Register for free to join our community of investors and share your ideas. You will also get access to streaming quotes, interactive charts, trades, portfolio, live options flow and more tools.
Thanks. What type of corp reorg is going on?
D - This is temporarily used to denote a corporate reorganization
I have a list of NASDAQ 5th letter descriptions on ticker symbols for A - Z. However, "D" is missing on the list. Very odd.
Anyone know what the "D" represents at the end of the ticker symbol?
Nice PPS move today. This has great long term potential.
Good article regarding ONCY!
https://americannewsgroup.com/2020/02/07/a-company-with-an-aim-to-cure-certain-types-of-cancers/
ONCY is a good stock investment but before trying to take down ONCY go to this website. Let us not condemn ONCY. ONCY is working to cure Breast Cancer.
https://nbcf.org.au/
5 Biotech Stocks to Buy for Blockbuster Potential
Trading Symbol: NASDAQ:ONCY
Median Price Target: $6.80 (516.18% upside potential)
Consensus Rating: Strong Buy
Oncolytics Biotech Inc. (NASDAQ:ONCY) (TSX:ONC) is a development stage biotech company developing an immuno-oncology virus (called pelareorep) that’s currently being studied for potential combination with Opdivo® from Bristol-Myers Squibb Company (NYSE:BMY), Roche’s Tecentriq, Pfizer’s & Merck KGaA’s Bavencio, as well as Keytruda® from Merck & Co., Inc. (NYSE:MRK).
For a more detailed report on Oncolytics Biotech, USA News Group has some very in-depth coverage of the company that can be viewed here.
Here is the full article.
https://equity-insider.com/2019/11/19/5-biotech-stocks-to-buy-for-blockbuster-potential/
Agency Taps Australia to Create a Coronavirus Vaccine With 'Unprecedented Speed'
8:55 am ET January 28, 2020 (PR Newswire) Print
USA News Group - According to the University of Queensland (UQ), the institution has been asked to develop a vaccine for the emerging Chinese coronavirus outbreak. The call comes in order to tap the University's recently developed rapid response technology.
As part of efforts to overcome new and deadly viruses, research and developments with different locations and companies may prove vital. Several companies are working to offer new treatments that may potentially influence the diagnostic and treatment options. As a result, these may produce some of the most valuable companies in this era. Leaders in biotech that may benefit from collaborations include Novavax, Inc. (NASDAQ: NVAX), NanoViricides, Inc. (NYSE: NNVC), and Oncolytics Biotech Inc. (NASDAQ: ONCY) (TSX: ONC).
One newcomer in this sector, Aethlon Medical, Inc., (NASDAQ:AEMD) is a medical technology company, bringing solutions to address unmet needs in health and biodefense worldwide. Aethlon Medical Inc. is developer of the patented Aethlon Hemopurifier, a clinical-stage immunotherapeutic device that removes exosomes and life-threatening viruses from the human circulatory system. Already, the device has been shown effective in wide application to combat highly infectious diseases like the Ebola virus and potentially, other emerging viruses. Aethlon is also known for its development of TauSome, an exosomal biomarker candidate to diagnose chronic traumatic encephalopathy in living individuals.
Getting Started on a Speedy Vaccine for Coronavirus
The University of Queensland says it had received a request to develop a vaccine from the Coalition for Epidemic Preparedness Innovations (CEPI). That organization is billed as an innovative partnership between public, private, philanthropic, and civil organizations. Its mandate is to develop vaccines to protect the world against significant outbreaks like the current corona virus emerging from China.
Professor Paul Young who heads the university's School of Chemistry and Molecular Biosciences, is quoted as saying that that UQ has novel technology for the rapid development of vaccines, which could provide a vaccine within six months.
Young stated, "The team hopes to develop a vaccine over the next six months, which may be used to help contain this outbreak." and added "The vaccine would be distributed to first responders, helping to contain the virus from spreading around the world."
At current count, 80 people have died, and more than 2,700 reported cases of the infection have been reported by authorities. It is known that the coronavirus originated in Wuhan, a city of about 11 million and reportedly came from the city's 'street meat' market.
Looking to Head Off Deadly Viruses
There are a number of approaches where biotechnology companies are applying new technologies to detect and halt deadly viruses. This may involve multiple locations and institutions or companies combing technologies to discovering new treatments. One such case is the approach taken by Aethlon Medical Inc. The company is developing its Hemopurifier(R)- one of the most intriguing, patent-protected therapeutic devices in the world - unveiled in 2014.
The unique blood purification device is a first-in-class therapeutic technology receiving two FDA Breakthrough designations, through multiple therapeutic targets: viral disease and cancer.
Hemopurifier(R) therapy has been administered to a number of individuals infected with deadly viruses including Ebola virus, Hepatitis C virus (HCV) and the Human Immunodeficiency virus (HIV). In the Ebola scenario, it was through a "remarkable response" to the Hemopurifier(R) therapy to a comatose patient (a Ugandan physician) with multiple organ failure that led to the accolades given by Time Magazine which named the device one of its 25 Best Inventions of 2014.
Pre-clinical Hemopurifier(R) studies have validated the broad-spectrum capture of numerous viral threats. These include Chikungunya, Dengue and West Nile virus, Vaccinia and Monkey pox, and models for human Smallpox infection. The Hemopurifier(R) also received the FDA's "Breakthrough Device" designation and subsequently entered the spotlight for its role of fighting cancer in cases of chemotherapy resistance, promotion of metastasis, and immune suppression.
All Hands On Deck To Fight the Spread of Coronavirus
In its public information, CEPI explained that it had already partnered with the University of Queensland earlier in January in order to develop what it calls a "molecular clamp" vaccine platform. This would enable "targeted and rapid vaccine production". Reports are that CEPI committed up to AU$15.4 million for UQ to develop the molecular clamp technology at that time. It seems certain that funding will now be put to immediate use.
