Register for free to join our community of investors and share your ideas. You will also get access to streaming quotes, interactive charts, trades, portfolio, live options flow and more tools.
NEWS -- Oncolytics Biotech® Announces Upcoming Presentations at the American Society of Clinical Oncology Annual Meeting
SAN DIEGO and CALGARY, AB, April 25, 2024 /PRNewswire/ -- Oncolytics Biotech® Inc. (NASDAQ: ONCY) (TSX: ONC), a leading clinical-stage company specializing in immunotherapy for oncology, today announced the acceptance of two abstracts at the 2024 American Society of Clinical Oncology (ASCO) Annual Meeting, which is taking place from May 31 – June 4, 2024, in Chicago, Illinois. Details on the abstracts and poster presentation are shown below.
Title: Phase 1/2 randomized, open-label, multicenter, Simon two-stage study of pelareorep combined with modified FOLFIRINOX +/- atezolizumab in patients with metastatic pancreatic ductal adenocarcinoma.
Presentation Type: Poster
Abstract Number: TPS4203
Session Title: Gastrointestinal Cancer – Gastroesophageal, Pancreatic, and Hepatobiliary
Session Date and Time: June 1, 2024, 1:30 – 4:30 p.m. CT
Title: Pelareorep driven blood TIL expansion in patients with pancreatic, breast and colon cancer.
Presentation Type: Online abstract
Abstract Number: e14625
Abstracts will be published on the ASCO Annual Meeting website at 5:00 p.m. ET on May 23, 2024.
About Oncolytics Biotech Inc.
Oncolytics is a clinical-stage biotechnology company developing pelareorep, an intravenously delivered immunotherapeutic agent. Pelareorep has demonstrated promising results in two randomized Phase 2 studies in metastatic breast cancer and Phase 1 and 2 studies in pancreatic cancer. It acts by inducing anti-cancer immune responses and promotes an inflamed tumor phenotype -- turning "cold" tumors "hot" -- through innate and adaptive immune responses to treat a variety of cancers.
Pelareorep has demonstrated synergies with multiple approved oncology treatments. Oncolytics is currently conducting and planning combination clinical trials with pelareorep in solid and hematological malignancies as it advances towards registrational studies in metastatic breast cancer and pancreatic cancer, both of which have received Fast Track designation from the FDA. For further information, please visit: https://www.oncolyticsbiotech.com or follow the company on social media on LinkedIn and on X @oncolytics.
Company Contact
Jon Patton
Director of IR & Communication
jpatton@oncolytics.ca
Investor Relations for Oncolytics
Timothy McCarthy
LifeSci Advisors
+1-917-679-9282
tim@lifesciadvisors.com
View original content: https://www.prnewswire.com/news-releases/oncolytics-biotech-announces-upcoming-presentations-at-the-american-society-of-clinical-oncology-annual-meeting-302127249.html
SOURCE Oncolytics Biotech® Inc.
NEWS -- Oncolytics Biotech® Advances Toward Registration-Enabling Trial for Pelareorep in Breast Cancer with Submission of Type C Meeting Request to FDA
SAN DIEGO and CALGARY, AB, April 11, 2024 /PRNewswire/ -- Oncolytics Biotech® Inc. (NASDAQ: ONCY) (TSX: ONC), a leading clinical-stage company specializing in immunotherapeutics for oncology, is pleased to announce the submission of a Type C meeting request to the FDA. This meeting aims to discuss the Company's planned registration-enabling trial for pelareorep in HR+/HER2- metastatic breast cancer (mBC).
"A key focus for Oncolytics in 2024 is defining the regulatory path for pelareorep in breast cancer treatment. We are optimistic that pelareorep, in combination with paclitaxel, could significantly enhance clinical outcomes for patients with HR+/HER2- metastatic breast cancer. Our position is strengthened by encouraging data from two randomized studies (BRACELET-1 and IND-213) and the AWARE-1 study, paving the way for the next phase of pelareorep's development and its registration. Ongoing discussions with our clinical collaborators and partners have helped us to prepare a robust, compelling briefing document," said Dr. Matt Coffey, President and Chief Executive Officer of Oncolytics. "We eagerly anticipate our discussion with the FDA to align on the design and objectives of the registrational trial for pelareorep in metastatic breast cancer, a critical step towards bringing this innovative treatment to patients. Having a well-defined plan for the registrational track study will also help advance our strategic partnering discussions. We hope to meet with the agency in Q2 2024 and look forward to a productive dialogue. With anticipated overall survival data from the BRACELET-1 study and productive discussions with the FDA, 2024 is poised to be a transformative year for Oncolytics and our stakeholders."
Thomas Heineman, M.D., Ph.D., Chief Medical Officer at Oncolytics, added, "The data from the randomized BRACELET-1 trial showcased compelling results for the pelareorep/paclitaxel combination therapy in HR+/HER2- metastatic breast cancer patients, with a nearly tripled confirmed response rate, a 50% improvement in median progression-free survival, and a hazard ratio of 0.29 compared to the paclitaxel alone control. Importantly, these data support the statistically significant near doubling of median overall survival in another randomized phase 2 study, IND-213, which also evaluated pelareorep and paclitaxel in HR+/HER2- metastatic breast cancer patients."
Dr. Heineman continued, "Our proposed study plans to evaluate pelareorep in patients with HR+/HER2- mBC who are eligible for chemotherapy after progressing on prior hormonal therapy, including a CDK 4/6 inhibitor. We also intend to evaluate potential biomarkers, including the expansion of tumor-infiltrating lymphocytes, using T cell receptor sequencing. We look forward to the opportunity to discuss our plans with the agency and align on the best approach to advance the development of pelareorep as a potential treatment option to improve outcomes for breast cancer patients."
About Oncolytics Biotech Inc.
Oncolytics is a clinical-stage biotechnology company developing pelareorep, an intravenously delivered immunotherapeutic agent. Pelareorep has demonstrated promising results in Phase 2 studies in breast and pancreatic cancers. It acts by inducing anti-cancer immune responses and promotes an inflamed tumor phenotype -- turning "cold" tumors "hot" -- through innate and adaptive immune responses to treat a variety of cancers.
Pelareorep has demonstrated synergies with multiple approved oncology treatments. Oncolytics is currently conducting and planning combination clinical trials with pelareorep in solid and hematological malignancies as it advances towards registrational studies in metastatic breast cancer and pancreatic cancer, both of which have received Fast Track designation from the FDA. For further information, please visit: https://www.oncolyticsbiotech.com or follow the company on social media on LinkedIn and on X @oncolytics.
This press release contains forward-looking statements, within the meaning of Section 21E of the Securities Exchange Act of 1934, as amended and forward-looking information under applicable Canadian securities laws (such forward-looking statements and forward-looking information are collectively referred to herein as "forward-looking statements"). Forward-looking statements contained in this press release include Oncolytics' belief as to the potential, mechanism of action and benefits of pelareorep as a cancer therapeutic; our plans for a meeting with the FDA in relation to the Company's planned registration-enabling trial for pelareorep in patients with HR+/HER-2 mBC and the anticipated timing thereof; our focus in 2024; our belief that 2024 is poised to be a transformative year for Oncolytics and its stakeholders; the design and areas of evaluation for our proposed study of pelareorep in patients with HR+/HER-2 mBC; our plans to advance towards registrational studies in metastatic breast cancer and pancreatic cancer; and other statements related to anticipated developments in Oncolytics' business and technologies. In any forward-looking statement in which Oncolytics expresses an expectation or belief as to future results, such expectations or beliefs are expressed in good faith and are believed to have a reasonable basis, but there can be no assurance that the statement or expectation or belief will be achieved. Such forward-looking statements involve known and unknown risks and uncertainties, which could cause Oncolytics' actual results to differ materially from those in the forward-looking statements. Such risks and uncertainties include, among others, the availability of funds and resources to pursue research and development projects, the efficacy of pelareorep as a cancer treatment, the success and timely completion of clinical studies and trials, Oncolytics' ability to successfully commercialize pelareorep, uncertainties related to the research and development of pharmaceuticals, uncertainties related to the regulatory process and general changes to the economic environment. We may incur expenses or delays relating to such events outside of our control, including public health crises such as pandemics and epidemics, which could have a material adverse impact on our business, operating results and financial condition. Investors should consult Oncolytics' quarterly and annual filings with the Canadian and U.S. securities commissions for additional information on risks and uncertainties relating to the forward-looking statements. Investors are cautioned against placing undue reliance on forward-looking statements. The Company does not undertake any obligation to update these forward-looking statements, except as required by applicable laws.
Company Contact
Jon Patton
Director of IR & Communication
mailto://jpatton@oncolytics.ca
Investor Relations for Oncolytics
Timothy McCarthy
LifeSci Advisors
+1-917-679-9282
mailto://tim@lifesciadvisors.com
View original content: https://www.prnewswire.com/news-releases/oncolytics-biotech-advances-toward-registration-enabling-trial-for-pelareorep-in-breast-cancer-with-submission-of-type-c-meeting-request-to-fda-302113665.html
SOURCE Oncolytics Biotech® Inc.
for the greater good of peoples need everyone should support this company at least with their spare money. Faster their product comes to market faster will be the help to many seriously suffering patients through their platform treatment and off label use in similar high risk cancer patients. explore their pipeline clinical trial data and the quality of their 110+ patent portfolio. help them to speed up their effort.
https://patents.justia.com/assignee/oncolytics-biotech-inc?page=7
ONCY.................................................https://stockcharts.com/h-sc/ui?s=ONCY&p=W&b=5&g=0&id=p86431144783
NEWS -- Oncolytics Biotech® to Participate in a Panel Presentation at Canaccord Genuity's Horizons in Oncology Virtual Conference

SAN DIEGO and CALGARY, AB, April 2, 2024 /CNW/ -- Oncolytics Biotech® Inc. (NASDAQ: ONCY) (TSX: ONC), a clinical-stage immunotherapeutics company focused on oncology, today announced that President and Chief Executive Officer Dr. Matt Coffey will participate in the Viral Approaches in Oncology panel at Canaccord Genuity's Horizons in Oncology Virtual Conference, which is taking place on April 15, 2024. Additional details on the panel presentation can be found below.
Date: Monday, April 15, 2024
Time: 9:00 a.m. ET
Panel Title: Viral Approaches in Oncology
A live webcast of the panel presentation will be available to registered attendees of the conference through the conference website. Company management will also be participating in virtual one-on-one investor meetings at the conference. To schedule a meeting, contact your Canaccord representative or email mailto://jpatton@oncolytics.ca.
About Oncolytics Biotech Inc.
Oncolytics is a clinical-stage biotechnology company developing pelareorep, an intravenously delivered immunotherapeutic agent. Pelareorep has demonstrated promising results in Phase 2 studies in breast and pancreatic cancers. It acts by inducing anti-cancer immune responses and promotes an inflamed tumor phenotype -- turning "cold" tumors "hot" -- through innate and adaptive immune responses to treat a variety of cancers.
Pelareorep has demonstrated synergies with multiple approved oncology treatments. Oncolytics is currently conducting and planning combination clinical trials with pelareorep in solid and hematological malignancies as it advances towards registrational studies in metastatic breast cancer and pancreatic cancer, both of which have received Fast Track designation from the FDA. For further information, please visit: https://www.oncolyticsbiotech.com or follow the company on social media on LinkedIn and on X @oncolytics.
Company Contact
Jon Patton
Director of IR & Communication
mailto://jpatton@oncolytics.ca
Investor Relations for Oncolytics
Timothy McCarthy
LifeSci Advisors
+1-917-679-9282
mailto://tim@lifesciadvisors.com
View original content to download multimedia: https://www.prnewswire.com/news-releases/oncolytics-biotech-to-participate-in-a-panel-presentation-at-canaccord-genuitys-horizons-in-oncology-virtual-conference-302105507.html
SOURCE Oncolytics Biotech® Inc.
View original content to download multimedia: http://www.newswire.ca/en/releases/archive/April2024/02/c3716.html
ONCY earning release tomorrow - Guess it is time for them to talk more on FDA approval, launch time line and the players
NEWS -- Oncolytics Biotech® Files Amendment to Initiate New Pancreatic Cancer GOBLET Cohort Supported by PanCAN

