Register for free to join our community of investors and share your ideas. You will also get access to streaming quotes, interactive charts, trades, portfolio, live options flow and more tools.
RGLS..........................................................https://stockcharts.com/h-sc/ui?s=RGLS&p=W&b=5&g=0&id=p86431144783
Regulus Therapeutics Announces Successful Completion of its Phase 1b Multiple-Ascending Dose (MAD) Clinical Trial of Farabursen (RGLS8429) for the Treatment of Autosomal Dominant Polycystic Kidney Disease (ADPKD)
Positive Topline Data from the Fourth Cohort of Patients
Consistent demonstration of polycystin (PC) biomarker impact confirming 300 mg fixed dose selection to achieve optimal kidney exposure
Patients receiving 300 mg farabursen demonstrated a mean halting of height-adjusted total kidney volume (htTKV) growth over 4 months
Exploratory analysis of patients from high-dose cohorts demonstrates statistical significance in htTKV change compared to a large cohort of historical placebo-treated patients, which meaningfully derisks the 12-month htTKV endpoint in the planned Phase 3 trial
Company on track for initiation of Phase 3 single pivotal trial in third quarter 2025
SAN DIEGO, March 27, 2025 /PRNewswire/ -- Regulus Therapeutics Inc. (Nasdaq: RGLS), a biopharmaceutical company focused on the discovery and development of innovative medicines targeting microRNAs (the "Company" or "Regulus"), today announced positive topline results from all patients in the fourth cohort in its Phase 1b MAD study of farabursen for the treatment of ADPKD.
The Phase 1b MAD study is a double-blind, placebo-controlled trial which evaluated the safety, tolerability, pharmacokinetics and pharmacodynamics (PK/PD) of farabursen in adult patients with ADPKD. The study evaluated farabursen treatment for three months followed by one month of follow up across three weight-based dose levels (1, 2 and 3 mg/kg) and one fixed dose level (300 mg). Primary endpoints were changes in urinary PC1 and PC2, with an exploratory examination of change in htTKV growth rate. PC1 and PC2 are the protein products of the PKD1 and PKD2 genes and their levels have been shown to inversely correlate with disease severity; htTKV has been shown to inversely correlate with kidney function.
In the fourth cohort, 26 subjects received a fixed dose of 300 mg of farabursen every other week for three months. Consistent with the previously announced interim analysis of efficacy data from the first 14 subjects of this fixed-dose cohort, the full cohort of 26 patients demonstrated similar mechanistic response based on urinary PC1 and PC2 levels as well as a mean halting of htTKV growth over the four-month study period.
Cohort 4 data highlights:
Increases in urinary PC1 and PC2 levels were similar to cohort 3 and reached a similar level of statistical significance compared to placebo (PC1 p=0.026; PC2 p=0.014).
Mean htTKV growth rate over 4 months was 0.05% (SE -0.86% to +0.92%) while placebo subjects in the trial experienced a mean growth rate of 2.58% (SE +1.09% to +4.10%).
Changes in htTKV were highly correlated with changes in renal cyst volume, suggesting farabursen directly impacts disease progression by limiting abnormal cyst growth.
In an exploratory analysis, the combination of patients treated with 3 mg/kg or 300 mg fixed dose farabursen (n=35) were compared to a combined historical control group of placebo-treated patients from prior pivotal ADPKD trials (n=550). Farabursen-treated patients experienced mean reduction in htTKV growth rate (-0.14%) compared to a mean increase of (+1.87%) in placebo-treated patients (p=0.0056).
Farabursen at 300 mg demonstrated a favorable safety and tolerability profile in this study, consistent with earlier cohorts.
"We are pleased that the results of cohort 4 demonstrate a level of mechanistic response, as measured by urinary PC1 and PC2, that indicates maximal anti-miR-17 activity. Along with the observed favorable safety and tolerability profile, these results give us confidence that 300 mg provides optimal target exposure and is the appropriate dose to take forward into Phase 3," said Preston Klassen, M.D., President and Head of Research & Development of Regulus Therapeutics. "Even more encouraging is the repeated demonstration across multiple cohorts in the trial that, on average, growth of the kidney in ADPKD patients is halted after only a relatively short treatment period with farabursen. Based on exploratory analysis of Cohorts 3 & 4, which deliver overlapping drug exposures, we are confident that the htTKV effects seen with farabursen in this trial are clinically meaningful and provide important insight into the probability of success for the 12-month htTKV endpoint in the planned Phase 3 trial."
