Register for free to join our community of investors and share your ideas. You will also get access to streaming quotes, interactive charts, trades, portfolio, live options flow and more tools.
RADNOR, Pa., Oct. 2, 2023 /PRNewswire/ -- NRx Pharmaceuticals, Inc. (Nasdaq: NRXP) ("NRx Pharmaceuticals", the "Company"), a clinical-stage biopharmaceutical company, today announced that FDA has given the company clearance to proceed with human trials to treat Chronic Pain under the Investigational New Drug (IND) application filed with the U.S. Food and Drug Administration (FDA) for the use of NRX-101. The IND application leverages pioneering research on the use of D-cycloserine (a key ingredient of NRX-101) in the treatment of chronic pain and the recent licensure by NRx of a US Patent for the use of D-cycloserine in the treatment of pain.
NRX Pharmaceuticals Announces Data Sharing Agreement Demonstrating Efficacy and Safety of Intravenous Ketamine for the Treatment of Suicidal Bipolar Depression
September 15 2023 - 06:46AM
PR Newswire (US)
Ketamine, an NMDA blocker, was highly effective in Bipolar subgroup (p<0.001)
NRx plans to present the data to FDA with a goal of identifying a path to an NDA
RADNOR, Pa., Sept. 15, 2023 /PRNewswire/ -- NRx Pharmaceuticals, Inc. (Nasdaq: NRXP) ("NRx Pharmaceuticals", the "Company"), a clinical-stage biopharmaceutical company, today announced that it has signed a data sharing agreement with the study leadership of a randomized, placebo-controlled trial of 156 patients hospitalized for Acute Suicidality and Depression in 7 French Government Hospitals.
This trial, conducted under International Good Clinical Practices and Helsinki standards demonstrated a dramatic and statistically-significant reduction in suicidal ideation and depression (P<.0001) among patients randomized to intravenous racemic ketamine, compared to those randomized to placebo. The trial reached its primary endpoint for all patients and demonstrated the largest effect among the subgroup with bipolar depression (84% vs 28% remission, drug vs. placebo; p<0.0001; Odds ratio 14).
The top line data from this trial were published in the British Medical Journal (BMJ 2022; 376 doi: https://doi.org/10.1136/bmj-2021-067194) and additional data reports are planned. The efficacy demonstrated confirms earlier, smaller trials conducted in the United States and elsewhere.
As disclosed previously, NRx met with the FDA in January 2023 at which time the FDA requested randomized, placebo-controlled data in support of intravenous ketamine for acute suicidality in the inpatient setting. Such trials are extraordinarily complex to organize and generally require Government support. In this case, NRx approached the Fondation FundaMental, led by Prof. Marion Leboyer, MD, PhD, who served on the NRx Scientific Advisory Board and facilitated establishing the current Data Sharing Agreement.
Under this agreement, NRx has translated the clinical study report, which will be submitted to FDA and is converting the electronic, patient level data files to a form suitable for FDA review. NRx plans to meet with FDA in the coming months to discuss a regulatory path for the use of ketamine to treat patients with Bipolar Depression and Acute Suicidal Ideation and Behavior.
"We at NRx are honored that the study leadership has agreed to share these landmark data so that they may be submitted to and reviewed by the US FDA," said Dr. Jonathan Javitt, Founder and Chief Scientist of NRx Pharmaceuticals. "We look forward to a fruitful ongoing collaboration with Prof. Abbar and his study team leadership for the benefit of patients everywhere."
Looks like they knocked out of the park with the French study news
NRx Pharmaceuticals Announces Agreement with LifeSci Associates to Provide Financial Services and Support
Industry veteran Richard Narido joins the NRx Pharmaceuticals management team
RADNOR, Pa., Sept. 14, 2023 /PRNewswire/ -- NRx Pharmaceuticals, Inc. (Nasdaq: NRXP) ("NRx Pharmaceuticals" or the "Company"), a clinical-stage biopharmaceutical company, today announced that it has entered into an agreement (the "Agreement") with LS Associates, a division of LifeSci Advisors, LLC ("LSA"), pursuant to which LSA will provide certain consulting services to the Company, including but not limited to, arranging for the provision of the Company's Interim Chief Financial Officer. In connection with the Agreement, the Company appointed Richard Narido to serve as Interim Chief Financial Officer of the Company. As Interim Chief Financial Officer, Mr. Narido will serve as the Company's principal financial officer and principal accounting officer.
