Register for free to join our community of investors and share your ideas. You will also get access to streaming quotes, interactive charts, trades, portfolio, live options flow and more tools.
New 52 Wk Low of $1.59
In light of recent news releases and the potential mind blowing and life changing treatments that NRXP has developed, it's just as mind blowing that we sit at a 52 week low of $1.59 with an equally bind blowing OS of JUST 10,749,518. I can't even believe I found this today. Somebody pinch me. Is this for real? I've already loaded up what I can at the moment with more funds on the way. What are the other 54 followers here think?
Ascendiant Capital Maintains Buy on NRX Pharmaceuticals, Raises Price Target to $44
Benzinga
1:58 PM ET Sep-12-2024
Ascendiant Capital analyst Edward Woo maintains NRX Pharmaceuticals (NRXP.NaE) with a Buy and raises the price target from $43 to $44.
$NRXP News: NRx Pharmaceuticals, Inc. (Nasdaq:NRXP) Featured on Psychiatrist.com
"How NRx Could Upend the Fight Against Depression and Suicide"
RADNOR, Pa., Sept. 11, 2024 /PRNewswire/ -- NRx Pharmaceuticals, Inc. (Nasdaq: NRXP), a clinical-stage biopharmaceutical company, ("NRx," the "Company"), today highlighted that the Company's pipeline products NRX-100 and NRX-101 were featured in an article published on Psychiatrist.com. This prominent outlet is the home of the Journal of Clinical Psychiatry, the official journal of the American Society of Clinical Psychopharmacology.
The article, entitled "How NRx Could Upend the Fight Against Depression and Suicide," can be found here. The author notes:
"Clinical relevance: NRX Pharmaceuticals is developing a pair of promising new treatments for bipolar depression and suicidal ideation."
"NRX-101 is a twice-daily fixed-dose oral combination of D-cycloserine and lurasidone the company developed to treat suicidal treatment-resistant bipolar depression. Researchers found this combination showed a higher efficacy than lurasidone alone for reducing akathisia and suicidality."
"NRX-100 is a proprietary preservative-free formulation of IV ketamine. Researchers have studied it as a treatment for acute suicidal crises in depression."
For more information, please visit hppt://www.nrxpharma.com.
About NRx Pharmaceuticals, Inc.
NRx Pharmaceuticals is a clinical-stage biopharmaceutical company developing therapeutics based on its NMDA platform for the treatment of central nervous system disorders, specifically suicidal bipolar depression, chronic pain, and PTSD. The Company is developing NRX-101, an FDA-designated investigational Breakthrough Therapy for suicidal treatment-resistant bipolar depression and chronic pain. NRx plans to file an NDA for Accelerated Approval for NRX-101 in patients with bipolar depression and suicidality or akathisia. NRX-101 additionally has potential to act as a non-opioid treatment for chronic pain, as well as a treatment for complicated UTI.
NRx has recently announced plans to submit a New Drug Application for NRX-100 (IV ketamine) for the treatment of suicidal depression, based on results of well-controlled clinical trials conducted under the auspices of the US National Institutes of Health and newly obtained data from French health authorities, licensed under a data sharing agreement. NRx was awarded Fast Track Designation for development of ketamine (NRX-100) by the US FDA as part of a protocol to treat patients with acute suicidality.
Notice Regarding Forward-Looking Statements
The information contained herein includes forward-looking statements within the meaning of Section 21E of the Securities Exchange Act of 1934, as amended, and Section 27A of the Securities Act of 1933, as amended. These statements include, among others, statements regarding the proposed public offering and the timing and the use of the proceeds from the offering. Forward-looking statements generally include statements that are predictive in nature and depend upon or refer to future events or conditions, and include words such as "may," "will," "should," "would," "expect," "plan," "believe," "intend," "look forward," and other similar expressions among others. These statements relate to future events or to the Company's future financial performance, and involve known and unknown risks, uncertainties and other factors that may cause the Company's actual results to be materially different from any future results, levels of activity, performance or achievements expressed or implied by these forward-looking statements. You should not place undue reliance on forward-looking statements since they involve known and unknown risks, uncertainties and other factors which are, in some cases, beyond the Company's control and which could, and likely will, materially affect actual results, levels of activity, performance or achievements. Any forward-looking statement reflects the Company's current views with respect to future events and is subject to these and other risks, uncertainties and assumptions relating to the Company's operations, results of operations, growth strategy and liquidity. More detailed information about the Company and the risk factors that may affect the realization of forward-looking statements is set forth in the Company's most recent Annual Report on Form 10-K and other filings with the Securities and Exchange Commission. Investors and security holders are urged to read these documents free of charge on the SEC's website at http://www.sec.gov. Except as may be required by applicable law, The Company assumes no obligation to publicly update or revise these forward-looking statements for any reason, or to update the reasons actual results could differ materially from those anticipated in these forward-looking statements, whether as a result of new information, future events or otherwise.
For further information:
CORPORATE CONTACTS:
Jeremy Feffer, LifeSci Advisors, Inc.
jfeffer@lifesciadvisors.com
Matthew Duffy
Chief Business Officer, NRx Pharmaceuticals
Co-CEO, HOPE Therapeutics, Inc.
mduffy@nrxpharma.com
https://c212.net/c/img/favicon.png?sn=CL03022&sd=2024-09-11 View original content to download multimedia:https://www.prnewswire.com/news-releases/nrx-pharmaceuticals-inc-nasdaqnrxp-featured-on-psychiatristcom-302244304.html
SOURCE NRx Pharmaceuticals, Inc.
$NRXP Another great update! "How NRx Could Upend the Fight Against Depression and Suicide"
A recent article details how NRX Pharmaceuticals is developing a pair of promising new treatments for bipolar depression & suicidal ideation.
Read More - https://psychiatrist.com/news/how-nrx-could-upend-the-fight-against-depression-and-suicide/ #PharmaNews #depression #NRX101 $NRXP @nasdaq #StocksonWatch
$NRXP NEWS! @NRxPharma NRx Pharmaceuticals, Inc. (Nasdaq:NRXP) and HOPE Therapeutics, Inc. Announce Participation at the H.C. Wainwright 26th Annual Global Investment Conference September 9-11, 2024 https://finance.yahoo.com/news/nrx-pharmaceuticals-inc-nasdaq-nrxp-115500521.html?soc_src=social-sh&soc_trk=tw&tsrc=twtr via @YahooFinance #BreakingNews @Nasdaq
Good morning $NRXP Pre-market news out!
News: HOPE Therapeutics, Inc. and NRx Pharmaceuticals, Inc. (Nasdaq:NRXP) Announce Potential Acquisition and Financing Agreements for $30 Million in Currently-Operating Interventional Psychiatry Clinics
PR Newswire
Mon, Aug 26, 2024, 7:55 AM EDT
In this article:
NRXP
+1.96%
NRXPW
+35.91%
$30 million Term Sheet from qualified lender for non-dilutive financing for first clinic acquisitions; anticipated closing within 60 days
Executed Non-Binding Term Sheet with initial clinic partners generating annual revenue of over $10 million, with additional partners to be selected under this initial funding agreement
Company projects annualized revenues of $100 million through continued acquisition by mid 2025
Additional information to be presented at upcoming HC Wainwright Annual Global Investment Conference in New York, September 9-11, 2024
MIAMI, Aug. 26, 2024 /PRNewswire/ -- HOPE Therapeutics, Inc., a wholly-owned subsidiary of NRx Pharmaceuticals, Inc. (Nasdaq: NRXP) ("HOPE," the "Company"), a medical and technology driven company, today announced signing a non-binding Term Sheet for non-dilutive, nonconvertible debt acquisition funding of its first interventional psychiatry clinics (ketamine clinic acquisitions), together with the signing of a Term Sheet for five currently operational clinics in the Western United States. In addition to the currently-signed non-binding Term Sheet, the Company has been offered non-binding lending commitments it believes are sufficient to assemble/acquire a network of operational clinics with revenues in excess of $100 million. The Company anticipates potential operations in the United States, France, and the United Kingdom.
The non-dilutive acquisition funding announced today is in addition to the over $60 million in potential equity funding previously offered upon public listing of HOPE Therapeutics shares on a public exchange.
Ketamine is increasingly used to treat suicidal depression, treatment resistant depression (TRD) and Post Traumatic Stress Disorder (PTSD). Until now there has been no unified entity organized around delivering consistent outpatient care for suicidal depression, TRD and PTSD that combines pharmaceutical therapy, FDA approved and proven medical technologies such as Transcranial Magnetic Stimulation (TMS), digital therapeutics and access to clinical trial protocols for the newest potential treatments, delivered by properly licensed medical professionals to optimize care of people with these conditions. Collectively, these conditions represent a crisis that results in the death of someone around the globe every minute. HOPE aims to bring a unified, patient-centric and science-based approach to the care of patients and their families.
"HOPE is dedicated to providing an accessible platform to help caregivers address the critical needs of those suffering from suicidal thoughts, TRD, PTSD and related challenges. We are delighted to take the critical first steps towards developing a network of clinics that can provide the highest possible level of care and demonstrate best-practices for mental health professionals around the world," said Jonathan Javitt and Matthew Duffy, Co-CEOs of HOPE Therapeutics. "These clinics, and others under review, are planned to provide the foundation for a network generating revenue of approximately $100 million annually in the coming year."
About HOPE Therapeutics, Inc.
HOPE Therapeutics, Inc. (www.hopetherapeutics.com) is a healthcare delivery company developing a best-in-class network of clinics that currently offer ketamine and other lifesaving therapies to patients with suicidal depression and related disorders, together with a digital therapeutic-enabled platform designed to augment and preserve the clinical benefit of NMDA-targeted drug therapy.
About NRx Pharmaceuticals, Inc.
NRx Pharmaceuticals is a clinical-stage biopharmaceutical company developing therapeutics based on its NMDA platform for the treatment of central nervous system disorders, specifically suicidal bipolar depression, chronic pain, and PTSD. The Company is developing NRX-101, an FDA-designated investigational Breakthrough Therapy for suicidal treatment-resistant bipolar depression and chronic pain. NRx plans to file an NDA for Accelerated Approval for NRX-101 in patients with bipolar depression and suicidality or akathisia. NRX-101 additionally has potential to act as a non-opioid treatment for chronic pain, as well as a treatment for complicated UTI.
NRx has recently announced plans to submit a New Drug Application for NRX-100 (IV ketamine) for the treatment of suicidal depression, based on results of well-controlled clinical trials conducted under the auspices of the US National Institutes of Health and newly obtained data from French health authorities, licensed under a data sharing agreement. NRx was awarded Fast Track Designation for development of ketamine (NRX-100) by the US FDA as part of a protocol to treat patients with acute suicidality.
Notice Regarding Forward-Looking Statements
The information contained herein includes forward-looking statements within the meaning of Section 21E of the Securities Exchange Act of 1934, as amended, and Section 27A of the Securities Act of 1933, as amended. These statements include, among others, statements regarding the proposed public offering and the timing and the use of the proceeds from the offering. Forward-looking statements generally include statements that are predictive in nature and depend upon or refer to future events or conditions, and include words such as "may," "will," "should," "would," "expect," "plan," "believe," "intend," "look forward," and other similar expressions among others. These statements relate to future events or to the Company's future financial performance, and involve known and unknown risks, uncertainties and other factors that may cause the Company's actual results to be materially different from any future results, levels of activity, performance or achievements expressed or implied by these forward-looking statements. You should not place undue reliance on forward-looking statements since they involve known and unknown risks, uncertainties and other factors which are, in some cases, beyond the Company's control and which could, and likely will, materially affect actual results, levels of activity, performance or achievements. Any forward-looking statement reflects the Company's current views with respect to future events and is subject to these and other risks, uncertainties and assumptions relating to the Company's operations, results of operations, growth strategy and liquidity. More detailed information about the Company and the risk factors that may affect the realization of forward-looking statements is set forth in the Company's most recent Annual Report on Form 10-K and other filings with the Securities and Exchange Commission. Investors and security holders are urged to read these documents free of charge on the SEC's website at http://www.sec.gov. Except as may be required by applicable law, The Company assumes no obligation to publicly update or revise these forward-looking statements for any reason, or to update the reasons actual results could differ materially from those anticipated in these forward-looking statements, whether as a result of new information, future events or otherwise.
View original content to download multimedia:https://www.prnewswire.com/news-releases/hope-therapeutics-inc-and-nrx-pharmaceuticals-inc-nasdaqnrxp-announce-potential-acquisition-and-financing-agreements-for-30-million-in-currently-operating-interventional-psychiatry-clinics-302230157.html
SOURCE NRx Pharmaceuticals, Inc.
$NRXP $1.99 +14.37% on Target Price Revision; Reiterate BUY with PT Adjusted to $19 by #HCWainwright; Company Planning to File Two NDAs in 2024
Read Report - https://nrxpharma.com/wp-content/uploads/2024/08/735aea4a-b494-44a9-b241-76f07d7428e7.pdf
#PharmaNews #mentalillness #mentalhealth #mentalhealthmatters #depression #ketamine #stress #PTSD #bipolar #NRX100 #NRX101 $NRXP
NRx Pharmaceuticals (NASDAQ:NRXP) Reports Second Quarter and Year to Date 2024 Financial Results and Provides Business Update
Company is now funded for and focused on New Drug Applications (NDAs) for NRX-100 (ketamine) and NRX-101
Audit of HOPE Therapeutics is now complete, SEC filing of spinout this quarter
Key Milestones
Secured $10.8 - $16.3 million in convertible-debt funding from an institutional investor; funds targeted to support FDA New Drug Applications for NRX-100 (ketamine) and NRX-101. Replacement funding entails substantial reduction in interest rate, conversion discount, and other financial terms compared to prior debt
Retirement of Streeterville debt and settlement of litigation at a substantial discount to litigation claims
NRX-100 NDA for suicidal depression based on data from four clinical trials in nearly 1000 participants demonstrating highly significant efficacy compared to placebo, active comparator, and electroshock therapy
Ketamine findings have just been confirmed in published 43,000 person cohort study1
Phase 2b/3 trial of NRX-101 in suicidal patients with bipolar depression demonstrated depression efficacy comparable to standard of care and significant reduction of akathisia (P=0.025) and time to sustained remission from suicidality (P=.05). Presented at the annual meeting of the American Society of Clinical Psychopharmacology. Profile demonstrates possible best in class bipolar depression medication
Company plans to file a New Drug Application (NDA) for Accelerated Approval under Breakthrough Therapy Designation and Priority Review of NRX-101 in treatment of bipolar depression in people akathisia or suicidality, based on the Phase 2b/3 and STABIL-B data
Stability data continues to mature on the three manufacturing lots required for the NRX-100 (IV ketamine) NDA filing and the Company announced alignment with FDA on its Pediatric Study Plan for NRX-100, also a requirement for filing an NDA
HOPE Therapeutics, the Company's wholly owned subsidiary, is focused on developing a best-in-class network of clinics that currently offer ketamine and other lifesaving therapies to patients with suicidal depression and related disorders. HOPE is planned to be spun out as a separate company to be owned by NRx, current NRx shareholders, and new investors. This effort will be funded apart from NRx.