"With full data from cohort 4 now analyzed, we are pleased with the positive results, in line with our expectations based on previous cohorts. These data suggest that farabursen has the potential to be an important addition to the limited therapeutic options for patients with ADPKD," said Jay Hagan, CEO of Regulus Therapeutics. "I would like to thank all the patients, physicians, and support staff who have been vital to the ongoing development of farabursen. We are excited to move forward with initiation of the pivotal Phase 3 trial later this year."
In January, the Company announced that it held a meeting with U.S. Food and Drug Administration (FDA) and had confirmed alignment with the FDA on the acceptability of the program's CMC, non-clinical and clinical pharmacology plans and the key elements of a Phase 3 trial design as a single pivotal study, including a 12-month htTKV endpoint planned to support Accelerated Approval and a 24-month eGFR endpoint to support Full Approval.
More information about the MAD clinical trial is available at clinicaltrials.gov (NCT05521191).
About ADPKD
Autosomal dominant polycystic kidney disease (ADPKD), caused by mutations in the PKD1 or PKD2 genes, is among the most common human monogenic disorders and a leading cause of end-stage renal disease. The disease is characterized by the development of multiple fluid filled cysts primarily in the kidneys, and to a lesser extent in the liver and other organs. Excessive kidney cyst cell proliferation, a central pathological feature, ultimately leads to end-stage renal disease in approximately 50% of ADPKD patients by age 60. Approximately 160,000 individuals are
RGLS...................................https://stockcharts.com/h-sc/ui?s=RGLS&p=W&b=5&g=0&id=p86431144783
$RGLS: Finally boosting........... now $1.90
Its about time this showed up.
Now up 50% in premarket
LFGgggggggggggggggggggggg
GO $RGLS
*************************************************
Announces Successful Completion of its Phase 1b Multiple-Ascending Dose (MAD) Clinical Trial of Farabursen (RGLS8429) for the Treatment of Autosomal Dominant Polycystic Kidney Disease (ADPKD)
🔹Successful halting of kidney volume growth
🔹FDA alignment on Phase 3 trial design with potential Accelerated Approval pathway
RGLS..................................https://stockcharts.com/h-sc/ui?s=RGLS&p=W&b=5&g=0&id=p86431144783
RGLS.................................................https://stockcharts.com/h-sc/ui?s=RGLS&p=W&b=5&g=0&id=p86431144783
RGLS8429: Phase 1b MAD study ongoing
• Presented topline data in September 2023 from the first cohort of patients in the
Phase 1b MAD study indicating increases in both PC1 and PC2 urine biomarkers after
treatment
• In March 2024, presented topline data from the second cohort:
• Greater biological activity of RGLS8429 was observed at 2 mg/kg based on urinary polycystin levels
compared to 1 mg/kg and placebo
• The largest reductions in htTKV were seen in patients with the highest increase in PC1 and PC2
• Completed enrollment in the third cohort and top-line data anticipated mid-2024
• Initiated enrollment in the fourth and final cohort
FWIW
Jones Trading analyst Catherine Novack initiates coverage on Regulus Therapeutics ( RGLS ) with a Buy rating and announces Price Target of $8.
FWIW
Jones Trading analyst Catherine Novack initiates coverage on Regulus Therapeutics ( RGLS ) with a Buy rating and announces Price Target of $8.
Regulus is currently dosing patients in the third cohort of the MAD study patients with either 3mg/kg of RGLS8429 or a placebo every other week for three months. Enrolment in this cohort has been completed and top-line data from the same is expected in mid-2024.
RGLS. Getting some interest today ...not sure what the catalyst is ...impending data ?
Kiwi
Well they had positive topline data in March for cohort 2 and expect cohort 3 data by July or so. Higher dosage in cohort 3. 3mg per kg. Also starting cohort 4. Will keep an eye on this. No position yet.
RGLS . I have a small position in this Co
Reasons for my position ?
Theres currently only 1 med available specific to ADPKD kidney disease and its poorly tolerated ( makes you pee a lot ) and not very effective.
There are around 160,000 ADPKD patients in the US and around 55% of them will have kidney failure and need dialysis by age 55.
My wife rounds dialysis clinics and writes reports on roughly 120 dialysis patients each month ..There is a high mortality rate for dialysis patients ...you can google or perplexity the numbers if you wish.
RGLS 's med slows the formation of cysts in the kidney filtration system and so far appears very safe and well tolerated.
This is P 1 data . P2 trials expected in 2025 will an expected application for accelerated approval .
So early days
Vivo Capital owns roughly 8% of the outstanding shares ( IIRC ) . Vivo specializes in a lot of niche kidney specific drugs in development ...UNCY for example
Kiwi
RGLS........................https://stockcharts.com/h-sc/ui?s=RGLS&p=W&b=5&g=0&id=p86431144783
$RGLS in with 15,000 shares!! See ya at $9 share shortly!!