"I am pleased to welcome Richard to the team at NRx Pharmaceuticals. He brings great experience and energy to our dynamic company," said Stephen Willard, Chief Executive Officer of NRx Pharmaceuticals. "Additionally, we are pleased to have immediate access to LSA's broad team of financial professionals, who will be available to us as needed," he continued.
Prior to his appointment as the Company's Interim Chief Financial Officer, Mr. Narido served as the Chief Financial Officer of Lucira Health ("Lucira") until Pfizer Inc.'s acquisition of Lucira in April 2023. From July 2018 to March 2021, Mr. Narido served in various roles at Assembly Biosciences, Inc., including most recently as Executive Director, Finance, Controllership and Treasury. From June 2014 to June 2018, Mr. Narido served in various roles at Bio-Rad Laboratories, Inc., including as Americas Head of Finance, Global Commercial Operations. Prior to June 2018, Mr. Narido held various finance roles, including Global Head Finance Reporting and Accounting for Novartis Vaccines and Diagnostics and several industry-related positions, including Business Unit Controller for McKesson Corporation. Mr. Narido started his career with PricewaterhouseCoopers's Financial Audit and Assurance practice. Mr. Narido holds a Bachelor of Science degree from the University of San Francisco and a Master of Science degree from the Pepperdine Graziadio Business School.
The Company also today announced that it accepted the resignation of Seth Van Voorhees, Ph.D., former Chief Financial Officer of the Company, which was tendered on September 11, 2023, as he has decided to pursue other professional opportunities. His resignation will be effective as of September 30, 2023, in order to facilitate a smooth transition. "We thank Seth for his many contributions to the Company, and we wish him well in his future endeavors," Mr. Willard commented.
About NRX-101
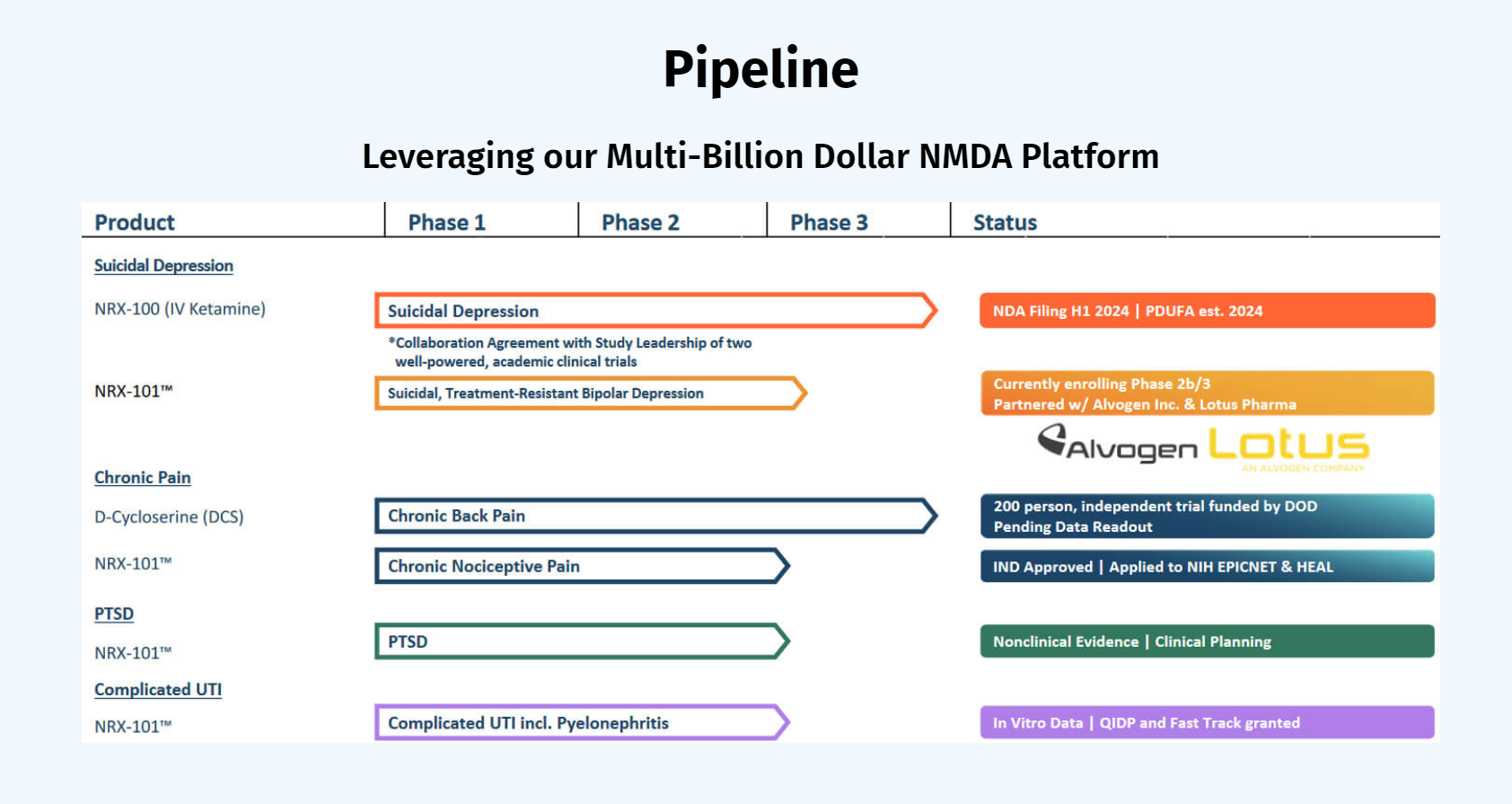
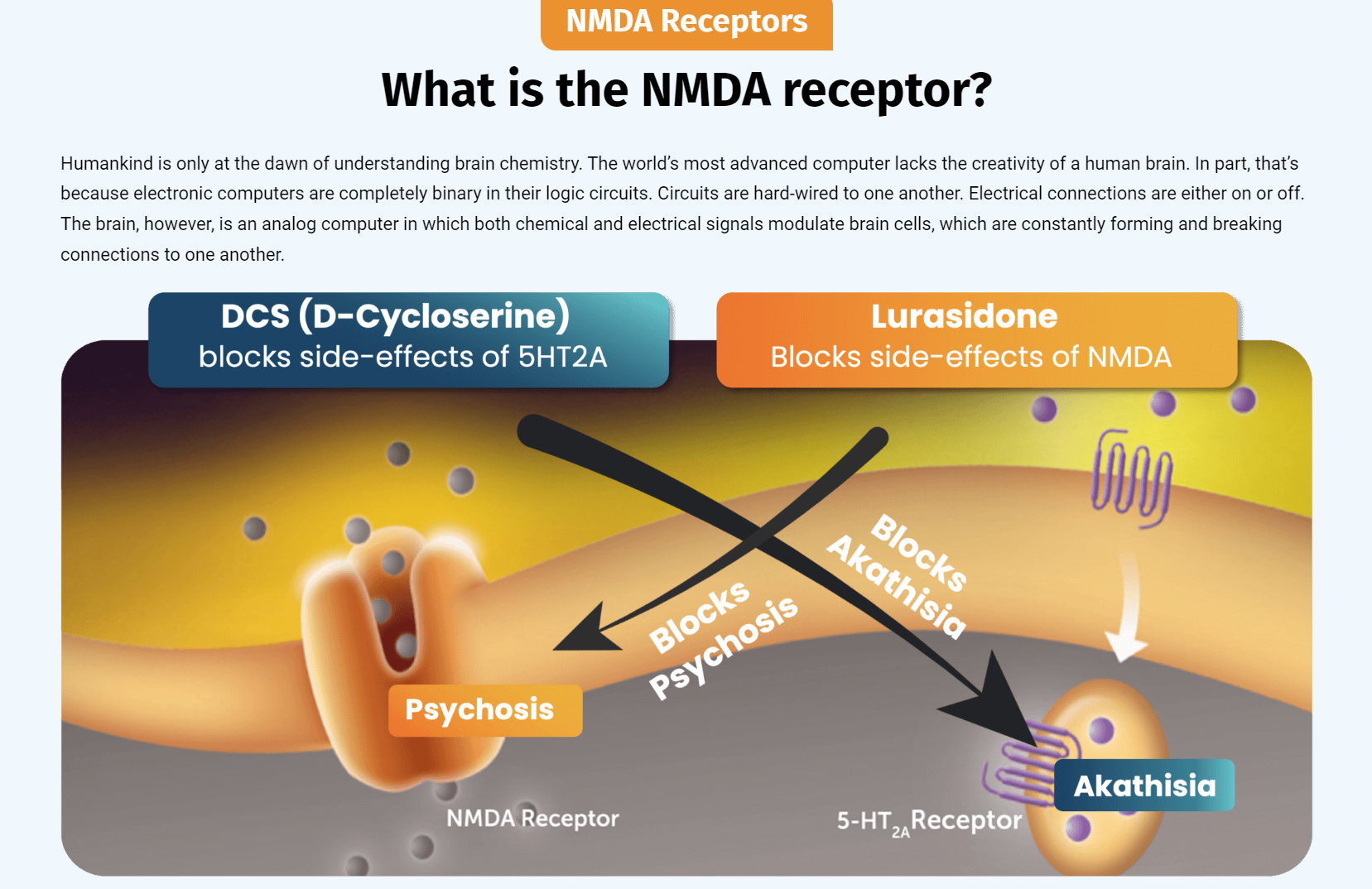
NRX-101, a fixed dose combination of D-cycloserine and lurasidone, has been granted Fast Track Designation, Breakthrough Therapy Designation, a Special Protocol Agreement, and a Biomarker Letter of Support from the FDA for S-TRBD. Additionally, the product is being developed in chronic pain and PTSD.