US$5M grant supports study of pelareorep in combination with modified FOLFIRINOX +/- atezolizumab
Testing with the most common therapies could facilitate broad use of pelareorep in pancreatic cancer patients
SAN DIEGO and CALGARY, AB, March 5, 2024 /CNW/ -- Oncolytics Biotech® Inc. (NASDAQ: ONCY) (TSX: ONC), a clinical-stage immunotherapeutics company focused on oncology, today announced it has submitted an amendment to GOBLET to initiate a new Phase 1/2 cohort evaluating pelareorep in combination with modified FOLFIRINOX (mFOLFIRINOX) with and without atezolizumab (Tecentriq®) in patients with newly diagnosed metastatic pancreatic ductal adenocarcinoma (PDAC). This amendment will be reviewed by the Paul Ehrlich Institute (PEI; Germany's regulatory body) for approval before patient enrollment can begin. The cohort, the fifth of the GOBLET gastrointestinal cancer study, is being supported by the US$5 million Therapeutic Accelerator Award from the Pancreatic Cancer Action Network (PanCAN), an innovative program established to accelerate the development of new treatments for pancreatic cancer. Evaluation of this novel treatment approach will complement Oncolytics' ongoing development of pelareorep, atezolizumab, gemcitabine, and nab-paclitaxel in PDAC, which is expected to advance to a registrational study later this year.
"We are enthusiastic to have the support of PanCAN to expand the evaluation of pelareorep in pancreatic cancer and explore mFOLFIRINOX as another combination that could improve outcomes for patients. Notably, this patient population is newly diagnosed patients who are receiving first-line treatment. Chemotherapies, including either mFOLFIRINOX or gemcitabine and nab-paclitaxel, are the backbone treatment regimens of pancreatic cancer therapy1. Evaluating pelareorep in combination with these widely used regimens is an important step in our broad clinical development program," said Dr. Matt Coffey, President and Chief Executive Officer of Oncolytics. "Last fall, we reported a 62% objective response rate for the GOBLET PDAC cohort studying pelareorep in combination with the checkpoint inhibitor atezolizumab in addition to gemcitabine and nab-paclitaxel (link to the PR, link to the poster). This response is about three times that of historical controls and forms the basis of the registrational program, expected to begin this year. Therefore, we are enthusiastic about this new mFOLFIRINOX pancreatic cancer cohort and look forward to enrolling the first patient as soon as possible."
Dirk Arnold, M.D., Ph.D., Director of Asklepios Tumorzentrum Hamburg, and primary investigator of the GOBLET trial, commented, "One of the reasons for pancreatic cancer's poor survival rate is that it effectively evades the immune system and can induce an immunosuppressive tumor microenvironment (TME)2. Pelareorep is an attractive combination partner because of its ability to address both issues by activating the innate and adaptive immune systems while driving the remodeling of the tumor microenvironment. Positive results from the Phase 2 study evaluating pelareorep combined with atezolizumab and chemotherapy reported last fall support the potential use of these agents together. I am hopeful that the combination of pelareorep and mFOLFIRINOX (with or without atezolizumab) will yield positive response data and advance the development of new treatment options for patients with pancreatic cancer."
"This study is designed to evaluate whether pelareorep can enhance outcomes in patients receiving mFOLFIRINOX, one of the most commonly used metastatic pancreatic cancer treatments. Combining pelareorep with mFOLFIRINOX represents an expansion of our existing pancreatic cancer program and maximizes the potential of pelareorep-based combination therapies to benefit pancreatic cancer patients," commented Thomas Heineman, M.D., Ph.D., Chief Medical Officer at Oncolytics. "The mFOLFIRINOX cohort utilizes a screened selection design within a Simon two-stage approach that will also allow evaluation of the contribution of atezolizumab to the pelareorep/mFOLFIRINOX combination. In addition, this study is designed to provide valuable translational assessments, such as the expansion of tumor-infiltrating lymphocytes (TILs) in the blood, which has been associated with tumor responses. We look forward to building on PanCAN's strong relationships with the pancreatic cancer community and furthering our collaboration with AIO-Studien-gGmbH (AIO) on the GOBLET study."
References
1. Botta G, et al. SWI/SNF complex alterations as a biomarker of immunotherapy efficacy in pancreatic cancer. JCI Insight. 2021;6(18):e150453. https://doi.org/10.1172/jci.insight.150453.
2. Yoon JH, et al. Immunotherapy for pancreatic cancer. World J Clin Cases. 2021 May 6;9(13):2969-2982. doi: 10.12998/wjcc.v9.i13.2969. PMID: 33969083; PMCID: PMC8080736.
About GOBLET cohort 5
The mFOLFIRINOX cohort of the Phase 1/2 GOBLET study is designed to evaluate newly diagnosed PDAC patients treated with pelareorep + mFOLFIRINOX with or without atezolizumab. There will be a three-patient safety run-in to evaluate the tolerability of each treatment arm - pelareorep + mFOLFIRINOX + atezolizumab and pelareorep + mFOLFIRINOX. A total of fifteen patients may be randomized to each arm in Stage one of the Simon-two stage design. The co-primary endpoints of the cohort are objective response rate and safety. The success criteria for Stage 1 is defined as six or more responses in one or both of the treatment groups. Successful completion of Stage one will support expansion into Stage two, which can include one or both treatment regimens, and would enroll 17 additional evaluable patients. A total of 13 or more responses from Stage 1 and 2 combined are required to achieve the success criteria. Translational data will also be generated.
About GOBLET
The GOBLET (Gastrointestinal tumOrs exploring the treatment comBinations with the oncolytic reovirus peLarEorep and anTi-PD-L1) study is a phase 1/2 multiple indication study in advanced or metastatic gastrointestinal tumors. The study is being conducted at 12 centers in Germany and is being managed by AIO-Studien-gGmbH. The co-primary endpoints of the study are objective response rate (ORR) and/or disease control rate assessed at week 16 and safety. Key secondary and exploratory endpoints include additional efficacy assessments and evaluation of potential biomarkers (T cell clonality and CEACAM6). The study employs a Simon two-stage design with Stage 1 comprising five treatment groups:
1. Pelareorep in combination with atezolizumab, gemcitabine, and nab-paclitaxel in 1st line advanced/metastatic pancreatic cancer patients;
2. Pelareorep in combination with atezolizumab in 1st line MSI (microsatellite instability)-high metastatic colorectal cancer patients;
3. Pelareorep in combination with atezolizumab and TAS-102 in 3rd line metastatic colorectal cancer patients
4. Pelareorep in combination with atezolizumab in 2nd line advanced and unresectable anal cancer patients; and
5. Pelareorep in combination with mFOLFIRINOX with and without atezolizumab in newly diagnosed metastatic PDAC patients.
Any cohort meeting pre-specified efficacy criteria in Stage 1 may be advanced to Stage 2 and enroll additional patients.
About AIO
AIO-Studien-gGmbH (AIO) emerged from the study center of the internal oncology working group within the German Cancer Society (DKG). AIO operates with a non-profit purpose of promoting science and research with a focus on medical oncology. Since its foundation, AIO has become a successful sponsor and study management company and has established itself both nationally and internationally.
About Oncolytics Biotech Inc.
Oncolytics is a clinical-stage biotechnology company developing pelareorep, an intravenously delivered immunotherapeutic agent. Pelareorep has demonstrated promising results in Phase 2 studies in breast and pancreatic cancers. It acts by inducing anti-cancer immune responses and promotes an inflamed tumor phenotype -- turning "cold" tumors "hot" -- through innate and adaptive immune responses to treat a variety of cancers.
Pelareorep has demonstrated synergies with multiple approved oncology treatments. Oncolytics is currently conducting and planning combination clinical trials with pelareorep in solid and hematological malignancies as it advances towards registrational studies in metastatic breast cancer and pancreatic cancer, both of which have received Fast Track designation from the FDA. For further information, please visit: https://www.oncolyticsbiotech.com or follow the company on social media on LinkedIn and on X @oncolytics.
This press release contains forward-looking statements, within the meaning of Section 21E of the Securities Exchange Act of 1934, as amended and forward-looking information under applicable Canadian securities laws (such forward-looking statements and forward-looking information are collectively referred to herein as "forward-looking statements"). Forward-looking statements contained in this press release include Oncolytics' belief as to the potential, mechanism of action and benefits of pelareorep as a cancer therapeutic; our stated goals, objectives and mission; our belief that pelareorep in combination with mFOLFIRINOX could improve outcomes for pancreatic cancer patients and advance the development of new treatment options for patients with pancreatic cancer; our plans to build on PanCAN's strong relationships with the pancreatic cancer community and further our collaboration with AIO-Studien-gGmbH on our GOBLET study; our plans to advance towards registrational studies in metastatic breast cancer and pancreatic cancer; and other statements related to anticipated developments in Oncolytics' business and technologies. In any forward-looking statement in which Oncolytics expresses an expectation or belief as to future results, such expectations or beliefs are expressed in good faith and are believed to have a reasonable basis, but there can be no assurance that the statement or expectation or belief will be achieved. Such forward-looking statements involve known and unknown risks and uncertainties, which could cause Oncolytics' actual results to differ materially from those in the forward-looking statements. Such risks and uncertainties include, among others, the availability of funds and resources to pursue research and development projects, the efficacy of pelareorep as a cancer treatment, the success and timely completion of clinical studies and trials, Oncolytics' ability to successfully commercialize pelareorep, uncertainties related to the research and development of pharmaceuticals, uncertainties related to the regulatory process and general changes to the economic environment. In particular, we may be impacted by business interruptions resulting from COVID-19 coronavirus, including operating, manufacturing supply chain, clinical trial and project development delays and disruptions, labour shortages, travel and shipping disruption, and shutdowns (including as a result of government regulation and prevention measures). We may incur expenses or delays relating to such events outside of our control, which could have a material adverse impact on our business, operating results and financial condition. Investors should consult Oncolytics' quarterly and annual filings with the Canadian and U.S. securities commissions for additional information on risks and uncertainties relating to the forward-looking statements. Investors are cautioned against placing undue reliance on forward-looking statements. The Company does not undertake any obligation to update these forward-looking statements, except as required by applicable laws.
Company Contact
Jon Patton
Director of IR & Communication
+1-858-886-7813
mailto://jpatton@oncolytics.ca
Investor Relations for Oncolytics
Timothy McCarthy
LifeSci Advisors
+1-917-679-9282
mailto://tim@lifesciadvisors.com
View original content to download multimedia: https://www.prnewswire.com/news-releases/oncolytics-biotech-files-amendment-to-initiate-new-pancreatic-cancer-goblet-cohort-supported-by-pancan-302079547.html
SOURCE Oncolytics Biotech® Inc.
View original content to download multimedia: http://www.newswire.ca/en/releases/archive/March2024/05/c7756.html
NEWS -- Oncolytics Biotech® to Host Conference Call to Discuss Fourth Quarter and Full Year Financial Results and Recent Operational Highlights

Conference call and webcast to take place on Thursday, March 7, 2024, at 4:30 p.m. ET
SAN DIEGO and CALGARY, AB, March 4, 2024 /CNW/ -- Oncolytics Biotech® Inc. (NASDAQ: ONCY) (TSX: ONC), a clinical-stage immunotherapeutics company focused on oncology, today announced that it will host a conference call and webcast on Thursday, March 7, 2024, at 4:30 p.m. ET to discuss a corporate update and financial results for the fourth quarter and full year 2023.
Conference Call & Webcast
Date: Thursday, March 7, 2024
Time: 4:30 p.m. ET
Dial In – North American Toll-Free: (888) 664-6383
Dial In – International: (416) 764-8650
RapidConnect: to join the conference call without operator assistance, please click here
Conference ID (if needed): 6244-5815
Webcast: please click here
A webcast of the call will also be available on the Investor Relations page of Oncolytics' website, available by clicking here, and will be archived for three months. A dial-in replay will be available for one week and can be accessed by dialing (888) 390-0541 (North America) or (416) 764-8677 (International) and using replay code: 445-815#.
About Oncolytics Biotech Inc.
Oncolytics is a clinical-stage biotechnology company developing pelareorep, an intravenously delivered immunotherapeutic agent. Pelareorep has demonstrated promising results in Phase 2 studies in breast and pancreatic cancers. It acts by inducing anti-cancer immune responses and promotes an inflamed tumor phenotype -- turning "cold" tumors "hot" -- through innate and adaptive immune responses to treat a variety of cancers.
Pelareorep has demonstrated synergies with multiple approved oncology treatments. Oncolytics is currently conducting and planning combination clinical trials with pelareorep in solid and hematological malignancies as it advances towards registrational studies in metastatic breast cancer and pancreatic cancer, both of which have received Fast Track designation from the FDA. For further information, please visit: https://www.oncolyticsbiotech.com or follow the company on social media on LinkedIn and on X @oncolytics.
Company Contact
Jon Patton
Director of IR & Communication
+1-858-886-7813
mailto://jpatton@oncolytics.ca
Investor Relations for Oncolytics
Timothy McCarthy
LifeSci Advisors
+1-917-679-9282
mailto://tim@lifesciadvisors.com
View original content: https://www.prnewswire.com/news-releases/oncolytics-biotech-to-host-conference-call-to-discuss-fourth-quarter-and-full-year-financial-results-and-recent-operational-highlights-302078051.html
SOURCE Oncolytics Biotech® Inc.
View original content: http://www.newswire.ca/en/releases/archive/March2024/04/c8901.html
Patents Assigned to Oncolytics Biotech, Inc. till 2020, 2021-24 newly filed list not yet public
https://patents.justia.com/assignee/oncolytics-biotech-inc
2024 Outlook: Dr. Coffey: Looking ahead to 2024, we expect to initiate the first Phase 3 study for pelareorep in pancreatic cancer. The transition to a late-stage biopharmaceutical company will mark an important inflection point for investors, clinical collaborators and potential partners by providing a line of sight towards the path to regulatory approval and achieving our mission of developing pelareorep as an immunotherapeutic agent for cancer. We are excited to launch a new Phase 1/2 pancreatic cancer study investigating, for the first time, pelareorep in combination with modified FOLFIRINOX (mFOLFIRINOX). Finally, our discussions with regulators on a pivotal Phase 3 trial have been productive to date, and we expect to provide guidance on the registration path for metastatic breast cancer in the first half of 2024. We look forward to updating our stakeholders on our progress as the year unfolds."
NEWS -- Oncolytics Biotech® Announces Presentation at the Cancer Advocacy Group of Louisiana's 3rd Annual NeauxCancer Conference
PR Newswire

