Register for free to join our community of investors and share your ideas. You will also get access to streaming quotes, interactive charts, trades, portfolio, live options flow and more tools.
Another Fine Canadian Company ...https://finance.yahoo.com/news/village-farms-international-named-fastest-110000817.html
I agree, this mgmt is getting things done wisely. I like how they bought Shelter Cannabis and sold a vacant lot for 1.9 million. I like the fact they
have a good cash balance. Hoping this quarter And the next will show us what they are really worth.
Agreed as well ; I am liking the New Management . After a Dim Future for a while things are looking up here !
The Positive combined with this low share Price and I'm back buying after I thought it would eventually be a Tax wright off .
looking forward to this next Quarterly Report.
Would be nice if some of this seemingly good news would translate into a SP bump ...
News out . Happy they decided to sell the Tilray shares for cash.
https://www.otcmarkets.com/stock/MEDIF/news/MediPharm-Labs-Update-on-9M-Settlement-Agreement---All-Tilray-Shares-Sold-and-Converted-to-Cash?id=418769
Opinions on the Tilray commitments ??
News is deceiving , article title sounds bad but is really good for MEDIF
Here is the link , but I pasted highlights below.
https://www.otcmarkets.com/stock/MEDIF/news/MediPharm-Labs-Settles-an-Outstanding-Claim-for-9M?id=415985
Settlement Highlights
Upon the acquisition of HEXO Corp. by Tilray Brands (NASDAQ: TLRY), MediPharm and Tilray sought a resolution that was favorable to each party and established a long-term supply relationship in connection with the settlement agreement. Under the agreement, MediPharm will receive a total value consideration of $9 million including:
$3 million immediate cash payment;
$4.5 million in common shares of Tilray;
$1 million in Tilray cannabis products, including high-quality flower and extractable bio-mass; and
$500,000 supply agreement to provide Tilray with MediPharm products and services over four years.
This long-term supply agreement will allow MediPharm to establish a positive ongoing business relationship with a global leader in the cannabis industry. The deal is also expected to benefit Tilray through access to MediPharm's unique pharmaceutical products and services both domestically and internationally, including products produced under the company's US FDA Foreign Drug Site registration and Canadian Pharmaceutical Drug Establishment License.
That's 1.9 million for all you bean counters. Its still a great move.
Two million dollar discount on VIVO purchase when you can sell the vacant land you don't need.
https://www.otcmarkets.com/stock/MEDIF/news/MediPharm-Labs-Completes-Sale-of-Vacant-Land-for-Cash-Proceeds-of-19-million?id=412951
Thanks for the heads up Bro ... 30% gain last 2 days .
Jeeez , new ATL .....Anybody buying here ?
Medipharm Labs Provides Update on VIVO integration and Board Appointee.
Thinning out the employees on many levels. Getting ready for a lean mean International Cannabis machine.
https://www.otcmarkets.com/stock/MEDIF/news/MediPharm-Labs-Provides-Update-on-VIVO-Integration-and-Board-Appointee?id=396478
MEDIF: effective April 5,2023: Plan of Arrangement. Shareholders of VIVO will receive 0.2910 of one common share of MediPharm for each VIVO Share held. Fractional shares will be rounded down to the nearest whole number.
FINRA will delete the VVCIF symbol:
https://otce.finra.org/otce/dailyList?viewType=Deletions
Medipharm Completes Acquisition of VIVO Cannabis.
link:
https://www.otcmarkets.com/stock/MEDIF/news/MediPharm-Labs-Corp-Completes-Acquisition-of-VIVO-Cannabis-Inc-and-Confirms-Final-Ownership-Ratio?id=395401
Medipharm sets date for 4th QTR and full year 2022, Shareholder meeting info also
https://www.otcmarkets.com/stock/MEDIF/news/MediPharm-Labs-Sets-Date-to-Report-Fourth-Quarter-and-Full-Year-2022-Financial-Results?id=394779
MediPharm Labs Corp. Announces Voting Results From Special Meeting of Shareholders
Looks good for the merger.
Link:
https://www.otcmarkets.com/stock/MEDIF/news/MediPharm-Labs-Corp-Announces-Voting-Results-From-Special-Meeting-of-Shareholders?id=394044
MediPharm Labs Provides Update on Clinical Trial Progress Including FDA Approval of Partner Study
https://www.otcmarkets.com/stock/MEDIF/news/MediPharm-Labs-Provides-Update-on-Clinical-Trial-Progress-Including-FDA-Approval-of-Partner-Study?id=391692
MediPharm Labs Corp. Announces Mailing and Filing of Joint Circular for Special Meeting of Shareholders to approve Arrangement with VIVO Cannabis Inc.
https://www.otcmarkets.com/stock/MEDIF/news/MediPharm-Labs-Corp-Announces-Mailing-and-Filing-of-Joint-Circular-for-Special-Meeting-of-Shareholders-to-approve-Arrang?id=390542
VIVO Cannabis Inc. Announces Mailing and Filing of Joint Circular for Special Meeting of Shareholders to approve Arrangement with MediPharm Labs Corp.
https://www.otcmarkets.com/stock/VVCIF/news/VIVO-Cannabis-Inc-Announces-Mailing-and-Filing-of-Joint-Circular-for-Special-Meeting-of-Shareholders-to-approve-Arrangem?id=390450
They're never gonna get that $$$ that HEXO owes them , are they ???
Thanks for posting . I wondered what they would do with all that money on hand.
I'm was hoping for UPLIST news, but I'll take a VIVO aquisition.
Merry Christmas to all, Fingers crossed for 2023
MediPharm Labs Corp. to Acquire VIVO Cannabis Inc.
Two global leaders in the medical wellness cannabis industry expected to combine complementary strengths of diversified revenue in multiple markets and channels to create a pro-forma Combined Company with over $50M in annualized revenue, based on Q3 2022.(1)(2)(3)(4)
Pro-forma Combined Company is expected to have positive EBITDA(5) synergies of between $7M to $9M on an annualized basis within 12 months closing of the Transaction.(1)(2)(3)(4)
Transaction is expected to accelerate MediPharm Labs’ path to profitability, with possibility to reach positive EBITDA(5) and cash flow targeted in the first half of 2024.(1)(2)(3)
BARRIE, Ontario, December 22, 2022 (CISION NEWSWIRE) -- MediPharm Labs Corp. TSX: LABS) (OTCQX: MEDIF) (FSE: MLZ) (“MediPharm”, “MediPharm Labs” or the “Company”) and VIVO Cannabis Inc. (TSX: VIVO) (OTCQX: VVCIF) (“VIVO”) today announced that MediPharm and VIVO have entered into a definitive arrangement agreement (the “Arrangement Agreement”) whereby MediPharm has agreed to acquire VIVO in an all-equity business combination transaction (the “Transaction”). The Transaction is expected to combine two highly complementary businesses, creating a unique and market differentiating international medical cannabis leader. Upon the completion of the Transaction, existing MediPharm shareholders are expected to own between 65% and 79% of the combined company resulting from the Transaction (the “Combined Company”) and VIVO shareholders are expected to own between 35% and 21% of the Combined Company.
Under the terms of the Arrangement Agreement, holders of common shares of VIVO (“VIVO Shares”) will receive between 0.2110 and 0.4267 common shares of MediPharm (the “MediPharm Shares”) for each VIVO Share held, subject to adjustment (the “Exchange Ratio”). The Exchange Ratio at closing will be determined by the amount of interim working capital of VIVO (the “Interim Working Capital”), taking into account any funds advanced by MediPharm to VIVO up to a maximum of $3.75 million, by way of a promissory note (the “Note”). The Interim Working Capital will allow VIVO to continue operations in the ordinary course throughout the proposed closing period. Holders of VIVO Shares will be entitled to receive such number of common shares of the Combined Company as is equivalent to 35% of the issued and outstanding common shares of the Combined Company (or an Exchange Ratio of 0.4267), which may be reduced depending on the Interim Working Capital of VIVO prior to closing, to a minimum of 21% of the issued and outstanding common shares of the Combined Company (or an Exchange Ratio of 0.2110).(1)
Key Transaction Highlights(1)
Leading Pharmaceutical Cannabis Company: The acquisition of VIVO will add established Australian and German medical cannabis brand Beacon Medical, an industry-leading medical cannabis clinic business Harvest Medicine, and a longstanding Canadian medical sales platform Canna Farms Medical.
Direct to Patient Sales:(1)(3) VIVO’s medical sales channel, Canna Farms Medical, was the first Licenced Producer in British Columbia and has supported over 60,000 patients since 2014.(6) Following the Transaction, it is anticipated that this platform will provide patients with a more diverse product portfolio that includes existing MediPharm products. Direct to patient sales generally result in a better gross margin with the ability to bypass provincial distributors. VIVO’s clinic business Harvest Medicine will allow real-time product feedback and clinical insights on MediPharm products.
