Register for free to join our community of investors and share your ideas. You will also get access to streaming quotes, interactive charts, trades, portfolio, live options flow and more tools.
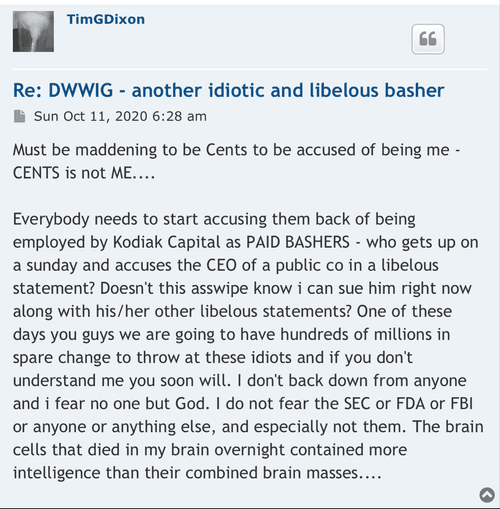
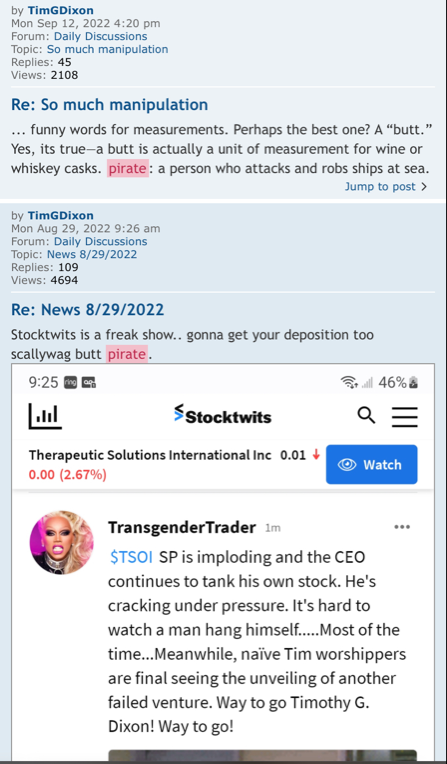
Exactly bleed every cent out of investors. It’s the name of the game
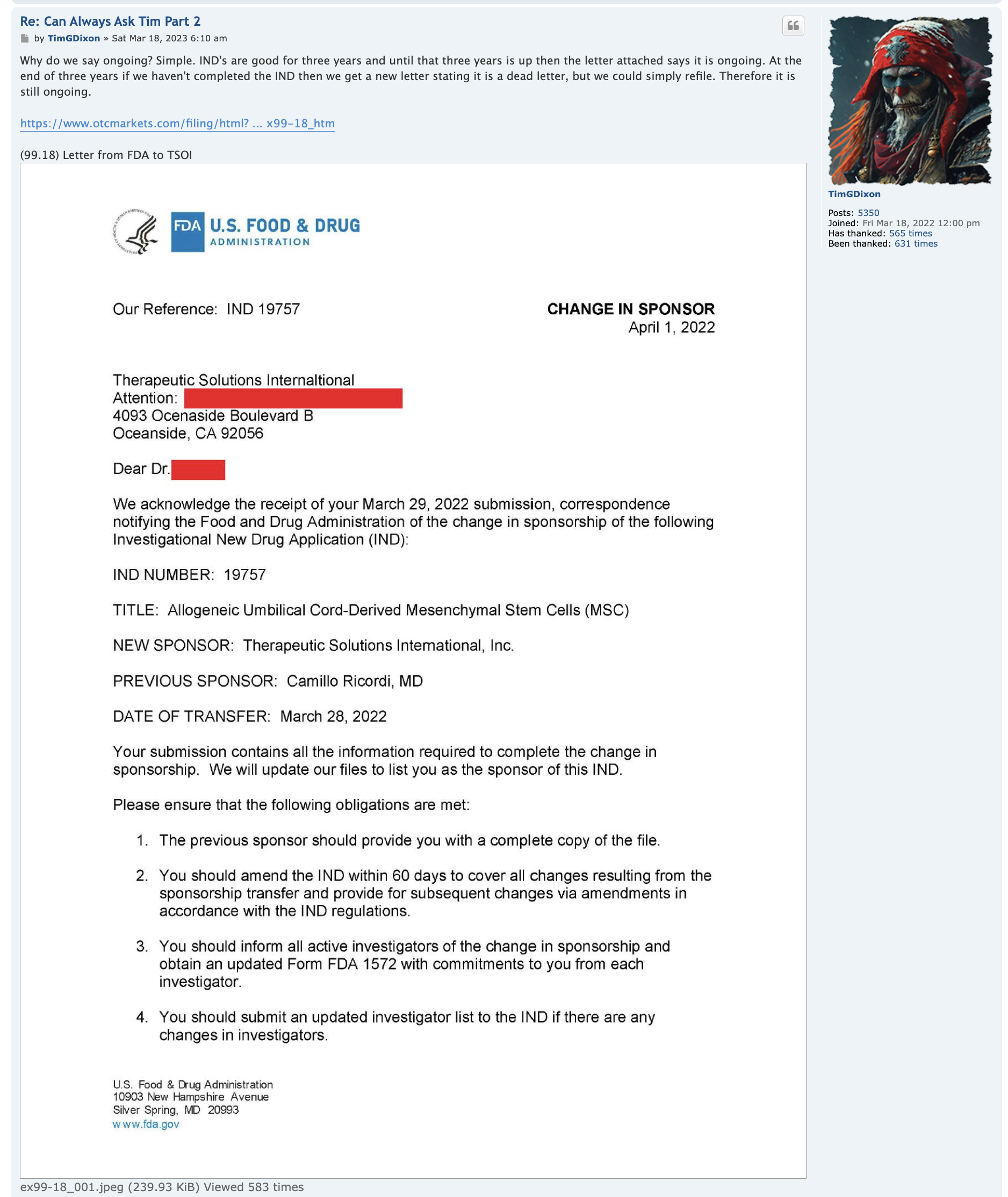
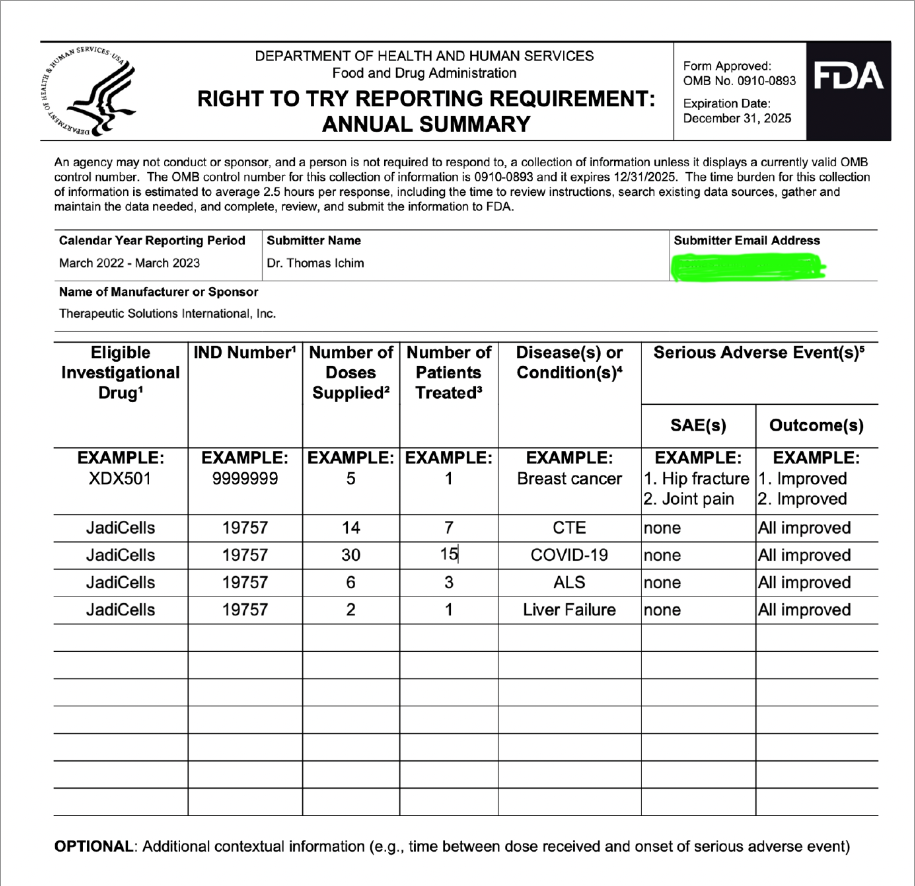
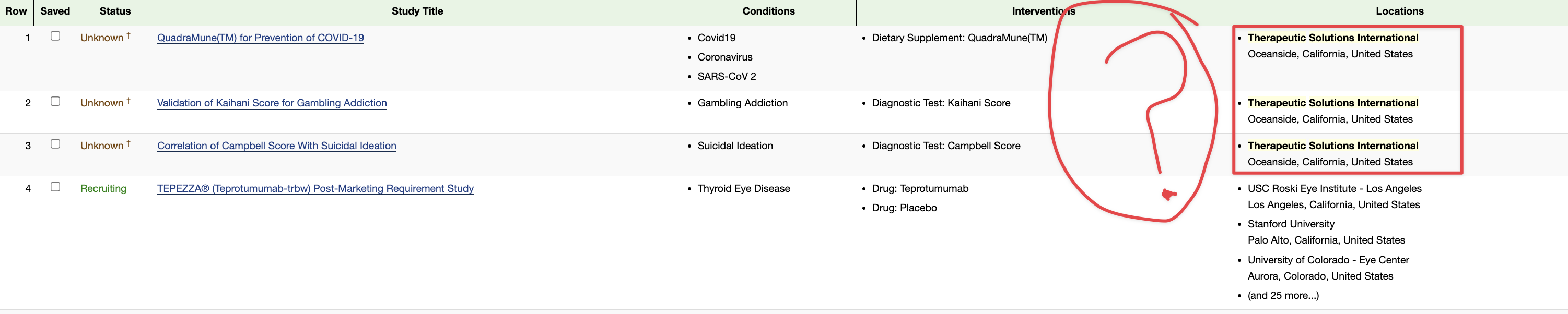
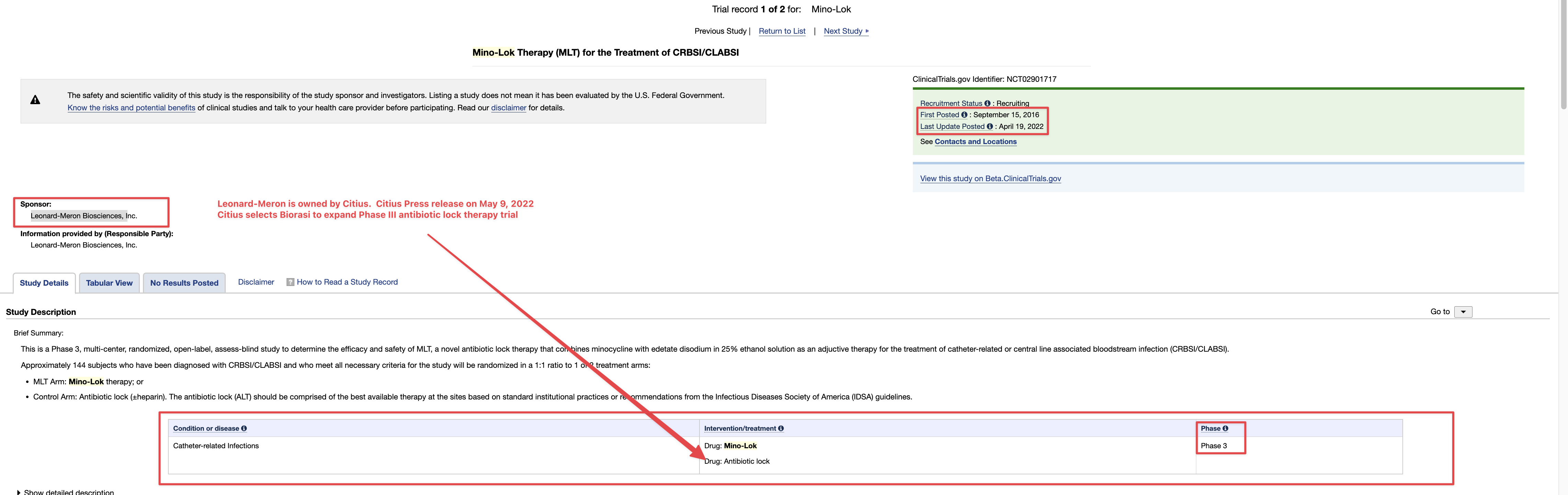
TSOI INDs
here is a start :
Therapeutic Solutions International Receives IND Number 28508 for Chronic Obstructive Pulmonary Disease Clinical Trial and Enters Binding Discussions with FDA for Initiation of Phase I/II Clinical Trial
https://www.businesswire.com/news/home/20220523005584/en/Therapeutic-Solutions-International-Receives-IND-Number-28508-for-Chronic-Obstructive-Pulmonary-Disease-Clinical-Trial-and-Enters-Binding-Discussions-with-FDA-for-Initiation-of-Phase-III-Clinical-Trial
Company Responds to Rapidly Growing COVID-19 Cases by Increasing Mechanisms to Make Lung Healing Cells Available
ELK CITY, Idaho, July 18, 2022--(BUSINESS WIRE)--Therapeutic Solutions International announced today granting of Emergency IND # 28685
https://www.yahoo.com/now/therapeutic-solutions-international-granted-emergency-130000290.html
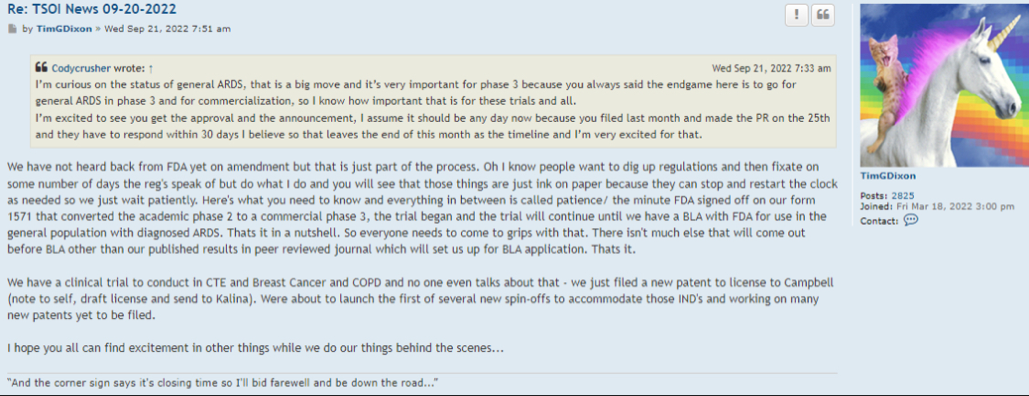
why would Dixon change now, just because of a Phase III Trial awaiting hopefully an approved amendment by the FDA that would enhance enrollment (shall we say ????)