Appointed Dr. Dennis McBride, a Neuroscience, Information Technology and Medical Technology Veteran, to its Board of Directors
Management to host a conference call August 14, 2024, at 4:30 PM ET
RADNOR, Pa., Aug. 14, 2024 /PRNewswire/ -- NRx Pharmaceuticals, Inc. (Nasdaq: NRXP) ("NRx Pharmaceuticals", the "Company"), a clinical-stage biopharmaceutical company, today announced its financial results for the quarter and year to date ended June 30, 2024, and provided a business update.
"NRx has continued to execute on our plans to file two NDA's this year. As we near maturation of NRX-100 stability data, we also achieved an important milestone in aligning with FDA on our pediatric study plan. Together with strong clinical data from four clinical trials, we believe this application will be quite robust. Additionally, the important data generated from two trials conducted by NRx with NRX-101 in suicidal bipolar depression sets the stage for a second NDA for Accelerated Approval later this year. Finally, work continues to spin out Hope Therapeutics and distribute shares to NRx stockholders. We believe reaching these important milestones will generate significant value in the company and reward our shareholders," said Jonathan Javitt, MD, MPH, Chairman and Chief Scientist of NRx Pharmaceuticals. "Facilitated by funding to allow us to achieve these goals and while replacing the prior expensive and toxic debt on our balance sheet, we are in a position to deliver therapy that can meet considerable unmet medical need in millions of patients across the country. We are dedicated to bringing hope to life and I thank our team and shareholders for their ongoing hard work and support."
Second Quarter Clinical, Regulatory and Corporate Highlights
Funding for FDA filings of NRX-100 and NRX-101
The Company has executed a Convertible Debt instrument with Anson Funds of Toronto for $10.8 - $16.3 million in funding designed to retire existing debt and to support FDA New Drug filings of NRX-100 and NRX-101 in the fourth quarter of 2024. Terms have been disclosed in 8K filings but are at an interest rate and conversion rate substantially lower than current corporate indebtedness. The new funding has no provision for "extraordinary redemptions" triggered by appreciation in NRx share price.
Retirement of current debt and settlement of litigation
Concurrent with the Anson investment, the Company has settled its outstanding litigation with Streeterville Capital, LLC at a substantial discount to the amounts claimed in litigation.
Progress towards an NDA for NRX-100 (IV ketamine) in the treatment of suicidal depression
Intravenous ketamine has now become a standard of care for acute treatment of suicidal depression, in the absence of an FDA-labeled product. Intranasal Esketamine is approved by the FDA (SPRAVATO®) but has not demonstrated a benefit on suicidality and is not approved for use in patients with bipolar depression. Attempts to use intranasal racemic ketamine for suicidal depression have failed.
The Company has formed data-sharing partnerships to license clinical trial data from a French Government-funded trial and two NIH-funded trials all of which demonstrate efficacy of racemic Intravenous ketamine against depression and two of which demonstrate statistically significant benefit vs suicidality. The Company's role is to reformat these data into the required presentation required for review by the FDA.
In contrast to nasal ketamine, Intravenous racemic ketamine demonstrates dramatic and immediate reduction of suicidality in patients with both Major Depressive Disorder and Bipolar Depression. Grunebaum and colleagues demonstrated a rapid and statistically significant reduction in Suicidal Ideation (SSI) at day 1 (p=0.0003) and in depression (P=0.0234), as measured by the Profile of Mood States (POMS) among patients randomized to IV Ketamine compared to those randomized to midazolam. This trial was published in the American Journal of Psychiatry Grunebaum, et.al.2. Abbar and colleagues similarly published 84% remission from suicidality on the Columbia Suicide Severity Rating Scale (C-SSRS) in patients treated with ketamine, vs. 28% in those treated with placebo (P<.0001). This trial was published in the British Medical Journal3. Data are expected to be transmitted to FDA by in 2024.
In November 2023, the Company initiated manufacture of ketamine together with Nephron Pharmaceuticals, Inc. (West Columbia, SC) to develop a single patient presentation of ketamine. Nine months of real-time stability is ongoing, the minimum stability time required for a New Drug Application. This presentation of ketamine does not contain the preservative included in multi-dose vials, (Benzanthonium Chloride), that is designed to preserve sterility in the vial when multiple doses are drawn for multiple patients. The Company is not aware of any data to support the safety of this preservative for repeated IV administration. Data were collected 20 years ago that demonstrated the toxic effect of this class of preservatives when applied repeatedly to the surface of the eye, which led to the current generation of preservative-free eye drops. NRX-100 will therefore be launched as a preservative-free presentation.
A long-term challenge with ketamine is that the current formulation (KETALAR®) is highly acidic. While it is suitable for intravenous use, it cannot be administered subcutaneously. In March 2024 the Company demonstrated the formulation of a pH neutral patentable form of IV ketamine that it anticipates will have widespread applicability both in treatment of depression and chronic pain.
Development of NRX-101 for Suicidal Treatment-Resistant Suicidal Bipolar Depression
The Company presented final data from the recently completed phase 2b/3 trial of NRX-101 in suicidal bipolar depression4 at the American Society of Clinical Psychopharmacology's annual meeting. These data demonstrated a significantly improved safety profile versus the standard of care, as demonstrated by a clinically significant reduction in akathisia (P=0.025) and time to sustained remission from suicidality (P=0.05). Akathisia is an adverse event seen with antidepressant medications considered by many experts to be a precursor to suicide. Given the vital need for safer medications in this at-risk population, we plan to submit an NDA for Accelerated Approval to the US FDA for treatment of bipolar depression patients with suicidality or akathisia, based on these data as well as additional data from our STABIL-B5 trial.
Incorporation of HOPE Therapeutics
In February we first presented the contours of HOPE Therapeutics, a subsidiary that will focus on the delivery of advance psychiatric treatments, including ketamine-focused treatment for depression and suicidality. Unlike the core business of NRx Pharmaceuticals, that is focused on biotechnology Research and Development, HOPE is organized around consolidating existing best-in-class clinics into a nationwide network. This has been done previously and without much success with clinics that are not necessarily psychiatrist-led. As currently designed, the HOPE consolidation is likely to be funded as a separate entity, through bond offerings and, thus, to be non-dilutive to NRx shareholders. Over the past quarter, HOPE leadership has identified the clinics that are most likely to participate in the first $100 million consolidation, has completed the audit required for a public listing of HOPE shares, and has identified appropriate underwriters for a future bond offering.
With Hope ownership as an asset of NRx, this will further strengthen the NRx balance sheet and aims to further enhance NRx shareholder value.
NRX-101 for Treatment of Chronic Pain:
In 2023, the Company licensed US Patent 8,653,120 for the use of DCS in chronic pain and filed a now-accepted Investigational New Drug (IND) application with the FDA to initiate commercial drug development of NRX-101 in chronic pain. Data lock has now been achieved in a 200-person randomized prospective trial funded by the US DOD (NCT 03535688) in which patients with chronic pain were randomly assigned to DCS 400mg/day vs. placebo. Should these results support efficacy of DCS in the treatment of chronic low back pain, they are expected to provide a Breakthrough Therapy path towards treatment of chronic pain with DCS and DCS-containing medicines.
Treatment of Urinary Tract Infection (UTI) and Urosepsis:
Although treatment of UTI is quite different from use of NRX-101 to treat Central Nervous System disorders, D-cycloserine was originally developed as an antibiotic because of its role in disrupting the cell wall of certain pathogens. During Q3 2023, NRx tested NRX-101 and its components against resistant pathogens that appear on the Congressionally mandated Qualified Infectious Disease Product (QIDP) list and proved in vitro effectiveness against antibiotic-resistant E. coli, Pseudomonas, and Acinetobacter. Accordingly, NRx was granted QIDP designation, Fast Track Designation, and Priority Review by the US FDA in January 2024.
In recent years, increased antibiotic resistance to common pathogens that cause urinary tract infections and urosepsis (i.e., sepsis originating in the urinary tract) has resulted in a marked increase in cUTI, hospitalization, and death from urosepsis. The US Center for Disease Control and Prevention reports that more than 1.7 million Americans contract sepsis each year, of whom at least 350,000 die during their hospitalization or are discharged to hospice (CDC Sepsis Ref.)6. There are approximately 3 million patients per year who contract cUTI in the US annually (Lodise, et. al.)7. Additionally, should NRX-101 succeed in clinical trials, the Company will consider developing a follow-on product that is anticipated to achieve another 20 years of patent exclusivity.
A key challenge in the treatment of cUTI is the tendency of advanced antibiotics to cause C. difficile infection, which is fatal in 10% of those who contract it over the age of 65 and results in prolonged hospitalization in many more. The Company recently announced data demonstrating that NRX-101 does not compromise the intestinal microbiome, unlike common antibiotics including Clindamycin and Ciprofloxacin. Should these findings be documented in human patients, NRX-101 would represent the only treatment for cUTI that does not cause C. Difficile infection.
In The Company does not anticipate funding this initiative with core NRx assets and is exploring structures for partnership opportunities. Should the Company or its partners succeed in serving 10% of the cUTI market, the Company believes that the revenue from NRX-101 has the potential to be hundreds of million annually, based on 3 million cases per year in the US and potential pricing of over $3,500/course of therapy.
Financial Results for the Quarter and Year to Date 2024
For the three months ended June 30, 2024, we at NRx Pharmaceuticals reduced our net loss from $8.7 million in the second quarter of 2023 to $7.9 million in 2024, representing nearly a 10% improvement year over year. For that same period, we reduced research and development expenses from $3.9 million in 2023 to $2.8 million in 2024. The $1.1 million decrease is related primarily to a decrease of $2.4 million in clinical trial and development expenses, offset by an increase of $1.3m related to the Alvogen warrants. Also in that 3 month period we recorded an increase in general and administrative expenses, from $4.1 million in 2023 to $4.2 million in 2024. The increase of $0.1 million is related primarily to an increase in consultants and legal fees partially offset by lower insurance expenses.
For the six months ended June 30, 2024, NRx Pharmaceuticals reduced its net loss to $14.4 million compared to $19.8 million in the prior year. These efficiencies represent an improvement in net loss of $5.4 million year over and a $1.32, or 47%, improvement in net loss per share year over year. Over that six-month period we recorded $4.6 million of research and development expenses compared to $7.5 million for the same period in 2023 representing a 39% decrease year over year. The decrease of $2.9 million is related primarily to a decrease of $4.1 million in clinical trial and development expenses, $0.3 million related to fees paid to regulatory and process development consultants while offset by $1.3 million related to the Alvogen warrants and $0.4 million related to fees paid to regulatory and development consultants. Also in that six-month period, we decreased G&A by $1.4 million, from $9.9 million in 2023 to $8.5 million in 2024, nearly a 15% decrease year over year.
As of June 30, 2024, we had $1.9 million in cash and cash equivalents. As previously stated, we recently announced we secured up to $16.3 Million Senior Secured Debt Financing from Anson Funds. This financing is expected to support 2024 filing of New Drug Applications for NRX-100 (ketamine) and NRX-101 and to support launch of HOPE Therapeutics as well as retire historical debt with more favorable terms, and a lower annual interest rate.
NRx continues to implement operational efficiencies to extend cash runway and maintain focus on our path to generating revenue and value for our shareholders.
Please see detailed financials on our Form 10-Q, filed with the SEC and available on our website.
Conference Call and Webcast Details
A live webcast of the conference call will be available on the Company's website at 4:30 p.m. ET today, at https://ir.nrxpharma.com/events. An archive of the webcast will be available on the Company's website for 30 days. Participants that are unable to join the webcast can access the conference call via telephone by dialing domestically 1-800-717-1738 or internationally 1-646-307-1865.
About NRx Pharmaceuticals, Inc.
NRx Pharmaceuticals is a clinical-stage biopharmaceutical company developing therapeutics based on its NMDA platform for the treatment of central nervous system disorders, specifically suicidal bipolar depression, chronic pain, and PTSD. The Company is developing NRX-101, an FDA-designated investigational Breakthrough Therapy for suicidal treatment-resistant bipolar depression and chronic pain. NRx plans to file an NDA for Accelerated Approval for NRX-101 in patients with bipolar depression and suicidality or akathisia. NRX-101 additionally has potential to act as a non-opioid treatment for chronic pain, as well as a treatment for complicated UTI.
NRx has recently announced plans to submit a New Drug Application for NRX-100 (IV ketamine) for the treatment of suicidal depression, based on results of well-controlled clinical trials conducted under the auspices of the US National Institutes of Health and newly obtained data from French health authorities, licensed under a data sharing agreement. NRx was awarded Fast Track Designation for development of ketamine (NRX-100) by the US FDA as part of a protocol to treat patients with acute suicidality.
About HOPE Therapeutics, Inc.
HOPE Therapeutics, Inc. (http://www.hopetherapeutics.com) is a care delivery company developing a best-in-class network of clinics that currently offer ketamine and other lifesaving therapies to patients with suicidal depression and related disorders, together with a digital therapeutic-enabled platform designed to augment and preserve the clinical benefit of NMDA-targeted drug therapy.
Notice Regarding Forward-Looking Statements
The information contained herein includes forward-looking statements within the meaning of Section 21E of the Securities Exchange Act of 1934, as amended, and Section 27A of the Securities Act of 1933, as amended. These statements include, among others, statements regarding the proposed public offering and the timing and the use of the proceeds from the offering. Forward-looking statements generally include statements that are predictive in nature and depend upon or refer to future events or conditions, and include words such as "may," "will," "should," "would," "expect," "plan," "believe," "intend," "look forward," and other similar expressions among others. These statements relate to future events or to the Company's future financial performance, and involve known and unknown risks, uncertainties and other factors that may cause the Company's actual results to be materially different from any future results, levels of activity, performance or achievements expressed or implied by these forward-looking statements. You should not place undue reliance on forward-looking statements since they involve known and unknown risks, uncertainties and other factors which are, in some cases, beyond the Company's control and which could, and likely will, materially affect actual results, levels of activity, performance or achievements. Any forward-looking statement reflects the Company's current views with respect to future events and is subject to these and other risks, uncertainties and assumptions relating to the Company's operations, results of operations, growth strategy and liquidity. More detailed information about the Company and the risk factors that may affect the realization of forward-looking statements is set forth in the Company's most recent Annual Report on Form 10-K and other filings with the Securities and Exchange Commission. Investors and security holders are urged to read these documents free of charge on the SEC's website at http://www.sec.gov. Except as may be required by applicable law, The Company assumes no obligation to publicly update or revise these forward-looking statements for any reason, or to update the reasons actual results could differ materially from those anticipated in these forward-looking statements, whether as a result of new information, future events or otherwise.
