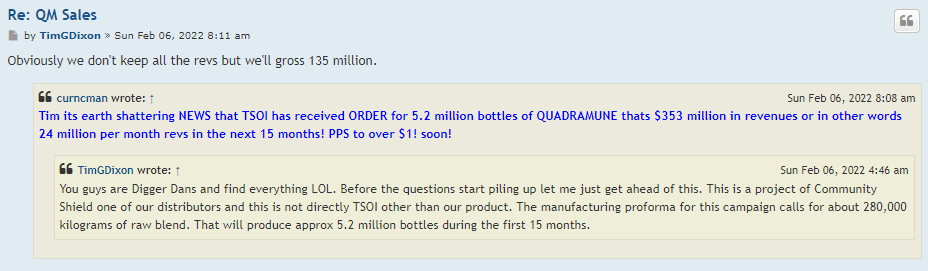
In my opinion this is a one-man show he’s told us that many times I am an item are the only board members and that’s going to stay that way. And personally I think that’s too bad just like shooting yourself in the foot sometimes. For this stock to go up we have got to have revenue coming in way more than what Quadrum you can bring them and that group of things.
Next thing I would like to know is how the spin offs are going to help nobody knows about us to begin with that’s one of the problems. I bet once or twice a week I hear on the news media about some product or new way of medicine even if it’s not been approved by the FDA yet still being touted over the air letting people know what’s being worked on in and some of the successes. When I went to a town that serves over 200,000 people to the supplement store I asked them if they was carrying Quadry moon. It’s a nice store and they’ve got tons of supplements in there and they said to me Quadra watt. I told him to contact community shield out of California I think that’s who the distributor is.
Long...Train...Runnin...
i'm looking for a re-awakening
like a 'this old house' rebuild to the final unveiling
Hopefully some good news can drive this up further.
Will these gains be sold off end of day today, or sold off tomorrow? Has to be one or the other, given there’s been no substantive news to drive the buying.
#MightyFineDay
TSOI Therapeutic Solutions International Inc (PK)
0.0154
0.00365 (31.06%)
Volume: 5,939,195
Day Range: 0.0115 - 0.0161
Bid: 0.0155
Ask: 0.0156
Last Trade Time: 2:27:59 PM EDT
Total Trades: 143
I hope the squeeze lasts a long time TSOI.
interesting indeed ...
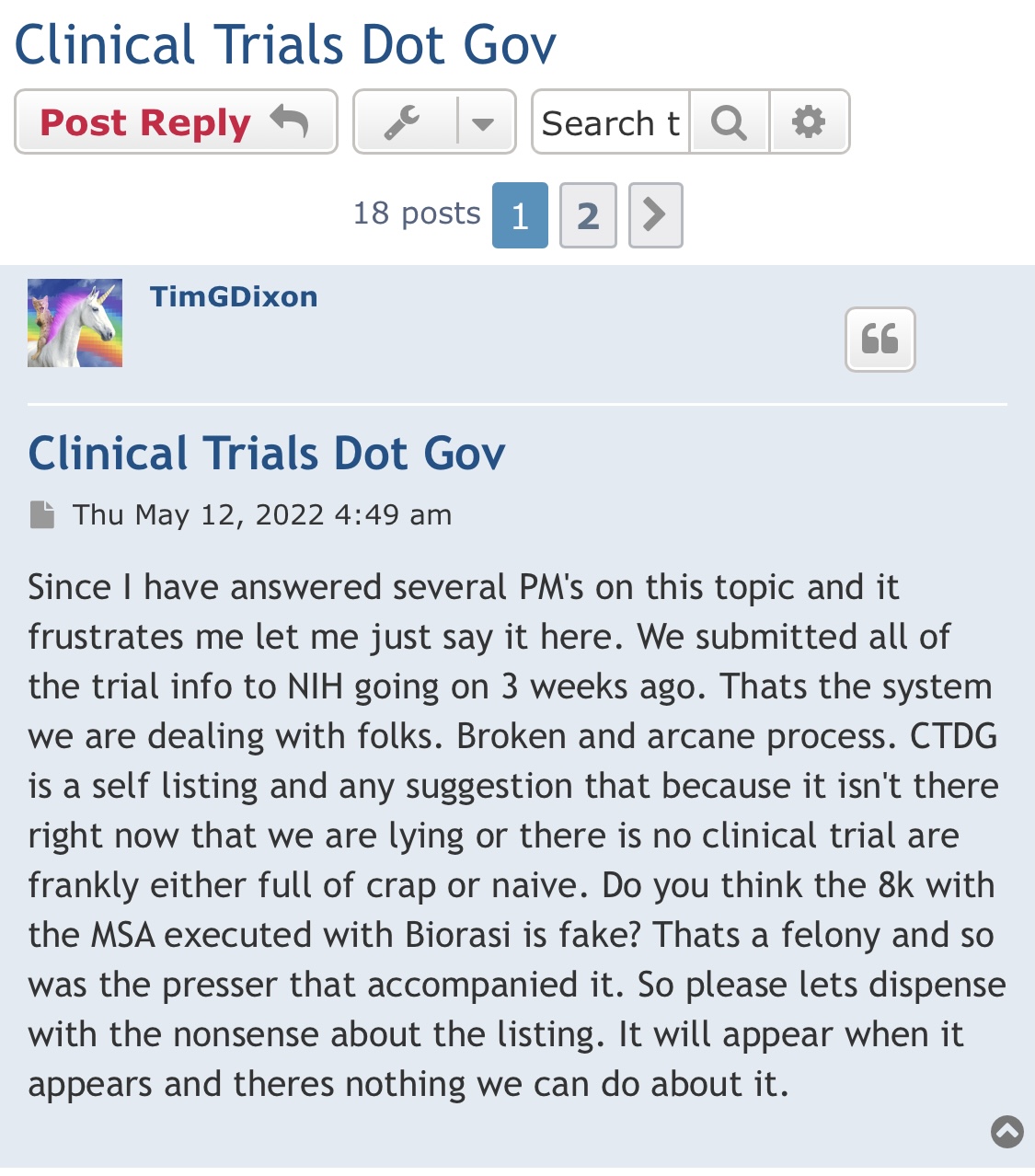
Patent filings = 66
that's some stuff comin our way as investors that seek
a multi year plan for the future in Lung repair, w/ COPD, and other lung issues.
and why you and i invested in TSOI , on a Bio Solution to restoration in several Bio tech fields.
well i hope the PPS shows an upward swing,
cuz a swing in this stock PPS is long overdue
3.5mm shares purchased in the last hour and half...Huge volume compared to where this normally trades
Pegged it again Bill
brains and longevity :)
there are cycles to this.
Pickin a pick aint for the queasy in Bio Phama.