(e) Use of Proceeds. The Company will use the net proceeds from the offering for any corporate purpose at the sole discretion of the Company.
Subject: FILE NO S7-24-20.
From: Tim Dixon
Affiliation:
Feb. 05, 2021
To Whom It May Concern,
The Company I run, Therapeutic Solutions International, Inc. (TSOI) has relied upon the availability of convertible debentures with the utilization of rule 144 as a means of financing the operations of TSOI. Had this not been available I would not have been able to advance the Company otherwise and most likely would not have been able to stay fully reporting. The loss of such instruments would be devastating to smaller companies like TSOI who cannot attract the big investment bankers the big boards keep in their pockets like loose change. Please consider carefully the impact this will have upon innovation and new technologies coming to market to benefit all of humanity.
Sincerely yours,
Timothy G Dixon, President & CEO
Therapeutic Solutions International, Inc.
Note 8 – Convertible Notes Payable
At various times during the six months ended June 30, 2022, the Company entered into convertible promissory notes with principal amounts totaling $337,500 with a third party for which the proceeds were used for operations. The Company received net proceeds of $315,000, and a $22,500 original issuance discount was recorded. The convertible promissory notes incur interest at rates from 10% to 12% per annum and mature on dates ranging from January 1, 2023 to June 27, 2023. The convertible promissory notes are convertible to shares of the Company’s common stock 180 days after issuance. The conversion price per share is equal to 63% of the average of the