Register for free to join our community of investors and share your ideas. You will also get access to streaming quotes, interactive charts, trades, portfolio, live options flow and more tools.
SNGX.....out today with a 2% loss
I agree. Thanks for the info.
SNGX: It could very well hit that TODAY --- even after the Firm's pathetic dumping of more shares to Market yesterday, after the initial glorious run-up. (Wall Street makes Las Vegas, et al., look like 'child's-play'!!)
How is that $20 close working out?
Seems like you missed the mark. Wondering if it could hit $20 in 2 weeks.
congrats, i got in too high today, holding.
its an odd time to put….
..
out that type of news right as it was about to hit 15. i have seen these rebound well after such odd timing on that type of news.
lets see what happens.
Thank you. I was wondering what happened.
On July 9, 2024, Soligenix, Inc. (the “Company”) entered into a warrant inducement agreement (the “Inducement Agreement”) with certain holders (the “Holders”) of the Company’s existing Warrant to Purchase Shares of Common Stock (“Existing Warrants”) to purchase shares of common stock, par value $0.001 per share (the “Common Stock”), of the Company. Pursuant to the Inducement Agreement, the Holders agreed to exercise for cash their Existing Warrants to purchase up to 703,125 shares of Common Stock at an exercise price of $6.00 per share during the period from the date of the Inducement Agreement until 1:30 p.m., Eastern Time, on July 9, 2024. The aggregate gross proceeds to be received by the Company will depend on the number of Existing Warrants actually exercised by the Holders. If all of the Existing Warrants are exercised in connection with the Inducement Agreement, the Company would anticipate receiving aggregate gross proceeds of up to approximately $4,218,750 from the exercise of the Existing Warrants before deducting financial advisory fees and other expenses payable by us. There is, however, no guarantee that all of the Existing Warrants will be exercised by the Holder in accordance with the Inducement Agreement.
5.75 TO 12.00 WORKS...BE CAREFUL!!
JUMPIN JESUS..THE POWER OF A TRIAL 3......LEFT SOME ON THIS TABLE!!!
SNGX: Told ya not to worry --- $20 Close today, very likely.
it wants to break new highs, it appears.
Less than 1M shares in the O/S, volume already over 50 times that, and it's 10:15...
Robot wash trading, and when they turn those robots off, folks are going to be stuck with grossly overpriced stock.
SNGX: Hope it does better than that "dip" ya got yesterdary, Bro.
TOUCHING 900 EMA ON 5 SECONDS CHART SIGNALS EVENTUAL UPTREND, WE SHALL SEE. I AM STILL HOLDING MINE SINCE
OUR HONORABLE, IMPORTANT AND DIGNIFIED "INVEST-" ASSERTED TO THE MOON ON 1000% TARGET.
SNGX: Who knew?????
SNGX: Sarcasm, or compliment??? Or some linguistical item in between those two??? (HAH-HAH-HAH!!!)
VERY BULLISH ON THIS
It figures you would be here
SNGX: I just rented an Elon Muskrat personal ROCKET, to see if I can CATCH the price here before it hits 1,000% in the Pre-M!!! (And of course, don't forget that Mr. MONK, "did it again"!!!)
Whoops, I did it, again
Expanded HyBryte™ Treatment Demonstrating Positive Outcomes in Early-Stage Cutaneous T-Cell Lymphoma
Source: PR Newswire (US)
Study Supported by $2.6 Million FDA Orphan Products Development Grant
Another @Zardiw early runner pick. I think he meant up.
Well, just do a quick search if your memory is faulty, as you bring your memory into question.
Quick search and you find all of them. You seem to keep track.
I don't recall any........
Z
No kidding? How many DDAmanda subscribers do you have?
There aren't or there really aren't? lol.
Well, there really aren't any negative opinions on #DDAmanda, or I would have posted them...........There's a few guys that didn't make money, but that's their fault, not the software's.
Z
Amazing the testimonials that sound like DDAmanda marketing.
What about the less favorable opinions of your DDAmanda members?
Your claimed testimonials don't change fact that you and #DDAmanda recommend really lame stocks as your picks.
You post after the break out, but claim your DDAmanda finds them before.
But you cannot show us 20 in a row DDAmanda posts of stocks a day or two prior to breakout.
Rather, you pick some, and some of these you then pump the crap out of.
Seems DDAmanda requires finding a stock to load up on and pump.
.
Really.......well this is what members say about #DDAmanda:
Testimonials
----------- Actual Unsolicited Feedback/Comments received from users:
These all came out of the blue. We didn't even ask people what they thought.
"Hans, i just made a killing off GWAV and CRKN thanks to ddamanda. Your scanner is no joke!!"
"I love you guys..."