It requires real DD background checks
back and forth ,
to and fro, and you have to have your trading hat on when it accelerates
Low vol skweeze or something koming
GL2U cvytrader in all of your trades
:)
Thank you Cents, you've been doing it for several years here now hope I get to meet you at the BBQ
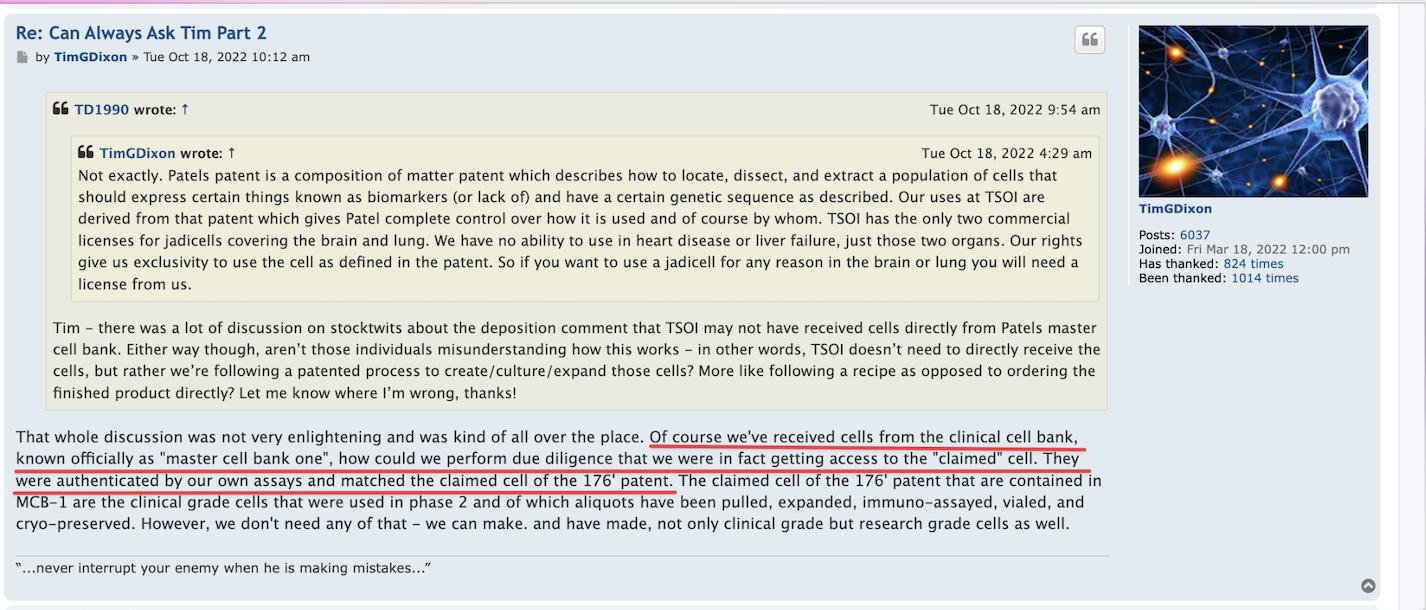
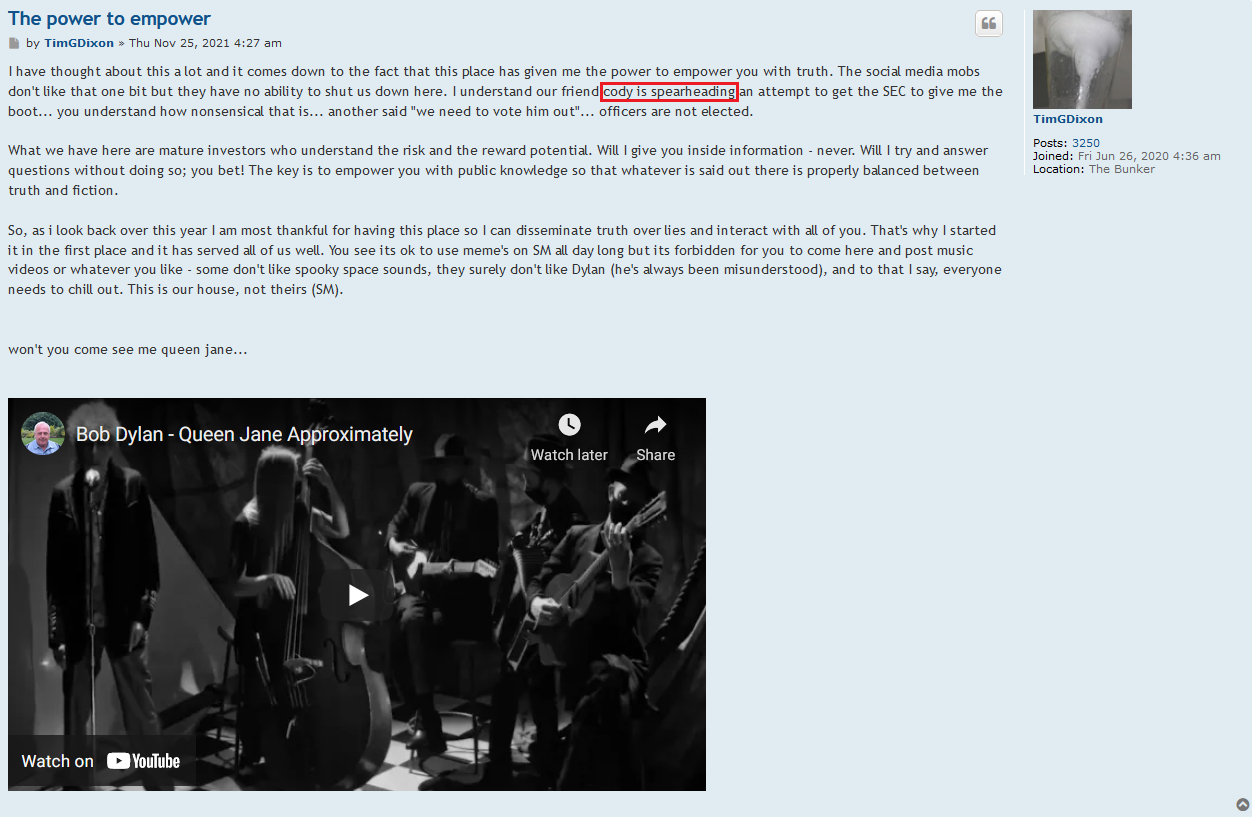
..wait...what? What are you speaking of...they are selling useless patent applications? Could elaborate on what part of the PR is Fluff....They announced the source of the funding, a non-toxic funding source where a reputable company is investing in this company by purchasing shares? How is that fluff?
these are the TSOI trademarks,
so don't whine and complain when you get free DD
https://uspto.report/company/Therapeutic-Solutions-I-N-T-Inc
That stupid funding company must of had an extra 10 million dollars to lose! Makes sense to me! Up goes TSOI…
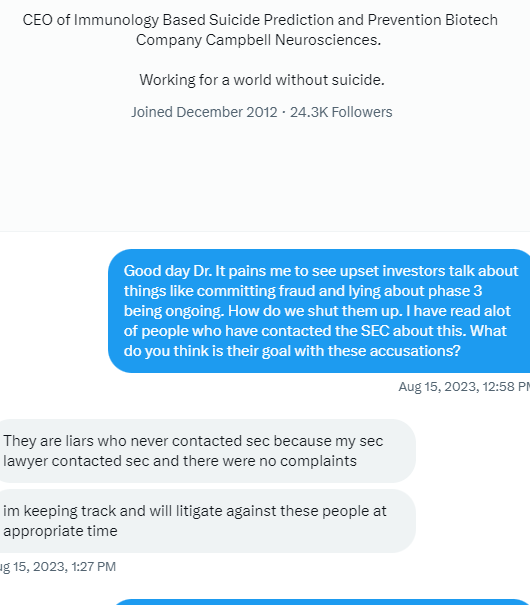
TSOI: You are NOT wrong, Sir. This SCAM Company sells their SHARES, & their useless Patent Applications, & their fluff PR's about how they just got MORE FUNDING from some stupid FUNDING COMPANY.