CEPI is also committed to working on a number of fronts at the same time, including other organizations, and companies around the globe. CEPI also just announced it would be working with two other partners to develop a vaccine - the US based pharmaceuticals company Inovio, along with the advanced stage biotechnology firm Moderna, Inc.
All of these efforts are aimed at bringing a speedy solution to detecting and dealing with the coronavirus and other potential pandemics. It is yet unclear which approaches will prove most useful. Leading companies are moving to accelerate development of therapies and drugs that can guard from new and deadly viruses. A number of advanced companies in the biotech sector could benefit from the breakthroughs including:
Novavax, Inc. (NASDAQ: NVAX) together with its subsidiary, Novavax AB, a late-stage biotechnology company, focuses on the discovery, development, and commercialization of vaccines to prevent serious infectious diseases. The company announced that it has initiated development of a vaccine candidate for the Wuhan-version of the coronavirus that has spread from China to other Asian nations and was recently confirmed to have infected at least one person in the United States.
NanoViricides, Inc. (NYSE: NNVC) a nano-biopharmaceutical company, discovers, develops, and commercializes drugs for the treatment of viral infections. The company recently disclosed that it has successfully completed genetic toxicology testing required to support the IND application for NV-HHV-101 moving towards human clinical trials. NV-HHV-101 is NanoViricides' lead drug candidate with its first indication as dermal topical cream for the treatment of shingles rash.
Oncolytics Biotech Inc. (NASDAQ: ONCY) (TSX: ONC) is a biotech company developing an immuno-oncology virus (called pelareorep) being studied for potential combination with Opdivo(R) from Bristol-Myers Squibb Company, Roche's Tecentriq, Pfizer's & Merck KGaA's Bavencio, as well as Keytruda(R) from Merck & Co., Inc. Oncolytics Biotech recently announced positive multiple myeloma data presented at the 61st Annual Meeting & Exposition of the American Society of Hematology.
Oncolytics Biotech® Announces Statistically Significant Data Identifying CEACAM6 as a Prospective Prognostic Biomarker for Pelareorep in the Treatment of Pancreatic Adenocarcinoma
Download as PDF January 27, 2020
Over 80% improvement in progression free survival in patients with low levels of CEACAM6
SAN DIEGO and CALGARY, Alberta, Jan. 27, 2020 /PRNewswire/ -- Oncolytics Biotech® Inc. (NASDAQ: ONCY) (TSX: ONC), currently developing pelareorep, an intravenously delivered immuno-oncolytic virus, today announced that a poster presentation highlighting statistically significant data identifying CEACAM6 as a prospective biomarker for pelareorep in the treatment of pancreatic cancer. The presentation was delivered at the 2020 Gastrointestinal Cancers Symposium sponsored by ASCO in San Francisco.
"We have identified another biomarker candidate for pelareorep," said Dr. Rita Laeufle, Chief Medical Officer of Oncolytics Biotech. "These results correlate CEACAM6 levels with long term benefit in patients with pancreatic cancer. We are working with industry and academic colleagues to verify this important finding not only in pancreatic cancer but potentially in other GI indications where this biomarker is linked to clinical outcomes."
The poster, CEACAM6 is a candidate biomarker for Reolysin® (pelareorep) sensitivity in pancreatic adenocarcinoma (PDAC), highlights data from the randomized study NCI 8601: Carboplatin and Paclitaxel With or Without Viral Therapy in Treating Patients With Recurrent or Metastatic Pancreatic Cancer. Data in the presentation associate low levels of the gene CEACAM6 with prolonged progression free survival (PFS) in pelareorep-treated patients with pancreatic cancer. PFS increased over 80%, from 5.72 months to 10.32 months (p=0.05).
"I am very encouraged to see additional data come from this important study by the Ohio State University Comprehensive Cancer Center," said Dr. Matt Coffey, President and Chief Executive Officer of Oncolytics Biotech. "This statistically significant result highlights the potential for CEACAM6 to become an additional prognostic biomarker for pelareorep and could provide considerable clinical value as we investigate its potential in pancreatic and other GI cancers."
CEACAM6 is differentially expressed in pancreatic adenocarcinoma cells. High levels of CEACAM6 are known to block viral trafficking in virally infected cells, thereby decreasing viral replication. Study investigators speculated that altered CEACAM6 levels may be predictive for pelareorep sensitivity and may serve as a biomarker.
Key data and conclusions:
CEACAM6 was the most differentially expressed gene, with an eight-fold decrease in levels of mRNA, in long-term responders compared to early progressors in patients receiving pelareorep.
Low levels of CEACAM6 mRNA expression were associated with prolonged PFS in pelareorep-treated patients (p=0.05). This treatment effect was not seen in patients that were not treated with pelareorep (p=0.35).
In pelareorep treated patients, CEACAM6 mRNA expression level was very influential with a hazard ratio of 1.54 (p=0.01), suggesting that one unit increase in CEACAM6, corresponds to an increase in the risk of progression and/or death by 54% in this arm. There was no significant relationship seen in patients that were not treated with pelareorep
CEACAM6 may be included as a candidate biomarker of resistance to pelareorep and, in theory, could inhibit viral trafficking in tumor cells
The poster presentation was co-authored by Dr. Anne Noonan, Department of Medical Oncology, Ohio State University Wexner Medical Center, Richard Solove Research Institute and James Cancer Hospital, and Dr. Tanios Bekaii-Saab Senior Associate Consultant, Division of Hematology/Oncology, Department of Internal Medicine, Mayo Clinic, Phoenix, Arizona. It can be found on the Posters & Publications page of the company's website: https://www.oncolyticsbiotech.com/technology/posters-publications
https://finance.yahoo.com/news/oncolytics-biotech-announces-statistically-significant-120000886.html
Pelareorep scores ahead of other OV (oncolytic virus) therapies in three key aspects of delivery, safety, and efficacy. Unlike other OVs which require intratumoral delivery, Pelareorep is administered intravenously. This will allow nurses to administer Pelareorep with chemotherapy drugs the same way they infuse other cancer therapies. Intratumoral delivery requires specialized training and hence is costlier for the oncologist.
