Register for free to join our community of investors and share your ideas. You will also get access to streaming quotes, interactive charts, trades, portfolio, live options flow and more tools.
$NRXP News: NRx Pharmaceuticals, Inc. (NASDAQ:NRXP) to Participate in the EF Hutton Annual Global Conference on May 15, 2024
RADNOR, Pa., May 8, 2024 /PRNewswire/ -- NRx Pharmaceuticals, Inc. (Nasdaq: NRXP) ("NRx Pharmaceuticals", the "Company"), a clinical-stage biopharmaceutical company, today announced that it will participate in the EF Hutton Annual Global Conference, which will take place on May 15, 2024, at The Plaza Hotel in New York City.
Prof. Jonathan Javitt, MD, MPH, the Company's Chairman and Chief Scientist, and Matthew Duffy, the Company's Chief Business Officer, will hold one-on-one meetings with investors throughout the day. Interested parties can register to attend here.
About NRx Pharmaceuticals
NRx Pharmaceuticals is a clinical-stage biopharmaceutical company developing therapeutics based on its NMDA platform for the treatment of central nervous system disorders, specifically suicidal bipolar depression, chronic pain and PTSD. The Company is developing NRX-101, an FDA-designated investigational Breakthrough Therapy for suicidal treatment-resistant bipolar depression and chronic pain. NRx has partnered with Alvogen and Lotus around the development and marketing of NRX-101 for the treatment of suicidal bipolar depression. NRX-101 additionally has potential to act as a non-opioid treatment for chronic pain, as well as a treatment for complicated UTI.
NRx has recently announced plans to submit a New Drug Application for HTX-100 (IV ketamine), through Hope Therapeutics, in the treatment of suicidal depression, based on results of well-controlled clinical trials conducted under the auspices of the US National Institutes of Health and newly obtained data from French health authorities, licensed under a data sharing agreement. NRx was awarded Fast Track Designation for development of ketamine (NRX-100) by the US FDA as part of a protocol to treat patients with acute suicidality.
About HOPE Therapeutics, Inc.
HOPE Therapeutics, Inc. (www.hopetherapeutics.com) is a Specialty Pharmaceutical Company, wholly-owned by NRX Pharmaceuticals focused on development and marketing of an FDA-approved form of intravenous ketamine for the treatment of acute suicidality and depression together with a digital therapeutic-enabled platform designed to augment and preserve the clinical benefit of NMDA-targeted drug therapy.
https://c212.net/c/img/favicon.png?sn=CL08030&sd=2024-05-08 View original content to download multimedia:https://www.prnewswire.com/news-releases/nrx-pharmaceuticals-inc-nasdaqnrxp-to-participate-in-the-ef-hutton-annual-global-conference-on-may-15-2024-302139427.html
SOURCE NRx Pharmaceuticals, Inc.
$NRXP News: NRx Pharmaceuticals (Nasdaq:NRXP) Announces Final Clinical Trial Results: Superior Safety Combined with Similar Efficacy in the Trial of NRX-101 Compared to Lurasidone in Suicidal Bipolar Depression
Both drugs demonstrated > 50% response for treating depression. NRX-101 demonstrated a mean 76% reduction in symptoms of akathisia compared to lurasidone that was sustained over 42 days (Effect Size .37; P=0.025), using prespecified analytic methodology memorialized in FDA Special Protocol Agreement. Levels of akathisia with NRX-101 were essentially zero at day 42
This safety advantage was previously reported in the Company's published STABIL-B trial
Akathisia is identified as a life-threatening side effect of nearly all antidepressants, reported in 10-15% of treated patients and is closely linked to suicide in FDA black box warning
Akathisia was seen in 2% of participants treated with NRX-101 vs. 11% treated with lurasidone
Company plans to seek accelerated approval of NRX-101 for use in patients with bipolar depression at risk of akathisia while continuing to broaden the indication to all patients with bipolar depression and perhaps schizophrenia
Study will be presented at the American Society of Clinical Psychopharmacology (ASCP) meeting May 28-31, 2024 (Miami) together with study investigators, accompanied by a broadcast scientific presentation on akathisia and antidepressant safety, and investor Q&A
RADNOR, Pa., May 6, 2024 /PRNewswire/ -- NRx Pharmaceuticals, Inc. (Nasdaq: NRXP) ("NRx Pharmaceuticals", the "Company"), a clinical stage pharmaceutical company, today announced a statistically significant safety advantage of NRX-101 compared to the standard of care comparator in its recently completed clinical trial in patients with suicidal bipolar depression. Therefore, the Company believes that demonstration of reduced akathisia in the setting of comparable antidepressant efficacy constitutes a basis for Accelerated FDA Approval of NRX-101. The full clinical trial results will be presented at the upcoming meeting to the American Society of Clinical Psychopharmacology held May 28-31, 2024 in Miami. NRx will gather Key Opinion Leaders to educate the public on the importance and potentially life-saving implication of this finding.
Last week, the Company released preliminary top-line data as required by SEC disclosure rules. The Company believes that today's findings based on mixed model regression analysis as specified in the Company's Special Protocol Agreement with the FDA, when combined with the prior STABIL-B trial1, demonstrate a basis for seeking accelerated drug approval of NRX-101 based on improved safety related to akathisia and suicidality in the setting of comparable antidepressant efficacy.
Trial participants had identical mean scores on the Barnes Akathisia Rating Scale (BARS) at baseline with subsequent decrease in the NRX-101 treated group versus an increase in the lurasidone-treated group, yielding a 76% relative mean difference between the groups. The difference was apparent at the first post-randomization visit and continued throughout the trial. (Fig 1) Over the 42 days of observation, an effect size of .37 was identified with a statistically significant P value of 0.025 on the Mixed Model for Repeated Measures (MMRM) methodology agreed to with FDA in the 2018 Special Protocol Agreement. Akathisia as ascertained by a 1 point increase in the BARS was seen in 11% of participants randomized to lurasidone (comparable to previous reports in the literature) and seen in only 2% of those treated with NRX-101, an akathisia level that was previously reported for the placebo arm of the lurasidone registration trial.
Akathisia was a prespecified key safety endpoint of the Company's clinical trial. Hence this finding is not a "post-hoc" observation. As previously noted, this clinical trial of 91 participants with suicidal bipolar depression who were not pre-treated with ketamine demonstrated that NRX-101 and lurasidone were comparable in their antidepressant effect. A 33% but statistically non-significant sustained decrease in suicidality was also seen favoring NRX-101. As noted above, improved antidepressant efficacy is not required to seek drug accelerated drug approval based on a statistically-significant safety benefit.
Based on this safety finding, NRx plans to seek Accelerated Approval of NRX-101 for treatment of bipolar depression in patients at risk for akathisia who are at highest risk of suicide, while continuing to develop evidence to support broader indications both in treatment of depression and schizophrenia. Should these data be confirmed in additional large scale trials, the Company believes that physicians and patients will universally prefer antidepressant and antipsychotic drugs with a reduced akathisia risk. The NRx patent portfolio supports the development of a broad range of combined NMDA/serotonergic drugs for treatment of depression and psychosis.
There is a recent regulatory precedent for the approval of psychiatry drugs that demonstrate comparable efficacy with improved safety. A combination of olanzapine and samidorphan (LYBALVI®) was approved based on comparable effect on schizophrenia symptoms with evidence of less weight gain favoring LYBALVI. Thus, public assertions by journalists and short-sellers that NRx has no path to market based on the finding of comparable efficacy in this trial are utterly baseless and may be designed to mislead investors.
"More than 7 million Americans suffer from bipolar depression with a potential market opportunity in excess of $20 billion. No prior drug to treat bipolar depression has demonstrated superiority on side effects most closely linked to suicide. Patients with bipolar depression have a 50% lifetime risk of a suicidal attempt and a 20% lifetime risk of dying from suicide. On this basis of these superiority findings, previously seen in the STABIL-B trial, we plan to seek Accelerated Approval from FDA for treatment of patients with bipolar depression who are at risk for akathisia, as we and our partners continue to broaden the indication to the treatment of all patients with bipolar depression." said Dr. Jonathan Javitt, NRx's Chairman and Chief Scientist. "Patients and key opinion leaders alike have told us clearly that an antidepressant with comparable antidepressant effect and reduced risk of akathisia and other risk factors for suicidality would be unambiguously preferred in the marketplace."
Background on Akathisia
Akathisia is a known extrapyramidal side effect of all serotonin-targeted antidepressants and antipsychotic drugs. It can be demonstrated in laboratory models, using rodent behavioral data.2 Prof. Daniel Javitt, the inventor of NRX-101 first discovered the ability of NMDA antagonist drugs to reduce akathisia in 2009, which forms the basis of the Company's composition of matter patent portfolio. In his 2012 patent application Javitt stated 3
A major limitation in use of antipsychotic and antidepressant medications is the liability to produce behavioral side effects, especially anxiety, agitation, and akathisia, all of which are associated with generating or exacerbating suicidality in psychotic or depressed patients. These behavioral side effects can be differentiated from symptoms of the illness by consideration of both time course and specific patterns of symptoms.
The side effect is clearly recognized in an FDA-mandated black box warning applied to lurasidone and all medications in its class of drugs, with a specific warning about suicidality as follows:
"The following symptoms, anxiety, agitation, panic attacks, insomnia, irritability, hostility, aggressiveness, impulsivity, akathisia (psychomotor restlessness), hypomania, and mania have been reported in adult and pediatric patients being treated with antidepressants for major depressive disorder as well as for other indications, both psychiatric and non-psychiatric. Although a causal link between the emergence of such symptoms and either the worsening of depression and/or the emergence of suicidal impulses has not been established, there is concern that such symptoms may represent precursors to emerging suicidality"4.
In most clinical trials, safety endpoints are not a basis for drug approval, although adverse safety findings may prevent drug approval. Typically, new drugs are compared to placebo in order to prove a difference in efficacy. In this case, the trial was conducted against the standard of care drug, not against placebo and, therefore, safety differences are highly important. Akathisia is a labeled negative side-effect of lurasidone and all similar drugs, and is closely linked to suicidal behavior.56 It is a side effect about which patients must be warned[7] and has figured prominently in class action lawsuits brought against manufacturers of serotonin-targeted antidepressants and antipsychotics. Currently, there is no FDA-approved treatment for akathisia and it is considered a medical emergency by many treating psychiatrists. Akathisia causes extreme anxiety to patients, inability to control motor movement, and impulsive acts, all to often leading to suicide and may be the key driver of the observed increase in suicidal ideation and behavior associated with all serotonin-targeted antidepressants and antipsychotic drugs.
"These studies support our original observation that D-cycloserine reverses the akathisia-like behaviors induced by lurasidone in rodent models. It is exciting to see those findings demonstrated in a second trial with unequivocal statistical significance," said Prof. Daniel Javitt, co-founder of NRx Pharmaceuticals. "Hopefully, this opens a path to bringing a lifesaving drug to millions of patients with suicidal depression whose only approved long-term treatment is electroconvulsive therapy."
About NRx Pharmaceuticals
NRx Pharmaceuticals is a clinical-stage biopharmaceutical company developing therapeutics based on its NMDA platform for the treatment of central nervous system disorders, specifically suicidal bipolar depression, chronic pain and PTSD. The Company is developing NRX-101, an FDA-designated investigational Breakthrough Therapy for suicidal treatment-resistant bipolar depression and chronic pain. NRx has partnered with Alvogen and Lotus around the development and marketing of NRX-101 for the treatment of suicidal bipolar depression. NRX-101 additionally has potential to act as a non-opioid treatment for chronic pain, as well as a treatment for complicated UTI.
NRx has recently announced plans to submit a New Drug Application for HTX-100 (IV ketamine), through Hope Therapeutics, in the treatment of suicidal depression, based on results of well-controlled clinical trials conducted under the auspices of the US National Institutes of Health and newly obtained data from French health authorities, licensed under a data sharing agreement. NRx was awarded Fast Track Designation for development of ketamine (NRX-100) by the US FDA as part of a protocol to treat patients with acute suicidality.
About HOPE Therapeutics, Inc.
HOPE Therapeutics, Inc. (www.hopetherapeutics.com) is a Specialty Pharmaceutical Company, wholly-owned by NRX Pharmaceuticals focused on development and marketing of an FDA-approved form of intravenous ketamine for the treatment of acute suicidality and depression together with a digital therapeutic-enabled platform designed to augment and preserve the clinical benefit of NMDA-targeted drug therapy.
