Register for free to join our community of investors and share your ideas. You will also get access to streaming quotes, interactive charts, trades, portfolio, live options flow and more tools.
Where's a place to get all the news like this?
Where's a place to get all the news like this?
RONTO, ON / ACCESSWIRE / April 22, 2016 / Theralase Technologies Inc. ("Theralase" or the "Company") (TSX V: TLT) (TLTFF), a leading biotech company focused on commercialization of medical devices to eliminate pain and development of Photo Dynamic Compounds ("PDCs") to destroy cancer, announced today that it has reached a milestone, demonstrating 6 month accelerated stability and 9 month long term stability of it lead anti-cancer PDC, TLD-1433.
Stability studies are an essential component of pharmaceutical development, allowing evaluation of a drug's stability under the influence of a variety of environmental factors such as: temperature, humidity and light. Data from these studies enable recommended storage conditions, retest intervals and shelf lives to be established.
Demonstrating accelerated and long term stability of a drug to be used in human clinical testing is essential to prove that the potency and/or efficacy of the drug is not affected during prolonged shelf life.
Under accelerated and long term stability storage conditions, the drug is evaluated by High Performance Liquid Chromatography to separate, identify and quantify each chemical component to a very high degree of resolution to assess if any change occurs in the chemical composition over time.
Long-term stability is completed over three years, with reporting at 0, 3, 6, 9, 12, 18, 24 and 36 months. Accelerated stability is completed over six months, with reporting at 0, 3 and 6 months.
TLD-1433 has now demonstrated stability at 6 months under accelerated conditions and 9 months under long-term stability conditions, satisfying Health Canada guidelines, to allow use in treating patients in a clinical study.
The Company is currently pursuing an Investigational Testing Authorization ("ITA") of its proprietary laser system to activate the PDCs for the indication of Non-Muscle Invasive Bladder Cancer ("NMIBC").
Theralase is focused on commencing and successfully completing a Phase Ib clinical trial for patients afflicted with NMIBC utilizing its novel, next generation light activated, anti-cancer drug, TLD-1433 for the primary endpoints of safety and tolerability, with a secondary endpoint of pharmacokinetics (movement of drug within tissue) and an exploratory endpoint of efficacy.
Theralase (TSXV: TLT) (TLTFF: OTC) Partners with University of Toledo in Cancer Research
(via Thenewswire.ca)
Toronto, Ontario / TNW-ACCESSWIRE / June 18, 2014 -- Theralase Technologies Inc. ("Theralase") (TSXV: TLT) (TLTFF: OTC Pink(R)) announced today that it has executed an agreement with the University of Toledo to conduct preclinical research into the safety and effectiveness of Theralase's Photo Dynamic Compounds (PDCs) in the destruction of bladder cancer in a rat model. The study is entitled, "The use of novel Photo Dynamic Therapy (PDT) in a rat bladder tumor model."
The first phase of this preclinical study will be to optimize the effectiveness of various Theralase PDCs in the destruction of rat bladder tumor cells (AY-27) using various PDC concentrations and laser light doses.
The second phase will be to complete two in vivo rat tumor model studies. The first set of experiments will examine PDC uptake and distribution in rat bladder tumors. The second set of experiments will focus on destroying rat bladder cancer safely and effectively through the instillation of PDCs into rat bladders exhibiting cancerous tumours and then light activating them.
The outcome of these experiments will provide independent confirmation and optimization of the lead PDC selected for bladder cancer destruction and prove the safety and efficacy of the Theralase lead PDC in the destruction of bladder cancer in a live animal model.
Dr. Arkady Mandel, Chief Scientific Officer at Theralase Inc. stated, "I am interested in reviewing the results of an independent confirmation and optimization of the safety and efficacy of the Theralase PDC in destroying bladder cancer in an orthotopic (occurring at the normal place) rat model. The University of Toledo is a great choice for this research, as their group is well known for expertise in the urological applications of PDT. The results of this preclinical research will allow the Company to progress to Good Manufacturing Practice (GMP) manufacture of the lead PDC. We are delighted to have the experience and knowledge of the University of Toledo on board to execute this pivotal preclinical research, as they will be instrumental in helping us prove the destruction of rat bladder cancer with PDCs in 2014, preparing us for clinical application in 2015."
Roger Dumoulin-White, President and CEO of Theralase stated that, "Theralase is delighted that we have executed a research agreement with the University of Toledo. Completion of this work is pivotal for Theralase to independently validate and optimize our research findings in the PDT destruction of bladder cancer. Results of the University of Toledo's work will determine the uptake and distribution of the PDCs into bladder cancer tumors versus healthy bladder and the amount of PDCs required to safely and effectively destroy bladder cancer in a live animal model. This data will be instrumental in helping to determine the proper amount of PDC required for human application. I look forward to reporting on the results of this exciting research later this year."
About Theralase Technologies Inc.
Theralase Technologies Inc. ("Theralase") (TSXV: TLT) (TLTFF: OTC Pink(R)) designs, manufactures and markets patented, superpulsed laser technology used in eliminating pain and destroying cancer. Theralase technology is safe and effective in eliminating pain, reducing inflammation and accelerating tissue regeneration in numerous nerve, muscle and joint conditions. Theralase is developing patented Photo Dynamic Compound (PDC) technology that is able to target and destroy cancers, bacteria and viruses when light activated.
Theralase's (TLTFF) (TLT.V), Cancer Technology Demonstrates Vaccine-Like Properties
Whitefish, MT / June 16, 2014 / Conventional cancer treatment involves surgically removing cancerous tumors and then killing the remaining cells with toxic chemotherapy drugs. Unfortunately, these approaches involve invasive surgeries and the indiscriminant killing of cells that lead to the immune system being compromised. The success rates for these treatments, particularly in later stage cancers, also leave a lot to be desired.
Companies like Galena Biopharma (GALE), Inovio Pharmaceuticals (NYSE MKT: INO), and ImmunoCellular Therapeutics (IMUC) have tried to empower the patient's own immune system to selectively target cancer cells rather than killing indiscriminately. The idea is to create a kind of cancer vaccine that separates proteins from cancer cells and immunizes patients against those proteins to stimulate an immune reaction.
In this article, we'll take a look at a different non-invasive approach that's achieves similar vaccine-like properties using the power of light.
Killing Cancer with Light
Theralase Technologies Inc. (TLTFF) (TLT.V), a designer, manufacturer, and marketer of patented super-pulsed laser technologies used in eliminating pain and destroying cancer, has taken a different variation of that approach by introducing Photo Dynamic Compounds ("PDCs") into cancerous cells and then killing those cells by exposing them to cold laser light.
In preclinical animal testing at Princess Margaret Cancer Centre, University Health Network ("UHN"), the company injected mice with 350,000 colon cancer cells to produce tumors that grew to 5mm in size. They were subsequently treated with an intra-tumoral injection of the firm's lead PDC and exposed to Near InfraRed ("NIR") light to activate the PDCs and destroy the vast majority of tumors.
Vaccine-like Properties
The same mice that received the initial PDCs were re-injected with the same number of colon cancer cells 13 to 23 days later. Interestingly, with no further treatment intervention, 40% of the mice demonstrated either a small tumor regrowth that quickly regressed or in 60% of the mice, no tumor regrowth at all. The results suggest a short-term immune-mediated ("memory response") tumor rejection with vaccine-like properties.
Approximately 10 months after that injection, the same mice were injected for a third time with 350,000 additional colon cancer cells. 100% of the mice showed no signs of tumor regrowth, even at a 3-month follow-up, suggesting the presence of a long-term anti-tumor ("memory response") immunity responsible for complete tumor rejection. Mice in control experiments did not survive longer than a month following the cancer cell injection.
Potential Implications
The potential for short-term and long-term anti-cancer memory response – or vaccine-like properties, would represent a major breakthrough in cancer research and provide substantial treatment benefit and survival advantage to cancer patients. In addition to the immunity, the patented therapy's potential to rapidly kill patient-specific cancer cells and regress tumors provides the immediate efficacy needed.
"This is one of the first preclinical trials to show that it's possible to generate long-term anticancer memory response," said Theralase CSO Dr. Arkady Mandel. "For the first time in our research program, we have demonstrated that NIR Photo Dynamic Therapy (PDT) leads not only to long standing clearance of colon cancer cells, but also provides long lasting protection against further tumor cell challenge in young and older mice."
Looking Ahead
Theralase Technologies plans on continuing its pre-clinical studies to confirm the findings for a range of induced and spontaneous animal tumor models. Once the results have been adequately tested on animals, researchers hope to replicate the characteristics in humans and demonstrate the same efficacy. Successful results in humans could have immense implications on the war on cancer.
With a market capitalization of just $17.6 million, investors have the opportunity to buy into a promising cancer-fighting technology, as well as an existing pain management franchise that already generates revenue. Clinical trials on humans are slated for early 2015 and the potential for strategic partnerships and additional clinical results along the way could catalyze the stock higher.
Theralase (TSXV: TLT) (TLTFF) Stock Upgraded on US OTC Market
Toronto, Ontario / TNW-ACCESSWIRE / June 12, 2014 / Theralase Technologies Inc. ("Theralase") (TSXV: TLT) (TLTFF: OTC Link(R)) announced that effective June 3, 2014, its common shares, which trade in the United States Over The Counter (OTC) market under the trading symbol TLTFF, have been upgraded on a venue change from the Grey Market to the OTC Link Market.
OTC Markets facilitates electronic trading with its SEC registered Alternative Trading System known as OTC Link(R) ATS. This system is owned by OTC Link(R), a subsidiary company of OTC Markets Group, that is a fully registered broker-dealer and member of FINRA. The OTCQX(R), OTCQB(R) and OTC Pink(R) platforms traded $135 billion of transactions in 2012 with an average daily dollar volume of $600 million, comprising 10,000 corporate securities; which includes 650 U.S. banks, 2,300 companies that report to the Securities and Exchange Commission (SEC), 1,600 companies that pay dividends, 1,400 American Depository Receipts (ADRs) and 1,400 foreign companies. Together these corporations boast an aggregate market capitalization of approximately $11.6 trillion.
Roger Dumoulin-White, President and CEO of Theralase stated that, "As Theralase continues to execute on its strategic initiatives and accelerate our growth in both our therapeutic and anti-cancer divisions, there is a growing interest south of the border to invest in our common stock. By changing venues from Grey Market to OTC Link(R), the Company is able to provide further transparency and financial reporting to our US based investors allowing them access to more information and hence a greater comfort in investing in Theralase. As Theralase delivers on its corporate milestones in both divisions in 2014 and 2015, it would be reasonable to expect the interest in acquiring Theralase common stock to increase among US based investors, leading Theralase to pursue full financial reporting and disclosure to the SEC to allow an eventual listing on a major US exchange, such as NASDAQ."
About Theralase Technologies Inc.
Theralase Technologies Inc. ("Theralase") (TSXV: TLT) (TLTFF: OTC Link(R)) designs, manufactures and markets patented, superpulsed laser technology used in eliminating pain and destroying cancer. Theralase technology is safe and effective in eliminating pain, reducing inflammation and accelerating tissue regeneration in numerous nerve, muscle and joint conditions. Theralase is developing patented Photo Dynamic Compound (PDC) technology that is able to target and destroy cancers, bacteria and viruses when light activated.
Theralase (TSXV: TLT) (TLTFF: OTCBB) CEO Interviewed by CBC TV about Progress in Anti-Cancer and Pain Research
Roger Dumoulin-White talks to Amanda Lang about Theralase's strategy to deliver value to shareholders and patients
Toronto, Ontario / TNW-ACCESSWIRE / June 5, 2014 / Theralase Technologies Inc. ("Theralase") (TSXV: TLT) (TLTFF: OTCBB) announced today that CBC's senior business correspondent and anchor on The Lang and O'Leary Exchange, Amanda Lang, interviewed Theralase President and CEO, Roger Dumoulin-White about the Company and how the Company is breaking significant new ground in anti-cancer and pain management technology.