ONCY is a Buyout waiting to happen. A large company will have a the money to get this research moved months ahead and get FDA approval.
Oncolytics Biotech(R) Announces Publication of an Abstract for the 2020 Gastrointestinal Cancers Symposium Highlighting CEACAM6 as a Potential Prognostic Biomarker Candidate for Pancreatic Cancer
SAN DIEGO and CALGARY, Alberta , Jan. 22, 2020 /CNW/ -- Oncolytics Biotech® Inc. (ONCY) (ONC.TO), currently developing pelareorep, an intravenously delivered immuno-oncolytic virus, today stated that the previously announced abstract for a poster to be presented at the 2020 Gastrointestinal Cancers Symposium sponsored by ASCO in San Francisco , has been published. The abstract highlights new biomarker data from the randomized study NCI 8601: Carboplatin and Paclitaxel With or Without Viral Therapy in Treating Patients With Recurrent or Metastatic Pancreatic Cancer.
The abstract, CEACAM6 is a candidate biomarker for Reolysin® (pelareorep) sensitivity in pancreatic adenocarcinoma (PDAC), was co-authored by Dr. Anne Noonan , Department of Medical Oncology, Ohio State University Wexner Medical Center, Richard Solove Research Institute and James Cancer Hospital, and Dr. Tanios Bekaii-Saab Senior Associate Consultant, Division of Hematology/Oncology, Department of Internal Medicine, Mayo Clinic, Phoenix, Arizona .
Data in the abstract associate low levels of the gene CEACAM6 with prolonged progression free survival (PFS) in pelareorep-treated patients with pancreatic cancer, with PFS improving from 5.72 months to 10.32 months (p=0.05). This effect was not seen in non-pelareorep treated patients. Consequently, CEACAM6 may serve as a prognostic biomarker for sensitivity of pancreatic tumors to pelareorep treatment. Additional data will be announced following the poster presentation.
Abstracts are available on the ASCO meeting library website at https://meetinglibrary.asco.org/. The poster will be added to the Oncolytics corporate website shortly after the presentation.
Abstract ID: 285103
Abstract Number: 746
Poster Board: M13
Abstract Title: CEACAM6 as a candidate biomarker for pelareorep sensitivity in pancreatic adenocarcinoma (PDAC)
Session Information: Poster Session B: Hepatobiliary Cancer, Neuroendocrine/Carcinoid, Pancreatic Cancer, and Small Bowel Cancer
Session Date & Time: January 24, 2020 from 12:00 PM-1:30 PM & 4:30 PM-5:30 PM
Big week for ONCY lots of good news coming out heavy volume day so far today.
BusinessWire, GlobeNewswire and PR Newswire News
Oncolytics Biotech Inc ONCY:NASDAQ
Biotech Sector's Technological Advancements Winning Major Battles in the War on Cancer
PR Newswire
9:00 AM ET
USA News Group - In the war against cancer, it appears that all hands are on deck, with contributions coming from across the biotech sector. As the 2020s begin, new approvals and promising data are encouraging the market to pay more attention to the developers of new treatments, including Oncolytics Biotech Inc. (NASDAQ:ONCY) (TSX:ONC), GlaxoSmithKline plc (NYSE:GSK), Aethlon Medical, Inc. (NASDAQ:AEMD), Clovis Oncology, Inc. (NASDAQ:CLVS), and Merck & Co., Inc. (NYSE:MRK).
Market researchers see big things to come from investment into the oncological war, with recent forecasts such as: the cancer gene therapy market surpassing $3.2 billion by 2026; the global breast cancer drug market offering a $40 billion opportunity; the noninvasive cancer diagnostics market hitting $195 billion by 2025; and the immunotherapy market estimated to reach $115 billion by 2023.
Key to the current optimism for drugs and therapies that are being developed, either from scratch or as enhancements.
PELAREOREP'S 1-2 COMBO PUNCH AGAINST CANCER
One thriving example an important enhancement therapy is pelareorep from Oncolytics Biotech Inc. (NASDAQ:ONCY) (TSX:ONC)--an immuno-oncolytic virus (OV) being combined so me of the world's top selling anti-cancer drugs, including Keytruda ($7.2B in 2018 sales for Merck), Opdivo ($6.7B in 2018 sales for Bristol-Myers Squibb), Tecentriq ($766M in 2018 sales for Roche) and Bavencio ($75.5M in 2018 sales for Pfizer/Merck).
Recently Reuters published an analysis that revealed a record 89 therapies are currently undergoing human trials involving the pairing of treatments with antibodies with toxic agents to fight cancer--which Reuters calls "guided-missile" cancer drugs.
Like a guided missile, pelareorep selectively infects tumor cells, leading to the creation of inflamed tumors. T hese then engage the body's immune system to create of tumor reactive T cells.
This process is designed to enhance the likes of the market's best oncology drugs out there, as listed above. It does this by expanding existing T cell clones priming the immune system for checkpoint blockade. To date, the drug has synergized with all checkpoint inhibitor combinations tested.
So far, based on the final advice from the FDA following their EOP2 meeting, the company's been granted Special Protocol Assessment (SPA). It's been recommended that identification of biomarker should be done before Phase III. Confirmation of a single phase 3 study is required for approval.
Already across 13 clinical studies and a broad range of cancers, up to 96% of tumor samples tested positive for replicating pelareorep virus after intravenous delivery. To date, pelareorep is the only oncolytic virus with meaningful clinical data demonstrating intravenous delivery to tumor tissue.
To date, it's been used to treat 1,100 patients, over 900 of which were administered intravenously--So far, no maximum tolerated dose (MTD) has been reached.
Worldwide, Oncolytics Biotech Inc. (ONCY-ONC) has accumulated 398 patents issued, including 48 US and 21 Canadian, and more than 21 additional applications pending.
