Register for free to join our community of investors and share your ideas. You will also get access to streaming quotes, interactive charts, trades, portfolio, live options flow and more tools.
* NRxP added to the Nasdaq Biotechnology Index
* NRxP reapplies to FDA for BTD
* RLFTF issues positive PR on NRxP
Can't deny it - Good things are happening.
December 29, 2021 11:17 AM
NRXP Stock Alert: What Is Going on With NRx Pharmaceuticals Today?
InvestorPlace - Stock Market News, Stock Advice & Trading Tips
NRx Pharmaceuticals (NASDAQ:NRXP) stock is advancing in early trading today after the company announced that it was seeking breakthrough therapy designation from the U.S. Food and Drug Administration for its Covid-19 treatment, Zyesami, for the second time.
In mid-morning trading, NRXP stock rose 4% to $4.68.
The New Data on Zyesami
According to NRx, Covid-19 patients treated with the drug were 2.8 times more likely to avoid both death and respiratory failure 28 days and 60 days after they began using the medicine than those given placebos.
Patients treated with Zyesami after Gilead’s (NASDAQ:GILD) Remdesivir failed to stop their respiratory failure were four times more likely to survive than those who received placebos.
Among all 196 Covid-19 patients who received NRx’s drug, the survival rate was double those who were given placebos. But much more encouraging was the data on 70% of those whose condition was worsening after being treated with Gilead’s remdesivir drug and other approved drugs. Among that group, the patients who received Zyesami were four times more likely to survive at the 60-day mark than those who were given placebos.
Finally, the patients who were on ventilators were ten times more likely to survive if they were given Zyesami.
Background on NRXP Stock
The FDA had previously decided not to give breakthrough designation to Zyesami or grant an emergency-use authorization for the drug. The agency, however, had suggested that NRx could reapply for breakthrough designation if new data on the drug showed that it could be meaningfully more effective than other approved therapies.
NRxP is now seeking to have the agency grant breakthrough designation to its drug as a “treatment of COVID-19 respiratory failure in patients who progress despite treatment with remdesivir and other approved therapies.”
The FDA grants breakthrough designation to drugs in order to reduce the time needed for them to be enhanced by companies and evaluated by the agency. The status is only given to drugs that have shown the potential, in clinical trials, to “treat a serious condition” significantly more effectively than “available” drugs.
Worth noting is that NRXP stock has lost about 80% of its value since peaking at nearly $25 in July 2021. The shares now have a market capitalization of just under $300 million.
Israeli-modified erectile dysfunction drug shows some promise treating COVID-19
Innovator says very early findings suggest adapted form of aviptadil can cause improvement in lungs and blood oxygen, but hard evidence still needed
An Israeli innovator says he has repurposed an erectile dysfunction treatment for coronavirus, and reports that it is showing promise, having significantly improved the health of six patients.
Jonathan Javitt has developed a form of aviptadil — a drug that is used almost exclusively for injection into the penis along with another drug phentolamine, to help men dealing with erectile disfunction — to treat severe coronavirus. This version of the drug for intravenous injection to COVID-19 patients, RLF-100, is now undergoing clinical trials in five American hospitals.
No results from these trials are available, but Javitt has released a report on six patients who received the drug outside of the trials, claiming that they had taken strong steps toward recovery.
“As far as I know, this is the first drug to treat COVID with which the [lung] X-ray gets better, the blood oxygen gets better, and cytokines drop very quickly,” he said. Cytokines are the small proteins that COVID patients sometimes produce in huge numbers, causing harm to their bodies.
Medics work with COVID-19 patients at the isolation ward of Sheba Medical Center in Ramat Gan on July 29, 2020. (JACK GUEZ / AFP)
“If you ask me whether this is the cure to COVID, my answer is that I don’t know, but it shows promise, and I hope so,” he told The Times of Israel.
Javitt, CEO of the US-Israeli NeuroRx Inc, partnered with Geneva-based Relief Therapeutics Holdings to produce the drug. An American doctor who moved to Israel, Javitt started working on it in March, when a capital fund that invests in both companies contacted him to say that it had the rights to the research, and wondered whether it might be useful in the fight against the coronavirus.
The research consisted of data on the safety and toxicology of aviptadil, which is essential for regulatory approval of any new drug based on it. Aviptadil is a synthetic form of vasoactive intestinal peptide (VIP), a short chain of amino acids that occurs naturally in the human body and protects the lungs.
Past research has illustrated that VIP, in addition to being used to treat erectile dysfunction, mostly in northern Europe, is a potent anti-inflammatory, though it is not currently in widespread use for this purpose. Javitt, with Yves Sagot of Relief Therapeutics, started exploring possible relevance to COVID-19, and made a formulation of the drug that they expected to work on the disease. They obtained permission from America’s Food and Drug Administration to carry out clinical trials, and also to administer it to some patients for emergency use.
Javitt said: “They reported that if you give the virus to human lung cells in a laboratory, the virus will enter the cells and kill them. If you give them this drug, it suppresses the replication of the virus and if the virus can’t make millions of copies of itself, it’s not harmful to you.”
As clinical trials for 70 people continue, 25 people have been given the drug under emergency use rules at Houston Methodist Hospital. Follow-up has been conducted on the first six people who received it on this basis, on average, 14 days after treatment. Javitt said that at the time of follow-up, all patients showed an improvement in blood oxygenation. He added that there was an average 56 percent reduction in inflammatory markers, and four out of six patients showed complete remission from respiratory failure.
Javitt said that despite his enthusiasm, he is realistic about the limitations of the research so far. “Of course, this is small number, and of course, testing that includes patients given placebos is needed,” he said.
https://www.timesofisrael.com/erectile-dysfunction-drug-shows-promise-treating-covid-israeli-innovator-says/
Good luck and GOD bless,
Great question... Is it the inherent slow grind of a typical government organization. They do not have to worry about making a profit! It is amazing how they actually sped up the approval of three vaccines... yet, I do not think the Biden admin is too interested in pressuring anyone to speed things up.
But Rand Paul has some interesting observations...
https://www.dailymail.co.uk/news/article-10350179/Rand-Paul-says-Fauci-responsible-THOUSANDS-COVID-deaths.html
Rand Paul says Fauci is responsible for THOUSANDS of COVID deaths across the US because he 'deemphasized therapeutics' while focusing entirely on vaccines
The Kentucky Republican accused Fauci of having a 'bias' toward vaccines that dates back to the AIDS crisis
'I would venture to say that thousands of people die in our country every month now because [Fauci's] deemphasized the idea that there are therapeutics'
The senator also claimed that thousands had died because Fauci did not take into account natural immunity in prioritizing vaccinations
The CDC recommends that all people be fully vaccinated regardless of previous infection
Great question... Is it the inherent slow grind of a typical government organization. They do not have to worry about making a profit! It is amazing how they actually sped up the approval of three vaccines... yet, I do not think the Biden admin is too interested in pressuring anyone to speed things up.