Notice Regarding Forward-Looking Statements
The information contained herein includes forward-looking statements within the meaning of Section 21E of the Securities Exchange Act of 1934, as amended, and Section 27A of the Securities Act of 1933, as amended. These statements include, among others, statements regarding the proposed public offering and the timing and the use of the proceeds from the offering. Forward-looking statements generally include statements that are predictive in nature and depend upon or refer to future events or conditions, and include words such as "may," "will," "should," "would," "expect," "plan," "believe," "intend," "look forward," and other similar expressions among others. These statements relate to future events or to the Company's future financial performance, and involve known and unknown risks, uncertainties and other factors that may cause the Company's actual results to be materially different from any future results, levels of activity, performance or achievements expressed or implied by these forward-looking statements. You should not place undue reliance on forward-looking statements since they involve known and unknown risks, uncertainties and other factors which are, in some cases, beyond the Company's control and which could, and likely will, materially affect actual results, levels of activity, performance or achievements. Any forward-looking statement reflects the Company's current views with respect to future events and is subject to these and other risks, uncertainties and assumptions relating to the Company's operations, results of operations, growth strategy and liquidity. More detailed information about the Company and the risk factors that may affect the realization of forward-looking statements is set forth in the Company's most recent Annual Report on Form 10-K and other filings with the Securities and Exchange Commission. Investors and security holders are urged to read these documents free of charge on the SEC's website at http://www.sec.gov. Except as may be required by applicable law, The Company assumes no obligation to publicly update or revise these forward-looking statements for any reason, or to update the reasons actual results could differ materially from those anticipated in these forward-looking statements, whether as a result of new information, future events or otherwise.
1 Nierenberg A, Lavin P, Javitt DC, et. al. NRX-101 vs lurasidone for the maintenance of initial stabilization after ketamine in patients with severe bipolar depression with acute suicidal ideation and behavior; a randomized prospective phase 2 trial. Int J Bipolar Dis 2023;11:28-38, doi.org/10.1186/s40345-023-00308-5
2 Walf, A. A., & Frye, C. A. (2007). The use of the elevated plus maze as an assay of anxiety-related behavior in rodents. Nature Protocols, 2(2), 322-328. https://doi.org/10.1038/nprot.2007.44
3 Javitt DC. Composition and method for treatment of depression and psychosis in humans. US Patent 10583138B2. Granted March 10, 2020.
4 Trivedi et al., J Clin Psychiatry, 72:765-774, 2011
5 Chow LC, Kahouh NK, Bostwick JR, et. al., Akathisia and newer second-generation antipsychotic drugs: A review of current evidence. Pharmacotherapy 202;40(6):565-574 doi: 10.1002/phar.2404
6 Uwai Y, Nabekura T. Risk factors for suicidal behavior/ideation and hostility/aggression in patients with bipolar disorders: An analysis using the Japanese adverse drug event report database. J Psychiatric Res 2022;153:99-103. https://doi.org/10.1016/j.jpsychires.2022.07.005
7 https://www.webmd.com/schizophrenia/side-effects-of-lurasidon
https://c212.net/c/img/favicon.png?sn=CL05849&sd=2024-05-06 View original content to download multimedia:https://www.prnewswire.com/news-releases/nrx-pharmaceuticals-nasdaqnrxp-announces-final-clinical-trial-results-superior-safety-combined-with-similar-efficacy-in-the-trial-of-nrx-101-compared-to-lurasidone-in-suicidal-bipolar-depression-302136430.html
SOURCE NRx Pharmaceuticals, Inc.
$AGBA Trump, Triller, and the return of Ryan Kavanaugh
~ After billionaire status and bankruptcy, Ryan Kavanaugh is seeking a new path to moguldom with Triller, the dark horse TikTok rival endorsed by the Trumps.
https://fastcompany.com/90563686/trump-triller-and-the-return-of-ryan-kavanaugh
$NCL News: Northann Corp Enters Strategic Relationship with Rexil Group of Companies Netherlands to Promote Sustainable Technology and Products
ELK GROVE, Calif., April 24, 2024 (GLOBE NEWSWIRE) -- Northann Corp (NYSE American: NCL), a leading innovator in 3D-printed home improvement solutions, is pleased to announce the signing of a strategic relationship with Golden Gate BCE B.V., a subsidiary of Rexil Group of Companies Netherlands, a prominent distributor in the Netherlands. This collaboration aims to enhance the presence of sustainable technology and products throughout Rexil Group of Companies Netherlands and its subsidiaries distribution channels.
Under this agreement, Rexil Group of Companies Netherlands will promote Northann Corp's environmentally friendly products, Blue11 and SuperOak, to its extensive network of customers in the Netherlands. This partnership aligns with Northann Corp's commitment to sustainability and innovation, furthering its mission to transform spaces through cutting-edge solutions.
"We are thrilled to partner with Rexil Group of Companies Netherlands to bring our sustainable technology and products to a wider audience in the Netherlands," said Lin Li, CEO and President of Northann Corp. "This collaboration underscores our dedication to environmental stewardship and showcases our commitment to providing innovative solutions to our customers."
Northann Corp’s partnership with Rexil Group of Companies Netherlands represents a significant step forward for the Company in its efforts to expand its global presence and promote sustainable practices within the industry. Both companies share a common vision for driving positive change through innovation and sustainability, making this a synergistic collaboration for both parties.
About Northann Corp.
Northann specializes in 3D-printed flooring solutions under its flagship brand, "Benchwick." The Company's operations span the full spectrum of additive manufacturing, from sourcing recycled ocean plastics to the final production of intricate flooring designs. Northann offers its 3D printing ecosystem as an extensive range of proprietary solutions, including Infinite Glass, DSE, TruBevel, and MattMaster, primarily through its sales network in North America and Europe. The Company aims to redefine the essence of modern flooring and wall panels by offering stylish, durable, and ecologically conscious solutions.
For more information about Northann, please visit www.northann.com.
Forward Looking Statements
This press release contains forward-looking statements. Forward-looking statements include statements concerning plans, objectives, goals, strategies, future events or performance, and underlying assumptions and other statements that are other than statements of historical facts. When the Company uses words such as "may, "will, "intend," "should," "believe," "expect," "anticipate," "project," "estimate" or similar expressions that do not relate solely to historical matters, it is making forward-looking statements. Forward-looking statements are not guarantees of future performance and involve risks and uncertainties that may cause the actual results to differ materially from the Company's expectations discussed in the forward-looking statements. These statements are subject to uncertainties and risks including, but not limited to, other factors discussed in the “Risk Factors” section of the registration statement filed with the SEC. For these reasons, among others, investors are cautioned not to place undue reliance upon any forward-looking statements in this press release. Additional factors are discussed in the Company's filings with the SEC, which are available for review at www.sec.gov. The Company undertakes no obligation to publicly revise these forward-looking statements to reflect events or circumstances that arise after the date hereof.
For investor and media inquiries, please contact:
Investor Relations
Northann Corp.
Email: ir@northann.com
Phone: +1 (916) 573-3803
Skyline Corporate Communications Group, LLC
Scott Powell, President
1177 Avenue of the Americas, 5th Floor New York, NY, 10036
Office: (646) 893-5835 x2
Email: info@skylineccg.com
$IVDN (16 million float) News on Rising Sales of Superior Insulation Product: Innovative Designs Vendor Growth
PITTSBURGH, PA, April 10, 2024 (GLOBE NEWSWIRE) -- via NewMediaWire -- Innovative Designs, Inc. (OTCQB: IVDN) continues to gain market share through its growing vendor base. Since the beginning of this fiscal year (November 1, 2023), Innovative Designs has added over 30 new accounts that have purchased its Insultex House Wrap®. This has resulted in a comp sales increase of 318% as compared to the same time period last year (November 2022 – April 2023). With 7 months remaining in FY2024, sales for the first 5 months are approaching 88% of last year's total.
Randy Kimbler, a National Distributor of Insultex, commented, “The introduction of Insultex® House Wrap to several major building supply companies has been very well-received. The product is being placed in the retail locations of these organizations, as well as many local building supply companies. The R-6 insulation value provided by this very thin product has proven to be an affordable solution for builders facing economic challenges in states where energy codes require R-5 Continuous insulation for new construction.”
About Innovative Designs, Inc.
Innovative Designs, Inc. manufactures the Insultex® House Wrap and Arctic Armor® Line, under the "i.d.i.gear" label featuring INSULTEX®. Patented INSULTEX® is the thinnest, lightest and warmest insulator in the market today. For more information, please visit http://www.idigear.com or http://www.insultexhousewrap.com.
Disclaimer
Certain statements in this press release constitute "forward-looking" statements as defined by federal law. Such statements are based on assumptions, but there is no assurance that actual outcomes will not be materially different as those implied. Any such statements are made in reliance on the "Safe Harbor" protections provided under the Private Securities Reform Act of 1995 and are subject to various factors, including the risks and matters discussed in the Company's SEC filings available at http://www.sec.gov.
CONTACT:
Innovative Designs, Inc.
Joseph Riccelli, CEO
412-799-0350
joer@idigear.com
Website: http://www.insultexhousewrap.com
Link: https://finance.yahoo.com/news/innovative-designs-vendor-growth-173800119.html
$NCL News: Northann Corp.'s Benchwick Brand to Showcase SuperOak Hybrid Wood Planks Alongside Blue11 Collection at NWFA in New Orleans, April 16-18, 2024
ELK GROVE, Calif., April 17, 2024 (GLOBE NEWSWIRE) -- Northann Corp. (NYSE American: NCL) today announced that its Benchwick brand will unveil its latest patented innovation, SuperOak Hybrid Wood Planks, before showcasing its award-winning Blue11 Collection, at the National Wood Flooring Association (NWFA) Expo in New Orleans, April 16-18, 2024, at the New Orleans Ernest N. Morial Convention Center - Hall F & G.
Combining the natural beauty of wood with the durability and sustainability of modern materials, SuperOak Hybrid Wood Planks represent a breakthrough in flooring technology. The product will be in stock across the U.S. starting in May, ensuring customers have access to this revolutionary flooring solution.
"We are thrilled to introduce our Benchwick SuperOak Hybrid Wood Planks, as well as showcase our award-winning Blue11 Collection, at NWFA," said Lin Li, CEO and President of Northann Corp. "SuperOak demonstrates our dedication to pushing the boundaries of flooring technology."
Attendees of NWFA are invited to visit Northann at booth number 2219 to explore the SuperOak and Blue11 Collection and learn more about Benchwick's cutting-edge flooring solutions.
For more information about Northann Corp. and its Benchwick brand, visit www.northann.com.
About Northann Corp.
Northann specializes in 3D-printed flooring solutions under its flagship brand, "Benchwick." The Company's operations span the full spectrum of additive manufacturing, from sourcing recycled ocean plastics to the final production of intricate flooring designs. Northann offers its 3D printing ecosystem as an extensive range of proprietary solutions, including Infinite Glass, DSE, TruBevel, and MattMaster, primarily through its sales network in North America and Europe. The Company aims to redefine the essence of modern flooring and wall panels by offering stylish, durable, and ecologically conscious solutions.
For more information about Northann, please visit www.northann.com.
Forward Looking Statements
This press release contains forward-looking statements. Forward-looking statements include statements concerning plans, objectives, goals, strategies, future events or performance, and underlying assumptions and other statements that are other than statements of historical facts. When the Company uses words such as "may, "will, "intend," "should," "believe," "expect," "anticipate," "project," "estimate" or similar expressions that do not relate solely to historical matters, it is making forward-looking statements. Forward-looking statements are not guarantees of future performance and involve risks and uncertainties that may cause the actual results to differ materially from the Company's expectations discussed in the forward-looking statements. These statements are subject to uncertainties and risks including, but not limited to, other factors discussed in the “Risk Factors” section of the registration statement filed with the SEC. For these reasons, among others, investors are cautioned not to place undue reliance upon any forward-looking statements in this press release. Additional factors are discussed in the Company's filings with the SEC, which are available for review at www.sec.gov. The Company undertakes no obligation to publicly revise these forward-looking statements to reflect events or circumstances that arise after the date hereof.
For investor and media inquiries, please contact:
Investor Relations
Northann Corp.
Email: ir@northann.com
Phone: +1 (916) 573-3803
Skyline Corporate Communications Group, LLC
Scott Powell, President
1177 Avenue of the Americas, 5th Floor New York, NY, 10036
Office: (646) 893-5835 x2
Email: info@skylineccg.com
https://www.globenewswire.com/newsroom/ti?nf=OTA4OTg3MCM2MjAyMzk1IzIyNTM4NDA=
https://ml.globenewswire.com/media/ZWViZDNhYWYtNmMzYS00OGZiLTlmNjItMGI4MTE3NDQ2MTEyLTEyNjUzOTM=/tiny/Northann-Corp-.png
Source: Northann Corp.