The reovirus issued patent claims cover compositions of matter comprising reovirus (through 2028 and extendable to 2033), and all pharmaceutical uses of it.
GUIDED MISSILE PARTNERSHIPS
Pharma giants, such as GlaxoSmithKline plc (NYSE:GSK), and Merck & Co., Inc. (NYSE:MRK) are throwing a lot of weight into these guided missile approaches--and there could be a lot more to come.
Between 2000 and 2018, US regulators only approved five antibody-drug conjugates (ADCs). In 2019, those same regulators approved three more, making it the largest one-year total ever. The tide is changing.
GlaxoSmithKline is currently testing its belantamab mafodotin against multiple myeloma. Most recently, the treatment's pivotal DREAMM-2 trial supported the drug's use for heavily-treated multiple myelom a.
Oncolytics Biotech Inc. (ONCY-ONC) has also recently highlighted pelareorep's unique ability to activate the immune system in late stage myeloma, with multiple myeloma data expected at ASCO 2020.
FN Media Group Presents USA News Group Market Commentary
Los Angeles, CA – January 16, 2020 – USA News Group – A new study out suggests that T cells are a subset of lymphocytes (white blood cells), which play a key role in the body’s immune response. This new information could prove to be a key in unlocking “helper” cells that could aid in cancer therapy.
Companies across the biotech space continue to seek new and novel therapies to screen for and treat a wide variety of blood borne cancers. New information in the space is key to providing links between drug therapies and strategies. Companies in this space are expecting to see significant increases in revenue and support for new technologies supported by leaders in the sector including Alnylam Pharmaceuticals, Inc. (NASDAQ: ALNY), Oncolytics Biotech Inc. (NASDAQ: ONCY) (TSX: ONC), and Bristol-Myers Squibb Company (NYSE: BMY).
Oncolytics Biotech Inc. is a development stage biotech company focused on an immuno-oncology virus (called pelareorep) currently being studied for potential combination with lead treatments Opdivo® from Bristol-Myers Squibb Company, Roche’s Tecentriq, Pfizer’s & Merck KGaA’s Bavencio, Oncolytics has recently highlighted the four ongoing studies with Pfizer, Merck KGaA, Merck, Bristol-Myers Squibb and Roche. The studies are in support of checkpoint inhibitors, targeting metastatic breast cancer, early stage breast cancer, multiple myeloma and pancreatic cancer.
https://marketwirenews.com/news-releases/new-study-indicates-helper-cells-may-kill-cancerous--8656739352178508.html
Great article
Great article: Already across 13 clinical studies and a broad range of cancers, up to 96% of tumor samples tested positive for replicating pelareorep virus after intravenous delivery. To date, pelareorep is the only oncolytic virus with meaningful clinical data demonstrating intravenous delivery to tumor tissue.
BusinessWire, GlobeNewswire and PR Newswire News
Oncolytics Biotech Inc ONCY:NASDAQ
'Guided-Missile' Cancer Drugs are the Biotech Sector's First Major Story of the 2020s
PR Newswire
8:45 AM ET
USA News Group - With a new decade still less than a month underway, it appears the 2020s should see some major breakthroughs in the fight against cancer. A recent analysis from Reuters uncovered that a record 89 therapies are underway in human trials that feature the pairing of treatments with antibodies with toxic agents to fight cancer--affectionately dubbed "guided-missile" cancer drugs.
Cancer treatments are being targeted for drastic improvements by several biotech developers large and small, including Oncolytics Biotech Inc. (NASDAQ:ONCY) (TSX:ONC), Roche Holding AG (OTC:RHHBY), Seattle Genetics, Inc. (NASDAQ:SGEN), ImmunoMedics, Inc. (NASDAQ:IMMU), and AstraZeneca PLC (NYSE:AZN).
More technically known as antibody-drug conjugates (or ADCs), treatments are engineered to zero in on tumors and then attack with up to 10,000 times the potency of standard chemotherapy, while minimizing damage to healthy tissue.
MAXIMIZING CANCER-FIGHTING POTENCY
One of the best examples of this method is pelareorep--an immuno-oncolytic virus (OV)--from Oncolytics Biotech Inc. (NASDAQ:ONCY) (TSX:ONC).
Pelareorep is currently being studied for combinations with some of the world's top selling anti-cancer drugs, including Keytruda ($7.2B in 2018 sales for Merck), Opdivo ($6.7B in 2018 sales for Bristol-Myers Squibb), Tecentriq ($766M in 2018 sales for Roche) and Bavencio ($75.5M in 2018 sales for Pfizer/Merck). To date, the drug has synergized with all checkpoint inhibitor combinations tested.
It's potential developments such as pelareorep that could be the embodiment of what makes a "guided-missile" cancer drug hit its target.
Already across 13 clinical studies and a broad range of cancers, up to 96% of tumor samples tested positive for replicating pelareorep virus after intravenous delivery. To date, pelareorep is the only oncolytic virus with meaningful clinical data demonstrating intravenous delivery to tumor tissue.
The potential for these new treatments is quite large, as the global oncology drug market is projected to grow at a rate of 7.6% CAGR to hit $176.5 billion by 2025 with the therapeutic cancer vaccine market alone projected to surpass $15 billion by 2025.
ARE RISING APPROVALS A SIGN OF THINGS TO COME?
The rise of these types of treatments over the last two decades has been arguably slow--But that could be changing very soon.
Only five ADCs won approval between 2000 and 2018. But in 2019, US regulators approved three ADCs--the most ever in a single year.
The key turning point has been results from studies on last-ditch treatments showing they helped patients whose survival outlook was bleak.
Kadcyla from Roche Holding AG (OTC:RHHBY), approved in 2013 for breast cancer, so far is the only one to have surpassed $1 billion in annual sales. This came after data last year showed it boosted disease-free survival for some patients compared with the standard treatment, the company's other drug, Herceptin.