Diversified Revenue Profile with Strong Canadian Base: (1)(3) The pro-forma Combined Company is expected to provide fulsome Canadian market coverage with cultivation and manufacturing expertise, and a full suite of dried flower & derivative products with both established medical and adult-use wellness distribution channels.
Expanding International Medical Cannabis Opportunity:(1)(2)(3)(4) The pro-forma Combined Company’s international distribution will cover European and Asia-Pacific markets through established, revenue-generating agreements. The VIVO Napanee Ontario facility is EU-GMP certified for cultivating and packaging flower and the MediPharm Barrie Ontario facility is GMP certified for flower alternative format medical products. With two distinct international platforms, the pro-forma Combined Company is expected to open many new product offerings for existing distribution channels and geographies. The pro-forma Combined Company would have annualized international revenue of over $20M, based on Q3 2022.
Revenue and Cost Synergies Realizable in the Near-Term:(1)(2)(3)(4) Using forecasts derived collaboratively by both management teams, along with revenue and cost synergy estimates, the pro-forma Combined Company aims to find positive EBITDA(5) synergies to the magnitude of between $7M to $9M on an annualized basis, and could reach positive EBITDA and cash flow in the first half of 2024.
Balance Sheet Strength:(1)(2)(3)(4) Anticipated combined cash position of approximately $30 million (as reported September 30, 2022 and including the subsequent sale of MediPharm Labs Australia Pty Ltd.), less than $2.5M in debt on closing, and unencumbered ownership of all major assets. This strength is expected to provide confidence in the Combined Company’s balance sheet to execute on its strategic growth roadmap, despite the macro backdrop of capital markets that continue to soften.
Management Commentary(1)
“MediPharm Labs has been actively pursuing M&A opportunities in the industry since June of 2022. When we first met with the management of VIVO, it was immediately apparent that this was a natural fit from a strategy, values, approach and financial perspective. Both companies have a primary medical wellness vs. recreational focus. Both have a strong history in the medical cannabis sector, investing in GMP production, clinical trials and building diversified medical revenue streams internationally. As many cannabis companies solely focused on the Canadian recreational space, both VIVO and MediPharm saw the future in cannabis wellness products and in pharmaceutical drugs containing cannabis. We were mutually focused on the global opportunities for GMP facilities as international regulations evolved with ever higher quality and regulatory standards. Through this business combination, we have identified the potential for millions in cost and revenue synergies to solidify our leadership for the long term”(1), said David Pidduck, Chief Executive Officer, and Director of MediPharm. “We look forward to expanding our offerings within each others’ respective channels, including medical patients, wellness consumers, and through our respective global partners. We have the chance to offer even more options for individuals using cannabis to potentially improve their quality of life.”
“VIVO has been exploring options to continue its goals of growth and profitability, of being a best-in-class provider of medical cannabis. By leveraging our broad patient base and EU-GMP investments to date and combining our business with MediPharm we achieve just that. In the current capital markets both inside and outside of our industry, capital investment opportunities are extremely limited and we were attracted to MediPharm as a partner given their cash position of over $19.5M, at the end of Q3, and virtually no debt. As a Combined Company we can service the small outstanding amount of VIVO debt, continue international operations and invest in the future to grow the Combined Company and achieve profitability sooner than by going at it alone”, said Ray Laflamme, Chief Executive Officer, and Chairman of the Board of VIVO. “This transaction brings a great opportunity to our employees, shareholders and patients. The clinical trial initiatives at MediPharm with their standardized non-flower pharmaceutical cannabis products align well with our patient-first values and I am excited about the future of what this Combined Company will achieve. Together we are an even stronger, a more diversified and a more credible global medical cannabis player.”
Terms of the Transaction
The Transaction is to be carried out by way of a court-approved plan of arrangement under the Canada Business Corporations Act. The Transaction will require the approval of: (a) (i) two-thirds of the votes cast by shareholders of VIVO, and, if required, (ii) a simple majority of the votes cast by minority VIVO shareholders in accordance with Multilateral Instrument 61-101 – Protection of Minority Security Holders in Special Transactions, at a special meeting of VIVO shareholders expected to take place in the first quarter of 2023 (the “VIVO Meeting”); and (b) a majority of the votes cast by shareholders of MediPharm at a special meeting of MediPharm shareholders expected to take place in the first quarter of 2023 (the “MediPharm Meeting”).
MediPharm has entered into voting and support agreements with each of its directors and officers and each person that, to the knowledge of MediPharm, holds at least 5% of the MediPharm Shares, pursuant to which these parties have agreed, subject to certain rights of withdrawal, to vote in favour of the Arrangement and not to dispose of their MediPharm Shares.
VIVO has entered into voting and support agreements with each of its directors and officers and each person that, to the knowledge of VIVO, holds at least 5% of the VIVO Shares, pursuant to which these parties have agreed, subject to certain rights of withdrawal, to vote in favour of the Arrangement and not to dispose of their VIVO Shares.
Completion of the Transaction is subject to court and regulatory approvals, including the approval of the Toronto Stock Exchange, which are currently expected to be received during the first half of 2023.(1) The transaction is expected to close during the first half of 2023.(1)
The Arrangement Agreement contains certain customary provisions, including covenants in respect of non-solicitation of alternative acquisition proposals for VIVO and a termination fee of $1M payable to either party in certain circumstances. There can be no assurance that any payments will be made with respect of the Note.
Further details with respect to the Transaction will be included in an information circular to be mailed to VIVO shareholders in connection with the VIVO Meeting and to MediPharm shareholders in connection with the MediPharm meeting. A copy of the Arrangement Agreement and information circular will be filed on each of MediPharm’s and VIVO’s SEDAR profiles at www.sedar.com.
Fairness Opinions
The MediPharm board of directors obtained a fairness opinion from Hyperion Capital Inc. on December 21, 2022 (the “Hyperion Opinion”) stating that, as of the date of the Hyperion Opinion and subject to the assumptions, limitations and qualifications contained in the Hyperion Opinion, the consideration to be paid by MediPharm pursuant to the Transaction is fair, from a financial point of view, to MediPharm shareholders. The VIVO board of directors obtained an independent fairness opinion from ATB Capital Markets Inc. on December 20, 2022 (the “ATB Opinion”) stating that, as of the date of the ATB Opinion and subject to the assumptions, limitations and qualifications contained in the ATB Opinion, the consideration to be received by VIVO shareholders pursuant to the Transaction is fair, from a financial point of view, to VIVO shareholders.
Recommendation of the MediPharm Board
The board of directors of MediPharm has reviewed and approved the Transaction. After obtaining the Hyperion Opinion and consulting with its financial and legal advisors, among other considerations, the board of directors of MediPharm have unanimously: (i) determined that the Transaction is in the best interests of MediPharm; (ii) resolved to recommend that MediPharm shareholders vote in favor of the Transaction; and (iii) determined that the consideration to be paid by MediPharm pursuant to the Transaction is fair, from a financial point of view, to MediPharm shareholders.
Recommendation of the VIVO Board
The board of directors of VIVO has reviewed and approved the Transaction. After obtaining the ATB Opinion and consulting with its financial and legal advisors, among other considerations, the independent members of the board of directors of VIVO have unanimously: (i) determined that the Transaction is in the best interests of VIVO; (ii) resolved to recommend that VIVO shareholders vote in favor of the Transaction; and (iii) determined that the consideration to be received by VIVO shareholders pursuant to the Transaction is fair, from a financial point of view, to VIVO shareholders.
Financial and Legal Advisors
Hyperion Capital Inc. is acting as financial advisor to MediPharm and provided the Hyperion Opinion to the MediPharm board of directors. Aird & Berlis LLP is acting as legal counsel to MediPharm.
Stoic Advisory Inc. is acting as financial advisor to VIVO. ATB Capital Markets Inc. acted as financial advisor for the restructuring of VIVO's convertible debentures and provided the ATB Opinion to the VIVO board of directors. Bennett Jones LLP is acting as legal counsel to VIVO.
Notes:
This is forward-looking information and based on a number of assumptions. See “Cautionary Note Regarding Forward-Looking Information“ and “Assumptions”.
Based on both costs and revenue opportunities identified by MediPharm and VIVO management. Revenue opportunity assumed that both existing products may be sold into the existing sales channels of both VIVO and MediPharm. Costs savings estimated depends on the eliminating duplicated public company expenses and redundant corporate infrastructure.
This target, and the related assumptions, involve known and unknown risks and uncertainties that may cause actual results to differ materially. While MediPharm and VIVO believe there is a reasonable basis for this target, such target may not be met. Actual results may vary and differ materially from the targets. See “Assumptions”.