$AVRW New and Perfect Skincare Products. #OTCQB subisdiary@Seratopical
Solutions "Harmony Toner" Available on the Seratopical Website! Great video below with Facial Plastic Surgeon Dr. Michael Persky https://seralabshealth.com/products/ $JNJ $CVS $AMZN
$TREIF Keep on watch!!! hearing a huge update coming very soon....
$NRXP News: NRx Pharmaceuticals (Nasdaq:NRXP) Reports Fourth Quarter and Full Year 2023 Financial Results and Provides Business Update
Four potential near-term milestones, including data from two clinical trials, an NDA filing and an upcoming share dividend
-- 50% reduction in corporate overhead and 25% reduction in overall net loss in 2023, compared to 2024 with $0.20 per share improvement in negative earnings. Additions to working capital of $8 million in Q1 2024.
-- Company forecasts first commercial revenue in 2024 from sales of ketamine and related technologies. Company received advance of first milestone payments in 2024 for ongoing development of NRX-101 from Alvogen and Lotus Pharmaceuticals, Inc. (1975.TW)
-- Company announces new partnership around the first drug to potentially modify the underlying cause of schizophrenia
-- Data lock this week and top-line data expected this month, after completed enrollment of the Phase 2b/3 trial of NRX-101 in Treatment Resistant Bipolar Depression (TRBD); trial demonstrated 94% rater concordance, far in excess of industry norms and exceeded industry norms in medication compliance
-- Two new Investigational New Drug applications (INDs) accepted by the US Food and Drug Administration (FDA) for NRX-101 in Chronic Pain and Complicated UTI.
-- Data lock expected this week in 200-person DOD-funded trial of D-cycloserine (DCS), the key component of NRX-101, to treat chronic pain, conducted by Northwestern University
-- Grant of Qualified Infectious Disease Product (QIDP), Fast Track and Priority Review designations for NRX-101 in the treatment of Complicated Urinary Tract Infection (cUTI); Publication last week of QIDP-qualifying data in a peer-reviewed journal. NRx is reviewing partnership options
-- Established HOPE Therapeutics to develop and launch IV Ketamine together with related technologies with FDA New Drug Application to be submitted this year. In advance of FDA approval, HOPE is partnered with national 503b and 503a pharmacies to address the ketamine shortage declared by FDA. HOPE is planned to be spun out as a separate company to be owned by NRx, current NRx shareholders via a tax-free dividend, and new investors; Term Sheets received from prospective anchor investors for $60 million of new investment, once publicly listed
-- HOPE is presenting data from four randomized, prospective trials demonstrating safety and efficacy in 800 patients of IV Ketamine in treating severe and suicidal depression as the clinical basis for New Drug Application (NDA) for HTX-100 (IV Ketamine); expecting stability and CMC data sufficient for NDA filing by June 2024.
-- Added over $8 million in working capital, including an advance of a $5.1 million milestone payment from partners Alvogen, Inc. and Lotus Pharmaceuticals
-- Elected nationally recognized attorney in highly regulated industries, and healthcare specialist, Janet Rehnquist, Esq., to the Company's Board of Directors
-- Management has taken actions to address NASDAQ listing compliance and naked shorting of NRx securities
-- Management to host a conference call, April 1, 2024, at 8:30 AM ET
RADNOR, Pa., April 1, 2024 /PRNewswire/ -- NRx Pharmaceuticals, Inc. (Nasdaq: NRXP) ("NRx Pharmaceuticals", the "Company"), a clinical-stage biopharmaceutical company, today announced its financial results for the quarter and year ended December 31, 2023 and provided a business update.
"2023 was a pivotal year for NRx in which we advanced from a single clinical trial in a single indication to a dramatically streamline its operations with a 50% reduction in overhead costs, a 25% reduction in overall costs, and a $0.20 per share improvement in negative earnings, while completing our clinical trial objectives. We expect data from two key trials this month and predict our first commercial revenue by the end of 2024. Over the past year the Company has navigated the most challenging business environment in the history of the biotechnology industry. Despite unprecedented headwinds, we negotiated a critical commercial partnership for our lead compound in bipolar depression, while retaining rights to the far larger indications of chronic pain and PTSD," said Stephen Willard, J.D., Chief Executive Officer and Director of NRx Pharmaceuticals. "We augmented our intellectual property portfolio to include the use of our lead compound in chronic pain and anticipate results of a 200-person efficacy trial that could open a multibillion dollar opportunity in this therapeutic area. We have acquired sufficient data on safety and efficacy of ketamine to support a New Drug Application for IV ketamine in acute suicidality. We established the foundation of a specialty pharmaceutical business around ketamine that we expect to yield positive cash flow by the end of 2024. Finally, we received unanticipated data supporting the use of our lead compound to treat complicated Urinary Tract Infection and Pyelonephritis, a condition that affects 3 million Americans and results in more than 15,000 deaths annually. We believe that NRx is poised for substantial growth in 2024 and look forward sharing further results in our conference call."
Fourth Quarter Clinical, Regulatory and Corporate Highlights
Development of NRX-101 for Treatment-Resistant Suicidal Bipolar Depression
The Company has announced today that it expects data lock this week and release of top line data this month in its Phase 2b/3 trial of NRX-101 in Suicidal Bipolar Depression. In 2023, the Company published results of a phase 2 trial demonstrating that oral NRX-101 extends the effect of IV ketamine in reducing both suicidality and depression in patients presenting to the hospital. This trial is designed to determine whether oral NRX-101 can reduce depression and suicidality in outpatients, which would be a massive broadening of the potential market for the drug. This potential expansion was guided by the Company's January 2023 meeting with the FDA in which NRx was advised to seek approval for NRX-101 as a drug for bipolar depression as a chronic, intermittent disease, a far broader indication.
With the completion of data collection, an unprecedented data integrity standard (94% agreement between site raters and central raters) has been achieved across the completed cohort of patients.
In 2023, the Company completed manufacture and Chemical Manufacturing Controls for NRX-101. This initiative is expected to yield stability data sufficient to support a shelf life in excess of two years at time of potential drug launch (should the clinical trials be successful). The completion of this manufacturing milestone allowed the Company to decrease its ongoing expenditure associated with manufacturing and development of chemical manufacturing controls.
During Q4 and in early 2024, the Company has continued to solidify its working relationship with Alvogen and Lotus, and begun working in unison to plan the final development and commercialization of NRX-101. These partners recently advanced $5 million of the first milestone to the Company. As previously announced, a successful readout from this trial and FDA interaction will trigger an additional $4 million milestone payment together with transfer of future development costs to our partner. The partnership provides for potential milestones of $329 million and a royalty reaching 15% on Net Sales.
NRX-101 for Treatment of Chronic Pain:
The Company has previously detailed the scientific basis for treatment of chronic pain with DCS as outlined in a 2016 scientific paper published by Schnitzer, et. al. and in the White Paper posted by the Company's Scientific Leadership (Sappko, et. al.). In 2023, the Company licensed US Patent 8,653,120 for the use of DCS in chronic pain and filed a now-accepted Investigational New Drug (IND) application with the FDA to initiate commercial drug development of NRX-101 in chronic pain.
Chronic pain affects more than 50 million American adults, compared to the approximately 3 million who report thoughts of suicide on an annual basis. There has been no new non-opioid class of drugs to treat nociceptive pain in the past two decades and NRX-101 has the potential to be the first N-methyl-D-aspartate (NMDA)-antagonist drug to seek approval for this indication. Today, ketamine is used off label to treat nociceptive pain, despite its clear limitations (addiction, neurotoxicity, hallucination, and the need for IV administration.)
The Company is advised that data lock will occur this week in a 200-person randomized prospective trial funded by the US DOD (NCT 03535688) in which patients with chronic pain were randomly assigned to DCS 400mg/day vs. placebo. Top line results will follow. Should these results support efficacy of DCS in the treatment of chronic low back pain, they are expected to provide a Breakthrough Therapy path towards treatment of chronic pain with DCS and DCS-containing medicines.
Spin out of HOPE Therapeutics and progress towards an NDA for NRX-100 (ketamine) in the treatment of suicidal depression.
When NRx met with the FDA in January 2023, the agency strongly encouraged the Company to develop NRX-100 (IV Ketamine) as a labeled drug, rather than rely on prior stabilization of suicidality and depression achieved via the common clinical practice of infusing generic ketamine compounded in licensed pharmacies. Shortly thereafter, the FDA issued the first of two advisory letters warning physicians against using compounded forms of ketamine and began a program of rigorous inspections of such pharmacies. Although there was once an expectation that intranasal administration of ketamine would be effective in treating suicidality, the attempts to demonstrate the clinical efficacy of nasal racemic ketamine for acute suicidality have not succeeded.
Accordingly, in Q3 the Company finalized a scientific collaboration with Prof. Marion Leboyer of Paris, France and Prof. Mocrane Abbar of Lyon, France in order to incorporate the results of a 156-person inpatient trial of intravenous ketamine vs. placebo for the stabilization of patients admitted for acute suicidality (the KETIS trial). The findings of the trial demonstrate a statistically significant reduction in both suicidality (the primary endpoint) and depression (the secondary endpoint) among patients treated with intravenous ketamine compared to those treated with placebo. (Link)
In the fourth quarter, the company similarly licensed data from Columbia University. In this trial, Dr. Michael Grunebaum and colleagues demonstrated a rapid and statistically significant reduction in Suicidal Ideation (SSI) at day 1 (p=0.0003) and in depression (P=0.0234), as measured by the Profile of Mood States (POMS) among patients randomized to IV Ketamine compared to those randomized to midazolam. This trial was published in the American Journal of Psychiatry https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5880701/.
The patient-level deidentified data from both studies have now been received by the Company and are being assembled in the electronic format required by the FDA. The Company believes that these randomized, blinded prospective trials encompassing nearly 240 participants, when submitted for review at a patient level could be sufficient to demonstrate preliminary safety and efficacy of intravenous ketamine in acutely suicidal patients. Data are expected to be transmitted to FDA by the end of 2Q23.
Submission of an NDA for the use of IV Ketamine is dependent upon submission of a manufacturing file documenting the manufacture of a presentation of ketamine suitable for single-patient use in the treatment of suicidal depression. In November 2023, the Company initiated manufacture of ketamine together with Nephron Pharmaceuticals, Inc. (West Columbia, SC) to develop a single patient presentation of ketamine. This formulation is expected to overcome some of the formulation deficiencies of existing forms of ketamine (developed for anesthesia) and is expected to have diversion-resistant and tamper-resistant features.
On March 30, 2024, the Company booked first commercial delivery of ketamine manufactured to 503b pharmacy standards from Nephron. HOPE will be distributing this presentation to qualified ketamine clinics in coordination with Nephron under Nephron's 503b pharmacy license in light of the current FDA-declared drug shortage of ketamine, starting this month (April 2024).
Treatment of Urinary Tract Infection (UTI) and Urosepsis:
Although treatment of UTI is quite different from use of NRX-101 to treat Central Nervous System disorders, D-cycloserine was originally developed as an antibiotic because of its role in disrupting the cell wall of certain pathogens. This is true of a number of drugs used in psychiatry today. D-cycloserine fell out of favor as an antibiotic in the 1970s because of the CNS effects caused by its NMDA-blocking properties and because of the widespread availability of effective first and second-generation antibiotics.
During Q3 2023, NRx tested NRX-101 and its components against resistant pathogens that appear on the Congressionally-mandated Qualified Infectious Disease Product (QIDP) list and proved in vitro effectiveness against antibiotic-resistant E. coli, Pseudomonas, and Acinetobacter. Accordingly, NRx was granted QIDP designation, Fast Track Designation, and Priority Review by the US FDA.
In recent years, increased antibiotic resistance to common pathogens that cause urinary tract infections and urosepsis (i.e., sepsis originating in the urinary tract) has resulted in a marked increase in cUTI, hospitalization, and death from urosepsis. The US Center for Disease Control and Prevention reports that more than 1.7 million Americans contract sepsis each year, of whom at least 350,000 die during their hospitalization or are discharged to hospice (CDC Sepsis Ref.). There are approximately 3 million patients per year who contract cUTI in the US annually (Lodise, et. al.). Additionally, should NRX-101 succeed in clinical trials, the Company will consider developing a follow-on product that is anticipated to achieve another 20 years of patent exclusivity.
Qualification for QIDP affords a sponsor five years of additional market exclusivity from FDA, regardless of patent status.
The Company does not anticipate funding this initiative with core NRx assets and is exploring structures for partnership opportunities. Should the Company or its partners succeed in serving 10% of the cUTI market, the Company believes that the revenue from NRX-101 has the potential to hundreds of million annually, based on 3 million cases per year (Lodise, et. al.) in the US and potential pricing of over $3,500/course of therapy.
Cash runway and financing
The Company continues to believe cash on hand is sufficient to fund operations through potential delivery of the upcoming milestones described herein.