three (3) lowest trading prices of the Company’s common stock during the fifteen (15) trading days immediately preceding the applicable conversion date. The trading price is defined within the agreement as the closing bid price on the applicable trading market. The Company has the option to prepay the convertible notes in the first 180 days from closing subject to prepayment penalties ranging from 120% to 145% of principal balance plus interest, depending upon the date of prepayment. The convertible promissory notes include various default provisions for which the default interest rate increases to 22% per annum with the outstanding principal and accrued interest increasing by 150%. The Company was required to reserve at June 30, 2022 a total of 167,223,808 common shares in connection with these promissory notes.
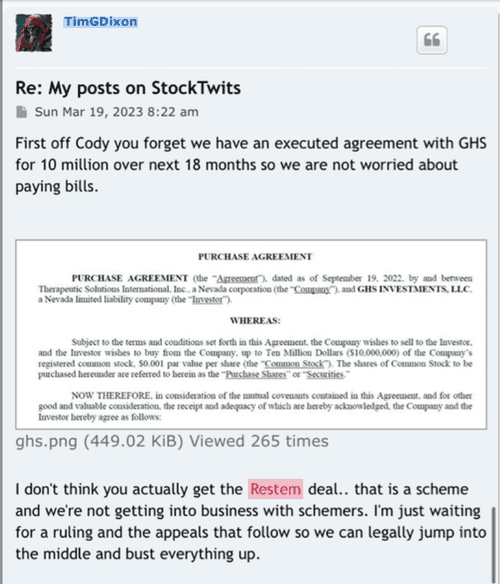
Pg12 of 8k exhibit on purchase agreement
(e) Use of Proceeds. The Company will use the net proceeds from the offering for any corporate purpose at the sole discretion of the Company.
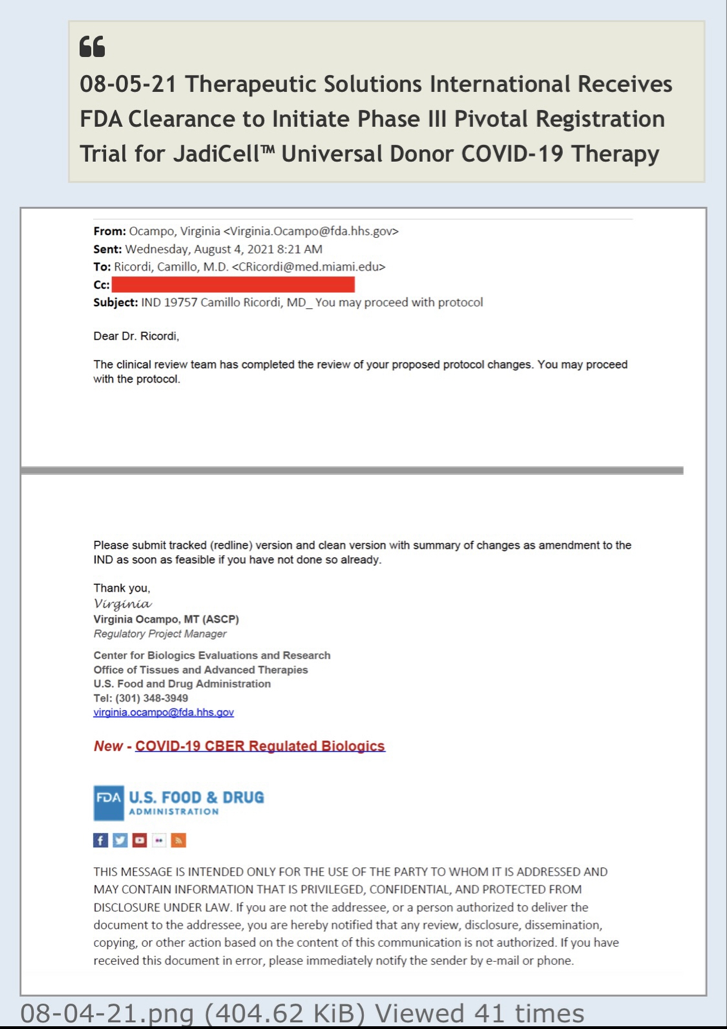
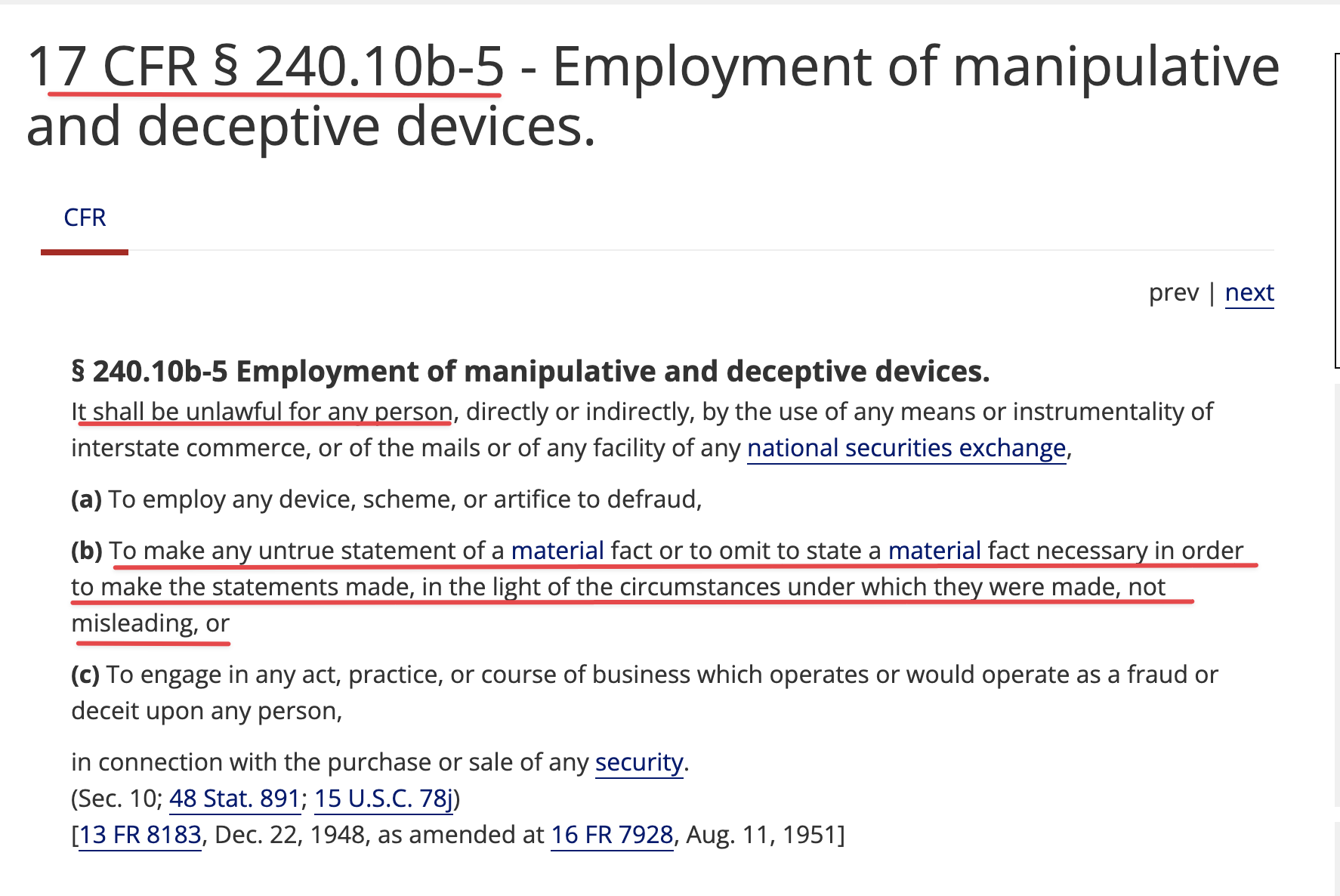
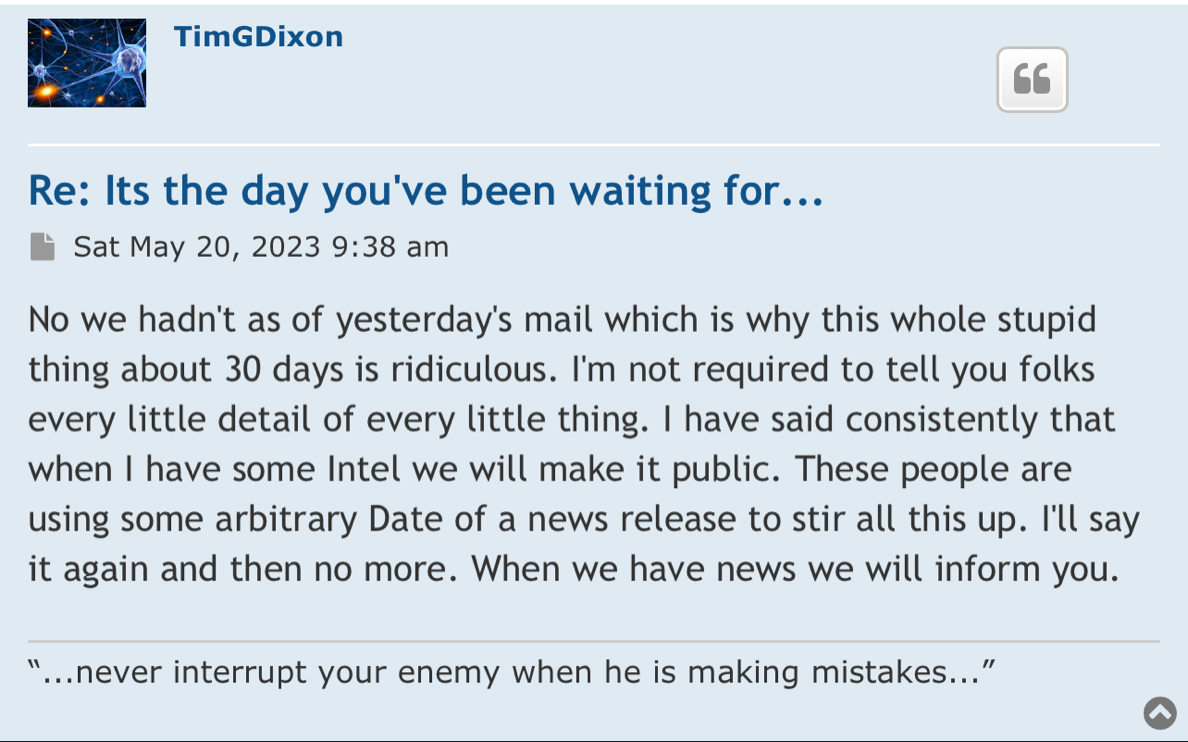
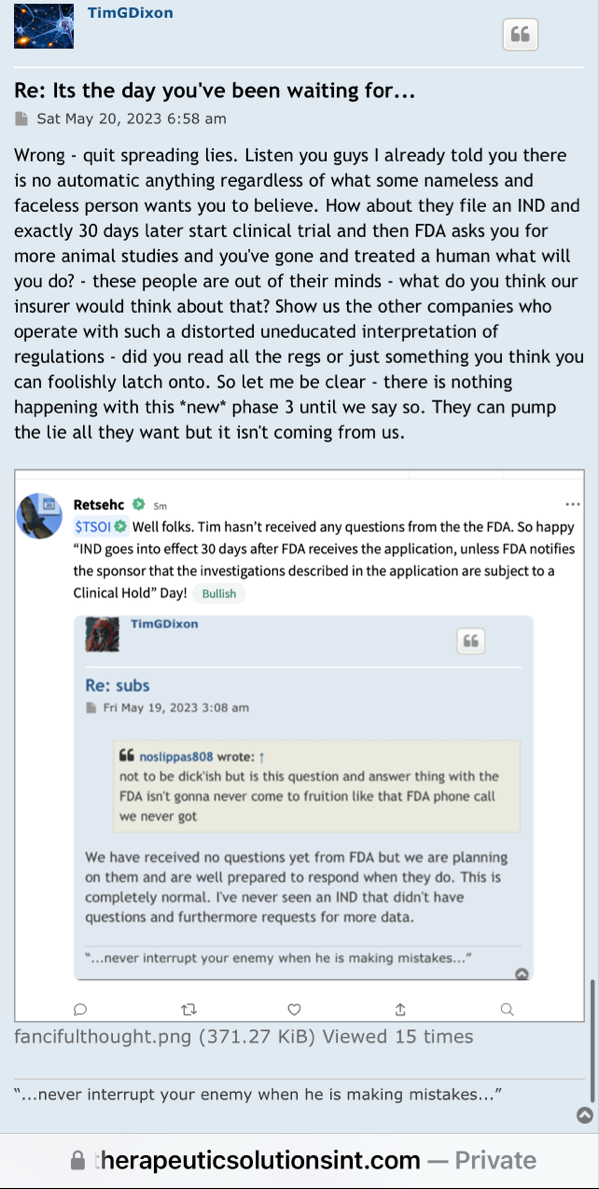
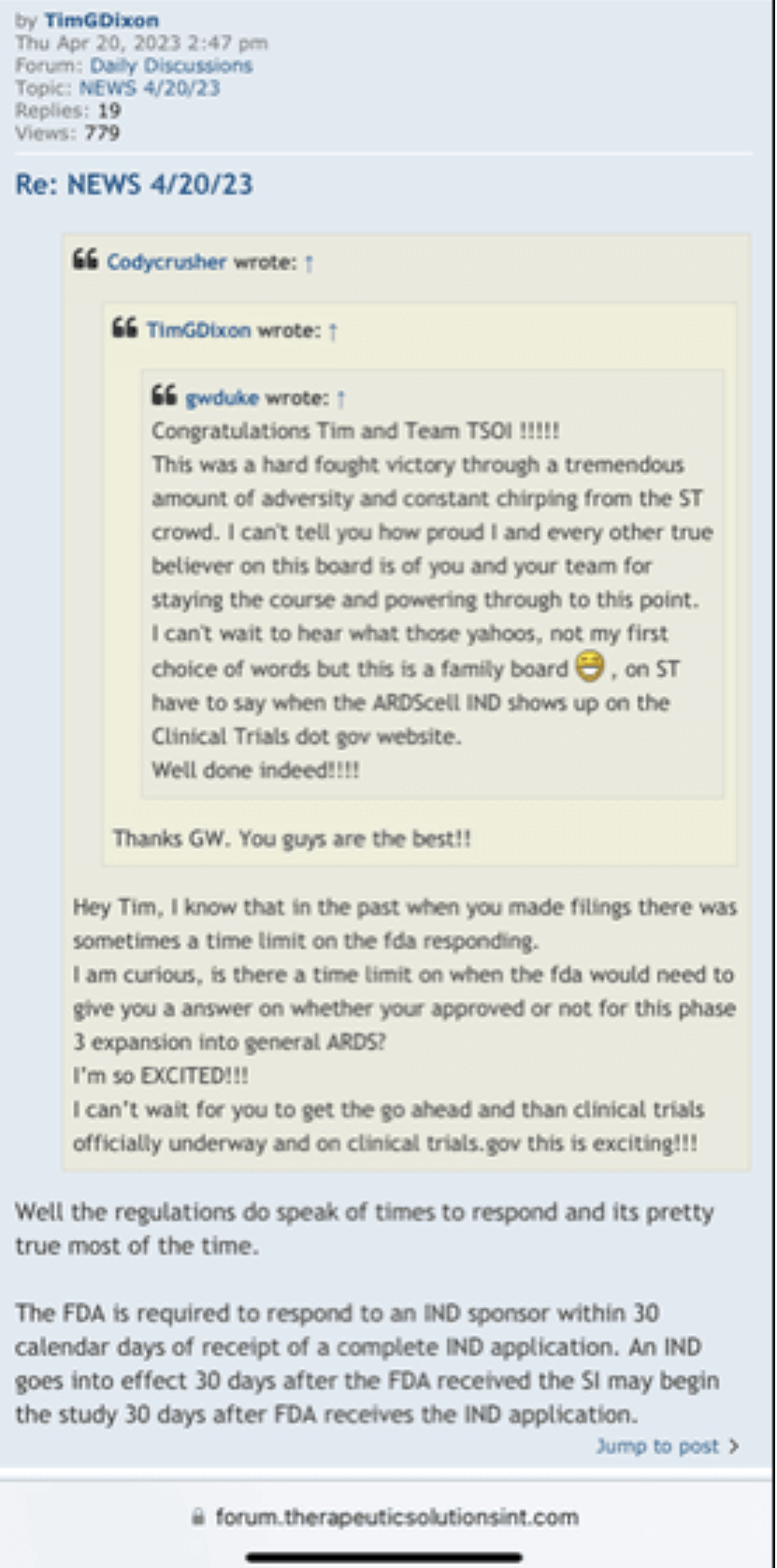
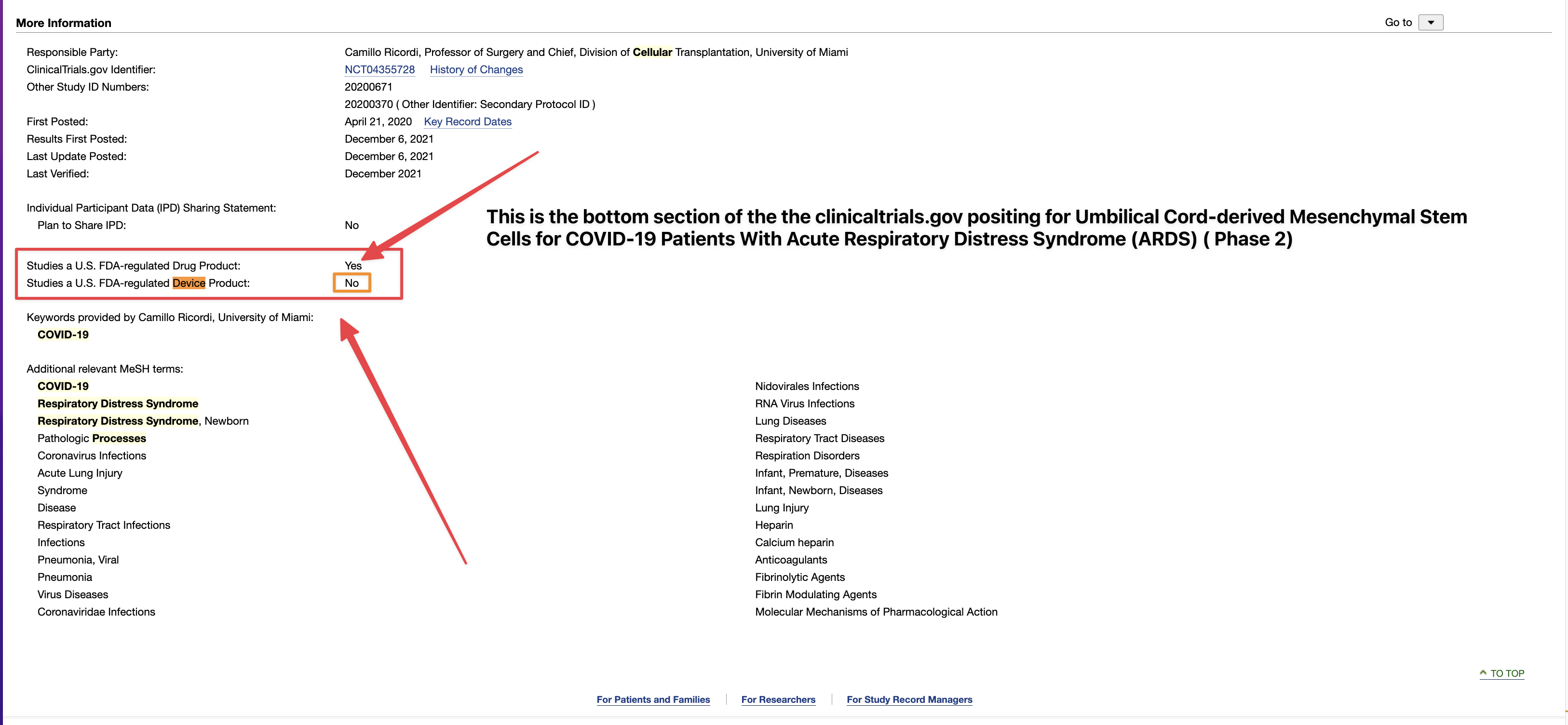
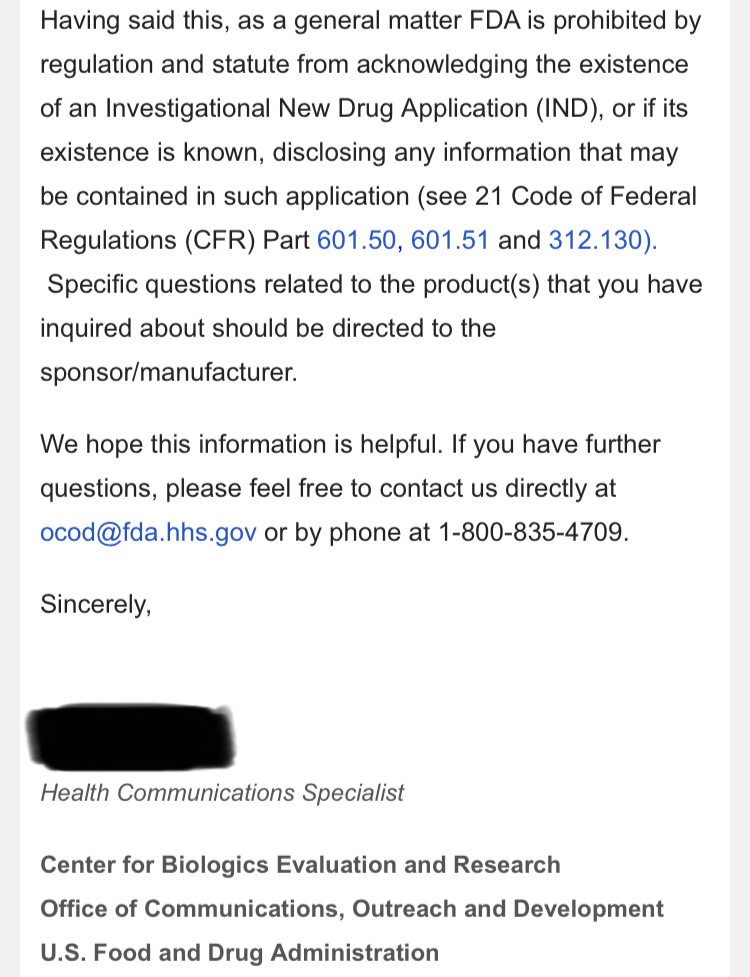
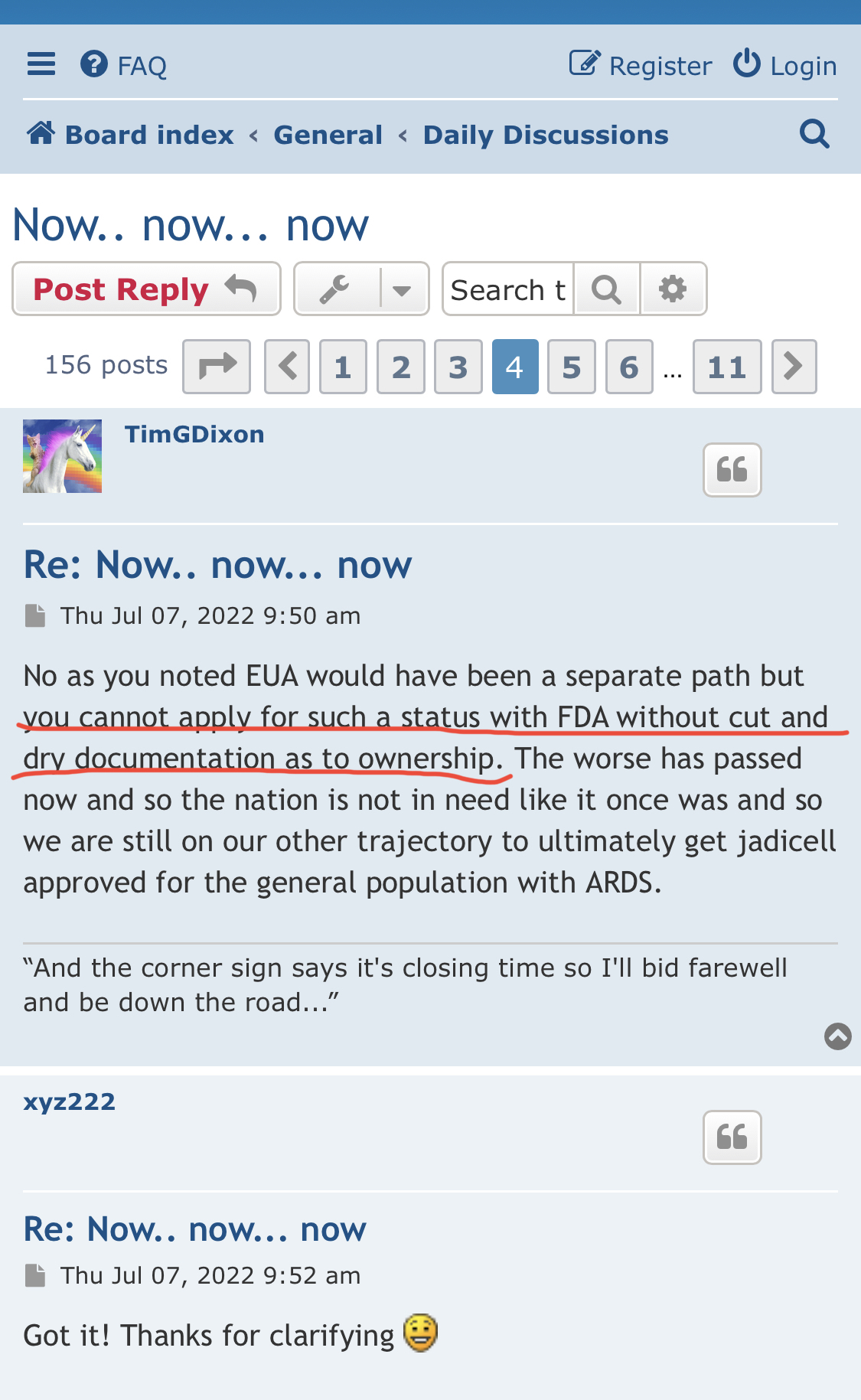
Review Time for initial submission of an Investigational New Drug application is 30 days from the date FDA receives the IND. An IND applicant may proceed with a clinical investigation once the applicant has been notified by FDA that the investigation may proceed or after 30 days if the IND is not placed on Clinical Hold.
