Register for free to join our community of investors and share your ideas. You will also get access to streaming quotes, interactive charts, trades, portfolio, live options flow and more tools.
Under $1….I wonder how that offering went lol
bought 25,000 shares at 1.14 average
ready to DUMP after the CC tomorrow morning
This is perhaps the best explanation of the share price action here I have ever heard
Dilution too little money chasing too many shares
I’m not sure how to read those filings. Did they get all they were looking for in that offering? Seems weird $1.60 a share and this is barely holding $1.20
Israeli stonks are notorious for pump and dumps
A pump will begin two nanoseconds after the moomoo session opens for orders at 4am when it’s 11am in Tel Aviv
How long the pump lasts varies
Others may disagree with what I think is the usual senario for Israeli stonks
QNRX was done well before the 7am platforms open for orders
Sometimes the pump can continue after 7am when it’s 2pm in Tel Aviv
Most times, though put a for in it it’s done and the Tel Aviv players go out for dinner
I got some more 19’s. I don’t think an offering at 1.60 was issued for no reason. Insiders think this is going higher. Hit $2’s premarket.
I think we can see a nice move. We will see
Not bad. This can run.
I think it did
Did this hit $2 in premarket?
Wash trade bot running
Wash trade bot running
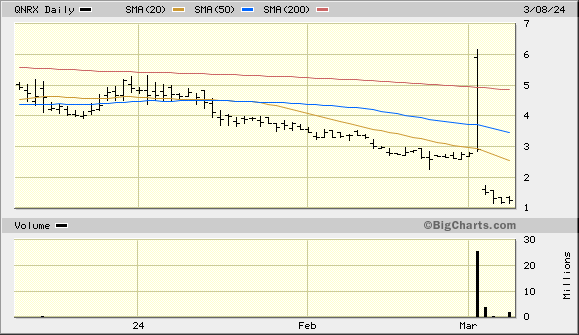
Thanks. Curious to see what happens. This ran to $6 recently
No, to create a wash trade run
Is that just to drop the stock?
Hong Kong, mainland Chinese, and Malaysian stonks are notorious for announcing an offering and then withdrawing it
What does that mean if they withdraw? It runs?
You just might
And then this Israeli stonk witndraws the offering and it sets off a wash trade bot rally
Offering closes tomorrow…. Wonder what happens to the price. We gonna see $1 and under?
Bid sitting at .94 - I always bid 30-50% off offering price. Either it comes to me or I pass.
It would be crazy if this goes to .28 again after the split
Looks like they are going to do a rinse and repeat.
Maybe $1 is coming
This is lower than what the offering is. Why wouldn’t anyone just buy open market??
Quoin Pharmaceuticals Announces FDA Clearance to Recruit Teen Subjects into Both Ongoing Netherton Syndrome Clinical Studies
$QNRX
Quoin Pharmaceuticals Announces FDA Clearance to Recruit Teen Subjects into Both Ongoing Netherton Syndrome Clinical Studies $QNRX https://t.co/gVQbnnmOKC
— Health Stocks News (@health_stocks) March 4, 2024
What difference does it make? You gotta own 60K shares just to be left with one.
Sht like this doesn't even happen on the stinky pinkies, I'd like to know how these crooks are not in jail and even more concerning is that NASDAQ leaves it listed - Shows Nasdaq is just a more expensive scam operation than pinks
Do we know what they are doing with fractional shares leftover after the split?
My god man WTF are these theives thinking?
increase our registered
share capital from 500,000,000,000 ordinary shares, no par value, to 6,000,000,000,000 ordinary shares, no par value;
3. To adopt an amendment of our Articles of Association to effect a reverse split of our ordinary shares at a ratio of 1-for-60,000
Increase shares from 500 BILLION TO 6 TRILLION AND PULL A 60k TO 1 REVERSE THEFT?
Trying to time this bag of garbage before the bag rips and lose everything lol I need some hel Beijing what we gonna do?????
well BB maybe ill set a limit for $15 hope for a quick spike before this implodes worse then oceangate
|
Followers
|
8
|
Posters
|
|
|
Posts (Today)
|
0
|
Posts (Total)
|
135
|
|
Created
|
12/15/21
|
Type
|
Free
|
| Moderators | |||
| Volume | |
| Day Range: | |
| Bid Price | |
| Ask Price | |
| Last Trade Time: |