Financial Results for the Quarter and Year Ended December 31, 2023
For the three months ended December 31, 2023, we at NRx Pharmaceuticals reduced our net loss from $10.2 million in the final quarter of 2022 to $4.3 million in 2023, representing nearly a 60% improvement year over year. For that same period, we reduced research and development expenses from $4.5 million in 2022 to $2.5 million in 2023, while substantially improving and finalizing our clinical trial enrollment. The $2.0 million decrease is related primarily to a decrease of $1.1 million in clinical trial expenses, $0.6 million in stock-based compensation, and $0.2 million in consulting and personnel wage costs. Also in that 3 month period we recorded a 67% reduction in general and administrative expenses, from $5.4 million in 2022 to $1.8 million in 2023. The decrease of $3.6 million is related primarily to a decrease of $1.3 million in insurance expenses, $1.3 million in stock-based compensation, $0.5 million in employee expenses, $0.2 million in legal and professional consulting fees, and $0.2 million in franchise tax expenses.
For the year ended December 31, 2023, NRx Pharmaceuticals reduced its net loss to $30.2 million compared to $39.8 million in the prior year. These efficiencies represent an improvement in net loss of nearly $10.0 million year over and a 20 cent, or 34%, improvement in net loss per share year over year. Over that annual period we recorded $13.4 million of research and development expenses compared to $17.0 million for the same period in 2022 representing a 21% decrease year over year. The decrease of $3.6 is related primarily to a decrease of $2.1 million in clinical trial and development expenses, $0.9 million related to fees paid to regulatory and process development consultants, $0.8 million in stock-based compensation while offset by a $0.2 million increase in patent costs as our patent portfolio has expanded.
Please note that the improvement in G&A expenses is even larger than the improvement in other areas. We decreased G&A by $13.1 million, from $27.3 million in 2022 to $14.2 million in 2023, nearly a 50% decrease year over year.
As of December 31, 2023, we had $4.6 million in cash and cash equivalents. Over the first three months of 2024 we improved our access to working capital by $8 million total, representing $2.9 million from equity sales and $5.1 million from the Alvogen milestone advance, while reducing our corporate indebtedness by 50% as shown in our financial statements.
We continue to implement operational efficiencies to extend runway and focus on our path to generating revenue. We believe that the near-term delivery of clinical trial data and the planned the launch of HOPE Therapeutics will be defining events in the second quarter of 2024.
Conference Call and Webcast Details
A live webcast of the conference call will be available on the Company's website at 8:30 a.m. ET today, at https://ir.nrxpharma.com/events. An archive of the webcast will be available on the Company's website for 30 days. Participants that are unable to join the webcast can access the conference call via telephone by dialing domestically 1-877-704-4453 or internationally 1-201-389-0920.
About NRx Pharmaceuticals
NRx Pharmaceuticals is a clinical-stage biopharmaceutical company developing therapeutics based on its NMDA platform for the treatment of central nervous system disorders, specifically suicidal bipolar depression, chronic pain and PTSD. The Company is developing NRX-101, an FDA-designated investigational Breakthrough Therapy for suicidal treatment-resistant bipolar depression and chronic pain. NRx has partnered with Alvogen and Lotus around the development and marketing of NRX-101 for the treatment of suicidal bipolar depression. NRX-101 additionally has potential to act as a non-opioid treatment for chronic pain, as well as a treatment for complicated UTI.
NRx has recently announced plans to submit a New Drug Application for HTX-100 (IV ketamine), through Hope Therapeutics, in the treatment of suicidal depression, based on results of well-controlled clinical trials conducted under the auspices of the US National Institutes of Health and newly obtained data from French health authorities, licensed under a data sharing agreement. NRx was awarded Fast Track Designation for development of ketamine (NRX-100) by the US FDA as part of a protocol to treat patients with acute suicidality.
About HOPE Therapeutics, Inc.
HOPE Therapeutics, Inc. (www.hopetherapeutics.com) is a Specialty Pharmaceutical Company, wholly-owned by NRX Pharmaceuticals focused on development and marketing of an FDA-approved form of intravenous ketamine for the treatment of acute suicidality and depression together with a digital therapeutic-enabled platform designed to augment and preserve the clinical benefit of NMDA-targeted drug therapy.
Notice Regarding Forward-Looking Statements
The information contained herein includes forward-looking statements within the meaning of Section 21E of the Securities Exchange Act of 1934, as amended, and Section 27A of the Securities Act of 1933, as amended. These statements include, among others, statements regarding the proposed public offering and the timing and the use of the proceeds from the offering. Forward-looking statements generally include statements that are predictive in nature and depend upon or refer to future events or conditions, and include words such as "may," "will," "should," "would," "expect," "plan," "believe," "intend," "look forward," and other similar expressions among others. These statements relate to future events or to the Company's future financial performance, and involve known and unknown risks, uncertainties and other factors that may cause the Company's actual results to be materially different from any future results, levels of activity, performance or achievements expressed or implied by these forward-looking statements. You should not place undue reliance on forward-looking statements since they involve known and unknown risks, uncertainties and other factors which are, in some cases, beyond the Company's control and which could, and likely will, materially affect actual results, levels of activity, performance or achievements. Any forward-looking statement reflects the Company's current views with respect to future events and is subject to these and other risks, uncertainties and assumptions relating to the Company's operations, results of operations, growth strategy and liquidity. More detailed information about the Company and the risk factors that may affect the realization of forward-looking statements is set forth in the Company's most recent Annual Report on Form 10-K and other filings with the Securities and Exchange Commission. Investors and security holders are urged to read these documents free of charge on the SEC's website at http://www.sec.gov. Except as may be required by applicable law, The Company assumes no obligation to publicly update or revise these forward-looking statements for any reason, or to update the reasons actual results could differ materially from those anticipated in these forward-looking statements, whether as a result of new information, future events or otherwise.
https://c212.net/c/img/favicon.png?sn=CL76439&sd=2024-04-01 View original content to download multimedia:https://www.prnewswire.com/news-releases/nrx-pharmaceuticals-nasdaqnrxp-reports-fourth-quarter-and-full-year-2023-financial-results-and-provides-business-update-302104468.html
SOURCE NRx Pharmaceuticals, Inc.
$NRXP News: NRx Pharmaceuticals (NASDAQ:NRXP) Initiates Strategy to Combat Short Sales
Initiative is intended to protect shareholder value through continued compliance with Nasdaq listing rules and elimination of naked short sales positions in the Company's securities
The Company is contemplating a reverse split, if needed to maintain NASDAQ listing compliance, to be accompanied by change in corporate name and CUSIP number
Company has retained former SEC enforcement leadership to notify leading brokerages of the need to close all naked short positions in the Company's securities and to prevent future accumulation of naked short positions in the Company's new security
RADNOR, Pa., March 12, 2024 /PRNewswire/ -- NRx Pharmaceuticals, Inc. (Nasdaq: NRXP) ("NRx Pharmaceuticals", the "Company" today initiated actions to combat short sellers in the Company's stock.
The Company announced a proposal to simultaneously change the CUSIP under which the Company's shares are traded, together with changing the name of the Company to NRx Therapeutics, Inc. The Company plans to accompany these actions with a required exchange of the underlying stock certificates. This certificate change is expected to be seamless for those investors holding NRXP shares in electronic form and similarly for investors who may have established short positions in NRXP by borrowing the underlying shares from a registered shareholder. Any party holding a short position in the Company's shares who has not complied with the legal requirement to borrow the underlying shares of stock (i.e. "a naked short" may be unable to exchange that position for a position in the new security.
The Company is working with attorneys who formerly served in leadership positions at the SEC Division of Enforcement to correspond with corporate counsel and compliance heads at leading brokerages to emphasize the current legal prohibitions against naked short sales. As identified in previous announcements, the Company was advised by ShareIntel in September 2023 that substantial naked short positions in the Company's securities had been identified at major brokerage firms.
"We believe it is in the best interests of our shareholders to have fully-compliant trading in the markets," said Janet Rehnquist, Esq., who chairs the Company's Compliance Committee. "As we focus on the development of potentially life-saving drugs to combat suicidal depression, PTSD, and Chronic Pain, it is critical that we take all steps possible to maximize the value of those who have invested in our Company and provided the resources to address these critical unmet medical needs."
About NRx Pharmaceuticals
NRx Pharmaceuticals is a clinical-stage biopharmaceutical company developing therapeutics based on its NMDA platform for the treatment of central nervous system disorders, specifically suicidal bipolar depression, chronic pain and PTSD. The Company is developing NRX-101, an FDA-designated investigational Breakthrough Therapy for suicidal treatment-resistant bipolar depression and chronic pain. NRx has partnered with Alvogen and Lotus around the development and marketing of NRX-101 for the treatment of suicidal bipolar depression. NRX-101 additionally has potential to act as a non-opioid treatment for chronic pain, as well as a treatment for complicated UTI.
NRx has recently announced plans to submit a New Drug Application for HTX-100 (IV ketamine), through Hope Therapeutics, in the treatment of suicidal depression, based on results of well-controlled clinical trials conducted under the auspices of the US National Institutes of Health and newly obtained data from French health authorities, licensed under a data sharing agreement. NRx was awarded Fast Track Designation for development of ketamine (NRX-100) by the US FDA as part of a protocol to treat patients with acute suicidality.
Notice Regarding Forward-Looking Statements
The information contained herein includes forward-looking statements within the meaning of Section 21E of the Securities Exchange Act of 1934, as amended, and Section 27A of the Securities Act of 1933, as amended. These statements include, among others, statements regarding the proposed public offering and the timing and the use of the proceeds from the offering. Forward-looking statements generally include statements that are predictive in nature and depend upon or refer to future events or conditions, and include words such as "may," "will," "should," "would," "expect," "plan," "believe," "intend," "look forward," and other similar expressions among others. These statements relate to future events or to the Company's future financial performance, and involve known and unknown risks, uncertainties and other factors that may cause the Company's actual results to be materially different from any future results, levels of activity, performance or achievements expressed or implied by these forward-looking statements. You should not place undue reliance on forward-looking statements since they involve known and unknown risks, uncertainties and other factors which are, in some cases, beyond the Company's control and which could, and likely will, materially affect actual results, levels of activity, performance or achievements. Any forward-looking statement reflects the Company's current views with respect to future events and is subject to these and other risks, uncertainties and assumptions relating to the Company's operations, results of operations, growth strategy and liquidity. More detailed information about the Company and the risk factors that may affect the realization of forward-looking statements is set forth in the Company's most recent Annual Report on Form 10-K and other filings with the Securities and Exchange Commission. Investors and security holders are urged to read these documents free of charge on the SEC's website at http://www.sec.gov. Except as may be required by applicable law, The Company assumes no obligation to publicly update or revise these forward-looking statements for any reason, or to update the reasons actual results could differ materially from those anticipated in these forward-looking statements, whether as a result of new information, future events or otherwise.
View original content to download multimedia:https://www.prnewswire.com/news-releases/nrx-pharmaceuticals-nasdaqnrxp-initiates-strategy-to-combat-short-sales-302086668.html
SOURCE NRx Pharmaceuticals, Inc.
$NRXP News: NRx Pharmaceuticals (NASDAQ: NRXP) Announces Last Patient, Last Visit in its Phase 2b/3 Trial of NRX-101 in Suicidal Treatment Resistant Bipolar Depression
Marks a major step in the development of what could be the first drug approved for Suicidal Bipolar Depression
The study database is being cleaned and locked; statistical analysis and top-line data to follow shortly thereafter
Study maintained 95% concordance rate between study sites and central raters on primary endpoint. No unexpected Serious Adverse Events were reported.
Positive data and FDA comment would trigger the next $4 million milestone payment from partners Alvogen and Lotus and their assumption of development costs; agreement provides for up to $329 million in milestone payments and a royalty on Net Sales in the mid-teens
RADNOR, Pa., March 4, 2024 /PRNewswire/ -- NRx Pharmaceuticals, Inc. (Nasdaq: NRXP) ("NRx Pharmaceuticals", the "Company"), a clinical-stage biopharmaceutical company, today announced that the 74th and last evaluable patient has completed their day 42 visit in its Phase 2b/3 study of NRX-101, the Company's patented combination of the NMDA antagonist D-cycloserine and lurasidone, in Suicidal Treatment Resistant Bipolar Depression. The database is being cleaned, finalized, and locked; statistical analysis will then be performed, with top-line data to follow shortly thereafter. As previously disclosed, positive data from this trial triggers a milestone payment from Alvogen. Alvogen will then be responsible for further development and commercialization costs for this program.
NRX-101 has been awarded Breakthrough Therapy Designation, Fast Track Designation, a Biomarker Letter of Support, and a Special Protocol Agreement by the FDA for treatment of suicidal bipolar depression. It is the only oral medication to have demonstrated reduced suicidal ideation in patients with bipolar depression, a lethal disease that claims the lives of one in five who live with it.
