Register for free to join our community of investors and share your ideas. You will also get access to streaming quotes, interactive charts, trades, portfolio, live options flow and more tools.
LOL!!!! That's the very same thing I always say about you!
Any fool who listens to Jim Cramer deserves to lose. Yes, he suckers you in then he goes short. This is well known for many years.
Any fool who listens to Jim Cramer deserves to lose. Yes, he suckers you in then he goes short. This is well known for many years.
I expect a rally coming in the next few months as the study reveals more success.
I was dumb enough to get suckered into this one by Jim Cramer. It's garbage!
Look at the chart. Over the last two days, it has had HUGE volume (for it) and down 13% today.
No one can convince me good news is coming from this company.
I lost over 75% here and was happy I got out at $3.54
Small pharma companies are akin to buying fools gold.
SNGX: Nice Cancer news!!! (Sorely needed on Planet Earth!! THANKS!!)
is the news today a Recycled one from July 9 2024?
$3.96 + 25% SNGX good news again can it retake $5s then break to $7 always a chance
....PRINCETON, N.J., Jan. 14, 2025 /PRNewswire/ -- Soligenix, Inc. (Nasdaq: SNGX) (Soligenix or the Company), a late-stage biopharmaceutical company focused on developing and commercializing products to treat rare diseases where there is an unmet medical need, announced an interim update on the open-label, investigator-initiated study (IIS) evaluating extended HyBryte™ (synthetic hypericin) treatment for up to 12 months in patients with early-stage cutaneous T-cell lymphoma (CTCL). The trial is sponsored by Ellen Kim, MD, Director, Penn Cutaneous Lymphoma Program, Vice Chair of Clinical Operations, Dermatology Department, and Professor of Dermatology at the Hospital of the University of Pennsylvania who was a leading enroller in the Phase 3 FLASH (Fluorescent Light Activated Synthetic Hypericin) study for the treatment of early-stage CTCL. To date, nine patients have been enrolled and treated with HyBryte™ over a time period of up to 54 weeks. Patients have responded positively to HyBryte™ therapy, with over 70% (5 of the 6 subjects who have completed at least 18 weeks of therapy) already achieving "Treatment Success". Treatment Success is predefined in the study's protocol as a greater than or equal to 50% improvement in the cumulative mCAILS (modified Composite Assessment of Index Lesion Severity) score compared to Baseline. Of the five Treatment Successes, three were achieved within the first 12 weeks of treatment, with two patients achieving a "complete response" by 18 weeks. Of the remaining patients, two have recently started the study and two had to drop from the study for logistical reasons (e.g., need to care for an elderly parent), with one showing a substantial improvement (>30%) by their Week 18 visit. In addition, HyBryte™ appears to be safe and well tolerated in all patients.
"The complete response rate, consistent treatment response and safety profile across multiple clinical studies to date with HyBryte™ has been exciting to see," noted Dr. Kim, Principal Investigator of the IIS and Lead Investigator of the FLASH2 study. "In the first Phase 3 FLASH study, HyBryte™ was shown to be efficacious with a benign safety profile compared to the current therapies of steroids, chemotherapeutics and ultraviolet light in this chronic orphan disease. With limited treatment options, especially in the early stages of their disease, CTCL patients are often searching for alternative treatments. In our U.S. Food and Drug Administration (FDA)-funded study, initial results evaluating the expanded use of HyBryte™ in a "real world" treatment setting remain very promising, further supporting and extending results from the previous positive Phase 2 and 3 clinical trials. We look forward to continuing to work with the FDA to complete this study while we participate in the confirmatory Phase 3 placebo-controlled FLASH2 study."
"We are pleased with these recent study results and that the FDA is continuing to financially support the HyBryte™ program, giving patients an opportunity to access the therapy in an open-label setting," stated Christopher J. Schaber, PhD, President and Chief Executive Officer of Soligenix. "CTCL is an incredibly difficult to treat orphan disease and remains an area of unmet medical need with a very limited number of safe and effective treatment options. Following the initial Phase 3 FLASH study, which demonstrated the safety and efficacy of shorter courses of HyBryte™ therapy, we are pleased to see that continuing treatment for longer time periods is resulting in the anticipated improved outcomes for patients. As the body of compelling data continues to grow in support of this novel therapy, we look forward to continuing to work with Dr. Kim on this important study as well as advancing enrollment in the 80-patient confirmatory Phase 3 FLASH2 replication study. We will plan to provide additional updates on the IIS as data becomes available."