Link to the show: http://www.cbc.ca/revenuegroup/the-lang-oleary-exchange.html
Roger Dumoulin-White, President and CEO of Theralase, stated, "My appearance on Canada's top business program confirms that Theralase is emerging as a major research and manufacturing company in two of the most important sectors of the healthcare industry - seeking a cure for cancer and alleviating pain. It is estimated that $91 Billion is spent on the war on cancer and $100 Billion on pain in the United States, annually.
For the last 20 years, Theralase has designed and manufactured patented therapeutic laser technology, which is used for the elimination of pain, reduction of inflammation and dramatic acceleration of tissue healing. Theralase has sold over 800 systems in Canada and over 400 systems in the US and international markets to licenced healthcare practitioners such as: medical doctors, chiropractors, physical therapists and athletic therapists.
Theralase has been so successful in healing nerve, muscle and joint conditions in clinical practice that Theralase's scientists decided to investigate a new application for lasers, the destruction of cancer. Theralase's anti-cancer technology focuses on using specially designed molecules called Photo Dynamic Compounds or PDCs that are able to localize to the DNA of cancer cells and then, when activated by light, destroy the cancer cells.
Theralase recently completed research that demonstrated that its PDC technology is not only able to destroy the primary tumour, but also discovered a "memory response" for the technology. In these animal studies, once the primary tumour was destroyed, repeated injections of cancer cells at a few weeks post-treatment, or even 10 months post-treatment, did not generate cancerous tumours and in fact prevented the recurrence of cancer.
Roger Dumoulin-White, President and CEO of Theralase, stated, "Amanda Lang is extremely knowledgeable and understood the application and benefits of Theralase's therapeutic laser and anti-cancer technology for both the elimination of pain and the destruction of cancer, respectively. Our therapeutic laser technology has been clinically proven to eliminate pain, reduce inflammation and accelerate tissue repair, healing millions of patient's nerve, muscle and joint conditions, effectively. We are very excited about the possibilities for Theralase's anti-cancer technology. If it shows the same efficacy in humans that it has in animals, then our PDC anti-cancer technology and the implications of this "memory response" discovery are nothing short of game changing for both Theralase and for cancer patients."
About The Lang and O'Leary Exchange:
The Lang and O'Leary Exchange is a Canadian business news television series, which airs weekdays on CBC Television and CBC News Network. Hosted by CBC's senior business correspondent Amanda Lang and entrepreneur/investor Kevin O'Leary, the series presents a summary of the day's major business stories in a manner similar to Lang and O'Leary's earlier Business News Network series SqueezePlay.
About Theralase Technologies Inc.
Founded in 1994, Theralase Technologies Inc. ("Theralase") (TSXV: TLT) (TLTFF: OTCBB) designs, manufactures and markets patented superpulsed laser technology used in eliminating pain and destroying cancer. Theralase technology is safe and effective in eliminating pain, reducing inflammation and accelerating tissue regeneration of numerous nerve, muscle and joint conditions. Theralase is actively developing patented technology that is able to target and destroy cancers, bacteria and viruses when activated by light.
Additional information is available at www.theralase.com and www.sedar.com .
Theralase Laser Technology (TSXV:TLT) (OTC:TLTFF), Used By Star Athletes for Pain Management
Whitefish, MT / June 2, 2014 / Theralase Technologies Inc. (TSXV:TLT) (OTC:TLTFF), a key player in the life sciences industry is breaking ground with its laser pain treatments for celebrity athletes. Baseball legend Roy Halladay and tennis star James Blake are just two of many celebrity sports athletes that have extensively used and vouch for Theralase’s TLC-1000 Cold Laser System, a system developed and optimized over the last 20 years.
Extensive clinical research proves that the TLC-1000 is able to reduce pain and accelerate tissue healing, dramatically improving the lives of both professional athletes and the everyday person experiencing muscle pain, joint pain or fatigue. The Theralase super pulsed laser system can penetrate up to 4? into tissue, to promote cellular regeneration at the source of the injury and is the only laser on the market known to activate all three cellular pathways.
Theralase has over 800 systems installed and in use in Canada, with an additional 400 more systems in the US and internationally. To date, over 1 million patients have been successfully treated, and that number is growing daily.
“I had to withdraw from the Rogers Masters in Montreal due to a severe abdominal muscle strain,” said James Blake, #14 ranked professional tennis player in 2007. “For the next two weeks, I had two laser treatments per day with the Theralase(r) 1000 cluster laser, which accelerated the tissue healing and reduced the pain... Theralase(r) laser treatments were very helpful in accelerating my recovery time.”
In addition to healing injuries, many athletes use the technology to maintain musculoskeletal health. Roy Halladay, Cy Young Award Winner and former Major League Baseball all-star, used the technology in this way during his professional baseball career, saying, “I use the Theralase(r) laser to keep my throwing arm healthy. It reduces fatigue and allows me to play at my peak, game after game
Theralase (TSXV: TLT) (OTCBB: TLTFF) Releases 1Q2014 Financial Results
May 30, 2014 -- Theralase Technologies Inc. ("Theralase") (TSXV: TLT) (OTCBB: TLTFF) released its first quarter 2014 financial results today, demonstrating a slight increase in revenue for the three month period ended March 31, 2014, edging up 5% year over year, while successfully advancing its patented next generation therapeutic laser and cancer destruction technologies.
Total revenue for the three-month period ended March 31, 2014 increased 5% from $342,900 to $361,179 year over year.
The net loss for the three-month period ended March 31, 2014 was $344,074 (including $15,897 of net non-cash expenses) compared to a net loss of $332,435 in the same period in 2013 (including $56,829 of net non-cash expenses), a 3.5% increase.
The net loss reflects the ongoing commitment of Theralase to invest in the next generation of therapeutic laser and cancer destruction technologies, from existing therapeutic laser sales.
Selling and marketing expenses increased 12% from $109,391 to $122,278 for the same period in 2013, due primarily to increased spending on product marketing and travel costs.
Administrative expenses decreased 4% from $249,767 to $240,373 for the same period in 2013, due to reductions in administrative personnel and stock based compensation.
Research and development costs decreased 1% from $199,597 to $197,792 for the same period in 2013. The slight decrease reflects the approaching completion of the development of the patented TLC-2000 therapeutic laser technology and the ramp-up in the research and development costs associated with the cancer destruction technology.
Roger Dumoulin-White, President and CEO of Theralase stated that, "The Company is on-track to launch the next generation therapeutic laser technology in 4Q2014 in Canada. The patented TLC-2000 biofeedback therapeutic laser technology has been researched and designed to revolutionize the therapeutic laser industry, by providing patient specific treatments that adapt to a patient's physical characteristics delivering best-in-class performance in the elimination of pain, reduction in inflammation and dramatic acceleration of tissue healing. The medical community and especially the patients who suffer from pain and inflammation on a daily basis have been waiting for a technology of this efficacy and performance to enter the market for a long time."
Mr. Dumoulin-White went on to say, "Our patented cancer technology is demonstrating significant success in preclinical research and is on track for clinical evaluation in humans for bladder cancer as early as 1Q2015. If our PDC technology, with its recent advances in providing an immune-mediated "memory response" in the destruction of cancer is proven effective in cancer patients, the implications of this discovery are nothing short of game changing for both Theralase and for the cancer patients, who are inflicted with this deadly disease. The ability to destroy the original cancer and at the same time program the body's immune system to prevent its recurrence, after only a single treatment, is nothing short of miraculous. The scientific, clinical and engineering teams at UHN, Acadia and Theralase are all fully dedicated to bringing the Theralase anti-cancer PDC technology to the forefront of clinical treatment, as soon as possible, in order to help eradicate the world of cancer and assist cancer patients."
In order to help facilitate the launch of these two ground breaking technologies in 2014 and 2015, the Company closed a $3.15 M private placement on November 7, 2013, issuing 21,000,000 Units to investors at a price of $0.15 per Unit. Each Unit consisted of one common share in the capital of the Company and one non-transferable common share purchase warrant. Each whole Warrant will entitle the purchaser to purchase one additional common share in the capital of the Company until November 7, 2015 at a price of $0.20.
About Theralase Technologies Inc.
Theralase Technologies Inc. designs, manufactures and markets patented, superpulsed laser technology used in healing injured tissue and destroying cancer. Theralase technology is safe and effective in eliminating pain, reducing inflammation and dramatically accelerating tissue regeneration of numerous nerve, muscle and joint injuries. Theralase is actively developing patented technology that is able to target and destroy cancers, bacteria and viruses when light activated.
The complete interim consolidated financial statements and MD&A for the three month period ended March 31, 2014 may be viewed at www.theralase.com and www.sedar.com .
Theralase (TLT.V) (TLTFF:OTCBB) Discovers Anti-Cancer Memory Response
Toronto, Ontario / ACCESSWIRE / May 29, 2014 / Theralase Technologies Inc. ("Theralase") (TLT.V) (TLTFF:OTCBB) announced today that in preclinical animal testing, performed at Princess Margaret Cancer Centre, University Health Network ("UHN"), it has discovered that its lead Photo Dynamic Compound (PDC), intended for the destruction of cancer, has demonstrated an ability to render animals immune to repeated exposures of the same cancer.
This initial data has been accepted for presentation at the 37th Annual American Society for Photobiology taking place in San Diego, California in June 2014.
In previous research conducted at UHN by Theralase, mice were injected with 350,000 colon cancer cells (murine cell line CT26.CL25) to produce tumours that were allowed to grow to approximately five millimeters in size. They were treated with an intra-tumoural injection of Theralase's lead PDC (3 mg/kg TLDOsH2IP) and then illuminated by Near Infrared (NIR) light (808 nm, 600 J cm-2) to activate the PDC. The vast majority of tumours were completely destroyed, with the PDC treatment demonstrating prolonged tumour regression.
In this latest research, the same mice who received the initial, successful Photo Dynamic Therapy (PDT) were re-injected with the same number of colon cancer cells, 13 to 23 days later. With no further treatment intervention, mice in these experiments, demonstrated either a small tumour regrowth, which quickly regressed, or in the majority of animals, no tumour regrowth at all, suggesting a short-term immune-mediated (immune "memory response") tumour rejection.
To further prove the resilience of the PDT treatment, these same animals were then injected a third time with an additional 350,000 colon cancer cells at ten months post PDT treatment. None of these animals showed any sign of tumour regrowth, even at 3 months post follow up, suggesting the presence of a long-term anti-tumour immunity, responsible for complete tumour rejection.
To strengthen the data, control experiments were conducted where age matched mice without prior tumour exposure or PDT treatment were injected with the same number of colon cancer cells, where the majority of these mice proceeded to develop tumours and did not survive more than 1 month following the injection.
These initial results are now being further researched by Theralase and UHN scientists to confirm the immune-mediated (immune "memory response") tumour rejection in additional subject animals. This potential short term and long term anti-cancer memory response suggests a major breakthrough in cancer research and may provide substantial treatment benefit and survival advantage to cancer patients. Technology that is able to rapidly and effectively destroy "patient-specific" cancer cells, prevent their recurrence and provide long lasting protection against local and distant metastasis, offers immense clinical benefit to cancer patients and the facilities that treat their disease.
Dr. Arkady Mandel, Chief Scientific Officer of Theralase stated, "This is one of the first preclinical trials to show that it's possible to generate a long-term anticancer memory response. For the first time in our research program, we have demonstrated that NIR PDT leads not only to long standing clearance of colon cancer cells, but also provides long lasting protection against further tumour cell challenge in young (eight to ten weeks old) and older (ten to eleven month old) mice. It is our first step toward the long-term goal of developing an affordable and practical vaccine to prevent cancer recurrence. The next steps are to further validate this research with additional animals and then find the best way of translating this research into a human clinical trial. To complete our preclinical and clinical development in this ground breaking work, we are collaborating with experts in medical biophysics, immunology and clinical oncology at UHN and with other internationally acclaimed clinical research institutes to further advance this remarkable platform technology."