But Rand Paul has some interesting observations...
https://www.dailymail.co.uk/news/article-10350179/Rand-Paul-says-Fauci-responsible-THOUSANDS-COVID-deaths.html
Rand Paul says Fauci is responsible for THOUSANDS of COVID deaths across the US because he 'deemphasized therapeutics' while focusing entirely on vaccines
The Kentucky Republican accused Fauci of having a 'bias' toward vaccines that dates back to the AIDS crisis
'I would venture to say that thousands of people die in our country every month now because [Fauci's] deemphasized the idea that there are therapeutics'
The senator also claimed that thousands had died because Fauci did not take into account natural immunity in prioritizing vaccinations
The CDC recommends that all people be fully vaccinated regardless of previous infection
Does anyone know how long it takes for a decision on BreakThrough Designation?
Can’t believe this is below $5. Goodbye nasdaq.
** WAS ACTIV3B ENROLLMENT GOAL INCREASED 20%***
NEW VIDEO ON ACTIV 3B VERY INTERESTING
BUT DOCTOR REPRESENTING NIH SAYS ENROLLMENT GOAL IS OVER 800????
JAVITT SAID 640 IN LAST PR, WHY NOW OVER 800? AM I MISSING SOMETHING??
NRx Pharmaceuticals Files Breakthrough Therapy Designation Request for ZYESAMI® (aviptadil) in Patients at Immediate Risk of Death from COVID-19 Despite Treatment with Remdesivir and Other Approved Therapies
https://ir.nrxpharma.com/news-events/press-releases/detail/105/nrx-pharmaceuticals-files-breakthrough-therapy-designation
Might be a positive day.
https://abcnews.go.com/Business/wireStory/eu-approves-5th-covid-19-vaccine-bloc-novavax-81856001
Another High Hurdle for anyone developing a potential new vax
PW....Thats a Very Interesting Question.....
We do know that some dislike following business procedures.
Illegal insider trading? Dr JJ the source of info to help his friends? I'm not accusing. I'm only asking.
**** ISRAEL FUND SELLS NRX????***
INTERESTING TIMING ON THEIR SALE.... Q3 2021??? WHILE WE WERE BEING TOLD TO “EXPECT EUA” ISRAEL FUND SELLING?? WONDER WHY??
https://www.google.com/amp/s/finance.yahoo.com/amphtml/news/timothy-plan-israel-common-values-113802982.html
Multiple huge blocks of 1M+ shares privately (at predetermined price) traded after-hours this month. We expect private placement for up to 6.6M shares. The only one capable of doing this is its CEO & Chairman.
I guess you can’t make enough money off preventative medicine.
Do you think at some point, someone will realize that it’s going to take a multisystem approach to stop this pandemic? The vaccines alone can’t do it. Why not also protect the cells once the virus has entered the body, giving the body a better chance to fight off this infection when the body is still relatively healthy enough to put up a fight, instead of waiting until the body is at its weakest point to try to initiate some form of weak response? It doesn’t take a genius to figure this out.
Over 2.5 million shares of NRXP were bought today!!!
On Monday Dec. 20, 2021 NRXP will become part of the NASDAQ Biotechnology Index
How many shares of NRXP do you think will trade on Monday Dec. 20, 2021???
Good luck and GOD bless,
NRx Pharmaceuticals Added to the Nasdaq® Biotech Index
https://ir.nrxpharma.com/news-events/press-releases/detail/104/nrx-pharmaceuticals-addedtothenasdaq-biotech-index
Good question. I can only speculate that there are more people vaccinated than not especially for those out there traveling a lot. So the ratio of new infections should reflect the effectiveness of any vaccine against any variant. But on initial look, it might appear as the article points out, that the first offered vaccines are not as effective with this newest variant.
Bob, does this mean that the unvaxxed folks have a form of natural immunity towards Omicron?
Aside from ZYESAMI... there is BriLife... and perhaps just in time.
Interesting how if you wait on initial news reports.. you learn so much more! This new variant sounds a bit worrisome... except that it appears so far that it may not be as severe.. at least for the healthy.
The difference between the first US "Man Engineered" vaccine and BriLife being studied by NRXP is interesting in that BriLife seems to be manufactured by traditional means and contains all the genetic material so that our bodies can recognize any possible variant. It appears.. so far that the US man-made vaccines are limited in scope with regards to preparing our body's immune system to recognize variants.
From and earlier News Release:
"BriLife presents the entire spike protein complex of the coronavirus to the body's immune system and can simultaneously present multiple variants of the spike protein."
We shall see.
Article:
https://www.reuters.com/world/us/most-reported-us-omicron-cases-have-hit-fully-vaccinated-cdc-2021-12-10/
Most reported U.S. Omicron cases have hit the fully vaccinated -CDC
By Mrinalika Roy
Dec 10 (Reuters) -
Most of the 43 COVID-19 cases caused by the Omicron variant identified in the United States so far were in people who were fully vaccinated, and a third of them had received a booster dose, according to a U.S. report published on Friday.
The U.S. Centers for Disease Control and Prevention (CDC) said that of the 43 cases attributed to Omicron variant, 34 people had been fully vaccinated. Fourteen of them had also received a booster, although five of those cases occurred less than 14 days after the additional shot before full protection kicks in.
While the numbers are very small, they add to growing concerns that current COVID-19 vaccines may offer less protection against the highly transmissible new variant.
The Omicron variant of the coronavirus has been found through testing in about 22 states so far after first being identified in southern Africa and Hong Kong in late November.
Among the Omicron cases, 25 were in people aged 18 to 39 and 14 had traveled internationally. Six people had previously been infected with the coronavirus.
Most of them only had mild symptoms such as coughing, congestion, and fatigue, the report said, and one person was hospitalized for two days. Other symptoms reported less frequently including nausea or vomiting, shortness of breath or difficulty breathing, diarrhea and loss of taste or smell.
The CDC said that while many of the first reported Omicron cases appear to be mild, a lag exists between infection and more severe outcomes. Symptoms would also be expected to be milder in vaccinated persons and those with previous SARS-CoV-2 infection.
The first known U.S. Omicron case was identified on Dec 1 in a fully vaccinated person who had traveled to South Africa. The CDC said that the earliest date of symptom onset was Nov. 15 in a person with a history of international travel.