1 Pan, Y., Gorenflo, M.P., Davis, P.B. et al. Suicidal ideation following ketamine prescription in patients with recurrent major depressive disorder: a nation-wide cohort study. Transl Psychiatry 14, 327 (2024). https://doi.org/10.1038/s41398-024-03033-4
2 Grunebaum, et. al., Ketamine for Rapid Reduction of Suicidal Thoughts. Am J Psychiatry. 2018 Apr 1: 175(4): 327-335
3 Abbar, et. al. Ketamine for Acute Treatment of Severe Suicidal Ideation, BMJ 2022; 376
4 Nierenberg, et. al., A Randomized, Double-Blind Controlled Comparison of NRX-101 (D-cycloserine/ lurasidone) to Lurasidone for Adults with Bipolar Depression and Subacute Suicidal Ideation or Behavior. Am Soc Clin Psych Annual Meeting 2024.
5 Nierenberg et al. International Journal of Bipolar Disorders (2023) 11:28 https://doi.org/10.1186/s40345-023-00
6 https://www.cdc.gov/sepsis/what-is-sepsis.html
7 Open Forum Infectious Diseases, Volume 9, Issue 7, July 2022, ofac315, https://doi.org/10.1093/ofid/ofac315
https://c212.net/c/img/favicon.png?sn=CL84005&sd=2024-08-14 View original content to download multimedia:https://www.prnewswire.com/news-releases/nrx-pharmaceuticals-nasdaqnrxp-reports-second-quarter-and-year-to-date-2024-financial-results-and-provides-business-update-302222664.html
SOURCE NRx Pharmaceuticals, Inc.
$NRXP $1.85 HOD $1.92 on NEWS! NRx Pharmaceuticals, Inc. (NASDAQ: NRXP) to Report Second Quarter and Year to Date 2024 Financial Results
https://ir.nrxpharma.com/2024-08-13-NRx-Pharmaceuticals,-Inc-NASDAQ-NRXP-to-Report-Second-Quarter-and-Year-to-Date-2024-Financial-Results-on-August-14,-2024
#NRxPharmaceuticals #NRxPharma #PharmaNews #NRX100 #NRX101 $NRXP
NRx Pharmaceuticals, Inc. (NASDAQ: NRXP) to Report Second Quarter and Year to Date 2024 Financial Results on August 14, 2024
RADNOR, Pa., Aug. 13, 2024 /PRNewswire/ -- NRx Pharmaceuticals, Inc. (Nasdaq: NRXP) ("NRx Pharmaceuticals", the "Company"), a clinical-stage biopharmaceutical company, today announced that it will release its second quarter and year to date 2024 financial results after the market closes on Wednesday, August 14, 2024, via press release, which will be available on the Company's website at https://ir.nrxpharma.com/. The Company will host a conference call to discuss the financial results as well as provide a corporate update at 4:30pm ET the same day.
A live webcast of the conference call will be available on the Company's website at https://ir.nrxpharma.com/events . Participants that are unable to join the webcast can access the conference call via telephone by dialing domestically +1-800-717-1738 or internationally +1-646-307-1865.
About NRx Pharmaceuticals, Inc.
NRx Pharmaceuticals is a clinical-stage biopharmaceutical company developing therapeutics based on its NMDA platform for the treatment of central nervous system disorders, specifically suicidal bipolar depression, chronic pain, and PTSD. The Company is developing NRX-101, an FDA-designated investigational Breakthrough Therapy for suicidal treatment-resistant bipolar depression and chronic pain. NRx plans to file an NDA for Accelerated Approval for NRX-101 in patients with bipolar depression and suicidality or akathisia. NRX-101 additionally has potential to act as a non-opioid treatment for chronic pain, as well as a treatment for complicated UTI.
NRx has recently announced plans to submit a New Drug Application for NRX-100 (IV ketamine) for the treatment of suicidal depression, based on results of well-controlled clinical trials conducted under the auspices of the US National Institutes of Health and newly obtained data from French health authorities, licensed under a data sharing agreement. NRx was awarded Fast Track Designation for development of ketamine (NRX-100) by the US FDA as part of a protocol to treat patients with acute suicidality.
About HOPE Therapeutics, Inc.
HOPE Therapeutics, Inc. (www.hopetherapeutics.com) is a care delivery company developing a best-in-class network of clinics that currently offer ketamine and other lifesaving therapies to patients with suicidal depression and related disorders, together with a digital therapeutic-enabled platform designed to augment and preserve the clinical benefit of NMDA-targeted drug therapy.
https://c212.net/c/img/favicon.png?sn=CL82891&sd=2024-08-13 View original content to download multimedia:https://www.prnewswire.com/news-releases/nrx-pharmaceuticals-inc-nasdaq-nrxp-to-report-second-quarter-and-year-to-date-2024-financial-results-on-august-14-2024-302221376.html
SOURCE NRx Pharmaceuticals, Inc.
$NRXP News: NRx Pharmaceuticals, Inc. (NASDAQ:NRXP) Announces Up to $16 Million Senior Secured Debt Financing from Anson Funds
This financing is expected to support 2024 filing of New Drug Applications forNRX-100 (ketamine) and NRX-101 and to support launch of HOPE Therapeutics
Streeterville Capital has agreed to a settlement of all claims to be paid from the proceeds of this financing
RADNOR, Pa., Aug. 13, 2024 /PRNewswire/ -- NRx Pharmaceuticals, Inc. (Nasdaq: NRXP) ("NRx Pharmaceuticals" or the "Company"), a clinical-stage biopharmaceutical company, today announced that it has obtained up to approximately $16 million in convertible debt financing (the "Financing") from an institutional investor. The Company intends to use the net proceeds from the Financing to support the 2024 New Drug Application filing its two lead products, NRX-100 and NRX-101 and to retire existing debt from Streeterville Capital. Concurrent with this financing, Streeterville has agreed to stay its arbitration and to release all claims upon receipt of the agreed settlement funds. The interest rate and other costs of capital are substantially lower than prior debt.
"We are thrilled to have attracted a forward-looking science-focused investor to our company. The proceeds from their investment will support our FDA filing of two lifesaving drugs that address critical unmet medical needs for patients with suicidal depression. In the process, we are retiring problematic debt from our balance sheet on favorable terms, and at a lower annual interest rate," said Jonathan Javitt, MD, MPH, Chairman and Chief Scientist of NRx Pharmaceuticals. "We are pleased to welcome Anson Funds to NRx Pharmaceuticals and delighted that they have chosen to share our mission of bringing Hope to Life."
"NRx Pharmaceuticals has a promising pipeline with potential to transform the lives of patients and their loved ones. We are pleased to be working with the NRx Pharmaceuticals team to support these drugs in their registration and – hopefully – approval phase," said Amin Nathoo, Principal, Anson Funds.
The Notes have an interest rate of 6% per annum with a term of 15 months and will be convertible into shares of the Company's common stock. The investors will receive warrant coverage equivalent to 50% of their investment, exercisable into shares of the Company's common stock. The conversion price of the Notes and the exercise price of the Warrants will be each subject to customary adjustments and adjustments for certain corporate transactions, and the issuance of shares of Common Stock underlying the Notes and Warrants will be subject to stockholder approval as required by the listing rules of the Nasdaq Capital Market. The Company will file a Current Report on Form 8-K with the U.S. Securities and Exchange Commission, which will describe the terms of the Notes and Warrants, and include copies of transaction documents relating to the Notes, the Warrants and the Financing.
EF Hutton LLC acted as the exclusive placement agent for the Financing.
The Notes and Warrants have not been registered under the Securities Act of 1933, as amended, and may not be resold in the United States except pursuant to an effective registration statement with the Securities and Exchange Commission or an exemption from registration under the Securities Act and any applicable state securities laws.
This press release does not constitute an offer to sell or the solicitation of an offer to buy, nor will there be any sales of these securities in any jurisdiction in which such offer, solicitation or sale would be unlawful prior to registration or qualification under the securities laws of such jurisdiction.
About NRx Pharmaceuticals, Inc.
NRx Pharmaceuticals is a clinical-stage biopharmaceutical company developing therapeutics based on its NMDA platform for the treatment of central nervous system disorders, specifically suicidal bipolar depression, chronic pain, and PTSD. The Company is developing NRX-101, an FDA-designated investigational Breakthrough Therapy for suicidal treatment-resistant bipolar depression and chronic pain. NRx plans to file an NDA for Accelerated Approval for NRX-101 in patients with bipolar depression and suicidality or akathisia. NRX-101 additionally has potential to act as a non-opioid treatment for chronic pain, as well as a treatment for complicated UTI.
NRx Pharmaceuticals has recently announced plans to submit a New Drug Application for NRX-100 (IV ketamine) for the treatment of suicidal depression, based on results of well-controlled clinical trials conducted under the auspices of the US National Institutes of Health and newly obtained data from French health authorities, licensed under a data sharing agreement. NRx Pharmaceuticals was awarded Fast Track Designation for development of ketamine (NRX-100) by the US FDA as part of a protocol to treat patients with acute suicidality.
About HOPE Therapeutics, Inc.
HOPE Therapeutics, Inc. (http://www.hopetherapeutics.com) is a care delivery company developing a best-in-class network of clinics that currently offer ketamine and other lifesaving therapies to patients with suicidal depression and related disorders, together with a digital therapeutic-enabled platform designed to augment and preserve the clinical benefit of NMDA-targeted drug therapy.
Notice Regarding Forward-Looking Statements
This press release contains forward-looking statements which involve substantial risks and uncertainties. Forward-looking statements are often identifiable by the words "anticipate," "believe," "continue," "could," "estimate," "expect," "intend," "may," "might," "objective," "ongoing," "plan," "predict," "project," "potential," "should," "will," or "would," or the negative of these terms, or other comparable terminology intended to identify statements about the future. These statements involve known and unknown risks, uncertainties and other factors that may cause the Company's actual results, levels of activity, performance or achievements to be materially different from the information expressed or implied by these forward-looking statements. Although the Company believes that it has a reasonable basis for making each forward-looking statement contained in this press release, the Company cautions that these statements are based on a combination of facts and factors currently known by the Company and its expectations of the future, about which the Company cannot be certain. Forward-looking statements are subject to considerable risks and uncertainties, as well as other factors that may cause the Company's actual results, levels of activity, performance or achievements to be materially different from the information expressed or implied by these forward-looking statements. These risks and uncertainties include, without limitation, risks and uncertainties related to whether or not the Company will be able to raise capital through the sale of its securities; the Company's ability to consummate the offering; the Company's ability to repay the Note; the Company being able to fulfill the conditions for a second closing and a third closing and receive the remainder of the financing amount; the Company being able to receive the consent of its stockholders to the Financing; market conditions; satisfaction of customary closing conditions related to future closings; the Company's ability to maintain adequate liquidity and financing sources; various risks related to the Company's business operations; and other risks and uncertainties, including those described within the section entitled "Risk Factors" in the Company's Annual Report on Form 10-K for the year ended December 31, 2023, and other filings with the Securities and Exchange Commission.
Investors and security holders are urged to read these documents free of charge on the SEC's website at http://www.sec.gov. Except as may be required by applicable law, The Company assumes no obligation to publicly update or revise these forward-looking statements for any reason, or to update the reasons actual results could differ materially from those anticipated in these forward-looking statements, whether as a result of new information, future events or otherwise.
https://c212.net/c/img/favicon.png?sn=CL82597&sd=2024-08-13 View original content to download multimedia:https://www.prnewswire.com/news-releases/nrx-pharmaceuticals-inc-nasdaqnrxp-announces-up-to-16-million-senior-secured-debt-financing-from-anson-funds-302220794.html
SOURCE NRx Pharmaceuticals, Inc.
$NRXP $1.995 Holding strong HOD $2.15 with Target Price Revision; Reiterate BUY with PT Adjusted to $19 by #HCWainwright; Company Planning to File Two NDAs in 2024
Read Report - https://nrxpharma.com/wp-content/uploads/2024/08/735aea4a-b494-44a9-b241-76f07d7428e7.pdfNew analyst report from Vernon Bernardino, NRx Pharmaceuticals Managing Director at H.C. Wainwright & Co., LLC
— NRx Pharmaceuticals (@NRxPharma) August 5, 2024
Read Report - https://t.co/T8n97Qz9mA#PharmaNews #mentalillness #mentalhealth #mentalhealthmatters #depression #ketamine #stress #PTSD #bipolar #NRX100 #NRX101 $NRXP pic.twitter.com/Eu9Zlk0JRi
$NRXP (Nasdaq: NRXP) The Accelerated Approval to Treat Bipolar Depression and Akathisia Could Yield Over $150 in Revenue Per Share; Planned HOPE Subsidiary Spinoff to Continue: NRx Pharmaceuticals, Inc.
@Benzinga https://benzinga.com/pressreleases/24/07/ab40021653/accelerated-approval-to-treat-bipolar-depression-and-akathisia-could-yield-over-150-in-revenue-pe
$NRXP -NRx Pharmaceuticals (NASDAQ:NRXP) Publishes Shareholder Update Letter https://ir.nrxpharma.com/index.php?s=43&item=243 @Nasdaq @NRxPharma #revenues #profits #shareholder #updates #2025
$NRXP $2.30 +6.98% NRx Pharmaceuticals is focused on the high unmet need for lifesaving treatments for people with severe bipolar depression and PTSD in the presence of suicidality.
Follow our journey.
http://NRxPharma.com
#ketamine #PTSD #bipolar #NRX100 #NRX101 $NRXP
$NRXP Accelerated Approval to Treat Bipolar Depression and Akathisia Could Yield Over $150 in Revenue Per Share; Planned HOPE Subsidiary Spinoff to Continue: NRx Pharmaceuticals, Inc. (Nasdaq: NRXP)
@Benzinga https://benzinga.com/pressreleases/24/07/ab40021653/accelerated-approval-to-treat-bipolar-depression-and-akathisia-could-yield-over-150-in-revenue-pe
#PharmaNews #NRX100 #NRX101 $NRXP
$NRXP Press out this week. @Nasdaq https://ir.nrxpharma.com/press-releases
$NRXP News Highlights: FDA response highlights the importance of addressing suicidal depression in adolescents age 9-17
Alignment on initial Pediatric Study Plan (iPSP) is a gating requirement for the upcoming filing of an NRX-100 New Drug Application (NDA) for suicidal depression
NRx remains on track to file the NDA for NRX-100 in Q4 2024 with anticipated PDUFA date in Q2 2025
https://ir.nrxpharma.com/2024-07-29-HOPE-Therapeutics,-Inc-and-NRx-Pharmaceuticals,-Inc-Nasdaq-NRXP-Announce-Alignment-with-FDA-on-Pediatric-Study-Plan-for-NRX-100-ketamine
#PharmaNews #mentalillness #mentalhealth #depression #ketamine #stress #PTSD #bipolar #NRX100 #NRX101 $NRXP
$NRXP Great news today. Thanks!