TSOI wants to try & change the box, question is does retail continue to feed the (bid) need or support while not stacking the ask
better Start for Therapeutic Solutions International Inc (PK)
0.0138 0.00205 (17.45%)
Volume: 497,828
Day Range: 0.0115 - 0.0138
Bid: 0.0126 Ask: 0.0138
Last Trade Time: 10:05:39 AM EDT
Total Trades: 16
Glad you recognize it. You have yourself a blessed day.
So that must mean TSOI was a dilution machine prior to August 9, 2021 since TSOI has never been profitable! Gotta love that DD! What an investment catalyst! Thanks for clarifying!
It’s just a dilution machine. Thanks for clarifying
And none of those things existed before August 9, 2021? Obviously! Gotta live that TSOI DD…
Right and just award themselves 10s of million more shares every time the wind changes. Well we know what’s for sure around TSOI. Dilution. Dilution misdirection lies and patents. Those are the sure things with TSOI
And at the same time, Tim and Tom lose at the same time during the bleeding out! Sounds like a win for everyone! Trade penny stocks and don’t fall in love with them…
Only difference is "Freely" NO Use of Rule 144
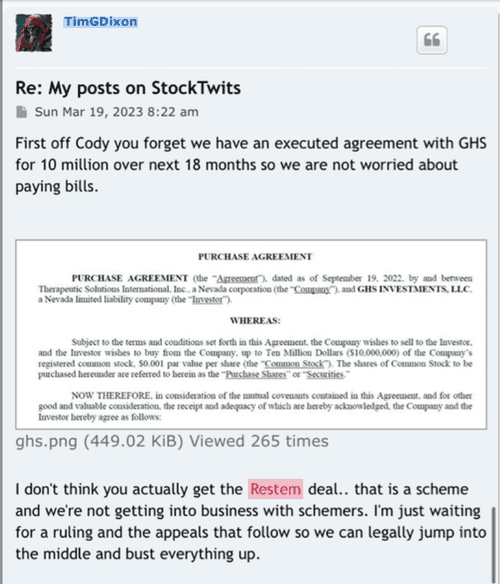
GHS Purchased Shares Freely Tradable w/o restrictions on resale
as per Purchase Agreement
(b) Settlement for Purchase Shares
All Purchase Shares issued hereunder will be DWAC Shares.
What does that mean, well as per Purchase Agreement