The Delta variant still accounts for more than 99% of all U.S. cases. But reports from South Africa show that the Omicron variant is very transmissible.
Even if most cases are mild, a highly transmissible variant could result in enough infections to overwhelm health systems, the CDC cautioned.
Laboratory studies released this week suggest that the Omicron variant will blunt the protective power of two doses of Pfizer (PFE.N) and BioNTech's COVID-19 vaccine, although a third dose may restore that protection.
The U.S. has authorized COVID-19 vaccine boosterdoses for all Americans age 16 and older.
Aside from ZYesami... there is BriLife... and perhaps just in time.
Interesting how if you wait on initial news reports.. you learn so much more! This new variant sounds a bit worrisome... except that it appears so far that it may not be as severe.. at least for the healthy.
The difference between the first US "Man Engineered" vaccine and BriLife being studied by NRXP is interesting in that BriLife seems to be manufactured by traditional means and contains all the genetic material so that our bodies can recognize any possible variant. It appears.. so far that the US man-made vaccines are limited in scope with regards to preparing our body's immune system to recognize variants.
We shall see.
Article:
https://www.reuters.com/world/us/most-reported-us-omicron-cases-have-hit-fully-vaccinated-cdc-2021-12-10/
Most reported U.S. Omicron cases have hit the fully vaccinated -CDC
By Mrinalika Roy
Dec 10 (Reuters) -
Most of the 43 COVID-19 cases caused by the Omicron variant identified in the United States so far were in people who were fully vaccinated, and a third of them had received a booster dose, according to a U.S. report published on Friday.
The U.S. Centers for Disease Control and Prevention (CDC) said that of the 43 cases attributed to Omicron variant, 34 people had been fully vaccinated. Fourteen of them had also received a booster, although five of those cases occurred less than 14 days after the additional shot before full protection kicks in.
While the numbers are very small, they add to growing concerns that current COVID-19 vaccines may offer less protection against the highly transmissible new variant.
The Omicron variant of the coronavirus has been found through testing in about 22 states so far after first being identified in southern Africa and Hong Kong in late November.
Among the Omicron cases, 25 were in people aged 18 to 39 and 14 had traveled internationally. Six people had previously been infected with the coronavirus.
Most of them only had mild symptoms such as coughing, congestion, and fatigue, the report said, and one person was hospitalized for two days. Other symptoms reported less frequently including nausea or vomiting, shortness of breath or difficulty breathing, diarrhea and loss of taste or smell.
The CDC said that while many of the first reported Omicron cases appear to be mild, a lag exists between infection and more severe outcomes. Symptoms would also be expected to be milder in vaccinated persons and those with previous SARS-CoV-2 infection.
The first known U.S. Omicron case was identified on Dec 1 in a fully vaccinated person who had traveled to South Africa. The CDC said that the earliest date of symptom onset was Nov. 15 in a person with a history of international travel.
The Delta variant still accounts for more than 99% of all U.S. cases. But reports from South Africa show that the Omicron variant is very transmissible.
Even if most cases are mild, a highly transmissible variant could result in enough infections to overwhelm health systems, the CDC cautioned.
Laboratory studies released this week suggest that the Omicron variant will blunt the protective power of two doses of Pfizer (PFE.N) and BioNTech's COVID-19 vaccine, although a third dose may restore that protection.
The U.S. has authorized COVID-19 vaccine boosterdoses for all Americans age 16 and older.
The genius of jj....
Israel is one of the most VAXXED country on the planet, if not the most highly VAXXED of all countries, and now on their Fourth VAXX
https://stuartbramhall.wordpress.com/2021/09/27/it-never-ends-israel-says-fourth-booster-vaccine-will-be-required-to-keep-covid-green-pass-active/
And jj thinks he will insert himself on top of four or five other "vaccinations" of Israels folks and other companies doing a huge business in Israel.
On top of testing his VAXX, he will need to test it against people that have already been VAXXED with a different VAXX four or five or six times, and ya know it might just be very time consuming and very expensive on top of regular testing.
All the while dealing with a massive host of legal problems for a very young and small company, now with a history of bad behavior, or the lawyers would not be all over Neuro
Good luck.
NRx Pharmaceuticals and Hungarian Health Officials Agree on Pathway for ZYESAMI® and BriLife COVID-19 Vaccine Trials
https://ir.nrxpharma.com/news-events/press-releases/detail/102/nrx-pharmaceuticals-and-hungarian-health-officials-agree-on
Good luck and GOD bless,
So I don't see how constantly running down/knocking NRXP is going to help/lift RLFT ??
Realize @ some level they are partners. Realize at some point the mngmt of RLFT chose NRXP and vice versa.
Maybe point some finger===>>>blame toward the RLFT mngmt?? If you hate their choice--that is on RLFT mngmt.
For better or worse, whether you like it or I like it, as of NOW they are partners. You might not like the boat--but you are in it. You do not have to paddle--you can paddle with your partner--or do what you do.
Hopefully a covid approval will benefit both companies and ALL shareholders.
https://stockcharts.com/h-sc/ui?s=NRXP&p=D&b=5&g=0&id=p27695432074
At this rate.......Four, Three, Two, One ???
jj needs to come clean with RELIEF.... and do whatever it takes to
settle with RELIEF, esp before the need for bankruptcy lawyers.
Will We Ever Get Rid of COVID-19?
https://www.quantamagazine.org/will-we-ever-eradicate-covid-19-20211130/
The whole world needs NRx Pharmacuticals now.
Good luck and GOD bless,
Is that Pre Christmas News about Bankruptcy, another FDA Rejection,
Another Failed Test, an addition Violation of the Contract w Relief, A Fourth (4th) lawsuit against Neuro or perhaps something else like a concession to RELIEF and Actually abiding by the extensive terms of the signed contract???
Repayment of the $$$$$$$ owed to Relief ?
LOL NOW ITS BEFORE CHRISTMAS.....
NOTICE THE PATTERN????
GOAL LINE MOVED OVER AND OVER AND NEVER GET ANYTHING ACROSS
Expect NEWS for NRXP by or before CHRISTmas 2021.
Good luck and GOD bless,
Intravaneous ZYESAMI® (Avipdatil), Inhaled ZYESAMI® (Aviptadil Acetate) and BriLife™ are needed in the USA and across the whole world ASAP (As Soon As Possible). ICUs and hospitals are being overwhelmed and thousands of people in hospital ICUs are dying each and every day from SEVERE COVID-19.
https://www.freep.com/story/news/2021/11/28/covid-19-michigan-hospital-beds/8783134002/
85% of Michigan's hospital beds are full, some patients are being turned away
Kate WellsMichigan Radio
More than 85% of all intensive care unit (ICU) beds and 85% of all hospital inpatient beds in Michigan are currently full, a dangerous and potentially deadly level of overcrowding as the state’s health system is strained by a fourth surge of COVID-19 cases.