SAN DIEGO and CALGARY, AB, Feb. 28, 2024 /PRNewswire/ -- Oncolytics Biotech® Inc. (NASDAQ: ONCY) (TSX: ONC), a clinical-stage immunotherapeutics company focused on oncology, today announced that Chief Executive Officer Dr. Matt Coffey will present a corporate overview at the Cancer Advocacy Group of Louisiana's 3rd Annual NeauxCancer Conference. The conference is taking place from February 29 - March 2, 2024, at the Ritz Carlton Hotel in New Orleans, LA. Additional details on the presentation can be found below.
Date: Friday, March 1, 2024
Time: 11:30 a.m. ET
Location: Ritz Carlton Hotel New Orleans, Acadia Room 2nd Floor
Webcast Link: Available by clicking here
Company management will also be participating in one-on-one investor meetings at the conference. To schedule a meeting, please submit a request to the conference organizers or email mailto://jpatton@oncolytics.ca
A live webcast of the Company's presentation will also be available on the Investor Relations page of Oncolytics' website (LINK).
Additional information about the event can be found on the official website by clicking here.
About Oncolytics Biotech Inc.
Oncolytics is a clinical-stage biotechnology company developing pelareorep, an intravenously delivered immunotherapeutic agent. Pelareorep has demonstrated promising results in phase 2 studies in breast and pancreatic cancers. It acts by inducing anti-cancer immune responses and promotes an inflamed tumor phenotype -- turning "cold" tumors "hot" -- through innate and adaptive immune responses to treat a variety of cancers.
Pelareorep has demonstrated synergies with multiple approved oncology treatments. Oncolytics is currently conducting and planning combination clinical trials with pelareorep in solid and hematological malignancies as it advances towards registrational studies in metastatic breast cancer and pancreatic cancer, both of which have received Fast Track designation from the FDA. For further information, please visit: https://www.oncolyticsbiotech.com or follow the company on social media on LinkedIn and on X @oncolytics.
Company Contact
Jon Patton
Director of IR & Communication
+1-858-886-7813
mailto://jpatton@oncolytics.ca
Investor Relations for Oncolytics
Timothy McCarthy
LifeSci Advisors
+1-917-679-9282
mailto://tim@lifesciadvisors.com
View original content to download multimedia: https://www.prnewswire.com/news-releases/oncolytics-biotech-announces-presentation-at-the-cancer-advocacy-group-of-louisianas-3rd-annual-neauxcancer-conference-302074097.html
SOURCE Oncolytics Biotech® Inc.
NEWS -- Oncolytics Biotech® Initiates Enrollment Expansion of GOBLET Anal Cancer Cohort
Successful Stage 1 data showed a near tripling of Objective Response Rate compared to checkpoint inhibitor monotherapy, including a Complete Response, and supports expansion
SAN DIEGO and CALGARY, Alberta, Feb. 14, 2024 /CNW/ -- Oncolytics Biotech® Inc. (NASDAQ: ONCY) (TSX: ONC), a clinical-stage immunotherapeutics company focused on oncology, today announced the expansion of enrollment for the anal cancer cohort of the GOBLET study evaluating pelareorep in combination with atezolizumab (Tecentriq®) in patients with second-line or later unresectable squamous cell carcinoma of the anal canal (SCCA). The study was expanded based on positive data from Stage 1 of the study, presented at the 2nd International Multidisciplinary Anal Cancer Conference (IMACC) in November 2023 (link to the PR, link to the poster).
"These exciting clinical data, which exceed the Simon two-stage success criteria, provide strong support to expand the evaluation of pelareorep in patients with advanced anal cancer. The results reported at IMACC 2023 showed that the combination of pelareorep and atezolizumab provided a 37.5% objective response rate, including one patient with a long-lasting complete response, and good overall tolerability. These data represent a meaningful contrast to recent clinical trial results which show that patients with second-line or later anal carcinoma treated with checkpoint inhibitor therapy experienced response rates of 10-14%1-3," said Dr. Matt Coffey, President and Chief Executive Officer of Oncolytics."
"We begin the Stage 2 expansion with substantial optimism for patients and the potential of pelareorep, especially considering the initial efficacy signal observed for pelareorep-based therapy in pancreatic cancer. There is currently no established standard therapy for patients with anal carcinoma who have failed first-line treatment. Continued positive results could potentially expand the opportunity for pelareorep beyond the lead indications of breast cancer and pancreatic cancer and open the door to a rapid regulatory pathway in this rare and significantly under-served patient population," continued Dr. Coffey.
Dirk Arnold, M.D., Ph.D., Director of Asklepios Tumorzentrum Hamburg, and primary investigator of the GOBLET trial, commented, "One of the most difficult challenges in my practice is the limited number of treatment options that are available for patients with advanced anal cancer who have progressed on first-line therapy. I am enthusiastic about the expansion of this cohort because it will enable the continued evaluation of the pelareorep/atezolizumab combination and could provide important confirmatory data that may lead to better treatment options for patients with this late-stage disease."
"We look forward to building on the oncology community's enthusiastic reception of the IMACC 2023 data by expanding enrollment in the anal carcinoma cohort and incorporating additional sites into the study," commented Thomas Heineman, M.D., Ph.D., Chief Medical Officer at Oncolytics. "Careful consideration of recently published clinical trial results indicates that a modest expansion of fewer than 20 patients will be sufficient to solidify the efficacy signal we have observed to date and lay the groundwork for a potential future registrational study in this population. We look forward to continuing our excellent collaboration with the clinical sites and investigators at AIO and hope to report additional results in 2025."
References
1. Rao S, et al. Phase II study of retifanlimab in patients (pts) with squamous carcinoma of the anal canal (SCAC) who progressed following platinum-based chemotherapy. Annals of Oncology. 2020 September. doi: https://doi.org/10.1016/j.annonc.2020.08.2272.
2. Marabelle A, et al. Pembrolizumab for previously treated advanced anal squamous cell carcinoma: results from the non-randomised, multicohort, multicentre, phase 2 KEYNOTE-158 study. Lancet Gastroenterol Hepatol. 2022 May;7(5):446-454. doi: 10.1016/S2468-1253(21)00382-4.
3. Lonardi S, et al. Randomized phase II trial of avelumab alone or in combination with cetuximab for patients with previously treated, locally advanced, or metastatic squamous cell anal carcinoma: the CARACAS study. J Immunother Cancer. 2021 November;9(11):e002996. doi: 10.1136/jitc-2021-002996. PMID: 34815354; PMCID: PMC8611452.
About GOBLET
The GOBLET (Gastrointestinal tumOrs exploring the treatment comBinations with the oncolytic reovirus peLarEorep and anTi-PD-L1) study is a phase 1/2 multiple indication study in advanced or metastatic gastrointestinal tumors. The study is being conducted at 12 centers in Germany and is being managed by AIO-Studien-gGmbH. The co-primary endpoints of the study are objective response rate (ORR) assessed at week 16 and safety. Key secondary and exploratory endpoints include additional efficacy assessments and evaluation of potential biomarkers (T cell clonality and CEACAM6). The study employs a Simon two-stage design with Stage 1 comprising four treatment groups:
1. Pelareorep in combination with atezolizumab, gemcitabine, and nab-paclitaxel in 1st line advanced/metastatic pancreatic cancer patients;
2. Pelareorep in combination with atezolizumab in 1st line MSI (microsatellite instability)-high metastatic colorectal cancer patients;
3. Pelareorep in combination with atezolizumab and TAS-102 in 3rd line metastatic colorectal cancer patients; and
4. Pelareorep in combination with atezolizumab in 2nd line advanced and unresectable anal cancer patients.
Any cohort meeting pre-specified efficacy criteria in Stage 1 may be advanced to Stage 2 and enroll additional patients.
About AIO
AIO-Studien-gGmbH (AIO) emerged from the study center of the internal oncology working group within the German Cancer Society (DKG). AIO operates with a non-profit purpose of promoting science and research with a focus on medical oncology. Since its foundation, AIO has become a successful sponsor and study management company and has established itself both nationally and internationally.
About Oncolytics Biotech Inc.
Oncolytics is a clinical-stage biotechnology company developing pelareorep, an intravenously delivered immunotherapeutic agent. Pelareorep has demonstrated promising results in Phase 2 studies in breast and pancreatic cancers. It acts by inducing anti-cancer immune responses and promotes an inflamed tumor phenotype -- turning "cold" tumors "hot" -- through innate and adaptive immune responses to treat a variety of cancers.
Pelareorep has demonstrated synergies with multiple approved oncology treatments. Oncolytics is currently conducting and planning combination clinical trials with pelareorep in solid and hematological malignancies as it advances towards registrational studies in metastatic breast cancer and pancreatic cancer. For further information, please visit: https://www.oncolyticsbiotech.com.
This press release contains forward-looking statements, within the meaning of Section 21E of the Securities Exchange Act of 1934, as amended and forward-looking information under applicable Canadian securities laws (such forward-looking statements and forward-looking information are collectively referred to herein as "forward-looking statements"). Forward-looking statements contained in this press release include Oncolytics' belief as to the potential, mechanism of action and benefits of pelareorep as a cancer therapeutic; our plan to expand the evaluation of pelareorep in patients with advanced anal cancer; our belief that continued positive results could potentially expand the opportunity for pelareorep beyond the lead indications of breast cancer and pancreatic cancer and open the door to a rapid regulatory pathway; our belief that the expansion of the anal cancer cohort will enable the continued evaluation of the pelareorep/atezolizumab combination and could provide important confirmatory data that may lead to better treatment options for patients with this late-stage disease; our belief in the potential for a future registrational study in anal cancer; our plans to continue our collaboration with the clinical sites and investigators at AIO-Studien-gGmbH and hope to report additional results in 2025; our plans to advance towards registrational studies in metastatic breast cancer and pancreatic cancer; and other statements related to anticipated developments in Oncolytics' business and technologies. In any forward-looking statement in which Oncolytics expresses an expectation or belief as to future results, such expectations or beliefs are expressed in good faith and are believed to have a reasonable basis, but there can be no assurance that the statement or expectation or belief will be achieved. Such forward-looking statements involve known and unknown risks and uncertainties, which could cause Oncolytics' actual results to differ materially from those in the forward-looking statements. Such risks and uncertainties include, among others, the availability of funds and resources to pursue research and development projects, the efficacy of pelareorep as a cancer treatment, the success and timely completion of clinical studies and trials, Oncolytics' ability to successfully commercialize pelareorep, uncertainties related to the research and development of pharmaceuticals, uncertainties related to the regulatory process and general changes to the economic environment. In particular, we may be impacted by business interruptions resulting from COVID-19 coronavirus, including operating, manufacturing supply chain, clinical trial and project development delays and disruptions, labour shortages, travel and shipping disruption, and shutdowns (including as a result of government regulation and prevention measures). We may incur expenses or delays relating to such events outside of our control, which could have a material adverse impact on our business, operating results and financial condition. Investors should consult Oncolytics' quarterly and annual filings with the Canadian and U.S. securities commissions for additional information on risks and uncertainties relating to the forward-looking statements. Investors are cautioned against placing undue reliance on forward-looking statements. The Company does not undertake any obligation to update these forward-looking statements, except as required by applicable laws.
Company Contact
Jon Patton
Director of IR & Communication
+1-858-886-7813
mailto://jpatton@oncolytics.ca
Investor Relations for Oncolytics
Timothy McCarthy
LifeSci Advisors
+1-917-679-9282
mailto://tim@lifesciadvisors.com
View original content: https://www.prnewswire.com/news-releases/oncolytics-biotech-initiates-enrollment-expansion-of-goblet-anal-cancer-cohort-302061219.html
SOURCE Oncolytics Biotech® Inc.
View original content: http://www.newswire.ca/en/releases/archive/February2024/14/c7011.html
Good hire of Patricia S. Andrews. Bad cash position. They need something big to happen, or they need to raise cash or sell…..Too much risk at $1 for me. It is a swing for the fences, but a swing they have. Hope I eat crow. Good luck all!
gonna be little while, imo. still play time for the shorts.
this could be $20 to $80 in a single day
there drug showing game changing result in pancreatic cancer in clinical trials - that is why so many big company now lined up behind
NEWS -- Oncolytics Biotech® Appoints Patricia S. Andrews to its Board of Directors

SAN DIEGO and CALGARY, AB, Jan. 9, 2024 /CNW/ -- Oncolytics Biotech® Inc. (NASDAQ: ONCY) (TSX: ONC), a clinical-stage immunotherapeutics company focused on oncology, today announced the appointment of Patricia S. Andrews to its Board of Directors (the "Board").
"I am pleased to welcome Pat Andrews to our Board of Directors," said Dr. Matt Coffey, President and Chief Executive Officer of Oncolytics. "Pat's extensive board and executive leadership experience navigating first registrational trials in oncology and completing transformational business development agreements with global pharmaceutical companies makes her a natural fit for Oncolytics. As we begin preparations to initiate pivotal studies with pelareorep in breast and pancreatic cancers and progress partnering discussions, we look forward to benefitting from her insights and strategic and operational experience."
"I am honored to join the Oncolytics board at this exciting time for the organization as it advances pelareorep toward registrational studies," said Ms. Andrews. "Promising recent results from the BRACELET-1 and GOBLET studies suggest that pelareorep has the potential to make an important contribution to cancer care, and I look forward to working with my fellow board members in addition to Dr. Coffey and the Executive Team to execute Oncolytics' clinical and corporate development plans."
Ms. Andrews is an accomplished biopharmaceutical executive and public company board member with a track record of success in corporate strategy, first-in-class and first-for-the-company new product commercializations, and business development. Ms. Andrews currently serves as a Director and Member of the Audit Committee at GlycoMimetics. During her tenure, the company planned and initiated its first Phase 3 study and is preparing for commercialization.
Ms. Andrews previously served as Chief Executive Officer for Sumitomo Pharma Oncology, Inc. (SMP Oncology), where she led the organization through the integration of its multiple predecessor companies. Under her leadership, the organization completed three Phase 3 trials and expanded the clinical pipeline from two programs to eight. Prior to SMP Oncology, Ms. Andrews served as Chief Commercial Officer for Incyte and led the organization through the launch preparations and introduction of Jakafi®, the company's first commercial product, a novel, first-in-class, first-in-disease agent for the treatment of myelofibrosis. While with Incyte, she also steered landmark licensing arrangements with Novartis and Eli Lilly that were critically important to fund the company's long-term trajectory.
Prior to Incyte, Ms. Andrews spent 17 years with Pfizer, ultimately serving as Vice President and General Manager of the U.S. Oncology Business Unit, a $900 million portfolio at the time. Under her leadership, Sutent® was launched, becoming the market leader in renal cell carcinoma, and a refreshed positioning of Camptosar® led to robust revenue growth after years of declining sales in colorectal cancer. Ms. Andrews is a member of the Boston chapter of the Board of Advisors for Life Science Cares, a collective impact organization dedicated to fighting poverty in local communities. She earned an MBA from the University of Michigan and her BA from Brown University.
About Oncolytics Biotech Inc.
Oncolytics is a clinical-stage biotechnology company developing pelareorep, an intravenously delivered immunotherapeutic agent. Pelareorep has demonstrated promising results in Phase 2 studies in breast and pancreatic cancers. It acts by inducing anti-cancer immune responses and promotes an inflamed tumor phenotype -- turning "cold" tumors "hot" -- through innate and adaptive immune responses to treat a variety of cancers.
Pelareorep has demonstrated synergies with multiple approved oncology treatments. Oncolytics is currently conducting and planning combination clinical trials with pelareorep in solid and hematological malignancies as it advances towards registrational studies in metastatic breast cancer and pancreatic cancer. For further information, please visit: https://www.oncolyticsbiotech.com.
This press release contains forward-looking statements, within the meaning of Section 21E of the Securities Exchange Act of 1934, as amended and forward-looking information under applicable Canadian securities laws (such forward-looking statements and forward-looking information are collectively referred to herein as "forward-looking statements"). Forward-looking statements contained in this press release include Oncolytics' belief as to the potential, mechanism of action and benefits of pelareorep as a cancer therapeutic; our plans to advance towards registrational studies in metastatic breast cancer and pancreatic cancer; our plans to progress partnering discussions; and other statements related to anticipated developments in Oncolytics' business and technologies. In any forward-looking statement in which Oncolytics expresses an expectation or belief as to future results, such expectations or beliefs are expressed in good faith and are believed to have a reasonable basis, but there can be no assurance that the statement or expectation or belief will be achieved. Such forward-looking statements involve known and unknown risks and uncertainties, which could cause Oncolytics' actual results to differ materially from those in the forward-looking statements. Such risks and uncertainties include, among others, the availability of funds and resources to pursue research and development projects, the efficacy of pelareorep as a cancer treatment, the success and timely completion of clinical studies and trials, Oncolytics' ability to successfully commercialize pelareorep, uncertainties related to the research and development of pharmaceuticals, uncertainties related to the regulatory process and general changes to the economic environment. In particular, we may be impacted by business interruptions resulting from COVID-19 coronavirus, including operating, manufacturing supply chain, clinical trial and project development delays and disruptions, labour shortages, travel and shipping disruption, and shutdowns (including as a result of government regulation and prevention measures). We may incur expenses or delays relating to such events outside of our control, which could have a material adverse impact on our business, operating results and financial condition. Investors should consult Oncolytics' quarterly and annual filings with the Canadian and U.S. securities commissions for additional information on risks and uncertainties relating to the forward-looking statements. Investors are cautioned against placing undue reliance on forward-looking statements. The Company does not undertake any obligation to update these forward-looking statements, except as required by applicable laws.
Company Contact
Jon Patton
Director of IR & Communication
+1-858-886-7813
mailto://jpatton@oncolytics.ca
Investor Relations for Oncolytics
Timothy McCarthy
LifeSci Advisors
+1-917-679-9282
mailto://tim@lifesciadvisors.com
View original content: https://www.prnewswire.com/news-releases/oncolytics-biotech-appoints-patricia-s-andrews-to-its-board-of-directors-302029215.html
SOURCE Oncolytics Biotech® Inc.
View original content: http://www.newswire.ca/en/releases/archive/January2024/09/c2494.html
NEWS -- Oncolytics Biotech® Recaps 2023 Accomplishments, Provides Outlook for 2024