The clinical study RW-HPN-MF-01, "Assessment of Treatment with Visible Light Activated Synthetic Hypericin Ointment in Mycosis Fungoides Patients" is designed as an open-label, multicenter clinical trial enrolling approximately 20 patients in the U.S. Patients have the potential to be treated for up to 12 months with twice a week dosing (visible light activation following ointment application by 24 ± 6 hours). The study also allows for potential transition to a "real-world" setting with home-use. The primary endpoint for the study is evaluating the number of treatment successes defined as ≥50% reduction in the cumulative mCAILS score from Baseline to end of the treatment.
About HyBryte™
HyBryte™ (research name SGX301) is a novel, first-in-class, photodynamic therapy utilizing safe, visible light for activation. The active ingredient in HyBryte™ is synthetic hypericin, a potent photosensitizer that is topically applied to skin lesions that is taken up by the malignant T-cells, and then activated by safe, visible light approximately 24 hours later. The use of visible light in the red-yellow spectrum has the advantage of penetrating more deeply into the skin (much more so than ultraviolet light) and therefore potentially treating deeper skin disease and thicker plaques and lesions. This treatment approach avoids the risk of secondary malignancies (including melanoma) inherent with the frequently employed DNA-damaging drugs and other phototherapy that are dependent on ultraviolet exposure. Combined with photoactivation, hypericin has demonstrated significant anti-proliferative effects on activated normal human lymphoid cells and inhibited growth of malignant T-cells isolated from CTCL patients. In a published Phase 2 clinical study in CTCL, patients experienced a statistically significant (p=0.04) improvement with topical hypericin treatment whereas the placebo was ineffective. HyBryte™ has received orphan drug and fast track designations from the FDA, as well as orphan designation from the European Medicines Agency (EMA).
The published Phase 3 FLASH trial enrolled a total of 169 patients (166 evaluable) with Stage IA, IB or IIA CTCL. The trial consisted of three treatment cycles. Treatments were administered twice weekly for the first 6 weeks and treatment response was determined at the end of the 8th week of each cycle. In the first double-blind treatment cycle (Cycle 1), 116 patients received HyBryte™ treatment (0.25% synthetic hypericin) and 50 received placebo treatment of their index lesions. A total of 16% of the patients receiving HyBryte™ achieved at least a 50% reduction in their lesions (graded using a standard measurement of dermatologic lesions, the CAILS score) compared to only 4% of patients in the placebo group at 8 weeks (p=0.04) during the first treatment cycle (primary endpoint). HyBryte™ treatment in this cycle was safe and well tolerated.
In the second open-label treatment cycle (Cycle 2), all patients received HyBryte™ treatment of their index lesions. Evaluation of 155 patients in this cycle (110 receiving 12 weeks of HyBryte™ treatment and 45 receiving 6 weeks of placebo treatment followed by 6 weeks of HyBryte™ treatment), demonstrated that the response rate among the 12-week treatment group was 40% (p<0.0001 vs the placebo treatment rate in Cycle 1). Comparison of the 12-week and 6-week treatment responses also revealed a statistically significant improvement (p<0.0001) between the two timepoints, indicating that continued treatment results in better outcomes. HyBryte™ continued to be safe and well tolerated. Additional analyses also indicated that HyBryte™ is equally effective in treating both plaque (response 42%, p<0.0001 relative to placebo treatment in Cycle 1) and patch (response 37%, p=0.0009 relative to placebo treatment in Cycle 1) lesions of CTCL, a particularly relevant finding given the historical difficulty in treating plaque lesions in particular.
