Register for free to join our community of investors and share your ideas. You will also get access to streaming quotes, interactive charts, trades, portfolio, live options flow and more tools.
News: $RDUS Radius Health Appoints Jessica Hopfield, Ph.D., to Board of Directors
WALTHAM, Mass., Jan. 25, 2019 (GLOBE NEWSWIRE) -- Radius Health, Inc. (“Radius” or the “Company”) (Nasdaq: RDUS), a science-driven fully integrated biopharmaceutical company that is committed to developing and commercializing innovative endocrine therapeutics in ...
In case you are interested https://marketwirenews.com/news-releases/radius-health-appoints-jessica-hopfield-ph-d-to-board-of-directors-7557440.html
http://www.streetedition.net/radius-health-rdus-december-short-interest-increases-by-22-7/6259886/
shorts UP and so it PPS, turn around time with analysts changing to strong buy and buy and hold
$RDUS recent news/filings
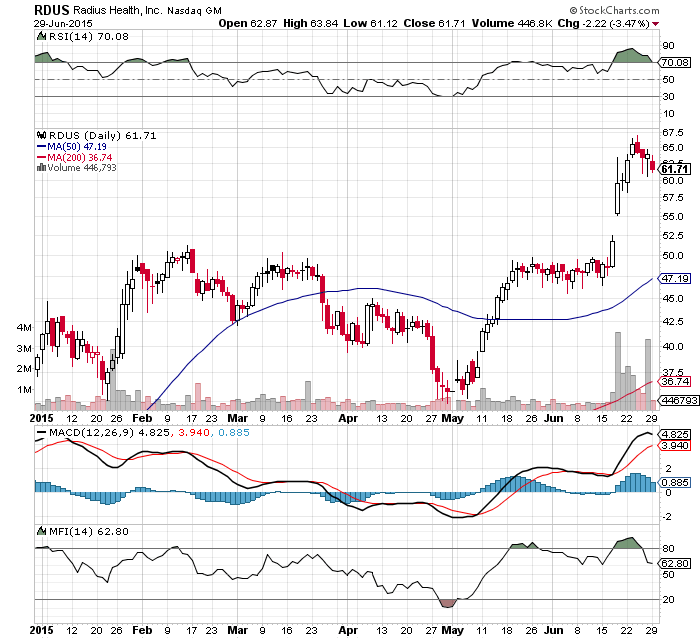
bullish 65.41
basic chart ## source: stockcharts.com
basic chart ## source: stockscores.com
big daily chart ## source: stockcharts.com
big weekly chart ## source: stockcharts.com
$RDUS DD Notes ~ http://www.ddnotesmaker.com/RDUS
## STOCK DETAILS ##
After Hours Quote (nasdaq.com): http://www.nasdaq.com/symbol/RDUS/after-hours
Option Chain (nasdaq.com): http://www.nasdaq.com/symbol/RDUS/option-chain
Historical Prices (yahoo.com): http://finance.yahoo.com/q/hp?s=RDUS+Historical+Prices
Company Profile (yahoo.com): http://finance.yahoo.com/q/pr?s=RDUS+Profile
Industry (yahoo.com): http://finance.yahoo.com/q/in?s=RDUS+Industry
## COMPANY NEWS ##
Market Stream (nasdaq.com): http://www.nasdaq.com/symbol/RDUS/stream
Latest news (otcmarkets.com): http://www.otcmarkets.com/stock/RDUS/news - http://finance.yahoo.com/q/h?s=RDUS+Headlines
## STOCK ANALYSIS ##
Analyst Research (nasdaq.com): http://www.nasdaq.com/symbol/RDUS/analyst-research
Guru Analysis (nasdaq.com): http://www.nasdaq.com/symbol/RDUS/guru-analysis
Stock Report (nasdaq.com): http://www.nasdaq.com/symbol/RDUS/stock-report
Competitors (nasdaq.com): http://www.nasdaq.com/symbol/RDUS/competitors
Stock Consultant (nasdaq.com): http://www.nasdaq.com/symbol/RDUS/stock-consultant
Stock Comparison (nasdaq.com): http://www.nasdaq.com/symbol/RDUS/stock-comparison
Investopedia (investopedia.com): http://www.investopedia.com/markets/stocks/RDUS/?wa=0
Research Reports (otcmarkets.com): http://www.otcmarkets.com/stock/RDUS/research
Basic Tech. Analysis (yahoo.com): http://finance.yahoo.com/q/ta?s=RDUS+Basic+Tech.+Analysis
Barchart (barchart.com): http://www.barchart.com/quotes/stocks/RDUS
## FUNDAMENTALS ##
Call Transcripts (nasdaq.com): http://www.nasdaq.com/symbol/RDUS/call-transcripts
Annual Report (companyspotlight.com): http://www.companyspotlight.com/library/companies/keyword/RDUS
Income Statement (nasdaq.com): http://www.nasdaq.com/symbol/RDUS/financials?query=income-statement
Revenue/EPS (nasdaq.com): http://www.nasdaq.com/symbol/RDUS/revenue-eps
SEC Filings (nasdaq.com): http://www.nasdaq.com/symbol/RDUS/sec-filings
Edgar filings (sec.gov): http://www.sec.gov/cgi-bin/browse-edgar?action=getcompany&CIK=0000320193&owner=exclude&count=40
Latest filings (otcmarkets.com): http://www.otcmarkets.com/stock/RDUS/filings
Latest financials (otcmarkets.com): http://www.otcmarkets.com/stock/RDUS/financials
Short Interest (nasdaq.com): http://www.nasdaq.com/symbol/RDUS/short-interest
Dividend History (nasdaq.com): http://www.nasdaq.com/symbol/RDUS/dividend-history
RegSho (regsho.com): http://www.regsho.com/tools/symbol_stats.php?sym=RDUS&search=search
OTC Short Report (otcshortreport.com): http://otcshortreport.com/index.php?index=RDUS
Short Sales (otcmarkets.com): http://www.otcmarkets.com/stock/RDUS/short-sales
Key Statistics (yahoo.com): http://finance.yahoo.com/q/ks?s=RDUS+Key+Statistics
Insider Roster (yahoo.com): http://finance.yahoo.com/q/ir?s=RDUS+Insider+Roster
Income Statement (yahoo.com): http://finance.yahoo.com/q/is?s=RDUS
Balance Sheet (yahoo.com): http://finance.yahoo.com/q/bs?s=RDUS
Cash Flow (yahoo.com): http://finance.yahoo.com/q/cf?s=RDUS+RDUSh+Flow&annual
## HOLDINGS ##
Major holdings (cnbc.com): http://data.cnbc.com/quotes/RDUS/tab/8.1
Insider transactions (yahoo.com): http://finance.yahoo.com/q/it?s=RDUS+Insider+Transactions
Insider transactions (secform4.com): http://www.secform4.com/insider-trading/RDUS.htm
Insider transactions (insidercrow.com): http://www.insidercow.com/history/company.jsp?company=RDUS
Ownership Summary (nasdaq.com): http://www.nasdaq.com/symbol/RDUS/ownership-summary