Most recently, bladder cancer drug Padcev from Seattle Genetics, Inc. (NASDAQ:SGEN) received expedited approval in December. This was based on evidence that 44% of patients who had failed immunotherapy showed improvement, including in some cases showing no evidence of cancer when they were assessed after treatment.
Also approved in December was breast cancer drug Enhertu from AstraZeneca PLC (NYSE:AZN), following positive results showing the drug helped patients who had failed numerous treatments survive a median of more than 16 months before their disease worsened.
Earlier in the summer, was lymphoma drug Polivy from Roche Holding AG getting approval after producing complete response rates, with no signs of disease, in 40% of patients when combined with two other therapies.
Now on deck could be ImmunoMedics, Inc. (NASDAQ:IMMU), whose market cap has gained more than 60% to approximately $4.3 billion over the last six months. In late December, the US Food and Drug Administration (FDA) decided to review ImmunoMedics' ADC that targets triple-negative breast cancer, specifically which is hard to treat and has poor prognosis.
ASSISTED TARGETING FOR THE 'GUIDED MISSILES'
Helping these new treatments succeed, will be complimentary biotech developments, such as pelareorep from Oncolytics Biotech Inc. (ONCY-ONC).
Most recently, ROTH Capital Partners sponsored a Key Opinion Leader call discussing recent data presented at the 61st American Society of Hematology Annual Meeting and Exposition.
During the call, which featured two of Oncolytics' multiple myeloma clinical investigators, Dr. Craig Hofmeister M.D. and Dr. Flavia Pichiorri Ph.D., the speakers highlighted that carfilzomib promotes reovirus infection, that pelareorep upregulates PD-L1, and that delivery of additional data from ongoing pelareorep studies in multiple myeloma is planned for presentation at ASCO in June 2020.
"I think that carfilzomib promotes pelareorep infection by suppressing the innate antiviral response and our data suggest that it does not get in the way of T-cell activation," said Dr. Hofmeister. "Pelareorep infection, not proteasome inhibition, can upregulate PD-L1 expression on myeloma cells and the adaptive immune system can then assist in clearing infected tumor cells. The combination in fact enhances the body's immune attack on infected myeloma cells."
The call also discussed the competitive landscape for refractory multiple myeloma and the paucity of available therapies to treat these patients.
"Reovirus is the only strategy I see in the market that is completely different and may be able to activate the immune system of these patients," said Dr. Pichiorri. "It would be a salvage therapy for now, but so many patients need a salvage therapy after being refractory to other therapies."
PROGRESSION OF THE MOVEMENT
Pelareorep to date has been involved with 1,100 patients treated, over 900 of which were administered intravenously--So far, no maximum tolerated dose (MTD) has been reached.
Oncolytics Biotech Inc. (ONCY-ONC) has accumulated 398 patents issued worldwide, including 48 US and 21 Canadian, with over 21 more pending applications worldwide. The reovirus issued patent claims cover compositions of matter comprising reovirus (through 2028 and extendable to 2033), and all pharmaceutical uses of it.
Getting ahead of a breakout, Oncolytics Biotech Inc. (ONCY-ONC) has already established a commercial scale manufacturing agreement with SAFC (part of Merck Millipore Sigma), with a final formulation produced at 100 liter-scale under cGMP--which is more than 50,000 standard doses per production run.
While it helped pioneer ADCs Kadcyla and Polivy, Roche Holding AG appears to be backing off of the ADC bandwagon for now.
"We have shifted our technology priorities," Roche CEO Severin Schwan told Reuters. "Maybe others will be luckier, but we failed to master the complexity."
This is where groups like AstraZeneca PLC are looking to pick up the ball. Already the Enhertu developer has struck a $7 billion deal with Japan's Daiichi Sankyo ( receiving $1.35 billion up-front)--and potentially more to come if it challenges Roche's drugs' dominance in breast cancer.
With 89 therapies underway with human trials, the era of the guided-missile cancer drug could be officially upon us in 2020
Good catch. That most likely is why it was shorted so hard today cause there will be restrictions tomorrow. Thanks for the update.
Thank You Timetravelerdoes, It's Good to be Here. I'am not going anywhere especially with a huge Press released to be release at anytime. I'am expecting for Positive update data results on clinical trial studies to be release hopefully this week. Plus possibility of a buyout by one of Oncolytics Big Pharma Partners. Bristol Myers I believe will be the one to buyout Oncolytics Biotech they're working with them in the trials. Oncolytics were giving 15mills to work on the trials. Either way Something is going to be release very soon.
Plus tomorrow is a ssr day. Short Sell Restriction could help in re-couping the share price. Looking for great things to happen for Oncolytics Biotech Inc this month in January.
Good Luck.
Great price to pick up more shares before the NEWS coming next week! Well done mms.
Looks like some profit taking and flipping. Buying is now coming back strong vs selling. Good news is only a week away.
GOOD NEWS! Oncolytics Biotech® Announces Key Opinion Leader Call Conducted by ROTH Capital Partners to Discuss Multiple Myeloma
PR Newswire PR Newswire•January 9, 2020
SAN DIEGO and CALGARY, Alberta, Jan. 9, 2020 /PRNewswire/ -- Oncolytics Biotech® Inc. (NASDAQ: ONCY) (TSX: ONC), currently developing pelareorep, an intravenously delivered immuno-oncolytic virus, today announced that ROTH Capital Partners (ROTH) will conduct a Key Opinion Leader call today at 10:30 am ET for their institutional clients. The call, "Proteasome Inhibitors Augment Pelareorep in Multiple Myeloma" will feature Dr. Flavia Pichiorri Ph.D. and Dr. Craig Hofmeister M.D., both of whom are working on ongoing multiple myeloma studies with Oncolytics' oncolytic virus, pelareorep.