BLASTOFF ROCKETSHIP IMMINENT!!
Respectfully,
Maverick
$RGLS
RGLS
RNAZ Is next Cancer breakthrough this week
Regulus Therapeutics Announces Oversubscribed $100 Million Private Placement of Equity
I took a much larger position after RGLS crashed after stopping trials on it first drug candidate in favor of the one they are pursuing now. Did you know Regulus was started by a collaboration of two BPs and the scientists of Regulus came from the research divisions of those BPs?
Hi Jimmy, yes I agree. Investors starting to flock here.Looking sweet!
very strong clinical science
Very strong today!
Not good news Friday after 4PM. Still PPS not down that much. Will it drift lower next week.
RGLS
Interesting, PPS didn't drop after R/S. Trading in same range.
RGLS
Interesting, PPS didn't drop after R/S. Trading in same range.
RGLS
R/S 10 = 1. less than expected. A/S reduced from 400mil to 300mil.. Watch.
RGLS
News: Regulus Therapeutics Announces Receipt of FDA Orphan Drug Designation for RGLS8429 for the Treatment of Autosomal Dominant Polycystic Kidney Disease (ADPKD)
SAN DIEGO, June 21, 2022 /PRNewswire/ -- Regulus Therapeutics Inc. (Nasdaq: RGLS), a biopharmaceutical company focused on the discovery and development of innovative medicines targeting microRNAs (the "Company" or "Regulus"), today announced that the U.S. Food and Drug Administration (FDA) has granted orphan drug designation (ODD) to RGLS8429 for the treatment of Autosomal Dominant Polycystic Kidney Disease (ADPKD).
"Patients living with ADPKD currently have limited treatment options and approximately half of patients develop end-stage renal disease by age 60 requiring dialysis or transplantation," commented Jay Hagan, CEO of Regulus Therapeutics. "ADPKD is a disease of high unmet need affecting nearly 160,000 Americans. We look forward to advancing RGLS8429 through the clinic with the goal of improving future treatment options for patients in need."
The FDA's Office of Orphan Products Development grants orphan designation status to drugs and biologics that are intended for the safe and effective treatment, diagnosis or prevention of rare diseases, or conditions that affect fewer than 200,000 people in the U.S. Orphan designation status is intended to facilitate drug development for rare diseases and may provide several benefits to drug developers, including financial incentives, to support clinical development and the potential for up to seven years of market exclusivity in the U.S. upon regulatory approval.
About ADPKD
Autosomal Dominant Polycystic Kidney Disease (ADPKD), caused by mutations in the PKD1 or PKD2 genes, is among the most common human monogenic disorders and a leading cause of end-stage renal disease. The disease is characterized by the development of multiple fluid filled cysts primarily in the kidneys, and to a lesser extent in the liver and other organs. Excessive kidney cyst cell proliferation, a central pathological feature, ultimately leads to end-stage renal disease in approximately 50% of ADPKD patients by age 60. Approximately 160,000 individuals are diagnosed with the disease in the United States alone, with an estimated global prevalence of 4 to 7 million.
About RGLS8429
RGLS8429 is a novel, next generation oligonucleotide for the treatment of ADPKD designed to inhibit miR-17 and to preferentially target the kidney. Administration of RGLS8429 has shown robust data in preclinical models, where clear improvements in kidney function, size, and other measures of disease severity have been demonstrated along with a superior pharmacologic profile in preclinical studies compared to Regulus' first-generation compound. Regulus is currently conducting a Phase 1 single-ascending dose study in healthy volunteers to assess safety, tolerability, and pharmacokinetics of RGLS8429.
About Regulus
Regulus Therapeutics Inc. (Nasdaq: RGLS) is a biopharmaceutical company focused on the discovery and development of innovative medicines targeting microRNAs. Regulus has leveraged its oligonucleotide drug discovery and development expertise to develop a pipeline complemented by a rich intellectual property estate in the microRNA field. Regulus maintains its corporate headquarters in San Diego, CA.