The third (optional) treatment cycle (Cycle 3) was focused on safety and all patients could elect to receive HyBryte™ treatment of all their lesions. Of note, 66% of patients elected to continue with this optional compassionate use / safety cycle of the study. Of the subset of patients that received HyBryte™ throughout all 3 cycles of treatment, 49% of them demonstrated a positive treatment response (p<0.0001 vs patients receiving placebo in Cycle 1). Moreover, in a subset of patients evaluated in this cycle, it was demonstrated that HyBryte™ is not systemically available, consistent with the general safety of this topical product observed to date. At the end of Cycle 3, HyBryte™ continued to be well tolerated despite extended and increased use of the product to treat multiple lesions.
Overall safety of HyBryte™ is a critical attribute of this treatment and was monitored throughout the three treatment cycles (Cycles 1, 2 and 3) and the 6-month follow-up period. HyBryte's™ mechanism of action is not associated with DNA damage, making it a safer alternative than currently available therapies, all of which are associated with significant, and sometimes fatal, side effects. Predominantly these include the risk of melanoma and other malignancies, as well as the risk of significant skin damage and premature skin aging. Currently available treatments are only approved in the context of previous treatment failure with other modalities and there is no approved front-line therapy available. Within this landscape, treatment of CTCL is strongly motivated by the safety risk of each product. HyBryte™ potentially represents the safest available efficacious treatment for CTCL. With very limited systemic absorption, a compound that is not mutagenic and a light source that is not carcinogenic, there is no evidence to date of any potential safety issues.
Following the first Phase 3 study of HyBryte™ for the treatment of CTCL, the FDA and the EMA indicated that they would require a second successful Phase 3 trial to support marketing approval. With agreement from the EMA on the key design components, the second, confirmatory study, called FLASH2, is expected to be initiated before the end of 2024. This study is a randomized, double-blind, placebo-controlled, multicenter study that will enroll approximately 80 subjects with early-stage CTCL. The FLASH2 studyreplicates the double-blind, placebo-controlled design used in the first successful Phase 3 FLASH study that consisted of three 6-week treatment cycles (18 weeks total), with the primary efficacy assessment occurring at the end of the initial 6-week double-blind, placebo-controlled treatment cycle (Cycle 1). However, this second study extends the double-blind, placebo-controlled assessment to 18 weeks of continuoustreatment (no "between-Cycle" treatment breaks) with the primary endpoint assessment occurring at the end of the 18-week timepoint. In the first Phase 3 study, a treatment response of 49% (p<0.0001 vs patients receiving placebo in Cycle 1) was observed in patients completing 18 weeks (3 cycles) of therapy. In this second study, all important clinical study design components remain the same as in the first FLASH study, including the primary endpoint and key inclusion-exclusion criteria. The extended treatment for a continuous 18 weeks in a single cycle is expected to statistically demonstrate HyBryte's™ increased effect over a more prolonged, "real world" treatment course. Given the extensive engagement with the CTCL community, the esteemed Medical Advisory Board and the previous trial experience with this disease, accelerated enrollment in support of this study is anticipated, including the potential to enroll previously identified and treated HyBryte™ patients from the FLASH study. Discussions with the FDA on an appropriate study design remain ongoing. While collaborative, the agency has expressed a preference for a longer duration comparative study over a placebo-controlled trial. Given the shorter time to potential commercial revenue and the similar trial design to the first FLASH study afforded by the EMA accepted protocol, this study is being initiated. At the same time, discussions with the FDA will continue on potential modifications to the development path to adequately address their feedback.
Additional supportive studies have demonstrated the utility of longer treatment times (Study RW-HPN-MF-01, see above), the lack of significant systemic exposure to hypericin after topical application (Study HPN-CTCL-02) and its relative efficacy and tolerability compared to Valchlor® (Study HPN-CTCL-04).
In addition, the FDA awarded an Orphan Products Development grant to support the investigator-initiated study evaluation of HyBryte™ for expanded treatment in patients with early-stage CTCL, including in the home use setting. The grant, totaling
About Cutaneous T-Cell Lymphoma (CTCL)
CTCL is a class of non-Hodgkin's lymphoma (NHL), a type of cancer of the white blood cells that are an integral part of the immune system. Unlike most NHLs which generally involve B-cell lymphocytes (involved in producing antibodies), CTCL is caused by an expansion of malignant T-cell lymphocytes (involved in cell-mediated immunity) normally programmed to migrate to the skin. These malignant cells migrate to the skin where they form various lesions, typically beginning as patches and may progress to raised plaques and tumors. Mortality is related to the stage of CTCL, with median survival generally ranging from about 12 years in the early stages to only 2.5 years when the disease has advanced. There is currently no cure for CTCL. Typically, CTCL lesions are treated and regress but usually return either in the same part of the body or in new areas.