The equity research team at ROTH will host and moderate the call for their clients, with a focus on recent data presented at the 61st American Society of Hematology Annual Meeting & Exposition, and how it could impact the design of future multiple myeloma trials with oncolytic viruses versus the standard of care.
Management plans to provide a recap of the call next week, including key messages and insights provided by Dr. Pichiorri and Dr. Hofmeister.
Dr. Flavia Pichiorri Ph.D., Judy and Bernard Briskin Center for Multiple Myeloma Research, City of Hope, Duarte, CA and Department of Hematologic Malignancies Translational Science, Beckman Research Institute, City of Hope, Duarte, CA.
Dr. Craig Hofmeister M.D., Winship Cancer Institute /Department of Hematology and Medical Oncology, Emory University School of Medicine, Atlanta, GA.
https://finance.yahoo.com/news/oncolytics-biotech-announces-key-opinion-120000962.html
Really good news coming on the 21st. Price target $9 but if a Buyout happens ONCY will be much higher!
Heading back up this week with more good news coming soon. Especially with the conference in Jan !
ONCY is letting us know they are going to sell some shares, they can raise up to 30 million depends on the share price I think they have very good news that could push the share price up past $8.00 dollars a share , not much dilution with these shares they want to sell 3.747 million shares.
35,685,543 Common Shares (non-diluted) after the issuance of up to 3,746,835 Common Shares, assuming a sales price of US$3.95 per Common Share, which was the closing price of the Common Shares on NASDAQ on January 2, 2020, for the US$30,000,000 that may be sold from time to time through the Agent, subject to a maximum of US$14,800,000 under this Prospectus Supplement. The actual number of Common Shares issued and outstanding will vary depending on the actual sales prices and aggregate dollar amount sold under the Offering.
ONCY target raised to $9
Oncolytics Biotech (NASDAQ:ONCY) had its price objective hoisted by Roth Capital from $6.80 to $9.00 in a report issued on Thursday, The Fly reports. They currently have a buy rating on the stock.
https://www.tickerreport.com/banking-finance/4922890/oncolytics-biotech-nasdaqoncy-pt-raised-to-9-00.html
Buying is coming on real strong going into power hour. Looks like Buying might overtake the selling by the close of today. Huge volume as Oncy is getting alot of attention. Profit takers are out by now and the shorts left after a couple hours this am. Keep it going Oncy !
Awesome how the Bulls kicked back in after the shorts bailed out. Well done Oncy Longs !
looks like shorts decided to take their loss and move on today. Looking forward to Outstanding news on the 21st of Jan
Abstracts will be published at 5:00 pm ET on Tuesday, January 21, 2020 on the ASCO meeting library website at https://meetinglibrary.asco.org/.
Abstract ID: 285103Abstract Number: 746Poster Board: M13Abstract Title: CEACAM6 as a candidate biomarker for pelareorep sensitivity in pancreatic adenocarcinoma (PDAC)Session Information: Poster Session B: Hepatobiliary Cancer, Neuroendocrine/Carcinoid, Pancreatic Cancer, and Small Bowel CancerSession Date & Time: January 24, 2020 from 12:00 PM-1:30 PM & 4:30 PM-5:30 PM
Very good article for ONCY.
Pelareorep is a non-pathogenic, proprietary isolate of the unmodified reovirus: a first-in-class intravenously delivered immuno-oncolytic virus for the treatment of solid tumors and hematological malignancies. The compound induces selective tumor lysis and promotes an inflamed tumor phenotype through innate and adaptive immune responses to treat a variety of cancers and has been demonstrated to be able to escape neutralizing antibodies found in patients.
https://seekingalpha.com/pr/17726370-oncolytics-biotech-r-announces-abstract-outlining-potential-new-biomarker-to-be-presented
Another Outstanding day ! Awesome !!
profit takers hitting early but ONCY is still about even on buying and selling today.
Good to have you back Cash Cow. This is going to go a lot higher. We are very fortunate to be invested at this price for ONCY.
Yes, the news has to be huge for ONCY to a 59% run today and never look back. Still holding after buying back 1.97 today.
Look out when the Blue Line crosses the red line ^^^
http://schrts.co/qSKjhYdN
Hmm, Ok, I like that. It's not impossible. If Merck or Pfizer comes out with a positive PR implicating ONCYD, the sky is the limit then on PPS. Such a monster spike to $33 may only happen via irrational exuberance, but that's ok. Time then to cash in the chips IMO.
Oncolytics Biotech® Announces Abstract Outlining Potential New Biomarker to be Presented at the 2020 Gastrointestinal Cancers Symposium
SAN DIEGO, CA and CALGARY, AB / ACCESSWIRE / December 12, 2019 / Oncolytics Biotech® Inc. (NASDAQ:ONCY)(TSX:ONC), currently developing pelareorep, an intravenously delivered immuno-oncolytic virus, today announced the acceptance of an abstract highlighting new biomarker data from the randomized study NCI 8601: Carboplatin and Paclitaxel With or Without Viral Therapy in Treating Patients With Recurrent or Metastatic Pancreatic Cancer. The data will be part of a poster presentation at the 2020 Gastrointestinal Cancers Symposium sponsored by ASCO, January 23 - 25, 2020, in San Francisco.
The abstract, CEACAM6 is a candidate biomarker for REOLYSIN® (pelareorep) sensitivity in pancreatic adenocarcinoma (PDAC), was co-authored by Dr. Anne Noonan, Department of Medical Oncology, Ohio State University Wexner Medical Center, Richard Solove Research Institute and James Cancer Hospital, and Dr. Tanios Bekaii-Saab Senior Associate Consultant, Division of Hematology/Oncology, Department of Internal Medicine, Mayo Clinic, Phoenix, Arizona.
https://ir.oncolyticsbiotech.com/press-releases/detail/489/oncolytics-biotechr-announces-abstract-outlining
Looks like we are getting HUGE News!! ONCY up over.60 in the past week !