DWAC Shares means shares of Common Stock that are (i) issued in electronic form. (ii)freely tradable and without restriction on resale and (iii) timely credited by the company to the Investor's or it's designee's specific Deposit Withdrawal at Custodian (DWAC) account with DTC under it's Fast Automated Securities Transfer (FAST) Program, or any similar program hereafter adopted by DTC performing substantially the same function.
Exactly bleed every cent out of investors. It’s the name of the game
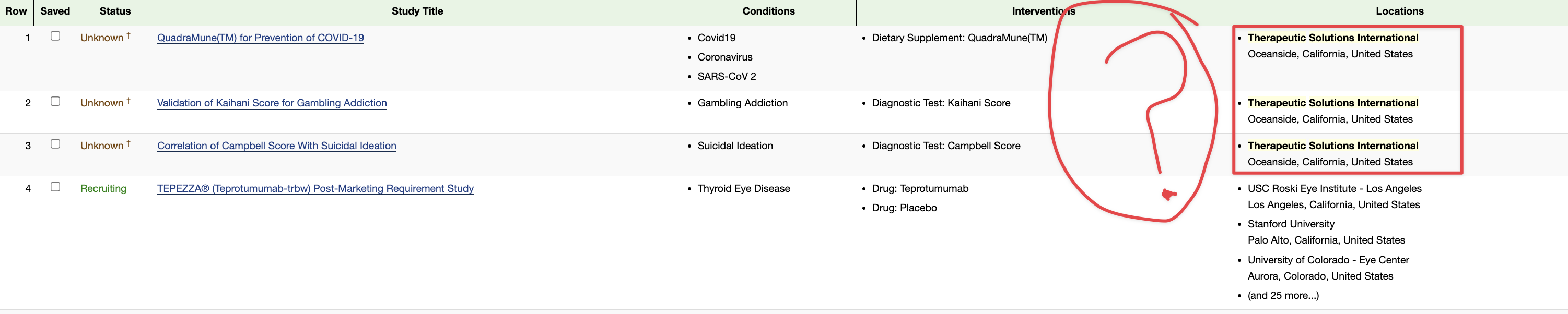
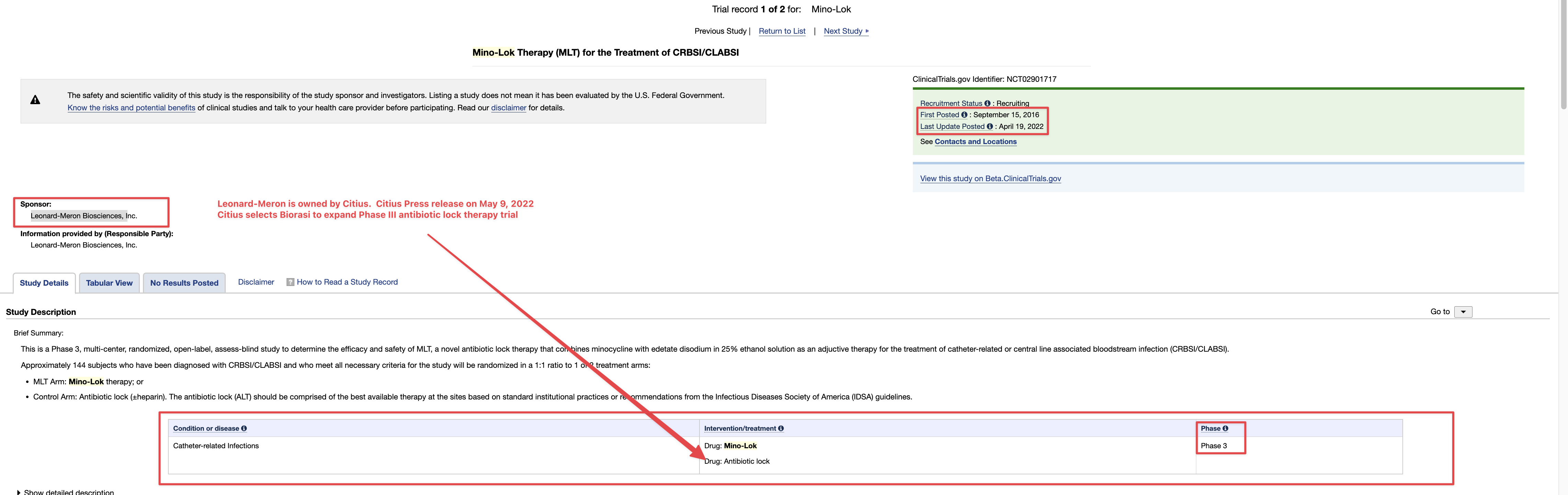
TSOI INDs
here is a start :
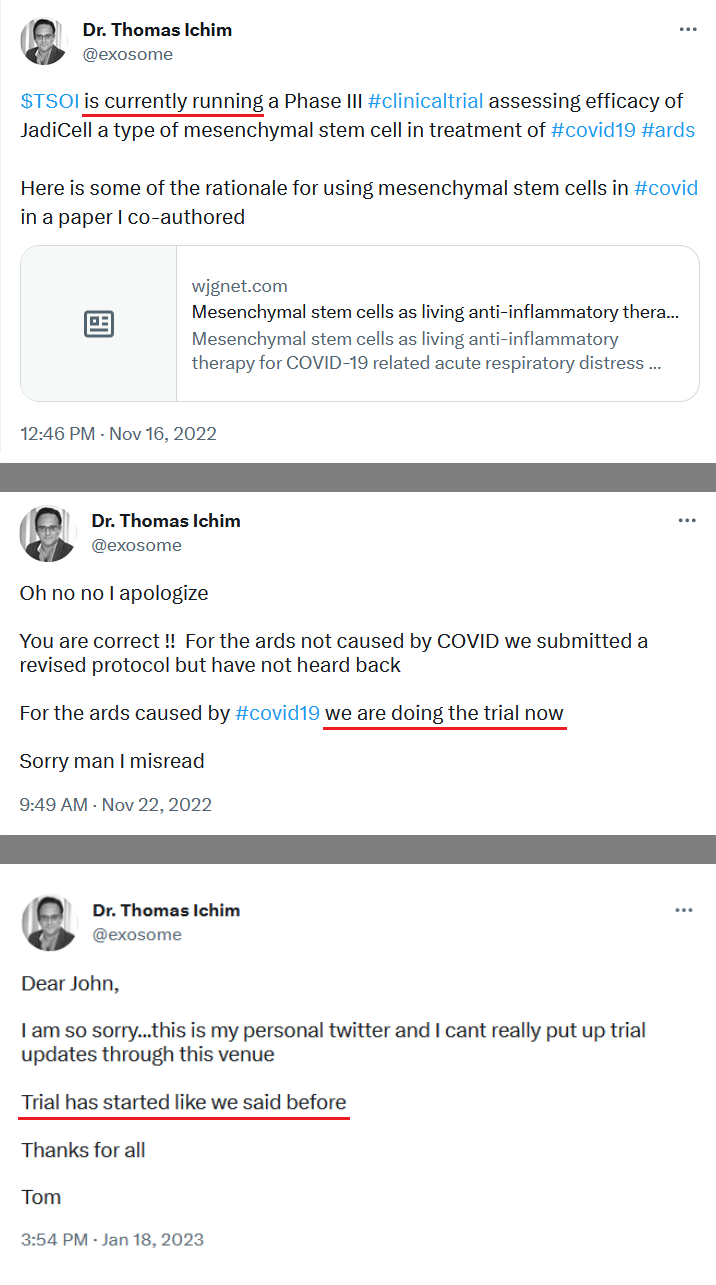
Therapeutic Solutions International Receives IND Number 28508 for Chronic Obstructive Pulmonary Disease Clinical Trial and Enters Binding Discussions with FDA for Initiation of Phase I/II Clinical Trial
https://www.businesswire.com/news/home/20220523005584/en/Therapeutic-Solutions-International-Receives-IND-Number-28508-for-Chronic-Obstructive-Pulmonary-Disease-Clinical-Trial-and-Enters-Binding-Discussions-with-FDA-for-Initiation-of-Phase-III-Clinical-Trial
Company Responds to Rapidly Growing COVID-19 Cases by Increasing Mechanisms to Make Lung Healing Cells Available
ELK CITY, Idaho, July 18, 2022--(BUSINESS WIRE)--Therapeutic Solutions International announced today granting of Emergency IND # 28685
https://www.yahoo.com/now/therapeutic-solutions-international-granted-emergency-130000290.html
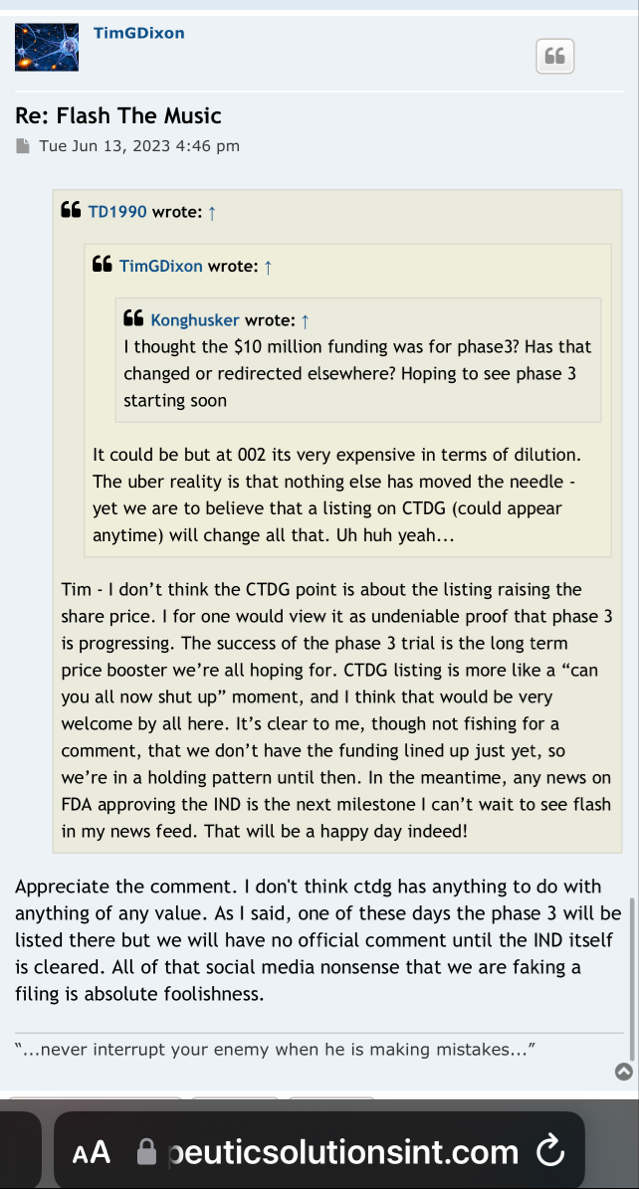
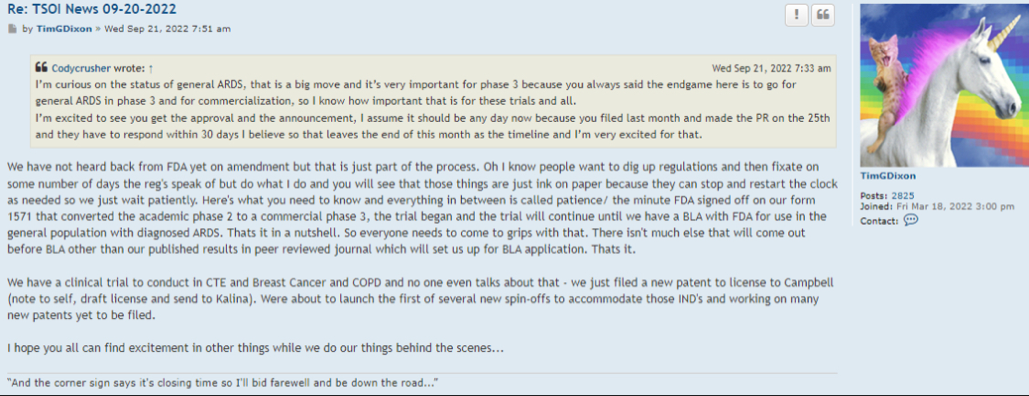
why would Dixon change now, just because of a Phase III Trial awaiting hopefully an approved amendment by the FDA that would enhance enrollment (shall we say ????)