At least eight hospitals are 100% full, according to the latest state data. West Michigan’s largest hospital system, Spectrum Health, has reached a system-record number of patients in both its hospitals and its ICUs.
Dr. Darryl Elmouchi, Spectrum’s president, stood at his computer Friday in his Grand Rapids office, next to a row of windows looking out on a cold, gray day. Normally, they’d take up to 50 transfer patients each day from smaller hospitals or rural emergency rooms, he said. But now, they can’t.
“We’ll get phone calls saying we’re the 15th hospital they’ve called, and can we please help? And very often right now, the answer is no,” he said. “Because we have to take care of those people in front of us before we can take care of people that are coming from a distance. And that's really heartbreaking, and it’s hard.”
St. Joseph Mercy Hospital in Ann Arbor and St. Joseph Mercy Livingston Hospital are both 100% full, a spokesperson confirmed Friday.
"St. Joseph Mercy Ann Arbor and St. Joseph Mercy Livingston have been managing day-to-day ICU operations either at capacity or above standard capacity throughout much of November,” Dr. David Vandenberg, chief medical officer at St. Joseph Mercy Ann Arbor and St. Joseph Mercy Livingston, said in a statement. “While patient safety remains our No. 1 priority, the unrelenting volume of COVID-19 patients with advanced illness makes managing their care very difficult on our medical teams.”
Hospitals are also being hit by waves of non-COVID-19 patients whose conditions have worsened after months of delayed care during the pandemic. Emergency room waits are getting longer, ambulances are overwhelmed by demand, and patients are being sent greater distances to find a hospital that can take them.
“You could be at a hospital in the U.P. and not have someone to accept you at a bigger, more capable health system right now because of this,” Elmouchi said. “...You can get in a car accident, you can have a heart attack, and you don’t get the care that you otherwise would have at the right time. Things can be delayed or changed as a result of this. Every health system, every hospital, every doctor across the state is trying to do their best for everybody. But it's hard to do that when you're stretched in capacity.”
More than 4,000 adults and 58 children are hospitalized for confirmed or suspected COVID-19 overall. And currently, 2,651 of the state’s 3,114 ICU beds are occupied, according to the most recent state data.
Overcrowding at these levels isn’t just inconvenient. It can kill people. Once hospitals hit 75% ICU capacity, more patients die for medically-preventable reasons, according to a CDC study published last week in Morbidity and Mortality Weekly.
By looking at the impacts of hospital strain between July 2020 and July 2021, researchers predicted that once the nation’s ICU beds were at 75% capacity, an estimated additional 12,000 excess deaths would occur two weeks later. “As hospitals exceed 100% ICU bed capacity, 80,000 excess deaths would be expected two weeks later,” the authors said.
But those are national models that are hard to drill down to a state level: Neither the Michigan Department of Health and Human Services nor the Michigan Health and Hospital Association tracks deaths linked to hospital overcrowding.
“MDHHS does not have a model for excess deaths, but we work with facilities to monitor morgue capacity and have mobile resources to support additional morgue capacity if needed and requested,” spokesperson Bob Wheaton said Friday via email.
Asked whether any health systems have adopted crisis standards of care (an emergency mode to help health care workers determine who gets lifesaving care when resources are scarce), Wheaton said while they “aren’t aware of any facilities in full crisis standards of care ... several have reported being between contingency and crisis.”
On Nov. 9, Munson Healthcare announced it was moving to “Pandemic Level Red Status” for the first time in the organization’s history. That means it will prioritize “pandemic-related care ... above all other issues,” including pausing some services and assessing “non-urgent surgeries on a case-by-case basis to shift staff and resources to where they are needed most.”
And across the state, some ER patients are being “placed in hallways or conference rooms,” while hospitals divert others away “because there is no physical room or medical staff available to accept more patients,” the Michigan Health and Hospital Association said in a letter from Chief Medical Officers across the state earlier this week.
“... Just as hospitals and the staff working inside are and have been working at capacity, our emergency medical services (EMS) are also stressed and overworked. There may be times when capacity in the system is not adequate to accommodate the usual response and speed of transport, especially for out-of-area transfers.”
The U.S. Department of Defense has agreed to a request from Gov. Gretchen Whitmer’s administration for federal teams to come assist Spectrum Health in Grand Rapids and Beaumont Hospital in Dearborn.
Meanwhile, health leaders continue to plead with the public: Please, get vaccinated.
“Every day folks say, ‘Well, but you know, people with vaccines can still get COVID,’ ” Elmouchi said. “They can. But our data, as recently as two days ago, shows that 91% of those that are hospitalized ... in our 14 hospitals are here unvaccinated. And so the key to this in my mind is not, we're never going to get rid of (COVID). But if we can minimize it and make it much less of a severe illness, we'll do better.”
It would be one thing if COVID-19 cases and hospitalizations showed signs of leveling off, Elmouchi said. But the trend lines keep rising, with the state poised to break a record number of COVID inpatients.
“The COVID numbers day by day just keep creeping up,” he said. “And at this point, there’s not an end in sight to that.”
Good luck and GOD bless,
This bears repeating:
Beware of FUD (FEAR, UNCERTENTY AND DOUBT)
Only believe FACTS with links.
https://www.nrxpharma.com
Good luck and GOD bless,
(Old) James 18 hours ago FROM Y@H@@ INTERESTING THOUGHTS.
Curious about how the coming mediation will work out. Assume for the moment Relief has a much stronger position with the Agreement and IP, and the JJ/NRX position is legally and factually very weak. Those are not radical assumptions. If mostly true, then actual regulatory progress in December may play a big role in the mediation:
Scenario 1: JJ fails to coax any encouraging word out FDA, new efficacy data does not appear from anywhere, and the old data from our trial is either not provided or does not measure up as advertised in the top line. If so, Relief should play hardball and cut JJ out of the project as payback for deceptions, total noncompliance, and failure across the board. Or,
Scenario 2: New data comes forth, FDA indicates favorable movement or reconsideration, some path becomes visible which can get us to market. In that fact pattern, both companies should have a long chat and work out a new, improved Agreement.
This strategy might possibly be Part of the plan for January. But if this is the case, it puts enormous pressure on JJ and NRXP. IMO, If NRXP cannot produce ANY evidence of regulatory progress THIS MONTH, then fire the Contractor for cause, terminate the CA, and find someone who can do the job. Such as newly hired CMO, Ms. Varawaller, who can likely run circles around JJ - blindfolded.