CTCL constitutes a rare group of NHLs, occurring in about 4% of the more than 1.7 million individuals living with the disease in the U.S. and Europe (European Union and United Kingdom). It is estimated, based upon review of historic published studies and reports and an interpolation of data on the incidence of CTCL that it affects approximately 31,000 individuals in the U.S. (based on SEER data, with approximately 3,200 new cases seen annually) and approximately 38,000 individuals in Europe (based on ECIS prevalence estimates, with approximately 3,800 new cases annually).
Phase 3 double success
Not many posts since then. "As for the industry’s initial reactions on the innovation front, the Pharmaceutical Research and Manufacturers of America (PhRMA) wants to “work with his administration to further strengthen our innovation ecosystem that enables the United States to lead the world in medicine development,” CEO Stephen Ubl said in a statement. “New medicines are transforming our ability to prevent, treat, and cure deadly disease, improving patient lives and helping to avoid the most expensive parts of our health care system.”
"“We are committed to working with the Trump administration and the new Congress to make our health care system work better for patients while preserving our unique ecosystem that enables greater innovation and lower costs for patients,” Ubl said.
Of course, a recent twist in Trump’s later campaign days was the introduction of Robert F. Kennedy Jr. as a prospect to head up healthcare. Kennedy, who scrapped his own campaign for the presidency in August, is widely known for his anti-vaccine rhetoric and conspiracies and was even temporarily banned from Instagram in 2021 for spreading misinformation about COVID-19 vaccines. At a rally in New York, Trump pledged to, if elected, allow Kennedy to “go wild on health” as well as “the medicines.”
Kennedy later delivered a grim message to the FDA on X, formerly Twitter, warning those who “work for the FDA and are part of this corrupt system” to “1. Preserve your records, and 2. Pack your bags.” Today, Fierce Biopharma
thx but that post was back in july. ![]()
Now 1071 for your understanding the fundamentals. New FDA Commissioner soon to be will make some much needed changes and this company and others will be recognized positively and not as a threat to BP to be hamstrung as is presently the situation. Oversold so I will add to my little starter.
SNGX.....out today with a 2% loss
I agree. Thanks for the info.
SNGX: It could very well hit that TODAY --- even after the Firm's pathetic dumping of more shares to Market yesterday, after the initial glorious run-up. (Wall Street makes Las Vegas, et al., look like 'child's-play'!!)
How is that $20 close working out?
Seems like you missed the mark. Wondering if it could hit $20 in 2 weeks.
congrats, i got in too high today, holding.
its an odd time to put….
..
out that type of news right as it was about to hit 15. i have seen these rebound well after such odd timing on that type of news.
lets see what happens.
Thank you. I was wondering what happened.
On July 9, 2024, Soligenix, Inc. (the “Company”) entered into a warrant inducement agreement (the “Inducement Agreement”) with certain holders (the “Holders”) of the Company’s existing Warrant to Purchase Shares of Common Stock (“Existing Warrants”) to purchase shares of common stock, par value $0.001 per share (the “Common Stock”), of the Company. Pursuant to the Inducement Agreement, the Holders agreed to exercise for cash their Existing Warrants to purchase up to 703,125 shares of Common Stock at an exercise price of $6.00 per share during the period from the date of the Inducement Agreement until 1:30 p.m., Eastern Time, on July 9, 2024. The aggregate gross proceeds to be received by the Company will depend on the number of Existing Warrants actually exercised by the Holders. If all of the Existing Warrants are exercised in connection with the Inducement Agreement, the Company would anticipate receiving aggregate gross proceeds of up to approximately $4,218,750 from the exercise of the Existing Warrants before deducting financial advisory fees and other expenses payable by us. There is, however, no guarantee that all of the Existing Warrants will be exercised by the Holder in accordance with the Inducement Agreement.