"This is the first clinical trial, to the company's knowledge, conducted among patients with suicidal bipolar depression in the outpatient setting. Our previous trial measured the ability of NRX-101 to maintain the anti-depressant and anti-suicidal effect of ketamine administered in the hospital setting. These patients, whose clinical need is urgent and extraordinary have routinely been excluded from the clinical trials of all previously-known anti-depressant drugs. said Dr. Jonathan Javitt, Founder, Chairman and Chief Scientist of NRx Pharmaceuticals. Although there were patients whose depression worsened and required hospitalization (we don't yet know whether they were on NRX-101 or comparator), patient safety was maintained, and no trial participant suffered a serious unexpected adverse outcome. Our thanks go out to our investigators, clinics, partners and, most importantly, our amazing patients and their families for seeing this study through to this important milestone," "
The Phase 2b/3 trial (www.clinicaltrials.gov NCT 03395392) is a randomized, prospective, multicenter, double-blind study comparing NRX-101 to lurasidone over six weeks. The Principal Investigator is Prof. Andrew Nierenberg of Harvard Massachusetts General Hospital. The primary efficacy endpoint is reduction in depression as measured on the MADRS scale and the secondary endpoint is reduction of suicidal ideation as measured by the Clinical Global Impression Suicidality Scale (CGI-SS). As previously disclosed, treatment compliance and concordance of local raters to central raters scores was in excess of 94%, well above the industry standard that is normally seen in CNS trials.
Top-line results are expected around the end of this quarter.
About NRx Pharmaceuticals
NRx Pharmaceuticals is a clinical-stage biopharmaceutical company developing therapeutics based on its NMDA platform for the treatment of central nervous system disorders, specifically suicidal bipolar depression, chronic pain and PTSD. The Company is developing NRX-101, an FDA-designated investigational Breakthrough Therapy for suicidal treatment-resistant bipolar depression and chronic pain. NRx has partnered with Alvogen and Lotus around the development and marketing of NRX-101 for the treatment of suicidal bipolar depression. NRX-101 additionally has potential to act as a non-opioid treatment for chronic pain, as well as a treatment for complicated UTI.
NRx has recently announced plans to submit a New Drug Application for HTX-100 (IV ketamine), through Hope Therapeutics, in the treatment of suicidal depression, based on results of well-controlled clinical trials conducted under the auspices of the US National Institutes of Health and newly obtained data from French health authorities, licensed under a data sharing agreement. NRx was awarded Fast Track Designation for development of ketamine (NRX-100) by the US FDA as part of a protocol to treat patients with acute suicidality.
About HOPE Therapeutics, Inc.
HOPE Therapeutics, Inc. (www.hopetherapeutics.com) is a Specialty Pharmaceutical Company, wholly-owned by NRX Pharmaceuticals focused on development and marketing of an FDA-approved form of intravenous ketamine for the treatment of acute suicidality and depression together with a digital therapeutic-enabled platform designed to augment and preserve the clinical benefit of NMDA-targeted drug therapy.
Notice Regarding Forward-Looking Statements
The information contained herein includes forward-looking statements within the meaning of Section 21E of the Securities Exchange Act of 1934, as amended, and Section 27A of the Securities Act of 1933, as amended. These statements include, among others, statements regarding the proposed public offering and the timing and the use of the proceeds from the offering. Forward-looking statements generally include statements that are predictive in nature and depend upon or refer to future events or conditions, and include words such as "may," "will," "should," "would," "expect," "plan," "believe," "intend," "look forward," and other similar expressions among others. These statements relate to future events or to the Company's future financial performance, and involve known and unknown risks, uncertainties and other factors that may cause the Company's actual results to be materially different from any future results, levels of activity, performance or achievements expressed or implied by these forward-looking statements. You should not place undue reliance on forward-looking statements since they involve known and unknown risks, uncertainties and other factors which are, in some cases, beyond the Company's control and which could, and likely will, materially affect actual results, levels of activity, performance or achievements. Any forward-looking statement reflects the Company's current views with respect to future events and is subject to these and other risks, uncertainties and assumptions relating to the Company's operations, results of operations, growth strategy and liquidity. More detailed information about the Company and the risk factors that may affect the realization of forward-looking statements is set forth in the Company's most recent Annual Report on Form 10-K and other filings with the Securities and Exchange Commission. Investors and security holders are urged to read these documents free of charge on the SEC's website at http://www.sec.gov. Except as may be required by applicable law, The Company assumes no obligation to publicly update or revise these forward-looking statements for any reason, or to update the reasons actual results could differ materially from those anticipated in these forward-looking statements, whether as a result of new information, future events or otherwise.
https://c212.net/c/img/favicon.png?sn=CL51263&sd=2024-03-04 View original content to download multimedia:https://www.prnewswire.com/news-releases/nrx-pharmaceuticals-nasdaq-nrxp-announces-last-patient-last-visit-in-its-phase-2b3-trial-of-nrx-101-in-suicidal-treatment-resistant-bipolar-depression-302078041.html
SOURCE NRx Pharmaceuticals, Inc.
$AVRW: Leading LA Plastic Surgeon Recommends #Seratopical Skin Care for Superior Anti-Aging Performance: (Stock Symbol: AVRW)
Link:
https://www.einpresswire.com/article/688477296/leading-la-plastic-surgeon-recommends-seratopical-skin-care-for-superior-anti-aging-performance-stock-symbol-avrw
From the article:
Expanding Marketing Plans in 2024 for Increasingly Successful Seratopical Skin Care, Personally Used and Endorsed by Nicole Kidman and Recommended by Leading LA Plastic Surgeon for Superior Anti-Aging Performance; New TikTok Shop to be Launched: Avenir Wellness Solutions, Inc. (Stock Symbol: AVRW)
Avenir Wellness Solutions™ ($AVRW) Skin Care Products are Sold at Major Retailers Including Walmart ($WMT), Target ($TGT), CVS Health ($CVS), and Amazon ($AMZN)
For more information on Avenir Wellness Solutions ($AVRW) visit: http://www.avenirwellness.com
Proprietary Nutraceutical & Topical Delivery Systems for Wellness and Anti-Aging Beauty Product Lines.
Company Currently Holds 15 Patents.
Seratopical Revolution Skin Care Products with Continue to be Promoted by Global Brand Ambassador and Strategic Partner Nicole Kidman.
LA-Based Facial Plastic Surgeon Dr. Michael Persky Directly Supporting Latest Seratopical DNA Complex Product with New Video Testimonials.
Media Exposure in Top-Tier Publications Including CNN Underscored, Page Six, PEOPLE Magazine and More.
Q3 Net Revenue Increased Sequentially from Q2 2023.
Sales Expected to Increase Significantly During Q4 and Into 2024.
Upcoming Launch of New TikTok Shop to Further Boost Sales
$NHMD---
$nhmd pic.twitter.com/JjRXxXd5ab
— rareone (@Rare_Oneeee) November 10, 2023
$TMGI Insider Activity
https://www.nasdaq.com/market-activity/stocks/tmgi/insider-activity

$SONX .10 GentleWave Video - New Painless Dental Technology:
What’s It Like to Experience the GentleWave® Procedure?
https://www.youtube.com/watch?v=cHjXGf5LmYo
$IVDN On the Rise -- Sales are just starting to pick up steam now. Review the latest news below which details the multiple new customer accounts for the company's superior, energy saving Insultex insulation, who are discovering this advanced product and placing orders for the first time.
LIG Assets, Inc. Announces Agreement With Insultex House Wrap
October 30, 2023
https://www.globenewswire.com/news-release/2023/10/30/2769422/0/en/LIG-Assets-Inc-Announces-Agreement-With-Insultex-House-Wrap.html
Innovative Designs Inc. Receiving Orders From Numerous New Client Accounts for Top Performing Insultex House Wrap With Unmatched R-6 Insulation Value
October 26, 2023
https://www.globenewswire.com/news-release/2023/10/26/2767843/0/en/Innovative-Designs-Inc-Receiving-Orders-From-Numerous-New-Client-Accounts-for-Top-Performing-Insultex-House-Wrap-With-Unmatched-R-6-Insulation-Value.html
Innovative Designs Adds New Accounts
October 2, 2023
https://www.globenewswire.com/news-release/2023/10/02/2752606/0/en/Innovative-Designs-Adds-New-Accounts.html
$NCL great short in the $9's I snagged some.
$SITS News: Southern ITS International, Inc. Subsidiary, Pure Oil & Gas, Inc., Reports on Oil Production Numbers and Announces a New 4-Well Drilling Project in Texas
Palm Desert, California, Nov. 14, 2023 (GLOBE NEWSWIRE) -- Southern ITS International, Inc. (Pink Open Market: SITS) proudly announces a significant achievement for its subsidiary, Pure Oil & Gas, Inc., as it began sales of oil produced by one of its wells in Texas. Production in October yielded three loads of oil—510 barrels—which has been collected and sold to BML Inc.
This success sets the stage for Pure Oil & Gas, Inc.’s next venture – a four-well drilling project in Texas.
The first new well is scheduled to begin drilling in the first week of December 2023, with production anticipated to commence within four weeks of the start of drilling operations. Funding for this new project has been secured.
Jeremy Larsen, President of Pure Oil & Gas, Inc. stated, “We are thrilled to announce our successful entry into the Texas oil reserves and look forward to the promising opportunities that lie ahead with our upcoming four-well drilling project. Our strategic location in Texas provides us with a valuable resource base, and we are confident that our collaboration with ICS Energy will contribute to the efficiency and success of our drilling operations.”
Pure Oil & Gas, Inc. has partnered with ICS Energy, LLC, an experienced driller-operator with extensive expertise in the regional geological landscape. This collaboration aims to optimize operations and bolster Pure Oil & Gas's position in the energy sector.
About Pure Oil & Gas, Inc.
Pure Oil & Gas, Inc. focuses on leveraged oil and gas acquisitions as a key strategy for growth and establishing a strong position in the industry. Supported by its parent company, Southern ITS International, Inc., Pure Oil & Gas leverages financial flexibility provided by its parent to acquire leases, producing wells, oil services companies, and new oil industry technology.
About Southern ITS International, Inc.
Southern ITS International, Inc., through its subsidiary companies, currently has operations focused on a wide variety of fields, including oil and gas exploration and development, clothing, and e-commerce fulfillment. We intend to expand upon our current base of operational companies and to increase our ownership and/or control of a portfolio of highly successful businesses. As a holding company, Southern ITS International continues to be in the market to acquire a stake in various companies, both public and private, which will complement its current operations. Our mission includes establishing a robust Direct-to-Consumer (DTC) and Business-To-Business (B2B) network, harnessing the potential of e-commerce across an array of diverse sectors, including manufacturing, distribution, and product sales. We have put in place an experienced management team that is continuing to build a diverse portfolio, buying entire companies, or interests therein, involved in technology, oil and gas, manufacturing, real estate, and other sectors, which will then become operating subsidiaries of Southern ITS International.
Precautionary and Forward-Looking Statement
This release contains "forward-looking statements" within the meaning of Section 27A of the Securities Act of 1933, as amended, and Section 21E of the Securities Exchange Act of 1934, as amended, and such forward-looking statements are made pursuant to the safe harbor provisions of the Private Securities Litigation Reform Act of 1995. "Forward looking statements" describe future expectations, plans, results, or strategies and are generally preceded by words such as "may" "future," "plan" or "planned," "will" or "should," "expected," "anticipates," "draft," "eventually" or "projected." You are cautioned that such statements are subject to a multitude or risks and uncertainties that could cause future circumstances, events, or results to differ materially from those projected in the forward-looking statements, including the risks that actual results may differ materially from those projected in the forward-looking statements as a result of several factors, and other risks identified in the Company's disclosures or filings with the SEC or OTC Markets, Inc. You are further cautioned that penny stocks and stocks of smaller companies like Southern ITS International, Inc. are inherently volatile and risky, and that no investor should buy this stock unless they can afford the loss of their entire investment.
$BSEG - Our mission at Big Screen Entertainment Group is to create transformative stories that captivate, entertain, inspire and touch the hearts of audiences worldwide while forging enduring robust financial returns for our investors through innovation and ingenuity.
Headquartered in Los Angeles, California with partners in Michigan, Florida and China, our films have premiered at the world’s most prestigious film festivals and played at the largest theatrical chains in the world. Our relationships with top Hollywood studios and talent, as well as our corporate sales and distribution alliances, have positioned us for continued growth.
http://www.bigscreenentgroup.com/
$BLEG Branded Legacy, Inc. Secures $1M Line of Credit, Affirms No Reverse Split, and Previews Upcoming Investor Engagements
PR Newswire
MELBOURNE, Fla., Nov. 7, 2023
Branded Legacy strengthens financial stability with a new line of credit and commits to enhancing shareholder value by avoiding a reverse stock split.