(e) Use of Proceeds. The Company will use the net proceeds from the offering for any corporate purpose at the sole discretion of the Company.
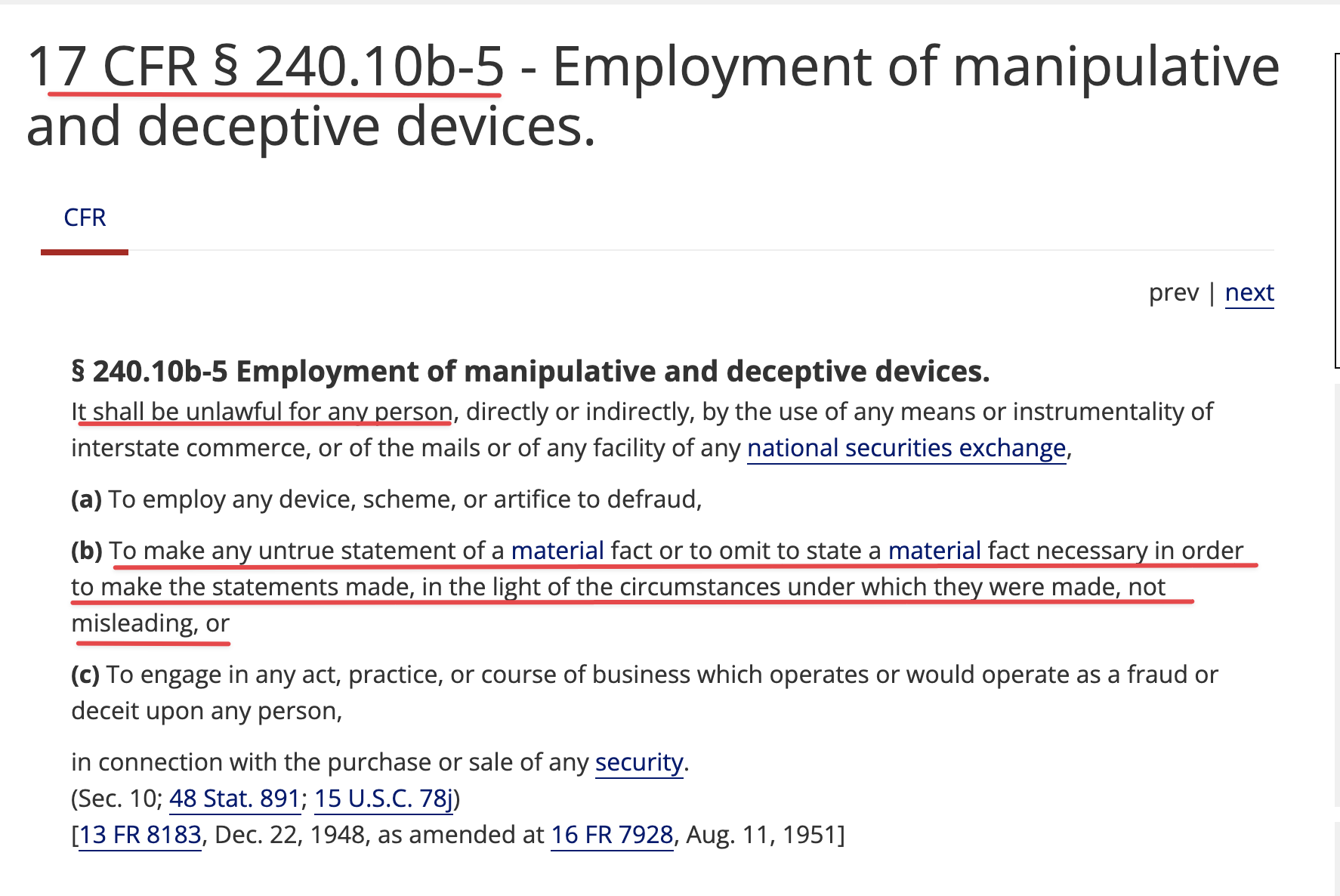
Subject: FILE NO S7-24-20.
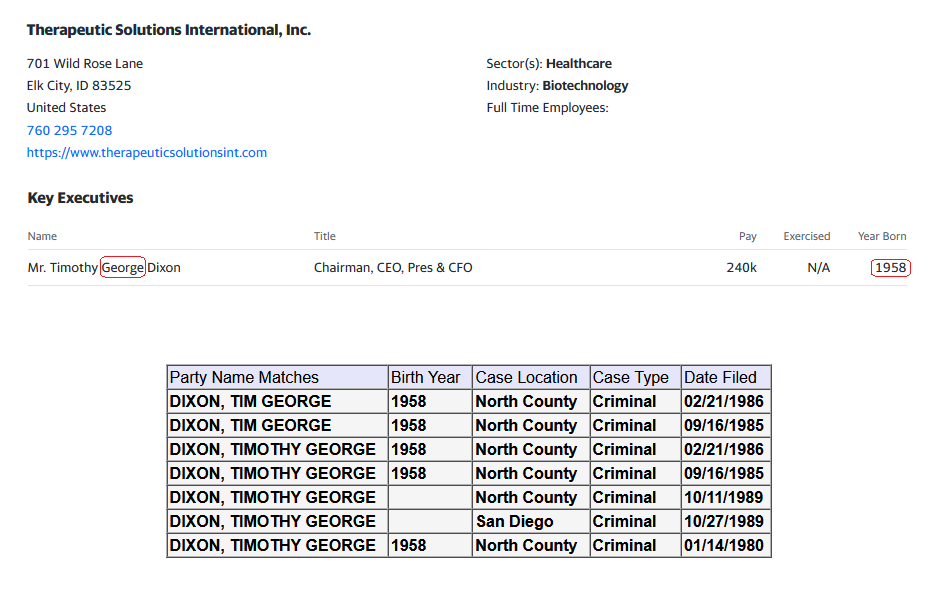
From: Tim Dixon
Affiliation:
Feb. 05, 2021
To Whom It May Concern,
The Company I run, Therapeutic Solutions International, Inc. (TSOI) has relied upon the availability of convertible debentures with the utilization of rule 144 as a means of financing the operations of TSOI. Had this not been available I would not have been able to advance the Company otherwise and most likely would not have been able to stay fully reporting. The loss of such instruments would be devastating to smaller companies like TSOI who cannot attract the big investment bankers the big boards keep in their pockets like loose change. Please consider carefully the impact this will have upon innovation and new technologies coming to market to benefit all of humanity.