"Yeah, love the program. So worth it!! "
"Dude.... I have been a member for a month now of both, and you have made me so much money it's not even funny... Multiple 100% plays within past 4 weeks, and bought into CAPV as soon as the filing came out last Monday, the best play yet... . Please for the love of everything... don't ever discontinue Amanda... :) Thanks again!"
"Awesome program by the way, after just one day of use it's worth every penny. Not to mention it paid for it self several times in just one day..."
"... it is well worth $175.00 and I’ve made probably $500k in the stocks that I found using DD..."
"Thanks DDA made me a lot of money and set me up to make more. Im self sufficient bc of dda"
"Bought this, found Tsnp dropped like 800$ I'm up 30K on it! DDamanda literally like the reviews said paid for itself in the first trade!"
"I have only been trading for 2 years this is the beginning of my 3rd and I have spent a lot of time looking at well everything I can look at to make trading decisions. I’ve noticed on iHub here and there mentions and references to the DDAmanda software and decided to give it a try. She “the software” may look like it was made in 2000 but I am pleasantly surprised with the features as an all in one screener.
Having almost everything I need to research a ticker on one page is a definite time saver.
Some of the features I enjoy the most are the factor rating, seeing 200 days of cash flow in or out of a stock at a glance. "
"Scan picked up GARB two days ago so I added at .0004 yesterday for a single day $1525 profit. I would say the scanner paid for itself on that trade."
"I wasn't even in for a week with DDAmanda and I made $10k off of TRNX. I just notice that if you do your scans properly, there is at least one stock daily where you can make good money off. So, I really like using this program. "
"Hello, you have a great product and I have been pleased with my experience of it."
"DDAmanda is awesome in any case. really helps in penny land to quickly identify possible runners facilitating the DD immensely. Love it!"
" I am absolutely digging DDAmanda. I think it’s brilliant."
"Love the program... and am telling everyone about it..."
"DDAmanda is no joke. Just got it. Narrowed down my preferred stocks, to a few stocks due to the $ volume pattern, and profited huge on SNPW and BMIX (not to mention my other picks) "
"I just made 15k on my first DDAmanda Pick."
"...just paid for my DD Amanda subscription for the next 3 years due to 1 find hahaha"
Our Best Success Story (We don't quite know what to make of this. The guy seems sincere):
"I started with DD Amanda and $1000. I grew that sum using the software to $100,000.
Like any wise investor (he said sarcastically), I put all of that into a stock in and slightly above trips that I also found with the software.
I held that for almost 2 years and didn’t trade at all. Sometimes I didn’t check on the stock for weeks at a time.
Well, to make a long story short, my account spiked to 30,000,000 and has settled around 10,000,000. Patience + DD Amanda has changed my life. Thanks Hans! "
"Loving DDA so far and recommending it to others."
"I have done well using the program."
"I use DDAmanda and two other scanning tools, and you can see that it provided a AITX daily trading history in a blink. I like easy.
While OS is important (it does show AS-OS-Float that other users can update and share notes on), it usually comes in as the fourth or fifth thing to consider for swing trades. DDAmanda, for me, is the fastest tool that throws everything up on a single screen to quickly determine if I should dig a little deeper.
The built in shortcuts that you don't usually see on the left side of the screen is where time is really saved -- SEC, OTC, Twitter, iHub All Post Search, Charts, etc.
If it wasn't worth the price I wouldn't be using it."
" ...your software has helped me SO MUCH..."
"I FUCKING LOVE AMANDA"
"...the shear quantity of information your service provided is stunning."
"..DDAmanda. She's very exciting and a very lucrative tool to research and discover the under currents for nearly all the stocks."
"What you have put together, in my view, us honestly extraordinary. "
"Your program is the best tool I've ever used in the past 21 years. "
"I think the software is a great tool!"
"Program is great......."
"The platform is a fantastic research and alert tool. .......I was very pleased with the platform and will continue to recommend it to others."
" Really a good piece of software"
"Love this program - Use it all the time. All your information is in one spot. Its way worth getting a subscription in you trade in the pinks."
"I made about $10k this past week using DDAmanda. Just wanted to say thanks for creating this beautiful creature and hope she continues to make you money actively/passively."
"I found CXCQ w/ DDAmanda. I am up almost $100,000 on paper. I would say that DDAmanda is probably going to pay for itself. ;)"
".... thank you so much for your excellent customer service. "
" It's an awesome program ....."
"Your program is fantastic. ....."
"...a VERY HAPPY DDAMANDA user :) "
"You have created a great software. "
".. a testament to your product, which by the way i did tell you that i loved ;) ...I just wanted to give you this feed back, as i appreciate you and your business model."
"..... DDAmanda. I love it, especially the cash flow and factor finder, ..."