(b) An IND goes into effect:
(1) Thirty days after FDA receives the IND, unless FDA notifies the sponsor that the investigations described in the IND are subject to a clinical hold under § 312.42; or
(2) On earlier notification by FDA that the clinical investigations in the IND may begin. FDA will notify the sponsor in writing of the date it receives the IND.
https://www.fda.gov/drugs/investigational-new-drug-ind-application/ind-applications-clinical-investigations-overview
Remember...
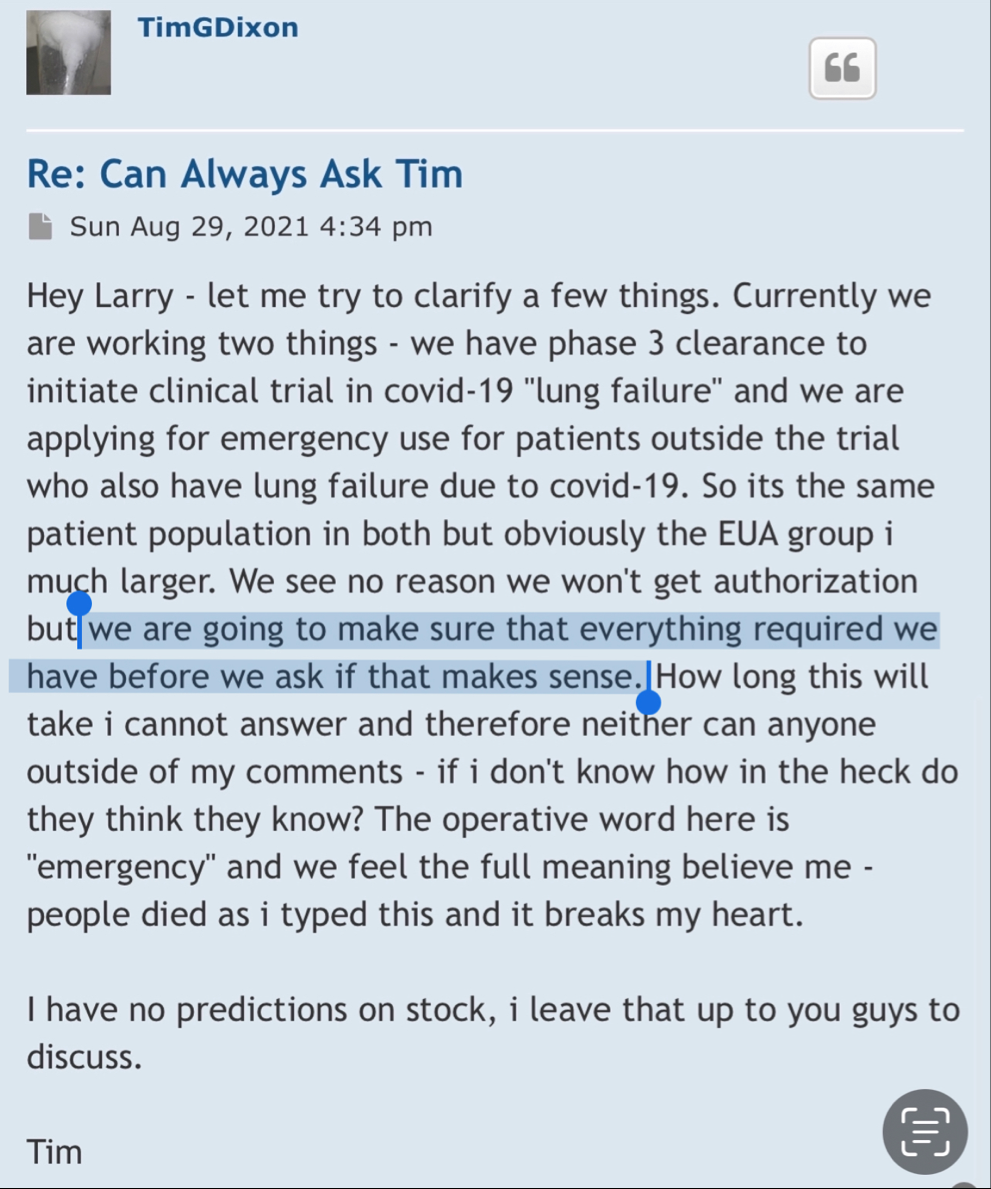
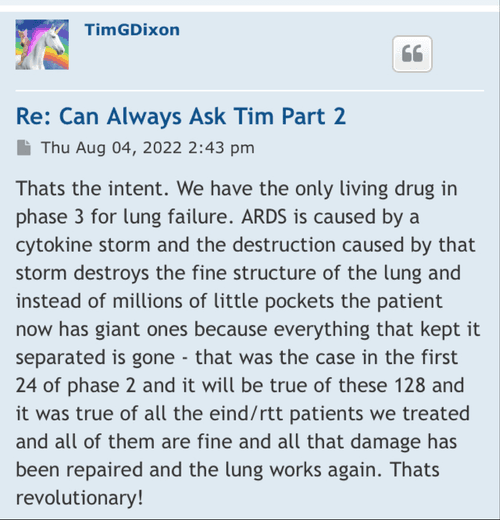
Post by TimGDixon » Wed Sep 07, 2022 7:51 am
Well there are timelines and then there are timelines. We would never proceed without clearance as a rule of thumb even if som regulation says no reply in 30 you can proceed. Always better to have them on your side. This is an amendment and so we'll have to let it play out.
An Office action is a document written by a patent examiner in the course of examination of a patent application. The Office action may cite prior art and gives reasons why the examiner has allowed (approved) the applicant's claims, and/or rejected the claims.
The USPTO issues a Notice of Allowance after an examiner determines that a patent application satisfies the requirements for patentability. The Notice of Allowance establishes the date by which the applicant must pay the issue fee, and may be accompanied by a statement of the examiner's reasons for allowance..
It gripes me that even Justia publishes a link to a long list of large numbers with an attached list of dates, and says the list represents “patents”. Justia has been around a long time, and should know the difference between a patent application and a granted patent….. some on that list have not been present in the PTO long enough to have received a “first office action” or a “notice of allowance” from an examiner.
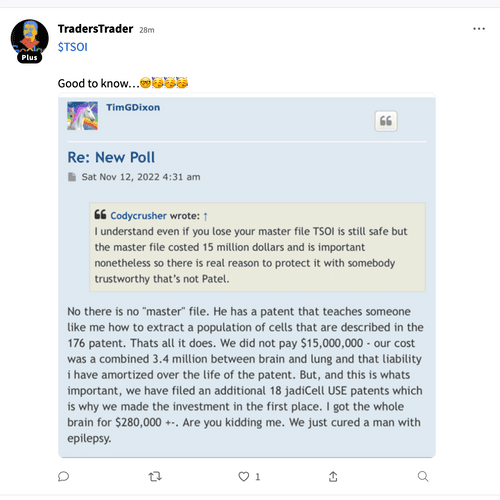
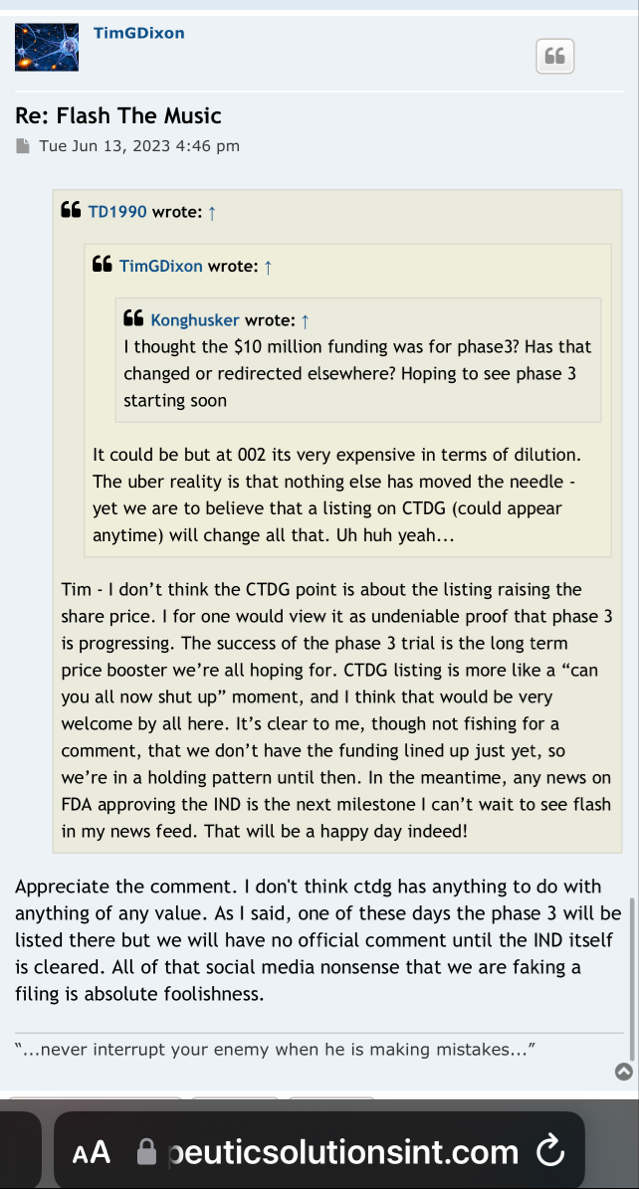
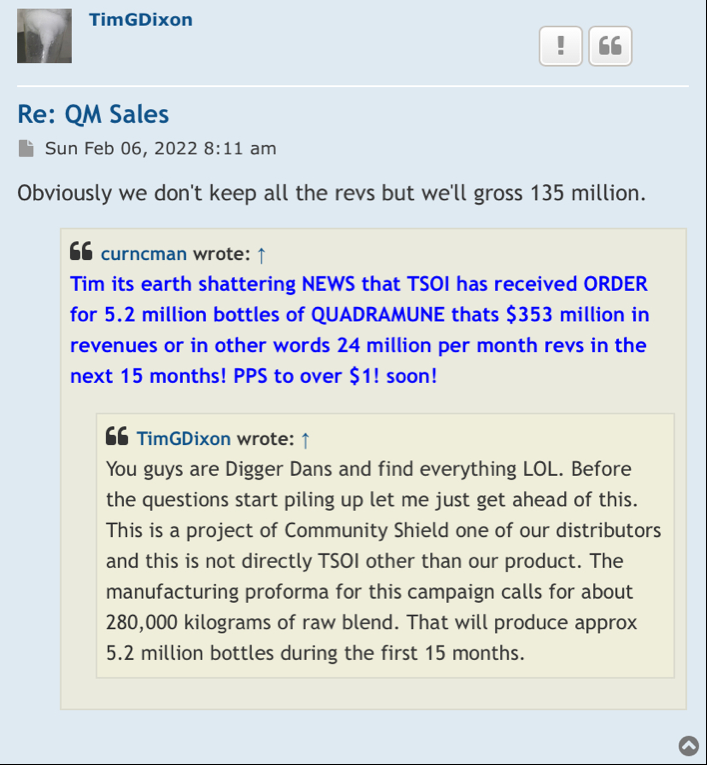
Lagging that to my understanding this 10 million in funding is strictly for phase 3 to help finish it out that’s the way I understood it. It was announced a couple of months ago that we had funding but he wouldn’t report to us who founded it until the 10K comes out the end of the year Originally the face three was supposedly to cost around 15 million up to 20 million so this 10 million is to help their original funding he had.
In my opinion this is why enrollment wasn’t started on phase 3 there wasn’t enough money in there to complete it now supposedly we’ve got it to finish the face or yeah but that money has to be spent on phase 3.
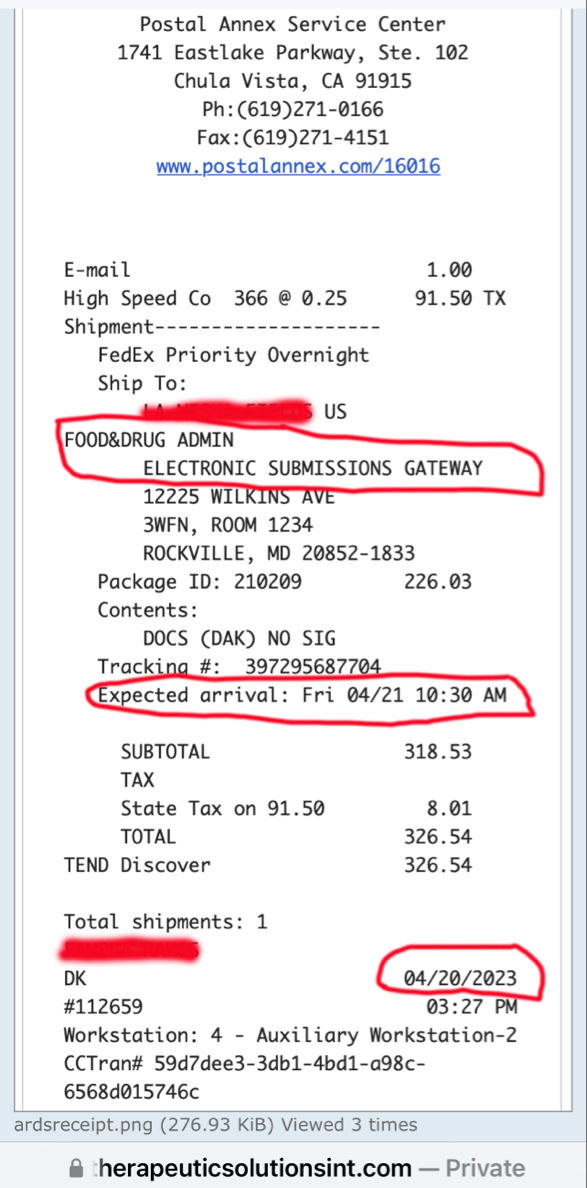
My next question is why haven’t we heard from him on the FDA about incorporating ARDS the rest of them into the COVID-19 phase 3 I thought we were supposed to know within 30 days which was up last week.
So now we all are waiting on the answer to that question with the FDA.