Sincerely yours,
Timothy G Dixon, President & CEO
Therapeutic Solutions International, Inc.
Note 8 – Convertible Notes Payable
At various times during the six months ended June 30, 2022, the Company entered into convertible promissory notes with principal amounts totaling $337,500 with a third party for which the proceeds were used for operations. The Company received net proceeds of $315,000, and a $22,500 original issuance discount was recorded. The convertible promissory notes incur interest at rates from 10% to 12% per annum and mature on dates ranging from January 1, 2023 to June 27, 2023. The convertible promissory notes are convertible to shares of the Company’s common stock 180 days after issuance. The conversion price per share is equal to 63% of the average of the three (3) lowest trading prices of the Company’s common stock during the fifteen (15) trading days immediately preceding the applicable conversion date. The trading price is defined within the agreement as the closing bid price on the applicable trading market. The Company has the option to prepay the convertible notes in the first 180 days from closing subject to prepayment penalties ranging from 120% to 145% of principal balance plus interest, depending upon the date of prepayment. The convertible promissory notes include various default provisions for which the default interest rate increases to 22% per annum with the outstanding principal and accrued interest increasing by 150%. The Company was required to reserve at June 30, 2022 a total of 167,223,808 common shares in connection with these promissory notes.
Pg12 of 8k exhibit on purchase agreement
(e) Use of Proceeds. The Company will use the net proceeds from the offering for any corporate purpose at the sole discretion of the Company.
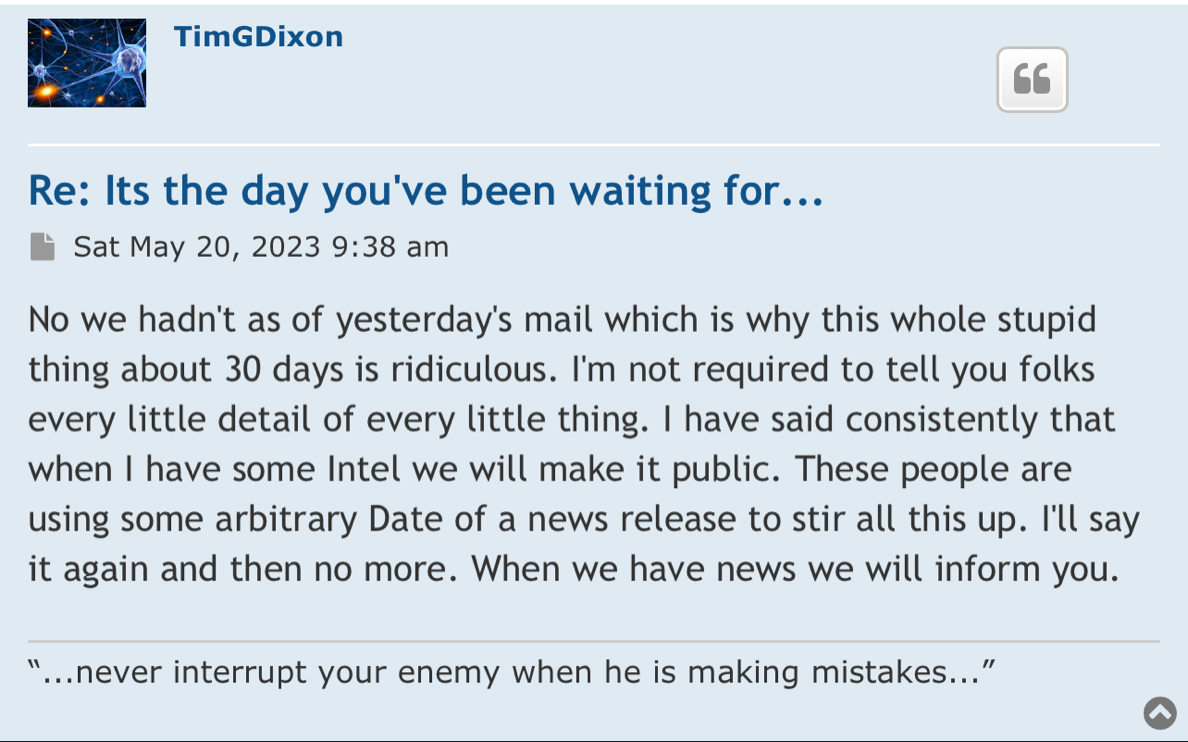
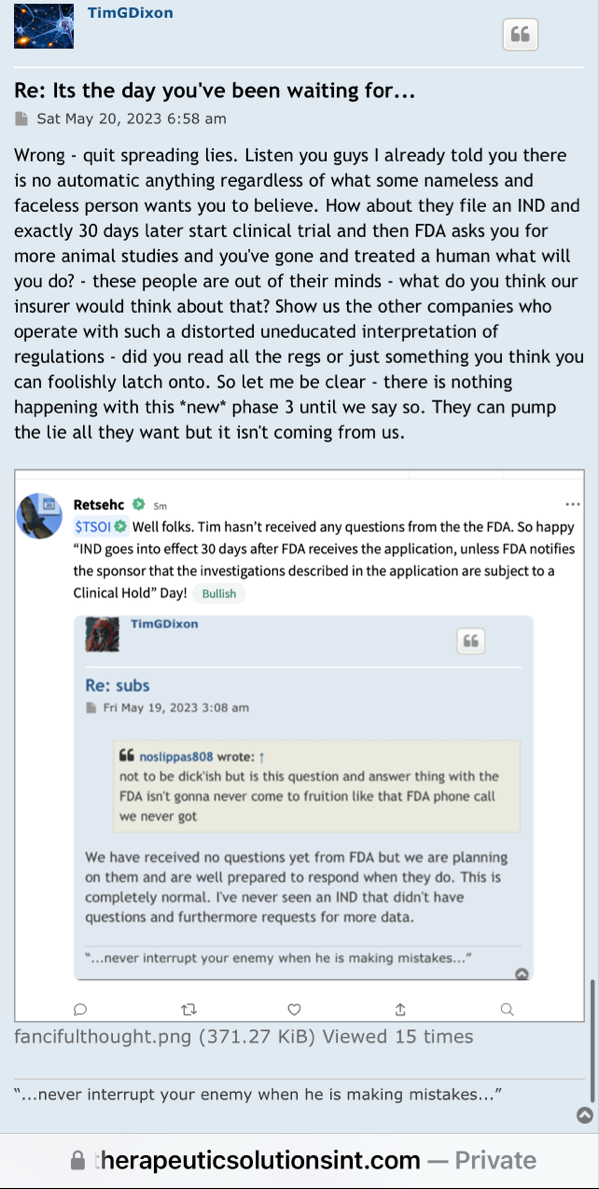
Review Time for initial submission of an Investigational New Drug application is 30 days from the date FDA receives the IND. An IND applicant may proceed with a clinical investigation once the applicant has been notified by FDA that the investigation may proceed or after 30 days if the IND is not placed on Clinical Hold.