Dr. Lothar Lilge PhD, Professor in the Department of Medical Biophysics at the University of Toronto, Senior Scientist, Princess Margaret Cancer Centre, University Health Network stated that, "We are delighted that we were able to partner in the preclinical research for this ground breaking discovery. The ability to effectively destroy cancer and simultaneously stimulate the immune system to target micro metastasis beyond the initial treatment volume is necessary to provide long term disease control. We will now proceed to confirm these findings for a range of induced and spontaneous animal tumour models."
Dr. Michael Jewett MD, clinician investigator and uro-oncologist at UHN stated, "I am excited about the possibilities of this discovery. If this newly discovered characteristic of the Theralase PDCs can be replicated in humans and demonstrate the same efficacy that it has in small animals, then the implications to the war on cancer could be immense. I look forward to furthering this preclinical work to the clinical stage to validate its efficacy in humans."
Roger Dumoulin-White, President and CEO of Theralase stated that, "If our PDC technology is proven effective in cancer patients, the implications of this discovery are nothing short of game changing for both Theralase and for cancer patients. The ability to destroy the original cancer and also program the body's immune system to prevent its recurrence, after only a single treatment, is nothing short of miraculous. Born a technical person at heart, I have always marvelled at the complexity of the human body and regarded it as the single most complex and intricate machine ever devised. The opportunity to bring technology to market that could help preserve the integrity of the human body and destroy such a deadly disease as cancer, fills me with hope and a great sense of accomplishment. The scientific, clinical and engineering teams at UHN, Acadia and Theralase are all fully dedicated to bringing the Theralase anti-cancer PDC technology to the forefront of clinical success, as soon as possible, in order to help eradicate the world of cancer and assist cancer patients stricken with this deadly disease."
About Theralase Technologies Inc.
Theralase Technologies Inc. designs, manufactures and markets patented, superpulsed laser technology used in eliminating pain and destroying cancer. Theralase technology is safe and effective in eliminating pain, reducing inflammation and accelerating tissue regeneration of numerous nerve, muscle and joint conditions. Theralase is actively developing patented technology that is able to target and destroy cancers, bacteria and viruses when light activated.
Additional information is available at www.theralase.com and www.sedar.com .
Theralase (TSXV: TLT) (TLTFF: OTCBB) Anti-Cancer Technology Validated in Prestigious US Chemistry Publication
Toronto, Ontario / TNW-ACCESSWIRE / May 27, 2014 / Theralase Technologies Inc. ("Theralase') (TSXV: TLT) (TLTFF: OTCBB) announced today that its latest research on Photo Dynamic Compound (PDC) technology, proven effective in the destruction of bacteria and cancer, was peer reviewed and invited to be published in the prestigious US Elsevier publication, Coordination Chemistry Reviews.
The new research presents how Theralase's new class of PDCs incorporates systems that act as dual Type I/II PDCs (able to work in oxygenated and non-oxygenated tissue), opening up the possibility of treating hypoxic (low oxygen) tumours with Photo Dynamic Therapy (PDT). These PDCs are remarkable in-vitro centromere binders (localizing to the nucleus of a cell) and photocleavers (ability to damage nucleus), thus destroying cells when exposed to light. They also exhibit no nucleic damage in the absence of light, supporting their high safety and tolerability. This PDT effect translates effectively to animals and has proven superior to the FDA approved PDC Photofrin(R), in this research. The ability to activate the Theralase PDCs from visible to Near Infra Red (NIR) light marks an unprecedented versatility that can be exploited to match treatment depth to tumour target depth, giving rise to PDCs for multi-wavelength activated PDT.
Photo Dynamic Therapy (PDT) is an elegant method for destroying cancer cells. PDCs accumulate in cells intended for destruction and when light activated destroy the intended cell; hence, PDT is best described as a combination therapy that offers selectivity through local interactions between a PDC, light and oxygen. Briefly, light absorption by the PDC produces a reactive excited state that can participate in electron (Type I) or energy (Type II) transfer to ground state molecular oxygen forming either superoxide radical anions or cytotoxic singlet oxygen, respectively. The production of a cytotoxic (cell killing) burst of Reactive Oxygen Species (ROS), notably singlet oxygen has proven effective in eliminating tumours and/or tumour vasculature. The primary advantage of light-based approaches in treating diseases, such as cancer, is that guided light delivery confines drug activity to malignant sites; thereby, reducing collateral damage to surrounding healthy tissue. Consequently, due to the high photostability of the Theralase PDC, very low drug doses can be used (nanograms) with activation at higher light doses, simultaneously eliminating the side effects caused by conventional systemic chemotherapeutics, such as cisplatin.
Dr. Arkady Mandel, Chief Scientific Officer of Theralase stated, "A number of successful efforts have been made by Theralase's research team to satisfy the clinical requirements and to improve the pharmaceutical and therapeutic properties of the original PDCs. The results of our collaborative research with Dr. Lothar Lilge's scientific team at Princess Margaret Cancer Centre, University Health (UHN) and Dr. Sherri McFarland's chemistry team at Acadia University (Acadia) reveal an unprecedented versatility and efficacy of the Theralase's PDCs. The research data published in the high impact journal Coordination Chemistry Reviews may lead to development of the first-line of patient specific PDT. I am delighted that Theralase has established a very strong partnership with UHN and Acadia to progress this pivotal technology to the next milestone, the completion of an orthotopic animal model for bladder cancer. Together with clinical guidance by Dr. Michael Jewett, a Professor of Surgery (Urology) at the University of Toronto and one of the lead clinician investigators and uro-oncologists at UHN, our research provides excellent preclinical support to Theralase to finalize the regulatory submissions and prepare us for a Phase 1/2a human clinical trial to evaluate the technology in patients inflicted with bladder cancer."
Dr. Lothar Lilge PhD, Professor in the Department of Medical Biophysics at the University of Toronto, Senior Scientist, Princess Margaret Cancer Centre, University Health Network stated that the, "Publication of our research in Coordination Chemistry Reviews validates the importance of the efforts of Acadia University and UHN, with the fully committed support of Theralase, in developing a novel approach for the destruction of solid tumours, preferably in a single administration, thus significantly improving the quality of life of cancer patients. The results obtained to date provide strong support and encouragement to complete the small outstanding preclinical work at UHN to properly position this exciting technology for human clinical studies at the earliest feasible point in time."
Dr. Sherri McFarland PhD, Professor of Chemistry at Acadia University stated that, "I am delighted to work with a strong partner such as Theralase in the development of these PDCs that I originally invented and optimized with the support of my partners. These PDCs have proven to be very potent compounds in the destruction of cancer cells in very small doses and I am excited to lead the development of the PDCs and be part of the team completing the preclinical work in 2014 to support human clinical studies in early 2015."
Roger Dumoulin-White, President and CEO of Theralase stated that, "Coordination Chemistry Reviews is a highly renowned publication known for publishing chemistry research of the highest calibre. It is an honour for this cutting-edge research to be recognized in a publication of this level, further lending credence to the importance of the cancer research we are undertaking with our partners, UHN and Acadia. Targeting cancer cells regardless of tissue depth, enables Theralase to customize its patented technology to be "patient specific" to destroy cancer regardless of where it may reside in the body."
About Theralase Technologies Inc.
Theralase Technologies Inc. designs, manufactures and markets patented, superpulsed laser technology used in eliminating pain and destroying cancer. Theralase technology is safe and effective in eliminating pain, reducing inflammation and accelerating tissue regeneration of numerous nerve, muscle and joint conditions. Theralase is actively developing patented technology that is able to target and destroy cancers, bacteria and viruses, when light activated
Zack's Research Initiates Coverage on Theralase (TLTFF) (TLT.V) With a Price Target of $1.00
Theralase Lights Up Cancer Therapeutics
Whitefish, MT / May 20, 2014 / Update: Zacks Small Cap Research recently initiated coverage of Theralase Technologies with a price target of $1.00 per share that represents a 270% premium to the May 6th stock price of $0.27 per share. The Full Zack’s Report can be read here. Investors in the cancer therapeutics space, including those in companies like Cell Therapeutics Inc. (CTIC) or Osiris Therapeutics Inc. (OSIR), may want to take a closer look at the company given these recent developments.
Cancer patients don’t have many good treatment options. Once the disease is detected, most oncologists will surgically remove as much as possible and then use powerful radiation and chemotherapy to kill as many cells in those areas as possible. The side effects from these treatments can be terrible and patients are at a higher risk of additional complications given the weakened state of the immune system.
Theralase Technologies Inc. (TLTFF) (TLT.V) aims to provide a better treatment option using the power of laser light and photodynamic compounds (“PDCs”). In this article, we’ll take a look at how these technologies could revolutionize cancer treatment as well as other areas like pain management.
SECFILINGS.COM Executive Interview | Theralase Technologies (TLTFF) []
Theralase Overcomes Key Hurdles
Theralase Technologies delivers light-sensitive PDCs directly into cancerous tumors, via injection, where they are selectively absorbed by cancer cells. A very specific type of laser light is then applied to the region via fiber optic wires, forcing the PDCs to become cytotoxic and inducing apoptosis – or cell death – in the cancer cells. Since no surgery is necessary, the process is more affordable and minimally invasive.
The company’s approach is unique in that it’s effective in hypoxic environments (low oxygen), seen in solid-core cancers like: brain, breast, lung and prostate and in hypoxic areas like bladder cancer. Hypoxic cancers are extremely aggressive, resistant to standard therapies, and are very difficult to destroy. Tumor hypoxia is also known to play a role in cancer metastasis and resistance to therapy.
The effectiveness of the approach has already been highlighted in numerous published studies. For example, a December 2013 study published in Photodiagnosis and Photodynamic Therapy found that the company’s TLD1411 and TDL1433 maintained photodynamic inactivation (“PDI”) potency under hypoxic conditions becoming even more effective in the low-oxygen environment.
Clear Pathway to Commercialization
Theralase Technologies has outlined a very clear path towards commercializing its unique technology platform with a uniquely qualified management team. President & CEO Roger Dumoulin-White founded the company back in 1995 and has 27+ years of senior management experience with private and public companies, while CSO Dr. Arkady Mandel was a key founder of therapeutic lasers in numerous clinical areas.
On April 3, 2014, the company announced that it executed a research agreement with Acadia University to supply PDCs necessary to commence toxicity studies and ramp up manufacturing. These steps are necessary to file Investigational New Drug (“IND”) applications with the U.S. FDA and Health Canada and begin Phase 1/2a human clinical trials for bladder cancer as early as Q1 2015.
The company hopes to expedite the process using the FDA’s new Breakthrough Therapy program initiated on July 9, 2012 with the Safety and Innovation Act. Management believes that the designation could lead to its PDC technology being available to bladder cancer patients as early as 2016.
Additional Play in Pain Management
Theralase Technologies’ superpulsed laser technology is already being used as a safe and effective way to eliminate pain, reduce inflammation, and accelerate tissue regeneration in nerve, muscle, and joint injuries. The company’s TLC-1000 is a best-in-class technology that has already helped millions of patients throughout Canada and there are plans to launch an even more effective technology, the next generation TLC-2000, in Q4 2014.
Using the technology, monochromatic laser light is delivered into the therapeutic window (wavelength range where laser light can be delivered deep into tissue without heat) in wavelengths ranging from 600 to 950 nanometers. Light particles from these lasers penetrate up to 10 centimeters into tissue and are absorbed into the elements of cell mitochondria, where they increase the rate of ATP production, nitric oxide production and rebalance what is called the sodium potassium pump. ATP production fuels the cells and actuates the healing process, nitric oxide production brings more blood, oxygen and fuel molecules to the injured area to further accelerate healing and rebalancing the sodium potassium pump eliminates pain. A very effective one, two, three punch for effective healing.