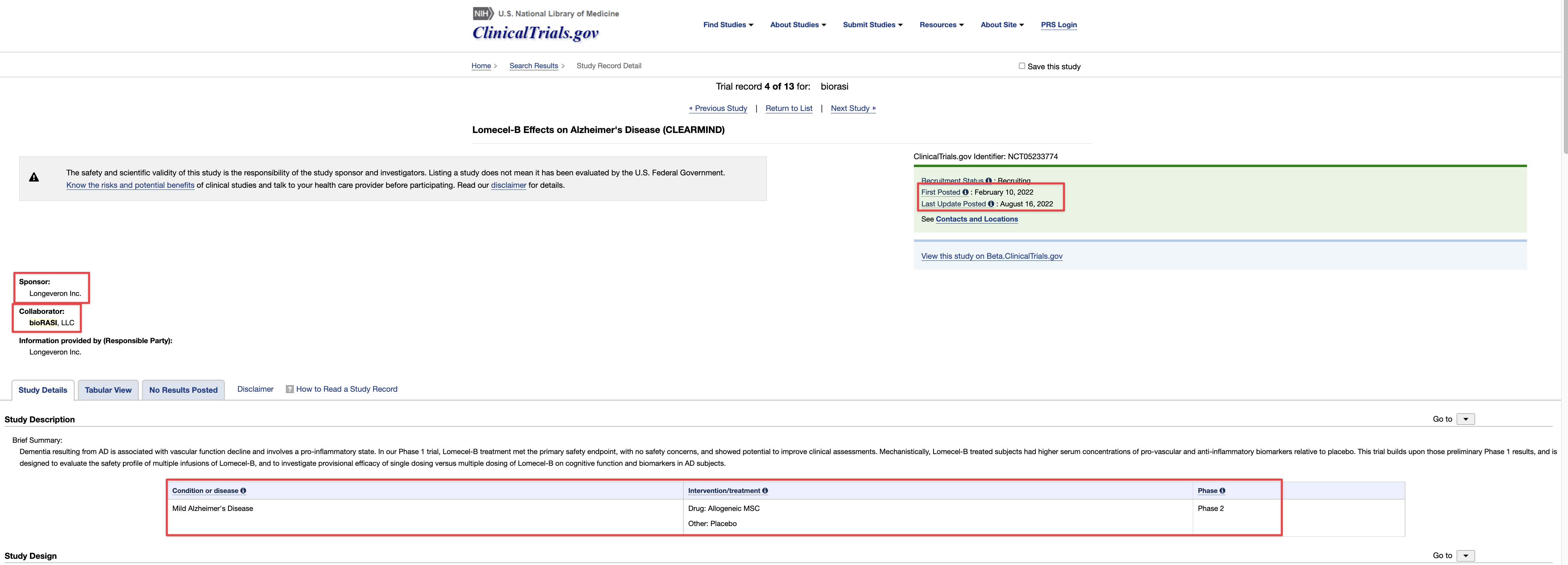
TSOI Therapeutic Solutions International Inc (PK)
0.0125 0.0015 (13.64%)
i wasn't in that stock,
so I don't know who made money, or who didn't .
the PRs are what they are..
I wasn't in the stock either, but archived filings are available in the EDGAR system that goes back to the year 2001 to see what companies were up to. Medistem announced the merger with Intrexon in December 2013. During the years 2012 and 2013, the Medistem stock traded from a low of 0.28 to a high of $3. The $1.35 cash/stock angered enough people that there were lawsuits in multiple states against the companies including scientists as defendants, which ended in a class settlement years later.
This is how to form realistic opinions by reviewing facts and not going by PR's alone. There is no point to ask for the story that occurred a decade ago, and what am I supposed to ask them? Did you guys scam retail investors when company was sold for only 26 million with valuable IP? LOL. Who really knows what happened or the truth. Were kickbacks involved? People invested were concerned that was the case.
By the way, Intrexon still exists to this day and is huge, but they rebranded as Precigen years ago. The CEO since 2020 is Helen Sabzevari, the same Sabzevari that has a history with Regen Biopharma, and I don't need to elaborate on Regen and Ichim, right? If TSOI ever mentions any deals with this company, it will be wise to be a little cautious considering the past.
It's important to learn from history and people that are interconnected. It may mean nothing, or it may mean something, and there wouldn't be as much digging if TSOI weren't misleading during the past year or so.
thanks
I'll leave you to the trademarks then.
i happened to know about it because
the same thing has been posted here several times.
Slim mentioned it , and i was able to dig up the info , and provide links.
i have no opinion on it, because i wasn't in the stock, before, during , or after the buyout .
i dug up the info, but if you want to form any realistic opinion on it, you should contact those that were involved , instead of reading anything into it.
Tally talk it’s just a diluted stock. The dilution continues. It blows
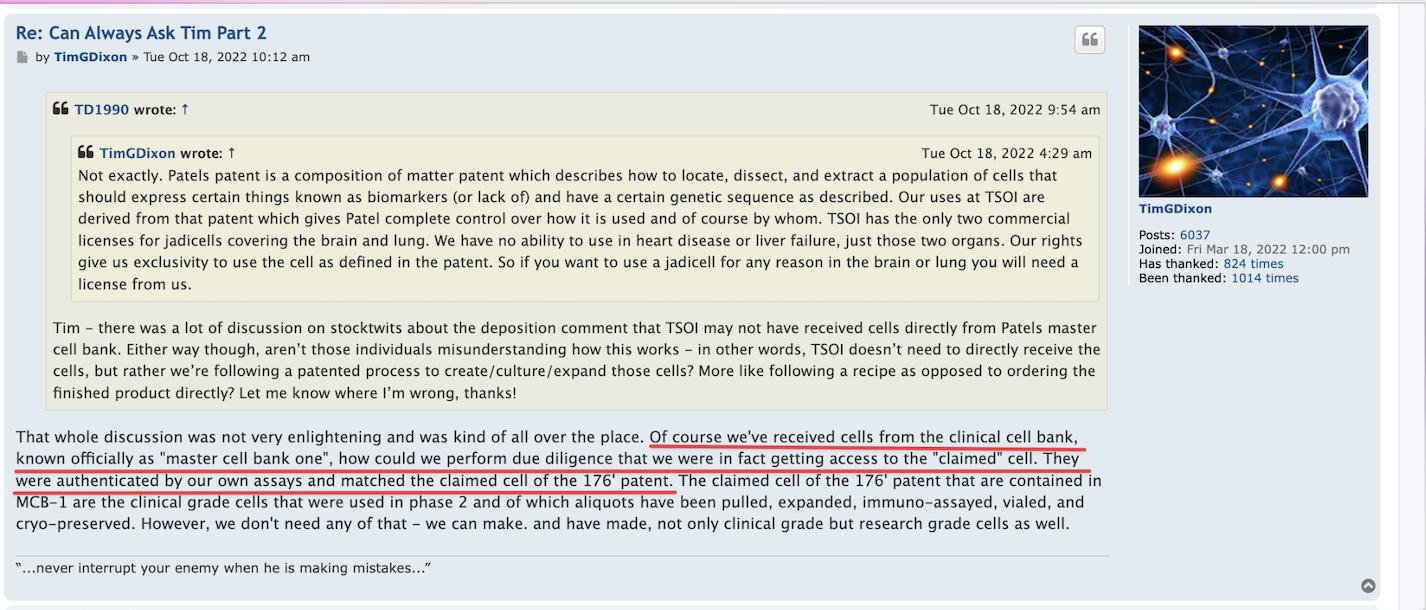
I see numerous published patent applications assigned to TSOI, and 3 granted patents with patent numbers in that list. Correct me if I am mistaken.
The funny thing with Medistem is when you look at the history, long term shareholders were pissed it sold for just $26 million, receiving only about $1.35 per share (0.27 cash + 1.08 stock that lost value) when it was trading $2 - $3 earlier causing many people a loss who believed it was undervalued. To be fair, Tim Dixon was not involved with that company, not that I know of, but some of the scientists are on the advisory board, in addition to Dr Ichim as director who also held a CEO position in that other company. It didn't sound like a good deal so why do people occasionally mention this acquisition? Let's hope the same does not happen with TSOI. Am I missing something?
Now that TSI has private funding does anyone think they’re still considering a big pharma partnership or will they go at it on their own? Until a strong revenue is generated on a steady basis 10 mil might not last that long with all they have in the works…even the price of a BBQ alone is going up on a daily basis.
from first word pharma
(and bringing us all up to date)
https://firstwordpharma.com/story/5560979
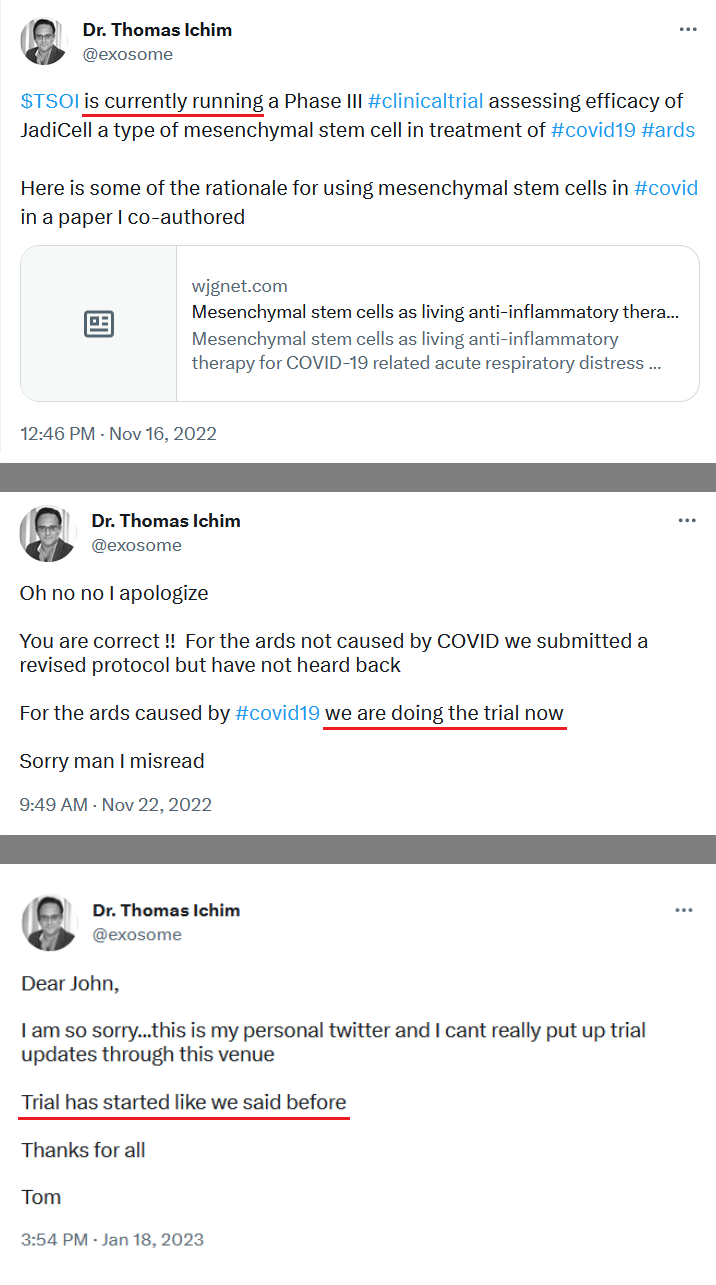
Therapeutic Solutions International Announces Launching of Phase III Clinical Trial for Treating COVID-19 Lung Damage Using its JadiCell Universal Donor Stem Cell Drug
Ref: Business Wire
Published: May 02, 2022
Landmark Study Aimed to Support FDA Registration of First Cell Therapy with Dual Anti-inflammatory and Lung Healing Mechanism of Action
OCEANSIDE, Calif.--(BUSINESS WIRE)-- Therapeutic Solutions International announced today the launching of a double-blind, randomized, placebo controlled, multi-center, multi-nation, clinical trial of 128 patients with COVID-19 associated lung failure.
The study will be comprised of two groups, JadiCell™ treatment group and control group. The primary endpoint of the study is comparing the proportion of patients alive and free of respiratory failure at Day 60 after treatment with JadiCells as compared to placebo.
The Company has licensed the issued patent covering composition of matter for JadiCell and FDA Right of Reference1,2, has identified and filed patents on novel mechanisms of action of JadiCell related to lung preservation and regeneration3,4,5, and acquired the FDA cleared IND and associated data package, which was the basis for Phase III clearance6, and has contracted Biorasi, a global, full-service CRO, to launch and run the clinical trial.
"Cell therapy is one of the most promising and demanding forms of medical intervention," said Chris O'Brien, CEO of Biorasi. "In contrast to traditional medicines, products such as JadiCell are living therapeutics, which require a very detail-oriented approach to their administration as well as patient follow-up. We are honored to partner with TSOI for this landmark clinical trial."
In previous studies the Company has demonstrated the superior activity of JadiCell to other types of stem cells including bone marrow, adipose, cord blood, and placenta. Furthermore, the JadiCell was shown to be 100% effective in saving the lives of COVID-19 patients under the age of 85 in a double-blind, randomized, placebo controlled clinical trial with patients in the ICU on a ventilator. In patients over the age of 85 the survival rate was 91%7.
"The launch of this clinical trial is a historical step for Therapeutic Solutions International, but also to patients with degenerative lung diseases in general," said Dr. James Veltmeyer, Principal Investigator of the Clinical Trial and Chief Medical Officer of TSOI. "Given the extremely positive effects that we have seen in the previously published double blind clinical trial, as well as our own Right to Try case reports, I am extremely enthusiastic to begin this last step before obtaining marketing approval by the FDA."
"Having worked with the JadiCell for several years now, I can state with full confidence that this is by far the most potent stem cell that we have seen to date," stated Dr. Thomas Ichim, Board Member of the Company. "Given its unparalleled ability to stimulate angiogenesis, suppress lung inflammation, augment healing of scar tissue in the lung, and stimulate endogenous lung stem cells, I have high confidence in the rapid success of our clinical trial."
"Having a second-to-none track record of rapid patient recruitment in advanced therapeutics across a variety of indications, we are enthusiastic to work with Biorasi initially to provide the basis for marketing approval for COVID-19 and subsequently to address other unmet medical needs in pulmonary medicine such as chronic obstructive pulmonary disease and idiopathic pulmonary fibrosis," said Timothy Dixon, President, and CEO of the Company.
here is another Slim
https://en.wikialpha.org/wiki/Boris_Reznik
Boris Reznik
Born Moscow
Nationality Russian
Citizenship Russia/United States
Occupation Entrepreneur
Known for Chairman of Venvalo Group.