(b) An IND goes into effect:
(1) Thirty days after FDA receives the IND, unless FDA notifies the sponsor that the investigations described in the IND are subject to a clinical hold under § 312.42; or
(2) On earlier notification by FDA that the clinical investigations in the IND may begin. FDA will notify the sponsor in writing of the date it receives the IND.
https://www.fda.gov/drugs/investigational-new-drug-ind-application/ind-applications-clinical-investigations-overview
Remember...
Post by TimGDixon » Wed Sep 07, 2022 7:51 am
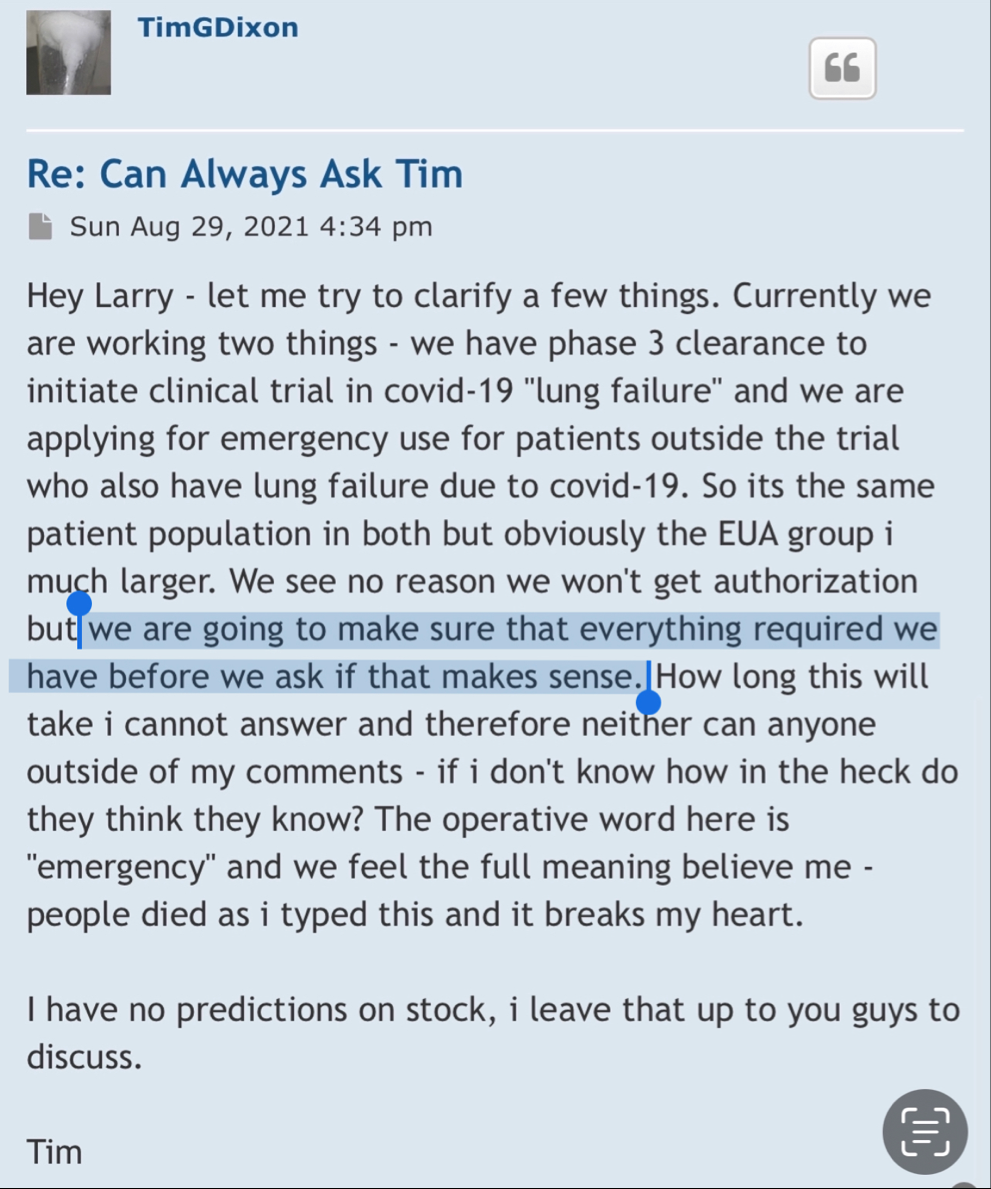
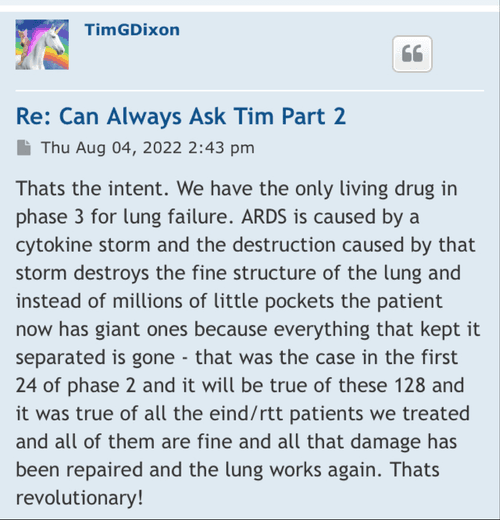
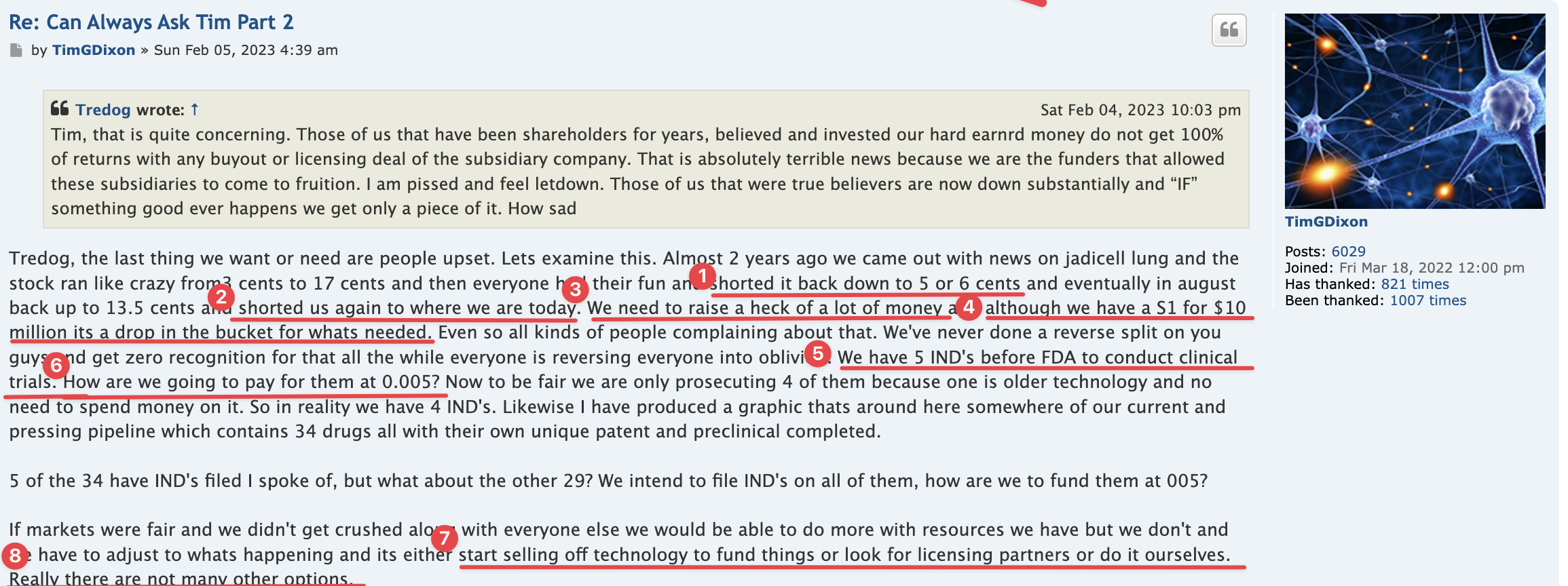
Well there are timelines and then there are timelines. We would never proceed without clearance as a rule of thumb even if som regulation says no reply in 30 you can proceed. Always better to have them on your side. This is an amendment and so we'll have to let it play out.