On April 15, 2014, the company announced the hiring of Mr. Derek Small as Director of Sales and Marketing to jumpstart revenues in these areas. With over 15 years of experience in both the U.S. and Canada, Mr. Small notably helped another U.S.-based aesthetic laser company grow sales from no revenue to $20 million in annual revenue within five years, taking increasingly senior roles.
Potential Investment Opportunity
Theralase Technologies represents an attractive investment opportunity for a variety of different reasons. In the cancer treatment space, the company’s unique approach could open the door to significant long-term revenue potential. Commercialization partnerships with major pharmaceutical companies in oncology, like GlaxoSmithKline plc. (NYSE:GSK), could also boost its near-term potential.
In the meantime, the company’s existing cold laser therapy division continues to generate revenue. The hiring of Mr. Small could significantly expand that revenue through 2014, particularly if his prior experience serves as any indicator. These revenues could make the stock attractive in the pain management space as an alternative to options like Pain Therapeutics Inc.’s (NASDAQ:PTIE) REMOXY.
Theralase (TSXV: TLT) Partners with Princess Margaret Cancer Centre
Toronto, Ontario / TNW-ACCESSWIRE / May 15, 2014 -- Theralase Technologies Inc. ("Theralase') (TSXV: TLT) (TLTFF: OTCBB) announced today that it has partnered with Princess Margaret Cancer Centre, University Health Network ("UHN") to complete preclinical research on its Photo Dynamic Compound (PDC) technology for the destruction of cancer.
Under the terms of the agreement, Theralase's scientific and engineering teams will work with UHN's scientific and medical cancer specialists to complete preclinical research for the anti-cancer technology in the destruction of cancer and to advance the technology to the clinical stage. Anticipated 2014 milestones include the destruction of bladder cancer in a rat model, verification of the lead PDC for bladder cancer, validation of manufactured PDC quality and toxicology analysis assistance.
Dr. Arkady Mandel, Chief Scientific Officer of Theralase stated, "I am delighted that we have partnered with UHN to complete this pivotal preclinical research. UHN is one of the top five cancer centres in the world and provides excellent clinical and scientific support to our existing teams to complete the preclinical work required to prepare us for a Phase 1/2a human clinical trial for bladder cancer.
Dr. Lothar Lilge, PhD, Senior Research Scientist at UHN stated, "The initial in-vitro and small animal in-vivo work using Theralase's anti-cancer PDC technology has been excellent, demonstrating elimination of a wide range of cancer cells at sub micromolar concentations (nanograms of PDC) when light activated and without toxicity in the absence of light. Eliminating subcutaneous tumours in in vivo small animals with this non-invasive and non-ionizing technology bodes very well for the anticipated clinical destruction of cancer in the future."
Dr. Michael Jewett MD, clinician investigator and uro-oncologist at Princess Margaret/UHN stated, "I am encouraged that Theralase has continued to pursue investigating the possibility of destroying bladder cancer with their anti-cancer PDC technology. Bladder cancer is a devastating disease with over 386,000 new cases annually worldwide and no new drug treatments approved since 1998. If, with the support of UHN, Theralase is able to prove its safety and efficacy in clinical trials, we have a game changer on our hands in the management and treatment of patients with bladder cancer."
Roger Dumoulin-White, President and CEO of Theralase stated that, "I am pleased that Theralase has chosen a strong partner like UHN to help us complete our preclinical research and help us to prepare for human clinical trials. UHN will be strategic for Theralase to help complete our preclinical work by early 2015 and allow us to commence human clinical trials with them and a large US cancer facility soon thereafter."
About Theralase Technologies Inc.
Theralase Technologies Inc. designs, manufactures and markets patented, superpulsed laser technology used in eliminating pain and destroying cancer. Theralase technology is safe and effective in eliminating pain, reducing inflammation and accelerating tissue regeneration of numerous nerve, muscle and joint conditions. Theralase is actively developing patented technology that is able to target and destroy cancers, bacteria and viruses when light activated
Theralase (TSXV: TLT) Releases 2013 Financials
Toronto, Ontario / TNW-ACCESSWIRE / April 30, 2014 / Theralase Technologies Inc. (herein "Theralase") (TSXV: TLT) (TLTFF: OTCBB) released its 2013 financial results today, demonstrating an increase in profitability by 24% year over year, while successfully advancing its patented next generation therapeutic laser and cancer destruction technologies.
Total revenue for the year ended December 31, 2013 decreased 34% from $1,824,313 to $1,203,620 year over year.
The net loss for the year ended December 31, 2013 was $1,152,209 (including $211,543 of net non-cash expenses) compared to a net loss of $1,509,569 in 2012 (including $322,915 of net non-cash expenses), demonstrating an improvement of 24% year over year.
The net loss is a reflection of the ongoing commitment of Theralase to invest in the next generation of therapeutic laser and cancer destruction technologies, from existing therapeutic laser sales.
Selling and marketing expenses decreased 31% from $626,380 to $433,622 for the same period in 2012. The decrease was due to reduced spending on salaries for sales personnel and a reduction in associated marketing costs.
Administrative expenses decreased 24% from $1,238,900 to $942,069 for the same period in 2012. The decrease in administrative expenditures was due to reductions in administrative personnel, stock based compensation and commercial rent.
Research and development costs decreased 40% from $873,335 to $527,233 for the same period in 2012. The decrease reflects the approaching completion of the patented next generation TLC-2000 therapeutic laser technology and the pending ramp-up in the research and development costs associated with the cancer destruction technology in 2014.
The Company is focused on achieving the following strategic initiatives in 2014:
-- Increasing product sales and market acceptance of the TLC-1000 laser technology in Canada, the US and international medical markets, supported by the latest independent scientific and clinical research, which continues to confirm that the Company's proprietary technology has a higher safety and effectiveness as compared to other competitive technologies.
-- Investing in scientific and clinical research aimed at unlocking the mechanisms of action as to how Theralase laser light can so dramatically heal tissue versus competitive technologies.
-- Launch its patented next generation TLC-2000 biofeedback laser technology in Canada and the US in 4Q2014.
-- Research and development of its patented Photo Dynamic Compound (PDC) technology proven effective pre-clinically in the destruction of cancer by completing preclinical research in 2014 and commencing a FDA Phase 1/2a human clinical trial in bladder cancer in 1Q2015.
Roger Dumoulin-White, President and CEO of Theralase stated that, "2013 was a pivotal year for Theralase, where we relocated our head office to a better corporate location, streamlined our operations and made advancements in both the therapeutic laser and anti-cancer divisions. 2014 will be a very exciting year for Theralase as we increase our revenues through expansion of our sales and marketing initiatives, launch our next generation therapeutic laser technology and prepare to commence human clinical trials in our anti-cancer division in early 2015. Due to the on-going requirement of capital to fund the Company's growth in 2014 in both divisions, the Company will continue to investigate financing options on both the debt and the equity side, in order to achieve its strategic initiatives and unlock shareholder value."
About Theralase Technologies Inc.
Theralase Technologies Inc. designs, manufactures and markets patented, superpulsed laser technology used in healing injured tissue and destroying cancer. Theralase technology is safe and effective in eliminating pain, reducing inflammation and accelerating tissue regeneration of numerous nerve, muscle and joint injuries. Theralase is actively developing patented technology that is able to target and destroy cancers, bacteria and viruses when light activated.
Stockwatch News
To Stockwatch News
Today at 7:55 AM
Theralase's PDC destroys cancer cells on NIR activation
Ticker Symbol: C:TLT
Theralase's PDC destroys cancer cells on NIR activation
Theralase Technologies Inc (C:TLT)
Shares Issued 66,530,642
Last Close 4/22/2014 $0.32
Thursday April 24 2014 - News Release
Mr. Roger Dumoulin-White reports
THERALASE ANTI-CANCER TECHNOLOGY VALIDATED AT INTERNATIONAL CONFERENCE
Theralase Technologies Inc.'s new research on its Photo Dynamic Compound technology, proven effective in the destruction of bacteria and cancer, has been peer reviewed and approved for presentation at the 16th Annual Laser Optics International Conference to be held in June, 2014.
The new research scientifically proves that Theralase's patented PDC technology can be activated by Near Infrared (NIR) laser light destroying cancer cells that reside deep into tissue, providing a higher kill rate in a single Photo Dynamic Therapy (PDT) treatment, than previously possible, setting a new standard in the industry. The scientific and medical community considers the research pivotal because the Theralase PDCs are able to provide a solution to a problematic issue in cancer treatment, the ability to destroy cancerous tumours via PDT deep into tissue.
Cancer is a major worldwide disease, causing great threats to human health. Tumour recurrence, metastasis (spread of cancer to distant body parts) and drug resistance are making the disease untreatable in many instances. There is therefore an urgent need to develop technology that is effective in destroying cancer before it has an opportunity to metastasize to distant body parts and render the patient incurable. Providing a technology that is able to eliminate ongoing cancer proliferation and destroy deep seated tumours with a higher kill rate in a single PDT treatment is ground breaking.
Dr. Arkady Mandel, Chief Scientific Officer of Theralase stated, "Conventional cancer therapies, including chemotherapy and radiation therapy, have many limitations such as toxic side effects, drug resistance and the fact that they quite often fail to completely destroy the tumour. PDT utilizes a class of minimally invasive PDCs with many advantages such as an ability to administer the treatment with minimal side effects, improved selectivity to target only the cancer cells and virtually zero toxicity to the body; however, light penetration is one of the major challenges in PDT. Light in the UV to visible range (300 to 700 nm) has limited tissue penetration depth restricting the therapeutic efficacy of PDT for large or deep tumours for competitive PDCs. The NIR window in the range of 700 to 970 nm, in which biological tissues have minimal light absorption, is ideal for targeted cancer therapy. Activation of the Theralase PDCs by NIR light now addresses this issue. Therefore the results of our preclinical research are of great scientific and potential clinical significance." Roger Dumoulin-White, President and CEO of Theralase stated that, "I am encouraged that the latest Theralase anti-cancer research continues to receive such high accolades from the international scientific and medical community. This validation of our technology from some of the most well known experts in the field supports the magnitude of our research and the fact that we are breaking new ground in cancer research. By being able to target cancer cells in deeper tissue than currently available allows Theralase the ability to use our patented technology to target cancer regardless of where it may lurk in the body."
We seek Safe Harbor.
© 2014 Canjex Publishing Ltd.
Theralase Research Accepted at International Conference
Toronto, Ontario – April 22, 2014 Theralase Technologies Inc. (TSXV: TLT) (TLTFF: OTCBB) announced today that its Photo Dynamic Compound (PDC) technology, proven effective in the destruction of bacteria and cancer, has been peer reviewed and approved for presentation at the renowned International Conference on Laser Applications in Life Sciences to take place in Ulm, Germany in June 2014.
Theralase’s ability to destroy two types of bacteria (Staphylococcus aureus and Methicillin Resistant Staphylococcus aureus (MRSA)) up to 99.99999% with a new class of PDCs has caught the attention of the global scientific and medical community after recent publication in the journal Photodiagnosis and Photodynamic Therapy.
The latest Theralase research is considered pivotal by the international community because the PDCs were able to destroy two types of bacteria in both normal and low oxygenated tissue in nanomolar concentrations (micrograms of the PDCs were lethal to bacteria when light activated) and were proven especially effective in low oxygen environments, where certain types of bacteria and cancer thrive.
Dr. Arkady Mandel, Chief Scientific Officer of Theralase stated that, “Theralase’s PDC technology addresses the challenge of bacteria that develop an immunity to destruction due to an acquired resistance to treatment, such as antibiotic drugs. The latest research confirms that the Theralase PDCs are a reliable alternative to antibiotics and have none of the acquired resistance limitations. This new approach provides a novel and effective treatment to destroying bacteria by improving selectivity, reducing treatment time and improving safety for clinical applications, which is critical for a broad international acceptance of the technology. Moreover, the fact that the Theralase PDCs are effective in low oxygen and have a higher stability than FDA approved PDCs may have a tremendous impact in limiting the further spread of antibiotic-resistant strains of bacteria and simultaneously provide superior health care worldwide.”