I signed up for Chaikin Power Pulse and here is what they say about NRXP .. I rarely join these services, but it was a free trial. I did not expect that rating. My META and TSLA are just listed as bullish.. But they are not recommending a buy yet on NRXP just listing it as a "wait".. So we shall see.
NRXP - VERY BULLISH
NRx Pharmaceuticals, Inc.
$0.25
0.01
(+4.17%)
As of 09/11/23 00:28AM EDT
INDUSTRY
Pharmaceuticals
EARNINGS
POWER GAUGE RATING
As of 09/11/23
NRXP is Very Bullish
Financials
Earnings
Technicals
Experts
The Chaikin Power Gauge Rating for NRXP is Very Bullish due to very positive expert activity, attractive financial metrics and strong earnings performance. The stock also has weak price/volume activity.
Expert activity for NRXP is very positive which is evidenced by insiders purchasing stock and relative strength of the stock's industry.
PRE-TRADE CHECKLIST
Strength
2/3
Power Gauge Rating Very Bullish
Rel. Strength vs SPY Weak
Industry Strength Strong
So they are claiming that NRX will do three things.. Miracle drug? or... are they just trying to get something to stick to the wall? I do not trust the FDA to keep their profit agenda and or politics out of all this.
If just one of these options works and is approved then it could be a good thing.