5.75 TO 12.00 WORKS...BE CAREFUL!!
JUMPIN JESUS..THE POWER OF A TRIAL 3......LEFT SOME ON THIS TABLE!!!
SNGX: Told ya not to worry --- $20 Close today, very likely.
it wants to break new highs, it appears.
Less than 1M shares in the O/S, volume already over 50 times that, and it's 10:15...
Robot wash trading, and when they turn those robots off, folks are going to be stuck with grossly overpriced stock.
SNGX: Hope it does better than that "dip" ya got yesterdary, Bro.
TOUCHING 900 EMA ON 5 SECONDS CHART SIGNALS EVENTUAL UPTREND, WE SHALL SEE. I AM STILL HOLDING MINE SINCE
OUR HONORABLE, IMPORTANT AND DIGNIFIED "INVEST-" ASSERTED TO THE MOON ON 1000% TARGET.
SNGX: Who knew?????
SNGX: Sarcasm, or compliment??? Or some linguistical item in between those two??? (HAH-HAH-HAH!!!)
VERY BULLISH ON THIS
It figures you would be here
SNGX: I just rented an Elon Muskrat personal ROCKET, to see if I can CATCH the price here before it hits 1,000% in the Pre-M!!! (And of course, don't forget that Mr. MONK, "did it again"!!!)
Whoops, I did it, again
Expanded HyBryte™ Treatment Demonstrating Positive Outcomes in Early-Stage Cutaneous T-Cell Lymphoma
Source: PR Newswire (US)
Study Supported by $2.6 Million FDA Orphan Products Development Grant
Another @Zardiw early runner pick. I think he meant up.
|
Followers
|
211
|
Posters
|
|
|
Posts (Today)
|
0
|
Posts (Total)
|
9821
|
|
Created
|
06/06/06
|
Type
|
Free
|
| Moderators | |||
| SNGX [NASD] |
| Soligenix, Inc. |
| Healthcare | Biotechnology | USA |
Soligenix, Inc. (Soligenix) is a late-stage biopharmaceutical company.
The Company is focused on developing and commercializing products to treat rare diseases.
The Company operates through two business segments:
Specialized BioTherapeutics and Public Health Solution. Soligenix’s Specialized BioTherapeutics business segment is developing
a photodynamic therapy (SGX301) utilizing topical synthetic hypericin activated with safe visible fluorescent light for the treatment of cutaneous T-cell lymphoma (CTCL).
The Public Health Solutions business segment includes active development programs for RiVax,
its ricin toxin vaccine candidate and SGX943, its therapeutic candidate for antibiotic-resistant and emerging infectious disease.
CTCL is its innate defense regulator technology, dusquetide (SGX942) for the treatment of oral mucositis in head and neck cancer.
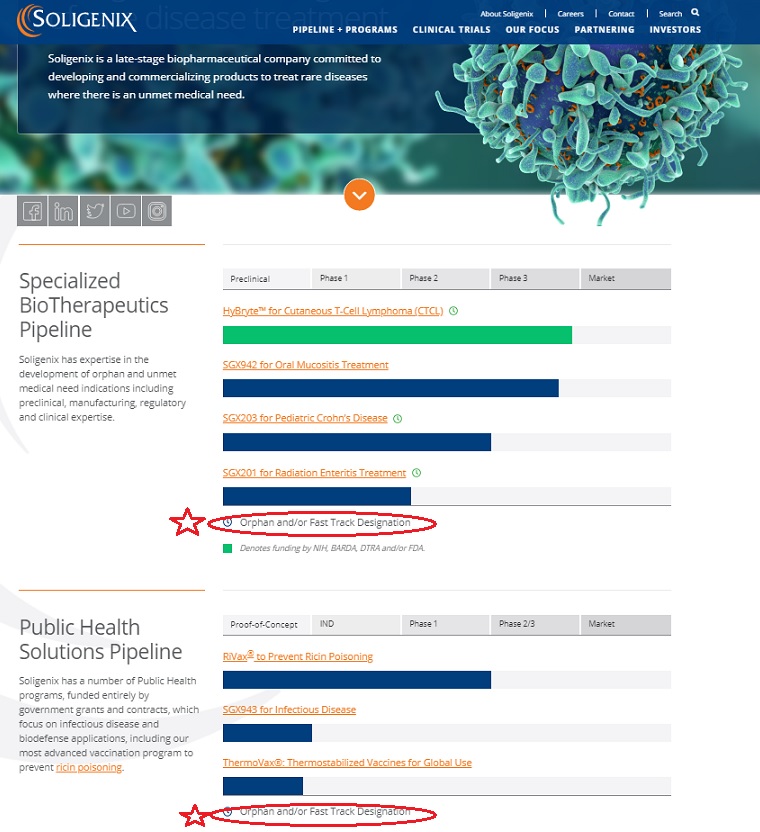
Soligenix has expertise in the development of orphan and unmet medical need indications including preclinical, manufacturing, regulatory and clinical expertise.