Roger Dumoulin-White, President and CEO of Theralase stated that, “I am delighted that our research in PDCs has attracted the international attention of the scientific and medical community. By being extremely effective in microgram concentrations, Theralase now has the opportunity for early partnership with large pharmaceutical organizations interested in alternatives to antibiotics for bacteria destruction. This is a billion dollar market in need of technology that is able to eliminate the bacterial load quickly and effectively, while minimizing risk to the patient.”
About Theralase Technologies Inc.
Theralase Technologies Inc. designs, manufactures and markets patented, superpulsed laser technology used in eliminating pain and destroying cancer. Theralase technology is safe and effective in eliminating pain, reducing inflammation and accelerating tissue regeneration of numerous nerve, muscle and joint injuries. Theralase is actively developing patented technology that is able to target and destroy cancers, bacteria and viruses when light activated.
Additional information is available at www.theralase.com and www.sedar.com
This press release contains forward-looking statements, which reflect the Company's current expectations regarding future events. The forward-looking statements involve risks and uncertainties. Actual results could differ materially from those projected herein. The Company disclaims any obligation to update these forward-looking statements.
Neither TSX Venture Exchange nor its Regulation Services Provider (as that term is defined in the policies of the TSX Venture Exchanges) accepts responsibility for the adequacy or accuracy of this release.
For More Information:
Roger Dumoulin-White
President & CEO
1-866-THE-LASE (843-5273)
416-699-LASE (5273) ext. 225
rwhite@theralase.com
Theralase (TSXV: TLT) Anti-Cancer Technology Validated at International Conference
Toronto, Ontario / TNW-ACCESSWIRE / April 24, 2014 / Theralase Technologies Inc. ("Theralase') (TSXV: TLT) (TLTFF: OTCBB) announced today new research on its Photo Dynamic Compound (PDC) technology, proven effective in the destruction of bacteria and cancer, has been peer reviewed and approved for presentation at the 16th Annual Laser Optics International Conference to be held in June 2014.
The new research scientifically proves that Theralase's patented PDC technology can be activated by Near Infrared (NIR) laser light destroying cancer cells that reside deep into tissue, providing a higher kill rate in a single Photo Dynamic Therapy (PDT) treatment, than previously possible, setting a new standard in the industry. The scientific and medical community considers the research pivotal because the Theralase PDCs are able to provide a solution to a problematic issue in cancer treatment, the ability to destroy cancerous tumours via PDT deep into tissue.
Cancer is a major worldwide disease, causing great threats to human health. Tumour recurrence, metastasis (spread of cancer to distant body parts) and drug resistance are making the disease untreatable in many instances. There is therefore an urgent need to develop technology that is effective in destroying cancer before it has an opportunity to metastasize to distant body parts and render the patient incurable. Providing a technology that is able to eliminate ongoing cancer proliferation and destroy deep seated tumours with a higher kill rate in a single PDT treatment is ground breaking.
Dr. Arkady Mandel, Chief Scientific Officer of Theralase stated, "Conventional cancer therapies, including chemotherapy and radiation therapy, have many limitations such as toxic side effects, drug resistance and the fact that they quite often fail to completely destroy the tumour. PDT utilizes a class of minimally invasive PDCs with many advantages such as an ability to administer the treatment with minimal side effects, improved selectivity to target only the cancer cells and virtually zero toxicity to the body; however, light penetration is one of the major challenges in PDT. Light in the UV to visible range (300 to 700 nm) has limited tissue penetration depth restricting the therapeutic efficacy of PDT for large or deep tumours for competitive PDCs. The NIR window in the range of 700 to 970 nm, in which biological tissues have minimal light absorption, is ideal for targeted cancer therapy. Activation of the Theralase PDCs by NIR light now addresses this issue. Therefore the results of our preclinical research are of great scientific and potential clinical significance."
Roger Dumoulin-White, President and CEO of Theralase stated that, "I am encouraged that the latest Theralase anti-cancer research continues to receive such high accolades from the international scientific and medical community. This validation of our technology from some of the most well known experts in the field supports the magnitude of our research and the fact that we are breaking new ground in cancer research. By being able to target cancer cells in deeper tissue than currently available allows Theralase the ability to use our patented technology to target cancer regardless of where it may lurk in the body."
About Theralase Technologies Inc.
Theralase Technologies Inc. designs, manufactures and markets patented, superpulsed laser technology used in eliminating pain and destroying cancer. Theralase technology is safe and effective in eliminating pain, reducing inflammation and accelerating tissue regeneration of numerous nerve, muscle and joint conditions. Theralase is actively developing patented technology that is able to target and destroy cancers, bacteria and viruses when light activated
Theralase (TSXV: TLT) Research Accepted at International Conference
(via Thenewswire.ca)
Toronto, Ontario / TNW-ACCESSWIRE / April 22, 2014 / Theralase Technologies Inc. (TSXV: TLT) (TLTFF: OTCBB) announced today that its Photo Dynamic Compound (PDC) technology, proven effective in the destruction of bacteria and cancer, has been peer reviewed and approved for presentation at the renowned International Conference on Laser Applications in Life Sciences to take place in Ulm, Germany in June 2014.
Theralase's ability to destroy two types of bacteria (Staphylococcus aureus and Methicillin Resistant Staphylococcus aureus (MRSA)) up to 99.99999% with a new class of PDCs has caught the attention of the global scientific and medical community after recent publication in the journal Photodiagnosis and Photodynamic Therapy.
The latest Theralase research is considered pivotal by the international community because the PDCs were able to destroy two types of bacteria in both normal and low oxygenated tissue in nanomolar concentrations (micrograms of the PDCs were lethal to bacteria when light activated) and were proven especially effective in low oxygen environments, where certain types of bacteria and cancer thrive.
Dr. Arkady Mandel, Chief Scientific Officer of Theralase stated that, "Theralase's PDC technology addresses the challenge of bacteria that develop an immunity to destruction due to an acquired resistance to treatment, such as antibiotic drugs. The latest research confirms that the Theralase PDCs are a reliable alternative to antibiotics and have none of the acquired resistance limitations. This new approach provides a novel and effective treatment to destroying bacteria by improving selectivity, reducing treatment time and improving safety for clinical applications, which is critical for a broad international acceptance of the technology. Moreover, the fact that the Theralase PDCs are effective in low oxygen and have a higher stability than FDA approved PDCs may have a tremendous impact in limiting the further spread of antibiotic-resistant strains of bacteria and simultaneously provide superior health care worldwide."
Roger Dumoulin-White, President and CEO of Theralase stated that, "I am delighted that our research in PDCs has attracted the international attention of the scientific and medical community. By being extremely effective in microgram concentrations, Theralase now has the opportunity for early partnership with large pharmaceutical organizations interested in alternatives to antibiotics for bacteria destruction. This is a billion dollar market in need of technology that is able to eliminate the bacterial load quickly and effectively, while minimizing risk to the patient."
About Theralase Technologies Inc.
Theralase Technologies Inc. designs, manufactures and markets patented, superpulsed laser technology used in eliminating pain and destroying cancer. Theralase technology is safe and effective in eliminating pain, reducing inflammation and accelerating tissue regeneration of numerous nerve, muscle and joint conditions. Theralase is actively developing patented technology that is able to target and destroy cancers, bacteria and viruses when light activated.
Theralase (TSXV: TLT) Advances Anti-Cancer Technology Platform
(via Thenewswire.ca)
Toronto, Ontario / TNW-ACCESSWIRE / April 3, 2014 / Theralase Technologies Inc. ("Theralase") (TSXV: TLT) (TLTFF: OTCBB) announced today that it has advanced its anti-cancer technology platform through an executed research agreement with Acadia University ("Acadia"). Under the terms of the agreement, Acadia will supply initial quantities of Theralase's lead Photo Dynamic Compounds (PDCs) and the Standard Operating Procedures (SOPs) of how to synthesize them.
The lead PDCs will be used by Theralase to commence toxicity analysis and manufacturing ramp up via Contract Manufacturing Organization (CMO); both mandatory prerequisites in the evolution towards an approved Investigational New Drug (IND) application from the FDA and Health Canada. With larger quantities of the PDCs in hand and an approved IND; Theralase would be in the position to commence a Phase 1/2a human clinical trial for the lead indication of bladder cancer, as early as 1Q2015.
The lead PDCs have advanced towards international patent protection under a filed Patent Cooperation Treaty (PCT) application. Once approved at this phase, the Company plans to provide wide spread patent coverage of the PDCs across numerous countries during the national phase.
Dr. Sherri McFarland, Professor of Chemistry at Acadia University, the originator of the PDCs under worldwide exclusive licence to Theralase stated, "I am truly excited to work with Theralase in the development of these PDCs and be associated with their lead scientists and clinical oncologists to develop the next generation of anti-cancer technology. From the raw research I have observed, this technology has the potential to not only destroy one type of cancer, but many. I am convinced that this technology has the opportunity to go mainstream as a primary treatment in the destruction of cancer, within the next few years."
Dr. David MacKinnon, Dean, Research and Graduate Studies at Acadia University stated, "We are pleased to have partnered with Theralase in the development of these PDCs for the destruction of cancer. It is rewarding to advance research originally developed in our chemistry labs from conception through to commercialization knowing that it may one day be a cure for cancer."
Dr. Arkady Mandel, Chief Scientific Officer of Theralase stated that, "Sherri's strong background as a medicinal chemist and specialist in photochemistry, coupled with my scientific and medical knowledge as to how laser light interacts at the molecular and cellular level, will bring a new platform of cutting-edge anti-cancer technologies to the forefront within the next few years."
Roger Dumoulin-White, President and CEO of Theralase stated that, "I am delighted that Acadia has partnered with Theralase in the commercialization of technology that may provide a new front line treatment for cancer in the not too distant future. With good partners, disruptive technologies are born, which can make marked impacts on devastating diseases such as cancer, in our lifetime. I am humbled to be part of the process."
About Theralase Technologies Inc.
Theralase Technologies Inc. designs, manufactures and markets patented, superpulsed laser technology used in eliminating pain and destroying cancer. Theralase technology is safe and effective in eliminating pain, reducing inflammation and accelerating tissue regeneration of numerous nerve, muscle and joint injuries. Theralase is actively developing patented technology that is able to target and destroy cancers, bacteria and viruses when light activated.
Tekmira and Theralase Leaders in Innovative Drug Therapies
Subscribe to get future updates from Theralase (TSX-V: TLT) HERE
Without aggressive research and regulatory approval, cancer could soon overtake heart disease as the leading cause of disease-related death in the United States. In Canada, cancer is already the number one killer, far and away outpacing heart disease. Small molecule drugs remain the standard of care, but the problem is that most are systemic and highly toxic or have dose-limiting qualities that minimize their efficacy and support the development of resistance. While the small molecule sector certainly still has upside, investors should be attentive to companies that are developing therapies with different mechanisms of action as the industry undergoes a dynamic shift towards accelerated approvals of innovative technologies.
The often-overlooked Canadian biotech space has grown into a $53-billion sector that offers a robust growth opportunity for investors. To that point, Tekmira Pharmaceuticals (TSX: TKM)(NASDAQ: TKMR) and Theralase Technologies (TSX-V: TLT)(OTCBB: TLTFF) are two dually-listed companies with large upside potential that have little competition to their pipelines, which are focused on RNA interference and photodynamic therapy, respectively.
RNA interference, or RNAi, was discovered essentially by accident just over a decade ago when Richard Jorgensen and colleagues found that by adding genes to petunias (in a bid to make them deeper purple), other native genes in the flower were silenced (turning the petunia white). This discovery of “switching off” genes via other cells has already led to promising research in viral conditions, such as hepatitis C and AIDS, and is taking root with new treatments for cancer on the horizon.
It’s discoveries like this and growing body of evidence for other treatments that makes the “Holy Grail” cure for cancer through new approaches, such as those of Tekmira and Theralase, even more attainable than ever before.