Boris Reznik is a Russian-born, American entrepreneur known as the founder and Chairman of multiple technologies based companies, such as American Turnkey, Optum Software, Biorasi, Medistem, Batu Biologics, Biotech Holdings.[1][2] He is currently also the Chairman of Venvalo Group, the value venture optimization firm investing in and working with emerging and mid-market companies in helping to optimize their value.[3]
Therapeutic Solutions International Engages Venvalo Group to Oversee JadiCell COVID-19 Phase III Clinical Trial and Emergency Use Authorization (EUA) Process
https://www.biospace.com/article/releases/therapeutic-solutions-international-engages-venvalo-group-to-oversee-jadicell-covid-19-phase-iii-clinical-trial-and-emergency-use-authorization-eua-process/
Published: Sep 10, 2021
ELK CITY, Idaho, Sept. 10, 2021 /PRNewswire/ -- Therapeutic Solutions International, Inc., (OTC Markets: TSOI), announced today the engagement of Venvalo Group to assist the Company on regulatory, strategic, and implementation aspects of the recently FDA-cleared Phase III clinical trial assessing ability of JadiCells to treat patients with advanced COVID-19.
"I have known Dr. Boris Reznik for many years and have seen first-hand his ability to rapidly grow companies and perform clinical trials in a variety of disease indications including COVID-19," said Dr. Camillo Ricordi, Principal Investigator of the initial clinical trial, whose promising results resulted in the current FDA approval to proceed with a multi-site, Phase III trial. "Being in the middle of the biggest biological threat that our generation has witnessed, I believe it is imperative to work with top quality groups to rapidly complete this trial and bring this new treatment to patients as quickly as possible."
In 2020 Dr. Ricordi led the international team that successfully completed the first FDA approved controlled trial to treat the most severe cases of COVID-19 with stem cell infusions1. The unprecedented results allowed for 100% patient survival at one month in subjects treated who were less than 85 years old, and 91% survival in subjects of all ages, compared to 42% survival in the control group.
"The work of Dr. Ricordi has significantly impacted the field of medicine in areas of Type 1 Diabetes, regenerative medicine, and transplant immunology," stated Dr. Boris Reznik, Chairman of Venvalo Group. "We are proud to work with this Medical Visionary and to perform the most rapid and effective clinical translation of this novel and life-saving technology as soon as we can."
"By leveraging the knowledge gained from the hundreds of clinical trials performed by Dr. Reznik at bioRASI, as well as the unsurpassed innovative abilities of Dr. Ricordi, we are excited to rapidly move forward with the current clinical trial, as well as the Emergency Use Application, which is currently under development," said Timothy Dixon, President and CEO of Therapeutic Solutions International.
Dr. Ichim was not the only one back then. there was another or a couple more in their science team.
https://www.bloomberg.com/profile/person/17589405
Feng Lin
Chief Scientific Officer, Therapeutic Solutions Intl Inc
CURRENT POSITION
Chief Scientific Officer, Therapeutic Solutions Intl Inc
TENURE AT CURRENT POSITION
9/2019-PRESENT
PREVIOUS POSITION
Dir:Chinese Operations, Medistem Inc
yes,
Intrexon Completes Acquisition of Medistem
https://www.prnewswire.com/news-releases/intrexon-completes-acquisition-of-medistem-248984811.html
GERMANTOWN, Md. and SAN DIEGO, March 7, 2014 /PRNewswire/ -- Intrexon Corporation ( NYSE: XON), a leader in synthetic biology, today announced that it has completed the acquisition of San Diego-based Medistem, Inc. ( OTCQB: MEDS), a pioneer in the development of Endometrial Regenerative Cells ("ERC" or "ERCs"), universal donor adult-derived stem cells with properties uniquely suited for therapeutic use with Intrexon's existing suite of synthetic biology technologies. Upon integration of these platforms, ERCs could be manufactured as genetically modified vehicles to secrete powerful therapeutic effectors in the body for clinical benefit to patients suffering from many different diseases.
In combination with Intrexon's diverse proprietary suite of technologies, including its UltraVector® gene engineering and assembly, RheoSwitch Therapeutic System® platform, and Cell Systems Informatics, Medistem's ERCs have the potential to be utilized as vehicles to deliver novel genetically controlled therapies for conditions such as cancer, inflammation, and a number of orphan diseases. Intrexon looks forward to developing these compelling technologies and making them available to existing and future collaborators.
Thomas Ichim, Ph.D., former President and Chief Science Officer of Medistem, has agreed to join the Intrexon team within its Cell Engineering Unit. Under the terms of the agreement, Medistem stockholders will receive in exchange for each share of Medistem common stock $0.27 in cash and $1.08 worth of Intrexon common stock, or approximately 0.03920 shares, based on the 20-day volume-weighted average price of Intrexon's common stock immediately prior to closing, subject to adjustment pursuant to the terms of the merger agreement.
Patents Assigned to Therapeutic Solutions International, Inc.
https://patents.justia.com/assignee/therapeutic-solutions-international-inc
PLURIPOTENT STEM CELL DERIVED DENDRITIC CELLS AND ENGINEERED DENDRITIC CELLS FOR CANCER IMMUNOTHERAPY
Publication number: 20220298491
Abstract: Disclosed are populations of dendritic cells generated from stem cells capable of inducing immunity towards cancer. In one embodiment said dendritic cells are generated from allogeneic inducible pluripotent stem cells, for some uses, said pluripotent stem cells are genetically engineered/edited to induce cancer specific immunity and/or resist immunosuppressive effect of tumor derived microenvironment. In one embodiment pluripotent stem cells are transfected with cancer stem cell antigens such as BORIS and/or NR2F6.
Type: Application
Filed: March 16, 2022
Publication date: September 22, 2022
Applicant: Therapeutic Solutions International, Inc.
Inventors: Thomas E. ICHIM, Famela RAMOS, James VELTMEYER, Timothy G. DIXON
THERAPEUTIC MONOCYTES FOR PREVENTION OF SUICIDAL IDEATION
Publication number: 20220280574
Abstract: The invention discloses compositions of matter, protocols, and therapeutic means for treatment of suicidal ideations and/or suppression of suicidal attempts. In one embodiment the invention provides the use of umbilical cord derived monocytes as a means of treatment. In another embodiment, monocytes are de-differentiated from adult monocytes using reprogramming means to create monocyte capable of producing anti-inflammatory as well as regenerative properties useful in reducing suicidal ideations and/or attempts.
Type: Application
Filed: March 4, 2022
Publication date: September 8, 2022
Applicant: Therapeutic Solutions International, Inc.
Inventors: Thomas E. Ichim, Timothy G. Dixon, Famela Ramos, Kalina O'Connor, James Veltmeyer
EX VIVO GENERATION OF IMMUNOCYTES RECOGNIZING BROTHER OF THE REGULATOR OF IMPRINTED SITES (BORIS) EXPRESSING CANCER STEM CELLS
Publication number: 20220267730
Abstract: Disclosed are means, methods and compositions of matter useful for induction of immunity towards cancer stem cells by providing a dendritic cell, wherein said dendritic cells express BORIS and/or peptides derived from BORIS, wherein said dendritic cell is cultured in the presence of one or more immunocytes. In one embodiment said dendritic cells are derived from umbilical cord blood sources and allogeneic to T cells, which are expanded ex vivo and used for the purposes of immunotherapy.
Type: Application
Filed: February 22, 2022
Publication date: August 25, 2022
Applicant: Therapeutic Solutions International, Inc.
Inventors: Thomas E. Ichim, Timothy G. Dixon, Feng LIN, Famela Ramos, James Veltmeyer
STIMULATION OF NATURAL KILL CELL MEMORY BY ADMINISTRATION OF DENDRITIC CELLS
Publication number: 20220249551
Abstract: Disclosed are means, methods and compositions of matter useful for induction of natural killer cell memory by administration of dendritic cells and/or exosomes thereof. In one embodiment a mammal suffering from cancer is administered allogeneic cord blood derived dendritic cells that are not pulsed exogenously. In one embodiment the dendritic cells are stimulated to possess chemotactic activity towards the tumor by culture of dendritic cell progenitors in hypoxia. Natural killer cell memory is induced, in part, by triggering of upregulation of cytokines associated with homeostatic expansion such as interleukin 7 and interleukin 15.
Type: Application
Filed: February 8, 2022
Publication date: August 11, 2022
Applicant: Therapeutic Solutions International, Inc.
Inventors: Thomas E. ICHIM, Timothy G. DIXON, James VELTMEYER, Famela RAMOS
STIMULATION OF DENDRITIC CELL ACTIVITY BY HOMOTAURINE AND ANALOGUES THEREOF
Publication number: 20220235325
Abstract: Disclosed are means, methods, and compositions of matter useful for enhancement of dendritic cell activity. In one embodiment the invention provides the use of GABA agonists such as homotaurine for stimulation of dendritic cell activity. In one embodiment said dendritic cell activity is enhancement of natural killer cell activity and/or of T cell activity. In one embodiment NK cell activity is ability to induce cytotoxicity in neoplastically transformed cells, whereas T cell activity is either cytokine production for CD4 cells or cytotoxicity for CD8 cells.
Type: Application
Filed: January 26, 2022
Publication date: July 28, 2022
Applicant: Therapeutic Solutions International, Inc.
Inventors: Thomas Ichim, Timothy G. Dixon, James Veltmeyer, Famela Ramos
IMMUNOTHERAPY FOR OPIOID ADDICTION
Publication number: 20220193127
Abstract: Disclosed are means, methods and compositions of matter useful for reduction of brain inflammation and prevention of opioid addiction and/or tolerance. In one embodiment the invention provides utilization of platelet rich plasma (PRP), alone, or admixed with regenerative/anti-inflammatory adjuvants, for reduction of neural inflammation. In one embodiments PRP is admixed with oxytocin and administered intranasally in a patient at risk of opioid addiction. In another embodiment, PRP is admixed with fortified and non-fortified nigella sativa oil, and/or pterostilbene and administered intranasally. Other embodiments include utilization of autologous stromal vascular fraction cells alone and/or admixed with regenerative/anti-inflammatory adjuvants.
Type: Application
Filed: December 20, 2021
Publication date: June 23, 2022
Applicant: Therapeutic Solutions International, Inc.
Inventors: Thomas Ichim, Timothy G. Dixon, Famela Ramos, Wais Kaihani, James Veltmeyer, Kalina O'Connor
TREATMENT OF MAJOR DEPRESSIVE DISORDER AND SUICIDAL IDEATIONS THROUGH STIMULATION OF HIPPOCAMPAL NEUROGENESIS UTILIZING PLANT-BASED APPROACHES
Publication number: 20220175701
Abstract: Disclosed are means and methods of treating major depressive disorder and/or other disorders that predispose to suicide by administration of nutraceutical means, wherein said nutraceuticals are administered at a frequency and/or concentration sufficient to induce proliferation of endogenous neural progenitor cells. In one embodiment said nutraceuticals are comprised of green tea extract, and/or Nigella sativa, and/or pterostilbene, and/or sulforaphane. In some embodiment's nutraceutical compositions are utilized to overcome treatment resistant of currently used antidepressants.
Type: Application
Filed: December 8, 2021
Publication date: June 9, 2022
Applicant: THERAPEUTIC SOLUTIONS INTERNATIONAL, INC.
Inventors: Thomas Ichim, Timothy G. Dixon, James Veltmeyer, Kalina O'Connor
PROTECTION AND REGENERATION OF NEUROLOGICAL FUNCTION BY USING STEM CELLS
Publication number: 20220125852
Abstract: Disclosed are therapeutic compounds, protocols, and compositions of matter useful for treatment of neurological conditions. In one embodiment the invention teaches the treatment of chronic traumatic encephalopathy (CTE) through protecting/regenerating the endothelial by administration of cells such as stem cells. In one embodiment stem cells are administered in order to protect the endothelium from apoptosis and to preserve the blood brain barrier. In another embodiment stem cells are administered together with endothelial progenitor cells in order to regenerate neural endothelium. In other embodiments preservation of brain integrity in conditions of degeneration is accomplished by administration of stem cells and/or endothelial cells.
Type: Application
Filed: October 27, 2021
Publication date: April 28, 2022
Applicant: Therapeutic Solutions International, Inc.
Inventors: Thomas E. Ichim, Timothy G. Dixon, Amit N. Patel, Famela Ramos, Kalina O'Connor
AUGMENTATION OF NATURAL KILLER CELL ACTIVITY AND INDUCTION OF CYTOTOXIC IMMUNITY USING LEUKOCYTE LYSATE ACTIVATED ALLOGENEIC DENDRITIC CELLS: STEMVACS
Publication number: 20220096542
Abstract: Stimulation of immunity would be beneficial in various chronic conditions such as viral infections and neoplasia. Autologous dendritic cell therapy has been widely described in the immunotherapy literature and has been approved by the FDA for treatment of prostate cancer. Unfortunately, the need to generate individual doses is costly and limited by ability of the patients to have sufficient starting cell numbers available to generate sufficient dendritic cells. Here we describe the process of preparing allogeneic dendritic cells utilizing a leukocyte lysate based approach. These data support development of StemVacs for conditions that would benefit from NK activation such as cancer and COVID-19.
Type: Application
Filed: June 30, 2021
Publication date: March 31, 2022
Applicant: THERAPEUTIC SOLUTIONS INTERNATIONAL, INC.
Inventors: Thomas E. ICHIM, Timothy G. DIXON, James VELTMEYER
Personalized Immunotherapies for Reduction of Brain Inflammation and Suicide Prevention
Publication number: 20220088086
Abstract: Disclosed are means, methods and compositions of matter useful for reduction of brain inflammation and prevention of suicidal ideations and suicidal attempts. In one embodiment the invention provides utilization of autologous platelet rich plasma, alone, or admixed with regenerative/anti-inflammatory adjuvants, for reduction of neural inflammation. In one embodiment autologous PRP is admixed with oxytocin and administered intranasally in a patient at risk of suicidal ideation. In another embodiment, PRP is admixed with fortified and non-fortified nigella sativa oil and administered intranasally. Other embodiments include utilization of autologous stromal vascular fraction cells alone and/or admixed with regenerative/anti-inflammatory adjuvants.
Type: Application
Filed: September 24, 2020
Publication date: March 24, 2022
Applicant: Therapeutic Solutions International, Inc.