".....Hello! Thanks I understand the software and it’s very easy to use, ..."
"....Amanda is awesome! ..."
"... very good customer service =]"
"....DDAmanda is an extremely useful tool..."
"DD Amanda has been a great tool for me."
"....this is Amazing...I love this.."
"... I really liked:
--- the speed of the program
--- the unbelievable advantages of the scanners one can run
--- being able to collect stock tickers and run then to see if the company has been front loaded before investing
--- the special note section was a key item
--- hitting any message board from the main screen was really cool..."
"Thanks for the great customer service! "
"Well, amanda has paid for herself for the next 12 months today..Ty sir"
".. i like how you guys have everything on one page.. seems user friendly.... "
"Amanda is basically my secret weapon… I get to see money moving around long before anyone else notices"
" ... as a new user, I made 10 times the subscription price on my first trade......."
"I can honestly say this is the best program around ....."
" ....Profited 2k because of her."
".... thanking my stars that i got such a good tool for a rookie investor (not a day trader and never intended) like me."
"...., the best thing I liked so far was Drag. Awesome. you know what, everything about the tool is awesome. I just loved it. "
" DDAmanda's great by the way, the ability to see back so far in the trading history is awesome...among countless other insights"
"... Ive learned alot from you and Amanda ..well worth the money..."
"..... The product is phenomenal and I will recommend it to others ..."
"Wow using Amanda is like playing with a magical wand. I don't know quite what I'm doing yep but when I do,
I'm turning everything into pumpkins??...kidding.. playing with the scanner is so much fun."
".... first week I picked something up on insider and made $10k...."
"....this software is unbelievable....."
" ... I have only good things to say ....."
"Many thanks for the great work you have done in sharing and developing this lovely piece of software."
"I love the tool though. DDAmanda been great"
"I am super excited to have lucked across this site!"
"I think your application has been extremely helpful thus far."
" Thank you for answering so quickly! That is great support. I am going to
take you up on this soon. "
"......Wonderful tool...."
"I have to say so far I am impressed...."
"I have enjoyed the service so far and profited greatly from it..."
"I am a big fan........ No complaints!.."
" I really like that dollar volume log, helps with trying 2 figure out notes ending. Also how users can update tickers with notes. Also the screeners you can setup of course. Really could find some early runners."
"Great DD Amanda play I doubled! Appreciate you!"
Z
I wouldn't buy this sh'tty DDAmanda @Zardiw stock pick. I've stated so already. This is your scam stock pick, not mine. Now you're so low to wish someone lose on your pick.
"1-16 isn't that bad.......it doesn't signify dilution"
No reverse dilutes. And is near always to get the price up.
Just Zardiw bullshit.
With another shi*ty #DDAmanda stock pick.
Because Jim Cramer is a bigtime shorter who dupes his audience. He is a champion fakester. Two peas in a pod here. I don't know but looks like it.
I hope you lose your ass on this stock.......if you even have a position that is.........
Z
|
Followers
|
211
|
Posters
|
|
|
Posts (Today)
|
0
|
Posts (Total)
|
9810
|
|
Created
|
06/06/06
|
Type
|
Free
|
| Moderators | |||
| SNGX [NASD] |
| Soligenix, Inc. |
| Healthcare | Biotechnology | USA |
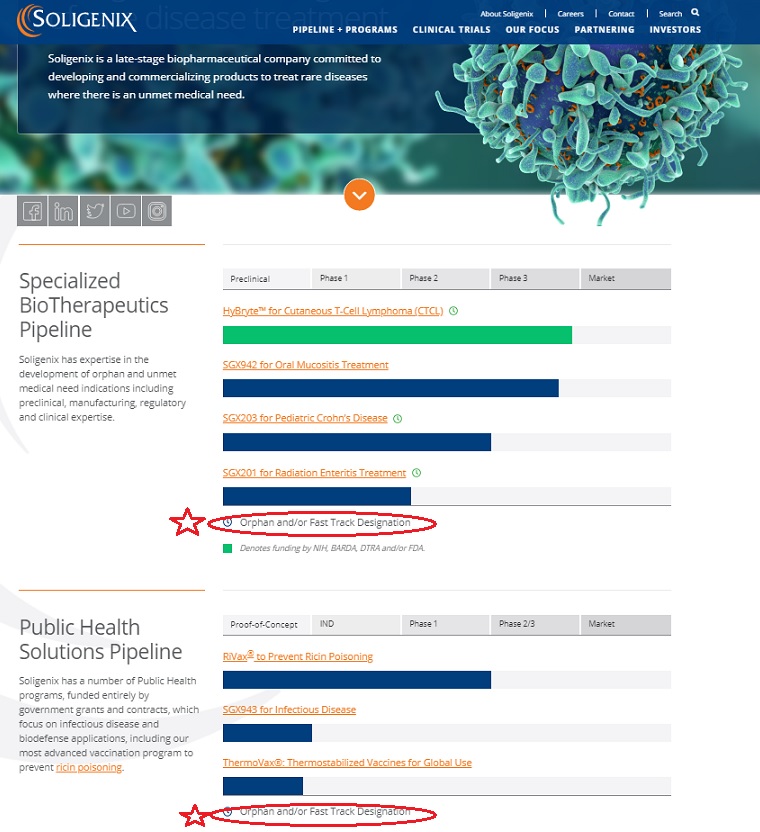
Soligenix, Inc. (Soligenix) is a late-stage biopharmaceutical company.