NRx Pharmaceuticals Announces the Licensure of a US Patent to Support Use of NRX-101™ for Chronic Pain
NRx Pharmaceuticals Reports Minutes of Recent U.S. Food and Drug Administration (FDA) Meeting on the Development of NRX-101 to Treat Severe Bipolar Depression in Patients with Suicidality
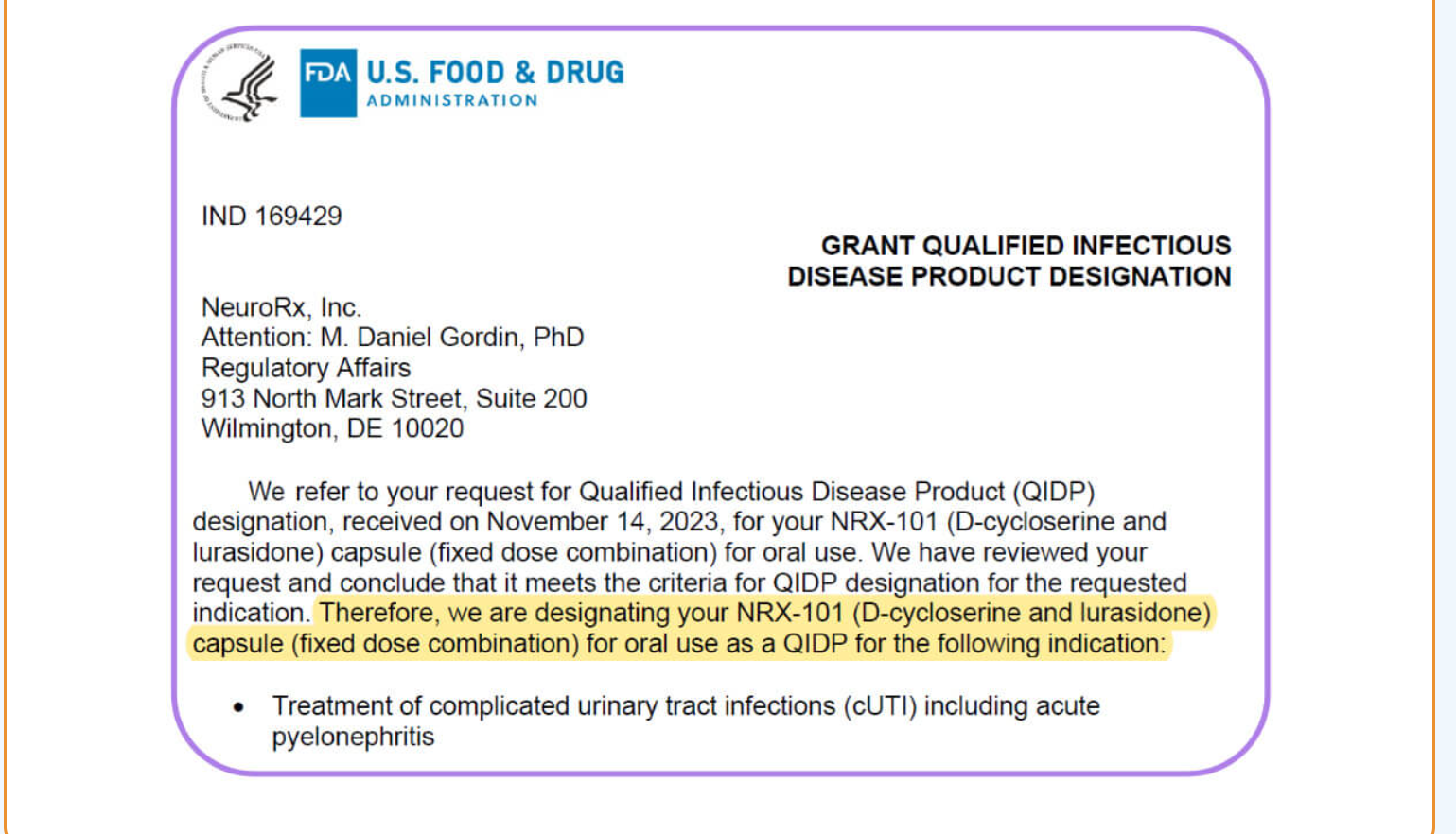
NRx Pharmaceuticals Announces Potent Antibacterial Activity of NRX-101 Against Common, Antibiotic Resistant Urinary Pathogens
This turned out to be real POS didn't it. Geez. Moon market never mentioned it again.
Moon made a new video finally. Didn't even mention this stock haha. What a loser. Talked about stocks that were already up 60% yesterday. I'm out 😭 f this one. Will watch for a while from the sidelines. Peace.
Historically when I have been the only one posting on the board the price is about to spike. I was the only one on the ni# board board when it was $1. Went over $30. Not saying it will happen here but every time that's been the case
Looks like 28s coming again. Geez this can't get going. And still no moon market. AWOL. Never seen him AWOL this long before. Must have got booted for stock manipulation
Now it's been 7 days. I'm just trying to figure of yesterday's news is bullish or bearish for this stock.
Good Deal , GLTY !
Good move. It just broke free! That was a close one. I bought back most of my shares about half hr ago when I saw it creeping up. I'm up over 27% on my other play today also. I sold some of that and bought back in here. What a day haha! GL.