SAN DIEGO, CA and CALGARY, AB, Jan. 4, 2024 /CNW/ -- Oncolytics Biotech® Inc. (NASDAQ: ONCY) (TSX: ONC), a clinical-stage immunotherapeutics company focused on oncology, reported on significant 2023 accomplishments and announced corporate priorities and anticipated clinical development milestones for 2024.
Overview: "2023 was an outstanding year for Oncolytics and the development of pelareorep as a potential cancer therapy. To date, we have studied pelareorep in more than 1,100 patients, and it has been shown to be well-tolerated with mild side effects. Promising clinical and translational results from our Phase 2 programs and mechanism of action learnings from a wide range of tumor types, as detailed below, provided consistent data and efficacy signals with durable responses that support the use of pelareorep as an immunotherapeutic agent, either as a monotherapy or in combination with other agents. These data readouts from multiple clinical studies at numerous sites and from diverse cancer indications that are clinically important make a compelling argument for pelareorep as a true backbone immunotherapy with the potential to help countless patients across a range of tumors," said Dr. Matt Coffey, President and Chief Executive Officer of Oncolytics. "We believe our cash balance and strong data, supported by our active business development program, position us well for 2024."
Positive Clinical and Translational Data: "Positive, early results from the Phase 1/2 GOBLET study in three gastrointestinal cancers - pancreatic, anal, and colorectal - showed that the combination of pelareorep and atezolizumab produced clinically meaningful improvements in response rates and survival timelines that are notably improved compared to historical results with no toxicity concerns. Translational data from studies in solid tumors, breast, pancreatic, and colorectal cancers showed that treatment with pelareorep remodels the tumor microenvironment and stimulates tumor-directed immune responses, affirming its mechanism of action as an immunotherapeutic agent. Together, these data provide a strong foundation to support advancing pelareorep into registrational-track studies, starting in 2024," continued Dr Coffey.
2024 Outlook: Dr. Coffey concluded by saying, "Looking ahead to 2024, we expect to initiate the first Phase 3 study for pelareorep in pancreatic cancer. The transition to a late-stage biopharmaceutical company will mark an important inflection point for investors, clinical collaborators and potential partners by providing a line of sight towards the path to regulatory approval and achieving our mission of developing pelareorep as an immunotherapeutic agent for cancer. We are excited to launch a new Phase 1/2 pancreatic cancer study investigating, for the first time, pelareorep in combination with modified FOLFIRINOX (mFOLFIRINOX). Finally, our discussions with regulators on a pivotal Phase 3 trial have been productive to date, and we expect to provide guidance on the registration path for metastatic breast cancer in the first half of 2024. We look forward to updating our stakeholders on our progress as the year unfolds."
Immunotherapeutic Mechanism of Action: Thomas Heineman, M.D., Ph.D., Chief Medical Officer at Oncolytics, said, "Translational data from multiple studies in 2023 have helped define pelareorep's role in shaping the tumor microenvironment and have provided a better understanding of its ability to induce the expansion of T cell populations. Notably, the positive association between tumor response and TIL clone expansion could become a useful marker of clinical outcomes in future studies and during patient care. Moreover, these findings further distinguish pelareorep's mechanism of action from that of other immunotherapeutic agents and provide support for its immunologic effects, which are largely driven by the introduction of its double-stranded RNA genome into cancer cells."
Financial Strength and Partnering Outlook: Kirk Look, Oncolytics' Chief Financial Officer, commented, "Our $40 million cash balance as of September 30, 2023, and the grant funding from The Pancreatic Cancer Action Network (PanCAN) provides us with over 12 months of runway to support our operations, including the initiation of our first Phase 3 study and the BRACELET-1 survival data. We continue to have active conversations with potential partners and work diligently with clinical collaborators, including Roche and Pfizer. We believe the positive, extended dataset, including continued and durable responses, survival results, and translational observations, will provide potential partners with a well-defined and unique target product profile that will add to our discussions."
Anticipated Milestones are expected to include:
NEWS -- Oncolytics Biotech® and SOLTI Present Positive Translational Data at SABCS
SAN DIEGO and CALGARY, AB, Dec. 7, 2023 /CNW/ -- Oncolytics Biotech® Inc. (NASDAQ: ONCY) (TSX: ONC), a clinical-stage immunotherapeutics company focused on oncology, today announced the presentation of additional translational data from the AWARE-1 breast cancer window-of-opportunity study conducted in combination with SOLTI-Innovative Cancer Research at The San Antonio Breast Cancer Symposium (SABCS).
"Translational data presented from the AWARE-1 study at SABCS showed that pelareorep induced the expansion of tumor-infiltrating lymphocytes, or TILs, in matched tumor biopsy and peripheral blood samples of newly diagnosed breast cancer patients. In addition, sequencing of the T cell receptors (TCRs) showed a more prominent expansion of existing TIL clones in the blood. We consider these results to be positive and important because they build on additional translational results reported this fall at two other medical meetings, The Society for Immunotherapy of Cancer (SITC) and The European Society for Medical Oncology (ESMO), from the AWARE-1 and GOBLET studies respectively, and provide further support for pelareorep's unique immunologic mechanism of action," said Dr. Matt Coffey, President and Chief Executive Officer of Oncolytics. "Taken together, these translational data affirm pelareorep's ability to enhance T cell infiltration into tumors and expand TILs in the peripheral blood, which have been correlated with tumor response. We intend to incorporate these learnings into the designs of our registrational studies in metastatic breast cancer and pancreatic cancer."
Thomas Heineman, M.D., Ph.D., Chief Medical Officer at Oncolytics, commented, "A review of the AWARE-1 study translational data presented at this year's SABCS and previous scientific conferences demonstrates that pelareorep treatment, especially in combination with atezolizumab, results in:
NEWS -- Oncolytics Provides Update on Pancreatic Cancer Program for Pelareorep
Updated plan follows PanCAN's strategic re-evaluation of the Precision PromiseSM Program
PanCAN US$5 million grant provides support for new GOBLET mFOLFIRINOX arm to proceed as planned
New Phase 3 strategy provides significant value-creation opportunities
SAN DIEGO, CA and CALGARY, AB, Nov. 9, 2023 /PRNewswire/ -- Oncolytics Biotech® Inc. (NASDAQ: ONCY) (Oncolytics) (TSX: ONC), a clinical-stage immunotherapeutics company focused on oncology, provided an update on the planned program for pelareorep in pancreatic ductal adenocarcinoma (PDAC).
Oncolytics' collaborator, The Pancreatic Cancer Action Network (PanCAN), a pancreatic cancer patient advocacy organization, advised the company that it is implementing a strategic re-evaluation of the Precision PromiseSM program and will not be adding any new investigational therapies to the platform until the re-evaluation is complete. Oncolytics and PanCAN had previously planned to initiate a Phase 3 trial using the Precision PromiseSM platform in H1 2024. Oncolytics now plans to conduct an adaptive Phase 3 program of pelareorep, gemcitabine, nab-paclitaxel, and atezolizumab, similar to the Precision Promise study and manage it directly within our Clinical Group and a contract research organization (CRO) with the goal of enrolling the first patient in mid-2024.
Additionally, Oncolytics and PanCAN confirmed that PanCAN will continue with its plans to grant US$5 million to Oncolytics as part of the Therapeutic Accelerator Award for the new Phase 2 GOBLET study arm evaluating the combination of pelareorep and modified FOLFIRINOX (mFOLFIRINOX) in pancreatic cancer patients.
Dr. Matt Coffey, President and Chief Executive Officer of Oncolytics, said, "Oncolytics remains steadfast in its commitment to initiate the Phase 3 program for pelareorep, starting in PDAC, in 2024. Having a compelling Phase 2 dataset in hand and strong connections with the pancreatic cancer community, Oncolytics is well positioned to effectively conduct the Phase 3 pancreatic cancer program. Trial preparations are actively underway, and we expect to initiate the enrollment of the first patient in mid-2024. The company plans to provide a further update on the design of the Phase 3 trial in H1 2024."
Dr. Coffey continued, "The opportunity to work with the strong scientific, regulatory and clinical leadership teams at PanCAN continues to be valuable for Oncolytics. We are grateful for our ongoing collaboration through the US$5 million Therapeutic Accelerator Award grant program in support of the new arm of the GOBLET study of pelareorep and mFOLFIRINOX, slated to begin in H1 2024."
"While we are undertaking a strategic re-evaluation of the program and are not adding any new therapies until the evaluation is complete, we remain committed to Oncolytics' efforts to accelerate therapies for pancreatic cancer patients through the US$5 million Therapeutic Accelerator Award Grant," said Julie Fleshman, President and CEO of PanCAN."
Dr. Coffey concluded by saying, "Looking ahead, we believe our updated plan to conduct the pelareorep Phase 3 pancreatic cancer program will enable us to be much closer to the conduct of the trial and the data. We believe this will be strategically valuable as we advance our partnering discussions and explore regulatory strategies to accelerate the development of pelareorep in order to bring this potentially important immunotherapeutic agent to people with cancer."
About Oncolytics Biotech Inc.
Oncolytics is a clinical-stage biotechnology company developing pelareorep, an intravenously delivered immunotherapeutic agent. Pelareorep has demonstrated promising results in Phase 2 studies in breast and pancreatic cancers. It acts by inducing anti-cancer immune responses and promotes an inflamed tumor phenotype -- turning "cold" tumors "hot" -- through innate and adaptive immune responses to treat a variety of cancers.
Pelareorep has demonstrated synergies with multiple approved oncology treatments. Oncolytics is currently conducting and planning combination clinical trials with pelareorep in solid and hematological malignancies as it advances towards registrational studies in metastatic breast cancer and pancreatic cancer. For further information, please visit: https://www.oncolyticsbiotech.com.
This press release contains forward-looking statements, within the meaning of Section 21E of the Securities Exchange Act of 1934, as amended and forward-looking information under applicable Canadian securities laws (such forward-looking statements and forward-looking information are collectively referred to herein as "forward-looking statements"). Forward-looking statements contained in this press release include statements regarding Oncolytics' belief as to the potential and benefits of pelareorep as a cancer therapeutic; our planned program for pelareorep in PDAC, including our plan to conduct an adaptive Phase 3 program managed directly within our clinical group and the anticipated benefits and timing thereof; our goals regarding the timing of enrollment in the study; our expectations regarding the US$5 million Therapeutic Accelerator Award from PanCAN and our planned usage thereof; our belief that we are well-positioned to effectively conduct the Phase 3 pancreatic cancer program; our plans for providing a further update on the design of the Phase 3 trial; our plans to advance partnering discussions and explore regulatory strategies to accelerate the development of pelareorep; our plans to advance towards a registration study in metastatic breast cancer and pancreatic cancer; and other statements related to anticipated developments in Oncolytics' business and technologies. In any forward-looking statement in which Oncolytics expresses an expectation or belief as to future results, such expectations or beliefs are expressed in good faith and are believed to have a reasonable basis, but there can be no assurance that the statement or expectation or belief will be achieved. Such forward-looking statements involve known and unknown risks and uncertainties, which could cause Oncolytics' actual results to differ materially from those in the forward-looking statements. Such risks and uncertainties include, among others, the availability of funds and resources to pursue research and development projects, the efficacy of pelareorep as a cancer treatment, the success and timely completion of clinical studies and trials, Oncolytics' ability to successfully commercialize pelareorep, uncertainties related to the research and development of pharmaceuticals, uncertainties related to the regulatory process and general changes to the economic environment. In particular, we may be impacted by business interruptions resulting from COVID-19 coronavirus, including operating, manufacturing supply chain, clinical trial and project development delays and disruptions, labour shortages, travel and shipping disruption, and shutdowns (including as a result of government regulation and prevention measures). It is unknown whether and how Oncolytics may be affected if the COVID-19 pandemic persists for an extended period of time. We may incur expenses or delays relating to such events outside of our control, which could have a material adverse impact on our business, operating results and financial condition. Investors should consult Oncolytics' quarterly and annual filings with the Canadian and U.S. securities commissions for additional information on risks and uncertainties relating to the forward-looking statements. Investors are cautioned against placing undue reliance on forward-looking statements. The Company does not undertake any obligation to update these forward-looking statements, except as required by applicable laws.
Company Contact
Jon Patton
Director of IR & Communication
+1-858-886-7813
mailto://jpatton@oncolytics.ca
Investor Relations for Oncolytics
Timothy McCarthy
LifeSci Advisors
+1-917-679-9282
mailto://tim@lifesciadvisors.com
View original content: https://www.prnewswire.com/news-releases/oncolytics-provides-update-on-pancreatic-cancer-program-for-pelareorep-301983006.html
SOURCE Oncolytics Biotech® Inc.
ONCY news.
Oncolytics Provides Update on Pancreatic Cancer Program for Pelareorep
https://finance.yahoo.com/news/oncolytics-provides-pancreatic-cancer-program-113000246.html
ONCY, reports Q3 financials.
https://finance.yahoo.com/news/oncolytics-biotech-reports-third-quarter-110000259.html
NEWS -- Oncolytics Presents Positive Updated Pancreatic Cancer Data from GOBLET Phase 1/2 Study at ESMO
7.2 months mPFS and 10.6 months interim median OS surpass historical outcomes by = 25%
T-cell expansion data correlate with tumor response, providing important proof-of-concept
SAN DIEGO and CALGARY, AB, Oct. 23, 2023 /PRNewswire/ -- Oncolytics Biotech® Inc. (NASDAQ: ONCY),( Oncolytics) (TSX: ONC), a clinical-stage immunotherapeutics company focused on oncology, today announced the poster presentation of positive, updated results from the Phase 1/2 GOBLET study evaluating pelareorep-based combination therapy in patients with pancreatic ductal adenocarcinoma (PDAC) at the European Society for Medical Oncology meeting (ESMO 2023), taking place in Madrid, Spain.
"We are very pleased to share such positive and consistent data on pelareorep from the PDAC arm of the GOBLET study, including an impressive overall response rate, 7.2 months of median progression-free survival, interim median overall survival of 10.6 months, and expansion of both pre-existing and new T-cell clones. These data build upon results from previous studies showing the clinical benefit of pelareorep combination therapy in PDAC and support the decision to move to a licensure-enabling study in pancreatic cancer," said Dr. Matt Coffey, President and Chief Executive Officer of Oncolytics. "Everything we do at Oncolytics is focused on advancing the development of our immunotherapy candidate, pelareorep, with a goal of providing improved care and longer survival for patients with pancreatic cancer and other tumor types. The data we are presenting at ESMO provide a solid foundation as we advance our pancreatic cancer program through the Precision PromiseSM Phase 3 trial in this indication."
Summary of Data and Findings from the PDAC Arm of the Phase 1/2 GOBLET Study:
Tumor Responses: Consistent with the abstract, data from the study outlined patient responses, including:
NEWS -- Oncolytics Achieves Success Criteria for Efficacy in the Third-Line Colorectal Cancer Cohort of the GOBLET Study
Results met efficacy criteria for enrollment expansion and provide support for pelareorep's mechanism of action
SAN DIEGO and CALGARY, AB, Oct. 23, 2023 /PRNewswire/ -- Oncolytics Biotech® Inc. (NASDAQ: ONCY), (Oncolytics) (TSX: ONC), a clinical-stage immunotherapeutics company focused on oncology, today announced the poster presentation of interim results from the Phase 1/2 GOBLET study evaluating a combination treatment of pelareorep in patients with third-line (3L) metastatic colorectal cancer (CRC) regardless of microsatellite instability status at the European Society for Medical Oncology meeting (ESMO 2023), taking place in Madrid, Spain.
"The results presented at ESMO met the criteria to advance the study to the next stage, with 4 of 14 enrolled patients demonstrating stable disease at week 16. These data demonstrated a 40% overall disease control rate and provided further encouraging data for pelareorep," said Dr. Matt Coffey, President and Chief Executive Officer of Oncolytics. "In a patient population that had failed multiple rounds of treatment, we continue to see pelareorep's ability to synergize with atezolizumab by generating an immune response, including the expansion of T cell clones. The translational data from this cohort are consistent with the observed clinical response, providing further support for pelareorep's mechanism of action as a potential backbone immunotherapy for patients with gastrointestinal and other forms of cancer."
"We designed the GOBLET study to evaluate pelareorep's ability to improve clinical outcomes in different gastrointestinal cancers, including at different disease stages, and to better understand pelareorep's mechanism of action by generating strong translational data. The data from this arm of the study demonstrate that pelareorep is taken up by tumor cells and stimulates T cell expansion even in heavily pre-treated colorectal cancer patients," said Thomas Heineman, M.D., Ph.D., Chief Medical Officer at Oncolytics. "These patients demonstrated a 40% disease control rate, a progression-free survival of 2.8 months, a median overall survival of 8.0 months, and a 12-month survival rate of 33%, exceeding historical results1-3. These findings are encouraging given that exhaustion of tumor-infiltrating lymphocytes, resulting from late stage of disease and extensive prior chemotherapy, may have limited their ability to expand in response to treatment. Notably, this is the second GOBLET study cohort in a row that has met its success criteria, further supporting pelareorep's ability to synergize with atezolizumab. These data also support pelareorep's immunologic mechanism of action and will inform our plans for further development."
GOBLET Study 3L Metastatic CRC Patient Overview:
Patients in the CRC cohort, presented at ESMO 2023, are undergoing third-line treatment with a combination of pelareorep, atezolizumab, and trifluridine/tipiracil. The 15 evaluable patients enrolled in the first stage of the study have been evaluated based on a September 18, 2023 data cut-off date. The enrolled patient population included patients with an ECOG score of =1 and confirmed colorectal cancer, regardless of microsatellite instability status.
References
1. Mayer et al. N Engl J Med 2015; 372:1909-1919. DOI: 10.1056/NEJMoa1414325
2. Moriwaki et al. The Oncologist 2018; 23(1):7-15. DOI: 10.1634/theoncologist.2017-0275
3. Bachet et al. ESMO Open. 2020 Jun;5(3):e000698. doi: 10.1136/esmoopen-2020-000698
Poster Information
Poster Title: Pelareorep + atezolizumab and chemotherapy in third-line (3L) metastatic colorectal cancer (mCRC) patients – Interim results from the GOBLET study
Final Publication Number (FPN): 619P
Poster Date: October 22, 2023
About GOBLET
The GOBLET (Gastrointestinal tumOrs exploring the treatment comBinations with the oncolytic reovirus peLarEorep and anTi-PD-L1) study is a phase 1/2 multiple indication study in advanced or metastatic gastrointestinal tumors. The study is being conducted at 12 centers in Germany and is being managed by AIO-Studien-gGmbH. The co-primary endpoints of the study are objective response rate (ORR) assessed at week 16 and safety. Key secondary and exploratory endpoints include additional efficacy assessments and evaluation of potential biomarkers (T cell clonality and CEACAM6). The study employs a Simon two-stage design with Stage 1 comprising four treatment groups expected to enroll a total of approximately 55 patients:
1. Pelareorep in combination with atezolizumab, gemcitabine, and nab-paclitaxel in 1st line advanced/metastatic pancreatic cancer patients (n=12);
2. Pelareorep in combination with atezolizumab in 1st line MSI (microsatellite instability)-high metastatic colorectal cancer patients (n=19);
3. Pelareorep in combination with atezolizumab and TAS-102 in 3rd line metastatic colorectal cancer patients (n=14); and
4. Pelareorep in combination with atezolizumab in 2nd line advanced and unresectable anal cancer patients (n=10).
Any cohort showing an ORR above a pre-specified threshold in Stage 1 may be advanced to Stage 2 and enroll additional patients.
About AIO
AIO-Studien-gGmbH (AIO) emerged from the study center of the internal oncology working group within the German Cancer Society (DKG). AIO operates with a non-profit purpose of promoting science and research with a focus on medical oncology. Since its foundation, AIO has become a successful sponsor and study management company and has established itself both nationally and internationally.
About Oncolytics Biotech Inc.
Oncolytics is a clinical-stage biotechnology company developing pelareorep, an intravenously delivered immunotherapeutic agent. Pelareorep has demonstrated promising results in Phase 2 studies in breast and pancreatic cancers. It acts by inducing anti-cancer immune responses and promotes an inflamed tumor phenotype -- turning "cold" tumors "hot" -- through innate and adaptive immune responses to treat a variety of cancers.
Pelareorep has demonstrated synergies with multiple approved oncology treatments. Oncolytics is currently conducting and planning combination clinical trials with pelareorep in solid and hematological malignancies as it advances towards registrational studies in metastatic breast cancer and pancreatic cancer. For further information, please visit: https://www.oncolyticsbiotech.com.
This press release contains forward-looking statements, within the meaning of Section 21E of the Securities Exchange Act of 1934, as amended and forward-looking information under applicable Canadian securities laws (such forward-looking statements and forward-looking information are collectively referred to herein as "forward-looking statements"). Forward-looking statements contained in this press release include statements regarding Oncolytics' belief as to the potential, mechanism of action, and benefits of pelareorep as a cancer therapeutic; our stated goals and objectives; and our plans to advance towards a registrational study in metastatic breast cancer and pancreatic cancer. In any forward-looking statement in which Oncolytics expresses an expectation or belief as to future results, such expectations or beliefs are expressed in good faith and are believed to have a reasonable basis, but there can be no assurance that the statement or expectation or belief will be achieved. Such forward-looking statements involve known and unknown risks and uncertainties, which could cause Oncolytics' actual results to differ materially from those in the forward-looking statements. Such risks and uncertainties include, among others, the availability of funds and resources to pursue research and development projects, the efficacy of pelareorep as a cancer treatment, the success and timely completion of clinical studies and trials, Oncolytics' ability to successfully commercialize pelareorep, uncertainties related to the research and development of pharmaceuticals, uncertainties related to the regulatory process and general changes to the economic environment. In particular, we may be impacted by business interruptions resulting from COVID-19 coronavirus, including operating, manufacturing supply chain, clinical trial and project development delays and disruptions, labour shortages, travel and shipping disruption, and shutdowns (including as a result of government regulation and prevention measures). It is unknown whether and how Oncolytics may be affected if the COVID-19 pandemic persists for an extended period of time. We may incur expenses or delays relating to such events outside of our control, which could have a material adverse impact on our business, operating results and financial condition. Investors should consult Oncolytics' quarterly and annual filings with the Canadian and U.S. securities commissions for additional information on risks and uncertainties relating to the forward-looking statements. Investors are cautioned against placing undue reliance on forward-looking statements. The Company does not undertake any obligation to update these forward-looking statements, except as required by applicable laws.
Company Contact
Jon Patton
Director of IR & Communication
+1-858-886-7813
mailto://jpatton@oncolytics.ca
Investor Relations for Oncolytics
Timothy McCarthy
LifeSci Advisors
+1-917-679-9282
mailto://tim@lifesciadvisors.com
View original content: https://www.prnewswire.com/news-releases/oncolytics-achieves-success-criteria-for-efficacy-in-the-third-line-colorectal-cancer-cohort-of-the-goblet-study-301964255.html
SOURCE Oncolytics Biotech® Inc.
NEWS -- Oncolytics Biotech® to Host Conference Call to Discuss Third Quarter Financial Results and Recent Operational Highlights
Conference call and webcast to take place on Friday, November 3, 2023, at 8:30 a.m. ET
SAN DIEGO and CALGARY, AB, Oct. 20, 2023 /PRNewswire/ -- Oncolytics Biotech® Inc. (NASDAQ: ONCY) (TSX: ONC), a clinical-stage immunotherapeutics company focused on oncology, today announced that it will host a conference call and webcast on Friday, November 3, 2023, at 8:30 a.m. ET to discuss a corporate update and financial results for the third quarter of 2023.