Institutional Holdings (nasdaq.com): http://www.nasdaq.com/symbol/RDUS/institutional-holdings
Insiders (SEC Form 4) (nasdaq.com): http://www.nasdaq.com/symbol/RDUS/insider-trades
Insider Disclosure (otcmarkets.com): http://www.otcmarkets.com/stock/RDUS/insider-transactions
## SOCIAL MEDIA AND OTHER VARIOUS SOURCES ##
PST (pennystocktweets.com): http://www.pennystocktweets.com/stocks/profile/RDUS
Market Watch (marketwatch.com): http://www.marketwatch.com/investing/stock/RDUS
Bloomberg (bloomberg.com): http://www.bloomberg.com/quote/RDUS:US
Morningstar (morningstar.com): http://quotes.morningstar.com/stock/s?t=RDUS
Bussinessweek (businessweek.com): http://investing.businessweek.com/research/stocks/snapshot/snapshot_article.asp?ticker=RDUS
StockTwits (stocktwits.com): http://stocktwits.com/symbol/RDUS
$RDUS DD Notes ~ http://www.ddnotesmaker.com/RDUS
$RDUS - $62.31 -6.87 (-9.93%) Radius Health submits Marketing Authorization Application for once-daily subcutaneous injection of abaloparatide, updates on timing for NDA submission
8:35 AM ET 11/17/15 | Briefing.com
Following the completion and reporting of the positive top line results for the Phase 3 ACTIVE trial and the first six months of ACTIVExtend, Radius has now submitted the MAA for the investigational drug abaloparatide-SC in the EU. The Company is now turning its focus to the completion of the work streams necessary for the submission of the New Drug Application to the FDA for the investigational drug abaloparatide-SC. For the MAA, the Company has submitted 6-month stability data for abaloparatide in accordance with European requirements. As additional data become available, they will be submitted in support of our targeted 3-year shelf life for the commercial product following receipt of regulatory approval.
Abaloparatide: In the US, the FDA requires that 12-month stability data be submitted at the time of the NDA. The 12-month time point for the abaloparatide stability samples will be reached in December 2015 and Radius expects to initiate data analysis in January 2016. To accommodate this analytic plan, the Company announced a change in the NDA submission timing to the end of the first quarter of 2016. Similar to the European submission, as additional stability data become available they will be submitted in support of Radius' targeted 3-year shelf life for the commercial product following receipt of regulatory approval. The Company believes that the stability data supporting both the MAA submission and the planned NDA submission will be sufficient to meet the regulatory standards for approval.Radius plans to commence a clinical evaluation for an optimized abaloparatide transdermal patch by the end of 2015.Radius confirmed that partnership discussions regarding abaloparatide commercialization are ongoing and that the Company expects to announce a partnership by the time of first commercial launch. Radius Health's ideal partner for abaloparatide would have experience in the development and commercialization of therapeutics to treat osteoporosis and related bone metabolic disorders.RAD1901:Radius plans to provide an update on the ongoing Phase 1 dose escalation study in metastatic breast cancer patients at the San Antonio Breast Cancer Symposium in December 2015. Radius expects to initiate an additional Phase I clinical trial in metastatic breast cancer patients in the European Union in December 2015. Radius intends to commence a Phase 2b trial for low-dose RAD1901 for vasomotor symptoms in December 2015.RAD140: Radius expects to commence a Phase 1 trial for the investigational drug RAD140 after making an Investigational New Drug submission in 2016.
Radius Health, Inc. (“Radius” or the “Company”) (Nasdaq:RDUS), a science-driven biopharmaceutical company focused on developing new therapeutics for patients with osteoporosis, hormone responsive metastatic breast cancer, and other postmenopausal conditions including vasomotor symptoms reported its financial results for the third quarter ended September 30, 2015, and provided recent corporate highlights. As of September 30, 2015, Radius had $500.8 million in cash, cash equivalents and marketable securities.
Lol...great, great day here. For what it's worth, various analysts all have price targets $85+ . The price collapse a few days ago was a gift, imo.
$RDUS - $65.14 +10.99 (+20.30%) What??? Nobody has anything negative to say today I guess
Tested support on the weekly chart today...$45 ought to be about it for lows, if not $35 should be the target. This went up ferociously so correcting that way ought to be expected no?