Sold some of my position. I don't like the way it's trading. Still have plenty of shares but I was over invested here. Other plays I want to buy. GL
So moon market hyped this like no timorrow. Even the title of video is 10x short squeeze coming. Then he promises more DD on the stock and instead disappears for the last 5 days.
Possibly. I'll load up more if so. I'm in pretty big here. Wish me luck haha.
I hear you. Looking better after hrs. today and I actually added in the .28 range today. So just hoping it can hold that 28
He made his money. That’s all he cared about. I can’t believe people pay him so he can preload and dump on them. Some pay $50 per month I think.
We have revisited the 52 week low today. It has to bounce from here or who knows where the bottom may be. Inside buys could be manipulation to entice buyers with a guarantee from the company to pay him back if it doesn't work.
Well that was disappointing. Geez. Another loser from moon market. Unsubscribing from that channel. Promised more DD but is hiding somewhere now
I was able to pick up some more this morning at .305. Pretty happy about it. GL.
Stocks tend to like to double bottom before they rebound
Should be a good week next week. May stay in this range Monday but I have a feeling by mid week this should be looking pretty good
Bought a ton After hours .325- .327 couldn't get it past .33
Been some inside buys lately and supposed several news events coming. This info. Is from moon market you tube channel. Low float and it's been hammered but apparently new developments coming. Could be big squeeze coming. There was support at .33 and then .30 on the chart. It no nced off the .30 and is now stuck in the .30-.33 range. If we can close above .33 then that would be a really bullish sign.
OK... what makes you think there will be lift off? I've been long this things for too long now.
Added from 33-30s today. Waiting for liftoff. Countdown has begun
AAPJ is a new gem 2023 // looks Low SS - wow and only .0032
The chart is showing potential for the sky and the SS is looking very good and the stock has a lot of catalysts and an insanely small structure for an OTC stock (at these prices .0032) and a lot of imagination and things are just beginning and this is where it's developing something and $AAPJ should take off in the future and where and how high the stock may go no one knows..... but we will see what this little hidden gem = brings us in the future
all only imho
$NRXP - In exchange for the license grant and the participation of NRx in the development, regulatory and commercial activities described below, Alvogen will pay NRx up to an aggregate of $330 million in cash milestone payments, including an initial $10 million cash payment
$NRXP ! Boom very good 8K out https://ih.advfn.com/stock-market/NASDAQ/nrx-pharmaceuticals-NRXP/stock-news/91243293/current-report-filing-8-k
$NRXP - Only the beginning
With the 8:30 am update this could squeeze very hard. Light float as well.
$NRPX - In exchange for the license grant and the participation of NRx in the development, regulatory and commercial activities described below, Alvogen will pay NRx up to an aggregate of $330 million in cash milestone payments, including an initial $10 million cash payment
imho
$NRXP - In exchange for the license grant and the participation of NRx in the development, regulatory and commercial activities described below, Alvogen will pay NRx up to an aggregate of $330 million in cash milestone payments, including an initial $10 million cash payment
$NRXP ! Boom very good 8K out https://ih.advfn.com/stock-market/NASDAQ/nrx-pharmaceuticals-NRXP/stock-news/91243293/current-report-filing-8-k
$NRXP - Only the beginning
With the 8:30 am update this could squeeze very hard. Light float as well.
$NRPX - In exchange for the license grant and the participation of NRx in the development, regulatory and commercial activities described below, Alvogen will pay NRx up to an aggregate of $330 million in cash milestone payments, including an initial $10 million cash payment
imho
NRx Announces Recommendation to Continue Enrollment NRX-101
https://ir.nrxpharma.com/2023-03-27-NRx-Pharmaceuticals-Announces-Positive-Recommendation-to-Continue-Enrollment-in-the-Ongoing-Trial-of-NRX-101-in-Patients-with-Suicidal-Treatment-Resistant-Bipolar-Depression
· No safety or futility signals were reported in the first 50 patients
· This patient population could represent up to 1 million bipolar depression patients in the US and represents a portion of the indication expansion recommended in recent correspondence with the FDA
· Trial has been upgraded to a Phase 2b/3 study that may be used for a registrational filing
We expect top-line data from this trial in the fourth quarter of 2023. The Company plans to discuss the path to approval in this population of people with Suicidal Treatment Resistant Bipolar Depression in the planned Comprehensive Breakthrough Therapy Meeting with FDA that is planned for the second quarter of 2023. Additionally, we are opening an Expanded Access Program as required for all Breakthrough Therapy Medicines to make this investigational medicine more broadly available and to accrue the safety database previously requested by the FDA.