TECHNICALS LOOK TO BE POINTING LOWER STILL FOR NRXP
https://stockcharts.com/h-sc/ui?s=NRXP&p=D&b=5&g=0&id=p27695432074
perhaps new lows, imho.
Watch the stock price and volume of NRXP right before the close of trading TODAY!!!
I think I sill take a nap and dream about the gifts I will get on Saint Nicholas Day 6 December 2021.
Good luck and GOD bless,
ASK YOURSELF WHY.....
@Jonathan_Javitt is withholding information from and REFUSING to be honest with shareholders...
JUST A FEW OF THE WITHHELD ITEMS: (there are MANY more)
#1 Status of Type A meeting
#2 Status of Georgia EUA
#3 Refusal to publish FDA denial
#4 Refusal to answer why Javitt listed as author on preprint wrote we had EUA approval in Ukraine, Caucus Region and India
#5 Refusal to disclose on what date they were first advised (directly or indirectly) That EUA’s & BTD Were going to be denied
#6 refusal to disclose on what date and why they hired a new statistician to re-analyze the data of Javitts botched trial yet again.
#7 Refusal to advise and disclose why they filed for a type a meeting a week before they supposedly were surprised by FDA denial
Great investors do not live or die based on one PR. Great investors always keep site of the Big Picture.
Beware of FUD (FEAR, UNCERTENTY AND DOUBT)
Only believe FACTS with links.
https://www.nrxpharma.com/brilife-covid19-vaccine-omicron-variant/
Good luck and GOD bless,
Dec. 3, 2021 News - Updates from the CEO
CEO Update: The BriLife™ COVID-19 Vaccine and the Omicron Variant
December 3, 2021
Yesterday, the Israel Institute for Biological Research (IIBR) announced that it is currently studying the new Omicron variant of the SARS-CoV-2 to assess the extent to which this variant is neutralized by antibodies produced by the BriLife™ COVID-19 vaccine.
Previously, the IIBR released a scientific report demonstrating effective antibodies against the Delta variant in a sample of patients who were vaccinated with BriLife.
The Omicron variant is, by far, the most complicated of all mutations studied thus far. While the Delta variant has 18 spike protein mutations, experts have identified 43 spike protein mutations for Omicron. It’s important to note that while the number of mutations does not necessarily define the severity of illness for those infected, a larger number of mutations may pose additional challenges to the immunity produced by vaccines targeting the original wild-type virus.
NRx will continue to provide updates as they become available about the BriLife COVID-19 vaccine, its development, and data produced from laboratory experiments and clinical trials. We are fortunate that the IIBR, as a government laboratory, is organized to receive new variants of the virus as they are detected and isolated.
Please check the NRx website often to ensure you receive the most current information about the BriLife COVID-19 vaccine and all of our efforts to advance the NRx pipeline. Click this link to subscribe to our mailing list. https://www.nrxpharma.com
Thank you for following our journey.
-Jonathan
Good luck and GOD bless,
SO LET ME GET THIS STRAIGHT...
THE NATION OF GEORGIA APPROVED EUA ALMOST 5 MONTHS AGO BUT WAS SO IMPRESSED WITH ZYESAMI AND WANTED IT SO BAD THEY HAVE ORDERED A GRAND TOTAL OF ZERO DOSES!!!
JAVITT/NRX REFUSE TO ANSWER VALID QUESTIONS ABOUT WHAT THEY KNOW FROM FDA ABOUT TYPE A MEETING...
JAVITT/NRX REFUSE TO ANSWER WHEN THE STARTED THE NEW DATA ANALYSIS BY NEW STATISTICIAN?
JAVITT/NRX REFUSE TO SAY WHEN THEY FIRST NEW (in any way) THAT EUA’s & BTD WERE GOIN G TO BE DENIED
AND PEOPLE WONDER WHY @Jonathan_Javitt is thought to be a FRAUD LIAR!!
” SOON MANY OTHER NATIONS WILL FOLLOW HUH JON”???
Georgejjl.. I READ IT.. QUESTION....
HOW HAS THE MARKET REACTED SINCE THAT WAS PUT OUT??
**** NEW CHALLENGE *****
SINCE NO ONE COULD WIN THE FIRST CHALLENGE OF FINDING EVIDENCE THAT TYPE A MEETING WAS ACCEPTED.... HERE IS NEW ONE... TRY AND FIND ANSWERS TO QUESTIONS BELOW... (hint you will be ignored by @Jonathan_Javitt and NRX WITHHOLDING INFO)
DEMAND TO KNOW THE LATEST ON THE TYPE A MEETING!!!!
DEMAND TO KNOW STATUS OF FDA DISCUSSION ABOUT 150 PATIENT DATA JAVITT WANTED TO SHOW THEM FROM BLINDED TRIAL
DEMAND TO KNOW IF FDA ACCEPTED JAVITT WANTING TO BRING IN DR’s ETC TO SPEAK TO FDA...
THE TRUTH IS OUT THERE... TRY AND GET ANSWERS AND YOU WILL SEE THEY ARE HIDING TRUTHS AND REFUSING TO ANSWER
Without a substantial concession to RELIEF and a quick honorable settlement by the Doc, it sure looks like another long down leg for NEURO.
The lawyers are not going away evidently.
All the Neuro Shareholders Happy now ??
A MUST READ!!!
Read the following slowly and thoroughly. There is a lot of information to digest and understand.
ZYESAMI® Update
Dr. Francis Collins, Director of the NIH, identified ZYESAMI as one of a small number of remaining candidates still being considered by the NIH to treat COVID-19 from a starting field of 600 or more candidate medicines. Dr. Collins stressed that medicines were selected based both on their potential for efficacy and their demonstrated potential for manufacturability. During the quarter, Dr. Anthony Fauci gave briefings that included ZYESAMI at the White House and Congress.
NIH has funded the ACTIV-3b TESICO trial, an FDA Phase 3 trial that has now enrolled more than half of its targeted 660 patients. The trial randomizes patients with COVID-19 respiratory failure to ZYESAMI vs. Veklury® (remdesivir) and placebo in a factorial design trial (NCT04843761). The independent Data Safety Monitoring Board met to assess safety and futility at the enrollment midpoint of more than 300 enrolled patients and reported no drug-related Serious Adverse Events. The trial was cleared for continued enrollment.
During the quarter, NRx developed and validated a patentable formulation, manufacturing method, and container closure system that enables NRx to produce ZYESAMI (aviptadil) at a commercial scale in lot sizes of up to 100,000 doses with shelf stability of 150 days or more. Based on this development:
NRx updated Module 3 of its FDA IND, providing the documentation necessary to show the full manufacturing capability of NRx to provide ZYESAMI upon potential regulatory approval. FDA reviewed the Module 3 and identified no “clinical hold” items.