Soligenix has expertise in the development of orphan and unmet medical need indications including preclinical, manufacturing, regulatory and clinical expertise.
| Preclinical | Phase 1 | Phase 2 | Phase 3 | Market |
|---|---|---|---|---|
| HyBryte™ for Cutaneous T-Cell Lymphoma (CTCL) | ||||
| | | | | |
| SGX942 for Oral Mucositis Treatment | ||||
| | | | | |
| SGX203 for Pediatric Crohn’s Disease | ||||
| | | | | |
| SGX201 for Radiation Enteritis Treatment | ||||
| | | | | |
Orphan and/or Fast Track Designation
Soligenix has a number of Public Health programs, funded entirely by government grants and contracts, which focus on infectious disease and biodefense applications, including our most advanced vaccination program to prevent ricin poisoning.
| Proof-of-Concept | IND | Phase 1 | Phase 2/3 | Market |
|---|---|---|---|---|
| RiVax® to Prevent Ricin Poisoning | ||||
| | | | | |
| SGX943 for Infectious Disease | ||||
| | | | | |
| ThermoVax®: Thermostabilized Vaccines for Global Use | ||||
| | | | | |
Orphan and/or Fast Track Designation


| Preclinical | Phase 1 | Phase 2 | Phase 3 | Market |
|---|---|---|---|---|
| HyBryte™ for Cutaneous T-Cell Lymphoma (CTCL) almost done [in phase 3] | ||||
| | | | | |
| SGX942 for Oral Mucositis Treatment almost done [in phase 3] | ||||
| | | | | |
| SGX203 for Pediatric Crohn’s Disease [phase 2 almost done] | ||||
| | | | | |
| SGX201 for Radiation Enteritis Treatment [phase 1 going into phase 2] | ||||
| Proof-of-Concept | IND | Phase 1 | Phase 2/3 | Market |
|---|---|---|---|---|
| RiVax® to Prevent Ricin Poisoning | ||||
| | | | | |
| SGX943 for Infectious Disease | ||||
| | | | | |
| ThermoVax®: Thermostabilized Vaccines for Global Use | ||||
| | | | | |
Orphan and/or Fast Track Designation
| PER IHUB MGMT |
02-07-2021
DISCLAIMER: ONLY FOR MICK
https://investorshub.advfn.com/boards/profilea.aspx?user=1012
*The Board Monitor and herewithin , are not licensed brokers and assume NO responsibility for actions,
investments,decisions, or messages posted on this forum.
CONTENT ON THIS FORUM SHOULD NOT BE CONSIDERED ADVISORY NOR SOLICITATION
AUTHORS MAY HAVE BUYS OR SELLS WITH THE COMPANIES MENTIONED IN TRADING POSTERS SHOULD DUE DILIGENT BUYING OR SELLING.
ALL POSTING SHOULD BE CONSIDERED FOR INFORMATION ONLY. WE DO NOT RECOMMEND ANYONE BUY OR SELL ANY SECURITIES POSTED HEREWITHIN.
ANY trade entered into risks the possibility of losing the funds invested.
• There are no guarantees when buying or selling any security.Any
| Volume | |
| Day Range: | |
| Bid Price | |
| Ask Price | |
| Last Trade Time: |