Tekmira has a pipeline of novel RNAi therapeutics as well as a lipid nanoparticle (LNP) drug delivery technology. The Burnaby, BC-based company has reached commercialization by licensing Marqibo, an FDA-approved liposomal formulation of vincristine for the treatment of certain acute lymphoblastic leukemia (ALL) patients, to Talon Therapeutics, which was subsequently acquired by Spectrum Pharmaceuticals (NASDAQ: SPPI) last July.
On its own or through partner Alnylam Pharmaceuticals (NASDAQ: ALNY), which uses Tekmira’s LNP technology in three trials, Tekmira has five drug candidates in the clinic, including Alnylam’s ALN-VSP for liver cancer and its own lead therapy TKM-PLK1 for solid tumors. PLK1 (short for polo-like kinase 1) is a protein known to be overexpressed is specific cancers. Tekmira hypothesizes – and data to date supports – that using the RNAi approach of TKM-PLK1 can inhibit the protein, disrupting tumor cell division and leading to apoptosis in the cancerous cells.
In August, Tekmira built upon encouraging data collected in a Phase 1 trial of TKM-PLK1 to initiate a Phase 1/2 trial enrolling patients with either advanced Gastrointestinal Neuroendocrine Tumors (GI-NET) or Adrenocortical Carcinoma (ACC). Interim data from this trial, which is scheduled to enroll about 20 patients, is expected in the second half of this year.
GI-NET carries a terrible prognosis, with one-quarter of those diagnosed with advanced neuroendocrine tumors dying within one year. With no FDA-approved drugs to treat, it is a prime candidate for accelerated pathways for development.
Tekmira also plans to soon initiate another Phase 1/2 trial of TKM-PLK1 for patients with primary liver cancer. This is a compelling part of Tekmira as it is strengthening its own cornerstones by sponsoring clinical trials focused on therapies, rather than being dependent on milestone and royalties payments centered on partners using its LNP technology.
Theralase Technologies is generating revenue already with its highly effective TLC-1000 system and building out additional products based on patented superpulse laser technologies for biostimulative and biodestructive clinical applications.
On the biostimulative front, the Toronto-based company sells its TLC-1000 cold laser system that can penetrate up to 4 inches of tissue to deliver subcutaneous light pulses that years of clinical research have proven to eliminate pain, reduce inflammation and promote cellular regeneration. In the simplest sense, the scaffolding of this technology is built upon the ability of cytochromes to absorb light energy and convert it to chemical energy to heal tissue without side effects.
The TLC-1000 system delivers efficacy in the range of 80% to 90% and Theralase is preparing to take that to the next level with the planned fourth-quarter launch of an enhanced version, dubbed TLC-2000. This system, according to Theralase CEO Roger Dumoulin-White, can increase efficacy up to 100% by using state-of-the-art technologies to define and deliver the precise amount and intensity of light energy necessary to treat patients on an individual basis. In the $100-billion pain management industry in the U.S. alone, for which traditional drug therapies and surgeries comprise only about $50 billion, Theralase could establish itself as a significant player in the business.
To date, Theralase has sold about 1,200 of the TLC-1000 systems (800 in Canada and 400 in the U.S.) that have successfully treated more than one million patients. Sales tallied $1.17 million through the first three quarters of 2013 and are projected to increase to about $6 million going forward as it launches the new product and transitions into an annuity-based model, as opposed to a one-time-sale model.
This steady revenue stream helps mitigate risk as Theralase moves strategically ahead with its true value proposition utilizing its expert photodynamic skill set to develop new treatments for cancer and bacterial infections.
Theralase has introduced a new method for selectively targeting cancer cells through the use of patented Photo Dynamic Compounds (PDCs), small molecules that are activated into tumor-killing form when exposed to very specific light sources. In order to provide the appropriate light energy, Theralase has custom designed the TLC-3000 medical laser system. In vitro and animal model studies, in part conducted at the world renowned Ontario Cancer Institute located at Princess Margaret Hospital, University Health Network, have demonstrated the unique potential and powerful results of the treatment across a broad array of cancer lines, including breast, brain, colon, pancreatic, prostate and bladder. In the research, low concentration doses have demonstrated up to 100% tumor destruction with no adverse side effects.
One particular mouse model study best exemplifies the potential of this novel therapy. In the study, about 350,000 cells from a bladder cancer line were injected into mice and tumors were allowed to grow to 5 millimeters in size. At that point, the mice underwent a single treatment of the PDCs and light therapy. In short, this process involves using a catheter to insert the PDCs into the bladder where they enter the cancer cell and lock onto the cell nucleus. The bladder is then drained and filled with distilled water to give it shape and a fiber optic bundle is inserted. The light activates the PDCs, which induces apoptosis, destroying the tumor from the inside out. The dead cells exit the body through urine. The single treatment eradicated 100% of the cancerous cells and had no side effects, according to the company. Follow-up 20 months after treatment showed the mouse models remained cancer-free, with no scar tissue. Considering 20 months is the life expectancy of a mouse, it seems fair to say that they were “cured” of cancer.
A one-treatment cure for cancer is almost unimaginable, yet this study showed it to be possible.
Of course, this is only one animal study and there is no guarantee that the robust efficacy will translate to humans, but it certainly offers hope and provides scientific evidence that is worthy of clinical trials. One of the best parts is that this Theralase process for bladder cancer takes less than three hours from start-to-finish, meaning that it holds the potential to be conducted on an outpatient basis should the therapy eventually garner regulatory approval.
Theralase is planning to commence clinical trials for bladder cancer in the first quarter of 2015. Bladder cancer is the fifth most common cancer in the U.S., with about 72,000 new diagnoses annually and 15,000 deaths attributed to the disease that carries a stark recurrence rate in the area of 80 percent. The indication represents an area of great unmet medical need, qualifying it for a potential FDA “Breakthrough Therapy” designation, a regulatory pathway passed into law in 2012 as a means to expedite development of innovative technologies for hard-to-treat diseases where patients have little to no options. Theralase has stated that this is a path that they intend to pursue should the Phase 1 clinical trial meet its endpoints.
Realistically, this only scratches the surface of Theralase. The company plans to expand its clinical pipeline by building on preclinical data targeting other localized cancer lines, such as breast and lung cancer. Further, a newly published scientific report showed that the company’s PDCs have been shown to destroy Staphylococcus aureus (s. aureus) and Methicillin Resistant Staphylococcus aureus (MRSA) in low oxygen atmospheres. This new discovery supports the intrinsic value of the Theralase platform technology, as cancerous and bacterial cells are aggressive and express strong drug resistance in hypoxic conditions.
Because Theralase is still preclinical with its cancer and bacterial therapies, the markets haven’t given any real valuation to these key components of Theralase’s business, holding the market capitalization around $25 million. It’s arguable that this valuation is low based solely upon Theralase’s commercialized product. Consider that generic drug giant Sun Pharma spent $230 million to acquire Dusa Pharmaceuticals in November 2008 to gain control of Dusa’s approved photodynamic treatment for a few skin conditions. The markets that Theralase is pursuing with its TLC-1000 and TLC-2000 are much larger. While the TLC biostimulative series is formidable and fair for comparatives, the real beauty of biotechs is the speculative nature of pipelines and the tremendous market opportunities that accompany novel therapeutics for large indications. It doesn’t get much bigger than the cancer and bacteria arenas and the markets have not yet baked-in this potential into Theralase’s valuation.
The life sciences equities on Bay and Wall Streets have been on fire for the past year, with U.S. biotechs essentially doubling in 2013, only to be topped by the 111% gain that Canadian stocks delivered. Both Tekmira and Theralase have been strong performers in the past four months and both have revenue streams to support further clinical research. It is companies like these that have assumed leadership positions with innovative approaches that are likely to continue to attract the attention of investors and other bigger pharmas in the future as they deliver this clinical data to validate their drug candidates.
Written by Robert James.
Theralase (TSXV: TLT) Developing Next Generation Medical Lasers
Toronto, Ontario / TNW-ACCESSWIRE / March 25, 2014 / Theralase Technologies Inc. (TSXV: TLT) (TLTFF: OTCBB) announced today the expansion of its engineering team to develop and commercialize its next generation of smart medical laser technologies. This highly trained engineering team will be instrumental in developing Theralase products to eliminate pain and heal tissue, a $100 Billion industry in the US and to destroy cancer, a $77 Billion industry in the US.
The first of these cutting-edge technologies under development is the patented TLC-2000, a technology that eliminates pain by automatically adjusting its laser parameters in direct relation to a patient's condition and their physical characteristics allowing the delivery of an optimized laser treatment, specific to a particular patient, each and every time. This dramatic advancement in pain medicine will allow much more effective patient specific treatments than currently available and also provide valuable treatment feedback information to the practitioner to further enhance the effectiveness of their patient's treatments. The second cutting-edge technology under development is the TLC-3000 anti-cancer medical laser, a patient specific cancer solution, which is able to deliver a specific dose of laser light through fibre optic bundles directly to cancerous tissues. The technology will be specifically designed to destroy tumours, wherever they may reside in the body, by activating patented Photo Dynamic Compounds (PDCs), already previously disclosed by the Company to be extremely effective in the destruction of cancer.
The newly expanded engineering team, managed by Wayne Embree, a senior engineering manager, who has been involved in leading engineering teams in the design and development of complex electronic technologies over the past 38 years. His team consists of 5 full time and 4 consultant engineering personnel, who are all resident experts in their respective fields of hardware, software, firmware and radio frequency communication design.
Mr. Embree, VP Engineering for Theralase, stated that, "I am excited to join Theralase and be associated with some of the leading minds in engineering, chemistry, biology, physics and medicine to develop the next generation of medical laser technology. The technology they have amassed to date is ground breaking and with its commercialization in the next few years, they will undoubtedly introduce to main stream medicine truly disruptive technology, which will have a dramatic effect on the way medicine is practiced for years to come."
Dr. Arkady Mandel, Chief Scientific Officer of Theralase stated that, "Wayne's strong engineering background coupled with my in-depth scientific and medical knowledge in how laser light interacts at the molecular, cellular and tissue levels in the human body, will bring a whole new platform of cutting-edge laser technologies to the forefront in the next few years."
Roger Dumoulin-White, President and CEO of Theralase stated that, "I am delighted that Wayne has joined the Theralase management team and am confident in his ability to commercialize the next generation medical laser technologies. His skill set will be an asset to the corporation as we launch these disruptive technologies, allowing the Company an ability to significantly increase its revenues."
About Theralase Technologies Inc.
Theralase Technologies Inc. designs, manufactures and markets patented, superpulsed laser technology used in eliminating pain and destroying cancer. Theralase technology is safe and effective in eliminating pain, reducing inflammation and accelerating tissue regeneration of numerous nerve, muscle and joint injuries. Theralase is actively developing patented technology that is able to target and destroy cancers, bacteria and viruses when light activated.
Additional information is available at www.theralase.com and www.sedar.com
Yorba Interview - The Traders Network
I found the link to both of the radio segments from yesterday's show:
Segment 1 http://www.yorbamedia.com/images/stories/audio/TN031214SEG1.mp3
Segment 2
http://www.yorbamedia.com/images/stories/audio/TN031214SEG2.mp3
Time to listen Yorba Interview The Traders Network 0n Theralase Technology Inc
DUE DILIGENCE COMPLIATION
For those that just joined us here...