I m with you 100 percent on your reply. Since july 27th, aug 24th, and subsequently Sept 21 when hillary tweeted, maintaining the bio portfolio has been like herding cats on fire running toward the grand canyon. Good is bad, bad is bad, and even a hair of optimism is doused with an ocean of ice water.
Regarding RDUS, i cant tell if you r serious or not since it is a 2.3 bill dollar company so not 'value' in the classic non bio investor sense. However, if the data was nt one of those false positive doo doo presentations wrapped in roses it could be value again based on the size of the osteoporosis market. Full disclosure ive never owned the stock and i m not all too familiar with it but it has corrected ferociously.
The biotech lemmings are following a lot of herd action. I hold 60-70 individual biotech stocks, and many are down in share price from 2-3 months ago, good news or no news notwithstanding. Sector rotation, media attention constantly screaming that biotechs are in a bubble[following Yellen and Hillary lead] are part cause---I do disagree, as I do not detect the bubble that did exist in 2000-2002 time frame. IBB and FBIOX are not immune to down turn either at the moment.
Thanks for calling my attention to RDUS on the Value board.
what s with the recent brutality of late? Data delivered no?
you'll have to wait until Tuesday
Ciciagt,
Great news!!
GLTY
Here's for Monday's pop...
http://www.bizjournals.com/boston/blog/bioflash/2015/10/radius-health-says-osteoporosis-drug-lowers-bone.html
A Waltham biotech that plans to seek approval of its first drug by the end of the year says that a late-stage trial of more than 2,400 postmenopausal women with osteoporosis shows its daily injection lowers bone fractures by 70 percent after a year-and-a-half.
Seems like textbook volatility to me as the rest of the market has experienced...good luck!
starbuxsux
Good For You! You did alright.
Looking at the 3 month graph
July Aug Sept
high 80 high 73 High 75
low 73 low 66 *** low 56
Just very inconsistent.
Shares of Radius Health, Inc. (NASDAQ:RDUS) rose by 10.17% in the past week and 18.78% for the last 4 weeks. In the past week, the shares have outperformed the S&P 500 by 17.32% and the outperformance increases to 77.43% for the last 4 weeks.
Radius Health, Inc. is up 25% in the last 3-month period. Year-to-Date the stock performance stands at 92.75%. The company shares have rallied 390.2% in the past 52 Weeks. On July 15, 2015 The shares registered one year high of $84.64 and one year low was seen on September 22, 2014 at $14.65. The 50-day moving average is $67.23 and the 200 day moving average is recorded at $55.79. S&P 500 has rallied 1.82% during the last 52-weeks.
Radius Health, Inc. (NASDAQ:RDUS) : On Friday heightened volatility was witnessed in Radius Health, Inc. (NASDAQ:RDUS) which led to swings in the share price. The shares opened for trading at $72.4 and hit $75 on the upside , eventually ending the session at $75, with a gain of 3.18% or 2.31 points. The heightened volatility saw the trading volume jump to 1,292,142 shares. The 52-week high of the share price is $84.64 and the company has a market cap of $3,209 million. The 52-week low of the share price is at $14.1 .
Radius Health, Inc. (NASDAQ:RDUS): 6 Analyst have given the stock of Radius Health, Inc. (NASDAQ:RDUS) a near short term price target of $73.83. The standard deviation reading, which is a measure by which the stock price is expected to swing away from the mean estimate, is at $18.86. The higher price target estimate is at $90 while the lower price estimates are fixed at $50.
On a different note, The Company has disclosed insider buying and selling activities to the Securities Exchange, The Securities and Exchange Commission has divulged in a Form 4 filing that the officer (SVP Global Oncology/Commercial) of Radius Health, Inc., Purandare Dinesh had purchased shares worth of $12,440 in a transaction dated on August 31, 2015. A total of 200 shares were purchased at a price of $62.2 per share. The information is based on open market trades at the market prices.Option exercises are not covered. Currently the company Insiders own 37.4% of Radius Health, Inc. Company shares. In the past six months, there is a change of 2.73% in the total insider ownership. Institutional Investors own 73.5% of Company shares. During last 3 month period, 7.07% of total institutional ownership has changed in the company shares.
Radius Health, Inc. is a science-driven biopharmaceutical company focused on developing therapeutics for patients with osteoporosis, as well as other serious endocrine-mediated diseases. The Companys lead product candidate is abaloparatide (BA058), a bone anabolic for the treatment of osteoporosis delivered via subcutaneous injection, which it refers to as Abaloparatide-SC. The Company is leveraging its investment in Abaloparatide-SC to develop Abaloparatide-TD. The Companys clinical product portfolio also includes a oral agent, RAD1901, a selective estrogen receptor down-regulator/degrader, or SERD. The Companys Abaloparatide is a synthetic peptide analog of parathyroid hormone-related protein (PTHrP), that the Company is developing as a bone anabolic treatment for osteoporosis. The Company is also developing RAD1901 at lower doses as a selective estrogen receptor modulator (SERM), for the treatment of vasomotor symptoms.
http://newswatchinternational.com/news/company-shares-of-radius-health-inc-nasdaqrdus-rally-10-17.html
I would love to hear why you claim RDUS looks like a scam company. Scam or no scam I rode this from $20's to $80's so she's a darling of mine.
Starbuxsux
Whats' wrong? I posted a comment problem?
Looks like another scam company.