Inventors: Thomas E. Ichim, Timothy G. Dixon, Famela Ramos, Kalina O'Connor
Nutraceuticals for the prevention, inhibition, and treatment of SARS-CoV-2 and associated COVID-19
Patent number: 11266707
Abstract: Disclosed herein are methods of treating or preventing complications associated with a SARS-CoV-2 infection, comprising: administration of a combination comprising: a) Green Tea and/or extract thereof; b) Blueberry and/or extract thereof; c) Nigella sativa and/or extract thereof; and d) broccoli and/or extract thereof in an amount and frequency sufficient to treat or prevent complications associated with said SARS-CoV-2 infection.
Type: Grant
Filed: May 4, 2020
Date of Patent: March 8, 2022
Assignee: Therapeutic Solutions International, Inc.
Inventors: Thomas E Ichim, Timothy G Dixon
NEUROPROTECTION AND NEUROREGENERATION BY PTEROSTILBENE AND COMPOSITIONS THEREOF
Publication number: 20220031793
Abstract: Disclosed are means, compositions of matter, and protocols useful for neuroprotection and neuroregeneration. In one embodiment the invention provides administration of pterostilbene alone or in combination with other ingredients to induce neuroprotection and/or neuroregeneration. The invention teaches protection/regeneration in conditions associated with neurological inflammation and/or other congenital or acquired neurodegenerative diseases.
Type: Application
Filed: July 28, 2021
Publication date: February 3, 2022
Applicant: THERAPEUTIC SOLUTIONS INTERNATIONAL, INC.
Inventors: Thomas E. ICHIM, Timothy G. DIXON, James VELTMEYER
ADDITIVE AND/OR SYNERGISTIC COMBINATIONS OF METFORMIN WITH NUTRACEUTICALS FOR THE PREVENTION, INHIBITION AND TREATMENT OF SARS-COV-2 AND ASSOCIATED COVID-19
Publication number: 20220023237
Abstract: Disclosed are compositions of matter, treatments and protocols useful for prevention of SARS-CoV-2 infection, as well as inhibition of viral propagation and acceleration of viral cure. In some embodiments the invention teaches the administration of a therapeutic combination of ingredients comprising of metformin, pterostilbene, nigella sativa, sulforaphane, and epigallocatechin-3-gallate (EGCG) to a mammal at risk of infection with SARS-CoV-2. In another embodiment, the invention teaches administration of said therapeutic combination to a mammal infected with said SARS-CoV-2. In some embodiments dosage of said therapeutic combination is based on inflammatory and/or immunological parameters observed in patients with COVID-19.
Type: Application
Filed: July 22, 2021
Publication date: January 27, 2022
Applicant: THERAPEUTIC SOLUTIONS INTERNATIONAL, INC.
Inventors: Thomas E. Ichim, James Veltmeyer, Timothy G. Dixon
Nutraceuticals for suppressing indolamine 2,3 deoxygenase
Patent number: 11229674
Abstract: Disclosed are compositions of matter, treatments and protocols useful for reduction of expression and/or activity of indolamine 2,3 deoxygenase (IDO). In some embodiments the invention teaches the administration of a therapeutic combination of ingredients comprising of pterostilbene, Nigella sativa, sulforaphane, and epigallocatechin-3-gallate (EGCG) to a mammal at possessing an increased expression and/or activity of said IDO in which reduction of number and/or activity is desired. In another embodiment, the invention teaches administration of said therapeutic combination to a mammal infected with viral and/or bacterial infections and/or neoplasia. In some embodiments dosage of said therapeutic combination is based on inflammatory and/or immunological parameters observed in patients.
Type: Grant
Filed: October 23, 2020
Date of Patent: January 25, 2022
Assignee: Therapeutic Solutions International, Inc.
Inventors: Thomas E. Ichim, Timothy G. Dixon, James Veltmeyer
Treatment of SARS-CoV-2 with Dendritic Cells for Innate and/or Adaptive Immunity
Publication number: 20210393681
Abstract: Disclosed are means, methods, and compositions of matter for prophylaxis and/or treatment of SARS-CoV-2 by administration of dendritic cells in a manner and frequency sufficient to induce activation of innate and/or adaptive immune responses. In one embodiment the invention teaches administration of dendritic cells pulsed with one or more innate immune stimulants in a manner endowing said dendritic cell with ability to induce augmentation of natural killer (NK) cell number and/or activity. In another embodiment the invention teaches the use of dendritic cells stimulated with innate immune activators in a manner to allow for uptake of viral particles and presentation of viral epitopes to T cells in order to stimulate immunological activation and/or memory responses.
Type: Application
Filed: June 22, 2020
Publication date: December 23, 2021
Applicant: Therapeutic Solutions International, Inc.
Inventors: Thomas E. Ichim, Timothy G. Dixon, James Veltmeyer
Nutraceuticals for Reducing Myeloid Suppressor Cells
Publication number: 20210386815
Abstract: Disclosed are compositions of matter, treatments and protocols useful for reduction of number and/or activity of myeloid suppressor cells (MSC). In some embodiments the invention teaches the administration of a therapeutic combination of ingredients comprising of pterostilbene, Nigella sativa, sulforaphane, and epigallocatechin-3-gallate (EGCG) to a mammal at possessing an increased number and/or activity of said MSC in which reduction of number and/or activity is desired. In another embodiment, the invention teaches administration of said therapeutic combination to a mammal infected with viral and/or bacterial infections and/or neoplasia. In some embodiments dosage of said therapeutic combination is based on inflammatory and/or immunological parameters observed in patients.
Type: Application
Filed: June 11, 2020
Publication date: December 16, 2021
Applicant: Therapeutic Solutions International, Inc.
Inventors: Thomas E. Ichim, Timothy G. Dixon, James Veltmeyer
Nutraceuticals for the Prevention, Inhibition, and Treatment of SARS-Cov-2 and Associated COVID-19
Publication number: 20210338763
Abstract: Disclosed herein are methods of treating or preventing complications associated with a SARS-CoV-2 infection, comprising: administration of a combination comprising: a) Green Tea and/or extract thereof; b) Blueberry and/or extract thereof; c) Nigella sativa and/or extract thereof; and d) broccoli and/or extract thereof in an amount and frequency sufficient to treat or prevent complications associated with said SARS-CoV-2 infection.
Type: Application
Filed: May 4, 2020
Publication date: November 4, 2021
Applicant: Therapeutic Solutions International, Inc.
Inventors: Thomas E. Ichim, Timothy G. Dixon
CELLULAR, ORGAN, AND WHOLE-BODY REJUVENATION UTILIZING CORD BLOOD PLASMA AND PTEROSTILBENE
Publication number: 20210128638
Abstract: Disclosed are methods, means, and protocols for stimulation of rejuvenation in single cells, organs, and organisms by administration of cord blood derived plasma, cord blood plasma concentrates, and cord blood derived exosomes together with pterostilbene. The invention describes the previously unexpected finding that addition of pterostilbene to cord blood enhances the rejuvenation properties of cord blood. Said rejuvenation properties include telomere preservation, reduction in beta galactosidase, and retention of cellular activities.
Type: Application
Filed: November 4, 2020
Publication date: May 6, 2021
Applicant: Therapeutic Solutions International, Inc.
Inventors: Thomas E. Ichim, Timothy G. Dixon
Augmentation of oncology immunotherapies by pterostilbene containing compositions
Patent number: 9682047
Abstract: Compositions and methods useful to enhancing, improving, or eliciting anti-tumor immune responses are disclosed. A pterostilbene containing composition is administered to a cancer patient at a sufficient concentration and frequency to induce de-repression of tumor targeting immune responses. The composition enhances antibody dependent cellular toxicity (ADCC) and augments efficacy of antigen specific immunotherapeutics such as trastuzumab and other monoclonal antibody therapies useful for treating cancer.
Type: Grant
Filed: July 7, 2016
Date of Patent: June 20, 2017
Assignee: Therapeutic Solutions International, Inc.
Inventors: Timothy G. Dixon, Gerry B. Berg, Robert F. Graham, Santosh Kesari, Thomas Ichim
the IPs keep rolling in now.
The TSOI R and D follow through
is factoring in
His patents will make the patient investor rich, and help people all over the world. I know that the ones I've tried work, That is all I need for proof that TSOI will prevail.
they're not worthless patents
there is some massive IP work that's gone into this company -imo
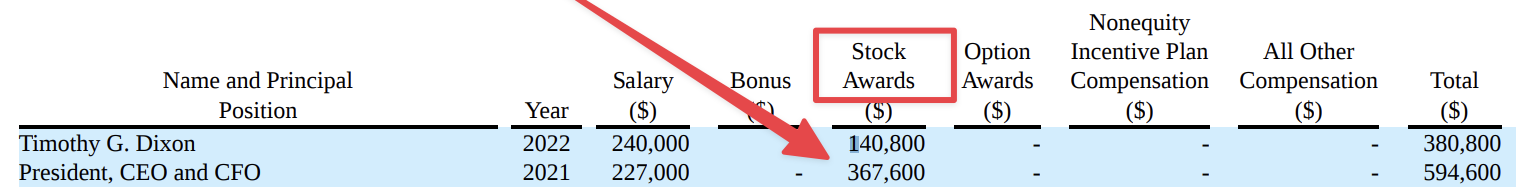
Worthless patents brought in investors on August 9, 2021! No doubt about that! That’s part of the loved DD!!!
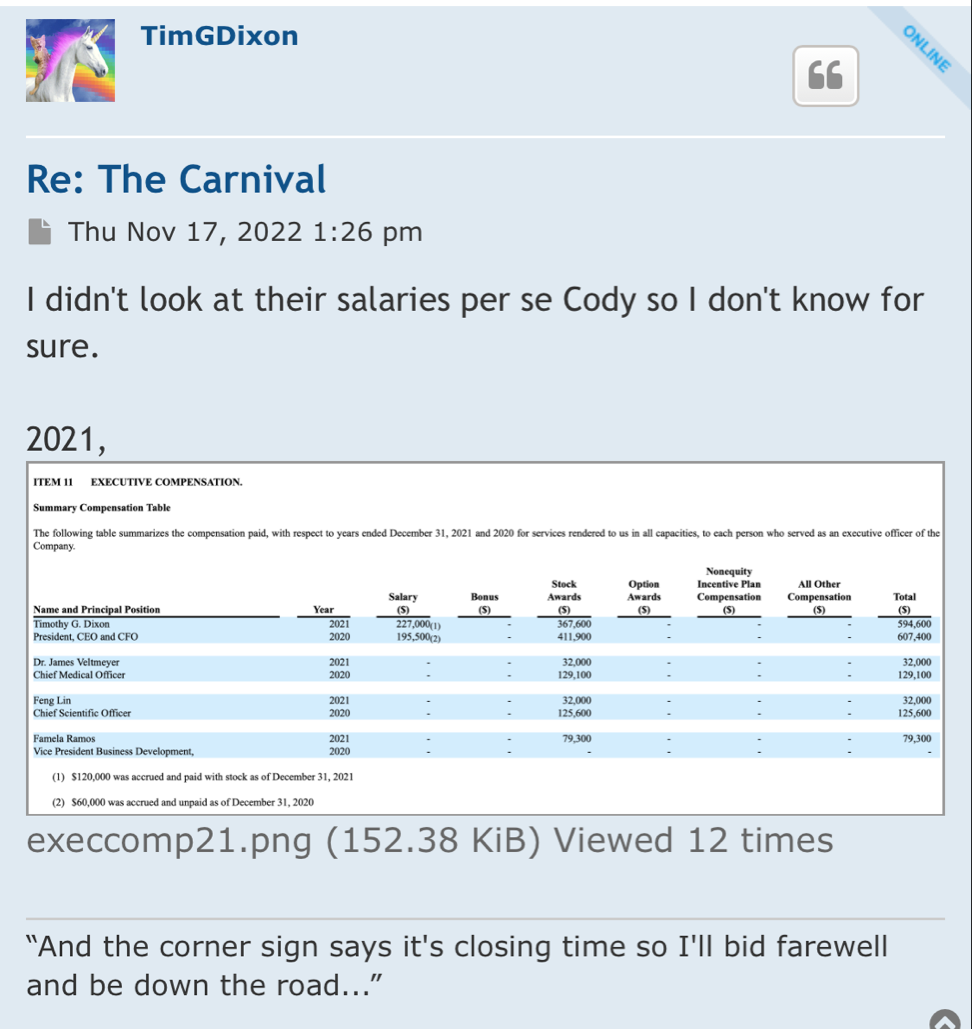
It’s just a diluted pig. Tim’s produced well patents (not worth shit to investors) and well dilution. He’s not gonna stop till he can’t anymore
Well I’m getting ready to go on a little vacation to Washington state. I’ve got the TSOI shareholders property address that I’m going to stop by and do a little fission in that beautiful creek running through it. I think the trees are beautiful and the creek is also beautiful and hope it’s got some nice fish in it I love to fish.
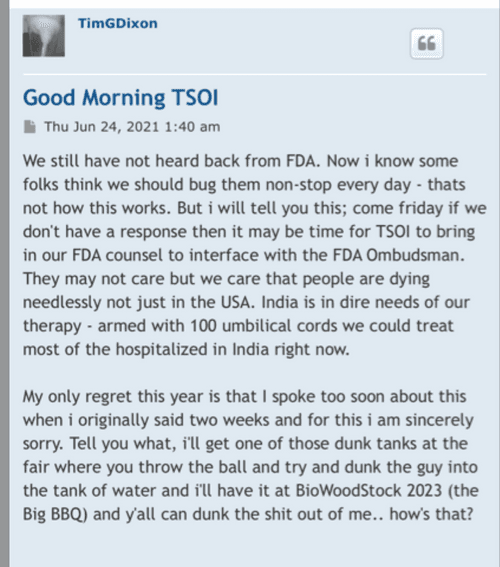
I’ll keep checking in to see if the FDA has reported back to us I think the 30 days are up today hopefully Mandy will hear something from the company. In the meantime I’ve got my heart set on fission.