Articles:
Bob Moriarty / 321gold.com –
http://www.321gold.com/editorials/moriarty/moriarty030314.html
Midas Letter -
http://www.midasletter.com/2014/02/theralase-technologies-inc-cure-cancer/
Smallcapnetwork -
http://www.smallcapnetwork.com/How-Using-Light-To-Cure-Cancer-Could-Fuel-Rapid-Growth-For-Theralase-Technologies-Inc/s/via/26025/article/view/p/mid/1/id/1/
Seeking Alpha –
http://seekingalpha.com/instablog/19040961-e-vestorsgroup/2601761-theralase-technologies-inc-the-greatest-story-never-told?source=kizur_seekingalpha
http://seekingalpha.com/instablog/742421-thewebguy/2684691-theralase-big-pharma-bait
Canadian Business –
http://www.canadianbusiness.com/technology-news/most-interesting-companies-at-cantech-2014/
Investing.com –
http://www.investing.com/analysis/with-patent-cliff-looming,-2-potential-takeover-targets-for-roche-204220
http://www.investing.com/analysis/theralase:-a-cancer-cure-to-market-by-2016-204637
Videos:
Cambridge House Investor Presentation -
Written up by Moriarty
Attached please find a copy of the news letter written by in my opinion the second most followed letter writer next to Jim Dynes (Midas Letter). This is a GREAT sign for Theralase ( TLTFF - TLT – TSX.V).
More to come. Please hit the links below for a copy.
www.321gold.com/editorials/moriarty/moriarty030314.html
http://www.midasletter.com/midas-letter-financial-radio-podcast/interview-theralase-technologies-inc-tsx-vtlt-ceo-roger-white/
Theralase: A Prime Roche Target
Just found this article about a big pharma company, Roche, and two potential targets for it to acquire based on its 'patent cliff', particularly its expiring Herceptin sales...
A patent cliff is the decline in revenues a pharmaceutical company experiences when the patents on one or more of their protected compounds expires. The lack of patent protection opens up the market to generic alternatives, which generic drug manufacturers sell at a discount to the branded option. This reduced cost redirects revenues from the manufacturers (generally big pharma) that bore the cost of research and development.
During 2014, the patent cliff concept will expose a number of incumbent pharmaceutical companies to potential revenue redirection. One of those companies is Roche Holdings (RHHBY), with the European patent for the company's breast cancer drug, Herceptin, due for expiry. Roche's Herceptin sales totaled $6.07B during 2013, 16% of the company's total annual sales.
What Can Roche Do About The Expiration?
One way to offset the risk of a patent cliff is to extend the life of the expiring drug through modification. Modification might involve combining a drug with another compound to improve efficacy, or simply altering the delivery of the drug. There are problems associated with these actions however. Conducting trials to seek enhanced efficacy can be costly and time consuming; further, there is no guarantee that a combination will be successful. Altering the delivery method necessitates improved release rates, which even if achieved offer no guarantee of approval and extended exclusivity.
An Alternative, More Aggressive Option
Another option is to acquire smaller biotech companies that are taking treatments through the FDA approval process, or to license treatments from these smaller companies. As the pharmaceutical community is likely already aware, Roche generates the majority of its revenues from oncology drugs. As such, and in light of its potential Herceptin losses, Roche will likely look to strengthen this area of it operations. Examples of Roche's activity of this sort include the company's acquisition of Piramed Limited, which at the time had a drug candidate in phase I clinical testing for treating breast and lung cancer; and more recently, Roche'spartnership with Inovio Pharmaceuticals Inc, (INO) that gave the company an exclusive license for Inovio's DNA-based, prostate cancer targeting vaccine, INO-5150. Below are a couple of potential targets that could help Roche bolster oncology revenues over the next two years.
Theralese: Potential Target Number One
The first company that could be the target of a Roche deal in the next two years is Theralase, Inc. (TLT.V) (TLTFF). Theralase currently generates revenues from the sale of its cold laser technology, which practitioners use to simulate the healing process in patients with muscle damage. In addition to this however, Theralase is currently developing a type of cancer treatment, which uses lasers to activate compounds that, when activated, kill cancer cells.
How Does The Treatment Work?
Theralase has a portfolio of patented photodynamic compounds ("PDCs") that, via intravenous introduction, attach to cancer cells. Practitioners then use one of Theralase's laser platforms to activate the compounds, which upon activation induce apoptosis, a type of programmed cell death that cancer cells do not undergo naturally.
Theralase's Trials to Date
To date, Theralase has conducted a mouse model trial that investigates the efficacy of its PDCs in the treatment of bladder cancer. Researchers at Princess Margaret Hospital injected cancerous cells into the bladder of a mouse. Following this, they injected TLD1633, one of the Theralase's patented PDCs, into the tumor site. Twenty-four hours post activation, the PDCs had induced apoptosis in the cancer cells, and the mouse lived for 20 months post treatments. Eighteen-twenty months is the average lifespan of a mouse, meaning the treatment completely cured the cancer.
Theralase's 2014-2016 Strategy
Having cured bladder cancer in a mouse, the company is now carrying out the same test in a rat, the aim being to validate the previous results. During 2015, Theralase expects to achieve breakthrough status for its PDC treatment and to complete a phase I/IIa human clinical study. If successful, Theralase expects to execute a strategic partnership with big pharma during 2016, the point at which a company like Roche will get involved.
Agenus: Potential Target Number Two
The second potential takeover or licensing target is Agenus, Inc. (AGEN). Agenus focuses on another area of cancer treatment, immunotherapy. The company's lead immunotherapy candidate is Prophage G-200, which is currently undergoing trials for use in the treatment of glioblastoma multiforme ("GMB"), a malignant, deadly brain cancer.
How Does The Treatment Work?
Two major factors make GBM a difficult cancer to treat using traditional methods. The first is the tumor's location. Its proximity to the brain makes surgery extremely risky. The second us the tumor's form. A GBM tumor has long, tentacle-type fingers that spread throughout the brain. Agenus' treatment removes much of this risk, as it only requires a small sample of the tumor. Once removed, the company uses the sample to manufacture a vaccine, which contains the tumor's unique antigenic fingerprint. Practitioners then introduce this vaccine intravenously into the patient, and it stimulates the patient's immune system to combat the tumor. As with that of Theralase, the Agenus treatment is highly targeted and does not have any of the negative side effects traditionally associated with standard of care treatment.
Prophage G-200 Trials to Date
At the end of last year, Agenus announced the results of a phase II clinical trial. Of 41 patients enrolled, 90% were alive at six months, compared to 36% for a placebo and 56% for current chemotherapy standard of care.
Agenus' Forward Strategy
Agenus currently has 23 programs in clinical development, a combination of its own pipeline and a number of strategic partnerships. Four of these programs are currently in Phase 3 clinical trials and nearing data readouts. The company expects these data readouts during the first half of 2014. In addition to its pipeline, Agenus also seeks value through expansion. Last week, the company announced the completion of its acquisition of 4-Antibody AG, a private European-based biopharmaceutical company.
Conclusion
Over the next two years, the incumbent pharmaceutical companies will likely seek acquisition targets and licensing opportunities to offset potential patent related revenue losses. For Roche, a company that generates the majority of its revenues from oncology treatments, these acquisitions will likely include development stage cancer treatments. If they can prove the efficacy of their respective treatments, both Theralase and Agenus would offer Roche exposure to cutting edge cancer treatments over a range of disease instances.
http://www.investing.com/analysis/with-patent-cliff-looming,-2-potential-takeover-targets-for-roche-204220
TLT Positive Cash flow / double digit growth
Theralase has a very experienced and diverse management team with a strong background in medicine, engineering, technology and finance. Management has successfully negotiated a number of strategic partnership agreements with organizations such as University Health Network, Ontario Centers of Excellence for Photonics, Virginia Tech, The Scripts Research Institute, University of Buffalo, and Mayo Clinic.Potential market in the US can reach a share of billions, pain market excess $100 Billion annually and is growing rapidly.
Theralase designs, manufactures and markets patented FDA approved super-pulsed laser technology used in healing injured tissue and destroying cancer. Recent lab results have shown that Theralase was able to completely destroy subcutaneous bladder cancer tumorsin mouse models. (Mice have survived 20 months cancer free after only one PDC treatment)
In addition Dr. Andrews personally said that quote himself. It's from the press release dated July 6, 2011:
http://www.theralase.com/press/Theralase_Technologies_Announces_MSAB_Renewals_2011.pdf
He PERSONALLY endorses and uses this technology in his rehab clinics and facilities.
Here is also a great article from ESPN that details the life and career of Dr. Andrews:
http://sports.espn.go.com/espn/news/story?id=3024046
"Andrews has been on the cutting edge of new surgical technologies and rehab techniques that enable players to play longer and recover from injuries faster."
Notable Patients
Dr. James Andrews' patient list reads like a cross-sport hall of fame. He's a rundown of some of the most notable athletes who have been treated by Andrews:
Football players
• Bo Jackson: shoulder, 1984; hip, 1992
• Doug Williams: knee, 1988
• Bruce Smith: knees, 1990 and 1991
• Troy Aikman: elbow and shoulder, 1991
• Michael Irvin: shoulder, 1994
• Emmitt Smith: shoulder, 1994
• Trent Green: knee, 1999 and 2001
• Chad Pennington: shoulder, 2005 (twice)
• Deuce McAllister: knee, 2005
• Daunte Culpepper: knee, 2005 and 2006
• Takeo Spikes: achilles tendon, 2005
• Donovin Darius: ACL, 2005; shoulder, 2006
• Drew Brees: shoulder, 2006
• Byron Leftwich: ankle, 2006
• Donovan McNabb: knee, 2006
• Matt Hasselbeck: shoulder, 2007
• Joey Porter: knee, 2007
• Kenny Irons: knee, 2007
• Isaiah Kacyvenski: knee, 2007
• D.J. Shockley: knee, 2007
Baseball players
• Roger Clemens: 1985, shoulder, 1985
• David Wells: 1985, elbow, 1985
• Jimmy Key: elbow, 1988; shoulder, 1994, 1995
• Jose Rijo: elbow, 1995; five more elbow ops, 1996-2003
• Steve Karsay: elbow, 1995; shoulder, 2003
• Kerry Wood: elbow, 1999
• John Smoltz: elbow, 2000 and 2003
• Carl Pavano: elbow, 2001 and 2006
• Jon Lieber: elbow, 2002
• A.J. Burnett : elbow, 2003
• Andy Pettitte: elbow, 2004
• Gary Sheffield: shoulder, 2004
• Jim Thome: elbow, 2005
• Mark Prior: shoulder, 2007
• Anibal Sanchez: shoulder, 2007
• Freddie Garcia: shoulder, 2007
• Chris Ray: elbow, 2007
Basketball players
• Charles Barkley: shoulder, 1990
• Michael Jordan: shoulder (therapy, not surgery), 1994
• Penny Hardaway: knee, 1996
• Randy Livingston: knee, 1996
• Scottie Pippen: elbow, 2001
• Allen Iverson: elbow, 2001
• Aaron McKie: shoulder, 2001
• Chris Webber: knee, 2003
• Shaun Livingston: knee, 2007
Golfers
• Jack Nicklaus: knee, 1984
• Jerry Pate: shoulders, 1985, 1986, 2003, 2006
• Mark McCumber: shoulder, 1996
I haven't found a link where HE specifically names players or athletes that use the Theralase technology, but as I mentioned before he personally endorses it and uses it at his rehab clinics and facilities.
I DID HOWEVER find a video from the Toronto Blue Jays from an in-game Sportsnet broadcast where the announcers are all over it:
http://www.dailymotion.com/video/xe1xoj_theralse-blue-jays-2008-432-240_tech
Dr. James Andrews personally referred the Blue Jays into using the Theralase laser technology for player rehabilitation!
It's a good time to take a position before the release of the TLC-2000.
Management should be negotiating upgrade contracts and new business as we speak.
http://www.midasletter.com/2014/02/theralase-technologies-inc-cure-cancer/
http://thenewswire.ca/archives?tnwarchive2=release_id%3D11114
http://biotechnologyfocus.ca/scientific-paper-reports-theralase-compounds-destroy-bacteria-in-low-oxygen-environments/
Biotechnology Focus, Scientific Paper Reports
Two of the top scientic researchers from Princess Margaret Cancer Centre, University Health Network both are working with Theralase Dr. Lothar Lilge, senior scientist and Dr. Michael Jewett, clinical principal scientific investigator are looking forward to phase 1/2 clinical trials coming up.