Radius Health Announced That it Has Appointed Catherine Friedman to the Board of Directors
Aug 18, 2015 07:00:00 (ET)
Radius Health Announced That it Has Appointed Catherine Friedman to the Board of Directors
WALTHAM, Mass., Aug. 18, 2015 (GLOBE NEWSWIRE) -- Radius Health, Inc. (Nasdaq:RDUS), a science-driven biopharmaceutical company developing new therapeutics for patients with advanced osteoporosis as well as other serious endocrine-mediated diseases including hormone responsive metastatic breast cancer, today announced that Catherine Friedman, an independent consultant and former Co-Head of the Biotechnology Practice at Morgan Stanley, has been elected to its Board of Directors. Cathy has also been appointed Chair of the Audit Committee of the Board of Directors, replacing Dr. Muenchbach, who has resigned from the Board effective November 3, 2015, but will remain on the Board until that date in order to ensure a smooth transition.
"We are pleased to have attracted someone with Cathy's depth and breadth of financial experience to the Radius Board as we prepare for the future commercialization of our investigational drug abaloparatide-SC, following regulatory submissions and subject to favorable review by the authorities," said Radius Health CEO, Robert E. Ward. "We also would like to thank Martin for his important contributions over the years, in particular in building Radius' solid financial foundation, which enables us to complete development and prepare the regulatory submissions for abaloparatide-SC, while advancing the rest of our pipeline and building our organization."
Catherine Friedman has served as an independent consultant serving public and private growth companies since 2006. Prior to that, Ms. Friedman held the position of Managing Director at Morgan Stanley from 1997 to 2006 and head of West Coast Healthcare and co-head of the Biotechnology Practice at Morgan Stanley from 1993 to 2006. She originally joined Morgan Stanley in 1982 and worked in its New York, London and Silicon Valley offices. Since 2007, Ms. Friedman has been a director of XenoPort Inc., where she serves as Chair of the Audit and Governance Committee, Enteromedics, where she serves as the Chair of the Audit Committee and Governance Committee, and in 2014, she joined the Board of Theravance and serves on its Audit and Governance Committees. Ms. Friedman joined the board of GSV Capital, a publicly traded investment fund, in 2013 and serves as a member of the Valuation Committee and as the chair of the Audit Committee. Ms. Friedman is a member of the Board of Trustees for Sacred Heart Schools in Atherton. She is a graduate of Harvard University and received an MBA from the University of Virginia Darden School of Business.
About Radius Health
Radius is a science-driven biopharmaceutical company developing new therapeutics for patients with advanced osteoporosis as well as other serious endocrine-mediated diseases including hormone responsive cancers. Radius' lead development candidate is the investigational drug abaloparatide for subcutaneous injection, currently in Phase 3 development for potential use in the reduction of fracture risk in postmenopausal women with severe osteoporosis. The Radius clinical portfolio also includes an investigational abaloparatide transdermal patch for potential use in osteoporosis and the investigational drug RAD1901 for potential use in hormone driven, or hormone resistant, metastatic breast cancer, including breast cancer brain metastases. www.radiuspharm.com
Forward Looking Statements
This press release contains forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995. All statements contained in this press release that do not relate to matters of historical fact should be considered forward-looking statements, including statements regarding the commercialization of abaloparatide-SC, our ability to complete the development of abaloparatide-SC, advance other drug product candidates and build our company; and the progress and success of abaloparatide-SC in the regulatory process.
These forward-looking statements are based on management's current expectations. These statements are neither promises nor guarantees, but involve known and unknown risks, uncertainties and other important factors that may cause our actual results, performance or achievements to be materially different from any future results, performance or achievements expressed or implied by the forward-looking statements, including, but not limited to, the following: we have no product revenues; our need for additional funding, which may not be available; we are not currently profitable and may never become profitable; risks related to raising additional capital; our limited operating history; quarterly fluctuation in our financial results; our dependence on the success of abaloparatide-SC, and our inability to ensure that abaloparatide-SC will obtain regulatory approval or be successfully commercialized; risks related to clinical trials, including having most of our products in early stage clinical trials and uncertainty that results will support our product candidate claims; the risk that adverse side effects will be identified during the development of our product candidates; product candidates for which we obtain marketing approval, if any, could be subject to restrictions or withdrawal from the market and we may be subject to penalties; failure to achieve market acceptance of our product candidates; risks related to the use of our limited resources on particular product candidates and not others; delays in enrollment of patients in our clinical trials, which could delay or prevent regulatory approvals; the dependence of our drug development program upon third-parties who are outside of our control; the risk that a regulatory or government official will determine that third-parties with a financial interest in the outcome of the Phase 3 study of abaloparatide-SC affected the reliability of the data from the study; our reliance on third parties to formulate and manufacture our product candidates; failure to establish additional collaborations; our lack of experience selling, marketing and distributing products and our lack of internal capability to do so; failure to compete successfully against other drug companies; developments by competitors may render our products or technologies obsolete or non-competitive; risks related to the fact that our drugs may sell for inadequate prices or patients may be unable to obtain adequate reimbursement; the effects of product liability lawsuits on commercialization of our products; failure to comply with obligations of our intellectual property licenses; failure to protect our intellectual property or failure to secure necessary intellectual property related to abaloparatide-SC, abaloparatide-TD, RAD1901 and/or RAD140; our or our licensors' inability to obtain and maintain patent protection for technology and products; risks related to our compliance with patent application and maintenance requirements; failure to protect the confidentiality of our trade secrets; risks related to our infringement of third parties' rights; risks associated with intellectual property litigation, including expending substantial resources and distracting personnel from their normal responsibilities; risks associated with healthcare reform; our failure to comply with healthcare laws and regulations; our exposure to claims associated with the use of hazardous materials and chemicals; as we become involved in drug commercialization, risk related to our inability to successfully manage our growth and expanded operations; risks relating to business combinations and acquisitions; our reliance on key executive officers and advisors; our inability to hire additional qualified personnel; volatility in the price of our common stock; capital appreciation is the only source of gain for our common stock; risks related to increased costs and compliance initiatives associated with operating as a public company; our directors, executive officers and principal stockholders have substantial influence over us and could delay or prevent a change in control; future sales and issuances of our common stock could depress the price of our common stock; risks related to securities or industry analysts ceasing to publish research about us or publishing inaccurate or unfavorable information about us, which could cause the price of our common stock to decline; provisions in our charter documents and Delaware law that could discourage takeover attempts; and our ability to use our net operating loss carryforwards and certain other tax attributes may be limited. These and other important factors discussed under the caption "Risk Factors" in our Quarterly Report on Form 10-Q filed with the Securities and Exchange Commission, or SEC, on August 6, 2015, and our other reports filed with the SEC could cause actual results to differ materially from those indicated by the forward-looking statements made in this press release. Any such forward-looking statements represent management's estimates as of the date of this press release. While we may elect to update such forward-looking statements at some point in the future, we disclaim any obligation to do so, even if subsequent events cause our views to change. These forward-looking statements should not be relied upon as representing our views as of any date subsequent to the date of this press release.