$NRXP News: HOPE Therapeutics, Inc. and NRx Pharmaceuticals, Inc. (Nasdaq:NRXP) Announce Alignment with FDA on Pediatric Study Plan for NRX-100 (ketamine)
FDA response highlights the importance of addressing suicidal depression in adolescents age 9-17
Alignment on initial Pediatric Study Plan (iPSP) is a gating requirement for the upcoming filing of an NRX-100 New Drug Application (NDA) for suicidal depression
NRx remains on track to file the NDA for NRX-100 in Q4 2024 with anticipated PDUFA date in Q2 2025
RADNOR, Pa., July 29, 2024 /PRNewswire/ -- NRx Pharmaceuticals, Inc. (Nasdaq: NRXP) ("NRx Pharmaceuticals", the "Company"), a clinical stage pharmaceutical company, today announced a communication from the US Food and Drug Administration (FDA) providing feedback and alignment on NRx's proposed initial Pediatric Study Plan (iPSP) for NRX-100 (ketamine) in the treatment of suicidal depression. Congress required the submission of an iPSP as a precondition to filing a New Drug Application in the 2012 Food and Drug Administration Safety and Innovation Act (FDASIA).[1]
Suicide is a growing crisis among adolescents in the United States. According to the US Centers for Disease Control, 10% of high school students attempted suicide in the past year and 22% of high school students reported having seriously considered suicide. This percentage is highest among females (30%), American Indians/Alaska Natives (27%), and lesbian, gay, or bisexual teens (45%) (CDC, 2023). [2]
In support of its upcoming NDA filing, NRx will be submitting existing data supporting the safety and efficacy of ketamine to treat suicidal depression in adults. FDA has now documented its recognition that suicide is a serious and growing public health concern in adolescents as well. Based on the guidance received, NRx and HOPE Therapeutics will commit to conducting a clinical trial of NRX-100 in adolescents aged 9-17 with suicidal depression, but will not be required to study the effects of NRX-100 in younger age groups, following initial approval of NRX-100 in adults. Additional neurotoxicity studies will be conducted in juvenile animal subjects to support the safety of intravenous ketamine in this younger population.
"Youth suicide has reached crisis proportions in the United States with a 62% increase over the past two decades, disproportionately affecting minorities.[3] We appreciate FDA's recognition of the urgent unmet medical need related to suicidal depression in adolescents and look forward to expanding the mission of NRx and HOPE Therapeutics to serve America's youth in preventing needless deaths from suicidal depression, said Prof. Jonathan Javitt, Chairman of NRx Pharmaceuticals and Co-CEO of HOPE Therapeutics."
About NRx Pharmaceuticals, Inc.
NRx Pharmaceuticals is a clinical-stage biopharmaceutical company developing therapeutics based on its NMDA platform for the treatment of central nervous system disorders, specifically suicidal bipolar depression, chronic pain, and PTSD. The Company is developing NRX-101, an FDA-designated investigational Breakthrough Therapy for suicidal treatment-resistant bipolar depression and chronic pain. NRx plans to file an NDA for Accelerated Approval for NRX-101 in patients with bipolar depression and suicidality or akathisia. NRX-101 additionally has potential to act as a non-opioid treatment for chronic pain, as well as a treatment for complicated UTI.
NRx has recently announced plans to submit a New Drug Application for NRX-100 (IV ketamine) for the treatment of suicidal depression, based on results of well-controlled clinical trials conducted under the auspices of the US National Institutes of Health and newly obtained data from French health authorities, licensed under a data sharing agreement. NRx was awarded Fast Track Designation for development of ketamine (NRX-100) by the US FDA as part of a protocol to treat patients with acute suicidality.
About HOPE Therapeutics, Inc.
HOPE Therapeutics, Inc. (www.hopetherapeutics.com) is a care delivery company developing a best-in-class network of clinics that currently offer ketamine and other lifesaving therapies to patients with suicidal depression and related disorders, together with a digital therapeutic-enabled platform designed to augment and preserve the clinical benefit of NMDA-targeted drug therapy.
Notice Regarding Forward-Looking Statements
The information contained herein includes forward-looking statements within the meaning of Section 21E of the Securities Exchange Act of 1934, as amended, and Section 27A of the Securities Act of 1933, as amended. These statements include, among others, statements regarding the proposed public offering and the timing and the use of the proceeds from the offering. Forward-looking statements generally include statements that are predictive in nature and depend upon or refer to future events or conditions, and include words such as "may," "will," "should," "would," "expect," "plan," "believe," "intend," "look forward," and other similar expressions among others. These statements relate to future events or to the Company's future financial performance, and involve known and unknown risks, uncertainties and other factors that may cause the Company's actual results to be materially different from any future results, levels of activity, performance or achievements expressed or implied by these forward-looking statements. You should not place undue reliance on forward-looking statements since they involve known and unknown risks, uncertainties and other factors which are, in some cases, beyond the Company's control and which could, and likely will, materially affect actual results, levels of activity, performance or achievements. Any forward-looking statement reflects the Company's current views with respect to future events and is subject to these and other risks, uncertainties and assumptions relating to the Company's operations, results of operations, growth strategy and liquidity. More detailed information about the Company and the risk factors that may affect the realization of forward-looking statements is set forth in the Company's most recent Annual Report on Form 10-K and other filings with the Securities and Exchange Commission. Investors and security holders are urged to read these documents free of charge on the SEC's website at http://www.sec.gov. Except as may be required by applicable law, The Company assumes no obligation to publicly update or revise these forward-looking statements for any reason, or to update the reasons actual results could differ materially from those anticipated in these forward-looking statements, whether as a result of new information, future events or otherwise.
1 https://www.hhs.gov/guidance/sites/default/files/hhs-guidance-documents/Pediatric-Study-Plans--Content-of-and-Process-for-Submitting-Initial-Pediatric-Study-Plans-and-Amended-Pediatric-Study-Plans.pdf
2 https://www.cdc.gov/healthyyouth/data/yrbs/pdf/YRBS_Data-Summary-Trends_Report2023_508.pdf
3 https://www.apa.org/monitor/2023/07/psychologists-preventing-teen-suicide
https://c212.net/c/img/favicon.png?sn=CL71115&sd=2024-07-29 View original content to download multimedia:https://www.prnewswire.com/news-releases/hope-therapeutics-inc-and-nrx-pharmaceuticals-inc-nasdaqnrxp-announce-alignment-with-fda-on-pediatric-study-plan-for-nrx-100-ketamine-302208338.html
SOURCE NRx Pharmaceuticals, Inc.
$NRXP -Accelerated Approval to Treat Bipolar Depression and Akathisia Could Yield Over $150 in Revenue Per Share; Planned HOPE Subsidiary Spinoff: NRx Pharmaceuticals, Inc. (Nasdaq: NRXP)
https://icont.ac/4ZxIW
Really good news today for NRXP shareholders today. The planned spin-off dividend for the company's HOPE subsidiary can now move ahead.
From the news:
"As we have previously shared with the public, HOPE Therapeutics is in the process of developing a best-in-class network of clinics that currently offer ketamine and other lifesaving therapies to patients with suicidal depression. This arbitration decision enables us to keep our promise to shareholders to spin out 49% of HOPE Therapeutics shares held by NRx to our shareholders, subject to approval by our Board of Directors and compliance with applicable law. We appreciate the support and loyalty of our shareholders as we work to bring HOPE to life," said Prof. Jonathan Javitt, Founder and Chairman of NRx Pharmaceuticals and Co-CEO of HOPE Therapeutics.
$NRXP Download the July 2024 corporate presentation for @NRxPharma https://www.nrxpharma.com/wp-content/uploads/NRx-Corporate-Presentation.pdf
#Depression #SuicidePrevention #FDA @Nasdaq
NEWS: HOPE Therapeutics, Inc. and NRx Pharmaceuticals (Nasdaq:NRXP) Announce Arbitration Order Enabling HOPE Therapeutics Spinoff | NASDAQ
PUBLISHED
JUL 29, 2024 8:30AM EDT
Petition for a temporary restraining order brought by Streeterville Capital, LLC seeking injunctive relief to prevent spinoff of 49% of HOPE Therapeutics shares to current NRx Shareholders has been denied by Utah arbitrator.
Petition by Streeterville Capital, LLC seeking injunctive relief to prevent sales of NRx shares has been denied by Utah arbitrator.
NRx to proceed with spinoff of HOPE Therapeutics as previously announced.
RADNOR, Pa., July 29, 2024 /PRNewswire/ -- NRx Pharmaceuticals, Inc. (Nasdaq: NRXP) ("NRx Pharmaceuticals", the "Company"), a clinical stage pharmaceutical company, today announced the issuance of an order by a Utah arbitrator denying the petition of Streeterville Capital, LLC to enjoin NRx's planned spinoff of 49% of the shares in HOPE Therapeutics to current shareholders of NRx pharmaceuticals. While the proposed spinoff remains subject to compliance with certain disclosure and other requirements under the Securities Act of 1933, as amended, and the Securities Exchange Act of 1934, as amended, the spinoff is intended to provide NRx shareholders with an opportunity to participate in the anticipated value created as a result of the spinoff and to enable a potential listing of HOPE Therapeutics (currently a wholly-owned subsidiary of NRx) on a national securities exchange. The arbitrator also denied Streeterville's petition to enjoin NRx from selling additional shares of NRx stock to finance ongoing operations.
(PRNewsfoto/NRx Pharmaceuticals)
"As we have previously shared with the public, HOPE Therapeutics is in the process of developing a best-in-class network of clinics that currently offer ketamine and other lifesaving therapies to patients with suicidal depression. This arbitration decision enables us to keep our promise to shareholders to spin out 49% of HOPE Therapeutics shares held by NRx to our shareholders, subject to approval by our Board of Directors and compliance with applicable law. We appreciate the support and loyalty of our shareholders as we work to bring HOPE to life," said Prof. Jonathan Javitt, Founder and Chairman of NRx Pharmaceuticals and Co-CEO of HOPE Therapeutics.
About NRx Pharmaceuticals
NRx Pharmaceuticals is a clinical-stage biopharmaceutical company developing therapeutics based on its NMDA platform for the treatment of central nervous system disorders, specifically suicidal bipolar depression, chronic pain and PTSD. The Company is developing NRX-101, an FDA-designated investigational Breakthrough Therapy for suicidal treatment-resistant bipolar depression and chronic pain. NRx has partnered with Alvogen and Lotus around the development and marketing of NRX-101 for the treatment of suicidal bipolar depression. NRX-101 additionally has potential to act as a non-opioid treatment for chronic pain, as well as a treatment for complicated UTI.
NRx has recently announced plans to submit a New Drug Application for NRX-100 (IV ketamine) for the treatment of suicidal depression, based on results of well-controlled clinical trials conducted under the auspices of the US National Institutes of Health and newly obtained data from French health authorities, licensed under a data sharing agreement. NRx was awarded Fast Track Designation for development of ketamine (NRX-100) by the US FDA as part of a protocol to treat patients with acute suicidality.
About HOPE Therapeutics, Inc.
HOPE Therapeutics, Inc. (www.hopetherapeutics.com) is a care delivery company developing a best-in-class network of clinics that currently offer ketamine and other lifesaving therapies to patients with suicidal depression, together with a digital therapeutic-enabled platform designed to augment and preserve the clinical benefit of NMDA-targeted drug therapy.
Notice Regarding Forward-Looking Statements
The information contained herein includes forward-looking statements within the meaning of Section 21E of the Securities Exchange Act of 1934, as amended, and Section 27A of the Securities Act of 1933, as amended. These statements include, among others, statements regarding the proposed public offering and the timing and the use of the proceeds from the offering. Forward-looking statements generally include statements that are predictive in nature and depend upon or refer to future events or conditions, and include words such as "may," "will," "should," "would," "expect," "plan," "believe," "intend," "look forward," and other similar expressions among others. These statements relate to future events or to the Company's future financial performance, and involve known and unknown risks, uncertainties and other factors that may cause the Company's actual results to be materially different from any future results, levels of activity, performance or achievements expressed or implied by these forward-looking statements. You should not place undue reliance on forward-looking statements since they involve known and unknown risks, uncertainties and other factors which are, in some cases, beyond the Company's control and which could, and likely will, materially affect actual results, levels of activity, performance or achievements. Any forward-looking statement reflects the Company's current views with respect to future events and is subject to these and other risks, uncertainties and assumptions relating to the Company's operations, results of operations, growth strategy and liquidity. More detailed information about the Company and the risk factors that may affect the realization of forward-looking statements is set forth in the Company's most recent Annual Report on Form 10-K and other filings with the Securities and Exchange Commission. Investors and security holders are urged to read these documents free of charge on the SEC's website at http://www.sec.gov. Except as may be required by applicable law, The Company assumes no obligation to publicly update or revise these forward-looking statements for any reason, or to update the reasons actual results could differ materially from those anticipated in these forward-looking statements, whether as a result of new information, future events or otherwise.
HOPE Therapeutics, Inc. (PRNewsfoto/NRx Pharmaceuticals, Inc.)
Cision View original content to download multimedia:https://www.prnewswire.com/news-releases/hope-therapeutics-inc-and-nrx-pharmaceuticals-nasdaqnrxp-announce-arbitration-order-enabling-hope-therapeutics-spinoff-302208316.html
SOURCE NRx Pharmaceuticals, Inc.
https://www.nasdaq.com/press-release/hope-therapeutics-inc-and-nrx-pharmaceuticals-nasdaq-nrxp-announce-arbitration-order #NRxPharma #News
$NRXP Potential paths to revenue and profitability in 2025; NRx Pharmaceuticals (NASDAQ:NRXP) Publishes Shareholder Update Letter https://ir.nrxpharma.com/index.php?s=43&item=243 @Nasdaq @NRxPharma #revenues #profits #shareholder #updates #2025
$NRXP News: NRx Pharmaceuticals (NASDAQ:NRXP) to Proceed with Two New Drug Applications in 2024; NRX-101 has Been Returned to the Company for Filing
New Drug Application (NDA) for Accelerated Approval planned for NRX-101 in people with bipolar depression and akathisia in 2024, based on two positive trials 1 2 and Breakthrough Therapy Designation. Potential revenue in 2025
NDA for NRX-100 (IV ketamine) in suicidal depression in advanced preparation for submission in 2024; based on four positive trials 3 4 5 6 and Fast Track Designation. Potential revenue in 2025
Gaining these approvals has the potential to yield more than $150 in revenue per NRXP share in the near term, at current share count
Planning for HOPE Therapeutics share distribution progresses; audit nearing completion – critical step towards a public listing
RADNOR, Pa., June 28, 2024 /PRNewswire/ -- NRx Pharmaceuticals, Inc. (Nasdaq: NRXP) ("NRx Pharmaceuticals", the "Company"), a clinical-stage biopharmaceutical company, today announced that advice from regulatory counsel, which includes former senior officials from the Food and Drug Administration, supports filing two New Drug Applications (NDAs) in 2024: an application for Accelerated Approval for NRX-101 to treat bipolar depression in patients with akathisia and an application for approval of NRX-100 (IV ketamine) for treatment of suicidal depression.