Conference Call & Webcast
Date: Friday, November 3, 2023
Time: 8:30 a.m. ET
Dial-In – North American Toll-Free: (888) 664-6383
Dial-In – International: (416) 764-8650
RapidConnect: to join the conference call without operator assistance, please click here
Conference ID (if needed): 6026-0546
Webcast: please click here
A webcast of the call will also be available on the Investor Relations page of Oncolytics' website, available by clicking here, and archived for three months. A dial-in replay will be available for one week and can be accessed by dialing (888) 390-0541 (North America) or (416) 764-8677 (International) and using replay code: 260-546#.
About Oncolytics Biotech Inc.
Oncolytics is a clinical-stage biotechnology company developing pelareorep, an intravenously delivered immunotherapeutic agent. Pelareorep has demonstrated promising results in Phase 2 studies in breast and pancreatic cancers. It acts by inducing anti-cancer immune responses and promotes an inflamed tumor phenotype -- turning "cold" tumors "hot" -- through innate and adaptive immune responses to treat a variety of cancers.
Pelareorep has demonstrated synergies with multiple approved oncology treatments. Oncolytics is currently conducting and planning combination clinical trials with pelareorep in solid and hematological malignancies as it advances towards registrational studies in metastatic breast cancer and pancreatic cancer. For further information, please visit: https://www.oncolyticsbiotech.com.
Company Contact
Jon Patton
Director of IR & Communication
+1-858-886-7813
mailto://jpatton@oncolytics.ca
Investor Relations for Oncolytics
Timothy McCarthy
LifeSci Advisors
+1-917-679-9282
mailto://tim@lifesciadvisors.com
Logo: https://mma.prnewswire.com/media/1808285/4295352/Oncolytics_Biotech_Grey.jpg
View original content: https://www.prnewswire.com/news-releases/oncolytics-biotech-to-host-conference-call-to-discuss-third-quarter-financial-results-and-recent-operational-highlights-301962804.html
SOURCE Oncolytics Biotech® Inc.
NEWS -- Oncolytics Biotech® To Present Two Posters on the Pelareorep-Based GOBLET Study at ESMO 2023
Abstracts Overview
Preliminary updated PDAC data show 6-month overall survival rate of 82%
Results from pelareorep combination in mCRC met the Stage 1 success criteria
SAN DIEGO and CALGARY, AB, Oct. 16, 2023 /CNW/ -- Oncolytics Biotech® Inc. (NASDAQ: ONCY) (TSX: ONC), a clinical-stage immunotherapeutics company focused on oncology, today announced the publication of two abstracts for posters to be presented during the upcoming European Society for Medical Oncology (ESMO) Congress 2023, taking place from October 20th to 24th at the IFEMA Madrid Conference Center in Madrid, Spain.
Abstracts Overview
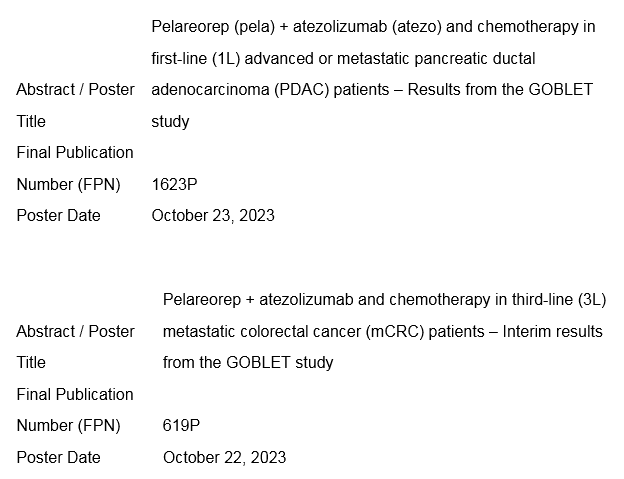
Abstracts Summaries
Abstract 1623P
ONCY news, Oncolytics Biotech® To Present Two Posters on the Pelareorep-Based GOBLET Study at ESMO 2023
https://finance.yahoo.com/news/oncolytics-biotech-present-two-posters-110000707.html
hit the ask and mm fill ya half way between bid n ask. course only they can do that seeing as ONCY trades in 1 cent increments..
NEWS -- THE PANCREATIC CANCER ACTION NETWORK SELECTS ONCOLYTICS BIOTECH® INC. TO RECEIVE $5 MILLION THERAPEUTIC ACCELERATOR AWARD TO DEVELOP LEADING-EDGE TREATMENTS
Innovative Multimillion-Dollar Grant Was Created in 2022 to Drive Biopharma Industry to Speed Development of Pancreatic Cancer Therapies
BOSTON and SAN DIEGO and CALGARY, AB, Sept. 26, 2023 /PRNewswire/ -- In an effort to continue accelerating new treatment options for people with pancreatic cancer, the Pancreatic Cancer Action Network (PanCAN) today announced Oncolytics Biotech, Inc. (NASDAQ: ONCY) (TSX: ONC) as the recipient of its second annual PanCAN Therapeutic Accelerator Award. Oncolytics received this award in recognition of its promising work on pelareorep, an investigational immunotherapy treatment that introduces double-stranded RNA into cancer cells, which stimulates anticancer inflammatory effects, including innate and adaptive immune responses and the activation and recruitment of T cells into the tumor. This $5 million grant will enable Oncolytics to continue the next stage of its research focused on a clinical trial with Oncolytics' proprietary reovirus pelareorep in combination with modified FOLFIRINOX chemotherapy with or without an immune checkpoint inhibitor. If results are encouraging, the treatment combination may be advanced to late-stage clinical development through the PanCAN Precision PromiseSM adaptive clinical trial platform.
The Pancreatic Cancer Action Network (PanCAN) is dedicated to fighting the world’s toughest cancer. In our urgent mission to save lives, we attack pancreatic cancer on all fronts: research, clinical initiatives, patient services and advocacy. Our effort is amplified by a nationwide network of grassroots support. We are determined to improve outcomes for today’s patients and those diagnosed in the future. (PRNewsfoto/Pancreatic Cancer Action Network)
The combination of pelareorep, gemcitabine, nab-paclitaxel, and atezolizumab was granted Fast Track designation by the FDA last year and has already been selected for inclusion in the Precision Promise clinical trial platform, which is anticipated to open in early 2024. Testing pelareorep in combination with FOLFIRINOX expands the approach so all patients with advanced pancreatic cancer could have the opportunity to benefit from this innovative immunotherapeutic approach.
The announcement of the Therapeutic Accelerator Award is being made in advance of PanCAN's annual Scientific Summit in Boston, which brings together PanCAN's Community for Progress, including research grant recipients, Scientific and Medical Advisors, industry partners and special guests to share ideas and build collaboration. Oncolytics was selected to receive the 2023 PanCAN Therapeutic Accelerator Award for $5 million through a rigorous, competitive process involving scientific, business and programmatic review from leading experts in the field.
Pancreatic cancer is notoriously aggressive and hard to treat. Current estimates suggest that the disease will be the second leading cause of cancer-related deaths in the United States before 2030. Most people with pancreatic cancer are diagnosed at a late stage when their tumor is inoperable, leaving these patients with few treatment options. And because the disease is significantly more resistant to chemotherapy than other cancers, it can even be difficult to treat in its earliest stages. Despite the fact that there is an urgent need for better outcomes for pancreatic cancer, research is often not prioritized because pancreatic cancer affects a relatively small population compared to other cancers, and clinical research can be risky and expensive.
"Today's five-year survival rate of 12% is too low and we need new treatment options now," said PanCAN Chief Science Officer Lynn Matrisian, PhD, MBA. "PanCAN launched our own clinical program to de-risk companies' investment through both the Therapeutic Accelerator Award and the Precision Promise platform to facilitate rapid advances in this disease."
PanCAN continues to take bold actions to improve the lives of everyone impacted by pancreatic cancer, including the investment of more than $208 million since 2003 to advance scientific research. PanCAN created the multimillion-dollar Therapeutic Accelerator Award in 2022 as a research investment focused on supporting companies working on new pancreatic cancer therapies that plan to conduct early-stage (Phase 1 and 2) clinical trials for these therapies.
"Oncolytics is so pleased to receive this innovative award and have the opportunity to work with PanCAN," said Dr. Matt Coffey, President and Chief Executive Officer of Oncolytics Biotech Inc. "This generous grant will enable early and late-stage patients with pancreatic cancer to potentially benefit from another commonly employed chemotherapy backbone, as FOLFIRINOX and gemcitabine + nab-paclitaxel are the most frequently used chemotherapy standards of care in both the first and second-line setting. By teaming up with PanCAN, we believe we may be able to expedite development and provide pancreatic cancer patients with a bespoke immunotherapeutic treatment option."
Oncolytics plans to utilize the grant funding to study pelareorep in combination with modified FOLFIRINOX chemotherapy in patients with metastatic pancreatic cancer who have not received prior cancer treatment. In addition, a separate group of patients will receive this combination along with a checkpoint inhibitor to verify synergies previously observed in this patient population. The goal of this research is to evaluate the safety and efficacy of these combination therapies. Efficacy will be assessed by measuring the effect of the treatments on tumor size, progression-free survival, and overall survival. In addition, immune responses to treatment will be assessed.
The funding offered through the PanCAN Therapeutic Accelerator Award represents an innovative and groundbreaking way for a non-profit to accelerate drug development. If the result of the clinical trial is positive, the hope is that the selected investigational treatment combination will be incorporated into the PanCAN Precision Promise adaptive clinical trial. While Precision Promise provides an effective approach for late-stage research on new pancreatic cancer therapies, many companies are not investing in the early-stage research necessary for late-stage studies and potential drug approval in this disease.
To learn more about PanCAN's research investments, visit https://pancan.org.
About the Pancreatic Cancer Action Network
The Pancreatic Cancer Action Network (PanCAN) leads the way in accelerating critical progress for pancreatic cancer patients. PanCAN takes bold action by funding life-saving research, providing personalized patient services and creating a community of supporters and volunteers who will stop at nothing to create a world in which all pancreatic cancer patients will thrive. For 18 years in a row, PanCAN has earned a Four-Star Rating from Charity Navigator – the highest rating an organization can receive. This rating designates PanCAN as an official "Give with Confidence" charity, indicating strong financial health, ongoing accountability and transparency.
About Oncolytics Biotech Inc.
Oncolytics is a clinical-stage biotechnology company developing pelareorep, an intravenously delivered immunotherapeutic agent. Pelareorep has demonstrated promising results in phase 2 studies in breast and pancreatic cancers. It acts by inducing anti-cancer immune responses and promotes an inflamed tumor phenotype -- turning "cold" tumors "hot" -- through innate and adaptive immune responses to treat a variety of cancers.
Pelareorep has demonstrated synergies with multiple approved oncology treatments. Oncolytics is currently conducting and planning combination clinical trials with pelareorep in solid and hematological malignancies as it advances towards registrational studies in metastatic breast cancer and pancreatic cancer. For further information, please visit: https://www.oncolyticsbiotech.com.
Media Contacts:
Charaighn Sesock
Public Relations
Pancreatic Cancer Action Network
Direct: 559-972-4877
mailto://charaighn@gmail.com
Jillian Scholten
Senior Manager, Public Relations
Pancreatic Cancer Action Network
Direct: 310-706-3360
Email: mailto://jscholten@pancan.org
Jon Patton
Director of IR & Communication
Oncolytics Biotech, Inc.
858-886-7813
mailto://jpatton@oncolytics.ca
View original content to download multimedia: https://www.prnewswire.com/news-releases/the-pancreatic-cancer-action-network-selects-oncolytics-biotech-inc-to-receive-5-million-therapeutic-accelerator-award-to-develop-leading-edge-treatments-301938335.html
SOURCE Pancreatic Cancer Action Network
NEWS -- Oncolytics Biotech® Announces Fireside Chat at the Cantor Global Healthcare Conference