Certain financial information included in this press release is neither audited nor reviewed. Where possible, the information has been constructed by management from available audited or audit reviewed financial statements. Where no audited or audit reviewed information has been available, additional management accounting information has been utilized to construct financial information. Readers are cautioned not to place undue reliance on such information.
This is a non-IFRS reporting measure. For a reconciliation of this to the nearest IFRS measure, see “Non- IFRS Measures” below.
Based on patient count details collected and provided by licence holder CannaFarms, a wholly owned subsidiary of VIVO.
About MediPharm Labs
Founded in 2015, MediPharm Labs specializes in the development and manufacture of purified, pharmaceutical-quality cannabis concentrates, active pharmaceutical ingredients (API) and advanced derivative products utilizing a Good Manufacturing Practices certified facility with ISO standard-built clean rooms. MediPharm Labs has invested in an expert, research driven team, state-of-the-art technology, downstream purification methodologies and purpose-built facilities with five primary extraction lines for delivery of pure, trusted and precision-dosed cannabis products for its customers. Through its wholesale and white label platforms, MediPharm Labs formulates, develops (including through sensory testing), processes, packages and distributes cannabis extracts and advanced cannabinoid-based products to domestic and international markets.
In 2021, MediPharm Labs received a Pharmaceutical Drug Establishment Licence from Health Canada, becoming the only company in North America to hold a domestic Good Manufacturing Licence for the extraction of natural cannabinoids. The Company
About VIVO Cannabis
VIVO Cannabis® is recognized for trusted, quality medical cannabis products and services. It holds production, sales and research licences from Health Canada and operates world-class indoor cultivation facilities. VIVO has a collection of brands, each targeting different customer segments, including Canna Farms™, Beacon Medical®, Fireside™, and Lumina™. Harvest Medicine™, VIVO’s patient-centric network of medical cannabis clinics, has serviced over 200,000 patient visits. VIVO focuses its international efforts on Germany and Australia. For more information visit: www.vivocannabis.com
Assumptions
In developing the financial guidance set forth above, MediPharm and VIVO made the following assumptions and relied on the following factors and considerations:
The targets are based on MediPharm and VIVO’s historical results including annualized revenue from its interim financial results for the period ended September 30, 2022, as adjusted for subsequent events including completion of the Transaction.
Revenue sustainability and growth depend on a variety of factors, including among other things, location, competition, legal and regulatory requirements. Prices are projected forward at recently realized wholesale and direct to patient prices.
Cost of goods sold, before taking into account the impact of value changes in biological assets (which are non-cash in nature), and, accordingly, are excluded from calculations of EBITDA, have been projected based on estimated costs of production and capacity available from a similar supply chain.
The immediate reduction of public company professional and service fees, such as but not limited to, errors and omissions insurance, audit services, listing expenses and external legal fees.
Implied redundancy of employee roles in the Combined Company, mainly in corporate functions. Impacted employee severance fees are calculated on current employment agreements and Employment Standards Act (Ontario).
No changes to existing medical cannabis legislation and regulations in Canada, Germany, Australia and Brazil.
All VIVO and MediPharm regulatory licenses remain in good standing with domestic and international regulators, particular Good Manufacturing Practices (GMP).
Non-IFRS Measures
This news release contains references to certain non-IFRS financial measures, including “EBITDA”, which means earnings before interest, taxes, depreciation, and amortization and is used as an indicator of the Company’s overall profitability. These measures do not have any standardized meaning according to International Financial Reporting Standards (“IFRS”) and therefore may not be comparable to similar measures presented by other companies. There are no comparable IFRS financial measures presented in MediPharm or VIVO’s unaudited condensed interim consolidated financial statements. The most directly comparable measure to EBITDA calculated in accordance with IFRS is operating income (loss). MediPharm and VIVO believe that the non-IFRS measure presented herein provides information useful to shareholders and investors in understanding our performance and may assist in the evaluation of the Combined Company’s business relative to that of its peers. For more information, please see the most recent MD&A of each of MediPharm and VIVO available on www.sedar.com.
Cautionary Note Regarding Forward-Looking Information
This news release contains “forward-looking information” and “forward-looking statements” (collectively, “forward-looking statements”) within the meaning of the applicable Canadian securities legislation. All statements, other than statements of historical fact, are forward-looking statements and are based on expectations, estimates and projections as at the date of this news release. Any statement that involves discussions with respect to predictions, expectations, beliefs, plans, projections, objectives, assumptions, future events or performance (often but not always using phrases such as “expects”, or “does not expect”, “is expected”, “anticipates” or “does not anticipate”, “plans”, “budget”, “scheduled”, “forecasts”, “estimates”, “believes” or “intends” or variations of such words and phrases or stating that certain actions, events or results “may” or “could”, “would”, “might” or “will” be taken to occur or be achieved) are not statements of historical fact and may be forward-looking statements. In this news release, forward-looking statements relate to, among other things, statements regarding: the Transaction; the terms and conditions pursuant to which the Transaction will be completed, if at all; the anticipated timing for receipt of necessary court and regulatory approvals for the Transaction; the anticipated timing for completion of the Transaction; the Combined Company; the future financial and operational performance of the Combined Company; the Combined Company’s key business segments, product offerings, pro-forma and overall financial performance; future development of products of the Combined Company; potential future revenue and cost synergies resulting from the Transaction; statements about the Combined Company’s profitability and ability to grow the business going forward following the Transaction; the Combined Company establishing itself as an international pharmaceutical company; a leading position in the projected multibillion-dollar global cannabis pharmaceutical market; becoming the go-to partner for pharmaceutical companies around the globe; potential for material revenue growth for years to come; and the Combined Company's transition towards pharmaceutical and medical markets reaching new heights. Forward-looking statements are necessarily based upon a number of estimates and assumptions that, while considered reasonable, are subject to known and unknown risks, uncertainties, and other factors which may cause the actual results and future events to differ materially from those expressed or implied by such forward-looking statements. Such factors include, but are not limited to: the ability of MediPharm and VIVO to receive all necessary court, shareholder and regulatory approvals for the Transaction; general business, economic, competitive, political and social uncertainties; and other factors discussed in each of MediPharm’s and VIVO’s public filings, available on SEDAR at www.sedar.com. There can be no assurance that such statements will prove to be accurate, as actual results and future events could differ materially from those anticipated in such statements. Accordingly, readers should not place undue reliance on the forward-looking statements and information contained in this news release. Except as required by law, each of MediPharm and VIVO assumes no obligation to update the forward-looking statements of beliefs, opinions, projections, or other factors, should they change.
For further information:
MediPharm Labs Investor Relations
Telephone: 1 416.913.7425 ext. 1525
Email: investors@medipharmlabs.com
Website: www.medipharmlabs.com
VIVO Investor Relations
Michael Bumby, Chief Financial Officer
Email: ir@vivocannabis.com
Website: www.vivocannabis.com
Instagram: https://www.instagram.com/vivo_cannabis/
LinkedIn: https://www.linkedin.com/company/vivo-cannabis-inc/
Facebook: https://www.facebook.com/vivocanna/
Twitter: https://twitter.com/vivo_cannabis
When the Enthusiastic 3 year long becomes the realistic 10 year long....
Decent quarterly results . Thanks for all the updates Excalibur / You DA Man !
Posted Second quarter Results link.
https://www.otcmarkets.com/stock/MEDIF/news/MediPharm-Labs-Reports-Second-Quarter-Results?id=368585
Medipharm sets date to report second Quarter.
https://www.otcmarkets.com/stock/MEDIF/news/MediPharm-Labs-Sets-Date-to-Report-Second-Quarter-2022-Financial-Results?id=368201
I just replied to a guy on the Hexo board claiming they have 14 plus million. Told him Medif won.
I was there, lost and just came back in after news of dried cannabis and prerolls with buying Shelter Cannabis. Then the Australian Labs sale. I think we have a come back on this. They still hold one of the worlds few licenses to manufacture Pharma quality products.
Yeah , well , considering that MEDIF was trading in the dollars range at the time of the BS pulled by NEXO , I wouldn't chalk this up as a win .
Does NEXO even have the $$$ to pay the "judgement" ??? MEDIF is up a PENNY on the news ... ![]()
YUP, and the counter suit has been dismissed. WE WON
MUSIC to my ears been waiting a long time. HEXO lost the battle
MediPharm Labs Awarded Payment Of $9.8M By Ontario Court Of Justice
https://www.otcmarkets.com/stock/MEDIF/news/MediPharm-Labs-Awarded-Payment-of-98M-in-Connection-to-a-2020-Statement-of-Claim-Against-a-Previous-Customer?id=366187
Welcome news today . Is this the $ that HEXO stiffed us for back in the day ?