(PRNewsfoto/NRx Pharmaceuticals)
(PRNewsfoto/NRx Pharmaceuticals)
While efficacy and safety data are now in hand, filing of the above applications is dependent upon completion of 12-month stability data in manufactured lots as required by FDA regulations.
As disclosed in an 8K filing, NRx will be filing the NRX-101 application without a commercial partner. The addressable market for the accelerated approval indication is such that a compact and efficient salesforce can be constructed by a small company, such as NRx, and current executives at NRx have previously held primary responsibility for launch of similar-sized pharmaceutical assets.
NRx continues work to enable the distribution of shares in Hope Therapeutics. This distribution is dependent upon completion of a public audit and successful review of an SEC Form 10.
"The NRx team has worked diligently since the end of the COVID pandemic to achieve these milestones for our shareholders and most importantly the patients we have always sought to help," said Dr. Jonathan Javitt, Chairman and Chief Scientist of NRx. "Given the near-term market opportunity represented by an accelerated approval process, NRx anticipates a higher potential return to NRx investors associated with an NRx-led initiative than that which would likely be achieved with a large commercial partner."
NRX-101 for Bipolar Depression
NRX-101 is the Company's patented (Composition of Matter), oral combination of the NMDA antagonist D-cycloserine and lurasidone for bipolar depression. Data from two active control clinical trials vs. the standard of care, lurasidone, have shown comparable antidepressant efficacy with clinically important reductions in suicidality and/or akathisia. To the Company's knowledge, no other oral agent has demonstrated such a valuable profile.
Up to 15% of people treated with drugs in lurasidone's class develop akathisia7; this would constitute an estimated $3.7 billion initial market for NRX-101, with no approved medicines for akathisia. This is a population the Company can readily address without a large commercial partner, given the relatively small number of psychiatrists who treat high-risk patients. The broad bipolar market constitutes 7 million people and an opportunity greater than $20 billion per year. With a best-in-class product profile, the Company projects NRX-101 sales in excess of $2 billion.
NRX-101 was awarded Breakthrough Therapy Designation, Fast Track Designation, a Biomarker Letter of Support, and a Special Protocol Agreement by the FDA for treatment of suicidal bipolar depression.
NRX-100 (IV ketamine) for Suicidal Depression
Intravenous ketamine is widely accepted as a standard of care for acute treatment of suicidal depression, in the absence of an FDA-labeled product; the only treatment currently approved by FDA is electroconvulsive therapy (ECT).
According to the CDC, 3.5 million Americans make a plan for suicide each year.8 This represents a $3-5 billion market at expected pricing.
Based on the data in the trials referenced above, the Company's regulatory counsel has encouraged the Company to file an NDA for suicidal depression for NRX-100. This application has been in development and awaits 12-month stability data for filing, which is expected in 2024.
About NRx Pharmaceuticals
NRx Pharmaceuticals is a clinical-stage biopharmaceutical company developing therapeutics based on its NMDA platform for the treatment of central nervous system disorders, specifically suicidal bipolar depression, chronic pain and PTSD. The Company is developing NRX-101, an FDA-designated investigational Breakthrough Therapy for suicidal treatment-resistant bipolar depression and chronic pain. NRX-101 additionally has potential to act as a non-opioid treatment for chronic pain, as well as a treatment for complicated UTI.
NRx has recently announced plans to submit a New Drug Application for NRX-100 (IV ketamine) in the treatment of suicidal depression, based on results of well-controlled clinical trials conducted under the auspices of the US National Institutes of Health and newly obtained data from French health authorities, licensed under a data sharing agreements. NRx was awarded Fast Track Designation for development of ketamine (NRX-100) by the US FDA as part of a protocol to treat patients with acute suicidality.
About HOPE Therapeutics, Inc.
HOPE Therapeutics, Inc. (www.hopetherapeutics.com) is a Specialty Pharmaceutical Company, wholly-owned by NRX Pharmaceuticals focused on development and marketing of an FDA-approved form of intravenous ketamine for the treatment of acute suicidality and depression together with a digital therapeutic-enabled platform designed to augment and preserve the clinical benefit of NMDA-targeted drug therapy.
Notice Regarding Forward-Looking Statements
The information contained herein includes forward-looking statements within the meaning of Section 21E of the Securities Exchange Act of 1934, as amended, and Section 27A of the Securities Act of 1933, as amended. These statements include, among others, statements regarding the proposed public offering and the timing and the use of the proceeds from the offering. Forward-looking statements generally include statements that are predictive in nature and depend upon or refer to future events or conditions, and include words such as "may," "will," "should," "would," "expect," "plan," "believe," "intend," "look forward," and other similar expressions among others. These statements relate to future events or to the Company's future financial performance, and involve known and unknown risks, uncertainties and other factors that may cause the Company's actual results to be materially different from any future results, levels of activity, performance or achievements expressed or implied by these forward-looking statements. You should not place undue reliance on forward-looking statements since they involve known and unknown risks, uncertainties and other factors which are, in some cases, beyond the Company's control and which could, and likely will, materially affect actual results, levels of activity, performance or achievements. Any forward-looking statement reflects the Company's current views with respect to future events and is subject to these and other risks, uncertainties and assumptions relating to the Company's operations, results of operations, growth strategy and liquidity. More detailed information about the Company and the risk factors that may affect the realization of forward-looking statements is set forth in the Company's most recent Annual Report on Form 10-K and other filings with the Securities and Exchange Commission. Investors and security holders are urged to read these documents free of charge on the SEC's website at http://www.sec.gov. Except as may be required by applicable law, The Company assumes no obligation to publicly update or revise these forward-looking statements for any reason, or to update the reasons actual results could differ materially from those anticipated in these forward-looking statements, whether as a result of new information, future events or otherwise.
1 Nierenberg A, Lavin P, Javitt DC, et. al. NRX-101 vs lurasidone for the maintenance of initial stabilization after ketamine in patients with severe bipolar depression with acute suicidal ideation and behavior; a randomized prospective phase 2 trial. Int J Bipolar Dis 2023;11:28-38, STABIL-B
2 Nierenberg, et. al., A Randomized, Double-Blind Controlled Comparison of NRX-101 (D-cycloserine/lurasidone) to Lurasidone for Adults with Bipolar Depression and Subacute Suicidal Ideation or Behavior. Am Soc Clin Psych Annual Meeting 2024. ASCP Poster
3 Fava, M et. al., Double-blind, placebo-controlled, dose-ranging trial of intravenous ketamine as adjunctive therapy in treatment-resistant depression (TRD) Mol Psychiatry. 2020; 25(7): 1592–1603.
4 Grunebaum, et. al., Ketamine for Rapid Reduction of Suicidal Thoughts. Am J Psychiatry. 2018 Apr 1: 175(4): 327-335
5 Abbar, et. al. Ketamine for Acute Treatment of Severe Suicidal Ideation, BMJ 2022; 376
6 Anand, et. al, Ketamine is non-inferior to ECT for non-psychotic treatment resistant depression. NEJM 2023 388(25):2315-2325
7 Chow, C, et. Al., Akathisia and Newer Second-Generation Antipsychotic Drugs: A Review of Current Evidence
Pharmacotherapy 2020;40(6):565–574
8 CDC Suicide Data.
HOPE Therapeutics, Inc. (PRNewsfoto/NRx Pharmaceuticals, Inc.)
HOPE Therapeutics, Inc. (PRNewsfoto/NRx Pharmaceuticals, Inc.)
CisionCision
Cision
View original content to download multimedia:https://www.prnewswire.com/news-releases/nrx-pharmaceuticals-nasdaqnrxp-to-proceed-with-two-new-drug-applications-in-2024-nrx-101-has-been-returned-to-the-company-for-filing-302185390.html
SOURCE NRx Pharmaceuticals, Inc.
$NRXP $2.70 +1.12% NRX Pharmaceuticals is focused on the high unmet need for lifesaving treatments for people with severe bipolar depression and PTSD in the presence of suicidality.
Follow our journey.
http://NRxPharma.com
#ketamine #PTSD #bipolar #NRX100 #NRX101 $NRXP
$NRXP Retweeted: "Heat health awareness is vitally important for individuals living with a mental health condition and their loved ones."
Learn about the impacts and ways to reduce risks: https://bit.ly/4cuvjeO
Heat health awareness is vitally important for individuals living with a mental health condition and their loved ones.
— NAMI (@NAMICommunicate) June 20, 2024
Learn about the impacts and ways to reduce risks: https://t.co/DAzX0cde5r pic.twitter.com/4zbGZ4glWq
$NRXP Another great article on #FactoidFriday #mentalillness #mentalhealthawarenessmonth #mentalhealthmatters #bipolar #NRX101 #NRX100 $NRXP
Learn More - https://nrxpharma.com/nrx-100-nrx-101/#NRX100-101
$NRXP NRxPharma (Nasdaq) holds the only known commercial IND for NRX-100 (IV Ketamine) to treat suicidal depression and is developing NRX-101 (D-Cycloserine & Lurasidone), the first daily, oral treatment for Treatment-Resistant, Suicidal Bipolar Depression
https://compasslivemedia.com/nrxp/
$NRXP news: NRx Pharmaceuticals, a clinical stage pharmaceutical company, today announced that management will participate in the H.C. Wainwright 5th Annual Neuro Perspectives Virtual Conference on June 27, 2024.
Read More - https://ir.nrxpharma.com/2024-06-20-NRx-Pharmaceuticals,-Inc-Nasdaq-NRXP-to-Participate-in-the-H-C-Wainwright-5th-Annual-Neuro-Perspectives-Virtual-Conference-on-June-27,-2024
#PharmaNews #NRX100 #NRX101 $NRXP
NEWS: NRx Pharmaceuticals (NASDAQ:NRXP) Appoints Neuroscience, Information Technology and Medical Technology Veteran to its Board of Directors
PR Newswire
Tue, Jun 18, 2024, 8:30 AM EDT
In this article:NRXP
Dr. Dennis McBride brings extensive experience in Neuroscience and its interface with Information and Medical Technology
Retired at a rank that is the civilian equivalent of a senior flag officer from the United States Navy
RADNOR, Pa., June 18, 2024 /PRNewswire/ -- NRx Pharmaceuticals, Inc. (Nasdaq: NRXP) ("NRx Pharmaceuticals", the "Company"), a clinical-stage biopharmaceutical company, today announced that the Company has added Dr. Dennis McBride to its Board of Directors. Dr. McBride brings deep experience in Neuroscience, Medical and Information Technology and digital therapeutics to the Company.
(PRNewsfoto/NRx Pharmaceuticals)
(PRNewsfoto/NRx Pharmaceuticals)
"I am delighted to welcome Dennis to the Board of NRx. His unique background in both Neuroscience and Medical Technology will be an important asset to the company as we seek to develop more advanced treatments for patients," said Dr. Jonathan Javitt, Chairman and Chief Scientist of NRx. "I have worked with Dennis previously on a highly successful digital therapeutic and know the quality he brings to our company."
"I am proud to join the Board of NRx at such an exciting time for the company – the opportunities have never been greater in Neuroscience to advance and improve novel therapies," commented Dr. McBride. "I look forward to using my experience to help advance its strategy and further the development of NRx's impressive pipeline of CNS products and leading its initiative in digital therapeutics."
Dr. Dennis McBride has led numerous national and international initiatives in neuroscience and its interface with information technology, national security, and medical technology/drug development both within the federal government and in the private sector, three of which are now multi-billion-dollar enterprises. He has formative experience in CNS-focused digital therapeutics, having participated with NRx founders in developing now military-proven digital therapeutic technology for reduction of stress and depression. He was instrumental to the founding of InQTel and other private sector-focused initiatives.
Dr. McBride dedicated his Navy career to Aerospace Medicine and ergonomics, during which he served in leadership roles at six nationally-prominent laboratories, including the Defense Advanced Research Projects Agency (DARPA), Naval Aerospace Medical Research Lab, Naval Research Lab, the Office of Naval Research, and the Naval Medical Research Institute. Upon retiring as a highly decorated Navy Captain, he assumed leadership of the Potomac Institute for Policy Studies, where he continues to serve as President Emeritus. He then joined the National Defense University as a Professor to lead the Center for Technology and National Security Policy, completing his term as a Senior Executive-4 (Civilian equivalent to Vice Admiral). Most recently, he served a tour of duty in the Office of the Secretary of Defense. Dr. McBride has served as an adviser to Cabinet Secretaries, US Congressional Committees, and to corporate C-Suite executives. His educational background includes the University of Georgia, Naval Aerospace Medical Institute, the University of Southern California, the London School of Economics, and Harvard Business School, earning a Ph.D. in experimental psychology and four master's degrees.
About NRx Pharmaceuticals
NRx Pharmaceuticals is a clinical-stage biopharmaceutical company developing therapeutics based on its NMDA platform for the treatment of central nervous system disorders, specifically suicidal bipolar depression, chronic pain and PTSD. The Company is developing NRX-101, an FDA-designated investigational Breakthrough Therapy for suicidal treatment-resistant bipolar depression and chronic pain. NRx has partnered with Alvogen and Lotus around the development and marketing of NRX-101 for the treatment of suicidal bipolar depression. NRX-101 additionally has potential to act as a non-opioid treatment for chronic pain, as well as a treatment for complicated UTI.
NRx has recently announced plans to submit a New Drug Application for HTX-100 (IV ketamine), through Hope Therapeutics, in the treatment of suicidal depression, based on results of well-controlled clinical trials conducted under the auspices of the US National Institutes of Health and newly obtained data from French health authorities, licensed under a data sharing agreement. NRx was awarded Fast Track Designation for development of ketamine (NRX-100) by the US FDA as part of a protocol to treat patients with acute suicidality.
About HOPE Therapeutics, Inc.
HOPE Therapeutics, Inc. (www.hopetherapeutics.com) is a Specialty Pharmaceutical Company, wholly-owned by NRX Pharmaceuticals focused on development and marketing of an FDA-approved form of intravenous ketamine for the treatment of acute suicidality and depression together with a digital therapeutic-enabled platform designed to augment and preserve the clinical benefit of NMDA-targeted drug therapy.