SAN DIEGO and CALGARY, AB, Sept. 19, 2023 /CNW/ -- Oncolytics Biotech® Inc. (NASDAQ: ONCY) (TSX: ONC), a clinical-stage immunotherapeutics company focused on oncology, today announced its participation in an analyst-led fireside chat at the Cantor Global Healthcare Conference 2023 with Chief Executive Officer Dr. Matt Coffey. The conference is taking place September 26-28, 2023 at the InterContinental Barclay Hotel in New York, NY. Additional details on the fireside chat can be found below.
Date: Wednesday, September 27, 2023
Time: 8:35 a.m. ET
Location: InterContinental Barclay Hotel New York, Track 1
Webcast Link: Available by clicking here
Company management will also be participating in one-on-one investor meetings at the conference. To schedule a meeting, please submit a request on the conference website, contact your Cantor Fitzgerald representative, or email mailto://jpatton@oncolytics.ca.
A live webcast of the Company's presentation will also be available on the Investor Relations page of Oncolytics' website (LINK) and will be archived for three months.
About Oncolytics Biotech Inc.
Oncolytics is a clinical-stage biotechnology company developing pelareorep, an intravenously delivered immunotherapeutic agent. Pelareorep has demonstrated promising results in phase 2 studies in breast and pancreatic cancers. It acts by inducing anti-cancer immune responses and promotes an inflamed tumor phenotype -- turning "cold" tumors "hot" -- through innate and adaptive immune responses to treat a variety of cancers.
Pelareorep has demonstrated synergies with multiple approved oncology treatments. Oncolytics is currently conducting and planning combination clinical trials with pelareorep in solid and hematological malignancies as it advances towards registrational studies in metastatic breast cancer and pancreatic cancer. For further information, please visit: https://www.oncolyticsbiotech.com
Company Contact
Jon Patton
Director of IR & Communication
+1-858-886-7813
mailto://jpatton@oncolytics.ca
Investor Relations for Oncolytics
Timothy McCarthy
LifeSci Advisors
+1-917-679-9282
mailto://tim@lifesciadvisors.com
NEWS -- Oncolytics Biotech® Announces Full Exercise of Over-Allotment Option from Public Offering
CALGARY, AB, Sept. 7, 2023 /PRNewswire/ -- Oncolytics Biotech® Inc. ("Oncolytics" or the "Company") (NASDAQ: ONCY) (TSX: ONC) today announced that in connection with the previously announced underwritten public offering of US$15,000,750 (the "Offering") and the underwriting agreement dated August 1, 2023 (the "Underwriting Agreement"), Leede Jones Gable Inc. (the "Underwriter") has exercised their option (the "Over-Allotment Option"), in full, to purchase 1,000,050 units (the "Optioned Units") at US$2.25 for aggregate gross proceeds of US$2,250,112. The Company intends to use the proceeds from the Offering to continue the advancement of its pelareorep clinical programs in metastatic breast and pancreatic cancers, as well as general corporate and working capital purposes.

Each Optioned Unit consists of one common share of the Company (each a "Common Share") and one Common Share purchase warrant (each a "Warrant"). Each Warrant entitles the holder thereof to purchase one Common Share at an exercise price of US$2.81 at any time up to 60 months following the Closing, subject to acceleration in certain circumstances. Pursuant to the Underwriting Agreement, in consideration for the services rendered by the Underwriter in connection with the Offering, the Company paid to the Underwriter a cash commission equal to 7.0% of the aggregate gross proceeds raised from the Offering and issued to the Underwriter such number of compensation warrants (the "Compensation Warrants") as is equal to 7.0% of the aggregate number of Optioned Units sold in the Offering. Each Compensation Warrant is exercisable into one Common Share (an "Underwriter's Warrant Share") at an exercise price of US$2.25 per Underwriter's Warrant Share at any time up to 60 months following the Closing.
The Offering is being made by way of a prospectus supplement to the Company's short form base shelf prospectus filed on August 1, 2023 in each of the provinces and territories of Canada, except Quebec, pursuant to National Instrument 44-101 – Short Form Prospectus Distributions and National Instrument 44-102 - Shelf Distributions.
The securities referred to in this news release have not been, nor will they be, registered under the United States Securities Act of 1933, as amended, and may not be offered or sold within the United States or to, or for the account or benefit of, U.S. persons absent U.S. registration or an applicable exemption from the U.S. registration requirements. This press release does not constitute an offer for sale of securities, nor a solicitation for offers to buy any securities in the United States, nor in any other jurisdiction in which such offer, solicitation or sale would be unlawful. Any public offering of securities in the United States must be made by means of a prospectus containing detailed information about the company and management, as well as financial statements.
About Oncolytics Biotech Inc.
Oncolytics is a biotechnology company developing pelareorep, an intravenously delivered immunotherapeutic agent. This compound induces anti-cancer immune responses and promotes an inflamed tumor phenotype -- turning "cold" tumors "hot" -- through innate and adaptive immune responses to treat a variety of cancers.
Pelareorep has demonstrated synergies with multiple approved oncology treatments. Oncolytics is currently conducting and planning combination clinical trials with pelareorep in solid and hematological malignancies as it advances towards registrational studies in metastatic breast cancer and pancreatic cancer. For further information, please visit: https://www.oncolyticsbiotech.com.
This press release contains forward-looking statements, within the meaning of Section 21E of the Securities Exchange Act of 1934, as amended and forward-looking information under applicable Canadian securities laws (such forward-looking statements and forward-looking information are collectively referred to herein as "forward-looking statements"). Forward-looking statements contained in this press release include statements regarding Oncolytics' belief as to the potential and benefits of pelareorep as a cancer therapeutic; the Company's anticipated use of proceeds from the Offering: the terms of the Warrants, the Compensation Warrants; the Company's plans to advance towards a registrational study in metastatic pancreatic cancer; and other statements related to anticipated developments in Oncolytics' business and technologies. In any forward-looking statement in which Oncolytics expresses an expectation or belief as to future results, such expectations or beliefs are expressed in good faith and are believed to have a reasonable basis, but there can be no assurance that the statement or expectation or belief will be achieved. Such forward-looking statements involve known and unknown risks and uncertainties, which could cause Oncolytics' actual results to differ materially from those in the forward-looking statements. Such risks and uncertainties include, among others, the availability of funds and resources to pursue research and development projects, the efficacy of pelareorep as a cancer treatment, the success and timely completion of clinical studies and trials, Oncolytics' ability to successfully commercialize pelareorep, uncertainties related to the research and development of pharmaceuticals, uncertainties related to the regulatory process and general changes to the economic environment. In particular, the Company may be impacted by business interruptions resulting from COVID-19 coronavirus, including operating, manufacturing supply chain, clinical trial and project development delays and disruptions, labour shortages, travel and shipping disruption, and shutdowns (including as a result of government regulation and prevention measures). It is unknown whether and how Oncolytics may be affected if the COVID-19 pandemic persists for an extended period of time. The Company may incur expenses or delays relating to such events outside of its control, which could have a material adverse impact on the Company's business, operating results and financial condition. Investors should consult Oncolytics' quarterly and annual filings with the Canadian and U.S. securities commissions for additional information on risks and uncertainties relating to the forward-looking statements. Investors are cautioned against placing undue reliance on forward-looking statements. The Company does not undertake any obligation to update these forward-looking statements, except as required by applicable laws.
Company Contact
Jon Patton
Director of IR & Communication
+1-858-886-7813
mailto://jpatton@oncolytics.ca
Investor Relations for Oncolytics
Timothy McCarthy
LifeSci Advisors
+1-917-679-9282
mailto://tim@lifesciadvisors.com
View original content: https://www.prnewswire.com/news-releases/oncolytics-biotech-announces-full-exercise-of-over-allotment-option-from-public-offering-301920771.html
SOURCE Oncolytics Biotech® Inc.
NEWS -- Oncolytics Biotech® to Participate in a Fireside Chat at the H.C. Wainwright 25th Annual Global Investment Conference

Oncolytics Biotech® Inc. Logo (PRNewsfoto/Oncolytics Biotech® Inc.)
SAN DIEGO and CALGARY, AB, Sept. 6, 2023 /PRNewswire/ -- Oncolytics Biotech® Inc. (NASDAQ: ONCY) (TSX: ONC) today announced that Chief Executive Officer Dr. Matt Coffey will participate in a fireside chat at the H.C. Wainwright 25th Annual Global Investment Conference, which is taking place September 11-13, 2023 at the Lotte New York Palace Hotel in New York, NY. Additional details on the fireside chat can be found below.
Date: Tuesday, September 12, 2023
Time: 9:30 a.m. ET
Location: Lotte New York Palace Hotel, Adams Room 4th Floor
Webcast Link: Available by clicking here
Company management will also be participating in one-on-one investor meetings at the conference. To schedule a meeting, please submit a request on the conference website, contact your H.C. Wainwright representative, or email mailto://jpatton@oncolytics.ca.
A live webcast of the Company's presentation will also be available on the Investor Relations page of Oncolytics' website (LINK) and will be archived for three months.
About Oncolytics Biotech Inc.
Oncolytics is a biotechnology company developing pelareorep, an intravenously delivered immunotherapeutic agent. This compound induces anti-cancer immune responses and promotes an inflamed tumor phenotype -- turning "cold" tumors "hot" -- through innate and adaptive immune responses to treat a variety of cancers.
Pelareorep has demonstrated synergies with multiple approved oncology treatments. Oncolytics is currently conducting and planning combination clinical trials with pelareorep in solid and hematological malignancies as it advances towards registrational studies in metastatic breast cancer and pancreatic cancer. For further information, please visit: https://www.oncolyticsbiotech.com.
Investor Relations for Oncolytics
Timothy McCarthy
LifeSci Advisors
+1-917-679-9282
mailto://tim@lifesciadvisors.com
Company Contact
Jon Patton
Director of IR & Communication
+1-858-886-7813
mailto://jpatton@oncolytics.ca
View original content to download multimedia: https://www.prnewswire.com/news-releases/oncolytics-biotech-to-participate-in-a-fireside-chat-at-the-hc-wainwright-25th-annual-global-investment-conference-301918192.html
SOURCE Oncolytics Biotech® Inc.
NEWS -- Oncolytics Biotech® Reports Second Quarter 2023 Financial Results and Operational Highlights
SAN DIEGO and CALGARY, AB, Aug. 14, 2023 /PRNewswire/ -- Oncolytics Biotech® Inc. (NASDAQ: ONCY) (TSX: ONC) today announced recent operational highlights and financial results for the second quarter ended June 30, 2023. All dollar amounts are expressed in Canadian currency unless otherwise noted.
"Our core programs in pancreatic and breast cancer are both progressing rapidly towards registrational studies on the back of the impressive clinical data showing the potential of pelareorep as a backbone immunotherapy," said Dr. Matt Coffey, President and Chief Executive Officer. "Data reported at the American Society of Clinical Oncology (ASCO) Annual Meeting from our BRACELET-1 randomized phase 2 trial demonstrated robust improvements in objective response rate (ORR) and progression-free survival (PFS) with a hazard ratio of 0.29 (95% CI: 0.09, 0.98). This has unlocked the potential for including PFS as a dual endpoint in our registrational trial, which could considerably reduce the time to a pivotal readout. At the same time, pelareorep was selected for inclusion in the ongoing Precision Promise pancreatic cancer platform trial. Precision Promise, created by the Pancreatic Cancer Action Network, provides us with the opportunity to reduce the time and costs needed for a potential approval, and we are honored to have been selected for participation in this novel and exclusive trial."
To view rest of Oncolytics' Press Release including Financial Charts, follow link below:
https://www.prnewswire.com/news-releases/oncolytics-biotech-reports-second-quarter-2023-financial-results-and-operational-highlights-301899635.html
NEWS -- Oncolytics Biotech® Successfully Raises US$15 Million to Advance Breast and Pancreatic Cancer Clinical Programs