MediPharm Labs Awarded Payment of $9.8M in Connection to a 2020 Statement of Claim Against a Previous Customer
https://www.otcmarkets.com/stock/MEDIF/news/MediPharm-Labs-Awarded-Payment-of-98M-in-Connection-to-a-2020-Statement-of-Claim-Against-a-Previous-Customer?id=366187
News out - MediPharm selling Australian labs, there is a decimal point between 6 and the 2. Sounds low to me, must be a sign of the times.
https://www.otcmarkets.com/stock/MEDIF/news/MediPharm-Labs-Enters-into-62M-Purchase-Agreement-for-the-Sale-of-Australian-Facility?id=364216
BREAKING: Brazil Supreme Court Authorizes Citizens To Grow Medicinal Cannabis
6:39 pm ET June 14, 2022 (Benzinga) Hot Stories Print
What Happened?
The Sixth Panel of the Superior Court of Justice of Brazil authorized three people to grow marijuana for medicinal purposes, reported O Antagonista. “The Superior Court of Justice decided Tuesday, June 14 to grant two safe conducts that advance the regulation of artisanal marijuana cultivation in Brazil.”
Why It Matters?
The case should serve as a precedent for lower courts and the advance of cannabis legalization in the potentially huge market of 200 million people. The decision contemplates the use of cannabidiol (CBD) for personal use and allows marijuana cultivation only for medicinal purposes, with a prescription. In addition, this gives home growers a legal precedent that can evolve into more comprehensive legislation as in the case of Argentina, where hemp and medicinal cannabis production are already legally produced in the country.
The use of medical marijuana was legalized in Brazil in 2015, but until now patients could only obtain imported medicinal cannabis products with strict authorization from ANVISA (National Sanitary Surveillance Agency). In 2019, Brazil became the third Latin American country to regulate the sale of medical marijuana products after Uruguay and Colombia.
Manufacturers have to import the semi-finished product and can only operate after receiving a special certificate from ANVISA. The importation of whole plants is still prohibited.
CBD products and those containing less than 0.2% THC can be prescribed normally. Products with 0.2% THC or more can only be prescribed for terminal patients or in cases where the patient is not responding to traditional treatments.
“Achieving cannabis regulation through (ANVISA) could imply that Brazil becomes a key player in the Latin American cannabis market, not only because of its geographic and climatic diversity but also because of how significant the market would be for the Brazilian population,” said Silvia Muñoz, former head of Government Affairs for LATAM at the International Cannabis and Cannabinoids Institute in the Czech Republic during an interview a few years ago.
Which Cannabis Companies Are Already In Business In Brazil?
MediPharm Labs Corp. (TSX: LABS) (OTCQX: MEDIF) confirmed in September 2021 a partnership with, XLR8 BRAZIL, a distributor based in Rio de Janeiro. This latest move will strengthen the company's delivery services to the largest medical cannabis market in Latin America.
The two-year agreement would start from the authorization of the product. Pursuant to it, MedPharm Labs chose to provide a wide range of cannabis concentrate formats for the formulated products that XLR8 BRASIL will subsequently distribute.
“In our opinion, Brazil is destined to be a world power in terms of medical cannabis,” said Thiago Callado, CEO of XLRE.
Several months later, in November, the CBD company Panacea Life Sciences, Inc. (OTC: PLSH) confirmed that it was forming a partnership with MyPharma2Go to enter the growing cannabis market in Brazil.
Brazil "is the largest country in South America, with a population of more than 200 million people," noted Nick Cavarra, executive vice president of sales and marketing for Panacea.
In 2022, ANVISA approved the cannabis-based drug, Cannabis Sativa Extract Ease Labs, which joins ten other drugs already approved by ANVISA in this category. Four of them are derived from the whole plant, while the other six contain only CBD.
The American cannabis company Medical Marijuana, inc. (OTC: MJNA) launched its first pharmaceutical subsidiary, HM Pharma, in Brazil in May 2022. Medical Marijuana, Inc.'s subsidiary, HempMeds Brasil, was the first company to legally import products into Brazil in 2015 using the country's compassionate use laws.
“HempMeds Brazil has imported more than 150 thousand prescription products to Brazil through the compassionate model since 2015, and we continue to grow at an average rate of 50% per year. Our market leadership reflects the quality of our products, customer care, and relationships with physicians,” said Matheus Patelli, CEO of HM Pharma.
Cannabis company Avicanna Inc. (TSX:AVCN) (OTCQX: AVCNF) completed its first commercial export of 20 kg of full-spectrum high-CBD psychoactive cannabis extract to Brazil in 2021, through its majority-owned Colombian subsidiary Santa Marta Golden Hemp S.A.S. Avicanna products were developed in Canada and manufactured in Colombia. In 2022, the cannabis firm signed an exclusive license and supply agreement with a South American pharmaceutical company to market its proprietary products. According to information obtained exclusively by El Planteo, the pharmaceutical company that signed the agreement with Avicanna is based in Brazil. Avicanna can earn up to CA 1.3 million ($1.03 million) in license fees if certain short-term milestones are reached.
Image Via El Planteo.
© 2022 Benzinga.com. Benzinga does not provide investment advice. All rights reserved.
Study: Medical Marijuana Reduces Pain, Depression, Increases Life Quality in
Cancer Patients
https://www.newsmax.com/health/health-news/cancer-marijuana-medical-pain/2022/05/24/id/1071322
MediPharm Labs Continues to Lead with Innovation - Launches CBG and Water-Soluble Products
T.LABS |
View original content to download multimedia:
https://www.prnewswire.com/news-releases/medipharm-labs-continues-to-lead-with-innovation--launches-cbg-and-water-soluble-products-301517368.html
David Pidduck is the new CEO of MediPharm Labs
https://seekingalpha.com/news/3824667-david-pidduck-is-the-new-ceo-of-medipharm-labs?source=copyToPasteboard
Wow, is this c-suite change another signal of failure?
HAPPY BIRTHDAY PRESIDENT DONALD J TRUMP FROM CHARLIE WARD & ALL OF THE TRUTH COMMUNITY
WATCH
https://www.bitchute.com/video/VztdCmvZLgU/
CT Thanks; How many more good music artists has been hit like Justin Bieber???
DR. JUDY MIKOVITS PhD - THE PLAN TO INJECT HUMANITY WITH CANCER VIRUSES!
WATCH
https://www.bitchute.com/video/QMT4KXNWow9c/
Justin Bieber recently came out to tell fans he’s come down with Ramsay Hunt
Syndrome, which caused partial paralysis in his face — it turns out RHS is a
known vaccine side effect.

https://thecountersignal.com/justin-bieber-paralysis-is-a-known-vaccine-side-effect/
STATEMENT BY PRESIDENT DONALD J. TRUMP
Quote:
could not copy the entire story-- here is the link
:
https://cdn.nucleusfiles.com/e7/e746fae9-7a5f-462d-a0dd-dd9a11895451/statement-by-president-donald-j.-trump.pdf?
M_P; $500 TRILLION LAWSUIT AGAINST THE FEDERAL GOVT AND OVER 140
MONOPOLISTS (REMOVED BY YOUTUBE IN 5HRS)
WATCH
https://www.bitchute.com/video/QFcfDjifRl6u/
Leaked medicare data shows a 50% rise in all cause mortality of the elderly for the first time ever.
https://www.bitchute.com/video/DdxZLSGHmKLB/
They are right. Covid kills...the Covid vaccine is killing the elderly by the thousands. Source: Fat News.
CLIF HIGH - THE JABBED ARE ABOUT TO GET A RUDE AWAKENING
https://www.bitchute.com/video/bBcmJoO3BRdh/
"Organoids "Jab victim discharging biological creatures woman reports horror after Moderna shot
https://www.bitchute.com/video/BCrvrznQFaME/
SUBMIT TO THE BIOWEAPON OR REMAIN OUR HOSTAGE FOREVER --
BRENT JOHNSON - NWO Totalitarian Dictators Blackmail Anti-Human Genocide
killer agenda super communistic evil khazarian
WATCH
https://www.bitchute.com/video/l881ILlbk6UJ/
BREAKING!!! COVID VACCINE CLAIMS OVERWHELM COURTS
WATCH
https://www.bitchute.com/video/fxqw0Y7twx3o/
MASS DEPOPULATION ACCORDING TO PFIZER! - DOCUMENT LEAK
PROVES VACCINE IS CAUSING DIE OFF!