Notice Regarding Forward-Looking Statements
The information contained herein includes forward-looking statements within the meaning of Section 21E of the Securities Exchange Act of 1934, as amended, and Section 27A of the Securities Act of 1933, as amended. These statements include, among others, statements regarding the proposed public offering and the timing and the use of the proceeds from the offering. Forward-looking statements generally include statements that are predictive in nature and depend upon or refer to future events or conditions, and include words such as "may," "will," "should," "would," "expect," "plan," "believe," "intend," "look forward," and other similar expressions among others. These statements relate to future events or to the Company's future financial performance, and involve known and unknown risks, uncertainties and other factors that may cause the Company's actual results to be materially different from any future results, levels of activity, performance or achievements expressed or implied by these forward-looking statements. You should not place undue reliance on forward-looking statements since they involve known and unknown risks, uncertainties and other factors which are, in some cases, beyond the Company's control and which could, and likely will, materially affect actual results, levels of activity, performance or achievements. Any forward-looking statement reflects the Company's current views with respect to future events and is subject to these and other risks, uncertainties and assumptions relating to the Company's operations, results of operations, growth strategy and liquidity. More detailed information about the Company and the risk factors that may affect the realization of forward-looking statements is set forth in the Company's most recent Annual Report on Form 10-K and other filings with the Securities and Exchange Commission. Investors and security holders are urged to read these documents free of charge on the SEC's website at http://www.sec.gov. Except as may be required by applicable law, The Company assumes no obligation to publicly update or revise these forward-looking statements for any reason, or to update the reasons actual results could differ materially from those anticipated in these forward-looking statements, whether as a result of new information, future events or otherwise.
View original content to download multimedia: https://www.prnewswire.com/news-releases/nrx-pharmaceuticals-nasdaqnrxp-appoints-neuroscience-information-technology-and-medical-technology-veteran-to-its-board-of-directors-302175147.html
SOURCE NRx Pharmaceuticals, Inc.
$NRXP Innovative Bipolar Depression Treatment by NRx Pharmaceuticals Featured at ASCP June 2024 Meeting -Healthcare Industry Today
https://healthcareindustrytoday.com/article/718753903-innovative-bipolar-depression-treatment-by-nrx-pharmaceuticals-featured-at-ascp-june-2024-meeting-nasdaq-nrxp
#PharmaNews #mentalillness #mentalhealth #depression #ketamine #stress #PTSD #bipolar #NRX100 #NRX101 $NRXP
$NRXP @NRxPharma NRx Pharmaceuticals reposted American Psychiatric Association
@APApsychiatric as the recent tragic death of Professional golfer Grayson Murray put a spotlight on importance on raising mental health awareness https://t.co/yqnKNKETPQ
$NRXP $3.50 +3.55% NRx Pharmaceuticals, Inc. Highlights Breakthrough Oral Antidepressant’s Efficacy in Reducing Suicidality in Bipolar Depression at ASCP June 2024 Meeting - Boston Herald
https://markets.financialcontent.com/bostonherald/article/getnews-2024-6-10-nrx-pharmaceuticals-highlights-breakthrough-oral-antidepressants-efficacy-in-reducing-suicidality-in-bipolar-depression-at-ascp-june-2024-meeting-nrx-pharmaceuticals-inc-nasdaq-nrxp
#PharmaNews #mentalhealth #depression #suicide #PTSD #bipolar #NRX100 #NRX101 $NRXP
$NRXP NRX Pharmaceuticals is focused on the high unmet need for lifesaving treatments for people with severe bipolar depression and PTSD in the presence of suicidality.
Follow our journey.
http://NRxPharma.com
#ketamine #PTSD #bipolar #NRX100 #NRX101 $NRXP
$NRXP More great news: NRx Pharmaceuticals Showcases First Oral Antidepressant Proven to Reduce Suicidality in Bipolar Depression at ASCP June 2024 Meeting -@APNews
Read More - https://apnews.com/press-release/ein-presswire-newsmatics/pain-management-medication-clinical-trials-d6c73b6690e6e7176e525f0a8c52fbab
#PharmaNews #mentalhealth #depression #ketamine #stress #PTSD #bipolar #NRX100 #NRX101 $NRXP
$NRXP $3.27 +1.87% - NRx Pharmaceuticals reports Q1 results https://seekingalpha.com/news/4106135-nrx-pharmaceuticals-reports-q1-results?source=tweet @NRxPharma
Link to Today's Press Release: https://seekingalpha.com/symbol/NRXP/press-releases
NRx Pharmaceuticals (NASDAQ:NRXP) Publishes Shareholder Update Letter
PR Newswire
Mon, Jun 10, 2024, 8:30 AM EDT
In this article:
NRXP
The June 2024 meeting of the American Society for Clinical Psychopharmacology (ASCP) focused heavily on increasing use of intravenous ketamine and intranasal S-ketamine as the emerging standard of care for treating severe depression and suicidality
Presenters from 3 open label studies at the ASCP suggested that intravenous ketamine is at least equivalent and may have advantages over intranasal S-ketamine
NRx Pharmaceuticals has now reached the 9-month stability point with its ketamine formulation (NRX-100) and has initiated 3 manufacturing lots for future drug release. Nonclinical safety for short term use of NRX-100 has recently been published and submitted to FDA
FDA leadership, in public comments at ASCP, focused on the need for nonclinical safety data for intravenous ketamine as a condition of ketamine approval
The short-term need for intravenous ketamine as an already-approved, schedule 3 drug, is heightened by recent regulatory decisions that may delay the path of potent, schedule 1 psychedelic drugs that may require more complicated clinical trial designs.
RADNOR, Pa., June 10, 2024 /PRNewswire/ -- NRx Pharmaceuticals, Inc. (Nasdaq: NRXP) ("NRx Pharmaceuticals", the "Company"), a clinical-stage biopharmaceutical company, today announced that the Company posted a new Shareholder Update Letter on its website NRx Shareholder Update and further invites interested parties to subscribe to their email alert service to stay up to date on company's progress here: NRx Email Alerts . (Note: not all updates will be included in a Press Release in the future).
Today's update highlights potential implications of the Company's recent activities at the annual meeting of the American Society of Clinical Psychopharmacology. The key points include:
Intravenous and intranasal ketamine were highlighted as emerging standards of care for severe depression and suicidality
Planned NDA filing for NRX-100, our preservative free IV ketamine, for Suicidal Depression in 2024, is based on well controlled trials against both placebo and active comparator. Fast Track Designation was previously granted
An independent FDA advisory panel recently voted against MDMA, a potent, class I psychedelic, refocusing attention on already-approved Schedule 3 drugs such as ketamine for treatment of suicidal depression. The FDA panel and emerging guidance highlights the complexity of clinical trials of DEA Schedule 1 hallucinogens that do not have already-approved human uses
NRx anticipates that an important issue for longer term use of ketamine in depression will be the current multidose vial presentation that contains potentially toxic preservatives previously acceptable for one time use but less suitable for repeated use. NRX-100 is planned as a single-dose, preservative-free medication.
Please subscribe to the Company's email for future updates. NRX Email Alerts Not all of these will be the subject of a Press Release in the future.
About NRx Pharmaceuticals
NRx Pharmaceuticals is a clinical-stage biopharmaceutical company developing therapeutics based on its NMDA platform for the treatment of central nervous system disorders, specifically suicidal bipolar depression, chronic pain and PTSD. The Company is developing NRX-101, an FDA-designated investigational Breakthrough Therapy for suicidal treatment-resistant bipolar depression and chronic pain. NRx has partnered with Alvogen and Lotus around the development and marketing of NRX-101 for the treatment of suicidal bipolar depression. NRX-101 additionally has potential to act as a non-opioid treatment for chronic pain, as well as a treatment for complicated UTI.
NRx has recently announced plans to submit a New Drug Application for NRX-100 (IV ketamine), in the treatment of suicidal depression, based on results of well-controlled clinical trials conducted under the auspices of the US National Institutes of Health and newly obtained data from French health authorities, licensed under a data sharing agreement. NRx was awarded Fast Track Designation for development of ketamine (NRX-100) by the US FDA as part of a protocol to treat patients with acute suicidality.
About HOPE Therapeutics, Inc.
HOPE Therapeutics, Inc. (www.hopetherapeutics.com) is a Specialty Pharmaceutical Company, wholly-owned by NRX Pharmaceuticals, focused on development and marketing of an FDA-approved form of intravenous ketamine for the treatment of acute suicidality and depression, together with a digital therapeutic-enabled platform designed to augment and preserve the clinical benefit of NMDA-targeted drug therapy.
Notice Regarding Forward-Looking Statements
The information contained herein includes forward-looking statements within the meaning of Section 21E of the Securities Exchange Act of 1934, as amended, and Section 27A of the Securities Act of 1933, as amended. These statements include, among others, statements regarding the proposed public offering and the timing and the use of the proceeds from the offering. Forward-looking statements generally include statements that are predictive in nature and depend upon or refer to future events or conditions, and include words such as "may," "will," "should," "would," "expect," "plan," "believe," "intend," "look forward," and other similar expressions among others. These statements relate to future events or to the Company's future financial performance, and involve known and unknown risks, uncertainties and other factors that may cause the Company's actual results to be materially different from any future results, levels of activity, performance or achievements expressed or implied by these forward-looking statements. You should not place undue reliance on forward-looking statements since they involve known and unknown risks, uncertainties and other factors which are, in some cases, beyond the Company's control and which could, and likely will, materially affect actual results, levels of activity, performance or achievements. Any forward-looking statement reflects the Company's current views with respect to future events and is subject to these and other risks, uncertainties and assumptions relating to the Company's operations, results of operations, growth strategy and liquidity. More detailed information about the Company and the risk factors that may affect the realization of forward-looking statements is set forth in the Company's most recent Annual Report on Form 10-K and other filings with the Securities and Exchange Commission. Investors and security holders are urged to read these documents free of charge on the SEC's website at http://www.sec.gov. Except as may be required by applicable law, The Company assumes no obligation to publicly update or revise these forward-looking statements for any reason, or to update the reasons actual results could differ materially from those anticipated in these forward-looking statements, whether as a result of new information, future events or otherwise.
HOPE Therapeutics, Inc.
View original content to download multimedia:https://www.prnewswire.com/news-releases/nrx-pharmaceuticals-nasdaqnrxp-publishes-shareholder-update-letter-302168228.html
SOURCE NRx Pharmaceuticals, Inc.
$NRXP NEWS: NRx Pharmaceuticals today announced that the Company posted a new Shareholder Update Letter on its website.
Read more - https://ir.nrxpharma.com/2024-06-10-NRx-Pharmaceuticals-NASDAQ-NRXP-Publishes-Shareholder-Update-Letter
#PharmaNews #mentalillness #mentalhealth #mentalhealthmatters #depression #ketamine #stress #PTSD #bipolar #NRX100 #NRX101 $NRXP
$NRXP $3.60 +1.12% @NRxPharma NRx Pharmaceuticals reposted
American Psychiatric Association
@APApsychiatric
Death of golfer Grayson Murray puts spotlight on importance of raising mental health awareness https://t.co/yqnKNKETPQ
$NRXP $3.87 +1.57% -NRX Pharmaceuticals is focused on the high unmet need for lifesaving treatments for people with severe bipolar depression and PTSD in the presence of suicidality.
Follow our journey.
nrxpharma.com/
#ketamine #PTSD #bipolar #NRX100 #NRX101 $NRXP #bullish
$NRXP NRx-101 is the first investigational drug in FDA trials for suicidal bipolar depression, awarded fast track designation, breakthrough therapy designation, and a special protocol agreement by the FDA. @NRxPharma
https://www.nrxpharma.com/ $BMY $JNJ $RENB
$NRXP $3.96 +3.39% NRx Pharmaceuticals (Nasdaq:NRXP) Presents Landmark Trial of NRX-101 in Suicidal Bipolar Depression At the American Society of Clinical Psychopharmacology Annual Meeting
Read More - https://ir.nrxpharma.com/2024-05-28-NRx-Pharmaceuticals-Nasdaq-NRXP-Presents-Landmark-Trial-of-NRX-101-in-Suicidal-Bipolar-Depression-At-the-American-Society-of-Clinical-Psychopharmacology-Annual-Meeting-NRX-101-is-the-First-Oral-Antidepressant-Demonstrated-to-Reduce-Suicidality-
#PharmaNews #mentalhealth #depression #suicide #bipolar #NRX101 $NRXP
$NRXP NEWS: NRx Pharmaceuticals (Nasdaq:NRXP) Presents Landmark Trial of NRX-101 in Suicidal Bipolar Depression At the American Society of Clinical Psychopharmacology Annual Meeting: NRX-101 is the First Oral Antidepressant Demonstrated to Reduce Suicidality in Bipolar Depression
PR Newswire
Tue, May 28, 2024, 8:30 AM EDT
In this article:
Poster presentation of "A Randomized, Double-Blind Controlled Comparison of NRX-101 (D-cycloserine/lurasidone) to Lurasidone for Adults with Bipolar Depression and Subacute Suicidal Ideation or Behavior"
NRX-101 demonstrated a similar antidepressant effect (MADRS reduction ~50%) compared to lurasidone, the Standard of Care drug
NRX-101 demonstrated a 58% relative reduction in time to sustained remission from suicidality (P=.05) compared to lurasidone
NRX-101 demonstrated a 76% reduction in symptoms of akathisia (p=0.03), a side effect linked to suicide, compared to lurasidone
This represents the second trial of NRX-101 demonstrating reduction in suicidality and akathisia associated with NRX-101 compared to lurasidone
RADNOR, Pa., May 28, 2024 /PRNewswire/ -- NRx Pharmaceuticals, Inc. (Nasdaq: NRXP) ("NRx Pharmaceuticals", "NRx", the "Company"), a clinical stage pharmaceutical company, today announced presentation of its Phase 2b/3 trial of NRX-101, entitled "A Randomized, Double-Blind Controlled Comparison of NRX-101 (D-cycloserine/lurasidone) to Lurasidone for Adults with Bipolar Depression and Subacute Suicidal Ideation or Behavior" at the American Society of Clinical Psychopharmacology (ASCP) in Miami Beach, FL. The lead author is Prof. Andrew Nierenberg, Director, Dauten Family Center for Bipolar Treatment Innovation, Massachusetts General Hospital and Professor of Psychiatry, Harvard Medical School.
Akathisia rating by study day: a consistent effect is seen commencing at first post-randomization visit and continued throughout the study (Mixed Model for Repeated Measures Regression effect size =0.037, P=0.03)
Akathisia rating by study day: a consistent effect is seen commencing at first post-randomization visit and continued throughout the study (Mixed Model for Repeated Measures Regression effect size =0.037, P=0.03)
"Presentation of these data at this highly respected conference is another important step toward bringing a life-saving product to patients in tremendous need," said Dr. Jonathan Javitt, Chairman and Chief Scientist of NRx. "We believe that NRX-101 may offer a paradigm changing approach to treatment of Bipolar Depression, with a product highly effective in both treating depression and reducing suicidality and associated side effects. We will continue working to bring hope to life with life-saving medications."
The presentation will be held at 11:15 AM, Wednesday May 29, 2024.