________
I'm still in this one because I've got a bunch of options expiring in January 2024 lol. Not sure on that $25, oof.
But no matter what, best wishes on the drug helping people in the end.
Inhaled aviptadil for the possible treatment of COVID-19 in patients at high risk for ARDS: study protocol for a randomized, placebo-controlled, and multicenter trial
https://rdcu.be/cV1X7
Bottom line... It is starting to look like it all depends on how they apply the testing and how they look at the test results. India approved its emergency use authorization and one of the involved doctors who was at the top of the testing told me in an answer to my email question that it is most effective withing the first days... and he said it works.
This article below claims it helps significantly if used during a certain time period. How much of this is political? How much of this was just incorrect procedures during testing? Something is strange here....
https://pubmed.ncbi.nlm.nih.gov/36044317/
From the National Library of Medicine
2022 Aug 31.
The Use of IV Vasoactive Intestinal Peptide (Aviptadil) in Patients With Critical COVID-19 Respiratory Failure: Results of a 60-Day Randomized Controlled Trial
Jihad Georges Youssef 1 2, Philip Lavin 3, David A Schoenfeld 4, Richard A Lee 5, Rainer Lenhardt 6, David J Park 7, Javier Perez Fernandez 8, Melvin L Morganroth 9, Jonathan C Javitt 10 11, Dushyantha Jayaweera 12
Affiliations expand
PMID: 36044317 DOI: 10.1097/CCM.0000000000005660
Abstract
Objectives: Respiratory failure is a lethal complication of COVID-19 that has remained resistant to drug therapy. Vasoactive intestinal peptide (VIP) is shown in nonclinical studies to upregulate surfactant production, inhibit cytokine synthesis, prevent cytopathy, and block replication of the severe acute respiratory syndrome coronavirus 2 virus in pulmonary cells. The study aims to determine whether Aviptadil (synthetic VIP) can improve survival and recovery in patients with COVID-19 respiratory failure compared with placebo and demonstrate biological effects in such patients.
Design: A multicenter, placebo-controlled trial.
Setting: Ten U.S. hospitals: six tertiary-care hospitals and four community hospitals.
Patients: A total of 196 patients with COVID-19 respiratory failure.
Interventions: Participants were randomized 2:1 to receive 3 days of IV Aviptadil or placebo.
Measurements and main results: The primary end point (alive and free from respiratory failure at day 60) did not reach statistical significance (odds ratio [OR], 1.6; 95% CI, 0.86-3.11) for patients treated with Aviptadil when controlling for baseline ventilation status as prespecified in the protocol. There was, however, a statistically significant two-fold odds of improved survival (OR, 2.0; 95% CI, 1.1-3.9) at 60 days (p = 0.035). There was significant improvement in respiratory distress ratio and reduced interleukin 6 cytokine release (p = 0.02) by day 3.Subgroup analysis identified a statistically significant likelihood of achieving primary end point among those treated with high-flow nasal oxygen at baseline (p = 0.039). Subjects on mechanical ventilation also experienced a 10-fold increased odds of survival with drug versus placebo (p = 0.031).
Conclusions: The primary end point did not reach statistical significance, indicating that there was no difference between Aviptadil versus placebo. However, Aviptadil improves the likelihood of survival from respiratory failure at day 60 in critical COVID-19 across all sites of care. Given the absence of drug-related serious adverse events and acceptable safety profile, we believe the benefit versus risk for the use of Aviptadil is favorable for patient treatment.
|
Followers
|
54
|
Posters
|
|
|
Posts (Today)
|
0
|
Posts (Total)
|
1169
|
|
Created
|
12/15/20
|
Type
|
Free
|
| Moderators ProfitScout Trooperstocks | |||











Both drugs demonstrated > 50% response for treating depression. NRX-101 demonstrated a mean 76% reduction in symptoms of akathisia compared to lurasidone that was sustained over 42 days (Effect...