WATCH
https://www.bitchute.com/video/Ga7fvxiL1jFa/
Zardiw; Sarah Palin Pulls Ahead In Race For Alaska Representative
Photo of Carmine Sabia Carmine SabiaJune 12, 2022

https://conservativebrief.com/sarah-p-63700/
CT; M_P; VACCINES ARE MAKING PEOPLE NEUROLOGICALLY NUMB
WATCH
https://www.bitchute.com/video/aT3AxrAOGWeT/
US Is "Beyond Bankrupt" - Kim Dotcom Fears "Controlled Demolition" Enabling A "New Dystopian Future"
Tyler Durden's Photo
BY TYLER DURDEN
SATURDAY, JUN 11, 2022 - 04:44 AM
https://www.zerohedge.com/markets/its-worse-many-can-imagine-kim-dotcom-sees-controlled-demolition-enabling-new-dystopian
https://rumble.com/v1896jo-real-love.html
https://www.whatdoesitmean.com/index.htm
God Bless.
Ps.
Bill Holter – A Parallel Economy Is Emerging Which Wreak Havoc On The [CB]
System - X22 Report - VIDEO
https://x22report.com/aiovg_videos/bill-holter-a-parallel-economy-is-emerging-which-wreak-havoc-on-the-cb-system/
Jan. 6 Was ‘An Inside Job to Entrap People Who Attended Trump’s Speech’:
Investigative Journalist.. :+O
https://www.theepochtimes.com/jan-6-was-an-inside-job-to-entrap-people-who-attended-trumps-speech-investigative-journalist_4524391.html

Study: Medical Marijuana Reduces Pain, Depression, Increases Life Quality in
Cancer Patients
https://www.newsmax.com/health/health-news/cancer-marijuana-medical-pain/2022/05/24/id/1071322
HAPPY BIRTHDAY PRESIDENT DONALD J TRUMP FROM CHARLIE WARD & ALL OF THE TRUTH COMMUNITY
WATCH
https://www.bitchute.com/video/VztdCmvZLgU/
CT Thanks; How many more good music artists has been hit like Justin Bieber???
DR. JUDY MIKOVITS PhD - THE PLAN TO INJECT HUMANITY WITH CANCER VIRUSES!
WATCH
https://www.bitchute.com/video/QMT4KXNWow9c/
Justin Bieber recently came out to tell fans he’s come down with Ramsay Hunt
Syndrome, which caused partial paralysis in his face — it turns out RHS is a
known vaccine side effect.

https://thecountersignal.com/justin-bieber-paralysis-is-a-known-vaccine-side-effect/
STATEMENT BY PRESIDENT DONALD J. TRUMP
Quote:
could not copy the entire story-- here is the link
:
https://cdn.nucleusfiles.com/e7/e746fae9-7a5f-462d-a0dd-dd9a11895451/statement-by-president-donald-j.-trump.pdf?
M_P; $500 TRILLION LAWSUIT AGAINST THE FEDERAL GOVT AND OVER 140
MONOPOLISTS (REMOVED BY YOUTUBE IN 5HRS)
WATCH
https://www.bitchute.com/video/QFcfDjifRl6u/
Leaked medicare data shows a 50% rise in all cause mortality of the elderly for the first time ever.
https://www.bitchute.com/video/DdxZLSGHmKLB/
They are right. Covid kills...the Covid vaccine is killing the elderly by the thousands. Source: Fat News.
CLIF HIGH - THE JABBED ARE ABOUT TO GET A RUDE AWAKENING
https://www.bitchute.com/video/bBcmJoO3BRdh/
"Organoids "Jab victim discharging biological creatures woman reports horror after Moderna shot
https://www.bitchute.com/video/BCrvrznQFaME/
SUBMIT TO THE BIOWEAPON OR REMAIN OUR HOSTAGE FOREVER --
BRENT JOHNSON - NWO Totalitarian Dictators Blackmail Anti-Human Genocide
killer agenda super communistic evil khazarian
WATCH
https://www.bitchute.com/video/l881ILlbk6UJ/
BREAKING!!! COVID VACCINE CLAIMS OVERWHELM COURTS
WATCH
https://www.bitchute.com/video/fxqw0Y7twx3o/
MASS DEPOPULATION ACCORDING TO PFIZER! - DOCUMENT LEAK
PROVES VACCINE IS CAUSING DIE OFF!
WATCH
https://www.bitchute.com/video/Ga7fvxiL1jFa/
Zardiw; Sarah Palin Pulls Ahead In Race For Alaska Representative
Photo of Carmine Sabia Carmine SabiaJune 12, 2022

https://conservativebrief.com/sarah-p-63700/
CT; M_P; VACCINES ARE MAKING PEOPLE NEUROLOGICALLY NUMB
WATCH
https://www.bitchute.com/video/aT3AxrAOGWeT/
US Is "Beyond Bankrupt" - Kim Dotcom Fears "Controlled Demolition" Enabling A "New Dystopian Future"
Tyler Durden's Photo
BY TYLER DURDEN
SATURDAY, JUN 11, 2022 - 04:44 AM
https://www.zerohedge.com/markets/its-worse-many-can-imagine-kim-dotcom-sees-controlled-demolition-enabling-new-dystopian
https://rumble.com/v1896jo-real-love.html
https://www.whatdoesitmean.com/index.htm
God Bless.
Ps.
Bill Holter – A Parallel Economy Is Emerging Which Wreak Havoc On The [CB]
System - X22 Report - VIDEO
https://x22report.com/aiovg_videos/bill-holter-a-parallel-economy-is-emerging-which-wreak-havoc-on-the-cb-system/
Jan. 6 Was ‘An Inside Job to Entrap People Who Attended Trump’s Speech’:
Investigative Journalist.. :+O
https://www.theepochtimes.com/jan-6-was-an-inside-job-to-entrap-people-who-attended-trumps-speech-investigative-journalist_4524391.html

David Pidduck is the new CEO of MediPharm Labs https://seekingalpha.com/news/3824667-david-pidduck-is-the-new-ceo-of-medipharm-labs?source=copyToPasteboard
Wow, is this c-suite change another signal of failure?
I am still holding and hoping.
MediPharm Labs Continues to Lead with Innovation - Launches CBG and Water-Soluble Products
T.LABS | 1 day ago
View original content to download multimedia:
https://www.prnewswire.com/news-releases/medipharm-labs-continues-to-lead-with-innovation--launches-cbg-and-water-soluble-products-301517368.html
MediPharm Labs Sets Date to Report Fourth Quarter and Full Year 2021 Financial Results
Tue, March 29, 2022, 7:00 AM·
4 min read
TORONTO, March 29, 2022 /CNW/ - MediPharm Labs Corp. (TSX: LABS) (OTCQX: MEDIF) (FSE: MLZ) ("MediPharm" or the "Company") a pharmaceutical company specialized in precision-based cannabinoids, is pleased to announce it will release fourth quarter financial results for the three and twelve month period ended December 31, 2021 after markets close on Thursday, March 31, 2022.
MediPharm's executive management team will also host a conference call and audio webcast on Friday, April 1, 2022, at 8:30 a.m. eastern time to discuss the Company's financial results and outlook.
Audio Conference Call Dial in Details:
Toll-free number: +1 (888) 330-2454 / International number: +1 (236) 789-2714 / Conference ID: 4921762
Participants are asked to dial in approximately 15 minutes before the start of the call.
Audio Webcast:
An audio webcast will be available in the Events section of the MediPharm website https://www.medipharmlabs.com/investors or by visiting the following link here.
For those who are unable to participate on the live conference call or webcast, a replay will be available approximately one hour after completion of the call.
About MediPharm Labs Corp.
Founded in 2015, MediPharm specializes in the development and manufacture of purified, pharmaceutical-quality cannabis concentrates, API and advanced derivative products utilizing a Good Manufacturing Practices certified facility with ISO standard-built clean rooms. MediPharm has invested in an expert, research driven team, state-of-the-art technology, downstream purification methodologies and purpose-built facilities with five primary extraction lines for delivery of pure, trusted and precision-dosed cannabis products for its customers. Through its wholesale and white label platforms, MediPharm formulates, develops (including through sensory testing), processes, packages and distributes cannabis extracts and advanced cannabinoid-based products to domestic and international markets.
In 2021, MediPharm received a Drug Establishment Licence from Health Canada, becoming the only company in North America to hold a domestic Good Manufacturing Practices licence for the extraction of natural cannabinoids. The Company carries out its operations in compliance with all applicable laws in the countries in which it operates.