Holding News is due. Company stated that will be releasing information on clinical data in 4th quarter of 2019. I'm waiting.
I am looking for a 1 billion market cap or $33.00 dollars a share.
I'm looking for #10/share 1 year from now.
What a good day !!! up .25 at the close with heavy volume. Looking for news coming this week.
Anyone know the insider shares held and the institutional shares that are held, that number would tell a lot. Interesting Bio company
Huge volume so far today and what is the reason for the run up? Can't find any news but we should be double the avg volume by 12:30 est. really nice jump in share price.
It's Been a Particularly Exciting Year for Biotech Stocks Across the Board
Innovative companies in the industry, like Oncolytics Biotech (NASDAQ: ONCY) are adopting changes that are helping to transform the sector. Oncolytics develops treatments for cold tumor identification through Pelareorep. Clinical trials have provided us with a multitude of observations and conclusions thus far regarding the effectiveness of pelareorep in normal cells versus cancerous cells.
https://www.prnewswire.com/news-releases/its-been-a-particularly-exciting-year-for-biotech-stocks-across-the-board-300965923.html
Why did ONCY do a R/S back in May 2018? To get relisted? What do you feel is the risk of them doing it again in the near future?
another good day. looks like maybe some good news is on the way.
Very nice run up today! Really good work Oncy!
ONCY Bulls believe this is one Great investment. Staying GREEN again!
|
Followers
|
73
|
Posters
|
|
|
Posts (Today)
|
0
|
Posts (Total)
|
1874
|
|
Created
|
10/09/01
|
Type
|
Free
|
| Moderators | |||


See the official company website for the most current information. www.oncolyticsbiotech.com
CURRENT SUMMARY - March 9th 2014
OVERVIEW

 Dr Kevin Harrington - UK
Dr Kevin Harrington - UK  , Dr. James A Bonner - US
, Dr. James A Bonner - US
Publication of data for the PH I/II Ovarian cancer trial also sponsored by the NCI and conducted by Principal Investigator Dr. David Cohn 
COMPANY PROGRESS
The ubiquitous reovirus

Reovirus, an acronym for Respiratory Enteric Orphan virus, is generally believed to inhabit the respiratory and bowel systems in humans. Reovirus is found naturally in sewage and water supplies. By age 12, half of all children show evidence of reovirus exposure and by adulthood, most people have been exposed. However, the disease is non-pathogenic, meaning there are typically no symptoms from infections. The link to its cancer-killing ability was established after the reovirus was discovered to reproduce well in various cancer cell lines.

Using improved microscope technology, a team including Purdue's Timothy S. Baker and a colleague at Harvard has determined the structure of a reovirus (short for "respiratory enteric orphan" virus) down to the 7.6-angstrom scale, better than twice the 18-angstrom resolution previously available. http://www.purdue.edu/UNS/html4ever/031215.Baker.reovirus.html
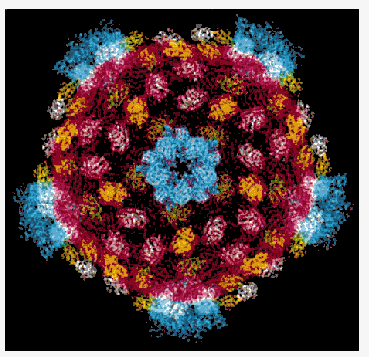
http://news.chess.cornell.edu/archive/highlights/rvrscr.htm
Synchrotron radiation is the only tool available for the determination of very large molecular structures at high resolution such as the reovirus core. One of the largest structures solved to-date has been reported from work carried out at MacCHESS by Karin Reinish in the Harrison group at Harvard. The reovirus core is a macromolecular assembly with a molecular mass of 52 million. The core synthesizes, modifies, and exports viral messenger RNA. The core contains five of the eight proteins that make up a complete virion and is about 700 Angstroms in diameter. They crystallize in a centered cubic space group with unit cell dimensions of 1255 Angstrom with crystal growth requiring 9 to 12 months. Using the CHESS F1 facility, one of the two Biosafety Level 2 facilities in the US, scientists have been able to "see" into the three-dimensional structure of the core using the tools of x-ray crystallography.
The reovirus core particle shows the subunits in different colors. There are 120 copies of the part in red that forms the shell and that packages the RNA. This part defines the symmetry and size of the particle. Other subunits, shown in yellow, green and white stabilize the shell. The blue parts form turret-like structures around the fivefold axes that exports mature mRNA into the cytoplasm of the infected cell.
THE PRODUCT

Mechanism of Action

The reovirus, or Respiratory Enteric Orphan virus, has been demonstrated to replicate specifically in tumor cells that have a constitutively activated Ras pathway. Activating mutations of Ras and mutations along the Ras pathway occur in approximately two-thirds of all tumors. Tumors bearing an activated Ras pathway are deficient in their ability to activate an anti-viral response mediated by the host cellular protein, PKR. Since PKR is responsible for preventing reovirus replication, tumor cells that lack the activity of PKR are susceptible to reovirus infection and eventual cell death. As normal cells do not possess Ras activation, these cells are able to thwart reovirus infection by the activity of PKR. In a tumor cell with an activated Ras pathway, the reovirus is able to freely replicate and kill the host tumor cell. Progeny virus particles are then able to infect and kill surrounding cancer cells. This cycle of infection, replication and cell death is believed to be repeated until there are no longer any tumor cells carrying an activated Ras pathway available.
The Company's technologies are based on discoveries made in the 1990s in the Department of Microbiology and Infectious Diseases at the University of Calgary. The potential products are being developed using the naturally occurring reovirus for treatment of cancers in humans.
The KRAS Opportunity
In mid-2009, the U.S. FDA approved revisions to labeling of the epidermal growth factor receptor (EGFR) class of antibodies, indicating that colorectal patients who have KRAS mutations in their tumours do not respond to EGFR-inhibiting antibodies and that the use of this class of pharmaceuticals is not recommended for these patients.