NRx established an EU/UK compliant manufacturing facility and, in October, passed the qualified person (Q.P.) audit required to release medicine in the European Union and the United Kingdom.
Based on the above manufacturing developments, commercial-scale ZYESAMI will now be incorporated into the NIH ACTIV-3b trial in the U.S., U.K., E.U., and Brazil.
Based on the above manufacturing developments, NRx is preparing a New Drug Application for ZYESAMI to be submitted under the Accelerated Approval (biomarker) pathway with the FDA.
NIH has introduced ZYESAMI to E.U. and U.K. regulators via the ACTIV-3b trial, and NRx has retained regulatory counsel to submit emergency use and drug approval requests in those regulatory jurisdictions.
NRx continues funding the development and providing of ZYESAMI in two additional clinical trials that evaluate the potential of ZYESAMI for inhaled use: a phase 2b/3 trial sponsored by NRx in the U.S. and the BARDA-funded I-SPY trial.
In 2022 NRx anticipates launching a clinical trial of ZYESAMI for the treatment of sepsis-related ARDS for which Stony Brook University was previously awarded an FDA Orphan Drug Designation. NRx is actively exploring the initiation of clinical trials for ZYESAMI in other lethal diseases that affect the lung, for which effective treatments are currently lacking.
NRx has partnered with the PolyPeptide Group to develop a proprietary large-scale manufacturing process for aviptadil drug substance (API), capable of yielding 5 million doses per manufacturing batch. Prior to this development, aviptadil was only manufactured in a high-cost process yielding 100,000 doses per batch. This historic process was discontinued by its sponsor as no longer environmentally sustainable. During the quarter, NRx received the first million dose batch of aviptavil drug substance and has secured delivery of 7 million doses of aviptadil drug substance through Q2 2022.
NRx has entered into a collaboration with IQVIA™ (NYSE:IQV), a leading global provider of advanced analytics, technology solutions and clinical research services to the life sciences industry to lead the pharmacovigilance services and medical information in preparation for potential regulatory actions regarding ZYESAMI.
NRx has partnered with MannKind Corporation (Nasdaq:MNKD) to develop a ZYESAMI inhaler for respiratory conditions.
NRx announced that it has signed an agreement with Cardinal Health (NYSE: CAH) to provide third-party logistics and distribution of ZYESAMI upon regulatory approval.
https://www.nrxpharma.com/nrx-pharmaceuticals-reports-third-quarter-2021-business-update-and-financial-results-2/
Good luck and GOD bless,
NRx Pharmaceuticals' stock jumps following new analysis of its potential Covid-19 drug