CAUTIONARY NOTE REGARDING FORWARD-LOOKING INFORMATION:
This news release contains "forward-looking information" and "forward-looking statements" (collectively, "forward-looking statements") within the meaning of the applicable Canadian securities legislation. All statements, other than statements of historical fact, are forward-looking statements and are based on expectations, estimates and projections as at the date of this news release. Any statement that involves discussions with respect to predictions, expectations, beliefs, plans, projections, objectives, assumptions, future events or performance (often but not always using phrases such as "expects", or "does not expect", "is expected", "anticipates" or "does not anticipate", "plans", "budget", "scheduled", "forecasts", "estimates", "believes" or "intends" or variations of such words and phrases or stating that certain actions, events or results "may" or "could", "would", "might" or "will" be taken to occur or be achieved) are not statements of historical fact and may be forward-looking statements. Forward-looking statements are necessarily based upon a number of estimates and assumptions that, while considered reasonable, are subject to known and unknown risks, uncertainties, and other factors which may cause the actual results and future events to differ materially from those expressed or implied by such forward-looking statements. Such factors include, but are not limited to: general business, economic, competitive, political and social uncertainties; the inability of MediPharm to obtain adequate financing; the delay or failure to receive regulatory approvals; and other factors discussed in MediPharm's filings, available on the SEDAR website at www.sedar.com. There can be no assurance that such statements will prove to be accurate, as actual results and future events could differ materially from those anticipated in such statements. Accordingly, readers should not place undue reliance on the forward-looking statements and information contained in this news release. Except as required by law, MediPharm assumes no obligation to update the forward-looking statements of beliefs, opinions, projections, or other factors, should they change.
They have a long way to go. I am still holding ![]()
MediPharm Labs Enters United States Pharmaceutical Market with Submission of FDA DMF
Debt paid 18 months early / now that's my kind of management !
at this point its Vegas or bust ! / thanks Funman .
Love this: MediPharm Labs has no other material debt and full ownership of its assets, which includes two GMP facilities – one in the province of Ontario, Canada and the other in the state of Victoria, Australia. Other major assets include manufacturing infrastructure and equipment.
MediPharm Labs Completes Payment of All Convertible Debt
January 05, 2022 07:00 ET | Source: MediPharm Labs Corp.
https://www.globenewswire.com/news-release/2022/01/05/2361498/0/en/MediPharm-Labs-Completes-Payment-of-All-Convertible-Debt.html
...
TORONTO, Jan. 05, 2022 (GLOBE NEWSWIRE) -- MediPharm Labs Corp. (TSXV: LABS) (OTCQX: MEDIF) (FSE: MLZ) (“MediPharm Labs” or the “Company”) a pharmaceutical company specialized in precision-based cannabinoids, is pleased to announce that it has completed all payments required under its 2020 $41 million unsecured convertible debt.
“MediPharm continues on its plan and path to profitability, and paying off our convertible debt in full is a major milestone on that path,” said Bryan Howcroft, CEO, MediPharm Labs. “Being essentially debt free with a strong cash position adds even more longevity to our business. As part of our growing nascent industry, this advantageous position differentiates us from some of our peers who may not have the runway to realize the industry’s full potential.”
On June 8, 2020, the Company closed a private placement with an US institutional investor for gross proceeds of $37.8 million through the issuance of two senior unsecured convertible notes. Starting in October 2020, the notes amortized through bi-monthly installment payments payable, in cash or shares, on the first and tenth business day of each month prior to the maturity date of June 8, 2023. During the interim period between payment dates, the holder of the notes had the option to convert accelerated installment amounts, in whole or in part at an installment conversion price calculated in accordance with the terms of the notes. As of December 31, 2021, the entire debt has been repaid in full through both cash installments and share accelerations. This extinguishes the debt in full 18 months before the maturity date.
MediPharm Labs has no other material debt and full ownership of its assets, which includes two GMP facilities – one in the province of Ontario, Canada and the other in the state of Victoria, Australia. Other major assets include manufacturing infrastructure and equipment.
The Company is now debt agreement free with a clean balance sheet and strong cash position. This position paired with a robust plan to reduce overhead, operating expenses and general and administrative expenses gives MediPharm Labs longevity to execute on its sales contracts and pipeline.
Makes no money. Completely changed over top management last year.
Tiger Money Wednesday, 01/05/22 01:19:10 PM
Re: FUNMAN post# 6676
Post # 6677 of 6677
Does this company make any money? Sorry for the lack of dd question but any insight would be helpful. Thanks
Does this company make any money? Sorry for the lack of dd question but any insight would be helpful. Thanks
|
Followers
|
73
|
Posters
|
|
|
Posts (Today)
|
0
|
Posts (Total)
|
6726
|
|
Created
|
10/05/18
|
Type
|
Free
|
| Moderators | |||
TORONTO, Jan. 29, 2021 (GLOBE NEWSWIRE) -- MediPharm Labs Corp. (TSX: LABS) (OTCQX: MEDIF) (FSE:MLZ) (“MediPharm Labs” or the “Company”) a global leader in specialized, research-driven pharmaceutical-quality cannabis extraction, distillation and derivative products, is pleased to announce Greg Hunter will join MediPharm Labs as Chief Financial Officer (CFO), effective February 8, 2021.
Greg Hunter brings over 20 years of experience as a business executive holding various senior finance and leadership roles across multiple industries including healthcare distribution, telecommunications, pharmaceuticals, biotechnology, medical device and consumer packaged goods. Greg also brings a track record and deep expertise in capital management, audit, compliance, tax, treasury, ERP, manufacturing, contract management and pricing strategy.
“As MediPharm continues to focus on accelerating the growth and execution of our global pharmaceutical, health and wellness business, Greg’s background in growing large pharmaceutical operations combined with a track record in strategic capital allocation and cost management is a strong addition to our Company,” said Keith Strachan, President and Interim CEO, MediPharm Labs. “As we look to seize new growth opportunities and expand our product offerings, his addition will ensure that we are well positioned to execute on our plans and deliver profitable growth in the future.”
Most recently, Greg was Chief Financial Officer of Medical Pharmacies Group Limited, a leading pharmacy and medical equipment manufacturer and distributor in Canada. Previously in the pharmaceuticals industry, Greg held various senior management roles with Baxter International Inc. including serving as CFO of Baxter’s Canadian subsidiary. Greg also previously held various senior operational and finance roles at Janssen-Ortho Inc., a Johnson and Johnson company.
Keith Strachan added, “I would also like to thank Olga Utkutug for her many contributions and leadership as Interim CFO. We look forward to her continued support as a key member of our finance team.”
Greg holds an MBA from McMaster University, an Honors B.Sc. in Microbiology and Immunology from University of Western Ontario and is a Chartered Professional Accountant.
In connection with Greg’s appointment as CFO, the Company granted 600,000 stock options to him with an exercise price set at the close of business on January 28, 2021. Each grant has a five-year term expiring January 28, 2026, and vests in five equal instalments, the first of which vests immediately with the four other instalments vesting on the dates which are six, twelve, eighteen and twenty-four months from the grant date. The stock options are subject to any necessary regulatory approvals.
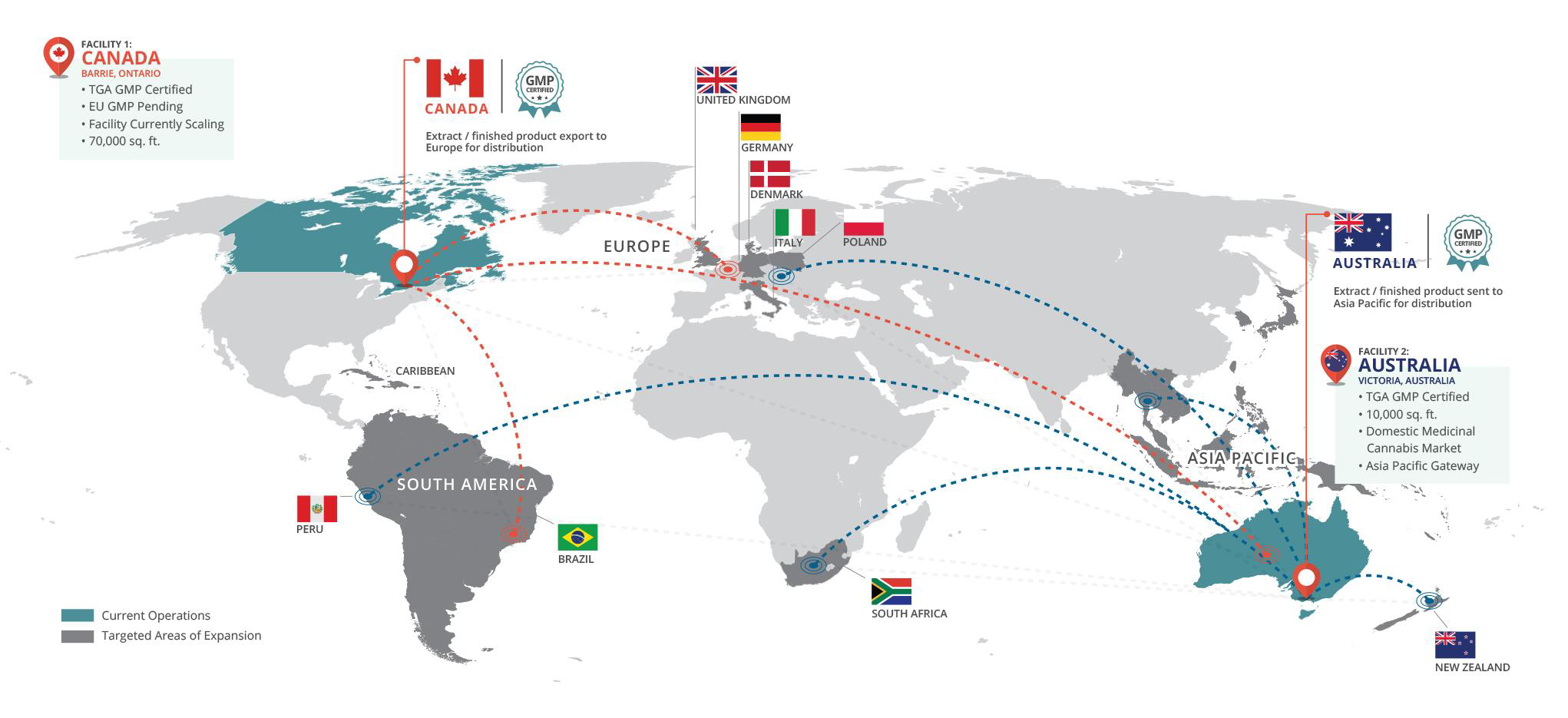
BAD VILBEL, Germany and TORONTO, Oct. 05, 2020 (GLOBE NEWSWIRE) -- As one of the leading European Consumer Healthcare and Generics companies, STADA, with a product presence in 120 countries, has signed an exclusive supply agreement with MediPharm Labs Inc. (“MediPharm”), a wholly owned subsidiary of MediPharm Labs Corp. (TSX: LABS) (OTCQX: MEDIF) (FSE:MLZ) (“MediPharm Labs” or the “Company”) a global leader in specialized, research-driven pharmaceutical-quality development and manufacture of cannabis API and derivative products to provide medical cannabis products for the European pharma sector.