MELBOURNE, Fla. , Nov. 7, 2023 /PRNewswire/ -- Branded Legacy, Inc (OTC.PK: BLEG), a biotechnology holdings company, has secured a $1M line of credit under a fixed interest rate, underscoring its commitment to strategic growth without shareholder dilution. This financial maneuver is aligned with the company's disciplined approach to expansion and reflects confidence in its operational and fiscal policies.
Dave Oswald, CEO of Branded Legacy, affirms, "This line of credit is pivotal, providing us with the flexibility to advance our operations and affirming our commitment to maintaining shareholder value. It's a strategic choice that supports our growth ambitions while preserving our corporate integrity."
In its ongoing efforts to enhance shareholder value, Branded Legacy has retired 1.6 billion shares and reduced its preferred classes to three. Independent Director Steven Augustine emphasizes the leadership's pledge to shareholder alignment, stating, "Our team is resolutely invested in Branded Legacy's future, evidenced by our unanimous decision against share liquidation, further securing our collective long-term interests
In line with these developments, Branded Legacy is excited to announce plans for an open house at its expansive 11,000 square-foot facility, providing an opportunity to experience the company's operations up close. Details will be released in the coming weeks.
Additionally, the investor packet, previously mentioned in the last teleconference, is nearing completion after thorough review by the finance team and consideration of recent acquisitions.
Dates and schedules are being aligned for the next investor tele-conference, slated for Q4, which will provide an in-depth update on the company's direction and initiatives.
Reflecting on our last communication, we not only shared the retirement of 1.6 billion common shares but also our ongoing efforts to enhance shareholder value. Today's announcement of securing a $1M credit line, coupled with our commitment to a stable share structure and executive alignment with shareholder interests, underscores our dedication to a prosperous future for Branded Legacy.
About Branded Legacy
$BLEG Branded Legacy Inc. is a forward-thinking biotechnology company dedicated to driving innovation in plant-derived medicines and biotechnological breakthroughs. The company's strategic partnerships and milestone achievements highlight its dedication to transforming patient care and contributing to the advancement of healthcare solutions.
$SUIC ON ALERT - SUIC's IHart Bags Big Orders - Top 3 E-Commerce Platforms With Over 70 Million Monthly Visits, Leading Convenience Stores 7-Eleven & Family Mart With 8000 Outlets, a Government Agency, a TV Network, Hotels, Supplier Channels and More https://finance.yahoo.com/news/suics-ihart-bags-big-orders-100000254.html
$RDRSF RDARS Inc. president and CEO Charles Zwebner tells Proactive's Stephen Gunnion the company is preparing for a large scaling up of sales of its innovative autonomous drone security system in 2024 after the company commenced commercialization in June 2023.
$BLFR News: BlueFire Equipment Corp (BLFR) Partners with Eventus Advisory Group, LLC. to Work Towards NASDAQ Uplisting
Woodlands, TX., Oct. 31, 2023 (GLOBE NEWSWIRE) -- BlueFire Equipment Corp. (OTC: BLFR) ("BLFR" or the "Company"), a specialist in emerging industry acquisitions, is pleased to announce its strategic partnership with Eventus Advisory Group, LLC ("Eventus"), a management consulting firm specializing in finance, accounting, SEC compliance and strategic CFO advisory support services. This collaboration marks a significant step towards applying for an uplisting to NASDAQ as part of the Company's short-term plan shared on October 19, 2023.
On October 26, 2023, the Company engaged Eventus to assist the Company to efficiently uplist to NASDAQ by the end of 2024 as well as enhancing financial operations and ensuring compliance with regulatory standards. The core objective of this engagement is to facilitate the Company in achieving timely and efficient financial reporting, NASDAQ and SEC compliance, and technical accounting guidance. Eventus will play a crucial role in supporting the Company's strategic initiatives, including facilitating support for acquisitions (M&A Transactions), filing a Form 10-12G or S-1 with the Securities and Exchange Commission (SEC), and ensuring compliance with the Securities Exchange Act of 1934 ('34 Act) once either, the FORM 10-12G or the S-1, is declared effective.
The Company looks forward to leveraging Eventus' specialized services to scale its financial operations effectively and meet its continuous reporting obligations under the '34 Act. This partnership positions the Company for sustained growth and long-term financial success.
Nickolas S. Tabraue, BLFR's Interim CEO and Director of the Board states, "Working with Eventus is going to be critical for us to achieve NASDAQ uplisting within 2024.Management is confident that there will not be a need for a reverse split to meet NASDAQ's $5.00 minimum bid requirement based on the Company's recent merger in September 2023; new partnership with a Joint Operating Agreement being negotiated and finalized to increase production within current operating assets; acquiring additional operating assets to increase productivity, revenue, and cashflow. We are currently working on a major producing asset anticipated to close by the end of 2023 and a second within Q1/Q2 2024." Mr. Tabraue continues with, "I look forward to sharing updates as we actualize the aforementioned ambitions."
About Eventus Advisory Group, LLC.
Eventus is renowned for its expertise in delivering comprehensive finance, accounting, and compliance solutions to both public and private enterprises. Specializing in services such as Accounting, Controllership, CFO support, and SEC reporting, Eventus empowers businesses to optimize their internal finance teams and processes, addressing unique financial challenges and fostering growth.
To learn more, please visit: https://eventusag.com
AboutBlueFire Equipment Corp. (BLFR)
BLFR, after its first acquisitions in the oil and gas industry Screaming Eagle Partners, LLC. operating in the state of Texas, has gained traction and momentum to focus on increasing its acquisitions within the energy sector.
SAFE HARBOR ACT: Forward-looking statements are included within the meaning of Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, as amended. All statements regarding the Company's expected future financial position, results of operations, cash flows, financing plans, business strategy, products and services, competitive positions, growth opportunities, plans and objectives of management for future operations or listing on an exchange -- including words such as "anticipate," "if," "believe," "plan," "estimate," "expect," "intend," "may," "could," "should," "will" and other similar expressions -- are forward-looking statements and involve risks, uncertainties and contingencies, many of which are beyond the Company's control and may cause actual results, performance or achievements to differ materially from anticipated results, performance or achievements. The Company is under no obligation to (and expressly disclaims any such obligation to) update or alter its forward-looking statements, whether as a result of new information, future events or otherwise. There are no assurances that the Company will complete additional acquisitions or will be successful in being approved for a NASDAQ listing. No information in this press release should be construed in any manner whatsoever as an indication of the future performance of the Company's revenues, financial condition or stock price.
Company Contact:
Nickolas S. Tabraue
Interim CEO, Chief Compliance and Investor Relations Officer, and Director of the Board
info@BLFR.info
Phone (786) 375-7281
Overdue MAGA & MAHA = Goodness Of GOD (LIVE) - Jenn Johnson | VICTORY
Bethel Music
4.83M subscribers
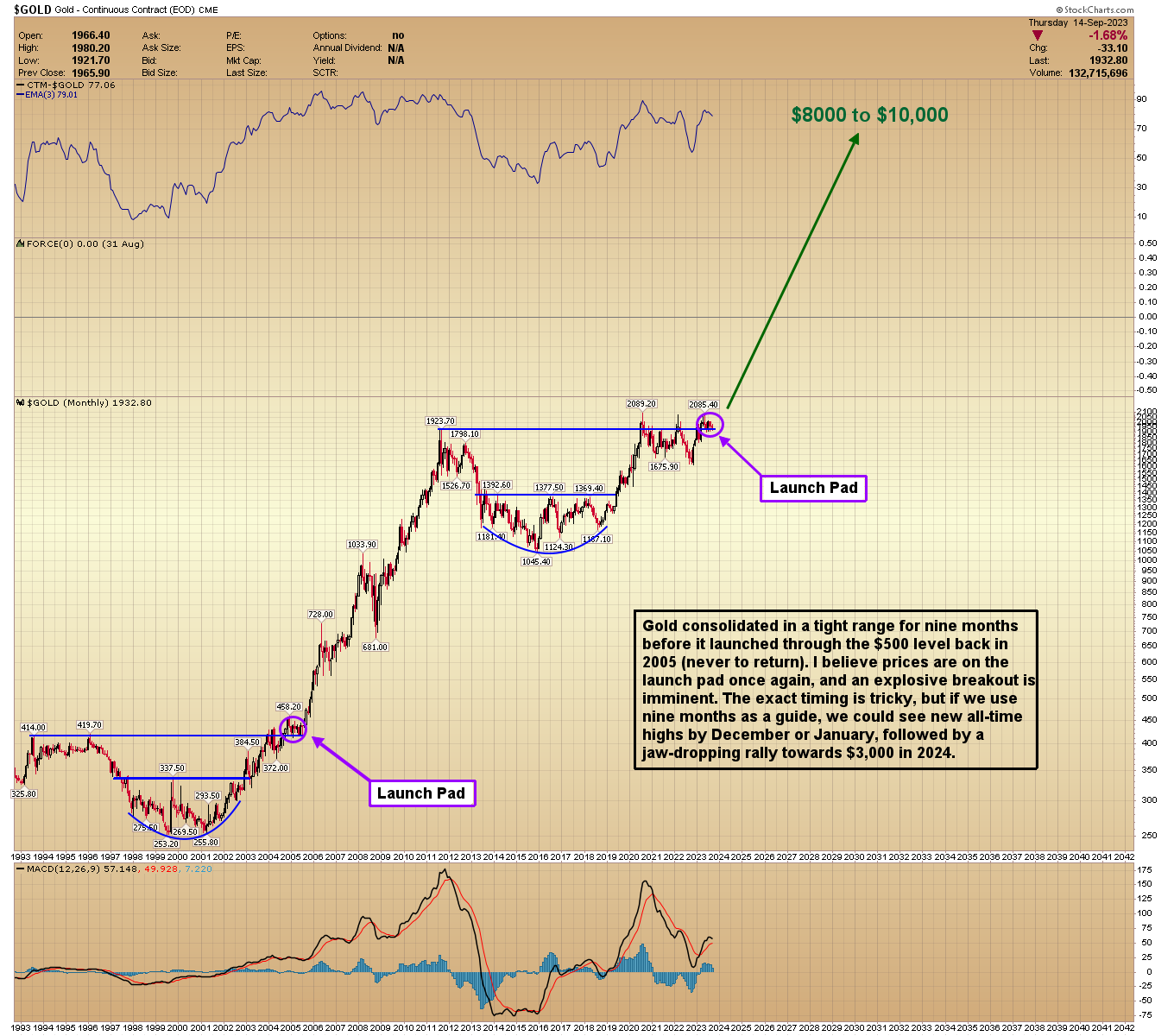



$IVDN (16 Million Float): Large-Scale Firm Added as One of Multiple New Sales Accounts for Superior Insulation with R-6 Value: Stock Symbol: IVDN
https://www.einpresswire.com/article/659804232/large-scale-firm-added-as-one-of-multiple-new-sales-accounts-for-superior-insulation-with-r-6-value-stock-symbol-ivdn via @ein_news
From the article:
On October 2nd IVDN announced that the company continues to add new sales accounts across the United States. Three of the most recent additions are as follows:
Guerdon, LLC is the leading manufacturer of large-scale, commercial modular construction projects in the Western US and Canada. With their factory and corporate office located in Boise, Idaho they serve 10 western states including California, Colorado, Idaho, Montana, Nevada, North Dakota, Oregon, Utah, Washington, and Wyoming as well as Canada. They are currently working on a project in Alaska.
The Guerdon team and facilities are equipped to handle a wide range of projects including Multi-family Modular Buildings, Modular Hotels & Hospitality facilities, Assisted Living & Senior Housing Centers, Student Housing Modular Buildings, Modular Workforce Housing or Man Camps, and more. Learn more about Guerdon at: https://www.guerdonmodularbuildings.com/
Skehan Home Center, located in Ossipee, New Hampshire, is a family-owned business that has been in operation since 1985. Their Outside Sales Staff is a group of experienced professionals that make job site and office visits. Learn more about Skehan at: https://skehanhomecenter.com/.
Outside In Construction is located in Alton, New Hampshire. Outside In Construction is a family business that has been providing service for Laconia, NH, Wolfeboro, NH & the Lakes Region of New Hampshire for 23 years and is a Design and build specialized building and remodeling firm. Learn more about Outside in Constriction at: https://www.oiconstruction.com/.