The Company is focused on developing and commercializing products to treat rare diseases.
The Company operates through two business segments:
Specialized BioTherapeutics and Public Health Solution. Soligenix’s Specialized BioTherapeutics business segment is developing
a photodynamic therapy (SGX301) utilizing topical synthetic hypericin activated with safe visible fluorescent light for the treatment of cutaneous T-cell lymphoma (CTCL).
The Public Health Solutions business segment includes active development programs for RiVax,
its ricin toxin vaccine candidate and SGX943, its therapeutic candidate for antibiotic-resistant and emerging infectious disease.
CTCL is its innate defense regulator technology, dusquetide (SGX942) for the treatment of oral mucositis in head and neck cancer.
Soligenix has expertise in the development of orphan and unmet medical need indications including preclinical, manufacturing, regulatory and clinical expertise.
Soligenix has expertise in the development of orphan and unmet medical need indications including preclinical, manufacturing, regulatory and clinical expertise.
| Preclinical | Phase 1 | Phase 2 | Phase 3 | Market |
|---|---|---|---|---|
| HyBryte™ for Cutaneous T-Cell Lymphoma (CTCL) | ||||
| | | | | |
| SGX942 for Oral Mucositis Treatment | ||||
| | | | | |
| SGX203 for Pediatric Crohn’s Disease | ||||
| | | | | |
| SGX201 for Radiation Enteritis Treatment | ||||
| | | | | |
Orphan and/or Fast Track Designation
Soligenix has a number of Public Health programs, funded entirely by government grants and contracts, which focus on infectious disease and biodefense applications, including our most advanced vaccination program to prevent ricin poisoning.
| Proof-of-Concept | IND | Phase 1 | Phase 2/3 | Market |
|---|---|---|---|---|
| RiVax® to Prevent Ricin Poisoning | ||||
| | | | | |
| SGX943 for Infectious Disease | ||||
| | | | | |
| ThermoVax®: Thermostabilized Vaccines for Global Use | ||||
| | | | | |
Orphan and/or Fast Track Designation


| Preclinical | Phase 1 | Phase 2 | Phase 3 | Market |
|---|---|---|---|---|
| HyBryte™ for Cutaneous T-Cell Lymphoma (CTCL) almost done [in phase 3] | ||||
| | | | | |
| SGX942 for Oral Mucositis Treatment almost done [in phase 3] | ||||
| | | | | |
| SGX203 for Pediatric Crohn’s Disease [phase 2 almost done] | ||||
| | | | | |
| SGX201 for Radiation Enteritis Treatment [phase 1 going into phase 2] | ||||
| Proof-of-Concept | IND | Phase 1 | Phase 2/3 | Market |
|---|---|---|---|---|
| RiVax® to Prevent Ricin Poisoning | ||||
| | | | | |
| SGX943 for Infectious Disease | ||||
| | | | | |
| ThermoVax®: Thermostabilized Vaccines for Global Use | ||||
| | | | | |
Orphan and/or Fast Track Designation
| PER IHUB MGMT |
02-07-2021
DISCLAIMER: ONLY FOR MICK
https://investorshub.advfn.com/boards/profilea.aspx?user=1012
*The Board Monitor and herewithin , are not licensed brokers and assume NO responsibility for actions,
investments,decisions, or messages posted on this forum.
CONTENT ON THIS FORUM SHOULD NOT BE CONSIDERED ADVISORY NOR SOLICITATION
AUTHORS MAY HAVE BUYS OR SELLS WITH THE COMPANIES MENTIONED IN TRADING POSTERS SHOULD DUE DILIGENT BUYING OR SELLING.
ALL POSTING SHOULD BE CONSIDERED FOR INFORMATION ONLY. WE DO NOT RECOMMEND ANYONE BUY OR SELL ANY SECURITIES POSTED HEREWITHIN.
ANY trade entered into risks the possibility of losing the funds invested.
• There are no guarantees when buying or selling any security.Any
| Volume | |
| Day Range: | |
| Bid Price | |
| Ask Price | |
| Last Trade Time: |