CONTACT: Investor Relations
Barbara Ryan
Clermont Partners
Partner
203-274-2825
Bryan@radiuspharm.com
(MORE TO FOLLOW) Dow Jones Newswires
August 18, 2015 07:00 ET (11:00 GMT)
$RDUS - Radius Health, Inc. Announces Pricing of Public Offering
8:03 PM ET 7/22/15 | GlobeNewswire
Radius Health, Inc. (NASDAQ:RDUS) (the "Company"), a science-driven biopharmaceutical company focused on developing new therapeutics for patients with advanced osteoporosis as well as other serious endocrine-mediated diseases, including hormone responsive metastatic breast cancer, today announced that it has priced its public offering of 4,054,054 shares of its common stock at a public offering price of $74.00 per share. In addition, the Company has granted the underwriters an option to purchase up to an additional 608,108 shares of its common stock, exercisable for 30 days.
J.P. Morgan, BofA Merrill Lynch and Deutsche Bank Securities are acting as joint book-running managers for the offering. Cowen and Company is acting as lead manager.
The offering is being made pursuant to an effective shelf registration statement on Form S-3 filed with the Securities and Exchange Commission on January 20, 2015. This press release shall not constitute an offer to sell, or the solicitation of an offer to buy, nor shall there be any sale of shares of these securities in any state or jurisdiction in which such offer, solicitation or sale would be unlawful prior to registration or qualification under the securities laws of any such state or jurisdiction.
A preliminary prospectus supplement describing the terms of the offering has been filed with the Securities and Exchange Commission and forms a part of the effective registration statement. Copies of the final prospectus supplement and accompanying prospectus relating to the offering may be obtained, when available, by contacting J.P. Morgan Securities LLC, Attention: Broadridge Financial Solutions, 1155 Long Island Avenue, Edgewood, New York 11717, or by telephone at 866-803-9204, or email at prospectus-eq_fi@jpmchase.com; BofA Merrill Lynch, Attention: Prospectus Department, 222 Broadway, New York, NY 10038, email: dg.prospectus_requests@baml.com; or Deutsche Bank Securities Inc., Attention: Prospectus Group, 60 Wall Street, New York, NY 10005-2836, via telephone at 800-503-4611 or via e-mail: prospectus.cpdg@db.com.
CONTACT: Investor Relations
Barbara Ryan
Investor Relations
Radius Health, Inc.
203-274-2825
bryan@radiuspharm.com
http://www.globenewswire.com/newsroom/ti?nf=MTMjMTAxNDI4MDEjMzExNDk=
OK that's it much to crowded here,,
Most active board!!
$RDUS - $83.03 +3.13 (+3.92%) WEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEE!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!
Got in a few weeks ago and so happy i did, just a few shares..But very happy great story the meetings yesterday should help sounds like great pipeline..
$RDUS - $80.08 +6.73 (+9.18%) WOW! JUST WOW!
THANKS for the up date
Radius Health Announces Multiple Presentations at the American Society for Bone and Mineral Research 2015 Annual Meeting
7:00 AM ET 7/14/15 | GlobeNewswire
Radius Health, Inc. (Nasdaq:RDUS), a science-driven biopharmaceutical company focused on developing new therapeutics for patients with osteoporosis as well as other serious endocrine-mediated diseases, including hormone responsive breast cancer, announced today that it will present new data from multiple studies of abaloparatide in women with postmenopausal osteoporosis at the American Society for Bone and Mineral Research (ASBMR) 2015 Annual Meeting, October 9-12, 2015, at the Washington State Convention Center in Seattle, Washington, USA.
The company will be presenting a responder analysis of the effects of abaloparatide and teriparatide on bone mineral density in postmenopausal women with osteoporosis, the effects of abaloparatide on major osteoporotic fracture incidence as well as pharmacokinetic data from the transdermal patch optimization program. Abstracts summarizing the abaloparatide data are published on the ASBMR website and are available to conference registrants and ASBMR members at: http://www.asbmr.org/ASBMR-abstracts.