$SMME News: SmartMetric the Maker of Fingerprint Biometric Secured and Activated Credit Cards Is Nearing Shipping of Its Latest Premium Card for the Credit Card Industry
BUSINESS WIRE | 08/10/2023
NEW YORK--(BUSINESS WIRE)-- SmartMetric, Inc. (OTC: SMME), having completed the upgrading of the internal hybrid solid state internal battery inside the SmartMetric card the company is pleased to announce it has achieved a 1/3 battery size reduction while retaining the power capacity features against its previous battery.
“Having a smaller rechargeable battery gives us a lower profile of the battery that flows through to a less overall thickness of our electronics that sit inside of our biometric credit cards,” said SmartMetric’s President and CEO, Chaya Hendrick.
“Over the past decade we have been constantly refining the size and all importantly the thickness of the components in our card so as to provide the lowest profile for the card's internal electronics. Each improvement we make resulting in smaller dimensions of our components directly results in greater thickness of the lamination layers of the card which in turn provides for a better overall look and finish of the end card product,” said Chaya Hendrick.
When SmartMetric first started on building its prototype biometric credit card over a decade ago, the overall thickness of the electronics was four times the thickness of a standard credit card. The company says that its overall thickness and profile of its electronics including the board and its internal battery is now less than one third the thickness of a standard credit card.
“It has always been a battle of constant innovation around achieving lower and lower profile for our electronics to achieve the smallest and slimmest size of our components that sit inside our biometric credit card. Something we are proud in achieving what is undoubtedly the smallest size dimension of its internal electronics of any biometric card created,” said Chaya Hendrick.
The SmartMetric rechargeable battery inside the biometric credit card allows the card to be used across all standard credit card readers at stores, gas pumps and even ATMs. Unlike non powered biometric cards that rely on drawing power from card readers in order to perform a biometric identification function thereby limiting the cards used and making it unworkable in most restaurants, gas pumps and ATMs.
Situations where the card is taken from the table in a restaurant or swallowed whole by the card reading device such as at a gas pump or ATM makes a card that does not have an internal rechargeable battery unworkable. That is why we have spent so much time and effort in including a rechargeable power source inside our biometric card that also includes a rapid rechargeability each time the card is inserted inside a credit card reader after it has already been turned on by the card holder’s fingerprint, according to SmartMetric.
The latest battery for the SmartMetric biometric card is going through final licensing and approvals for shipment via air from the SmartMetric assembly plant.
The SmartMetric biometric fingerprint recognition technology built inside of the credit and debit card uses embedded biometric technology to positively recognize the card holder and then only after a positive fingerprint recognition, turn on the card's EMV contact and contactless payments chip.
Market research has shown that 70% of current credit card users are willing to pay $70.00 for a biometric secured credit card. The single largest motivation is wanting to feel more secure. The same research showed that nearly 70% of existing 100’s of millions of credit card users would prefer to use a biometric credit card.
According to an article published by Finder.com1, the number of credit card accounts open in the United States is 564,500,000. This is an all-time high for the United States.
The average American owns three credit cards. 83% of Americans own at least one credit card. 14% of Americans own at least 10 credit cards.
To view the SmartMetric Biometric Card please follow this link - Video of the SmartMetric Biometric Card. To view the company website: www.smartmetric.com
1 2023 Credit card debt and spending statistics in the US | finder.com
Safe Harbor Statement: Forward-Looking Statements in this press release, which are not historical facts, are forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995. Also such forward-looking statements are within the meaning of that term in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934. Our actual results, performance or achievements may differ materially from those expressed or implied by these forward-looking statements. In some cases, you can identify forward-looking statements by the use of words such as "may," "could," "expect," "intend," "plan," "seek," "anticipate," "believe," "estimate," "predict," "potential," "continue," "likely," "will," "would" and variations of these terms and similar expressions, or the negative of these terms or similar expressions. Such forward-looking statements are necessarily based upon estimates and assumptions that, while considered reasonable by us and our management, are inherently uncertain. Factors that may cause actual results to differ materially from current expectations include, among others, if we are unable to access the capital necessary to fund current operations or implement our plans for growth; changes in the competitive environment in our industry and the markets where we operate; our ability to access the capital markets; and other risks discussed in the Company's filings with the U.S. Securities and Exchange Commission, including our Annual Report on Form 10-K, which filings are available from the SEC. We caution you not to place undue reliance on any forward-looking statements, which are made as of the date of this press release. We undertake no obligation to update publicly any of these forward-looking statements to reflect actual results, new information or future events, changes in assumptions or changes in other factors affecting forward-looking statements, except to the extent required by applicable laws. If we update one or more forward-looking statements, no inference should be drawn that we will make additional updates with respect to those or other forward-looking statements. Investors and security holders are urged to carefully review and consider each of SmartMetric Inc. public filings with the SEC, including but not limited to, if applicable, Annual Reports on Form 10-K, proxy statements, Current Reports on Form 8-K and Quarterly Reports on Form 10-Q.
https://cts.businesswire.com/ct/CT?id=bwnews&sty=20230810018537r1&sid=acqr8&distro=nx&lang=en
View source version on businesswire.com: https://www.businesswire.com/news/home/20230810018537/en/
SmartMetric, Inc.
Chaya Hendrick
Tel: (702) 990-3687 Mobile: 305.607.3910 Pacific Time
ceo@smartmetric.com
http://www.smartmetric.com
Source: SmartMetric, Inc.
KSCP + 9.2% today..
$MGIH 1.2mil float, 90% insider ownership can't sell shares until October. The company has been in business for nearly 50 years and won numerous awards in their industry. Check out their awesome finances - go read their filings!
KSCP, way undervalued and under radars. take a look see. do some DD..
https://finance.yahoo.com/quote/KSCP?p=KSCP&.tsrc=fin-srch
$VNUE VNUE, INC'S LIVESTREAM PLATFORM STAGEIT PRESENTS CELTIC THUNDER HOME ENTERTAINMENT SERIES, FREEDOM & NATIONS-LIBERTY https://finance.yahoo.com/news/vnue-incs-livestream-platform-stageit-174400272.html
$SNPW announces that its wholly owned subsidiary, Sun Pacific Power Corp (“SPPC”) has executed an exclusive contract to purchase and distribute solar panels in the USA on behalf of its partner GEP New Energy Co. Ltd. https://finance.yahoo.com/news/snpw-wholly-owned-subsidiary-sun-131500485.html
Exclusive Interview with Nancy Duitch, CEO of Avenir Wellness Solutions, Inc. (Stock Symbol: $AVRW) https://www.einpresswire.com/article/648860349/exclusive-interview-with-nancy-duitch-ceo-of-avenir-wellness-solutions-inc-stock-symbol-avrw
$ASRE Astra Energy Inc. Executes MOU with Tanzanian Government for the Development of a 350-Megawatt Combined Cycle Power Plant https://finance.yahoo.com/news/astra-energy-inc-executes-mou-132200112.html
$AMIH Technology-Driven Solutions for $194B Health & Wellness Market
$VNUE NEWS: Music Technology Firm Delivering Innovative Marketing Options: Recording Matchbox Twenty Tour: VNUE: Stock Symbol: VNUE
https://apnews.com/press-release/ein-presswire-newsmatics/ticketmaster-entertainment-inc-music-peter-frampton-concerts-rob-thomas-430fc0982f94935ce871d8c75876f27f
From the article:
VNUE is currently on the road with Matchbox Twenty, the multi-platinum, hit-making pop rock band, to record each date on the band’s 2023 “Slow Dream” tour. VNUE is also releasing “instant” collectible double CD sets and collectible download cards through its exclusive partner, DiscLive (disclive.net), as well as digital downloads.
Additionally, VNUE partnered with Ticketmaster so that fans may have the option of purchasing the “instant live” CD sets when they buy their tickets, as well as from the DiscLive.net website.
$NILIF Tesla Co-founder’s Startup Gets $2 Billion From the U.S. to Boost EV Battery Production…As This Junior Explorer Announces a Potentially Major Lithium Discovery in Nevada… https://tgd8hjs.com/3J67C1/4THFX9F/
$EVVL Acquisition News: Evil Empire Designs Inc (EVVL) Finalizes Agreement to Purchase Trendmark Industries
LAS VEGAS, NV / ACCESSWIRE / June 26, 2023 / Evil Empire Designs, Inc. (OTC PINK:EVVL) ("Evil Empire Designs" or the "Company") today announced that it has entered into a definitive Share Exchange Agreement with Trendmark Industries, Inc. ("Trendmark"), and the sole stockholder of Trendmark, under which Evil Empire Designs will acquire 100% of the outstanding shares of Trendmark. The purchase will give Evil Empire Designs its own manufacturing capabilities and includes proprietary molds, inventory and equipment, as well as certain intellectual property that compliments the Company's current product offering. The Company anticipates the new product line being available for sale on its website (http://www.evilempiredesigns.com) shortly after closing.
As part of the purchase, Evil Empire Designs has loaned Trendmark $50,000 and will issue the current Trendmark shareholder an aggregate of 10,000,000 shares of the Company's common stock. Trendmark management will also be required to operate Trendmark and participate in Evil Empire's sales, marketing and business planning for a term of three years.
"This acquisition not only allows us to bring the manufacturing of our designs in-house, saving us time and money, but also brings with it a host of complimentary products. Additionally, we won't be subject to supply chain interruptions because most of our products will be manufactured with parts made in the United States," commented Sheila Cunningham, CEO of Evil Empire Designs. "A huge bonus was being able to retain current management. They have years of experience in the motorcycle business, have been quite successful, and we anticipate they will facilitate opening new sales avenues for our designs. We believe this is an extremely accretive transaction for not only Evil Empire Designs but for our shareholders as we execute on our business strategy and seek to maximize value for our shareholders."
Closing of the Share Exchange Agreement is subject to customary closing conditions, and Evil Empire Designs anticipates closing of the transactions under the Share Exchange Agreement before June 30, 2023.
Evil Empire Designs also announced that it has decided to not pursue a prospective merger or acquisition of TOL Designs.
About Evil Empire Designs
At Evil Empire our mission is to design and produce the highest quality aftermarket parts that appeal to middle and upper class motorcycle enthusiasts to enhance the look of their American, V-Twin, Metric or Harley motorcycles, allowing them to express their individuality.
Evil Empire designs is committed to providing our customers with products and services that meet, conform to, and exceed their individual motorcycle needs, ensuring their design, values and investment expectations are being met.
Cautionary Note Regarding Forward-Looking Statements
This release by Evil Empire Designs Inc. ("Evil Empire") may contain "forward-looking statements" within the meaning of the Private Securities Litigation Reform Act of 1995. These statements can be identified by words such as "expects," "plan," "believes," "will," "achieve," "anticipate," "would," "should," "subject to," or words of similar meaning, and by the fact that they do not relate strictly to historical or current facts. Although Evil Empire management believes that such forward-looking statements are reasonable, it cannot guarantee that such expectations are, or will be, correct. These forward-looking statements involve several risks and uncertainties, which could cause the Company's future results to differ materially from those anticipated. Potential risks and uncertainties include, among others, general economic conditions and conditions affecting the industries in which the Company operates; the uncertainty of regulatory requirements and approvals; and the ability to obtain necessary financing on acceptable terms or at all. These risks, uncertainties and other factors include, among others: risks related to Evil Empire's plans for its intellectual property, including its strategies for monetizing, licensing, expanding, and defending its patent portfolio; risks associated with Evil Empire's product sales, including the market and demand for products sold by Evil Empire and its ability to successfully develop and launch new products that are attractive to the market; the success of product, joint development and licensing partnerships; the competitive landscape of Evil Empire's industry; and general economic, political and market conditions, including quarantines, factory slowdowns or shutdowns, and travel restrictions resulting from the COVID-19 pandemic. The military conflict between Russia and Ukraine may increase the likelihood of supply interruptions. All forward-looking statements reflect management's present assumptions, expectations and beliefs regarding future events and are subject to known and unknown risks, uncertainties and other factors that could cause actual results to differ materially from those expressed in or implied by any forward-looking statements. These and other risks and uncertainties are described in Evil Empire's annual and quarterly reports and the other filings it makes with the U.S. Securities and Exchange Commission from time to time, including any subsequently filed quarterly and current reports. In light of these risks, uncertainties and other factors, these forward-looking statements should not be relied on as predictions of future events. These forward-looking statements represent Evil Empire's assumptions, expectations and beliefs only as of the date they are made, and except as required by law, Evil Empire undertakes no obligation to revise or update any forward-looking statements for any reason.