W89 A Randomized, Double-Blind Controlled Comparison of NRX-101 (D-cycloserine/lurasidone) to Lurasidone for Adults with Bipolar Depression and Subacute Suicidal Ideation or Behavior
CONCLUSIONS of the Poster are:
NRX-101 and lurasidone both demonstrated > 50% response for treating bipolar depression with no difference seen on primary efficacy endpoint (MADRS)
A clinically meaningful difference was observed on both primary and secondary safety endpoints favoring NRX-101
NRX-101 was associated with 58% relative reduction in time to sustained remission from suicidality as measured by the Columbia Suicide Severity Rating Scale (C-SSRS) when stratified by sex, mood stabilizer use, antipsychotic use, lifetime suicide event (P=0.05).
NRX-101 was associated with a relative 76% reduction in symptoms of akathisia compared to lurasidone that was sustained over 42 days (Effect Size 0.37; P=0.03) on the Barnes Akathisia Rating Scale
Akathisia was seen in 2% of participants treated with NRX-101 vs. 11% treated with lurasidone.
NRX-101 showed superiority to lurasidone in akathisia starting at day 7 and continuing through day 42/ET.
No treatment-related serious adverse event was observed in either group. No safety issues were detected except for MedDRA General disorders: NRX-101 - 18.2% vs lurasidone - 0% (p=0.002).
Based on these findings, together with the earlier STABIL-B trial, the Company believes that NRX-101 has potential to become a standard of care drug for treating bipolar depression, an addressable population of 7 million patients in the US and many times that around the world.
This study represents the second trial conducted under FDA Good Clinical Practices guidelines to demonstrate large and meaningful advantages of NRX-101 vs lurasidone on akathisia and suicidality and clears the path for a registration trial of NRX-101 vs. placebo to treat bipolar depression together with earlier accelerated approval for those with akathisia. An additional academic trial conducted by Chen and co-workers similarly demonstrated a statistically-significant reduction in suicidality associated with D-cycloserine, the active ingredient in NRX-101, compared to various standard of care antidepressants.
To the Company's knowledge, this trial and its prior STABIL-B study represent the only clinical trials in which an oral antidepressant has been demonstrated to cause meaningful reductions in suicidality and akathisia. All currently approved antidepressant drugs carry FDA-mandated "black box" warnings on their labels indicating that they may increase the risk of suicide. Similarly, akathisia – a side effect in which patients are agitated and frequently experience involuntary movement – is a side effect that occurs in 10-15% of patients who take the lurasidone class of drugs and is closely linked to suicide. As shown in the clinical trial, those randomized to lurasidone experienced a substantial increase in akathisia from baseline, whereas those randomized to NRX-101 demonstrated a statistically-significant reduction in akathisia (see Figure).
About NRx Pharmaceuticals
NRx Pharmaceuticals is a clinical-stage biopharmaceutical company developing therapeutics based on its NMDA platform for the treatment of central nervous system disorders, specifically suicidal bipolar depression, chronic pain and PTSD. The Company is developing NRX-101, an FDA-designated investigational Breakthrough Therapy for suicidal treatment-resistant bipolar depression and chronic pain. NRx has partnered with Alvogen and Lotus around the development and marketing of NRX-101 for the treatment of suicidal bipolar depression. NRX-101 additionally has potential to act as a non-opioid treatment for chronic pain, as well as a treatment for complicated UTI.
NRx has recently announced plans to submit a New Drug Application for HTX-100 (IV ketamine), through Hope Therapeutics, in the treatment of suicidal depression, based on results of well-controlled clinical trials conducted under the auspices of the US National Institutes of Health and newly obtained data from French health authorities, licensed under a data sharing agreement. NRx was awarded Fast Track Designation for development of ketamine (NRX-100) by the US FDA as part of a protocol to treat patients with acute suicidality.
Notice Regarding Forward-Looking Statements
The information contained herein includes forward-looking statements within the meaning of Section 21E of the Securities Exchange Act of 1934, as amended, and Section 27A of the Securities Act of 1933, as amended. These statements include, among others, statements regarding the proposed public offering and the timing and the use of the proceeds from the offering. Forward-looking statements generally include statements that are predictive in nature and depend upon or refer to future events or conditions, and include words such as "may," "will," "should," "would," "expect," "plan," "believe," "intend," "look forward," and other similar expressions among others. These statements relate to future events or to the Company's future financial performance, and involve known and unknown risks, uncertainties and other factors that may cause the Company's actual results to be materially different from any future results, levels of activity, performance or achievements expressed or implied by these forward-looking statements. You should not place undue reliance on forward-looking statements since they involve known and unknown risks, uncertainties and other factors which are, in some cases, beyond the Company's control and which could, and likely will, materially affect actual results, levels of activity, performance or achievements. Any forward-looking statement reflects the Company's current views with respect to future events and is subject to these and other risks, uncertainties and assumptions relating to the Company's operations, results of operations, growth strategy and liquidity. More detailed information about the Company and the risk factors that may affect the realization of forward-looking statements is set forth in the Company's most recent Annual Report on Form 10-K and other filings with the Securities and Exchange Commission. Investors and security holders are urged to read these documents free of charge on the SEC's website at http://www.sec.gov. Except as may be required by applicable law, The Company assumes no obligation to publicly update or revise these forward-looking statements for any reason, or to update the reasons actual results could differ materially from those anticipated in these forward-looking statements, whether as a result of new information, future events or otherwise.
View original content to download multimedia:https://www.prnewswire.com/news-releases/nrx-pharmaceuticals-nasdaqnrxp-presents-landmark-trial-of-nrx-101-in-suicidal-bipolar-depression-at-the-american-society-of-clinical-psychopharmacology-annual-meeting-nrx-101-is-the-first-oral-antidepressant-demonstrated-to-re-302156419.html
SOURCE NRx Pharmaceuticals, Inc.
NRx Pharmaceuticals (Nasdaq:NRXP) Presents Landmark Trial of NRX-101 in Suicidal Bipolar Depression At the American Society of Clinical Psychopharmacology Annual Meeting: NRX-101 is the First Oral Antidepressant Demonstrated to Reduce Suicidality in Bipolar Depression
Poster presentation of "A Randomized, Double-Blind Controlled Comparison of NRX-101 (D-cycloserine/lurasidone) to Lurasidone for Adults with Bipolar Depression and Subacute Suicidal Ideation or Behavior"
NRX-101 demonstrated a similar antidepressant effect (MADRS reduction ~50%) compared to lurasidone, the Standard of Care drug
NRX-101 demonstrated a 58% relative reduction in time to sustained remission from suicidality (P=.05) compared to lurasidone
NRX-101 demonstrated a 76% reduction in symptoms of akathisia (p=0.03), a side effect linked to suicide, compared to lurasidone
This represents the second trial of NRX-101 demonstrating reduction in suicidality and akathisia associated with NRX-101 compared to lurasidone
RADNOR, Pa., May 28, 2024 /PRNewswire/ -- NRx Pharmaceuticals, Inc. (Nasdaq: NRXP) ("NRx Pharmaceuticals", "NRx", the "Company"), a clinical stage pharmaceutical company, today announced presentation of its Phase 2b/3 trial of NRX-101, entitled "A Randomized, Double-Blind Controlled Comparison of NRX-101 (D-cycloserine/lurasidone) to Lurasidone for Adults with Bipolar Depression and Subacute Suicidal Ideation or Behavior" at the American Society of Clinical Psychopharmacology (ASCP) in Miami Beach, FL. The lead author is Prof. Andrew Nierenberg, Director, Dauten Family Center for Bipolar Treatment Innovation, Massachusetts General Hospital and Professor of Psychiatry, Harvard Medical School.
"Presentation of these data at this highly respected conference is another important step toward bringing a life-saving product to patients in tremendous need," said Dr. Jonathan Javitt, Chairman and Chief Scientist of NRx. "We believe that NRX-101 may offer a paradigm changing approach to treatment of Bipolar Depression, with a product highly effective in both treating depression and reducing suicidality and associated side effects. We will continue working to bring hope to life with life-saving medications."
The presentation will be held at 11:15 AM, Wednesday May 29, 2024.
W89 A Randomized, Double-Blind Controlled Comparison of NRX-101 (D-cycloserine/lurasidone) to Lurasidone for Adults with Bipolar Depression and Subacute Suicidal Ideation or Behavior
CONCLUSIONS of the Poster are:
NRX-101 and lurasidone both demonstrated > 50% response for treating bipolar depression with no difference seen on primary efficacy endpoint (MADRS)
A clinically meaningful difference was observed on both primary and secondary safety endpoints favoring NRX-101NRX-101 was associated with 58% relative reduction in time to sustained remission from suicidality as measured by the Columbia Suicide Severity Rating Scale (C-SSRS) when stratified by sex, mood stabilizer use, antipsychotic use, lifetime suicide event (P=0.05).NRX-101 was associated with a relative 76% reduction in symptoms of akathisia compared to lurasidone that was sustained over 42 days (Effect Size 0.37; P=0.03) on the Barnes Akathisia Rating Scale
Akathisia was seen in 2% of participants treated with NRX-101 vs. 11% treated with lurasidone.
NRX-101 showed superiority to lurasidone in akathisia starting at day 7 and continuing through day 42/ET.
No treatment-related serious adverse event was observed in either group. No safety issues were detected except for MedDRA General disorders: NRX-101 - 18.2% vs lurasidone - 0% (p=0.002).
Based on these findings, together with the earlier STABIL-B trial, the Company believes that NRX-101 has potential to become a standard of care drug for treating bipolar depression, an addressable population of 7 million patients in the US and many times that around the world.
This study represents the second trial conducted under FDA Good Clinical Practices guidelines to demonstrate large and meaningful advantages of NRX-101 vs lurasidone on akathisia and suicidality and clears the path for a registration trial of NRX-101 vs. placebo to treat bipolar depression together with earlier accelerated approval for those with akathisia. An additional academic trial conducted by Chen and co-workers similarly demonstrated a statistically-significant reduction in suicidality associated with D-cycloserine, the active ingredient in NRX-101, compared to various standard of care antidepressants.
To the Company's knowledge, this trial and its prior STABIL-B study represent the only clinical trials in which an oral antidepressant has been demonstrated to cause meaningful reductions in suicidality and akathisia. All currently approved antidepressant drugs carry FDA-mandated "black box" warnings on their labels indicating that they may increase the risk of suicide. Similarly, akathisia – a side effect in which patients are agitated and frequently experience involuntary movement – is a side effect that occurs in 10-15% of patients who take the lurasidone class of drugs and is closely linked to suicide. As shown in the clinical trial, those randomized to lurasidone experienced a substantial increase in akathisia from baseline, whereas those randomized to NRX-101 demonstrated a statistically-significant reduction in akathisia (see Figure).
About NRx Pharmaceuticals
NRx Pharmaceuticals is a clinical-stage biopharmaceutical company developing therapeutics based on its NMDA platform for the treatment of central nervous system disorders, specifically suicidal bipolar depression, chronic pain and PTSD. The Company is developing NRX-101, an FDA-designated investigational Breakthrough Therapy for suicidal treatment-resistant bipolar depression and chronic pain. NRx has partnered with Alvogen and Lotus around the development and marketing of NRX-101 for the treatment of suicidal bipolar depression. NRX-101 additionally has potential to act as a non-opioid treatment for chronic pain, as well as a treatment for complicated UTI.
NRx has recently announced plans to submit a New Drug Application for HTX-100 (IV ketamine), through Hope Therapeutics, in the treatment of suicidal depression, based on results of well-controlled clinical trials conducted under the auspices of the US National Institutes of Health and newly obtained data from French health authorities, licensed under a data sharing agreement. NRx was awarded Fast Track Designation for development of ketamine (NRX-100) by the US FDA as part of a protocol to treat patients with acute suicidality.
Notice Regarding Forward-Looking Statements
The information contained herein includes forward-looking statements within the meaning of Section 21E of the Securities Exchange Act of 1934, as amended, and Section 27A of the Securities Act of 1933, as amended. These statements include, among others, statements regarding the proposed public offering and the timing and the use of the proceeds from the offering. Forward-looking statements generally include statements that are predictive in nature and depend upon or refer to future events or conditions, and include words such as "may," "will," "should," "would," "expect," "plan," "believe," "intend," "look forward," and other similar expressions among others. These statements relate to future events or to the Company's future financial performance, and involve known and unknown risks, uncertainties and other factors that may cause the Company's actual results to be materially different from any future results, levels of activity, performance or achievements expressed or implied by these forward-looking statements. You should not place undue reliance on forward-looking statements since they involve known and unknown risks, uncertainties and other factors which are, in some cases, beyond the Company's control and which could, and likely will, materially affect actual results, levels of activity, performance or achievements. Any forward-looking statement reflects the Company's current views with respect to future events and is subject to these and other risks, uncertainties and assumptions relating to the Company's operations, results of operations, growth strategy and liquidity. More detailed information about the Company and the risk factors that may affect the realization of forward-looking statements is set forth in the Company's most recent Annual Report on Form 10-K and other filings with the Securities and Exchange Commission. Investors and security holders are urged to read these documents free of charge on the SEC's website at http://www.sec.gov. Except as may be required by applicable law, The Company assumes no obligation to publicly update or revise these forward-looking statements for any reason, or to update the reasons actual results could differ materially from those anticipated in these forward-looking statements, whether as a result of new information, future events or otherwise.
https://c212.net/c/img/favicon.png?sn=CL24282&sd=2024-05-28 View original content to download multimedia:https://www.prnewswire.com/news-releases/nrx-pharmaceuticals-nasdaqnrxp-presents-landmark-trial-of-nrx-101-in-suicidal-bipolar-depression-at-the-american-society-of-clinical-psychopharmacology-annual-meeting-nrx-101-is-the-first-oral-antidepressant-demonstrated-to-re-302156419.html
SOURCE NRx Pharmaceuticals, Inc.