Forward-Looking Statements
Statements contained in this presentation regarding matters that are not historical facts are "forward-looking statements" within the meaning of the Private Securities Litigation Reform Act of 1995, including statements associated with the Company's RGLS8429 program, including the potential outcome of clinical development. Because such statements are subject to risks and uncertainties, actual results may differ materially from those expressed or implied by such forward-looking statements. Words such as "believes," "anticipates," "plans," "expects," "intends," "will," "goal," "potential" and similar expressions are intended to identify forward-looking statements. These forward-looking statements are based upon Regulus' current expectations and involve assumptions that may never materialize or may prove to be incorrect. Actual results and the timing of events could differ materially from those anticipated in such forward-looking statements as a result of various risks and uncertainties, which include, without limitation, risks associated with the process of discovering, developing and commercializing drugs that are safe and effective for use as human therapeutics and in the endeavor of building a business around such drugs, and the risk additional toxicology data may be negative. In addition, while Regulus expects the COVID-19 pandemic to adversely affect its business operations and financial results, the extent of the impact on Regulus' ability to achieve its preclinical and clinical development objectives and the value of and market for its common stock, will depend on future developments that are highly uncertain and cannot be predicted with confidence at this time, such as the ultimate duration of the pandemic, travel restrictions, quarantines, social distancing and business closure requirements in the U.S. and in other countries, and the effectiveness of actions taken globally to contain and treat the disease. These and other risks are described in additional detail in Regulus' filings with the Securities and Exchange Commission, including under the "Risk Factors" heading of Regulus most recently quarterly report on Form 10-Q. All forward-looking statements contained in this press release speak only as of the date on which they were made. Regulus undertakes no obligation to update such statements to reflect events that occur or circumstances that exist after the date on which they were made.
View original content to download multimedia:https://www.prnewswire.com/news-releases/regulus-therapeutics-announces-receipt-of-fda-orphan-drug-designation-for-rgls8429-for-the-treatment-of-autosomal-dominant-polycystic-kidney-disease-adpkd-301571856.html
SOURCE Regulus Therapeutics Inc.
Copyright 2022 PR Newswire
Good news on FDA orphan drug,
RGLS
Another nice move.
RGLS
Lol! I'm not in, never been, but I'm hopeful that no one took the bait on this "news" release. I hope everyone does great here, but I won't be one of them.
Anything these days for this company IMO was news. I would’ve taken the company newsletter of reporting a flat tire in the parking lot as news at this point. Lol
#DDAmanda Chart on: $RGLS :
You can scan for these before they run.
Contact/Text: 760 702-2009
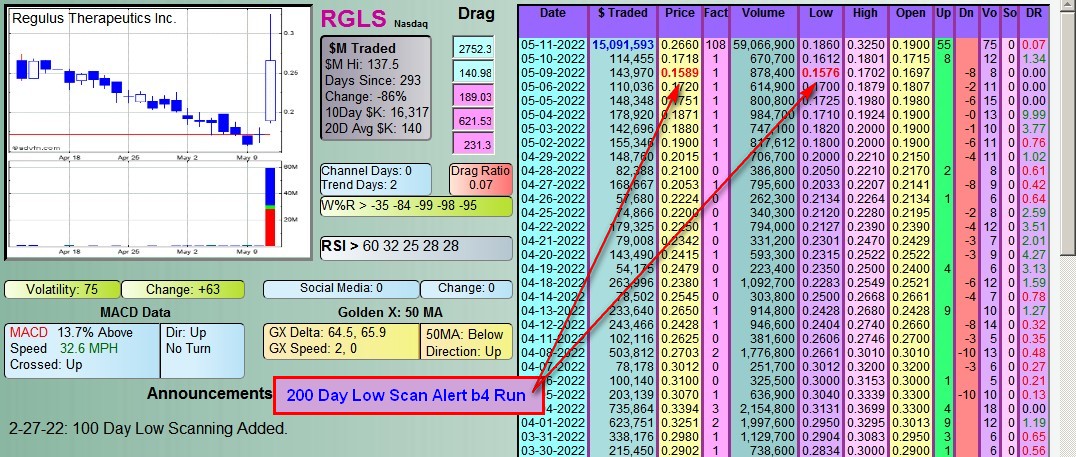
What the Fact (Factor) Column is:
The Factor is a proprietary indicator used for scanning in #DDAmanda.
It's defined as Today's $Traded divided by the average daily $Traded (20 day avg).
SO, if a stock has say a 10 Factor that day, it means she traded 10 Times the $ she normally trades.
That's significant, and many times indicates that a run in the stock is coming.

UP 80% on this little news. Holding some ...trading some.
RGLS
It's not even news. They "accepted" the application. I could go to Burger King right now and they'd "accept" my application!!
$pop$... 50%
RGLS
Nice news.
RGLS
Bid for new low..
RGLS
Premarket large bid. No sales. Buyout rumor?
RGLS
And now enrollments are completed for the Alport syndrome trial.
|
Followers
|
42
|
Posters
|
|
|
Posts (Today)
|
0
|
Posts (Total)
|
474
|
|
Created
|
10/11/12
|
Type
|
Free
|
| Moderators | |||
| Volume | |
| Day Range: | |
| Bid Price | |
| Ask Price | |
| Last Trade Time: |