Contact Information:
sheila@evilempiredesigns.com
SOURCE: Evil Empire Designs
View source version on accesswire.com:
https://www.accesswire.com/763622/Evil-Empire-Designs-Inc-EVVL-Finalizes-Agreement-to-Purchase-Trendmark-Industries
$GPHOF Graphite One Inc. is pioneering the only full-circle domestic supply chain for graphite in the United States, positioning the company as a leader in a critical industry with growing demand.
https://www.tgd8hjs.com/3J67C1/4TG32JS/
$IVDN: Increasing Orders and Insider Share Purchase; Patented Evacuated Cell Insulation; Innovative Designs: Stock Symbol: IVDN
https://www.einpresswire.com/article/637864225/increasing-orders-and-insider-share-purchase-patented-evacuated-cell-insulation-innovative-designs-stock-symbol-ivdn
From the article:
- $IVDN has a Very Small Stock Structure of Only 36 Million OS / 16 Million Float
- Sole Maker of Patented Insultex® Insulation Delivering Energy Saving Performance Far Superior to All Competition.
- Greater Energy Savings from Insultex® Insulation Delivers Essential Benefits of Economic Gains for the User and Carbon Reduction for the Environment.
- Significant New Orders for Insultex House Wrap® from Repeat Customers.
- Insider Buying from Board Member with Lockheed Martin & NASA Background.
- New US Patent Issued for Insultex® Manufacturing Process.
- International Distributor Agreement Signed with a Minimum Order Valued at $2 Million Per Year.
$LPTV Loop Media employs a dual growth strategy to drive penetration in the market.
$LPTV
Loop Media Inc
2.76
0.21 (8.24%)
Volume: 56,979
Day Range: 2.49 - 2.76
Last Trade Time: 12:11:56 PM EDT
@Mangoceuticals Tweeted about the upcoming launch of their mango flavored rapid dissolve ED product
“#Mangoceuticals, Inc. Announces Upcoming Launch of its Sildenafil Based Mango Flavored Rapid Dissolve ED Product” $MGRX & @mango_rx are excited to bring you todays breaking news! See PR here:https://t.co/C04dfDs18Q #Sildenafil #MangoRX @barstoolsports @gasdigital… pic.twitter.com/XXaz5blafN
— MANGOCEUTICALS INC (@Mangoceuticals) June 21, 2023
$KRTL Medflow, an AI-powered telemedicine solution provider, combines best-in-class facial recognition biometrics with next-generation zero-trust security architecture to accurately verify patient identities. Their comprehensive suite of telemedicine tools, designed with security at the forefront, includes features like end-to-end encryption, facial recognition with ID scan, and HIPAA compliance. The platform allows healthcare providers to review and legally sign medical and legal documentation live with the patient, issue prescriptions, and capture patient vitals such as heart rate, blood pressure, breathing, and stress indicators, all using the patient's computer or mobile phone camera.
https://www.marketwatch.com/press-release/krtl-partnered-to-bring-comprehensive-cloud-and-endpoint-security-and-ai-powered-telemedicine-solutions-2023-05-30?mod=mw_quote_news_seemore
$MGRX recent press release $MMMW new tech press release today.
$KRTL ~ KRTL International Corp., a wholly owned subsidiary of KRTL Holding Group Inc. (OTC: KRTL), is an organization focused on green technology.
$KRTL on Twitter - https://twitter.com/KRTLHolding
$KRTL Axiom utilized the data to complete a full-service package including data acquisition, processing, analysis, technical reporting, and claim management.
$KRTL Medflow, an AI-powered telemedicine solution provider, combines best-in-class facial recognition biometrics with next-generation zero-trust security architecture to accurately verify patient identities. Their comprehensive suite of telemedicine tools, designed with security at the forefront, includes features like end-to-end encryption, facial recognition with ID scan, and HIPAA compliance. The platform allows healthcare providers to review and legally sign medical and legal documentation live with the patient, issue prescriptions, and capture patient vitals such as heart rate, blood pressure, breathing, and stress indicators, all using the patient's computer or mobile phone camera.
https://www.marketwatch.com/press-release/krtl-partnered-to-bring-comprehensive-cloud-and-endpoint-security-and-ai-powered-telemedicine-solutions-2023-05-30?mod=mw_quote_news_seemore
$MGRX +9.86% at 1.56 to start the week and the chart looks like it's turning Bullish here!
https://finance.yahoo.com/quote/MGRX/press-releases?p=MGRX

$RGGI and $MMMW cutting edge technologies news.
|
Followers
|
285
|
Posters
|
|
|
Posts (Today)
|
1
|
Posts (Total)
|
45088
|
|
Created
|
02/23/10
|
Type
|
Free
|
| Moderator WANG | |||
| Assistants ProfitChaser J U ICE greenbayslacker | |||
 " />
" /> 
Stockcharts:
http://stockcharts.com/
Google News - Businesshttp://news.google.com/?ned=us&topic=b
Google News - Home page http://news.google.com/
Yahoo News http://www.yahoo.com/
Yahoo Finance http://finance.yahoo.com/
CNBC http://www.cnbc.com
Forbes http://www.forbes.com/home_usa
Barrons http://online.barrons.com/public/main
Wall Street Journal http://online.wsj.com/public/us?mod=topnav_2_0433
Bloomberg http://www.bloomberg.com/news/index.html?Intro=intro_news
TheStreet.Com http://www.thestreet.com/
MarketWatch.com http://www.marketwatch.com/
USAToday Business http://markets.usatoday.com/custom/usatoday-com/html-markets.asp
Businessveek http://www.businessweek.com/
Investor's Business Daily http://www.investors.com/
EquityFeed
(MicroCapFeed, NasdaqFeed).......http://www.equityfeed.com/
All OTC/Pink Market Action......... http://www.microcapmarkets.com/data_main_nav.jsp?market=OTCBB
StockFetcher............................... http://www.stockfetcher.com/
QuoteTracker.............................. http://www.quotetracker.com/
Commodity Prices....................... http://money.cnn.com/markets/commodities/
Education.................................... http://www.investopedia.com/university/technical/technical3.asp
Candlesticks................................ http://www.candlestickshop.com/
Bullish Candles........................... #MSG-2990648
Candlestick Patterns................... http://www.candlestickforum.com/PPF/Parameters/1_25_/candlestick.asp
Chartpatterns............................... http://www.chartpatterns.com/
Types of Market Orders................ http://www.sec.gov/answers/orderbd.htm
Free L2 quotes.............................http://datasvr.tradearca.com/arcadataserver/JArcaBook.php?Symbol=ms
/> SEC Form Types........................... http://www.gsionline.com/support/formtypes.html
Financial Statements - Primer...... http://www.gsionline.com/support/formtypes.html
Financial Statements - Detail........ http://www.moneychimp.com/articles/financials/fundamentals.htm
Ultimate DD................................... http://www.finitesite.com/irishbull/
LIst of DTC eligible securities
http://www.tradingdirect.com/Static/StandAlone/non_dtcc_list.pdf
If your broker clears through Penson, they can either not allow to trade or charge huge fees to do so
HAPPY TRADING AND LET'S MAKE SOME $$$$$$$$$ ![]()
SOLAR CTDC DSTI LDK FSLR SOLF JASO CSUN ESLR STP HOKU EMKR AXTI CSIQ
Some Hurricane stocks
WEGI ECCI BUGS WWAT ABIX PBLS RGMI CTCK CHDT INFN CHYS HOM GV PDGE GLBL VIFL DXPE AERTA AATK MTRX CLWT PHII SPN PESI FUEL HYRF CHB CPST SDIX NYER MVCO EEI LMS PLUG WMSI AGSI DW SGR IDSA OBCI IIIN TUG CAV OMI PAM CLHB FLE OMNI WPCS NVH PATK WEL SIRF DECT GLOV WWIN DXPE MPWG PRB IPUR WMT HBSC TVIN WCC HAL TRMA STRL MODT TRR THO AW STHK JCTCF TPO STRN MICG BEL MHCO IEVM EGHT CSLR AQUA PURE MNC EEGI WTCH CYDF SANZ IPII TSYS BTMD CYBL FRZ PCL FLDR MTRX KMGB ESV JEC IBTGF SFTV SPRL DESC PWTC VLPI PHBT FDSI ELNK REPR FCEL BLDP SWKJ RWL EONC ERFW HMSG LSTR WTAF BTMD MDFI SYEV
The Regional Bank List
AANB ABCB ABNYY.PK AIB AMFI AMNB ANNB AROW ASBC AUBN AWBC BANF BAP BARI BBT BBV BERK BFR BHB BK BKBK BKSC BLX BMO BMRC BMTC BNCC.PK BOH BOKF BPOP BTFG BUSE BXS CAC CACB CASS CATY CBAN CBBO CBC CBIN CBKN CBON CBSH CBU CCBG CCFH CCNE CCOW CFFI CFIC CFNL CFR CHCO CHCO CHFC CIB CMA CNAF CNB CNBC CNBKA CNBT COBT COBH COBZ COF COLB CORS CPF CRRB CSBK CTBI CTBK CVBF CVBK CVLY CWBC CWBC CWLZ CYN DEAR DRL ECBE EUBK EVBS EWBC FBNC FBP FBSS FCF FCNCA FFIN FFKT FHN FISI FITB FLIC FMAR FMBI FMER FMFC FNB FNLC FRBK FRGB FRME FSBK FSNM FTBK FULT FUNC FWBN FWV GABC GBCI GLOB.OB GNTY.PK GRAN GSBC HABC HBAN HBHC HDB HNBC HTBKE HU IBCA IBCP IBK IBK IBKC IBN IBNK IBOC IFC INDB IROQ JFBC KEY KFED LION LKFN LNBB MBFI MBHI MBVT METB MI MPB MSFG MSL MTB NABZY.PK NARA NBBC NBG NBTB NCC NFB NKSH NOVB NPBC NRIM NSFC NTRS NWFL OFG OKSB ONB OSBC OVBC OZRK PBKS PCBC PCBK PEBO PFBI PFI PNBC PNBK PNC PNTE PRK PRSP PVTB RBCAA RBPAA RF RGFC.PK RKNG SAL SAN SASR SAVB SBCF SBIB SBNY SBP SBSI SCB SCBT SFNCA SGB SIVB SIXR SLFI SNBC SNBJ SNV SRCE STBC STBA STD STI STL STT SUB SUBK SUPR SUSQ TBNS TRMK TRST TRUED TSFG UB UBB UBCP UBSC UBSH UBSI UCBH UCBH UMBF UMPQ UNTY USB USBI VIST VLY WABC WAIN WASH WB WBCO WBK WCBO WFC WHI WIBC WL WSBC WTFC WTNY ZION
http://www.bullsector.com/bank.html
The Bank Stock List
^BKX AANB ABCB ABNYY.PK AFBC AIB AMFI ANNB ANZBY.PK AROW ASBC AUBN BAC BANF BAP BARI BBT BBV BCS BCSB BERK BFR BK BKBK BKSC BKUNA BLX BMRC BMTC BNCC.PK BOH BOKF BPFH BPOP BRBI.PK BSRR BTFG BUSE BYFC CAC CACB CASS CATY CBAN CBBO CBKN CBSH CBU CCBG CCBP CCNE CCOW CFR CHFC CMA CNB CNBC CNBKA COBH COLB CORS CPF CRBC CSNT CTBI CVBF CVBK CVLY CWBC CYN DEAR ECBE ESBK EVBS EVRT EWBC FBNC FBSS FCF FCNCA FDT FED FFBC FFIN FITB FKFS FLIC FMAR FMBI FMER FMFC FNB FNBN FNLC FPFC FRGB FRME FSNM FULT FWV GABC GBCI GRAN HABC HBAN HBC HBEK HBHC HNBC HWBK HWEN.OB IBCA IBCP IBOC IBN ICBC INDB IRE JFBC JPM JXSB LARK LBAI LION LKFN LNBB LSBX MASB MBRG MBVT MBWM MCBC MCBI MI MNBB MSFG MTB NABZY.PK NARA NBG NBN NBTB NCC NFB NKSH NOVB NPBC NRIM NSFC NTRS NWFL OKSB ONB ONFC OPOF OSBC OZRK PACW PBKS PBNI.PK PCBC PCBK PEBO PFBX PMBC PNBC PNC PNTE PRK PVTB RBCAA RBPAA RGFC.PK RY SAN SAVB SBCF SBIB SBSI SCB SCBT SLFI SNBC SNV SRCE STD STI STL SUBI SUBK SUPR SUSQ SXNB SYBT TCB TCBK TD TFIN THFF TMP TFIN TMP TRMK TRST TSFG TRUED UB UBB UBCP UBSH UBSI UCBH UCBH UMPQ UMBF UNIB UNTY USBI VIST WABC WAIN WAL WASH WB WBCO WCBO WFC WBK WHI WIBC WL WSBC WTFC WTNY ZION
|
Posts Today
|
1
|
|
Posts (Total)
|
45088
|
|
Posters
|
|
|
Moderator
|
|
|
Assistants
|
| Volume | |
| Day Range: | |
| Bid Price | |
| Ask Price | |
| Last Trade Time: |