New data demonstrates no impact of NRX-101 on gut or vaginal flora – considered primary causes of pseudomembranous colitis due to C difficile and vaginal yeast infections NRX-101 previously...
Formulation based on prior patents by NRx founder Achieved pH neutral formulation of ketamine, potentially enabling both intravenous (IV) and subcutaneous (SQ) administration Company expects...
Data transferred for independent statistical analysis Top-line data expected in April 2024 RADNOR, Pa., April 8, 2024 /PRNewswire/ -- NRx Pharmaceuticals, Inc. (Nasdaq: NRXP) ("NRx...
Four potential near-term milestones, including data from two clinical trials, an NDA filing and an upcoming share dividend -- 50% reduction in corporate overhead and 25% reduction in overall net...
Four potential near-term milestones, including data from two clinical trials, an NDA filing and a share dividend RADNOR, Pa., March 28, 2024 /PRNewswire/ -- NRx Pharmaceuticals, Inc. (Nasdaq: NRXP) ("
NRx Pharmaceuticals, Inc. (Nasdaq: NRXP) ("NRx Pharmaceuticals", the "Company"), a clinical-stage biopharmaceutical company, today announced that it will release its fourth quarter and full year...
NRx Pharmaceuticals, Inc. (Nasdaq:NRXP) ("NRx Pharmaceuticals", the "Company"), a clinical-stage biopharmaceutical company, today announced that Dr. Jonathan Javitt, Chairman and Chief Scientist...
NRx Pharmaceuticals, Inc. (Nasdaq: NRXP) ("NRx Pharmaceuticals", the "Company"), a clinical-stage biopharmaceutical company, today announced that it will release its fourth quarter and full year...
61.4% (56,781,354) of eligible shares voted 94.4% of votes were cast in favor of the resolution RADNOR, Pa., March 21, 2024 /PRNewswire/ -- NRx Pharmaceuticals, Inc. (Nasdaq: NRXP) ("NRx...
NRx Pharmaceuticals Board of Directors has authorized its Chairman, CEO, and management to take all necessary steps to affect the Dividend and Royalty Rights to NRXP Shareholders and applicable...
Initiative is intended to protect shareholder value through continued compliance with Nasdaq listing rules and elimination of naked short sales positions in the Company's securities The Company is...
Company to receive first allocation of ketamine for sale by month end Partners preparing to ship IV Ketamine to full range of customers via 503a and 503b pharmacies NRx Pharmaceuticals and HOPE...
Marks a major step in the development of what could be the first drug approved for Suicidal Bipolar Depression The study database is being cleaned and locked; statistical analysis and top-line...
NRx Pharmaceuticals received approximately $1.0 million in cash from an existing investor Shares were sold at $0.38, a 26.7% premium the recent share offering, along with one common 5-year warrant...
In the news release, NRx Pharmaceuticals, Inc. Announces Pricing of $1.5 Million Underwritten Public Offering of Common Stock, issued 27-Feb-2024 by NRx Pharmaceuticals, Inc. over PR Newswire, we...
NRx Pharmaceuticals, Inc. (Nasdaq: NRXP), ("NRx Pharmaceuticals" or the "Company"), a clinical-stage biopharmaceutical company, today announced that it intends to offer to sell shares of its...
Presentation available on NRx Pharmaceuticals website at https://www.nrxpharma.com/hope-therapeutics/ KEY UPDATES ARE AS FOLLOWS: NRx management is proposing to award 50% of founding shares in...
DISCLAIMER:
Nothing in the contents transmitted on this board should be construed as an investment advisory, nor should it be used to make investment decisions.
There is no express or implied solicitation to buy or sell securities.
The author(s) may have positions in the stocks or financial relationships with the company or companies discussed and may trade in the stocks mentioned.
Readers are advised to conduct their own due diligence prior to considering buying or selling any stock. All information should be considered for information purposes only.
No stock exchange has approved or disapproved of the information here.
| Volume | |
| Day Range: | |
| Bid Price | |
| Ask Price | |
| Last Trade Time: |