Abstracts related to Radius Health include:
Abstract Title: Eighteen Months of Treatment with Abaloparatide Followed by Six Months of Treatment with Alendronate in Postmenopausal Women with Osteoporosis - Results of the ACTIVExtend Trial
Presenter: Felicia Cosman MD, Professor of Clinical Medicine, Columbia University, New York, Clinical Director, National Osteoporosis Foundation
Presentation Number: 1142
Presentation Type: Oral Presentations
Presentation Date/Time: October 12 10:15 AM - 10:30 AM
Session: Plenary Orals: John H. Carsten's Memorial Session: Osteoporosis Session
Session Date/Time: October 12 10:00 AM - 11:30 AM
Room/Details: Room 6 E, Washington State Convention Center
Abstract Title: Response Rates for Hip, Femoral Neck and Lumbar Spine BMD are Higher for Patients Treated with Abaloparatide when Compared to Placebo or Teriparatide - Results of the ACTIVE Trial
Presentation Number: FR0333
Presentation Type: Plenary Sessions
Presentation Date/Time: October 9 5:30 PM - 7:00 PM
Session: Welcome Reception & Plenary Poster Session
Session Date/Time: October 9 5:30 PM - 7:00 PM
Location: Discovery Hall - Hall 4B, Washington State Convention Center
Abstract Title: Effects of Abaloparatide on Major Osteoporotic Fracture Incidence in Postmenopausal Women with Osteoporosis - Results of the Phase 3 ACTIVE Trial
Presentation Number: 1053
Presentation Type: Oral Presentations
Presentation Date/Time: October 10 3:00 PM - 3:15 PM
Session: Concurrent Orals: Osteoporosis Therapy and Management
Session Date/Time: October 10 2:30 PM - 4:00 PM
Location: Hall 4A, Washington State Convention Center
Abstract Title: Transdermal Delivery of Abaloparatide: Optimization of the Pharmacokinetic Profile in Cynomologus Monkeys
Presentation Number: SA0335
Presentation Type: Poster Sessions
Presentation Date/Time: October 10 12:30 PM - 2:30 PM
Session: Poster Session I & Poster Tours
Session Date/Time: 10/10/15 12:30 PM - 2:30 PM
Location: Discovery Hall - Hall 4B, Washington State Convention Center
About the Investigational Drug Abaloparatide
Radius' investigational drug abaloparatide is a synthetic peptide analog of human parathyroid hormone-related protein (hPTHrP), a naturally occurring bone-building hormone that the company believes has the potential to increase bone mineral density by stimulating new bone formation. Abaloparatide-SC is an investigational drug currently completing Phase 3 development for potential use as a daily self-administered injection for the treatment of patients with postmenopausal osteoporosis at high risk of fracture. Radius also is developing the investigational drug abaloparatide-TD for potential use as a short wear-time transdermal patch designed to administer abaloparatide without the need for subcutaneous injection based on 3M's patented Microstructured Transdermal System technology.
About Radius Health
Radius is a science-driven biopharmaceutical company developing new therapeutics for patients with advanced osteoporosis as well as other serious endocrine-mediated diseases. Radius' lead development candidate is the investigational drug abaloparatide (BA058) for subcutaneous injection, which is completing Phase 3 development for the reduction of fracture risk in postmenopausal women with severe osteoporosis. The Radius clinical portfolio also includes an investigational abaloparatide transdermal patch for the treatment of osteoporosis and the investigational drug RAD1901 for the treatment of hormone driven, or hormone resistant, metastatic breast cancer. www.radiuspharm.com
Forward-Looking Statements
This press release contains forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995. All statements contained in this press release that do not relate to matters of historical fact should be considered forward-looking statements, including without limitation, statements regarding our presentations at ASBMR.
These forward-looking statements are based on management's current expectations. These statements are neither promises nor guarantees, but involve known and unknown risks, uncertainties and other important factors that may cause our actual results, performance or achievements to be materially different from any future results, performance or achievements expressed or implied by the forward-looking statements, including, but not limited to, the following: our dependence on the success of abaloparatide-SC, and our inability to ensure that abaloparatide-SC will obtain regulatory approval in the timeframe anticipated or at all or be successfully commercialized; we have no product revenues; our need for additional funding, which may not be available; we are not currently profitable and may never become profitable; restrictions imposed on our business by our credit facility, and risks related to default on our obligations under our credit facility; risks related to raising additional capital; our limited operating history; quarterly fluctuation in our financial results; risks related to clinical trials, including having most of our products in early stage clinical trials and uncertainty that results will support our product candidate claims; the risk that adverse side effects will be identified during the development of our product candidates; product candidates for which we obtain marketing approval, if any, could be subject to restrictions or withdrawal from the market and we may be subject to penalties; failure to achieve market acceptance of our product candidates; risks related to the use of our limited resources on particular product candidates and not others; delays in enrollment of patients in our clinical trials, which could delay or prevent regulatory approvals; the dependence of our drug development program upon third-parties who are outside our control; the risk that a regulatory or government official will determine that third-parties with a financial interest in the outcome of the Phase 3 study of abaloparatide-SC affected the reliability of the data from the study; our reliance on third parties to formulate and manufacture our product candidates; failure to establish additional collaborations; our lack of experience selling, marketing and distributing products and our lack of internal capability to do so; failure to compete successfully against other drug companies; developments by competitors may render our products or technologies obsolete or non-competitive; risks related to the fact that our drugs may sell for inadequate prices or patients may be unable to obtain adequate reimbursement; effects of product liability lawsuits on commercialization of our products; failure to comply with obligations of our intellectual property licenses; failure to protect our intellectual property or failure to secure necessary intellectual property related to abaloparatide-SC, abaloparatide-TD, RAD-1901 and/or RAD-140; our or our licensors' inability to obtain and maintain patent protection for technology and products; risks related to our compliance with patent application requirements; failure to protect the confidentiality of our trade secrets; risks related to our infringement of third parties' rights; risks related to employees' disclosure of former employers' trade secrets; risks associated with intellectual property litigation, including expending substantial resources and distracting personnel from their normal responsibilities; risks associated with healthcare reform; our failure to comply with healthcare laws and regulations; our exposure to claims associated with the use of hazardous materials and chemicals; inability to successfully manage our growth; risks relating to business combinations and acquisitions; our reliance on key executive officers and advisors; our inability to hire additional qualified personnel; volatility in the price of our common stock; capital appreciation is the only source of gain for our common stock; risks related to increased costs and compliance initiatives associated with operating as a public company; our directors, executive officers and principal stockholders have substantial control over us and could delay or prevent a change in control; future sales of our common stock could depress the price of our common stock; inaccurate or unfavorable information about us could cause the price of our common stock to decline; provisions in our charter documents and Delaware law could discourage takeover attempts; and our ability to use our net operating loss carryforwards and certain other tax attributes may be limited. These and other important factors discussed under the caption "Risk Factors" in our Annual Report on Form 10-K filed with the Securities and Exchange Commission, or SEC, on March 10, 2015, and our most recent quarterly and other reports filed with the SEC could cause actual results to differ materially from those indicated by the forward-looking statements made in this press release. Any such forward-looking statements represent management's estimates as of the date of this press release. While we may elect to update such forward-looking statements at some point in the future, we disclaim any obligation to do so, even if subsequent events cause our views to change. These forward-looking statements should not be relied upon as representing our views as of any date subsequent to the date of this press release.