Scientific paper reports Theralase compounds destroy bacteria in low oxygen environments
theralaseUnique characteristics of new photo dynamic compounds has application in destruction of lung, breast, brain, bladder and prostate cancers
Theralase Technologies Inc. reports that a recently published scientific paper indicates that its new family of Photo Dynamic Compounds (PDCs) are able to destroy two types of bacteria (Staphylococcus aureus (s. aureus) and Methicillin Resistant Staphylococcus aureus (MRSA)) in low oxygen atmospheres.
The results are considered pivotal because they validate Theralase PDCs efficacy in both normal and low oxygen environments and shows that they are able to be used in both bacteria and cancer destruction. According to Dr. Arkady Mandel, chief scientific officer of Theralase Inc., the technology could offer a new paradigm for destruction of low oxygenated cancerous tumours because of its ability to be effective in low oxygen environments, specifically against non-muscle invasive bladder cancer.
“This form of disease represents up to 75 per cent of newly diagnosed bladder cancer cases accounting for more than 386,000 cases and 150,000 deaths annually worldwide. The abnormal decrease or the lack of oxygen supply to cells and tissues is called hypoxia and commonly presents in solid cancers, such as brain, bladder, breast, lung and prostate. Hypoxic cancers are extremely aggressive, resistant to standard therapies (chemotherapy and radiotherapy), and thus very difficult to destroy. Tumour hypoxia is known to play a role in cancer metastasis (spread) and resistance to therapy, as well as the ability of cancer cells to escape destruction by the immune system.”
According to Dr. Mandel, the evidence supporting the Theralase PDC technology represents a potential solution for such hypoxic cancers.
Dr. Lothar Lilge, senior scientist at the Princess Margaret Cancer Centre, University Health Network further comments that, “In well oxygenated environments, these new PDCs generate singlet oxygen, a dominant cytotoxic substance with close to 100 per cent efficacy, yet under hypoxia (low oxygen) conditions, they display the remarkable ability to switch to a Type 1 or oxygen independent cytotoxic substance owing to their ability to simultaneously act as an excited state oxidant and reductant. The intrinsic positive charge of the Ru(II) metal combined with the oxygen independent light activated cytotoxicity demonstrated by this family of PDCs opens a new strategy for destroying both gram-positive and gram-negative bacteria regardless of oxygenation level. Therefore, this has a significant impact on the destruction of cancer cells by PDC technology, as a considerable fraction of cancer will survive in low oxygen environments and according to the literature this is one of the causes for recurrence of cancer post therapy.”
http://biotechnologyfocus.ca/scientific-paper-reports-theralase-compounds-destroy-bacteria-in-low-oxygen-environments/
Read more at http://www.stockhouse.com/companies/bullboard/v.tlt/theralase-technologies-inc#ly1qcrPkjZ5Br4eZ.99
Theralase's PDC destroy 2 types of bacteria, study says
2014-02-13 07:48 ET - News Release
Dr. Arkady Mandel reports
THERALASE DEMONSTRATES BACTERIA DESTRUCTION IN LOW OXYGEN ENVIRONMENTS. UNIQUE CHARACTERISTICS OF NEW PHOTO DYNAMIC COMPOUNDS HAS APPLICATION IN DESTRUCTION OF LUNG, BREAST, BRAIN, BLADDER AND PROSTATE CANCERS
A recently published scientific paper demonstrated that Theralase Technologies Inc.'s new family of photodynamic compounds has been proven to significantly destroy two types of bacteria (Staphylococcus aureus and methicillin-resistant Staphylococcus aureus) in low-oxygen atmospheres. The results are considered pivotal because the Theralase PDC efficacy has been validated in both normal and low-oxygen environments. Since the Theralase PDC platform technology is able to be used in both bacteria and cancer destruction, the described technology is offering a new paradigm for destruction of low-oxygenated cancerous tumours. (Photodiagnosis and Photodynamic Therapy, Dec. 10 (4), 615-25.)
"The ability for the PDC technology to be effective in low-oxygen environments is considered to be an essential factor in the recurrence and progression of non-muscle-invasive bladder cancer. This form of disease represents up to 75 per cent of newly diagnosed bladder cancer cases, accounting for more than 386,000 cases and 150,000 deaths annually worldwide," said Dr. Arkady Mandel, chief scientific officer of Theralase. Dr. Mandel continued: "The abnormal decrease or the lack of oxygen supply to cells and tissues is called hypoxia and commonly presents in solid cancers, such as brain, bladder, breast, lung and prostate. Hypoxic cancers are extremely aggressive, resistant to standard therapies (chemotherapy and radiotherapy), and thus very difficult to destroy. Tumor hypoxia is known to play a role in cancer metastasis (spread) and resistance to therapy, as well as the ability of cancer cells to escape destruction by the immune system. The evidence supporting the Theralase PDC technology represents a potential solution for hypoxic cancers. In our work, we described a family of Theralase PDCs that have shown an ability to switch their photoreactivity from a type 2 reaction (oxygen-dependent) to a type 1 (free-radical-mediated) reaction. This is strategic to the company in that a type 1 reaction is unique and opens the opportunity of using the PDCs beyond sterilization and the treatment of superficial cancerous lesions to the treatment of harder-to-treat tumours."
Dr. Lothar Lilge, senior scientist at the Princess Margaret Cancer Centre, University Health Network, stated: "In well-oxygenated environments, these new PDCs generate singlet oxygen, a dominant cytotoxic substance with close to 100-per-cent efficacy, yet under hypoxia (low-oxygen) conditions, they display the remarkable ability to switch to a type 1 or oxygen-independent cytotoxic substance owing to their ability to simultaneously act as an excited-state oxidant and reductant. The intrinsic positive charge of the Ru(II) metal combined with the oxygen-independent light-activated cytotoxicity demonstrated by this family of PDCs opens a new strategy for destroying both gram-positive and gram-negative bacteria regardless of oxygenation level. Therefore, this has a significant impact on the destruction of cancer cells by PDC technology, as a considerable fraction of cancer will survive in low-oxygen environments and, according to the literature, this is one of the causes for recurrence of cancer posttherapy."
Dr. Michael Jewett, clinical principal scientific investigator at Princess Margaret Cancer Centre, University Health Network, stated: "I am extremely encouraged by the results of this scientific work and the implications that it may have in the destruction of bladder transitional-cell carcinoma, as the bladder is traditionally known as a very low-oxygen environment. There has not been a new drug approved for bladder cancer since 1998, and I am looking forward to working with the Theralase team to initiate a phase 1/2 clinical trial in Q1 2015."
|
Followers
|
3
|
Posters
|
|
|
Posts (Today)
|
0
|
Posts (Total)
|
28
|
|
Created
|
02/26/14
|
Type
|
Free
|
| Moderators | |||
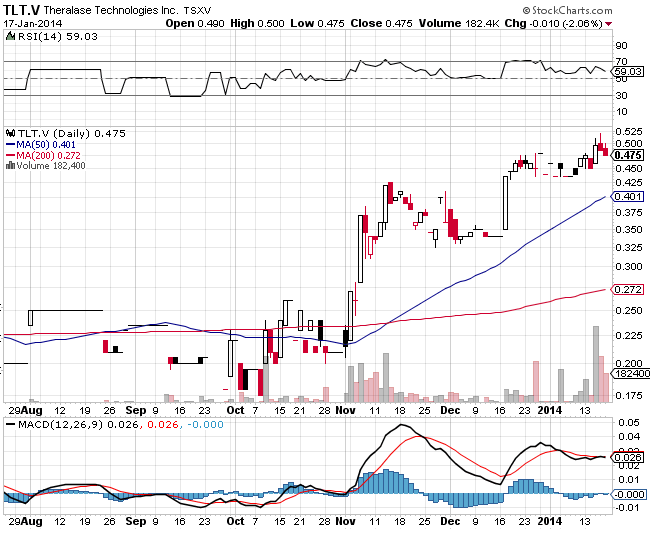
Technically speaking, Theralase is very bullish. Average volume has doubled this past month, and new 200-day highs have been reached. The MACD line is on the zero and signal lines, potentially setting up what I believe to be the next breakout. Short-term indicators appear bullish as well. The share price crossed the 21-day MA last week, and strong momentum moved the share price up to a new 52-week high.
ANTI-CANCER PLATFORM TECHNOLOGY
Theralase has patented anti-cancer drugs known as Photo Dynamic Compounds (PDCs) which localize the DNA of cancer cells and when irradiated, destroy the DNA resulting in apoptosis (natural cell death). While PDCs are used in Photodynamic Therapy (PDT) as a potential treatment regime for a number of conditions, Theralase has discovered that they hold particular promise as treatment to particular cancers. Theralase PDCs have proven successful in destroying breast, colon, brain and bladder cancer cells. Recent lab results have shown that Theralase was able to completely destroy subcutaneous bladder cancer tumors in mouse models. "The achievement of this important milestone signifies that Theralase's leading drug candidate is effective in the destruction of cancer in a live animal model and can prevent the cancer from recurring," commented Roger White of Theralase.
Key Market Statistics:
Theralase PDC Characteristics:
(668,000x ALA, 198x PHOTOFRIN)
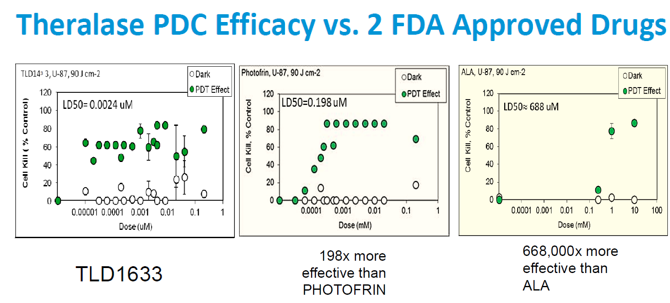
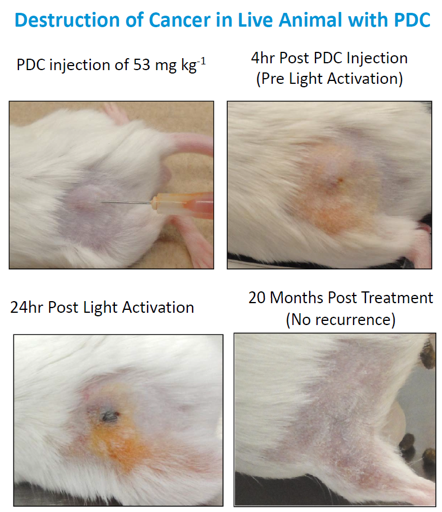
We are VERY IMPRESSED, and VERY EXCITED about these results! In 2012, annual spending for bladder cancer treatment in the US was $3.9 billion, and the current standard of care has been unchanged with no new drugs approved since 1998. Bladder cancer is the most expensive cancer to treat, and has a recurrence rate up to 80%. All of the mice which showed an initial response have remained cancer free, healed without scarring, and have developed normally after only one Theralase PDC treatment. Needless to say, the early results from Theralase look extremely positive. Imagine being able to not only provide treatment for bladder cancer, but to actually cure it, and stop it from coming back! The company is also working on developing further PDCs that can be used to treat a wide variety of other cancers (breast, colon, brain and lung), as well as bacteria and viruses.
To help explain how the procedure works we sketched a diagram:
Theralase is currently completing the validation of the orthotopic rat model, a dose toxicity study, GMP drug manufacture, and FDA Investigational New Drug (IND) application. The next phase is to complete an FDA phase 1/2a human clinical study with FDA breakthrough status, and then to execute a strategic partnership agreement with big pharmaceutical (pharma) company (upfront payments, co-development funds, annual recurring revenue streams).
Read More: http://static.cdn-seekingalpha.com/instablog/19040961-e-vestorsgroup/2601761-theralase-technologies-inc-the-greatest-story-never-told| Volume | |
| Day Range: | |
| Bid Price | |
| Ask Price | |
| Last Trade Time: |