CALGARY, Alberta, Aug. 8, 2023 /CNW/ -- Oncolytics Biotech® Inc. ("Oncolytics" or the "Company") (NASDAQ: ONCY) (TSX: ONC) today announced the closing (the "Closing") of the previously announced underwritten public offering of US$15,000,750 (the "Offering"). An institutional investor was a significant participant in the Offering. The Company intends to use the proceeds from the Offering to continue the advancement of its pelareorep clinical programs in metastatic breast and pancreatic cancers, as well as general corporate and working capital purposes.
Leede Jones Gable Inc. acted as underwriter and bookrunner (the "Underwriter") in accordance with the terms of an underwriting agreement between the Company and the Underwriter dated as of August 1, 2023 (the "Underwriting Agreement"), pursuant to which Oncolytics issued 6,667,000 units (the "Equity Units") for gross proceeds to the Company of US$15,000,750 at a price of US$2.25 per Equity Unit. Each Equity Unit consists of one common share of the Company (each a "Common Share") and one Common Share purchase warrant (each a "Warrant"). Each Warrant entitles the holder thereof to purchase one Common Share at an exercise price of US$2.81 at any time up to 60 months following the Closing, subject to acceleration in certain circumstances.
In addition, pursuant to the Underwriting Agreement, the Company granted the Underwriter an option (the "Over-Allotment Option"), exercisable in part or in whole at the Underwriter's sole discretion, at any time beginning on the Closing until 30 days following the Closing, to purchase up to that number of additional Equity Units, Common Shares or Warrants, or any combination thereof, as is equal to 15% of the aggregate number of Equity Units sold in the Offering to cover the Underwriter's over-allotment position, if any, and for market stabilization purposes.
Pursuant to the Underwriting Agreement, in consideration for the services rendered by the Underwriter in connection with the Offering, at the Closing, the Company paid to the Underwriter a cash commission equal to 7.0% of the aggregate gross proceeds raised from the Offering and issued to the Underwriter such number of compensation warrants (the "Compensation Warrants") as is equal to 7.0% of the aggregate number of Equity Units sold in the Offering. Each Compensation Warrant is exercisable into one Common Share (an "Underwriter's Warrant Share") at an exercise price of US$2.25 per Underwriter's Warrant Share at any time up to 60 months following the Closing.
The Offering is being made by way of a prospectus supplement to the Company's short form base shelf prospectus filed on August 1, 2023 in each of the provinces and territories of Canada, except Quebec, pursuant to National Instrument 44-101 – Short Form Prospectus Distributions and National Instrument 44-102 - Shelf Distributions.
The securities referred to in this news release have not been, nor will they be, registered under the United States Securities Act of 1933, as amended, and may not be offered or sold within the United States or to, or for the account or benefit of, U.S. persons absent U.S. registration or an applicable exemption from the U.S. registration requirements. This press release does not constitute an offer for sale of securities, nor a solicitation for offers to buy any securities in the United States, nor in any other jurisdiction in which such offer, solicitation or sale would be unlawful. Any public offering of securities in the United States must be made by means of a prospectus containing detailed information about the company and management, as well as financial statements.
About Oncolytics Biotech Inc.
Oncolytics is a biotechnology company developing pelareorep, an intravenously delivered immunotherapeutic agent. This compound induces anti-cancer immune responses and promotes an inflamed tumor phenotype -- turning "cold" tumors "hot" -- through innate and adaptive immune responses to treat a variety of cancers.
Pelareorep has demonstrated synergies with multiple approved oncology treatments. Oncolytics is currently conducting and planning combination clinical trials with pelareorep in solid and hematological malignancies as it advances towards registrational studies in metastatic breast cancer and pancreatic cancer. For further information, please visit: https://www.oncolyticsbiotech.com.
This press release contains forward-looking statements, within the meaning of Section 21E of the Securities Exchange Act of 1934, as amended and forward-looking information under applicable Canadian securities laws (such forward-looking statements and forward-looking information are collectively referred to herein as "forward-looking statements"). Forward-looking statements contained in this press release include statements regarding Oncolytics' belief as to the potential and benefits of pelareorep as a cancer therapeutic; the Company's anticipated use of proceeds from the Offering: the terms of the Warrants, the Compensation Warrants and the Over-Allotment Option; the Company's plans to advance towards a registrational study in metastatic pancreatic cancer; and other statements related to anticipated developments in Oncolytics' business and technologies. In any forward-looking statement in which Oncolytics expresses an expectation or belief as to future results, such expectations or beliefs are expressed in good faith and are believed to have a reasonable basis, but there can be no assurance that the statement or expectation or belief will be achieved. Such forward-looking statements involve known and unknown risks and uncertainties, which could cause Oncolytics' actual results to differ materially from those in the forward-looking statements. Such risks and uncertainties include, among others, the availability of funds and resources to pursue research and development projects, the efficacy of pelareorep as a cancer treatment, the success and timely completion of clinical studies and trials, Oncolytics' ability to successfully commercialize pelareorep, uncertainties related to the research and development of pharmaceuticals, uncertainties related to the regulatory process and general changes to the economic environment. In particular, the Company may be impacted by business interruptions resulting from COVID-19 coronavirus, including operating, manufacturing supply chain, clinical trial and project development delays and disruptions, labour shortages, travel and shipping disruption, and shutdowns (including as a result of government regulation and prevention measures). It is unknown whether and how Oncolytics may be affected if the COVID-19 pandemic persists for an extended period of time. The Company may incur expenses or delays relating to such events outside of its control, which could have a material adverse impact on the Company's business, operating results and financial condition. Investors should consult Oncolytics' quarterly and annual filings with the Canadian and U.S. securities commissions for additional information on risks and uncertainties relating to the forward-looking statements. Investors are cautioned against placing undue reliance on forward-looking statements. The Company does not undertake any obligation to update these forward-looking statements, except as required by applicable laws.
Company Contact
Jon Patton
Director of IR & Communication
+1-858-886-7813
mailto://jpatton@oncolytics.ca
Investor Relations for Oncolytics
Timothy McCarthy
LifeSci Advisors
+1-917-679-9282
mailto://tim@lifesciadvisors.com
View original content: https://www.prnewswire.com/news-releases/oncolytics-biotech-successfully-raises-us15-million-to-advance-breast-and-pancreatic-cancer-clinical-programs-301895716.html
SOURCE Oncolytics Biotech® Inc.
View original content: http://www.newswire.ca/en/releases/archive/August2023/08/c2445.html
NEWS -- Oncolytics Biotech® to Participate in a Fireside Chat at Canaccord Genuity's 43rd Annual Growth Conference

SAN DIEGO and CALGARY, AB, Aug. 4, 2023 /PRNewswire/ -- Oncolytics Biotech® Inc. (NASDAQ: ONCY) (TSX: ONC) today announced that Chief Executive Officer Dr. Matt Coffey will participate in a fireside chat at Canaccord Genuity's 43rd Annual Growth Conference, which is taking place August 7-10, 2023 at the InterContinental Boston in Boston, MA. Additional details on the fireside chat can be found below.
Date: Thursday, August 10, 2023
Time: 12:00 p.m. ET
Location: InterContinental Boston, Abigail Adams C Room
Webcast Link: Available by clicking here
Company management will also be participating in one-on-one investor meetings at the conference. To schedule a meeting, please submit a request on the conference website, contact your Canaccord representative, or email jpatton@oncolytics.ca.
A live webcast of the Company's presentation will also be available on the Investor Relations page of Oncolytics' website (LINK) and will be archived for three months.
About Oncolytics Biotech Inc.
Oncolytics is a biotechnology company developing pelareorep, an intravenously delivered immunotherapeutic agent. This compound induces anti-cancer immune responses and promotes an inflamed tumor phenotype -- turning "cold" tumors "hot" -- through innate and adaptive immune responses to treat a variety of cancers.
Pelareorep has demonstrated synergies with multiple approved oncology treatments. Oncolytics is currently conducting and planning combination clinical trials with pelareorep in solid and hematological malignancies as it advances towards registrational studies in metastatic breast cancer and pancreatic cancer. For further information, please visit: https://www.oncolyticsbiotech.com.
Company Contact
Jon Patton
Director of IR & Communication
+1-858-886-7813
mailto://jpatton@oncolytics.ca
Investor Relations for Oncolytics
Timothy McCarthy
LifeSci Advisors
+1-917-679-9282
mailto://tim@lifesciadvisors.com
View original content to download multimedia: https://www.prnewswire.com/news-releases/oncolytics-biotech-to-participate-in-a-fireside-chat-at-canaccord-genuitys-43rd-annual-growth-conference-301893276.html
SOURCE Oncolytics Biotech® Inc.
NEWS -- Oncolytics Biotech® to Host Conference Call to Discuss Second Quarter Financial Results and Recent Operational Highlights

Conference call and webcast to take place on Monday, August 14, 2023, at 8:30 a.m. ET
SAN DIEGO and CALGARY, AB, Aug. 4, 2023 /PRNewswire/ -- Oncolytics Biotech® Inc. (NASDAQ: ONCY) (TSX: ONC) today announced that it will host a conference call and webcast on Monday, August 14, 2023, at 8:30 a.m. ET to discuss a corporate update and financial results for the second quarter of 2023.
Conference Call & Webcast
Date: Monday, August 14, 2023
Time: 8:30 a.m. ET
Dial-In – North American Toll-Free: (888) 664-6383
Dial-In – International: (416) 764-8650
RapidConnect: to join the conference call without operator assistance, please click here
Conference ID (if needed): 5411-4798
Webcast: please click here
A webcast of the call will also be available on the Investor Relations page of Oncolytics' website, available by clicking here, and will be archived for three months. A dial-in replay will be available for one week and can be accessed by dialing (888) 390-0541 (North America) or (416) 764-8677 (International) and using replay code: 114-798#.
About Oncolytics Biotech Inc.
Oncolytics is a biotechnology company developing pelareorep, an intravenously delivered immunotherapeutic agent. This compound induces anti-cancer immune responses and promotes an inflamed tumor phenotype -- turning "cold" tumors "hot" -- through innate and adaptive immune responses to treat a variety of cancers.
Pelareorep has demonstrated synergies with multiple approved oncology treatments. Oncolytics is currently conducting and planning combination clinical trials with pelareorep in solid and hematological malignancies as it advances towards registrational studies in metastatic breast cancer and pancreatic cancer. For further information, please visit: https://www.oncolyticsbiotech.com.
Company Contact
Jon Patton
Director of IR & Communication
+1-858-886-7813
mailto://jpatton@oncolytics.ca
Investor Relations for Oncolytics
Timothy McCarthy
LifeSci Advisors
+1-917-679-9282
mailto://tim@lifesciadvisors.com
View original content: https://www.prnewswire.com/news-releases/oncolytics-biotech-to-host-conference-call-to-discuss-second-quarter-financial-results-and-recent-operational-highlights-301893245.html
SOURCE Oncolytics Biotech® Inc.
Intresting to see if they reveal who bought deal is by? Will it be oversubscribed?
Not great, but maybe best deal to add stability for now and move forward.
Lots of catalysts still between now and year end.
NEWS -- Oncolytics Biotech® Announces US$15 Million Bought Deal Offering of Units

Not for distribution to U.S. Newswire Services or Dissemination in the United States
CALGARY, AB, July 31, 2023 /PRNewswire/ -- Oncolytics Biotech® Inc. ("Oncolytics" or the "Company") (NASDAQ: ONCY) (TSX: ONC) today announced that it has entered into an agreement with Leede Jones Gable Inc. as underwriter and bookrunner (the "Underwriter"), pursuant to which the Underwriter has agreed to purchase, on a bought-deal basis, 6,667,000 units (the "Equity Units") for gross proceeds to the Company of US$15,000,750 (the "Offering") at a price of US$2.25 per Equity Unit.
Each Equity Unit will consist of one common share of the Company (a "Common Share") and one Common Share purchase warrant (each whole purchase warrant, a "Warrant"). Each Warrant will entitle the holder thereof to purchase one Common Share at an exercise price of US$2.81 (the "Exercise Price") at any time up to 60 months following the Closing (as defined below).
The Company has granted the Underwriter an option (the "Over-Allotment Option"), exercisable in part or in whole at the Underwriter's sole discretion, at any time beginning on the Closing until 30 days following the Closing, to purchase up to that number of additional Equity Units, Common Shares or Warrants, or any combination thereof, as is equal to 15% of the aggregate number of Equity Units sold in the Offering to cover over-allotments, if any.
The Equity Units will be offered by way of a prospectus supplement to the Company's short form base shelf prospectus to be filed in all provinces of Canada except Quebec pursuant to National Instrument 44-101 – Short Form Prospectus Distributions and National Instrument 44-102 - Shelf Distributions.
The Company intends to use the net proceeds from the Offering to continue its pelareorep clinical programs in metastatic breast cancer and pancreatic cancer and general corporate and working capital purposes.
The closing of the Offering is expected to occur on or about August 4, 2023 (the "Closing") and is subject to the Company receiving all necessary regulatory approvals, including the approval of the Exchange.
The securities referred to in this news release have not been, nor will they be, registered under the United States Securities Act of 1933, as amended, and may not be offered or sold within the United States or to, or for the account or benefit of, U.S. persons absent U.S. registration or an applicable exemption from the U.S. registration requirements. This press release does not constitute an offer for sale of securities, nor a solicitation for offers to buy any securities in the United States, nor in any other jurisdiction in which such offer, solicitation or sale would be unlawful. Any public offering of securities in the United States must be made by means of a prospectus containing detailed information about the company and management, as well as financial statements.
About Oncolytics Biotech Inc.
Oncolytics is a biotechnology company developing pelareorep, an intravenously delivered immunotherapeutic agent. This compound induces anti-cancer immune responses and promotes an inflamed tumor phenotype -- turning "cold" tumors "hot" -- through innate and adaptive immune responses to treat a variety of cancers.
Pelareorep has demonstrated synergies with multiple approved oncology treatments. Oncolytics is currently conducting and planning combination clinical trials with pelareorep in solid and hematological malignancies as it advances towards registrational studies in metastatic breast cancer and pancreatic cancer. For further information, please visit: https://www.oncolyticsbiotech.com.
This press release contains forward-looking statements, within the meaning of Section 21E of the Securities Exchange Act of 1934, as amended and forward-looking information under applicable Canadian securities laws (such forward-looking statements and forward-looking information are collectively referred to herein as "forward-looking statements"). Forward-looking statements contained in this press release include statements regarding Oncolytics' belief as to the potential and benefits of pelareorep as a cancer therapeutic; the Offering and the timing thereof and anticipated use of proceeds therefrom; our plans to advance towards a registrational study in metastatic pancreatic cancer; and other statements related to anticipated developments in Oncolytics' business and technologies. In any forward-looking statement in which Oncolytics expresses an expectation or belief as to future results, such expectations or beliefs are expressed in good faith and are believed to have a reasonable basis, but there can be no assurance that the statement or expectation or belief will be achieved. Such forward-looking statements involve known and unknown risks and uncertainties, which could cause Oncolytics' actual results to differ materially from those in the forward-looking statements. Such risks and uncertainties include, among others, the availability of funds and resources to pursue research and development projects, the efficacy of pelareorep as a cancer treatment, the success and timely completion of clinical studies and trials, Oncolytics' ability to successfully commercialize pelareorep, uncertainties related to the research and development of pharmaceuticals, uncertainties related to the regulatory process and general changes to the economic environment. In particular, we may be impacted by business interruptions resulting from COVID-19 coronavirus, including operating, manufacturing supply chain, clinical trial and project development delays and disruptions, labour shortages, travel and shipping disruption, and shutdowns (including as a result of government regulation and prevention measures). It is unknown whether and how Oncolytics may be affected if the COVID-19 pandemic persists for an extended period of time. We may incur expenses or delays relating to such events outside of our control, which could have a material adverse impact on our business, operating results and financial condition. Investors should consult Oncolytics' quarterly and annual filings with the Canadian and U.S. securities commissions for additional information on risks and uncertainties relating to the forward-looking statements. Investors are cautioned against placing undue reliance on forward-looking statements. The Company does not undertake any obligation to update these forward-looking statements, except as required by applicable laws.
Company Contact
Jon Patton
Director of IR & Communication
+1-858-886-7813
mailto://jpatton@oncolytics.ca
Investor Relations for Oncolytics
Timothy McCarthy
LifeSci Advisors
+1-917-679-9282
mailto://tim@lifesciadvisors.com
Logo: https://mma.prnewswire.com/media/1762876/Oncolytics_Biotech_New_Logo.jpg
View original content:https://www.prnewswire.com/news-releases/oncolytics-biotech-announces-us15-million-bought-deal-offering-of-units-301889041.html
SOURCE Oncolytics Biotech® Inc.
Let's hope these guys don't go the way of PMCB - they too focused on pancreatic cancer "targeting method". So far IMO ONCY seems better positioned then PMCB ever was... didn't get burned by PMCB but it was close - didn't make any $$ either. LOL Cheers FX
10 to 40$ a share possible if buyout.
Breast Cancer, Pancreatic Cancer P3 announcement around September 20th, CAR-T BD possible announcement, Colorectal, Pancreatic and Anal Cancer Data in October. Asian Deal with Adlai Nortye P3 in Breast cancer triggers 80 million dollar payment.
Depending on which ones are good news/ vs bad news or don't work.
But lots of catalysts to move forward.
Announcement could come at anytime. BE READY. Good luck everyone. I'm already up a lot here so don't care reallly it's all free money for me. $$$$$$$$$$$$$$$$$$
What’s going on with the stock any predictions.
Cheers
Sorry been too busy. I own way too many shares of this but been buying for a long time. Pretty happy with share price movement and PanCan announcement. Good Luck everyone
2023 Anticipated Milestones and Catalysts
Overall response rate and progression-free survival data from phase 2 BRACELET-1 metastatic breast cancer study: Q2 2023
Preclinical data from the combination of pelareorep and CAR T cell therapy: Q2 2023
Updated data in advanced/metastatic pancreatic ductal adenocarcinoma (PDAC) from the GOBLET study: H2 2023
Update on the metastatic colorectal and anal cancer cohorts from the GOBLET study: H2 2023
Guidance for the registration paths for HR+/HER2- metastatic breast cancer and advanced/metastatic PDAC: H2 2023
highest volume day since march 2021 and there have only been a couple handful of days with volume like this since the stock started regaining in share price back in December 2019.
This volume bodes well for this run. things could get pretty wild here imo.
Only other comparable company on the same Phase 3 trial is FibroGen Inc with a market cap over $1.1 billion.
A Multi-center Trial to Evaluate Multiple Regimens in Metastatic Pancreatic Cancer
https://classic.clinicaltrials.gov/ct2/show/NCT04229004
Next stop $3.00 then $4.00 then its pretty much blue sky.
Also Phase 1 Clinical trials for viral loaded CAR-T cells announcement is also imminent. This new treatment process has shown to have 80% to 100% total cure rate for the brain cancer model used on rodents. I wonder who our partners will be for this? :D
This clinical trial annoucement will be the cherry on top of the upcoming announcement for the Phase 3 trial for breast cancer. (who is going to be the partner for this? :D)
The coming weeks and months is ONCY's time to pop off!
ONCY IS A PHASE 3 BIOTECH COMPANY WITH AN ADDITIONAL IMMINENT PHASE THREE ANNOUNCEMENT.
Cheers to all here. I invested in the 2019 and sold in 2020. recently bought back in earlier this month. Cheers to the longs here too!
I am here. Here is a comment for appreciation and to bring attention to ONCY.
NEWS -- Oncolytics Biotech's® Pelareorep Selected for Inclusion in Precision PromiseSM Pivotal Phase 3 Platform Trial