An Office action is a document written by a patent examiner in the course of examination of a patent application. The Office action may cite prior art and gives reasons why the examiner has allowed (approved) the applicant's claims, and/or rejected the claims.
The USPTO issues a Notice of Allowance after an examiner determines that a patent application satisfies the requirements for patentability. The Notice of Allowance establishes the date by which the applicant must pay the issue fee, and may be accompanied by a statement of the examiner's reasons for allowance..
|
Followers
|
523
|
Posters
|
|
|
Posts (Today)
|
2
|
Posts (Total)
|
65717
|
|
Created
|
10/04/08
|
Type
|
Free
|
| Moderators BigBadWolf johnnytrader33 JMC$ Yooperman Hogwarts | |||












































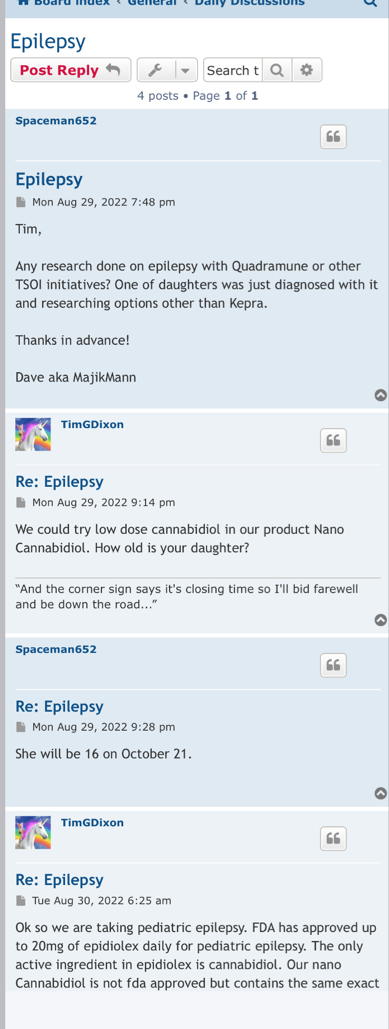







Preclinical Data Suggests QuadraMune™ Prevents Stress-Induced Suppression of Neurogenesis More Effectively than Prozac
OCEANSIDE, Calif., Dec. 9, 2020 /PRNewswire/ -- Therapeutics Solution International, Inc., (OTC Markets: TSOI), announced today new data suggesting the possibility that QuadraMune™ may mediate neuroprotective activity through preserving the ability of regenerative brain cells to proliferate subsequent to psychological stress.
The experiments, which involved exposing mice to established stressors, demonstrated that specific areas of the brain associated with production of new brain cells are damaged by stress. In agreement with previously published research, administration of fluoxetine (Prozac™) protected the brain from stress-induced damage. Surprisingly, QuadraMune™ administration appeared superior to Prozac™ at stimulating proliferation of new brain cells.
"QuadraMune™ which is currently in a clinical trial for prevention of COVD-191, has also been demonstrated to possess anti-inflammatory activity in other clinical trials, suppressing cytokines such as IL-62, which are known to be involved in depression3 and suicide4" said Kalina O'Connor, Director of Campbell Neurosciences and co-inventor on the patent. "Given major depressive disorder causes a significant risk for suicide, we are highly interested in exploring the use of QuadraMune™ for preventing suicide."
"Although much enthusiasm has been generated over the planned distribution of the COVID vaccine, at present little is being done to address mental health issues that are being exacerbated by the current pandemic" said Dr. James Veltmeyer, co-inventor of the patent, and Chief Medical Officer of the Company. "If current results are reproducible, the possibility that a nutraceutical would concurrently boost immunity while preserving mental health is highly enticing."
"It has not escaped us that COVID-19 is associated with increased inflammatory cytokines in the blood of patients, cytokines that also predispose to depression" said Famela Ramos, Vice President of Business Development for the Company. "It may be that the recent increase in suicides and suicide attempts is related biologically to activities of the coronavirus. It will be interesting to examine whether QuadraMune™ may modify putative negative mental effects of the virus."
"An estimated 17.3 million adults in the United States had at least one major depressive episode. This number represented 7.1% of all U.S. adults" stated Timothy Dixon, President and CEO of the Company. "We believe the Mission of our Company is not just providing a return on investment to our shareholders, but also increasing the quality of life for Americans. We are extremely pleased to report this unexpected finding with significant potential implications to advancing non-toxic means of helping patients with this terrible condition."
1 QuadraMune(TM) for Prevention of COVID-19 - Full Text View - ClinicalTrials.gov
2 Therapeutic Solutions International Announces Positive Preclinical and Clinical Evaluation of Nutritional Supplement QuadraMune™, Designed to Protect Against COVID-19 | BioSpace
3 Ting et al. Role of Interleukin-6 in Depressive Disorder. Int J Mol Sci. 2020 Mar 22;21(6):2194.
4 O'Donovan et al. Suicidal ideation is associated with elevated inflammation in patients with major depressive disorder. Depress Anxiety. 2013 Apr;30(4):307-14.
| Volume | |
| Day Range: | |
| Bid Price | |
| Ask Price | |
| Last Trade Time: |