$NRXP Oral Antidepressants for Reduction in Suicidality Could Deliver New Standard of Care for Bipolar Depression: NASDAQ NRXP https://www.einpresswire.com/article/707706109/oral-antidepressants-for-reduction-in-suicidality-could-deliver-new-standard-of-care-for-bipolar-depression-nasdaq-nrxp via @ein_news
|
Followers
|
55
|
Posters
|
|
|
Posts (Today)
|
0
|
Posts (Total)
|
1152
|
|
Created
|
12/15/20
|
Type
|
Free
|
| Moderators ProfitScout jedijazz | |||





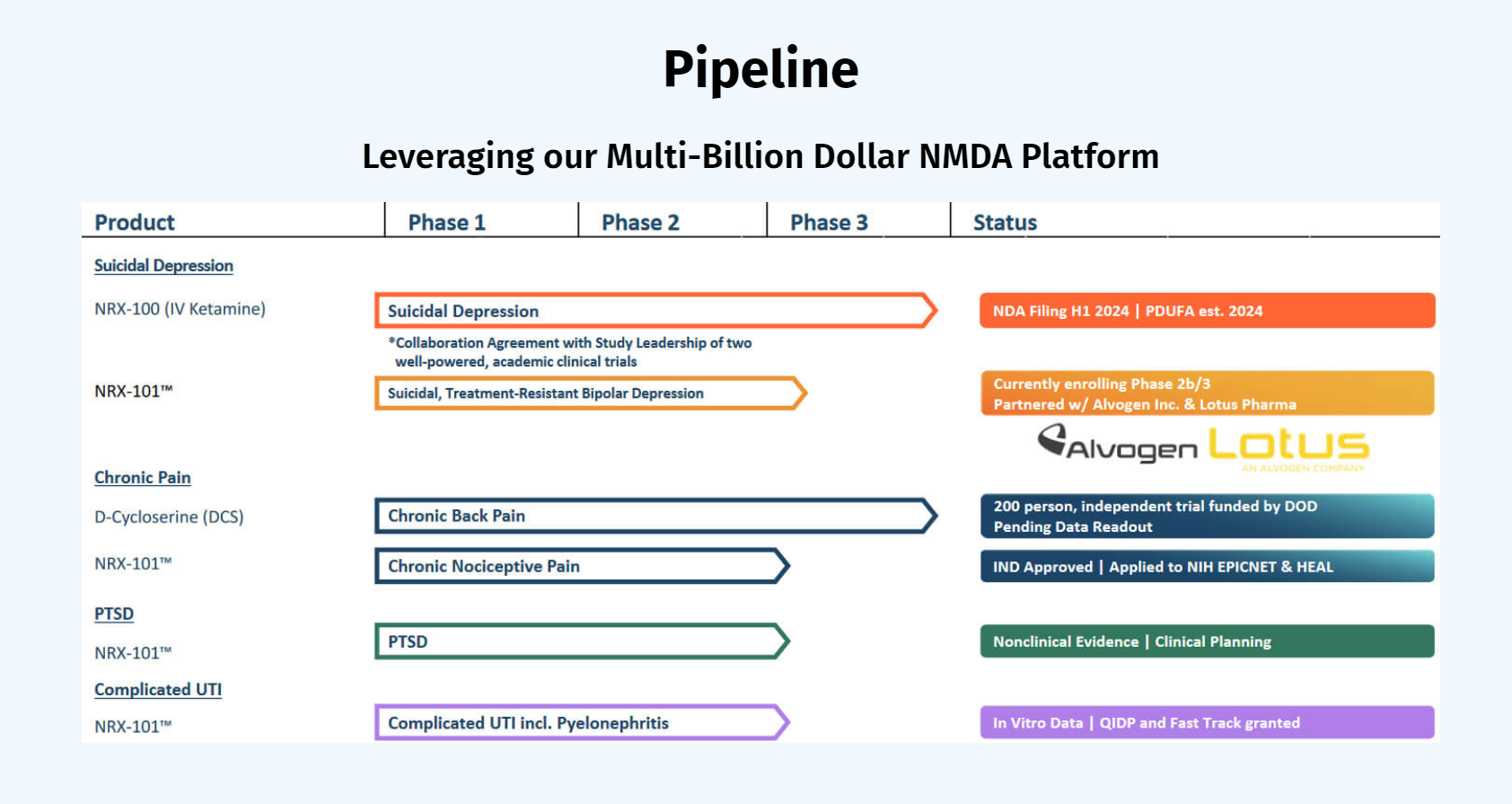
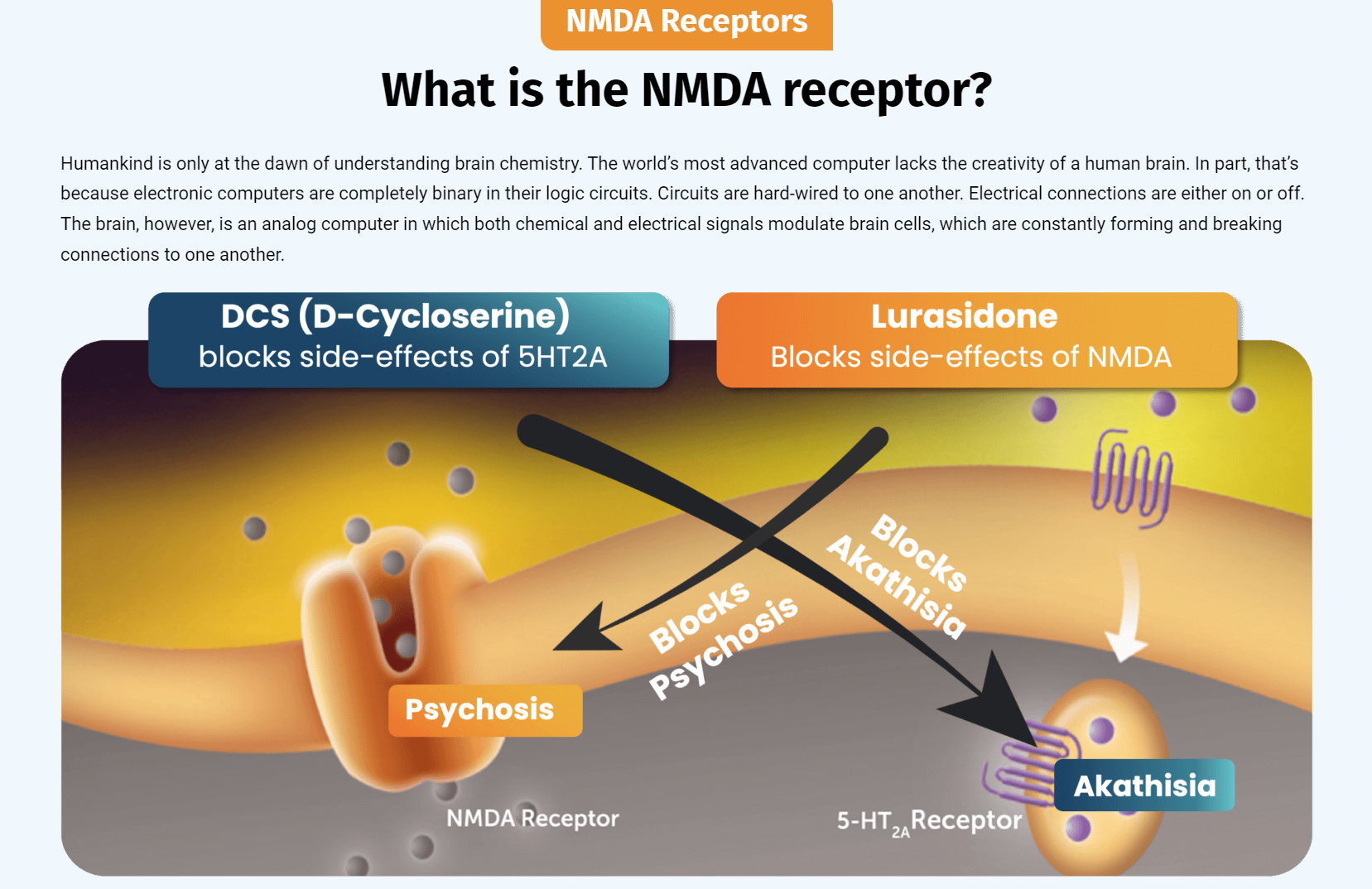

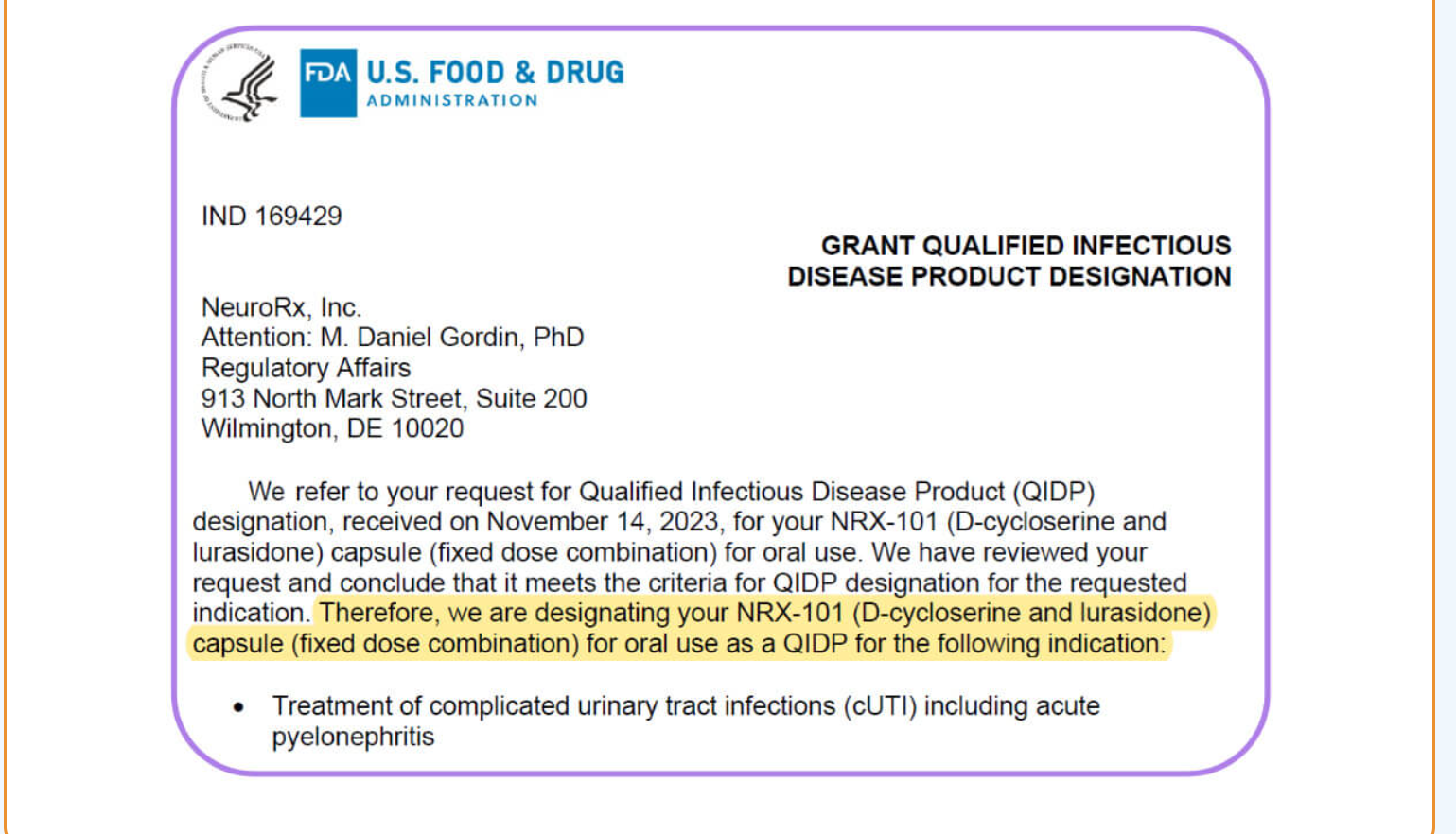





Both drugs demonstrated > 50% response for treating depression. NRX-101 demonstrated a mean 76% reduction in symptoms of akathisia compared to lurasidone that was sustained over 42 days (Effect...

New data demonstrates no impact of NRX-101 on gut or vaginal flora – considered primary causes of pseudomembranous colitis due to C difficile and vaginal yeast infections NRX-101 previously...
Formulation based on prior patents by NRx founder Achieved pH neutral formulation of ketamine, potentially enabling both intravenous (IV) and subcutaneous (SQ) administration Company expects...
Data transferred for independent statistical analysis Top-line data expected in April 2024 RADNOR, Pa., April 8, 2024 /PRNewswire/ -- NRx Pharmaceuticals, Inc. (Nasdaq: NRXP) ("NRx...
Four potential near-term milestones, including data from two clinical trials, an NDA filing and an upcoming share dividend -- 50% reduction in corporate overhead and 25% reduction in overall net...
Four potential near-term milestones, including data from two clinical trials, an NDA filing and a share dividend RADNOR, Pa., March 28, 2024 /PRNewswire/ -- NRx Pharmaceuticals, Inc. (Nasdaq: NRXP) ("
NRx Pharmaceuticals, Inc. (Nasdaq: NRXP) ("NRx Pharmaceuticals", the "Company"), a clinical-stage biopharmaceutical company, today announced that it will release its fourth quarter and full year...
NRx Pharmaceuticals, Inc. (Nasdaq:NRXP) ("NRx Pharmaceuticals", the "Company"), a clinical-stage biopharmaceutical company, today announced that Dr. Jonathan Javitt, Chairman and Chief Scientist...
NRx Pharmaceuticals, Inc. (Nasdaq: NRXP) ("NRx Pharmaceuticals", the "Company"), a clinical-stage biopharmaceutical company, today announced that it will release its fourth quarter and full year...
61.4% (56,781,354) of eligible shares voted 94.4% of votes were cast in favor of the resolution RADNOR, Pa., March 21, 2024 /PRNewswire/ -- NRx Pharmaceuticals, Inc. (Nasdaq: NRXP) ("NRx...
NRx Pharmaceuticals Board of Directors has authorized its Chairman, CEO, and management to take all necessary steps to affect the Dividend and Royalty Rights to NRXP Shareholders and applicable...
Initiative is intended to protect shareholder value through continued compliance with Nasdaq listing rules and elimination of naked short sales positions in the Company's securities The Company is...
Company to receive first allocation of ketamine for sale by month end Partners preparing to ship IV Ketamine to full range of customers via 503a and 503b pharmacies NRx Pharmaceuticals and HOPE...
Marks a major step in the development of what could be the first drug approved for Suicidal Bipolar Depression The study database is being cleaned and locked; statistical analysis and top-line...
NRx Pharmaceuticals received approximately $1.0 million in cash from an existing investor Shares were sold at $0.38, a 26.7% premium the recent share offering, along with one common 5-year warrant...
In the news release, NRx Pharmaceuticals, Inc. Announces Pricing of $1.5 Million Underwritten Public Offering of Common Stock, issued 27-Feb-2024 by NRx Pharmaceuticals, Inc. over PR Newswire, we...
NRx Pharmaceuticals, Inc. (Nasdaq: NRXP), ("NRx Pharmaceuticals" or the "Company"), a clinical-stage biopharmaceutical company, today announced that it intends to offer to sell shares of its...
Presentation available on NRx Pharmaceuticals website at https://www.nrxpharma.com/hope-therapeutics/ KEY UPDATES ARE AS FOLLOWS: NRx management is proposing to award 50% of founding shares in...
DISCLAIMER:
Nothing in the contents transmitted on this board should be construed as an investment advisory, nor should it be used to make investment decisions.
There is no express or implied solicitation to buy or sell securities.
The author(s) may have positions in the stocks or financial relationships with the company or companies discussed and may trade in the stocks mentioned.
Readers are advised to conduct their own due diligence prior to considering buying or selling any stock. All information should be considered for information purposes only.
No stock exchange has approved or disapproved of the information here.
| Volume | |
| Day Range: | |
| Bid Price | |
| Ask Price | |
| Last Trade Time: |