https://www.bizjournals.com/philadelphia/news/2021/11/29/nrx-pharmaceuticals-covid-19-therapy-zyesami-study.html?ana=yahoo
Good luck and GOD bless,
https://stockcharts.com/h-sc/ui?s=NRXP&p=D&b=5&g=0&id=p27695432074
Sure looks headed back down again to perhaps new lows, a big wack today again
Looks like no progress on reconciliation with Relief in the news.
The Doc may run out of $$$$$$$$$ and companies that want to extend credit, however the lawyers might be willing to litigate pending payment from the bankruptcy court and remaining assets.
Maybe the attorneys will be agreeable to the Contract Agreement Terms, as it seems the Doc may be impossible to satisfy
with any signed agreement. IMHO, The Doc would be much better off becoming an employee of Relief, and have some kind of stock options granted based on performance, instead of, what seems like, his own mindless out of control contract breaking self will that has produced very little to nothing so far.
|
Followers
|
54
|
Posters
|
|
|
Posts (Today)
|
0
|
Posts (Total)
|
1168
|
|
Created
|
12/15/20
|
Type
|
Free
|
| Moderators ProfitScout Trooperstocks | |||





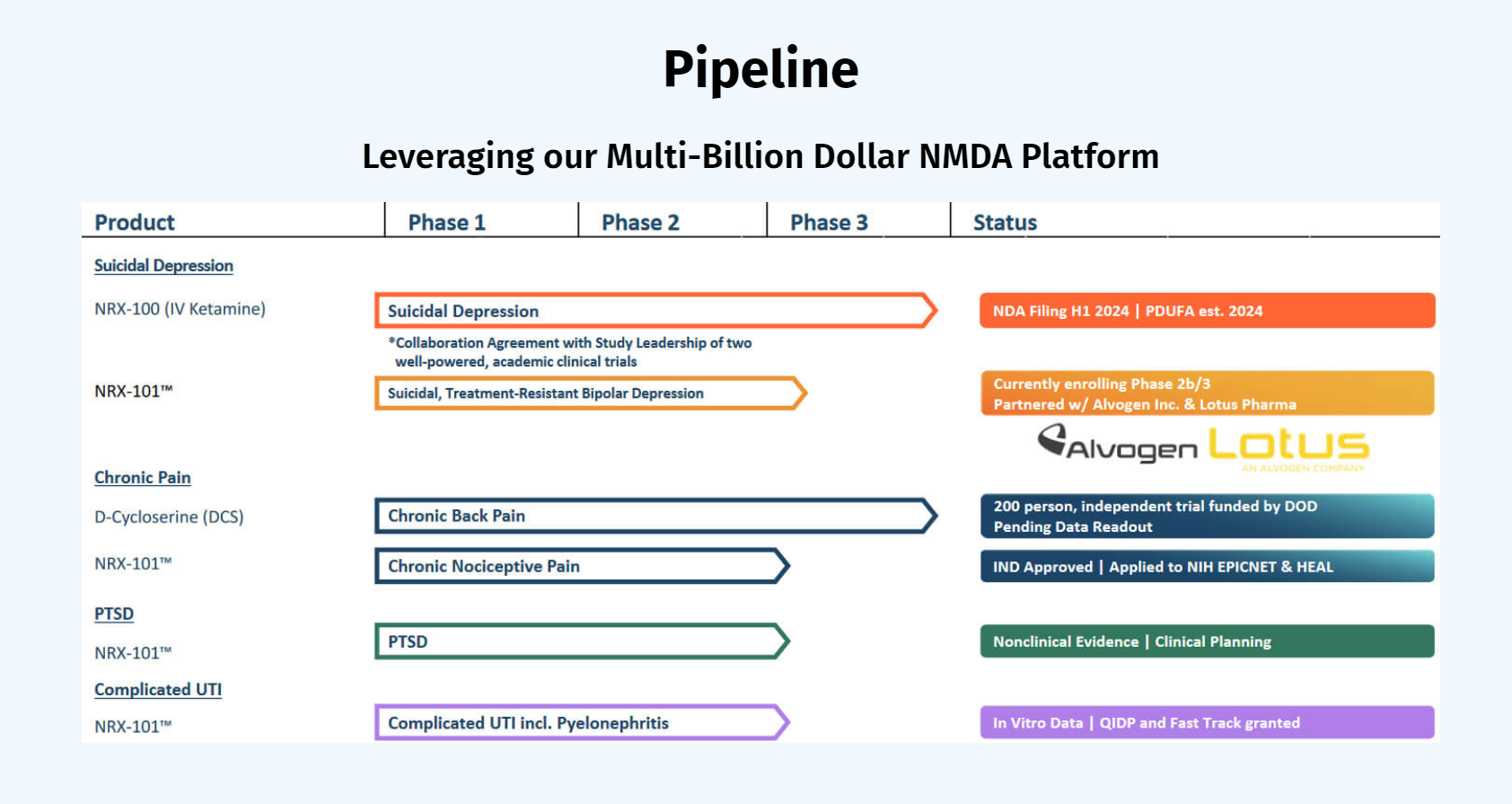
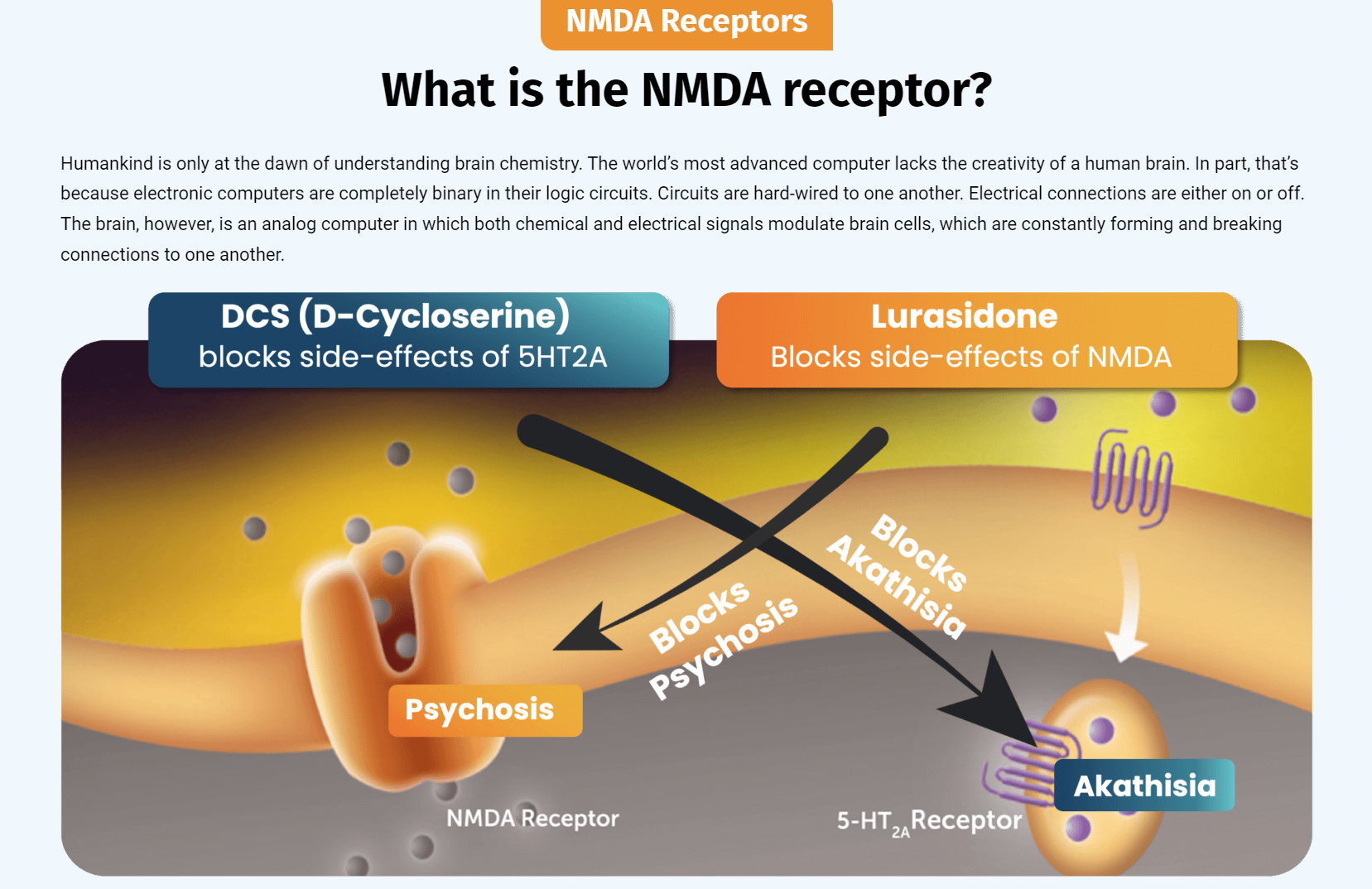

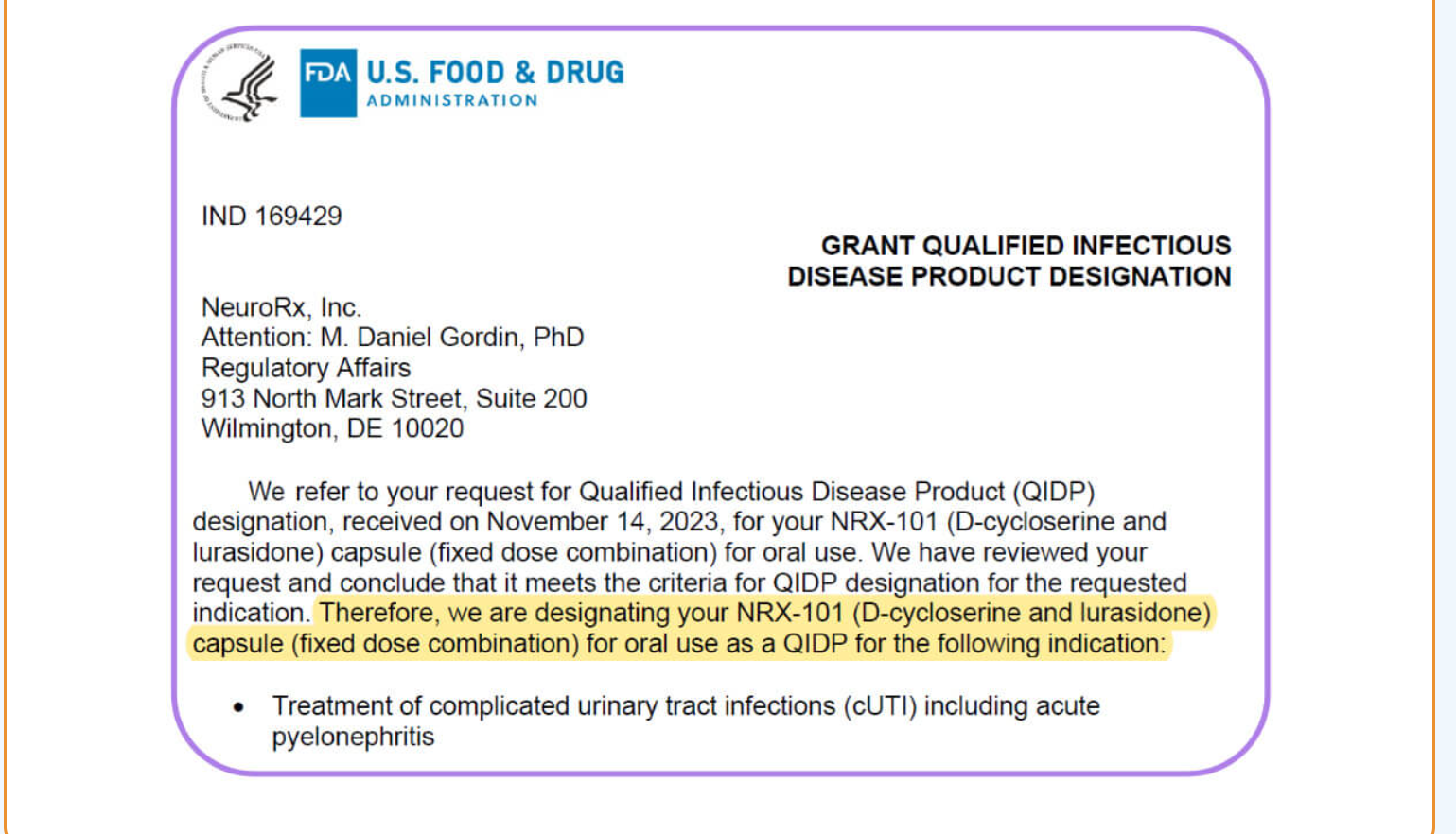





Both drugs demonstrated > 50% response for treating depression. NRX-101 demonstrated a mean 76% reduction in symptoms of akathisia compared to lurasidone that was sustained over 42 days (Effect...

New data demonstrates no impact of NRX-101 on gut or vaginal flora – considered primary causes of pseudomembranous colitis due to C difficile and vaginal yeast infections NRX-101 previously...
Formulation based on prior patents by NRx founder Achieved pH neutral formulation of ketamine, potentially enabling both intravenous (IV) and subcutaneous (SQ) administration Company expects...
Data transferred for independent statistical analysis Top-line data expected in April 2024 RADNOR, Pa., April 8, 2024 /PRNewswire/ -- NRx Pharmaceuticals, Inc. (Nasdaq: NRXP) ("NRx...
Four potential near-term milestones, including data from two clinical trials, an NDA filing and an upcoming share dividend -- 50% reduction in corporate overhead and 25% reduction in overall net...
Four potential near-term milestones, including data from two clinical trials, an NDA filing and a share dividend RADNOR, Pa., March 28, 2024 /PRNewswire/ -- NRx Pharmaceuticals, Inc. (Nasdaq: NRXP) ("
NRx Pharmaceuticals, Inc. (Nasdaq: NRXP) ("NRx Pharmaceuticals", the "Company"), a clinical-stage biopharmaceutical company, today announced that it will release its fourth quarter and full year...
NRx Pharmaceuticals, Inc. (Nasdaq:NRXP) ("NRx Pharmaceuticals", the "Company"), a clinical-stage biopharmaceutical company, today announced that Dr. Jonathan Javitt, Chairman and Chief Scientist...