TSOI is a Bio-Pharma company
what do you think an IND is ? it's an Investigational New Drug
since TSOI has several INDs in the mix,
ie: INDs =Drugs
Drugs=Pharma
A biopharmaceutical, also known as a biological medical product,[1] or biologic, is any pharmaceutical drug product manufactured in, extracted from, or semisynthesized from biological sources .
https://en.wikipedia.org/wiki/Biopharmaceutical
JadiCells qualify under that definition. TSOI is now running the Phase 3 clinical trial .
the JadiCell MSCs are extracted from the umbilical cord
TSOI is far from a Biotech Pharma company …
Therapeutic Solutions International, Inc. is involved in the medical sciences market. We are focused on three different areas within the same market. They are outlined as follows: 1. Dietary Supplements 2. Immunotherapy Our business plan is twofold: 1. Produce and refine current product lines and develop them further, and 2. To focus on leveraging immunological processes to diagnose and intervene in niche areas of unmet medical needs.
The average Debt that Biotech/Pharma cos carry with Phase 3 trials in 100's of millions. Know that and check again!
FREE TRADING (No restrictions) w/ 20% discount to the lowest closing bid price (posted) over the preceding 10 consecutive day evaluation period
Oh I now expect many more of the investing public will be asking MORE questions & relaying what really is happening w/ & between TSOI & GHS
PLURIPOTENT STEM CELL DERIVED DENDRITIC CELLS AND ENGINEERED DENDRITIC CELLS FOR CANCER IMMUNOTHERAPY
https://patents.justia.com/patent/20220298491
(New TSOI patent published 9/22/22)
Abstract
Disclosed are populations of dendritic cells generated from stem cells capable of inducing immunity towards cancer. In one embodiment said dendritic cells are generated from allogeneic inducible pluripotent stem cells, for some uses, said pluripotent stem cells are genetically engineered/edited to induce cancer specific immunity and/or resist immunosuppressive effect of tumor derived microenvironment. In one embodiment pluripotent stem cells are transfected with cancer stem cell antigens such as BORIS and/or NR2F6.
Claims
1. A method of generating a dendritic cell from a stem cell possessing enhanced ability to induce anticancer immunity, wherein said dendritic cell is obtained by the process of: a) selecting a stem cell population; b) genetically modifying said stem cell population to endow enhanced anticancer activity towards said stem cell; c) differentiating said stem cell into a dendritic cell population.
2. The method of claim 1, wherein said stem cell is an inducible pluripotent stem cell.
3. The method of claim 2, wherein said inducible pluripotent stem cell is generated by transfection of mammalian cells with a pluripotency factor, wherein said pluripotency factor gene is one or more genes selected from the group consisting of oct3/4, sox2, klf4, c-myc, lin28, nanog, glis-1, bcl2, bclxl, AIRE, HIF-1 alpha, survivin, livin and bclx.
4. The method of claim 3, wherein, mammalian cells are further provided with: a. a third nucleic acid encoding from 2 to 7 distinct gRNAs, each gRNA comprising a DNA-binding segment and a polypeptide-binding segment, wherein the DNA-binding segment binds the promoter region of a second endogenous pluripotency factor gene; and b. a fourth nucleic acid encoding from 2 to 7 distinct gRNAs, each gRNA comprising a DNA-binding segment and a polypeptide-binding segment, wherein the DNA-binding segment binds the promoter region of a third endogenous pluripotency factor gene; wherein the transcriptional modulator binds the polypeptide-binding segment of the gRNAs encoded by the third and fourth nucleic acids.
5. The method of claim 4, wherein: (i) the DNA-binding segment of each the gRNAs encoded by the first nucleic acid is complementary to at least a portion of the promoter region of a mammalian oct3/4 gene; (ii) the DNA-binding segment of each the gRNAs encoded by the third nucleic acid is complementary to at least a portion of the promoter region of a mammalian sox2 gene; and (iii) the DNA-binding segment of each the gRNAs encoded by the fourth nucleic acid is complementary to at least a portion of the promoter region of a mammalian klf4 gene.
6. The method of claim 1, wherein said pluripotent stem cell is transfected with a tumor antigen in order to induce immunity towards said tumor antigen.
7. The method of claim 6, wherein said tumor antigen is CTCFL.
8. The method of claim 6, wherein said tumor antigen is PDGFR-beta.
9. The method of claim 6, wherein said tumor antigen is PAP.
10. The method of claim 6, wherein said tumor antigen is MAD-CT-2.
11. The method of claim 6, wherein said tumor antigen is Tie-2.
12. The method of claim 6, wherein said tumor antigen is PSA.
13. The method of claim 6, wherein said tumor antigen is protamine.
14. The method of claim 6, wherein said tumor antigen is legumain.
15. The method of claim 6, wherein said tumor antigen is endosialin.
16. The method of claim 6, wherein said tumor antigen is PSMA.
17. The method of claim 6, wherein said tumor antigen is carbonic anhydrase IX.
18. The method of claim 6, wherein said tumor antigen is STn.
19. The method of claim 6, wherein said tumor antigen is Page4.
20. The method of claim 6, wherein said tumor antigen is proteinase 3.
CROSS-REFERENCE TO RELATED APPLICATIONS
[0001] This application claims priority to U.S. Provisional Application Ser. No. 63/161,526, filed on Mar. 16, 2021, entitled "Pluripotent Stem Cell Derived Dendritic Cells and Engineered Dendritic Cells for Cancer Immunotherapy", which is incorporated herein by reference in its entirety
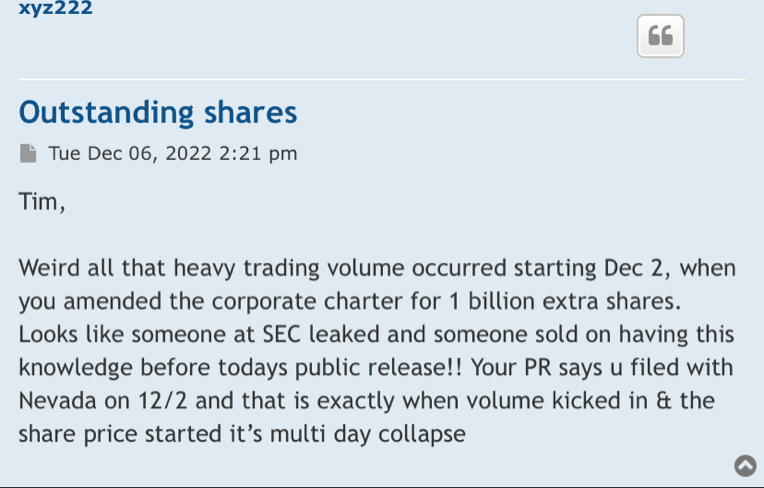
i didn't hear anyone else saying they were done asking questions about the purchase agreement, here or on their forum
GHS Purchased Shares Freely Tradable w/o restrictions on resale surprised that none of the investing public caught this or why it has not been brought up prior. hell(o) it's real DD that can affect one's investment. Timothy G Dixon has also not mentioned it on his private forum while touting other sections that he thinks might be well received or might deflect any from really looking closely @ the Purchase Agreement.
https://investorshub.advfn.com/boards/read_msg.aspx?message_id=170028726
TSOI GHS Purchased Shares Freely Tradable w/o restrictions on resale Appears to be another falsehood trying to be inserted into the conversation, more deflection or...Wonder Why as I have stated pull up the Purchase Agreement, enlarge & read carefully
they buyin in restricted shares @ par value .
(b) Settlement for Purchase Shares
All Purchase Shares issued hereunder will be DWAC Shares.
DWAC Shares means shares of Common Stock that are (i) issued in electronic form. (ii)freely tradable and without restriction on resale and (iii) timely credited by the company to the Investor's or it's designee's specific Deposit Withdrawal at Custodian (DWAC) account with DTC under it's Fast Automated Securities Transfer (FAST) Program, or any similar program hereafter adopted by DTC performing substantially the same function.[
nice to see ETRF move that 2.5m+ bid up to 01 instead of 0095
"... geared to GO!!"
Good advice. Recharge.
TimGDixon wrote: Fri Sep 23, 2022 6:51 am
Time to go home...
Tough to leave those places we all go & slow-down, unwind and RECHARGE….
Lots to do between now and next spring, at least your geared to GO!!
Travel Safe my friend !!
the bad MM's never have many shares that run through they just like to scare investors
with the exception of GTSM
Re: Good morning from Idaho
Post by TimGDixon » Fri Sep 23, 2022 7:03 am
curncman wrote: ?Fri Sep 23, 2022 6:59 am
Heading back to San Diego?
Heading somewhere... maybe china lake or maybe temecula or pauma valley...
“And the corner sign says it's closing time so I'll bid farewell and be down the road..
https://forum.therapeuticsolutionsint.com/viewtopic.php?t=334&start=270
.”
hard(ly) @ work or????? although his (actually should be shareholders) fishing shack seems to be completed or damn near
3 retail mm's at .02 //others JANE ASCM, GTSM OTCX taking us down
all I can do is go with the flow TSOI
whatever your opinion of Tim,
the R and D continues , and the patents keep rollin in .
some day the odds will play into the equation ,
and bring it all home .
Always does much like TimmyBoi’s actions/words are & have been quite telling to date
time will tell .
earlier funders were not much fun.
mea culpa,
i hadn't read the 8K fully until yesterday . there were a lot of questions on the forum that were answered by Tim after market hours yesterday.
GHS gets to buy the shares @par value
Par Value 0.001
What happens if you own 5% of a company?
When a person or group acquires 5% or more of a company's voting shares, they must report it to the Securities and Exchange Commission. Among the questions Schedule 13D asks is the purpose of the transaction, such as a takeover or merger.
1. Certain definitions (e) Commitment Shares means 5 million shares(5,000,000) of the Company's common stock issued upon the initial Closing as an equity incentive.
(h) DWAC Shares means shares of Common Stock that are (i) issued in electronic form (ii)freely tradable & transferable & without restriction on resale (iii) timely credited by the Company to the Investor's or it's designee's specified Deposit Withdrawal @ Custodian (DWAC) account with DTC under its Fast Automated Securities transfer (FAST) Program, or any similar program hereafter adopted by the DTC performing substantially the same function.
yes, but what i posted before
https://investorshub.advfn.com/boards/read_msg.aspx?message_id=170018681
was about the when,
here is the explanation:
by TimGDixon » Thu Sep 22, 2022 5:38 pm
That's not really how this works. If you look at exhibit in 8k, it contains an exhibit (A) that is used to cause a sale by the Company to the Investor based on the terms of the Purchase Agreement.
EXHIBIT A
FORM OF PURCHASE NOTICE
________, 202__
To: GHS Investments, LLC
In accordance with Section 2 of the purchase agreement, dated September [ ], 2022 (the “Purchase Agreement”),
between Therapeutic Solutions International, Inc. (the “Company”) and GHS Investments, LLC (the “Investor”), the
Company hereby provides notice to the Investor of a sale by the Company to the Investor of Purchase Shares in the
amount set forth in this Purchase Notice. Capitalized terms used herein have the meanings set forth in the Purchase
Agreement.
Purchase Amount: $___________
Purchase Price per share: $____________
Number of Purchase Shares: __________
Very truly yours,
Therapeutic Solutions International, Inc.
By:_________________________
Name:
Title:
|
Followers
|
523
|
Posters
|
|
|
Posts (Today)
|
2
|
Posts (Total)
|
65717
|
|
Created
|
10/04/08
|
Type
|
Free
|
| Moderators BigBadWolf johnnytrader33 JMC$ Yooperman Hogwarts | |||



















































Preclinical Data Suggests QuadraMune™ Prevents Stress-Induced Suppression of Neurogenesis More Effectively than Prozac
OCEANSIDE, Calif., Dec. 9, 2020 /PRNewswire/ -- Therapeutics Solution International, Inc., (OTC Markets: TSOI), announced today new data suggesting the possibility that QuadraMune™ may mediate neuroprotective activity through preserving the ability of regenerative brain cells to proliferate subsequent to psychological stress.
The experiments, which involved exposing mice to established stressors, demonstrated that specific areas of the brain associated with production of new brain cells are damaged by stress. In agreement with previously published research, administration of fluoxetine (Prozac™) protected the brain from stress-induced damage. Surprisingly, QuadraMune™ administration appeared superior to Prozac™ at stimulating proliferation of new brain cells.
"QuadraMune™ which is currently in a clinical trial for prevention of COVD-191, has also been demonstrated to possess anti-inflammatory activity in other clinical trials, suppressing cytokines such as IL-62, which are known to be involved in depression3 and suicide4" said Kalina O'Connor, Director of Campbell Neurosciences and co-inventor on the patent. "Given major depressive disorder causes a significant risk for suicide, we are highly interested in exploring the use of QuadraMune™ for preventing suicide."
"Although much enthusiasm has been generated over the planned distribution of the COVID vaccine, at present little is being done to address mental health issues that are being exacerbated by the current pandemic" said Dr. James Veltmeyer, co-inventor of the patent, and Chief Medical Officer of the Company. "If current results are reproducible, the possibility that a nutraceutical would concurrently boost immunity while preserving mental health is highly enticing."
"It has not escaped us that COVID-19 is associated with increased inflammatory cytokines in the blood of patients, cytokines that also predispose to depression" said Famela Ramos, Vice President of Business Development for the Company. "It may be that the recent increase in suicides and suicide attempts is related biologically to activities of the coronavirus. It will be interesting to examine whether QuadraMune™ may modify putative negative mental effects of the virus."
"An estimated 17.3 million adults in the United States had at least one major depressive episode. This number represented 7.1% of all U.S. adults" stated Timothy Dixon, President and CEO of the Company. "We believe the Mission of our Company is not just providing a return on investment to our shareholders, but also increasing the quality of life for Americans. We are extremely pleased to report this unexpected finding with significant potential implications to advancing non-toxic means of helping patients with this terrible condition."
1 QuadraMune(TM) for Prevention of COVID-19 - Full Text View - ClinicalTrials.gov
2 Therapeutic Solutions International Announces Positive Preclinical and Clinical Evaluation of Nutritional Supplement QuadraMune™, Designed to Protect Against COVID-19 | BioSpace
3 Ting et al. Role of Interleukin-6 in Depressive Disorder. Int J Mol Sci. 2020 Mar 22;21(6):2194.
4 O'Donovan et al. Suicidal ideation is associated with elevated inflammation in patients with major depressive disorder. Depress Anxiety. 2013 Apr;30(4):307-14.
| Volume | |
| Day Range: | |
| Bid Price | |
| Ask Price | |
| Last Trade Time: |