CONTACT: Investor Relations
Barbara Ryan
Clermont Partners
Partner
203-274-2825
bryan@radiuspharm.com
http://www.globenewswire.com/newsroom/ti?nf=MTMjMTAxNDE1NjAjMzExNDk=
$RDUS recent news/filings
bullish
strong green CMF indicator and climbing acc/dist line indicates people are buyers
higher highs, higher lows uptrend
basic chart ## source: stockcharts.com
basic chart ## source: stockscores.com
big daily chart ## source: stockcharts.com
big weekly chart ## source: stockcharts.com
$RDUS DD Notes ~ http://www.ddnotesmaker.com/RDUS
## STOCK DETAILS ##
After Hours Quote (nasdaq.com): http://www.nasdaq.com/symbol/RDUS/after-hours
Option Chain (nasdaq.com): http://www.nasdaq.com/symbol/RDUS/option-chain
Historical Prices (yahoo.com): http://finance.yahoo.com/q/hp?s=RDUS+Historical+Prices
Company Profile (yahoo.com): http://finance.yahoo.com/q/pr?s=RDUS+Profile
Industry (yahoo.com): http://finance.yahoo.com/q/in?s=RDUS+Industry
## COMPANY NEWS ##
Market Stream (nasdaq.com): http://www.nasdaq.com/symbol/RDUS/stream
Latest news (otcmarkets.com): http://www.otcmarkets.com/stock/RDUS/news - http://finance.yahoo.com/q/h?s=RDUS+Headlines
## STOCK ANALYSIS ##
Analyst Research (nasdaq.com): http://www.nasdaq.com/symbol/RDUS/analyst-research
Guru Analysis (nasdaq.com): http://www.nasdaq.com/symbol/RDUS/guru-analysis
Stock Report (nasdaq.com): http://www.nasdaq.com/symbol/RDUS/stock-report
Competitors (nasdaq.com): http://www.nasdaq.com/symbol/RDUS/competitors
Stock Consultant (nasdaq.com): http://www.nasdaq.com/symbol/RDUS/stock-consultant
Stock Comparison (nasdaq.com): http://www.nasdaq.com/symbol/RDUS/stock-comparison
Investopedia (investopedia.com): http://www.investopedia.com/markets/stocks/RDUS/?wa=0
Research Reports (otcmarkets.com): http://www.otcmarkets.com/stock/RDUS/research
Basic Tech. Analysis (yahoo.com): http://finance.yahoo.com/q/ta?s=RDUS+Basic+Tech.+Analysis
Barchart (barchart.com): http://www.barchart.com/quotes/stocks/RDUS
DTCC (dtcc.com): http://search2.dtcc.com/?q=Apple%2C+Inc.&x=10&y=8&sp_p=all&sp_f=ISO-8859-1
Spoke company information (spoke.com): http://www.spoke.com/search?utf8=%E2%9C%93&q=Apple%2C+Inc.