REOLYSIN, Oncolytics' proprietary isolate of the reovirus, preferentially replicates in cancer cells that have an activated RAS pathway. Approximately two-thirds of all cancers have an activated RAS pathway, including most metastatic disease. A large number of mutations, including mutations in EGFR, Her2 or KRAS along the RAS pathway lead to RAS pathway activation. A significant clinical opportunity for REOLYSIN is in the treatment of patients with metastatic cancers who have a mutated KRAS gene and are unlikely to respond to treatment with anit-EGFR monoclonal antibodies.
Current Clinical Trials Roadmap
Company published list by Trial Number http://www.oncolyticsbiotech.com/clinical.html
List of US based clinical trials on the official ClinicalTrials.gov website...Search Term: Reolysin
List of EU based clinical trials on the official EU .... https://www.clinicaltrialsregister.eu/ctr-search/search?query=reovirus
Key Published Scientific Data
Links collected here on the company website
Oncolytic Reovirus Effectively Targets Breast Cancer Stem Cells by Patrick Lee's lab published March 17, 2009 in Mol Therapy
DAILY and WEEKLY Stock Charts
| Trading Data | Share Structure | ||
| | |||
| Exchange Symbol: | TSX: ONC | Estimated As at Jun, 2012 | |
| NASDAQ: ONCY | Outstanding: | 76.6 million | |
| ***Cash On Hand Reported to be approx CAD $31.25 Mil on June 30th, 2012 including the bought deal of CAD $18+Mil conversion announced in Feb 2012. Monthly Burn Rate has increased to $3.1 Mil - From Q2 2012 6mo Income Statement. Current and Past Financials can be found by clicking here! | |||
- All numbers are stated in Canadian Dollars
History
Curing Cancer? Patrick Lee's Path to the Reovirus Treatment
http://cogsci.uwaterloo.ca/Articles/Lee.htm
by Paul Thagard from the Philosophy Department of Waterloo in 2002
Dalhousie virologist Patrick Lee:
http://bhcri.ca/dr-patrick-lee























| The waterfall graph above shows the percentage of tumour regression on a per patient basis at the lesion injected with REOLYSIN®, measured against baseline. |
| *Bar graph is ordered by best response at injected lesion, starting from tumour regression of 100% as shown by first two bars on far left of graph. Results do not reflect the order in which patients were treated, or the dosage received. http://www.oncolyticsbiotech.com/English/clinical-trials/clinical-trials-news/clinical-trials-details/2013/REO-001/ |
Selected Highlights
Since January 1, 2014, selected highlights announced by the Company include:
Clinical Program
Completion of patient enrollment in an ongoing, NCIC Clinical Trials Group sponsored randomized Phase II study of REOLYSIN® in patients with advanced or metastatic colorectal cancer (IND 210). The Company awaits preliminary data from this study;
Reporting completion of enrollment and interim overall and KRAS-mutated patient data from an NCI-sponsored randomized Phase II study of REOLYSIN® in combination with carboplatin and paclitaxel in patients with recurrent or metastatic pancreatic cancer (NCI-8601). The Company awaits final data from this study, which will be available once all remaining patients have progressed;
Completion of patient enrollment in an ongoing, NCI-sponsored randomized Phase II study of REOLYSIN® in combination with paclitaxel in patients with persistent or recurrent ovarian, fallopian tube or primary peritoneal cancer (GOG-186H). The Company awaits preliminary data from this study;
Reporting final data from the Company's randomized, double-blinded clinical study examining REOLYSIN® in combination with carboplatin and paclitaxel in patients with second-line, platinum-refractory, taxane-naïve head and neck cancers;
Presentation by the Company's collaborators of preliminary clinical data demonstrating that intravenously delivered REOLYSIN® can cross the blood brain barrier to access tumours in the brains of humans;
Regulatory
Application for Orphan Drug Designation from the U.S. Food and Drug Administration (FDA) for REOLYSIN® in the treatment of ovarian and pancreatic cancers. The Company was granted designations in ovarian, fallopian tube, primary peritoneal and pancreatic cancer subsequent to year end;
Application to the European Medicines Agency for Orphan Designation for REOLYSIN® in the treatment of pancreatic and ovarian cancers;
Subsequent to year end, the Company applied to the U.S. FDA for a fifth Orphan Drug Designation for high grade gliomas in paediatric patients;
Basic Research
Presentation of a poster entitled "Combination Therapy with Reovirus and PD-1 Blockade Effectively Establishes Tumour Control Via Innate and Adaptive Immune Responses" by the Company's research collaborators, Vile et al., at the AACR Tumor Immunology and Immunotherapy Conference;
A series of presentations made by the Company's research collaborators at the 8th Annual International Conference on Oncolytic Virus Therapeutics held in Oxford, UK, covering:
Preclinical research examining the synergies associated with treatment in animal models with GM-CSF prior to administering REOLYSIN®;
Preclinical research focused on identifying biomarkers predictive of sensitivity/resistance to reovirus in head and neck cancer cell lines; and
Preclinical research into the treatment of hepatocellular carcinoma associated with infection by Hepatitis B and Hepatitis C;
Governance
The nomination and election of Ms. Linda Hohol and Ms. Angela Holtham to the Company's Board of Directors;
Financial
Entry into and subsequent amendments to a share purchase agreement with Lincoln Park Capital Fund, LLC;
Entry into a $20 million "At-the-Market" equity distribution agreement with Canaccord Genuity Inc.; and
At December 31, 2014 the Company reported $16.2 million in cash, cash equivalents and short-term investments. At March 13, 2015, the Company had approximately $27.5 million in cash, cash equivalents and short-term investments.
http://ih.advfn.com/p.php?pid=nmona&article=65886481

| Volume | |
| Day Range: | |
| Bid Price | |
| Ask Price | |
| Last Trade Time: |