Adaptive clinical trial designed to accelerate registration pathways for pancreatic cancer therapies and expected to reduce cost of a Phase 3 study for pelareorep by ~50% compared to a traditional trial
If successful, new clinical study expected to support approval of pelareorep in combination with a checkpoint inhibitor, gemcitabine, and nab-paclitaxel in first-line metastatic pancreatic cancer
Data presented at SITC 2022 showed a near tripling of overall response rate for pelareorep + gemcitabine + nab-paclitaxel + a PD-L1 inhibitor compared to historical control trials
SAN DIEGO, CA and CALGARY, AB, June 22, 2023 /CNW/ -- Oncolytics Biotech® Inc. (NASDAQ: ONCY) (TSX: ONC) today announced pelareorep has been selected for inclusion as a new investigational treatment in Precision PromiseSM, an innovative adaptive Phase 3 clinical trial. The Precision Promise study is designed to evaluate pelareorep in combination with a checkpoint inhibitor and the chemotherapeutic agents gemcitabine and nab-paclitaxel. If successful, the clinical study is expected to support approval of the studied combination as a treatment for first-line metastatic pancreatic ductal adenocarcinoma (PDAC).
Precision Promise has a primary endpoint of overall survival and can include multiple investigational treatments as well as control arms evaluating: (1) gemcitabine plus nab-paclitaxel or (2) mFOLFIRINOX. Each investigational therapy is subject to pre-specified interim analyses prior to proceeding to the registrational portion of the trial. This design, which was developed with guidance from the U.S. Food and Drug Administration, minimizes the number of participants needed to generate licensure-enabling data, thereby accelerating late-stage development by up to two years and reducing costs compared to non-platform trials.
"We are delighted at being selected by the Precision Promise panel of experts," said Dr. Matt Coffey, President and Chief Executive Officer of Oncolytics Biotech. "Our next step is to engage with stakeholders to finalize the protocol for Precision Promise's pelareorep-containing investigational treatment so that we can enter into this study. We are thrilled to have the opportunity to leverage Precision Promise, which we expect will allow us to reduce the time and costs needed to reach a potential approval."
Julie Fleshman, JD, MBA, President and CEO of PanCAN commented, "With a five-year survival rate of 12%, pancreatic cancer patients cannot afford to wait for new treatment options. This urgent unmet need was the driving inspiration behind the Precision Promise platform trial, which was designed specifically to identify, accelerate, and de-risk the development of promising pancreatic cancer treatments. We are thrilled to be bringing pelareorep into Precision Promise as a new investigational therapy to study against the current standard of care."
Dr. Thomas Heineman, Chief Medical Officer of Oncolytics Biotech, commented, "Prior trials in pancreatic cancer show pelareorep-based combinations outperforming historical controls on key metrics such as one and two-year survival and objective response rate. In addition, mechanistic data from these studies highlight how pelareorep's immunologic mechanism of action allows it to synergize with chemotherapy and checkpoint inhibition in this indication. I look forward to working with the Precision Promise team of investigators to seek to confirm the therapeutic value of pelareorep in a randomized setting so that we can potentially provide pancreatic cancer patients with a new treatment option."
Oncolytics expects to finalize the definitive agreements within the next 90 days and open the Precision Promise investigational treatment of pelareorep, checkpoint inhibitor, gemcitabine, and nab-paclitaxel in early 2024.
Pelareorep in Pancreatic Cancer
Pelareorep's inclusion in Precision Promise is supported by prior clinical data in pancreatic cancer that suggests it synergizes with checkpoint inhibition and chemotherapy. These prior data include phase 1/2 results showing a 69% objective response rate (ORR, n = 13) in a cohort of first-line advanced/metastatic PDAC patients treated with pelareorep combined with atezolizumab, gemcitabine, and nab-paclitaxel. This compares to an average ORR of ~25% reported in relevant historical control trials1-4. In addition, a phase 2 study of pelareorep plus gemcitabine that included 29 evaluable chemotherapy-naïve pancreatic cancer patients showed a median overall survival (mOS) of 10.2 months and a one-year survival rate of 45%, compared to historical control trials showing mOS of approximately 6.7 months and one-year survival rates of approximately 20 – 22%, respectively1,5-6. A subsequent phase 2 study of pelareorep plus checkpoint inhibition without chemotherapy in pancreatic cancer patients who progressed after first-line treatment showed a 42% disease control rate (n = 12), post-treatment increases in PD-L1+ cells, and a correlation between clinical response and increased activation of anti-cancer CD8+ T cells.
References
1. Von Hoff D et al. N Engl J Med 2013; 369:1691-1703 DOI: 10.1056/NEJMoa1304369
2. O'Reilly et al. Eur J Cancer. 2020 June; 132: 112–121. DOI:10.1016/j.ejca.2020.03.005
3. Karasic et al. JAMA Oncol. 2019 Jul 1; 5(7):993-998. DOI: 10.1001/jamaoncol.2019.0684
4. Tempero et al. Ann Oncol. 2021 May; 32(5):600-608. DOI: 10.1016/j.annonc.2021.01.070
5. Conroy et al. N Engl J Med 2011; 364:1817-1825. DOI: 10.1056/NEJMoa1011923
6. Goldstein et al. JNCI J Natl Cancer Inst (2015) 107(2): DOI:10.1093/jnci/dju413
About the Pancreatic Cancer Action Network
The Pancreatic Cancer Action Network (PanCAN) leads the way in accelerating critical progress for pancreatic cancer patients. PanCAN takes bold action by funding life-saving research, providing personalized patient services and creating a community of supporters and volunteers who will stop at nothing to create a world in which all pancreatic cancer patients will thrive. For 18 years in a row, PanCAN has earned a Four-Star Rating from Charity Navigator – the highest rating an organization can receive. This rating designates PanCAN as an official "Give with Confidence" charity, indicating strong financial health, ongoing accountability and transparency.
About Oncolytics Biotech Inc.
Oncolytics is a biotechnology company developing pelareorep, an intravenously delivered immunotherapeutic agent. This compound induces anti-cancer immune responses and promotes an inflamed tumor phenotype -- turning "cold" tumors "hot" -- through innate and adaptive immune responses to treat a variety of cancers.
Pelareorep has demonstrated synergies with multiple approved oncology treatments. Oncolytics is currently conducting and planning combination clinical trials with pelareorep in solid and hematological malignancies as it advances towards registrational studies in metastatic breast cancer and pancreatic cancer. For further information, please visit: https://www.oncolyticsbiotech.com.
This press release contains forward-looking statements, within the meaning of Section 21E of the Securities Exchange Act of 1934, as amended and forward-looking information under applicable Canadian securities laws (such forward-looking statements and forward-looking information are collectively referred to herein as "forward-looking statements"). Forward-looking statements contained in this press release include statements regarding Oncolytics' belief as to the potential and benefits of pelareorep as a cancer therapeutic; the design and anticipated benefits of the Precision Promise study, including our belief that the study, if successful, will support approval of the studied combination as a treatment for first-line metastatic pancreatic ductal adenocarcinoma; our plan to engage with stakeholders to finalize the protocol for Precision Promise's pelareorep-containing investigational treatment so that we can enter into the study; our expectation that we will be in a position to finalize the definitive agreements within the next 90 days and open the Precision Promise investigational treatment of pelareorep, checkpoint inhibitor, gemcitabine, and nab-paclitaxel in early 2024; our plans to advance towards a registrational study in metastatic pancreatic cancer; and other statements related to anticipated developments in Oncolytics' business and technologies. In any forward-looking statement in which Oncolytics expresses an expectation or belief as to future results, such expectations or beliefs are expressed in good faith and are believed to have a reasonable basis, but there can be no assurance that the statement or expectation or belief will be achieved. Such forward-looking statements involve known and unknown risks and uncertainties, which could cause Oncolytics' actual results to differ materially from those in the forward-looking statements. Such risks and uncertainties include, among others, the availability of funds and resources to pursue research and development projects, the efficacy of pelareorep as a cancer treatment, the success and timely completion of clinical studies and trials, Oncolytics' ability to successfully commercialize pelareorep, uncertainties related to the research and development of pharmaceuticals, uncertainties related to the regulatory process and general changes to the economic environment. In particular, we may be impacted by business interruptions resulting from COVID-19 coronavirus, including operating, manufacturing supply chain, clinical trial and project development delays and disruptions, labour shortages, travel and shipping disruption, and shutdowns (including as a result of government regulation and prevention measures). It is unknown whether and how Oncolytics may be affected if the COVID-19 pandemic persists for an extended period of time. We may incur expenses or delays relating to such events outside of our control, which could have a material adverse impact on our business, operating results and financial condition. Investors should consult Oncolytics' quarterly and annual filings with the Canadian and U.S. securities commissions for additional information on risks and uncertainties relating to the forward-looking statements. Investors are cautioned against placing undue reliance on forward-looking statements. The Company does not undertake any obligation to update these forward-looking statements, except as required by applicable laws.
Company Contact
Jon Patton
Director of IR & Communication
+1-858-886-7813
mailto://jpatton@oncolytics.ca
Investor Relations for Oncolytics
Timothy McCarthy
LifeSci Advisors
+1-917-679-9282
mailto://tim@lifesciadvisors.com
View original content: https://www.prnewswire.com/news-releases/oncolytics-biotechs-pelareorep-selected-for-inclusion-in-precision-promisesm-pivotal-phase-3-platform-trial-301857678.html
SOURCE Oncolytics Biotech® Inc.
View original content: http://www.newswire.ca/en/releases/archive/June2023/22/c8207.html
NEWS -- Oncolytics Biotech® Announces Updated Randomized Phase 2 Data from BRACELET-1 Metastatic Breast Cancer Trial that Show Pelareorep Driving Robust Increases in Progression-Free Survival and Confirmed Overall Response Rate
Pelareorep-paclitaxel combination reduced risk of disease progression by 71% (hazard ratio of 0.29) compared to paclitaxel monotherapy
37.5% confirmed overall response rate with pelareorep-paclitaxel vs. 13.3% with paclitaxel monotherapy
12-month progression-free survival rate of 32.8% for pelareorep-paclitaxel compared to 0% for paclitaxel monotherapy and 0% for pelareorep-paclitaxel-avelumab
Oncolytics' HR+/HER2- breast cancer program now phase 3-ready and advancing to a registrational study of pelareorep-paclitaxel combination
Data to be discussed during a key opinion leader webinar today, June 5th at 8:00 a.m. ET (registration link here)

SAN DIEGO and CALGARY, AB, June 5, 2023 /CNW/ -- Oncolytics Biotech® Inc. (NASDAQ: ONCY) (TSX: ONC) today announced updated results from BRACELET-1, a randomized phase 2 trial in HR+/HER2- metastatic breast cancer, which include data featured in an oral presentation at the 2023 American Society of Clinical Oncology (ASCO) Annual Meeting, as well as additional new data and analyses.
BRACELET-1 enrolled 48 patients, including 45 that were randomized and well-balanced across three cohorts evaluating: (1) paclitaxel monotherapy; (2) paclitaxel in combination with pelareorep; and (3) paclitaxel plus pelareorep in combination with the anti-PD-L1 checkpoint inhibitor, avelumab (Bavencio®). A three-patient safety run-in was also conducted with patients receiving pelareorep, paclitaxel, and avelumab prior to randomization. All participants enrolled in the trial had previously progressed on at least one hormone-based therapy with a CDK 4/6 inhibitor. No patients in BRACELET-1 received chemotherapy for metastatic disease prior to enrolling in the trial.
Updated data from BRACELET-1 showed a median progression-free survival (mPFS) of 9.5 months in the paclitaxel plus pelareorep cohort vs. 6.3 months in the paclitaxel monotherapy cohort for a hazard ratio of 0.29 as of a March 3, 2023 cut-off date. Confirmed overall response rate (ORR) in these cohorts was 37.5% and 13.3%, respectively. As previously reported, ORR at week-16 (the trial's primary endpoint) in the pelareorep plus paclitaxel and paclitaxel monotherapy cohorts was 31.3% and 20%, respectively. Overall survival data from the trial continue to mature.
"BRACELET-1's positive results complement prior phase 2 data showing a statistically significant increase in overall survival when pelareorep was combined with paclitaxel by demonstrating similar robust improvements in PFS and ORR in less heavily pre-treated patients," said Dr. Matt Coffey, President and Chief Executive Officer. "Given this exciting finding, our next step is to discuss our data with the FDA to investigate incorporating dual PFS and OS endpoints into our breast cancer program's registrational study. Including a PFS endpoint could substantially reduce the time to a pivotal readout from the registrational trial, thereby accelerating pelareorep's path to potential approval as we work to address the urgent needs of HR+/HER2- breast cancer patients."
A summary of response and PFS data from all 48 patients enrolled in BRACELET-1 is shown below.
Additional updated BRACELET-1 data 1:
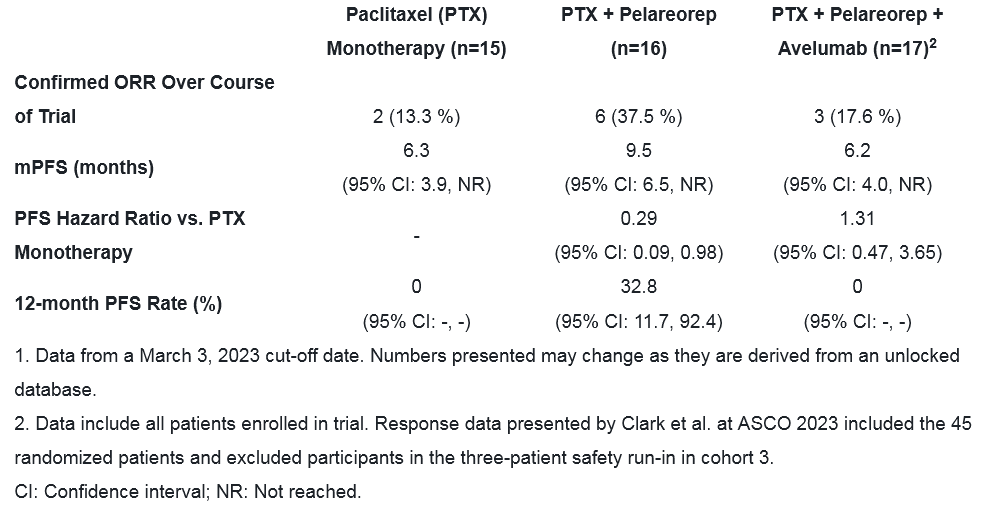
Key biomarker and safety findings from BRACELET-1 include:
|
Followers
|
20
|
Posters
|
|
|
Posts (Today)
|
0
|
Posts (Total)
|
324
|
|
Created
|
12/27/19
|
Type
|
Free
|
| Moderators | |||
| Volume | |
| Day Range: | |
| Bid Price | |
| Ask Price | |
| Last Trade Time: |