Corporation WIKI (corporationwiki.com): http://www.corporationwiki.com/search/results?term=Apple%2C+Inc.&x=0&y=0
WHOIS (domaintools.com): http://whois.domaintools.com/http://www.apple.com/pr
Alexa (alexa.com): http://www.alexa.com/siteinfo/http://www.apple.com/pr#
Corporate website internet archive (archive.org): http://web.archive.org/web/*/http://www.apple.com/pr
## FUNDAMENTALS ##
Call Transcripts (nasdaq.com): http://www.nasdaq.com/symbol/RDUS/call-transcripts
Annual Report (companyspotlight.com): http://www.companyspotlight.com/library/companies/keyword/RDUS
Income Statement (nasdaq.com): http://www.nasdaq.com/symbol/RDUS/financials?query=income-statement
Revenue/EPS (nasdaq.com): http://www.nasdaq.com/symbol/RDUS/revenue-eps
SEC Filings (nasdaq.com): http://www.nasdaq.com/symbol/RDUS/sec-filings
Edgar filings (sec.gov): http://www.sec.gov/cgi-bin/browse-edgar?action=getcompany&CIK=0000320193&owner=exclude&count=40
Latest filings (otcmarkets.com): http://www.otcmarkets.com/stock/RDUS/filings
Latest financials (otcmarkets.com): http://www.otcmarkets.com/stock/RDUS/financials
Short Interest (nasdaq.com): http://www.nasdaq.com/symbol/RDUS/short-interest
Dividend History (nasdaq.com): http://www.nasdaq.com/symbol/RDUS/dividend-history
RegSho (regsho.com): http://www.regsho.com/tools/symbol_stats.php?sym=RDUS&search=search
OTC Short Report (otcshortreport.com): http://otcshortreport.com/index.php?index=RDUS
Short Sales (otcmarkets.com): http://www.otcmarkets.com/stock/RDUS/short-sales
Key Statistics (yahoo.com): http://finance.yahoo.com/q/ks?s=RDUS+Key+Statistics
Insider Roster (yahoo.com): http://finance.yahoo.com/q/ir?s=RDUS+Insider+Roster
Income Statement (yahoo.com): http://finance.yahoo.com/q/is?s=RDUS
Balance Sheet (yahoo.com): http://finance.yahoo.com/q/bs?s=RDUS
Cash Flow (yahoo.com): http://finance.yahoo.com/q/cf?s=RDUS+RDUSh+Flow&annual
## HOLDINGS ##
Major holdings (cnbc.com): http://data.cnbc.com/quotes/RDUS/tab/8.1
Insider transactions (yahoo.com): http://finance.yahoo.com/q/it?s=RDUS+Insider+Transactions
Insider transactions (secform4.com): http://www.secform4.com/insider-trading/RDUS.htm
Insider transactions (insidercrow.com): http://www.insidercow.com/history/company.jsp?company=RDUS
Ownership Summary (nasdaq.com): http://www.nasdaq.com/symbol/RDUS/ownership-summary
Institutional Holdings (nasdaq.com): http://www.nasdaq.com/symbol/RDUS/institutional-holdings
Insiders (SEC Form 4) (nasdaq.com): http://www.nasdaq.com/symbol/RDUS/insider-trades
Insider Disclosure (otcmarkets.com): http://www.otcmarkets.com/stock/RDUS/insider-transactions
## SOCIAL MEDIA AND OTHER VARIOUS SOURCES ##
PST (pennystocktweets.com): http://www.pennystocktweets.com/stocks/profile/RDUS
Market Watch (marketwatch.com): http://www.marketwatch.com/investing/stock/RDUS
Bloomberg (bloomberg.com): http://www.bloomberg.com/quote/RDUS:US
Morningstar (morningstar.com): http://quotes.morningstar.com/stock/s?t=RDUS
Bussinessweek (businessweek.com): http://investing.businessweek.com/research/stocks/snapshot/snapshot_article.asp?ticker=RDUS
StockTwits (stocktwits.com): http://stocktwits.com/symbol/RDUS
$RDUS DD Notes ~ http://www.ddnotesmaker.com/RDUS
Confidential Treatment Order (ct Order)
Date : 07/07/2015 @ 12:35PM
Source : Edgar (US Regulatory)
Stock : Radius Health, Inc. (MM) (RDUS)
Quote : 70.4 0.21 (0.30%) @ 8:10PM
Why Radius Health Inc Stock Skyrocketed Today
Shares of pharmaceutical company Radius Health soared today after posting great clinical outcome news.
Although we don't believe in timing the market or panicking over market movements, we do like to keep an eye on big changes -- just in case they're material to our investing thesis.
What: Shares of Radius Health (NASDAQ: RDUS), a pharmaceutical company focused on developing products designed to treat osteoporosis, were up more than 17% today on heavy trading volume after the company posted positive clinical data related to their investigational drug abaloparatide.
So what: In the release, the company noted that patients who previously completed the 18 month long phase 3 ACTIVE clinical trial that were now participating in the follow on ACTIVExtend trial experienced no new vertebral fractures through the first 6 month of the follow on trial. In total, the 25 month long study period of abaloparatide-SC, which is the injectable form of the companies drug abaloparatide, continued to show positive clinical outcomes including huge reductions in vertebral, non-vertebral, clinical and major osteoporotic fractures.
The company believes that these trials show that the drug has met the primary and secondary endpoints, which are essential to helping the company prepare the drug for regulatory submission.
Now what: Investors were correct to cheer the news today, which may help the company get one step closer to bringing their first drug to market. Osteoporosis is a huge problem, as the International Osteoporosis Foundation estimates that more than 200 million people worldwide have the disease, which is responsible for more then 8.9 million fractures annually. Treating the disease is big business, as in 2014 alone global sales of osteoporosis therapies totaled $6.4 billion, with $2.3 billion of that number coming from injectable drugs.
Radius is trying to enter a big market, but investors should remember that Radius is still a high risk company that is currently burning more than $60 million in operating cash per year as it works to develop abaloparatide. However, with more than $230 million in cash on the balance sheet, the company appears to have plenty of reserves for the next few years as they work to bring their first drug to market.
Looking ahead, analysts are bullish on the potential for abaloparatide, and the peak sales estimates I have seen are pretty close to or maybe a little above a billion dollars. While there is still plenty of work to do before the company will see any revenue from this drug, today's announcement is certainly a great sign for the company.
Stockcharts @ RDUS
Like this stock opportunity so took a starter position today!
Radius Health Up on Positive Data on Fracture Candidate - Analyst Blog
http://finance.yahoo.com/news/radius-health-positive-data-fracture-155003307.html
Investor 100
|
Followers
|
9
|
Posters
|
|
|
Posts (Today)
|
0
|
Posts (Total)
|
116
|
|
Created
|
11/07/12
|
Type
|
Free
|
| Moderators | |||
| Volume | |
| Day Range: | |
| Bid Price | |
| Ask Price | |
| Last Trade Time: |