NRx Pharmaceuticals, Inc. (Nasdaq: NRXP) ("NRx Pharmaceuticals", the "Company"), a clinical-stage biopharmaceutical company, today announced that it will release its fourth quarter and full year...
61.4% (56,781,354) of eligible shares voted 94.4% of votes were cast in favor of the resolution RADNOR, Pa., March 21, 2024 /PRNewswire/ -- NRx Pharmaceuticals, Inc. (Nasdaq: NRXP) ("NRx...
NRx Pharmaceuticals Board of Directors has authorized its Chairman, CEO, and management to take all necessary steps to affect the Dividend and Royalty Rights to NRXP Shareholders and applicable...
Initiative is intended to protect shareholder value through continued compliance with Nasdaq listing rules and elimination of naked short sales positions in the Company's securities The Company is...
Company to receive first allocation of ketamine for sale by month end Partners preparing to ship IV Ketamine to full range of customers via 503a and 503b pharmacies NRx Pharmaceuticals and HOPE...
Marks a major step in the development of what could be the first drug approved for Suicidal Bipolar Depression The study database is being cleaned and locked; statistical analysis and top-line...
NRx Pharmaceuticals received approximately $1.0 million in cash from an existing investor Shares were sold at $0.38, a 26.7% premium the recent share offering, along with one common 5-year warrant...
In the news release, NRx Pharmaceuticals, Inc. Announces Pricing of $1.5 Million Underwritten Public Offering of Common Stock, issued 27-Feb-2024 by NRx Pharmaceuticals, Inc. over PR Newswire, we...
NRx Pharmaceuticals, Inc. (Nasdaq: NRXP), ("NRx Pharmaceuticals" or the "Company"), a clinical-stage biopharmaceutical company, today announced that it intends to offer to sell shares of its...
Presentation available on NRx Pharmaceuticals website at https://www.nrxpharma.com/hope-therapeutics/ KEY UPDATES ARE AS FOLLOWS: NRx management is proposing to award 50% of founding shares in...
DISCLAIMER:
Nothing in the contents transmitted on this board should be construed as an investment advisory, nor should it be used to make investment decisions.
There is no express or implied solicitation to buy or sell securities.
The author(s) may have positions in the stocks or financial relationships with the company or companies discussed and may trade in the stocks mentioned.
Readers are advised to conduct their own due diligence prior to considering buying or selling any stock. All information should be considered for information purposes only.
No stock exchange has approved or disapproved of the information here.
| Volume | |
| Day Range: | |
| Bid Price | |
| Ask Price | |
| Last Trade Time: |