“Working with MediPharm will meet the needs of pharmacists and patients and deliver on STADA’s purpose of caring for people’s health as a trusted partner,” commented STADA CEO Peter Goldschmidt. “This partnership with MediPharm demonstrates STADA’s ambition to be the go-to-partner for Generics, Consumer Health and Specialty Products.”
Under the terms of the exclusive partnership, MediPharm will supply GMP certified medical cannabis products to STADA, as well as manufacturing, logistics, and regulatory support. STADA will be responsible for commercializing the cannabis products, initially in Germany as well as marketing and medical education utilizing a pharmaceutically experienced field force.
“This partnership is exactly the type of business MediPharm has been pursuing since its inception and a validation of our strategy to deliver GMP-certified cannabis products that can be used in multiple new products in multiple markets around the world,” said Pat McCutcheon, CEO, MediPharm Labs. “This mandate has meaningful revenue potential for MediPharm Labs and we couldn’t be more excited to partner with a company that has the reputation and resources that STADA commands. As a powerhouse in sales and distribution of pharmaceuticals and non-prescription consumer health products, STADA has the ability to become a transformative force in European medical cannabis markets and we are thrilled to be their exclusive partner.”
The partners will initially focus on Germany, before potentially expanding to other European countries and territories. The partnership also signals MediPharm’s entry into the global pharmaceutical industry within a major European market.
With over 83 million inhabitants benefitting from broad access to healthcare services, Germany currently represents an estimated three-quarters of the current EU medical cannabis market.
The Medical Cannabis Network reports the medical cannabis market in Germany is currently valued at between €150m and €175m, despite only around 10% of the 20,000 pharmacies in Germany selling medical cannabis products today. Furthermore, only a limited number of doctors in Germany currently prescribe medical cannabis. With greater awareness and education around the benefits of medical cannabis, the Medical Cannabis Network estimates that Germany’s dominance in the European cannabis market could expand to €1.5bn by 2025.(1)
This is a further milestone for the global cannabis industry as STADA, in partnership with MediPharm, forges the way as a large pharmaceutical company to commercialize medical cannabis products.
Details of the products that STADA will bring to market through this partnership with MediPharm Labs will be unveiled in the coming months in the respective relevant communication channels.
June 25th, 2020
TORONTO, June 25, 2020 (GLOBE NEWSWIRE) -- MediPharm Labs Corp. (TSX: LABS) (OTCQX: MEDIF) (FSE:MLZ) (“MediPharm Labs” or the “Company”) a global leader in specialized, research-driven pharmaceutical-quality cannabis extraction, distillation and derivative products, today announced a further expansion of its innovative, pharma-quality family of branded products with the retail introduction of CBD25:5 Release Formula (“CBD25:5”).
MediPharm Labs announces the launch of CBD25:5 Release Formula, a 25:5 CBD:THC formulated oil to harness the properties of these two cannabinoids for patients and adult consumers.

“We’ve positioned ourselves to lead the market in well-defined Cannabis 2.0 categories on our own and through our growing white-label supply business,’” said Pat McCutcheon, Chief Executive Officer, MediPharm Labs. “Today’s announced product launch, coupled with other recently formed partnerships, demonstrate just how far we’ve come in realizing our ambition to transition our focus to commercializing and distributing a robust portfolio of pharma-quality products.”
Cannabis 2.0 Update
In late December 2019, the Company commenced shipments of Cannabis 2.0 products into three of Canada’s provincial jurisdictions – British Columbia, Manitoba and Saskatchewan.
Since then, MediPharm Labs has received multiple reorders and added several notable new customers:
-MediPharm Labs is able to supply and provide tailored product formulations and solutions to its Licensed Producer and CPG customers who serve the four product categories recognized under Schedule 4 of the Cannabis Act: cannabis extracts (for ingestion and inhalation), cannabis topicals, edible cannabis (beverages and food products) and cannabis oils. This broad exposure to Cannabis 2.0 categories is a key business strength.
WATCH THESE VIDEOS:
extraction:
https://youtu.be/HAzs2xhbHgM
soft gel manufacturing:
https://www.youtube.com/watch?v=t9tKO_cFLnY&feature=youtu.be
December 30, 2019
"...MediPharm Labs Inc. (“MediPharm”) has received a licence amendment from Health Canada allowing for production to begin in the recently expanded area of its specialized manufacturing facility in Barrie, Ontario.
This amendment increases MediPharm’s licensed facility footprint by around three times, or 16,746 sq. ft., to a total of approximately 25,000 sq. ft., allowing it to productively use more of its manufacturing space for cannabis activities – including automated downstream production and packaging, cannabis quality control and testing, research and development and secure storage to support the fulfillment and distribution of new product format formulations and orders.
MediPharm Labs Canadian Facility Expansion
In 2019, MediPharm Labs commenced a phased plan to expand its licensed space and scale its operations in Canada within its 70,000 sq.ft. facility in Barrie, Ontario. Under new regulations, the Company was required to complete construction prior to applying for an expansion licence. Today’s announcement means MediPharm received its Health Canada licence amendment just four months after completing construction.
The recently completed first phase of expansion added 16,746 square feet, all of it now licensed. This represents a three-fold increase in total licensed footprint suitable for cannabis activities to a total of approximately 25,000 sq. ft.
The now-completed first phase of the expansion includes:

July 16 update: MediPharm Labs Provides Operational Update and Increases Average Weekly Production of Active Cannabinoid Component to 75 Million Milligrams
Growing Private Label Production and White Label Platform: MediPharm Labs has increased production of private label cannabis concentrate products and distillate supply – including CBD or THC dominant as well as balanced formulations – to meet the growing demand for specialty concentrate based consumer end products ahead of legalization of vapeables, edibles and topicals in the fall of 2019. Production and quality operations teams ramped up weekly production output to approximately 75 million milligrams of active cannabinoid component concentrate at the end of Q2 2019.
To date, MediPharm Labs has signed four significant contracts for the sale of private label cannabis concentrate including to Canopy Growth, Cronos Group and AusCann. The Company also launched its White Label platform to service direct-to-consumer brands, CPG companies and large LPs in Canada. In June 2019, MediPharm announced its first white label deal to produce a minimum of approximately two million vape pens with Ace Valley, subject to purchase orders from provincial distributors.
Increasing Production Volumes: The number of dry cannabis suppliers and concentrate purchasers in Canada continues to increase. In turn, this has resulted in higher demand that supports MediPharm Labs’ differentiated, private label business model.
The Company announced it successfully acquired 9,000 KG of dried cannabis in the last three weeks of June 2019 expected to be processed and sold in Q3 2019, and 5,000 KG in the last two weeks of March 2019 that were processed and sold in Q2 2019.
Enhanced Governance and Quality: To date, the Company has purchased dried cannabis from over 23 suppliers across the country. MediPharm Labs has conducted in-depth qualifications of all of its dry cannabis and product suppliers.
Potential suppliers to MediPharm Labs are subjected to rigorous scrutiny before entering into agreements which require an enhanced Quality Agreement beyond Health Canada standards. This enhanced supplier qualification aligns with our pharmaceutical-like standards for production of cannabis oil and derivative products. All suppliers and customers remain in good standing with Health Canada Licensing and Inspection division.
Broadening Domestic and Global Distribution: During the quarter, MediPharm Labs became the only third-party concentrate manufacturer to begin shipping white label products, including bottled oil, to Provincial cannabis distributors. MediPharm Labs’ white label products can be found in three provinces including Ontario, British Columbia and Alberta. The Company expects to rollout distribution of Health Canada approved products to all remaining provinces during H2 2019.
MediPharm Labs successfully completed its first international export of commercial volume cannabis concentrate to AusCann in Australia in June 2019. The Company continues to focus on accessing and distributing its private label cannabis concentrate to global markets, including across Europe, and is working on various commercial terms.
Expanding Footprint in Canada: At MediPharm Labs’ facility in Barrie, Ontario, the Company is adding over 25,000 square feet of purpose-built production space for filling and packing automation, new product manufacturing, cannabinoid isolation activities and specialized R&D projects. Taking a phased approach, operations in this new space are expected to come as each phase becomes approved by Health Canada during the second half of 2019.
Building Scale and Increasing Capacity: MediPharm Labs has successfully completed several equipment automation and innovation projects. The success of these optimization projects has resulted in an increased in capacity of dry cannabis processing to 300,000 KG annually.
As a global leader in extraction with a focus on providing high quality cannabinoid concentrates, MediPharm Labs recently completed a 14-month project for an additional, fully customized large-scale extraction line. The extractor has completed its factory testing in Europe and is being shipped to the Company’s Barrie, Ontario facility. Upon completion of installation, operator training, EU GMP qualification and regulatory prestart activities, operational capacity of specialized and automated dry cannabis processing will exceed 500,000 KG annually.
New Product Development: MediPharm Labs is continuing to evolve its product mix. The bulk of its production and revenue to date has been in high quality winterized resin. This critical input ingredient will become the key building block for future high-growth and high-margin products:
Soft Gel Caps: MediPharm Labs has focused on building scale across operations and product lines including bottled oil and gel caps. An industrial scale soft gel capsule project is underway. Equipment has been deployed, equipment training has started, and formulation work is being conducted.
Vapourizers: The Company is building the foundation to be one of the largest vaporizer cartridge manufacturers in Canada. With new regulations finalized and set to come into effect on October 17, 2019 MediPharm Labs has finalized various formulations and started vapourizer pen cartridge filling research and development trials to ensure readiness to serve current and future white-label partners.
Australia Gaining Momentum: MediPharm Labs Australia received its manufacturing licence in May from the Australian Office of Drug Control. Construction of the facility is well underway and nearing completion with equipment ordered for installation in H2 2019. MediPharm Labs Australia is expected to have 75,000 KG of annual capacity.
Financing: The Company completed a $75 million bought deal financing to fund capital expenditures at its Canadian and Australian facilities, for domestic and international expansions, research and development and general corporate purposes. Signed a $20 million debt facility term sheet with a Schedule 1 Bank.
MediPharm Labs Announces Addition to $1.2B MJ ETFMG Alternative Harvest ETF (USA)
Tuesday 21 May 2019

TORONTO, May 22, 2019 (GLOBE NEWSWIRE) -- MediPharm Labs Corp. (TSXV: LABS) (OTCQX: MEDIF) (FSE:MLZ) (“MediPharm Labs”) a leader in specialized, research-driven cannabis extraction and cannabinoid isolation, is pleased to announce it has been selected by The Mount Sinai Hospital, New York City (“Mount Sinai”), to participate in a clinical trial dedicated to developing a non-addictive oral gelcap medication for the treatment of opioid use disorder through anti-anxiety intervention utilizing hemp-derived CBD combined with a proprietary formula (the “Formula”). This will be a U.S. and international large-scale, multi-site clinical trial that will include at least 500 patients spanning the United States, Canada, Australia, Europe and Jamaica.
The clinical trial will be led by renowned principal researcher, Dr. Yasmin L. Hurd, PhD, the Ward-Coleman Chair of Translational Neuroscience at the Icahn School of Medicine at Mount Sinai, New York City, and Director of the Center for Addictive Disorders for the Mount Sinai Behavioral Health System...
...MediPharm Labs will be the exclusive manufacturer of a proprietary hemp-derived CBD oral gelcap medication utilizing the Formula provided by Timeless Herbal for all phases of the clinical trials related to this study that will allow researchers to test an investigational product containing CBD active ingredient.
MAY 17, 2019
Emerald Health Therapeutics, Inc. (“Emerald”) (TSXV: EMH; OTCQX: EMHTF) has shipped 6,000 40 ml units of its SYNC 25 CBD oil to the British Columbia Liquor Distribution Branch (BCLDB). The Emerald-branded pure CBD oil offers consumers a non-THC, smoke-free product alternative.
“We are pleased to offer our new Emerald-branded wellness product SYNC 25 to the recreational marketplace in British Columbia,” said Dr. Avtar Dhillon, President and Executive Chairman of Emerald. “The introduction of SYNC 25 marks the next step in our product growth strategy to develop innovative and quality consumer products that respond to a growing demand while providing higher profit margins.”
The SYNC 25 CBD oil product was extracted by MediPharm Labs Inc...
May 15, 2019
MediPharm Labs Corp. (TSXV: LABS) (OTCQX: MEDIF) (FSE:MLZ) (“MediPharm Labs”) a leader in specialized, research-driven cannabis extraction and cannabinoid isolation, is pleased to announce that its wholly-owned subsidiary, MediPharm Labs Inc. (“MediPharm”), has signed a committed term sheet for a $20 million senior secured revolving credit facility ("Credit Facility") from a Canadian Schedule 1 Bank. The Credit Facility is intended to provide MediPharm Labs access to non-dilutive capital to fund planned growth, as well as for general corporate and working capital purposes.
The Credit Facility consists of a $15 million operating loan with a one-year term and a $5 million non-revolving equipment term loan with a three-year term. The Credit Facility, once closed, will bear interest at the Schedule 1 Bank’s prime lending rate plus 1.85% per annum.
“This commitment marks yet another important milestone that will provide access to non-dilutive capital to support our plans for accelerated growth as we continue to ramp up operations ahead of expected legalization of broader concentrate-based products this fall,” said, Patrick McCutcheon, CEO, MediPharm Labs. “We are pleased to have a leading financial institution support MediPharm Labs as we continue to innovate and expand our private and white label offerings to serve a growing demand for high-quality, adult-use and medical cannabis products.”

14 MAY 2019
Cronos Group and MediPharm Labs Enter Additional Multi-Year Tolling Arrangement
TORONTO, May 14, 2019 (GLOBE NEWSWIRE) -- Cronos Group Inc. (NASDAQ: CRON) (TSX: CRON) (“Cronos Group” or the “Company”), is pleased to announce that it has entered into a multi-year supply agreement with MediPharm Labs Corp. (TSXV: LABS) (OTCQX: MEDIF) (“MediPharm Labs”).
MediPharm Labs will supply Cronos Group with approximately $30 million of high-quality private label cannabis concentrate over 18-months, and, subject to certain renewal and purchase options, potentially up to $60 million over 24-months. In addition, Cronos Group and MediPharm Labs have entered into a multi-year tolling agreement, where Cronos Group will supply bulk cannabis to MediPharm Labs’ state of the art extraction facility in Barrie, Ontario, to fulfill certain additional processing needs of the Company.




2019 Year-to-Date Highlights
(Mr. McCutcheon continued)... “Looking ahead, we are now working on an ambitious, well-planned agenda for 2019 that will enable MediPharm Labs to extend our first-mover advantage. We are ramping up production, adding capacity, targeting EU GMP certification, expanding product offerings, developing R&D and IP, signing new sales agreements, and executing on our M&A and international growth pipeline.”
“Most importantly, we see all of this as a starting point. We expect to accelerate our growth globally as the size of our addressable market increases and we strengthen our foothold domestically with the expected legalization of vapeables, edibles, beverages and topicals providing a strong growth trajectory in Canada. We will continue to capitalize on the numerous opportunities available to us through effective capital deployment and continued expert execution to create shareholder value for the long term.”
2019 Strategic Priorities

For further information, please contact:
Laura Lepore, VP, Investor Relations
Telephone: 705-719-7425 ext 216
Email: investors@medipharmlabs.com
Website: www.medipharmlabs.com
| Volume | |
| Day Range: | |
| Bid Price | |
| Ask Price | |
| Last Trade Time: |
